Abstract
Introduction
Sodium homeostasis is intimately associated with blood pressure (BP) rhythm, and potassium excretion is closely associated with sodium excretion in the general population. However, the association between circadian sodium and potassium pattern excretion and nocturnal BP in patients with chronic kidney disease (CKD) is not elucidated.
Methods
We evaluated the correlation between the day-to-night ratio of urinary sodium and potassium excretion rate, nocturnal blood pressure, and nocturnal BP dipping in a CKD cohort.
Results
A total of 3152 (56.76% males, mean age 47.63 years) individuals with CKD were included in the study. Patients in quartile 1 (with the lowest ratio) exhibited a 12 mmHg or 9 mmHg higher nocturnal systolic blood pressure (SBP) and blunted SBP dipping than those in quartile 4 when urinary sodium or potassium excretion rate was divided into day-to-night ratios (both P < 0.001). In multivariate analyses, lower day-to-night ratio of urinary sodium was independently linked to higher nocturnal SBP and blunted SBP dipping (linear regression coefficient (95% confidence interval [CI]): −6.89 (−9.48 to −4.31), and −3.64 (−5.48 to −1.80), respectively; both P < 0.001). Similarly, compared with the highest quartile of day-to-night ratio of urinary potassium excretion rate, linear regression coefficient (95% CI) for the lowest quartile was −5.60 (−8.13 to −3.07) for nocturnal SBP, and −2.47 (−4.28 to −0.67) for SBP dipping (both P < 0.001). Moreover, urine flow rate and concentrates of sodium or potassium in the urine were positively associated with urinary sodium or potassium excretion during daytime (P < 0.001).
Conclusion
A higher nocturnal BP and a blunted nocturnal BP dipping were both independently linked to a lower excretion of sodium or potassium during the day in patients with CKD. Furthermore, a decreased urine flow rate and a diminished capacity to concentrate sodium or potassium in the urine appear to be the key contributors to a low day-to-night ratio of urinary sodium excretion or potassium rate.
Keywords: blood pressure, chronic kidney disease, circadian rhythm, potassium, sodium, urine
Graphical abstract
Hypertension is a worldwide cause of death.1,2 Elevated nighttime BP and the absence of nocturnal BP dipping have been identified as risk factors for death and cardiovascular disease (CVD) not only in the general population,3, 4, 5 but also in the CKD population.6,7 BP rhythm is endogenously generated, and has its cycle.8 Multiple factors such as day-night variation in activity9 and heart rate10 will contribute to normal BP rhythms, which is regulated by the sympathetic nervous system and several other neurohormonal systems.11,12 Importantly, accumulating evidence suggest that sodium homeostasis is closely associated with BP rhythm.13
Impaired natriuretic efficiency has been linked to hypertension,14,15 and may also be responsible for the loss of diurnal BP variationn.13 A blunted nocturnal dip was observed in participants with a lower day-to-night ratio of sodium excretion.16 Nondippers, on the other hand, have sodium excretion that is more equally distributed throughout the 24-hour cycle.17,18 This suggests that increased nocturnal BP is necessary for decreased daytime sodium excretion to facilitate a compensatory rise in sodium excretion and restore balance.19,20 Other studies found that nephron loss caused reactive system failure, resulting in elevated, arrhythmic BP,21,22 and that >80% of patients with renal disease displayed nondipping.23 Therefore, we were particularly interested in quantifying the variation in nocturnal BP between groups of patients with CKD with different day-to-night ratios of sodium excretion. It has not, as far as we know, been elucidated. Studies on association between circadian rhythm of potassium excretion, which is closely related to sodium excretion, and nocturnal BP are scarce. Therefore, we aim to clarify the association between circadian rhythm of sodium and potassium excretion and nocturnal BP, using separated daytime and nighttime urine tests in individuals with CKD.
Methods
Study Population
The study took place in the Fifth Affiliated Hospital of Sun Yat-sen University, Zhuhai City, China. A total of 3152 patients with a proven CKD diagnosis from 2018 to 2022 were included. CKD was defined as an estimated glomerular filtration rate (eGFR) <60 ml/min per 1.73 m2 for ≥3 months,24 or with evidence of kidney damage (albuminuria or pathologic or structural abnormalities confirmed by kidney biopsy or imageology examination).25 All participants provided written informed consent, and the study protocol was approved by the institutional review board and ethical committee of the Fifth Affiliated Hospital of Sun Yat-sen University (Zhuhai, China) (ID: K55-1). Exclusion criteria included patients <14 years old, dialyzed or transplanted, an acute medical situation (such as myocardial infarction or acute stroke within the last 6 months), and incomplete data of daytime or nighttime sodium and potassium excretion. The study was carried out in accordance with the Declaration of Helsinki, regional registry regulations, and Chinese clinical study ethics.
Measurements and Definitions
Patient data, including clinical features, medical history, and medication usage were collected based on patient interviews, medical records, and physical examinations, and were recorded by trained clinical research physicians. Diabetes mellitus was defined if diagnosis was stated in medical records, as glucose-lowering medication use, as fasting blood glucose level ≥126 mg/dl, a nonfasting glucose ≥200 mg/dl, or an HbA1c ≥7%. Hyperlipidemia was identified as the use of lipid-lowering medications or as a diagnosis noted in medical records. History of CVD at baseline included myocardial stroke, angina pectoris, and infarction.
Omron HEM 907XL devices (Kyoto, Japan) were used to monitor office BP 3 times using a validated cuff-oscillometric device in accordance with guidelines from the European Society of Hypertension and the European Society of Cardiology.25,26 Ambulatory blood pressure monitoring (ABPM) was performed with a validated Mobil-O-Graph PWA monitor (IEM Healthcare, Stolberg, Germany) after the out-patient follow-up. The monitor recorded BP at intervals of 15 and 30 minutes during the daytime and nighttime, respectively. All patients were encouraged to maintain their usual activities and refrain from strenuous physical activity during the monitoring period. Valid measurements were defined as a minimum 70% valid measurements at ≥24-hour duration and ≥20 successful daylight recordings and ≥7 successful nighttime recordings. Daytime and nighttime periods were personalized according to bedtime and wakeup timings.27,28 Hypertension control was defined according to office BP (<140/90 mmHg), mean 24-hour ambulatory BP (<130/80 mmHg), and daytime BP (<135/85 mmHg) and nocturnal BP (<120/70 mmHg).10 Nondipper or reduced dipper (a nocturnal BP decline of 10% of daytime values) and riser (or reverse dipper) (a nocturnal BP rise) status were defined according to international guidelines.10 Separate 24-hour urine collections were made for the day and the night. The average duration of urine collections for daytime and nighttime was 15 and 9 hours, respectively.
Fasting blood drawn, ABPM recording, and urine collection were performed on the same day. A Cobas c702 automatic biochemical analyzer (Roche, Basel, Switzerland) was used to quantify the concentrations of sodium and potassium in plasma and urine. Urine flow rate and sodium and potassium excretion rates (urinary sodium concentration × urine flow rate and urinary potassium concentration × urine flow rate, respectively) were measured independently for daytime and overnight. The excretion rate of sodium or potassium was determined by its urine concentration multiplied by the flow rate of the urine. A Cobas c702 automatic biochemical analyzer (Roche, Basel, Switzerland) was used to measure the serum creatinine levels using an enzymatic method. We determined the eGFR value based on serum creatinine using the Chronic Kidney Disease Epidemiology Collaboration equation.24 Urinary albumin-to-creatinine ratio was measured in the central laboratory by immunoturbidimetry and was reported in mg/g creatinine (TBA-FX8, Canon, Japan). Following the Kidney Disease Improving Global Outcomes guidelines, patients were categorized based on eGFR (≥60 or <60 ml/min per 1.73 m2) or urine albumin-to-creatinine ratio (microalbuminuria [<30 mg/g] or high albuminuria [30–300 mg/g] or macroalbuminuria [>300 mg/g]).29 Patients were classified into high and low 24-hour sodium excretion group, and high and low 24-hour potassium excretion group according to the corresponding median.
Statistical Methods
The data were divided into quartiles (Q1 to Q4) on the basis of the day-to-night ratio of urinary sodium excretion or day-to-night ratio of urinary potassium excretion. The Kolmogorov-Smirnov test was used to test the normal distribution of data. A nonparametric test for continuous data or a χ2 test for categorical variables was utilized to assess trends across quartiles. SBP, diastolic blood pressure (DBP), pulse rate, and pulse pressure readings at night and fluctuations between daytime and nocturnal values (nocturnal dipping) were employed as dependent variables. We used general linear model to evaluate the correlation between dependent variables and day-to-night ratio of urinary sodium (or potassium) excretion (4 groups), adjusting for age (continuous), sex, body mass index (continuous), smoking status (current, ex-smoker, or never), diabetes (yes/no), blood glucose levels, dyslipidemia (yes/no), CVD (yes/no), use of antihypertensive medications (yes/no), use of glucocorticoids and immunosuppressive agents (yes/no), eGFR (continuous), albuminuria (microalbuminuria, high albuminuria, or macroalbuminuria), and 24-hour excretion of sodium and potassium (continuous). Multivariable logistic regression models adjusted for variables were used to assess the association between dependent variables (high nocturnal BP [yes/no] and nocturnal BP risers [yes/no]) and day-tonight ratio of urinary sodium (or potassium) excretion. To evaluate model performance, continuous net reclassification improvement >0 and integrated discrimination improvement were calculated.30 Fully adjusted model (model 5) was compared with model 5 minus day-to-night ratio of urinary sodium or potassium excretion. An interaction term was added between day-to-night ratio and eGFR to analyze the interactions with eGFR in the models of high nocturnal BP and nocturnal BP risers. Statistics were considered significant for P values <0.05. Data was analyzed using SPSS (version 25.0, SPSS Inc), Graph Pad Prism 8 and R (version 4.2.2).
Results
Baseline Characteristics
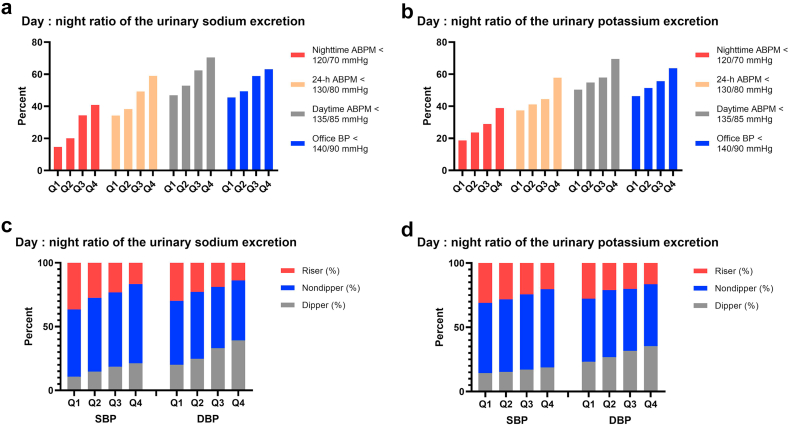
This analysis included 3152 patients (mean age, 47.63 years; 56.76% men). The patients were divided into quartiles based on the day-to-night ratio of urinary sodium and potassium excretion rate for the purposes of clinical correlation (Table 1). Patients in Q4 excreted 3.63 times as much sodium during daytime as they do during nighttime, whereas patients in Q1 excreted sodium that is 27% lower during daytime than during nighttime. 24-hour sodium excretion in Q2 and Q3 were higher than those in Q1 and Q4 (Table 1). Patients in Q1 were older than those in Q2, Q3, and Q4 (P < 0.05). The body mass index, fasting blood glucose, and percentage of diabetes mellitus and CVD were higher in Q1 than in Q4 (P < 0.05). Patients in Q1 had a lower eGFR than those in Q4 (P < 0.001, Table 1). A considerable rise was seen in the proportion of patients with BP (office BP, daytime BP, nocturnal BP, and 24-h BP) under control from Q1 to Q4 (all P < 0.001, Figure 1). However, as the day-to-night ratio of urinary sodium excretion increased, the percentage of risers pattern on ABPM decreased (P < 0.001, Figure 1). The use of antihypertensive agents in patients, and the percentage of macroalbuminuria decreased significantly from Q1 to Q4 (both P < 0.001). When compared to the patients in the lower quartile of day-to-night ratio of urinary potassium excretion rate, those in the higher quartile were younger, had a lower prevalence of hypertension and lower BP (office and 24-h BP), and had a higher eGFR (all P < 0.001).
Table 1.
Characteristics of patients with CKD according to quartile of day-to-night ratio of the urinary sodium and potassium excretion rate
|
Variable |
Day-to-night ratio of the urinary sodium excretion |
Day-to-night ratio of the urinary potassium excretion |
||||||
|---|---|---|---|---|---|---|---|---|
| Q1 ≤1.00 (n = 788) |
Q2 1.01–1.52 (n = 788) |
Q3 1.53–2.52 (n = 788) |
Q4 >2.52 (n = 788) |
Q1 ≤1.44 (n = 788) |
Q2 1.45–1.94 (n = 788) |
Q3 1.95–2.62 (n = 787) |
Q4 >2.62 (n = 789) |
|
| D/Na | 0.73 (0.56–0.87) | 1.24 (1.11–1.38) | 1.91 (1.72–2.16) | 3.63 (2.93–5.33) | 1.14 (0.90–1.29) | 1.69 (1.56–1.81) | 2.24 (2.09–2.40) | 3.39 (2.90–4.42) |
| Age (years) | 52.73 ± 13.32 | 49.62 ± 13.85 | 46.08 ± 14.19 | 42.07 ± 14.88 | 49.96 ± 14.53 | 50.18 ± 14.10 | 46.65 ± 14.22 | 43.72 ± 14.66 |
| ≥60 years (%) | 35.41 | 26.27 | 18.15 | 14.47 | 29.82 | 29.19 | 19.82 | 15.46 |
| Men (%) | 59.01 | 57.36 | 56.09 | 54.57 | 61.04 | 57.11 | 56.16 | 52.72 |
| Risk factors | ||||||||
| BMI (kg/m2) | 24.24 (21.87–26.61) | 23.93 (21.48–26.58) | 23.74 (21.16–26.45) | 23.56 (20.98-26.12) | 24.02 (21.57–26.64) | 23.96 (21.41–26.56) | 23.79 (21.50–26.37) | 23.67 (21.00–26.22) |
| Obesity (BMI ≥ 30 kg/m2) | 8.50 | 8.12 | 6.73 | 6.60 | 8.38 | 7.49 | 8.01 | 6.08 |
| Diabetes mellitus (%) | 30.08 | 22.72 | 19.54 | 14.34 | 25.51 | 23.73 | 20.08 | 17.36 |
| Dyslipidemia (%) | 91.50 | 92.51 | 92.26 | 92.64 | 92.01 | 93.15 | 92.63 | 91.00 |
| Cardiovascular disease (%) | 18.02 | 17.39 | 11.29 | 10.66 | 16.62 | 15.99 | 12.71 | 12.04 |
| Antihypertensive medication (%) | 77.79 | 70.81 | 59.52 | 58.88 | 73.73 | 70.30 | 66.20 | 56.78 |
| RAS blocking agents (%) | 31.35 | 35.03 | 34.52 | 37.31 | 32.49 | 32.23 | 36.34 | 37.14 |
| Calcium channel blockers (%) | 58.12 | 45.81 | 33.63 | 28.68 | 52.41 | 47.72 | 39.52 | 26.62 |
| Other antihypertensive medicationsb (%) | 31.35 | 23.73 | 14.47 | 12.06 | 25.63 | 23.48 | 19.44 | 13.05 |
| Immunosuppressive agentsc (%) | 7.23 | 6.85 | 8.38 | 10.53 | 6.85 | 7.49 | 7.62 | 11.03 |
| Current smoking (%) | 3.81 | 2.79 | 1.90 | 3.30 | 3.55 | 3.05 | 2.67 | 2.53 |
| Weekly alcohol drinking (%) | 13.20 | 13.20 | 13.45 | 12.69 | 13.58 | 13.32 | 14.10 | 11.53 |
| BP | ||||||||
| Office SBP (mmHg) | 137.00 (122.00–152.00) | 134.00 (120.00–151.00) | 129.00 (116.00–144.00) | 127.00 (114.00–141.00) | 136.00 (122.00–153.00) | 133.50 (118.00–149.00) | 130.00 (118.00–146.00) | 126.00 (114.00–141.00) |
| Office DBP (mmHg) | 86.00 (76.00–95.00) | 85.00 (76.00–95.00) | 83.00 (75.00–91.00) | 82.00 (74.00–92.00) | 86.00 (76.00–95.00) | 85.00 (76.00–95.00) | 83.00 (76.00–93.00) | 81.00 (74.00–91.00) |
| 24-h SBP (mmHg) | 127.00 (117.00–139.75) | 123.00 (114.00–134.00) | 120.00 (110.25–130.00) | 117.00 (109.00–126.00) | 125.00 (115.00–138.00) | 123.00 (113.25–134.00) | 121.00 (112.00–131.00) | 118.00 (109.00–126.00) |
| 24-h DBP (mmHg) | 83.00 (75.25–91.00) | 81.00 (74.00–89.00) | 79.00 (71.00–87.00) | 76.00 (70.00–84.00) | 82.00 (74.00–90.00) | 81.00 (74.00–89.00) | 80.00 (72.00–88.00) | 77.00 (70.00–84.00) |
| eGFR by CKD-EPId (ml/min per 1.73 m2) | 47.00 (23.00–80.00) | 57.00 (28.00–90.89) | 75.00 (41.00–104.00) | 94.03 (61.36–112.00) | 53.21 (23.44–92.00) | 54.13 (27.87–89.13) | 72.00 (40.00–100.00) | 91.00 (59.00–109.00) |
| eGFR by CKD-EPI <60 (ml/min per 1.73 m2) | 61.17 | 51.65 | 37.82 | 23.10 | 54.82 | 54.57 | 38.63 | 25.73 |
| 24-h V (L) | 1.75 (1.33–2.24) | 1.86 (1.41–2.38) | 1.79 (1.36–2.35) | 1.78 (1.27–2.37) | 1.67 (1.18–2.14) | 1.83 (1.4–2.31) | 1.88 (1.45–2.4) | 1.84 (1.33–2.41) |
| Na excretion (mmol/24h) | 121.75 (80.77–164.58) | 125.18 (88.04–177.04) | 128.32 (88.88–176.89) | 119.11 (78.11–168.88) | 117.39 (76.34–163.20) | 124.78 (84.50–166.97) | 126.59 (88.46–179.73) | 126.57 (87.15–178.05) |
| K excretion (mmol/24h) | 31.08 (23.40–41.58) | 32.50 (24.86–40.81) | 33.62 (26.02–42.14) | 32.48 (23.87–41.44) | 29.69 (21.66–39.21) | 31.62 (24.89–40.77) | 33.25 (26.44–40.94) | 33.94 (25.94–44.94) |
| Plasma Na (mmol/l) | 141.00 (139.00–142.60) | 141.00 (139.00–142.30) | 141.00 (139.00–142.65) | 141.00 (139.00–142.30) | 141.00 (139.00–142.70) | 141.00 (139.00–142.60) | 141.00 (139.00–142.20) | 141.00 (139.00–142.40) |
| Plasma K (mmol/l) | 3.90 (3.60–4.30) | 3.90 (3.60–4.22) | 3.81 (3.60–4.15) | 3.79 (3.51–4.00) | 3.90 (3.57–4.30) | 3.90 (3.60–4.20) | 3.81 (3.59–4.10) | 3.80 (3.60–4.10) |
| Albuminuria category (%) | ||||||||
| A1 (normal to mildly increased) | 13.20 | 18.02 | 24.27 | 23.60 | 15.61 | 16.12 | 21.50 | 25.86 |
| A2 (moderately increased) | 21.83 | 26.27 | 23.00 | 26.90 | 22.72 | 25.13 | 24.05 | 26.11 |
| A3 (severely increased) | 64.97 | 55.71 | 52.73 | 49.49 | 61.68 | 58.76 | 54.45 | 48.04 |
ABPM, ambulatory blood pressure monitoring; BP, blood pressure; BMI, body mass index; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration equation; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; Na, sodium; K, potassium; RAS, renin-angiotensin system; SBP, systolic blood pressure.
Results are medians (interquartile range) or percentage unless otherwise specified.
Missing values for the following variables: plasma Na (n = 9), plasma K (n = 11).
D/N indicates day/night ratio of urinary sodium or potassium excretion rate; This day-to-night ratio was used to divide the 3152 subjects into 4 quartiles.
Other hypertension medications include a-blockers, b-blockers, and vasodilator (only 12 patients routinely used diuretics).
Any exposure to glucocorticoids or immunosuppressive agents within 12 weeks.
GFR was evaluated using EPI equation.
Figure 1.
Baseline percentage of high BP (a, b) and circadian profile of BP (c, d) by quartile of day-to-night ratio of the urinary sodium and potassium excretion rate. ABPM, ambulatory blood pressure monitoring; BP, blood pressure; DBP, diastolic blood pressure; SBP, systolic blood pressure.
ABPM Findings on the Basis of Day-to-Night Ratio of Urinary Sodium and Potassium Excretion
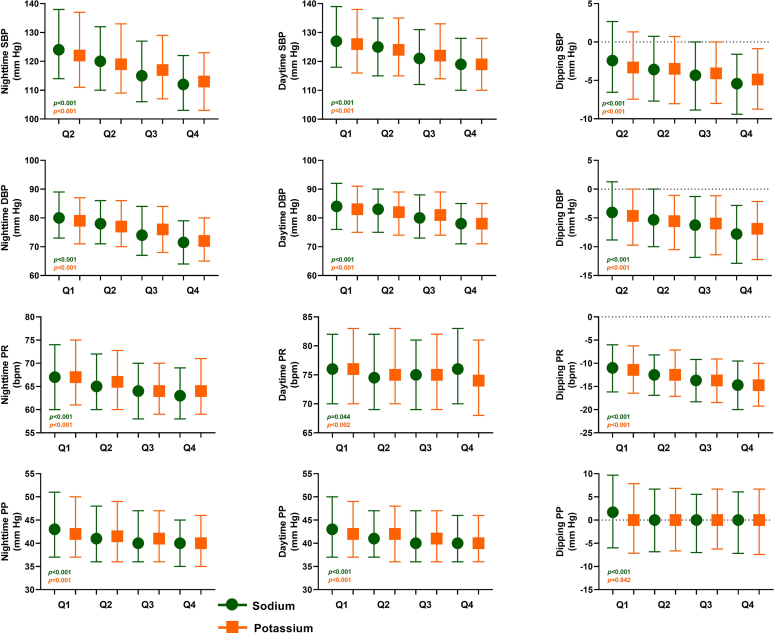
SBP and DBP reduced across increasing quartiles of the day-to-night ratio of urinary sodium excretion, for both daytime and nighttime values (all P < 0.05, Figure 2, Supplementary Table S1). A gradual decrease in pulse pressure values from Q1 to Q4 was observed due to the larger difference for SBP than for DBP (both P < 0.05). The nocturnal SBP and DBP dipping in Q1 were substantially lower than in the other quartiles (both P < 0.05). The same was true for ABPM data across quartiles of the day-to-night ratio of urinary potassium excretion rate (Figure 2, Supplementary Table S1).
Figure 2.
BP, PR, PP, and nocturnal dipping, by quartiles of day-to-night ratio of urinary sodium and potassium excretion rate. The dots and squares represent means in different quartiles of day-to-night ratio of the urinary sodium excretion and day-to-night ratio of the urinary potassium excretion, respectively. Bars represent interquartile ranges (25th and 75th quartile); dipping represents the nocturnal BP decrease (nocturnal BP minus daytime BP), expressed in percentage of the daytime BP. BP, blood pressure; bpm, beats per minute; DBP, diastolic blood pressure; PP, pulse pressure; PR, pulse rate; SBP, systolic blood pressure.
ABPM Findings on the Basis of Presence of CKD and Sodium and Potassium Excretion Categories
Both eGFR categories for nocturnal SBP and nocturnal DBP showed a significant decline from Q1 to Q4 (all P < 0.001, Table 2). When compared with Q1, the median decrease in nighttime SBP among patients in Q4 was 12.0 mmHg in the whole group, 10.0 mmHg in the group with eGFR ≥60 ml/min per 1.73 m2, and 8.5 mmHg in the group with eGFR <60 ml/min per 1.73 m2 (all P < 0.05, Table 2, Figure 2). For daytime SBP, this decrease was 8.0, 7.0, and 5.0 mmHg in the 3 groups, respectively (all P < 0.05). Among the excretion of day-to-night ratio of urinary potassium, the pattern of nocturnal and daytime BP values across quartiles and eGFR groups was similar in magnitude and direction to that in the day-to-night ratio of urinary sodium excretion rate, but differences were smaller (Table 2).
Table 2.
Nighttime and daytime BP values and dipping proportion according to eGFR and day-to-night ratio of the urinary sodium and potassium excretion status
|
n (%) |
Day-to-night ratio of the urinary sodium excretion |
Day-to-night ratio of the urinary potassium excretion |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| eGFR ≥60 ml/min per 1.73 m2 |
eGFR < 60 ml/min per 1.73 m2 |
eGFR ≥60 ml/min per 1.73 m2 |
eGFR <60 ml/min per 1.73 m2 |
|||||||||||||||||
| Q1 306 (17.16) |
Q2 381 (21.37) |
Q3 490 (27.48) |
Q4 606 (33.99) |
P Value | Q1 482 (35.21) |
Q2 407 (29.73) |
Q3 298 (21.77) |
Q4 182 (13.29) |
P Value | Q1 356 (19.97) |
Q2 358 (20.08) |
Q3 483 (27.09) |
Q4 586 (32.87) |
P Value | Q1 432 (31.56) |
Q2 430 (31.41) |
Q3 304 (22.21) |
Q4 203 (14.83) |
P Value | |
| Nighttime SBP (mmHg) | 120.00 (109.00–131.00) | 117.00 (107.00–126.00) | 111.00 (103.75–122.00) | 110.00 (102.00–118.00) | <0.001 | 128.50 (116.75–142.00) | 123.00 (113.00–136.00) | 121.50 (111.00–134.00) | 120.00 (108.75–133.25) | <0.001 | 117.00 (106.00–127.75) | 113.50 (105.00–126.25) | 113.00 (105.00–122.00) | 111.00 (102.00–120.00) | < 0.001 | 127.00 (116.00–142.00) | 124.00 (113.00–137.00) | 124.00 (113.00–137.00) | 119.00 (109.00–132.00) | <0.001 |
| Nighttime DBP (mmHg) | 78 .00 (70.00–85.00) | 76.00 (68.00–82.00) | 72.00 (65.00–80.00) | 70.00 (63.00–77.00) | <0.001 | 82.00 (74.00–91.00) | 80.00 (72.00–89.00) | 79.50 (71–87) | 78 (69.75–86.25) | <0.001 | 75.00 (67.25–83.00) | 74.00 (66.75–82.00) | 73.00 (66.00–81.00) | 70.00 (64.00–78.00) | < 0.001 | 82.00 (74.00–91.00) | 79.00 (72.00–88.00) | 81.00 (72.00–88.75) | 78.00 (70.00–87.00) | <0.001 |
| Daytime SBP (mmHg) | 124.00 (113.00–132.00) | 122.00 (112.00–131.00) | 118.00 (110.00–127.00) | 117.00 (108.00–125.00) | <0.001 | 131.00 (120.00–143.00) | 126.00 (118.00–138.00) | 127.00 (118.00–139.00) | 126.00 (117.00–139.00) | 0.008 | 123.00 (112.00–130.75) | 120.00 (111.00–129.00) | 119.00 (112.00–128.00) | 117.00 (109.00–125.25) | < 0.001 | 131.00 (120.00–143.75) | 127.00 (119.00–139.00) | 128.00 (118.00–140.00) | 125.00 (117.00–139.00) | 0.004 |
| Daytime DBP (mmHg) | 82.00 (73.00–89.00) | 81.00 (73.00–87.00) | 77.00 (71.00–84.00) | 76.00 (70.00–83.00) | <0.001 | 85.00 (77.00–93.00) | 85.00 (78.00–92.00) | 85.00 (77.00–92.00) | 84.00 (75.75–91.00) | 0.169 | 80.00 (72.00–88.00) | 79.50 (72–86.25) | 79.00 (72.00–86.00) | 76.00 (71.00–83.00) | < 0.001 | 85.00 (78.00–93.00) | 84.00 (77.00–91.00) | 86.00 (77.00–93.00) | 84.00 (75.00–91.00) | 0.142 |
| Dippersa (%) | 10.78 | 15.52 | 19.02 | 20.03 | 0.003 | 10.81 | 14.00 | 17.91 | 25.41 | <0.001 | 16.57 | 15.64 | 17.43 | 18.35 | 0.757 | 12.53 | 14.92 | 16.50 | 19.80 | 0.110 |
| Nondippersa (%) | 56.21 | 59.21 | 61.15 | 64.24 | 0.119 | 50.31 | 56.51 | 53.48 | 54.56 | 0.304 | 57.58 | 57.82 | 63.28 | 62.95 | 0.186 | 52.20 | 55.48 | 51.16 | 54.95 | 0.617 |
| Risersa (%) | 33.01 | 25.26 | 19.84 | 15.73 | <0.001 | 38.88 | 29.48 | 28.72 | 19.89 | <0.001 | 25.84 | 26.54 | 19.29 | 18.70 | 0.004 | 35.27 | 29.60 | 32.34 | 25.25 | 0.061 |
BP, blood pressure; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Data are given as n (%) for categorical variables and medians (interquartile range) for continuous variables.
Percent of SBP circadian patterns (dippers, nondippers, and risers).
When compared with Q1, the median increase of nighttime SBP in patients in Q4 was 2.5 mmHg stratified by 24-hour sodium excretion, 2.0 mmHg by 24-hour potassium excretion (Supplementary Table S2). However, for day-to-night ratio of urinary sodium and potassium, nighttime SBP and nighttime DBP decreased from Q1 to Q4 in both 24-hour sodium excretion categories and in both 24-hour potassium excretion categories (all P < 0.001, Supplementary Table S3).
Multivariable Analysis of Day-to-Night Ratio of Urinary Sodium and Potassium Excretion With Nocturnal BP
After fully adjusting for demographic factors, lifestyles, and clinical variables (including diabetes mellitus, blood glucose levels, CVD, eGFR, BP medications, glucocorticoids and immunosuppressive agents, and 24-hour excretion of sodium and potassium), the general linear model revealed that nighttime SBP and DBP in Q1 were 6.89 mmHg and 4.25 mmHg higher than Q4 in accordance with the day-to-night ratio of urinary sodium excretion status (both P < 0.001, Table 3). Patients’ nighttime SBP and nighttime DBP decreased by 5.60 mmHg and 3.07 mmHg in Q4 compared to Q1 according to day-to-night ratio of urinary potassium excretion rate (both P < 0.001, Table 3). In addition, multivariable models for dipping in BP showed similar trends as nocturnal BP. Compared with the highest quartile of day-to-night ratio of urinary sodium and potassium excretion rate, linear regression coefficient (95% CI) in the lowest quartile was −3.64 (−5.48 to −1.80), and −2.47 (−4.28 to −0.67) for SBP dipping, respectively (both P < 0.001). Significance still existed when the administration of diuretics and renin-angiotensin system inhibitors were included in the multivariable regression analysis (all P < 0.001, data not shown). Similar association were observed in nocturnal BP between day-to-night ratio of urinary potassium excretion.
Table 3.
Associations of day-to-night ratio of urinary sodium and potassium excretion rate with nocturnal SBP among patients with CKD
| Dependent Variable | Model | Day-to-Night Ratio of Urinary Sodium Excretion |
Day-to-Night Ratio of Urinary potassium Excretion |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 ≤ 1.00 | Q2 1.01–1.52 |
Q3 1.53–2.52 |
Q4 > 2.52 | P Value | Q1 ≤ 1.44 | Q2 1.44–1.9 |
Q3 1.95–2.62 |
Q4 > 2.62 | P Value | ||
| Nocturnal BP (mmHg)/ Nocturnal PR (bpm) | |||||||||||
| SBP | 1 | Ref | −6.37 (−8.82 to −3.93) | −10.34 (−12.79 to −7.90) | −13.75 (−16.19 to −11.30) | < 0.001 | Ref | −4.01 (−6.48 to −1.54) | −6.36 (−8.83 to −3.90) | −10.61 (−13.08 to −8.15) | < 0.001 |
| 2 | Ref | −5.50 (−7.92 to −3.07) | −8.47 (−10.922 to −13.26) | −10.76 (−13.26 to −8.26) | < 0.001 | Ref | −4.02 (−6.45 to −1.59) | −5.25 (−7.69 to −2.82) | −8.53 (−10.99 to −6.07) | < 0.001 | |
| 3 | Ref | −4.34 (−6.77 to −1.90) | −5.41 (−7.90 to −9.52) | −6.93 (−9.52 to −4.34) | < 0.001 | Ref | −3.65 (−6.08 to −1.22) | −3.87 (−6.32 to −1.41) | −5.47 (−7.99 to −2.96) | < 0.001 | |
| 4 | Ref | −4.46 (−6.89 to −2.02) | −5.55 (−8.04 to −3.05) | −6.89 (−9.47 to −4.30) | < 0.001 | Ref | −3.73 (−6.16 to −1.30) | −4.03 (−6.26 to −1.57) | −5.52 (−8.05 to −2.99) | < 0.001 | |
| 5 | Ref | −4.41 (−6.85 to −1.98) | −5.53 (−8.02 to −3.03) | −6.89 (−9.48 to −4.31) | < 0.001 | Ref | −3.75 (6.18 to −1.31) | −4.02 (−6.48 to −1.57) | −5.60 (−8.13 to −3.07) | < 0.001 | |
| DBP | 1 | Ref | −2.58 (−3.77 to −1.38) | −5.56 (−6.76 to −4.37) | −8.35 (−9.54 to −7.15) | < 0.001 | Ref | −1.59 (−2.80 to −0.38) | −2.98 (−4.19 to −1.77) | −6.46 (−7.67 to −5.25) | < 0.001 |
| 2 | Ref | −2.26 (−3.45 to −1.08) | −4.91 (−6.11 to −3.72) | −7.32 (−8.54 to −6.09) | < 0.001 | Ref | −1.50 (−2.69 to −0.30) | −2.46 (−3.66 to −1.26) | −5.51 (−6.72 to −4.31) | < 0.001 | |
| 3 | Ref | −1.46 (−2.58 to −0.33) | −2.65 (−3.80 to −1.50) | −4.26 (-5.56 to −3.07) | < 0.001 | Ref | −1.25 (−2.37 to −0.13) | −1.32 (−2.45 to −0.18) | −2.92 (−4.08 to −1.76) | < 0.001 | |
| 4 | Ref | −1.56 (−2.68 to −0.44) | −2.77 (−3.92 to −1.63) | −4.24 (−5.43 to −3.05) | < 0.001 | Ref | −1.33 (−2.45 to −0.21) | −1.47 (−2.60 to −0.34) | 3.00 (−4.16 to −1.83) | < 0.001 | |
| 5 | −1.52 (−2.63 to −0.40) | −2.75 (−3.89 to −1.61) | −4.25 (−5.23 to −3.06) | < 0.001 | −1.35 (−2.46 to −0.23) | -1.47 (-2.59- -0.34) | −3.07 (−4.23 to −1.91) | < 0.001 | |||
| PR | 1 | Ref | −1.37 (−2.37 to −0.36) | −2.83 (−3.83 to −1.83) | −2.75 (−3.75 to −1.75) | < 0.001 | Ref | −1.31 (−2.31 to −0.32) | −2.62 (−3.62 to −1.63) | −4.15 (−5.15 to −3.16) | < 0.001 |
| 2 | Ref | −1.33 (−2.34 to −0.32) | −2.75 (−3.76 to −1.73) | −2.62 (−3.66 to −1.59) | < 0.001 | Ref | −1.33 (−2.33 to −0.33) | −2.60 (−3.60 to −1.60) | −4.10 (−5.11 to −3.09) | < 0.001 | |
| 3 | Ref | −0.98 (−1.97 to 0.00) | −1.54 (−2.55 to −0.53) | −0.99 (−2.04 to −0.06) | 0.038 | Ref | −1.2 (−2.18 to −0.22) | −2.03 (−3.02 to −1.05) | −2.88 (−3.90 to −1.87) | < 0.001 | |
| 4 | Ref | −0.96 (−1.95 to 0.03) | −1.50 (−2.51 to −0.48) | −0.96 (−2.01 to 0.09) | 0.043 | Ref | −1.12 (−2.10 to −0.14) | −1.94 (−2.94 to −0.95) | −2.74 (−3.77 to −1.72) | < 0.001 | |
| 5 | Ref | −0.95 (−1.94 to 0.04) | −1.49 (−2.50 to −0.48) | −0.96 (−2.01 to 0.09) | 0.043 | Ref | −1.13 (−2.11 to −0.15) | −1.94 (−2.93 to −0.95) | −2.76 (−3.79 to −1.74) | < 0.001 | |
| Nocturnal BP/PR dipping (%) | |||||||||||
| SBP | 1 | Ref | −2.59 (−4.24 to −0.93) | −3.51 (−5.16 to −1.85) | −4.50 (−6.16 to −2.85) | < 0.001 | Ref | −1.53 (−3.19 to −0.13) | −2.25 (−3.91 to −0.59) | −2.87 (−4.53 to −1.21) | < 0.001 |
| 2 | Ref | -2.39 (-4.05- -0.74) | −3.08 (−4.75 to −1.41) | −3.81 (−5.52 to −2.10) | < 0.001 | Ref | −1.62 (−3.27 to 0.03) | −2.05 (−3.71 to −0.39) | −2.47 (−4.14 to 0.79) | 0.004 | |
| 3 | Ref | -2.30 (-4.04- -0.57) | −2.93 (−4.71 to −0.57) | −3.64 (−5.47 to −1.8) | < 0.001 | Ref | −1.72 (−3.45 to 0.01) | −2.11 (−3.86 to −0.37) | −2.40 (−4.19 to −0.61) | 0.008 | |
| 4 | Ref | -2.33 (-4.06- -0.59) | −2.96 (−4.74 to −1.18) | −3.63 (−5.48 to −1.79) | < 0.001 | Ref | −1.75 (−3.48 to −0.01) | −2.16 (−3.91 to −0.40) | −2.42 (−4.23 to −0.62) | 0.008 | |
| 5 | Ref | -2.30 (-4.04- -0.56) | −2.94 (−4.72 to −1.17) | −3.64 (−5.48 to −1.80) | < 0.001 | Ref | −1.76 (−3.49 to −0.02) | −2.15 (-3.91 to −0.40) | −2.47 (−4.28 to −0.67) | 0.007 | |
| DBP | 1 | Ref | -1.41 (-2.19- -0.63) | −2.61 (3.38 to −1.83) | −3.96 (−4.74 to −3.18) | < 0.001 | Ref | −0.84 (−1.62 to −0.05) | −1.63 (−2.42 to −0.84) | −2.37 (−3.16 to −1.58) | < 0.001 |
| 2 | Ref | −1.15 (−1.92 to −0.39) | −2.05 (−2.82 to −1.27) | −3.06 (−3.85 to −2.27) | < 0.001 | Ref | −.092 (−1.68 to −0.15) | −1.36 (−2.13 to −0.58) | −1.84 (−2.62 to −1.06) | < 0.001 | |
| 3 | Ref | −0.96 (2.56 to −0.95) | −1.75 (2.56 to −0.95) | −2.61 (−3.45 to −1.78) | < 0.001 | Ref | −0.89 (−1.68 to −0.11) | −1.20 (−1.99 to −0.41) | −1.55 (−2.37 to −0.74) | < 0.001 | |
| 4 | Ref | −1.00 (−1.78 to −0.21) | −1.80 (−2.60 to −0.99) | −2.61 (−3.45 to 1.78) | < 0.001 | Ref | −0.94 (−1.73 to −0.15) | −1.28 (−2.07 to −0.48) | −1.62 (−2.44 to −0.80) | < 0.001 | |
| 5 | Ref | −0.96 (−1.74 to −0.17) | −1.78 (−2.58 to −0.98) | −2.62 (−3.45 to −1.79) | < 0.001 | Ref | −0.96 (−1.74 to −0.17) | −1.27 (−2.06 to −0.48) | −1.69 (−2.51 to −0.88) | < 0.001 | |
| PR | 1 | Ref | −1.34 (−2.11 to −0.57) | −2.50 (−3.26 to −1.73) | −3.82 (−4.59 to −3.05) | < 0.001 | Ref | −1.16 (−0.193 to −0.39) | −2.51 (−3.28 to −1.74) | −3.44 (−4.21 to −2.67) | < 0.001 |
| 2 | Ref | −0.87 (−1.61 to −0.13) | −1.50 (−2.25 to −0.75) | −2.23 (−2.80 to −1.46) | < 0.001 | Ref | −1.19 (−1.92 to −0.46) | −2.00 (−2.74 to −1.25) | −2.48 (−3.23 to −1.73) | < 0.001 | |
| 3 | Ref | −0.52 (−1.25 to −0.22) | 0.55 (−1.30 to −0.21) | −0.83 (−1.61 to −0.04) | 0.048 | Ref | −1.04 (−1.77 to −0.31) | −1.50 (−2.24 to −0.76) | −1.42 (−2.18 to −0.66) | < 0.001 | |
| 4 | Ref | −0.51 (−1.24 to −0.23) | −0.52 (−1.27 to −0.24) | −0.80 (−1.58 to −0.01) | 0.058 | Ref | −0.96 (−1.69 to −0.23) | −1.41 (−2.16 to −0.67) | −1.26 (−2.02 to −0.49) | < 0.001 | |
| 5 | Ref | −0.51 (−1.25 to 0.23) | −0.52 (−1.28 to 0.24) | −0.80 (−1.58 to −0.01) | 0.059 | Ref | −0.96 (−1.69 to −0.22) | −1.41 (−2.16 to −0.67) | −1.25 (−2.01 to −0.48) | < 0.001 | |
Results are regression coefficient (95% CI). Nocturnal BP dipping is day-night difference in percentage of day value. M1 indicates unadjusted model; M2, model adjusted for age and sex; M3, the model adjusted for age, sex, eGFR, BMI, tobacco smoking, diabetes, fasting blood glucose, dyslipidemia, CVD, antihypertensive treatment, and albuminuria category; M4, model 3 plus 24h Na, 24h K; M5, model 4 plus glucocorticoids.
95% CI, 95% confidence interval. P values for trend were calculated by treating quartiles as a continuous variable in each model. BP, blood pressure; bpm, beats per minute; DBP, diastolic blood pressure; PR, pulse rate; SBP, systolic blood pressure.
Missing values for the following variables: PR dipping (n = 4).
Multivariable logistic analyses showed that day-to-night ratio of urinary sodium or potassium excretion was negatively related with the risk of high nocturnal SBP, and nocturnal BP risers (all P < 0.01, Supplementary Table S4). Continuous net reclassification improvements (> 0) were 0.28 (95% CI, 0.21–0.38), 0.35 (95% CI, 0.26–0.44), 0.23 (95% CI, 0.14–0.32), and 0.21 (95% CI, 0.12–0.32) for high nocturnal SBP, high nocturnal DBP, SBP risers, and DBP risers, respectively (model 5 vs. model 5 minus day-to-night ratio of urinary sodium) (all P < 0.05). Integrated discrimination improvement also showed that model 5 was superior to model 5 without day-to-night ratio of urinary sodium excretion (integrated discrimination improvement: 0.0099 (95% CI, 0.0061–0.0138), 0.0151 (95% CI, 0.0103–0.0200), 0.0113 (95% CI, 0.0071–0.0156), and 0.0082 (95% CI, 0.0044–0.0120), all P < 0.05). The net reclassification improvement analysis also revealed that day-to-night ratio of urinary potassium excretion had an improved classification accuracy in patients with and without high nocturnal SBP, high nocturnal DBP, SBP risers, and DBP risers, leading to a continuous net reclassification improvement (> 0) of 0.21 (95% CI, 0.13–0.30), 0.27 (95% CI, 0.17–0.35), 0.15 (95% CI, 0.06–0.24), and 0.14 (95% CI, 0.05–0.24), respectively (model 5 vs. model 5 minus day-to-night ratio of urinary potassium excretion) (all P < 0.05). Corresponding integrated discrimination improvements were 0.0034 (95% CI, 0.0012–0.0057), 0.0063 (95% CI, 0.0029–0.0096), 0.0038 (95% CI, 0.0014–0.0062), and 0.0047 (95% CI, 0.0020–0.0073), showing significant improvements (all P < 0.05). Interactions between day-to-night ratio of urinary sodium excretion and eGFR and eGFR categories were not significant (all P > 0.10). Significant interactions was not observed between day-to-night ratio of urinary potassium excretion or eGFR and eGFR categories (all P > 0.10, data not shown).
Characteristics of Excretion of Sodium and Potassium in Urine
The total 24-hour sodium excretion in Q2 and Q3 were higher than those in Q1 and Q4 (Table 1), and progressive increased excretion of sodium during the day and decreased excretion of sodium during the night from Q1 to Q4 (both P < 0.001, Table 4). The patients in Q4 had a high urine flow rate and a high sodium concentration in their urine during the daytime (both P < 0.001, Table 4). During daytime, the urine flow rate and urinary sodium concentration in Q4 were approximately 65.59% and 20% higher than in Q1, respectively (Table 4). Furthermore, from Q1 to Q3, the urinary sodium concentration was much greater at night than during the day; however, in Q4, it was lower at night than during the day. The sodium excretion rate in Q1 was the lowest during the day and the greatest during the night of the 4 quartiles: the median of day-to-night ratio of urinary sodium excretion multiplied by the flow rate (ml/h) in Q1 to Q4 were 0.44, 0.75, 1.15, and 2.18, respectively (P < 0.001). Similar trends were observed across quartiles of the day-to-night ratio of urinary potassium excretion rate (Table 4).
Table 4.
Daytime and nighttime urinary excretion and concentrations by quartiles of day-to-night ratio of urinary sodium and potassium excretion rate
|
Variable |
Day-to-night ratio of the urinary sodium excretion |
Day-to-night ratio of the urinary potassium excretion |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q1 ≤ 1.00 (n = 788) |
Q2 1.01-1.52 (n = 788) |
Q3 1.53-2.52 (n = 788) |
Q4 > 2.52 (n = 788) |
P Value | Q1 ≤ 1.44 (n = 788) |
Q2 1.44-1.94 (n = 788) |
Q3 1.95-2.62 (n = 787) |
Q4 > 2.62 (n = 789) |
P Value | |
| Day | ||||||||||
| Day Va (L) | 0.84 (0.59–1.13) | 1.11 (0.80–1.48) | 1.19 (0.89–1.61) | 1.39 (0.95–1.85) | < 0.001 | 0.80 (0.55–1.13) | 1.08 (0.8–1.48) | 1.23 (0.91–1.60) | 1.34 (0.99–1.83) | < 0.001 |
| Day V† (ml/h) | 0.93 (0.66–1.26) | 1.23 (0.89–1.64) | 1.32 (0.99–1.79) | 1.54 (1.06–2.06) | < 0.001 | 0.89 (0.61–1.26) | 1.20 (0.89–1.64) | 1.37 (1.01–1.78) | 1.49 (1.10–2.03) | < 0.001 |
| Day Na excretion (mmol) | 46.52 (29.04–67.26) | 69.00 (48.35–96.37) | 83.97 (58.36–116.48) | 94.66 (61.93–133.78) | < 0.001 | 50.61 (32.00–77.33) | 67.18 (45.60–97.58) | 79.52 (54.29–113.90) | 90.92 (60.87–129.60) | < 0.001 |
| Day UNa (mmol/l) | 59.00 (38.00–83.28) | 64.37 (44.42–90.00) | 69.78 (48.87–96.66) | 71.00 (50.00–99.65) | < 0.001 | 66.08 (42.49–93.64) | 63.61 (43.70–89.87) | 66.00 (46.00–90.10) | 68.90 (48.00–97.34) | 0.032 |
| Day K excretion (mmol) | 17.26 (11.77–23.33) | 20.48 (15.72–26.76) | 22.53 (16.90–28.96) | 23.46 (17.32–31.48) | < 0.001 | 15.29 (10.73–20.12) | 19.94 (15.65–25.80) | 22.95 (18.08–28.52) | 26.62 (20.23–35.35) | < 0.001 |
| Day UK (mmol/l) | 21.74 (14.12–30.78) | 19.15 (13.90–25.71) | 18.87 (13.61–26.10) | 18.12 (12.70–25.26) | < 0.001 | 19.15 (12.70–27.50) | 18.60 (13.40–26.30) | 19.00 (13.90–26.10) | 20.30 (14.30–28.20) | 0.015 |
| Night | ||||||||||
| Night Va(l) | 0.86 (0.63–1.15) | 0.71 (0.55–0.93) | 0.56 (0.40–0.78) | 0.36 (0.23–0.53) | < 0.001 | 0.76 (0.53–1.05) | 0.70 (0.50–0.93) | 0.61 (0.43–0.82) | 0.44 (0.26–0.65) | < 0.001 |
| Night Vb (ml/h) | 1.59 (1.17–2.13) | 1.31 (1.02–1.72) | 1.04 (0.75–1.44) | 0.67 (0.43–0.98) | < 0.001 | 1.41 (0.98–1.94) | 1.30 (0.93–1.72) | 1.13 (0.80–1.52) | 0.81 (0.48–1.20) | < 0.001 |
| Night Na excretion (mmol) | 71.61 (47.89–100.79) | 55.75 (29.23–28.56) | 44.08 (30.25–60.98) | 22.78 (13.21–35.91) | < 0.001 | 60.90 (35.41–90.18) | 52.52 (32.49–75.80) | 45.82 (27.15–65.17) | 32.24 (17.03–51.68) | < 0.001 |
| Night UNa (mmol/l) | 85.00 (61.00–109.00) | 80.19 (58.64–104.04) | 78.00 (54.00–106.49) | 66.14 (41.00–96.00) | < 0.001 | 82.00 (55.00–107.00) | 78.16 (56.00–101.00) | 76.00 (51.50–103.00) | 73.60 (50.00–107.89) | 0.164 |
| Night K excretion (mmol) | 13.34 (9.82–17.97) | 11.36 (8.73–15.11) | 10.28 (7.85–13.06) | 8.24 (5.61–11.22) | < 0.001 | 14.17 (10.26–19.10) | 11.74 (9.21–15.52) | 10.32 (8.14–12.70) | 7.52 (5.14–9.95) | < 0.001 |
| Night UK (mmol/l) | 15.80 (11.50–21.40) | 16.10 (11.75–21.92) | 17.64 (13.00–25.38) | 22.52 (14.70–33.71) | < 0.001 | 18.72 (13.34–27.04) | 17.70 (12.41–24.95) | 16.52 (12.30–24.00) | 17.11 (12.20–25.52) | 0.001 |
| D/N | ||||||||||
| D/N Va | 0.95 (0.70–1.24) | 1.46 (1.2–1.81) | 2.07 (1.66–2.67) | 3.60 (2.55–5.35) | < 0.001 | 1.03 (0.73–1.47) | 1.55 (1.17–2.04) | 1.95 (1.48–2.73) | 3.08 (2.17–4.71) | < 0.001 |
| D/N Na Va | 0.73 (0.56–0.87) | 1.24 (1.11–1.38) | 1.91 (1.72–2.16) | 3.63 (2.93–5.33) | < 0.001 | 0.88 (0.60–1.24) | 1.29 (0.98–1.79) | 1.74 (1.30–2.50) | 2.87 (1.96–4.37) | < 0.001 |
| D/N K Va | 1.28 (0.98–1.65) | 1.77 (1.47–2.19) | 2.19 (1.77–2.78) | 2.85 (2.23–4.10) | < 0.001 | 1.14 (0.90–1.29) | 1.69 (1.56–1.81) | 2.24 (2.09–2.40) | 3.39 (2.90–4.42) | < 0.001 |
| D/N Vb | 0.57 (0.42–0.75) | 0.88 (0.72–1.08) | 1.24 (1.00–1.60) | 2.16 (1.53–3.21) | < 0.001 | 0.62 (0.44–0.88) | 0.92 (0.70–1.23) | 1.17 (0.89–1.64) | 1.85 (1.30–2.82) | < 0.001 |
| D/N Na Vb | 0.44 (0.33–0.52) | 0.75 (0.67–0.83) | 1.15 (1.03–1.30) | 2.18 (1.76–3.20) | < 0.001 | 0.53 (0.36–0.74) | 0.77 (0.59–1.07) | 1.05 (0.78–1.50) | 1.72 (1.17–2.62) | < 0.001 |
| D/N K Vb | 0.77 (0.59–0.99) | 1.06 (0.88–1.31) | 1.31 (1.06–1.67) | 1.71 (1.34–2.46) | < 0.001 | 0.68 (0.54–0.78) | 1.02 (0.94–1.08) | 1.34 (1.26–1.44) | 2.03 (1.74–2.65) | < 0.001 |
D/N, daytime/nighttime.
urine volumes represent in l.
urine flow rates in mL/h. Na, sodium; K, potassium; UNa, urinary sodium; UK, urinary potassium.
Discussion
In this study, we found that poor excretion of daytime sodium and potassium correlated with increased nocturnal BP and blunted nocturnal BP dipping in individuals with CKD, irrespective of the level of eGFR and 24-hour excretion. After considering any confounding factors, this connection remained substantial. We found a negative correlation between nocturnal BP and daytime urine flow rate and urine sodium and potassium concentration, suggesting that low sodium and potassium excretion in the daytime was because of a reduced urine flow rate and a decreased capability of concentrating sodium and potassium in the urine. Our study adds to the sparse scientific literature describing correlation between the circadian fluctuations of sodium and potassium in urine and rhythm of BP in patients with CKD. The median day-to-night ratio of sodium and potassium excretion rate differed between the first and fourth quartiles by approximately 5-fold and 3-fold ranges, and there were 12-mmHg and 9-mmHg differences between the lowest and highest quartiles of nocturnal SBP, respectively. These findings highlight the clinical value of day-to-night ratio of urinary sodium and potassium excretion rate on BP in individuals with CKD.
Patients with CKD with very high quartile of the day-to-night ratio of urinary sodium excretion rate had lower nocturnal SBP than those with lower quartiles. Even after full adjustment for demographic factors, lifestyles, and clinical variables (including diabetes mellitus, blood glucose levels, CVD, eGFR, BP medications, glucocorticoids and immunosuppressive agents, and 24-hour sodium and potassium excretion), this association remained the same. Nocturnal SBP amounted to 6.89 mmHg lower pressure among Q4 patients than those in Q1. In addition, daytime SBP became lower as the quartile increased. Indeed, most BP variables (nighttime SBP, daytime SBP, dipping SBP, nighttime DBP, daytime DBP, dipping DBP) were negatively and independently associated with higher quartile. In our study, the antihypertensive medication use did not contribute to BP pattern change.
Previous study found that day-to-night ratio of urinary sodium excretion rate was linked to circadian ambulatory BP in general population.16 However, prior research on the association of circadian rhythm of urinary sodium excretion with abnormal rhythm of BP in patients with CKD is scarce. Zhang et al.31 described a positive relationship between night-to-day ratio of urinary sodium excretion and increased nocturnal clinic BP in patients with CKD; however, they did not analyze the ABPM or nocturnal BP. Notably, among the ABPM components, nighttime BP levels are significantly positively associated with greater risks of death and a composite cardiovascular outcome,3, 4, 5 and nondipping patterns are accompanied by worst prognosis.32,33 Evidence has shown that patients with simultaneously nondipping and nocturnal hypertension tended to have worst cardiovascular risk.34 Similarly, risers BP pattern was independent of long-term predictors of the adverse cardiovascular outcome.35,36 In the present study, we found that patients in Q1 were more likely to be risers and that nocturnal BP was negatively correlated with day-to-night ratio of urinary sodium excretion. Consistent with our study, several studies have reported a positive relation between nocturnal fall in BP and excretion of sodium during daytime.
The data supported the hypothesis that insufficient daytime sodium excretion requires increased nighttime BP to facilitate a compensatory rise in sodium excretion and restore balance.19,20 In line with the hypothesis are the studies that posit improving the circadian BP profile played a role in recovering nocturnal dip in renal patients.37 Our results suggest that decreased urinary daytime sodium excretion requiring increased nocturnal BP also existed in patients with CKD. To be noted, the relationship between dietary sodium intake and BP38 might be overestimated because of the “salt sensitive” people.39 Studies found that young normotensive African Americans, who had a smaller diurnal change of fluid and sodium excretion,40 were more likely than Whites to exhibit a high risk for sodium-sensitive hypertension and a blunted dipping pattern of BP.41,42
Furthermore, we found that the nocturnal BP dipping was positively associated with daytime urinary potassium excretion rate in the present study. In addition, the association was independent of and consistent with sodium. The circadian rhythm of potassium excretion and sodium excretion in urine has been paralleled in a previous study.43 The tubular excretion of potassium in the distal tubule and cortical collecting duct, accounting for most of the urinary potassium excretion, is determined by the amount of sodium delivered to the nephrons.44 The sodium excretion into urine was proportional to the amount of sodium delivered to the nephrons. Unsurprisingly, therefore, increased urinary potassium excretion paralleled with enhanced urinary sodium excretion. For circadian rhythms of urinary sodium and potassium excretions, the association was similar.17,45 In contrast, the secretion of potassium through renal outer medullary potassium channel increased as the amount of potassium delivered to distal nephron enhanced.46,47 This will cause the suppression of tubular sodium reabsorption through epithelial sodium channel and sodium/chloride cotransporter in the distal nephron46,47; and therefore, increased urinary sodium excretion. Compared with other electrolytes and creatinine, the variations of excretion rate of potassium during daytime and nighttime are more because the potassium excreted during daytime accounts for the majority of the total 24-hour excretion.48 Moreover, the secretion of potassium can selectively enhance urine flow rate during the active phase, which is daytime for humans but night for rats. It could provide a possible explanation for the increase in the day-to-night ratio of the sodium excretion rate.49 Our work expands on this data by demonstrating that circadian rhythms of BP, circadian rhythm of urinary sodium and potassium excretion may interact in CKD participants.
Studies have found a link between 24-hour urinary sodium and potassium and BP in patients with CKD in the past decade.25,50 However, this is the first study to demonstrate how the BP varies according to the circadian pattern of sodium and potassium excretion in a group of patients with CKD through ABPM. Previous studies found a positive relationship between urinary sodium excretion and BP in patients with CKD, but unlike contradictory conclusions in general population,25,50 or contrary conclusion in studies on potassium excretion.51 Increased 24-hour urinary sodium excretion was strongly correlated with higher nighttime SBP in our study; however, the correlation between 24-hour urinary potassium excretion and nighttime SBP was not significant. Further analyses showed that nighttime SBP, nighttime DBP, and percentage of risers decreased from Q1 to Q4 whether in high or low 24-hour sodium (or potassium) excretion group. This finding suggests that patients with low day-to-night ratio of urinary excretion rate may still have elevated nocturnal BP and be risers even at comparatively low excretion of 24-hour urinary sodium (or potassium). This may reflect that the optimum excretions of 24-hour sodium and potassium are different among patients with CKD. Therefore, in addition to 24-hour urinary excretion, the day-to-night ratio of urinary excretion deserves our emphasis. Because the parameter is not measured routinely in the clinic, important information may be missed.
According to the theory put forth by Fukuda et al.,19 diminished sodium excretory capacity may be caused by enhanced tubular sodium reabsorption and reduced GFR. The finding that an abnormal BP dipping was associated with an elevated sodium reabsorption or a declined renal function52 was congruent with this hypothesis. Also consistent is our result that patients with impaired renal function were more likely to have increased nocturnal BP and to be risers. More interestingly, we found a pattern for the decrease in nocturnal BP and risers in patients with lower day-to-night ratio of sodium and potassium excretion rate not only in lower GFR, but also in higher GFR. A previous study reported a strong correlation between urinary sodium excretion and nocturnal systolic BP, with a significant interaction between urinary sodium excretion and eGFR on nighttime systolic BP.53 In this study, significant interactions between day-to-night ratio of urinary sodium and potassium excretion with eGFR were not found. Further investigation in urine revealed a low excretion of sodium and a low concentration of urinary sodium during daytime in Q1 when compared to the 3 other quartiles, suggesting that a greater daytime sodium reabsorption is tubular. For the similar 24-hour sodium excretion, urine flow rate and urinary sodium concentration were significantly lower in Q1 than those in Q4 during daytime. Our results suggest that circadian rhythm of sodium and potassium in urine are not confined to reduced GFR, but that enhanced tubular sodium reabsorption is also associated with declined capability to excrete electrolyte during daytime.
This study has several limitations. First, no causal inferences can be made, and the possibility of residual confounding cannot be excluded because this is an observational study. Second, the time of sodium and potassium intake has not been assessed. We cannot rule out that Q1 patients ate more salt and potassium during nighttime than during daytime when compared to the other 3 quartiles. However, because the definition of nighttime in our study is dependent on the time patients going to-bed, the possibility of eating in the evening is relatively low. On the other hand, although it is easily subject to bias because of the lack of information on sodium and potassium contents in many foods, inaccurate reporting by patients, recall bias, and other reasons,54 detailed eating habits (including diet information and use of tea or drinks) should better be included in future studies. Third, the higher nocturnal BP may be due to the elevated urine volume during nighttime, resulting in more times of getting-up and poor sleep quantity and quality. We sought to overcome this limitation by excluding patients with BP data missing for 2 hours continuously and those who got up during the night and had trouble going back to asleep. Fourth, this study included only Chinese people, therefore our results may not be generalized to the Western and other populations.
To summarize, a reduced excretion of daytime sodium or potassium was independently linked to a higher nocturnal BP and a blunted nocturnal BP dipping in patients with CKD. Furthermore, a reduced urine flow rate and a diminished capacity to concentrate sodium and potassium in the urine were the main contributors to a low day-to-night ratio of urinary sodium excretion and excretion of potassium. Therefore, separated daytime and nighttime measurements of urinary sodium and potassium should be recommended for patients with CKD.
Disclosure
The authors have declared no conflicting interest.
Acknowledgments
This work was funded by the Five-Five Project of the Fifth Affiliated Hospital of Sun Yat-sen University. The authors would like to appreciate all individuals (physicians, nurses, institutional staff, and patients) involved in this study.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Author Contributions
Conceptualization, design, acquisition of data was done by LingL; Methodology was by LinL, JK, and BC; Original draft preparation was by LingL; Data collection was by YX; Review and editing was by LingL and CW; Supervision was by CW.
Footnotes
Table S1. BP, PR, PP, and nocturnal dipping, by quartiles of day-to-night ratio of urinary sodium and potassium excretion rate.
Table S2. Characteristics of patients with CKD according to quartiles of 24-hour urinary excretion of sodium and potassium.
Table S3. Nighttime and daytime BP values and dipping proportion according to 24-hour urinary sodium and potassium excretion categories and day-to-night ratio of the urinary sodium and potassium excretion status.
Table S4. Multivariate logistic regression analysis for nocturnal SBP according to day-to-night ratio of urinary sodium and potassium excretion rate categories among patients with CKD.
STROBE Statement (PDF).
Supplementary Material
Table S1. BP, PR, PP, and nocturnal dipping, by quartiles of day-to-night ratio of urinary sodium and potassium excretion rate.
Table S2. Characteristics of patients with CKD according to quartiles of 24-hour urinary excretion of sodium and potassium.
Table S3. Nighttime and daytime BP values and dipping proportion according to 24-hour urinary sodium and potassium excretion categories and day-to-night ratio of the urinary sodium and potassium excretion status.
Table S4. Multivariate logistic regression analysis for nocturnal SBP according to day-to-night ratio of urinary sodium and potassium excretion rate categories among patients with CKD.
STROBE Statement (PDF)
References
- 1.Lim S.S., Vos T., Flaxman A.D., et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen T.N., Chow C.K. Global and national high blood pressure burden and control. Lancet. 2021;398:932–933. doi: 10.1016/S0140-6736(21)01688-3. [DOI] [PubMed] [Google Scholar]
- 3.Yang W.Y., Melgarejo J.D., Thijs L., et al. Association of office and ambulatory blood pressure with mortality and cardiovascular outcomes. JAMA. 2019;322:409–420. doi: 10.1001/jama.2019.9811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kario K., Hoshide S., Mizuno H., et al. Nighttime blood pressure phenotype and cardiovascular prognosis: practitioner-based nationwide JAMP study. Circulation. 2020;142:1810–1820. doi: 10.1161/CIRCULATIONAHA.120.049730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dolan E., Stanton A., Thijs L., et al. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension. 2005;46:156–161. doi: 10.1161/01.HYP.0000170138.56903.7a. [DOI] [PubMed] [Google Scholar]
- 6.Borrelli S., Garofalo C., Gabbai F.B., et al. Dipping status, ambulatory blood pressure control, cardiovascular disease, and kidney disease progression: a multicenter cohort study of CKD. Am J Kidney Dis. 2023;81:15–24.e11. doi: 10.1053/j.ajkd.2022.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Fu X., Ren H., Xie J., et al. Association of nighttime masked uncontrolled hypertension with left ventricular hypertrophy and kidney function among patients with chronic kidney disease not receiving dialysis. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.14460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shea S.A., Hilton M.F., Hu K., Scheer F.A. Existence of an endogenous circadian blood pressure rhythm in humans that peaks in the evening. Circ Res. 2011;108:980–984. doi: 10.1161/CIRCRESAHA.110.233668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheward W.J., Naylor E., Knowles-Barley S., et al. Circadian control of mouse heart rate and blood pressure by the suprachiasmatic nuclei: behavioral effects are more significant than direct outputs. PLoS One. 2010;5 doi: 10.1371/journal.pone.0009783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurtz T.W., Lujan H.L., DiCarlo S.E. The 24 h pattern of arterial pressure in mice is determined mainly by heart rate-driven variation in cardiac output. Physiol Rep. 2014;2 doi: 10.14814/phy2.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishihara M., Hirooka Y. [Effect of sympathetic nervous system on circadian rhythm of blood pressure] Nihon Rinsho. 2014;72:1374–1378. [PubMed] [Google Scholar]
- 12.Smolensky M.H., Haus E. Circadian rhythms and clinical medicine with applications to hypertension. Am J Hypertens. 2001;14:280s–290s. doi: 10.1016/s0895-7061(01)02175-6. [DOI] [PubMed] [Google Scholar]
- 13.Ivy J.R., Bailey M.A. Nondipping blood pressure: predictive or reactive failure of renal sodium handling? Physiology (Bethesda) 2021;36:21–34. doi: 10.1152/physiol.00024.2020. [DOI] [PubMed] [Google Scholar]
- 14.Coffman T.M. The inextricable role of the kidney in hypertension. J Clin Invest. 2014;124:2341–2347. doi: 10.1172/JCI72274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ivy J.R., Bailey M.A. Pressure natriuresis and the renal control of arterial blood pressure. J Physiol. 2014;592:3955–3967. doi: 10.1113/jphysiol.2014.271676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bankir L., Bochud M., Maillard M., Bovet P., Gabriel A., Burnier M. Nighttime blood pressure and nocturnal dipping are associated with daytime urinary sodium excretion in African subjects. Hypertension. 2008;51:891–898. doi: 10.1161/HYPERTENSIONAHA.107.105510. [DOI] [PubMed] [Google Scholar]
- 17.Dyer A.R., Martin G.J., Burton W.N., Levin M., Stamler J. Blood pressure and diurnal variation in sodium, potassium, and water excretion. J Hum Hypertens. 1998;12:363–371. doi: 10.1038/sj.jhh.1000601. [DOI] [PubMed] [Google Scholar]
- 18.Staessen J.A., Birkenhäger W., Bulpitt C.J., et al. The relationship between blood pressure and sodium and potassium excretion during the day and at night. J Hypertens. 1993;11:443–447. doi: 10.1097/00004872-199304000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Fukuda M., Goto N., Kimura G. Hypothesis on renal mechanism of non-dipper pattern of circadian blood pressure rhythm. Med Hypotheses. 2006;67:802–806. doi: 10.1016/j.mehy.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 20.Sachdeva A., Weder A.B. Nocturnal sodium excretion, blood pressure dipping, and sodium sensitivity. Hypertension. 2006;48:527–533. doi: 10.1161/01.HYP.0000240268.37379.7c. [DOI] [PubMed] [Google Scholar]
- 21.Huang X.M., Yuan J.P., Zeng X.R., Peng C.X., Mei Q.H., Chen W.L. Effects of chronotherapy of benazepril on the diurnal profile of RAAS and clock genes in the kidney of 5/6 nephrectomy rats. J Huazhong Univ Sci Technolog Med Sci. 2013;33:368–374. doi: 10.1007/s11596-013-1126-7. [DOI] [PubMed] [Google Scholar]
- 22.Motohashi H., Tahara Y., Whittaker D.S., et al. The circadian clock is disrupted in mice with adenine-induced tubulointerstitial nephropathy. Kidney Int. 2020;97:728–740. doi: 10.1016/j.kint.2019.09.032. [DOI] [PubMed] [Google Scholar]
- 23.Sinha A.D., Agarwal R. The complex relationship between CKD and ambulatory blood pressure patterns. Adv Chronic Kidney Dis. 2015;22:102–107. doi: 10.1053/j.ackd.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levey A.S., Stevens L.A., Schmid C.H., et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim H.W., Park J.T., Yoo T.H., et al. Urinary potassium excretion and progression of CKD. Clin J Am Soc Nephrol. 2019;14:330–340. doi: 10.2215/CJN.07820618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X., Lian R., Zhu Y., et al. Masked morning hypertension correlated with target organ damage in non-dialysis patients with chronic kidney disease. J Hypertens. 2020;38:1794–1801. doi: 10.1097/HJH.0000000000002461. [DOI] [PubMed] [Google Scholar]
- 27.Williams B., Mancia G., Spiering W., et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 28.Kario K., Shin J., Chen C.H., et al. Expert panel consensus recommendations for ambulatory blood pressure monitoring in Asia: the HOPE Asia Network. J Clin Hypertens (Greenwich) 2019;21:1250–1283. doi: 10.1111/jch.13652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease [article online] Published 2013. https://www.sciencedirect.com/journal/kidney-international-supplements/vol/3/issue/1 [DOI] [PubMed]
- 30.Pencina M.J., D’Agostino R.B., Sr, Steyerberg E.W. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J., Rao J., Liu M., et al. Abnormal circadian rhythm of urinary sodium excretion correlates closely with hypertension and target organ damage in Chinese patients with CKD. Int J Med Sci. 2020;17:702–711. doi: 10.7150/ijms.42875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de la Sierra A., Redon J., Banegas J.R., et al. Prevalence and factors associated with circadian blood pressure patterns in hypertensive patients. Hypertension. 2009;53:466–472. doi: 10.1161/HYPERTENSIONAHA.108.124008. [DOI] [PubMed] [Google Scholar]
- 33.Muxfeldt E.S., Cardoso C.R., Salles G.F. Prognostic value of nocturnal blood pressure reduction in resistant hypertension. Arch Intern Med. 2009;169:874–880. doi: 10.1001/archinternmed.2009.68. [DOI] [PubMed] [Google Scholar]
- 34.de la Sierra A., Gorostidi M., Banegas J.R., Segura J., de la Cruz J.J., Ruilope L.M. Nocturnal hypertension or nondipping: which is better associated with the cardiovascular risk profile? Am J Hypertens. 2014;27:680–687. doi: 10.1093/ajh/hpt175. [DOI] [PubMed] [Google Scholar]
- 35.O’Brien E., Parati G., Stergiou G., et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31:1731–1768. doi: 10.1097/HJH.0b013e328363e964. [DOI] [PubMed] [Google Scholar]
- 36.Tadic M., Cuspidi C., Celic V., et al. The prognostic importance of right ventricular remodeling and the circadian blood pressure pattern on the long-term cardiovascular outcome. J Hypertens. 2020;38:1525–1530. doi: 10.1097/HJH.0000000000002432. [DOI] [PubMed] [Google Scholar]
- 37.Velasquez M.T., Beddhu S., Nobakht E., Rahman M., Raj D.S. Ambulatory blood pressure in chronic kidney disease: ready for prime time? Kidney Int Rep. 2016;1:94–104. doi: 10.1016/j.ekir.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He F.J., MacGregor G.A. A comprehensive review on salt and health and current experience of worldwide salt reduction programmes. J Hum Hypertens. 2009;23:363–384. doi: 10.1038/jhh.2008.144. [DOI] [PubMed] [Google Scholar]
- 39.Franco V., Oparil S. Salt sensitivity, a determinant of blood pressure, cardiovascular disease and survival. J Am Coll Nutr. 2006;25(suppl):247s–255s. doi: 10.1080/07315724.2006.10719574. [DOI] [PubMed] [Google Scholar]
- 40.Bankir L., Perucca J., Weinberger M.H. Ethnic differences in urine concentration: possible relationship to blood pressure. Clin J Am Soc Nephrol. 2007;2:304–312. doi: 10.2215/CJN.03401006. [DOI] [PubMed] [Google Scholar]
- 41.Gretler D.D., Fumo M.T., Nelson K.S., Murphy M.B. Ethnic differences in circadian hemodynamic profile. Am J Hypertens. 1994;7:7–14. doi: 10.1093/ajh/7.1.7. [DOI] [PubMed] [Google Scholar]
- 42.Agyemang C., Bhopal R., Bruijnzeels M., Redekop W.K. Does nocturnal blood pressure fall in people of African and South Asian descent differ from that in European white populations? A systematic review and meta-analysis. J Hypertens. 2005;23:913–920. doi: 10.1097/01.hjh.0000166827.94699.f9. [DOI] [PubMed] [Google Scholar]
- 43.Miura T., Fukuda M., Naito T., et al. Circadian rhythm of urinary potassium excretion in patients with CKD. Clin Nephrol. 2012;78:169–173. doi: 10.5414/cn107399. [DOI] [PubMed] [Google Scholar]
- 44.Kamel K.S., Quaggin S., Scheich A., Halperin M.L. Disorders of potassium homeostasis: an approach based on pathophysiology. Am J Kidney Dis. 1994;24:597–613. doi: 10.1016/s0272-6386(12)80220-4. [DOI] [PubMed] [Google Scholar]
- 45.Dyer A.R., Stamler R., Grimm R., et al. Do hypertensive patients have a different diurnal pattern of electrolyte excretion? Hypertension. 1987;10:417–424. doi: 10.1161/01.hyp.10.4.417. [DOI] [PubMed] [Google Scholar]
- 46.Huang C.L., Kuo E. Mechanisms of disease: WNK-ing at the mechanism of salt-sensitive hypertension. Nat Clin Pract Nephrol. 2007;3:623–630. doi: 10.1038/ncpneph0638. [DOI] [PubMed] [Google Scholar]
- 47.Hedayati S.S., Elsayed E.F., Reilly R.F. Non-pharmacological aspects of blood pressure management: what are the data? Kidney Int. 2011;79:1061–1070. doi: 10.1038/ki.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koopman M.G., Koomen G.C., Krediet R.T., de Moor E.A., Hoek F.J., Arisz L. Circadian rhythm of glomerular filtration rate in normal individuals. Clin Sci (Lond) 1989;77:105–111. doi: 10.1042/cs0770105. [DOI] [PubMed] [Google Scholar]
- 49.Perucca J., Bankir L. Dietary potassium supplementation increases urine volume and alters the circadian pattern of sodium excretion. The FASEB. 2007;21 A510-A510. [Google Scholar]
- 50.He J., Mills K.T., Appel L.J., et al. Urinary sodium and potassium excretion and CKD progression. J Am Soc Nephrol. 2016;27:1202–1212. doi: 10.1681/ASN.2015010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alencar de Pinho N., Kaboré J., Laville M., et al. Urinary sodium-to-potassium ratio and blood pressure in CKD. Kidney Int Rep. 2020;5:1240–1250. doi: 10.1016/j.ekir.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burnier M., Coltamai L., Maillard M., Bochud M. Renal sodium handling and nighttime blood pressure. Semin Nephrol. 2007;27:565–571. doi: 10.1016/j.semnephrol.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 53.Borrelli S., Mallamaci F., Chiodini P., et al. Salt intake correlates with night systolic blood pressure in non-dialytic chronic kidney disease. Nephrol Dial Transplant. 2022;37:1387–1389. doi: 10.1093/ndt/gfac028. [DOI] [PubMed] [Google Scholar]
- 54.Cobb L.K., Anderson C.A., Elliott P., et al. Methodological issues in cohort studies that relate sodium intake to cardiovascular disease outcomes: a science advisory from the American Heart Association. Circulation. 2014;129:1173–1186. doi: 10.1161/CIR.0000000000000015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.