Abstract
Virtually all cystic fibrosis (CF) patients become infected with Pseudomonas aeruginosa, and once the infection is established, the organism is rarely cleared. One of the P. aeruginosa virulence factors, exoenzyme S, has been shown to correlate with increased morbidity and mortality both in rat models of chronic pulmonary inflammation and in human CF patients. It has previously been shown that exoenzyme S is a potent stimulus for the proliferation of T cells in greater than 95% of adults, which could contribute to the pathogenesis of CF. The goal of this study was to determine the mechanism of T-cell stimulation by exoenzyme S in an effort to shed light on the immune response and contribute to understanding its role in P. aeruginosa pathogenesis. The current studies demonstrate that exoenzyme S stimulates naive T cells, since fetal blood lymphocytes proliferated and adult lymphocytes that expressed CD45RA proliferated. The percentage of T cells activated by exoenzyme S after a 4-h culture (as measured by CD69 surface expression) was intermediate in magnitude compared to levels induced by a panel of superantigens and mitogens. To determine the mechanism of activation, the requirement for accessory cells was investigated. The proliferative response to exoenzyme S was dependent on the presence of accessory cells but was not blocked by an anti-DR antibody. Exoenzyme S activated both CD4+ and CD8+ T cells, but CD4+ T cells were preferentially activated. The Vβ repertoire of donor T cells showed no preferential activation or preferential expansion after stimulation by exoenzyme S, suggesting that it is not a superantigen. Taken together, our data suggest that exoenzyme S is a T-cell mitogen but not a superantigen. Activation of a large percentage of T lymphocytes by exoenzyme S may produce a lymphocyte-mediated inflammatory response that should be considered in the pathogenesis of CF.
Pseudomonas aeruginosa is an opportunistic organism of immunocompromised individuals (8). P. aeruginosa can cause both acute and chronic infections such as those seen in nosocomial pneumonia, burn wounds, septicemia, and bronchiectasis in cystic fibrosis (CF) patients. Bronchopulmonary infections are the primary cause of morbidity and mortality in CF patients (34). It has been shown that the vast majority of CF patients by the age of 11 become colonized with P. aeruginosa (25) and that once established, this infection is rarely cleared (37). One of the P. aeruginosa virulence factors associated with disease is exoenzyme S (15, 16, 47). In this study, we have investigated the effect of P. aeruginosa exoenzyme S on T-cell activation and proliferation.
Exoenzyme S is a 49- to 53-kDa protein that is capable of transferring an ADP-ribose to various proteins in vitro (9) and stimulates human T cells to proliferate (32). Approximately 90% of clinical isolates from CF patients produce exoenzyme S, and 40% of the isolates produce the enzymatically active form (48). Moreover, increases in morbidity and mortality rates have been associated with exoenzyme S-producing strains compared to isogenic mutants in a rat model of chronic P. aeruginosa infection (47). Screening of clinical isolates among CF patients chronically colonized with P. aeruginosa showed that acute deterioration of pulmonary function was accompanied by increased exoenzyme S production (16). Despite the inability of antibiotics to completely eliminate P. aeruginosa from the airways of CF patients during pulmonary exacerbations, antibiotic treatment can lead to clinical improvement (37). Interestingly, it has also been shown that exoenzyme S levels (but not bacterial load) decrease following antibiotic treatment of clinical exacerbations correlating with clinical amelioration (15, 16). Taken together, these studies indicate that exoenzyme S plays a significant role in pulmonary exacerbations by P. aeruginosa.
Previous studies have established a role for cell-mediated immunity in host defense to P. aeruginosa. Whole-cell preparations of heat-killed P. aeruginosa induced peripheral lymphocytes to proliferate in vitro, suggesting that lymphocytes may be important in host defense (35). Further, T cells from CF patients in advanced stages of infection have been shown to be hyporesponsive to Pseudomonas bacterial challenge, suggesting that their inability to clear the organism may in part be due to impaired T-cell function (42). Research has therefore focused on identifying antigenic determinants of the microorganism that are recognized by T cells of normal healthy adults. We have recently reported that a purified preparation of exoenzyme S induces T cells to proliferate in over 95% of adults (32). The high frequency of adult responders suggests that exoenzyme S may be stimulating T cells as a mitogen or superantigen. If exoenzyme S were a mitogen or a superantigen, it might have quite different implications than if it were an antigen. Mitogens such as plant lectins induce massive T-lymphocyte proliferation of naive T cells (27). The response requires accessory cells but does not require major histocompatibility complex (MHC) molecules (19, 28). Superantigens are produced by some bacteria and viruses and are a unique type of mitogen because they stimulate T cells based on the expression of the Vβ chain of the T-cell receptor (TCR) (22). Superantigens induce T-cell proliferation by cross-linking MHC class II molecules of accessory cells with the Vβ chain of the TCR (12). Because of these mechanisms, the frequencies of responding T cells to mitogens and superantigens are approximately 1:10 and 1:20, respectively, compared to 1:104 to 1:105 for recall antigens (2, 13, 26). As a result, the large percentage of lymphocytes could initiate a deleterious inflammatory reaction rather than an antigen-specific immune response.
To investigate the mechanism of T-cell activation by exoenzyme S, proliferation of naive lymphocytes (fetal blood mononuclear cells [FBMC] and adult CD45RA+ cells) was determined. The frequency of responding T cells was determined by analyzing the percentage of cells expressing CD69 after activation. The mechanism of activation was determined by assessing the requirement for accessory cells and for MHC class II. Finally, the Vβ repertoire of activated and proliferating T cells was determined.
MATERIALS AND METHODS
Purification of P. aeruginosa exoenzyme S.
Purification was performed as previously described (49). Briefly, P. aeruginosa DG1 was grown in aerated S medium containing 1 g of NH4Cl, 3 g of Na2HPO4, 5 g of NaCl, 0.1 g of MgSO4, and 27 g of sodium succinate (per liter of distilled water) for 18 h at 32°C. Cultures were then centrifuged at 4°C for 20 min at 10,000 × g. Ammonium sulfate was added slowly to the culture supernatant to 60% saturation and incubated overnight at 4°C. Supernatants were then centrifuged at 15,000 × g for 30 min at 4°C to obtain the precipitated protein. The precipitate was dissolved in 100 ml of 0.05 M Tris hydrochloride buffer (pH 8.0) and dialyzed overnight at 4°C against 6 liters of the same buffer. The dialyzed material was then applied to a DEAE-Sephacel column (Pharmacia) previously equilibrated in Tris buffer. Elution was performed with a linear gradient of 0.01 to 1.01 M NaCl in Tris buffer. Protein-containing fractions were collected by measuring the A280. The fractions eluting at 0.4 to 0.5 M NaCl were then pooled, and additional NaCl was added to make the final concentration 1 M. This solution underwent acetone (previously cooled to −20°C) precipitation in an ice-salt bath, with the solution temperature never allowed to rise above 3°C. When the acetone concentration reached 33%, the solution was allowed to cool to 0°C and equilibrated for 15 min. The acetone solution was centrifuged at 5,000 × g for 20 min at 0°C, and the precipitate was redissolved in a small volume of Tris buffer and dialyzed overnight at 4°C in 6 liters of the same buffer. The dialyzed material was finally applied to a G-100 gel filtration column previously equilibrated in Tris buffer, and protein-containing fractions were detected by measuring the A280. Endotoxin levels were measured by using a Limulus amebocyte lysate kit (Associates of Cape Cod, Woods Hole, Mass.) according to the manufacturer’s protocol and determined to be less than 0.2 μg per μg of exoenzyme S. This concentration of lipopolysaccharide does not cause T-lymphocyte activation (CD69) or proliferation {tritiated thymidine ([3H]TdR) incorporation}.
Exoenzyme S purified as stated above does not contain ADP-ribosylation activity, migrates as a single, homogeneous band on sodium dodecyl sulfate-polyacrylamide gel electrophoresis and is not contaminated with other cell proteins, including flagellin. Recombinant exoenzyme S was purified from P. aeruginosa PA103 harboring the pUCPexoS expression vector, a kind gift from D. Frank and J. Barbieri (University of Wisconsin). The monoclonal antibody (MAb) against DG1 exoenzyme S (9.49.9) reacts with the 49- and 53-kDa proteins produced by P. aeruginosa 388 (46a). Further, MAb 9.49.9 neutralizes CD69 expression by DG1 exoenzyme S and also neutralizes CD69 expression by recombinant exoenzyme S (unpublished data).
Isolation of peripheral blood leukocyte populations.
Peripheral blood was obtained from healthy adults by venipuncture. Peripheral blood mononuclear cells (PBMC) were isolated by centrifugation (800 × g, 20 min) over a Ficoll-Hypaque density gradient (C-six Diagnostics Inc., Mequon, Wis.). Mononuclear cells were harvested and washed three times in Hanks’ balanced salt solution (Gibco, Burlington, Ontario, Canada). Blood was also collected from the umbilical vein of fresh human placenta, and FBMC were isolated similarly to PBMC. Residual erythrocytes were removed by a 3- to 5-min lysis treatment (0.15 M NH4Cl, 0.01 M NaHCO3, 0.001 M EDTA). Viable cells were then counted by trypan blue exclusion as visualized by light microscopy. Cells were resuspended in medium containing RPMI 1640 (Gibco), 5% human AB serum (BioWhittaker, Walkersville, Md.), penicillin (100 U/ml), streptomycin (100 μg/ml), amphotericin B (0.25 μg/ml), 2 mM l-glutamine, 1 mM sodium pyruvate, and 0.1 mM nonessential amino acids (all from Gibco).
To isolate T cells, PBMC were plated in petri plates (Corning Glass Works, New York, N.Y.) in the presence of RPMI 1640 for 1 h at 37°C and nonadherent populations were collected by rinsing twice in medium. The nonadherent cells were then rosetted to 2-aminoethylisothiouronium bromide (AET)-treated sheep erythrocytes (SRBC; Cedarlane, Hornby, Ontario, Canada) as previously described (21), with minor modifications. Briefly, 20 ml of AET solution was added to 5 ml of washed SRBC for 15 min at 37°C. AET-treated SRBC were made to 5% by addition of 20% human serum–RPMI 1640. AET-treated SRBC were added to nonadherent cells (<10 × 106 cells/ml) at a 1:2 ratio. The suspension was incubated at 37°C for 10 min, centrifuged (500 × g, 10 min), and refrigerated overnight. Rosette-positive cells were finally passed through a nylon wool column (20), and nonadherent cells were collected. T cells isolated in this fashion were typically >95% CD3+ as analyzed by flow cytometry. Cells that were adherent to plastic after a 1-h incubation and irradiated (3,000 rads) were used as a source of accessory cells.
To obtain CD45RA- and CD45RO-enriched cells, nonadherent cells were depleted of CD45RO or CD45RA cells by immunomagnetic separation. Briefly, nonadherent cells were incubated with either anti-CD45RA (L48) or anti-CD45RO (UCHL-1) antibody (Becton Dickinson) for 30 min at 4°C under gentle agitation. The cells were then centrifuged (800 × g, 10 min), and the supernatant was discarded. The cells were washed three times in phosphate-buffered saline containing 2% fetal calf serum (Gibco). M-450 Dynabeads conjugated with goat anti-mouse antibody (Dynal, Oslo, Norway) were added at a bead-to-target cell ratio of 3:1 for 10 min at 4°C under gentle agitation. The labeled cells were removed with a magnet (Dynal). The enriched populations contained <3% contaminating cells of the reciprocal subset as analyzed by flow cytometry. Irradiated (3,000 rads) adherent cells (105) were added (1:1) to the enriched cells as a source of accessory cells.
Lymphocyte proliferation assays.
Exoenzyme S (10 to 0.001 μg/ml) was added to 2 × 105 PBMC (or FBMC) and incubated for 7 days (predetermined optimal day for proliferation, 32) in 96-well round-bottom plates (Nunc, Roskilde, Denmark). Eighteen hours before the end of incubation, 1 μCi of [3H]TdR was added. Cells were harvested on glass filters, and counts per minute was determined in a liquid scintillation counter. As controls, the superantigens (staphylococcal enterotoxin A [SEA], SEB, SEC-2, SEE, and toxic shock syndrome toxin 1 [TSST-1]; Toxin Technologies, Sarasota, Fla.) and the mitogenic lectins (concanavalin A and phytohemagglutinin [PHA]; Sigma, St. Louis, Mo.) were all used at 1 μg/ml and harvested on day 3. Tetanus toxoid (10−2 Leaf [Lf] units; Connaught, Willowdale, Ontario, Canada) was used as a recall antigen control and harvested on day 7.
For MHC molecule blocking experiments, anti-DR MAb L243 (Becton Dickinson) was washed three times in phosphate-buffered saline before use to remove azide. PBMC (2 × 105) were pretreated for 1 h with 3 μg of anti-DR or isotype control (Becton Dickinson) per ml and then stimulated with or without 1 μg of exoenzyme S per ml for 7 days. As a positive control, PBMC stimulated with TSST-1 were used to ensure that anti-DR can block class II-mediated responses.
Analysis of lymphocyte activation and lymphocyte subsets.
PBMC were stimulated in culture for 4 h. Cells were labeled with CD69-phycoerythrin (PE) and CD3-peridinin chlorophyll (PerCP) (Becton Dickinson). In some experiments, fluorescein isothiocyanate (FITC)-conjugated anti-Vβ MAbs (Vβ-2, -3, -5a, -5b, -5c, -6, -8, -12, and -13; all from T Cell Diagnostics) or FITC-conjugated anti-CD4 or anti-CD8 (Becton Dickinson) was used in conjunction with anti-CD69-PE and anti-CD3-PerCP for simultaneous three-color immunolabeling. FITC- and PE-conjugated isotype-matched antibodies (immunoglobulin G1 [IgG1]-FITC, IgG1-PE, and CD3-PerCP; Becton Dickinson) were used as control antibodies. The percentage of cells within each Vβ subset that expressed CD69 was determined at 4 h. Net values were calculated by subtracting the percentage of positive cells in the unstimulated group from the values of the experimental groups. After 7 days of culture, the total number of cells in each Vβ subset was also determined. Fluorescence analysis was performed by using Lysis II software on a FACScan fluorocytometer (Becton Dickinson). The percentage of activated CD4 and CD8 T cells was determined by two-color dot plot analysis. Activated T-cell subsets were determined by dividing the number of double-positive cells (quadrant 2) by the sum of double-positive cells (quadrant 2) and single-positive cells (quadrant 4) (i.e., 100 × CD4+ CD69+/CD4+ or 100 × CD8+ CD69+/CD8+).
Statistics.
Values are expressed as means ± standard errors of the means (SEM). Statistical analysis was performed by paired analysis of variance (ANOVA; Statview 512+; Brain Power Inc., Calabasa, Calif.), two-sample, two-tailed, paired Student’s t test, or repeated measures of ANOVA based on the pairwise Laird-Ware mixed model (Stataquest; Stata Corporation, College Station, Tex.). P < 0.05 was considered significant.
RESULTS
Fetal cord mononuclear cells proliferate in response to exoenzyme S.
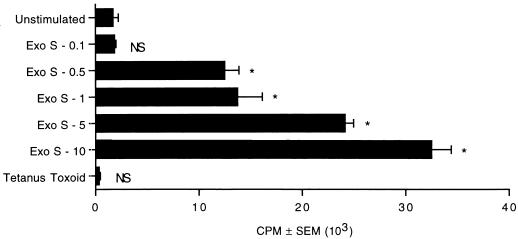
Previous experiments using DNA quantitation showed that exoenzyme S selectively induced fetal T cells but not B cells to enter cell cycle (data not shown). To determine whether exoenzyme S was capable of stimulating fetal cord lymphocytes to proliferate, FBMC (2 × 105) were cultured with various concentrations of exoenzyme S (0.1 to 10 μg/ml). FBMC showed a dose-dependent proliferation to exoenzyme S between 0.5 and 10 μg/ml, while tetanus toxoid (a recall antigen control) did not induce significant proliferation (Fig. 1). This result suggests that exoenzyme S is capable of stimulating naive T lymphocytes to proliferate.
FIG. 1.
FBMC proliferate in response to exoenzyme S. FBMC (2 × 105) were cultured with various concentrations of exoenzyme S (Exo S; 0.1 to 10 μg/ml) or 10−2 Lf units of tetanus toxoid for 7 days. The experiment was performed twice with similar results. ∗, P < 0.05 calculated by ANOVA compared with the unstimulated group; NS, nonsignificant difference compared with the unstimulated group.
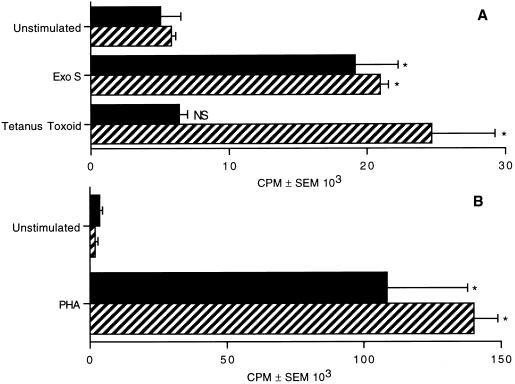
Naive and memory T cells proliferate in response to exoenzyme S.
CD45RA is expressed by naive T cells, while CD45RO is expressed by memory T cells (36). To determine whether T cells bearing the CD45RA isoform are capable of proliferating to exoenzyme S, we depleted nonadherent adult cells of either CD45RA+ or CD45RO+ cells. CD45RA- and CD45RO-enriched cells were stimulated with exoenzyme S (0.1 μg/ml) in the presence of irradiated adherent cells as a source of accessory cells. Exoenzyme S was capable of inducing both CD45RA-enriched and CD45RO-enriched populations to proliferate (Fig. 2A). This finding demonstrates that T cells expressing the phenotype of naive and memory cells proliferate in response to exoenzyme S. Conversely, tetanus toxoid could induce only CD45RO-enriched cells to proliferate, while the T-cell mitogen PHA induced significant proliferation in both CD45RA- and CD45RO-enriched cultures (Fig. 2B). Additionally, the ratio of activated (i.e., expressing CD69) CD45RA cells to CD45RO cells stimulated with exoenzyme S was similar to that in PHA-stimulated cultures (data not shown), while tetanus toxoid predominantly activated cells enriched for the memory phenotype.
FIG. 2.
Proliferation of T-cell subsets in response to exoenzyme S. CD45RA-enriched (solid bars) and CD45RO-enriched (striped bars) cells were cultured with irradiated accessory cells in the presence of 1 μg of exoenzyme S (Exo S) per ml or 10−2 Lf units of tetanus toxoid (A) or 1 μg of PHA per ml (B). Cultures stimulated with PHA were harvested on day 3, and cultures stimulated with exoenzyme S or tetanus toxoid were harvested on day 7. The experiment was repeated three times with similar results. ∗, P < 0.05 calculated by ANOVA compared with the corresponding unstimulated group. NS, nonsignificant difference compared with the corresponding unstimulated group.
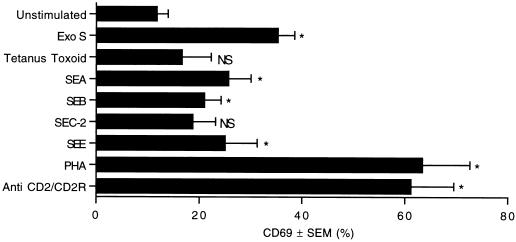
T-lymphocyte activation by exoenzyme S.
To compare the percentage of T cells that become activated by exoenzyme S to the percentage of T cells activated by antigens, superantigens, and mitogenic lectins, we measured the expression of an early T-cell activation marker. CD69 is an early inducible marker found on activated T cells (6), and its surface expression is detectable within 2 h of stimulation (44). To determine early T-cell activation events directly due to these stimuli and reduce nonspecific bystander activation, we measured CD69 expression after stimulation for 4 h. While the kinetics for induction and duration of CD69 expression by exoenzyme S, superantigens, and mitogens are different (unpublished data), these stimuli consistently induced significant T-cell activation at 4 h. Exoenzyme S stimulated a large percentage of peripheral T cells to express CD69 (Fig. 3). The percentage of T cells activated by exoenzyme S was 10 to 17% greater than that induced by any of the staphylococcal superantigens tested. However, fewer T cells were activated by exoenzyme S than by PHA or anti-CD2. The recall antigen, tetanus toxoid, induced minimal T-cell activation over background (unstimulated mean = 11.78% ± 2.13%, n = 19; tetanus toxoid mean = 16.64% ± 5.71%, n = 4).
FIG. 3.
Induction of CD69 expression on peripheral T lymphocytes. PBMC were cultured with 1 μg of exoenzyme S (Exo S) per ml, 10−2 Lf units of tetanus toxoid, 1 μg each of SEA, SEB, SEC-2, SEE, or PHA per ml, or 10 μl of anti-CD2/CD2R for 4 h. Samples were harvested and labeled with anti-CD69-PE/anti-CD3-PerCP. The percentage of CD3 cells expressing CD69 was determined. ∗, P < 0.05 using repeated measures of ANOVA based on the Laird-Ware mixed model compared with the unstimulated group; NS, nonsignificant difference compared with the unstimulated group (n = 19 for unstimulated and exoenzyme S; n = 13 for SEB; n = 8 for PHA; n = 5 for SEE; n = 4 for tetanus toxoid; n = 3 for SEA, SEC-2, and anti-CD2/CD2R).
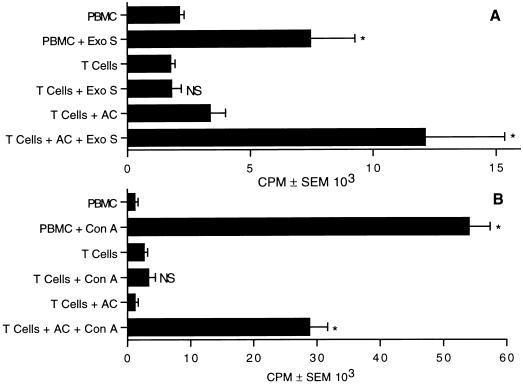
Accessory cells are necessary for the proliferative response to exoenzyme S.
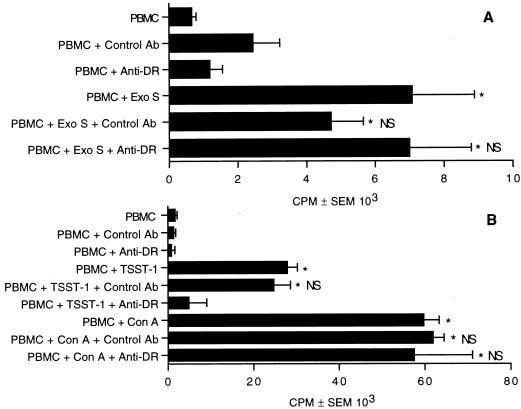
Previous experiments show that T cells are the predominant lymphocyte population that proliferate in response to exoenzyme S (32). Treatment of T cells with a phorbol ester and calcium ionophore induces significant lymphocyte proliferation (45); however, accessory cells are required for the proliferative response of T cells when stimulated with antigens or mitogens (28, 41). Experiments were designed to determine whether the proliferative response was dependent on accessory cells or whether exoenzyme S is capable of bypassing the cell surface molecules that are required for physiologic T-cell responses. T cells were stimulated with or without irradiated accessory cells. Exoenzyme S could not induce the T-cell-enriched population to proliferate, but addition of irradiated accessory cells reconstituted the response (Fig. 4A), suggesting that the proliferative response of T cells to exoenzyme S is dependent on accessory cells. To ensure that T cells were depleted of accessory cells, concanavalin A was used. T cells were incapable of proliferating to concanavalin A unless irradiated accessory cells were added, suggesting that a high degree of purity was attained (Fig. 4B) (41).
FIG. 4.
Antigen-presenting cells are required for T-cell proliferation to exoenzyme S. PBMC or purified T cells with and without accessory cells (AC) were incubated with 1 μg of exoenzyme S (Exo S; A) or 10 μg of concanavalin A (Con A; B) per ml. Cultures stimulated with concanavalin A were harvested on day 3, while cultures stimulated with exoenzyme S were harvested on day 7. The experiment was repeated three times with similar results. ∗, P < 0.05 calculated by ANOVA compared with the corresponding unstimulated group; NS, nonsignificant difference compared with the corresponding unstimulated group.
MHC molecules are not required for exoenzyme S-induced proliferation.
The previous experiments were able to show that accessory cells are required for T-cell proliferation to exoenzyme S. To determine whether MHC molecules were involved in the presentation of exoenzyme S, we pretreated PBMC with anti-DR or isotype control MAb for 1 h before the addition of stimulus and determined the proliferative response by [3H]TdR incorporation. Treatment with anti-DR or isotype control did not significantly abrogate the proliferative response to exoenzyme S (Fig. 5A), suggesting that MHC is not directly involved in the presentation of exoenzyme S to T cells. In control experiments, we confirmed that anti-DR antibody blocked the response to the superantigen TSST-1 but not concanavalin A (Fig. 5B) (39).
FIG. 5.
HLA-DR is not necessary for the proliferative response to exoenzyme S. PBMC were pretreated with anti-DR MAb or isotype control or left untreated for 1 h and then stimulated with 1 μg of exoenzyme S (Exo S; A) per ml for 7 days or with 0.1 μg of TSST-1 or 10 μg of concanavalin A (Con A) (B) per ml for 3 days. This experiment was performed three times with similar results. ∗, P < 0.05 calculated by ANOVA compared with the corresponding unstimulated group; NS, nonsignificant difference compared with stimulated PBMC.
Exoenzyme S preferentially activates CD4+ T cells.
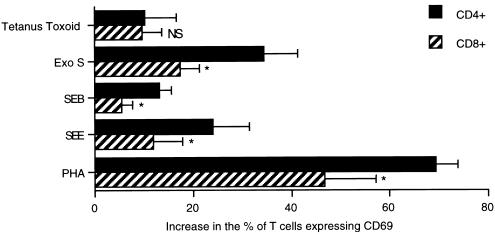
Preferential activation of T-cell subsets has been demonstrated for a number of stimuli. Superantigens such as streptococcal erythrogenic toxin A and SEB have been shown to preferentially activate CD4+ over CD8+ T cells (1, 23); other stimuli such as Candida albicans antigen preferentially activate CD8+ T cells (30), while the mitogen concanavalin A does not preferentially activate either subset (5). We therefore designed experiments to determine whether there was preferential activation of either T-cell subset after exoenzyme S stimulation. The expression of CD69 was analyzed on both CD4+ CD3+ and CD8+ CD3+ T-cell subsets, calculated as described in Materials and Methods. Exoenzyme S preferentially activated CD4+ over CD8+ T cells at a ratio of 2:1, which was similar to results for both SEB and SEE (Fig. 6). PHA induced 1.5 times more CD4+ T cells to express CD69 than CD8+ T cells, while tetanus toxoid did not show preferential activation of either subset. These data suggest that the activation profile of responding T cells to exoenzyme S is similar to that of responding T cells stimulated with mitogens and superantigens.
FIG. 6.
T-cell subset activation by exoenzyme S. PBMC cultures were stimulated with 10−2 Lf units of tetanus toxoid or with 1 μg of staphylococcal superantigens, PHA, or exoenzyme S (Exo S) per ml for 4 h. Samples were then harvested and labeled with FITC-conjugated anti-CD4 or anti-CD8 and anti-CD69-PE/anti-CD3-PerCP. The net percentage of CD4+ CD3+ or CD8+ CD3+ cells that expressed CD69 was determined as described in Materials and Methods. ∗, P < 0.05, using a paired Student t test compared to the corresponding CD4+ group; NS, nonsignificant difference compared with the corresponding CD4+ group (n = 4 for SEE; n = 5 for tetanus toxoid, SEB, and PHA; n = 6 for exoenzyme S).
Exoenzyme S does not induce oligoclonal activation of T cells.
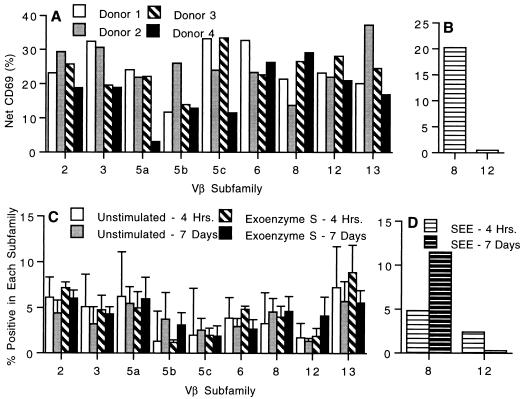
In contrast to mitogens, superantigens oligoclonally activate T cells based on the Vβ elements of the TCR (12). Experiments were performed to determine whether exoenzyme S was capable of preferentially activating T cells based on their TCR Vβ expression. Exoenzyme S activated T cells bearing all of the nine Vβ elements analyzed (Fig. 7A), while SEE, a control superantigen, caused a preferential activation of T cells bearing Vβ8 and failed to activate Vβ12 (Fig. 7B). Additionally, after 7 days of stimulation with exoenzyme S, there was no apparent increase or decrease in the percentage of T cells bearing any of the 9 Vβ elements analyzed (Fig. 7C). By contrast, SEE caused a significant increase in the percentage of T cells bearing Vβ8 and a concomitant decrease in the percentage of cells bearing Vβ12 (Fig. 7D). Therefore, exoenzyme S did not induce oligoclonal activation or proliferation in PBMC cultures, suggesting that it is not a superantigen.
FIG. 7.
Vβ-specific activation (A and B) and expansion (C and D) of human T lymphocytes. PBMC (2 × 105) were stimulated with 1 μg of exoenzyme S (A and C) or SEE (B and D) per ml for 4 h. Cells were labeled with anti-CD69-PE/anti-CD3-PerCP and one of the FITC-conjugated Vβ-specific MAbs. The net percentage of cells within each Vβ family that expressed CD69 was determined by subtracting the CD69 expression of unstimulated cultures from exoenzyme S (A)- or SEE (B)-stimulated cultures. Four separate experiments are shown. After 7 days, cultured cells were labeled with anti-Vβ-specific MAb. Vβ expression is shown after 4 h and 7 days of culture for unstimulated and exoenzyme S-stimulated (C) cultures as well as for SEE-stimulated cultures (D). The means ± standard error of the means of four separate experiments are shown.
DISCUSSION
We have made five observations regarding the T-cell response to P. aeruginosa exoenzyme S. (i) Exoenzyme S is capable of inducing immunologically naive T cells to proliferate. (ii) The percentage of T cells activated by exoenzyme S is much greater than the percentages stimulated by a recall antigen and by the superantigens tested but less than the percentage stimulated by PHA or cross-linking antibody. (iii) The proliferative response to exoenzyme S is dependent on accessory cells but independent of MHC. (iv) CD4+ T cells are preferentially activated over CD8+ T cells. (v) Exoenzyme S does not cause oligoclonal activation or proliferation of T cells, based on the expression of the Vβ element of the TCR.
This study was undertaken to determine whether exoenzyme S is a recall antigen or a mitogen or whether it has the characteristics of the unique subset of mitogens known as superantigens. We have provided four pieces of evidence that exoenzyme S is a T-cell mitogen. First, exoenzyme S stimulates fetal lymphocytes. Over 90 to 95% of human fetal lymphocytes express naive T-cell markers (7, 18), and mitogens induce fetal lymphocytes to proliferate whereas recall antigens do not (17, 43). Second, adult T cells that are immunologically naive express the CD45RA isoform of the CD45 molecule (36). CD45RA-enriched lymphocytes proliferated in response to exoenzyme S, which suggests that exoenzyme S is a mitogen. Third, exoenzyme S activated a large percentage of T cells; the percentage of T cells activated by exoenzyme S was far greater than that induced by a recall antigen and intermediate between a panel of superantigens and mitogens. Finally, T-cell proliferation to exoenzyme S is not blocked by an anti-DR antibody as is the case with other well-characterized mitogens (39). Previous data which show that greater than 95% of tested adults proliferated to exoenzyme S in vitro are also consistent with a T-cell mitogen (32).
Our data suggest that the mitogenic activity of exoenzyme S is specific for T lymphocytes. This inference is supported by our observations that T cells require an accessory cell, that B cells proliferate only in the presence of activated T cells (32), and that the 53-kDa form of exoenzyme S strain 388 does not enhance [3H]TdR incorporation in fibroblasts or epidermoid cells (33).
Our previous observations demonstrated that the T-cell response to exoenzyme S had characteristics of a mitogen but that it also had characteristics of a recall antigen. The features of the T-cell response which suggested that exoenzyme S was a recall antigen included slow kinetics and a relatively low magnitude of proliferation (32). The delayed kinetics and the relatively low magnitude of proliferation (32) may be explained by a number of different, nonexclusive possibilities. First, exoenzyme S could stimulate T cells but bypass the TCR-dependent pathway. That is, exoenzyme S may be capable of activating T cells in a manner similar to that for RANTES, which involves a kinase-independent pathway and results in many physiological changes but fails to induce a brisk proliferative response (4). We believe that this possibility is unlikely since the T-cell response to exoenzyme S required accessory cells, which is consistent with findings for other T-cell mitogens (28, 41) and superantigens (12, 22). Second, it may also be possible that exoenzyme S induces an aberrant signal which allows for early events of T-cell activation (i.e., acid and intracellular calcium release and CD69 expression) but which fail to optimally induce terminal events such as proliferation (10). This implies that the defect in signalling occurs distal to early signalling events and prior to commitment and entry into S phase (40). This activation-induced arrest may occur as a direct result of exoenzyme S on T cells or indirectly through an inhibition of costimulatory signals from the accessory cells. Last, exoenzyme S may activate T cells but also induce some of them to undergo programmed cell death (apoptosis), thus reducing the number of proliferating lymphocytes. The increased expression of early markers of T-cell activation (i.e., CD69) is consistent with the surface phenotype of some T cells in early stages of apoptosis (24). Studies to determine the mechanism of this relative defect in T-cell proliferation by exoenzyme S are ongoing.
We have demonstrated that exoenzyme S preferentially activates CD4+ T cells over CD8+ T cells, a phenomenon common to the mitogenic lectins and superantigens that we tested. Although CD4 cells were preferentially activated, it is unlikely that observed activation of CD8 cells is due to a bystander effect from CD4-derived cytokines since the activation was determined at 4 h, which is prior to production of T-cell growth factors by T cells (14). Previous studies have demonstrated that the superantigen-mediated interaction between the TCR and MHC class II is in some cases stabilized by the CD4 molecule and therefore a greater number of responding CD4+ T cells are activated, although both subtypes respond (1, 23, 38). Although PHA preferentially induced CD69 expression on CD4+ T cells more efficiently than CD8+ T cells, the difference was not as pronounced as for exoenzyme S or the superantigens. PHA can utilize the CD4 molecule to stimulate T cells (31), and this may explain the preferential expansion of CD4 cells. In contrast, expression of CD69 following stimulation with the recall antigen, tetanus toxoid, did not show preferential activation of either subset. We have no data to suggest that the T-cell response (activation or proliferation) to exoenzyme S utilizes the CD4 molecule; however, we cannot exclude this as a possible explanation for the preferential T-cell activation induced by exoenzyme S.
A number of bacterial mitogens stimulate T cells as superantigens. Superantigens bind and cross-link MHC class II on the accessory cell with a restricted repertoire of Vβ elements of the TCR, resulting in stimulation of T cells bearing these Vβ elements but not others. Since exoenzyme S is a bacterial mitogen, we were interested in determining whether it was a superantigen. Two pieces of evidence suggest that exoenzyme S is not a superantigen. First, antibodies to MHC class II block the response to superantigens such as TSST-1 (39) but did not block the response to exoenzyme S. It should be noted that our data do not exclude the possibility that exoenzyme S possesses a binding site that allows cross-linking of the TCR with MHC that is not hindered by the anti-MHC antibody that we used. Therefore, we performed experiments to determine whether there was oligoclonal activation or oligoclonal proliferation of Vβ subsets. We found that exoenzyme S activated all of the subsets tested rather than causing oligoclonal activation or expansion of Vβ subsets. Thus, our data are most consistent with exoenzyme S being a mitogen but not a superantigen.
The outcomes of the T-cell responses to mitogens, superantigens, and antigens are quite different. Although T-cell responses in vivo are complex, in general, mitogens and superantigens are both capable of inducing a nonspecific inflammatory response and superantigens can also promote a subsequent state of unresponsiveness (29) or programmed cell death (29, 46). In general, antigens stimulate T lymphocytes for B-cell help, for delayed hypersensitivity, and for production of memory cells. Thus, recall antigens are capable of stimulating an effective immunologic response resulting in enhanced host defense, while stimulation by mitogens and superantigens results in inflammation and, potentially, impaired host defense.
T-lymphocyte activation has been implicated in the onset of pulmonary inflammation in CF patients (3, 11). As a mitogen, exoenzyme S may contribute to this chronic state of inflammation by activating a large percentage of naive T cells as they are recruited to the lung. This could in turn cause the secretion of a number of proinflammatory cytokines. Certainly, the overproduction of T-cell-derived cytokines could contribute to respiratory exacerbations of disease in CF patients. Therefore, the T-cell-derived mediators could promote a chronic state of inflammation and impede host clearance of the organism in CF patients. Understanding the mechanism of T-cell activation by exoenzyme S will help direct future therapeutic strategies. This study suggests that strategies aimed at downregulating the T-cell response to exoenzyme S would benefit CF patients. These strategies could potentially subvert a large but ineffective inflammatory response, reducing pulmonary damage.
ACKNOWLEDGMENTS
This work was supported by grants from the Canadian Foundation for Cystic Fibrosis and the Alberta Lung Association. T.F.B. is a student of the Alberta Lung Association. C.H.M. is a Scholar of the Alberta Heritage Foundation for Medical Research.
We thank Laurie Bryant for assistance with flow cytometry and Roland Brandt for statistical assistance.
REFERENCES
- 1.Abe J, Forrester J, Nakahara T, Lafferty J A, Kotzin B L, Leung D Y. Selective stimulation of human T cells with streptococcal erythrogenic toxins A and B. J Immunol. 1991;146:3747–3750. [PubMed] [Google Scholar]
- 2.Andersson J, Nagy S, Bjork L, Abrams J, Holm S, Andersson U. Bacterial toxin-induced cytokine production studied at the single-cell level. Immunol Rev. 1992;127:69–96. doi: 10.1111/j.1600-065x.1992.tb01409.x. [DOI] [PubMed] [Google Scholar]
- 3.Azzawi M, Johnston P W, Majumdar S, Kay A B, Jeffrey P K. T lymphocytes and activated eosinophils in airway mucosa in fatal asthma and cystic fibrosis. Am Rev Respir Dis. 1992;145:1477–1482. doi: 10.1164/ajrccm/145.6.1477. [DOI] [PubMed] [Google Scholar]
- 4.Bacon K B, Premack B A, Gardner P, Schall T J. Activation of dual T cell signalling pathways by the chemokine RANTES. Science. 1995;269:1727–1730. doi: 10.1126/science.7569902. [DOI] [PubMed] [Google Scholar]
- 5.Biselli R, Matricardi P M, D’Amelio R, Fattorossi A. Multiparametric flow cytometric analysis of the kinetics of surface molecule expression after polyclonal activation of human peripheral blood T lymphocytes. Scand J Immunol. 1992;35:439–447. doi: 10.1111/j.1365-3083.1992.tb02879.x. [DOI] [PubMed] [Google Scholar]
- 6.Cebrián M, Yagüe E, Rincón M, López-Botet M, de Landázuri M O, Sánchez-Madrid F. Triggering of T cell proliferation through AIM, an activation inducer molecule expressed on activated human lymphocytes. J Exp Med. 1988;168:1621–1637. doi: 10.1084/jem.168.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clement L T, Vink P E, Bradley G E. Novel immunoregulatory functions of phenotypically distinct subpopulations of CD4+ cells in the human neonate. J Immunol. 1990;145:102–108. [PubMed] [Google Scholar]
- 8.Coburn J. Pseudomonas aeruginosa exoenzyme S. Curr Top Microbiol Immunol. 1992;175:133–143. doi: 10.1007/978-3-642-76966-5_7. [DOI] [PubMed] [Google Scholar]
- 9.Coburn J, Gill D M. ADP-ribosylation of p21ras and related proteins by Pseudomonas aeruginosa exoenzyme S. Infect Immun. 1991;159:4259–4262. doi: 10.1128/iai.59.11.4259-4262.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crabtree G R. Contingent regulatory events in T lymphocyte activation. Science. 1989;243:355–361. doi: 10.1126/science.2783497. [DOI] [PubMed] [Google Scholar]
- 11.Dagli E, Warner J A, Besley C R, Warner J O. Raised serum soluble interleukin-2 receptor concentrations in cystic fibrosis patients with and without evidence of lung disease. Arch Dis Child. 1992;67:479–481. doi: 10.1136/adc.67.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dellabona P, Peccoud J, Kappler J, Marrack P, Benoist C, Mathis D. Superantigens interact with MHC class II molecules outside of the antigen groove. Cell. 1990;62:1115–1121. doi: 10.1016/0092-8674(90)90388-u. [DOI] [PubMed] [Google Scholar]
- 13.Gebel H M, Scott J R, Parvin C A, Rodney G E. In vitro immunization to KLH. II. Limiting dilution analysis of antigen-reactive cells in primary and secondary cultures. J Immunol. 1983;130:29–32. [PubMed] [Google Scholar]
- 14.Gillis S, Ferm M M, Ou W, Smith K A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978;120:2027–2032. [PubMed] [Google Scholar]
- 15.Grimwood K, To M, Rabin H R, Woods D E. Inhibition of Pseudomonas aeruginosa exoenzyme expression by subinhibitory antibiotic concentrations. Antimicrob Agents Chemother. 1989;33:41–47. doi: 10.1128/aac.33.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grimwood K, To M, Semple R A, Rabin H R, Sokol P A, Woods D E. Elevated exoenzyme expression by Pseudomonas aeruginosa is correlated with exacerbations of lung disease in cystic fibrosis. Pediatr Pulmonol. 1993;15:135–139. doi: 10.1002/ppul.1950150302. [DOI] [PubMed] [Google Scholar]
- 17.Hanzel Z T, Levin S, Dolphin Z, Schlesinger M, Hahn T, Altman Y, Schecter B, Shenyour A, Trainin N. Immune competence in newborn lymphocytes. Pediatrics. 1980;65:491–496. [PubMed] [Google Scholar]
- 18.Hayward A R, Lee J, Beverley P C. Ontogeny of expression of UCHL1 antigen on TcR-1+ (CD4/8) and TcRδ+ T cells. Eur J Immunol. 1989;19:771–773. doi: 10.1002/eji.1830190430. [DOI] [PubMed] [Google Scholar]
- 19.Hunig T. The role of accessory cells in polyclonal T cell activation. III. No requirement for recognition of H-2 encoded antigens on accessory cells. Eur J Immunol. 1984;14:483–489. doi: 10.1002/eji.1830140602. [DOI] [PubMed] [Google Scholar]
- 20.Julius M H, Simpson E, Herzenberg L A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973;3:645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- 21.Kanof M E. Isolation of T cells using rosetting procedures. In: Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. Current protocols in immunology. New York, N.Y: John Wiley & Sons, Inc.; 1996. pp. 7.2.3–7.2.4. [Google Scholar]
- 22.Kappler J, Kotzin B, Herron L, Gelfand E W, Bigler R D, Boylston A, Carrel S, Posnett D N, Marrack P. Vβ-specific stimulation of T cells by staphylococcal toxins. Science. 1989;244:811–813. doi: 10.1126/science.2524876. [DOI] [PubMed] [Google Scholar]
- 23.Kawabe Y, Ochi A. Selective anergy of Vβ8+ T cells in Staphylococcus enterotoxin B-primed mice. J Exp Med. 1990;172:1065–1070. doi: 10.1084/jem.172.4.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kishimoto H, Surh C D, Sprent J. Upregulation of surface markers on dying thymocytes. J Exp Med. 1995;181:649–655. doi: 10.1084/jem.181.2.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konstan M W, Berger M. Infection and inflammation of the lung in cystic fibrosis. In: Davis P B, editor. Cystic fibrosis. 1st ed. New York, N.Y: Marcel Dekker Inc.; 1993. pp. 219–276. [Google Scholar]
- 26.Larsson E, Lindahl K F, Langhorne J, Coutinho A. Quantitative studies on concanavalin A-induced, TCGF-reactive cells. I. Correlation between proliferation and lectin dependent cytolytic activity. J Immunol. 1981;127:1081–1085. [PubMed] [Google Scholar]
- 27.Licastro F, Chiricolo M, Barbieri L, Stripe F, Falasca A, Rossi C A, Franceschi C. Effect of 32 purified animal and plant lectins on human T lymphocytes. In: Bog-Hansen T C, Spengler G A, editors. Lectins: biology-biochemistry, clinical, biochemistry. Vol. 3. Berlin, Germany: Walter de Gruyter; 1983. pp. 293–302. [Google Scholar]
- 28.Licastro F, Davis E J, Morini M C. Lectins and superantigens: membrane interactions of these compounds with T lymphocytes affect immune responses. Int J Biochem. 1993;25:845–852. doi: 10.1016/0020-711x(93)90239-b. [DOI] [PubMed] [Google Scholar]
- 29.MacDonald H R, Baschieri S, Lees R K. Clonal expression precedes anergy and death of Vβ8+ peripheral T cells responding to staphylococcal enterotoxin B in vivo. Eur J Immunol. 1991;21:1963–1966. doi: 10.1002/eji.1830210827. [DOI] [PubMed] [Google Scholar]
- 30.Maino V C, Suni M A, Ruitenberg J. Rapid flow cytometric method for measuring lymphocyte subset activation. Cytometry. 1995;20:127–133. doi: 10.1002/cyto.990200205. [DOI] [PubMed] [Google Scholar]
- 31.Mann D L, Lasane F, Popovic M, Arthur L O, Robey W G, Blattner W A, Newman M J. HTLV-III large envelope protein (gp120) suppresses PHA-induced lymphocyte blastogenesis. J Immunol. 1987;138:2640–2644. [PubMed] [Google Scholar]
- 32.Mody C H, Buser D E, Syme R M, Woods D E. Pseudomonas aeruginosa exoenzyme S induces proliferation of human T lymphocytes. Infect Immun. 1995;63:1800–1805. doi: 10.1128/iai.63.5.1800-1805.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olson J C, McGuffie E M, Frank D W. Effects of differential expression of the 49-kilodalton exoenzyme S by Pseudomonas aeruginosa on cultured eukaryotic cells. Infect Immun. 1997;65:248–256. doi: 10.1128/iai.65.1.248-256.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pitcher-Wilmott R W, Levinsky R J, Gordon I, Turner M W, Mathew D J. Pseudomonas infection, allergy, and cystic fibrosis. Arch Dis Child. 1982;57:582–586. doi: 10.1136/adc.57.8.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Porwoll J M, Gebel H M, Rodey G E, Markham R B. In vitro response of human T cells to Pseudomonas aeruginosa. Infect Immun. 1983;40:670–674. doi: 10.1128/iai.40.2.670-674.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prince H E, York J, Jensen E R. Phenotypic comparison of the three populations of human lymphocytes defined by CD45RO and CD45RA expression. Cell Immunol. 1992;145:254–262. doi: 10.1016/0008-8749(92)90329-n. [DOI] [PubMed] [Google Scholar]
- 37.Regelmann W E, Elliott G R, Warwick W J, Clawson C C. Reduction of sputum Pseudomonas aeruginosa density by antibiotics improves lung function in cystic fibrosis more than do bronchodilators and chest physiotherapy alone. Am Rev Respir Dis. 1990;141:914–921. doi: 10.1164/ajrccm/141.4_Pt_1.914. [DOI] [PubMed] [Google Scholar]
- 38.Scherer M T, Ignatowicz L, Winslow G M, Kappler J W, Marrack P C. Superantigens: bacterial and viral proteins that manipulate the immune response. Annu Rev Cell Biol. 1993;9:101–128. doi: 10.1146/annurev.cb.09.110193.000533. [DOI] [PubMed] [Google Scholar]
- 39.See R H, Krystal G, Chow A W. Receptors for toxic shock syndrome toxin-1 and Staphylococcal enterotoxin A on human blood monocytes. Can J Microbiol. 1992;38:937–944. doi: 10.1139/m92-151. [DOI] [PubMed] [Google Scholar]
- 40.Shenker B J, Datar S. Fusobacterium nucleatum inhibits human T-cell activation by arresting cells in the mid-G1 phase of the cell cycle. Infect Immun. 1995;63:4830–4836. doi: 10.1128/iai.63.12.4830-4836.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sopori M L, Hurt Y L, Cherian S, Kaplan A M, Diamantstein T. Differential requirement for accessory cells in polyclonal T-cell activation. Cell Immunol. 1987;105:174–186. doi: 10.1016/0008-8749(87)90066-9. [DOI] [PubMed] [Google Scholar]
- 42.Sorensen R U, Stern R C, Chase P A, Polmar S H. Changes in lymphocyte reactivity to Pseudomonas aeruginosa in hospitalized patients with cystic fibrosis. Am Rev Respir Dis. 1981;123:37–41. doi: 10.1164/arrd.1981.123.1.37. [DOI] [PubMed] [Google Scholar]
- 43.Stiehm E R, Winter H S, Bryson Y J. Cellular immunity in the human newborn. Pediatrics. 1979;64:814–821. [PubMed] [Google Scholar]
- 44.Testi R, Phillips J H, Lanier L L. Leu 23 induction as an early marker of functional CD3/T cell antigen receptor signalling. J Immunol. 1989;142:1854–1860. [PubMed] [Google Scholar]
- 45.Truneh A, Albert F, Golstein P, Schmitt-Verhulst A M. Early steps of lymphocyte activation bypassed by synergy between calcium ionophores and phorbol ester. Nature. 1985;313:318–320. doi: 10.1038/313318a0. [DOI] [PubMed] [Google Scholar]
- 46.Webb S, Morris C, Sprent J. Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 1990;63:1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- 46a.Woods, D. E. Unpublished data.
- 47.Woods D E, Sokol P A. Use of transposon mutants to assess the role of exoenzyme S in chronic pulmonary disease due to Pseudomonas aeruginosa. Eur J Clin Microbiol. 1985;4:163–169. doi: 10.1007/BF02013591. [DOI] [PubMed] [Google Scholar]
- 48.Woods D E, Schaffer M S, Rabin H R, Campbell G D, Sokol P A. Phenotypic comparison of Pseudomonas aeruginosa strains isolated from a variety of clinical sites. J Clin Microbiol. 1986;24:260–264. doi: 10.1128/jcm.24.2.260-264.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woods D E, Que J. Purification of Pseudomonas aeruginosa exoenzyme S. Infect Immun. 1987;55:579–586. doi: 10.1128/iai.55.3.579-586.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]