Abstract
Vibrio parahaemolyticus is an important food-borne enteropathogen that encounters various adverse conditions in its native environment or during infection. Effects of mild acid treatment on survival under stress conditions, enteropathogenicity, and protein production in this pathogen were investigated. Logarithmically grown cells, at pH 7.5 shifted to pH 5.0 for 30 min, were more resistant to subsequent acid challenge at pH 4.4. A two-phase adaptive procedure (pH 5.8 for 30 min; pH 5.0 for 30 min) was better than a single-phase procedure for enhancing the acid tolerance of this pathogen. The acid-adapted cells were cross-protected against the challenges of low salinity and thermal inactivation. One-dimensional polyacrylamide gel electrophoresis revealed that proteins with molecular masses of 6.4, 9.0, 13.6, 16.3, 18.9, 22.9, 24.4, 28.3, 33.9, 36.9, 41.2, 47.6, 58.1, 65.6, 80.5, 88.2, and 96.9 kDa were induced or significantly enhanced, while proteins of 25.3, 30.1, 30.7, and 91.7 kDa were significantly inhibited. Two-dimensional polyacrylamide gel electrophoresis revealed that 20 species of proteins were induced or significantly enhanced, while 26 species were inhibited. In assays conducted using the suckling mouse model, enteropathogenicity of the acid-adapted cells was significantly enhanced in terms of intestine/body weight ratio and in vivo recovery of infected cells.
Vibrio parahaemolyticus is a halophilic, gram-negative, straight to curved rod bacterium with a single polar flagellum (when grown in liquid medium) or peritrichous flagella (when grown on solid medium). It was first discovered in 1950 during a food poisoning outbreak in Osaka, Japan, and is now one of the most important food-borne pathogens in Taiwan, Japan, and other coastal regions. The high incidence of this pathogen undoubtedly results from the frequent consumption of marine foods in these regions. Clinical manifestations have included diarrhea, abdominal cramps, nausea, vomiting, headache, fever, and chills, with incubation periods ranging from 4 to 96 h (4, 16, 28).
Enterotoxigenicity of V. parahaemolyticus isolates can be determined with suckling mouse and adult mouse models, as with other enteropathogenic vibrios (13, 31, 41). However, no characteristic enterotoxin has been identified in this enteropathogen. Thermostable direct hemolysin is the major well-characterized virulence factor present in most of the clinical isolates of this pathogen (16, 39, 42). This hemolysin was found to be enterotoxigenic but less so than heat-labile enterotoxin of Escherichia coli or cholera toxin of V. cholerae (30, 39). Some virulence factors, including other heat-labile hemolysin(s), lethal toxin(s) (37), and vascular permeability factor(s) (14), have been identified but not well characterized. It is also unclear which virulence factors are regulated by environmental signals in this organism.
Enteric pathogens are exposed to substantial changes in their environment when they enter a mammalian host, and they have evolved a number of mechanisms to adapt to these changes. The pathogen can be deprived of certain nutrients, exposed to oxygen radicals and changes in pH, and bathed in degradative enzymes. In adapting to such a hostile environment, the pathogens synthesize stress proteins or other heat shock proteins, some of which are associated with pathogenesis of these pathogens (40). Environmental signals controlling the expression of coordinately regulated virulence determinants have been characterized in some enteric bacteria (27, 29). An acid tolerance response (ATR) has been demonstrated in several pathogenic bacteria, such as E. coli (36), Listeria monocytogenes (33), Streptococcus mutans (12), Aeromonas hydrophila (18), and Salmonella typhimurium (8). Intensive studies have been carried out on S. typhimurium (35).
The effects of high or low temperature, starvation, and other adverse conditions on the survival of V. parahaemolyticus have been investigated, and the presence of homologous GroEl-like proteins was identified (2, 19–21). However, protein production during the adaptive ATR has not been investigated in detail in this pathogen, and the effect of the ATR on virulence is still unclear. In this study, we examined the ATR in this pathogen and assayed the virulence of the stress-adapted cells in the suckling mouse model. The protein profile of this pathogen after mild acid treatment was analyzed by one-dimensional (1-D) and 2-D polyacrylamide gel electrophoresis (PAGE).
MATERIALS AND METHODS
Bacterial strain and cultivation.
V. parahaemolyticus ST550, a serotype K13 and KP+ strain isolated from clinical sample and originating in Japan, was used in this study. It was stocked in 10% glycerol at −85°C. It was cultured in Luria-Bertani medium (LB; Difco Laboratories, Detroit, Mich.)–3% NaCl (pH 7.5) at 37°C. Growth of bacteria was determined by measuring the absorbance at 600 nm or by the plate count method on Luria-Bertani agar (LA)–3% NaCl.
Acid adaptation and determination of survivors.
Fifty milliliters of LB–3% NaCl medium (pH 7.5), in a 250-ml Erlenmeyer flask, was inoculated with 0.1 ml of overnight culture and incubated at 37°C, with shaking at 160 rpm, until mid-exponential phase (3 h). To induce acid tolerance, the culture was acidified to pH 5.8, 5.5, or 5.0 by adding 12 N HCl.
The acid-adapted bacterial culture was challenged by acidifying the culture medium to pH 4.4 by adding 12 N HCl and incubated for various durations. For examination of the cross-protection against low salinity, the adapted culture was collected by centrifugation and resuspended in fresh LB without supplementary NaCl. To examine cross-protection against thermal inactivation, the adapted culture was incubated at 45°C.
The survivors of the experimental or control groups were counted after serial dilution in LB–3% NaCl, plated on LA–3% NaCl, and incubated at 37°C for 16 h.
Examination of stress-regulated proteins.
For the analysis of the protein profiles during the adaptation period, modified M9 medium (MM9) supplemented with 3% NaCl and 20 amino acids (each at 0.2 mg/ml) was used for the preparation of inoculum (3). Fifty milliliters of MM9–3% NaCl containing 18 amino acids (each at 0.2 mg/ml) without methionine and cysteine was inoculated with 0.1 ml of the inoculum and cultured at 37°C for 3 h. The culture was adjusted to pH 5.8, incubated for 30 min, and then adjusted to pH 5.0, and subsequently the labeling mix Pro-mix (specific activity, >1,000 Ci/mmol; containing 70% l-[35S]methionine and 30% l-[35S]cysteine; Amersham International, Buckinghamshire, England) was added at different intervals. As preparation for 1-D analysis, 15 μCi of labeling mix per ml was added, and the culture was incubated for 7 min. As preparation for 2-D analysis, 34 μCi of labeling mix per ml was added, and the culture was incubated for 15 min.
After isotope labeling, the bacterial cultures were immediately stored in an ice bath to stop reactions, and cells were collected by centrifugation. Samples subjected to 1-D electrophoresis were lysed in buffer containing, per 100 ml, 10 ml of TBS buffer (0.41 M Tris, 0.4 M boric acid, 1% sodium dodecyl sulfate [SDS] [pH 8.64]), 5 g of glucose, 185 mg of EDTA, 5 ml of 2-mercaptoethanol, 1.9 g of SDS, and 5 ml of glycerol (6). Samples subjected to 2-D electrophoresis were lysed in another lysis buffer containing, per 25 ml, 13.5 g of urea, 0.5 ml of Triton X-100, 0.5 ml of 2-mercaptoethanol, 0.5 ml of Pharmalyte 3-10, and 35 mg of phenylmethylsulfonyl fluoride. A homogeneous SDS–12.5% polyacrylamide gel (ExcelGel SDS; Pharmacia Biotech, Uppsala, Sweden) was used in 1-D electrophoresis. In 2-D electrophoresis, Immobiline DryStrip (pH 4 to 7) and ExcelGel SDS 12.5% polyacrylamide gels were used. The protein samples were resolved by 1-D and 2-D PAGE as instructed by the supplier (Pharmacia). Prestained SDS-PAGE broad-range standards (Bio-Rad Laboratories, Hercules, Calif.) were used. Autoradiography was performed with BioMax MR film (Eastman Kodak Co., Rochester, N.Y.), and the image was analyzed by Stratascan 7000 1-D and 2-D densitometry (Stratagene, La Jolla, Calif.).
Animal assays.
Enteropathogenicity of the acid-adapted cells was determined by the suckling mouse assay (41). The adapted cells were collected by centrifugation and resuspended in fresh LB–3% NaCl. Cell density was determined from the light absorption of the suspensions at 600 nm and also by the dilution plate counting method. Inocula at a cell density of 108, 0.25 × 108, or 0.1 × 108 CFU/ml were prepared. Aliquots (0.1 ml) of these inocula, with Evans blue, were inoculated intragastrically into 2- to 4-day-old suckling ICR mice and incubated at room temperature. Each group consisted of six mice. The mice were then sacrificed 3.0, 4.5, and 6.0 h postinfection for inoculation densities of 108, 0.25 × 108, and 0.1 × 108 CFU/ml, respectively, for experimental and control groups, and the intestine weight/whole body weight ratio was determined for each mouse. The intestine of each mouse was homogenized, and the population of V. parahaemolyticus was determined by serial dilution and plate counting method on thiosulfate-citrate-bile-salt sucrose (TCBS) agar (Difco) incubated at 37°C for 24 h. The incubation time preceding sacrifice was about 30 min prior to the average death time for each group, which was determined in preliminary experiment.
Statistics analysis.
Data were means of triplicate determinations. The data were analyzed by using an SPSSPC computer program with t test and analysis of variance.
RESULTS
ATR.
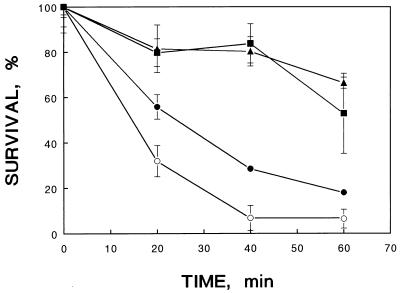
The logarithmically grown bacterial cells at pH 7.5 were acidified to pH 5.0 for 15, 30, and 60 min and subsequently subjected to low-pH challenge at pH 4.4. The adaptation period in a mild acid environment significantly enhanced the acid tolerance of this pathogen. The survival rate was greatest for cells previously acid adapted for 30 or 60 min (Fig. 1).
FIG. 1.
Effect of mild acid treatment time on the acid tolerance of V. parahaemolyticus ST550. The exponential-phase culture was adapted at pH 5.0 for 15 (•), 30 (▴), and 60 (▪) min, and the adapted cells were challenged at pH 4.4. ○, control, nonadapted cells.
The effect of different acidity levels on inducing the ATR was demonstrated. Cells adapted at pH 5.0, 5.5, or 5.8 for 30 min showed significantly greater survival rates than the nonadapted control cells, with pH 5.0 the most effective for inducing acid tolerance (data not shown).
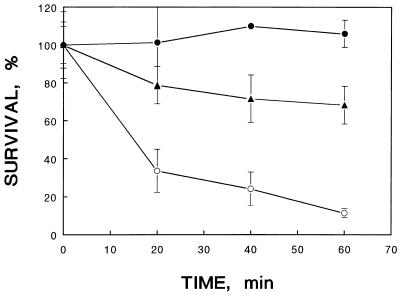
For acid adaptation, bacterial cultures were adapted at pH 5.0 for 1 h (single-phase adaptation) or at pH 5.8 for 30 min followed by pH 5.0 for another 30 min (two-phase adaptation). The survival rate after pH 4.4 challenge showed that two-phase acid adaptation provided significantly greater protection than single-phase adaptation (Fig. 2). This experiment was also repeated in MM9–3% NaCl medium containing 20 amino acids, in place of LB–3% NaCl medium. Logarithmically grown cells cultured in the synthetic medium were more susceptible to acid inactivation than those cultured in LB–3% NaCl medium. Nevertheless, the acid-adapted cells in this synthetic medium also showed significantly greater acid tolerance than the control group. In fact, 2,000-fold protection was observed when the cells were subjected to acid challenge for 40 min (data not shown).
FIG. 2.
Effect of single- or two-phase adaptation on the acid tolerance of V. parahaemolyticus ST550. The exponential-phase culture was adapted at pH 5.0 for 60 min (▴) or at pH 5.8 for 30 min followed by pH 5.0 for another 30 min (•) before being subjected to challenge at pH 4.4. ○, control, nonadapted cells.
Cross-protection of ATR.
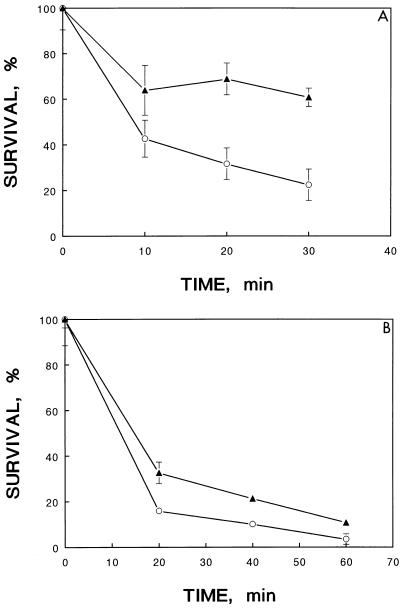
The acid-adapted cells were collected and challenged with low salinity and thermal inactivation at 45°C. Higher survival rates were observed for the acid-adapted cells after low-salinity (Fig. 3A) or thermal (Fig. 3B) inactivation as compared to the control group.
FIG. 3.
Cross-protection of the adapted acid-tolerant cells of V. parahaemolyticus ST550. (A) Against challenge at low salinity. The bacterial cells with or without acid adaptation were suspended in LB without supplementation of NaCl. (B) Against thermal inactivation at 45°C. The bacterial cells with or without acid adaptation were subjected to thermal inactivation at 45°C. ○, control, nonadapted cells; ▴, cells adapted at pH 5.0 for 30 min.
Protein profiles in the ATR.
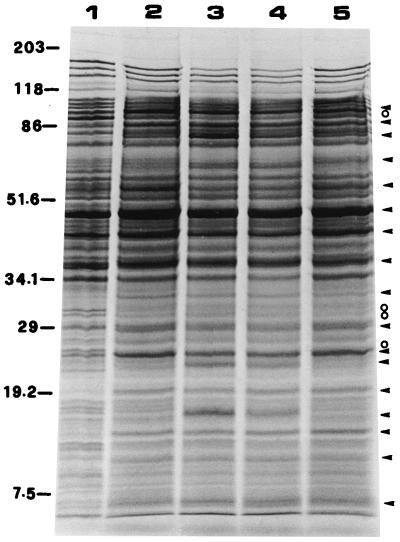
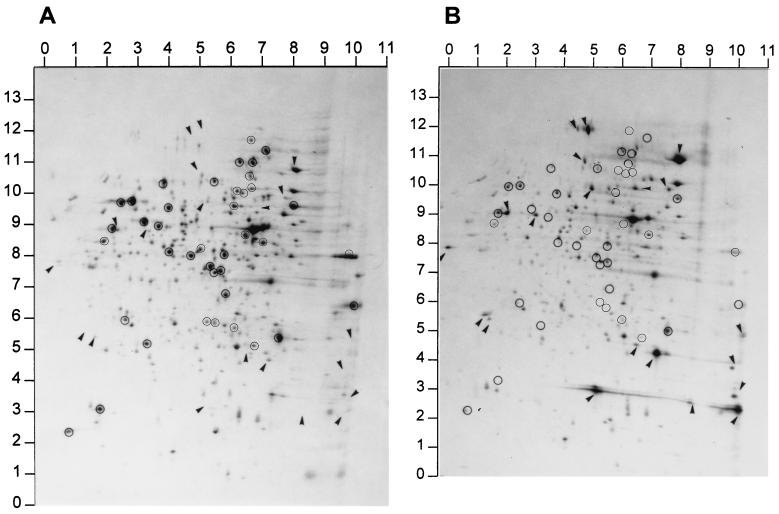
The protein profile of the two-phase acid adaptation response in V. parahaemolyticus was examined by isotope labeling followed by 1-D and 2-D PAGE. When analyzed by 1-D PAGE, several proteins were found to be regulated by the mild acid treatment. Seventeen proteins with molecular masses of 6.4, 9.0, 13.6, 16.3, 18.9, 22.9, 24.4, 28.3, 33.9, 36.9, 41.2, 47.6, 58.1, 65.6, 80.5, 88.2, and 96.9 kDa were induced or significantly enhanced. Two of the proteins (16.3 and 22.9 kDa) were induced after exposure to pH 5.0 for 7 min but were not detected after 21 min, while other induced proteins were detected at comparatively constant quantities throughout the adaptation period. A protein of 47.6 kDa appeared in large quantities in both the control and experimental groups. Proteins of 25.3, 30.1, 30.7, and 91.7 kDa were significantly inhibited (Fig. 4). Protein synthesis during the ATR was revealed in detail by 2-D PAGE, and the result showed that 20 species of proteins were induced while 26 species were repressed (Fig. 5). Proteins with coordinates 00 × 78, 48 × 116, 50 × 28, 71 × 40, 80 × 107, and 100 × 21 were greatly enhanced. The five major acid-enhanced proteins detected by 2-D PAGE may be equivalent to the proteins of 13.6, 18.9, 24.4, 88.2, and 96.9 kDa resolved by the 1-D PAGE (Fig. 4 and 5). Many of the repressed proteins detected by the 2-D PAGE were not discerned in the 1-D PAGE.
FIG. 4.
Analysis of protein synthesis in the ATR in V. parahaemolyticus ST550 by 1-D SDS-PAGE. The culture at exponential phase was shifted from pH 7.5 to pH 5.8 for 30 min and to pH 5.0 for 30 min. At different intervals after the shift to pH 5.0, protein synthesis was detected by adding labeling mix and incubating the mixture for 7 min. Lane 1, control (the pH 7.5 culture was labeled); lanes 2 to 5, protein was labeled 0 to 7, 7 to 14, 14 to 21, and 21 to 28 min, respectively, after the shift to pH 5.0. Circles and arrows designate the proteins that decreased and increased, respectively. Sizes are indicated in kilodaltons.
FIG. 5.
Analysis of protein synthesis in the ATR in V. parahaemolyticus ST550 by 2-D PAGE. (A) Cells grown at pH 7.5 and labeled for 15 min. (B) Cells grown at pH 7.5, shifted to pH 5.8 for 30 min and pH 5.0 for 30 min, and then labeled for 15 min. All proteins identified as regulated by the acid stress in panel B were compared with the proteins in panel A and assigned coordinates based on that comparison. Circles and arrows represent proteins that decreased and increased, respectively.
Effect of the ATR on the enteropathogenicity of V. parahaemolyticus.
Enteropathogenicity of the acid-adapted cells and nonadapted control cells was assayed in the suckling mouse model. Different infection dosages (0.1-ml aliquots of inocula of 108, 0.25 × 108, and 0.1 × 108 CFU/ml) were used, and the time to death of the animals increased with the decrease of dosage. Within the same inoculation level, the average time to death was not affected by acid adaptation (data not shown). However, the intestine/body weight ratio and the in vivo bacterial population were significantly affected. The suckling mice were sacrificed, and the enterotoxigenicity for each group was determined in terms of intestine/body weight ratio. Compared to nonadapted cells, the single-phase- and two-phase-adapted cells showed significantly greater enteropathogenicity (Table 1).
TABLE 1.
Effect of ATR on the enteropathogenicity of V. parahaemolyticus ST550, as determined by the suckling mouse modela
| Treatment | Inoculum density (108 CFU/ml) | Incubation time (h) | Intestine/ body wt (10−3) | Bacteria recovered (log CFU/g of intestine) |
|---|---|---|---|---|
| Culture medium | 41.2 ± 4.2 | 0 | ||
| Nonadapted control | 1 | 3.0 | 48.3 ± 3.7 | 3.65 ± 0.04 |
| Single-phase adaptation | 1 | 3.0 | 51.7 ± 2.6 | 4.02 ± 0.04* |
| Two-phase adaptation | 1 | 3.0 | 52.8 ± 4.2* | 4.11 ± 0.04*+ |
| Nonadapted control | 0.25 | 4.5 | 52.2 ± 3.4 | 4.37 ± 0.60 |
| Single-phase adaptation | 0.25 | 4.5 | 60.9 ± 3.8* | 5.16 ± 0.05* |
| Two-phase adaptation | 0.25 | 4.5 | 63.7 ± 4.1* | 5.27 ± 0.05* |
| Nonadapted control | 0.1 | 6.0 | 62.9 ± 4.1 | 5.29 ± 0.08 |
| Single-phase adaptation | 0.1 | 6.0 | 75.2 ± 4.5* | 5.56 ± 0.06 |
| Two-phase adaptation | 0.1 | 6.0 | 80.5 ± 3.7*+ | 5.75 ± 0.04 |
The nonadapted or adapted bacterial culture was infected at several different dosages given intragastrically to suckling mice, and the mice were sacrificed at several different incubation times. The intestine/body weight ratio and the population of the pathogen in the intestine of each mouse were also determined. Each group consisted of six mice, and blank medium was used as a negative control. *, significant when the adapted group was compared with the nonadapted control group at P < 0.05. +, significant when the single-phase-adapted group was compared with the two-phase-adapted group at the same dosage level at P < 0.05.
The intestines of these suckling mice were excised and homogenized, and the V. parahaemolyticus cells were counted on TCBS agar plates. The results also showed significantly higher bacterial counts for the adapted cells than for the nonadapted cells in each group (Table 1).
DISCUSSION
Effects of other environmental stresses, such as nutrition starvation, heat shock, and high level of metallic ions, on survival and physiological aspects have been studied in several Vibrio species (1, 17, 20, 21, 32, 34, 38). However, the ATR phenomenon has not been reported in Vibrio species. In previous V. parahaemolyticus studies, nutrition starvation has been investigated and shown to induce cross-protection against heat, osmotic, or hydrogen peroxide challenges (21) and to enable survival of this pathogen at low temperature (15). However, the enteropathogenic V. parahaemolyticus may also encounter high acidity in food-handling environments or in the human gastroenteric tract. In this study, we have demonstrated the ATR in this pathogen and have also showed that this phenomenon may be important in the pathogenesis of the organism.
Koga and Takumi showed that cadmium-adapted and heat-adapted cells of V. parahaemolyticus were cross-protected against thermal and osmotic stresses (20). Cross-protection by the ATR was also investigated in S. typhimurium against the challenge of heat, salt, an activated lactoperoxidase system, and the surface-active agents crystal violet and polymyxin B (24). In this report, we have also shown that the ATR in V. parahaemolyticus cross-protected significantly against challenge of low salinity and thermal inactivation (Fig. 3).
Although induction of the ATR in V. parahaemolyticus is similar to what is observed in S. typhimurium and A. hydrophila, the protection effect was not so dramatic in this pathogen. The ATR induced in A. hydrophila and S. typhimurium greatly enhanced the survival of these pathogens in highly acidic environments, such as pH 3.3 to 3.5 (9, 18). The adaptive acid tolerance response enhanced the survival of V. parahaemolyticus at a milder acidity, pH 4.4 (Fig. 1). Since V. parahaemolyticus is a comparatively vulnerable species and highly susceptible to environmental stresses, such an ATR phenomenon could significantly enhance its protection against acid or other stresses and may well increase its survival rate within the human host or in other adverse environments.
The stress-adapted cells of V. parahaemolyticus showed enhanced survival in adverse in vitro conditions. Such adaptation phenomena in this pathogen may also enhance in vivo survival, adhesion, colonization, or the production of other virulence-associated factors during infection. The preliminary virulence assay in this report indicated that the acid-tolerant cells of V. parahaemolyticus exhibited significantly higher enteropathogenic activity than the nonadapted cells, in terms of intestine/body weight ratio and in vivo bacterial density (Table 1). However, such changes were not strong enough to shorten the time to death in any of the experimental groups. The intestine/body weight ratio and bacterial density were higher in groups with lower infection dosages, and it is probably the case that longer incubation times for these groups enabled bacterial proliferation and pathogenic manifestation (Table 1). Regulation of virulence by the ATR has also been demonstrated in other pathogenic bacteria. In L. monocytogenes, an acid-tolerant mutant demonstrated increased virulence in an intraperitoneally infected mouse model (33). S. typhimurium strains containing two or three different ATR gene mutants were acid sensitive and also much less virulent (35).
Addition of the protein synthesis inhibitor chloramphenicol inhibited the adaptive response to environmental stresses, which indicates that synthesis of special proteins is required in these responses (9). In our study, chloramphenicol (20 μg/ml) was added to the logarithmically grown cells of this pathogen before or after the acid adaptation period at pH 5.8, followed by treatment at pH 5.0, and the survival rate was assayed. The result showed that chloramphenicol also inhibit the ATR of V. parahaemolyticus (data not shown). Thus, as reported in other studies, protein synthesis is important during stress adaptation in this pathogen. We analyzed the protein profile during the ATR and identified 46 special proteins as being regulated by mild acid treatment, 20 increased and 26 inhibited (Fig. 4 and 5). Similar results have been observed for the ATR in another member of the family Vibrioaceae, A. hydrophila, with 28 proteins increased and 10 decreased (19).
Since several stress proteins are highly conserved in many organisms, proteins of similar families may be produced during acid tolerance in V. parahaemolyticus (Fig. 4 and 5). Simply judging from mobility and position in 2-D PAGE, the protein with coordinates 80 × 107 may be homologous to the DnaK of Salmonella (7). Two proteins observed in high quantities, with molecular sizes of 47.6 and 58.1 kDa (Fig. 4), may be similar to DnaJ (41 kDa) and GroEL (63 kDa), respectively, of E. coli (22). These proteins are being identified in our laboratory by using immunological and molecular procedures. A 58-kDa Hsp60 (GroEL)-like protein has been demonstrated, by immunoblotting, in V. parahaemolyticus and in six other Vibrio species subjected to heat shock from 30 to 42°C (19).
The sustained ATR in virulent S. typhimurium required the presence of alternate sigma factor ςs encoded by rpoS for the synthesis of seven acid shock proteins (23). The ferric uptake regulator, Fur, is also involved in the ATR. In S. typhimurium, fur mutants failed to mount an effective ATR, and a clear subset of seven proteins were influenced by both acid and iron and were controlled by fur (10). In this Salmonella fur mutant, the uptake of iron was restored by a fur+-containing plasmid, but exposure to low pH was required in order to induce the ATR. Thus, the role of fur in the ATR of S. typhimurium is physiologically and genetically separable from its role in iron acquisition (11). In A. hydrophila, the level of iron in the culture medium also did not affect the ATR (18). These studies support the idea that Fur is a major global regulator. In our previous studies, we also demonstrated the presence of the Fur system and analyzed the fur gene in V. parahaemolyticus (5, 25, 26). Spontaneous iron-utilizing mutants of V. parahaemolyticus with different iron-regulated outer membrane profiles showed a significant decrease of virulence as assayed by animal models and lowered adherence to excised intestine of mouse (41). These results suggest that Fur and fur-regulated factors may have profound influences on the pathogenesis of this bacterium. Nevertheless, the influence of the Fur system on the ATR has not yet been investigated in this pathogen.
ACKNOWLEDGMENTS
This research was supported by the National Science Council (NSC85-2311-B-031-001) and by the Department of Health of the Republic of China (DOH86-HR-606).
We thank Carlos Javier for editing the English manuscript.
REFERENCES
- 1.Albertson N H, Nystroem T, Kjelleberg S. Macromolecular synthesis during recovery of the marine Vibrio sp. S14 from starvation. J Gen Microbiol. 1990;136:2201–2207. [Google Scholar]
- 2.Beuchat L R. Environmental factors affecting survival and growth of Vibrio parahaemolyticus. A review. J Milk Food Technol. 1975;38:476–480. [Google Scholar]
- 3.Chakravarti D, Ghosh A. Reversal by cyclic AMP of the urea-induced inhibition of synthesis of a catabolite-repressible enzyme in Vibrio cholerae. J Gen Microbiol. 1987;133:3265–3270. doi: 10.1099/00221287-133-11-3265. [DOI] [PubMed] [Google Scholar]
- 4.Chiou A, Chen L-H, Chen S-K. Foodborne illness in Taiwan, 1981–1989. Food Aust. 1991;43:70–71. [Google Scholar]
- 5.Dai J W, Lee Y S, Wong H C. Effects of iron limitation of production of a siderophore, outer membrane proteins, and hemolysin and on hydrophobicity, cell adherence, and lethality for mice of Vibrio parahaemolyticus. Infect Immun. 1992;60:2952–2956. doi: 10.1128/iai.60.7.2952-2956.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dascher C C, Poddar K S, Maniloff J. Heat shock response in mycoplasmas, genome-limited organisms. J Bacteriol. 1990;172:1823–1827. doi: 10.1128/jb.172.4.1823-1827.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foster J W. Salmonella acid shock proteins are required for the adaptive acid tolerance response. J Bacteriol. 1991;173:6896–6902. doi: 10.1128/jb.173.21.6896-6902.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foster J W. Low pH adaptation and the acid tolerance response of Salmonella typhimurium. Crit Rev Microbiol. 1995;21:215–237. doi: 10.3109/10408419509113541. [DOI] [PubMed] [Google Scholar]
- 9.Foster J W, Hall H K. Adaptive acidification tolerance response of Salmonella typhimurium. J Bacteriol. 1990;172:771–778. doi: 10.1128/jb.172.2.771-778.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster J W, Hall H K. Effect of Salmonella typhimurium ferric uptake regulator (fur) mutations on iron- and pH-regulated protein synthesis. J Bacteriol. 1992;174:4317–4323. doi: 10.1128/jb.174.13.4317-4323.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall H K, Foster J W. The role of fur in the acid tolerance response of Salmonella typhimurium is physiologically and genetically separable from its role in iron acquisition. J Bacteriol. 1996;178:5683–5691. doi: 10.1128/jb.178.19.5683-5691.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamilton I R, Buckley N D. Adaptation by Streptococcus mutans to acid tolerance. Oral Microbiol Immunol. 1991;6:65–71. doi: 10.1111/j.1399-302x.1991.tb00453.x. [DOI] [PubMed] [Google Scholar]
- 13.Hoashi K, Ogata K, Taniguchi H, Yamashita H, Tsuji K, Mizuguchi Y, Ohtomo N. Pathogenesis of Vibrio parahaemolyticus: intraperitoneal and orogastric challenge experiments in mice. Microbiol Immunol. 1990;34:355–366. doi: 10.1111/j.1348-0421.1990.tb01016.x. [DOI] [PubMed] [Google Scholar]
- 14.Honda T, Shimizu M, Takeda Y, Miwatani T. Isolation of a factor causing morphological changes of chinese hamster ovary cells from the culture filtrate of Vibrio parahaemolyticus. Infect Immun. 1976;14:1028–1033. doi: 10.1128/iai.14.4.1028-1033.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang X P, Chai T J. Survival of Vibrio parahaemolyticus at low temperatures under starvation conditions and subsequent resuscitation of viable, nonculturable cells. Appl Environ Microbiol. 1996;62:1300–1305. doi: 10.1128/aem.62.4.1300-1305.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joseph S W, Colwell R R, Kaper J B. Vibrio parahaemolyticus and related halophilic vibrios. Crit Rev Microbiol. 1983;10:77–123. doi: 10.3109/10408418209113506. [DOI] [PubMed] [Google Scholar]
- 17.Jyot J, Ghosh A. Induction of heat shock response in Vibrio cholerae. Microbiology. 1995;141:2101–2109. doi: 10.1099/13500872-141-9-2101. [DOI] [PubMed] [Google Scholar]
- 18.Karem K L, Foster J W, Bej A K. Adaptive acid tolerance response (ATR) in Aeromonas hydrophila. Microbiology. 1994;140:1731–1736. doi: 10.1099/13500872-140-7-1731. [DOI] [PubMed] [Google Scholar]
- 19.Koga T, Nakajyo Y, Komoto A. Detection of Hsp60 (GroEL)-like proteins in Vibrio parahaemolyticus and Vibrio species by western immunoblotting analysis. Lett Appl Microbiol. 1996;23:295–298. doi: 10.1111/j.1472-765x.1996.tb00193.x. [DOI] [PubMed] [Google Scholar]
- 20.Koga T, Takumi K. Nutrient starvation induces cross protection against heat, osmotic, or H2O2 challenge in Vibrio parahaemolyticus. Microbiol Immunol. 1995;39:213–215. doi: 10.1111/j.1348-0421.1995.tb02191.x. [DOI] [PubMed] [Google Scholar]
- 21.Koga T, Takumi K. Comparison of cross-protection against some environmental stresses between cadmium-adapted and heat-adapted cells of Vibrio parahaemolyticus. J Gen Appl Microbiol. 1995;41:263–268. [Google Scholar]
- 22.LaRossa R A, Van Dyk T K. Physiological roles of the DnaK and GroE stress proteins: catalysts of protein folding or macromolecular sponges? Mol Microbiol. 1991;5:529–534. doi: 10.1111/j.1365-2958.1991.tb00724.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee I S, Lin J, Hall H K, Bearson B, Foster J W. The stationary-phase sigma factor sigma S (RpoS) is required for a sustained acid tolerance response in virulent Salmonella typhimurium. Mol Microbiol. 1995;17:155–167. doi: 10.1111/j.1365-2958.1995.mmi_17010155.x. [DOI] [PubMed] [Google Scholar]
- 24.Leyer G J, Johnson E A. Acid adaptation induces cross-protection against environmental stresses in Salmonella typhimurium. Appl Environ Microbiol. 1993;59:1842–1847. doi: 10.1128/aem.59.6.1842-1847.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin H-C. Cloning of an iron-regulated outer membrane protein gene of Vibrio parahaemolyticus. Master’s thesis. Taipei, Taiwan, Republic of China: Soochow University; 1994. [Google Scholar]
- 26.Mao C-J. Cloning and characterization of a fur-like gene from the Vibrio parahaemolyticus. Master’s thesis. Taipei, Taiwan, Republic of China: Soochow University; 1996. [Google Scholar]
- 27.Mekalanos J J. Environmental signals controlling expression of virulence determinants in bacteria. J Bacteriol. 1992;174:1–7. doi: 10.1128/jb.174.1.1-7.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morris J G, Black R E. Cholera and other vibrioses in the United States. N Engl J Med. 1985;312:343–350. doi: 10.1056/NEJM198502073120604. [DOI] [PubMed] [Google Scholar]
- 29.Murray P J, Young R A. Stress and immunological recognition in host-pathogen interactions. J Bacteriol. 1992;174:4193–4196. doi: 10.1128/jb.174.13.4193-4196.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishibuchi M, Fasano A, Russell R G, Kaper J B. Enterotoxigenicity of Vibrio parahaemolyticus with and without genes encoding thermostable direct hemolysin. Infect Immun. 1992;60:3539–3545. doi: 10.1128/iai.60.9.3539-3545.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishibuchi M, Seidler R J, Rollins D M, Joseph S W. Vibrio factors cause rapid fluid accumulation in suckling mice. Infect Immun. 1983;40:1083–1091. doi: 10.1128/iai.40.3.1083-1091.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nyström T, Olsson R M, Kjelleberg S. Survival, stress resistance, and alterations in protein expression in the marine Vibrio sp. strain S14 during starvation for different individual nutrients. Appl Environ Microbiol. 1992;58:55–65. doi: 10.1128/aem.58.1.55-65.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Driscoll B, Gahan C G, Hill C. Adaptive acid tolerance response in Listeria monocytogenes: isolation of an acid-tolerant mutant which demonstrates increased virulence. Appl Environ Microbiol. 1996;62:1693–1698. doi: 10.1128/aem.62.5.1693-1698.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oliver J D, Stringer W F. Lipid composition of a psychrophilic marine Vibrio sp. during starvation-induced morphogenesis. Appl Environ Microbiol. 1984;47:461–466. doi: 10.1128/aem.47.3.461-466.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riesenberg Wilmes M R, Bearson B, Foster J W, Curtis R., III Role of the acid tolerance response in virulence of Salmonella typhimurium. Infect Immun. 1996;64:1085–1092. doi: 10.1128/iai.64.4.1085-1092.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rowbury R J. An assessment of environmental factors influencing acid tolerance and sensitivity in Escherichia coli, Salmonella spp. and other enterobacteria. Lett Appl Microbiol. 1995;20:333–337. doi: 10.1111/j.1472-765x.1995.tb01314.x. [DOI] [PubMed] [Google Scholar]
- 37.Sarkar B L, Kumar R, De S P, Pal S C. Hemolytic activity of and lethal toxin production by environmental strains of Vibrio parahaemolyticus. Appl Environ Microbiol. 1987;53:2696–2698. doi: 10.1128/aem.53.11.2696-2698.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simigielski A J, Wallace B J, Marshall K C. Changes in membrane functions during short-term starvation of Vibrio fluvialis strain NCTC 11328. Arch Microbiol. 1989;151:336–347. [Google Scholar]
- 39.Takeda Y. Thermostable direct hemolysin of Vibrio parahaemolyticus. Pharmacol Ther. 1983;19:123–146. doi: 10.1016/0163-7258(82)90044-4. [DOI] [PubMed] [Google Scholar]
- 40.Valone S E, Chikami G K, Miller V L. Stress induction of the virulence proteins (SpvA, -B, and -C) from native plasmid pSDL2 of Salmonella dublin. Infect Immun. 1993;61:705–713. doi: 10.1128/iai.61.2.705-713.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wong H C, Liu C C, Yu C M, Lee Y S. Utilization of iron sources and its possible roles in the pathogenesis of Vibrio parahaemolyticus. Microbiol Immunol. 1996;40:791–798. doi: 10.1111/j.1348-0421.1996.tb01144.x. [DOI] [PubMed] [Google Scholar]
- 42.Yamamoto T, Fujita K, Yokota T. Piliated Vibrio parahaemolyticus adherent to human ureteral mucosa. J Infect Dis. 1990;161:361–362. doi: 10.1093/infdis/161.2.361. [DOI] [PubMed] [Google Scholar]