Abstract
Transradial access (TRA) is a safe and comfortable approach and the preferred access for percutaneous coronary intervention. However, TRA is not widely used for peripheral interventions. Currently, there is a lack of data on patient selection, appropriate medical devices, complication prevention, and TRA adoption. Therefore, the Chinese Society of Interventional Oncology of the China Anti-Cancer Association organized nationwide experts to establish a Working Group of China Expert Consensus on TRA in percutaneous peripheral interventions in 2022, and jointly formulated this consensus to better promote the application of TRA in peripheral interventions to guide clinicians on patient selection, technical recommendations, and physician training. This consensus mainly focuses on the current situation, advantages and limitations of TRA in peripheral interventions, anatomical characteristics of the radial artery, patient selection, technical aspects, prevention and management of complications, radiation dose, and learning curve. A consensus was reached through a literature evaluation and by referring to the opinions of the expert group.
Keywords: Transradial access, Transfemoral access, Peripheral intervention
Transfemoral access (TFA) has become the standard access option for interventional radiologists since 1953, when Sven-Ivar Seldinger first reported the use of percutaneous femoral artery puncture for angiography.1 The use of percutaneous radial artery puncture for selective coronary angiography was first reported by Campeau in 1989.2 The first transradial access (TRA) for coronary stent implantation was performed in 1992 by Ferdinand Kiemenij, a Dutch physician, based on Lucien Campeau's experience and a book on radial artery anatomy.3 Numerous studies have shown that TRA can reduce access site complications, mortality, hospital burden, length of hospital stay, and patient comfort compared to TFA.4, 5, 6 Currently, TRA is the preferred choice for coronary interventions worldwide. Shiozawa reported a case series on the use of TRA in transarterial chemoembolization (TACE) in 2003.7 Since then, the use of TRA in peripheral interventions has gradually increased. In the past five years, this technique has been rapidly promoted in China, but there is still insufficient data, especially on patient selection, appropriate medical devices, and the prevention and management of complications. Therefore, the Chinese Society of Interventional Oncology and China Anti-Cancer Association organized nationwide experts to establish a Working Group of China Expert Consensus on TRA in percutaneous peripheral interventions in 2022 to drive the application of TRA in peripheral interventions and guide clinicians in the technical aspects of TRA and physician training.
1. Current application status of TRA in percutaneous peripheral interventions
The application of TRA in peripheral vascular interventions is relatively rare. According to a survey report, only 53.5 % of interventional radiologists in Europe and America routinely use TRA. The major factors hindering interventional radiologists from performing TRA include a long learning curve, lack of training, prolonged procedure time, potential cerebrovascular complications, and perceptions of prolonged radiation exposure time.8 With the growing knowledge of TRA, it is increasingly being used for peripheral interventions internationally. In China, an increasing number of centers are exploring TRA in TACE, hepatic arterial infusion chemotherapy, and interventional treatment for visceral aneurysms and gynecological diseases, and are gradually accumulating experience in the clinical application of TRA in peripheral interventions.9, 10, 11, 12, 13 Unlike coronary interventions, peripheral interventions involve many vessels, anatomical variations, and various interventional methods and even require repeated treatment and/or combined use of the femoral artery approach. Peripheral interventions involve a wider range of diseases, larger patient populations, and more physicians. Promoting and standardizing the techniques of TRA for peripheral interventions can benefit many patients.
Consensus
The application of TRA in peripheral interventions is limited, and this technique needs to be promoted and standardized.
2. Pros and cons of TRA in peripheral interventions
There are several advantages of using TRA for peripheral interventions. The radial artery is located more superficially than the femoral artery and is not surrounded by vital structures; therefore, the incidence of puncture-related adverse events is low. In addition, the hand has a dual blood supply from the ulnar and radial arteries, and injury to the radial artery does not usually seriously affect the blood supply to the hand. In aortic arches with complex anatomy, such as type II or III, the supra-aortic vessels can be catheterized via an ipsilateral TRA more easily and effectively than a TFA.14 For example, in hemoptysis, where the thyrocervical trunk, costocervical trunk, and internal thoracic artery are the 'culprit vessels,’ it may be easier to select these vessels via an ipsilateral transradial approach. Furthermore, TRA may facilitate selective catheterization and provide better support to the mesenteric, uterine, and prostatic arteries, where the angle to the aorta is straightforward. The incidence of hematoma and pseudoaneurysm after TRA has been shown to be lower than that after TFA in several studies15,16 (Evidence level A, Recommendation level I; see Table 1 for evaluation methods). Even if bleeding occurs at the puncture site, it can be detected early and treated easily. Furthermore, the incidence of distal limb ischemia during radial artery compression is lower than that during femoral artery compression.17 TRA is safer than TFA in patients with a high risk of bleeding, such as those with impaired coagulation, low platelet counts, or those requiring anticoagulation therapy.18 In patients who are pregnant, obese, or have iliofemoral arterial atherosclerotic disease, TRA can provide alternative access and reduce the incidence of access-related complications such as bleeding. TRA allows lower limb movement, which can reduce immobilization-related complications and facilitate subsequent treatment. For instance, periprocedural lower limb deep vein thrombosis is a concern for TFA interventions, whereas TRA could reduce the potential risk of early ambulation and rapid recovery. Compared to TFA, the application of TRA in uterine artery embolization improves patients' periprocedural quality of life, reduces procedure time and radiation exposure, and meets the requirements of positioning during gynecological surgery19,20 (Evidence level B, Recommendation level I). TRA does not hinder the positional requirements of orthopedic surgery immediately after preoperative embolization for orthopedic diseases. For emergency interventions, TRA is the preferred choice when patients are unable to cooperate with immobilization during and after the procedure. TRA allows patients to change their positions as needed when treatment-related abdominal pain, vomiting, and other discomforts occur, facilitating pain management. In addition, procedures via TRA do not expose the patients’ private zones, which significantly improves their experience. TRA allows for rapid postprocedural recovery and improves patient satisfaction13,21, 22, 23 (Evidence level A, Recommendation level I), and is suitable for interventions in day care units.
Table 1.
GRADE: Grading of Recommendations Assessment, Development and Evaluation system.
| Quality grade | Specific description |
|---|---|
| High (A) | Very sure that the true effect value is close to the estimated effect. |
| Middle (B) | There is a moderate degree of confidence in the value of effect; the real effect value may be close to the estimated effect, but there is still a possibility that the two are not the same. |
| Low (C) | There is limited confidence in the effect estimates; the true effect value may not be the same as the estimated effect. |
| Extremely low (D) | There is little confidence in the estimated effect; the true effect value may be quite different from the estimated effect. |
| Recommended strength | |
| Strong (I) | The desirable effects of an intervention clearly outweigh the undesirable effects or clearly not; consensus of more than 80% of experts. |
| Medium (II) | The desirable effects of an intervention outweigh the undesirable effects or clearly not; consensus of 60 %–80 % of experts. |
| Weak (III) | The pros and cons are uncertain, or the evidence is equal regardless of quality. |
In addition, hemostasis of the radial artery can be achieved without using a vascular closure device, which can reduce financial costs. Patients recover more quickly after TRA, with shorter hospital stays and less nursing care required, resulting in a reduced healthcare burden.24
TRA is generally suitable for peripheral interventions; however, there are some limitations that affect its clinical application. First, the diameter of the radial artery is relatively small, and the sheaths are generally recommended to be no more than 6 Fr, thus limiting their application for procedures requiring 7 Fr sheaths or above. Second, the radial artery and proximal vessels are more likely to have anatomical variations than the femoral artery, such as a high bifurcation of the radial artery requiring a sheath within the small radial artery, which might increase the risk of radial artery spasm. Anatomical anomalies include the presence of a radial loop whose reverse vascular alignment increases the risk of arterial perforation. Other factors that limit TRA application include a tortuous radial artery, subclavian artery tortuosity, and the type of aortic arch.25
Additionally, peripheral interventional devices specifically designed for TRA are lacking. Most catheters are either insufficiently long (e.g., MIK) or have a stiff tip with narrow angulation (e.g., MPA), which makes it difficult to access or be in coaxial alignment with the targeted vessel. Therefore, further development of suitable medical devices for treating TRA is required.
Consensus
-
(1)
TRA has the advantages of fewer severe local complications, reduced length of hospital stays, cost-effectiveness, and patient comfort.
-
(2)
TRA should be chosen carefully for peripheral interventions in patients with a small radial artery (particularly in females), anatomical variations or the need for a larger sheath (≥7 Fr). The decision to use TRA in these patients requires a comprehensive preprocedural assessment, and routine preparation for backup TFA is recommended.
3. Anatomical features of the radial artery
The radial and ulnar arteries, which are branches of the brachial artery, provide a dual blood supply to the hand. The radial artery descends along the lateral side of the forearm towards the wrist, where it can be palpated between the flexor carpi radialis tendon and anterior border of the radius. The distal portion of the radial artery has less variation and is a conventional puncture site. The radial artery gives rise to the palmar carpal branch to form a transverse anastomosis with the homologous branch arising from the ulnar artery and the superficial palmar branch, which passes through the thenar muscles and sometimes anastomoses with the end of the ulnar artery to complete the superficial palmar arch. The terminal branch of the radial artery and deep palmar branch of the ulnar artery form the deep palmar arch. The superficial and deep palmar arches constitute a dual blood supply to the hand. There are some anatomical variations in both the superficial and deep palmar arches, with incomplete anastomoses of the superficial and deep palmar arches observed in 18.7 % and 4.8 % of the population, respectively.26
The radial artery has several branches of communication before reaching the "anatomical snuffbox” area or the “Hegu” area on the back of the hand (distal radial artery puncture site), which can theoretically prevent hand ischemia even if the distal radial artery puncture site is occluded. The radial artery diameter is smaller in females than in males.27 In China, the average inner diameter of the left radial artery is approximately 2.44 mm in males and 2.07 mm in females, which is slightly smaller than that reported in other countries.28 The diameter ratio of the distal radial artery to the conventional radial artery in the wrist is approximately 0.8:1.0.
The Allen or Barbeau test provides a simple and rapid method to assess the circulation of the radial artery.29 In the Allen test, the subject is first asked to bend the elbow and make a tight fist for 30s. The examiner then compresses both the radial and ulnar arteries with both hands to block blood flow. The subject is then asked to open their fist, and the palm should blanch. At this point, the pressure on the ulnar artery is released. If there is sufficient collateral circulation, the hand flushes within approximately 3–12 s. If the hand does not flush within 12 s, the Allen test is considered positive. Patients with positive Allen test results are generally unsuitable for radial artery access. However, for patients with a positive Allen test result who still require radial artery puncture, Doppler ultrasonography should be used to perform a complete assessment of the hand vasculature to avoid serious complications associated with radial artery occlusion. In the Barbeau test, a pulse oximeter is placed on the thumb to measure plethysmography and oxygen saturation. Once the waveform is stable, continuous pressure is applied to the radial artery, and the oxygen saturation waveform is observed. The classification is as follows: Type A: The waveform remains unchanged within 2 min of radial artery compression and after release; Type B: The amplitude of the wave initially decreases but fully recovers after 2 min; Type C: During radial artery compression, the waveform disappeared and oxygen saturation could not be detected, but it partially recovers after 2 min of compression and oxygen saturation can be detected; Type D: The waveform remains absent and oxygen saturation cannot be detected even after 2 min of radial artery compression. TRA is not suitable for patients with Type D waveforms.
In addition, some anatomical variations, including high radial artery bifurcation or arterial loops, should be assessed during the procedure.30 The choice of catheter and left or right TRA can be made based on the aortic arch classification and variation in the origin of the subclavian artery. The left radial artery access has a shorter distance to the visceral vessels and does not require passing through the brachiocephalic trunk compared to the right radial artery access.
Consensus
-
(1)
The circulation, diameter, and anatomical variations of the radial artery should be carefully assessed before a TRA procedure.
-
(2)
The Allen and Barbeau tests are simple and practical for assessing radial artery circulation, and ultrasonography is recommended for radial artery anatomy and hemodynamic assessment in centers with available resources.
4. Patient selection
TRA is generally preferred over TFA in patients with coagulopathy, obesity, difficulty with immobilization, femoral tortuosity, and supra-arch disease. However, it is not recommended in patients with a positive Allen test or Barbeau test type D. The proximal radial artery should not be used in patients requiring coronary bypass surgery or in preparation for ipsilateral hemodialysis with an arteriovenous (AV) fistula. However, for patients with malfunctional AV fistulas, interventions can be completed via a TRA.
For patients who require continuous transarterial infusion, TRA can significantly improve patient comfort and reduce complications caused by prolonged immobilization compared to TFA. Considering that the radial artery is small, multiple punctures or a prolonged indwelling catheter may increase the risk of radial artery occlusion (RAO).13 For these cases, short-term anticoagulation therapy is recommended.
In addition to the feasibility of TRA, patient preferences should be respected, and patients should be fully informed about the benefits and risks of the procedure. Studies have shown that patients undergoing TACE or genitourinary interventions preferred TRA to TFA19,21,22 (Evidence level A; Recommendation level I). Moreover, TRA and TFA can be interchangeable according to specific conditions, and their combined use can be considered for patients requiring multiple-vessel catheterizations. Alternative approaches should be used if radial artery spasms impede subsequent procedures.
Consensus
-
(1)
TRA is suitable for most peripheral percutaneous interventions.
-
(2)
TRA is particularly suitable for patients with coagulopathy, obesity, immobilization difficulty, or iliofemoral artery occlusion.
-
(3)
TRA and TFA can be applied interchangeably or in combination during a procedure.
5. TRA technique recommendations
5.1. Preparing for puncture
Patients should be fully informed about the benefits and risks of TRA. Preprocedural education and counseling are helpful in relaxing patients, thus reducing the incidence of arterial spasms. The positions of the patient, operator, and digital subtraction angiography DSA machine can be chosen to facilitate subsequent procedures according to the operator's habits, specific procedures to be performed, and patient compliance.
Puncture site: The ideal puncture site is usually 2–3 cm proximal to the radial styloid, where the radial artery is straight with clear pulsation and a superficial position. The distal radial artery approach is to puncture the radial artery in the “snuff box” or the “Hegu” area.
5.2. Radial artery puncture and vascular protection
The choice of a steel or two-part needle depends on the operator's preference. Steel needle: It should be inserted at an angle of 30–45°, in alignment with the vessel. Introduce the guidewire gently if a pulsatile spurt of blood is observed (modified Seldinger method). Two-part needle: After puncturing through the arterial walls, the needle core is withdrawn, the cannula is slowly withdrawn backward, and the guidewire is advanced when blood spurts. Ultrasound-guided puncture can reduce the number of puncture attempts and puncture time, and increase the success rate of the first puncture31, 32, 33 (Evidence level B, Recommendation level I). Ultrasound guidance can be provided using a combination of in-plane (longitudinal) and out-of-plane (transverse) approaches. Furthermore, the selected artery can be easily differentiated from the vein by appropriate compression of the vessels or color Doppler, and ultrasonography can verify that the guidewire is within the radial artery.
Use of antispasmodic agents: Immediately after sheath insertion, a “cocktail” of vasodilators and anticoagulants is administered via the sheath to reduce the incidence of vasospasms and radial artery occlusion. The “cocktail” protocol typically includes 100–200 μg of nitroglycerin, 20 mg of lidocaine (or 2.5 mg of verapamil), and 2000–5000 IU of unfractionated heparin. In clinical practice, the use of heparin or nitroglycerin can be reduced or eliminated in patients with coagulopathy or hypotension, respectively. For patients who require prolonged catheter indwelling, it is recommended that the “cocktail” be re-administered just before removal of the sheath.
5.3. Devices selection and catheterization
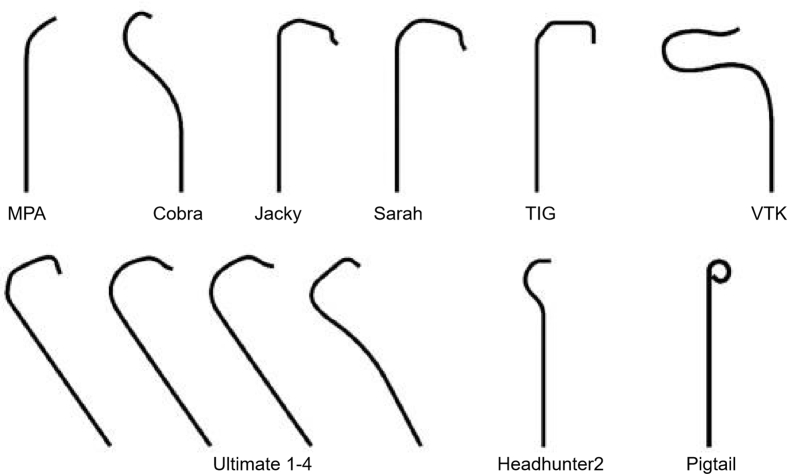
A puncture needle with a smaller caliber (e.g., 20G, 21G), guidewire, or introducer sheath can reduce the incidence of complications. Thin-walled sheaths allow the passage of larger-diameter catheters with smaller profiles. The hydrophilic coating of the sheath reduces the incidence of radial artery spasms.45 The catheter tip shape should be selected based on the type of aortic arch and anatomy of the target vessel (Fig. 1). For subdiaphragmatic vessels, the catheter should be ≥ 110 cm in length and stiff enough for satisfactory torque control; the microcatheter should be ≥ 135 cm in length. For patients requiring a sheath of ≥7 Fr, the radial artery diameter should be assessed using ultrasound before the procedure.34 For beginners, fluoroscopic monitoring of catheter advancement from the radial artery to the aorta is recommended to avoid vascular and cardiac injuries or even perforation.
Fig. 1.
Different catheter types in percutaneous peripheral intervention.
TRA catheterization of specific vessels differs from the femoral approach. For example, celiac arterial catheterization is achieved by rotating an RH catheter “anticlockwise” during TFA, whereas the catheter needs to be rotated “clockwise” during TRA.
5.4. Radial artery hemostasis
The patent hemostasis technique can reduce the incidence of postprocedural radial artery occlusion. Patent hemostasis is defined as the maintenance of antegrade flow in the radial artery throughout compression hemostasis. However, there is no standardized protocol for this technique. A specially designed radial artery hemostasis band can be used to achieve patent hemostasis and reduce the incidence of radial artery occlusion.35,36 The duration of compression is determined by the type of procedure, size of the sheath used, and patient coagulation status. It is recommended that the compression time should be as short as possible to reduce the incidence of radial artery occlusion. The use of an inflatable balloon-type radial artery compression device is described step-by-step as follows: first, inflate the balloon after the sheath is removed, deflate until oozing of blood from the puncture site is seen, re-inflate the balloon with 1–2 mL of air until complete hemostasis is achieved, and observe the pulse oxygen by the reverse Barbeau test to assess the blood supply to the hand.
5.5. Distal radial artery
Distal TRA (dTRA) has numerous advantages over conventional TRA. These advantages are that dTRA is relatively superficial with a shorter compression time and has fewer complications and greater patient comfort37 (Evidence level B, Recommendation level 2). However, the distal radial artery is smaller and takes a longer time to puncture than in a conventional TRA. Ultrasonography can facilitate distal radial arterial punctures. With a standardized hemostasis protocol, there is no significant difference in complications between dTRA and conventional TRA; however, the compression time is shorter for dTRA.38
5.6. Periprocedural nursing
Periprocedural nursing plays an important role, especially in preprocedural education and psychological counseling, postprocedural observation of puncture site bleeding and forearm hematoma, and the early detection of complications. In some centers, nurses are involved in the hemostasis process and observation of finger pulse oximetry.
6. Prevention and management of common complications related to TRA
Data on the complications of TRA during peripheral interventions are limited. Therefore, the prevention and treatment of complications are primarily based on relevant experiences with TRA coronary intervention. Current evidence shows that severe complications associated with the access site are less frequent with TRA than those with TFA39, 40, 41 (Evidence level A; Recommendation level I). However, multiple TRAs or prolonged indwelling catheters may increase the risk of RAO.10,13 The following sections describe TRA-related complications.
6.1. Prevention and management of RAO
RAO is the most common postprocedural complication of TRA,35 with an incidence ranging from less than 1 %–33 %.13,35 Most patients with RAO are asymptomatic; however, hand ischemia with pain, numbness, and limited movement might occur. Furthermore, in patients who require repeated peripheral interventions, RAO can affect subsequent access. Doppler ultrasound provides an objective assessment of the radial artery and is the standard technique for identifying the RAO.
Strategies to prevent RAO include: (i) Selection of the sheath and relevant medical devices of an appropriate size. The ratio of the outer diameter of the sheath to the internal diameter of the radial artery should be less than 1. A sheath with a smaller size or a thin-walled sheath should be used to reduce potential damage to the radial artery.42,43 (ii) Adequate anticoagulation: 2000–5000 IU of unfractionated heparin is recommended. For patients at a high risk of RAO (e.g., multiple punctures, prolonged indwelling catheters [>12 h], and small vessels [<1.6 mm]), short-term postprocedural anticoagulation (e.g., rivaroxaban tablets 10 mg/day for 7 days) may further reduce the incidence of RAO44 (Evidence level B, Recommendation level II). (iii) Use of patent hemostasis technique: The rate of RAO decreased from 12.0 % to 5.0 % in the early phase (<24 h) and from 7.0 % to 1.8 % in the late phase (30 days) when patent hemostasis was applied45 (Evidence level A, Recommendation level I). (iv) Reducing compression time: Prolonged compression is a major factor in RAO and minimizing the duration of compression is important for prevention. It is generally recommended that the compression time should not exceed 120 min or, in some cases, it should be less than 90 min with the use of a compression device46, 47, 48 (Evidence level A, Recommendation level I). (v) Use of vasodilators: Routine administration of antispasmodic agents after successful puncture (see 5.2 Radial artery puncture and vascular protection) may reduce the incidence of RAO39 (Evidence level B, Recommendation level I).
Anticoagulation therapy is administered for RAO that is detected early by subcutaneous injection of low-molecular-weight heparin for 1–4 weeks, oral rivaroxaban44,49 (Evidence level C, Recommendation level II), or by injection of a higher dose (5000 IU) of unfractionated heparin with compression of the ulnar artery for 1 h.50 (Evidence level C, Recommendation level II). Invasive treatment for radial recanalization, either in an antegrade or retrograde fashion, is used for symptomatic patients.51,52
6.2. Prevention and management of radial artery spasm
Radial artery spasm is a major cause of RAO and occurs more frequently in females, patients with small radial diameters or under psychological stress, and cases of improper catheter manipulation. For interventions that may require prolonged procedure time (>90 min), or that require frequent catheter exchanges and adjustments, it is recommended to inject the “cocktail” through the sheath into the artery intermittently.22,53 In cases of radial artery spasm, an appropriate dose of the “cocktail” or 200 μg of nitroglycerin should be injected immediately through the sheath and then wait. If the spasm is not resolved, alternative access is recommended.
6.3. Management of forearm bruising, hematoma, and compartment syndrome
Over-anticoagulation or bleeding can lead to bruising or hematoma in the forearm after TRA; in most cases, it resolves spontaneously within a week to a few weeks. Patients should be informed about the potential for the formation of pseudoaneurysms or compartment syndrome.54 Compartment syndrome is a rare but serious complication that can occur acutely or develop slowly over a few days, with an incidence of approximately 0.004 %. The main symptoms include paresthesia of the median, radial, or ulnar nerve distribution areas and/or pain associated with passive movement of the fingers. If any of these symptoms occur, compartmental pressure monitoring and emergency surgical decompression should be performed to avoid amputation.55
6.4. Management of pseudoaneurysms
Pseudoaneurysms of the radial artery occur in approximately 0.09 % of cases and may present as swelling near the puncture site, which may be pulsatile and/or locally erythematous and can be diagnosed by ultrasound. Ultrasound-guided local compression can be used for treatment.56 If this fails, ultrasound-guided percutaneous injection of 300–8000 U thrombin can be performed.57 Most radial artery pseudoaneurysms can be treated nonsurgically, and surgical treatment can be the last resort.55
6.5. Risk and prevention of stroke
There is a potential risk of stroke with TRA procedures due to thrombus or atherosclerotic plaque dislodgement into the intracranial vessels when navigating the wires and catheters along the left subclavian artery or right cephalic trunk. However, there is insufficient evidence to suggest that the incidence of stroke is higher with TRA than that with TFA.58 However, caution is generally advised when performing TRA in patients older than 70 years with a history of stroke and/or atherosclerosis.53 Patients with a prolonged indwelling catheter should be removed immediately after infusion to avoid thrombus dislodgement from the catheter tip.
Consensus
-
(1)
TRA showed fewer serious access-related bleeding complications than TFA. The use of small-size/thin-walled introducer sheaths, adequate anticoagulation, and patent hemostasis with a shorter compression time can reduce the incidence of complications.
-
(2)
Most RAOs are asymptomatic, and early detection and management are beneficial for the reuse of the same radial artery. Clinicians and patients should be alert to rare but severe complications such as compartment syndrome.
7. Radiation exposure from TRA
Radiation exposure to the operator and patient is a major concern when adopting a TRA.8 However, in the clinical setting, the principle of “as low as reasonably achievable” (ALARA) and proper shielding are still the key to minimizing radiation exposure for both the patients and doctors. Indeed, radiation exposure largely depends on superselective catheterization and the number of angiographies rather than the access site.
Previous studies have concluded that there are no significant differences between TRA and TFA in terms of fluoroscopy time, radiation dose, or actual radiation exposure to patients and operators (especially in high-volume centers with over 142 cases per year)59 (Evidence level A, Recommendation level I). In a systematic review and meta-analysis of nine cohort studies on liver cancer interventions, 1096 procedures were performed in 877 patients, of which 545 (49.7 %) were performed using TRA and 551 (50.3 %) using TFA. Again, there were no significant differences between the two groups in terms of fluoroscopy time or radiation dose.60 However, a study that focused on radiation exposure at different access sites for TACE demonstrated that doctors in the TRA group showed lower radiation exposure than those in the TFA group, despite no difference in radiation exposure among the patients.61 Similarly, another study showed lower radiation exposure among doctors in the TRA group during chemoembolization.22 The fluoroscopy time and dose-area product (DAP) were not significantly different between TRA and TFA for 90Y therapy for hepatocellular carcinoma (HCC).21 The Department of Interventional Radiology at the University of California, Los Angeles Medical Center investigated the radiation doses of TRA and TFA in abdominal and peripheral interventions at the center and showed that TRA had a longer fluoroscopy time (P = 0.033), but there was no significant difference in DAP (P = 0.186).62 Compared with the right radial access, the fluoroscopy time of visceral catheterization is shorter when using the left radial access. In addition, TACE via the left radial artery with the arm in an abducted position is effective in reducing the radiation dose to the operator and the risk of radiation exposure.63
For pelvic interventional therapy, the fluoroscopy time of uterine arterial embolization for fibroids was comparable between TFA and TRA, with a mean of 20.36 min (±9.48) in TFA and 12 min (±7.67) in TRA (P = 0.86).64 However, another randomized study of uterine arterial embolization reported reduced radiation exposure in the TRA group.64
The skill and proficiency of the operator is another factor that contributes to the reduction of radiation exposure. For seasoned operators, the radiation exposure during TRA and TFA procedures was similar. Therefore, the continuous use of TRA is recommended for doctors who perform interventional therapy.
Consensus
-
1)
Proper radiation protection and adherence to the ALARA principle are the key to minimizing radiation exposure for both patients and operators.
-
2)
Increasing the volume of TRA can reduce radiation exposure for both patients and operators.
-
3)
Current evidence suggests that there is no difference in radiation exposure between TRA and TFA.
8. Learning curve for TRA in peripheral interventional therapy
A prolonged learning curve is another factor that hinders the conversion of TFA to TRA. Interventional cardiologists can achieve the same outcomes as experienced physicians after 50 or more coronary intervention cases with TRA (Evidence level B, Recommendation level I).65,66 For beginners, the procedure time, fluoroscopy time, and access crossover rate were not significantly different between TRA and TFA learning. A recent study reported shorter procedure times and less radiation exposure in the TRA group after an operator performed >30 procedures.67 Furthermore, the radiation exposure and volume of contrast agent used were similar to those in the TFA group after an experienced TFA doctor attended two days of TRA training and performed a TRA 20 times (Evidence level C, Recommendation level II).68 The learning curve for the left TRA was shorter than that for the right TRA when performing interventional therapy below the aortic arch.69 In addition, the learning curve for radial puncture can be reduced using ultrasound guidance and practice on puncture training models.70
Consensus
-
1)
Experienced interventional radiologists could master the TRA technique quickly after specialized training.
-
2)
For beginners in peripheral intervention, it is recommended that they learn both the TRA and TFA simultaneously.
9. Future perspectives
Peripheral TRA interventions have the advantages of improving patient comfort and reducing serious complications, such as local bleeding. However, Peripheral interventions involve a broad spectrum of diseases, various target vessel locations and morphologies, and diverse medical devices. Comprehensive preprocedural assessment, proper selection of the radial artery access, standardized procedural practice, selection of appropriate medical devices, and patent hemostasis techniques are required to reduce the incidence of complications. In the future, the development of TRA-specific devices, standardized hemostasis protocols, and protection of the radial artery in patients with prolonged indwelling catheters is warranted. The learning curve and radiation exposure of TRA are similar to those of TFA. Promoting TRA use in peripheral interventional therapy by enhancing physician education and training, standardizing technical aspects, and improving operator skill proficiency will benefit more patients.
Consultant: Gaojun Teng (Zhongda Hospital Southeast University) gjteng@seu.edu.cn
Experts panel: Xiaoyi Ding (Ruijin Hospital, Shanghai Jiaotong University School of Medicine), Changlu Yu (Tianjin Third Central Hospital), Haipeng Yu (Tianjin Medical University Cancer Institute & Hospital), Jian Wang (Peking University First Hospital), Song Wang (The Affiliated Hospital of Qingdao University), Yanli Wang (The First Affiliated Hospital of Zhengzhou University), Haidong Zhu (Zhongda Hospital Southeast University), Xu Zhu (Beijing Cancer Hospital), Yimin Ren (The First Affiliated Hospital of Guangzhou Medical University), Ruibao Liu (Harbin Medical University Cancer Hospital), Xi Liu (The Second Affiliated Hospital of Chongqing Medical University), Sen Jiang (Shanghai Pulmonary Hospital), Zhichao Sun (The First Affiliated Hospital of Clinical Medicine of Zhejiang Chinese Medical University), Jiarui Li (The First Hospital of Jilin University), Xiao Li (Cancer Hospital Chinese Academic of Medical Sciences), Weizhu Yang (Fujian Medical University Union Hospital), Minjie Yang (Zhongshan Hospital, Fudan University), Xu He (Nanjing First Hospital), Yusheng Song (Ganzhou People's Hospital), Wen Zhang (Zhongshan Hospital, Fudan University), Guoliang Shao (Zhejiang Cancer Hospital), Haibo Shao (The First Hospital of China Medical University), Xiaoxi Meng (Shanghai Changzheng Hospital), Xiong Bin (The First Affiliated Hospital of Guangzhou Medical University), Hongjie Hu (Sir Run Run Shaw Hospital), Feng Duan (Chinese PLA General Hospital), Caifang Ni (The First Affiliated Hospital of Soochow University), Liwen Guo (Zhejiang Cancer Hospital), Mingsheng Huang (The Third Affiliated Hospital, Sun Yat-sen University), Yonghui Huang (The First Affiliated Hospital, Sun Yat-sen University), Weihua Dong (Shanghai Changzheng Hospital), Zhiping Yan (Zhongshan Hospital, Fudan University).
Secretary-general: Wen Zhang (Zhongshan Hospital, Fudan University).
Writing: Minjie Yang (Zhongshan Hospital, Fudan University), Sen Jiang (Shanghai Pulmonary Hospital), Yanli Wang (The First Affiliated Hospital of Zhengzhou University), Xiaoxi Meng (Shanghai Changzheng Hospital), Liwen Guo (Zhejiang Cancer Hospital), Wen Zhang (Zhongshan Hospital, Fudan University), Xin Zhou (Zhongshan Hospital, Fudan University).
Declaration of competing interest
Zhiping Yan is the associate editors-in-chief for Journal of Interventional Medicine and was not involved in the editorial review or the decision to publish this article. All authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Zhiping Yan, Email: yan.zhiping@zs-hospital.sh.cn.
Jiarui Li, Email: jiaruili1971@sina.com.
Weihua Dong, Email: dongweihua@smmu.edu.cn.
References
- 1.Seldinger S.I. Catheter replacement of the needle in percutaneous arteriography; a new technique. Acta radiol. 1953;39:368–376. doi: 10.3109/00016925309136722. [DOI] [PubMed] [Google Scholar]
- 2.Campeau L. Percutaneous radial artery approach for coronary angiography. Cathet Cardiovasc Diagn. 1989;16:3–7. doi: 10.1002/ccd.1810160103. [DOI] [PubMed] [Google Scholar]
- 3.Kiemeneij F., Laarman G.J. Percutaneous transradial artery approach for coronary stent implantation. Cathet Cardiovasc Diagn. 1993;30:173–178. doi: 10.1002/ccd.1810300220. [DOI] [PubMed] [Google Scholar]
- 4.Valgimigli M., Frigoli E., Leonardi S., et al. Radial versus femoral access and bivalirudin versus unfractionated heparin in invasively managed patients with acute coronary syndrome (MATRIX): final 1-year results of a multicentre, randomised controlled trial. Lancet. 2018;392:835–848. doi: 10.1016/S0140-6736(18)31714-8. [DOI] [PubMed] [Google Scholar]
- 5.Jolly S.S., Yusuf S., Cairns J., et al. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet. 2011;377:1409–1420. doi: 10.1016/S0140-6736(11)60404-2. [DOI] [PubMed] [Google Scholar]
- 6.Shoji S., Kohsaka S., Kumamaru H., et al. Cost reduction associated with transradial access in percutaneous coronary intervention: a report from a Japanese nationwide registry. Lancet Reg Health West Pac. 2022;28 doi: 10.1016/j.lanwpc.2022.100555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shiozawa S., Tsuchiya A., Endo S., et al. Transradial approach for transcatheter arterial chemoembolization in patients with hepatocellular carcinoma: comparison with conventional transfemoral approach. J Clin Gastroenterol. 2003;37:412–417. doi: 10.1097/00004836-200311000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Iezzi R., Posa A., Bilhim T., et al. Most common misconceptions about transradial approach in interventional radiology: results from an international survey. Diagn Interv Radiol. 2021;27:649–653. doi: 10.5152/dir.2021.20256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du N., Yang M.J., Ma J.Q., et al. Transradial access chemoembolization for hepatocellular carcinoma in comparation with transfemoral access. Transl Cancer Res. 2019;8:1795–1805. doi: 10.21037/tcr.2019.08.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang X., Luo Y., Tsauo J., et al. Transradial versus transfemoral access without closure device for transarterial chemoembolization in patients with hepatocellular carcinoma: a randomized trial. Eur Radiol. 2022;32:6812–6819. doi: 10.1007/s00330-022-09038-1. [DOI] [PubMed] [Google Scholar]
- 11.Jiang H., Chen Y., Liao H., et al. Operator radiation dose during trans-hepatic arterial chemoembolization: different patients' positions via transradial or transfemoral access. Diagn Interv Radiol. 2022;28:376–382. doi: 10.5152/dir.2022.211327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu T., Sun R., Huang Y., et al. Partial splenic embolization of patients with hypersplenism by transradial or transfemoral approach: a prospective randomized controlled trial. Acta Radiol. 2016;57:1201–1204. doi: 10.1177/0284185115622076. [DOI] [PubMed] [Google Scholar]
- 13.Wan Y., Chen B., Li N., et al. Transradial versus transfemoral access for patients with liver cancer undergoing hepatic arterial infusion chemotherapy: patient experience and procedural complications. J Vasc Intervent Radiol. 2022;33:956–963.e1. doi: 10.1016/j.jvir.2022.04.024. [DOI] [PubMed] [Google Scholar]
- 14.Burzotta F., Nerla R., Pirozzolo G., et al. Clinical and procedural impact of aortic arch anatomic variants in carotid stenting procedures. Cathet Cardiovasc Interv. 2015;86:480–489. doi: 10.1002/ccd.25947. [DOI] [PubMed] [Google Scholar]
- 15.Brueck M., Bandorski D., Kramer W., et al. A randomized comparison of transradial versus transfemoral approach for coronary angiography and angioplasty. JACC Cardiovasc Interv. 2009;2:1047–1054. doi: 10.1016/j.jcin.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 16.Bhat F.A., Changal K.H., Raina H., et al. Transradial versus transfemoral approach for coronary angiography and angioplasty - a prospective, randomized comparison. BMC Cardiovasc Disord. 2017;17:23. doi: 10.1186/s12872-016-0457-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adnan S.M., Romagnonli A.N., Elansary N.N., et al. Radial versus femoral arterial access for trauma endovascular interventions: a noninferiority study. J Trauma Acute Care Surg. 2020;89:458–463. doi: 10.1097/TA.0000000000002740. [DOI] [PubMed] [Google Scholar]
- 18.Titano J.J., Biederman D.M., Zech J., et al. Safety and outcomes of transradial access in patients with international normalized ratio 1.5 or above. J Vasc Intervent Radiol. 2018;29:383–388. doi: 10.1016/j.jvir.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Basile A., Rebonato A., Failla G., et al. Early post-procedural patients compliance and VAS after UAE through transradial versus transfemoral approach: preliminary results. Radiol Med. 2018;123:885–889. doi: 10.1007/s11547-018-0920-5. [DOI] [PubMed] [Google Scholar]
- 20.Nakhaei M., Mojtahedi A., Faintuch S., et al. Transradial and transfemoral uterine fibroid embolization comparative study: technical and clinical outcomes. J Vasc Intervent Radiol. 2020;31:123–129. doi: 10.1016/j.jvir.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 21.Liu L.B., Cedillo M.A., Bishay V., et al. Patient experience and preference in transradial versus transfemoral access during transarterial radioembolization: a randomized single-center trial. J Vasc Intervent Radiol. 2019;30:414–420. doi: 10.1016/j.jvir.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Yamada R., Bracewell S., Bassaco B., et al. Transradial versus transfemoral arterial access in liver cancer embolization: randomized trial to assess patient satisfaction. J Vasc Intervent Radiol. 2018;29:38–43. doi: 10.1016/j.jvir.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 23.Iezzi R., Pompili M., Posa A., et al. Transradial versus transfemoral access for hepatic chemoembolization: intrapatient prospective single-center study. J Vasc Intervent Radiol. 2017;28:1234–1239. doi: 10.1016/j.jvir.2017.06.022. [DOI] [PubMed] [Google Scholar]
- 24.Cooper C.J., El-Shiekh R.A., Cohen D.J., et al. Effect of transradial access on quality of life and cost of cardiac catheterization: a randomized comparison. Am Heart J. 1999;138:430–436. doi: 10.1016/s0002-8703(99)70143-2. [DOI] [PubMed] [Google Scholar]
- 25.Brunet M.C., Chen S.H., Peterson E.C. Transradial access for neurointerventions: management of access challenges and complications. J Neurointerventional Surg. 2020;12:82–86. doi: 10.1136/neurintsurg-2019-015145. [DOI] [PubMed] [Google Scholar]
- 26.Zarzecki M.P., Popieluszko P., Zayachkowski A., et al. The surgical anatomy of the superficial and deep palmar arches: a meta-analysis. J Plast Reconstr Aesthetic Surg. 2018;71:1577–1592. doi: 10.1016/j.bjps.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 27.Wahood W., Ghozy S., Al-Abdulghani A., et al. Radial artery diameter: a comprehensive systematic review of anatomy. J Neurointerventional Surg. 2022;14:1274–1278. doi: 10.1136/neurintsurg-2021-018534. [DOI] [PubMed] [Google Scholar]
- 28.Yan Z.X., Zhou Y.J., Zhao Y.X., et al. Anatomical study of forearm arteries with ultrasound for percutaneous coronary procedures. Circ J. 2010;74:686–692. doi: 10.1253/circj.cj-09-0577. [DOI] [PubMed] [Google Scholar]
- 29.Barbeau G.R., Arsenault F., Dugas L., et al. Evaluation of the ulnopalmar arterial arches with pulse oximetry and plethysmography: comparison with the Allen's test in 1010 patients. Am Heart J. 2004;147:489–493. doi: 10.1016/j.ahj.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 30.Vallespin J., Meola M., Ibeas J. Upper limb anatomy and preoperative mapping. J Vasc Access. 2021;22:9–17. doi: 10.1177/11297298211046827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen P., Makris A., Hennessy A., et al. Standard versus ultrasound-guided radial and femoral access in coronary angiography and intervention (SURF): a randomised controlled trial. EuroIntervention. 2019;15:e522–e530. doi: 10.4244/EIJ-D-19-00336. [DOI] [PubMed] [Google Scholar]
- 32.Flumignan R.L., Trevisani V.F., Lopes R.D., et al. Ultrasound guidance for arterial (other than femoral) catheterisation in adults. Cochrane Database Syst Rev. 2021;10(10) doi: 10.1002/14651858.CD013585.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seto A.H., Roberts J.S., Abu-Fadel M.S., et al. Real-time ultrasound guidance facilitates transradial access: RAUST (Radial Artery access with Ultrasound Trial) JACC Cardiovasc Interv. 2015;8:283–291. doi: 10.1016/j.jcin.2014.05.036. [DOI] [PubMed] [Google Scholar]
- 34.Sandoval Y., Bell M.R., Gulati R. Transradial artery access complications. Circ Cardiovasc Interv. 2019;12 doi: 10.1161/CIRCINTERVENTIONS.119.007386. [DOI] [PubMed] [Google Scholar]
- 35.Bernat I., Aminian A., Pancholy S., et al. Best practices for the prevention of radial artery occlusion after transradial diagnostic angiography and intervention: an international consensus paper. JACC Cardiovasc Interv. 2019;12:2235–2246. doi: 10.1016/j.jcin.2019.07.043. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y., Tang J., Ni J., et al. A comparative study of TR Band and a new hemostatic compression device after transradial coronary catheterization. J Interv Med. 2019;1:221–228. doi: 10.19779/j.cnki.2096-3602.2018.04.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corcos T. Distal radial access for coronary angiography and percutaneous coronary intervention: a state-of-the-art review. Cathet Cardiovasc Interv. 2019;93:639–644. doi: 10.1002/ccd.28016. [DOI] [PubMed] [Google Scholar]
- 38.Aminian A., Sgueglia G.A., Wiemer M., et al. Distal versus conventional radial access for coronary angiography and intervention: the DISCO RADIAL trial. JACC Cardiovasc Interv. 2022;15:1191–1201. doi: 10.1016/j.jcin.2022.04.032. [DOI] [PubMed] [Google Scholar]
- 39.Karrowni W., Vyas A., Giacomino B., et al. Radial versus femoral access for primary percutaneous interventions in ST-segment elevation myocardial infarction patients: a meta-analysis of randomized controlled trials. JACC Cardiovasc Interv. 2013;6:814–823. doi: 10.1016/j.jcin.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 40.Mehta S.R., Jolly S.S., Cairns J., et al. Effects of radial versus femoral artery access in patients with acute coronary syndromes with or without ST-segment elevation. J Am Coll Cardiol. 2012;60:2490–2499. doi: 10.1016/j.jacc.2012.07.050. [DOI] [PubMed] [Google Scholar]
- 41.Romagnoli E., Biondi-Zoccai G., Sciahbasi A., et al. Radial versus femoral randomized investigation in ST-segment elevation acute coronary syndrome: the RIFLE-STEACS (Radial versus Femoral Randomized Investigation in ST-Elevation Acute Coronary Syndrome) study. J Am Coll Cardiol. 2012;60:2481–2489. doi: 10.1016/j.jacc.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 42.Aminian A., Dolatabadi D., Lefebvre P., et al. Initial experience with the Glidesheath Slender for transradial coronary angiography and intervention: a feasibility study with prospective radial ultrasound follow-up. Cathet Cardiovasc Interv. 2014;84:436–442. doi: 10.1002/ccd.25232. [DOI] [PubMed] [Google Scholar]
- 43.Saito S., Ikei H., Hosokawa G., et al. Influence of the ratio between radial artery inner diameter and sheath outer diameter on radial artery flow after transradial coronary intervention. Cathet Cardiovasc Interv. 1999;46:173–178. doi: 10.1002/(SICI)1522-726X(199902)46:2<173::AID-CCD12>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 44.Liang D., Lin Q., Zhu Q., et al. Short-term postoperative use of rivaroxaban to prevent radial artery occlusion after transradial coronary procedure: the RESTORE randomized trial. Circ Cardiovasc Interv. 2022;15 doi: 10.1161/CIRCINTERVENTIONS.121.011555. [DOI] [PubMed] [Google Scholar]
- 45.Pancholy S., Coppola J., Patel T., et al. Prevention of radial artery occlusion-patent hemostasis evaluation trial (PROPHET study): a randomized comparison of traditional versus patency documented hemostasis after transradial catheterization. Cathet Cardiovasc Interv. 2008;72:335–340. doi: 10.1002/ccd.21639. [DOI] [PubMed] [Google Scholar]
- 46.Dangoisse V., Guédès A., Chenu P., et al. Usefulness of a gentle and short hemostasis using the transradial band device after transradial access for percutaneous coronary angiography and interventions to reduce the radial artery occlusion rate (from the prospective and randomized CRASOC I, II, and III studies) Am J Cardiol. 2017;120:374–379. doi: 10.1016/j.amjcard.2017.04.037. [DOI] [PubMed] [Google Scholar]
- 47.Edris A., Gordin J., Sallam T., et al. Facilitated patent haemostasis after transradial catheterisation to reduce radial artery occlusion. EuroIntervention. 2015;11:765–771. doi: 10.4244/EIJV11I7A153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pancholy S.B., Heck L.A., Patel T. Forearm arterial anatomy and flow characteristics: a prospective observational study. J Invasive Cardiol. 2015;27:218–221. [PubMed] [Google Scholar]
- 49.Zankl A.R., Andrassy M., Volz C., et al. Radial artery thrombosis following transradial coronary angiography: incidence and rationale for treatment of symptomatic patients with low-molecular-weight heparins. Clin Res Cardiol. 2010;99:841–847. doi: 10.1007/s00392-010-0197-8. [DOI] [PubMed] [Google Scholar]
- 50.Bernat I., Bertrand O.F., Rokyta R., et al. Efficacy and safety of transient ulnar artery compression to recanalize acute radial artery occlusion after transradial catheterization. Am J Cardiol. 2011;107:1698–1701. doi: 10.1016/j.amjcard.2011.01.056. [DOI] [PubMed] [Google Scholar]
- 51.Pancholy S.B. Transradial access in an occluded radial artery: new technique. J Invasive Cardiol. 2007;19:541–544. [PubMed] [Google Scholar]
- 52.Pancholy S. Recanalization and reuse of early occluded radial artery within 6 days after previous transradial diagnostic procedure. Cathet Cardiovasc Interv. 2012;79:348. doi: 10.1002/ccd.23146. ; author reply 349. [DOI] [PubMed] [Google Scholar]
- 53.Gayed A., Yamada R., Bhatia S., et al. Society of interventional radiology quality improvement standards on radial artery access. J Vasc Intervent Radiol. 2021;32:761. doi: 10.1016/j.jvir.2020.12.013. e1-761.e21. Erratum in: J Vasc Interv Radiol. 2021;32:1100. [DOI] [PubMed] [Google Scholar]
- 54.Tizón-Marcos H., Barbeau G.R. Incidence of compartment syndrome of the arm in a large series of transradial approach for coronary procedures. J Intervent Cardiol. 2008;21:380–384. doi: 10.1111/j.1540-8183.2008.00361.x. [DOI] [PubMed] [Google Scholar]
- 55.Hadad M.J., Puvanesarajah V., Deune E.G. Complications of transradial catheterization and cannulation. J Hand Surg Am. 2019;44:973–979. doi: 10.1016/j.jhsa.2019.06.018. [DOI] [PubMed] [Google Scholar]
- 56.Zegrí I., García-Touchard A., Cuenca S., et al. Radial artery pseudoaneurysm following cardiac catheterization: clinical features and nonsurgical treatment results. Rev Esp Cardiol. 2015;68:349–351. doi: 10.1016/j.rec.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 57.Garvin R.P., Ryer E.J., Yoon H.R., et al. Ultrasound-guided percutaneous thrombin injection of iatrogenic upper extremity pseudoaneurysms. J Vasc Surg. 2014;59:1664–1669. doi: 10.1016/j.jvs.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 58.Agostoni P., Biondi-Zoccai G.G., de Benedictis M.L., et al. Radial versus femoral approach for percutaneous coronary diagnostic and interventional procedures; Systematic overview and meta-analysis of randomized trials. J Am Coll Cardiol. 2004;44:349–356. doi: 10.1016/j.jacc.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 59.Jolly S.S., Cairns J., Niemela K., et al. Effect of radial versus femoral access on radiation dose and the importance of procedural volume: a substudy of the multicenter randomized RIVAL trial. JACC Cardiovasc Interv. 2013;6:258–266. doi: 10.1016/j.jcin.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 60.Chen Y.Y., Liu P., Wu Y.S., et al. Transradial vs transfemoral access in patients with hepatic malignancy and undergoing hepatic interventions: a systematic review and meta-analysis. Medicine (Baltimore) 2018;97 doi: 10.1097/MD.0000000000013926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Malenstein H., Maleux G., Vandecaveye V., et al. A randomized phase II study of drug-eluting beads versus transarterial chemoembolization for unresectable hepatocellular carcinoma. Onkologie. 2011;34:368–376. doi: 10.1159/000329602. [DOI] [PubMed] [Google Scholar]
- 62.Hung M.L., Lee E.W., McWilliams J.P., et al. A reality check in transradial access: a single-centre comparison of transradial and transfemoral access for abdominal and peripheral intervention. Eur Radiol. 2019;29:68–74. doi: 10.1007/s00330-018-5580-2. [DOI] [PubMed] [Google Scholar]
- 63.Shah R.M., Patel D., Abbate A., et al. Comparison of transradial coronary procedures via right radial versus left radial artery approach: a meta-analysis. Cathet Cardiovasc Interv. 2016;88:1027–1033. doi: 10.1002/ccd.26519. [DOI] [PubMed] [Google Scholar]
- 64.Mortensen C., Chung J., Liu D., et al. Prospective study on total fluoroscopic time in patients undergoing uterine artery embolization: comparing transradial and transfemoral approaches. Cardiovasc Intervent Radiol. 2019;42:441–447. doi: 10.1007/s00270-018-2100-3. [DOI] [PubMed] [Google Scholar]
- 65.Jayanti S., Juergens C., Makris A., et al. The learning curves for transradial and ultrasound-guided arterial access: an analysis of the SURF trial. Heart Lung Circ. 2021;30:1329–1336. doi: 10.1016/j.hlc.2021.02.006. [DOI] [PubMed] [Google Scholar]
- 66.Ball W.T., Sharieff W., Jolly S.S., et al. Characterization of operator learning curve for transradial coronary interventions. Circ Cardiovasc Interv. 2011;4:336–341. doi: 10.1161/CIRCINTERVENTIONS.110.960864. [DOI] [PubMed] [Google Scholar]
- 67.Liu Y., Wen X., Bai J., et al. A single-center, randomized, controlled comparison of the transradial vs transfemoral approach for cerebral angiography: a learning curve analysis. J Endovasc Ther. 2019;26:717–724. doi: 10.1177/1526602819859285. [DOI] [PubMed] [Google Scholar]
- 68.Iezzi R., Posa A., Merlino B., et al. Operator learning curve for transradial liver cancer embolization: implications for the initiation of a transradial access program. Diagn Interv Radiol. 2019;25:368–374. doi: 10.5152/dir.2019.18437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sciahbasi A., Romagnoli E., Trani C., et al. Evaluation of the "learning curve" for left and right radial approach during percutaneous coronary procedures. Am J Cardiol. 2011;108:185–188. doi: 10.1016/j.amjcard.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 70.Oh E.J., Lee J.H., Kwon E.J., et al. Simulation-based training using a vessel phantom effectively improved first attempt success and dynamic needle-tip positioning ability for ultrasound-guided radial artery cannulation in real patients: an assessor-blinded randomized controlled study. PLoS One. 2020;15 doi: 10.1371/journal.pone.0234567. [DOI] [PMC free article] [PubMed] [Google Scholar]