Abstract
The 800-m (m) run is part of Physical Education classes in Cameroon, after which arrhythmias may occur during recovery. Hence, this study aimed at determining relationship between 800-m run loads on cardiac autonomic recovery among school adolescents.
Forty-two male adolescents (aged [17 ± 1] years) performed 800-m. Post-exercise heart rate variability (HRV) was recorded during 5-min (min) (HRV5-min) and 15-min (HRV15-min) in time: Standard deviation of normal to normal (SDNN); Root mean square of successive differences (RMSSD) and frequency domain (LH: Low frequency, HF: High frequency, TP: Total power). Rating of Perceived Exertion (RPE) and blood lactate concentration (BLa) were measured after exercise. In HRV5-min, RPE was associated with SDNN (r = −0.44, p < 0.01) and RMSSD (r = −0.38, p < 0.05). BLa was correlated with SDNN (r = −0.38, p < 0.05) and RMSSD (r = −0.56, p < 0.001) in the time-domain, LF (r = −0.64, p < 0.001), HF (r = −0.58, p < 0.001) and TP (r = −0.61, p < 0.001) in frequency-domain. Moreover, RPE was correlated with LF (r = −0.44, p < 0.01), TP (r = −0.49, p < 0.01) while exercise duration with HF (r = −0.38, p < 0.05). In HRV15-min, BLa was correlated with RMSSD (r = −0.53, p < 0.001) and SDNN (r = −0.68, p < 0.001). RPE was negatively correlated SDNN (r = −0.53, p < 0.01) and RMSSD (r = −0.44, p < 0.01). BLa was associated with HF (r = −0.55, p < 0.001), TP (r = −0.50, p < 0.01) and RPE with LF (r = −0.51, p < 0.01), HF (r = −0.50, p < 0.01), TP (r = −0.49, p < 0.01). In addition, exercise duration was negatively linked to HF (r = −0.36, p < 0.05). This study outlined that in untrained adolescents an increase of 800-m loads is associated with a slow vagal indexes of HRV during the recovery.
Keywords: Heart rate variability, Recovery, 800-m load, Adolescents, Physical education classes
Abbreviations
- ANS
Autonomic nervous system
- HR
Heart rate
- HRV
Heart rate variability
- PE
Physical Education class
- BMI
Body mass index
- BP
Blood pressure
- RPE
Rating of Perceived Exertion
- BLa
Blood lactate concentration
- RR
Mean time between normal R waves of ECG
- RMSSD
Root mean square of successive differences
- SDNN
Standard deviation of normal to normal
- LF
Low frequency
- HF
High frequency
- TP
Total power
- HRV5-min
minutes of heart rate variability recording
- HRV15-min
15 min of heart rate variability recording
1. Introduction
Regular practice of dynamic exercise improves and maintains physical fitness, overall health and well-being of adolescents through strengthening muscles and the cardiovascular system, honing athletic skills, weight loss or maintenance, fighting against addictive practises as well as for enjoyment.1 Dynamic exercise is regulated by the autonomic nervous system (ANS) which induces marked changes in heart rate (HR) via either inhibition of parasympathetic tone or stimulation of sympathetic tone, thereby slow downing or accelerating HR, respectively.2,3
During a sustained exercise, HR raises in response to sympathetic activation and cardiodeceleration deactivation, whereas after exercise HR recovery occurs in response to simultaneous progressive cardioacceleration deactivation and a rapid parasympathetic reactivation., It is therefore reasonable to expect that these autonomic changes are dependent on the cardiac autonomic responsiveness, and that adaptive chronotropic changes to exercise-induced stress display this cardiac autonomic modulation and this depends on the intensity of exercise. During recovery after maximal exercise, HR decreases mainly because of parasympathetic reaction control while sympathetic control remains practically unchanged.4,5
Such interplay between sympathetic and vagal regulation of HR usually occurs during common autonomic challenges such as endurance exercises.6,7 The continuous assessment of ANS activity is possible by studying heart rate variability (HRV) which is considered as a standard, reliable and non-invasive approach. Post-exercise HR or HRV recovery is positively correlated with the sympathetic responsiveness during stress and its slow reduction and overall autonomic modulation of HRV.8
High values of HRV are generally interpreted as good indicator of increased of parasympathetic branch of ANS.9 It is well established that high cardiac vagal tone increases electrical stability of the heart9, 10, 11 and various HRV reduction had been shown to be predictive of cardiac mortality and morbidity.12,13 Physiological justification for this protective effect often invokes a key role for the ANS, in particular the parasympathetic branch.9
Physical Education class (PE) is an activity universally implemented in countries education systems all over the world. Its positive effects on health and school performances are well documented.14 Physical Education consists in practicing different physical exercises comprised mainly of races (e.g., speed, endurance), which are very strenuous in energy and associated with cardiovascular adaptation.15 Regarding the latter aspect, profound HR changes are commonly appear during endurance race related PE and physical training interventions.
In Cameroon, 800-m endurance races are included in the School-time PE program and national examinations. In practice, during evaluation and teaching session endurance races are perceived by schoolchildren as physically-exhausting and strenuous, and thus are generally performed at the end of PE session.16 Occurrence of deleterious cardiovascular events, such as sudden cardiac death, in schoolchildren during PE related endurance races have been noticed these last years in the Cameroon. It is commonly known that cardiovascular events mostly arise during post-exercise recovery phase.17, 18, 19, 20 Likewise, a study previously reported the appearance of several life-threatening cardiac rhythm and conduction disorders among school adolescents during recovery phase after endurance run.21
Several authors outlined the clinical value of measuring autonomous function during post-exercise recovery phase for prognostic of cardiovascular disorders. It was showed that delayed cardiovascular autonomic recovery was associated with cardiovascular events in general population.22, 23, 24 Therefore, parasympathetic recovery, after exercise test, is studied as an important indicator of prognosis and mortality.25,26
In adolescents, behavior of cardiovascular autonomic regulation during recovery is still elusive, especially in those performing 800-m endurance races at school. However, available data in adolescents pointed out that low HRV was strongly associated with a cluster of cardiovascular risk factors27 while delayed of post-exercise parasympathetic reactivation was a powerful marker of arrhythmias.28, 29, 30, 31 Post-exercise HRV recovery depends on many parameters as the type of exercise,32 training33 modalities, and exercise intensity.34,35 It is in this context of post-exercise arrhythmias and recovery disorders that, the present study was designed to analyze association between 800-m run load and post-exercise HRV recovery among Cameroonian male schoolchildren.
2. Materials and methods
2.1. Participants
This study took place at a local government high schools in the town of Douala, Cameroon. Forty-two male active adolescents practicing PE regularly non-trained in 800-m, took part in the study. Students with documented cardiovascular or respiratory disorders, and under medication were not included. Prior to field experimentations, parents/tutors of adolescents and school administration staff were informed about the aim, specific objectives and procedures of the study. Participants were asked to hydrate at least 4 h after the last meal, and avoid any strenuous exercises and consumption of coffee and alcohol beverages within 24 hours (h) before field experimentation.36 Experimentations were carried out between 4.00 p.m. and 5.00 p.m. at ambient temperature of 20–23 °C to control the influence of circadian rhythms.
2.2. Ethical approval statement
Adolescents were carefully informed about the experiment procedures and an appropriate signed informed consent document was gave by their parents/legal tutors. The study was approved by the University of Douala Institutional Ethic Committee for Human Research (NO: 1177CEI-UDo/11/2017/T). The protocol was complied with the human and animal experimentation policy statement guidelines of the American College of Sport Medicine and in accordance with the Helsinki Declaration as amended in Seoul in October 2008.
2.3. Experimental procedures
The experimental protocol consisted of mimicking the processes of evaluation of school adolescents during PE session with 800-m. The protocol was performed on a closed track of 200 m by two investigators, one located at the starting block and the other one at the arrival. Participants were invited to perform the protocol starting with free warm up majority constitutes by stretching and low run then followed by 800-m endurance run. Participants were grouped (two participants in each group) and encouraged by investigators during endurance race to obtain best performances. Upon ending the race, each adolescent was asked to recover in supine position for 15 min without chattering or carrying out abrupt movements in order to make stable parasympathetic reactivation and HRV signal in accordance to the Task Force recommendations.9
2.4. Parameters measurement
2.4.1. Anthropometry and blood pressure
Weight, percentage of fatty mass were measured using an electronic impedancemeter scale (Tanita BC-532, Kyoto, Japan) and body mass index (BMI) calculated. Blood pressure (BP) was measured before the race and after a resting period of 15 min using a BP monitor, Omron M6 (Kyoto, Japan).
2.4.2. Heart rate variability
HR (The R-R interval) series were recorded continuously using a valid and reliable heart rate monitor Polar RS800CX (Electro Oy, Kempele, Finland) with a sampling rate of 1 000 Hz.37 Before exercise, HR was recorded according to the recommendations of the task force, i.e. 7 min on supine position and 8 min on standing without movement (Fig. 1). The R-R interval series recorded data were extracted from a computer using the processing program Polar Protrainer 5.1 (Polar Electro, Kempele, Finland) and occasional ectopic beats (irregularity of the heart rhythm involving extra or skipped heartbeats; extra systoles and consecutive compensatory pause) were visually identified and manually replaced with interpolated adjacent HR interval values. Following data transfer, HR curves were exported in text format to Kubios HRV v2.2 (Biosignal Analysis and Medical Imaging Group, Joensuu, Finland)38 for the analysis of R-R intervals in time and frequency-domain. Recovery related to post-exercise HRV was studied during 5 min (HRV5-min) and 15- min (HRV15-min) according to recommendations of task force of 1996.9 The kinetic of HR and HRV recovery was studied 5-min immediately after the run and 10-min (5-15-min) after the previous phase of recovery.
Fig. 1.
Protocol of the study m: meter, min: minutes, HRV: Heart rate variability.
2.4.3. Rating of Perceived Exertion (RPE), performance and blood lactate concentration (BLa)
Perceived exertion of the protocol was evaluated using the Borg's rating perceived exertion 6–20 scale.39 A subjective verbal-anchored scale was shown to the participants after completion of the 800-m endurance run. Performance was recorded in seconds. BLa was measured between the third and the fifth minute after exercise in accordance of the recommendation40 using a Lactate Scout (SensLab GMBH, Germany).
2.5. Statistical analysis
Statistical analyses were performed using StatView 5.0 software (SAS institute, Inc., USA). Values were expressed as mean ± standard deviation (SD). The natural logarithm transformation (ln) was applied for absolute HRV parameters and to meet the normality requirements of parametric statistical analysis. Pearson's correlation coefficient was used to study link between BLa, rating perceived exertion (RPE), exercise duration (performance), and post-exercise HRV parameters. The student t-paired test was performed to compare means of HR and HRV components at each study phase of recovery. Statistical significance was set at p-value < 0.05.
3. Results
Mean values of participants’ anthropometric and hemodynamic parameters are presented in Table 1.
Table 1.
Age, anthropometric and hemodynamic characterization and exercise characteristics of participants.
| Parameters | Mean ± SD |
|---|---|
| Age (years) | 17 ± 1 |
| Height (cm) | 169.5 ± 8.6 |
| Weight (kg) | 58.4 ± 8.1 |
| Body fat (%) | 8.32 ± 3.84 |
| BMI (kg/m2) | 20.3 ± 2.3 |
| SBP (mmHg) | 118 ± 11 |
| DBP (mmHg) | 71 ± 8 |
| HR0 (bpm) |
79 ± 3 |
|
Exercise characteristics | |
| Distance (m) | 800 |
| RPE | 13 ± 1 |
| Exercise duration (s) | 205 ± 26 |
| Speed (m⋅s−1) | 3.96 ± 0.49 |
| BLa (mmol⋅L−1) | 10.8 ± 4.1 |
| HR peak (bpm) | 197 ± 10 |
SD: Standard deviation; BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; HR0: resting heart rate, RPE: Rating of Perceived Exertion; BLa: Blood lactate concentration.
Correlations between BLa, RPE, performance and post-exercise HRV HRV5-minare presented in Table 2 outline a significant correlation between exercise load and post-exercise HRV parameters in time-domain. Thus in HRV5-minof time-domain, RPE, exercise duration and BLa were negatively correlated with RR and SDNN (p < 0.05).
Table 2.
Correlation between exercise load and HRV5-min.
| BLa (mmol·L−1) | RPE | Exercise duration (s) | |
|---|---|---|---|
| Time-domain | |||
| RR (ln ms) | −0.47∗∗ | −0.27 | 0.22 |
| SDNN (ln ms) | −0.38∗ | −0.44∗∗ | −0.40∗∗ |
| RMSSD (ln ms) | −0.56∗∗∗ | −0.38∗ | 0.16 |
| Frequency-domain | |||
| LF (ln ms2) | −0.64∗∗∗ | −0.44∗∗ | −0.23 |
| HF (ln ms2) | −0.58∗∗∗ | −0.39∗ | −0.38∗ |
| TP (ln ms2) | −0.61∗∗∗ | −0.49∗∗ | 0.20 |
BLa: Blood lactate concentration; RPE: Rating of Perceived Exertion; RR: mean time between normal R waves of ECG; RMSSD: Root Mean Square of successive differences; SDNN: Standard Deviation of Normal to Normal; LF: Low Frequency, HF: High Frequency, TP: Total Power; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
A strong negative correlation was found between RMSSD and BLa (p < 0.001); exercise duration and SDNN (p < 0.01). Likewise, BLa was strongly associated with LF, HF and TP in frequency-domain (p < 0.001). In addition, RPE was negatively linked to LF and TP (p < 0.01) while exercise duration were negatively associated with HF (p < 0.05).
The correlations between BLa, RPE, performance and HRV15-mins post-exercise HRV recovery are summarized in Table 3. In time-domain, negative significant (p < 0.01) correlations were found between BLa and RR, and RMSSD. Moreover, BLa was strongly associated with SDNN (p < 0.001) while RPE and SDNN were correlated (p < 0.01). In frequency-domain, BLa and RPE were negatively associated (p < 0.01) to LF and HF and TP. In addition, exercise duration was slightly correlated with HF (p < 0.05).
Table 3.
Correlations between exercise load and post-exercise HRV15-min.
| BLa (mmol·L−1) | RPE | Exercise duration (s) | |
|---|---|---|---|
| Time-domain | |||
| RR (ln ms) | −0.52∗∗ | −0.40∗ | 0.22 |
| SDNN (ln ms) | −0.68∗∗∗ | −0.53∗∗ | −0.11 |
| RMSSD (ln ms) | −0.53∗∗ | −0.44∗∗ | 0.16 |
| Frequency-domain | |||
| LF (ln ms2) | −0.49∗∗ | −0.51∗∗∗ | −0.15 |
| HF (ln ms2) | −0.55∗∗∗ | −0.50∗∗ | −0.36∗ |
| TP (ln ms2) | −0.50∗∗ | −0.49∗∗ | 0.37 |
RPE: Rating of Perceived Exertion; BLa:Blood lactate concentration; RR mean: mean time between normal R waves of ECG; RMSSD: Root Mean Square of successive differences; SDNN: Standard Deviation of Normal to Normal; LF: Low Frequency, HF: High Frequency, TP: Total Power; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
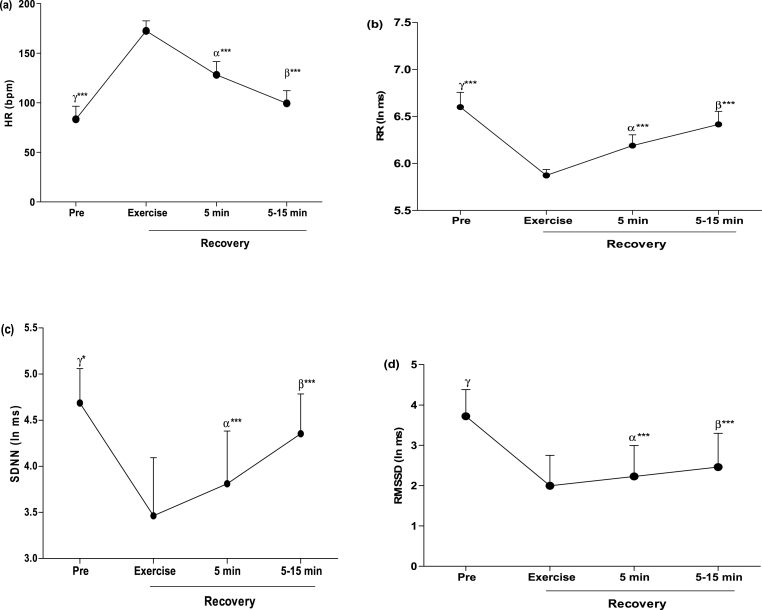
The recovery kinetic of post-exercise HR and HRV in time-domain as illustrated in Fig. 2 shows a significant (p < 0.001) recovery of HR and HRV after exercise. HR at 5- and 5-15-min post-exercise significantly (p < 0.001) decreased than the one reported at after 800-m run. On the other hand, RR, SDNN and RMSSD at 5-min and 5–15 min increased (p < 0.001) compared to that observed immediately after exercise.
Fig. 2.
The recovery kinetic of time-domain of HRV. (a): HR, (b): RR, (c): SDNN and (d): RMSSD. HR: heart rate, RR mean: mean time between normal R waves of ECG; RMSSD: Root Mean Square of successive differences; SDNN: Standard Deviation of Normal to Normal. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. α: comparison between end of exercise and 5-min post exercise, β; comparison between 5-min and 5-15-min post exercise, γ: comparison between pre exercise and 5-15-min post exercise.
Fig. 3 depicts kinetic of post-exercise recovery HRV in frequency-domain. LF, HF and TP recorded at 5-min and 5–15 min of recovery were significantly (p < 0.001) high compared to that noticed immediately after exercise (p < 0.001). These parameters of HRV in time and frequency domain increased (p < 0.05) after 800-m run during the recovery without reaching pre-exercise values.
Fig. 3.
The recovery kinetic of frequency-domain of HRV (a): LF, (b): HF, and (c): TP. LF: Low Frequency, HF: High Frequency, TP: Total Power, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, α: comparison between end of exercise and 5-min post exercise, β: comparison between 5-min and 5-15-min post exercise, γ: comparison between pre exercise and 5-15-min post exercise.
4. Discussion
We aimed to determine the effect of 800-m loads on post-exercise cardiac autonomic recovery and its kinetic among school adolescents in Douala, Cameroon.
Findings outline that BLa and RPE were negatively correlated with post-exercise HRV, and this is in accordance with previous authors.41, 42, 43, 44, 45, 46 Kaikkonen et al.46 assessed the effects of training loads on post-exercise 3-min HRV among athletes, and reported strong and negative correlation between HRV, BLa and RPE. Likewise, we found a negative correlation between BLa and 15-min of HRV as did Al Haddad et al.47 on nocturnal HRV after a supramaximal intermittent exercise. These findings suggest that BLa could be an interesting physiological and metabolic marker that slow HRV during post-exercise recovery of 15-min.
Negative associations were found between exercise duration and post-exercise short- and long -term HRV in time-domain (SDNN) and frequency-domain (HF). Some authors stated that increase in duration of moderate to high intensity exercise could elicit slow recovery of parasympathetic reactivation-related short/long term of HRV recording.44,48 However, it should be noted in the present study the absence of real competition between students during the protocol as observed during their evaluation process during PE would have highlighted important associations between physical performance and HRV following to acute cardiovascular adaptations constitutes a limitation to this study.
Most of statistically significant correlations between exercise characteristics and post-exercise HRV were seen in frequency domain. This confirms previous investigations that largely used frequency domain as proxy parameter for evaluating post-exercise cardiac autonomic recovery. Moreover, frequency-domain based HRV analysis is commonly used to appraise changing conditions and physiological tasks such as exercise test and recovery.49
RPE, BLa and exercise duration post-exercise were negatively associated to components of HRV (LF, HF and TP) which is probably related to increased sympathetic activity.
Physiologically, this result would be related to exercise load which is closely linked to the exponential elimination of metabolites resulting from the catabolism of energy substrates in the muscles associated with a slow decline in circulating cortisol and catecholamine concentration during post-exercise recovery. This is followed by a subsequent decrease in vagal indices. In addition, exercise intensity has been found to influence HRV both during and after immediate exercise41,43 and HRV15-mins recovery.42,49,50 Other mechanisms that may help explain the negative association between exercise intensity and HRV during the recovery phase in the present study is the recovery pattern of baroreflex sensitivity. Post-exercise recovery of set point and baroreceptor sensitivity is closely related to increased parasympathetic nervous system activity immediately after exercise cessation.51,52 It is established that post-exercise baroreflex recovery is also influenced by exercise intensity.52 It should be noted that the increase in intensity during exercise results in significant metabolite production which could delay baroreflex recovery by increasing the baroreflex set point with a detrimental effect on HRV recovery.53
Findings from the present study seem to indicate that exercise intensity can be a major factor shaping post-exercise HRV during immediate or HRV15-mins recovery in Cameroonian adolescents. Indeed, on time domain analysis, HRV vagal indices were significantly increased during 15-min recovery, and this is in line with results of Gladwell et al.54 in young healthy non-trained females, and Buchheit et al.55 in trained adolescents during 5-min recovery. Regarding frequency domain analysis, 5- and 15-min HRV values were significantly higher than that noted at the end of exercise. Similar results were reported in untrained individuals.49,54 Whatever an aerobic exercise testing in children or adolescents can make acute adaptation on ANS. But it's important to see effect exercise intensity on parasympathetic recovery, because a slow parasympathetic reactivation after a sub-maximal/maximal can because of cardiac electric conduction troubles that can harm adolescent health.
Despite negatives association between marker of 800-m run intensity, significant parasympathetic reactivation was observed in both time and frequency domain HRV during recovery phase (Fig. 2, Fig. 3). It is well known that physiological mechanisms, mainly loss of central command and baroreflex activation, selecting for fast changes in cardiac autonomic modulation occur in cardiac pre-load and contractility immediately after physical exercise is completed.55, 56, 57, 58, 59 Early increased vagal activation during recovery is associated with inhibition of sympathetic autonomic system which is predominant contributors to early HRV recovery,2,60 associated to the fast decrease of plasma cortisol and catecholamine's which have been produced to make in place physiological and metabolic adaptations due to exercise. Besides, recovery position could also explain this finding. It was documented that supine position, used in the present study, can speed up parasympathetic reactivation.61
5. Conclusion
The practice of PE in order to preserve its beneficial effects on health in adolescence is crucial, especially the practice of endurance races. However, aerobic exercise in adolescents makes acute physiological adaptations on ANS which must quickly be restored a few minutes after the cessation of an effort. Therefore, from the perspective that endurance races do not have adverse effects on the health of adolescents, this study has shown that psychological (RPE) metabolic (BLa) parameters of 800-m and exercise duration were negatively associated with post-exercise cardiac autonomic recovery. An increase in these parameters shows a slow vagal indexes recovery. Thus, 800-m endurance race loads could be significant factors modulating post-exercise HRV recovery in adolescents.
6. Limitations
The present study did not take into account the gender, competitive, physical performance, and the training aspects of adolescents on post-exercise neurovegetative regulation. For future studies it would be important to look for eventual links between cardiac morpho-functional changes using ECG and the kinetics of HRV recovery for identifying factors likely to be causes of a slow parasympathetic reactivation and how HRV kinetic differ between the youth with different activity or fitness levels.
Submission statement
All authors have read and agree with manuscript content and their position. The manuscript has not been published and is not under consideration for publication elsewhere.
Ethical approval statement
Participants gave informed consent document signed by their parents/legal tutors. The study was approved by the University of Douala Institutional Ethic Committee for Human Research (NO: 1177CEI-UDo/11/2017/T). The protocol was complied in accordance with the Helsinki Declaration as amended in Seoul in October 2008.
Authors’ contributions
MNJ: conceived the study, collected data, performed the data analysis, interpreted the results and drafted the manuscript. ET, LGG, WM: Collected datas, BLEC: performed the data analysis and drafted the manuscript, KFLP, CNO: performed data analysis, proofread English language, and revised manuscript for important intellectual content, GWR: drafted the manuscript; GLG, MMW: collected data; CAA, JG, BN, NOC: drafted the manuscript; ANPB, TA, supervised the study and draft the manuscript; BB, FR, MSH conceived et supervised the study.
Conflict of interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Acknowledgments
We thank the administrative staff and sport and physical education teachers of government secondary school of Cite des Palmiers. We also extend our acknowledgments to all the schoolchildren who accepted to participate in this study as well as their parents/guardians who gave their approval.
References
- 1.Kumar B., Robinson R., Till S. Physical activity and health in adolescence. Clin Med. 2015;15(3):267–272. doi: 10.7861/clinmedicine.15-3-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Savin W.M., Davidson D.M., Haskell W.L. Autonomic contribution to heart rate recovery from exercise in humans. J Appl Physiol Respir Environ Exerc Physiol. 1982;53(6):1572–1575. doi: 10.1152/jappl.1982.53.6.1572. [DOI] [PubMed] [Google Scholar]
- 3.Pierpont G.L., Stolpman D.R., Gornick C.C. Heart rate recovery post-exercise as an index of parasympathetic activity. J Auton Nerv Syst. 2000;80(3):169–174. doi: 10.1016/s0165-1838(00)00090-4. [DOI] [PubMed] [Google Scholar]
- 4.Lahiri M.K., Kannankeril P.J., Goldberger J.J. Assessment of autonomic function in cardiovascular disease: physiological basis and prognostic implications. J Am Coll Cardiol. 2008;51(18):1725–1733. doi: 10.1016/j.jacc.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 5.Ohuchi H., Suzuki H., Yasuda K., Arakaki Y., Echigo S., Kamiya T. Heart rate recovery after exercise and cardiac autonomic nervous activity in children. Pediatr Res. 2000;47(3):329–335. doi: 10.1203/00006450-200003000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Orizio C., Perini R., Comandè A., Castellano M., Beschi M., Veicsteinas A. Plasma catecholamines and heart rate at the beginning of muscular exercise in man. Eur J Appl Physiol Occup Physiol. 1988;57(5):644–651. doi: 10.1007/BF00418477. [DOI] [PubMed] [Google Scholar]
- 7.Tulppo M.P., Mäkikallio T.H., Takala T.E., Seppänen T., Huikuri H.V. Quantitative beat-to-beat analysis of heart rate dynamics during exercise. Am J Physiol. 1996;271(1 Pt 2):244–252. doi: 10.1152/ajpheart.1996.271.1.H244. [DOI] [PubMed] [Google Scholar]
- 8.Molina G.E., Da Cruz C.J.G., Fontana K.E., Soares E.M.K.V.K., Porto L.G.G., Junqueira L.F., Jr. Post-exercise heart rate recovery and its speed are associated with cardiac autonomic responsiveness following orthostatic stress test in men. Scand Cardiovasc J. 2021;55(4):220–226. doi: 10.1080/14017431.2021.1879394. [DOI] [PubMed] [Google Scholar]
- 9.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- 10.Bonilla I.M., Belevych A.E., Sridhar A., et al. Endurance exercise training normalizes repolarization and calcium-handling abnormalities, preventing ventricular fibrillation in a model of sudden cardiac death. J Appl Physiol. 2012;113(11):1772–1783. doi: 10.1152/japplphysiol.00175.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schomer A.C., Nearing B.D., Schachter S.C., Verrier R.L. Vagus nerve stimulation reduces cardiac electrical instability assessed by quantitative T-wave alternans analysis in patients with drug resistant focal epilepsy. Epilepsia. 2014;55(12):1996–2002. doi: 10.1111/epi.12855. [DOI] [PubMed] [Google Scholar]
- 12.Qiu S., Cai X., Sun Z., et al. Heart rate recovery and risk of cardiovascular events and all-cause mortality: a meta-analysis of prospective cohort studies. J Am Heart Assoc. 2017;6(5) doi: 10.1161/JAHA.117.005505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sydo N., Sydo T., Gonzalez Carta K.A., et al. Prognostic performance of heart rate recovery on an exercise test in a primary prevention population. J Am Heart Assoc. 2018;7(7) doi: 10.1161/JAHA.117.008143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bailey R. Physical education and sport in schools: a review of benefits and outcomes. J Sch Health. 2006;76(8):397–401. doi: 10.1111/j.1746-1561.2006.00132.x. [DOI] [PubMed] [Google Scholar]
- 15.Cavalcante P.A.M., Perilhão M.S., Da Silva A.A., Serra A.J., Júnior A.F., Bocalini D.S. Cardiac remodeling and physical exercise: a brief review about concepts and adaptations. Int J Sports Sci. 2016;6(2):52–61. doi: 10.5923/j.sports.20160602.06. [DOI] [Google Scholar]
- 16.Mekoulou N.J., Temfemo A., Assomo Ndemba P.B., et al. Is there an effect of the order of realization of sprint and endurance for intermittent test? JPES. 2016;16:982–987. doi: 10.7752/jpes.2016.03155. [DOI] [Google Scholar]
- 17.Siscovick D.S., Weiss N.S., Fletcher R.H., et al. The incidence of primary cardiac arrest during vigorous exercise. N Engl J Med. 1984;311(14):874–877. doi: 10.1056/NEJM198410043111402. [DOI] [PubMed] [Google Scholar]
- 18.Coumel P. Paroxysmal atrial fibrillation: a disorder of autonomic tone? Eur Heart J. 1994;15(Suppl A):9–16. doi: 10.1093/eurheartj/15.suppl_a.9. [DOI] [PubMed] [Google Scholar]
- 19.Huikuri H.V. Heart rate dynamics as a marker of vulnerability to atrial fibrillation. J Cardiovasc Electrophysiol. 2008;19(9):913–914. doi: 10.1111/j.1540-8167.2008.01197.x. [DOI] [PubMed] [Google Scholar]
- 20.Von Klot S., Mittleman M.A., Dockery D.W., et al. Intensity of physical exertion and triggering of myocardial infarction: a case-crossover study. Eur Heart J. 2008;29(15):1881–1888. doi: 10.1093/eurheartj/ehn235. [DOI] [PubMed] [Google Scholar]
- 21.Mekoulou Ndongo J., Assomo Ndemba P.B., Temfemo A., et al. Pre- and post-exercise electrocardiogram pattern modifications in apparently healthy school adolescents in Cameroon. Int J Adolesc Med Health. 2017;31(6):/j/ijamh.2019.31.issue-6/ijamh-2017-0071/ijamh-2017-0071.xml. doi:10.1515/ijamh-2017-0071. [DOI] [PubMed]
- 22.Cole C.R., Blackstone E.H., Pashkow F.J., Snader C.E., Lauer M.S. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341(18):1351–1357. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- 23.Cole C.R., Foody J.M., Blackstone E.H., Lauer M.S. Heart rate recovery after submaximal exercise testing as a predictor of mortality in a cardiovascularly healthy cohort. Ann Intern Med. 2000;132(7):552–555. doi: 10.7326/0003-4819-132-7-200004040-00007. [DOI] [PubMed] [Google Scholar]
- 24.Jouven X., Empana J.P., Schwartz P.J., Desnos M., Courbon D., Ducimetière P. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med. 2005;352(19):1951–1958. doi: 10.1056/NEJMoa043012. [DOI] [PubMed] [Google Scholar]
- 25.Dewey F.E., Freeman J.V., Engel G., et al. Novel predictor of prognosis from exercise stress testing: heart rate variability response to the exercise treadmill test. Am Heart J. 2007;153(2):281–288. doi: 10.1016/j.ahj.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Van de Vegte Y.J., Van der Harst P., Verweij N. Heart rate recovery 10 seconds after cessation of exercise predicts death. J Am Heart Assoc. 2018;5;7(8) doi: 10.1161/JAHA.117.008341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farah B.Q., Barros M.V., Balagopal B., Ritti-Dias R.M. Heart rate variability and cardiovascular risk factors in adolescent boys. J Pediatr. 2014;165(5):945–950. doi: 10.1016/j.jpeds.2014.06.065. [DOI] [PubMed] [Google Scholar]
- 28.Wang N.C., Chicos A., Banthia S., et al. Persistent sympathoexcitation long after submaximal exercise in subjects with and without coronary artery disease. Am J Physiol Heart Circ Physiol. 2011;301(3):912–920. doi: 10.1152/ajpheart.00148.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith L.L., Kukielka M., Billman G.E. Heart rate recovery after exercise: a predictor of ventricular fibrillation susceptibility after myocardial infarction. Am J Physiol Heart Circ Physiol. 2005;288(4):1763–1769. doi: 10.1152/ajpheart.00785.2004. [DOI] [PubMed] [Google Scholar]
- 30.Lieve K.V.V., Dusi V., Van der Werf C., et al. Heart rate recovery after exercise is associated with arrhythmic events in patients with catecholaminergic polymorphic ventricular tachycardia. Circ Arrhythm Electrophysiol. 2020;13(3) doi: 10.1161/CIRCEP.119.007471. [DOI] [PubMed] [Google Scholar]
- 31.Reed M.J., Robertson C.E., Addison P.S. Heart rate variability measurements and the prediction of ventricular arrhythmias. QJM. 2005;98(2):87–95. doi: 10.1093/qjmed/hci018. [DOI] [PubMed] [Google Scholar]
- 32.Ye Y., Tong T.K., Kong Z., Tao E.D., Ying X., Nie J. Cardiac autonomic disturbance following sprint-interval exercise in untrained young males: does exercise volume matter? J Exerc Sci Fit. 2022;20(1):32–39. doi: 10.1016/j.jesf.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peçanha T., De Paula-Ribeiro M., Nasario-Junior O., de Lima J.R. Post-exercise heart rate variability recovery: a time-frequency analysis. Acta Cardiol. 2013;68(6):607–613. doi: 10.1080/ac.68.6.8000008. [DOI] [PubMed] [Google Scholar]
- 34.Michael S., Graham K.S., Oam Davis GM. Cardiac autonomic responses during exercise and post-exercise recovery using heart rate variability and systolic time intervals-a review. Front Physiol. 2017;29(8):301. doi: 10.3389/fphys.2017.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michael S., Jay O., Halaki M., Graham K., Davis G.M. Submaximal exercise intensity modulates acute post-exercise heart rate variability. Eur J Appl Physiol. 2016;116(4):697–706. doi: 10.1007/s00421-016-3327-9. [DOI] [PubMed] [Google Scholar]
- 36.Wiklund U., Karlsson M., Oström M., Messner T. Influence of energy drinks and alcohol on post-exercise heart rate recovery and heart rate variability. Clin Physiol Funct Imag. 2009;29(1):74–80. doi: 10.1111/j.1475-097X.2008.00837.x. [DOI] [PubMed] [Google Scholar]
- 37.Barbosa M.P., da Silva N.T., de Azevedo F.M., Pastre C.M., Vanderlei L.C. Comparison of Polar® RS800G3™ heart rate monitor with Polar® S810i™ and electrocardiogram to obtain the series of RR intervals and analysis of heart rate variability at rest. Clin Physiol Funct Imag. 2016;36(2):112–117. doi: 10.1111/cpf.12203. [DOI] [PubMed] [Google Scholar]
- 38.Tarvainen M.P., Niskanen J.P., Lipponen J.A., Ranta-Aho P.O., Karjalainen P.A. Kubios HRV-heart rate variability analysis software. Comput Methods Progr Biomed. 2014;113(1):210–220. doi: 10.1016/j.cmpb.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 39.Borg G.A. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381. [PubMed] [Google Scholar]
- 40.Lacour J.R., Bouvat E., Barthélemy J.C. Post-competition blood lactate concentrations as indicators of anaerobic energy expenditure during 400-m and 800-m races. Eur J Appl Physiol Occup Physiol. 1990;61(3-4):172–176. doi: 10.1007/BF00357594. [DOI] [PubMed] [Google Scholar]
- 41.Casties J.F., Mottet D., Le Gallais D. Non-linear analyses of heart rate variability during heavy exercise and recovery in cyclists. Int J Sports Med. 2006;27(10):780–785. doi: 10.1055/s-2005-872968. [DOI] [PubMed] [Google Scholar]
- 42.Seiler S., Haugen O., Kuffel E. Autonomic recovery after exercise in trained athletes: intensity and duration effects. Med Sci Sports Exerc. 2007;39(8):1366–1373. doi: 10.1249/mss.0b013e318060f17d. [DOI] [PubMed] [Google Scholar]
- 43.Martinmäki K., Rusko H. Time-frequency analysis of heart rate variability during immediate recovery from low and high intensity exercise. Eur J Appl Physiol. 2008;102(3):353–360. doi: 10.1007/s00421-007-0594-5. [DOI] [PubMed] [Google Scholar]
- 44.Kaikkonen P., Rusko H., Martinmäki K. Post-exercise heart rate variability of endurance athletes after different high-intensity exercise interventions. Scand J Med Sci Sports. 2008;18(4):511–519. doi: 10.1111/j.1600-0838.2007.00728.x. [DOI] [PubMed] [Google Scholar]
- 45.Kaikkonen P., Hynynen E., Mann T., Rusko H., Nummela A. Can HRV be used to evaluate training load in constant load exercises? Eur J Appl Physiol. 2010;108(3):435–442. doi: 10.1007/s00421-009-1240-1. [DOI] [PubMed] [Google Scholar]
- 46.Kaikkonen P., Hynynen E., Mann T., Rusko H., Nummela A. Heart rate variability is related to training load variables in interval running exercises. Eur J Appl Physiol. 2012;112(3):829–838. doi: 10.1007/s00421-011-2031-z. [DOI] [PubMed] [Google Scholar]
- 47.Al Haddad H., Laursen P.B., Ahmaidi S., Buchheit M. Nocturnal heart rate variability following supramaximal intermittent exercise. Int J Sports Physiol Perform. 2009;4(4):435–447. doi: 10.1123/ijspp.4.4.435. [DOI] [PubMed] [Google Scholar]
- 48.Hynynen E., Vesterinen V., Rusko H., Nummela A. Effects of moderate and heavy endurance exercise on nocturnal HRV. Int J Sports Med. 2010;31(6):428–432. doi: 10.1055/s-0030-1249625. [DOI] [PubMed] [Google Scholar]
- 49.Terziotti P., Schena F., Gulli G., Cevese A. Post-exercise recovery of autonomic cardiovascular control: a study by spectrum and cross-spectrum analysis in humans. Eur J Appl Physiol. 2001;84(3):187–194. doi: 10.1007/s004210170003. [DOI] [PubMed] [Google Scholar]
- 50.Parekh A., Lee C.M. Heart rate variability after isocaloric exercise bouts of different intensities. Med Sci Sports Exerc. 2005;37(4):599–605. doi: 10.1249/01.mss.0000159139.29220.9a. [DOI] [PubMed] [Google Scholar]
- 51.Coote J.H. Recovery of heart rate following intense dynamic exercise. Exp Physiol. 2010;95(3):431–440. doi: 10.1113/expphysiol.2009.047548. [DOI] [PubMed] [Google Scholar]
- 52.Niemela T.H., Kiviniemi A.M., Hautala A.J., Salmi J.A., Linnamo V., Tulppo M.P. Recovery pattern of baroreflex sensitivity after exercise. Med Sci Sports Exerc. 2008;40(5):864–870. doi: 10.1249/MSS.0b013e3181666f08. [DOI] [PubMed] [Google Scholar]
- 53.Stuckey M.I., Tordi N., Mourot L., et al. Autonomic recovery following sprint interval exercise. Scand J Med Sci Sports. 2012;22(6):756–763. doi: 10.1111/j.1600-0838.2011.01320.x. [DOI] [PubMed] [Google Scholar]
- 54.Gladwell V.F., Sandercock G.R., Birch S.L. Cardiac vagal activity following three intensities of exercise in humans. Clin Physiol Funct Imag. 2010;30(1):17–22. doi: 10.1111/j.1475-097X.2009.00899.x. [DOI] [PubMed] [Google Scholar]
- 55.Miles D.S., Sawka M.N., Hanpeter D.E., Foster J.E., Jr., Doerr B.M., Frey M.A. Central hemodynamics during progressive upper- and lower-body exercise and recovery. J Appl Physiol Respir Environ Exerc Physiol. 1984;57(2):366–370. doi: 10.1152/jappl.1984.57.2.366. [DOI] [PubMed] [Google Scholar]
- 56.Plotnick G.D., Becker L.C., Fisher M.L. Changes in left ventricular function during recovery from upright bicycle exercise in normal persons and patients with coronary artery disease. Am J Cardiol. 1986;58(3):247–251. doi: 10.1016/0002-9149(86)90056-1. [DOI] [PubMed] [Google Scholar]
- 57.Arai Y., Saul J.P., Albrecht P., et al. Modulation of cardiac autonomic activity during and immediately after exercise. Am J Physiol. 1989;256(1 Pt 2):132–141. doi: 10.1152/ajpheart.1989.256.1.H132. [DOI] [PubMed] [Google Scholar]
- 58.O'Leary D.S. Autonomic mechanisms of muscle metaboreflex control of heart rate. J Appl Physiol. 1993;4(4):1748–1754. doi: 10.1152/jappl.1993.74.4.1748. [DOI] [PubMed] [Google Scholar]
- 59.Oida E., Moritani T., Yamori Y. Tone-entropy analysis on cardiac recovery after dynamic exercise. J Appl Physiol. 1997;82(6):1794–1801. doi: 10.1152/jappl.1997.82.6.1794. [DOI] [PubMed] [Google Scholar]
- 60.Kannankeril P.J., Le F.K., Kadish A.H., Goldberger J.J. Parasympathetic effects on heart rate recovery after exercise. J Invest Med. 2004;52(6):394–401. doi: 10.1136/jim-52-06-34. [DOI] [PubMed] [Google Scholar]
- 61.Barak O.F., Jakovljevic D.G., Popadic Gacesa J.Z., Ovcin Z.B., Brodie D.A., Grujic N.G. Heart rate variability before and after cycle exercise in relation to different body positions. J Sports Sci Med. 2010;9(2):176–182. [PMC free article] [PubMed] [Google Scholar]