Summary
Forced metastasis models, those in which the step of intravasation is bypassed, can be used to investigate the mechanisms underlying metastasis and evaluate potential therapeutic targets. Here, we present a protocol for using three forced models of lung and liver metastasis to generate metastasis within 3–4 weeks in approximately 99% of injected mice. We describe steps for cancer cell preparation, mouse analgesia and anesthesia; injecting through intrasplenic, intraportal, and intravenous techniques; and daily evaluation of metastasis.
For complete details on the use and execution of this protocol, please refer to Giannou et al.1
Subject areas: Cancer, Immunology, Model Organisms
Graphical abstract

Highlights
-
•
Steps to establish forced liver and lung metastasis in mouse models
-
•
Intrasplenic, intraportal, and intravenous injection for induction of metastasis
-
•
Experimental processing of harvested liver and lung
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Forced metastasis models, those in which the step of intravasation is bypassed, can be used to investigate the mechanisms underlying metastasis and evaluate potential therapeutic targets. Here, we present a protocol for using three forced models of lung and liver metastasis to generate metastasis within 3–4 weeks in approximately 99% of injected mice. We describe steps for cancer cell preparation, mouse analgesia and anesthesia; injecting through intrasplenic, intraportal, and intravenous techniques; and daily evaluation of metastasis.
Before you begin
Forced metastasis models are performed by injecting cancer cells into the tail vein2 (which then leads to the vena cava, then the right heart, and finally to the lung capillary system) or into the spleen3 (which is drained by the splenic vein, which merges into the portal vein that provides the liver with nutrient-rich blood from the intestinal tract) or directly into the portal vein. Despite not completely mimicking all aspects of metastasis, especially the part of intravasation, these models are commonly used due to their rather easy experimental execution, the short time span of metastasis development, the number of developed metastasis, and nearly full penetration of metastasis within that time span. While many of these protocols have been previously published,4,5,6 we describe improved protocols for intravenous, intrasplenic and intraportal injection of cancer cells in this report.
We also routinely use spontaneous models for liver and lung metastasis. Spontaneous models also include the step of extravasation into the investigated models, but are often more difficult to perform, have longer observation time spans and result in lower yield of metastatic foci, which might make it difficult to detect effects in different experimental groups.
Institutional permissions
These models describe experiments on living mice. All these experiments must be performed in accordance with local and/or national guidelines and must follow appropriate regulations. All experiments for this protocol were approved by the Institutional Review Board “Behörde für Justiz und Verbraucherschutz (Veterinärwesen/Lebensmittelsicherheit)” (Hamburg, Germany) under the sanction numbers N088/19, G056/16 and in compliance with the Yale Institutional Animal Care and Use Committee protocols (approved under the number 2012-10365).
Animal housing
Timing: 2 weeks
-
1.
Acquire 6- to 8-week-old mice and let them get used to your experimental area for 2 weeks.
CRITICAL: Make sure to use the right background mouse strain for your tumor cells of choice. For the following, we will describe all further steps using the murine colorectal cancer cell line, MC38, for C57BL/6 mice. Alternatively, the murine colorectal cancer cell line, CT26, can be used equivalently when doing experiments with BALB/c mice. Other cancer cell lines can also be used; however, they have not been tested by our lab so far.
CRITICAL: Make sure to always use sex- and age-matched experimental groups, since these factors may influence the outcome. Moreover, when using genetically modified mice, use wild-type littermates as control groups to minimize the effects of different genetic backgrounds as a reason for observed differences between experimental groups.
-
2.
24 h before starting rodent surgery, replace the drinking water of mice with analgetic drinking water (temperature 22°C–23°C and maximum storage 24 h).
CRITICAL: Change this analgetic drinking water daily.
Cell preparation
Timing: 1–2 weeks
-
3.
Bring 1 mL of cryopreserved MC38 cells that have been stored in a concentration of 1 million cells/mL in 10% DMSO/90% FBS to 37°C using a water bath.
-
4.
Transfer the solution of cells in 9 mL cell culture medium (see “materials and equipment”; temperature 4°C and maximum storage 1–2 weeks) and centrifuge at 300 × g for 5 min to remove the DMSO.
-
5.
Seed cells onto a 100 mm petri dish by diluting the MC38 cells with 10 mL cell culture medium warmed up to 37°C (see “materials and equipment”; temperature 4°C and maximum storage 1–2 weeks).
-
6.
Incubate the cells for 24 h in an incubator (5% CO2) at 37°C.
-
7.
Replace medium with 10 mL fresh cell culture medium and incubate the cells for another 24 h in a 5% CO2 incubator at 37°C.
-
8.
Check the cells daily under a microscope and proceed only when a confluence of 70% is reached.
-
9.Split the cells.
-
a.Remove and discard the medium completely. Since the cancer cells are adherent, they should stick to the bottom of the plate.
-
b.Rinse the plate with 10 mL PBS warmed to room temperature and discard what has been washed off.
-
c.Add 1 mL of EDTA-Trypsin warmed up to room temperature to the cells and incubate the cells for 3 min in an incubator (5% CO2) at 37°C.
-
d.Add 19 mL fresh cell culture medium (see “materials and equipment”; temperature 4°C and maximum storage 1–2 weeks) warmed up to 37°C and resuspend the cells.
-
e.Centrifuge the cells and resuspend the pellet in 20 mL medium warmed up to 37°C with a common 10 mL pipette.
-
f.Divide the 20 mL medium with the cells onto two fresh culture 100 mm plates.
-
g.Incubate the cells for 24 h more in an incubator (5% CO2) at 37°C.
-
a.
-
10.
The cells should be split at least 2‒3 times before using for in vivo experiments.
CRITICAL: The cells must be checked regularly for their identity to exclude cross-contamination and for the absence of mycoplasma.
-
11.
Split the cells (1:2) 24 h before conducting the rodent surgery.
-
12.
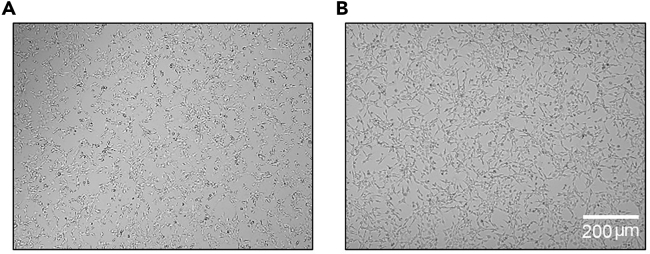
Before harvesting, check that a confluence of 50%–70% is reached (Figure 1).
CRITICAL: The cells must be confluent around 50%–70% to ensure that they are in their exponential growth phase. Otherwise, mice might not develop any metastasis.
-
13.Harvest the cells.
-
a.Discard the medium completely.
-
b.Rinse the plate with 10 mL PBS and remove what has been washed off.
-
c.Add 1 mL EDTA-Trypsin warmed up to 37°C to the cells and incubate the cells for 3 min in an incubator (5% CO2) at 37°C.
-
d.Add 9 mL fresh cell culture medium warmed up to 37°C and resuspend the cells.
 CRITICAL: Full culture medium must be added at this point since the included FBS stops the enzymatic activity of Trypsin.
CRITICAL: Full culture medium must be added at this point since the included FBS stops the enzymatic activity of Trypsin. -
e.Transfer the cells with cell culture medium warmed up to 37°C to a 50 mL Falcon and add 40 mL PBS.
-
f.Pellet by centrifugation at 300 × g for 5 min.
-
g.Discard the supernatant.
-
h.Resuspend the pellet in 1 mL PBS.
-
i.Take a 10 μL aliquot of the cells and dilute it with 80 μL PBS and 10 μL trypan blue.
-
j.Count the cells.Note: A general yield of 5–10 million cells per plate should be expected.
 CRITICAL: Ensure a viability of >90% of counted cancer cells. Viability can be determined with trypan blue solution (see above). Dead cells turn blue and can be observed using a microscope.
CRITICAL: Ensure a viability of >90% of counted cancer cells. Viability can be determined with trypan blue solution (see above). Dead cells turn blue and can be observed using a microscope. -
k.Dilute cells with PBS to a concentration of 3.5 million cells per mL for intrasplenic or intraportal injections and a concentration of 5 million cells per mL for intravenous injections.Note: Alternative cancer cell lines might require different cell concentrations for appropriate metastatic yield.
-
l.Aliquot the cells in 1 mL aliquots. Subsequently, transport and store the aliquots on ice.
 CRITICAL: Do not store cancer cells on ice for more than 2 hours. If more mice are to be injected, harvest cells multiple times.
CRITICAL: Do not store cancer cells on ice for more than 2 hours. If more mice are to be injected, harvest cells multiple times.
-
a.
Figure 1.
Cancer cells in culture
Pictures of cancer cells with confluency (A) 50 and (B) 70%. Scale bar = 200μm.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Experimental models: Cell lines | ||
| MC38 | Giannou et al.1 | N/A |
| Experimental models: Organisms/strains | ||
| C57BL/6J mice, wild type, adult, both sexes | The Jackson Laboratory | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| PBS | Dirk Hindorf Anprotec | AC-BS-0002 |
| FBS | Capricorn | CS-HI-1A |
| DMEM (GlutaMAX) | Gibco | 31966047 |
| Penicillin/Streptomycin | Gibco | 15070-063 |
| Metamizole | Ratiopharm GmbH | 9051799 |
| Buprenorphine | Temgesic | 00345928 |
| 0.05% Trypsin-EDTA | Gibco | 25300-054 |
| Trypan blue solution | Sigma-Aldrich | T8154-100ML |
| Other | ||
| Induction chamber for small animals | UNO Roestvaststaal BV | 180000232 |
| Ventilator for small animal use, e.g., UMV-03 UNO microventilator | UNO Roestvaststaal BV | 180000023 |
| Isoflurane vaporizer | UNO Roestvaststaal BV | 180000008 |
| Flowmeter CM2 | UNO Roestvaststaal BV | 180000002 |
| Heating plate | UNO Roestvaststaal BV | 180000028 |
| Laboratory stand with clamp | Zeiss | KL1500 LCD |
| Small surgical scissors | Fine Science Tools | 14060-09 |
| Student Adson forceps 1 × 2 teeth | Fine Science Tools | 91127-12 |
| Semken forceps curved 15 cm | Fine Science Tools | 11009-15 |
| Hand shaver machine | Aesculap | GT420 |
| Needle holder | Aesculap | BMO22R |
| Metal suture clips, 11 × 2 mm | Aesculap | BN511R |
| Metal suture clips applier | Aesculap | BN750R |
| Prolene 3-0 | Ethicon Inc. | VCP311H |
| Mersilene 2-0 | Ethicon Inc. | EH6854H |
| Injekt F, 1 mL syringe | B. Braun | 9166017V |
| 21-gauge needle, 0.80 × 40 mm | B. Braun | 4657527 |
| 26-gauge needle, 0.45 × 13 mm | B. Braun | 303800 |
| 32-gauge needle, 0.23 × 12 mm | Mesoram | 712306 |
| 30-gauge needle, 0.30 × 13 mm | BD Microlance | 304000 |
| Gauze compresses, 5 × 5 cm | Fink & Walter | 731021 |
| Cotton swabs, wood, 15 cm | NOBA Verbandmittel | 10859 |
| Betaisodona solution | Mundipharma GmbH | 6108022.00.01 |
| Isofluran-Piramal (isoflurane) 250 mL | Piramal Critical Care BV | N/A |
| Temgesic ampoules (buprenorphine hydrochloride, 0.3 mg/mL) | Indivior Europe Ltd. | N/A |
| Neubauer hemocytometer (cell counting) | Neubauer | 68052-14, 68052-15 |
| Bepanthen eyes and skin ointment | Bayer | 01578675 |
Materials and equipment
Analgetic drinking water
| Reagent | Final concentration | Amount |
|---|---|---|
| Autoclaved drinking water | 95% | 200 mL |
| Glucose | 5% | 10 g |
| Metamizole | <1% | 1000 mg |
The analgetic drinking water should be replaced daily.
Cell culture medium
| Reagent | Final concentration | Amount |
|---|---|---|
| DMEM medium | 90% | 450 mL |
| FBS | 10% | 50 mL |
| Penicillin/Streptomycin (5000 unit/mL Pen and 5000 μg/mL Strep) | 1% | 5 mL |
The cell culture medium must be prepared and stored under sterile conditions at 4°C. The medium can be stored for 1–2 weeks.
Step-by-step method details
Intrasplenic injection
Timing: 25–30 min per mouse
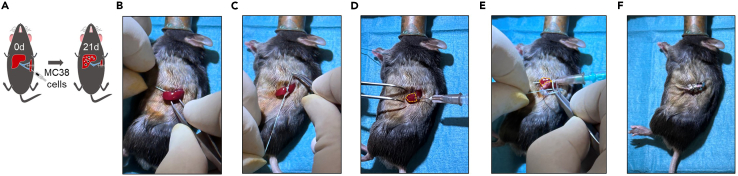
This part of the protocol describes an intrasplenic injection method used as a forced model of liver metastasis (Methods video S1). To prevent primary tumor growth in the spleen, a hemi-splenectomy is performed after injection (Figure 2A).
-
1.
Apply additional analgesia by injecting 0.2 mg metamizole per g body weight dissolved in PBS subcutaneously. Wait 15–30 min.
Note: Alternatively, buprenorphine can also be used. We recommend a subcutaneous injection of 0.1 μg buprenorphine per g body weight dissolved in PBS.
-
2.Prepare the cancer cells.
-
a.Resuspend the aliquoted cancer cells by mixing them 4 times by aspiration with a 21-gauge needle and a 1 mL syringe.
-
b.Aspirate 150 μL sterile PBS in a 1 mL syringe with a 26-gauge needle.
 CRITICAL: Avoid air bubbles.
CRITICAL: Avoid air bubbles. -
c.Carefully aspirate 100 μL of the resuspended cancer cells. Keep and transport the syringe with the needle facing downward to avoid mixing the cancer cells with the upper layer of PBS.
-
a.
-
3.
Anesthetize one mouse by using an appropriate induction chamber with an oxygen flow of 500 mL/min with 5% nebulized isoflurane until a respiratory rate of 1/s is achieved and the inter-toe reflex is lost.
-
4.
Shave the left abdominal part of the mouse.
-
5.
Maintain anesthesia by using the same oxygen flow with 2%–3% isoflurane provided via a mask.
-
6.
Position the mouse on its right side with the shaved part facing upwards.
-
7.
Use Bepanthen or comparable ointment to protect the eyes from drying out.
-
8.
Keep the mouse on a warming plate during the entire operation to prevent hypothermia.
-
9.
Apply betaisodona-solution on the shaved part thrice and let it dry for 3 min.
-
10.
Perform a 0.5 cm–1 cm incision with sharp scissors 0.5 cm under the thoracic cage. The muscle layer should become visible.
-
11.
Lift the muscle layer up using blunt forceps and perform a 0.5 cm–1 cm incision into the muscle layer so that the abdominal cavity and the spleen within become visible.
CRITICAL: Perform this step with care to avoid damaging the spleen, the colon, or the small intestine.
-
12.
Apply gentle pressure on the cranial and caudal side of the incision until the spleen pops out of the abdomen. Carefully insert the forceps between the caudal and cranial vascular bundles of the spleen (Figure 2B).
-
13.
Then perform a ligation in the middle of the spleen with Mersilene 2‒0, dividing the spleen into the cranial and caudal parts (Figure 2C).
CRITICAL: The ligation has to be tight to avoid subsequent reflux of injected cancer cells into the part of the spleen that was not injected.
-
14.
Carefully position and hold the caudal part of the spleen with the forceps in your left hand while inserting the needle tip of the prepared syringe at an angle of 30° with your right hand in the caudal part of the spleen, so that the lumen of the needle is completely in the splenic parenchyma (Figure 2D).
-
15.
Inject 250 μL of the prepared cell suspension into the spleen.
CRITICAL: A whitening and ballooning of the caudal part of the spleen has to be visible while the cranial part of the spleen must stay unchanged. Make sure that no injected suspension leaks due to accidental piercing of the needle through the spleen. In such a case, the mouse has to be euthanized.
-
16.
After injection, carefully pierce the needle through the caudal part of the spleen. This prevents unwanted dislocation of the needle tip inside the parenchyma and reduces the risk of bleeding during the next steps.
CRITICAL: Make sure that the needle is pierced through the spleen parenchyma and not through the caudal vascular bundle, since this would result in uncontrollable bleeding.
-
17.
Wait 3 min.
-
18.
Perform a ligation of the caudal vascular bundle with Prolene 3‒0. The pierced needle with the attached syringe can be used for careful maneuvering of the spleen around the suture (Figure 2E).
-
19.
Remove the caudal part of the spleen by first cutting directly below the intrasplenic suture and then the suture of the caudal splenic vascular bundle. The caudal part of the spleen should stay pierced on the needle tip throughout this step, since this grants as little cross-contamination with other tissue as possible.
-
20.
Check for hemostasis by excluding active bleeding from the rest of the spleen and the distal part of the pancreas.
-
21.
Position the spleen back into the abdomen.
-
22.
Close the abdominal wall with a continuous Z-suture with Prolene 3‒0.
-
23.
Close the opened skin with 3–5 metal clips (Figure 2F).
-
24.
Place the mouse back into its cage and monitor it until it is fully awake.
-
25.
Mice may be transferred to a heating rack for up to 24 h after surgery.
-
26.
Check the mice daily for complications, unwanted primary tumor growth or accelerated growth of metastasis.
-
27.
The analgetic drinking water can be changed into normal drinking water 48 h after surgery.
-
28.
Upon normal wound healing metal clamps should be removed 10–14 days after surgery.
-
29.
Mice should be euthanized 21 days after cancer cell injection.
Figure 2.
Intrasplenic injection of cancer cells
(A) Schematic view of intrasplenic injection of cancer cells.
(B) Expose the spleen.
(C) Ligate in the middle of the spleen.
(D) Carefully insert the needle tip into the caudal part of the spleen.
(E) Ligate the caudal vascular bundle while the needle is still pierced through the spleen.
(F) Close the skin using metal clips.
Intraportal injection
Timing: 25–30 min per mouse
This part of the protocol describes an intraportal injection method used as a second forced model of liver metastasis (Figure 3A) (Methods video S2). Despite being technically slightly more challenging than the intrasplenic injection method, the advantage of this model is that the spleen is left completely untouched.
-
30.
Apply additional analgesia by injecting 0.2 mg metamizole per g bodyweight dissolved in PBS subcutaneously. Wait 15–30 min.
Note: Alternatively, buprenorphine can also be used. We recommend a subcutaneous injection of 0.1 μg buprenorphine per g bodyweight dissolved in PBS.
-
31.Prepare the cancer cells.
-
a.Resuspend the aliquoted cancer cells by mixing them 4 times by aspiration with a 21-gauge needle and a 1 mL syringe.
-
b.Aspirate 150 μL sterile PBS in a 1 mL syringe with a 32-gauge needle.
 CRITICAL: The small gauge-size (32-gauge needle) of the needle is of critical importance, since it greatly reduces bleeding from the portal vein after removal of the needle.
CRITICAL: The small gauge-size (32-gauge needle) of the needle is of critical importance, since it greatly reduces bleeding from the portal vein after removal of the needle. CRITICAL: Avoid air bubbles.
CRITICAL: Avoid air bubbles. -
c.Carefully aspirate 100 μL of the resuspended cancer cells. Keep and store the syringe facing downward to avoid mixing of the cancer cells with the PBS.
-
a.
-
32.
Anesthetize one mouse by using an appropriate induction chamber with an oxygen flow of 500 mL/min with 5% nebulized isoflurane until a respiratory rate of 1/ s is achieved and the inter-toe reflex is lost.
-
33.
Shave the frontal abdominal part of the mouse.
-
34.
Maintain anesthesia by using the same oxygen flow with 2%–3% isoflurane provided via a mask.
-
35.
Position the mouse on its back with the shaved part facing upwards.
-
36.
Use Bepanthen or comparable ointment to protect the eyes from drying out.
-
37.
Keep the mouse on a warming plate during the entire operation to prevent hypothermia.
-
38.
Apply betaisodona-solution on the shaved part thrice and let it dry for 3 min.
-
39.
Perform a 4 cm midline incision with sharp scissors from the middle abdominal part until the xiphoid (Figure 3B). The muscle layer should become visible.
-
40.
Lift the muscle layer up using blunt forceps and perform a 4 cm incision into the muscle layer so that the abdominal cavity is opened.
CRITICAL: Perform this step with care to avoid damaging the small intestine during this step.
-
41.
Carefully bring the small and large intestine out of the abdomen to the left side of the mouse. Wrap the intestine in a sterile gauze soaked in 37°C PBS (Figure 3C).
CRITICAL: Ensure that the intestine is covered and hydrated throughout the whole procedure. A visible wet gauze is sufficient to ensure hydration of the intestine.
-
42.
Identify the portal vein and carefully insert the needle (Figure 3D).
-
43.
Inject 250 μL of the prepared suspension into the vein.
CRITICAL: A whitening of the vein should become visible for a short period of time. Make sure that no injected suspension leaks into the peritoneal cavity and that the portal vein is not accidentally pierced.
-
44.
After injection, wait 1 min with the needle still inside the vein.
-
45.
Remove the needle and immediately press a sterile cotton swap against the vein to stop the bleeding for 1 min (Figure 3E).
-
46.
Remove the swab and make sure that no further bleeding occurs. In case of light bleeding use of Ethicon Tabotamp is recommended by gently pressing it in front of the portal vein for up to 1 min.
-
47.
Place the intestine back inside the abdomen.
CRITICAL: Make sure not to accidentally rotate the intestine while moving it back since this might lead to a subsequent bowl obstruction or intestinal necrosis.
-
48.
Perform a continuous suture of the abdominal wall using Prolene 3‒0.
-
49.
Close the opened skin with 5–7 metal clips (Figure 3F).
-
50.
Place the mouse back into its cage and monitor it until it is fully awake.
-
51.
Mice may be transferred into a heating rack for up to 24 h after surgery.
-
52.
Check the mice daily for complications, unwanted primary tumor growth or accelerated growth of metastasis.
-
53.
The analgetic drinking water can be changed into normal drinking water 48 h after surgery.
-
54.
Upon normal wound healing metal clamps should be removed 10–14 days after surgery.
-
55.
Mice should be euthanized 21 days after surgery.
Figure 3.
Intraportal injection of cancer cells
(A) Schematic view of intraportal injection of cancer cells.
(B) Perform a 4 cm long, midline incision from the middle abdominal part until the xiphoid.
(C) Wrap the intestine in a sterile gauze soaked in 37°C warm PBS and place at the left side of the mouse.
(D) Carefully insert the needle into the portal vein.
(E) Press a sterile cotton swab against the vein to stop the bleeding for 1 min.
(F) Close the skin using metal clips.
Intravenous tail vein injection
Timing: 2–5 min per mouse
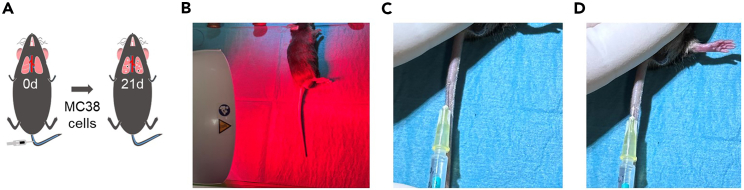
This part of the protocol describes the intravenous tail vein injection used as a forced model of lung metastasis (Figure 4A).
-
56.Prepare the cancer cells.
-
a.Resuspend the aliquoted cancer cells by mixing them 4 times by aspiration with a 21-gauge needle and a 1 mL syringe.
-
b.Carefully aspirate 100 μL of the resuspended cancer cells with a 30-gauge needle and a 1 mL syringe.
-
a.
CRITICAL: Avoid air bubbles.
-
57.
Anesthetize one mouse by using an appropriate induction chamber with an oxygen flow of 500 mL/min with 5% nebulized isoflurane until a respiratory rate of 1/s is achieved and the inter-toe reflex is lost.
-
58.
Maintain anesthesia by using the same oxygen flow with 2%–3% isoflurane provided via a mask.
-
59.
Dilate the tail vein by applying infrared light for approximately 20 s (Figure 4B).
CRITICAL: Avoid thermal damage to the tail by holding your finger next to the tail to evaluate if the heat becomes too strong.
Note: Alternatively, the tail can be positioned in a water bath heated to 37°C for 20 seconds.
-
60.
Position the mouse on its right or left side.
-
61.
Hold the tail with your non-dominant hand and stretch it into a straight line. The tail vein should be visible as a purple, straight line facing up (Figure 4C).
-
62.
Insert the tip of your needle at a 10° angle into the tail vein. The needle should slide very easily into the vein once you are inside the lumen of the vein (Figure 4D).
-
63.
Slowly inject 100 μL of your cell suspension.
CRITICAL: The injection has to feel very smooth and a short whitening of the tail vein has to be observed. If resistance is felt while injecting and if a whitening of an area around the injection site can be observed, intravenous injection was not successful.
-
64.
After injection, wait 30 s with the needle still inside the vein.
-
65.
Quickly remove the needle and immediately press a sterile gauze on the vein for 20 s.
-
66.
Remove the gauze and make sure that no further bleeding from the vein occurs.
-
67.
Place the mouse back into its cage and monitor it until it is fully awake.
-
68.
Check the mice daily for complications such as tail necrosis or accelerated growth of metastasis.
-
69.
Mice should be euthanized 21–28 days after cell injection.
Figure 4.
Intravenous injection of cancer cells
(A) Schematic view of intravenous injection of cancer cells.
(B) Apply infrared light to dilate the tail vein.
(C) A purple straight line is seen while the mouse tail is stretched.
(D) Smoothly insert the needle tip into the tail vein.
Liver harvest
Timing: 2 min per mouse
-
70.
Euthanize the mice according to your local guidelines (see Methods video S3).
-
71.
Disinfect the mice using 70% ethanol.
-
72.
Open the abdominal and the thoracic cavity.
-
73.
Cut the left ventricle of the heart.
-
74.
Push the small intestine to the left side so that the portal vein is visible.
-
75.
Insert a 21-gauge needle into the subhepatic vena cava inferior and flush with 5–10 mL PBS.
Note: During this step, the portal vein will expand, which makes it easier to perfuse it during the next step.
-
76.
Remove the needle from the subhepatic vena cava inferior and immediately hold a finger on the insertion place to prevent the portal vein from collapsing.
-
77.
Insert the needle into the portal vein and perfuse it with 5–10 mL PBS.
Note: After this step, the liver should turn fully “beige”.
-
78.
Gently pull down the stomach and cut the esophagus of the mouse as close to the stomach as possible. Then cut through the hepatoduodenal ligament.
-
79.
Finally, grab the ventral part of the diaphragm and cut it circularly close to the thoracic wall. The liver is now fully dissected from the mouse.
CRITICAL: For subsequent analysis of cellular compartments (e.g., with FACS), the gall bladder should be removed before further processing.
-
80.
Store the liver for up to 1 day in 20–24°C PFA 4% before proceeding to macroscopic or microscopic counting.
Lung harvest
Timing: 5 min per mouse
-
81.
Euthanize the mice according to your local guidelines. Avoid CO2 euthanasia if microscopic assessment of lung tissue is desired (see Methods video S3).
-
82.
Disinfect the mice using 70% ethanol.
-
83.
Open the thoracic cavity and the cervical part of the mice until the mandible.
-
84.
Cut the left ventricle of the heart.
-
85.
Insert a 21-gauge needle into the right ventricle of the heart and flush the mouse with 5–10 mL PBS.
Note: During this step, the lung will turn from pink to white.
-
86.
Dissect the ventral neck muscles to locate the trachea and prepare a ligature with Mersilene 2‒0 around the trachea, but leave the knot untied.
-
87.
Cut the ventral wall of the trachea above the prepared suture and insert the plastic cannula of a 22G vein catheter.
-
88.
Administer 800 μL 4% PFA intratracheally (for histological analysis and macroscopic counting).
Note: During this step, the lung will expand due to the replacement of air inside the airways with 4% PFA.
-
89.
Pull out the catheter and quickly ligate the prepared suture to trap the fluid inside the airways.
-
90.
Finally, cut the trachea above the knot and isolate the lung by carefully removing the aorta, esophagus, vena cava, and finally, the diaphragm.
-
91.
The lung and heart can now be removed together.
-
92.
Store the lung for up to one day at 20–24°C PFA 4% before proceeding to macroscopic or microscopic counting.
Expected outcomes
Using these three methods of forced metastasis, 99% of mice are expected to develop lung or liver metastasis. Development of primary tumors (such as tumors of the remaining part of the spleen or in the peritoneal cavity) is exceptionally rare. Moreover, they are an indicator of a technical problem and mice should not be used for subsequent analysis. Based on our experience, intra- and postoperative mortality combined lies between 0.5%‒1%. Of note, the average range of macroscopic metastasis varies from experiment to experiment. Therefore, a control group has to be used without exception for every single experiment that is being performed. From the 14th postoperative day, mice have to be watched exceptionally closely regarding metastatic development. In some cases, mice have to be sacrificed prematurely due to an aggravated speed of liver or lung metastasis development. From our experience, signs of aggravated metastasis burden are a tense abdomen, dyspnea, palpable peritoneal metastasis or a reduction in their general condition. However, regular MRI- or CT-scans might also present a possibility to assess metastasis burden.
Quantification and statistical analysis
Macroscopic counting of liver metastasis should be carried out in a blind fashion. Inspect every liver lobe from each accessible side in a structured fashion. For microscopic counting of liver metastasis, we recommend using a representative histological slide of the biggest liver lobe after H&E staining. The percentage of the area covered by metastatic lesions can be assessed using common software.
Macroscopic counting of lung metastasis should be carried out in a blind fashion. Inspect every lung lobe from each accessible side in a structured fashion. For microscopic counting of lung metastasis, we recommend using a representative histological slide of one lung lobe after H&E staining. The percentage of the area covered by metastatic lesions can be assessed using common software.
Limitations
Despite all the advantages of these three forced metastasis models outlined above, some limitations of these models deserve to be discussed. The nature of forced metastasis is that cells are forced into the circulation of the host. Thus, the first steps of metastasis, especially the seeding of cancer cells into the bloodstream, are bypassed. Therefore, these models can only be used to study the later phases of metastatic seeding. Furthermore, cancer cell lines have been used to mimic metastatic disease. However, cancer cell lines do not represent the full picture of metastatic cancer cells, which can behave quite heterogeneously and can be subdivided into specific subsets. Nonetheless, these described models could be used for the injection of tumor organoids, which can mimic more aspects of metastatic cancer than common cancer cell lines.
Troubleshooting
Problem 1
High intra- or postoperative mortality (related to steps 3‒13 in Before you begin; related to steps 1‒55).
Potential solution
-
•
Re-evaluate anesthetic protocol, reduce duration of operation, and reduce exposure to isoflurane.
-
•
Increase the duration of the recovery phase in heating cages.
-
•
Reduce the concentration of cancer cells.
-
•
Change buffers to exclude contamination of reagents used.
-
•
Check the technical accuracy of all performed sutures to exclude intraoperative bleeding.
Problem 2
No metastasis after 3 (or 4) weeks (related to steps 3‒13 in Before you begin).
Potential solution
-
•
Make sure that cancer cells were harvested during their exponential growth phase.
-
•
Make sure that the genetic background of the cancer cells matches the genetic background of the injected mice.
-
•
Exclude genetic contamination of used mouse strains.
-
•
Exclude contamination of cancer cells and/or mycoplasma infection.
-
•
Confirm sufficiently high viability of cancer cells before injection.
-
•
Increase the concentration of cancer cells.
Problem 3
Growth of primary tumor around the remaining spleen when performing intrasplenic injection (related to Steps 5‒29).
Potential solution
-
•
Ensure that the suture dividing the spleen into two parts is tight enough for future surgery.
-
•
Remove the spleen as close to the ligation as possible to exclude the possibility that spleen with tumor cells remains in situ.
-
•
Make sure that mice are immediately sacrificed if bleeding from the spleen after injection occurs or if cancer cells are not injected fully into the spleen.
-
•
Reduce the concentration of cancer cells.
Problem 4
Too many metastases. From our experience, signs of aggravated metastasis burden are a tense abdomen, dyspnea, palpable peritoneal metastasis or a reduction in their general condition. However, regular MRI- or CT-scans might also present a possibility to assess metastasis burden (related to steps 3‒13 in Before you begin; related to steps 1‒68).
Potential solution
-
•
Sacrifice future mice at an earlier time point.
-
•
Reduce the concentration of cancer cells.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Anastasios Giannou, a.giannou@uke.de.
Technical contact
Technical questions on executing this protocol should be directed to and will be answered by the technical contact, Anastasios Giannou, a.giannou@uke.de, and Jöran Lücke, j.luecke@uke.de.
Materials availability
This study did not generate new unique reagents.
Data and code availability
This study generated neither datasets nor code.
Acknowledgments
The authors thank Tom Blankenburg, Sandra Wende, and Cathleen Haueis for their excellent technical assistance. The graphical abstract was in part created with BioRender.com. This work was supported in part by the Deutsche Forschungsgemeinschaft (grants SFB841 and SFB1328 to S.H.), the European Research Council (CoG 865466 to S.H.), the Deutsche Krebshilfe (nr. 70114853 to A.D.G.), Else Kröner Memorial Stipendium (to A.D.G.), Erich und Gertrud Roggenbuck-Stiftung (to A.D.G.), Hamburger Krebsgesellschaft Stiftung (to A.D.G.), and the Jung Foundation for Science and Research (to A.D.G.). S.H. has an endowed Heisenberg Professorship awarded by the Deutsche Forschungsgemeinschaft.
Author contributions
J.L., T.Z., and A.D.G. performed all rodent surgeries and prepared the figures. J.L. and T.Z. wrote the manuscript. D.E.Z. and P.S. created the videos and edited the manuscript. J.R.I. and T.H. edited the manuscript and provided intellectual input. S.H. and A.D.G. provided scientific supervision and revised and finalized the manuscript.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xpro.2023.102696.
Contributor Information
Jöran Lücke, Email: j.luecke@uke.de.
Anastasios D. Giannou, Email: a.giannou@uke.de.
References
- 1.Giannou A.D., Kempski J., Shiri A.M., Lücke J., Zhang T., Zhao L., Zazara D.E., Cortesi F., Riecken K., Amezcua Vesely M.C., et al. Tissue resident iNKT17 cells facilitate cancer cell extravasation in liver metastasis via interleukin-22. Immunity. 2023;56:125–142.e12. doi: 10.1016/j.immuni.2022.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin-Orozco N., Muranski P., Chung Y., Yang X.O., Yamazaki T., Lu S., Hwu P., Restifo N.P., Overwijk W.W., Dong C. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31:787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X., Sun R., Hao X., Lian Z.X., Wei H., Tian Z. IL-17 constrains natural killer cell activity by restraining IL-15-driven cell maturation via SOCS3. Proc. Natl. Acad. Sci. USA. 2019;116:17409–17418. doi: 10.1073/pnas.1904125116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elkin M., Vlodavsky I. Tail vein assay of cancer metastasis. Curr. Protoc. Cell Biol. 2001;12:19.2.1–19.2.7. doi: 10.1002/0471143030.cb1902s12. [DOI] [PubMed] [Google Scholar]
- 5.Goddard E.T., Fischer J., Schedin P. A Portal Vein Injection Model to Study Liver Metastasis of Breast Cancer. J. Vis. Exp. 2016:54903. doi: 10.3791/54903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Brien M., Ernst M., Poh A.R. An intrasplenic injection model of pancreatic cancer metastasis to the liver in mice. STAR Protoc. 2023;4:102021. doi: 10.1016/j.xpro.2022.102021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study generated neither datasets nor code.