Abstract
Objectives
It has not been established whether vitamin D deficiency is associated with anthropometric state; therefore, this systematic review examined the relationship between serum vitamin D levels with anthropometrics and adiposity across different ages.
Methods
Studies that examined vitamin D deficiency with adiposity measures in different age groups were searched in the PubMed, Scopus, Embase, and Google Scholar databases until November 2023. Two investigators independently reviewed titles and abstracts, examined full-text articles, extracted data, and rated the quality in accordance with the Newcastle–Ottawa criteria.
Results
Seventy-two studies, with a total of 59,430 subjects, were included. Of these studies, 27 cross-sectional studies and one longitudinal study (with 25,615 participants) evaluated the possible link between 25(OH)D serum concentrations and anthropometric/adiposity indices in the pediatric population. Forty-two cross-sectional studies and two cohort investigations (with 33,815 participants) investigated the relationship between serum 25(OH)D levels and adiposity measures in adults and/or the elderly population. There is evidence supporting links between vitamin D deficiency and obesity, and revealed an inverse association between vitamin D and adiposity indicators, specifically in female subjects. However, the effects of several confounding factors should also be considered.
Conclusion
Most published studies, most of which were cross-sectional, reported a negative association between vitamin D and female adiposity indicators. Therefore, serum vitamin D levels should be monitored in overweight/obese individuals.
Keywords: vitamin D, 25(OH)D, anthropometric, adiposity
Introduction
Obesity results from excess fat accumulation and a positive energy balance, contributing to various chronic diseases and reduced life expectancy (1). Approximately 650 million adults, roughly 13% of the global adult population, were overweight or obese, with 340 million children and adolescents aged 5–19 years classified as overweight or obese in 2016 (2).
Micronutrient deficiencies, notably hypovitaminosis D, are common in obese patients (3, 4). Extensive observational studies like NHANES III and Framingham have linked obesity to an increased risk of hypovitaminosis D (5, 6). Vitamin D serves various functions, including maintaining calcium homeostasis and bone health, while also influencing metabolic processes, immunity, cellular proliferation, and differentiation, among other effects such as anti-inflammatory, antiatherogenic, cardioprotective, and neuroprotective impacts (7, 8). A global prevalence of widespread vitamin D deficiency has been identified, with deficiency rates rising by 13%, insufficiency rates reaching 40%, and notably higher rates observed in Asian countries (9, 10).
Obesity is commonly linked with reduced vitamin D levels regardless of various factors such as age, gender, season, study region, or smoking status (11). The coexistence of obesity and hypovitaminosis D represents a dual public health concern globally, prompting the need for investigating the underlying pathophysiology of this relationship. Mechanisms contributing to low vitamin D levels in obesity involve volumetric dilution, sequestration into adipose tissue, limited sunlight exposure, and reduced vitamin D synthesis in adipose tissue and the liver (11). Studies have suggested that low vitamin D levels may influence adipose tissue differentiation and growth, impacting obesity through gene expression regulation or by modulating parathyroid hormone (PTH), calcium, and leptin (11, 12, 13). While several observational studies have explored the link between vitamin D status and body weight, comprehensive evaluations of the relationship between serum vitamin D levels and anthropometric and adiposity indicators in both adults and children are lacking.
Hence, in this groundbreaking systematic review, we significantly contribute to the existing literature by taking a comprehensive and inclusive approach to evaluate the intricate relationship between serum 25(OH)D levels and adiposity. Unlike prior studies that primarily focused on specific age groups or relied on limited adiposity measures such as body mass index (BMI) and waist circumference (WC), our research spans diverse age groups and considers a broader set of indicators, including BMI, WC, HC (hip circumference), WHR (waist-to-hip ratio), and body fat mass percentage. Our findings reveal intriguing patterns across the life span, adding a valuable dimension to the understanding of vitamin D deficiency in the context of obesity. This holistic evaluation provides a nuanced perspective on the association between serum 25(OH)D levels and various aspects of adiposity, offering a more comprehensive overview compared to previous reviews. This research is important as it seeks to fill the existing gap in knowledge concerning the intricate association between vitamin D levels and obesity. By conducting a systematic review, the study intends to shed light on the underlying mechanisms and implications of this relationship, thereby contributing to the development of effective public health strategies.
Methods
We conducted a systematic review of studies that assessed the relationship between serum vitamin D levels with anthropometric and adiposity indices in children, adolescents, adults, and the elderly. Serum 25(OH)D was used as a proxy measure for vitamin D levels.
Search strategy
The PubMed, Scopus, Embase, and Google Scholar databases were used to identify relevant publications. Two authors (BA and SA) independently searched papers published until November 2023 using (‘25-hydroxy vitamin D’ OR ‘vitamin D’ OR ‘cholecalciferol’ OR ‘25(OH)D’) AND (‘BMI’ OR ‘body mass index’ OR ‘weight’ OR ‘obese’ OR ‘obesity’ OR ‘waist’ OR ‘waist circumference’ OR ‘adiposity’ OR ‘adipose’, OR ‘fat’) as keywords. No restrictions were imposed on publication time or language. The reference lists of relevant articles were also reviewed by the authors to determine whether any publications were missing. All of the studies included in this systematic review were published in English. Data extraction was done independently by two investigators (BA and MN). In the event of any disagreement, three authors (BA, MN, and FH) discussed it among them to resolve the disagreement. Owing to the differences in the comparisons of the included studies (differences in exposures, outcomes, participants, and settings), diversity of applied statistical tools in the comparisons of the included studies, and lack of data that could be pooled, we performed a qualitative systematic review. The systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2020) Statement (14).
Table 1 shows the PICOS (population, intervention/exposure, comparator, outcome, and setting) items used to conduct the systematic review. Owing to the methodological approach, no ethical approval was required.
Table 1.
PICOS (population, intervention/exposure, comparator, outcome, and setting) criteria used to perform the systematic review.
| PICOS | Criteria |
|---|---|
| Population | Healthy general population across all age groups |
| Intervention/exposure | Serum 25(OH)D |
| Comparator | Statistical tools (OR, HR, RR) |
| Outcome | Anthropometric/adiposity indices |
| Setting | Observational studies |
OR, odds ratios; HR, hazard ratios; RR, relative risk.
Eligibility criteria
Publications with abstracts that suggested vitamin D levels were investigated in relation to anthropometric and adiposity variables were reviewed in full. Studies met the inclusion criteria if they: i) had observational design; (ii) were carried out in apparently healthy individuals (without chronic diseases, such as diabetes, liver diseases, cancer, or chronic kidney disease); and (iii) used serum 25(OH)D levels as a proxy for vitamin D state. However, clinical trials, reviews, editorials, and studies on nonhuman models, were excluded. Sex and age ranges were not strictly defined in this systematic review.
Study selection
Each title and abstract collected during the initial search was independently evaluated by two authors after removing duplicates. To ensure that eligibility and exclusion criteria were met, the two authors assessed full-text articles. The researchers consulted each other whenever they disagreed.
Data extraction and quality assessment
The following information was recorded in a data mining sheet: first author, publication year, country, ethnicity, design of the study, sample size, sex of participants, age, study population, method of 25(OH)D measurements, cut offs for vitamin D status, anthropometric indices investigated in the study and their cut off points, adjustments, and main findings. We assessed the quality of observational studies using the Newcastle–Ottawa Scale (NOS) (15).
Results
Literature search and study selection process
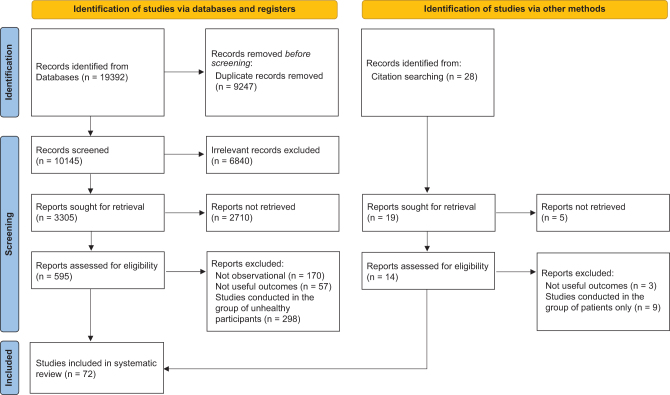
From databases, 19392 studies were initially found. Following the removal of 9247 duplicate articles, 7342 were excluded after scanning the titles/abstracts as they had no relevance to the present systematic review. After careful screening of 2803 full texts, we also excluded 2731 more studies because they evaluated the relationship between serum levels of 25(OH)D with an outcome other than anthropometric/adiposity indices, were clinical trials, animal or in vitro studies in design, editorial, and reviews, or the participants of the studies were unhealthy (with chronic diseases, such as diabetes, cancer, or chronic kidney disease). Ultimately, 72 studies (12, 13, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85) with 59,430 participants in total, published between 1981 and 2023, could be considered for the systematic review. Figure 1 illustrates the flowchart for selecting studies.
Figure 1.
PRISMA flow diagram for the selection process of the studies.
Characteristics of the studies conducted in children/adolescent population
The current systematic review identified 27 cross-sectional studies (16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42) and one longitudinal study (43) that assessed the potential relationship between serum 25(OH)D levels and anthropometric/adiposity indices in children. The studies about the relation between 25(OH)D serum level and anthropometric indices in pediatric were carried out in the following countries: four studies from Turkey, three from Spain, two from Denmark, five from the USA, two from Brazil, four from Italy and eight others from Iran, Korea, China, Thailand, England, Saudi Arabia, Sri Lanka, and Germany. The included studies were conducted between 2008 and 2021. Tgncluded studies had samples ranging from 51 to 12292 in this age group. Participant ages ranged from 3 months to 21 years.
All included studies involved both sexes, except three investigations (26, 39, 40) that were conducted in females (39, 40) and males (26) only. The included studies mostly considered the following potential confounders: sex, age, weight, BMI, cohort characteristics, Tanner stage, fat mass index, body fat mass percentage, BMI Z-score, parental education, pubertal breast stage, physical activity, dietary or supplemental vitamin D and calcium intake, race, ethnicity, season of blood collection, and height measures.
The characteristics of the articles are shown in Table 2.
Table 2.
Characteristics of the studies investigating the association between vitamin D and/or PTH with anthropometric indices in children/adolescents.
| First author (reference number), year | Country, ethnicity | Study design | Sample size | Sex | Age of participants (years) | Study population | Method of 25(OH)D measurement | Cutoffs for vitamin D status | Anthropometric indices investigated in the study and their cutoff points | Adjustments | Main findings | Study quality (NOS) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Küçükali (19), 2021 | Turkey; NM | Cross sectional | 162 (84/78) | F/M | 12–18 | 84 obese children and 78 healthy children | 25(OH)D: Liquid chromatography–tandem mass spectrometry with a commercial kit. PTH: colorimetric method using ready-made kits | Normal: 25(OH)D >20 ng/mL and PTH <65 pg/mL. | Obesity: BMI >95th percentile. Normal: 15th–85th percentiles. | – | Obese children had higher level of PTH. Bioavailable and free vitamin D were lower in the obese group. There was no difference in terms of total vitamin D between groups. | 4 |
| Durá-Travé (18), 2020 | Spain; Caucasian | Cross-sectional | 630 (282/348) | F/M | 7.2–15.2 | 282 sever obese participants and 348 healthy control children | Chemiluminescence immunoassay/two-site chemiluminescent enzyme-labeled immunometric assay in an Immulite analyzer | Normal: 25(OH)D ≥30 ng/mL, Insufficiency: 20–29 ng/mL, deficiency: <20 ng/mL. Hyperparathyroidism: PTH levels >65 pg/mL. | Severe obesity: BMI Z-score >3.0, 99th percentile). Control: Z-score between −1.0 (15th percentile) and +1.0 (85th percentile). | – | Suboptimal vitamin D status and high levels of PTH are a common feature in pediatric with severe obesity. | 5 |
| Geserick (43), 2020 | Germany; NM | Longitudinal | 2733 (317/2416) | F/M | 3 months–17 years | LIFE child study population | Electrochemiluminescence assays | Electrochemiluminescence assays | Weight and height. | – | Obese children had lower SDS for 25(OH)D and higher SDS for PTH than the control cohort. | 7 |
| Gün (17), 2020 | Turkey; – | Cross-sectional | 150 (92/58) | F/M | 5–17 | 92 obese children and 58 healthy controls without chronic diseases | 25(OH)D was measured using commercial kit | Severely lacking: 25(OH)D <5 ng/mL, <15 ng/mL as lacking, 15−20 ng/mL as deficiency. | Normal weight at BMI ≤85th percentile, overweight at 85th–95th percentile, and obese at ≥95th percentile. | – | The prevalence of vitamin D deficiency was higher in obese children compared to normal-weight and overweight children. | 4 |
| Durá-Travé (20), 2020 | Spain; Caucasian | Cross-sectional | 236 adolescents with severe obesity (BMI z-score > 3.0, 99th percentile) | F/M | 10.2–15.8 | Adolescents with severe obesity | 25(OH)D was measured by a high-specific chemiluminescence immunoassay/and PTH assessed by a highly specific solid-phase, two-site chemiluminescent enzyme-labeled immunometric assay | Normal: 25(OH)D ≥30 ng/mL, Insufficiency: 20–29 ng/mL, deficiency: < 20 ng/mL. | BMI, body composition, WC, WHR. | – | Subjects with vitamin D deficiency had significantly elevated values for BMI Z-score, WC, waist Z-score, body fat percentage, fat mass index, and PTH than those with normal vitamin D status. | 5 |
| Adikaram (21), 2019 | Sri Lanka; – | Cross-sectional | 202 | F/M | 5–15 | Children with BMI >2 SDS above the median for age and sex based on WHO | Immunoassays | Deficiency: 25(OH)D <20 ng/mL, Insufficiency: 25(OH)D: 20 –29 ng/mL. PTH >65 pg/mL. | Above +2 SDS for WC of relevant references and percentage fat mass >28.6 in boys and >33.7% in girls were considered as high. | – | Vitamin D deficiency was significantly high in children with obesity and showed negative correlations with indicators of adiposity. | 5 |
| Plesner (22), 2017 | Denmark; North European White | Cross-sectional | 3627 (1484/2143) | F/M | 6–18 | Children and adolescents with overweight/obesity and 2143 population-based controls | Electrochemiluminescence binding assay/ Electrochemiluminescent immunoassay | Normal: 25(OH)D ≥50 nmol/L), insufficiency: <50 nmol ≥ 30 nmol/L, Deficiency:<30 nmol/L. | Obese: BMI-SDS ≥2.33 or above the 99th percentile, overweight: BMI-SDS ≥1.28 < 2.33), lean: BMI-SDS <1.28 or below the 90th percentile. | – | Vitamin D deficiency was common among children and adolescents with obesity. The degree of obesity was independently associated with lower serum 25(OH)D concentrations. | 7 |
| Giudici (23), 2017 | Brazil; NM | Cross-sectional | 198 | F/M | 14–18 | Adolescents who were participated in the Health Survey–Sao Paulo study | High-performance liquid chromatography | Deficiency: 25(OH)D < 20 ng/mL, Insufficiency: 25(OH)D: 20 –29 ng/mL. | Weight, WC, hip circumference were measured. Weight status was determined according to BMI-for-age growth charts. Normal weight: between the 3rd and 85th percentiles; overweight: the 85th and 97th percentiles; obese: above 97th percentile. | – | 25(OH)D was lower in overweight and obese adolescents. | 4 |
| Alemzadeh (24), 2016 | USA; Caucasian, Hispanic, African American | Cross-sectional | 152 | F/M | 13.2–17.8 | Obese adolescents (BMI >95th percentile for age) | Nichols RIA/Nichols immunochemiluminometric assay | Normal: 25(OH)D ≥75 nM), insufficiency: 50–74.9 nM, Deficiency:<50 nmol/L. | BMI >95th percentile for age, body composition. | – | Hypovitaminosis D and vitamin D–deficient groups had higher BMI, fat mass, and iPTH, than vitamin D-sufficient group. Fat mass was negatively correlated with 25(OH)D (r = −0.40, P < 0.0001), it was positively correlated with iPTH (r = 0.46, P < 0.0001). | 4 |
| Saber (25), 2015 | Saudi Arabia; NM | Cross-sectional | 96 (60/36) | F/M | 3–13 | Healthy, overweight and obese children | Chemiluminescence immunoassay technology | – | Underweight, normal weight and overweight were defined as BMI below the 10th, between 10th and 85th and above the 85th percentile, respectively, according to WHO standards. | – | In obese children PTH level was significantly higher and 25(OH)D was lower than controls. 25(OH)D was negatively correlated with PTH and BMI. PTH was not positively correlated with BMI percentile. | 4 |
| Di Nisio (26), 2015 | Italy; NM | Cross-sectional | 108 | M | 11–14 | Boys were recruited in the Operative Unit of Medicine, District of Salerno, section of Sapri (SA, Italy) | Competitive chemiluminescent immunoassay/sandwich-type chemiluminescent immunoassay | Normal: 25(OH)D ≥30 ng/mL, Insufficiency: 20–29 ng/mL, deficiency: ≤ 20 ng/mL. | Normal weight, overweight, and severe obesity defined as CDC-BMI <85th percentile and ≥85th percentile, and ≥95th percentile. | Weight, cohort characteristics, Tanner stage | Lean and overweight groups had similar mean 25(OH)D levels and were below the sufficiency threshold. A considerable fraction of subjects featured by 25(OH)D insufficiency or deficiency was observed in both normal weight and overweight–obese groups. | 4 |
| Petersen (27), 2015 | Denmark; Danish | Cross-sectional | 782 | F/M | 8–11 | Date from the Optimal well-being, development and health for Danish children | Automated chemiluminescent immunoassay/chemiluminescent immunoassay on ADVIA Centaur XP | Vitamin D deficiency and insufficiency defined as serum 25(OH)D concentration <25 and ≤50 nmol/L, respectively. | Height, weight, BMI, WC, body composition. | FMI, BMI Z-score, parental education | Serum 25(OH)D was negatively associated with BMI Z-scores and FMI. The association with BMI became nonsignificant after adjustment. But the negative association with FMI remained after adjustment. | 5 |
| Reesukumal (28), 2015 | Thailand; Thai | Cross-sectional | 159 | F/M | 6–12 | Healthy children | Electrochemiluminescence immunoassay on Elecsys 2010 analyzers | Sufficiency: 25(OH)D level ≥75 nmol/L; hypovitaminosis D: 25(OH)D level <75 nmol/L; insufficiency: 25(OH)D level 50–74.9 nmol/L; vitamin D deficiency: 25(OH)D level <50 nmol/L. | BMI percentile groups (< 85th vs ≥ 85th percentile). | – | Children with hypovitaminosis D had a higher mean BMI percentile than the vitamin D-sufficient group. PTH levels in the children with hypovitaminosis D were significantly higher than in the children with normal levels of vitamin D. | 4 |
| Rusconi (29), 2015 | Italy; Caucasia, Africans, Asians, others | Cross-sectional | 120 | F/M | 10.2 ± 2.8 | Obese children with different values of vitamin D (25(OH)D <20 ng/mL (group Ι) and 25(OH)D >20 ng/mL (group ΙΙ)) | – | 25(OH)D <20 ng/mL (group Ι) and 25(OH)D > 20 ng/mL (group ΙΙ). | Weight, height, body composition. | – | The two groups were similar for BMI SDS and fat mass SDS, while showed differences for PTH. | 4 |
| Vierucci (30), 2014 | Italy; Italian | Cross-sectional | 427 | F/M | 10–21 | Healthy adolescents | Radioimmunoassay/immunoradiometric assay | Vitamin D deficiency < 20; insufficiency 20–30 ng/mL; sufficiency ≥ 30 ng/mL. PTH ≥65.0 ng/L defined as hyperparathyroidism. | Weight status was categorized in normal, overweight and obese according to criteria for subjects <18 years and according to the WHO for subjects aged 18–21 years. | – | Increased risk of hypovitaminosis D in overweight-obese adolescents compared to subjects with normal BMI, was shown. 25(OH)D levels were inversely related to PTH and BMI-SDS. | 5 |
| Chung (31), 2014 | Korea; Korean | Cross-sectional | 1212 | F/M | 4–15 | Children who visited Bundang CHA Medical Center for checkup of their health and growth status | Chemiluminescence immunoassay/electrochemiluminescence immunoassay | Deficiency: 25(OH)D, <20 ng/mL; insufficiency: 25(OH)D, 20–30 ng/mL, and sufficiency 25(OH)D, ≥30 ng/mL. | Normal weight defined by BMI 3rd to 84th percentile; overweight was defined by BMI ≥85th percentile for age and sex based on Korean standard growth curve. | – | The level of 25(OH)D was significantly lower in overweight group than in normal weight group. The PTH levels were significantly higher in vitamin D deficient group compared to vitamin D insufficiency and sufficiency group. | 6 |
| Vierucci (32), 2013 | Italy; Italian | Cross-sectional | 652 | F/M | Children (2.0–10.9 years) and adolescents (11.0–21.0 years) | Healthy children and adolescents | Radioimmunoassay/immunoradiometric assay | Vitamin D deficiency < 20; insufficiency 20–30 ng/mL; sufficiency ≥30 ng/mL, PTH ≥65.0 ng/L defined as hyperparathyroidism. | Weight status was categorized in normal, overweight and obese according to criteria for subjects <18 years and according to the WHO for subjects aged 18–21 years. | – | Significant increased risk of hypovitaminosis D in overweight and obese subjects compared to individuals with normal BMI was observed. But weight status did not affect PTH levels. | 5 |
| Oliveira (33), 2013 | Brazil; Brazilian | Cross-sectional | 160 (83/77) | F/M | 15–17 | Healthy adolescents | RIA kit/chemiluminescence immunoassay | Vitamin D deficiency and insufficiency were ≤10 ng/mL and between 10–30 ng/mL, respectively. The intact PTH level between 15 and 65 pg/mL being considered normal. | Weight, height, WC, %BF were measured. Nutritional status was assessed by BMI according the WHO recommendations. Excessive weight was defined as BMI above 1 SD. Abdominal adiposity was estimated using cutoff points corresponding to the 90th centiles. | – | Serum 25(OH)D levels were statistically lower in adolescents with weight excess, abdominal obesity, and higher levels of PTH. | 4 |
| Turer (37), 2013 | USA; White, African American, Latino, others | Cross-sectional | 12292 | F/M | 6–18 | Children who were enrolled in the 2003–2006 National Health and Nutrition Examination Survey | Radioimmunoassay | Deficiency: <20 ng/mL, insufficiency: 20–30 ng/mL | Height, weight, BMI. | Race, age, sex, season, TV use, PA, vitamin D, and milk intake | Vitamin D deficiency is highly prevalent in overweight and obese children. | 7 |
| Alemzadeh (35), 2012 | USA; Caucasian, Hispanic, African American | Cross-sectional | 133 | F/M | 13.1–17.9 | Obese adolescents | Nichols RIA/Nichols immunochemiluminometric assay | – | Age-adjusted BMI ≥95th percentile. | – | Fat mass was negatively correlated with 25(OH)D, but was positively correlated with PTH. | 4 |
| Buyukinan (36), 2012 | Turkey; Turkish | Cross-sectional | 106 | F/M | 8–16 | Children and adolescents with BMI ≥95th percentile | Electrochemiluminescence | Normal: 25(OH)D ≥30 ng/mL, insufficiency: 20–30, deficiency: < 20 ng/mL. PTH >65 pg/mL was hyperparathyroidism. | Height, weight, BMI, WC. Obesity was defined as BMI ≥ 95th. | – | No significant difference was found between the groups with different vitamin D levels in terms of weight SDS, height SDS, BMI SDS, and WC SDS. | 4 |
| Codoñer-Franch (34), 2012 | Spain; Caucasian | Cross-sectional | 105 (66/39) | F/M | 7–14 | Obese children | Electrochemiluminescence immunoassays | Vitamin D sufficient: >20 ng/m) or insufficient <20 ng/mL. | Weight, height, WC, and body composition were measured. Obese defined ad SDS-BMI ≥ 2), extremely obese: SDS-BMI >4. | Sex, age, and Tanner stage | Obese children had a significantly lower 25(OH)D and a higher iPTH than nonobese children. Insufficient serum levels of 25(OH)D were detected in 5% of normal children and in 30% of the obese children. | 4 |
| Razzaghy-Azar (38), 2010 | Iran; NM | Cross-sectional | 313 | F/M | 8–18 | Healthy children and adolescents who came to a routine growth monitoring clinic of the university hospital | Radioimmunoassay/immunoradiometric | Severely deficient <12.5 nmol/L; deficient ≥12.5 and <25 nmol/L; insufficient ≥25 and <50 nmol/L; sufficient, ≥50 nmol/L and ≤250 nmol/L and toxic >250 nmol/L. | Height, weight, BMI. | – | The level of 25(OH)D had a negative correlation with BMI-SDS and height-SDS in females, but these correlations were not significant in males. | 5 |
| Foo (39), 2009 | China; Chinese | Cross-sectional | 323 | F | 15.0 ± 0.4 | Apparently healthy adolescent girls | I-radioimmunoassay/immunometric assay | – | Height, weight, BMI, body composition. | Pubertal breast stage, physical activity, dietary calcium and vitamin D intake | A significant positive correlation was found between lean body mass and plasma 25(OH)D. No significant correlation was found between the %BF and vitamin D status. | 5 |
| Ashraf (40), 2009 | England; African-American | Cross-sectional | 51 | F | 14 ± 2 | Obese adolescent girls | Liquid chromatography–tandem mass spectrometry (LC-MS/MS)/two-site immunoradiometric assay | Vitamin D deficiency cutoff: <20 ng/mL | Height, weight, BMI. Obesity was defined as BMI ≥95th. | BMI | It was shown a trend toward a significant negative relationship between 25(OH)D and BMI, PTH. Level of PTH was significantly associated with BMI. | 4 |
| Lenders (41), 2009 | USA; Black or African American, Hispanic, Summer, North of Atlanta | Cross-sectional | 58 | F/M | 13.0–17.9 | Healthy obese adolescents | In-house competitive protein binding assay/enzyme immunoassay | Vitamin D deficiency was defined as 25(OH)D concentration <20 ng/mL. | Height, weight, BMI, body composition. | Age, sex, race, season, vitamin D intake, height measures, Tanner stage | 25(OH)D decreased by 0.46 ± 0.22 ng/mL per 1% increment in body fat mass, whereas PTH decreased by 0.78 ± 0.29 pg/mL per 1% increment in visceral adipose tissue. | 4 |
| Alemzadeh (42), 2008 | USA; Caucasian, Hispanic, African American | Cross-sectional | 127 | F/M | 6.0–17.9 | Obese children and adolescents | Nichols radioimmunoassay/Nichols immunochemiluminometric assay | Hypovitaminosis D, 25(OH)D <75 nmol/L; sufficiency, 25(OH)D ≥75 nmol/L; deficiency, 25(OH)D level <50 nmol/L; insufficiency, 25(OH)D of 50–74.9 nmol/L. | Height, weight, BMI, body composition. Obesity was defined as BMI > 95th percentile for age. | Age, sex, ethnicity, season | Hypovitaminosis D and vitamin D–deficient groups had higher BMI, fat mass, and iPTH, than vitamin D-sufficient group. Fat mass was negatively correlated with 25(OH)D, it was positively correlated with iPTH. | 4 |
| Çizmecioğlu (16), 2008 | Turkey; Turkish | Cross-sectional | 301 | F/M | 11–19 | Secondary and high school children | Competitive protein binding assay/chemiluminescence with an Immulite One analyzer | Deficiency: 25(OH)D <10 ng/mL; insufficiency as levels of 25(OH)D between 10 and 20 ng/mL, and a normal vitamin D level as >20 ng/mL. | Height, weight, BMI. | – | It was shown a negative correlation between serum vitamin D level and BMI in obese and overweight subjects whose vitamin D level was below 20 ng/mL. | 4 |
BMI, body mass index; F/M, female/male; FMI, fat mass index; iPTH, intact parathyroid hormone; NOS, Newcastle–Ottawa Scale; PA, physical activity; PTH, parathyroid hormone; SDS, standard deviation score; WC, waist circumference; WHO, World Health Organization.
Characteristics of the studies conducted in adult and/or elderly population
Our systematic search identified 42 cross-sectional studies (12, 13, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 79, 83, 84, 85) and two cohort investigations (60, 78) (with 33,815 participants) that investigated the potential relationship between 25(OH)D serum concentrations and anthropometric/adiposity indices in adults and/or elderly population. The studies about the link between 25(OH)D serum level and anthropometric indices in adults and/or elderly population were carried out in the following countries: 9 studies from USA, 3 from UK, 3 from Spain, 2 from Turkey, 4 from Saudi Arabia, 2 from Iran, 2 from India, 2 from Italy, 2 from Germany, and 18 others from China, UAE, Pakistan, Australia, Finland, England, Portugal, Malaysia, Bulgaria, Austria, Canada, Denmark, Greece, New Zealand, and the Netherlands. The included studies were conducted between 1981 and 2023. The included studies had samples ranging from 26 to 3113 in this age group. The age of participants was >18 years old. Among the included studies, 27 articles (44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 84, 85) involved both sexes, and 17 investigations conducted in females (12, 13, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83) and males (69, 70, 71) only. The included studies mostly considered the following potential confounders: age, sex, season of blood sampling, smoking, vitamin D status, body fat mess, weight, height, BMI, IGF-1, PTH, UVB, alcohol, tobacco, sports, use of multivitamin supplements, menopausal status/HRT use, physical activity, socioeconomic status, income, job, obesity, education level, lifestyle, sun exposure, lean mass, nutrient intake, residence type, race, ethnicity, serum calcium, magnesium and phosphate, GFR, 25(OH)D, CRP, WC, month of blood collection, marital status, district, area, latitude, phosphorus, serum alanine aminotransferase, creatinine, and IL-6.
The characteristics of the articles are shown in Table 3.
Table 3.
Characteristics of the studies investigating the association between vitamin D and/or PTH with anthropometric indices in adult and/or elderly population.
| First author (reference number), year | Country, ethnicity | Study design | Sample size | Sex | Age of participants (years) | Study population | Method of 25(OH)D measurement | Cutoffs for vitamin D status | Anthropometric indices investigated in the study and their cutoff points | Adjustments | Main findings | Study quality (NOS) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tejada‑Romero (12), 2022 | Spain; NM | Cross-sectional | 679 (493/186) | F | 60.6 ± 13.6 | Healthy postmenopausal women | Immunochemical luminescence/immunochemical luminescence | – | Weight, height, BMI. Obesity was defined as a BMI ≥30. | Age | Obese women had lower levels of 25(OH)D and higher PTH than nonobese. | 6 |
| Shan (13), 2022 | China; Chinese | Cross-sectional | 1505 | F | 30.04 (23.98–37.81) | Women of childbearing age | Liquid chromatography–tandem mass spectroscopy/electrochemiluminescence immunoassay | – | Height, weight, WC, BMI. | Season, district, area, latitude, age, BMI, PTH, P, ALT, CRE, IL-6, hs-CRP | Insufficient 25(OH)D was significantly related to the risk of elevated WC after adjusting for confounders. | 7 |
| Gariballa (48), 2022 | UAE; NM | Cross-sectional | 648 | F/M | ≥18 | Community free living adults | Electrochemiluminescence | Deficiency:< 20 ng/mL; insufficiency: 20–32 ng/mL; sufficient: >32 ng/mL | Height, weight, BMI. | – | There was no difference in BMI between groups, but, it was higher in vitamin D deficient subjects aged ≥50 and females <50 years. | 6 |
| Saleem (72), 2021 | Pakistan; NM | Cross-sectional | 397 (264/133) | F | 20–50 | Healthy women with normal fasting glucose | Radioimmunoassay/spectrophotometer | Deficient: serum vitamin D <12 ng/mL; sufficient: vitamin D >30 ng/mL | Weight, height, BMI, WC, and HC. Obesity was defined as BMI ≥30 kg/m2. | – | PTH levels were negatively correlated, though nonsignificantly with vitamin D. The mean BMI, WC, HC, WHR, and PTH were not significantly different in vitamin D-deficient as compared to nondeficient obese women. | 4 |
| Djafari (49), 2021 | Iran; NM | Cross-sectional | 178 | F/M | 60–83 | Elderly individuals | Enzymatic method using commercial kits | – | Weight, height, BMI, WC, HC, and fat mass. | Age, sex, income, PA, job, smoking, vitamin D and calcium supplement use | 25(OH)D was higher in the highest category of BAI compared to the lowest one. An inverse association between BAI with 25(OH)D was reported. No significant association between the %BF with PTH and 25(OH)D was observed. | 4 |
| Menon (69), 2021 | India; Indian | Cross-sectional | 224 | M | 20.74 ± 1.43 | Healthy military training | Chemiluminescence analyzer | Sufficient: vitamin D ≥30 ng/mL; insufficient: 20–30 ng/mL; deficient: ≤20 ng/mL | Height, weight, BMI, body composition. | – | There was no correlation of 25(OH) cholecalciferol with BMI or FMI. | 4 |
| Yaylali (73), 2021 | Turkey; NM | Cross-sectional | 126 (73/53) | F | 17–55 | Healthy premenopausal women | High performance liquid chromatography/electrochemiluminescence immunoassay | – | Height, weight, WC, BMI, body composition. Obesity was defined as BMI >30 kg/m2. | – | A positive association was observed between PTH and visceral fat. Vitamin D levels were inversely associated with visceral fat. | 4 |
| Sharma (74), 2020 | Australia; NM | Cross-sectional | 76 (50/26) | F | 61.9–68.5 | Healthy normal and overweight postmenopausal women | Chemiluminescent immunoassays | – | Weight, height, BMI, WC, HC, and body composition. Normal:BMI <25 kg/m2, and overweight: BMI >27 kg/m2. | – | Women in the highest visceral adipose tissue quartile had significantly lower 25(OH)D. | 4 |
| Albassam (75), 2019 | Saudi Arabia; NM | Cross-sectional | 265 (179/86) | F | 18–70 | Middle-aged Saudi women | Electrochemiluminescence immunoassay/Milliplex MAP Human Bone Magnetic Bead Panel | – | Weight, height, BMI, WC, HC, NC, body composition. | Age and menopausal status | NC was inversely associated with 25(OH)D and PTH. In the nonobese, WHR was inversely associated with PTH. | 5 |
| Saarnio (50), 2018 | Finland; Caucasian | Cross-sectional | 595 | F/M | 37–47 | Healthy men and women | IDS enzyme immunoassay kit/immunoluminescence-based method by Immulite1000 | – | Weight, height, BMI. | Age, physical activity, smoking | In women, 25(OH)D levels did not differ among the BMI groups. In men, 25(OH)D was lower in obese men than in normal-weight. Altogether, obese subjects had lower 25(OH)D than normal-weight. In women, PTH was significantly higher in obese compared to normal weight Altogether, the difference was significant. | 5 |
| Kord‑Varkaneh (51), 2018 | Iran; NM | Cross-sectional | 178 | F/M | 60–83 | Elderly subjects | Enzymatic method | – | Weight, height, BMI, WC. | Age, sex, PA, smoking, marital, supplement use | No significant correlation between 25(OH)D with BMI and WC. | 4 |
| Raposo (52), 2017 | Portugal; NM | Cross-sectional | 500 | F/M | 53 (41–67) | Adults registered in primary health-care centers | Chemiluminescent immunoassay/electrochemiluminescent immunoassay | Deficiency <12 ng/mL; inadequacy ≥12 and <20 ng/mL; sufficiency ≥20 ng/mL | Weight, height, WC, HC. Participants were classified into: underweight (BMI <18.5 kg/m2), normal range (≥18.5 to <25), pre-obese (≥25 to <30 kg/m2) and obese (≥30 kg/m2) categories. | Age and sex | The serum 25(OH)D levels were negatively associated with BMI. Positive associations between PTH with BMI and WC were found. | 5 |
| Trevisan (76), 2017 | Italy, Caucasian | Cross-sectional | 218 | F | ≥65 | Healthy older women | Radioimmunoassay/two-step immunoradiometric assay | 25(OH)D level <50 nmol/L was considered inadequate; PTH value >60 pg/mL defined hyperparathyroidism | Weight, height, BMI, body composition. | Age, 25(OH)D, PTH, month of blood collection | Fat mass showed a significant negative association with 25(OH)D. Binary logistic analysis revealed a protective effect of adiposity on secondary hyperparathyroidism. | 4 |
| Walsh (53), 2016 | UK, Caucasian | Cross-sectional | 233 | F/M | 25–40 and 55–75 | Healthy men and women in different weight groups | Immunoassay/direct measurement by immunoassay | NM | Weight, height, BMI. | Age, sex | Serum 25(OH)D was inversely correlated with BMI. PTH did not differ by BMI group. | 4 |
| Shafinaz (54), 2016 | Malaysia; Malays, Chinese, Indians, others | Cross-sectional | 858 | F/M | <30 to >50 | Permanent teachers who worked in the selected government secondary schools | Electro chemiluminescence immunoassay | Serum 25(OH)D <20 ng/mL was considered as vitamin D deficient | Weight, height, BMI, WC, body composition. Underweight: BMI <17.5 kg/m2; normal weight: 17.5–22.9 kg/m2; overweight: BMI 23.0–27.9 kg/m2; obese: BMI ≥ 28.0 kg/m2. | Age, sex, ethnicity, sun avoidance score, SBP, BMI, WC, %BF | A significant negative association between serum 25(OH)D with BMI and %BF was observed. Higher BMI and larger WC were significantly associated with lower serum 25(OH)D level. | 6 |
| Al-Daghri (55), 2015 | Saudi Arabia; NM | Cross-sectional | 830 | F/M | 18–50 | Apparently healthy individuals | ELISA | Sufficient vitamin D >50 nmol/L, insufficient: 25–50 nmol/L and deficient: <25 nmol/L | Weight, height, BMI, WC, HC, arm circumference. | Age, BMI, glucose, cholesterol, TG, LDL, HDL | Vitamin D insufficiency was significantly associated with abdominal obesity in males. | 6 |
| Wright (56), 2015 | India; Caucasian, Black, Asian, Hispanic | Cross-sectional | 336 | F/M | 35–65 | Middle-aged overweight and obese healthy adults | Radioimmunoassays | – | Weight, height, BMI, WC, HC, body composition. | Age, sex, race, season, supplement use, PTH | Total and central adiposity but not peripheral adiposity predicted low plasma 25(OH)D. | 4 |
| George (57), 2015 | USA; Caucasian | Cross-sectional | 714 | F/M | 18–65 | Healthy adults | High-performance liquid chromatography (HPLC) kit/chemiluminescent assay | – | Weight, height, BMI, WC, body composition. | Age, sex, height, ethnicity, serum calcium, magnesium and phosphate, GFR, smoking | 25(OH)D was not associated significantly with BMI and WC. | 6 |
| Tosunbayraktar (58), 2015 | Turkey; NM | Cross-sectional | 90 | F/M | 18–63 | Healthy individuals with various BMIs | – | Deficiency: 25(OH)D <20 ng/mL; and ≥20 ng/mL as sufficiency | Weight, height, BMI, WC, HC, body composition. | – | Overweight and obese groups had higher PTH and lower 25(OH)D levels than normal weight group. The overweight group had higher 25(OH)D and lower PTH than obese group. | 4 |
| Sorkin (77), 2014 | USA; white and black | Cross-sectional | 239 | F | 46–78 | Sedentary postmenopausal women without diabetes | RIA/immunoradiometric assay | – | – | Race, age, and time | 25(OH)D was inversely related to visceral abdominal fat, %BF, and PTH. | 4 |
| Shinkov (59), 2014 | Bulgaria; NM | Cross-sectional | 1952 | F/M | F: 49.5 ± 14.2 M: 47.2 ± 14.1 | Healthy adult population group | Liquid chromatography–tandem mass spectrometry/chemiluminescence | Sufficient vitamin D >50 nmol/L, insufficient: 25–50 nmol/L and deficient: <25 nmol/L | Weight, height, BMI. | Sex, age, education, residence type, BMI and smoking | 25(OH)D levels were significantly lower in obese females than in the normal weight females. In the males, the 25(OH)D levels did not differ among the BMI groups. The increase in BMI by 1 kg/m2 was associated with an increase in the prevalence of vitamin D deficiency in the young females by 1%. | 7 |
| Shapses (78), 2013 | USA; NM | Retrospective | 383 | F | 24–75 | Premenopausal women with regular menstruating and postmenopausal women were at least 2 years since their last menstruation | RIA | – | Weight, height, BMI, body composition. | – | Higher serum levels of PTH and lower 25(OH)D in the obese subjects compared to leaner subjects were observed. | 5 |
| González-Molero (60), 2013 | Spain; NM | Prospective | 961 | F/M | 18–68 | Participants in Pizarra cohort study | Electrochemiluminescence | Deficiency: 25(OH)D ≤20 ng/mL | Weight, height, BMI, WC, HC. | Age, sex, season, iPTH, and presence of diabetes | Neither obesity at baseline nor the development of obesity were significantly associated with vitamin D status. In nonobese subjects 25(OH)D ≤17 ng/mL was associated with an increased risk of developing obesity in the next 4 years. | 5 |
| Jungert (61), 2012 | Germany; NM | Cross-sectional | 131 | F/M | 66–96 | Independently living participants of the longitudinal study on nutrition and health status. | Direct electrochemiluminescence immunoassay | 25(OH)D <25.0 nmol/L as deficient, 25.0–49.9 nmol/L as insufficient and ≥50.0 nmol/L as adequate | Weight, height, BMI, WC, HC, body composition. | Age, lifestyle, iPTH | 25(OH)D3 was inversely associated with BMI, HC, and BF in women. Total BF was a negative predictor of 25(OH)D3 in women even after controlling for confounders, whereas the associations between BMI, HC, and 25(OH)D3 lost statistical significance after adjusting for iPTH. | 4 |
| Ardawi (70), 2012 | Saudi Arabia; NM | Cross-sectional | 834 | M | 20–74 | Men were recruited at random during a health survey from 40 primary health-care centers | Competitive chemiluminescence immunoassays/electrochemiluminescent assay | Deficiency: 25(OH)D <50 nmol/L | Weight, height, BMI, WC, HC. BMI ≥25–<30 kg/m2), and an obese group (BMI ≥30 kg/m2). | Age, obesity, education, season, lifestyle, sun exposure | Vitamin D deficiency was common among older and obese men with no education and sedentary lifestyle sampled during summer and spring. | 6 |
| Puntus (62), 2011 | Austria; NM | Cross-sectional | 1009 | F/M | 25–76 | Healthy adults | Enzyme-based protein binding assay/immunoradiometric assay | – | Weight, height, BMI. BMI 18.5–25 kg/m2 (normal), BMI 25–30 kg/m2 (overweight), BMI >30 kg/m2 (obese). | Age | Increasing BMI was associated with a significant fall of 25(OH)D in pre- and postmenopausal women, but with a significant rise in PTH in women before menopause. | 7 |
| Sukumar (79), 2011 | USA; Caucasian | Cross-sectional | 211 | F | 25–71 | Normal weight and overweight/obese women | Radioimmunoassay | – | Weight, height, BMI. Body composition. Normal-weight: BMI <25, overweight and obese-class I: BMI 25–35, obese-class II–III: BMI >35. | Lean mass, PA, nutrient intake | Higher BMI was associated with greater levels of PTH, but lower 25(OH)D. | 4 |
| Ardawi (80), 2011 | Saudi Arabia; NM | Cross-sectional | 1172 | F | 50.9 ± 12.6 | Women were recruited at random during a health survey from 40 primary health-care centers | Direct competitive chemiluminescence immunoassays/direct sandwich chemiluminescence immunoassays | 25(OH)D ≤75 nmol/L as deficiency/insufficiency; >75 nmol/L as sufficient; hyperparathyroidism: iPTH >7.0 pmol/L | Weight, height, BMI, WC, HC. BMI ≥25–<30 kg/m2), and an obese group (BMI ≥30 kg/m2). | Age, obesity, education, season, lifestyle, sun exposure | Serum 25(OH)D was lower and intact PTH higher in the upper quintiles of BMI and WHR. | 7 |
| Hayek (63), 2011 | Canada; NM | Cross-sectional | 2168 | F/M | >18 years | Inuit adults participated in the International Polar Year Inuit Health Survey | Chemiluminesent assays | Serum 25(OH)D was compared to different cutoffs: >75, >50 nmol/L, 75–37.5 nmol/L, and <37.5 nmol/L | Weight, height, BMI, WC, Body composition. For men, a healthy WC was defined as <102 cm and for women <88 cm. | – | Healthy WC was a significant predictor of better 25(OH)D level in adults. | 7 |
| Frost (71), 2010 | Denmark; Caucasian | Cross-sectional | 783 | M | 20–29 | Young men | Radioimmunoassay/nonimmunoflourometric assay | Deficiency: 25(OH)D <50 nm; insufficiency 25(OH)D <75 nm. | Weight, height, BMI, WHR, Body composition. | Age, alcohol, tobacco, sports, use of multivitamin supplements, and season, BMI | An inverse relationship between 25(OH)D and BMI was found in participants with a high BMI only, and increase in BMI of 1 kg/m2 corresponded to a decrease in 25(OH)D of 1.7 nm. PTH was inversely associated with BFM in vitamin D-insufficient subjects. | 6 |
| Valiña-Tóth (64), 2010 | USA; African American | Cross-sectional | 98 | F/M | ≥35 years | Healthy, overweight, adult | Liquid chromatography–tandem spectrometry/electrochemiluminescence method | Deficiency: 25(OH)D ≤50 nmol/L; insufficiency: 51-74 nmol/L; optimal: ≥75 nmol/l | Weight, height, BMI, WHR, Body composition. | Age, sex and seasons | PTH was directly correlated with total, truncal and extremity FM, while 25(OH)D was related inversely to truncal FM. | 4 |
| Muscogiuri (65), 2010 | Italy; NM | Cross-sectional | 39 | F/M | 41.4 ± 12.4 | Subjects with no known history of diabetes mellitus | Chemiluminescence immunoassay radioimmunoassay/ enzyme chemiluminescence immunoassay | – | Weight, height, BMI. | – | There was a correlation between 25(OH)D and BMI (r = −0.58; P = 0.01), and PTH (r = −0.44; P < 0.01). BMI was the most powerful predictor of 25(OH)D level. | 4 |
| Moschonis (81), 2009 | Greece; NM | Cross-sectional | 112 | F | 60.3 ± 5.0 | Postmenopausal healthy women | Chemiluminescence immunoassays | – | Weight, height, BMI, WC, Body composition. | Age, UVB, PTH, and IGF-1, PA | No significant associations were observed between anthropometric indices of body mass and serum 25(OH)D levels | 4 |
| Rueda (66), 2008 | Spain; NM | Cross-sectional | 298 | F/M | 42.9±10.6 | Severely obese patients | Radioimmunoassay/chemiluminescence immunoassay | Insufficient: 25(OH)D < 20 pg/mL; PTH reference range: 10–65 pg/mL | Weight, height, BMI, Body composition. | Age, sex, BMI, %FM, season | Insufficient 25(OH)D was associated with higher BMI. Elevated PTH levels were linked to higher BMI and higher FM percentage. | 4 |
| Macdonald (82), 2008 | UK; Caucasian | Cross-sectional from Aberdeen Prospective Osteoporosis Screening Study | 3113 | F | 54.8 (2.3) | Postmenopausal women | HPLC | 25(OH)D ≥28 ng/mL with those <28 ng/mL | Weight, height, BMI. | Age, weight, height, menopausal status/HRT use, physical activity and socioeconomic status | 25(OH)D was lower and PTH higher in the top quintile of BMI. | 7 |
| Bolland (83), 2006 | New Zealand; NM | Cross-sectional | 116 | F | 62.6 ± 5.9 | Healthy community-dwelling postmenopausal women | Radioimmunoassay/Allegro assay | Vitamin D insufficiency; 25(OH)D <50 nmol/L | Weight, height, BMI, body composition. | Vitamin D insufficiency, body fat mess | PTH was positively correlated with weight, regional and total fat mass, and %BF, and negatively correlated with 25(OH)D. On multivariate analysis, PTH was positively related to %BF. For 25(OH)D, there were negative correlations with PTH, total fat mass, trunk fat, and pelvic fat. On multivariate analysis, 25(OH)D was negatively related to pelvic fat mass. | 4 |
| Snijder (67), 2005 | Netherlands; NM | Cross-sectional | 453 | F/M | ≥66 | Older men and women | Competitive binding protein assay/immunoradiometric assay | Deficiency: 25(OH)D <10 ng/mL; insufficiency: < 20 ng/mL | Weight, height, BMI, WC, WHR, body composition. | Age, season, smoking, sex | Higher BMI, WC, and BF were associated with lower 25(OH)D and with higher PTH. | 5 |
| Parikh (68), 2004 | USA; Caucasian, African-American, other | Cross-sectional | 302 (154/148) | F/M | Obese: 37.6 ± 9.4; nonobese: 36.6 ± 11.4 | Healthy adults | Competitive binding assay/ two-site immunoenzymometric assay | NM | Weight, height, BMI, body composition. | NM | Serum PTH was positively correlated with both BMI and BF. 25(OH)D was negatively correlated with BMI and BF. | 5 |
| Ford (44), 2005 | USA; white, African American, Mexican American | Cross-sectional | 8421 | F/M | ≥20 | Noninstitutionalized civilian U.S. population | Radioimmunoassay | Deficiency: 25(OH)D < 25 ng/mL | Abdominal obesity was defined as WC ≥102 cm for males and ≥88 cm for females. | Age, sex, smoking, serum factors, PA | Inverse association was present for quintiles of 25(OH)D levels and abdominal adiposity. | 7 |
| Reis (45), 2007 | USA, Southern California; NM | Cross-sectional | 1070 | F/M | 44–96 | All adults living in the southern California community of Rancho Bernardo | CLIA | NM | Weight, height, BMI, WC. | Age, season, and major lifestyle factors | There was no significant association between 25(OH)D and abdominal obesity. | 4 |
| Bell (46), 1985 | USA; white | Cross-sectional | 26 (12/14) | F/M | 20–35 | Normal white subjects | Competitive protein binding assay/ radioimmunoassay | NM | Weight, height, BMI. | NM | PTH was higher and 25(OH)D was lower in the obese than in the nonobese individuals. | 4 |
| Compston (47), 1981 | England, NM | Cross-sectional | 42 (22/20) | F/M | Obese: 22–49; nonobese: 19–52 | Obese patients before intestinal bypass r gastric partitioning versus healthy normal controls | Competitive protein-binding assay | NM | Weight, height, BMI. | NM | The mean plasma 25(OH)D level was significantly lower in the obese group than in age-matched controls. | 4 |
| Vilaca (84), 2022 | UK, NM | Cross-sectional | 200 | F/M | 25–40 or 55–75 | Community-dwelling men and women from South Yorkshire | Autoanalyzers | NM | Weight, height, BMI, body composition. | Age, sex | Compared with normal weight, obese individuals had lower 25(OH)D. | 5 |
| Avila Castillo (85), 2023 | Germany, Caucasian | Cross-sectional | 1032 (533/499) | F/M | 40–79 | Adult population of LIFE-Adult-Study | Chemiluminescent enzyme immunometric assay | Deficiency: 25(OH)D <20 ng/mL | BMI, WC, HC, WHR, %BF. | NM | Low levels of 25(OH)D are linked to higher BMI. | 7 |
%BF, percentage of body fat; ALT, alanine aminotransferase; BAI, body adiposity index; BMI, body mass index; CRE, creatinine; F/M, female/male; FMI, fat mass index; HC, hip circumference; GFR, glomerular filtration rate; hs-CRP, high-sensitivity C-reactive protein; IGF-1, insulin-like growth factor 1; iPTH, intact parathyroid hormone; NC, neck circumference; NM, not mentioned; NOS, Newcastle–Ottawa Scale;P, phosphorus; PA, physical activity; PTH, Parathyroid hormone; SDS, standard deviation score; WC, waist circumference; WHR, waist-to-hip ratio.
Serum 25(OH)D and anthropometric measurements
Different methods were used to assess serum vitamin D concentrations, including chemiluminescent immunoassay (CLIA), electrochemiluminescence immunoassay (ECLIA), radioimmunoassay (RIA), enzyme-linked immune sorbent assay (ELISA), immunoassay (IA), enzyme IA (EIA), protein-binding assay (PBA), chromatography, although three articles did not report the using method.
Anthropometric status was assessed based on weight, BMI, WC, WHR, and body composition.
The relationship between vitamin D with anthropometric and adiposity indicators in children/adolescents population
In a study by Durá-Travé (18), among 282 participants with severe obesity and 348 healthy control children group, vitamin D deficiency was more frequent (P < 0.05) in the obesity group (44.5 vs 11.5% and 22.4 vs 3.9%, respectively). In Geserick et al.’s study (43) obese children had significantly lower 25(OH)D SDS (standard deviation score) levels (−0.43) than the reference group. According to the Codoñer-Franch et al. (34) obese children had a significantly lower 25(OH)D level (P = 0.002) compared with nonobese children. The obese group had significantly higher vitamin D deficiency rates compared to the control group in another study (P < 0.001) (17). Furthermore, a study (30) demonstrated an increased risk of hypovitaminosis D in overweight/obese adolescents (OR 3.89) compared to those with a normal BMI. Serum 25(OH)D levels were inversely related to BMI-SDS (r = −0.141, P = 0.007).
According to a cross-sectional study by Saber et al. (25) obese children had significantly lower 25(OH)D levels (21.02 vs 29.45 ng/mL) than controls. There was a negative correlation between serum 25(OH)D with PTH, weight, and BMI (r = −0.45, −0.55, −0.47, P < 0.01, respectively).
A cross-sectional research by Lenders et al. on 58 healthy obese adolescents (41) found 25(OH)D decreased by 0.46 ± 0.22 ng/mL per 1% increment in body fat mass (P = 0.05).
The cross-sectional study by Alemzadeh et al. (42) among 127 subjects aged 13.0 ± 3.0 years, found that hypovitaminosis D and vitamin D-deficient subjects had higher BMI, fat mass, and iPTH compared to vitamin D-sufficient subjects (P < 0.01). Also, fat mass was negatively correlated with 25(OH)D (r = −0.40, P < 0.0001) regardless of seasonal and racial/ethnic factors. In other works, by the same research group (24, 35) between obese adolescents, a negative correlation was found between fat mass and 25(OH)D (P < 0.001).
As reported by Chung et al. (31), the level of 25(OH)D in overweight children was significantly lower than that of normal weight children (17.1 ± 5.1 ng/mL vs 19.1 ± 6.1 ng/mL, P < 0.001). There was an independent association between overweight and vitamin D deficiency (OR 2.21; 95% CI 1.62–3.01).
According to Ashraf et al. (40), there is a trend toward a significant relationship between 25(OH)D with BMI (r = −0.24; P = 0.087) and PTH (r = −0.24; P = 0.088).
According to Buyukinan et al.’s study (36), children and adolescents with a BMI ≥95th percentile did not show any significant differences in terms of weight, height, BMI, and waist circumference between the three groups with deficient, insufficient, and normal vitamin D levels.
There was also a cross-sectional study, by Durá-Travé et al. (20), showing that adolescents with severe obesity who had vitamin D deficiency had significantly (P < 0.05) elevated BMI Z-scores, waist Z-scores, body fat percentages, fat mass indexes, and PTH values than those with normal vitamin D state. The serum 25(OH)D levels were negatively correlated (P < 0.05) with body fat percentage, fat mass index, and PTH.
Lean and overweight boys (based on CDC-BMI percentiles) had similar mean 25(OH)D levels (P = 0.160) and were below sufficiency thresholds according to an analysis by Di Nisio et al. (2015) (26). However, there was a large proportion of subjects with insufficient or deficient 25(OH)D in both groups (normal weight: 45/59, 76 %; overweight/obese: 45/49, 92%, P = 0.03).
In the study by Foo et al. (39), in adolescent girls, lean body mass and plasma 25(OH)D levels were significantly correlated (r = 0.446; P = 0.001). However, the correlation between body fat percentage and vitamin D status was not significant (r = 0.104; P > 0.05). According to Giudici et al. (23), compared to normal-weight participants, those with overweight had a lower 25(OH)D. All measures of BMI, weight, and WC were negatively associated with 25(OH)D (P < 0.05). Other researchers (16) found that serum vitamin D levels were negatively correlated with BMI in overweight/obese subjects with vitamin D levels <20 ng/mL (r = −0.186, P < 0.01).
Bioavailable and free vitamin D were lower in the obese group. However, the total vitamin D level between the two groups did not differ, according to Küçükali et al. (19). In another study by Turer et al., vitamin D deficiency is highly prevalent in overweight and obese children. (37). In a study by Oliveira et al. (33) serum 25(OH)D levels were statistically lower in adolescents with excess weight, abdominal obesity, and a high level of PTH (P < 0.05).
Another cross-sectional study by Petersen et al. (27) showed a negative relationship between serum 25(OH)D and BMI Z-scores and fat mass index (P = 0.001). However, the association with BMI Z-scores became nonsignificant when the model was adjusted for parental education (−0.03, 95% CI −0.07, −0.001, P = 0.14).
Plesner et al. (22) found that 16.5% of obese children and adolescents showed vitamin D deficiency, with an OR 3.41 (CI 2.27–5.71; P < 0.0001) in comparison with peers with normal weight. An increase in risk of hypovitaminosis D was observed in overweight (OR 5.02) and obese (OR 5.36) subjects compared to normal weight subjects (32).
There was a difference in BMI percentile between children with hypovitaminosis D and children who have sufficient vitamin D (56.7 ± 33.9 vs 42.6 ± 36.0; P = 0.04) according to a cross-sectional study (28). According to a multivariate analysis, high BMI percentile and high PTH levels were the parameters related to 25(OH)D concentration <75 nmol/L.
Another evaluation (21) among children with obesity could not observe any significant associations between vitamin D deficiency and the anthropometric or metabolic derangements.
In the study by Rusconi et al. (29), 59 had 25(OH)D <20 ng/mL (group Ι) and 61 had 25(OH)D > 20 ng/mL (group ΙΙ) were recruited. The two groups were similar for BMI SDS and fat mass SDS.
Razzaghy-azar et al. (38) reported that 25(OH)D level had a negative correlation with BMI-SDS and height-SDS in girls (P = 0.01 and 0.039, respectively), but these correlations were not significant in boys.
The relationship between vitamin D with anthropometric and adiposity indicators in adult/elderly population
Albassam et al. (75) found a negative correlation (R = −0.17; P < 0.05) between neck circumference, a surrogate for upper subcutaneous fat, and 25(OH)D in both obese and nonobese participants.
Gonzalez-Molero et al. (60) found that neither obesity at baseline (OR 0.98, 95% CI 0.69–1.40, P = 0.93) nor obesity after undergoing a second evaluation (OR 0.80, 95% CI 0.48–1.33, P = 0.39) was significantly related to vitamin D status. Yaylali et al. (73) demonstrated that vitamin D levels were inversely associated with visceral fat (P = 0.002, r = −0.366). It has also been shown (12) that obese women have lower 25(OH)D values than nonobese women.
In another analysis by Jungert et al. (61), BMI, hip circumference, and body fat were negatively correlated with 25(OH)D, but not in men. Using multiple regression analyses, total body fat was shown to be a negative predictor of 25(OH)D concentrations in women even after adjusting for confounders (β = −0.247; P = 0.016), whereas after adjusting for iPTH, there was no statistically significant association between BMI, hip circumference, and 25(OH)D. In men, 25(OH)D was not influenced by anthropometric or body composition variables.
The overweight and obese groups in Tosunbayraktar et al.’s study (58) had lower 25(OH)D levels when compared to the normal BMI group (P = 0.01). Participants in the overweight group had higher levels of 25(OH)D than those in the obese group (P < 0.05).
A study by Ardawi et al. (80) showed that serum 25(OH)D was lower (P = 0.001) in upper quintiles of BMI and WHR. Another cross-sectional study (70) found vitamin D deficiency was prevalent among older and obese men with no education and sedentary lifestyles. Among obese, euglycemic women, 90 (40.7%) were deficient in vitamin D, according to a study (72). There were no significant differences in mean age, BMI, WC, hip circumference, WHR, and PTH between vitamin D-deficient and nondeficient obese women.
Based on Bolland et al.’s findings (83) 25(OH)D was negatively correlated with total fat mass, trunk fat, and pelvic fat. On multivariate analysis, 25(OH)D was negatively related to pelvic fat mass (P = 0.014; partial r 2 = 0.05). According to Compston et al. (1981) (47) obese individuals had significantly lower plasma 25(OH)D levels than age-matched controls.
In another study by Djafari et al. (49), among elderly persons, 25(OH)D (P = 0.030) was higher in the highest category of body adiposity index (BAI) compared to the lowest one. Additionally, linear regression demonstrated a negative association between BAI with 25(OH)D (β = −0.039, P = 0.029).
Healthier WC was also associated with better 25(OH)D concentrations among adults (63). Other studies found no significant correlation between 25(OH)D with WC (45, 51) and BMI (51). According to Macdonald et al. (82), women in the top quintile of BMI had lower 25(OH)D (P < 0.01). There was a significant correlation between 25(OH)D and BMI (r = 0.58; P = 0.01). In addition, BMI was highly predictive of 25(OH)D level (r = −0.52; P < 0.01).
According to another study (69), 25(OH)D is not correlated with BMI or fat mass index in healthy male adults. No significant association was found between anthropometric indices and serum 25(OH)D levels in Moschonis et al.'s study (2009) (81) on nonosteoporotic, postmenopausal women.
Among women with different BMI levels, Saarnio et al. (50) found no difference in 25(OH)D levels. It was found that 25(OH)D was lower in obese man as compared to normal weight man (48.0 ± 2.4 nmol/L vs 56.4 ± 2.0 nmol/L, P = 0.003), but there was no difference between normal weight and overweight group or overweight and obese group. When obesity was examined in both sexes, obese subjects had lower 25(OH)D than normal-weight subjects (50.7 ± 1.6 vs 57.0 ± 1.0 nmol/L, respectively; P = 0.003). A significant difference was also found between overweight and obese groups (P = 0.023). It was found by Walsh et al. (53) that serum 25(OH)D was inversely related to BMI.
A study among male participants, by Frost et al. (71) showed that 25(OH)D levels were lower in those with a high BMI; those with a BMI over 25 kg/m2 had a lower 25(OH)D (61.4 (27.8) nm vs 66.7 (27.5) nm, P = 0.015). In participants with a BMI >25 kg/m2, an increase in BMI of 1 kg/m2 led to a decrease in 25(OH)D of 1.7 nm (95% CI: −2.8; −0.6, P = 0.002), whereas in participants with a BMI <25 kg/m2, BMI and 25(OH)D were unrelated (coef: 0.7 (95% CI: −0.6; −0.2)). Body fat mass was inversely related to PTH only in individuals with vitamin D insufficiency. Further, 25(OH)D were associated with lean body mass in adjusted analyses and in participants with low vitamin D levels.
An analysis by George et al. (57) showed 25(OH)D was not correlated with BMI (P = 0.38) and WC (P = 0.99). In the Sharma et al. study (74), women with a higher visceral adipose tissue quartile had significantly lower 25(OH)D levels (P = 0.05).
A negative correlation was found between serum 25(OH)D with BMI (r = −0.4; P < 0.0001) and body fat mass (r = −0.41; P < 0.0001) among healthy adults (68).
Analysis of the subjects aged ≥65 years, in the Longitudinal Aging Study Amsterdam (67), revealed that after adjusting for potential confounders, higher BMI, WC, and sum of skin folds were statistically significantly related to lower 25(OH)D (standardized β values were −0.136, −0.137, and −0.140, respectively; all P < 0.05) and with higher PTH (0.166, 0.113, and 0.114, respectively; all P < 0.05). In comparison to anthropometric measurements, total body fat percentage had a stronger relationship with 25(OH)D (−0.261).
A secondary analysis of data from middle-aged healthy adults with excess weight (56) indicated that total and abdominal adiposity, but not peripheral adiposity, predicted low plasma 25(OH)D total fat mass index (FMI): P = 0.018; android FMI: P = 0.052; gynoid FMI: P = 0.15; appendicular FMI: P = 0.07). An additional retrospective analysis (78) on women found lower levels of 25(OH)D among obese individuals (P < 0.001).
Bell et al. (46), in their study among 12 obese and 14 nonobese White subjects, demonstrated that mean serum 25(OH)D (8 ± 1 vs 20 ± 2 ng/mL, P < 0.001) was significantly lower in the obese than in the nonobese subjects.
There was a significant inverse association between abdominal obesity and the quintiles of 25(OH)D levels in another cross-sectional study (44) involving both sexes.
Valiña-Tóth et al. (64) showed in a study of healthy overweight adults that 25(OH)D inversely related to truncal fat mass (P = 0.02). Another study (79), among women with BMI 18–57 kg/m2, showed that higher BMI was related with lower 25(OH)D levels (r > −0.27, P < 0.001).
Serum 25(OH)D was found to be inversely related to visceral abdominal fat and percentage fat in a cross-sectional study of overweight and obesity (77).
After adjusting for the confounders, Shan et al. (13) found that low 25(OH)D levels were significantly related to elevated WC among women (OR = 1.612 (1.014–2.561). According to Puntus et al. (62) increasing BMI significantly reduced 25(OH)D levels in pre- and postmenopausal women (P < 0.001 and P < 0.05, respectively).
Further research (76) revealed a significant negative relationship between fat mass and 25(OH)D among fit older women (β = −3.76, P < 0.001).
Researchers examined a total of 830 healthy adults (55) and found that vitamin D insufficiency was significantly linked to abdominal obesity in males (OR 2.75 (CI: 1.1–7.1); P < 0.05).
Shinkov et al. (59) reported that 25(OH)D levels were significantly lower in obese females than in the normal weight females (34.6 ± 16.2 vs 38.2 ± 17.8 nmol/L, P = 0.014), but, in the males, the 25(OH)D levels did not differ among the BMI groups.
A significant negative correlation was reported by Shafinaz et al. (54) between serum 25(OH)D level and body fat percentage (β = −0.14). Multivariate linear regression analysis found that higher BMI and larger WC were significantly associated with lower serum 25(OH)D levels (P 0.05).
A study by Gariballa et al. (48) found that, although BMI did not differ statistically significantly between groups, it was higher among vitamin D deficient older subjects and women <50 years, respectively, compared to individuals with adequate vitamin D or optimal concentrations (P = 0.05).
Another study (52) found a negative correlation between serum 25(OH)D levels and BMI (β: −0.150; 95% CI: −2.262, −0.037). A study (66) in severely obese subjects found that insufficient 25(OH)D corresponded to higher BMI (insufficient: 47.2 ± 5.6 vs not insufficient: 45.9 ± 4.7 kg/m2; P = 0.047).
Another cross-sectional investigation (84) among community-dwelling men and women reported that compared with normal weight, obese individuals had lower 25(OH)D levels (P < 0.05).
In 2023, Avila Castillo et al. (85), in their study on the 1032 adult population of the LIFE-Adult-Study, concluded that low levels of 25(OH)D were linked to higher BMI, while fat mass areas showed a negative correlation with 25(OH)D concentrations only in women.
Discussion
The present systematic review investigated the correlation between serum 25(OH)D levels and anthropometric and adiposity measurements in healthy individuals of various ages. Some previous meta-analyses included studies that reported BMI (86) and WC (87) as an index of weight status in adults only, while our systematic review has considered different adiposity measures in both adults and children. The results of most of the included studies, but not all papers, showed lower 25(OH)D levels and a higher prevalence of vitamin D insufficiency and deficiency in subjects with higher weight, BMI, WC, WHR, and fat mass percentage, in all age groups. In fact, 25(OH)D levels were inversely associated with BMI, WC, HC, WHR, and body fat mass percentage, specifically in female subjects.
Adiposity indicators were inversely associated with vitamin D status in numerous studies but not in all studies. The association between low vitamin D levels and obesity may be attributed to several factors. First, individuals with obesity might face challenges in acquiring sufficient sun exposure owing to limited mobility or specific clothing choices (88, 89, 90). Second, vitamin D is stored in fat compartments and adipose tissues, particularly in the abdominal area, making its release less accessible in obese individuals (88, 91). Third, individuals with obesity often have an increased demand for vitamin D to support their body weight, but the bioavailability of 25(OH)D is reduced, making it challenging to meet these elevated requirements (88, 91). Fourth, as the concentrations of active vitamin D metabolites increase, they initiate a negative feedback control over hepatic 25(OH)D synthesis. This feedback mechanism led to a reduction in serum 25(OH)D levels, providing an additional explanation for the observed association (91). It is important to note that the full acceptance of these mechanisms is still under investigation (92). Conversely, reducing fat mass has been shown to elevate 25(OH)D levels by releasing stored vitamin D into circulation. In a systematic review, Mallard et al. (93) concluded that weight reduction slightly elevated the 25(OH)D level by 1.5 ng/mL. They proposed that the release of vitamin D from fat and fat-free mass after weight loss was responsible for 25(OH)D elevation. Therefore, there is contradictory evidence regarding this issue, and the accurate relationship between vitamin D and adiposity indicators remains unclear.
According to the results of the studies included in the systematic review, overweight and obese individuals of different ages have similar chances of becoming vitamin D-deficient. Hence, age does not appear to have a significant impact on this association.
As a result of the heterogeneity in study characteristics, findings on vitamin D status and adiposity were inconsistent, with the inverse association being more prominent in females. It has been speculated that ethnicity, sex, and age may have a mediating effect on the relationship between 25(OH)D levels and anthropometric measures. It is likely that the differences in associations between females and males stem from the fact that women have a higher percentage of body fat and a different body composition than men. With the same BMI, men have less body fat than women. Thus, men store less vitamin D in adipose tissue and more remains in the blood. Furthermore, serum 25(OH)D levels are not stable throughout the year due to inadequate levels of 25(OH)D in the adipose tissue. In addition, vitamin D-binding protein could also contribute to sex differences in vitamin D status (94). It has been demonstrated that vitamin D-binding protein and adiposity are negatively correlated in men and positively correlated in women (94).
In reviewing the studies included in the systematic review, critical issues were raised, which could contribute to bias and confounding. There are several limitations, including heterogeneity in participant characteristics, the diversity of methods used to determine vitamin D levels and the analytical challenges involved, the use of variable definitions of hypovitaminosis D, the absence of adjustment for various confounding factors that influence vitamin D levels, and the reliability of various adiposity measures for describing obesity. The results of these studies may also be affected by a number of other factors related to the population studied, such as cultural and religious factors, dressing codes that mandate covering the majority of the body surface, and behavioral and lifestyle differences (95). It is also possible that variations in socioeconomic and developmental status could cause heterogeneity among studies that influence nutrition and lifestyle. Various socioeconomic and developmental factors influence the prevalence of obesity and vitamin D level (96, 97). Moreover, the amount of air pollution may affect vitamin D status, especially in urban areas where UVB wavelengths of sunlight are mostly blocked by pollutants (98). As a result of differences in health policies regarding the fortification of food with vitamin D in Europe and the USA and in national recommendations regarding vitamin D supplement use, vitamin D intake may differ significantly between countries (99). Vitamin D levels are also affected by genetic factors; however, without population-based genetic analyses, it is difficult to quantify their impact (100).
On the other hand, it has been suggested that low serum 25(OH)D concentrations reduce calcium absorption. PTH is secreted in response to low serum calcium concentrations, which stimulates the production of 1,25(OH)2D. The (nearly) normal serum levels of 1,25(OH)2D are maintained at the expense of high serum PTH concentrations, known as ‘secondary hyperparathyroidism’. Considering that serum 25(OH)D is the substrate for serum 1,25(OH)2D, serum 25(OH)D levels tend to decrease when serum 1,25(OH)2D increases. PTH may contribute to fat accumulation by increasing insulin resistance and inhibiting lipolysis (101). Additionally, vitamin D may regulate uncoupling proteins, which may play a role in energy metabolism (102). Despite this, Walsh et al. (53) found that PTH was not affected by BMI or sex and was not correlated with BMI. In obese individuals, there may be alterations in the relationship between 25(OH)D and PTH levels (53). To optimally determine the vitamin D status, the 25(OH)D threshold for maximum suppression of PTH has been suggested (103). A previous study showed that patients with a BMI ≥30 kg/m2 had a lower threshold for suppressing PTH levels (5 ng/mL) than patients with a BMI <30 kg/m2 (10 ng/mL) (104). This suggests that a very low 25(OH)D level is required to activate the PTH axis, leading to secondary hyperparathyroidism and bone loss (104).
The BMI has been used as an indicator of obesity in the majority of studies included in this systematic review. However, although BMI is the most widely accepted method for defining obesity, it is not an accurate measure of fat mass and distribution of body fat. Compared with subcutaneous fat, excess visceral fat confers a greater risk of metabolic and cardiovascular diseases for the same BMI value (105, 106). Several more meaningful measures of adiposity have been developed to resolve this methodological issue, including fat mass, WC, and WHR.
The present systematic review investigated the correlation between serum 25(OH)D and anthropometric and adiposity measurements in healthy individuals of various ages. However, some limitations must be considered. As not all included studies separately reported the relationship between serum vitamin D and adiposity measures in men and women, accurate estimates for men and women could not be provided. Different cutoff points were used for defining obesity and vitamin D deficiency in the included studies. This systematic review also examined the relationship between obesity and vitamin D deficiency using cross-sectional studies, which made the causality findings more difficult. Finally, we did not have access to the complete data of all related papers.
Conclusion
Our systematic review highlights the prevalence of vitamin D deficiency among overweight/obese individuals and its inverse correlation with adiposity measures. Despite this association, it is essential to acknowledge the influence of various confounding factors, including dietary intake, physical activity, educational level, and seasonal variations, which could impact the serum 25(OH)D levels. Further prospective investigations are warranted to establish a causal relationship between vitamin D levels and obesity, shedding light on the underlying mechanisms. Additionally, the findings underscore the importance of monitoring serum vitamin D levels in individuals with excess weight. Considering the variability in climate and dietary patterns across different regions, standardizing the 25(OH)D cut-off points would benefit from additional research. Increased awareness of the interplay between obesity and vitamin D levels can prompt adjustments in clinical approaches among nutritionists and health-care professionals. Because the field of vitamin D research is dynamic and continues to evolve, and new studies may indeed provide further insights into the relationship between serum 25(OH)D levels and adiposity. In addition, the scarcity of cohort studies underscores the need for further longitudinal investigations to elucidate the causative mechanisms linking vitamin D with adiposity. So, we recommend that future researchers consider conducting updated systematic reviews to integrate the latest evidence on this important topic.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the study reported.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
All authors have given consent for the paper to be published by the corresponding author.
Author contribution statement
B.A. and F.H. designed and wrote the manuscript. S.H. and M.M. performed interpretation of results and critical revision of the manuscript. F.A. and M.V. critically revised the manuscript. All authors read and approved the final version.
References
- 1.Blüher M. Obesity: global epidemiology and pathogenesis. Nature Reviews. Endocrinology 201915288–298. ( 10.1038/s41574-019-0176-8) [DOI] [PubMed] [Google Scholar]
- 2.WHO. Obesity and overweight. Geneva, Switzerland: World Health Organization. (available at: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight) [Google Scholar]
- 3.Via M. The malnutrition of obesity:micronutrient deficiencies that promote diabetes. ISRN Endocrinology 20122012103472. ( 10.5402/2012/103472) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tamer G Mesci B Tamer I Kilic D & Arik S. Is vitamin D deficiency an independent risk factor for obesity and abdominal obesity in women? Endokrynologia Polska 201263196–201. [PubMed] [Google Scholar]
- 5.Yetley EA. Assessing the vitamin D status of the US population. American Journal of Clinical Nutrition 200888558S–64S. ( 10.1093/ajcn/88.2.558S) [DOI] [PubMed] [Google Scholar]
- 6.Cheng S, Massaro JM, Fox CS, Larson MG, Keyes MJ, McCabe EL, Robins SJ, O'Donnell CJ, Hoffmann U, Jacques PF, et al.Adiposity, cardiometabolic risk, and vitamin D status: the Framingham Heart Study. Diabetes 201059242–248. ( 10.2337/db09-1011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prietl B Treiber G Pieber TR & Amrein K. Vitamin D and immune function. Nutrients 201352502–2521. ( 10.3390/nu5072502) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park JE Pichiah PBT & Cha YS. Vitamin D and metabolic diseases: growing roles of vitamin D. Journal of Obesity and Metabolic Syndrome 201827223–232. ( 10.7570/jomes.2018.27.4.223) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hossein-nezhad A & Holick MF. Vitamin D for health: a global perspective. Mayo Clinic Proceedings 201388720–755. ( 10.1016/j.mayocp.2013.05.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cashman KD. Vitamin D. Deficiency: defining, prevalence, causes, and strategies of addressing. Calcified Tissue International 202010614–29. ( 10.1007/s00223-019-00559-4) [DOI] [PubMed] [Google Scholar]
- 11.de Azevedo FR & Caramelli B. Hypovitaminosis D and obesity - coincidence or consequence? European Endocrinology 20139128–131. ( 10.17925/EE.2013.09.02.128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Tejada-Romero MJG Saavedra-Santana P de la Rosa-Fernández F Suárez-Ramírez N Martín-Martínez A Del Rosario FM & Sosa-Henríquez M. Effect of obesity on fragility fractures, BMD and vitamin D levels in postmenopausal women. Influence of type 2 diabetes mellitus. Acta Diabetologica 2022591201–1208. ( 10.1007/s00592-022-01923-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shan X Zhao X Li S Song P Man Q Liu Z Hu Y & Yang L. Association of Serum 25(OH)D with metabolic syndrome in Chinese women of childbearing age. Nutrients 2022142301. ( 10.3390/nu14112301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al.The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021372n71. ( 10.1136/bmj.n71) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wells GA Shea B O’Connell D Peterson J Welch V Losos M & Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ontario, Canada: University of Ottawa, 2012. (available at: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp) [Google Scholar]
- 16.Çizmecioğlu FM Etiler N Görmüş U Hamzaoğlu O & Hatun Ş. Hypovitaminosis D in obese and overweight schoolchildren. Journal of Clinical Research in Pediatric Endocrinology 2008189–96. ( 10.4008/jcrpe.v1i2.43) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gün E Uzun H Bolu S Arslanoğlu İ & Kocabay K. Serum 25-hydroxyvitamin D is associated with insulin resistance independently of obesity in children ages 5–17. Primary Care Diabetes 202014741–746. ( 10.1016/j.pcd.2020.06.006) [DOI] [PubMed] [Google Scholar]
- 18.Durá-Travé T Gallinas-Victoriano F Malumbres-Chacon M Ahmed-Mohamed L Chueca-Guindulain MJ & Berrade-Zubiri S. Are there any seasonal variations in 25-hydroxyvitamin D and parathyroid hormone serum levels in children and adolescents with severe obesity? European Journal of Pediatrics 20211801203–1210. ( 10.1007/s00431-020-03857-4) [DOI] [PubMed] [Google Scholar]
- 19.Karacan Küçükali G Gülbahar Ö Özalkak Ş Dağlı H Ceylaner S Aycan Z & Savaş Erdeve Ş. Is bioavailable vitamin D better than total vitamin D to evaluate vitamin D status in obese children? Journal of Clinical Research in Pediatric Endocrinology 202113391–399. ( 10.4274/jcrpe.galenos.2020.2021.0230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Durá-Travé T Gallinas-Victoriano F Peñafiel-Freire DM Urretavizcaya-Martinez M Moreno-González P & Chueca-Guindulain MJ. Hypovitaminosis D and cardiometabolic risk factors in adolescents with severe obesity. Children 2020710. ( 10.3390/children7020010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adikaram SGS Samaranayake DBDL Atapattu N Kendaragama KMDLD Senevirathne JTN & Wickramasinghe VP. Prevalence of vitamin D deficiency and its association with metabolic derangements among children with obesity. BMC Pediatrics 201919186. ( 10.1186/s12887-019-1558-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plesner JL Dahl M Fonvig CE Nielsen TRH Kloppenborg JT Pedersen O Hansen T & Holm JC. Obesity is associated with vitamin D deficiency in Danish children and adolescents. Journal of Pediatric Endocrinology and Metabolism 20183153–61. ( 10.1515/jpem-2017-0246) [DOI] [PubMed] [Google Scholar]
- 23.Giudici KV Fisberg RM Marchioni DML Peters BSE & Martini LA. Crosstalk between bone and fat tissue: associations between vitamin D, osteocalcin, Adipokines, and markers of glucose metabolism among adolescents. Journal of the American College of Nutrition 201736273–280. ( 10.1080/07315724.2016.1274923) [DOI] [PubMed] [Google Scholar]
- 24.Alemzadeh R & Kichler J. Uric acid-induced inflammation is mediated by the parathyroid hormone: 25-hydroxyvitamin D ratio in obese adolescents. Metabolic Syndrome and Related Disorders 201614167–174. ( 10.1089/met.2015.0099) [DOI] [PubMed] [Google Scholar]
- 25.Saber LM Mahran HN Baghdadi HH & Al Hawsawi ZM. Interrelationship between bone turnover markers, calciotropic hormones and leptin in obese Saudi children. European Review for Medical and Pharmacological Sciences 2015194332–4343. [PubMed] [Google Scholar]
- 26.Di Nisio A De Toni L D'Addato E Pizzo MR Sabatino P & Foresta C. 25-hydroxyvitamin D insufficiency discriminates cardiovascular risk factors accumulation in peri-pubertal boys undergoing overweight screening. Endocrine 201653530–537. ( 10.1007/s12020-015-0725-4) [DOI] [PubMed] [Google Scholar]
- 27.Petersen RA, Dalskov SM, Sørensen LB, Hjorth MF, Andersen R, Tetens I, Krarup H, Ritz C, Astrup A, Michaelsen KF, et al.Vitamin D status is associated with cardiometabolic markers in 8–11-year-old children, independently of body fat and physical activity. British Journal of Nutrition 20151141647–1655. ( 10.1017/S0007114515003372) [DOI] [PubMed] [Google Scholar]
- 28.Reesukumal K Manonukul K Jirapongsananuruk O Krobtrakulchai W Hanyongyuth S Chatsiricharoenkul S & Pratumvinit B. Hypovitaminosis D in healthy children in Central Thailand: prevalence and risk factors. BMC Public Health 201515248. ( 10.1186/s12889-015-1588-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rusconi RE De Cosmi V Gianluca G Giavoli C & Agostoni C. Vitamin D insufficiency in obese children and relation with lipid profile. International Journal of Food Sciences and Nutrition 201566132–134. ( 10.3109/09637486.2014.959902) [DOI] [PubMed] [Google Scholar]
- 30.Vierucci F Del Pistoia M Fanos M Erba P & Saggese G. Prevalence of hypovitaminosis D and predictors of vitamin D status in Italian healthy adolescents. Italian Journal of Pediatrics 20144054. ( 10.1186/1824-7288-40-54) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung IH Kim HJ Chung S & Yoo EG. Vitamin D deficiency in Korean children: prevalence, risk factors, and the relationship with parathyroid hormone levels. Annals of Pediatric Endocrinology and Metabolism 20141986–90. ( 10.6065/apem.2014.19.2.86) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vierucci F Del Pistoia M Fanos M Gori M Carlone G Erba P Massimetti G Federico G & Saggese G. Vitamin D status and predictors of hypovitaminosis D in Italian children and adolescents: a cross-sectional study. European Journal of Pediatrics 20131721607–1617. ( 10.1007/s00431-013-2119-z) [DOI] [PubMed] [Google Scholar]
- 33.Oliveira RM Novaes JF Azeredo LM Cândido AP & Leite IC. Association of vitamin D insufficiency with adiposity and metabolic disorders in Brazilian adolescents. Public Health Nutrition 201417787–794. ( 10.1017/S1368980013001225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Codoñer-Franch P Tavárez-Alonso S Simó-Jordá R Laporta-Martín P Carratalá-Calvo A & Alonso-Iglesias E. Vitamin D status is linked to biomarkers of oxidative stress, inflammation, and endothelial activation in obese children. Journal of Pediatrics 2012161848–854. ( 10.1016/j.jpeds.2012.04.046) [DOI] [PubMed] [Google Scholar]
- 35.Alemzadeh R & Kichler J. Parathyroid hormone is associated with biomarkers of insulin resistance and inflammation, independent of vitamin D status, in obese adolescents. Metabolic Syndrome and Related Disorders 201210422–429. ( 10.1089/met.2012.0056) [DOI] [PubMed] [Google Scholar]
- 36.Buyukinan M Ozen S Kokkun S & Saz EU. The relation of vitamin D deficiency with puberty and insulin resistance in obese children and adolescents. Journal of Pediatric Endocrinology and Metabolism 20122583–87. ( 10.1515/jpem-2011-0426) [DOI] [PubMed] [Google Scholar]
- 37.Turer CB Lin H & Flores G. Prevalence of vitamin D deficiency among overweight and obese US children. Pediatrics 2013131e152–e161. ( 10.1542/peds.2012-1711) [DOI] [PubMed] [Google Scholar]
- 38.Razzaghy-Azar M & Shakiba M. Assessment of vitamin D status in healthy children and adolescents living in Tehran and its relation to iPTH, gender, weight and height. Annals of Human Biology 201037692–701. ( 10.3109/03014460903527348) [DOI] [PubMed] [Google Scholar]
- 39.Foo LH Zhang Q Zhu K Ma G Trube A Greenfield H & Fraser DR. Relationship between vitamin D status, body composition and physical exercise of adolescent girls in Beijing. Osteoporosis International 200920417–425. ( 10.1007/s00198-008-0667-2) [DOI] [PubMed] [Google Scholar]
- 40.Ashraf A Alvarez J Saenz K Gower B McCormick K & Franklin F. Threshold for effects of vitamin D deficiency on glucose metabolism in obese female African-American adolescents. Journal of Clinical Endocrinology and Metabolism 2009943200–3206. ( 10.1210/jc.2009-0445) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lenders CM, Feldman HA, Von Scheven E, Merewood A, Sweeney C, Wilson DM, Lee PD, Abrams SH, Gitelman SE, Wertz MS, et al.Relation of body fat indexes to vitamin D status and deficiency among obese adolescents. American Journal of Clinical Nutrition 200990459–467. ( 10.3945/ajcn.2008.27275) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alemzadeh R Kichler J Babar G & Calhoun M. Hypovitaminosis D in obese children and adolescents: relationship with adiposity, insulin sensitivity, ethnicity, and season. Metabolism: Clinical and Experimental 200857183–191. ( 10.1016/j.metabol.2007.08.023) [DOI] [PubMed] [Google Scholar]
- 43.Geserick M Vogel M Eckelt F Schlingmann M Hiemisch A Baber R Thiery J Körner A Kiess W & Kratzsch J. Children and adolescents with obesity have reduced serum bone turnover markers and 25-hydroxyvitamin D but increased parathyroid hormone concentrations - Results derived from new pediatric reference ranges. Bone 2020132115124. ( 10.1016/j.bone.2019.115124) [DOI] [PubMed] [Google Scholar]
- 44.Ford ES Ajani UA McGuire LC & Liu S. Concentrations of serum vitamin D and the metabolic syndrome among U.S. adults. Diabetes Care 2005281228–1230. ( 10.2337/diacare.28.5.1228) [DOI] [PubMed] [Google Scholar]
- 45.Reis JP von Mühlen D Kritz-Silverstein D Wingard DL & Barrett-Connor E. Vitamin D, parathyroid hormone levels, and the prevalence of metabolic syndrome in community-dwelling older adults. Diabetes Care 2007301549–1555. ( 10.2337/dc06-2438) [DOI] [PubMed] [Google Scholar]
- 46.Bell NH Epstein S Greene A Shary J Oexmann MJ & Shaw S. Evidence for alteration of the vitamin D-endocrine system in obese subjects. Journal of Clinical Investigation 198576370–373. ( 10.1172/JCI111971) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Compston JE Vedi S Ledger JE Webb A Gazet JC & Pilkington TR. Vitamin D status and bone histomorphometry in gross obesity. American Journal of Clinical Nutrition 1981342359–2363. ( 10.1093/ajcn/34.11.2359) [DOI] [PubMed] [Google Scholar]
- 48.Gariballa S Yasin J Abluwi G & Al Essa A. Vitamin D deficiency associations with metabolic, bone turnover and adverse general health markers in community free living adults. BMC Endocrine Disorders 20222217. ( 10.1186/s12902-021-00926-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Djafari F Eslahi M Zandi N Pazoki B Reza Amini M & Shab-Bidar S. The inverse association of body adiposity index and bone health in the older adults: a report from a developing country. International Journal of Clinical Practice 202175e14718. ( 10.1111/ijcp.14718) [DOI] [PubMed] [Google Scholar]
- 50.Saarnio E, Pekkinen M, Itkonen ST, Kemi V, Karp H, Ivaska KK, Risteli J, Koivula MK, Kärkkäinen M, Mäkitie O, et al.Low free 25-hydroxyvitamin D and high vitamin D binding protein and parathyroid hormone in obese Caucasians: a complex association with bone? PLoS One 201813e0192596. ( 10.1371/journal.pone.0192596) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kord-Varkaneh H Tangestani H Mansouri S Rahimi-Foroushani A & Shab-Bidar S. Association of body mass index and waist circumference with osteocalcin and C-terminal telopeptide in Iranian elderly: results from a cross-sectional study. Journal of Bone and Mineral Metabolism 201937179–184. ( 10.1007/s00774-018-0912-5) [DOI] [PubMed] [Google Scholar]
- 52.Raposo L Martins S Ferreira D Guimarães JT & Santos AC. Vitamin D, parathyroid hormone and metabolic syndrome - the PORMETS study. BMC Endocrine Disorders 20171771. ( 10.1186/s12902-017-0221-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walsh JS Evans AL Bowles S Naylor KE Jones KS Schoenmakers I Jacques RM & Eastell R. Free 25-hydroxyvitamin D is low in obesity, but there are no adverse associations with bone health. American Journal of Clinical Nutrition 20161031465–1471. ( 10.3945/ajcn.115.120139) [DOI] [PubMed] [Google Scholar]
- 54.Shafinaz IS & Moy FM. Vitamin D level and its association with adiposity among multi-ethnic adults in Kuala Lumpur, Malaysia: a cross sectional study. BMC Public Health 201616232. ( 10.1186/s12889-016-2924-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Al-Daghri NM, Al-Saleh Y, Aljohani N, Alokail M, Al-Attas O, Alnaami AM, Sabico S, Alsulaimani M, Al-Harbi M, Alfawaz H, et al.Vitamin D deficiency and cardiometabolic risks: a juxtaposition of Arab adolescents and adults. PLoS One 201510e0131315. ( 10.1371/journal.pone.0131315) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wright CS Weinheimer-Haus EM Fleet JC Peacock M & Campbell WW. The apparent relation between plasma 25-hydroxyvitamin D and insulin resistance is largely attributable to central adiposity in overweight and obese adults. Journal of Nutrition 20151452683–2689. ( 10.3945/jn.115.220541) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.George JA Norris SA Toman M Snyman T & Crowther NJ. Visceral adiposity is a predictor of parathyroid hormone levels in healthy adults. Journal of Endocrinological Investigation 201639447–453. ( 10.1007/s40618-015-0400-x) [DOI] [PubMed] [Google Scholar]
- 58.Tosunbayraktar G Bas M Kut A & Buyukkaragoz AH. Low serum 25(OH)D levels are assocıated to hıgher BMI and metabolic syndrome parameters in adult subjects in Turkey. African Health Sciences 2015151161–1169. ( 10.4314/ahs.v15i4.15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shinkov A Borissova AM Dakovska L Vlahov J Kassabova L & Svinarov D. Winter 25-hydroxyvitamin D levels in young urban adults are affected by smoking, body mass index and educational level. European Journal of Clinical Nutrition 201569355–360. ( 10.1038/ejcn.2014.163) [DOI] [PubMed] [Google Scholar]
- 60.González-Molero I, Rojo-Martínez G, Morcillo S, Gutierrez C, Rubio E, Pérez-Valero V, Esteva I, Ruiz de Adana MS, Almaraz MC, Colomo N, et al.Hypovitaminosis D and incidence of obesity: a prospective study. European Journal of Clinical Nutrition 201367680–682. ( 10.1038/ejcn.2013.48) [DOI] [PubMed] [Google Scholar]
- 61.Jungert A Roth HJ & Neuhäuser-Berthold M. Serum 25-hydroxyvitamin D3 and body composition in an elderly cohort from Germany: a cross-sectional study. Nutrition and Metabolism 2012942. ( 10.1186/1743-7075-9-42) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Puntus T Schneider B Meran J Peterlik M & Kudlacek S. Influence of age and gender on associations of body mass index with bone mineral density, bone turnover markers and circulating calcium-regulating and bone-active sex hormones. Bone 201149824–829. ( 10.1016/j.bone.2011.06.003) [DOI] [PubMed] [Google Scholar]
- 63.El Hayek J Egeland G & Weiler H. Older age and lower adiposity predict better 25-hydroxy vitamin D concentration in Inuit adults: international Polar Year Inuit Health Survey, 2007–2008. Archives of Osteoporosis 20116167–177. ( 10.1007/s11657-011-0062-z) [DOI] [PubMed] [Google Scholar]
- 64.Valiña-Tóth AL Lai Z Yoo W Abou-Samra A Gadegbeku CA & Flack JM. Relationship of vitamin D and parathyroid hormone with obesity and body composition in African Americans. Clinical Endocrinology 201072595–603. ( 10.1111/j.1365-2265.2009.03676.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Muscogiuri G Sorice GP Prioletta A Policola C Della Casa S Pontecorvi A & Giaccari A. 25-hydroxyvitamin D concentration correlates with insulin-sensitivity and BMI in obesity. Obesity 2010181906–1910. ( 10.1038/oby.2010.11) [DOI] [PubMed] [Google Scholar]
- 66.Rueda S Fernández-Fernández C Romero F Martínez de Osaba J & Vidal J. Vitamin D, PTH, and the metabolic syndrome in severely obese subjects. Obesity Surgery 200818151–154. ( 10.1007/s11695-007-9352-3) [DOI] [PubMed] [Google Scholar]
- 67.Snijder MB van Dam RM Visser M Deeg DJ Dekker JM Bouter LM Seidell JC & Lips P. Adiposity in relation to vitamin D status and parathyroid hormone levels: a population-based study in older men and women. Journal of Clinical Endocrinology and Metabolism 2005904119–4123. ( 10.1210/jc.2005-0216) [DOI] [PubMed] [Google Scholar]
- 68.Parikh SJ Edelman M Uwaifo GI Freedman RJ Semega-Janneh M Reynolds J & Yanovski JA. The relationship between obesity and serum 1,25-dihydroxy vitamin D concentrations in healthy adults. Journal of Clinical Endocrinology and Metabolism 2004891196–1199. ( 10.1210/jc.2003-031398) [DOI] [PubMed] [Google Scholar]
- 69.Menon AS Kapoor R Anayath S & Garg MK. Vitamin D, body mass composition and metabolic risk factors in healthy young Indians. Medical Journal, Armed Forces India 202177485–489. ( 10.1016/j.mjafi.2020.05.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ardawi MS Sibiany AM Bakhsh TM Qari MH & Maimani AA. High prevalence of vitamin D deficiency among healthy Saudi Arabian men: relationship to bone mineral density, parathyroid hormone, bone turnover markers, and lifestyle factors. Osteoporosis International 201223675–686. ( 10.1007/s00198-011-1606-1) [DOI] [PubMed] [Google Scholar]
- 71.Frost M Abrahamsen B Nielsen TL Hagen C Andersen M & Brixen K. Vitamin D status and PTH in young men: a cross-sectional study on associations with bone mineral density, body composition and glucose metabolism. Clinical Endocrinology 201073573–580. ( 10.1111/j.1365-2265.2010.03847.x) [DOI] [PubMed] [Google Scholar]
- 72.Saleem N Rizvi NB & Elahi S. Prevalence of vitamin D deficiency and its association with insulin resistance in obese women with normal fasting glucose. BioMed Research International 202120212259711. ( 10.1155/2021/2259711) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yaylali GF Dedeoglu O Topsakal S Herek D & Senol H. Relationships among bone metabolic markers, body fat composition and carotid intima-media thickness in premenopausal obese women. Acta Medica Okayama 202175373–379. ( 10.18926/AMO/62233) [DOI] [PubMed] [Google Scholar]
- 74.Sharma DK Anderson PH Morris HA & Clifton PM. Visceral fat is a negative determinant of bone health in obese postmenopausal women. International Journal of Environmental Research and Public Health 2020173996. ( 10.3390/ijerph17113996) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Albassam RS Sabico S Alnaami AM Khattak MNK Lei KY Al-Daghri NM Reginster JY & Alokail MS. Bone metabolism markers are associated with neck circumference in adult Arab women. Osteoporosis International 201930845–852. ( 10.1007/s00198-018-04830-6) [DOI] [PubMed] [Google Scholar]
- 76.Trevisan C, Veronese N, Berton L, Carraro S, Bolzetta F, De Rui M, Miotto F, Inelmen EM, Coin A, Perissinotto E, et al.Factors influencing serum-Hydroxivitamin D Levels and other bone metabolism parameters in healthy older women. Journal of Nutrition, Health and Aging 201721131–135. ( 10.1007/s12603-016-0746-6) [DOI] [PubMed] [Google Scholar]
- 77.Sorkin JD Vasaitis TS Streeten E Ryan AS & Goldberg AP. Evidence for threshold effects of 25-hydroxyvitamin D on glucose tolerance and insulin resistance in black and white obese postmenopausal women. Journal of Nutrition 2014144734–742. ( 10.3945/jn.114.190660) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shapses SA Lee EJ Sukumar D Durazo-Arvizu R & Schneider SH. The effect of obesity on the relationship between serum parathyroid hormone and 25-hydroxyvitamin D in women. Journal of Clinical Endocrinology and Metabolism 201398E886–E890. ( 10.1210/jc.2012-3369) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sukumar D Schlussel Y Riedt CS Gordon C Stahl T & Shapses SA. Obesity alters cortical and trabecular bone density and geometry in women. Osteoporosis International 201122635–645. ( 10.1007/s00198-010-1305-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ardawi MS Qari MH Rouzi AA Maimani AA & Raddadi RM. Vitamin D status in relation to obesity, bone mineral density, bone turnover markers and vitamin D receptor genotypes in healthy Saudi pre- and postmenopausal women. Osteoporosis International 201122463–475. ( 10.1007/s00198-010-1249-7) [DOI] [PubMed] [Google Scholar]
- 81.Moschonis G Tanagra S Koutsikas K Nikolaidou A Androutsos O & Manios Y. Association between serum 25-hydroxyvitamin D levels and body composition in postmenopausal women: the postmenopausal Health Study. Menopause 200916701–707. ( 10.1097/gme.0b013e318199d5d5) [DOI] [PubMed] [Google Scholar]
- 82.Macdonald HM Mavroeidi A Barr RJ Black AJ Fraser WD & Reid DM. Vitamin D status in postmenopausal women living at higher latitudes in the UK in relation to bone health, overweight, sunlight exposure and dietary vitamin D. Bone 200842996–1003. ( 10.1016/j.bone.2008.01.011) [DOI] [PubMed] [Google Scholar]
- 83.Bolland MJ Grey AB Ames RW Horne AM Gamble GD & Reid IR. Fat mass is an important predictor of parathyroid hormone levels in postmenopausal women. Bone 200638317–321. ( 10.1016/j.bone.2005.08.018) [DOI] [PubMed] [Google Scholar]
- 84.Vilaca T Evans A Gossiel F Paggiosi M Eastell R & Walsh JS. Fat, adipokines, bone structure and bone regulatory factors associations in obesity. European Journal of Endocrinology 2022187743–750. ( 10.1530/EJE-22-0530) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Avila Castillo A Hagemann T Hoffmann A Baber R Biemann R Wirkner K Krupka S Stumvoll M Blüher M & Klöting N. Associations between vitamin D, immunoglobulin E concentrations, and obesity. Frontiers in Nutrition 2023101147407. ( 10.3389/fnut.2023.1147407) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Saneei P Salehi-Abargouei A & Esmaillzadeh A. Serum 25-hydroxy vitamin D levels in relation to body mass index: a systematic review and meta-analysis. Obesity Reviews 201314393–404. ( 10.1111/obr.12016) [DOI] [PubMed] [Google Scholar]
- 87.Hajhashemy Z Shahdadian F Ziaei R & Saneei P. Serum vitamin D levels in relation to abdominal obesity: a systematic review and dose-response meta-analysis of epidemiologic studies. Obesity Reviews 202122e13134. ( 10.1111/obr.13134) [DOI] [PubMed] [Google Scholar]
- 88.Golzarand M Hollis BW Mirmiran P Wagner CL & Shab-Bidar S. Vitamin D supplementation and body fat mass: a systematic review and meta-analysis. European Journal of Clinical Nutrition 2018721345–1357. ( 10.1038/s41430-018-0132-z) [DOI] [PubMed] [Google Scholar]
- 89.Brock K, Huang WY, Fraser DR, Ke L, Tseng M, Stolzenberg-Solomon R, Peters U, Ahn J, Purdue M, Mason RS, et al.Low vitamin D status is associated with physical inactivity, obesity and low vitamin D intake in a large US sample of healthy middle-aged men and women. Journal of Steroid Biochemistry and Molecular Biology 2010121462–466. ( 10.1016/j.jsbmb.2010.03.091) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moy FM. Vitamin D status and its associated factors of free living Malay adults in a tropical country, Malaysia. Journal of Photochemistry and Photobiology 2011104444–448. ( 10.1016/j.jphotobiol.2011.05.002) [DOI] [PubMed] [Google Scholar]
- 91.Wortsman J Matsuoka LY Chen TC Lu ZR & Holick MF. Decreased bioavailability of vitamin D in obesity. American Journal of Clinical Nutrition 200072690–693. ( 10.1093/ajcn/72.3.690) [DOI] [PubMed] [Google Scholar]
- 92.Engelman CD Fingerlin TE Langefeld CD Hicks PJ Rich SS Wagenknecht LE Bowden DW & Norris JM. Genetic and environmental determinants of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels in Hispanic and African Americans. Journal of Clinical Endocrinology and Metabolism 2008933381–3388. ( 10.1210/jc.2007-2702) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mallard SR Howe AS & Houghton LA. Vitamin D status and weight loss: a systematic review and meta-analysis of randomized and nonrandomized controlled weight-loss trials. American Journal of Clinical Nutrition 20161041151–1159. ( 10.3945/ajcn.116.136879) [DOI] [PubMed] [Google Scholar]
- 94.Bolland MJ Grey AB Ames RW Horne AM Mason BH Wattie DJ Gamble GD Bouillon R & Reid IR. Age-, gender-, and weight-related effects on levels of 25-hydroxyvitamin D are not mediated by vitamin D binding protein. Clinical Endocrinology 200767259–264. ( 10.1111/j.1365-2265.2007.02873.x) [DOI] [PubMed] [Google Scholar]
- 95.Chouraqui JP, Turck D, Briend A, Darmaun D, Bocquet A, Feillet F, Frelut ML, Girardet JP, Guimber D, Hankard R, et al.Religious dietary rules and their potential nutritional and health consequences. International Journal of Epidemiology 20215012–26. ( 10.1093/ije/dyaa182) [DOI] [PubMed] [Google Scholar]
- 96.Drewnowski A. Obesity, diets, and social inequalities. Nutrition Reviews 200967(Supplement 1) S36–S39. ( 10.1111/j.1753-4887.2009.00157.x) [DOI] [PubMed] [Google Scholar]
- 97.Lips P. Worldwide status of vitamin D nutrition. Journal of Steroid Biochemistry and Molecular Biology 2010121297–300. ( 10.1016/j.jsbmb.2010.02.021) [DOI] [PubMed] [Google Scholar]
- 98.Agarwal KS Mughal MZ Upadhyay P Berry JL Mawer EB & Puliyel JM. The impact of atmospheric pollution on vitamin D status of infants and toddlers in Delhi, India. Archives of Disease in Childhood 200287111–113. ( 10.1136/adc.87.2.111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Prentice A. Vitamin D deficiency: a global perspective. Nutrition Reviews 200866(Supplement 2) S153–S164. ( 10.1111/j.1753-4887.2008.00100.x) [DOI] [PubMed] [Google Scholar]
- 100.Sepulveda-Villegas M Elizondo-Montemayor L & Trevino V. Identification and analysis of 35 genes associated with vitamin D deficiency: a systematic review to identify genetic variants. Journal of Steroid Biochemistry and Molecular Biology 2020196105516. ( 10.1016/j.jsbmb.2019.105516) [DOI] [PubMed] [Google Scholar]
- 101.Frost M Abrahamsen B Nielsen TL Hagen C Andersen M & Brixen K. Vitamin D status and PTH in young men: a crosssectional study on associations with bone mineral density, body composition and glucose metabolism. Clinical Endocrinology 201073573–580. ( 10.1111/j.1365-2265.2010.03847.x) [DOI] [PubMed] [Google Scholar]
- 102.Wong KE Szeto FL Zhang W Ye H Kong J Zhang Z Sun XJ & Li YC. Involvement of the vitamin D receptor in energy metabolism: regulation of uncoupling proteins. American Journal of Physiology 2009296E820–E828. ( 10.1152/ajpendo.90763.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lotito A Teramoto M Cheung M Becker K & Sukumar D. Serum parathyroid hormone responses to vitamin D supplementation in overweight/obese adults: a systematic review and meta- analysis of randomized clinical trials. Nutrients 20179241. ( 10.3390/nu9030241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Salazar DA, Ferreira MJS, Neves JS, Pedro JMP, Guerreiro VA, E Silva Viana S, Mendonça F, Silva MM, Belo SP, Sande AV, et al.Variable thresholds of vitamin D plasma levels to suppress PTH: the effect of weight and bariatric surgery. Obesity Surgery 2020301551–1559. ( 10.1007/s11695-019-04351-z) [DOI] [PubMed] [Google Scholar]
- 105.Chait A & den Hartigh LJ. Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Frontiers in Cardiovascular Medicine 2020722. ( 10.3389/fcvm.2020.00022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang Y Rimm EB Stampfer MJ Willett WC & Hu FB. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. American Journal of Clinical Nutrition 200581555–563. ( 10.1093/ajcn/81.3.555) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.



 This work is licensed under a
This work is licensed under a