Abstract
Despite the availability of adrenal hormone replacement therapy, patients with adrenal insufficiency can be affected by reduced fertility and parity. Patients with well-managed adrenal insufficiency are expected to have uneventful pregnancies and favourable outcomes, but an increased risk of maternal and neonatal complications has been reported in some cases. Many physiological changes occur to the hypothalamic–pituitary–adrenal (HPA) axis during pregnancy, often making a new diagnosis and management of adrenal insufficiency challenging. The management of adrenal insufficiency also needs to reflect the physiologic changes of pregnancy, often requiring increased doses of glucocorticoid as pregnancy progresses and in some circumstances mineralocorticoid replacement (in primary adrenal insufficiency patients only), especially in the third trimester. To date, there are no prospective data guiding management of adrenal insufficiency in pregnancy. In this review, we focus on the impact of adrenal insufficiency on fertility and parity based on the aetiology of adrenal insufficiency and provide a practical approach to the management of patients with adrenal insufficiency before and during pregnancy.
Keywords: adrenal insufficiency, cortisol, androgens, fertility, pregnancy
Introduction
Adrenal insufficiency (AI) is characterised by inadequate adrenal corticosteroid hormone production and is classified as primary, secondary or tertiary (1). Primary adrenal insufficiency (PAI) is caused by pathological processes affecting the normal structure and function of the adrenal gland, leading to a deficiency in glucocorticoid, mineralocorticoid and adrenal androgens (2). Secondary adrenal insufficiency (SAI) occurs due to impairment of the hypothalamus–pituitary–adrenal (HPA) axis, with insufficient adrenocorticotrophic hormone (ACTH) stimulation of the adrenal cortex (2) leading to deficiency in cortisol and adrenal androgens. This is mostly commonly caused by pituitary tumours and the resultant treatment including surgery and radiotherapy (3). Tertiary adrenal insufficiency (TAI) occurs as a result of suppression of HPA axis activity, as a result of exposure to supraphysiological exogenous glucocorticoids or chronic opioid use (4), leading to ACTH deficiency and subsequent adrenal atrophy (1) with resultant deficiency in cortisol and adrenal androgens. PAI and SAI are relatively uncommon and typically managed in specialist endocrine services, while TAI is the most prevalent form due to widespread exogenous glucocorticoid use (5). The clinical presentation of AI can be subtle in the initial stages of the disease and progress insidiously, often leading to a delay in diagnosis. Patients may present with subtle symptoms such as fatigue, reduced appetite, nausea, or arthralgia. Patients with PAI commonly have accompanying hyperpigmentation, unintentional weight loss, and orthostatic hypotension (6). If undiagnosed, and left untreated, adrenal insufficiency can present with an acute life-threatening adrenal crisis (7). Undiagnosed AI, therefore, leads to significant morbidity and mortality (8, 9).
All patients with AI require glucocorticoid (GC) replacement; however, in contrast to PAI, patients with ACTH deficiency (SAI and TAI) do not require mineralocorticoid replacement due to preservation of the renin-angiotensin aldosterone system. Patients with all forms of adrenal insufficiency also lack adrenal androgens (except patients with congenital adrenal hyperplasia (CAH)); however, studies of adrenal androgen replacement with dehydroepiandrosterone (DHEA) have reported inconsistent results (10).
Despite available replacement therapy, patients with AI continue to have morbidities including adverse metabolic profiles (11), reduced quality of life (12), risk of adrenal crisis (2), and premature mortality (13, 14).
In patients of reproductive age, AI may have consequences on fertility, reproduction, and pregnancy outcomes (15, 16). Patients with AI on optimal GC replacement pre-conception are expected to have uneventful pregnancies with favourable outcome; however, increased risk of maternal and neonatal complications is reported (17). The diagnosis of AI in pregnancy is rare and challenging. Symptom overlap with normal pregnancy and physiological alterations in cortisol secretion and cortisol binding globulin during pregnancy affect both clinical and biochemical evaluation (18). Furthermore, there is a paucity of evidence-based data on optimal adrenal hormone adjustment during pregnancy in patients with AI. In this review we focus on the impact of AI on fertility and pregnancy in patients with adrenal insufficiency and management strategies before, during, and after pregnancy.
Physiological changes in the HPA axis during pregnancy
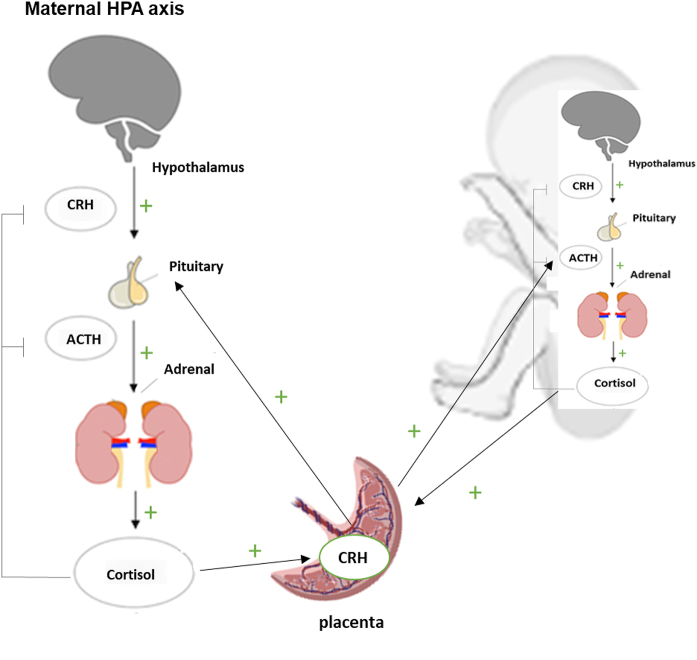
Physiological changes in pregnancy result in significant alterations in endocrine hormone profiles. This allows adaptation to the demands of pregnancy and aims to ensure adequate fetal growth. Pregnancy is associated with the development of the placenta, which serves as an additional endocrine gland throughout pregnancy. All placental hormones impact the function of maternal endocrine glands, including the maternal HPA axis (19, 20). The feto-placental unit drives a state of sustained physiological hypercortisolism, Fig. 1. Total plasma cortisol, plasma free cortisol, and 24-h urinary free cortisol progressively rise with advancing gestation, peaking in the third trimester (21). In parallel to the rise in total plasma cortisol, there is a progressive rise in plasma CBG concentrations following oestrogen stimulation in pregnancy. Mean CBG concentrations are 1.9-, 2.5-, and 3.0-fold elevated during the first, second, and third trimesters, respectively (18, 21, 22, 23, 24). Total plasma cortisol and CBG remain elevated 2–3 months post partum, compared to plasma free cortisol, salivary cortisol, and 24-h urinary free cortisol which return to baseline levels by 2-3 months post-partum (21, 25, 26, 27).
Figure 1.
Schematic representation of the maternal–placental–fetal steroidogenic unit. In the maternal compartment, increases in placental CRH promote increased synthesis and release of ACTH and cortisol. Maternal cortisol in turn stimulates placental CRH production, generating a positive feedback loop. Placental CRH stimulates production of fetal cortisol, both by increasing fetal adrenal responsiveness to ACTH and by direct stimulation of the adrenal. Increased fetal cortisol would in turn stimulate placental CRH production, constituting a second positive feedback loop. ACTH, adrenocorticotrophic hormone; CRH, corticotrophin-releasing hormone. Figure produced using biorender.com.
Several mechanisms have been proposed to explain the rise in plasma free cortisol during pregnancy. Studies have demonstrated an increase in plasma CRH and ACTH concentrations during the second and third trimesters (21, 26). This is likely a result of CRH and ACTH production by the placenta, which is autonomous and not subject to normal glucocorticoid feedback control. In contrast to the negative feedback effects of cortisol on hypothalamic CRH, cortisol stimulates placental CRH release. This is one of the rare positive feedback regulatory loops in physiology and is terminated at delivery (20). The rise in ACTH during pregnancy is not proportional to that of CRH. CRH levels increase by several 100-fold (28), while ACTH levels increase three-fold between 12- and 37-weeks’ gestation (26). At delivery, ACTH increases 15-fold and normalises by 24 h post-partum (21, 26). Studies have suggested that the adrenal glands have increased responsiveness to ACTH during pregnancy. This has been demonstrated by a greater rise in unbound cortisol in response to synthetic ACTH as pregnancy advances (25). Another potential explanation for the elevation in plasma free cortisol in pregnancy is that the maternal HPA axis may be reset at a higher level and therefore be less sensitive to the suppressive effects of glucocorticoids during gestation. Allolio et al. demonstrated correlations between serum progesterone and salivary cortisol during pregnancy. They suggest that elevated plasma free cortisol levels may result from the anti-glucocorticoid effects of elevated progesterone concentrations in pregnancy (27).
The fetus is protected from maternal hypercortisolaemia by placental 11β-hydroxysteroid dehydrogenase (11β-HSD) type 2 enzyme, which inactivates cortisol to cortisone (29). Dexamethasone is not metabolised by 11β-HSD type 2 and therefore can be used for lung maturation in pregnancy. It should not be used for the treatment of adrenal insufficiency during pregnancy due to significant fetal exposure.
The activity of the renin–angiotensin–aldosterone system is increased in pregnancy. Plasma renin activity increases early in the first trimester of normal pregnancy, reaching values almost 3- to 7-fold greater than the normal range by the third trimester. As a result, plasma renin activity cannot be used in pregnancy to monitor mineralocorticoid replacement. Plasma aldosterone concentrations also increase 5- to 7-fold during the first trimester and 10- to 20-fold by the 38th week of gestation (24). However, the effect of increased aldosterone is offset by the effect of increased progesterone levels. Progesterone production rises continuously during pregnancy and has been shown to act as a potent mineralocorticoid receptor antagonist (30, 31).
Fertility in adrenal insufficiency
For patients with AI, fertility outcomes reported depend on the underlying aetiology of AI.
The maturation and function of reproductive organs is primarily controlled by the hypothalamic–pituitary–gonadal (HPG) axis. Pulsatile secretion of gonadotrophin-releasing hormone (GnRH) from the hypothalamus stimulates the synthesis and secretion of follicle-stimulating hormone (FSH) and luteinising hormone (LH) within the anterior pituitary gland, which in turn promote ovarian folliculogenesis and steroidogenesis (16). Control of this reproductive axis occurs at all levels, and multiple cross talk exists between the activity of the HPG axis and the pituitary–adrenal axis (32).
Female fertility in PAI
Autoimmune Addison’s disease is the most common cause of primary adrenal insufficiency (up to 90% in developed countries), followed by infections, haemorrhage, metastases, or bilateral adrenalectomy (2). Addison’s disease is more prevalent in women than men, with a peak in the third and fourth decade of life (33). It occurs in isolation in approximately 30–40% of cases of PAI (34); however, greater than 50% of patients with Addison’s disease develop concomitant autoimmune diseases, as part of an autoimmune polyendocrine syndrome (APS), Table 1 (35). A major component of APS is hypergonadotropic hypogonadism (premature ovarian insufficiency). Approximately 10–20% of woman with Addison’s disease develop premature ovarian insufficiency (POI) (36, 37), defined by loss of ovarian function before the age of 40 years (38). The high prevalence of Addison’s disease and POI is due the presence of cross-reacting autoantibodies to steroid-producing cells (StCA), targeting antigenic factors including 17alpha hydroxylase/17,20-lyase (17-OH), P-450 side chain cleavage enzyme (P-450scc), 21-hydroxylase (21-OH), and 3β-hydroxysteroid dehydrogenase (HSD) antibodies (39). In a 10-year prospective study, De Bellis et al. studied time-related variations in StCA titres, ovarian function, and ovarian reserve (using serum concentrations of anti-Mullerian hormone) in 33 women with Addison’s disease with normal ovulatory cycles. They concluded that high basal StCA titre was a good predictor of subsequent development of autoimmune POI (39, 40). Interestingly, they observed that when StCA titres increased, oligomenorrhoea associated with high gonadotropin levels and subfertility developed, but AMH levels were still detectable albeit significantly lower than those previously found when normal ovulatory menses were still present. Hence, measurement of AMH concentrations may be useful in the early clinical phase of POI as the finding of decreased but still detectable values may indicate the persistence of a residual follicular pool (40), which is particularly relevant for woman wishing to conceive or explore fertility preservation. Primary ovarian insufficiency may precede Addison’s disease (36); however, it is unclear if all women with clinically idiopathic POI should be tested for autoantibodies to 21-hydroxylase (21OHAb) and further research is required (41).
Table 1.
| APS-I/APECED | APS-II | APS-III | APS-IV | |
|---|---|---|---|---|
| Main characteristics | Addison’s disease Hypoparathyroidism Chronic mucocutaneous candidiasis |
Addison’s disease Autoimmune thyroid disease Type 1 diabetes mellitus |
Autoimmune thyroid disease in addition to associated conditions listed below | ≥2 organ-specific autoimmune diseases (which do not fall into type I–III) |
| Associated conditions | Chronic active hepatitis Pernicious anaemia Vitiligo POI (39–72%) Type 1 diabetes mellitus Malabsorption syndrome Graves’ disease Autoimmune hypothyroidism IgA deficiency Alopecia Asplenism Ectodermal dysplasia Keratitis Pure red cell aplasia |
Pernicious anaemia Vitiligo Celiac disease POI (10–25%) Dermatitis herpetiformis Graves’ disease IgA deficiency Alopecia Myasthenia gravis Idiopathic thrombocytopenia Parkinson’s disease Serositis Stiff-man syndrome Idiopathic heart block Hypophysitis |
APS 3a – type 1 diabetes mellitus APS 3b – pernicious anaemia APS-3c – albinism and/or alopecia or autoimmune diseases of other organs |
APS-3B pernicious anaemia |
APECED, autoimmune polyendocrinopathy–candidiasis–ectodermal dystrophy; POI, premature ovarian insufficiency.
A recent survey of 269 Norwegian women with Addison’s disease reported that fertility remained significantly reduced after the exclusion of all women with POI. The standardised incidence ratio (SIR) for childbirth was 0.97 in women before being diagnosed with PAI which fell to 0.69 after the diagnosis had been established. This remained significantly reduced at 0.72 when excluding all women with POI (15). One explanation may be adrenal androgen deficiency, a hallmark of PAI (15, 23). Androgens play an essential role in ensuring adequate follicular steroidogenesis (42) and androgen receptor (AR) expression has been identified in human follicles through immunohistochemistry studies (43, 44). There is mounting evidence that androgens may have a specific action in pre-antral and small antral follicles, prior to serving as a substrate for oestradiol synthesis in larger follicles (45, 46). This has led to the investigation of the role of exogenous DHEA replacement in woman with infertility (47). Two recent meta-analysis reported conflicting conclusions as to the role of DHEA supplementation in women with diminished ovarian reserve (47, 48, 49), with placebo-controlled studies showing no benefit.
Other explanations for reduced fertility in women with Addison’s disease include coexistent diseases such as autoimmune thyroid disease (thyroid peroxidase antibody positivity) and type 1 diabetes mellitus, which may independently impact fertility (50, 51). Despite physiological glucocorticoid replacement therapy, patients with Addison’s disease report psychological morbidity and reduced quality of life compared to matched controls (12). Furthermore, standard GC replacement therapy in the form of immediate-release hydrocortisone fails to replicate the physiological cortisol circadian rhythm, and together with the uncertainties of GC dose adjustment and the absence of reliable biomarkers, patients receiving GC therapy may be over- or underexposed to cortisol over a 24-h period (52), with a resultant impact on the HPG axis (53). The burden of disease and associated loss of vitality and energy reported in this population may negatively impact on a woman’s desire to plan, and seek fertility (8, 15). Thus, the observed reduced birth rate in Addison’s disease likely may represent an interplay of pathological, social, and psychological processes.
Male fertility in PAI
There is a paucity of literature assessing male fertility in patients with primary adrenal insufficiency. In recent years, there is an expanding list of genetic causes of PAI (including CAH), which can have associated reproductive implications (54). X-linked adrenoleukodystrophy (X-ALD) is a rare inherited disorder of beta-oxidation with accumulation of very long-chain fatty acids (VLCFAs) in various tissues (55), including the nervous system, adrenal gland, and testes. The proposed mechanism of testicular dysfunction is the cytotoxic effect of VLCFAs on the Leydig cells which can disrupt androgen-receptor binding capabilities, leading to testicular atrophy. Interestingly, in one study of 17 men with ALD there was similar fertility compared to the general population despite biochemical evidence of testicular dysfunction in approximately half of the men tested (56). More rarely, testicular adrenal rest tumours (TARTs), benign masses originating from ACTH stimulation of pluripotent testicular steroidogenic cells, have also been reported in male patients with X-linked ALD, with associated infertility (57). X-linked adrenal hypoplasia congenita (X-AHC) characterised by PAI with onset predominantly at birth or in early childhood is rare, occurring in 1:70,000 to 1:600,000 boys (58). It is caused by mutations in the orphan nuclear receptor DAX1, now known as NR0B1, that plays an essential role in the development and function of the adrenal gland and HPG axis (59). It is frequently associated with hypogonadotropic hypogonadism and spermatogenesis failure detected after puberty (59), with increased recognition of adult-onset cases reported (60).
Elucidating a molecular diagnosis in these patients has important clinical implications for their management, enabling individualised care, prediction of co-morbidities including fertility, and genetic counselling for families.
Congenital adrenal hyperplasia
CAH is a broad group of genetic conditions in which there is disrupted adrenal steroidogenesis (61). The most common cause is 21-hydroxylase deficiency (21-OHD) due to mutations in the 21-hydroxylase (CYP21A2) gene (62). Other virilising forms include 3β-hydroxysteroid dehydrogenase (3β-HSD) and 11β-hydroxylase (11β-OH) deficiencies associated with mutations in the HSD3B2 and CYP11B1 genes, respectively (63). In the case of 21-OHD, the clinical features comprise a continuum ranging from salt-losing (most severe phenotype) and simple virilising forms, termed classic CAH, to the milder or late-onset non-classic CAH. Disease severity reflects residual enzyme activity, with salt-wasting CAH represents a life-threatening condition characterised by both cortisol and aldosterone deficiency. Patients with simple virilising CAH preserve aldosterone secretion, while non-classic adrenal hyperplasia can often be asymptomatic, with sufficient cortisol and aldosterone secretion. Overall, CAH is the most frequently encountered genetic steroid disorder affecting fertility (64).
Pathophysiology of fertility issues in females with CAH
Multiple factors can impact fertility in CAH, as summarised in Table 2. Most publications assessing impaired fertility in CAH focus on patients with 21-hydroxylase deficiency. Therefore, unless otherwise indicated, in this review the term CAH refers to 21-hydroxylase deficiency. There is an association between the severity of the CAH genotype and the degree of ovarian dysfunction and infertility (62). Increased synthesis and secretion of adrenal androgens and progesterone plays a significant role in fertility in patients with CAH. If GC replacement is insufficient in women with CAH, androgen production from the adrenals will increase and suppress gonadotrophin secretion from the pituitary. This will lead to suppression of follicular development and compromise ovulation, leading to infertility (65). Overproduction of adrenal-derived progesterone results in reduced LH frequency, therefore causing reduced endometrial lining, impaired ovulation, and decreased embryo implantation (66). In contrast, elevated androgens may impair the ability of progesterone to reduce the activity of the GnRH/LH pulse generator (67, 68). Therefore, in a subset of patients, the neuroendocrine dysfunction in 21-OHD may include increased GnRH pulse frequency, leading to higher LH levels than FSH levels, resulting in ovarian cyst morphology (66).
Table 2.
Overview of different forms of Congenital Adrenal Hyperplasia and associated effects on fertility.
| Forms of CAH | Effects on fertility |
|---|---|
| 21-hydroxylase deficiency (61, 62, 63) | Classic
|
| 11β-hydroxylase deficiency (118) | Resembles 21-hydroxylase deficiency without significant progesterone elevation |
| 17α-hydroxylase deficiency/17,20-lyase deficiency (119, 120, 121) | Classic
|
| Congenital lipoid hyperplasia (StAR deficiency) (122, 123) | Classic
|
| 3β-HSD2 deficiency (124) |
|
| P450 oxidoreductase deficiency (PORD) (125) |
|
DSD, disorder of sexual development; HPG, hypothalamic–pituitary–gonadal axis; HSD, hydroxysteroid dehydrogenase; LH, luteinising hormone; TART, testicular adrenal rest tumour.
Psychological factors may also play a role in the overall reduced pregnancy rates in women with CAH. Delayed heterosexual milestones including dating and sexual initiation have been described in this population (69, 70). In adulthood, there is a reduced interest in getting married and performing the traditional childcare role, compared with age-matched controls (70, 71, 72). Some authors postulate that the reported lower interest in motherhood may be caused by the effects of prenatal androgen exposure on gender role behaviour (61). Vaginal introitus size, previous genital surgery, and dissatisfaction with their genitals are other potential reasons reported for lower fertility among women with CAH (73).
Overall, it is difficult to delineate from available literature the relative contribution of adrenal insufficiency, hyperandrogenism and genital ambiguity on the adverse reproductive outcomes in this population.
Female fertility in classic CAH
Traditionally, significantly reduced fertility rates have been reported in women with classic CAH (74). In a Swedish population-based study, significantly fewer women (25.4%) with CAH had given birth compared to 45.8% of a healthy control population (75). The severity of 21-hydroxylase mutation correlated with the reduced number of children born. However, it has been shown that with modern treatments pregnancy rates can be like that of the general population (76, 77).
If fertility is desired, the first goal is optimisation of glucocorticoid therapy to achieve regular ovulatory menses. Immediate-release hydrocortisone remains the preferred option for glucocorticoid replacement therapy in CAH (62). Dexamethasone should be avoided in pregnancy, as it traverses the placenta. Over recent years, modified-release hydrocortisone preparations have been developed and investigated as GC replacement therapy in patients with CAH (78, 79). A recent phase III trial including 122 patients with classic CAH revealed superior hormonal control using modified-release HC, Efmody® (development name Chronocort®), during the early morning and early afternoon compared with patients receiving standard GC therapy (80); however, its use has not been investigated in pregnancy or its impact on conception.
Optimising mineralocorticoid treatment has been shown to improve fertility in salt-wasting and simple virilising CAH patients (71, 77). If ovulation is not achieved, it is reasonable to consider ovulation induction medications, albeit there is a paucity of evidence (81, 82, 83). Due to the possible pregnancy complications and implications on the offspring, pre-conception genetic testing and counselling and pregnancy care with an obstetrician with a specialist interest are recommended (84). If pregnancy is not desired, contraception options should be offered.
Prenatal treatment of CAH is beyond the scope of this review article, but overall expert guidelines state that treatment is experimental and should only be performed in expert centres and for very specific clinical indications (61, 62, 84).
Female fertility in non-classic CAH
Patients with non-classic CAH exhibit a milder clinical phenotype, which often manifests in adolescent or adult life. Infertility is the presenting symptom leading to diagnosis in as many of 13% of patients with non-classic CAH (85). In a retrospective analysis of 95 patients with non-classic CAH pursuing conception, 89.5% conceived, with 57.2% of pregnancies occurring when neither treatment for infertility nor suppressive glucocorticoid therapy for CAH was initiated (86). Not surprisingly, patients conceiving without intervention were less likely to exhibit features of significant clinical or biochemical hyperandrogenism including hirsutism and elevated testosterone and androstenedione. There is an increased risk of miscarriage in women with non-classic CAH, which is reduced with glucocorticoid therapy (86, 87, 88). Thus, guidance recommends that women with non-classic CAH with subfertility may benefit from glucocorticoid therapy to conceive and maintain pregnancy (62).
There is a significant phenotypic overlap between non-classic CAH and polycystic ovary syndrome (PCOS), which can lead to misdiagnosis. Patients with PCOS experience androgen excess, insulin resistance, and anovulation, like patients with non-classic CAH. It is important to rule out a diagnosis of non-classic CAH in patients with the diagnosis of PCOS, due to the divergent therapy and the genetic implications pre-conception.
Male fertility in CAH
Male patients with CAH may present with impaired gonadal function and infertility, due to a several pathological processes. Adrenal androgen and progesterone overproduction causes gonadotropin suppression and secondary gonadal failure, impairing fertility (61). Testicular adrenal rest tumours (TARTs) have been described in these patients. The prevalence of TARTs is variable and increases with age (89). TARTs do not have malignant potential but can result in obstructive azoospermia by mechanical obstruction of seminiferous tubules and irreversible peritubular fibrosis (61). Elevated FSH indicates testicular damage in men with TARTs and those with large TARTs may have low morning testosterone indicating Leydig cell dysfunction (62). Expert guidelines suggest surveillance screening with testicular ultrasound from adolescence every 1–2 years in asymptomatic males, and more frequently in symptomatic patients (62). Optimisation of glucocorticoid treatment can shrink early-stage TARTs and prevent progressive enlargement, resulting in infertility. If unresponsive to glucocorticoid therapy, testicular sperm extraction may be offered to the patient (90).
It is important to out rule the rare case of a Leydig cell tumour in the case of testicular enlargement in patients with CAH (similar risk to non-CAH population) as these have malignant potential. Leydig cell tumours are predominantly unilateral compared to TARTs which are usually bilateral and exhibit characteristic histologic structures called Reinke crystalloids, which are never found in TARTs (91). Of note, TARTS do not need to be biopsied in most cases.
Several controlled studies of fertility in men with CAH report a lower child rate compared to an age-matched healthy population (92, 93, 94). A multicentre cross-sectional study of adults with CAH in the United Kingdom, including 65 men, reported only 24 (37%) had sought fertility and 16 of 24 (67%) had been successful, including two children conceived after fertility treatment (89). In a large Swedish population study of men with CAH, only those born before neonatal screening had a reduced child rate, suggesting potentially improved fertility outcomes for men with CAH in the future (95). In the same study, men with non-classic CAH had a similar number of offspring compared to controls (95).
Fertility in patients with hypopituitarism
A recent systematic review highlighted the paucity of evidence as regards fertility and pregnancy in patients with hypopituitarism (16). The hypothalamic–pituitary–gonadal (HPG) axis is primarily responsible for regulating human reproduction. Disturbances in the secretion of anterior pituitary hormones or in the morphology of reproductive organs can interfere with HPG axis activity and have been linked to infertility (16).
The HPA axis exerts profound effects on the reproductive axis, with corticotrophin-releasing hormone (CRH) inhibiting hypothalamic GnRH secretion. Glucocorticoids can exert negative feedback loops on CRH and ACTH secretion and have inhibitory effects at the level of the GnRH neuron, the pituitary gonadotroph, and the gonads themselves. This results in inhibition of pituitary LH synthesis and secretion, and in ovarian oestrogen and progesterone secretion, and renders oestrogen target tissues, such as the endometrium, resistant to the gonadal steroid action (32). These effects of the HPA axis on the HPG axis are evident in the clinical state of ‘hypothalamic’ amenorrhoea associated with stress, depression, over exercise and eating disorders. Patients with undiagnosed ACTH deficiency may present with subfertility, with introduction of glucocorticoid therapy resuming an ovulatory menstrual cycle (96, 97).
Overall, the impairment of any anterior pituitary hormone axis may interfere with fertility. Hall et al. reported a pregnancy rate of 47% in women with hypopituitarism, which is approximately half of that reported in hypogonadotropic hypogonadism (HH). This indicates pituitary hormone deficiency beyond gonadotrophins has a major adverse effect on achieving pregnancy and successful pregnancy outcomes (98). Normal thyroid function is important to maintain healthy reproduction, with disorders of thyroid often linked to fertility issues (99). Growth hormone influences ovarian function by directly binding to specific receptors and indirectly via insulin growth factor 1 (IGF1). The insulin–IGF1 system affects several ovarian functions including ovulation (100, 101). The addition of growth hormone treatment to ovulation induction or in vitro fertilisation has been shown to improve outcomes in specific populations (16, 102).
Woman with hypopituitarism receiving adequate hormone replacement therapy may conceive spontaneously but frequently require assisted reproductive techniques (ART) (16). Ovarian stimulation techniques have evolved significantly over the last number of decades, with a positive impact on woman with hypopituitarism seeking fertility (103, 104). Replacing GH deficiency while seeking conception and effects of GH deficiency on pregnancy outcomes have been a central focus in several studies (105, 106, 107, 108). Observational data from the KIMS cohort (Pfizer International Metabolic Database) describing 173 pregnancies among 5092 female patients found neither GH replacement therapy at conception or during pregnancy appeared to impact pregnancy complications or live births (105). Growing evidence supports its use in selected women with hypopituitarism, particularly if previous attempts of fertility without GH replacement therapy have been unsuccessful; however, the decision to continue during pregnancy should be individualised to the patient (16). It should be noted that during pregnancy GH is produced by the placenta and the advice to hypopituitary patients receiving GH is that they can discontinue GH during pregnancy (109).
Pregnancy in adrenal insufficiency
Diagnosis of adrenal insufficiency in pregnancy
The onset of adrenal insufficiency in pregnancy is rare, yet it can lead to significant maternal and fetal morbidity if undiagnosed. According to a recent review of published case reports of AI in pregnancy, only 17.7% of women (n = 64) were diagnosed during pregnancy, with a similar distribution in all trimesters (18). Early diagnosis can be challenging as pregnancy can overlap with some of the symptoms of adrenal insufficiency, such as fatigue, nausea, vomiting, and abdominal discomfort. Hyperpigmentation is a more specific sign of primary adrenal insufficiency (110). In contrast to pregnancy-associated chloasma, hyperpigmentation in Addison’s disease typically involves areas of increased mechanical friction, including creases of hands, extensor surfaces, scars, nipples, and mucous membranes. Hyponatremia can be seen in regular pregnancy; however, a decrease in plasma sodium of more than 5 mmol/L is excessive and should prompt suspicion of underlying adrenal insufficiency. Clinical suspicion should be particularly high in patients with a personal or family history of other autoimmune conditions. A detailed history should be taken, including symptoms of pituitary disease and a careful medication history (20). In the case of a significant post-partum haemorrhage, where signs of circulatory collapse persist despite adequate volume repletion, the possibility of acute Sheehan’s syndrome should be considered.
Physiological hypercortisolism in pregnancy impacts the interpretation of diagnostic biochemistry. The use of paired early morning cortisol and ACTH is recommended as a screening investigation to diagnose adrenal insufficiency. However, as total cortisol levels increase during pregnancy, trimester specific cut-off values are recommended. On the basis of data presented by Jung et al., random morning cortisol levels of <300 nmol/L, <450 nmol/L, and <600 nmol/L should raise the suspicion of AI when measured during the first, second, and third trimesters of pregnancy, respectively (21). The short Synacthen test remains the test of choice to diagnose AI in pregnancy. However, cosyntropin is a category C drug as classified by the U.S. Food and Drug Administration, and the British National Formulary currently lists pregnancy as a contraindication for its use. This can be historically explained using repeated Synacthen injections to induce sustained hypercortisolaemia in autoimmune conditions in pregnancy. In a small cohort of healthy pregnant women the peak total cortisol response after ACTH administration was significantly higher in comparison to the non-pregnant state (median of 1000 nmol/L in the second and third trimester versus 700 nmol/L post partum) (24). Thus, it is important that higher total cortisol cut-off ranges are used for confirming a diagnosis AI in pregnancy; diagnostic cut-offs of 700 nmol/L, 800 nmol/L, and 900 nmol/L for the first, second, and third trimesters, respectively, have been recommended (17, 23). Insulin tolerance, metyrapone and CRH testing are not advised in pregnancy because of potential adverse effects on mother and fetus. Salivary cortisol/cortisone has emerged as an alternative tool in the assessment of cortisol exposure and is used as a diagnostic tool in Cushing’s disease (111) and more recently investigated in the diagnosis of adrenal insufficiency (112). As cortisol in saliva is only present in its biologically active ‘free’ form, then it would be unaffected by the rise in CBG during pregnancy and prove a promising alternative to serum cortisol in the assessment of cortisol dynamics during pregnancy; however, this would require validated pregnancy reference ranges.
Management of AI during pregnancy
During pregnancy, women with adrenal insufficiency require specialist input and ideally should be managed with joint obstetric and endocrine expertise. Pre-conception or early pregnancy counselling is paramount to ensure patients receive clear education about the risks and prevention strategies to avoid an adrenal crisis. Education on sick-day rules should be given at each clinic visit. Sick-day rule advice includes the need to double or treble the daily glucocorticoid dose in case of fever, infection requiring antibiotics, or minor surgery under local anaesthetic, and the need for IV or IM hydrocortisone in the case of trauma, major surgery, persistent vomiting, and active labour (22). All women and their partners should receive an emergency hydrocortisone self-injection kit and receive training in its use. All women should have a medical alert bracelet or steroid emergency card (23). These strategies to reduce the risk of adrenal crisis are paramount.
The aim of treatment for adrenal insufficiency in pregnancy is to achieve a physiological glucocorticoid replacement dose to ensure maternal and fetal wellbeing. Hydrocortisone is the glucocorticoid formulation of choice. Hydrocortisone is efficiently inactivated in the placenta by 11β-HSD2 to cortisone and therefore has no impact on the fetus; unlike dexamethasone which reaches the fetus without being inactivated (29) and is therefore not advised in pregnancy for the treatment of AI.
Little evidence exists on the optimum hydrocortisone replacement dosage during pregnancy (22). Based on the physiological increase in total and free cortisol values during pregnancy, the current guidance recommends increasing hydrocortisone doses by 20–40% from 24 weeks’ gestation (22, 23). Women with AI should be monitored closely for clinical symptoms and signs of glucocorticoid over- and under-replacement, with at least one review per trimester (17, 22). Undesirable effects of over-replacement include gestational diabetes and shorter length of gestation. In contrast, under-replacement might increase the risk of hyperemesis, and electrolyte imbalance and places the woman at risk of developing an acute adrenal crisis.
All woman with a new diagnosis of PAI during pregnancy should be initiated on mineralocorticoid replacement therapy. During pregnancy, the dosage of mineralocorticoid replacement is adjusted on an individual basis. The increase in hydrocortisone dose during pregnancy exerts some mineralocorticoid activity; however, as progesterone production continuously rises during pregnancy and exerts an anti-mineralocorticoid effect, increases in fludrocortisone dose may be required and this can be guided by clinical evidence of mineralocorticoid deficiency (22). Due to increasing plasma renin activity throughout pregnancy, plasma renin cannot be used in pregnancy to monitor mineralocorticoid replacement. Clinical parameters such as signs of orthostatic hypotension, changes in serum electrolytes, and volume depletion should instead be used to guide dose adjustment.
In women with CAH, pregnancy outcomes are good with placental aromatase activity protecting the fetus from maternal androgens (61). Adjustments in glucocorticoid (and fludrocortisone) dose are similar to pregnancies in women with primary adrenal insufficiency.
For woman with ACTH deficiency and a pituitary mass, observing for symptoms and signs of tumour enlargement is needed, such as headache, new visual field deficit, and additional hormonal deficiencies (16). If worsening mass effect of pituitary pathology is suspected with impact on visual function, MRI without gadolinium may be indicated. Guidance on GC replacement during pregnancy in patients with hypopituitarism is derived predominately from studies in patients with Addison’s disease (22); however, patients with ACTH deficiency often require lower doses of GC (than those required in Addison’s disease) due to elevated CRH and ACTH levels during pregnancy. It is recommended to use a personalised hydrocortisone regimen during pregnancy, avoiding over or under replacement, with the current practice to increase the HC dose by 20–40% in the third trimester (113).
Delivery and labour
Most deliveries are uncomplicated for both mother and child. Glucocorticoid cover during labour and delivery is equivalent to those recommended for major surgery and should be initiated after the onset of the second stage of labour. The woman should receive parenteral hydrocortisone 100 mg initially, followed by 50 mg every 6 h, throughout labour and for both vaginal delivery and caesarean section. A continuous infusion of 200 mg hydrocortisone over 24 h is an alternative option. Post-delivery, the stress dose is required for 24–48 h and then titrated down according to the clinical condition (23).
Post-partum care and lactation
Children of mothers with autoimmune Addison’s disease or hypopituitarism do not require an assessment of their adrenal function after birth. There is no contraindication to breastfeeding as less than 0.5% of hydrocortisone, and 0.07–0.23% of prednisolone is excreted in breast milk (18). However, lactation itself can be affected by pituitary insufficiency due to prolactin or oxytocin deficiency.
Outcomes of pregnancy in AI
Given the advances in obstetric care and glucocorticoid replacement therapy, good maternal and fetal outcomes in most women with adrenal insufficiency can be expected (114). Unfavourable maternal outcomes in women diagnosed with AI include increased caesarean delivery rates. In an analysis of 31 pregnancies in 27 women with hypopituitarism, caesarean sections were seen in 89% versus only 11.5% in IVF control pregnancies (115). Similarly, a Swedish population-based cohort study found the rate of caesarean delivery to be double among mothers with Addison’s disease (114). The high rate of caesarean section was felt to be partly due to the increased risk of malpresentation and partly due to the obstetrician’s recommendation. Hagenfeldt et al. reported that all women with CAH who had undergone previous genital surgery were offered an elective caesarean section (76). Therefore, the rate of caesarean section in women with CAH was 89% versus just 9% in the control group.
A high incidence of adrenal crisis is seen among patients with AI in pregnancy, with a reported rate of 7% in a recent multicentre retrospective survey (17). Triggers of adrenal crisis in pregnancy include hyperemesis, infection, non-adherence to glucocorticoid replacement therapy, and the stress of labour and delivery. In the recent literature, there is no report of maternal death with a direct relation to the presence of adrenal insufficiency (116).
Miscarriage rates depend on the underlying aetiology of adrenal insufficiency. Bothou et al. reported data from 128 pregnancies in 113 women with adrenal insufficiency from 19 centres. The percentage of miscarriages was significantly higher among patients with CAH (43.8%, 14/32) than among patients with Addison’s disease (27.3%, 15/55) and was considerably lower in patients with secondary AI (15.6%, 5/32) or due to other diseases (25%, 2/8). Most miscarriages (75%) occurred in the first trimester. With regard to other fetal outcomes, intrauterine growth restriction is the most common complication (18). The rate of children who are small for gestational age is also found to be higher in women with adrenal insufficiency, with poor placental function being proposed as a potential cause (18, 115). Furthermore, children are more likely to be born preterm (114). Bothou et al. noted preterm births in women with all causes of AI; however, preterm births were most frequent among patients with CAH (17). It is hypothesised that CRH and the HPA axis play an important role in the timing of parturition; CRH has been suggested as a ‘placental clock’ for delivery. Therefore, dysfunction of the HPA axis in adrenal insufficiency may contribute to this increased risk.
Conclusion
In summary, there are various pathophysiological consequences of AI that can both directly and indirectly affect fertility and complicate pregnancy. Glucocorticoid dosing should be optimised pre-conception as overtreatment with steroids can both suppress the HPG axis and lead to weight gain and metabolic syndrome, further compromising fertility care. The alterations in HPA axis dynamics and physiological changes in pregnancy are complex; thus, diagnosis and management of AI in pregnancy remain challenging. Overall, appropriately managed patients can expect good maternal and fetal outcomes. However, clear education on the risk of adrenal crisis and close monitoring with specialist endocrine input are advised.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Funding
MWOR is supported by an HRB Emerging Clinician Scientist award (ECSA-2020-001), MS is supported by an RCSI-Strategic academic appointment award.
References
- 1.Dineen R Martin-Grace J Thompson CJ & Sherlock M. The management of glucocorticoid deficiency: current and future perspectives. Clinica Chimica Acta 2020505148–159. ( 10.1016/j.cca.2020.03.006) [DOI] [PubMed] [Google Scholar]
- 2.Hahner S Ross RJ Arlt W Bancos I Burger-Stritt S Torpy DJ Husebye ES & Quinkler M. Adrenal insufficiency. Nature Reviews Disease Primers 2021719. ( 10.1038/s41572-021-00252-7) [DOI] [PubMed] [Google Scholar]
- 3.Sherlock M Ayuk J Tomlinson JW Toogood AA Aragon-Alonso A Sheppard MC Bates AS & Stewart PM. Mortality in patients with pituitary disease. Endocrine Reviews 201031301–342. ( 10.1210/er.2009-0033) [DOI] [PubMed] [Google Scholar]
- 4.Li T Cunningham JL Gilliam WP Loukianova L Donegan DM & Bancos I. Prevalence of opioid-induced adrenal insufficiency in patients taking chronic opioids. Journal of Clinical Endocrinology and Metabolism 2020105e3766–e3775. ( 10.1210/clinem/dgaa499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woods CP, Argese N, Chapman M, Boot C, Webster R, Dabhi V, Grossman AB, Toogood AA, Arlt W, Stewart PM, et al.Adrenal suppression in patients taking inhaled glucocorticoids is highly prevalent and management can be guided by morning cortisol. European Journal of Endocrinology 2015173633–642. ( 10.1530/EJE-15-0608) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin-Grace J Dineen R Sherlock M & Thompson CJ. Adrenal insufficiency: physiology, clinical presentation and diagnostic challenges. Clinica Chimica Acta 202050578–91. ( 10.1016/j.cca.2020.01.029) [DOI] [PubMed] [Google Scholar]
- 7.Dineen R Thompson CJ & Sherlock M. Adrenal crisis: prevention and management in adult patients. Therapeutic Advances in Endocrinology and Metabolism 2019102042018819848218. ( 10.1177/2042018819848218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lovas K Loge JH & Husebye ES. Subjective health status in Norwegian patients with Addison's disease. Clinical Endocrinology 200256581–588. ( 10.1046/j.1365-2265.2002.01466.x) [DOI] [PubMed] [Google Scholar]
- 9.Bleicken B Hahner S Ventz M & Quinkler M. Delayed diagnosis of adrenal insufficiency is common: a cross-sectional study in 216 patients. American Journal of the Medical Sciences 2010339525–531. ( 10.1097/MAJ.0b013e3181db6b7a) [DOI] [PubMed] [Google Scholar]
- 10.Alkatib AA Cosma M Elamin MB Erickson D Swiglo BA Erwin PJ & Montori VM. A systematic review and meta-analysis of randomized placebo-controlled trials of DHEA treatment effects on quality of life in women with adrenal insufficiency. Journal of Clinical Endocrinology and Metabolism 2009943676–3681. ( 10.1210/jc.2009-0672) [DOI] [PubMed] [Google Scholar]
- 11.Ueland GA & Husebye ES. Metabolic complications in adrenal insufficiency. Frontiers of Hormone Research 201849104–113. ( 10.1159/000486004) [DOI] [PubMed] [Google Scholar]
- 12.Tiemensma J Andela CD Kaptein AA Romijn JA van der Mast RC Biermasz NR & Pereira AM. Psychological morbidity and impaired quality of life in patients with stable treatment for primary adrenal insufficiency: cross-sectional study and review of the literature. European Journal of Endocrinology 2014171171–182. ( 10.1530/EJE-14-0023) [DOI] [PubMed] [Google Scholar]
- 13.Bergthorsdottir R Leonsson-Zachrisson M Oden A & Johannsson G. Premature mortality in patients with Addison's disease: a population-based study. Journal of Clinical Endocrinology and Metabolism 2006914849–4853. ( 10.1210/jc.2006-0076) [DOI] [PubMed] [Google Scholar]
- 14.Quinkler M Ekman B Zhang P Isidori AM Murray RD & Investigators E-A. Mortality data from the European Adrenal Insufficiency Registry-Patient characterization and associations. Clinical Endocrinology 20188930–35. ( 10.1111/cen.13609) [DOI] [PubMed] [Google Scholar]
- 15.Erichsen MM Husebye ES Michelsen TM Dahl AA & Lovas K. Sexuality and fertility in women with Addison's disease. Journal of Clinical Endocrinology and Metabolism 2010954354–4360. ( 10.1210/jc.2010-0445) [DOI] [PubMed] [Google Scholar]
- 16.Vila G & Fleseriu M. Fertility and pregnancy in women with hypopituitarism: a systematic literature review. Journal of Clinical Endocrinology and Metabolism 2020105. ( 10.1210/clinem/dgz112) [DOI] [PubMed] [Google Scholar]
- 17.Bothou C, Anand G, Li D, Kienitz T, Seejore K, Simeoli C, Ebbehoj A, Ward EG, Paragliola RM, Ferrigno R, et al.Current management and outcome of pregnancies in women with adrenal insufficiency: experience from a multicenter survey. Journal of Clinical Endocrinology and Metabolism 2020105e2853–e2863. ( 10.1210/clinem/dgaa266) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anand G & Beuschlein F. Management of endocrine disease: fertility, pregnancy and lactation in women with adrenal insufficiency. European Journal of Endocrinology 2018178R45–R53. ( 10.1530/EJE-17-0975) [DOI] [PubMed] [Google Scholar]
- 19.Lindsay JR & Nieman LK. The hypothalamic-pituitary-adrenal axis in pregnancy: challenges in disease detection and treatment. Endocrine Reviews 200526775–799. ( 10.1210/er.2004-0025) [DOI] [PubMed] [Google Scholar]
- 20.Lee JH & Torpy DJ. Adrenal insufficiency in pregnancy: physiology, diagnosis, management and areas for future research. Reviews in Endocrine and Metabolic Disorders 20232457–69. ( 10.1007/s11154-022-09745-6) [DOI] [PubMed] [Google Scholar]
- 21.Jung C Ho JT Torpy DJ Rogers A Doogue M Lewis JG Czajko RJ & Inder WJ. A longitudinal study of plasma and urinary cortisol in pregnancy and postpartum. Journal of Clinical Endocrinology and Metabolism 2011961533–1540. ( 10.1210/jc.2010-2395) [DOI] [PubMed] [Google Scholar]
- 22.Bornstein SR, Allolio B, Arlt W, Barthel A, Don-Wauchope A, Hammer GD, Husebye ES, Merke DP, Murad MH, Stratakis CA, et al.Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism 2016101364–389. ( 10.1210/jc.2015-1710) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lebbe M & Arlt W. What is the best diagnostic and therapeutic management strategy for an Addison patient during pregnancy? Clinical Endocrinology 201378497–502. ( 10.1111/cen.12097) [DOI] [PubMed] [Google Scholar]
- 24.Suri D Moran J Hibbard JU Kasza K & Weiss RE. Assessment of adrenal reserve in pregnancy: defining the normal response to the adrenocorticotropin stimulation test. Journal of Clinical Endocrinology and Metabolism 2006913866–3872. ( 10.1210/jc.2006-1049) [DOI] [PubMed] [Google Scholar]
- 25.Nolten WE & Rueckert PA. Elevated free cortisol index in pregnancy: possible regulatory mechanisms. American Journal of Obstetrics and Gynecology 1981139492–498. ( 10.1016/0002-9378(8190331-8) [DOI] [PubMed] [Google Scholar]
- 26.Carr BR Parker CR Madden JD MacDonald PC & Porter JC. Maternal plasma adrenocorticotropin and cortisol relationships throughout human pregnancy. American Journal of Obstetrics and Gynecology 1981139416–422. ( 10.1016/0002-9378(8190318-5) [DOI] [PubMed] [Google Scholar]
- 27.Allolio B Hoffmann J Linton EA Winkelmann W Kusche M & Schulte HM. Diurnal salivary cortisol patterns during pregnancy and after delivery: relationship to plasma corticotrophin-releasing-hormone. Clinical Endocrinology 199033279–289. ( 10.1111/j.1365-2265.1990.tb00492.x) [DOI] [PubMed] [Google Scholar]
- 28.Sasaki A Shinkawa O Margioris AN Liotta AS Sato S Murakami O Go M Shimizu Y Hanew K & Yoshinaga K. Immunoreactive corticotropin-releasing hormone in human plasma during pregnancy, labor, and delivery. Journal of Clinical Endocrinology and Metabolism 198764224–229. ( 10.1210/jcem-64-2-224) [DOI] [PubMed] [Google Scholar]
- 29.Chapman K Holmes M & Seckl J. 11beta-hydroxysteroid dehydrogenases: intracellular gate-keepers of tissue glucocorticoid action. Physiological Reviews 2013931139–1206. ( 10.1152/physrev.00020.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oelkers WK. Effects of estrogens and progestogens on the renin-aldosterone system and blood pressure. Steroids 199661166–171. ( 10.1016/0039-128x(9600007-4) [DOI] [PubMed] [Google Scholar]
- 31.Ambrosi B Barbetta L & Morricone L. Diagnosis and management of Addison's disease during pregnancy. Journal of Endocrinological Investigation 200326698–702. ( 10.1007/BF03347034) [DOI] [PubMed] [Google Scholar]
- 32.Magiakou MA Mastorakos G Webster E & Chrousos GP. The hypothalamic-pituitary-adrenal axis and the female reproductive system. Annals of the New York Academy of Sciences 199781642–56. ( 10.1111/j.1749-6632.1997.tb52128.x) [DOI] [PubMed] [Google Scholar]
- 33.Erichsen MM, Lovas K, Skinningsrud B, Wolff AB, Undlien DE, Svartberg J, Fougner KJ, Berg TJ, Bollerslev J, Mella B, et al.Clinical, immunological, and genetic features of autoimmune primary adrenal insufficiency: observations from a Norwegian registry. Journal of Clinical Endocrinology and Metabolism 2009944882–4890. ( 10.1210/jc.2009-1368) [DOI] [PubMed] [Google Scholar]
- 34.Betterle C Dal Pra C Mantero F & Zanchetta R. Autoimmune adrenal insufficiency and autoimmune polyendocrine syndromes: autoantibodies, autoantigens, and their applicability in diagnosis and disease prediction. Endocrine Reviews 200223327–364. ( 10.1210/edrv.23.3.0466) [DOI] [PubMed] [Google Scholar]
- 35.Dalin F, Nordling Eriksson G, Dahlqvist P, Hallgren Å, Wahlberg J, Ekwall O, Soderberg S, Ronnelid J, Olcen P, Winqvist O, et al.Clinical and immunological characteristics of autoimmune Addison disease: a nationwide Swedish multicenter study. Journal of Clinical Endocrinology and Metabolism 2017102379–389. ( 10.1210/jc.2016-2522) [DOI] [PubMed] [Google Scholar]
- 36.Vogt EC Breivik L Royrvik EC Grytaas M Husebye ES & Oksnes M. Primary ovarian insufficiency in women with Addison's disease. Journal of Clinical Endocrinology and Metabolism 2021106e2656–e2663. ( 10.1210/clinem/dgab140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laureti S De Bellis A Muccitelli VI Calcinaro F Bizzarro A Rossi R Bellastella A Santeusanio F & Falorni A. Levels of adrenocortical autoantibodies correlate with the degree of adrenal dysfunction in subjects with preclinical Addison's disease. Journal of Clinical Endocrinology and Metabolism 1998833507–3511. ( 10.1210/jcem.83.10.5149) [DOI] [PubMed] [Google Scholar]
- 38.European Society for Human R, Embryology Guideline Group on POI, Webber L, Davies M, Anderson R, Bartlett J, Braat D, Cartwright B, Cifkova R, de Muinck Keizer-Schrama S, Hogervorst E, et al.ESHRE guideline: management of women with premature ovarian insufficiency. Human Reproduction 201631926–937. ( 10.1093/humrep/dew027) [DOI] [PubMed] [Google Scholar]
- 39.Falorni A Laureti S Candeloro P Perrino S Coronella C Bizzarro A Bellastella A Santeusanio F & De Bellis A. Steroid-cell autoantibodies are preferentially expressed in women with premature ovarian failure who have adrenal autoimmunity. Fertility and Sterility 200278270–279. ( 10.1016/s0015-0282(0203205-3) [DOI] [PubMed] [Google Scholar]
- 40.De Bellis A, Bellastella G, Falorni A, Aitella E, Barrasso M, Maiorino MI, Bizzarro E, Bellastella A, Giugliano D, Esposito K, et al.Natural history of autoimmune primary ovarian insufficiency in patients with Addison's disease: from normal ovarian function to overt ovarian dysfunction. European Journal of Endocrinology 2017177329–337. ( 10.1530/EJE-17-0152) [DOI] [PubMed] [Google Scholar]
- 41.Bensing S Giordano R & Falorni A. Fertility and pregnancy in women with primary adrenal insufficiency. Endocrine 202070211–217. ( 10.1007/s12020-020-02343-z) [DOI] [PubMed] [Google Scholar]
- 42.Ryan KJ Petro Z & Kaiser J. Steroid formation by isolated and recombined ovarian granulosa and tehcal cells. Journal of Clinical Endocrinology and Metabolism 196828355–358. ( 10.1210/jcem-28-3-355) [DOI] [PubMed] [Google Scholar]
- 43.Horie K Takakura K Fujiwara H Suginami H Liao S & Mori T. Immunohistochemical localization of androgen receptor in the human ovary throughout the menstrual cycle in relation to oestrogen and progesterone receptor expression. Human Reproduction 19927184–190. ( 10.1093/oxfordjournals.humrep.a137614) [DOI] [PubMed] [Google Scholar]
- 44.Suzuki T Sasano H Kimura N Tamura M Fukaya T Yajima A & Nagura H. Immunohistochemical distribution of progesterone, androgen and oestrogen receptors in the human ovary during the menstrual cycle: relationship to expression of steroidogenic enzymes. Human Reproduction 199491589–1595. ( 10.1093/oxfordjournals.humrep.a138757) [DOI] [PubMed] [Google Scholar]
- 45.Nielsen ME Rasmussen IA Kristensen SG Christensen ST Mollgard K Wreford Andersen E Byskov AG & Yding Andersen C. In human granulosa cells from small antral follicles, androgen receptor mRNA and androgen levels in follicular fluid correlate with FSH receptor mRNA. Molecular Human Reproduction 20111763–70. ( 10.1093/molehr/gaq073) [DOI] [PubMed] [Google Scholar]
- 46.Sen A & Hammes SR. Granulosa cell-specific androgen receptors are critical regulators of ovarian development and function. Molecular Endocrinology 2010241393–1403. ( 10.1210/me.2010-0006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wierman ME & Kiseljak-Vassiliades K. Should dehydroepiandrosterone be administered to women? Journal of Clinical Endocrinology and Metabolism 20221071679–1685. ( 10.1210/clinem/dgac130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qin JC Fan L & Qin AP. The effect of dehydroepiandrosterone (DHEA) supplementation on women with diminished ovarian reserve (DOR) in IVF cycle: evidence from a meta-analysis. Journal of Gynecology Obstetrics and Human Reproduction 2017461–7. ( 10.1016/j.jgyn.2016.01.002) [DOI] [PubMed] [Google Scholar]
- 49.Schwarze JE Canales J Crosby J Ortega-Hrepich C Villa S & Pommer R. DHEA use to improve likelihood of IVF/ICSI success in patients with diminished ovarian reserve: a systematic review and meta-analysis. JBRA Assisted Reproduction 201822369–374. ( 10.5935/1518-0557.20180046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poppe K Velkeniers B & Glinoer D. The role of thyroid autoimmunity in fertility and pregnancy. Nature Clinical Practice. Endocrinology and Metabolism 20084394–405. ( 10.1038/ncpendmet0846) [DOI] [PubMed] [Google Scholar]
- 51.Wiebe JC, Santana A, Medina-Rodriguez N, Hernandez M, Novoa J, Mauricio D, Wagner AM. & T1DGC. Fertility is reduced in women and in men with type 1 diabetes: results from the Type 1 Diabetes Genetics Consortium (T1DGC). Diabetologia 2014572501–2504. ( 10.1007/s00125-014-3376-8) [DOI] [PubMed] [Google Scholar]
- 52.Dineen RA, Martin-Grace J, Ahmed KMS, Taylor AE, Shaheen F, Schiffer L, Gilligan LC, Lavery GG, Frizelle I, Gunness A, et al.Tissue glucocorticoid metabolism in adrenal insufficiency: a prospective study of dual-release hydrocortisone therapy. Journal of Clinical Endocrinology and Metabolism 20231083178–3189. ( 10.1210/clinem/dgad370) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Geraghty AC & Kaufer D. Glucocorticoid regulation of reproduction. Advances in Experimental Medicine and Biology 2015872253–278. ( 10.1007/978-1-4939-2895-8_11) [DOI] [PubMed] [Google Scholar]
- 54.Buonocore F & Achermann JC. Primary adrenal insufficiency: new genetic causes and their long-term consequences. Clinical Endocrinology 20209211–20. ( 10.1111/cen.14109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aversa A Palleschi S Cruccu G Silvestroni L Isidori A & Fabbri A. Rapid decline of fertility in a case of adrenoleukodystrophy. Human Reproduction 1998132474–2479. ( 10.1093/humrep/13.9.2474) [DOI] [PubMed] [Google Scholar]
- 56.Stradomska TJ Kubalska J Janas R & Tylki-Szymanska A. Reproductive function in men affected by X-linked adrenoleukodystrophy/adrenomyeloneuropathy. European Journal of Endocrinology 2012166291–294. ( 10.1530/EJE-11-0490) [DOI] [PubMed] [Google Scholar]
- 57.Tresoldi AS, Betella N, Hasenmajer V, Pozza C, Vena W, Fiamengo B, Negri L, Cappa M, Lania AGA, Lenzi A, et al.Bilateral testicular masses and adrenal insufficiency: is congenital adrenal hyperplasia the only possible diagnosis? First two cases of TARTS described in Addison-only X-linked adrenoleukodystrophy and a brief review of literature. Journal of Endocrinological Investigation 202144391–402. ( 10.1007/s40618-020-01362-x) [DOI] [PubMed] [Google Scholar]
- 58.Lin L Gu WX Ozisik G To WS Owen CJ Jameson JL & Achermann JC. Analysis of DAX1 (NR0B1) and steroidogenic factor-1 (NR5A1) in children and adults with primary adrenal failure: ten years' experience. Journal of Clinical Endocrinology and Metabolism 2006913048–3054. ( 10.1210/jc.2006-0603) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mantovani G De Menis E Borretta G Radetti G Bondioni S Spada A Persani L & Beck-Peccoz P. DAX1 and X-linked adrenal hypoplasia congenita: clinical and molecular analysis in five patients. European Journal of Endocrinology 2006154685–689. ( 10.1530/eje.1.02132) [DOI] [PubMed] [Google Scholar]
- 60.Kyriakakis N Shonibare T Kyaw-Tun J Lynch J Lagos CF Achermann JC & Murray RD. Late-onset X-linked adrenal hypoplasia (DAX-1, NR0B1): two new adult-onset cases from a single center. Pituitary 201720585–593. ( 10.1007/s11102-017-0822-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Claahsen-van der Grinten HL, Speiser PW, Ahmed SF, Arlt W, Auchus RJ, Falhammar H, Fluck CE, Guasti L, Huebner A, Kortmann BBM, et al.Congenital adrenal hyperplasia-current insights in pathophysiology, diagnostics, and management. Endocrine Reviews 20224391–159. ( 10.1210/endrev/bnab016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Speiser PW, Arlt W, Auchus RJ, Baskin LS, Conway GS, Merke DP, Meyer-Bahlburg HFL, Miller WL, Murad MH, Oberfield SE, et al.Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism 20181034043–4088. ( 10.1210/jc.2018-01865) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Auer MK Nordenstrom A Lajic S & Reisch N. Congenital adrenal hyperplasia. Lancet 2023401227–244. ( 10.1016/S0140-6736(2201330-7) [DOI] [PubMed] [Google Scholar]
- 64.Reichman DE White PC New MI & Rosenwaks Z. Fertility in patients with congenital adrenal hyperplasia. Fertility and Sterility 2014101301–309. ( 10.1016/j.fertnstert.2013.11.002) [DOI] [PubMed] [Google Scholar]
- 65.Holmes-Walker DJ Conway GS Honour JW Rumsby G & Jacobs HS. Menstrual disturbance and hypersecretion of progesterone in women with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Clinical Endocrinology 199543291–296. ( 10.1111/j.1365-2265.1995.tb02034.x) [DOI] [PubMed] [Google Scholar]
- 66.Gomes LG Bachega TASS & Mendonca BB. Classic congenital adrenal hyperplasia and its impact on reproduction. Fertility and Sterility 20191117–12. ( 10.1016/j.fertnstert.2018.11.037) [DOI] [PubMed] [Google Scholar]
- 67.Pielecka J Quaynor SD & Moenter SM. Androgens increase gonadotropin-releasing hormone neuron firing activity in females and interfere with progesterone negative feedback. Endocrinology 20061471474–1479. ( 10.1210/en.2005-1029) [DOI] [PubMed] [Google Scholar]
- 68.Moore AM & Campbell RE. The neuroendocrine genesis of polycystic ovary syndrome: a role for arcuate nucleus GABA neurons. Journal of Steroid Biochemistry and Molecular Biology 2016160106–117. ( 10.1016/j.jsbmb.2015.10.002) [DOI] [PubMed] [Google Scholar]
- 69.Dittmann RW Kappes ME & Kappes MH. Sexual behavior in adolescent and adult females with congenital adrenal hyperplasia. Psychoneuroendocrinology 199217153–170. ( 10.1016/0306-4530(9290054-b) [DOI] [PubMed] [Google Scholar]
- 70.Meyer-Bahlburg HF. What causes low rates of child-bearing in congenital adrenal hyperplasia? Journal of Clinical Endocrinology and Metabolism 1999841844–1847. ( 10.1210/jcem.84.6.5718) [DOI] [PubMed] [Google Scholar]
- 71.Casteras A De Silva P Rumsby G & Conway GS. Reassessing fecundity in women with classical congenital adrenal hyperplasia (CAH): normal pregnancy rate but reduced fertility rate. Clinical Endocrinology 200970833–837. ( 10.1111/j.1365-2265.2009.03563.x) [DOI] [PubMed] [Google Scholar]
- 72.Jaaskelainen J Tiitinen A & Voutilainen R. Sexual function and fertility in adult females and males with congenital adrenal hyperplasia. Hormone Research 20015673–80. ( 10.1159/000048095) [DOI] [PubMed] [Google Scholar]
- 73.May B Boyle M & Grant D. A comparative study of sexual experiences: women with diabetes and women with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Journal of Health Psychology 19961479–492. ( 10.1177/135910539600100406) [DOI] [PubMed] [Google Scholar]
- 74.Slowikowska-Hilczer J, Hirschberg AL, Claahsen-van der Grinten H, Reisch N, Bouvattier C, Thyen U, Cohen Kettenis P, Roehle R, Kohler B, Nordenstrom A, et al.Fertility outcome and information on fertility issues in individuals with different forms of disorders of sex development: findings from the dsd-LIFE study. Fertility and Sterility 2017108822–831. ( 10.1016/j.fertnstert.2017.08.013) [DOI] [PubMed] [Google Scholar]
- 75.Hirschberg AL Gidlof S Falhammar H Frisen L Almqvist C Nordenskjold A & Nordenstrom A. Reproductive and perinatal outcomes in women with congenital adrenal hyperplasia: a population-based cohort study. Journal of Clinical Endocrinology and Metabolism 2021106e957–e965. ( 10.1210/clinem/dgaa801) [DOI] [PubMed] [Google Scholar]
- 76.Hagenfeldt K Janson PO Holmdahl G Falhammar H Filipsson H Frisen L Thoren M & Nordenskjold A. Fertility and pregnancy outcome in women with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Human Reproduction 2008231607–1613. ( 10.1093/humrep/den118) [DOI] [PubMed] [Google Scholar]
- 77.Hoepffner W Schulze E Bennek J Keller E & Willgerodt H. Pregnancies in patients with congenital adrenal hyperplasia with complete or almost complete impairment of 21-hydroxylase activity. Fertility and Sterility 2004811314–1321. ( 10.1016/j.fertnstert.2003.10.024) [DOI] [PubMed] [Google Scholar]
- 78.Jones CM, Mallappa A, Reisch N, Nikolaou N, Krone N, Hughes BA, O'Neil DM, Whitaker MJ, Tomlinson JW, Storbeck KH, et al.Modified-release and conventional glucocorticoids and diurnal androgen excretion in congenital adrenal hyperplasia. Journal of Clinical Endocrinology and Metabolism 20171021797–1806. ( 10.1210/jc.2016-2855) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Prete A Auchus RJ & Ross RJ. Clinical advances in the pharmacotherapy of congenital adrenal hyperplasia. European Journal of Endocrinology 2021186R1–R14. ( 10.1530/EJE-21-0794) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Merke DP, Mallappa A, Arlt W, Brac de la Perriere A, Linden Hirschberg A, Juul A, Newell-Price J, Perry CG, Prete A, Rees DA, et al.Modified-release hydrocortisone in congenital adrenal hyperplasia. Journal of Clinical Endocrinology and Metabolism 2021106e2063–e2077. ( 10.1210/clinem/dgab051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Laohaprasitiporn C Barbieri RL & Yeh J. Induction of ovulation with the sole use of clomiphene citrate in late-onset 21-hydroxylase deficiency. Gynecologic and Obstetric Investigation 199641224–226. ( 10.1159/000292273) [DOI] [PubMed] [Google Scholar]
- 82.Lekarev O Lin-Su K & Vogiatzi MG. Infertility and reproductive function in patients with congenital adrenal hyperplasia: pathophysiology, advances in management, and recent outcomes. Endocrinology and Metabolism Clinics of North America 201544705–722. ( 10.1016/j.ecl.2015.07.009) [DOI] [PubMed] [Google Scholar]
- 83.Krysiak R & Okopien B. The effect of metformin on androgen production in diabetic women with non-classic congenital adrenal hyperplasia. Experimental and Clinical Endocrinology and Diabetes 2014122568–571. ( 10.1055/s-0034-1382048) [DOI] [PubMed] [Google Scholar]
- 84.Nordenstrom A & Falhammar H. Management of endocrine disease: diagnosis and management of the patient with non-classic CAH due to 21-hydroxylase deficiency. European Journal of Endocrinology 2019180R127–R145. ( 10.1530/EJE-18-0712) [DOI] [PubMed] [Google Scholar]
- 85.New MI Ghizzoni L Meyer-Bahlburg H Khattab A Reichman D & Rosenwaks Z. Fertility in patients with nonclassical congenital adrenal hyperplasia. Fertility and Sterility 201911113–20. ( 10.1016/j.fertnstert.2018.11.023) [DOI] [PubMed] [Google Scholar]
- 86.Bidet M, Bellanne-Chantelot C, Galand-Portier MB, Golmard JL, Tardy V, Morel Y, Clauin S, Coussieu C, Boudou P, Mowzowicz I, et al.Fertility in women with nonclassical congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Journal of Clinical Endocrinology and Metabolism 2010951182–1190. ( 10.1210/jc.2009-1383) [DOI] [PubMed] [Google Scholar]
- 87.Moran C, Azziz R, Weintrob N, Witchel SF, Rohmer V, Dewailly D, Marcondes JA, Pugeat M, Speiser PW, Pignatelli D, et al.Reproductive outcome of women with 21-hydroxylase-deficient nonclassic adrenal hyperplasia. Journal of Clinical Endocrinology and Metabolism 2006913451–3456. ( 10.1210/jc.2006-0062) [DOI] [PubMed] [Google Scholar]
- 88.Feldman S Billaud L Thalabard JC Raux-Demay MC Mowszowicz I Kuttenn F & Mauvais-Jarvis P. Fertility in women with late-onset adrenal hyperplasia due to 21-hydroxylase deficiency. Journal of Clinical Endocrinology and Metabolism 199274635–639. ( 10.1210/jcem.74.3.1310999) [DOI] [PubMed] [Google Scholar]
- 89.Arlt W, Willis DS, Wild SH, Krone N, Doherty EJ, Hahner S, Han TS, Carroll PV, Conway GS, Rees DA, et al.Health status of adults with congenital adrenal hyperplasia: a cohort study of 203 patients. Journal of Clinical Endocrinology and Metabolism 2010955110–5121. ( 10.1210/jc.2010-0917) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kavoussi PK Summers-Colquitt RB Odenwald KC Kressin M Kavoussi KM Pool TB & Kavoussi SK. Sperm retrieval and concomitant tumor resection in azoospermic men with congenital adrenal hyperplasia and bilateral testicular adrenal rest tumors: a case report. Journal of Assisted Reproduction and Genetics 201633545–548. ( 10.1007/s10815-016-0665-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Engels M Span PN van Herwaarden AE Sweep FCGJ Stikkelbroeck NMML & Claahsen-van der Grinten HL. Testicular adrenal rest tumors: current insights on prevalence, characteristics, origin, and treatment. Endocrine Reviews 201940973–987. ( 10.1210/er.2018-00258) [DOI] [PubMed] [Google Scholar]
- 92.Jaaskelainen J Kiekara O Hippelainen M & Voutilainen R. Pituitary gonadal axis and child rate in males with classical 21-hydroxylase deficiency. Journal of Endocrinological Investigation 20002323–27. ( 10.1007/BF03343671) [DOI] [PubMed] [Google Scholar]
- 93.Falhammar H Nystrom HF Ekstrom U Granberg S Wedell A & Thoren M. Fertility, sexuality and testicular adrenal rest tumors in adult males with congenital adrenal hyperplasia. European Journal of Endocrinology 2012166441–449. ( 10.1530/EJE-11-0828) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bouvattier C, Esterle L, Renoult-Pierre P, de la Perriere AB, Illouz F, Kerlan V, Pascal-Vigneron V, Drui D, Christin-Maitre S, Galland F, et al.Clinical outcome, hormonal status, gonadotrope axis, and testicular function in 219 adult men born with classic 21-hydroxylase deficiency. A French national survey. Journal of Clinical Endocrinology and Metabolism 20151002303–2313. ( 10.1210/jc.2014-4124) [DOI] [PubMed] [Google Scholar]
- 95.Falhammar H Frisen L Norrby C Almqvist C Hirschberg AL Nordenskjold A & Nordenstrom A. Reduced frequency of biological and increased frequency of adopted children in males with 21-hydroxylase deficiency: a Swedish population-based national cohort study. Journal of Clinical Endocrinology and Metabolism 20171024191–4199. ( 10.1210/jc.2017-01139) [DOI] [PubMed] [Google Scholar]
- 96.Atkin SL Masson EA & White MC. Isolated adrenocorticotropin deficiency presenting as primary infertility. Journal of Endocrinological Investigation 199518456–459. ( 10.1007/BF03349745) [DOI] [PubMed] [Google Scholar]
- 97.Dewailly D Bourdelle-Hego MF Pouplard-Barthelaix A & Fossati P. Recovery of ovulatory menstrual cycles under hydrocortisone in two amenorrheic women with isolated corticotropin deficiency. Hormone Research 19882914–16. ( 10.1159/000180958) [DOI] [PubMed] [Google Scholar]
- 98.Hall R Manski-Nankervis J Goni N Davies MC & Conway GS. Fertility outcomes in women with hypopituitarism. Clinical Endocrinology 20066571–74. ( 10.1111/j.1365-2265.2006.02550.x) [DOI] [PubMed] [Google Scholar]
- 99.Krassas GE Poppe K & Glinoer D. Thyroid function and human reproductive health. Endocrine Reviews 201031702–755. ( 10.1210/er.2009-0041) [DOI] [PubMed] [Google Scholar]
- 100.Barreca A Artini PG Del Monte P Ponzani P Pasquini P Cariola G Volpe A Genazzani AR Giordano G & Minuto F. In vivo and in vitro effect of growth hormone on estradiol secretion by human granulosa cells. Journal of Clinical Endocrinology and Metabolism 19937761–67. ( 10.1210/jcem.77.1.8325961) [DOI] [PubMed] [Google Scholar]
- 101.Poretsky L Cataldo NA Rosenwaks Z & Giudice LC. The insulin-related ovarian regulatory system in health and disease. Endocrine Reviews 199920535–582. ( 10.1210/edrv.20.4.0374) [DOI] [PubMed] [Google Scholar]
- 102.Regan SLP Knight PG Yovich JL Arfuso F & Dharmarajan A. Growth hormone during in vitro fertilization in older women modulates the density of receptors in granulosa cells, with improved pregnancy outcomes. Fertility and Sterility 20181101298–1310. ( 10.1016/j.fertnstert.2018.08.018) [DOI] [PubMed] [Google Scholar]
- 103.Tranoulis A Laios A Pampanos A Yannoukakos D Loutradis D & Michala L. Efficacy and safety of pulsatile gonadotropin-releasing hormone therapy among patients with idiopathic and functional hypothalamic amenorrhea: a systematic review of the literature and a meta-analysis. Fertility and Sterility 2018109708–719.e8. ( 10.1016/j.fertnstert.2017.12.028) [DOI] [PubMed] [Google Scholar]
- 104.Martin KA Hall JE Adams JM & Crowley WF Jr. Comparison of exogenous gonadotropins and pulsatile gonadotropin-releasing hormone for induction of ovulation in hypogonadotropic amenorrhea. Journal of Clinical Endocrinology and Metabolism 199377125–129. ( 10.1210/jcem.77.1.8325934) [DOI] [PubMed] [Google Scholar]
- 105.Vila G Akerblad AC Mattsson AF Riedl M Webb SM Hana V Nielsen EH Biller BM & Luger A. Pregnancy outcomes in women with growth hormone deficiency. Fertility and Sterility 20151041210–7.e1. ( 10.1016/j.fertnstert.2015.07.1132) [DOI] [PubMed] [Google Scholar]
- 106.Wiren L Boguszewski CL & Johannsson G. Growth hormone (GH) replacement therapy in GH-deficient women during pregnancy. Clinical Endocrinology 200257235–239. ( 10.1046/j.1365-2265.2002.01572.x) [DOI] [PubMed] [Google Scholar]
- 107.Curran AJ Peacey SR & Shalet SM. Is maternal growth hormone essential for a normal pregnancy? European Journal of Endocrinology 199813954–58. ( 10.1530/eje.0.1390054) [DOI] [PubMed] [Google Scholar]
- 108.Giampietro A Milardi D Bianchi A Fusco A Cimino V Valle D Marana R Pontecorvi A & De Marinis L. The effect of treatment with growth hormone on fertility outcome in eugonadal women with growth hormone deficiency: report of four cases and review of the literature. Fertility and Sterility 200991930.e7–930.11. ( 10.1016/j.fertnstert.2008.09.065) [DOI] [PubMed] [Google Scholar]
- 109.Kaur H Muhlhausler BS Roberts CT & Gatford KL. The growth hormone-insulin like growth factor axis in pregnancy. Journal of Endocrinology 2021. ( 10.1530/JOE-21-0087) [DOI] [PubMed] [Google Scholar]
- 110.Margulies SL Corrigan K Bathgate S & Macri C. Addison's disease in pregnancy: case report, management, and review of the literature. Journal of Neonatal-Perinatal Medicine 202013275–278. ( 10.3233/NPM-190231) [DOI] [PubMed] [Google Scholar]
- 111.Berndt V Dahlqvist P de Verdier J Ryberg H & Ragnarsson O. The diagnostic value of salivary cortisol and salivary cortisone in patients with suspected hypercortisolism. Frontiers in Endocrinology (Lausanne) 2022131028804. ( 10.3389/fendo.2022.1028804) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Debono M Caunt S Elder C Fearnside J Lewis J Keevil B Dixon S & Ross R. Real world evidence supports waking salivary cortisone as a screening test for adrenal insufficiency. Clinical Endocrinology 202399517–524. ( 10.1111/cen.14975) [DOI] [PubMed] [Google Scholar]
- 113.Fleseriu M Hashim IA Karavitaki N Melmed S Murad MH Salvatori R & Samuels MH. Hormonal replacement in hypopituitarism in adults: an Endocrine Society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism 20161013888–3921. ( 10.1210/jc.2016-2118) [DOI] [PubMed] [Google Scholar]
- 114.Bjornsdottir S Cnattingius S Brandt L Nordenstrom A Ekbom A Kampe O & Bensing S. Addison's disease in women is a risk factor for an adverse pregnancy outcome. Journal of Clinical Endocrinology and Metabolism 2010955249–5257. ( 10.1210/jc.2010-0108) [DOI] [PubMed] [Google Scholar]
- 115.Kubler K Klingmuller D Gembruch U & Merz WM. High-risk pregnancy management in women with hypopituitarism. Journal of Perinatology 20092989–95. ( 10.1038/jp.2008.116) [DOI] [PubMed] [Google Scholar]
- 116.Schneiderman M Czuzoj-Shulman N Spence AR & Abenhaim HA. Maternal and neonatal outcomes of pregnancies in women with Addison's disease: a population-based cohort study on 7.7 million births. BJOG 20171241772–1779. ( 10.1111/1471-0528.14448) [DOI] [PubMed] [Google Scholar]
- 117.Neufeld M Maclaren N & Blizzard R. Autoimmune polyglandular syndromes. Pediatric Annals 19809154–162. ( 10.3928/0090-4481-19800401-07) [DOI] [PubMed] [Google Scholar]
- 118.Husebye ES Anderson MS & Kampe O. Autoimmune polyendocrine syndromes. New England Journal of Medicine 20183781132–1141. ( 10.1056/NEJMra1713301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Baş F, Toksoy G, Ergun-Longmire B, Uyguner ZO, Abalı ZY, Poyrazoğlu Ş, Karaman V, Avcı Ş, Altunoğlu U, Bundak R.et al.Prevalence, clinical characteristics and long-term outcomes of classical 11 β-hydroxylase deficiency (11BOHD) in Turkish population and novel mutations in CYP11B1 gene. Journal of Steroid Biochemistry and Molecular Biology 201818188–97. ( 10.1016/j.jsbmb.2018.04.001) [DOI] [PubMed] [Google Scholar]
- 120.Sun M, Mueller JW, Gilligan LC, Taylor AE, Shaheen F, Noczyńska A, T'Sjoen G, Denvir L, Shenoy S, Fulton P.et al. The broad phenotypic spectrum of 17α-hydroxylase/17,20-lyase (CYP17A1) deficiency: a case series. European Journal of Endocrinology 2021185729–741. ( 10.1530/eje-21-0152) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhou Y Xue X Shi P Lu Q & Lv S.. Multidisciplinary team management of 46,XY 17alpha-hydroxylase deficiency: a case report and literature review. Journal of International Medical Research 202149300060521993965. ( 10.1177/0300060521993965) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.CA Marsh & Auchus RJ.. Fertility in patients with genetic deficiencies of cytochrome P450c17 (CYP17A1): combined 17-hydroxylase/17,20-lyase deficiency and isolated 17,20-lyase deficiency. Fertility and Sterility 2014101317–322. ( 10.1016/j.fertnstert.2013.11.011) [DOI] [PubMed] [Google Scholar]
- 123.Bose HS, Sugawara T, Strauss JF 3rd, Miller WL. & International Congenital Lipoid Adrenal Hyperplasia Consortium. The pathophysiology and genetics of congenital lipoid adrenal hyperplasia. New England Journal of Medicine 19963351870–1878. ( 10.1056/nejm199612193352503) [DOI] [PubMed] [Google Scholar]
- 124.Khoury K Barbar E Ainmelk Y Ouellet A & Lehoux JG.. Gonadal function, first cases of pregnancy, and child delivery in a woman with lipoid congenital adrenal hyperplasia. Journal of Clinical Endocrinology & Metabolism 2009941333–1337. ( 10.1210/jc.2008-1694) [DOI] [PubMed] [Google Scholar]
- 125.Burckhardt MA Udhane SS Marti N Schnyder I Tapia C Nielsen JE Mullis PE Rajpert-De Meyts E & Flück CE.. Human 3beta-hydroxysteroid dehydrogenase deficiency seems to affect fertility but may not harbor a tumor risk: lesson from an experiment of nature. European Journal of Endocrinology 2015173K1–K12. ( 10.1530/eje-15-0599) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a