Abstract
Background
CT attenuation is affected by lung volume, dosage, and scanner bias, leading to inaccurate emphysema progression measurements in multicenter studies.
Purpose
To develop and validate a method that simultaneously corrects volume, noise, and interscanner bias for lung density change estimation in emphysema progression at CT in a longitudinal multicenter study.
Materials and Methods
In this secondary analysis of the prospective Genetic Epidemiology of Chronic Obstructive Pulmonary Disease (COPDGene) study, lung function data were obtained from participants who completed baseline and 5-year follow-up visits from January 2008 to August 2017. CT emphysema progression was measured with volume-adjusted lung density (VALD) and compared with the joint volume-noise-bias–adjusted lung density (VNB-ALD). Reproducibility was studied under change of dosage protocol and scanner model with repeated acquisitions. Emphysema progression was visually scored in 102 randomly selected participants. A stratified analysis of clinical characteristics was performed that considered groups based on their combined lung density change measured by VALD and VNB-ALD.
Results
A total of 4954 COPDGene participants (mean age, 60 years ± 9 [SD]; 2511 male, 2443 female) were analyzed (1329 with repeated reduced-dose acquisition in the follow-up visit). Mean repeatability coefficients were 30 g/L ± 0.46 for VALD and 14 g/L ± 0.34 for VNB-ALD. VALD measurements showed no evidence of differences between nonprogressors and progressors (mean, −5.5 g/L ± 9.5 vs −8.6 g/L ± 9.6; P = .11), while VNB-ALD agreed with visual readings and showed a difference (mean, −0.67 g/L ± 4.8 vs −4.2 g/L ± 5.5; P < .001). Analysis of progression showed that VNB-ALD progressors had a greater decline in forced expiratory volume in 1 second (−42 mL per year vs −32 mL per year; Tukey-adjusted P = .002).
Conclusion
Simultaneously correcting volume, noise, and interscanner bias for lung density change estimation in emphysema progression at CT improved repeatability analyses and agreed with visual readings. It distinguished between progressors and nonprogressors and was associated with a greater decline in lung function metrics.
Clinical trial registration no. NCT00608764
© RSNA, 2024
Supplemental material is available for this article.
See also the editorial by Goo in this issue.
Summary
This study develops and validates a method simultaneously correcting for confounding factors of volume, noise, and scanner change for precise assessment of emphysema progression at CT in multicenter studies.
Key Results
■ In a secondary analysis of the Genetic Epidemiology of Chronic Obstructive Pulmonary Disease study with 5-year follow-up (n = 4954), correcting for volume-noise-bias confounders improved emphysema progression repeatability in repeated CT compared with volume-adjusted lung density (VALD) (14 g/L vs 30 g/L).
■ Volume-noise-bias–adjusted lung density (VNB-ALD) agreed with visually assessed nonprogressors and progressors and distinguished them (mean, −0.67 g/L ± 4.8 vs −4.2 g/L ± 5.5; P < .001) compared with VALD (mean, −5.5 g/L ± 9.5 vs −8.6 g/L ± 9.6; P = .11).
■ Use of VNB-ALD instead of VALD might reduce the required number of participants by approximately 75%.
Introduction
Emphysema is a pathologic hallmark of chronic obstructive pulmonary disease (COPD), and CT has become the standard in the detection of the presence of COPD and in the monitoring of its progression. The most widespread density-based metrics of emphysema are the low attenuation area, which reflects the percentage of lung volume ratio below a certain attenuation threshold, typically between −910 and −950 HU, and the CT attenuation at the 15th percentile of the lung CT histogram (Perc15), expressed in grams per liter. Such metrics are exposed to similar confounders.
In longitudinal studies, the variability of quantitative CT due to differences in imaging acquisition and reconstruction parameters (eg, dose, peak voltage, reconstruction algorithm) can be mitigated by employing standardized protocols (1,2). This may, however, be challenging in multicenter studies due to intersite differences in scanner manufacturers and models. The typically slow rate at which emphysema progresses further complicates radiologic disease monitoring, as sites may replace and upgrade their equipment, and participants may experience a change in their body mass index or lung volume between visits. Mitigation of such confounding factors is critical to enable CT densitometry for longitudinal observational and interventional studies.
The confounders of emphysema metrics can be grouped into three categories: changes in lung volume (3), different noise characteristics (4,5), and interscanner biases in Hounsfield unit values (6). The aggregate result of these effects is a nonlinear transformation of the lung attenuation histogram and the low-attenuation area and Perc15 values. Previous attempts to address these confounding effects have been proposed in the literature (7). The so-called sponge model uses a linear model to adjust the percentile based on the observed-to-predicted volume ratio (3). Denoising techniques have been used to make the histograms more comparable (8), but they do not ensure identical noise characteristics. Some studies have employed density deviations of air in the trachea across scanners to reduce systematic bias in lung parenchyma (9,10). However, differences observed in the trachea may differ in magnitude compared with those in lung parenchyma. Additionally, these methods do not consider the nonlinear combination of the three effects (volume, noise, and interscanner density bias) jointly. Therefore, the results obtained are suboptimal.
Thus, the aim of this work was to develop and validate a method that simultaneously corrects volume, noise, and interscanner bias for lung density change estimation in emphysema progression at CT in a longitudinal multicenter study.
Materials and Methods
Cohort
This is a secondary analysis of the prospective Genetic Epidemiology of COPD (COPDGene) study (ClinicalTrials.gov identifier NCT00608764; http://www.copdgene.org/). COPDGene is a multicenter longitudinal observational study focused on the epidemiologic and genetic factors associated with COPD in individuals with a smoking history and self-designation as non-Hispanic White or African American (1). No informed consent was required. The inclusion criteria were COPDGene participants who completed baseline and 5-year follow-up visits from January 2008 to August 2017. This included participants with an additional reduced-dose CT scan at the follow-up visit. Participants with poor CT quality or without lung masks were excluded. This study will validate simultaneous correction for lung volume variability, noise, and interscanner bias in emphysema progression in the COPDGene cohort.
CT Image Acquisition
CT images were acquired using a standardized manufacturer-specific protocol with participants in the supine position at full inspiration. Acquisition parameters included two dosage protocols: a full radiation dose (120 kVp, fixed 200 mAs) for phase 1 (baseline) and phase 2 (follow-up) and a reduced radiation dose (120 kVp with dose modulation, with average dose between 40 and 80 mAs) for a subset of participants at phase 2 (8). Reconstruction was performed with filtered back projection and soft algorithm (reconstruction methods, manufacturers, and models are listed in Appendix S1).
Volume-Noise-Bias–adjusted Lung Density Emphysema Progression
This study proposes a volume-noise-bias–adjusted lung density (VNB-ALD) approach. It applies an analytic technique to modify the paired baseline lung attenuation histogram to match the acquisition conditions of the follow-up scan. The baseline histogram transformation involves rescaling according to the sponge model after performing bias correction and convolving with a Gaussian kernel with variance equal to the difference in noise variance between baseline and follow-up scans (see Appendix S2 for further details). VNB-ALD only requires the estimation of volume, noise change, and interscanner bias.
Lung volume was calculated using the Chest Imaging Platform (https://chestimagingplatform.org/). Noise variance per CT scan was calculated using an extension of the method proposed by Tian and Samei (5). The scanner effect is calculated using a fixed-effects model of the lung mass difference between follow-up and baseline. See Appendix S3 for further details on parameter estimation.
The differences between the observed follow-up and transformed baseline histogram can be attributed to pathologic progression. Emphysema progression was thus defined as the difference between the 15th percentiles of the follow-up lung histogram (expressed as grams per liter) and the transformed baseline histogram. The effect of this formulation is depicted in Figure 1, where the baseline histogram is transformed to align with the volume, noise, and bias characteristics of the follow-up scanner.
Figure 1:

Prediction of the baseline lung density histogram under the acquisition conditions of the 5-year follow-up scanner. The observed baseline histogram is represented in green. The observed follow-up histogram is shown as a solid red line. The prediction is depicted as the dashed red line. From the prediction, emphysema progression can be estimated as the difference between the observed follow-up and the predicted measurements. HU = Hounsfield units.
Lastly, to compare with the current standard of analysis, the volume-adjusted lung density (VALD) at the 15th percentile of the attenuation histogram was calculated using the sponge model (3), as recommended by the Quantitative Imaging Biomarkers Alliance guidelines (11). The radiologic progression of emphysema was calculated as the VALD at the 5-year interval follow-up minus the VALD at baseline. A negative value represents loss of lung tissue and progression of emphysema.
Visual Emphysema Progression Score
The visual assessment of emphysema progression was performed in a randomly selected subset of 102 participants with emphysema at baseline. CT lung volume differences between baseline and 5-year follow-up were less than 250 mL. Participants were matched for sex, smoking status, and spirometric stability. The visual outcome assessment involved three readers, each with more than 5 years of experience in lung imaging, who classified the participants’ anonymized CT scans as progressors or nonprogressors. Two readers (G.V.S.F., R.S.J.E.; 8 and 15 years of experience, respectively) independently reviewed all cases, while a third reader (A.A.D.; 22 years of experience), unaware of the interpretations of the first two, provided the majority opinion for discordant scans. The classification was performed using the full-dose baseline and follow-up scans after co-registration and visualized simultaneously with ITK-SNAP software (http://www.itksnap.org/).
Statistical Analyses
The intraparticipant lung volume variability, noise variability, and interscanner bias were analyzed with t tests. VALD and VNB-ALD repeatability under dosage change were analyzed using the repeated acquisitions in phase 2. Repeatability of progression measurements under joint change of scanner and dosage protocol was done using the full-dose phase 1 to full-dose phase 2 scan pairs as a reference and was compared with full-dose phase 1 to reduced-dose phase 2 pairs. The calculation of the repeatability coefficient is described in Appendix S4.
The analyzed clinical characteristics in all participants (n = 4954) were stratified into four groups based on their combined change of lung density measured with VALD and VNB-ALD. P values were calculated using analysis of variance or χ2 tests, as appropriate. Tukey and Bonferroni post hoc tests (5% error rate) were used for multiple comparisons. Analyses were performed using G*Power 3.1.9.3 (12) (https://psychologie.hhu.de/arbeitsgruppen/allgemeine-psychologie-und-arbeitspsychologie/gpower) and Python statistical packages statsmodels 0.14.0 (https://statsmodels.org) and Pingouin 0.5.3 (https://pingouin-stats.org). Statistical significance was indicated by an α level of .05.
Results
Characteristics of Cohort
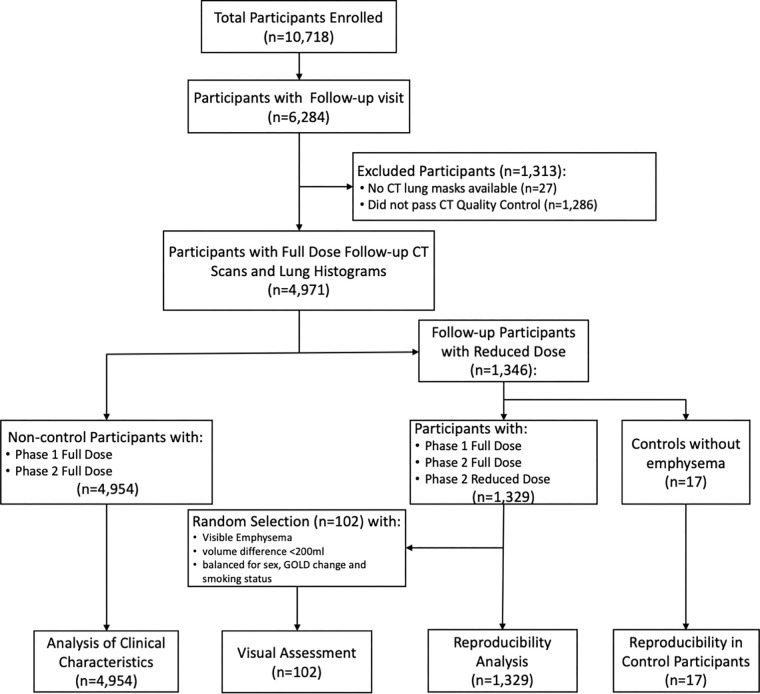
Demographics for participants are shown in Table 1 and are detailed in Table S2. COPDGene-specific data sets used in this study include 4954 COPDGene participants (mean age, 60 years ± 9 [SD]; 2511 male, 2443 female) with current or former smoking history (>10 pack-year cigarette smoking) with CT at baseline and 5-year follow-up available (Fig 2). The cohort included 3475 (approximately 70%) non-Hispanic White participants. A subset of 1329 participants had an additional low-radiation-dose phase 2 CT scan, including 17 control participants who had never smoked and were without COPD or visible emphysema.
Table 1:
Baseline Characteristics of the COPDGene Participants
Figure 2:
Flow diagram of participant selection. Participants were included from the Genetic Epidemiology of Chronic Obstructive Pulmonary Disease (COPDGene) study, including individuals with and those without spirometric chronic obstructive pulmonary disease. GOLD = Global Initiative for Chronic Obstructive Lung Disease.
Confounders in the COPDGene Cohort
Lung density confounder analysis in the COPDGene cohort is shown in Figure 3. Lung volume exhibited a mean intraparticipant difference in total lung capacity of 484 mL ± 493 across visits and 213 mL ± 350 within visit 2. CT noise metrics showed an increase in CT noise variance when the acquisition protocol changed to reduced dose (mean, 14.1 mL ± 3.4 vs 23.9 mL ± 3.0; P < .001). The full radiation dose protocol also exhibited differences across phases (mean, 14.8 mL ± 3.11 at baseline vs 14.1 mL ± 3.4 at follow-up; P < .001). The interscanner bias showed a lower value when the same scanner was used (mean, 11.0 mL ± 10.2 vs 13.8 mL ± 13.0; P < .001). The specific interscanner biases are provided in Table S1.
Figure 3:
Variability of (A) lung volume, (B) noise, and (C) interscanner bias in Genetic Epidemiology of Chronic Obstructive Pulmonary Disease (COPDGene) study for phases 1 and 2 with full-dose (FD) and reduced-dose (RD) protocols. (A) Intraparticipant volume absolute difference of total lung capacity (TLC) across phases and dose protocols. The absolute difference between TLC is over 480 mL across visits and 213 mL within visit 2. (B) Noise characteristics in each phase and dose protocol (differences between noise levels were assessed by using paired t tests). (C) Absolute differences due to interscanner bias. The differences for same scanner models were significantly smaller than when the scanner models differ (differences between groups assessed with t test).
Repeatability with Change of Scanner and Dosage Protocol
Figure 4 shows Bland-Altman plots for both methods. VALD results exhibited a positive average difference of 13.7 g/L (95% CI: −0.40, 28) for the difference in lung density Perc15 between full dose and reduced dose compared with full dose to full dose, which is attributable to increased noise variance that spreads the density distribution (refer to Fig S1 in Appendix S2 for examples). VNB-ALD method reduced the average difference by 66% (4.6 g/L; 95% CI: 6.0, 15.2). The repeatability coefficients were 30.3 g/L ± 0.46 for VALD and 13.9 g/L ± 0.34 for VNB-ALD. Repeatability analyses with the 17 control participants confirmed the presence of nonzero average differences, even in the absence of emphysema progression (Appendix S5).
Figure 4:
Consistency analysis of difference in CT attenuation at the 15th percentile of the lung CT histogram (Perc15). Bland-Altman plots represent the difference in Perc15 progression measured for full-dose configurations in both phases (∆Perc15FD FD) versus full dose in phase 1 and reduced dose in phase 2 (∆Perc15FD RD). Left: Conventional correction performed with the volume-adjusted lung density (VALD). Right: Proposed method considering the joint effect of volume, noise, and bias (VNB-ALD). Positive bias means more progression in the full-dose to reduced-dose pair of scans than in the full-dose to full-dose pair.
The reproducibility analysis performed just for dosage change is shown in Figure S5. No interscanner bias was expected, as both scans were acquired with the same scanner during phase 2. VALD exhibited a positive average difference (13.7 g/L; 95% CI: −0.40, 27.8) due to noise mimicking strong emphysema progression. Conversely, VNB-ALD reduced the effect of noise (−0.32 g/L; 95% CI: −2.2, 1.5). The repeatability coefficients obtained were 30.3 g/L ± 0.46 for VALD and 1.96 g/L ± 0.06 for VNB-ALD.
Visual Assessment
Lung density differences are shown in Figure 5 for both dosage protocols and are stratified by visually assessed progressors and nonprogressors (a negative difference means progression). For the full-dose phase 1 and full-dose phase 2 protocols (Fig 5A), mean VALD progression measurements showed no evidence of a difference between groups (nonprogressors, −5.5 g/L ± 9.5; progressors, −8.6 g/L ± 9.6; P = .10), while mean VNB-ALD measurements showed a difference (nonprogressors, −0.67 g/L ± 4.8; progressors, −4.2 g/L ± 5.5; P < .001). On the basis of this variability, using VNB-ALD instead of VALD in a clinical study assessing therapy efficacy for emphysema progression could reduce the required number of participants by approximately 75% (sample size of 92 vs 416 estimated with a t test for differences between means, assuming a 1:1 design and full response in the treatment arm).
Figure 5:
Comparison between methods for visually assessed emphysema progression for different dosage protocols. (A) Full-dose phase 1 to full-dose phase 2 protocol. (B) Full-dose phase 1 to reduced-dose phase 2 protocol. Volume adjusted lung density (VALD) shows a systematic bias toward emphysema progression for both groups and no evidence of a significant difference between visually assessed groups. Volume-noise-bias ALD (VNB-ALD) reduces the bias and finds statistical differences between visually assessed groups. Perc15 = CT attenuation at the 15th percentile of the lung CT histogram.
For full-dose to reduced-dose progression (Fig 5B), VALD showed no evidence of a difference between groups (nonprogressors, −17.4 g/L ± 9.3; progressors, −18.5 g/L ± 9.8; P = .56), while VNB-ALD showed a difference (nonprogressors, −3.7 g/L ± 5.5; progressors, −6.7 g/L ± 5.2; P = .007).
Figure 6 shows visual examples and their progression measurements. VNB-ALD showed more consistency across dosage protocols and visual emphysema progression. A detailed analysis of each case is provided in Appendix S6.
Figure 6:
Analysis of emphysema progression in five participants with different scanning scenarios. Participant 1 is a man with probable progression. Images were acquired with different inspiratory volumes and high noise due to high body mass index (BMI). Participant 2 is a woman without progression with different scanner and similar inspiratory volumes in both phases. Participant 3 is a woman with similar inspiratory volume and a different scanner model. Participant 4 is a man with progression with a different scanner model and similar inspiratory models. Participant 5 is a man with progression with different scanners and volume variability. Evidence of emphysema progression is circled. ΔVALD = progression of volume-adjusted lung density (negative values indicate progression), ΔVNB-ALD = progression of volume-noise-bias–adjusted lung density (negative values indicate progression), GOLD = Global Initiative for Chronic Obstructive Lung Disease.
Clinical Characteristics in Discordant Progression Groups between VALD and VNB-ALD
The clinical characteristics were stratified in four groups on the basis of the combined change of lung density measured with VALD and VNB-ALD, as follows: group 0, concordant preservation of lung density; group 1, VALD progressors and VNB-ALD nonprogressors; group 2, VALD nonprogressors and VNB-ALD progressors; and group 3, concordant progressors. The term progressors refers to individuals with a negative difference in adjusted lung densities, while nonprogressors refers to those with a positive difference.
Table 2 indicates that out of 4954 participants with lung density progression data, 1271 (26%) showed discordant progression between VALD and VNB-ALD (Appendix S7 shows the distribution of groups). The annualized VALD change in group 1 was −0.9 g/L per year ± 0.9 versus 1.7 g/L per year ± 1.1 in group 2. In contrast, the annualized VNB-ALD change in group 1 was 0.7 g/L per year ± 0.6 versus −1.0 g/L per year ± 0.8 in group 2. At baseline, group 2 participants were older than group 1 participants (61 years vs 59 years; Tukey-adjusted P = .002). There was no evidence of significant differences in sex (n = 332 vs n = 351; Bonferroni-adjusted P = .23) and race (n = 463 vs n = 458; Bonferroni-adjusted P > .99) between the two groups. Group 1 had a higher body mass index than group 2 (29.1 kg/m2 vs 28.1 kg/m2; Tukey-adjusted P = .014) and a lower prevalence of emphysema (31% vs 49%; Tukey-adjusted P < .001). There was no evidence of a significant difference in smoking status between groups (349 vs 351 participants with former smoking history; Bonferroni-adjusted P > .99). However, group 2 had lower forced expiratory volume in 1 second, percentage predicted (81% vs 85%; Tukey-adjusted P = .01), lower ratio of forced expiratory volume in 1 second to forced vital capacity (0.66 vs 0.72; Tukey-adjusted P < .001), higher total lung capacity (5.9 L vs 5.5 L; Tukey-adjusted P < .001), and a higher percentage of low-attenuation areas below −950 HU (9% vs 3%; Tukey-adjusted P < .001). No evidence of significant differences was observed in terms of quality of life (P = .54) and dyspnea (P > .99) between groups 1 and 2 (Table S2).
Table 2:
Summary of Baseline Characteristics and 5-year Follow-up Changes in Lung Density and Other Parameters for COPDGene Participants Stratified into Four Groups Based on their Combined Change in Lung Density Measured by VALD and VNB-ALD
During the follow-up period, 43% of participants changed scanner model between visits, with a higher proportion in group 2 (92%) versus group 1 (42%; adjusted P < .001). Group 2 showed a larger decline in FEV1 (−42.2 mL per year) compared with group 1 (−32.1 mL per year; adjusted P = .002) and a larger decrease in FEV1 percentage predicted (−3% vs −1%; adjusted P = .005). There was no evidence of significant differences in changes in body mass index (P = .014), smoking status (P > .99), quality of life (P = .23), and dyspnea (P > .99). Details are provided in Table S3.
Discussion
Accurate quantitative radiologic evaluations of emphysema progression face challenges like variations in lung volume, image noise, and bias. While previous efforts aimed to reduce these errors individually, primarily through volume-adjusted lung density (VALD), our study introduces a method called volume-noise-bias–adjusted lung density (VNB-ALD), which simultaneously corrects for the temporal change of these confounders.
Our study used longitudinal imaging and clinical data from the COPDGene study. These data cover a 5-year period and include protocolized baseline and follow-up CT scans and spirometric lung function measurements. The repeatability of the change in lung density when considering a change in scanner and protocol dose (from full dose to reduced dose) shows a strong improvement in favor of VNB-ALD (repeatability coefficient of 13.9 g/L for VNB-ALD vs 30.3 g/L for VALD). Remarkably, even under changes in dosage protocol and scanner model, VNB-ALD repeatability matches that achieved by the Quantitative Imaging Biomarkers Alliance under the same scanner and dosage conditions (11).
In 102 randomly selected COPDGene participants, a panel of experts performed a head-to-head classification of visual emphysema progressors and nonprogressors. VNB-ALD, but not VALD, distinguished between individuals with visual evidence of emphysema progression and those without progression (P < .001 vs P = .11). For nonprogressors, the mean estimated lung density change using VNB-ALD was −0.67 g/L ± 4.8, nearly one-tenth of the estimated loss of −5.5 g/L ± 9.5 using VALD. Conversely, visual progressors showed an estimated loss of −4.2 g/L ± 5.5 using VNB-ALD compared with −8.6 g/L ± 9.6 using VALD.
Lung density changes are widely used as a surrogate marker for assessing emphysema progression, as seen in clinical trials like EXACTLE (13), RAPID (14), and LEEP (15). However, our study suggests that the current trial design might underestimate the impact of imaging confounders, focusing solely on lung volume and overlooking measurement variability. Additionally, studies employing VALD may lack sufficient power to detect progression due to variability between progressors and nonprogressors. Using VNB-ALD instead of VALD in a clinical study assessing therapy efficacy for emphysema progression could reduce the required number of participants by approximately 75% (estimated sample size of 92 vs 416, assuming a 1:1 design and full response in the treatment arm).
Our second analysis focused on quantitatively assessing serial CT scans from 4954 participants. Estimates of annualized lung density changes were calculated using VALD and VNB-ALD. Four groups were identified according to concordant and discordant estimates of tissue loss. While most individuals (n = 3683) were classified similarly with both methods, 1271 participants were categorized differently. Examination of their clinical data revealed that VNB-ALD progressors (group 2) experienced a faster decline in lung function compared with VALD progressors (group 1). The group with the most rapid loss of lung function (group 2) showed an average annualized gain of lung tissue of 1.7 g/L according to the VALD method. In contrast, the group with the lowest rate of decline in lung function (group 1) was reported to lose nearly 1 g/L per year using the same technique. Interestingly, most participants with discordant progression between VNB-ALD and VALD switched scanners during visits (93% in group 2), potentially introducing biases to lung density changes. Prior studies have observed systematic biases in tracheal air (9,16,17). Our results suggest that accounting for the interdependence of noise, lung volume, and bias changes increases consistency between emphysema progression and lung function decline.
Previous observational studies failed to establish a clear link between lung tissue loss on CT scans and decline in spirometric lung function (18,19). This lack of association has been attributed to the complex interplay of factors, including airway disease, influencing spirometric results. Our findings suggest that confounding factors affecting longitudinal densitometry might have influenced these studies.
The primary limitation to our work is the lack of a ground truth to benchmark the performance of longitudinal lung density metrics from CT scans. We endeavored to address this gap with visual assessment of CT scans by experts. Although we have no reason to suspect hidden bias favoring VNB-ALD in the data, scaling this process to larger sample sizes is challenging. Another limitation is that COPDGene is confined to only two racial groups and is not geographically diverse. Further replication of our findings could shed light on this question.
In summary, we have developed and applied a method for longitudinally assessing lung density in persons with current or former smoking history, considering the effects of changes in lung volume, image noise, and bias. Our volume-noise-bias–adjusted lung density (VNB-ALD) method exhibited higher precision compared with the commonly used volume-adjusted lung density method and demonstrates a more clinically plausible correlation. Our findings support the use of quantitative emphysema as an imaging biomarker in clinical investigation and care. However, further research is required to fully investigate the clinical relevance of the VNB-ALD approach and its potential use in CT densitometry as an outcome measure for therapeutic trials in individuals with chronic obstructive pulmonary disease.
Supported by the National Heart, Lung, and Blood Institute (NHLBI K25HL143278) and the National Institutes of Health (R21HL156229, R01HL149877, U01 HL089897, U01 HL089856). The COPDGene study (NCT00608764) is supported by the COPD Foundation through contributions made to an industry advisory committee composed of AstraZeneca, Bayer Pharmaceuticals, Boehringer Ingelheim, Genentech, GlaxoSmithKline, Novartis, Pfizer, and Sunovion.
Data sharing: Data generated or analyzed during the study are available from the corresponding author by request.
Disclosures of conflicts of interest: G.V.S.F. No relevant relationships. A.A.D. Speaker fees from Boehringer Ingelheim; patent pending for novel assays to detect respiratory diseases and disorders. S.Y.A. Consulting fees from Vertex Pharmaceuticals, Verona Pharmaceuticals, and Triangulate Knowledge; ownership interest in Quantitative Imaging Solutions. D.B. No relevant relationships. M.S. No relevant relationships. J.D.C. Chair on the Board of Directors of the COPD Foundation. E.K.S. Grant support from Bayer and Northpond Laboratories. S.M.H. Institutional research support from Boehringer Ingelheim; institutional contracts with Calyx. G.R.W. Consulting fees from PulmonX, Vertex Pharmaceuticals, Janssen Pharmaceuticals, Sanofi, Intellia Therapeutics, and Pieris Therapeutics; on the Pieris Therapeutics advisory board; co-founder and equity share holder in Quantitative Imaging Solutions; spouse works for Biogen. D.A.L. U.S. patent application no. 16/412,120 (Systems and Methods for Classifying Severity of COPD). R.S.J.E. Grants from Lung Biotechnology, Insmed, and Boehringer Ingelheim; consulting fees from Leuko Labs and Mount Sinai; honorarium from Chiesi; three patents pending in the space of lung cancer risk assessment using machine learning technology; co-founder of and stockholder in Quantitative Imaging Solutions.
Abbreviations:
- COPD
- chronic obstructive pulmonary disease
- COPDGene
- Genetic Epidemiology of Chronic Obstructive Pulmonary Disease
- FEV1
- forced expiratory volume in 1 second
- Perc15
- CT attenuation at the 15th percentile of the lung CT histogram
- VALD
- volume-adjusted lung density
- VNB-ALD
- volume-noise-bias–adjusted lung density
References
- 1. Regan EA , Hokanson JE , Murphy JR , et al . Genetic epidemiology of COPD (COPDGene) study design . COPD 2010. ; 7 ( 1 ): 32 – 43 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sieren JP , Newell JD Jr , Barr RG , et al . SPIROMICS Protocol for Multicenter Quantitative Computed Tomography to Phenotype the Lungs . Am J Respir Crit Care Med 2016. ; 194 ( 7 ): 794 – 806 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Staring M , Bakker ME , Stolk J , Shamonin DP , Reiber JHC , Stoel BC . Towards local progression estimation of pulmonary emphysema using CT . Med Phys 2014. ; 41 ( 2 ): 021905 . [DOI] [PubMed] [Google Scholar]
- 4. Vegas-Sánchez-Ferrero G , Ledesma-Carbayo MJ , Washko GR , San José Estépar R . Harmonization of chest CT scans for different doses and reconstruction methods . Med Phys 2019. ; 46 ( 7 ): 3117 – 3132 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tian X , Samei E . Accurate assessment and prediction of noise in clinical CT images . Med Phys 2016. ; 43 ( 1 ): 475 – 482 . [DOI] [PubMed] [Google Scholar]
- 6. Chen-Mayer HH , Fuld MK , Hoppel B , et al . Standardizing CT lung density measure across scanner manufacturers . Med Phys 2017. ; 44 ( 3 ): 974 – 985 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baraghoshi D , Strand M , Humphries SM , et al . Quantitative CT Evaluation of Emphysema Progression over 10 Years in the COPDGene Study . Radiology 2023. ; 307 ( 4 ): e222786 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hatt CR , Oh AS , Obuchowski NA , Charbonnier JP , Lynch DA , Humphries SM . Comparison of CT Lung Density Measurements between Standard Full-Dose and Reduced-Dose Protocols . Radiol Cardiothorac Imaging 2021. ; 3 ( 2 ): e200503 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim SS , Seo JB , Kim N , et al . Improved correlation between CT emphysema quantification and pulmonary function test by density correction of volumetric CT data based on air and aortic density . Eur J Radiol 2014. ; 83 ( 1 ): 57 – 63 . [DOI] [PubMed] [Google Scholar]
- 10. Vegas-Sánchez-Ferrero G , Ledesma-Carbayo MJ , Washko GR , Estépar RSJ . Autocalibration method for non-stationary CT bias correction . Med Image Anal 2018. ; 44 : 115 – 125 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Quantitative Imaging Biomarkers Alliance . QIBA Profile: Computed Tomography: Lung Densitometry . Radiological Society of North America . https://qibawiki.rsna.org/images/a/a8/QIBA_CT_Lung_Density_Profile_090420-clean.pdf. Accessed November 17, 2022.
- 12. Faul F , Erdfelder E , Lang AG , Buchner A . G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences . Behav Res Methods . 2007. May ; 39 ( 2 ): 175 – 191 . [DOI] [PubMed] [Google Scholar]
- 13. Dirksen A , Piitulainen E , Parr DG , et al . Exploring the role of CT densitometry: a randomised study of augmentation therapy in alpha1-antitrypsin deficiency . Eur Respir J 2009. ; 33 ( 6 ): 1345 – 1353 . [DOI] [PubMed] [Google Scholar]
- 14. Chapman KR , Burdon JGW , Piitulainen E , et al . Intravenous augmentation treatment and lung density in severe α1 antitrypsin deficiency (RAPID): a randomised, double-blind, placebo-controlled trial . Lancet 2015. ; 386 ( 9991 ): 360 – 368 . [DOI] [PubMed] [Google Scholar]
- 15. Wise RA , Holbrook JT , Brown RH , et al . Losartan Effects on Emphysema Progression Randomized Clinical Trial: Rationale, Design, Recruitment, and Retention . Chronic Obstr Pulm Dis (Miami) 2021. ; 8 ( 4 ): 414 – 426 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Choi S , Hoffman EA , Wenzel SE , Castro M , Lin CL . Improved CT-based estimate of pulmonary gas trapping accounting for scanner and lung-volume variations in a multicenter asthmatic study . J Appl Physiol (1985) 2014. ; 117 ( 6 ): 593 – 603 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Parr DG , Stoel BC , Stolk J , Nightingale PG , Stockley RA . Influence of calibration on densitometric studies of emphysema progression using computed tomography . Am J Respir Crit Care Med 2004. ; 170 ( 8 ): 883 – 890 . [DOI] [PubMed] [Google Scholar]
- 18. McDonough JE , Yuan R , Suzuki M , et al . Small-airway obstruction and emphysema in chronic obstructive pulmonary disease . N Engl J Med 2011. ; 365 ( 17 ): 1567 – 1575 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bhatt SP , Soler X , Wang X , et al . Association between Functional Small Airway Disease and FEV1 Decline in Chronic Obstructive Pulmonary Disease . Am J Respir Crit Care Med 2016. ; 194 ( 2 ): 178 – 184 . [DOI] [PMC free article] [PubMed] [Google Scholar]