Abstract
Individuals diagnosed with chronic kidney disease (CKD) continue to increase globally. This group of patients experience a disproportionately higher risk of cardiovascular (CV) events compared to the general population. Despite multiple guidelines-based medical management, patients with CKD continue to experience residual cardiorenal risk. Several potential mechanisms explain this excessive CV risk observed in individuals with CKD. Several new drugs have become available that could potentially transform CKD care, given their efficacy in this patient population. Nevertheless, use of these drugs presents certain benefits and challenges that are often underrecognized by prescribing these drugs. In this review, we aim to provide a brief discussion about CKD pathophysiology, limiting our discussion to recent published studies. We also explore benefits and limitations of newer drugs, including angiotensin receptor/neprilysin inhibitors (ARNI), sodium glucose transporter 2 inhibitors (SGLT2i), glucagon-like peptides-1 (GLP-1) agonists and finerenone in patients with CKD. Despite several articles covering this topic, our review provides an algorithm where subgroups of patients with CKD might benefit the most from such drugs based on the selection criteria of the landmark trials. Patients with CKD who have nephrotic range proteinuria beyond 5000 mg/g, or those with poorly controlled blood pressure (systolic ≥160 mm Hg or diastolic ≥100 mm Hg) remain understudied.
Keywords: ARNI, chronic kidney disease, finerenone, inflammation, platelets, SGLT2
Burden
In the last 2 decades, the all-age death rate from CKD has nearly doubled and its all-age prevalence increased by 30% to 700 million people worldwide.1 Overall, it is much higher than that of diabetes, osteoarthritis, chronic obstructive pulmonary disease, asthma, or depressive disorders.1 CV diseases, including arrhythmias, heart failures, and thrombotic events account for >39% of deaths in patients with CKD.2,3 When compared to the non-CKD population, this CV risk increases with severity of kidney disease such that patients with CKD with estimated glomerular filtration rate (eGFR) ≥45 ml/min per 1.73 m2 or urine albumin-to-creatinine ratio (UACR) 30 to 300 mg/g are at a 2-fold higher CV risk; those with eGFR <45 ml/min per 1.73 m2 or UACR ≥300 mg/g are at 4 to 6 times higher CV risk.4, 5, 6, 7, 8 Accelerated plaque progression and rupture may account for the observed increase in the composite outcome of stroke, myocardial infarction, fatal coronary heart disease, and death among patients with CKD, which is more than twice the risk from diabetes mellitus alone.9 Moreover, 1 year after percutaneous coronary intervention, mortality is twice as high in patients with CKD with eGFR ≥45 ml/min per 1.73 m2 and 4 times as high in patients with CKD with eGFR <45 ml/min per 1.73 m2 as compared to patients with normal kidney function.5 In addition, higher rates of coronary in-stent thrombosis are observed in patients with CKD.4,7 Overall, patients with CKD who need dialysis is one of the most rapidly growing chronic diseases globally, with a patient population that includes individuals that are 75 years or older who are starting dialysis due to living longer with CV diseases.1 Previous reviews have discussed insights for cardiorenal issues in great details.10 This review will focus on the recent updates since the publication of last review on this topic.10
Mechanisms
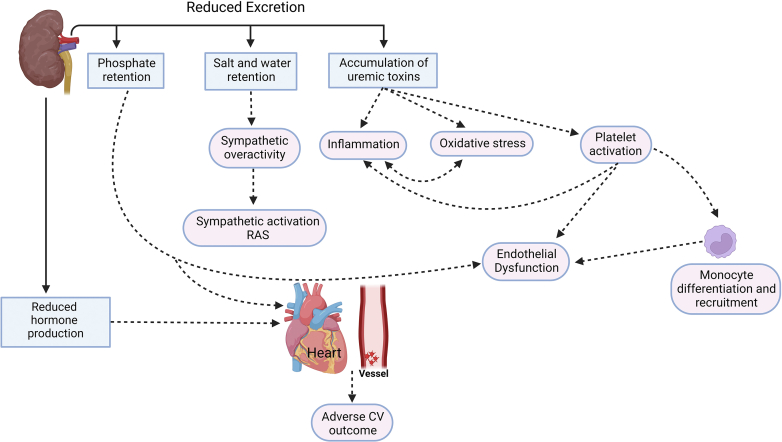
Excessive CV risks in patients with CKD involve mechanisms such as salt and water retention causing sympathetic overactivity and activation of renin-angiotensin-aldosterone system (RAAS). Uremic toxin accumulation leads to increased oxidative stress,11 inflammation,12,13 and increased platelet dysfunction,14, 15, 16 whereas phosphate retention contributes to vascular calcification17 and parathyroid mediated bone problems (Figure 1).18,19 In this section, we will limit our discussion to novel mechanisms pertaining to inflammation and platelet-related pathophysiology in CKD because other mechanisms were extensively reviewed by others,10,20, 21, 22, 23, 24 and managing immune cells and inflammation is an active area of research that would potentially generate new therapies for CKD management.
Figure 1.
Mechanisms for excessive CV events in patients with CKD arising from reduced excretion from the kidney or reduced hormone (erythropoietin and calcitriol) production from the kidney. Accumulation of salt and water leads to sympathetic overactivity and renin-angiotensin-aldosterone system activation that results in changes to the left ventricle and arterioles that affect systemic vascular resistance. Accumulation of uremic toxins leads to platelet activation causing endothelial dysfunction that generates oxidative stress and inflammation, which also involves liver and adipocytes. Finally, phosphate retention leads to endothelial dysfunction, which has effects on the parathyroid gland, bone, and vessels. Reduced production of calcitriol adds to parathyroid gland, bone, heart, and vessel problems. Reduced production of erythropoietin leads to anemia that affects heart and vessels. CKD, chronic kidney disease; CV cardiovascular; RAAS, renin-angiotensin systems
Inflammation in CKD
CKD is a proinflammatory state marked by higher levels of inflammatory molecules (e.g., interleukin-1 alpha and interleukin-1 beta) in the circulation.12 This heightened inflammation contributes to the progression of CKD and the CV risk of patients with CKD. In many ways, CKD parallels that of other systemic inflammatory response disorders or sepsis in its presentation of wide-ranging dysregulation of hemostasis and inflammation, which negatively impacts the CV network and kidneys. Studies have also reported altered monocytic differentiation state in the circulation of patients with CKD, with a significant increase in the percentage of circulating total nonclassical monocytes in patients with CKD (Figure 2).25,26 Monocytes are generally categorized into 1 of 3 categories: classical, nonclassical, and intermediate. Classical monocytes act as the primary phagocytic variety, nonclassical monocytes are the chief secretory cell type, and intermediate monocytes represent a transitional phenotype as the cell fluctuates between classical and nonclassical.27,28 Increase in percentage of circulating nonclassical monocytes in CKD possibly implies that these secretory cells might be producing proinflammatory cytokines in the circulation of patients with CKD. However, it is unclear whether these inflammatory characteristics are the result of preexisting disruptions in the inflammatory axis, which in turn initiates or exacerbates CKD-related inflammation or they are simply a phenomenon caused by an alternative driver of previously initiated CKD.
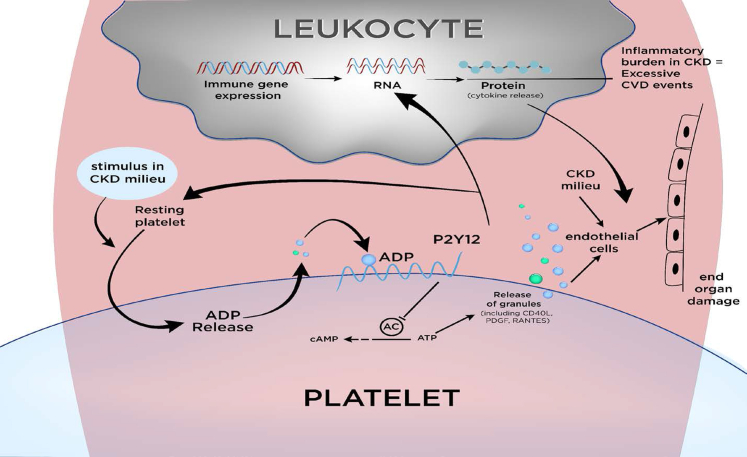
Figure 2.
Novel role of platelets in modulating inflammation in patients with CKD. Recently, interaction of platelets with leukocytes in the circulation was reported to modulate inflammation in preclinical studies. With stimulus, platelets interact with leukocytes in circulation via surface receptors. This interaction brings early changes in platelets marked by ADP release from preformed granules. ADP release subsequently acts on P2Y12 receptors to release more platelet granules that contain CD40L, PDGF, RANTES, and other molecules. Release of these molecules results in activation of endothelial cells as well as reprogramming of leukocytes for cytokine release and for monocyte differentiation. CD40L, CD40 ligand; CKD, chronic kidney disease; PDGF, platelet derived growth factor; RANTES, Regulated upon Activation, Normal T Cell Expressed and Presumably Secreted.
Platelet Dysfunction in CKD
CKD milieu can possibly stimulate resting platelets and initiate 2 pathophysiological processes, namely inflammatory cascade that subsequently is associated with thrombotic CV events, and endothelial activation that then drives end organ damage.20,29,30 Recently, interaction of platelets with leukocytes in the circulation was reported to modulate inflammation in preclinical studies (Figure 2). In addition, resting platelets can be activated with stimulus in CKD milieu.21,31,32 Furthermore, patients with CKD with albuminuria demonstrate stimulated platelets with increased aggregation via surface receptors.33 Although CKD milieu can activate platelets in the circulation, data are limited regarding dynamic modulation of platelet-mediated leukocytic changes that drive inflammation in CKD milieu.13 There is some data reporting the existence of platelet P2Y12 receptor-dependent inflammation in patients with CKD and the potent platelet P2Y12 antagonist ticagrelor lowering inflammation in patients with CKD.34, 35, 36 Platelets are also known to modulate endothelial cell function.37 Overall, there are preliminary studies suggesting platelet-mediated changes in inflammatory cascade of patients with CKD.
New Therapies
Problems arising from salt and water retention shown in Figure 1 are managed by widely used drugs angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and diuretics. The angiotensin-converting enzyme inhibitors and angiotensin receptor blockers reduce adverse cardiorenal outcomes in patients with CKD.38 Use of ACEi or ARB is graded IA by clinical practice guidelines for patients with CKD and for chronic heart diseases.39 Patients with CKD have multiple adverse outcomes due to metabolic derangements as shown in Figure 1. In recent years, several new drugs were approved by the US Food and Drud Administration after demonstrating reduced adverse cardiorenal outcomes with their use in landmark randomized controlled trials (RCTs).40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53 In this review article, we will mainly focus on the therapies that were recently introduced in the market.
Angiotensin Receptor/Neprilysin Inhibition
For patients with chronic heart failure (HF) and reduced ejection fraction, ACEi therapy has been used for over 2 decades to reduce their risk of death by 15% to 20%.42 Efficacy of ARB in patients with reduced ejection fraction has been inconsistent.42 Subsequent studies highlighted the role of neurohormonal activation as a result of ACEi or ARB monotherapy that contributes to residual CV risk.54 For patients with HF and preserved ejection fraction, several RCTs have failed to demonstrate consistent benefit of ACEi or ARB therapies in reducing adverse cardiac outcomes. Lack of efficacy of ACEi or ARB monotherapy in preserved ejection fraction setting is a result of reduced cyclic guanosine monophosphate in cardiac myocytes of these patients; most of these patients also have resistant hypertension as patients with CKD.55 Newer agents, such as ARNI, may provide added benefits to this patient population by counteracting neurohormonal activation from ACEi or ARB monotherapy, augmenting cyclic guanosine monophosphate levels in cardiac myocytes and inhibiting RAAS (Figure 3). Animal studies also demonstrate superiority of ARNI over ACEi or ARB in reducing inflammation and cardiorenal fibrosis. There are no data on its effects on platelet activation of CKD state. Because of these additional benefits of ARNI, it may also reduce blood pressure more than ACEi or ARB monotherapy.42,56 The first-in-class ARNI, a combination of sacubitril and valsartan, was found to be better than an ACEi or an ARB monotherapy in patients with HF based on the results from the PARADIGM-HF (prospective comparison of angiotensin receptor-neprilysin inhibitor with Angiotensin-converting enzyme inhibitor to Determine Impact on Global Mortality and morbidity in Hear Failure) trial42 and PARAGON-HF (prospective comparison of angiotensin receptor-neprilysin inhibitor with angiotensin-receptor blockers Global Outcomes in Heart Failure with preserved ejection fraction) trial (Supplementary Table S1)57; both RCTs reporting reduced CV-death and HF admissions by 25% to 30% with ARNI over ACEi or ARB monotherapy.42,57 ARNI also reduced blood pressure 3 to 5 mm Hg more than the monotherapy arm in these trials.42,57 In a pooled analysis of PARADIGM-HF and PARAGON-HF sacubitril/valsartan reduced the risk of serious adverse renal outcomes and decline in eGFR, compared to valsartan or enalapril monotherapies independent of baseline renal function.58
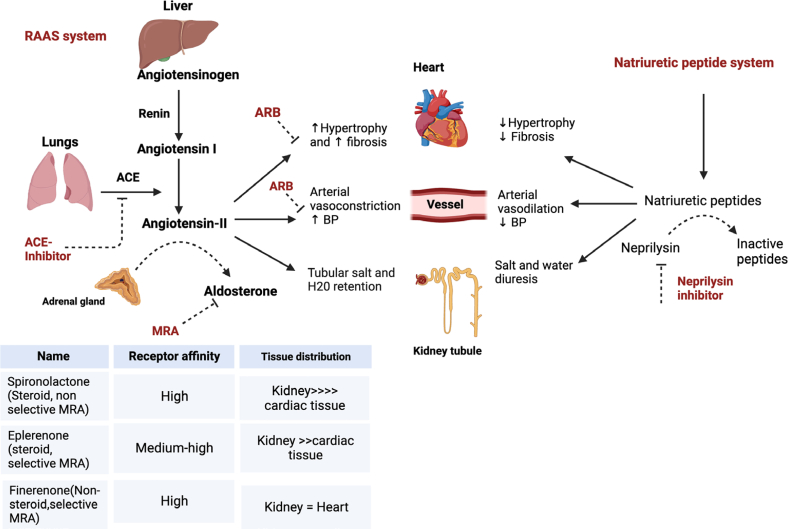
Figure 3.
Drugs (in red letters) acting on the RAAS, including newer drugs such as ARNI and mineralocorticoid receptor antagonists. Neprilysin is a neutral endopeptidase. It degrades endogenous vasoactive peptides (e.g., natriuretic peptides, bradykinin, and adrenomedullin). Levels of these neurohormones rise with ACE inhibitor or ARB use. Thus, neprilysin inhibitor counteracts on the neurohormonal activation arising from ACE inhibitor or ARB monotherapy that contributes residual adverse outcomes arising from neurohormone-mediated salt retention and sympathetic overactivity. ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor/neprilysin inhibitor; AT-1R, angiotensin-1 receptor; BP, blood pressure; RAAS, renin-angiotensin-aldosterone system.
Renal benefits of ARNI over ACEi or ARB monotherapy remain debatable in patients with HF. There are 3 observational studies and a small-scale RCT that generated confusing results. First, in a post hoc analysis of the PARADIGM-HF trial, there was a lesser annual decline in glomerular filtration rate in the sacubitril/valsartan arm compared to the ACEi monotherapy arm (−1.61 [95% confidence interval [CI]: −1.77 to −1.44] vs. −2.04 [95% CI: −2.21 to −1.88]). There was also a lesser annual decline in eGFR observed in tandem with a greater increase in albuminuria (1.20 mg/mmol [95% CI: 1.04–1.36] among ARNI users vs. 0.90 mg/mmol [95% CI: 0.77–1.03]) with ACEi users.59 Second, a recent retrospective study reported no additional benefit of ARNI over ACEi monotherapy in reducing renal outcomes of patients with CKD.60 Third, in PARAGON-HF trial, there was a 50% risk reduction in renal outcomes with ARNI over ARB monotherapy (hazard ration [HR] 0.50 [95% CI: 0.33–0.77]).58 Finally, in a smaller RCT (UK HARP-III trial), 414 patients with CKD with eGFR between 20 and 60 ml/min per 1.73 m2 were randomized to receive either an ARNI or an ARB monotherapy to evaluate renal outcomes over 12 months.61 This RCT did not show any benefit of using ARNI over ARB monotherapy in reducing the primary outcome of measured GFR among study participants.
There are no randomized studies to evaluate the benefit of ARNI over ACEi or ARB monotherapy in patients with CKD with eGFR <30 ml/min per 1.73 m2 regardless of presence of HF as a comorbidity; and 2 studies evaluating the effect of ARNI in patients with end-stage renal disease, one retrospective study showing improvement in the left ventricular ejection fraction with ARNI and, a trial showing improvement in left ventricle echocardiographic parameters after 1 year of ARNI use in patients with end-stage renal disease.62,63 Furthermore, there are no studies to evaluate the efficacy of ARNI over ACEi or ARB monotherapy in patients with CKD in the absence of HF.64 Before randomization in the PARADIGM-HF and PARAGON-HF trials (Supplementary Table S1),42,57 approximately 10% to 15% of participants dropped out of the studies because of the adverse effects of hyperkalemia, renal dysfunction, or hypotension during the run-in period. After randomization, nearly 2% of the study participants had renal dysfunction defined as end-stage renal disease, a decrease of ≥50% in eGFR from the value at randomization or a decrease in eGFR of >30 ml/min per 1.73 m2.42,57 Furthermore, nearly 1 in 5 participants had adverse events from the study due to hyperkalemia or elevated serum creatinine from baseline.42,57 This is complicated by confusing data regarding renal dysfunction on guideline-based medical management of HF. On one hand, it is thought that continuation of antihypertensive medicines during episodes of renal dysfunction or hypotension does not increase risk of CKD progression.65 On the other hand, HF data shows antihypertensive therapies increase risk of CKD progression with recurrent episodes of renal dysfunction and/or hypotension.66
Given this information, it may be reasonable to conclude that ARNIs over ACEi or ARB monotherapy should be used primarily for reducing CV events in patients with HF. This drug should be used with caution in patients with HF with comorbid CKD, and potentially avoided in subgroups with high normal potassium concentration >5.2 mmol/l, subgroups with eGFR <30 ml/min per 1.73 m2 and subgroups with systolic blood pressure <110 mm Hg (Figure 4). Data is also limited for individuals with poorly controlled blood pressure (systolic ≥160 mm Hg or diastolic ≥100 mm Hg). For those patients with HF with comorbid CKD who are prescribed ARNI, blood pressure and laboratory data should be monitored carefully for hypotension, renal dysfunction, and hyperkalemia. Nephrologists should also expect a drop in eGFR after starting therapy and that is expected to stabilize after 1 month of initiation. Blood pressure should also be monitored for hypotension where down-titration of other antihypertensive medicines may be required. There is no indication yet for using this drug class for patients with CKD without HF solely for the goal of preventing CKD progression.
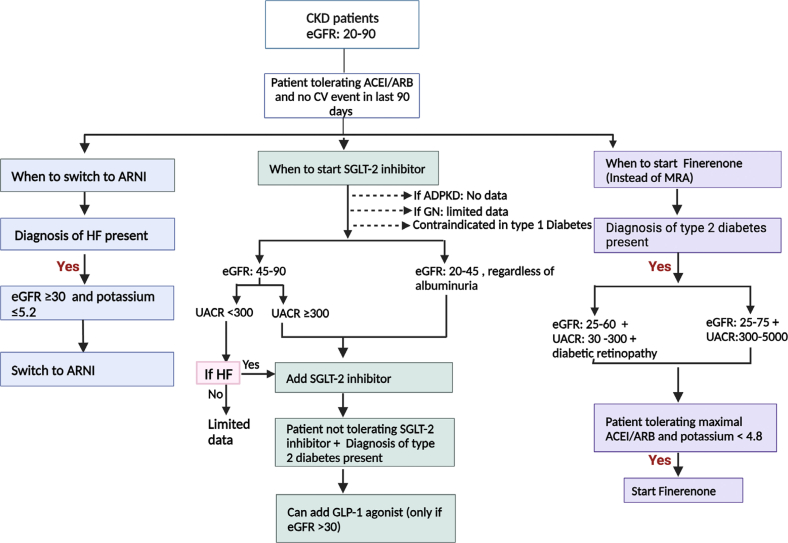
Figure 4.
An algorithm when ARNI, SGLT2i, and finerenone can be used based on the selection criteria used in landmark trials. First, ARNI should be limited to patients with heart failure with comorbid CKD if eGFR is >30 ml/min per 1.73 m2, they are tolerating RAASi, and do not have either serum potassium >5.2 mmol/l or systolic blood pressure <110 mm Hg or poorly controlled blood pressure (systolic ≥160 mm Hg or diastolic ≥100 mm Hg). Second, SGLT2i should be prescribed to patients with CKD if they are tolerating stable dose of RAASi, do not have CKD type 1 diabetes, or polycystic kidney disease. Data is limited for patients with glomerulonephritis. Furthermore, either eGFR should be 20 to 45 ml/min per 1.73 m2 regardless of albuminuria, 45 to 90 ml/min per 1.73 m2 with macroalbuminuria, or 45 to 90 ml/min per 1.73 m2 without macroalbuminuria but have heart failure for SGLT2i to be prescribed. Data for SGLT2i in patients with CKD and comorbid obesity (BMI >45 kg/m2) or in those with nephrotic range proteinuria (UACR >5000 mg/g) is limited. Third, GLP-1 agonist use is limited in patients with type 2 diabetic CKD with eGFR >30 ml/min per 1.73 m2 if they are tolerating stable dose of RAASi and cannot tolerate SGLT2i. Finally, finerenone use should be limited to patients with type 2 diabetic CKD who are tolerating maximal doses of ACEi or ARB, serum potassium concentration is <4.8 mmol/l and eGFR 25 to 60 ml/min per 1.73 m2 + microalbuminuria (30–500 mg/g) + diabetic retinopathy or, eGFR 25 to 75 ml/min per 1.73 m2 + macroalbuminuria (300–5000 mg/g). ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor/neprilysin inhibitor; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate expressed in ml/min per 1.73 m2; GLP-1, glucagon-like peptide 1; HF, heart failure; SGLT2i, sodium glucose transporter 2 inhibitor; UACR, urine albumin-to-creatinine ratio expressed in mg/g.
Sodium Glucose Transporter 2 Inhibitors
SGLT2i decrease glucose reabsorption in proximal tubules of kidneys; as a result, there is an increase in glucosuria and a reduction in plasma glucose concentration. SGLT2 receptor is distributed on the apical membranes of renal proximal tubular cells where filtered sodium and glucose from the glomerulus is reabsorbed. In animal models of type 1 and 2 diabetes mellitus, expression of these receptors increases by >50%; SGLT2i decreases SGLT2 expression in renal tubular cells and decreases glucose and sodium reuptake.67, 68, 69 SGLT2i also demonstrates antiinflammatory effects. In addition, in vitro studies on platelets harvested from healthy volunteers showed that SGLT2i use resulted in reduction in platelet activation by potentiating effects of nitric oxide and prostacyclin. These findings were translated to human studies where dapagliflozin reduced p-selectin expression of platelets in healthy volunteers.70,71 These pleiotropic effects of SGLT2i translate to improvement in CV outcomes with their use (Supplementary Table S2).40,41,43, 44, 45,47 A recent meta-analysis of landmark RCTs, which included 21,947 patients, reported that use of SGLT2i reduced risk of composite CV-death or hospitalization from HF by 23% (HR 0.77, 95% CI:0.72–0.82), of first hospitalization for HF by 28% (HR 0.72, 95% CI:0.67–0.78), and of all-cause mortality by 13% (HR 0.87, 95% CI: 0.79–0.95).72 In addition to these benefits, there was a mean weight loss by 0.7 kg and a mean reduction in systolic blood pressure by 2.5 mm Hg in participants receiving SGLT2i (vs. placebo).41 Because of these results, SGLT2is are one of the first-line agents used in patients with chronic HF with reduced and preserved ejection fraction (Class I) as endorsed by the clinical practice guidelines for HF.73 Mean eGFR of the study participants was close to 60 ml/min per 1.73 m2 and these trials excluded patients with eGFR <20 ml/min per 1.73 m2.41 Nearly two-thirds of the trial participants had adverse events, including hypotensive episodes, volume depletion, and urinary tract infections among others; this led to discontinuation of the study drug in nearly 15% to 20% of the study participants.41 Furthermore, SGLT2is are not recommended for glycemic control in patients with eGFR <30 ml/min per 1.73 m2 for empagliflozin and <45 ml/min per 1.73 m2 for dapagliflozin.74,75
In Supplementary Table S2, we summarize RCTs that evaluated effects of SGLT2i on renal outcomes in patients with CKD.46,48,49 The Dapagliflozin and Prevention of Adverse outcomes (DAPA)-CKD trial included patients with CKD (eGFR of 25–75 ml/min per 1.73 m2 and UACR 200 to 5000 mg/g), and reported use of dapagliflozin reduced the composite renal outcome by 39% in patients with CKD regardless of presence of type 2 diabetes mellitus (HR 0.61, 95% CI: 0.51–0.72).48 The EMPA-KIDNEY (The Study of Heart and Kidney Protection with Empagliflozin) collaborative group study included patients with CKD (eGFR of 20–45 ml/min per 1.73 m2 regardless of UACR, or eGFR 45–90 ml/min per 1.73 m2 plus UACR ≥200 mg/g), and reported reduction in progression of CKD or CV-death by 28% in patients randomized to the empagliflozin arm (vs. placebo) (HR 0.72, 95% CI: 0.64–0.82).49 In subgroup of patients with no overt albuminuria (UACR <300 mg/g), empagliflozin failed to improve progression of kidney disease or CV-death; this has generated ambiguity regarding use of empagliflozin in patients with CKD without overt albuminuria.49 All these RCTs required patients with CKD who were eligible to be on a stable dose of ACEi or ARB before randomization.
The DAPA-CKD and EMPA-KIDNEY trials excluded patients with morbid obesity (body mass index >45 kg/m2), poorly controlled blood pressure, and patients with CKD from polycystic kidney disease.48,49 The DAPA-CKD trial excluded patients with lupus nephritis and ANCA-associated vasculitis; however, it included patients with IgA nephropathy (n = 270) for whom it reduced CKD progression.48,76 EMPA-KIDNEY trial did not exclude any patients with vasculitis (n = not reported). More so, patients with recent CV event were also excluded. In our opinion, it is important to explicitly consider the populations to whom trial results will be applied and understand the limitations of extrapolations of these trials to general CKD management. Considering that use of these drugs has become more widespread in nephrology practice, there are some concerns of increased risk of urinary tract infections and of leg and foot amputations, mostly affecting toes, with SGLT2i use in patients with CKD.77 Post-marketing surveillance data will provide more insights into these risks for patients with CKD in the coming years. There is also a rising concern of high costs related to the use of these drugs. To offset these concerns, there is an ongoing discussion whether all types of patients with CKD should be prescribed SGLT2i given its cost or, whether it is more prudent to identify subgroups who will benefit the most. A recent study highlighted the variabilities in the therapeutic effects of SGLT2i; this drug class could be used in subgroups of patients with type 2 diabetes mellitus based on a multivariable risk prediction model that included HbA1c, UACR, and inflammatory burden (e.g., IL-6 levels). There is emerging data for a potential added benefit of ARNI in patients receiving SGLT2i therapy41,78; there were approximately 1 in 5 study participants on ARNI in the landmark SGLT2i RCT.41 Scientifically, SGLT2i + ARNI dual therapy may have added benefit but remains to be learned with post-marketing surveillance data. There is ongoing discussion about whether SGLT2i can be used in patients with CKD (eGFR <20 ml/min per 1.73 m2) or end-stage renal disease; these patients were excluded from the RCTs. There is limited experimental data regarding a direct effect of SGLT2i on the heart and the kidney irrespective of CKD severity. There is an ongoing Renal Lifecycle trial studying the efficacy of SGLT2i in these CKD subgroups.79
Given this information, it may be reasonable to conclude that SGLT2i could be used for any patient with CKD with eGFR 20 to 45 ml/min per 1.73 m2 regardless of UACR values, and for patients with CKD with eGFR 45 to 90 plus UACR >300 mg/g. We also propose to use it in patients with CKD with eGFR 45 to 90 ml/min per 1.73 m2 plus UACR <300 mg/g if they have history of HF. However, until more data is available regarding subgroups that are most likely to benefit from this treatment, clinicians should limit using SGLT2i based on the selection criteria for study enrollment of landmark trials. Patients with CKD (eGFR <20 ml/min per 1.73 m2, receiving dialysis or kidney transplant), morbid obesity (BMI >45 kg/m2), those who cannot tolerate ACEi or ARB therapy, or those with poorly controlled blood pressure should not be prescribed this drug class due to lack of data (Figure 4). Patients with CKD who have nephrotic range proteinuria (UACR >5000 mg/g) or those with poorly controlled blood pressure (systolic ≥160 mmHg or diastolic ≥100 mmHg) remain understudied. Patients with CKD arising polycystic kidney disease and type 1 diabetes mellitus should also avoid this drug class until more data becomes available (Figure 4). Nephrologists should also expect a drop in eGFR after starting therapy that is expected to stabilize after 1 month of initiation. Blood pressure should also be monitored for hypotension where down-titration of other antihypertensive medicines may be required.
Glucagon-Like Peptide-1 Receptor Agonists
GLP-1 binds to its receptor and activates adenylate cyclase to increase cytosolic cAMP and calcium, which induces insulin release from pancreas.80 GLP-1 receptor agonists potentiate the release of insulin and decreases glucagon secretion from pancreas. Its incretin-like effect increases early satiety.81 GLP-1 receptor agonists may affect complex signaling pathways such as extracellular matrix remodeling, platelets, and RAAS systems as demonstrated recently by use of network pharmacology methods analyzing large data sets. However preclinical or clinical studies remain to be performed to confirm these effects.82 In Supplementary Table S3, we summarize landmark RCTs evaluating effects of oral and injectable GLP1 receptor agonists in reducing CV events.50, 51, 52, 53,83 A recent meta-analysis of recently published RCTs reported reduction in CV events by 14% (HR 0.86, 95% CI: 0.80–0.93), in all-cause mortality by 12% (HR 0.88, 95% CI: 0.82–0.94 and in composite renal outcome by 21% (HR 0.79, 95% CI: 0.73–0.87).84 No RCT has evaluated efficacy of GLP-1 receptor agonists in reducing renal outcomes as the primary outcome measure. In a post hoc analysis of a landmark trial comparing tirzepatide to insulin glargine in patients with type 2 diabetes mellitus at high CV risk, tirzepatide reduced risk of incident CKD with more pronounced renal benefits in subgroups with eGFR <60 ml/min per 1.73 m2 (vs. ≥60 ml/min per 1.73 m2).85 There is an ongoing RCT, FLOW trial that is investigating the efficacy of GLP1 receptor agonists, semaglutide, in reducing adverse renal outcomes in patients with type 2 diabetes mellitus.86 In addition, combination therapies such as with SGLT2i and GLP-1 receptor agonists are being examined. The DURATION-8 trial evaluated efficacy of the combination of exenatide and dapagliflozin over monotherapy; it found better control of diabetes in individuals with type 2 diabetes mellitus.87 Ongoing RCTs (e.g., PRECIDENTED) are evaluating benefits of combination therapy in reducing cardiorenal outcomes.86 Data on this drug class is dynamically developing and we will learn more about use of this drug class in patients with CKD in the near future.
Finally, this class of drug has been associated with weight loss that can be quickly reversed when these medicines are stopped. Therefore, these medicines could be prescribed to achieve a tangible goal in adjunction to lifestyle changes. After those targets are met, we should caution patients for reversal of effects if lifestyle changes are not pursued. We also need to counsel the patients that this medication class will allow them to achieve their goals quicker, but in the end, lifestyle changes will need to continue for maintaining the clinical benefits.
Given this information, its use in patients with type 2 diabetic CKD is indicated primarily when SGLT2i is contraindicated, or as an adjuvant to SGLT2i in type 2 diabetic CKD (Figure 4). Its use in patients on dialysis remains unclear. Its use for glycemic control and for cardiorenal protection is limited in patients with type 2 diabetic CKD with eGFR <30 ml/min per 1.73 m2. There is no data to use this drug class in nondiabetic patients with CKD. However, its use is dynamically evolving with ongoing research.
Selective Mineralocorticoid Receptor Antagonist
Patients with type 2 diabetic CKD are treated with RAASi, SGLT2i, and hypoglycemic agents. Despite ACEi/ARB use, patients with type 2 diabetic CKD continue to have adverse cardiorenal outcomes. Nonselective steroidal mineralocorticoid receptor antagonists (MRA), spironolactone and eplerenone can be added as a therapeutic option but is poorly tolerated due to risk of side effects (e.g., acute kidney injury and hyperkalemia) especially when used in combination with ACEi/ARB. More recently, a nonsteroidal and selective MRA, finerenone, has become available.41,88 Finerenone offers a better side effect profile compared to spironolactone and eplerenone due to intraclass pharmacologic differences between the 3 drugs shown in Figure 3.89 In preclinical studies, finerenone was shown to have higher potency for antiinflammatory and antifibrotic effects than the other 2 MRAs.88,90 In addition, MRA play an important role in chronic tissue remodeling and CKD progression by reducing expression of immune cells in the end-organs. In pilot studies, it has also been shown to reduce albuminuria when added to ACEi/ARB in patients with type 2 diabetic CKD without worsening hyperkalemic events.90,91 The FIGARO-DKD (Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease) trial involved type 2 diabetics with eGFR 25 to 90 ml/min per 1.73 m2 plus UACR 30 mg/g to <300 mg/g, or with eGFR 60 ml/min per 1.73 m2 plus UACR 300 mg/g to <5000 mg/g. This trial showed that finerenone (vs. placebo) improved composite CV outcome by 18% (HR 0.82, 95% CI: 0.73–0.93) (Supplementary Table S4).90,92 The Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease (FIDELIO-DKD) trial evaluated efficacy of finerenone in patients with type 2 diabetic with UACR 30 mg/g to <300 mg/g plus eGFR 25 to <60 ml/min per 1.73 m2, or UACR 300 mg/g to <5000 mg/g plus eGFR ≥25 ml/min per 1.73 m2. This study showed that finerenone reduced progression of CKD and cardiorenal outcomes; an 18% reduction in composite renal outcomes compared to placebo (HR 0.82, 95% CI: 0.73–0.93) and an additional 13% reduction in a composite CV outcome (HR 0.87, 95% CI: 0.76–0.98) (Supplementary Table S4).90 Finally, in a pooled analyses of the 2 placebo-controlled trials (FIDELIO-DKD and FIGARO-DKD), finerenone reduced cardiorenal outcomes by approximately 15% to 20% in patients with type 2 diabetic CKD who are at risk of HF.93 Based on the subgroup analyses of these trials, it may be reasonable to suggest that the beneficial effects of finerenone might be more pronounced in patients with type 2 diabetic CKD who have baseline CV disease than those without it.93
Eligible patients in the FIGARO-DKD and FIDELIO-DKD trials were chosen based on selection criteria shown in Figure 4 and if they could tolerate maximal doses of ACEi/ARB and had a serum potassium concentration <4.8 mEq/l before randomization. Nearly 1 in 2 patients did not meet eligibility during the run-in period. After randomization, 18% developed hyperkalemia and 5% developed acute kidney injury. With the addition of finerenone to maximal dose of ACEi/ARB, there was an additional drop in mean systolic blood pressure by 3 mm Hg. There was also an acute decline in eGFR after starting finerenone that subsequently stabilized for the remaining follow-up. In the FIDELIO-DKD trial, only 4% of the participants were already on SGLT2i at the time of randomization. There is lack of clarity about whether patients with type 2 diabetic CKD who are on SGLT2i could benefit from the addition of finerenone because there are suggestions that hyperkalemia from finerenone use could be offset by combining it with SGLT2i94 along with potential reduction in CV outcomes.95 An ongoing study, Combination of Finerenone and Empagliflozin in Adults With Long-term Kidney Disease and Type 2 Diabetes (CONFIDENCE), is evaluating how combination therapy works and whether it is safe compared to each monotherapies.96 In addition, there is a concern about higher incidence of dialysis-dependent renal dysfunction with wide use of dual RAAS blockers (ACEi/ARB + MRA) as experienced almost 2 decades ago with gaining popularity of dual RAAS blockade in reduced ejection fraction patients.
Given this information, it may be reasonable to consider finerenone therapy in addition to ACEi/ARB for patients with type 2 diabetic CKD with eGFR 25 to 60 ml/min per 1.73 m2 + microalbuminuria + diabetic retinopathy or, eGFR 25 to 75 ml/min per 1.73 m2 + macroalbuminuria if serum potassium concentration remains <4.8 mmol/l on maximal ACEi/ARB dose (Figure 4). Patients with CKD who are started on this drug should be closely monitored for acute kidney injury and hyperkalemia. Nephrologists should also expect a drop in eGFR after starting finerenone therapy that is expected to stabilize after 1 month of initiation. Blood pressure should also be monitored for hypotension where down-titration of other antihypertensive medicines may be required. Benefits of combination therapy with SGLT2i and finerenone in patients with type 2 diabetic CKD remains to be established. Patients with CKD who have nephrotic range proteinuria (UACR >5000 mg/g) or those with poorly controlled blood pressure (systolic ≥160 mm Hg or diastolic ≥100 mm Hg) remain understudied.
Future
The past few decades have focused on fixing problems arising from salt and water retention that translates to improved cardiorenal outcomes by 10% to 30% in patients with CKD. More needs to be accomplished in the next several decades to better treat residual cardiorenal risk in patients with CKD despite guideline-based medical management, which is primarily driven by inflammation and platelet activation. Given that patients with CKD remain heterogeneous, there is a complex interplay of CKD pathophysiology that manifests as adverse outcomes (Figure 1). Many of the best-selling drugs today may not be effective in all patients with CKD, largely due to our lack of understanding of CKD pathophysiology and its variation among individuals. Understanding these mechanisms will also be important to identify appropriate therapeutic targets for achieving therapeutic effects in patients with CKD.97,98 Previous large CV outcomes trials recruited participants enriched for CV diseases where CKD was either underrepresented, failed to measure albuminuria, a strong predictor of CV risk; or included patients with CKD primarily based on eGFR cut-offs and excluding those with eGFR <30 ml/min per 1.73 m2.99 This creates a hurdle to understand CKD pathophysiology when clinicians continue to rely on CV literature to manage CKD. There are 2 problems with this extrapolation. First, we know that inflammation in CV diseases is not nearly as high as that in CKD.100,101 Second, platelet activation is one of the primary drivers of thrombotic CV events whereas chronic inflammation is one of the primary drivers of CKD.100, 101, 102 Moreover, several factors can determine inflammatory signals and platelet activation in patients with CKD, including baseline patient characteristics100 and genetics.103 Therefore, we need to move toward prescribing standard-of-care therapies to all patients with CKD that includes RAAS blockers and newer therapies in varying combinations discussed above. Subsequently, select subgroups who continue to exhibit heightened inflammation and/or platelet activation despite maximal guideline-based management who could be considered for escalation of care so as to reduce residual cardiorenal risks. It is therefore essential that nephrologists move on from the current “one size fits all” approach, toward more precise and better-informed solutions. Much work needs to be done in understanding these nuances for maximizing clinical benefits and reducing redundancies and risks of therapies. Moreover, future decades will focus on identifying subgroups of CKD who are more likely to reap these benefits, and on developing targeted escalation of interventions in CKD subgroups using novel therapeutic strategies to reduce residual cardiorenal risks.
Conclusion
CKD continues to grow worldwide. These patients are at disproportionately high risk of CV events. Complex interplay of deranged pathways is a hallmark of patients with CKD. Although RAASi have reduced cardiorenal outcomes of patients with CKD in the last few decades, burden of CKD continues to generate residual cardiorenal risk in this patient population. Newer agents are now available in the market and provide new hope to improve the lives of this patient population. It is important to select appropriate individuals for initiation of therapy with newer agents because these agents are expensive, have side effects, and should be used in clinical practice keeping in mind the selection criteria used in the landmark trials (algorithm shown in Figure 4). Despite these specific criteria for use of newer agents, risk of hypotension, acute kidney injury, and high serum potassium concentration remains and should be carefully monitored after treatment initiation. Combination therapy with newer agents is an area of growing knowledge and remains to be explored further for synergistic action and widespread clinical use. Patients with CKD who have nephrotic range proteinuria beyond 5000 mg/g and those with poorly controlled blood pressure remain understudied. It is important to explicitly consider the populations to whom trial results will be applied and understand the limitations of extrapolations of recent trials to general CKD management.
Disclosure
All the authors declared no competing interests.
Acknowledgments
We are grateful to UAMS Creative Services for creating Figure 2. Biorender.com was used to create other figures. We are also grateful to UAMS Science Communication Group for editing language in the manuscript. NJ is supported by the Dialysis Clinic Inc. (PTRF 2021-04 and C-4201) and administrative supplement to the Arkansas IDeA Network for Biomedical Research Excellence Program, awarded by the National Institute of General Medical Sciences at the National Institutes of Health (NIH). Institutional support for this review was provided to NJ by UL1 TR003107 and KL2 TR003108 from the National Center for Advancing Translational Sciences at the NIH. The views expressed here are those of the authors and do not necessarily represent the views of the Veterans Administration, the Dialysis Clinic Inc., or the National Institutes of Health.
Footnotes
Table S1. Landmark trials of angiotensin receptor/neprilysin inhibition.
Table S2. Landmark trials of sodium glucose cotransporter-2 inhibitors.
Table S3. Landmark studies that demonstrate the efficacy of glucagon-like peptide-1 receptor agonists.
Table S4. Landmark studies demonstrating efficacy of finerenone in chronic heart and kidney disease.
Supplementary Material
Table S1. Landmark trials of angiotensin receptor/neprilysin inhibition.
Table S2. Landmark trials of sodium glucose cotransporter-2 inhibitors.
Table S3. Landmark studies that demonstrate the efficacy of glucagon-like peptide-1 receptor agonists.
Table S4. Landmark studies demonstrating efficacy of finerenone in chronic heart and kidney disease.
References
- 1.Cockwell P., Fisher L.A. The global burden of chronic kidney disease. Lancet. 2020;395:662–664. doi: 10.1016/S0140-6736(19)32977-0. [DOI] [PubMed] [Google Scholar]
- 2.Foley R.N., Murray A.M., Li S., et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol. 2005;16:489–495. doi: 10.1681/ASN.2004030203. [DOI] [PubMed] [Google Scholar]
- 3.Foley R.N., Parfrey P.S., Sarnak M.J. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32(5 suppl 3):S112–S119. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 4.Zhu Z.B., Zhang R.Y., Zhang Q., et al. Moderate-severe renal insufficiency is a risk factor for sirolimus-eluting stent thrombosis. The RIFT study. Cardiology. 2009;112:191–199. doi: 10.1159/000149571. [DOI] [PubMed] [Google Scholar]
- 5.Best P.J., Lennon R., Ting H.H., et al. The impact of renal insufficiency on clinical outcomes in patients undergoing percutaneous coronary interventions. J Am Coll Cardiol. 2002;39:1113–1119. doi: 10.1016/s0735-1097(02)01745-x. [DOI] [PubMed] [Google Scholar]
- 6.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 suppl 1):S1–S266. [PubMed] [Google Scholar]
- 7.Machecourt J., Danchin N., Lablanche J.M., et al. Risk factors for stent thrombosis after implantation of sirolimus-eluting stents in diabetic and nondiabetic patients: the EVASTENT Matched-Cohort Registry. J Am Coll Cardiol. 2007;50:501–508. doi: 10.1016/j.jacc.2007.04.051. [DOI] [PubMed] [Google Scholar]
- 8.Best P.J., Steinhubl S.R., Berger P.B., et al. The efficacy and safety of short- and long-term dual antiplatelet therapy in patients with mild or moderate chronic kidney disease: results from the Clopidogrel for the Reduction of Events During Observation (CREDO) trial. Am Heart J. 2008;155:687–693. doi: 10.1016/j.ahj.2007.10.046. [DOI] [PubMed] [Google Scholar]
- 9.Debella Y.T., Giduma H.D., Light R.P., Agarwal R. Chronic kidney disease as a coronary disease equivalent--a comparison with diabetes over a decade. Clin J Am Soc Nephrol. 2011;6:1385–1392. doi: 10.2215/CJN.10271110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jankowski J., Floege J., Fliser D., Bohm M., Marx N. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation. 2021;143:1157–1172. doi: 10.1161/CIRCULATIONAHA.120.050686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dounousi E., Papavasiliou E., Makedou A., et al. Oxidative stress is progressively enhanced with advancing stages of CKD. Am J Kidney Dis. 2006;48:752–760. doi: 10.1053/j.ajkd.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 12.Oberg B.P., McMenamin E., Lucas F.L., et al. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 2004;65:1009–1016. doi: 10.1111/j.1523-1755.2004.00465.x. [DOI] [PubMed] [Google Scholar]
- 13.Speer T., Dimmeler S., Schunk S.J., Fliser D., Ridker P.M. Targeting innate immunity-driven inflammation in CKD and cardiovascular disease. Nat Rev Nephrol. 2022;18:762–778. doi: 10.1038/s41581-022-00621-9. [DOI] [PubMed] [Google Scholar]
- 14.Sloand J.A., Sloand E.M. Studies on platelet membrane glycoproteins and platelet function during hemodialysis. J Am Soc Nephrol. 1997;8:799–803. doi: 10.1681/ASN.V85799. [DOI] [PubMed] [Google Scholar]
- 15.Kozek-Langenecker S.A., Masaki T., Mohammad H., Green W., Mohammad S.F., Cheung A.K. Fibrinogen fragments and platelet dysfunction in uremia. Kidney Int. 1999;56:299–305. doi: 10.1046/j.1523-1755.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- 16.Muntner P., He J., Astor B.C., Folsom A.R., Coresh J. Traditional and nontraditional risk factors predict coronary heart disease in chronic kidney disease: results from the atherosclerosis risk in communities study. J Am Soc Nephrol. 2005;16:529–538. doi: 10.1681/ASN.2004080656. [DOI] [PubMed] [Google Scholar]
- 17.Leblond F., Guevin C., Demers C., Pellerin I., Gascon-Barre M., Pichette V. Downregulation of hepatic cytochrome P450 in chronic renal failure. J Am Soc Nephrol. 2001;12:326–332. doi: 10.1681/ASN.V122326. [DOI] [PubMed] [Google Scholar]
- 18.Mullangi R., Srinivas N.R. Clopidogrel: review of bioanalytical methods, pharmacokinetics/pharmacodynamics, and update on recent trends in drug-drug interaction studies. Biomed Chromatogr. 2009;23:26–41. doi: 10.1002/bmc.1128. [DOI] [PubMed] [Google Scholar]
- 19.Roberts M.A., Hare D.L., Ratnaike S., Ierino F.L. Cardiovascular biomarkers in CKD: pathophysiology and implications for clinical management of cardiac disease. Am J Kidney Dis. 2006;48:341–360. doi: 10.1053/j.ajkd.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Jain N., Corken A.L., Kumar A., Davis C.L., Ware J., Arthur J.M. Role of platelets in chronic kidney disease. J Am Soc Nephrol. 2021;32:1551–1558. doi: 10.1681/ASN.2020121806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baaten C., Sternkopf M., Henning T., Marx N., Jankowski J., Noels H. Platelet function in CKD: a systematic review and meta-analysis. J Am Soc Nephrol. 2021;32:1583–1598. doi: 10.1681/ASN.2020101440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gong S., Wang C., Xiong J., Zhao J., Yang K. Activated platelets, the booster of chronic kidney disease and cardiovascular complications. Kidney Dis (Basel) 2022;8:297–307. doi: 10.1159/000525090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Csaba P., Kovesdy M., Kamyar Kalantar-Zadeh M.D. UpToDate; 2021. Inflammation in Patients With Kidney Function Impairment. [Google Scholar]
- 24.Gremmel T., Müller M., Steiner S., et al. Chronic kidney disease is associated with increased platelet activation and poor response to antiplatelet therapy. Nephrol Dial Transplant. 2013;28:2116–2122. doi: 10.1093/ndt/gft103. [DOI] [PubMed] [Google Scholar]
- 25.Girndt M., Trojanowicz B., Ulrich C. Monocytes in uremia. Toxins (Basel) 2020;12 doi: 10.3390/toxins12050340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naicker S.D., Cormican S., Griffin T.P., et al. Chronic kidney disease severity is associated with selective expansion of a distinctive intermediate monocyte subpopulation. Front Immunol. 2018;9:2845. doi: 10.3389/fimmu.2018.02845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kapellos T.S., Bonaguro L., Gemund I., et al. Human monocyte subsets and phenotypes in major chronic inflammatory diseases. Front Immunol. 2019;10:2035. doi: 10.3389/fimmu.2019.02035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ziegler-Heitbrock L. Blood monocytes and their subsets: established features and open questions. Front Immunol. 2015;6:423. doi: 10.3389/fimmu.2015.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baaten C., Schroer J.R., Floege J., et al. Platelet abnormalities in CKD and their implications for antiplatelet therapy. Clin J Am Soc Nephrol. 2022;17:155–170. doi: 10.2215/CJN.04100321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vorchheimer D.A., Becker R. Platelets in atherothrombosis. Mayo Clin Proc. 2006;81:59–68. doi: 10.4065/81.1.59. [DOI] [PubMed] [Google Scholar]
- 31.Jain N., Li X., Adams-Huet B., et al. Differences in whole blood platelet aggregation at baseline and in response to aspirin and aspirin plus clopidogrel in patients with versus without chronic kidney disease. Am J Cardiol. 2016;117:656–663. doi: 10.1016/j.amjcard.2015.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kemec Z., Demir M., Gurel A., et al. Associations of platelet indices with proteinuria and chronic kidney disease. J Int Med Res. 2020;48 doi: 10.1177/0300060520918074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ji Y., Wang Y.L., Xu F., et al. Elevated soluble podoplanin associates with hypercoagulability in patients with nephrotic syndrome. Clin Appl Thromb Hemost. 2022;28 doi: 10.1177/10760296221108967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corken A., Ware J., Dai J., et al. Platelet-dependent inflammatory dysregulation in patients with Stages 4 or 5 chronic kidney disease: a mechanistic clinical study. Kidney360. 2022;3:2036–2047. doi: 10.34067/KID.0005532022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jain N., Phadnis M.A., Martin B.C., Mehta J.L. Potent antiplatelet therapy may reduce death from sepsis in patients on chronic dialysis. Am J Cardiol. 2022;162:209–211. doi: 10.1016/j.amjcard.2021.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Jain N., Corken A., Arthur J.M., et al. Ticagrelor inhibits platelet aggregation and reduces inflammatory burden more than clopidogrel in patients with stages 4 or 5 chronic kidney disease. Vascul Pharmacol. 2023;148 doi: 10.1016/j.vph.2023.107143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meza D., Shanmugavelayudam S.K., Mendoza A., Sanchez C., Rubenstein D.A., Yin W. Platelets modulate endothelial cell response to dynamic shear stress through PECAM-1. Thromb Res. 2017;150:44–50. doi: 10.1016/j.thromres.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y., He D., Zhang W., et al. ACE inhibitor benefit to kidney and cardiovascular outcomes for patients with non-dialysis chronic kidney disease Stages 3-5: a network meta-analysis of randomised clinical trials. Drugs. 2020;80:797–811. doi: 10.1007/s40265-020-01290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group KDIGO 2021 Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int. 2021;99:S1–S87. doi: 10.1016/j.kint.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Anker S.D., Butler J., Filippatos G., et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–1461. doi: 10.1056/NEJMoa2107038. [DOI] [PubMed] [Google Scholar]
- 41.Packer M., Anker S.D., Butler J., et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–1424. doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 42.McMurray J.J., Packer M., Desai A.S., et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 43.McMurray J.J.V., Solomon S.D., Inzucchi S.E., et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 44.Solomon S.D., McMurray J.J.V., Claggett B., et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387:1089–1098. doi: 10.1056/NEJMoa2206286. [DOI] [PubMed] [Google Scholar]
- 45.Bhatt D.L., Szarek M., Pitt B., et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med. 2021;384:129–139. doi: 10.1056/NEJMoa2030186. [DOI] [PubMed] [Google Scholar]
- 46.Perkovic V., Jardine M.J., Neal B., et al. Canagliflozin and renal outcomes in Type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 47.Bhatt D.L., Szarek M., Steg P.G., et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021;384:117–128. doi: 10.1056/NEJMoa2030183. [DOI] [PubMed] [Google Scholar]
- 48.Heerspink H.J.L., Stefansson B.V., Correa-Rotter R., et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 49.Herrington W.G., Staplin N., Staplin N., et al. Empagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2023;388:117–127. doi: 10.1056/NEJMoa2204233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marso S.P., Daniels G.H., Brown-Frandsen K., et al. Liraglutide and cardiovascular outcomes in Type 2 diabetes. N Engl J Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marso S.P., Bain S.C., Consoli A., et al. Semaglutide and cardiovascular outcomes in patients with Type 2 diabetes. N Engl J Med. 2016;375:1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 52.Gerstein H.C., Colhoun H.M., Dagenais G.R., et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121–130. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 53.Holman R.R., Bethel M.A., Mentz R.J., et al. Effects of Once-Weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377:1228–1239. doi: 10.1056/NEJMoa1612917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bomback A.S., Toto R. Dual blockade of the renin-angiotensin-aldosterone system: beyond the ACE inhibitor and angiotensin-II receptor blocker combination. Am J Hypertens. 2009;22:1032–1040. doi: 10.1038/ajh.2009.138. [DOI] [PubMed] [Google Scholar]
- 55.Jering K.S., Zannad F., Claggett B., et al. Cardiovascular and renal outcomes of mineralocorticoid receptor antagonist use in Paragon-HF. JACC Heart Fail. 2021;9:13–24. doi: 10.1016/j.jchf.2020.08.014. [DOI] [PubMed] [Google Scholar]
- 56.Pu Q., Amiri F., Gannon P., Schiffrin E.L. Dual angiotensin-converting enzyme/neutral endopeptidase inhibition on cardiac and renal fibrosis and inflammation in DOCA-salt hypertensive rats. J Hypertens. 2005;23:401–409. doi: 10.1097/00004872-200502000-00023. [DOI] [PubMed] [Google Scholar]
- 57.Solomon S.D., McMurray J.J.V., Anand I.S., et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381:1609–1620. doi: 10.1056/NEJMoa1908655. [DOI] [PubMed] [Google Scholar]
- 58.Mc Causland F.R., Lefkowitz M.P., Claggett B., et al. Angiotensin-neprilysin inhibition and renal outcomes in heart failure with preserved ejection fraction. Circulation. 2020;142:1236–1245. doi: 10.1161/CIRCULATIONAHA.120.047643. [DOI] [PubMed] [Google Scholar]
- 59.Damman K., Gori M., Claggett B., et al. Renal effects and associated outcomes during angiotensin-neprilysin inhibition in heart failure. JACC Heart Fail. 2018;6:489–498. doi: 10.1016/j.jchf.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 60.Hsiao F.C., Lin C.P., Yu C.C., Tung Y.C., Chu P.H. Angiotensin receptor-neprilysin inhibitors in patients with heart failure with reduced ejection fraction and advanced chronic kidney disease: a retrospective multi-institutional study. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.794707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haynes R., Judge P.K., Staplin N., et al. Effects of Sacubitril/Valsartan versus irbesartan in patients with chronic kidney disease. Circulation. 2018;138:1505–1514. doi: 10.1161/CIRCULATIONAHA.118.034818. [DOI] [PubMed] [Google Scholar]
- 62.Lee S., Oh J., Kim H., et al. Sacubitril/valsartan in patients with heart failure with reduced ejection fraction with end-stage of renal disease. ESC Heart Fail. 2020;7:1125–1129. doi: 10.1002/ehf2.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Niu C.Y., Yang S.F., Ou S.M., et al. Sacubitril/Valsartan in patients with heart failure and concomitant end-stage kidney disease. J Am Heart Assoc. 2022;11 doi: 10.1161/JAHA.122.026407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsukamoto S., Uehara T., Azushima K., Wakui H., Tamura K. Updates for cardio-kidney protective effects by angiotensin receptor-neprilysin inhibitor: requirement for additional evidence of kidney protection. J Am Heart Assoc. 2023;12 doi: 10.1161/JAHA.122.029565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ku E., Sarnak M.J., Toto R., et al. Effect of blood pressure control on long-term risk of end-stage renal disease and death among subgroups of patients with chronic kidney disease. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.012749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ettehad D., Emdin C.A., Kiran A., et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957–967. doi: 10.1016/S0140-6736(15)01225-8. [DOI] [PubMed] [Google Scholar]
- 67.Hou Y.C., Zheng C.M., Yen T.H., Lu K.C. Molecular mechanisms of SGLT2 inhibitor on cardiorenal protection. Int J Mol Sci. 2020;21:7833. doi: 10.3390/ijms21217833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zelniker T.A., Braunwald E. Mechanisms of cardiorenal effects of sodium-glucose cotransporter 2 inhibitors: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:422–434. doi: 10.1016/j.jacc.2019.11.031. [DOI] [PubMed] [Google Scholar]
- 69.van der Aart-van der Beek A.B., de Boer R.A., Heerspink H.J.L. Kidney and heart failure outcomes associated with SGLT2 inhibitor use. Nat Rev Nephrol. 2022;18:294–306. doi: 10.1038/s41581-022-00535-6. [DOI] [PubMed] [Google Scholar]
- 70.Lescano C.H., Leonardi G., Torres P.H.P., et al. The sodium-glucose cotransporter-2 (SGLT2) inhibitors synergize with nitric oxide and prostacyclin to reduce human platelet activation. Biochem Pharmacol. 2020;182 doi: 10.1016/j.bcp.2020.114276. [DOI] [PubMed] [Google Scholar]
- 71.Kohlmorgen C., Gerfer S., Feldmann K., et al. Dapagliflozin reduces thrombin generation and platelet activation: implications for cardiovascular risk reduction in type 2 diabetes mellitus. Diabetologia. 2021;64:1834–1849. doi: 10.1007/s00125-021-05498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vaduganathan M., Docherty K.F., Claggett B.L., et al. SGLT-2 inhibitors in patients with heart failure: a comprehensive meta-analysis of five randomised controlled trials. Lancet. 2022;400:757–767. doi: 10.1016/S0140-6736(22)01429-5. [DOI] [PubMed] [Google Scholar]
- 73.Heidenreich P.A., Bozkurt B., Aguilar D., et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2022;145:e895–e1032. doi: 10.1161/CIR.0000000000001063. [DOI] [PubMed] [Google Scholar]
- 74.Farxigra (Dapagliflozin) AstraZeneca Pharmaceuticals LP; 2014. Prescribing information.https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/202293s003lbl.pdf [Google Scholar]
- 75.Jardiance (empagliflozin). Prescribing information. Boehringer Ingelheim Pharmaceuticals, Inc; 2014. https://content.boehringer-ingelheim.com/DAM/7d9c411c-ec33-4f82-886f-af1e011f35bb/jardiance-us-pi.pdf [Google Scholar]
- 76.Wheeler D.C., Toto R.D., Stefansson B.V., et al. A pre-specified analysis of the DAPA-CKD trial demonstrates the effects of dapagliflozin on major adverse kidney events in patients with IgA nephropathy. Kidney Int. 2021;100:215–224. doi: 10.1016/j.kint.2021.03.033. [DOI] [PubMed] [Google Scholar]
- 77.U.S. Food and Drug Administration FDA drug safety communication: FDA confirms increased risk of leg and foot amputations with the diabetes medicine canagliflozin (Invokana, Invokamet, Invokamet XR) 2017. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-confirms-increased-risk-leg-and-foot-amputations-diabetes-medicine
- 78.Packer M., Anker S.D., Butler J., et al. Influence of neprilysin inhibition on the efficacy and safety of empagliflozin in patients with chronic heart failure and a reduced ejection fraction: the EMPEROR-Reduced trial. Eur Heart J. 2021;42:671–680. doi: 10.1093/eurheartj/ehaa968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gerstein H.C., Sattar N., Rosenstock J., et al. Cardiovascular and renal outcomes with Efpeglenatide in Type 2 diabetes. N Engl J Med. 2021;385:896–907. doi: 10.1056/NEJMoa2108269. [DOI] [PubMed] [Google Scholar]
- 80.Wei Y., Mojsov S. Tissue-specific expression of the human receptor for glucagon-like peptide-I: brain, heart and pancreatic forms have the same deduced amino acid sequences. FEBS Lett. 1995;358:219–224. doi: 10.1016/0014-5793(94)01430-9. [DOI] [PubMed] [Google Scholar]
- 81.Little T.J., Pilichiewicz A.N., Russo A., et al. Effects of intravenous glucagon-like peptide-1 on gastric emptying and intragastric distribution in healthy subjects: relationships with postprandial glycemic and insulinemic responses. J Clin Endocrinol Metab. 2006;91:1916–1923. doi: 10.1210/jc.2005-2220. [DOI] [PubMed] [Google Scholar]
- 82.Deng G., Ren J., Li R., et al. Systematic investigation of the underlying mechanisms of GLP-1 receptor agonists to prevent myocardial infarction in patients with type 2 diabetes mellitus using network pharmacology. Front Pharmacol. 2023;14 doi: 10.3389/fphar.2023.1125753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Husain M., Birkenfeld A.L., Donsmark M., et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381:841–851. doi: 10.1056/NEJMoa1901118. [DOI] [PubMed] [Google Scholar]
- 84.Sattar N., Lee M.M.Y., Kristensen S.L., et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021;9:653–662. doi: 10.1016/S2213-8587(21)00203-5. [DOI] [PubMed] [Google Scholar]
- 85.Heerspink H.J.L., Sattar N., Pavo I., et al. Effects of tirzepatide versus insulin glargine on kidney outcomes in type 2 diabetes in the SURPASS-4 trial: post-hoc analysis of an open-label, randomised, phase 3 trial. Lancet Diabetes Endocrinol. 2022;10:774–785. doi: 10.1016/S2213-8587(22)00243-1. [DOI] [PubMed] [Google Scholar]
- 86.Novo Nordisk . ClinicalTrials Gov. National Library of Medicine; 2023. A research study to see how semaglutide works compared to placebo in people with type 2 diabetes and chronic kidney disease (FLOW)https://clinicaltrials.gov/study/NCT03819153?tab=results Updated. [Google Scholar]
- 87.Frias J.P., Guja C., Hardy E., et al. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a 28 week, multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 2016;4:1004–1016. doi: 10.1016/S2213-8587(16)30267-4. [DOI] [PubMed] [Google Scholar]
- 88.Pitt B., Filippatos G., Agarwal R., et al. Cardiovascular events with finerenone in kidney disease and Type 2 diabetes. N Engl J Med. 2021;385:2252–2263. doi: 10.1056/NEJMoa2110956. [DOI] [PubMed] [Google Scholar]
- 89.Barfacker L., Kuhl A., Hillisch A., et al. Discovery of BAY 94-8862: a nonsteroidal antagonist of the mineralocorticoid receptor for the treatment of cardiorenal diseases. ChemMedChem. 2012;7:1385–1403. doi: 10.1002/cmdc.201200081. [DOI] [PubMed] [Google Scholar]
- 90.Bakris G.L., Agarwal R., Anker S.D., et al. Effect of finerenone on chronic kidney disease outcomes in Type 2 diabetes. N Engl J Med. 2020;383:2219–2229. doi: 10.1056/NEJMoa2025845. [DOI] [PubMed] [Google Scholar]
- 91.Barrera-Chimal J., Estrela G.R., Lechner S.M., et al. The myeloid mineralocorticoid receptor controls inflammatory and fibrotic responses after renal injury via macrophage interleukin-4 receptor signaling. Kidney Int. 2018;93:1344–1355. doi: 10.1016/j.kint.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 92.Filippatos G., Anker S.D., Agarwal R., et al. Finerenone reduces risk of incident heart failure in patients with chronic kidney disease and Type 2 diabetes: analyses from the Figaro-DKD trial. Circulation. 2022;145:437–447. doi: 10.1161/CIRCULATIONAHA.121.057983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Agarwal R., Filippatos G., Pitt B., et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur Heart J. 2022;43:474–484. doi: 10.1093/eurheartj/ehab777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Agarwal R., Joseph A., Anker S.D., et al. Hyperkalemia risk with finerenone: results from the FIDELIO-DKD trial. J Am Soc Nephrol. 2022;33:225–237. doi: 10.1681/ASN.2021070942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tsukamoto S., Morita R., Yamada T., et al. Cardiovascular and kidney outcomes of combination therapy with sodium-glucose cotransporter-2 inhibitors and mineralocorticoid receptor antagonists in patients with type 2 diabetes and chronic kidney disease: a systematic review and network meta-analysis. Diabetes Res Clin Pract. 2022;194 doi: 10.1016/j.diabres.2022.110161. [DOI] [PubMed] [Google Scholar]
- 96.Green J.B., Mottl A.K., Bakris G., et al. Design of the COmbinatioN effect of FInerenone and EmpaglifloziN in participants with chronic kidney disease and type 2 diabetes using a UACR Endpoint study (CONFIDENCE) Nephrol Dial Transplant. 2023;38:894–903. doi: 10.1093/ndt/gfac198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lowenstern A., Storey R.F., Neely M., et al. Platelet-related biomarkers and their response to inhibition with aspirin and P2Y12-receptor antagonists in patients with acute coronary syndrome. J Thromb Thrombolysis. 2017;44:145–153. doi: 10.1007/s11239-017-1516-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gao C., Tomaniak M., Takahashi K., et al. Ticagrelor monotherapy in patients with concomitant diabetes mellitus and chronic kidney disease: a post hoc analysis of the GLOBAL LEADERS trial. Cardiovasc Diabetol. 2020;19:179. doi: 10.1186/s12933-020-01153-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Poliakova A.G. Use of reflexotherapy in patients with sequelae of injuries. Ortop Travmatol Protez. 1988;1988:50–54. [PubMed] [Google Scholar]
- 100.Amdur R.L., Feldman H.I., Gupta J., et al. Inflammation and progression of CKD: the CRIC study. Clin J Am Soc Nephrol. 2016;11:1546–1556. doi: 10.2215/CJN.13121215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sorriento D., Iaccarino G. Inflammation and cardiovascular diseases: the most recent findings. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20163879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Khodadi E. Platelet function in cardiovascular disease: activation of molecules and activation by molecules. Cardiovasc Toxicol. 2020;20:1–10. doi: 10.1007/s12012-019-09555-4. [DOI] [PubMed] [Google Scholar]
- 103.Ollier W.E. Cytokine genes and disease susceptibility. Cytokine. 2004;28:174–178. doi: 10.1016/j.cyto.2004.07.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.