Abstract
Background
Despite immunization, patients on antineoplastic and immunomodulating agents have a heightened risk of COVID-19 infection. However, accurately attributing this risk to specific medications remains challenging.
Methods
An observational cohort study from December 11, 2020 to September 22, 2022, within a large healthcare system in San Diego, California, USA was designed to identify medications associated with greatest risk of postimmunization SARS-CoV-2 infection. Adults prescribed WHO Anatomical Therapeutic Chemical (ATC) classified antineoplastic and immunomodulating medications were matched (by age, sex, race, and number of immunizations) with control patients not prescribed these medications yielding a population of 26 724 patients for analysis. From this population, 218 blood samples were collected from an enrolled subset to assess serological response and cytokine profile in relation to immunization.
Results
Prescription of WHO ATC classified antineoplastic and immunomodulatory agents was associated with elevated postimmunization SARS-CoV-2 infection risk (HR 1.50, 95% CI 1.38 to 1.63). While multiple immunization doses demonstrated a decreased association with postimmunization SARS-CoV-2 infection risk, antineoplastic and immunomodulatory treated patients with four doses remained at heightened risk (HR 1.23, 95% CI 1.06 to 1.43). Risk variation was identified among medication subclasses, with PD-1/PD-L1 inhibiting monoclonal antibodies, calcineurin inhibitors, and CD20 monoclonal antibody inhibitors identified to associate with increased risk of postimmunization SARS-CoV-2 infection. Antineoplastic and immunomodulatory treated patients also displayed a reduced IgG antibody response to SARS-CoV-2 epitopes alongside a unique serum cytokine profile.
Conclusions
Antineoplastic and immunomodulating medications associate with an elevated risk of postimmunization SARS-CoV-2 infection in a drug-specific manner. This comprehensive, unbiased analysis of all WHO ATC classified antineoplastic and immunomodulating medications identifies medications associated with greatest risk. These findings are crucial in guiding and refining vaccination strategies for patients prescribed these treatments, ensuring optimized protection for this susceptible population in future COVID-19 variant surges and potentially for other RNA immunization targets.
Keywords: COVID-19; Immunogenicity, Vaccine; Immunotherapy; Immune Checkpoint Inhibitors; Immunomodulation
WHAT IS ALREADY KNOWN ON THIS TOPIC
Patients prescribed PD-1/PD-L1 directed immunotherapy are generally believed to generate an adequate immune response to COVID-19 vaccination.
WHAT THIS STUDY ADDS
Using an unbiased approach, we systematically evaluated all antineoplastic and immunomodulating agents to pinpoint those most significantly associated with increased SARS-CoV-2 infection risk following immunization. Notably, patients treated with PD-1/PD-L1 monoclonal antibody inhibitors exhibited an elevated likelihood of experiencing breakthrough infections.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The response to immunization against COVID-19, other infectious agents, or neoantigens should be closely assessed for clinical outcomes in addition to surrogates of immunity such as cytokine or antibody response in patients prescribed immunosuppressants. Additionally, patients prescribed PD-1/PD-L1 monoclonal antibody inhibitors, calcineurin inhibitors, and CD20 monoclonal antibody inhibitors may need additional counseling regarding increased infection risk after immunization based on these results.
Introduction
Patients prescribed antineoplastic and immunomodulating medications face an elevated risk of developing COVID-19, as well as more severe consequences of the disease. Given that this large class of medications includes various mechanisms of immune modulation, the degree of infection risk likely differs on a spectrum depending on the specific antineoplastic and immunomodulating agent used. However, the broad nature of this medication class results in a lack of understanding of which medications are associated with the greatest risk. This variability in medication mechanisms has led to inconsistent reports with some studies finding no increase in severe outcomes of COVID-19 by medication subclass, while others indicate a significant increase in risk of severe infection and death.1–5 Thus, it is currently difficult for clinicians to ascribe the individual risk associated with a particular medication, making discussion of risk mitigation strategies challenging. As a result, physicians and patients are limited to broad characterizations of immunosuppressants as a whole.
Although COVID-19 immunization reduces the risk of infection, patients with immune dysfunction due to antineoplastic or immunomodulating treatment remain at increased risk of breakthrough infection.6–10 We sought to understand which medications were more likely to increase postimmunization infection risk. Current investigations of breakthrough infection in individuals receiving immunosuppressing therapy are limited by the following factors: analysis of a single therapy,11 12 evaluation of postimmunization antibody response without a concurrent analysis of clinical infection,13 14 and assessment by disease group, such as solid organ transplant patients or patients with cancer with hematological malignancies, where a population with heterogeneous disease states receives a wide range of different medications.15 16 We address these limitations by assessing all medications included in the WHO Anatomical Therapeutic Chemical (WHO ATC) classification of antineoplastic and immunomodulating agents, examining the risk of postimmunization infection compared with a control population not receiving these medications. In parallel, we assessed serial blood samples in a subset of patients and controls to measure serological response to multiple SARS-CoV-2 Spike protein epitopes as well as postimmunization circulating cytokine levels for correlation with clinical risk. These data help healthcare providers and patients make informed decisions regarding COVID-19 risk reduction strategies during treatment with these medicines and may also have implications in future COVID-19 variant surges and for other infections targeted by RNA immunization.
Methods
Study design
We conducted an observational cohort study at the Scripps Health, the primary healthcare system for greater than 2.4 million patients and specialty referral site for another 1.5 million patients, designed to assess the outcome of SARS-CoV-2 infection following COVID-19 immunization in patients immunosuppressed by antineoplastic and immunomodulatory medications (“treatment”) compared with patients not on these medications (“control”). Included subjects were ≥18 years of age, received at least two COVID-19 immunizations (manufactured by Pfizer-BioNTech, Moderna, Janssen, or AstraZeneca), had no documentation of SARS-CoV-2 infection prior to initiation of immunization series, and were active in the healthcare system as defined by at least one healthcare appointment within 180 days of immunization. All COVID-19 immunizations were confirmed by Scripps Health through the California Immunization Registry.
Immunosuppressing medications were identified by WHO ATC classification system, L code “Antineoplastic and Immunosuppressing”. From all medications in WHO ATC L code, topical tretinoin, topical fluorouracil, celecoxib, megestrol, and medroxyprogesterone were excluded. Patients were defined as actively receiving antineoplastic or immunomodulating medication at time of initial immunization by having a prescription for one of the above-described medications within the 6 months preceding initial COVID-19 immunization. Patients not having a WHO ATC L code medication active on their medication list were eligible as matched controls.
The outcome of SARS-CoV-2 infection was determined as a positive SARS-CoV-2 infection PCR test documented in the electronic medical record (Epic Systems, Verona, Wisconsin, USA), including both internal and external laboratories. Patients with a SARS-CoV-2 infection positive test within 14 days following initial immunization were excluded.
Patients were classified based on disease criteria during outpatient encounters from July 1, 2020 to June 30, 2021. Those with hematological or oncological diseases had encounters noting a cancer diagnosis. Rheumatological disease cases were determined by encounters specifically within the rheumatology department. Identification of solid organ transplant recipients was based on encounters within the transplant department. Lastly, bone marrow transplant patients were identified using procedure codes for bone marrow transplantation within the same time frame.
In parallel, we invited patients prescribed WHO ATC L medicines and control patients to provide serial assessment of blood samples to assess vaccine immune response. Invitation was through direct study investigator communication in oncology, rheumatology, and transplant surgery clinics, posted study information flyers, and online patient portal information to patients on these medicines. A volunteer subset of these patients and control patients were enrolled digitally, in a paperless process using a QR code or link to the study consent and a survey confirming demographics, reason for WHO ATC L medication, and immunization types and dates. All participants gave informed consent to participate before taking part in the study. Study data were collected and managed using REDCap electronic data capture hosted at Scripps Health.17 18
Serological assessment
Anti-SARS-CoV-2 antibodies were assessed using the Maverick SARS-CoV-2 multiantigen panel (Genalyte, San Diego, California, USA), according to the manufacturer’s protocol as previously described.19 This panel detects antibodies to five SARS-CoV-2 antigens: nucleocapsid, Spike S1 subunit, Spike S1 receptor binding domain, Spike full length, and Spike S2 subunit. Briefly, 10 µL of each serum sample was added to the sample well plate array to assess baseline resonance.20 21 Subsequently, the samples were challenged over the respective antigens to assess specific IgM and IgG antibodies. Response measurements are reported in antibody response units.
Circulating cytokines were assessed in patient serum samples using the Luminex xMAP Immunoassay, a 38-plex magnetic cytokine kit, according to the manufacturers protocol (Millipore Sigma, Burlington, Massachusetts, USA). Briefly, 25 µL of patient serum was mixed 1:1 with magnetic beads and allowed to incubate overnight. The detection antibody was added and allowed to incubate for 1 hour. The phycoerythrin-streptavidin conjugate was added and allowed to incubate for 30 min prior to washing with subsequent quantification of fluorescent intensity on Luminex 200TM instrument.
Statistical analysis
To discern demographic and clinical characteristic differences between groups, all categorical variables were summarized as frequencies and percentages and compared between arms by χ2 tests or Fisher’s exact test. All continuous variables were summarized as means and SD and compared between arms by t-tests if normally distributed or medians and IQRs compared by Mann-Whitney U tests. Patients taking WHO ATC L medications were propensity matched to control patients by age, body mass index (BMI), sex, self-reported race and ethnicity, and number of immunizations received. For cumulative incidence assessment, log-rank test was used to assess HR. Cox proportional hazard analysis was used to assess the effect of multiple immunizations. To assess serological antibody response between WHO ATC L medicine-treated and control patients, two-way analysis of variance (ANOVA) was conducted, and when significance identified, Tukey’s multiple comparison test assessed effects at indicated time points. Differences in serological cytokine concentrations were assessed using restricted maximum likelihood mixed effect model with two-way ANOVA. Statistical significance was set at p<0.05 unless otherwise indicated. Propensity matching, cumulative incidence subgroup analysis, Cox proportional analysis, Restricted Maximum Likelihood (REML)-mixed effects model, and ANOVA were conducted using R. Figures were made using GraphPad Prism (V.9.5.1, Boston, Massachusetts, USA).
Results
Observational cohort
From December 11, 2020 to September 22, 2022, a total of 13 362 patients taking WHO ATC L medicines and 197 151 control patients not prescribed these medications were identified for matched analysis. Propensity matching the WHO ATC L subjects 1:1 produced a population of 26 724 patients that was median age 68, 63% female, 80% white, with an average BMI 26; 36% of patients received 4 immunizations (table 1).
Table 1.
Baseline demographics of patients
| Characteristic | Matched control patients (n=13 362) |
WHO ATC L-treated patients (n=13 362) |
| Age—median (IQR)—years | 68 (48–88) | 68 (48–88) |
| BMI—median (IQR)—kg/m2 | 26.5 (19.8–33.2) | 26.5 (19.5–33.5) |
| Sex—no (%) | ||
| Male | 4953 (37.1) | 4836 (36.2) |
| Female | 8408 (62.9) | 8525 (63.8) |
| Other | 1 (0.0) | 1 (0.0) |
| Race—no (%) | ||
| White | 10 724 (80.3) | 10 607 (79.4) |
| Asian | 1212 (9.1) | 1179 (8.8) |
| African American/Black | 288 (2.2) | 347 (2.6) |
| Native Hawaiian/Pacific Islander | 57 (0.4) | 63 (0.5) |
| American Indian/Native American | 37 (0.3) | 44 (0.3) |
| Other/unknown | 1044 (7.8) | 1122 (8.4) |
| Ethnicity—no (%) | ||
| Hispanic/Latino | 1501 (11.2) | 1608 (12.0) |
| Not Hispanic/Latino | 11 579 (86.7) | 11 429 (85.5) |
| Other/unknown | 282 (2.1) | 325 (2.4) |
| Immunizations received—no (%) | ||
| 2 | 3245 (24.3) | 3333 (24.9) |
| 3 | 5352 (40.1) | 5174 (38.7) |
| 4 | 4765 (35.7) | 4855 (36.3) |
| Vaccination 1 manufacturer—no (%) | ||
| Pfizer-BioNTech | 6049 (45.3) | 6084 (45.5) |
| Moderna | 6965 (52.1) | 6873 (51.4) |
| Janssen | 257 (1.9) | 314 (2.4) |
| AstraZeneca | 1 (0.0) | 2 (0.0) |
| Vaccination 2 manufacturer—no. (%) | ||
| Pfizer-BioNTech | 6967 (52.1) | 6108 (45.7) |
| Moderna | 7030 (52.6) | 6958 (52.1) |
| Janssen | 86 (0.6) | 102 (0.8) |
| AstraZeneca | 1 (0.0) | 1 (0.0) |
| Vaccination 3 manufacturer—no (%) | ||
| Pfizer-BioNTech | 4103 (30.7) | 4159 (31.1) |
| Moderna | 4317 (32.3) | 4492 (33.6) |
| Janssen | 14 (0.1) | 16 (0.1) |
| None | 3245 (24.3) | 3333 (24.9) |
| Vaccination 4 manufacturer—no (%) | ||
| Pfizer-BioNTech | 506 (3.8) | 538 (4.0) |
| Moderna | 2161 (16.2) | 2131 (16.0) |
| Janssen | 3 (0.0) | 2 (0.0) |
| None | 8597 (64.3) | 8507 (63.7) |
ATC, Anatomical Therapeutic Chemical; BMI, body mass index.
The majority received immunizations manufactured by Pfizer or Moderna. Treated and control patients initiated immunization series at similar times and experienced SARS-CoV-2 infection positive results across similar surge dates (online supplemental figure 1).
jitc-2023-008233supp001.pdf (2.1MB, pdf)
The risk of postimmunization SARS-CoV-2 infection varies depending on type of immunosuppressive medication taken
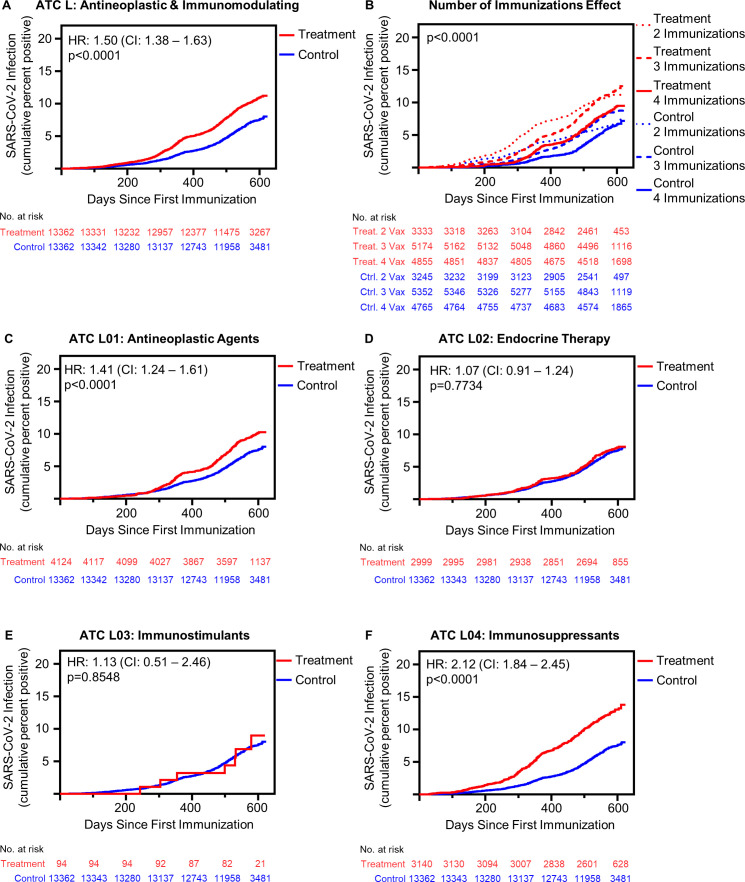
Active prescription of WHO ATC L medicines, the combined category of antineoplastic and immunomodulating medications, was associated with increased risk of postimmunization SARS-CoV-2 infection compared with matched controls (HR 1.50, 95% CI 1.38 to 1.63, p<0.0001) (figure 1A). Receiving 3 and 4 immunizations mitigated the risk of SARS-CoV-2 infection among WHO ATC L-treated patients; however, these patients remained at risk greater than the control group (figure 1B). Specifically, even among patients receiving four immunizations, WHO ATC L agents are associated with an increased risk of SARS-CoV-2 infection (HR 1.23, 95% CI 1.06 to 1.43, p=0.006) (online supplemental table 1). The risk of postimmunization SARS-CoV-2 infection remained greater in treated patients regardless of vaccine manufacturer (online supplemental figure 2 and table 2). Additionally, the risk of postimmunization SARS-CoV-2 infection remained greater in treated patients across immunodeficient disease types (online supplemental figure 3).
Figure 1.
Antineoplastic and immunomodulating agents associated with increased risk of postimmunization SARS-CoV-2 infection in a class dependent manner. (A) Cumulative incidence of SARS-CoV-2 infection following initial immunization in all patients receiving antineoplastic and immunomodulating agents (treatment, n=13 362) compared with matched control patients (n=13 362) (p<0.001 by log-rank). (B) Effect of multiple immunizations on SARS-CoV-2 infection incidence postimmunization (treatment: 2 immunizations n=3333, 3 immunizations n=5174, 4 immunizations n=4855; control: 2 immunizations n=3245, 3 immunizations n=5352, 4 immunizations n=4765) (p<0.001 by log-rank square). (C) SARS-CoV-2 infection cumulative incidence following initial immunization in all patients receiving L01: antineoplastic agents, patients receiving multiple medications excluded from analysis (L01 antineoplastic agent receiving patients n=4124) (p<0.0001 by log-rank). (D) SARS-CoV-2 infection cumulative incidence following initial immunization in all patients receiving L02: endocrine therapy, patients receiving multiple medications excluded from analysis (L02 endocrine therapy receiving patients n=2999) (p=0.7734 by log-rank). (E) SARS-CoV-2 infection cumulative incidence following initial immunization in all patients receiving L03: immunostimulants, patients receiving multiple medications excluded from analysis (L03 immunostimulant receiving patients n=94) (p=0.8548 by log-rank). (F) SARS-CoV-2 infection cumulative incidence following initial immunization in all patients L04: immunosuppressants, patients receiving multiple medications excluded from analysis (L04 immunosuppressant receiving patients n=3140) (p<0.0001 by log-rank). ATC, Anatomical Therapeutic Chemical.
The risk of SARS-CoV-2 infection varies depending on the type of antineoplastic or immunomodulating medication taken. The ATC broadly classifies these medications into four subgroups: L01 “antineoplastic agents”, L02 “endocrine therapy”, L03 “immunostimulants”, and L04 “immunosuppressants.” The risk of SARS-CoV-2 infection differed among these subgroups, with L01 antineoplastic and L04 immunosuppressant medications increasing risk (n=4124, HR 1.41, 95% CI 1.24 to 1.61, p<0.0001, and n=3140, HR 2.12, 95% CI 1.84 to 2.45, p<0.0001, respectively), while L02 endocrine therapy and L03 immunostimulants did not (n=2999, HR 1.07, 95% CI 0.91 to 1.24, p=0.7734, and n=94, HR 1.13, 95% CI 0.51 to 2.46, p=0.8548, respectively) (figure 1C–F). Receiving three or four immunizations reduced the risk of postimmunization SARS-CoV-2 infection in patients receiving L01 antineoplastic agents and L04 immunosuppressant agents compared with receiving only two immunization doses (online supplemental table 3 and figure 4). Among all patients who received four immunization doses, there was no risk difference between those receiving L01 antineoplastic agents and matched controls (HR 1.09, 95% CI 0.89 to 1.35, p=0.006); however, for patients receiving L04 immunosuppressant agents, risk was greater relative to controls (HR 1.43, 95% CI 1.15 to 1.77, p=0.0012) (online supplemental table 3).
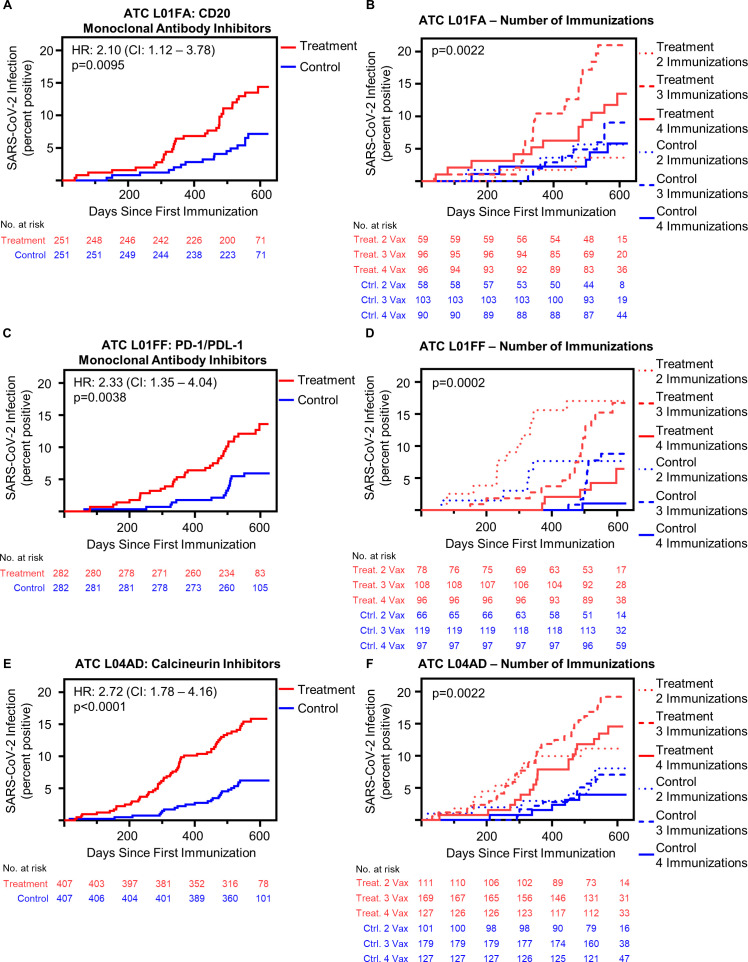
Given the infection risk differences seen among subgroups, we sought to better understand which therapeutic classes conferred the greatest risk of postimmunization SARS-CoV-2 infection. In an exploratory subclass analysis, comparing patients receiving a single WHO ATC L medication type with the full control group, significant risk differences were observed in patients receiving PD-1/PD-L1 inhibiting monoclonal antibodies, antiestrogen therapy, antiandrogen therapy, TNF-α inhibitors, interleukin inhibitors, and calcineurin inhibitors (online supplemental figure 5). Of particular interest, patients receiving calcineurin inhibitors had persistently elevated risk of SARS-CoV-2 infection regardless of the number of immunizations received (online supplemental figure 5). We then undertook matched analysis of patients treated with PD-1/PD-L1 inhibiting monoclonal antibodies, antiestrogen therapy, calcineurin inhibitors and CD20 monoclonal antibody inhibitors (demographics of these analyses, (online supplemental tables 4–7). Patients prescribed CD20 monoclonal antibody inhibitors had an increased risk of postimmunization SARS-CoV-2 infection compared with matched control patients (HR 2.10, 95% CI 1.12 to 3.78, p=0.0095) (figure 2A). Separating this group by immunization number, those who received three or four doses appeared to have decreased infection risk, although statistical significance was not reached (figure 2B, online supplemental table 8). Patients receiving PD-1/PD-L1 inhibiting monoclonal antibodies had significantly increased risk of postimmunization SARS-CoV-2 infection compared with control patients (HR 2.33, 95% CI 1.35 to 4.04, p=0.038) (figure 2C). This effect appears driven by the increased risk observed in patients who received only two immunizations (figure 2D, online supplemental table 9). Calcineurin inhibitors were associated with increased risk of COVID-19 following immunization (HR 2.72, 95% CI 1.78 to 4.16, p<0.0001) (figure 2E), which was significantly higher at all vaccination levels compared with controls and was not statistically incrementally decreased after three or four immunizations (figure 2F and online supplemental table 10). Matched analysis identified no significant difference in the risk of postimmunization SARS-CoV-2 infection in those receiving antiestrogen treatment (online supplemental figure 6).
Figure 2.
CD20 monoclonal antibody inhibitors, PD-1/PD-L1 monoclonal antibody inhibitors and calcineurin inhibitors increase risk of postimmunization COVID-19, which is variably overcome by repeat immunizations. (A) Cumulative incidence of SARS-CoV-2 infection following initial immunization in all patients receiving L01FA: CD20 monoclonal antibody inhibitors (n=251) compared with matched control patients (n=251) (p=0.0095 by log-rank). (B) Effect of multiple immunizations on SARS-CoV-2 infection cumulative incidence following initial immunization in patients receiving L01FA: CD20 monoclonal antibody inhibitors compared with matched control patients (immunosuppressed: 2 immunizations n=59, 3 immunizations n=96, 4 immunizations n=96; immunocompetent: 2 immunizations n=58, 3 immunizations n=103, 4 immunizations n=90) (p=0.0022 by log-rank) (online supplemental table 7) demonstrates HRs). (C) Cumulative incidence of SARS-CoV-2 infection following initial immunization in all patients receiving L01FF: PD-1/PD-L1 monoclonal antibody inhibitors (n=282) compared with matched control patients (n=282) (p=0.0038 by log-rank). (D) Effect of multiple immunizations on SARS-CoV-2 infection cumulative incidence following initial immunization in patients receiving L01FF: PD-1/PD-L1 monoclonal antibody inhibitors compared with matched control patients (immunosuppressed: 2 immunizations n=78, 3 immunizations n=108, 4 immunizations n=96; immunocompetent: 2 immunizations n=66, 3 immunizations n=119, 4 immunizations n=97) (online supplemental table 8 demonstrates p values). (E) Cumulative incidence of SARS-CoV-2 infection following initial immunization in all patients receiving L04AD calcineurin inhibitors (n=407) compared with matched control patients (n=407) (p<0.0001 by log-rank). (F) Effect of multiple immunizations on COVID-19 cumulative incidence following initial immunization in patients receiving L04AD calcineurin inhibitors compared with matched control patients (immunosuppressed: 2 immunizations n=111, 3 immunizations n=169, 4 immunizations n=127; immunocompetent: 2 immunizations n=101, 3 immunizations n=179, 4 immunizations n=127) (online supplemental table 9 demonstrates p values). ATC, Anatomical Therapeutic Chemical.
We also investigated the effects of combination treatment of antineoplastic and immunomodulatory agents on the risk of postimmunization SARS-CoV-2 infection. Given our findings of significantly increased risk associated with PD-1/PD-L1 inhibiting monoclonal antibodies used as single agents, we evaluated the use of these agents in combination with the combined group of chemotherapies, defined as WHO ATC L antineoplastic subgroups L01A (alkylating agents), L01B (antimetabolites), L01C (plant alkaloids and other natural products), and L01D (cytotoxic antibiotics and related substances). There is no significant difference in the risk of risk of postimmunization SARS-CoV-2 infection in those prescribed PD-1/PD-L1 inhibiting monoclonal antibodies as single agents compared with combination (online supplemental figure 7). Additionally, we assessed the effect of these agents in combination with antineoplastic protein kinase inhibitors (L01E). There was no significant difference in postimmunization SARS-CoV-2 infection risk in those prescribed PD-1/PD-L1 inhibiting monoclonal antibodies as single agents compared with those prescribed these agents in combination with antineoplastic protein kinase inhibitors (online supplemental figure 7). We also sought to evaluate the effects of chemotherapy with antiestrogen therapy and HER2 therapy. We identified no significant difference in the risk of postimmunization SARS-CoV-2 infection in those prescribed antiestrogen therapy as a single agent compared with in combination with chemotherapy (online supplemental figure 8). The use of HER2 agents in combination with chemotherapy was not associated with an increased risk of postimmunization SARS-CoV-2 infection (online supplemental figure 8).
Antineoplastic and immunomodulatory medications are associated with decreased seroconversion postimmunization
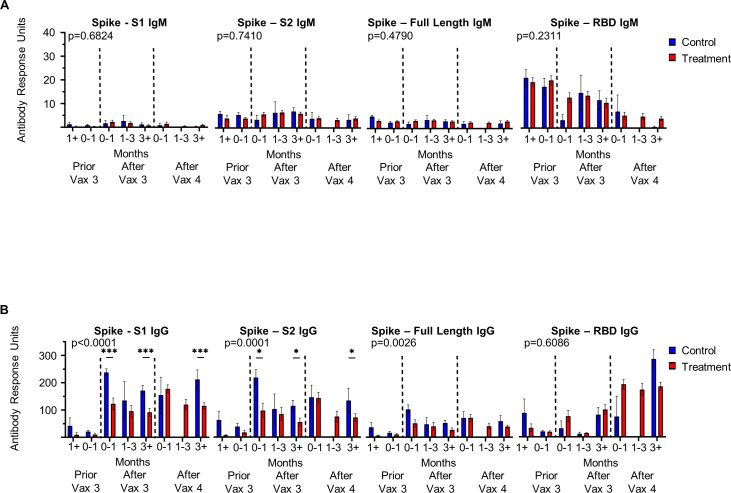
From this population of treated and control patients, we invited patients to provide serial blood samples alongside third and fourth doses of COVID-19 immunization, which resulted in 87 patients providing 218 blood samples (participant demographics online supplemental table 11). Using the Genalyte multiantigen panel, we assessed IgM and IgG antibody response to multiple SARS-CoV-2 antigens, including multiple epitopes on the Spike protein, the target of COVID-19 immunizations, as well as nucleocapsid, which is not present in available vaccines thus far and therefore represents natural viral infection. We found no differences in IgM antibody response between those receiving treatment with antineoplastic and immunomodulating agents and control patients (figure 3A). However, a significant difference is observed in levels of IgG against both Spike S1 subunit and Spike S2 subunit in antineoplastic and immunomodulating agent treated patients versus controls (p<0.0001 and p=0.0001, respectively) (figure 3B). There were no differences in the rate of natural viral infection in this population as determined by development of IgM and IgG against SARS-CoV-2 nucleocapsid (p=0.2321 and p=0.3187, respectively) (online supplemental figure 9). As we identified calcineurin inhibitors to confer significantly increased risk of postimmunization SARS-CoV-2 infection, we further assessed antibody levels in this subgroup of patients. Again, no significant differences were identified in IgM response (online supplemental figure 10); however, calcineurin inhibitors impaired IgG response to Spike full length and Spike S2 subunit (online supplemental figure 10). These findings suggest immunoglobulin class switching is impaired by immunosuppressive medications.
Figure 3.
Antineoplastic and immunomodulating agents impair immunoglobulin class switching to SARS-CoV-2 Spike subunits. (A) IgM and (B) IgG antibody response to respective SARS-CoV-2 epitopes at time points following third (Vax 3) and fourth (Vax 4) COVID-19 immunization in patients receiving antineoplastic and immunomodulating agents (treatment) and immunocompetent patients (control) (Immunocompetent: greater than 1 month prior to Vax 3 n=4, 0–1 month prior to Vax 3 n=9, 0–1 month following Vax 3 n=9, 1–3 months following Vax 3 n=3, greater than 3 months following Vax 3 n=17, 0–1 month following Vax 4 n=2, 1–3 months following Vax 4 n=0, greater than 3 months following Vax 4 n=4; Immunosuppressed: greater than 1 month prior to Vax 3 n=5, 0–1 month prior to Vax 3 n=18, 0–1 month following Vax 3 n=20, 1–3 months following Vax 3 n=19, greater than 3 months following Vax 3 n=36, 0–1 month following Vax 4 n=28, 1–3 months following Vax 4 n=17, greater than 3 months following Vax 4 n=27). Dashed lines indicate Vax three and Vax 4. Antibody response reported as antibody response units. When significance observed by ANOVA, significant pairwise comparisons indicated by *p<0.05, ***p<0.001 by Tukey’s multiple comparison. ANOVA, analysis of variance; RBD, receptor binding domain.
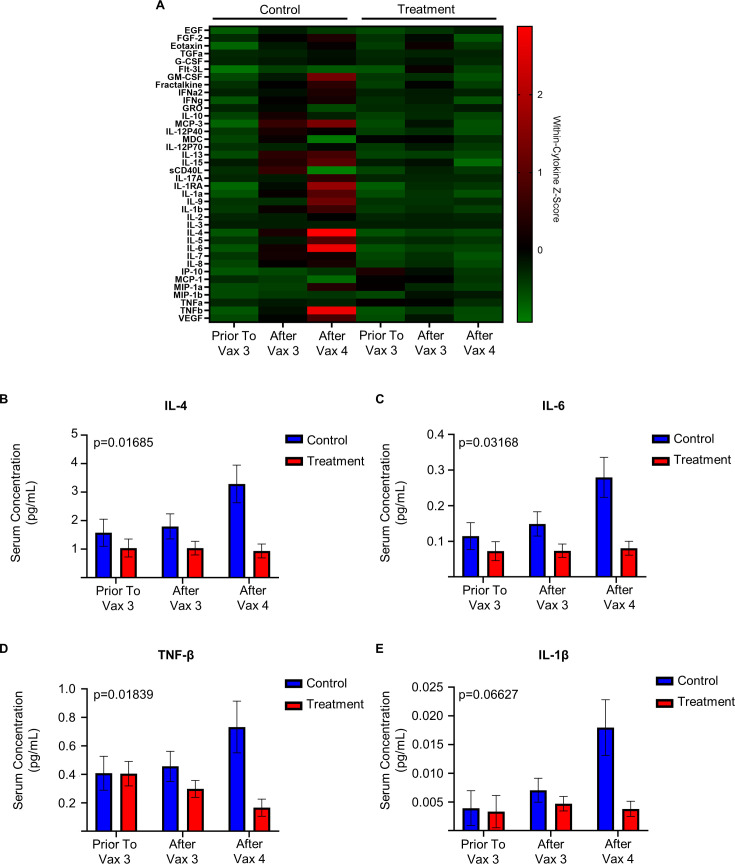
Finally, we observe a differential serum cytokine profile in those receiving treatment with antineoplastic and immunomodulating agents and control patients (figure 4A). Specifically, differences are observed in the circulating levels of IL-4, IL-6, TNF-β, and IL-1β in those treated with antineoplastic and immunomodulating agents (figure 4B–E).
Figure 4.
Antineoplastic and immunomodulating agents are associated with decreased serum cytokine concentration after immunization in patients receiving antineoplastic and immunomodulating agents. (A) Serum cytokine concentration of patients receiving antineoplastic and immunomodulating agents (treatment) and participants not receiving these medications (control) relative to third (Vax 3) and fourth (Vax 4) COVID-19 immunization (Treatment prior to Vax 3 n=10, after Vax 3 n=70, after Vax 4 n=68; control prior to Vax 3 n=9, after Vax 3 n=28, after Vax 4 n=6). Within cytokine Z-score demonstrated from normalized Luminex Immunoassay. (B) Serum concentration (pg/mL, mean estimated by Restricted Maximum Likelihood (REML)-mixed effect model, error bars represent SEM) of IL-4 of treatment and control participants. (p=0.01685 by ANOVA main interaction effect). (C) Serum concentration (pg/mL, estimated by REML-mixed effect model) of IL-6 of treatment and control participants. (p=0.03168 by ANOVA main interaction effect). (D) Serum concentration (pg/mL, estimated by REML-mixed effect model) of TNF-β of treatment and control participants (p=0.01839 by ANOVA main interaction effect). (E) Serum concentration (pg/mL, estimated by REML-mixed effect model) of IL-1β of treatment and control participants. (p=0.06627 by ANOVA main interaction effect, p=0.0245 ANOVA treatment effect). ANOVA, analysis of variance.
Discussion
Here, we confirm an increased risk of SARS-CoV-2 breakthrough infection in patients prescribed antineoplastic and immunomodulating agents despite immunization, risk that is reduced by but nonetheless persists to a total of 4 vaccine doses. Important for treatment planning and patient counseling, this hazard varied based on the particular subclass of WHO ATC L code immunosuppressant prescribed. This study focused on the association between antineoplastic and immunomodulating medications and the elevated risk of postimmunization SARS-CoV-2 infection. This approach represents an improvement over previous studies by offering a unique analysis of the risks associated with immunosuppressive medications, rather than a broad categorization based on disease type, which often encompasses a wide range of therapeutic interventions. The matching process, length of follow-up, and examination of specific medication subclasses add considerable depth to the understanding of increased risk of breakthrough infection and impaired surrogates of immunity in this patient population.1 2 22 23 It additionally builds on understanding of immunosuppression for other viral immunizations.24 25
Among subclasses, an increased incidence of postimmunization SARS-CoV-2 infection is seen particularly among those taking PD-1/PD-L1 monoclonal antibody inhibitors, CD20 monoclonal antibody inhibitors, and calcineurin inhibitors. Risk mitigation behaviors change with patient impression of protection conveyed by immunization, as demonstrated by immunocompromised patients increasing their levels of social participation after vaccination.26 However, the results of the present study suggest that immunization against COVID-19 does not achieve the same protective levels in these immunocompromised cohorts and that patients treated with PD-1/PD-L1 monoclonal antibody inhibitors, CD20 monoclonal antibody inhibitors, and calcineurin inhibitors should be identified for additional counseling regarding avoidance strategies. Although increased severity of COVID-19 has been observed with CD20 inhibition, immune checkpoint inhibitor therapy and calcineurin inhibitors are not associated with increased risk of COVID-19 Intensive Care Unit admission or COVID-19-related death.27–29 By concentrating on the risk of SARS-CoV-2 infection postimmunization, our research highlights that these patients exhibit an impaired protective immune response, necessitating tailored clinical strategies.
It is pertinent to note that during the study period, there was no cross-over of patients between different subclasses of medication in the subclass analysis. Considering the frequent coprescription of PD-1/PD-L1 monoclonal antibody inhibitors with other antineoplastic agents, we delved deeper into the impact of combination treatments. Our findings revealed no significant variation in postimmunization SARS-CoV-2 infection risk when these agents were used in tandem with cytotoxic chemotherapy or protein kinase inhibitors. This suggests that the increased risk of breakthrough infections is primarily linked to the use of PD-1/PD-L1 monoclonal antibody inhibitors, regardless of combination therapy. Given these insights, it becomes imperative to prioritize patients on these particular drug regimens for enhanced counseling about potential postvaccination vulnerabilities and the continued importance of risk mitigation strategies.
In this study, patients were consistently assessed based on their prescription status rather than actual medication consumption. This approach was taken to ensure that any temporary discontinuation, interruption, or dose reduction did not affect their classification. By evaluating the medication prescription status, we aimed to provide a controlled perspective on the risk associated with specific medication subclasses. However, it is important to recognize the inherent limitations in this methodology, especially when extrapolating to real-world scenarios. In real-world settings, it is not uncommon for patients, under the guidance of their physicians, to alter or modify their treatment plan. Our reliance on prescription status as a marker might not capture these nuanced changes, potentially introducing a confounding element. Additionally, during the pandemic personal fears or simple preferences might have led to sporadic adherence to the prescribed regimen. Our methodology, centered on prescription status, may not fully account for these self-guided choices. Additionally, the unprecedented shift to telemedicine healthcare delivery during the pandemic resulted in systemic shifts that may have influenced prescription patterns and patient adherence, somewhat compromising our study’s external validity to future vaccine response research. By assessment of the medication prescription status, we aimed to offer a controlled perspective on the risk associated with specific medication subclasses, while acknowledging these intricacies associated with patient behavior and the COVID-19 pandemic.
The mechanism of immune inhibition differs among the medications we identified as associated with increased risk of breakthrough infection. Despite differing mechanisms of action, CD20 monoclonal antibody inhibitors and calcineurin inhibitors are all known to have low rates of COVID-19 immunization antibody response, which suggests humoral impairment causes the increased rate of postimmunization infection.22 30–35 Durable humoral response depends on the concurrent activation of lymphocytes to develop high-affinity, class switched antibodies.36 This class switch, from IgM or IgD to the expression of IgG, IgE, or IgA, improves effector function for viral inhibition. While we observed no difference in postimmunization IgM levels in treatment versus control patients, a difference in IgG antibody response to Spike S1 and Spike S2 subunit was observed. This suggests that highly regulated mechanisms underlying class switch, such as cytokine stimulation, intracellular signaling, cellular proliferation, and epigenetic controls are also affected by these immunosuppressant subclasses, further supported by our finding of a different circulating cytokine expression profile in treated versus control participants. Although a plethora of cytokines have been reported to mediate class switch recombination, IL-4 and IL-21 are of particular significance in potentiating the switching to IgG, consistent with our finding of decreased postimmunization circulating IL-4 in patients treated with antineoplastic and immunomodulating agents, with an associated decrease in IgG antibody response.37 In those treated with WHO ATC L therapies, we also observed decreased circulating levels of IL-6 and TNF following immunization, both of which are necessary for antibody secreting cell proliferation and survival.36 38 Our findings, as based on correlations, should be considered preliminary as we did not prospectively modulate IL-4, IL-6, nor TNF, but rather observed the association with the WHO ATC L medication class.
IL-1β is primarily a mediator of innate immune response, yet it serves as a key mediator of inflammatory response to mRNA immunization by stimulating the release of other proinflammatory cytokines.33 The WHO ATC L category encompasses diverse immunosuppressive and immunomodulating agents, each potentially influencing IL-1β levels differently based on their mechanism of action. For instance, while aromatase inhibitors may not alter serum IL-1β levels, drugs like the IL-1 receptor antagonist anakinra can directly inhibit IL-1β activity.34 While IL-1β plays a crucial role in mRNA vaccine response and innate immunity, its levels can differ based on the specific immunocompromised state. The lower expression level of IL-1β in our treatment group suggests a possible deficiency with initial innate response to immunization, which in turn reduces long-term humoral response. However, it is essential to note that in non-vaccinated individuals, baseline IL-1β levels primarily hinge on health status and any prevailing inflammatory or immune processes. Absent an immune challenge, such as vaccination, healthy non-vaccinated individuals would maintain relatively stable IL-1β levels.35 36 It is essential to approach this correlation with caution, understanding that observed associations do not confirm causality, and other factors may also play a role in determining antibody responses. Importantly, we found differences in both circulating cytokines and IgG antibody response to Spike protein epitopes in a subgroup of immunosuppressed patients exhibiting a greater rate of postimmunization infection.
We are the first to report an increased risk of SARS-CoV-2 infection among patients treated with PD-1/PD-L1 monoclonal antibody inhibitors who have received immunization. Other investigators have reported a marked postvaccine surge in serological cytokine response, notably IL-6 and other cytokine release syndrome-related cytokines, postimmunization that seemingly did not correspond with heightened rates of antibody generation.1 22 30 39 40 This highlights the mechanism of checkpoint inhibitor therapy as a release on immune inhibition and stands unique to the antineoplastic and immunomodulating class as a whole, where we observe decreased cytokine and antibody response. Yet, this hyperinflammatory response did not result in increased adverse events in patients receiving COVID-19 immunization.40 Reported increase in IL-6 contrasts with our analysis of patients treated with WHO ATC L therapies in whom we observed decreased IL-6. This parallels the finding that patients receiving ICI therapy did not experience increased severity of natural COVID-19 infection, as measured by hypoxia and respiratory collapse caused by hyperinflammation.29 In a recent meta-analysis where the overall quality of evidence was rated as low, patients receiving immune checkpoint inhibitors had similar rates of seroconversion to patients with cancer who were not on active treatment.41 While PD-1/PD-L1 directed therapy may amplify certain arms of the immune response, specifically leading to a surge in cytokines, they might not uniformly bolster all aspects of immunity crucial for effective viral defense. Thus, our clinical outcome results clarify this dichotomy of previously published identification of augmented cytokine response but unaugmented antibody response which ultimately is associated in an increased risk of postimmunization SARS-CoV-2 infection among those prescribed PD-1/PD-L1 monoclonal antibody inhibitors. This observation underscores the potential pitfalls in solely relying on antibody or cytokine responses as a measure of immunity. Achieving a perceived “adequate” response could misleadingly reassure patients on immunotherapy, who might be inherently at higher risk. Hence, until surrogate immune responses are linked definitively to clinical outcomes across medication classes, clinicians should exercise prudence when communicating such preliminary findings to patients.
This stands unique compared with influenza immunization, where patients on PD-1/PD-L1 monoclonal antibody inhibitors have positive efficacy outcomes of influenza immunization, and thus may point to differences in the biology of response to RNA immunization, which was the majority mechanism of immunization in our population.25 Yet, our results support the positive efficacy outcomes of influenza immunization among patients with cancer.42 Further, we observe that receiving multiple immunizations reduces the risk associated with immunotherapy. Thus, although an altered immune response occurs, patients prescribed these medications still maintain some benefit from immunization.
Similarly, although a diminished antibody response occurs following multiple immunization doses in those treated with CD20 monoclonal antibody inhibitors, patients treated with these agents develop T-cell response.42 43 We find patients prescribed CD20 monoclonal antibody inhibitors to remain at increased risk of postimmunization COVID-19, clarifying that these patients remain at risk of infection despite T-cell response. Solid organ transplant patients primarily treated with a calcineurin inhibitor also develop T-cell response to immunization alongside diminished antibody response.44 45 We find patients treated with calcineurin inhibitors to remain at increased risk of postimmunization SARS-CoV-2 infection even after three or four doses of immunization. Even in the context of PD-1/PD-L1 monoclonal antibody inhibitors, the presence of a hyperactivated T-cell response does not necessarily translate to a decreased risk of infection postimmunization. Drawing from these observations, it can be inferred that while the T-cell response might play a crucial role in modulating the severity of the disease on infection, the B-cell-mediated antibody response seems to have a more pronounced effect in preventing the infection in the first place. This highlights the unique and distinct roles the two arms of the adaptive immune response play in the context of SARS-CoV-2 immunization, emphasizing the importance of a balanced immune response.
Patients on antineoplastic and immunomodulating medications were targeted with behavioral counseling throughout the duration of the pandemic.9 10 46 We find no reports of preferential counseling that targeted specific subclasses of immunocompromising medications. Our study helps identify certain medications within immunosuppressants at large that portend increased risk of postimmunization SARS-CoV-2 infection. Targeted interventions focused on these prescribed medications may improve postimmunization outcomes for this vulnerable patient population. For example, pausing these medications, exchanging medication around the timing of immunization, or delaying immunization until medication can be discontinued may improve postimmunization infection outcomes.22 35
Our study draws strength from beginning with the full WHO ATC L medication class, and unbiasedly assessing the risk of postimmunization SARS-CoV-2 infection first in all patients on antineoplastic and immunomodulating agents. We identify an associated risk of postimmunization SARS-CoV-2 infection that is not overcome by multiple immunizations. In our study, 87 patients, treated with a range of antineoplastic and immunomodulating agents, contributed 218 blood samples for antibody and cytokine analysis. However, for the subset giving blood samples, there were notable demographic differences between our control and experimental groups. Specifically, the treated group was older in median age and had a higher proportion receiving the Moderna COVID-19 immunization. The constraints of the pandemic impacted our enrolment, with many hesitant or unable to participate in person. By the time of our serological assessments, many patients had received their first two immunizations. Thus, our study was designed to analyze antibody responses and cytokine profiles after the third and fourth doses. While our serological sample size was modest compared with the 13 362 control and 13 362 treated patients evaluated for SARS-CoV-2 infection outcomes, the serological insights remain valuable. Although results should be interpreted with measured care given the demographic differences, our findings provide a foundation for further assessment of potential biomarkers of vaccine response, as it is anticipated this mechanism of immunization will be adopted for other diseases. Additionally, this study draws strength from its large sample size, our matched control cohort drawn from a population of over 2 00 000 patients within a large, diverse healthcare system. Despite capturing a broad spectrum of prescriptions reflective of community dynamics, it is pertinent to highlight the predominant Caucasian demographic with a median age in the mid-60s, actively engaged with the healthcare system. This may limit the generalizability to populations with different cultural or community norms, vaccine hesitancy, or restricted healthcare access. Extrapolation to groups less represented in our study, such as African Americans, Native Americans, and patients with age <40, necessitates caution.
We also recognize the absence of an unvaccinated study group, which would have provided a direct comparison to isolate the benefits or potential detriments of vaccination from the effects of these treatments. Without this directed comparison, our conclusions rely on existing literature that has shown patients with cancer, solid organ transplant recipients and those on immunosuppressive treatments generally face heightened risk of COVID-19. Our study expands on this to pinpoint medications of particular risk.
These findings highlight a medication-dependent increased risk of postimmunization infection for patients receiving antineoplastic and immunomodulating agents. They offer critical guidance for physician-patient decisions regarding booster effectiveness and additional risk mitigation during future COVID-19 variant surges, and they identify medications that may reduce the effectiveness of RNA-based vaccines against other infectious agents. Accordingly, our results will help clinicians practice more personalized care, tailoring interventions based on each patient’s treatment plan.
Footnotes
Twitter: @AmitabhCPandey
LJN and ACP contributed equally.
Contributors: Conceptualization and design: The conception and design of the study were carried out by JN, JC, SK, SB, RF, MQ, LJN and ACP. Data collection and analysis: The acquisition and analysis of data were performed by JN, JC, SK, SB, RF, LS, LP, TH, SK, RF, LH, PR, KSE, AM, BB, CM, LJN and ACP. Writing–original draft preparation: The initial manuscript was drafted by JN, JC, LJN and ACP. Writing–review and editing: The manuscript was critically revised for important intellectual content by JN, JC, LP, LH, LJN and ACP. Funding acquisition: Funding for the project was secured by JN, CM, MQ, LJN and ACP. Guarantor: ACP.
Funding: The project described in this article was supported by the Scripps Research CTSA awards UL1TR002550 and KL2TR002552 from the National Center for Advancing Translational Sciences of the National Institutes of Health and by philanthropic donations for COVID-19 research at Scripps Health.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of Scripps Health or the National Institutes of Health.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. The datasets generated and/or analyzed during the current study are not publicly available but can be made available from the corresponding author on reasonable request. Requests for access to these data should be addressed to ACP and LJN. Access to the data will be considered by the corresponding author in accordance with ethical guidelines and where permissible in line with data protection and confidentiality agreements. Please note that requests may be subject to certain conditions and approvals, as the data may contain information that could compromise research participant privacy/consent.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Scripps Health Institutional Review Board: IRB-21-7766. Participants gave informed consent to participate in the study before taking part.
References
- 1. Robilotti EV, Babady NE, Mead PA, et al. Determinants of COVID-19 disease severity in patients with cancer. Nat Med 2020;26:1218–23. 10.1038/s41591-020-0979-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sahota A, Tien A, Yao J, et al. Incidence, risk factors, and outcomes of COVID-19 infection in a large cohort of solid organ transplant recipients. Transplantation 2022;106:2426–34. 10.1097/TP.0000000000004371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gao Y, Chen Y, Liu M, et al. Impacts of immunosuppression and immunodeficiency on COVID-19: a systematic review and meta-analysis. J Infect 2020;81:e93–5. 10.1016/j.jinf.2020.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sparks JA, Wallace ZS, Seet AM, et al. Associations of baseline use of biologic or targeted synthetic DMARDs with COVID-19 severity in rheumatoid arthritis: results from the COVID-19 global rheumatology alliance physician registry. Ann Rheum Dis 2021;80:1137–46. 10.1136/annrheumdis-2021-220418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Suárez-García I, Perales-Fraile I, González-García A, et al. In-hospital mortality among immunosuppressed patients with COVID-19: analysis from a national cohort in Spain. PLOS ONE 2021;16:e0255524. 10.1371/journal.pone.0255524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603–15. 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-Cov-2 vaccine. N Engl J Med 2021;384:403–16. 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sun J, Zheng Q, Madhira V, et al. Association between immune dysfunction and COVID-19 breakthrough infection after SARS-Cov-2 vaccination in the US. JAMA Intern Med 2022;182:153–62. 10.1001/jamainternmed.2021.7024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-Cov-2 mRNA vaccine series in solid organ transplant recipients. JAMA 2021;325:2204–6. 10.1001/jama.2021.7489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Krasselt M, Wagner U, Nguyen P, et al. Humoral and cellular response to COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases under real-life conditions. Rheumatology (Oxford) 2022;61:SI180–8. 10.1093/rheumatology/keac089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moor MB, Suter-Riniker F, Horn MP, et al. Humoral and cellular responses to mRNA vaccines against SARS-Cov-2 in patients with a history of CD20 B-cell-depleting therapy (Rituxivac): an investigator-initiated, single-centre, open-label study. Lancet Rheumatol 2021;3:e789–97. 10.1016/S2665-9913(21)00251-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Iancovici L, Khateeb D, Harel O, et al. Rheumatoid arthritis patients treated with Janus kinase inhibitors show reduced humoral immune responses following BNT162b2 vaccination. Rheumatology (Oxford) 2022;61:3439–47. 10.1093/rheumatology/keab879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deepak P, Kim W, Paley MA, et al. Effect of immunosuppression on the immunogenicity of mRNA vaccines to SARS-Cov-2: a prospective cohort study. Ann Intern Med 2021;174:1572–85. 10.7326/M21-1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haggenburg S, Hofsink Q, Lissenberg-Witte BI, et al. Antibody response in immunocompromised patients with hematologic cancers who received a 3-dose mRNA-1273 vaccination schedule for COVID-19. JAMA Oncol 2022;8:1477–83. 10.1001/jamaoncol.2022.3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Choueiri TK, Labaki C, Bakouny Z, et al. Breakthrough SARS-Cov-2 infections among patients with cancer following two and three doses of COVID-19 mRNA vaccines: a retrospective observational study from the COVID-19 and cancer consortium. Lancet Reg Health Am 2023;19:100445. 10.1016/j.lana.2023.100445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hermel DJ, Cham J, Spierling Bagsic SR, et al. An observational study of hospitalized COVID-19 patients with cancer in San Diego County. Future Oncology 2022;18:719–25. 10.2217/fon-2021-1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (Redcap)—A metadata-driven methodology and Workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harris PA, Taylor R, Minor BL, et al. The REDcap consortium: building an international community of software platform partners. J Biomed Inform 2019;95:103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cham J, Pandey AC, New J, et al. 6 month serologic response to the Pfizer-BioNTech COVID-19 vaccine among healthcare workers. PLoS One 2022;17:e0266781. 10.1371/journal.pone.0266781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ikegami S, Benirschke RC, Fakhrai-Rad H, et al. Target specific serologic analysis of COVID-19 convalescent plasma. PLoS One 2021;16:e0249938. 10.1371/journal.pone.0249938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sterlin D, Mathian A, Miyara M, et al. IgA dominates the early neutralizing antibody response to SARS-Cov-2. Sci Transl Med 2021;13:eabd2223. 10.1126/scitranslmed.abd2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Figueiredo JC, Merin NM, Hamid O, et al. Longitudinal SARS-Cov-2 mRNA vaccine-induced humoral immune responses in patients with cancer. Cancer Res 2021;81:6273–80. 10.1158/0008-5472.CAN-21-3554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ehmsen S, Asmussen A, Jeppesen SS, et al. Increased antibody titers and reduced seronegativity following fourth mRNA COVID-19 vaccination in patients with cancer. Cancer Cell 2022;40:800–1. 10.1016/j.ccell.2022.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cowan M, Chon WJ, Desai A, et al. Impact of immunosuppression on recall immune responses to influenza vaccination in stable renal transplant recipients. Transplantation 2014;97:846–53. 10.1097/01.TP.0000438024.10375.2d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Spagnolo F, Boutros A, Croce E, et al. Influenza vaccination in cancer patients receiving immune Checkpoint inhibitors: a systematic review. Eur J Clin Invest 2021;51:e13604. 10.1111/eci.13604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heesen G, Heinemann S, Müller F, et al. Social participation and mental health of immunocompromised individuals before and after COVID-19 vaccination-results of a longitudinal observational study over three time points. Front Psychiatry 2022;13:1080106. 10.3389/fpsyt.2022.1080106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Calderón-Parra J, Cuervas-Mons V, Moreno-Torres V, et al. Influence of chronic use of corticosteroids and calcineurin inhibitors on COVID-19 clinical outcomes: analysis of a nationwide registry. Int J Infect Dis 2022;116:51–8. 10.1016/j.ijid.2021.12.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Singh N, Madhira V, Hu C, et al. Rituximab is associated with worse COVID-19 outcomes in patients with rheumatoid arthritis: a retrospective, nationally sampled cohort study from the U.S. national COVID cohort collaborative (N3C). Semin Arthritis Rheum 2023;58:152149. 10.1016/j.semarthrit.2022.152149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tan R, Yun C, Seetasith A, et al. Impact of immune checkpoint inhibitors on COVID-19 severity in patients with cancer. Oncologist 2022;27:236–43. 10.1093/oncolo/oyab083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oosting SF, van der Veldt AAM, GeurtsvanKessel CH, et al. COVID-19 vaccination in patients receiving chemotherapy, immunotherapy, or chemoimmunotherapy for solid tumours: a prospective, multicentre, non-inferiority trial. Lancet Oncol 2021;22:1681–91. 10.1016/S1470-2045(21)00574-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Madelon N, Lauper K, Breville G, et al. Robust T-cell responses in anti-Cd20-treated patients following COVID-19 vaccination: a prospective cohort study. Clin Infect Dis 2022;75:e1037–45. 10.1093/cid/ciab954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vijenthira A, Gong I, Betschel SD, et al. Vaccine response following anti-CD20 therapy: a systematic review and meta-analysis of 905 patients. Blood Adv 2021;5:2624–43. 10.1182/bloodadvances.2021004629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stoll S, Desai S, Levit E. A retrospective evaluation of seroconversion after COVID-19 during the early Omicron wave in fully vaccinated multiple sclerosis patients receiving anti-CD20 therapies. Mult Scler Relat Disord 2023;71:104574. 10.1016/j.msard.2023.104574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Midtvedt K, Vaage JT, Heldal K, et al. Fourth dose of the SARS-Cov-2 vaccine in kidney transplant recipients with previously impaired humoral antibody response. Am J Transplant 2022;22:2704–6. 10.1111/ajt.17091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Osmanodja B, Ronicke S, Budde K, et al. Serological response to three, four and five doses of SARS-Cov-2 vaccine in kidney transplant recipients. JCM 2022;11:2565. 10.3390/jcm11092565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nutt SL, Hodgkin PD, Tarlinton DM, et al. The generation of antibody-secreting plasma cells. Nat Rev Immunol 2015;15:160–71. 10.1038/nri3795 [DOI] [PubMed] [Google Scholar]
- 37. Avery DT, Bryant VL, Ma CS, et al. IL-21-induced Isotype switching to IgG and IgA by human naive B cells is differentially regulated by IL-4. J Immunol 2008;181:1767–79. 10.4049/jimmunol.181.3.1767 [DOI] [PubMed] [Google Scholar]
- 38. Dubois B, Massacrier C, Vanbervliet B, et al. Critical role of IL-12 in dendritic cell-induced differentiation of naive B lymphocytes. J Immunol 1998;161:2223–31. [PubMed] [Google Scholar]
- 39. Rogiers A, Pires da Silva I, Tentori C, et al. Clinical impact of COVID-19 on patients with cancer treated with immune checkpoint inhibition. J Immunother Cancer 2021;9:e001931. 10.1136/jitc-2020-001931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Walle T, Bajaj S, Kraske JA, et al. Cytokine release syndrome-like serum responses after COVID-19 vaccination are frequent and clinically inapparent under cancer immunotherapy. Nat Cancer 2022;3:1039–51. 10.1038/s43018-022-00398-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ruiz JI, Lopez-Olivo MA, Geng Y, et al. COVID-19 vaccination in patients with cancer receiving immune checkpoint inhibitors: a systematic review and meta-analysis. J Immunother Cancer 2023;11:e006246. 10.1136/jitc-2022-006246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jyssum I, Kared H, Tran TT, et al. Humoral and cellular immune responses to two and three doses of SARS-Cov-2 vaccines in rituximab-treated patients with rheumatoid arthritis: a prospective, cohort study. Lancet Rheumatol 2022;4:e177–87. 10.1016/S2665-9913(21)00394-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mrak D, Tobudic S, Koblischke M, et al. SARS-Cov-2 vaccination in rituximab-treated patients: B cells promote humoral immune responses in the presence of T-cell-mediated immunity. Ann Rheum Dis 2021;80:1345–50. 10.1136/annrheumdis-2021-220781 [DOI] [PubMed] [Google Scholar]
- 44. Bertrand D, Hamzaoui M, Lemée V, et al. Antibody and T cell response to SARS-Cov-2 messenger RNA BNT162b2 vaccine in kidney transplant recipients and hemodialysis patients. J Am Soc Nephrol 2021;32:2147–52. 10.1681/ASN.2021040480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schrezenmeier E, Rincon-Arevalo H, Jens A, et al. Temporary antimetabolite treatment hold BOOSTS SARS-Cov-2 vaccination–specific humoral and cellular immunity in kidney transplant recipients. JCI Insight 2022;7:e157836. 10.1172/jci.insight.157836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Embi PJ, Levy ME, Naleway AL, et al. Effectiveness of 2-dose vaccination with mRNA COVID-19 vaccines against COVID-19-associated hospitalizations among immunocompromised adults - nine States, January-September 2021. MMWR Morb Mortal Wkly Rep 2021;70:1553–9. 10.15585/mmwr.mm7044e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2023-008233supp001.pdf (2.1MB, pdf)
Data Availability Statement
Data are available on reasonable request. The datasets generated and/or analyzed during the current study are not publicly available but can be made available from the corresponding author on reasonable request. Requests for access to these data should be addressed to ACP and LJN. Access to the data will be considered by the corresponding author in accordance with ethical guidelines and where permissible in line with data protection and confidentiality agreements. Please note that requests may be subject to certain conditions and approvals, as the data may contain information that could compromise research participant privacy/consent.