Abstract
Introduction
Rituximab is a first-line treatment for membranous nephropathy. Nephrotic syndrome limits rituximab exposure due to urinary drug loss. Rituximab underdosing (serum level <2 μg/ml at month-3) is a risk factor for treatment failure. We developed a machine learning algorithm to predict the risk of underdosing based on patients’ characteristics at rituximab infusion. We investigated the relationship between the predicted risk of underdosing and the cumulative dose of rituximab required to achieve remission.
Methods
Rituximab concentrations were measured at month-3 in 92 sera from adult patients with primary membranous nephropathy, split into a training (75%) and a testing set (25%). A forward-backward machine-learning procedure determined the best combination of variables to predict rituximab underdosing in the training data set, which was tested in the test set. The performances were evaluated for accuracy, sensitivity, and specificity in 10-fold cross-validation training and test sets.
Results
The best variables combination to predict rituximab underdosing included age, gender, body surface area (BSA), anti-phospholipase A2 receptor type 1 (anti-PLA2R1) antibody titer on day-0, serum albumin on day-0 and day-15, and serum creatinine on day-0 and day-15. The accuracy, sensitivity, and specificity were respectively 79.4%, 78.7%, and 81.0% (training data set), and 79.2%, 84.6% and 72.7% (testing data set). In both sets, the algorithm performed significantly better than chance (P < 0.05). Patients with an initial high probability of underdosing experienced a longer time to remission with higher rituximab cumulative doses required to achieved remission.
Conclusion
This algorithm could allow for early intensification of rituximab regimen in patients at high estimated risk of underdosing to increase the likelihood of remission.
Keywords: artificial intelligence, immunomonitoring, machine learning, nephrotic syndrome, primary membranous nephropathy, rituximab
Graphical abstract
See Commentary on Page 1
Primary membranous nephropathy is an autoimmune disease affecting the kidney glomerulus which represents the most common cause of nephrotic syndrome in nondiabetic Caucasian adults.1 The spontaneous course of the disease varies from spontaneous remission to progressive chronic kidney disease.2 The identification of autoantibodies in membranous nephropathy, directed in 70% to 80% of cases against the PLA2R1, has led to proposing the use of B-cell depleting drugs as treatment.3, 4, 5 Rituximab is a chimeric anti-CD20 monoclonal IgG1 antibody designed to attach CD20 on B-cells, leading to their death. It has been approved for the treatment of non-Hodgkin lymphoma, rheumatoid arthritis, and autoantibody-driven vasculitis by the US Food and Drug Administration and the European Medicines Agency. It is also used as an off-label drug in an increasing array of autoimmune diseases such as antineutrophil cytoplasmic autoantibody-associated vasculitis and pemphigus vulgaris.6 It is now considered as a first-line option with a relevant benefit/risk ratio for membranous nephropathy.7 However, several factors may limit the efficacity of rituximab in patients with membranous nephropathy; these include chronic and irreversible glomerular damage, the appearance of rituximab targeted antibodies, and what is our concern here: a lower exposure to rituximab.8, 9, 10, 11, 12, 13 Indeed, in nephrotic patients, the clearance of rituximab is greater than in other rituximab-treated diseases, due to proteinuria leading to urinary drug loss.11,14 Therefore, uncertainties remain about the optimal dose of rituximab that should be used upon treatment initiation. The optimal titration and dosing regimen of rituximab as well as its therapeutic drug monitoring in patients with membranous nephropathy are currently debated.8 Indeed, the target concentrations and the optimal rituximab regimen to attain them vary according to the pathology.15, 16, 17, 18 In the GEMRITUX study comparing rituximab (2 infusions of 375 mg/m2 on day 1 and 8) combined with a nonimmunosuppressive antiproteinuric treatment to nonimmunosuppressive antiproteinuric treatment alone, B-cells were not fully depleted 6 months after rituximab treatment, suggesting a suboptimal dosage. This lack of B-cell depletion may explain the lack of significant difference in the 6-month clinical remission rate between the nonimmunosuppressive antiproteinuric treatment-rituximab group versus nonimmunosuppressive antiproteinuric treatment alone group.19 More recently, a high-dose rituximab regimen of 2 infusions of 1000 mg given 2 weeks apart proved more effective in inducing clinical remission than the GEMRITUX regimen.20 In the high-dose regimen, serum rituximab levels at month-3 were higher than in the GEMRITUX regimen with more effective B-cell depletion, which may explain the better clinical outcome.20 Repeated doses of rituximab up to cumulative doses of 3000 mg to 6000 mg, have safely demonstrated efficacy to induce remission.21 Serum rituximab level <2 μg/ml at month-3 from the initiation of therapy was an independent risk factor for treatment failure at month-6 and month-12 in a cohort of 68 patients with primary membranous nephropathy.10 Serum albumin at baseline was a predictive factor for underdosing (defined by a serum rituximab level <2 μg/ml at month-3) because patients with baseline serum albumin below 22.5 g/l had an 8.66-fold higher risk of undetectable rituximab at month-3.10 This conveys the notion that, the more severe the nephrotic syndrome, the more undertreated the patient will be. However, optimizing empirically rituximab regimen to yield concentrations at month-3 above the threshold of 2 μg/ml remains a challenging task.
Artificial intelligence combined with machine learning algorithms is increasingly used in medicine,22 and particularly in clinical pharmacology to support therapeutic drug monitoring, especially when classical pharmacometrics approaches are hard to apply. Machine learning algorithms help predict the initial dose to administer, the resulting exposure to a drug, as well as the interval between 2 infusions.23, 24, 25 In order to surpass the difficulties of optimizing rituximab treatment in nephrotic patients, we first developed a machine learning algorithm to predict the categorical target range of concentrations of rituximab (< or ≥ 2 μg/ml) 3 months after the first infusion, based on the patients’ baseline and 15 days treatment characteristics. Second, we retrospectively explored the relationship between the cumulative dose of rituximab required to achieve clinical remission and the algorithm’s initial prediction for each patient.
Methods
Patients’ Data
A total of 73 adult patients with primary membranous nephropathy participated in a prospective multicenter cohort in France, at Departments of Nephrology in Besancon, Marseille and Nice University Hospitals. The patients were enrolled between July 1, 2015 and January 31, 2020 and were followed-up for 2 years after inclusion. The inclusion criteria were as follows: (i) membranous nephropathy diagnosed by biopsy or serological assay; (ii) primary membranous nephropathy defined by the absence of concomitant autoimmune disease, negative hepatitis B and C serologies, and negative cancer workup; (iii) rituximab treatment according to the Kidney Disease: Improving Global Outcomes guidelines7 (i.e., in cases of persistent nephrotic syndrome or life-threatening complications of nephrotic syndrome); and (iv) monitoring of rituximab levels 3 months after treatment. Patients who have received any other immunosuppressive therapy within 6 months before rituximab were not included. Ninety-two cures of 2 rituximab infusions each were administered (84 cures of two 1000 mg infusions and 8 cures of two 375 mg/m2 infusions). Patients receiving multiple cures were considered independent “pharmacokinetically” because the lag time between the 2 infusions was greater than 9 months and rituximab levels were undetectable before reinjections. According to Kidney Disease: Improving Global Outcomes guidelines, in case of persistent nephrotic syndrome and persistent immunological activity 6 months after rituximab initiation, patients were treated again with a new course of rituximab until achievement of clinical and immunological remissions.7 The study protocol complied with the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the appropriate institutional review committees. Written informed consent was obtained from participants prior to inclusion in the study (NCT02199145 and NCT04326218).
Rituximab Immunomonitoring
Serum rituximab levels were measured 3 months after rituximab infusions by enzyme-linked immunosorbent assay according to the manufacturer’s instructions (LISA-TRACKER Duo Rituximab; Theradiag, Croissy Beaubourg, France). The limit of detection defined by the manufacturer was 2 μg/ml.
Detection of Anti-M-Type Phospholipase A2 Receptor 1 (PLA2R1) Antibodies
Serum levels of total immunoglobulin G anti-PLA2R1 antibodies were measured by the enzyme-linked immunosorbent assay developed by EUROIMMUN (Medizinische Labordiagnostika AG, Lübeck, Germany). Participants were considered as anti-PLA2R1 positive when levels were >14 RU/ml.
Machine Learning Approaches
Missing Data and Preparation of the Data
All preprocessing (imputation, upsampling, normalization of numeric variables, and factorization of categorical variables), machine learning and statistical analyses were performed using the Tidymodels framework in the R software (R version 4.1.2).26 To evaluate the impact of the “upsampling” step, we compare the performance of clustering. Descriptive statistics included quantitative variables (median and interquartile ranges) and qualitative variables (percentages).
Missing data were imputed using the K-nearest neighbors method implemented in R package “Recipes”27 including in the meta package “Tidymodels”.28 The imputation of data was only performed on variables with a proportion of missing data lower than of 30%. The distribution of quantitative variables before and after the imputation was tested with a Wilcoxon test. The distribution of qualitative variables before and after the imputation was tested with a Chi-square test. Variables whose distribution has been modified by imputation were not further exploited. A sensitivity analysis of imputation was performed on the final model to distinguish the potential contribution of imputation to algorithm performance.
After imputation, body mass index and BSA were calculated using the following formula29,30:
The following patient characteristics were gathered (21 variables): age; gender (female/male); weight; height; body mass index; BSA; use of drugs including angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, furosemide, or hydrochlorothiazide; rituximab dosage received for 2 infusions; proteinuria on day-0; proteinuria on day-15; serum albumin on day-0; serum albumin on day-15; serum creatinine on day-0; serum creatinine on day-15; glomerular filtration rate on day-0; glomerular filtration rate on day-15; CD19+ cell count on day-0; and anti-PLA2R1 antibody titer on day-0; aiming to be input into the machine learning algorithm.
Machine Learning Analysis
Among the 92 available rituximab concentrations, data splitting was performed by random selection and allotment into the training group of data (75%, i.e., 68 rituximab serum concentrations from 55 patients), to develop the algorithm or the testing data set (25%, i.e., 24 rituximab serum concentrations from 22 patients), to evaluate the algorithm performance. An “upsampling” step was performed, on the training data set only, which consisted of increasing the proportion of patients with rituximab dosage ≥2 μg/ml to a 50/50 ratio, to balance the classes. The polynomial Support Vector Machine algorithm was trained to predict the categorical target range of rituximab level (<2 μg/ml or ≥2 μg/ml) 3 months (± 1 week) after treatment initiation as function of probability. The patient was classified as “<2 μg/ml” if the probability was greater than 50%. If this is not the case, the patient is assigned to the other class.
A forward inclusion and a backward elimination procedure were applied to determine the best combination of clinical and biological variables, to increase the accuracy in the training data set. The first combination used in this forward inclusion was age, gender, and BSA in accordance with the previous rituximab population pharmacokinetics models.31,32
The accuracy was determined according to the following formula:
where, TP is true positive, TN is true negative, FP is false positive, and FN is false negative.
A 10-fold cross-validation was applied to the training data set to tune the hyperparameters, then another one to assess the model’s performances. Next, the performance of the best model to predict the categorical target range of rituximab levels (<2 μg/ml or ≥2 μg/ml) was evaluated using accuracy, sensitivity, specificity, positive predictive value, negative predictive value, and F-score, metrics allowing to appreciate the global performances of the algorithm, in the testing data set.
To distinguish the contribution of the algorithm from that of chance, the <2 μg/ml category was arbitrarily applied to all patients, and the fictive accuracy was calculated to determine the “no information rate” in the training and testing data set. The calculated accuracies, according to the 2 methods, were compared using a binomial test.
Retrospective Analysis of the Relationship Between Time to Remission or Cumulative Dose of Rituximab Required to Achieve Remission and the Algorithm’s Initial Prediction for Each Patient
Retrospective analysis to explore the relationships between: (i) machine learning algorithm prediction and time to remission or (ii) machine learning algorithm prediction and cumulative dose of rituximab required to achieve remission were performed in patients from the training set. We extracted the percentage risk of rituximab underdosing (i.e., rituximab level <2 μg/ml at 3 months) estimated by our algorithm for each patient. These latter were arbitrarily classified into 3 categories: unlikely (<50%), moderately likely (50%–75%), and highly likely (>75%). Clinical remission was defined according to the Kidney Disease: Improving Global Outcomes guidelines7 (i.e., remission was characterized by urinary protein-to-creatinine ratio <3.5 g/d, accompanied by an improvement or normalization of the serum albumin concentration and preserved kidney function). The cumulative dosage of rituximab received represents the overall dosages of rituximab received by the patient during the different courses of treatment. Comparisons between the received cumulative dosage of rituximab or the time to remission between each of the 3 categories were performed using a Kruskal-Wallis test. Comparison of time to remission between the 3 categories was carried out using a log-rank test and the Kaplan Meier method.
Results
Patients’ Data
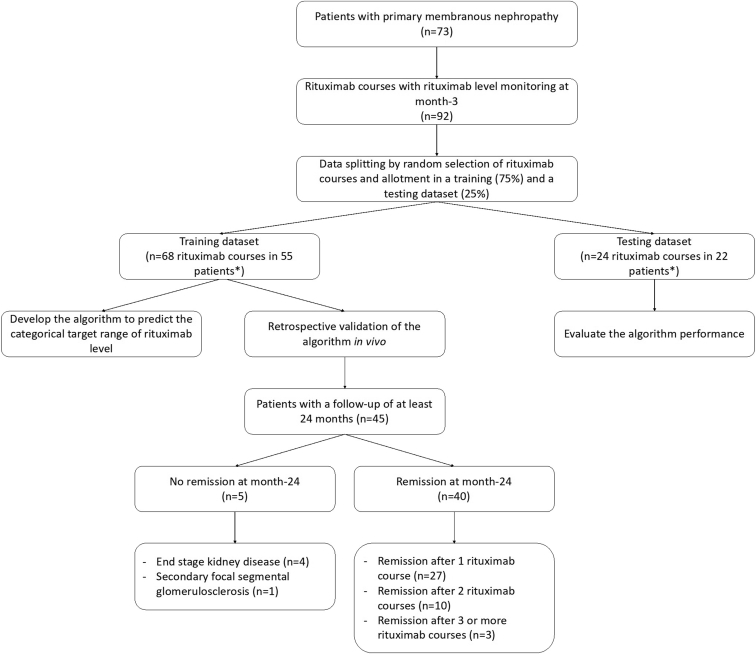
A total of 73 patients were enrolled in this study (Figure 1). Most patients were men (69.8%). The median (interquartile range) age was 59 (40–70) years. The median (interquartile range) weight and height were 74.8 (68.4–84.0) kg and 172 (165–178) cm, respectively. The most common drug used as a concomitant treatment was furosemide (>75% of patients), followed by angiotensin-converting enzyme inhibitors (63% of patients). The serum albumin level and urinary protein-to-creatinine ratio (median [interquartile range]) at the first rituximab infusion were 22.0 g/l (17.0–29.0) and 5.0 g/g (4.1–7.8), respectively. A total of 92 rituximab levels were obtained in these patients at month-3 post-rituximab treatment, 59.8% of which had rituximab levels <2 μg/ml. The training data set and the testing data set were composed of 68 and 24 patients’ rituximab levels from 55 and 22 patients, respectively (Figure 1). Four of them were included in both data sets. The patients’ characteristics before and after imputation are described in Table 1. The accuracy obtained after changing of imputed values (sensitive analysis of imputation) allowed to obtain an accuracy of 0.75. No variable had more than 30% of missing data (Table 1). No significant difference in patients’ characteristics was observed either before or after imputation (Table 1).
Figure 1.
Study flow chart. Four patients were included in both data sets. Patients receiving multiple cures were considered independent “pharmacokinetically” because the lag time between the 2 infusions was greater than 9 months and rituximab levels were undetectable before reinjections.
Table 1.
Comparison of patients’ characteristics before and after imputation
| Characteristics | Missing Data (%) | Before Imputation | After Imputation | P-Value |
|---|---|---|---|---|
| Age (yrs) | 0 | 59 (40–70) | No imputation | - |
| Gender (%) | 0 | M: 69.6 | No imputation | - |
| Weight (kg) | 3.3 | 75 (68.4–84) | 74.8 (68.4–84.0) | 0.95 |
| Height (cm) | 6.5 | 172 (165–178) | 172 (165–178) | 1.0 |
Use of
|
|
|
|
|
| Categorical rituximab concentrations (%) | 0 | <2 μg/ml: 59.8 ≥2 μg/ml: 40.2 |
No imputation | - |
| Proteinuria (g/g) | ||||
|
0 | 5.7 (4.1–7.8) | No imputation | - |
|
23.9 | 4.9 (3.0–7.9) | 5.4 (3.2–7.8) | 0.65 |
| Serum albumin (g/l) | ||||
|
0 | 22.0 (17.0–29.0) | No imputation | - |
|
23.9 | 22.4 (18.0–28.4) | 22.7 (18.8–27.9) | 0.95 |
| Serum creatinine (μmol/l) | ||||
|
2.2 | 120.0 (88.2–149.0) | 120.0 (88.8–149.0) | 0.91 |
|
20.7 | 118.0 (97.0–158.0) | 118.0 (97.0–158.0) | 0.98 |
| Glomerular filtration rate estimated by CKD-EPI formula (ml/min per 1.73 m2) | ||||
|
9.8 | 53 (40–73) | 54 (40–71) | 0.87 |
|
21.7 | 55 (34–74) | 57 (40–73) | 0.68 |
| CD19+ cell count (cells/μl) on day-0 | 26.1 | 169.0 (85.8–261.0) | 188.0 (116.0–209.0) | 0.72 |
| Anti-PLA2R1 antibody titer (RU/ml) on day-0 | 9.8 | 95.0 (40.5–217.0) | 114.0 (47.8–212.0) | 0.77 |
CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; PLA2R1, phospholipase A2 receptor type 1.
The data are presented as median (Interquartile range). The comparisons of data imputation were performed using Wilcoxon test (quantitative) and chi-square test (qualitative).
Characteristics of Interest for Predicting Rituximab Underdosing at Month-3
The accuracy obtained with the best variable combinations was 79.4% in the training data set comprising age, gender, BSA, anti-PLA2R1 antibody titer on day-0, serum albumin on day-0, serum albumin on day-15, serum creatinine on day-0, and serum creatinine on day-15. Different combinations of variables were tested, relying on the accuracy in the training data set (Table 2). We evaluated the impact of the upsampling step performed in this study. The algorithm trained without the upsampling step was unable to group patients. All patients were classified in the <2 μg/ml class, confirming the imbalance in the data.
Table 2.
Evaluation of different combinations of variables to predict the rituximab class concentrations
| Association of Variables | Accuracy |
|---|---|
| Age + Gender + BSA | 0.4559 |
| Age + Gender + BSA + GFR on D0 | 0.5294 |
| Age + Gender + BSA + GFR on D0 + CD19 on D0 | 0.5 |
| Age + Gender + BSA + GFR on D0 + anti-PLA2R1 antibody titer on D0 | 0.5441 |
| Age + Gender + BSA + GFR on D0 + anti-PLA2R1 antibody titer on D0 + weight | 0.5417 |
| Age + Gender + BSA + GFR on D0 + anti-PLA2R1 antibody titer on D0 + height | 0.5441 |
| Age + Gender + BSA + GFR on D0 + anti-PLA2R1 antibody titer on D0 + BMI | 0.4559 |
| Age + Gender + BSA + GFR on D0 + anti-PLA2R1 antibody titer on D0 + serum albumin on M0 | 0.6324 |
| Age + Gender + BSA + anti-PLA2R1 antibody titer on D0 + serum albumin on D0 | 0.6471 |
| Age + Gender + BSA + anti-PLA2R1 antibody titer on D0 + serum albumin on D0 + serum creatinine on D0 | 0.6618 |
| Age + Gender + BSA + anti-PLA2R1 antibody titer on D0 + serum albumin on D0 + serum creatinine on D0 + use of angiotensin-converting enzyme inhibitors | 0.6471 |
| Age + Gender + BSA + anti-PLA2R1 antibody titer on D0 + serum albumin on D0 + serum creatinine on D0 + use of angiotensin II receptor blockers | 0.6471 |
| Age + Gender + BSA + anti-PLA2R1 antibody titer on D0 + serum albumin on D0 + serum creatinine on D0 + use of furosemide | 0.6471 |
| Age + Gender + BSA + anti-PLA2R1 antibody titer on D0 + serum albumin on D0 + serum creatinine on D0 + use of hydrochlorothiazide | 0.6471 |
| Age + Gender + BSA + anti-PLA2R1 antibody titer on D0 + serum albumin on D0 + serum creatinine on D0 + serum albumin on D15 | 0.72 |
| Age + Gender + BSA + serum albumin on D0 + serum creatinine on D0 + serum albumin on D15 + serum creatinine on D15 | 0.72 |
| Age + Gender + BSA + anti-PLA2R1 antibody titer on D0 + serum albumin on M0 + serum creatinine on D0 + serum albumin on D15 + serum creatinine on D15 | 0.794 |
| Age + Gender + BSA + anti-PLA2R1 antibody titer on D0 + serum albumin on D0 + serum creatinine on D0 + serum albumin on D15 + serum creatinine on D15 + number of visits | 0.748 |
| Age + Gender + BSA + anti-PLA2R1 antibody titer on D0 + serum albumin on D0 + serum creatinine on D0 + serum albumin on D15 + serum creatinine on D15 + proteinuria on D0 | 0.7647 |
| Age + Gender + BSA + anti-PLA2R1 antibody titer on D0 + serum albumin on D0 + serum creatinine on D0 + serum albumin on D15 + serum creatinine on D15 + proteinuria on D15 | 0.75 |
| All predictors (N = 21) | 0.7353 |
BSA, body surface area; D0, day-0; D15, day-15; GFR, glomerular filtration rate; PLA2R1, phospholipase A2 receptor type 1.
Performances of the Machine Learning Algorithm in the Training Data Set
The algorithm yielded comparable sensitivity and specificity (78.7% and 81.0%, respectively) in the training data set (Table 3). A significant difference was observed between the accuracy algorithm and “no information rate” in the training data set (P = 0.04), confirming the prediction performances of our algorithm. The confusion matrix of training data set is presented in Figure 2a.
Table 3.
Performances of outcome prediction in training and testing data sets
| Performance Assessment Criteria | Training Data Set | Testing Data Set |
|---|---|---|
| Accuracy (%) | 79.4 | 79.2 |
| No information rate (%) | 69.1 | 54.2 |
| Sensitivity (%) | 78.7 | 84.6 |
| Specificity (%) | 81.0 | 72.7 |
| Positive predictive value (%) | 90.2 | 78.6 |
| Negative predictive value (%) | 63.0 | 80.0 |
| F-score (%) | 84.1 | 81.5 |
Figure 2.
Ability of machine learning algorithm to predict the categorical target range of rituximab levels in the training (a) and testing (b) data set.
Evaluation of Performances in the Testing Data Set
Sensitivity, specificity, and positive predictive value in the testing data set were similar to the ones of the training data set (Table 3). However, the negative predictive value was higher in the testing (80%) than in the training data set (63%). A significant difference was observed between the accuracy algorithm and ‘no information rate’ in the testing data set (P = 0.01). The confusion matrix of the testing data set is presented in Figure 2b.
Retrospective Analysis of the Relationship Between Time to Remission or Cumulative Dose of Rituximab Required to Achieve Remission and the Algorithm’s Initial Prediction for Each Patient
We analyzed the outcome of 45 patients from the training data set for whom we had at least 24 months of follow-up after the first course of rituximab to validate the clinical relevance of our algorithm, (Figures 1 and 3). The characteristics of the patients are presented in Table 4. Patients were retreated according to Kidney Disease: Improving Global Outcomes guidelines in case of persistent nephrotic syndrome and immunological activity 6 months after rituximab treatment. After 24 months of follow-up, 4 patients developed end-stage renal disease and 1 patient developed persistent nephrotic syndrome due to secondary focal segmental glomerulosclerosis. We retrospectively analyzed the time to achieve clinical remission, or the cumulative dose of rituximab finally received to achieve clinical remission according to the algorithm’s initial prediction for each patient. Patients with an initial high probability of rituximab underdosing (>50%) had a longer time to remission (underdosing probability <50%: 4.2 ± 1.8 months vs. 50%–75%: 8.4 ± 4.6 months vs. >75%: 10.7 ± 5.9 months; P = 0.002) (Figure 3a and b). Likewise, they required higher cumulative rituximab doses to achieve clinical remission (underdosing probability <50%: 2.0 ± 0.0 g vs. 50%–75%: 2.7 ± 0.9 g vs. >75%: 3.6 ± 1.6 g; P = 0.001) (Figure 3c and d).
Figure 3.
Clinical impact of the probability of rituximab underdosing on the time to reach remission (a and b) and the rituximab cumulative dose received (c and d). Patients were classified into 3 categories according to the percentage risk of rituximab underdosing estimated by our algorithm: unlikely (<50%) in green, moderately likely (50%–75%) in orange and very likely (>75%) in red. In Figure 3d, each diamond represents individual data from one of the 40 patients.
Table 4.
Clinical characteristics of patients included in the retrospective analysis of the relationship between time to remission and cumulative dose of rituximab required to achieve remission as function of machine learning algorithm prediction
| Variables | Patients (N = 45) |
|---|---|
| Characteristics at rituximab infusion | |
| Age (yrs) | 56 (42–67) |
| Gender (male/female) | 30/15 |
| Weight (kg) | 74.0 (66.0–87.3) |
| Height (cm) | 172 (165–178) |
| Body mass index (kg/m2) | 26 (23–28) |
| Serum albumin (g/l) | 22.1 (16.0–27.8) |
| Urinary protein-to-creatinine ratio (g/g) | 5.6 (4.1–8.7) |
| Serum creatinine (μmol/l) | 117 (88–169) |
| Glomerular Filtration Rate estimated by CKD-EPI formula (ml/min per 1.73 m2) | 54 (36–82) |
| Etiology | |
| Anti-PLA2R1-associated membranous nephropathy | 41 (91%) |
| Anti-THSD7A-associated membranous nephropathy | 1 (2%) |
| Antigen responsible not identified | 3 (7%) |
| Anti-PLA2R1 titer (RU/ml) | 153 (62–255) |
| Rituximab protocol | |
| 375 mg/m2 D0–D8 | 3 (7%) |
| 1000 mg D0–D15 | 42 (93%) |
| Supportive therapy | 45 (100%) |
| Serum rituximab level at month-3 | |
| <2 μg/ml | 27 (60%) |
| >2 μg/ml | 18 (40%) |
| CD19+ cell count | |
| CD19+ cell count at month-3 (cells/μl) | 2 (0–5) |
| CD19+ cell count at month-6 (cells/μl) | 28 (6–56) |
| Outcome | |
| Remission at month-6 | 24 (53%) |
| Remission at month-12 | 36 (80%) |
| Remission at month-24 | 40 (89%) |
| End stage kidney disease at month-24 | 4 (9%) |
| Cumulative rituximab dose | 2 (2–4) |
| Time to remission (mos) | 6 (3–11) |
CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; D0: day-0; D8: day-8; D15: day-15; PLA2R1: phospholipase A2 receptor type 1, THSD7A: Thrombospondin Type 1 Domain Containing 7A
Discussion
Off-label use of drugs, such as rituximab, is often at the forefront of health improvement of patients with rare diseases. Rituximab is a safe and effective treatment of membranous nephropathy, although uncertainties still remain about the optimal dosage to be employed. In this context, the lack of homogeneity of rituximab indications and dosages does not allow yet to develop standardized methods for therapeutic drug monitoring. The trough levels of rituximab 3 months after the beginning of the treatment efficiently predicts the likelihood of clinical remission.10 However, this biomarker is not available in all centers and does not allow to estimate the risk of rituximab underdosing before 3 months, while the disease may worsen or chronic lesions may develop. Earlier estimation of the residual rituximab level at month-3 after rituximab treatment, as well as tailoring the initial dose to the patient's characteristics, could increase the likelihood of remission. We developed the first machine learning algorithm aiming to predict the categorical target concentration range of rituximab at month-3, relying on clinical and biological data obtained at or around treatment initiation. The performances of this algorithm display relevant accuracy, sensitivity, and specificity. The algorithm was validated retrospectively by showing that the risk of underdosing estimated by our algorithm was correlated with the number of rituximab courses required to achieve clinical remission and time to achieved remission. The probability of underdosing initially proposed for each patient in the training data set appears related to the number of rituximab courses received and a fortiori to difficulties to achieve remission (Figure 3). Although remission is defined by some of the variables used in the algorithm, these variables are both validated prognostic markers and correlate with the risk of rituximab underdosing. Indeed, the severity of nephrotic syndrome (particularly low serum albumin levels) is an independent risk factor for rituximab underdosing, and rituximab underdosing is an independent risk factor for treatment failure at month-6 and month-12.9,10 If these preliminary results are confirmed, this algorithm could be used as a tool for early detection of patients at risk of rituximab underdosing, in order to personalize patient management by reinforcing treatment to limit the risk of underdosing and thus maximize the probability of remission. In centers with routine access to monitoring of rituximab level, our algorithm does not replace monitoring at month-3 as a prognostic marker. However, this algorithm is a relevant tool for patient follow-up in centers that do not have routine access to rituximab monitoring and provides valuable prognostic information. Such a contribution in daily practice needs to be confirmed.
Machine learning is often approached cautiously because of its “black box” aspect, of some uncertainties regarding the rationale of choice of variables of interest as well as the learning process underlying the capture of relevant information. In this study, the forward inclusion and the backward elimination procedure were used to select relevant variables according to the improvement of accuracy. Some of the clinical and biological parameters such as age, gender or BSA, are known to influence pharmacokinetic parameters such as the clearance, the volume of distribution in population models of rituximab pharmacokinetics, when applied to other diseases such as rheumatoid arthritis or diffuse large B-cell lymphoma.31,32 Serum albumin and creatinine levels at initiation of the treatment provide information on both the status of renal function at the start of rituximab therapy and the activity of the disease.33 The inclusion of albumin and creatinine levels on day-15 also reflects the patients’ renal status prior to the second injection. However, the iteration of variables’ assessment might alter the prediction of rituximab level at month-3. Therefore, the availability of biomarkers on day-15 could be a limitation, particularly because they contribute 15% to the model accuracy. The anti-PLA2R1 antibody titer is a marker of membranous nephropathy activity.34, 35, 36 However, some centers do not have access to anti-PLA2R1 antibody titer by enzyme-linked immunosorbent assay, and anti-PLA2R1 antibodies are not involved in all patients with membranous nephropathy, making our algorithm unusable. Therefore, we developed and tested an algorithm without this variable. The performances were slightly reduced as compared to the main algorithm (Supplementary Figure S1).
Different population pharmacokinetic models have described rituximab pharmacokinetics in other populations (e.g., pediatrics) or diseases (e.g., leukemia).31,32,37, 38, 39, 40, 41 These models, consider the clinico-biological characteristics of the patient (e.g., age, gender, or pathology). They allow the following: (i) to determine an a priori dosage of rituximab based on each patient’s clinical and biological data, (ii) to determine the probability of target attainment as a function of doses, or (iii) to estimate the overall rituximab exposure a posteriori in a specific population similar to the one that allowed the development of the model. No method of individualizing rituximab dosing in adult patients with nephrotic syndrome to improve the likelihood of remission has been developed so far. One reason among others prevails for that: the dynamics of the renal status, influenced by the course of the disease and the response to treatment. Nonetheless, the development of our algorithm based on a few variables paves the way for the development of a possible mechanistic model.
This study has several limitations. The algorithm needs to be validated externally before any routine use. Like other population pharmacokinetic models, this algorithm can only be used in patients similar to the ones which were included in its development (i.e., adult patients with primary membranous nephropathy). Only 1 patient in the testing data set was given a dosage of 375 mg/m2; thereby limiting the use of this algorithm to patients with this dosage and should be assessed prior to use in patients at this dosage.
Finally, the ability of machine learning algorithm to accurately categorize patients with membranous nephropathy appears interesting for personalized patient care. Indeed, we could propose a personalized therapeutic regimen according to the estimated risk of underdosing. In patients at high risk of underdosing, an increased initial dose of rituximab (e.g., to 3000 or even 4000 mg in 3 or 4 infusions) could be effective in preventing the risk of underdosing, thereby increasing the likelihood of remission. This could decrease the complications of persistent nephrotic syndrome (e.g., thromboembolic complication, cardiovascular morbidity, and renal failure), and increase the patients’ quality of life (e.g., by reducing hospitalizations due to complications or sick leave) by accelerating the time to remission. This personalized treatment based on our algorithm will shortly be evaluated in a multicenter randomized controlled trial. We propose an interactive R shiny application to healthcare professionals, to facilitate the use (for research purposes only) of the machine learning algorithm developed in this study (https://lecteurs.shinyapps.io/Rituximab/).
Disclosure
All the authors have declared no competing interest. SylvainB works for EXACURE, a private society for artificial intelligence. He acted as an expert, contributing his mathematical knowledge and actively participating in the development of this algorithm without any remuneration.
Author Contributions
AD, MT, SylvainB, MCG, and BSP contributed to the conception and design of the study, as well as the analysis and interpretation of data. AD, MDD, DM, SylvainB, and AG contributed to the production of the algorithm. All the authors participated in drafting of the article and approved the final version submitted.
Footnotes
Figure S1. Ability of machine learning algorithm without anti-PLA2R1 antibody titer to predict the categorical target range of rituximab levels in the training (A) and testing (B) data set.
Supplementary Material
Figure S1. Ability of machine learning algorithm without anti-PLA2R1 antibody titer to predict the categorical target range of rituximab levels in the training (A) and testing (B) data set.
References
- 1.Couser W.G. Primary membranous nephropathy. Clin J Am Soc Nephrol. 2017;12:983–997. doi: 10.2215/CJN.11761116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schieppati A., Mosconi L., Perna A., et al. Prognosis of untreated patients with idiopathic membranous nephropathy. N Engl J Med. 1993;329:85–89. doi: 10.1056/NEJM199307083290203. [DOI] [PubMed] [Google Scholar]
- 3.Fervenza F.C., Cosio F.G., Erickson S.B., et al. Rituximab treatment of idiopathic membranous nephropathy. Kidney Int. 2008;73:117–125. doi: 10.1038/sj.ki.5002628. [DOI] [PubMed] [Google Scholar]
- 4.Fervenza F.C., Abraham R.S., Erickson S.B., et al. Rituximab therapy in idiopathic membranous nephropathy: a 2-year study. Clin J Am Soc Nephrol. 2010;5:2188–2198. doi: 10.2215/CJN.05080610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Remuzzi G., Chiurchiu C., Abbate M., Brusegan V., Bontempelli M., Ruggenenti P. Rituximab for idiopathic membranous nephropathy. Lancet. 2002;360:923–924. doi: 10.1016/S0140-6736(02)11042-7. [DOI] [PubMed] [Google Scholar]
- 6.Lim S.H., Beers S.A., French R.R., Johnson P.W.M., Glennie M.J., Cragg M.S. Anti-CD20 monoclonal antibodies: historical and future perspectives. Haematologica. 2010;95:135–143. doi: 10.3324/haematol.2008.001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rovin B.H., Adler S.G., Barratt J., et al. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021;100:S1–S276. doi: 10.1016/j.kint.2021.05.021. [DOI] [PubMed] [Google Scholar]
- 8.Hartinger J.M., Kratky V., Hruskova Z., Slanar O., Tesar V. Implications of rituximab pharmacokinetic and pharmacodynamic alterations in various immune-mediated glomerulopathies and potential anti-CD20 therapy alternatives. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.1024068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teisseyre M., Cremoni M., Boyer-Suavet S., et al. Advances in the management of primary membranous nephropathy and rituximab-refractory membranous nephropathy. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.859419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teisseyre M., Cremoni M., Boyer-Suavet S., et al. Rituximab immunomonitoring predicts remission in membranous nephropathy. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.738788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyer-Suavet S., Andreani M., Cremoni M., et al. Rituximab bioavailability in primary membranous nephropathy. Nephrol Dial Transplant. 2019;34:1423–1425. doi: 10.1093/ndt/gfz041. [DOI] [PubMed] [Google Scholar]
- 12.Boyer-Suavet S., Andreani M., Lateb M., et al. Neutralizing anti-rituximab antibodies and relapse in membranous nephropathy treated with rituximab. Front Immunol. 2019;10:3069. doi: 10.3389/fimmu.2019.03069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teisseyre M., Brglez V., Cremoni M., et al. Risk factors associated with the occurrence of anti-rituximab antibodies in membranous nephropathy. Clin J Am Soc Nephrol. 2023;18:785–787. doi: 10.2215/CJN.0000000000000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fogueri U., Cheungapasitporn W., Bourne D., Fervenza F.C., Joy M.S. Rituximab exhibits altered pharmacokinetics in patients with membranous nephropathy. Ann Pharmacother. 2019;53:357–363. doi: 10.1177/1060028018803587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berinstein N.L., Grillo-López A.J., White C.A., et al. Association of serum rituximab (IDEC-C2B8) concentration and anti-tumor response in the treatment of recurrent low-grade or follicular non-Hodgkin’s lymphoma. Ann Oncol. 1998;9:995–1001. doi: 10.1023/A:1008416911099. [DOI] [PubMed] [Google Scholar]
- 16.Igarashi T., Kobayashi Y., Ogura M., et al. Factors affecting toxicity, response and progression-free survival in relapsed patients with indolent B-cell lymphoma and mantle cell lymphoma treated with rituximab: a Japanese phase II study. Ann Oncol. 2002;13:928–943. doi: 10.1093/annonc/mdf155. [DOI] [PubMed] [Google Scholar]
- 17.Liu C., Xu Y., Liu Q., Zhu H., Wang Y. Application of machine learning based methods in exposure-response analysis. J Pharmacokinet Pharmacodyn. 2022;49:401–410. doi: 10.1007/s10928-022-09802-2. [DOI] [PubMed] [Google Scholar]
- 18.Springer J.M., Funk R.S. Defining a therapeutic window for rituximab maintenance therapy in ANCA-associated vasculitis: a longitudinal observational study. J Clin Rheumatol. 2021;27:215–217. doi: 10.1097/RHU.0000000000001688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dahan K., Debiec H., Plaisier E., et al. Rituximab for severe membranous nephropathy: a 6-month trial with extended follow-up. J Am Soc Nephrol. 2017;28:348–358. doi: 10.1681/ASN.2016040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seitz-Polski B., Dahan K., Debiec H., et al. High-dose rituximab and early remission in PLA2R1-related membranous nephropathy. Clin J Am Soc Nephrol. 2019;14:1173–1182. doi: 10.2215/CJN.11791018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dahan K., Johannet C., Esteve E., Plaisier E., Debiec H., Ronco P. Retreatment with rituximab for membranous nephropathy with persistently elevated titers of anti-phospholipase A2 receptor antibody. Kidney Int. 2019;95:233–234. doi: 10.1016/j.kint.2018.08.045. [DOI] [PubMed] [Google Scholar]
- 22.Goecks J., Jalili V., Heiser L.M., Gray J.W. How machine learning will transform biomedicine. Cell. 2020;181:92–101. doi: 10.1016/j.cell.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Destere A., Marquet P., Gandonnière C.S., et al. A hybrid model associating population pharmacokinetics with machine learning: a case study with iohexol clearance estimation. Clin Pharmacokinet. 2022;61:1157–1165. doi: 10.1007/s40262-022-01138-x. [DOI] [PubMed] [Google Scholar]
- 24.Ponthier L., Ensuque P., Destere A., et al. Optimization of vancomycin initial dose in term and preterm neonates by machine learning. Pharm Res. 2022;39:2497–2506. doi: 10.1007/s11095-022-03351-6. [DOI] [PubMed] [Google Scholar]
- 25.Woillard J.-B., Salmon Gandonnière C., Destere A., et al. A machine learning approach to estimate the glomerular filtration rate in intensive care unit patients based on plasma iohexol concentrations and covariates. Clin Pharmacokinet. 2020;60:223–233. doi: 10.1007/s40262-020-00927-6. [DOI] [PubMed] [Google Scholar]
- 26.R Core Team R: a language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R-project.org/
- 27.Kuhn M., Wickham H. Hvitfeldt E: recipes: preprocessing and feature engineering steps for modeling. https://CRAN.R-project.org/package=recipes
- 28.Kuhn M., Wickham H. Tidymodels: a collection of packages for modeling and machine learning using tidyverse principles. https://www.tidymodels.org
- 29.Nuttall F.Q. Body mass index: obesity, BMI, and health: a critical review. Nutr Today. 2015;50:117–128. doi: 10.1097/NT.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mosteller R.D. Simplified calculation of body-surface area. N Engl J Med. 1987;317 doi: 10.1056/NEJM198710223171717. 1098-1098. [DOI] [PubMed] [Google Scholar]
- 31.Rozman S., Grabnar I., Novaković S., Mrhar A., Jezeršek Novaković B. Population pharmacokinetics of rituximab in patients with diffuse large B-cell lymphoma and association with clinical outcome: pharmacokinetics of rituximab and clinical outcome. Br J Clin Pharmacol. 2017;83:1782–1790. doi: 10.1111/bcp.13271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ng C.M., Bruno R., Combs D., Davies B. Population pharmacokinetics of rituximab (anti-CD20 monoclonal antibody) in rheumatoid arthritis patients during a Phase II clinical trial. J Clin Pharmacol. 2005;45:792–801. doi: 10.1177/0091270005277075. [DOI] [PubMed] [Google Scholar]
- 33.Kodner C. Nephrotic syndrome in adults: diagnosis and management. Am Fam Phys. 2009;80:1129–1134. [PubMed] [Google Scholar]
- 34.Hofstra J.M., Beck L.H., Beck D.M., Wetzels J.F., Salant D.J. Anti-phospholipase A2 receptor antibodies correlate with clinical status in idiopathic membranous nephropathy. CJASN. 2011;6:1286–1291. doi: 10.2215/CJN.07210810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hofstra J.M., Debiec H., Short C.D., et al. Antiphospholipase A2 receptor antibody titer and subclass in idiopathic membranous nephropathy. J Am Soc Nephrol. 2012;23:1735–1743. doi: 10.1681/ASN.2012030242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jullien P., Seitz Polski B., Maillard N., et al. Anti-phospholipase A2 receptor antibody levels at diagnosis predicts spontaneous remission of idiopathic membranous nephropathy. Clin Kidney J. 2017;10:209–214. doi: 10.1093/ckj/sfw121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bensalem A., Mulleman D., Paintaud G., et al. Non-linear rituximab pharmacokinetics and complex relationship between rituximab concentrations and anti-neutrophil cytoplasmic antibodies (ANCA) in ANCA-associated vasculitis: the RAVE trial revisited. Clin Pharmacokinet. 2020;59:519–530. doi: 10.1007/s40262-019-00826-5. [DOI] [PubMed] [Google Scholar]
- 38.Li J., Zhi J., Wenger M., et al. Population pharmacokinetics of rituximab in patients with chronic lymphocytic leukemia. J Clin Pharmacol. 2012;52:1918–1926. doi: 10.1177/0091270011430506. [DOI] [PubMed] [Google Scholar]
- 39.Blasco H., Chatelut E., de Bretagne I.B., Congy-Jolivet N., Le Guellec C. Pharmacokinetics of rituximab associated with CHOP chemotherapy in B-cell non-Hodgkin lymphoma. Fundam Clin Pharmacol. 2009;23:601–608. doi: 10.1111/j.1472-8206.2009.00714.x. [DOI] [PubMed] [Google Scholar]
- 40.Gibiansky E., Gibiansky L., Chavanne C., Frey N., Jamois C. Population pharmacokinetic and exposure-response analyses of intravenous and subcutaneous rituximab in patients with chronic lymphocytic leukemia. CPT Pharmacometr Syst Pharmacol. 2021;10:914–927. doi: 10.1002/psp4.12665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Y., Shen Q., Dong M., Xiong Y., Xu H., Li Z. Population pharmacokinetics of rituximab in pediatric patients with frequent-relapsing or steroid-dependent nephrotic syndrome. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.725665. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.