Abstract
Introduction
Life participation has been established as a critically important core for trials in kidney transplantation. We aimed to validate a patient-reported outcome measure for life participation in kidney transplant recipients.
Methods
A psychometric evaluation of the Standardized Outcomes in Nephrology life participation (SONG-LP) measure was conducted in adult kidney transplant recipients. The measure includes 4 items of life participation (leisure, family, work, and social) each with a 5-point Likert scale. Each item is scored from 0 (never) to 4 (always) and the summary measure score the average of each item.
Results
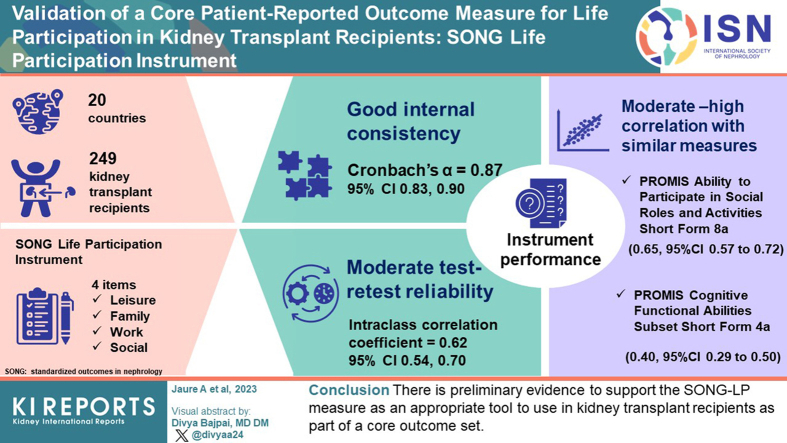
A total of 249 adult kidney transplant recipients from 20 countries participated. The SONG-LP instrument demonstrated internal consistency (Cronbach’s α = 0.87; 95% confidence intervals [CI]: 0.83–0.90, baseline) and test-retest reliability over 1 week (intraclass correlation coefficient of 0.62; 95% CI: 0.54–0.70). There was moderate to high correlation (0.65; 95% CI: 0.57–0.72) with the PROMIS Ability to Participate in Social Roles and Activities Short Form 8a that assessed a similar construct, and moderate correlation with measures that assessed related concepts (i.e., EQ5D 0.57; 95% CI: 0.49–0.65), PROMIS Cognitive Functional Abilities Subset Short Form 4a (0.40; 95% CI: 0.29–0.50).
Conclusion
The SONG-LP instrument is a simple, internally consistent, reliable measure for kidney transplant recipients and correlates with similar measures. Routine incorporation in clinical trials will ensure consistent and appropriate assessment of life participation for informed patient-centered decision-making.
Keywords: core outcomes, kidney transplantation, life participation, patient-centered outcomes, patient-reported outcome measures, quality of life
Graphical abstract
Kidney transplant recipients are at an increased risk of various treatment side effects and complications. These include diabetes, cancer, infection, cardiovascular disease, gastrointestinal disorders, pain, and cognitive and psychological problems,1, 2, 3, 4, 5 which can severely impair their quality of life and ability to participate in daily activities.4,6, 7, 8
Through the global SONG– Kidney Transplant Initiative consensus process involving more than 1100 patients, caregivers, and health professionals from over 70 countries, life participation was established as a critically important core outcome to be reported in trials in kidney transplantation.9, 10, 11 However, life participation, defined as the “ability to participate in meaningful activities of daily living, including work, study, and social recreational activities”9,10,12 is rarely assessed in trials. A systematic review of outcome measures used to assess life participation in kidney transplant recipients found the following: (i) no outcome measures assessed life participation specifically (12 measures assessed broader constructs that included 1 or more questions on life participation), (ii) current measures were too resource intensive (costly) such that it would not be feasible to be used as a core outcome measure in trials, (iii) the measures were burdensome for patients to complete (lengthy, questionable relevance of content, and archaic language), and (iv) the measures were widely heterogenous in content with uncertainty regarding the most relevant and important content for transplant recipients to assess life participation directly (instead of life participation embedded within a combination of related outcomes).13
To endorse and recommend a core patient-reported outcome for life participation, the following criteria were considered: (i) simple and easy to use, (ii) short (<5 items), and (iii) assesses content that is known to be relevant and important to kidney transplant recipients, caregivers, and health professionals. No existing measure fulfilled these criteria. We conducted a consensus workshop12 on developing content for the SONG-LP instrument with input from all relevant stakeholder groups (kidney transplant recipients) to ensure that this instrument assessed relevant and important dimensions of life participation for kidney transplant recipients. The instrument was developed using a rigorous methodological framework to ensure that it was a psychometrically robust measure, assessed content most important and relevant for transplant recipients, their caregivers, their clinicians, and researchers.12 As a result, compared with other measures that assess life participation or related concepts, the SONG-LP measure assesses dimension of direct importance and relevance to kidney transplant recipients and is shorter and simpler to support implementation in trials. Although developed initially for kidney transplant recipients, the SONG-LP instrument has potential for use in other populations.
A core outcome measure for life participation that includes content of relevance to patients and that is feasible to implement in all trials can facilitate consistent and meaningful measurement of life participation in kidney transplant recipients.11,14,15 Here, we present the SONG-LP measure and report the initial psychometric evaluation of the core patient-reported outcome for life participation in kidney transplant recipients.
Methods
Selection and Recruitment of Participants
Participants were eligible to participate if they were aged 18 years or older with a functioning kidney transplant, able to read and write in English, and able to provide informed consent. Participants with cognitive impairment who could not complete a patient-reported outcome measure on their own were excluded. An invitation to participate in the study was sent to 532 contacts on the SONG database. This database consists of patients with chronic kidney disease across 70 countries who registered to participate in the SONG initiative since its inception in 2015. Because of the COVID-19 pandemic, it was not feasible to broaden recruitment through kidney transplant centers. We estimated a sample size of 250 participants to allow the estimation of the intraclass correlation coefficient with a precision of 0.06 for the 95% CI, assuming an expected intraclass correlation coefficient of 0.7 for the agreement between the SONG-LP measured 1 week apart. Ethics approval was obtained from The University of Sydney.
Measures
SONG Life Participation
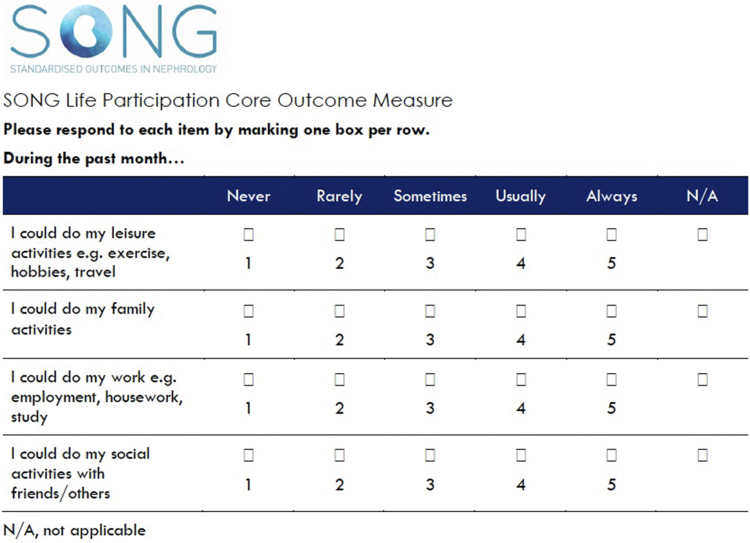
The SONG-LP instrument was developed based on a systematic review of all measures of life participation used in kidney transplantation13 and with the involvement of patients, caregivers and health professionals.12 Cognitive interviews were conducted with 20 kidney transplant recipients to ensure satisfactory comprehension, retrieval of relevant cognitive information, process of judgment, and response scale usability.16 The SONG-LP is shown in Figure 1 and consists of 4 items that are scored on a 5-point Likert scale of frequency (i.e., “Never” to “Always”). The recall period is 1 month. The Flesch-Kincaid Readability Grade Level is Grade 6 (US), which is categorized as easy to read. Individual item scores ranged from 0 (“Never”) to 4 (“Always”). An overall score for life participation was obtained by the average of the responses across the 4 questions that results in a scale ranging from 0 to 4, with higher scores indicating higher participation. A “non-applicable” (N/A) option was included for each question. For the overall life participation score, when a N/A was indicated, the average score was calculated based on the scales for the remaining items. The scoring assigns the same weight to each response category within an item and to each item in calculating the summary score.
Figure 1.
SONG life participation instrument. SONG, standardized outcomes in nephrology. N/A, not applicable.
PROMIS Item Bank v2.0 Ability to Participate in Social Roles and Activities (PROMIS-APS) Short Form 8a17
We used the PROMIS-APS measure to assess the convergent validity of the SONG-LP total scores. The PROMIS-APS measure assesses the construct of life participation, specifically “perceived ability to perform one’s usual social roles and activities.” Although there is limited evidence for the PROMIS-APS as a standalone measure, it has been evaluated in the kidney transplantation population as part of a greater 57-item instrument assessing various constructs such as physical function, anxiety, depression, social functioning, and fatigue.18 This measure has also been used and evaluated for psychometric robustness in other patient populations.19,20 The PROMIS-APS appears to have face validity because the items and wording closely reflect how kidney transplant recipients describe life participation and it was the best available measure for life participation.12 Of note, we used this as the basis to develop the SONG-LP measure. The items were changed based on input from kidney transplant recipients (e.g., kidney transplant recipients preferred the questions to be framed in a positive manner rather than in a deficit manner). Therefore, we hypothesized that the PROMIS-APS scores would correlate closely with the SONG-LP scores.
PROMIS Item Bank v2.0 Cognitive Functional Abilities Subset Short Form 6a
This measure assesses cognitive deficit and the degree to which it impairs daily functioning.21 We hypothesized that self-reported cognitive impairment should correlate with self-reported life participation.4,22,23
PROMIS Item Bank v2.0 Emotional Support Short Form 4a
The PROMIS Emotional Support instrument assesses the “perceived feelings of being cared for and valued as a person; having confidant relationships.”24 We anticipated correlation between levels of life participation and emotional support.
PROMIS Item Bank v2.0 Instrumental Support Short Form 4a
The PROMIS Instrumental Support assesses the respondent’s “perceived availability of assistance with material, cognitive, or task performance,”25 and it was expected that this construct would correlate with life participation.
EQ-5D-5L
The EQ-5D-5L measure assesses quality of life using 5 dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression.26 We selected this measure because it was short and simple to complete, has been shown to have a high accuracy of completion and comprehensible to kidney transplant recipients,27 and has been used and evaluated in transplant recipients.28, 29, 30 We hypothesized that there would be moderate correlation with SONG-LP as the EQ-5D-5L includes 1 construct (usual activities) and other related constructs (e.g., mobility). Specifically, the EQ-5D-5L provides examples of usual activities to include work, study, housework, family, or leisure activities), which are also captured in the SONG-LP instrument. Considering that the largest proportion of respondents were from Australia followed by the United Kingdom, United States of America, and Canada, the EQ-5D-5L utility values were calculated using the Australian value set.31 Although different validation sets will result in different utility scores, the differences between countries of similar cultural background are small.32
Self-Rated Kidney Function
Self-rated kidney function was a de novo self-scored question that participants used to describe their level of kidney function. The question was “I think my kidney function over the past month has been [response],” with the response being indicated on a Likert scale from 1 (poor) to 5 (excellent). We hypothesized the self-rated kidney function may correlate with life participation.
Data Collection
At baseline (time point 1), each participant completed a questionnaire of demographic and clinical characteristics, and the five measures outlined above: SONG life participation, PROMIS Item Bank v2.0 Ability to Participate in Social Roles and Activities (PROMIS-APS) Short Form 8a, PROMIS Item Bank v2.0 Cognitive Functional Abilities Subset Short Form 6a, PROMIS Item Bank v2.0 Emotional Support Short Form 4a, PROMIS Item Bank v2.0 Instrumental Support Short Form 4a, the EQ-5D-5L, and the self-reported level of kidney function. The order of the surveys was randomized to minimize ordering bias. After 1 week (time point 2), all participants completed the SONG-LP instrument and the self-rated kidney function questions. The surveys were administered online using Research Electronic Data Capture, a secure web-based application for building and managing surveys. The survey was open from August 2021 to November 2021.
Data Analysis
Data analysis was conducted in R (version 4.0.3). Demographic and clinical continuous variables were described as median and interquartile range. Categorical variables were summarized by counts and percentages. The scores of the different instruments were presented as means and SDs.
We assessed the reliability of the SONG-LP measure by comparing the internal consistency, and test retest between time points 1 and 2. Aspects of construct and content validity were also evaluated. Under construct validity, we were able to include convergent validity, acceptability, and hypothesis testing (i.e., the degree to which the instrument is associated with factors that may also be associated with life participation). The psychometric properties were assessed based on the following criteria:
Reliability
Test-retest
An intraclass coefficient between 0.5 and 0.75 comparing time point 1 and the score 1 week later at time point 2 was considered to indicate moderate stability, whereas above 0.75 indicates good to excellent stability.15,33
Internal Consistency
Cronbach’s alpha ≥ 0.70 indicated adequate scale consistency.15
Content Validity
Content validity has been demonstrated and supported by prior work12 and cognitive interviews.
Construct Validity
Convergent Validity
For convergent validity, we defined a spearman’s rho greater than 0.7 as indicating high correlation, 0.3 to 0.7 indicated moderate correlation, and less than 0.3 indicated low correlation.14,34 Because the SONG-LP measure was adapted from PROMIS-Ability to Participate in Social Activities, we anticipated a high positive correlation between these 2 measures. Given the similarities and overlap in constructs assessed between the SONG-LP measure and PROMIS-Cognitive Functional Abilities Subset Short Form 6a, Emotional Support Short Form 4a, Instrumental Support Short Form 4a, EQ-5D-5L, and self-rated kidney function, we expected to see positive moderate correlations
Hypothesis Testing
The ability to participate in the activities covered by SONG-LP are expected to be affected by a transplant recipient’s clinical condition and time since transplant. Creatinine, time since transplant, and perceived kidney function were identified as relevant variables, and it was hypothesized that these may be associated with SONG-LP scores.
Acceptability
Acceptability is a measure of the level of data quality, assessed by completeness and score distributions. We defined missing data for instrument scores <5% and floor and ceiling effects <10% as criteria for assessing acceptability.
Results
Of the 250 participants required as per the sample size calculation, 249 participants completed the survey at baseline (time point 1) and 231 (93%) completed the survey at time point 2 (Figure 2). There were participants from 20 countries, including Australia (n = 117, 47%), United Kingdom (n = 59, 24%) and United States (n = 38, 15%). The median (interquartile range) age of participants was 60 years (50–68 years). Approximately half of the participants were female (n = 128, 51%). Other demographic and clinical characteristics of the participants are provided in Table 1.
Figure 2.
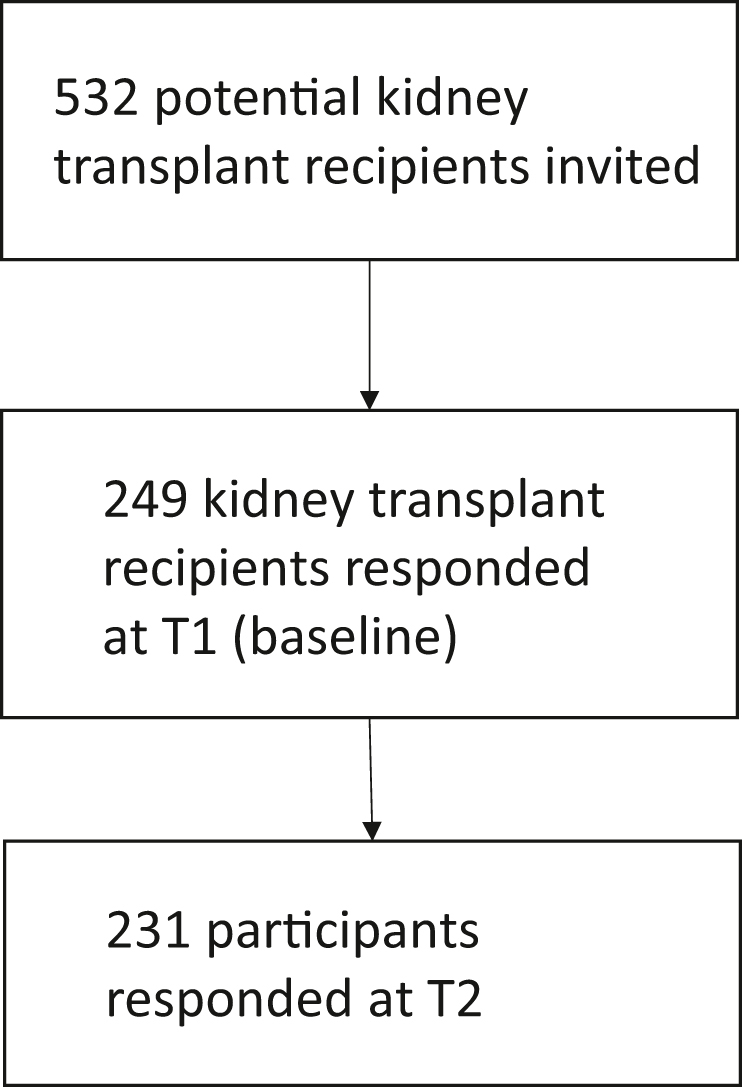
Participant flow chart.
Table 1.
Demographic and clinical characteristics of the participants at baseline (N = 249)
| Demographics | Median (IQR) or N (%) |
|---|---|
| Agea | 60 (50, 68) |
| Sex | |
| Male | 120 (48) |
| Female | 128 (51) |
| Other | 1 (1) |
| Country | |
| Australia | 117 (47) |
| United Kingdom | 59 (24) |
| USA | 38 (15) |
| Canada | 11 (4) |
| Otherb | 24 (10) |
| Donor | |
| Deceased | 142 (57) |
| Living | 107 (43) |
| Marital status | |
| Married/Partnered | 184 (74) |
| Divorced/Separated | 14 (6) |
| Widowed | 14 (6) |
| Single | 35 (14) |
| Other | 2 (1) |
| Employmentc | |
| Full time | 70 |
| Part time | 40 |
| Casual | 13 |
| Unemployed | 11 |
| Unemployed due to COVID-19 | 4 |
| Retired | 101 |
| Student | 9 |
| Other | 14 |
| Education level | |
| School certificate (yr 10) | 12 (5) |
| HSC (yr 12) | 27 (11) |
| Diploma/TAFE | 53 (21) |
| University degree | 110 (44) |
| Other | 47 (19) |
| Time since transplant (N = 240) | |
| <1 yr | 26 (11) |
| 1–5 yrs | 96 (40) |
| 6–10 yrs | 43 (18) |
| 10+ yrs | 75 (31) |
| Creatinine (mg/dl)d (N = 191)a | 1.32 (1.05, 1.60) |
HSC, high school; IQR, interquartile range; TAFE, technical and further education.
Median (IQR).
Denmark (n = 3), New Zealand (n = 3), India (n = 2), Italy (n = 2), South Africa (n = 2), The Netherlands (n = 2), Belgium (n = 1), Brazil (n = 1), Czech Republic (n = 1), Finland (n = 1), France (n = 1), Hong Kong (n = 1), Ireland (n = 1), Mexico (n = 1), Spain (n = 1), and Switzerland (n = 1).
Some participants selected more than one employment option, explaining why percentages cannot be calculated.
Self reported.
Acceptability
There was a completion rate of 100% at time point 1 for all the measures, and 93% of the participants completed all of the measures at time point 2 (Supplementary Table S1) The reasons for missing data cannot be ascertained because the survey was administered online. The nonresponders at time point 2 had a lower mean score at time 1 compared to the responders; however, the difference was not statistically significant (3.9 vs. 4.1, P = 0.273).
The scores were found to be across the scale range with no apparent skew. The floor effect (SONG-LP score of 0) was <1% whereas the ceiling effect (SONG-LP score of 4) was 34% and 27% for baseline and time point 2, respectively (Supplementary Table S1). The high ceiling effect is seen in the frequency of item scale responses (Supplementary Figure S1) where 40% to 50 % of participant responses were for the “always” category for all 4 items. This is consistent with the frequency of responses for the EQ5D-5L “usual activities” item where 59% indicated “no problems” (Supplementary Figure S2).
Reliability
Test-retest reliability for the SONG-LP instrument between times 1 and 2 showed moderate stability, with an intraclass correlation coefficient of 0.62 (95% CI: 0.53–0.69) (Table 2). The Cronbach’s alpha was 0.87 (95% CI: 0.83–0.90) at time 1, and 0.85 (95% CI: 0.81–0.88) at time 2, indicating an acceptable to good level of internal consistency in both measurements (Table 2).
Table 2.
Mean (SD) scores for SONG Life Participation, PROMIS Item Bank; Reliability (internal consistency and test-retest) for the SONG Life Participation Instrument and Spearman’s Rho correlations between SONG life participation and validity of the SONG measure compared to other instruments (N = 249)
| Results | SONG life |
PROMIS Item Bank v 2.0 |
EQ-5D-5La | Self-rated kidney |
|||
|---|---|---|---|---|---|---|---|
| Participation | APSa | CFAa | ESa | ISa | functiona | ||
| Mean scores | 3.1 (0.9)a 3.0 (1.0) b |
29.2 (8.0) | 23.9 (5.9) | 16.8 (3.7) | 16.7 (4.3) | 0.76 (0.22) | |
| Cronbach’s α (95% CI) | 0.87 (0.83, 0.90)a 0.85 (0.81, 0.88)b |
- | - | - | - | - | - |
| ICCc | 0.62 (0.54, 0.70) | - | - | - | - | - | - |
| Spearman’s correlation (95% CI) | - | 0.65 (0.57, 0.72) | 0.40 (0.29, 0.50) | 0.26 (0.14, 0.37) | 0.17 (0.04, 0.29) | 0.57 (0.49, 0.65) | 0.26 (0.14, 0.38) |
APS, ability to participate in social roles and activities short form 8a; CI, confidence interval; CFA, cognitive functional abilities subset short form 6a; ES, emotional support short form 4a; IS, instrumental support short form 4a; ICC, intraclass coefficient; -, not applicable; SD, standard deviation; SONG, standardized outcomes in nephrology.
Calculated at time point 1.
Calculated at time point 2.
Based on 231 participants who completed the survey at time point 2 (1 week after).
Convergent Validity
The SONG-LP instrument demonstrated good convergence validity with high correlations with the PROMIS Ability to Participate in Social Roles and Activities (0.65; 95% CI: 0.57–0.72) and the EQ-5D-5L instrument (0.57; 95% CI: 0.49–0.65). There was a lower correlation between the SONG-LP instrument and the PROMIS Emotional Support instrument (0.26; 95% CI: 0.14–0.37) and the PROMIS Instrumental Support instrument (0.17; 95% CI: 0.04–0.29). The PROMIS Cognitive Functional Abilities instrument was also found to have a moderate correlation with the SONG-LP instrument (0.40; 95% CI: 0.29–0.50) (Table 2).
Hypothesis Testing
There was a low correlation between the SONG-LP Instrument and creatinine levels (−0.16; 95% CI: −0.29 to −0.01). No significant difference was observed between the SONG-LP instrument mean scores across the categories of time since transplant groups (P = 0.842) (Table 3). There was also a low correlation between perceived kidney function and the SONG-LP instrument (0.26; 95% CI: 0.14–0.38; N = 249).
Table 3.
SONG life participation, PROMIS Item Bank score, EQ-5D-5L means (SD) for intervals of time since transplantation (N = 240)
| Results | SONG life participation | P-valuea | PROMIS Item Bank V 2.0 |
EQ-5D-5L | Self-rated kidney function | |||
|---|---|---|---|---|---|---|---|---|
| APS | CFA | ES | IS | |||||
| Time since transplantation | 0.842 | |||||||
| <1 yr | 3.12 (0.87) | 26.4 (8.21) | 21.6 (6.8) | 16.6 (3.60) | 16.2 (4.5) | 0.76 (0.21) | 3.85 (1.29) | |
| 1–5 yrs | 3.16 (0.79) | 29.3 (8.25) | 24.0 (6.1) | 17.0 (3.6) | 16.4 (4.5) | 0.76 (0.22) | 4.31 (0.84) | |
| 6–10 yrs | 3.01 (0.93) | 27.5 (7.48) | 24.6 (4.5) | 16.6 (3.5) | 17.1 (3.9) | 0.71 (0.22) | 4.14 (1.06) | |
| >10 yrs | 3.12 (1.02) | 31.0 (7.29) | 24.4 (6.2) | 16.9 (3.9) | 17.2 (4.2) | 0.79 (0.20) | 4.20 (0.94) | |
APS, ability to participate in social roles and activities short form 8a; CFA, cognitive functional abilities subset short form 6a; ES, emotional support short form 4a; IS, instrumental support short form 4a; SD, standard deviation; SONG, standardized outcomes in nephrology.
1-way ANOVA.
1-way ANOVA.
Discussion
Life participation has been established by kidney transplant recipients, caregivers, and health professionals to be a critically important core outcome to be reported in all trials in kidney transplantation. This outcome was considered critically important for patients, caregivers, clinicians, and researchers to know about, irrespective of whether an intervention was specifically aimed at impacting life participation. The SONG-LP instrument was developed based on a systematic review of all measures of life participation used in kidney transplantation and an international consensus workshop with the involvement of more than 1000 kidney transplant recipients, caregivers, and health professionals from over 70 countries.9,12 The items in the SONG-LP instrument encompass leisure, family activities, work, and social activities, which collectively reflect aspects of life participation that are most important and relevant to kidney transplant recipients.12
The SONG-LP meets some of the reliability and validity criteria articulated in the COSMIN-COMET recommendations34 and US Food and Drug Administration guidelines for measurement.35 The SONG-LP instrument is easy to complete given the high completion rate, has moderate test-retest reliability, and good internal consistency; and convergent validity has been demonstrated. Acceptability has also been demonstrated with respect to missing data and low floor effects. There was however a high ceiling effect that was consistent with the EQ5D-5L “usual activities” item and suggests that life participation may not have been problematic for a large proportion of this population. Further work is needed from a broader cross-section, ranging from kidney transplantation recipients who are clearly unwell to well, to see whether it is an underlying problem with the SONG-LP measure.
The SONG-LP instrument demonstrated moderate to high correlation with the PROMIS Item Bank version 2.0 APS and the EQ-5D-5L. We suggest that this is to be expected because the PROMIS Version 2.0 APS items reflect similar concepts and dimensions to life participation as captured by the SONG-LP instrument, and the EQ-5D-5L include an item (usual) that directly assesses the construct of life participation and with similar examples (work, study, housework, family, and leisure activities). The low to moderate correlation with the PROMIS measures of cognitive function, emotional support and instrumental support may be because these constructs do not directly reflect the same concept as life participation.
It was difficult to determine a priori definitive clinical characteristics to examine known-groups validity because there are a plethora of factors that may impact life participation in kidney transplant recipients. There was weak correlation between life participation (as measured by the SONG-LP instrument) with clinical variables, including kidney function and time since transplant. Although some studies suggest that kidney function may be associated with quality of life, which is a concept that is related to life participation,36,37 a range of complications, treatment side effects, comorbidities, and psychosocial challenges can also impair life participation.8 Further work is needed to investigate the patterns and relationships between clinical factors (e.g., infection, cardiovascular disease, diabetes, and other comorbidities) or medication side effects and life participation. Time since transplant did not correlate strongly with perceived life participation and this may be due to fluctuating symptoms and comorbidities over time. The perceived health (based on quality of life measured using the EQ-5D) of long-term kidney transplant recipients has been shown to be comparable to those who had been more recently transplanted,38 suggesting that time since transplant may have limited association with life participation.
We recognize that it was not feasible to assess all psychometric properties, including responsiveness and known-group validity. Similarly, the minimally important difference or clinically meaningful change in life participation has yet to be defined. The SONG-LP was available only in English and subsequent studies are proposed to assess cross-cultural validity. In addition, we will conduct further studies to ascertain the minimally important difference and to generate evidence to define other psychometric properties. The scoring used for SONG-LP was an average of scale responses, and this places an equal weight on each of the 4 items. This may not reflect patient preferences who may place greater importance on activities with family than work and development of a preference-based scoring algorithm may be warranted.
We also acknowledge that recruitment was conducted using the SONG database and the potential limitations regarding selection bias and generalizability. Furthermore, 19 participants did not complete the follow-up survey, which has marginally reduced the precision of our estimates and may have slightly increased the bias of the psychometric measures. Most participants were from high income countries and had high educational attainment. Only those with access to the Internet could complete the study, and it is possible that only people who have higher education from countries in which English is not the official language participated. We also plan to evaluate the potential use of the SONG-LP in other populations in chronic kidney disease.
Given the paucity of patient-reported outcome measures designed to assess life participation and validation that is relevant to the kidney transplantation population, the development of a new measure was undertaken. This initial evidence to support validity and reliability of the SONG-LP instrument demonstrates that it is an appropriate patient-reported outcome measure for kidney transplant recipients. Although our work provides evidence of validity and some psychometric properties, further studies are required to build the knowledge of the psychometric properties to support validity and reliability of the SONG-LP instrument. Validation is an iterative process whereby further work with the SONG-Kidney Transplant life participation measure will accumulate psychometric evidence for use in the kidney transplantation population. Furthermore, because it was designed to be used as a core outcome measure, we advise that trials in which life participation is a primary outcome may need to include more comprehensive measures of life participation in conjunction with or in place of the SONG-LP instrument. The use of the SONG-LP instrument across trials in kidney transplant recipients can provide a standardized measure of life participation that is relevant and meaningful for kidney transplant recipients. This will contribute to an evidence-base in which kidney transplant recipients, caregivers, and health professionals can compare and consider the effect of interventions on life participation for better decision-making and outcomes.
Disclosure
All the authors have declared no conflicting interests.
Acknowledgments
We thank all the kidney transplant recipients who participated in this study. This project is supported by a National Health and Medical Research Council Centers for Research Excellence Grant 2007026. AT is supported by a NHMRC Investigator Award 20191009. AKV receives grant support from a Queensland Advancing Clinical Research Fellowship and an NHMRC Emerging Leader Grant (1196033). The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript.
Footnotes
Table S1. Data completeness and targeting.
Figure S1. Frequency of SONG Life Participation items scale responses.
Figure S2. Frequency of EQ5D-5L “usual activities” items scale responses.
Supplementary Material
Table S1. Data completeness and targeting.
Figure S1. Frequency of SONG Life Participation items scale responses.
Figure S2. Frequency of EQ5D-5L “usual activities” items scale responses.
References
- 1.Marcen R. Immunosuppressive drugs in kidney transplantation: impact on patient survival, and incidence of cardiovascular disease, malignancy and infection. Drugs. 2009;69:2227–2243. doi: 10.2165/11319260-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 2.Weinrauch L.A., D’Elia J.A., Weir M.R., et al. Infection and malignancy outweigh cardiovascular mortality in kidney transplant recipients: post hoc analysis of the FAVORIT trial. Am J Med. 2018;131:165–172. doi: 10.1016/j.amjmed.2017.08.038. [DOI] [PubMed] [Google Scholar]
- 3.Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2010;9(Suppl 3):S1–S155. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 4.Ziengs A.L., Buunk A.M., van Sonderen L., et al. Long-term cognitive impairments in kidney transplant recipients: impact on participation and quality of life. Nephrol Dial Transplant. 2022;38:491–498. doi: 10.1093/ndt/gfac1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Troen A.M., Scott T.M., D’Anci K.E., et al. Cognitive dysfunction and depression in adult kidney transplant recipients: baseline findings from the FAVORIT Ancillary Cognitive Trial (FACT) J Ren Nutr. 2012;22:268–276. doi: 10.1053/j.jrn.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinter J., Hanson C.S., Chapman J.R., et al. Perspectives of older kidney transplant recipients on kidney transplantation. Clin J Am Soc Nephrol. 2017;12:443–453. doi: 10.2215/CJN.05890616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ettenger R., Albrecht R., Alloway R., et al. Meeting report: FDA public meeting on patient-focused drug development and medication adherence in solid organ transplant patients. Am J Transplant. 2018;18:564–573. doi: 10.1111/ajt.14635. [DOI] [PubMed] [Google Scholar]
- 8.Dukes J.L., Seelam S., Lentine K.L., Schnitzler M.A., Neri L. Health-related quality of life in kidney transplant patients with diabetes. Clin Transpl. 2013;27:e554–e562. doi: 10.1111/ctr.12198. [DOI] [PubMed] [Google Scholar]
- 9.Sautenet B., Tong A., Manera K.E., et al. Developing consensus-based priority outcome domains for trials in kidney transplantation: a multinational Delphi survey with patients, caregivers, and health professionals. Transplantation. 2017;101:1875–1886. doi: 10.1097/TP.0000000000001776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tong A., Gill J., Budde K., et al. Toward establishing core outcome domains for trials in kidney transplantation: report of the standardized outcomes in nephrology-kidney transplantation consensus workshops. Transplantation. 2017;101:1887–1896. doi: 10.1097/TP.0000000000001774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tong A., Manns B., Wang A.Y.M., et al. Implementing core outcomes in kidney disease: report of the Standardized Outcomes in Nephrology (SONG) implementation workshop. Kidney Int. 2018;94:1053–1068. doi: 10.1016/j.kint.2018.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ju A., Josephson M.A., Butt Z., et al. Establishing a Core Outcome Measure for Life Participation: a Standardized Outcomes in Nephrology-Kidney Transplantation Consensus Workshop report. Transplantation. 2019;103:1199–1205. doi: 10.1097/TP.0000000000002476. [DOI] [PubMed] [Google Scholar]
- 13.Ju A., Chow B.Y., Ralph A.F., et al. Patient-reported outcome measures for life participation in kidney transplantation: a systematic review. Am J Transplant. 2019;19:2306–2317. doi: 10.1111/ajt.15267. [DOI] [PubMed] [Google Scholar]
- 14.Gagnier J.J., Lai J., Mokkink L.B., Terwee C.B. COSMIN reporting guideline for studies on measurement properties of patient-reported outcome measures. Qual Life Res. 2021;30:2197–2218. doi: 10.1007/s11136-021-02822-4. [DOI] [PubMed] [Google Scholar]
- 15.Mokkink L.B., Prinsen C.A.C., Patrick D.L., et al. COSMIN methodology for systematic reviews of patient-reported outcome measures (PROMs) user manual. https://cosmin.nl/wp-content/uploads/COSMIN-syst-review-for-PROMs-manual_version-1_feb-2018.pdf [DOI] [PMC free article] [PubMed]
- 16.Willis G.B. Sage Publications Inc; 2005. Cognitive Interviewing: A Tool for Improving Questionnaire Design. [Google Scholar]
- 17.Hahn E.A., Kallen M.A., Jensen R.E., et al. Measuring social function in diverse cancer populations: evaluation of measurement equivalence of the patient reported outcomes measurement information system (PROMIS) ability to participate in social roles and activities short form. Psychol Test Assess Model. 2016;58:403–421. [PMC free article] [PubMed] [Google Scholar]
- 18.Tang E., Ekundayo O., Peipert J.D., et al. Validation of the Patient-Reported Outcomes Measurement Information System (PROMIS)-57 and -29 item short forms among kidney transplant recipients. Qual Life Res. 2019;28:815–827. doi: 10.1007/s11136-018-2058-2. [DOI] [PubMed] [Google Scholar]
- 19.Tamminga S.J., van Vree F.M., Volker G., et al. Changes in the ability to participate in and satisfaction with social roles and activities in patients in outpatient rehabilitation. J Patient Rep Outcomes. 2020;4:73. doi: 10.1186/s41687-020-00236-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cano-García L., Mena-Vazquez N., Manrique-Arija S., Redondo-Rodriguez R., Romero-Barco C.M., Fernández-Nebro A. Ability to participate in social activities of rheumatoid arthritis patients compared with other rheumatic diseases: a cross-sectional observational study. Diagnostics. 2021;11:2258. doi: 10.3390/diagnostics11122258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patient-reported outcomes measurement information system (PROMIS). Health Measures. https://staging.healthmeasures.net/images/PROMIS/manuals/PROMIS_Cognitive_Function_Scoring_Manual.pdf
- 22.Elliott C., Frith J., Pairman J., Jones D.E., Newton J.L. Reduction in functional ability is significant postliver transplantation compared with matched liver disease and community dwelling controls. Transpl Int. 2011;24:588–595. doi: 10.1111/j.1432-2277.2011.01240.x. [DOI] [PubMed] [Google Scholar]
- 23.Kim M.Y., Sen D., Drummond R.R., et al. Cognitive dysfunction among people with systemic lupus erythematosus is associated with reduced participation in daily life. Lupus. 2021;30 doi: 10.1177/09612033211006187. 1110-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patient-Reported Outcomes Measurement Information System (PROMIS) Emotional support available. Health Measures. https://staging.healthmeasures.net/images/PROMIS/manuals/PROMIS_Emotional_Support_Scoring_Manual.pdf Published 2020.
- 25.Patient-Reported Outcomes Measurement Information System (PROMIS) Instrumental support. Health Measures. https://staging.healthmeasures.net/images/PROMIS/manuals/PROMIS_Instrumental_Support_Scoring_Manual.pdf Published 2017.
- 26.EuroQol. EQ-5D-5L. https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/ Published 2021.
- 27.Mitchell P.M., Caskey F.J., Scott J., Sanghera S., Coast J. Response process validity of three patient reported outcome measures for people requiring kidney care: a think-aloud study using the EQ-5D-5L, ICECAP-A and ICECAP-O. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2019-034569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li B., Cairns J.A., Draper H., et al. Estimating health-state utility values in kidney transplant recipients and waiting-list patients using the EQ-5D-5L. Value Health. 2017;20:976–984. doi: 10.1016/j.jval.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sawada A., Hiragi S., Tamura H., et al. Evaluation of the quality of life and health-related quality of life of patients with end-stage kidney disease resulting from kidney transplantation using the kidney disease quality of life-short form and EuroQOL-5 dimension-5 level questionnaires. Transplant Proc. 2021;53:881–884. doi: 10.1016/j.transproceed.2020.09.018. [DOI] [PubMed] [Google Scholar]
- 30.Cleemput I K.K., Moons P., Vanrenterghem Y., Van Hooff J.P., Squifflet J.P., De Geest S. The construct and concurrent validity of the EQ-5D in a renal transplant population. Value Health. 2004;7:499–509. doi: 10.1111/j.1524-4733.2004.74013.x. [DOI] [PubMed] [Google Scholar]
- 31.Norman R., Cronin P., Viney R. A pilot discrete choice experiment to explore preferences for EQ-5D-5L health states. Appl Health Econ Health Policy. 2013;11:287–298. doi: 10.1007/s40258-013-0035-z. [DOI] [PubMed] [Google Scholar]
- 32.Devlin N., Roudijk B., Ludwig K., editors. Value Sets for Eq-5D-5L: A Compendium, Comparative Review & User Guide. Springer; 2022. [PubMed] [Google Scholar]
- 33.Koo T.K., Li M.Y. A guideline of selecting and reporting intraclass correlation coeffecients for reliability research. J Chiropr Med. 2016;15:155–163. doi: 10.1016/j.jcm.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prinsen C.A., Vohra S., Rose M.R., et al. How to select outcome measurement instruments for outcomes included in a “Core Outcome Set”-a practical guideline. Trials. 2016;17:449. doi: 10.1186/s13063-016-1555-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patient-reported outcome measures: use in medical product development to support labeling claims. United States Food and Drug Administration. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-reported-outcome-measures-use-medical-product-development-support-labeling-claims Published 2009.
- 36.Peipert J.D., Caicedo J.C., Friedewald J.J., et al. Trends and predictors of multidimensional health-related quality of life after living donor kidney transplantation. Qual Life Res. 2020;29:2355–2374. doi: 10.1007/s11136-020-02498-2. [DOI] [PubMed] [Google Scholar]
- 37.Neri L., Dukes J., Brennan D.C., et al. Impaired renal function is associated with worse self-reported outcomes after kidney transplantation. Qual Life Res. 2011;20:1689–1698. doi: 10.1007/s11136-011-9905-8. [DOI] [PubMed] [Google Scholar]
- 38.Schulz T., Niesing J., Homan van der Heide J.J., Westerhuis R., Ploeg R.J., Ranchor A.V. Perceived health after kidney transplantation: a cross-sectional comparison of long-term and short-term cohorts. Transplant Proc. 2013;45:2184–2190. doi: 10.1016/j.transproceed.2013.03.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.