Abstract
We have found the in vivo-regulated nirB promoter (PnirB) to be effective for directing expression of a number of antigens in salmonella in vivo. We wished to determine if other in vivo-regulated promoters have utility for antigen expression in salmonella and to compare the effectiveness of these promoters with that of PnirB. To this end, we have devised a scheme that allows the promoter element of the PnirB-fragment C plasmid pTETnir15 to be swapped with other promoters of interest. We demonstrate the usefulness of this system by replacing PnirB with PhtrA to create plasmid pTEThtrA1. htrA is a stress response gene that is required for virulence of salmonella in mice and survival within macrophages. Expression of fragment C in Salmonella typhimurium BRD509 (aroA aroD) harboring pTEThtrA1 (strain BRD937) correlated with growth temperature in vitro. A comparison was made of the immune responses to fragment C elicited in mice immunized orally with BRD937 or BRD847 (BRD509/pTETnir15) or subcutaneously with purified fragment C plus alhydrogel. High levels of anti-fragment C antibodies that persisted for at least 12 weeks were present in all groups of mice. Vaccination with BRD937 was the most effective means of immunization: the serum immunoglobulin G (IgG), IgA, and IgM anti-fragment C titers were higher in the BRD937-immunized mice throughout the duration of the study than in mice in the other groups. The kinetics of the serum anti-fragment C responses were different in different groups. The response was most rapid in the BRD937 group, with the titers almost at peak levels at 2 weeks postimmunization. Only the mice immunized with BRD937 or BRD847 developed an intestinal IgA response to fragment C. Again, the response was superior in the BRD937 group. The peak of the intestinal response was delayed with respect to the serum response. Analysis of the IgG subtype response to fragment C revealed a dominant IgG2a response in the salmonella-immunized mice, indicating a type 1 helper T-cell response to fragment C, whereas the major subtype in the group parenterally immunized with fragment C plus alhydrogel was IgG1. The IgG1/IgG2a ratio was much higher in sera of BRD937-immunized mice than in sera of BRD847-immunized mice. At 15 to 20 weeks after immunization, the mice immunized with BRD937 or BRD847 were solidly immune to tetanus toxin and salmonella. The immune responses to fragment C seen in mice immunized with BRD937 are the strongest we have observed and indicate that the htrA promoter may be very useful for expressing foreign antigens in salmonella vaccine strains.
Attenuated Salmonella strains are being evaluated as live vaccines against salmonella infections and as live carriers for heterologous antigens (5, 9, 35). There are a number of advantages to using salmonella as an antigen delivery system. First, the vaccine can be given orally, leading to immunization of the mucosa-associated lymphoid tissue, thus removing the need to use needles for vaccination. Moreover, by expressing multiple heterologous antigens in a single salmonella vector, it may be possible to immunize against multiple diseases with a single vaccine and ideally with a single immunization (22, 35). It has proved possible to immunize experimental animals against a range of different pathogens, including unrelated bacteria, viruses, and parasites, with salmonella expressing an appropriate antigen (5, 9, 35). However, in only very few instances has it proved possible to protect animals from salmonella and the disease of interest with a single oral immunization.
The major problem encountered with using salmonella as a vector has been obtaining strains that stably express high levels of the heterologous antigen in vivo for a sufficient time to develop an appropriate immune response. Multicopy plasmids encoding foreign antigens under the control of unregulated promoters are often rapidly lost from salmonella in vivo (8, 12, 35). A number of approaches have been used in attempts to improve the efficacy of salmonella vectors. Integration of the foreign antigen gene into the chromosome of salmonella overcomes the problem of stability but because of the drop in gene copy number, the amount of protein produced is greatly reduced and concomitantly the immune response is usually much weaker (35, 43).
Curtiss and coworkers (13, 31) have pioneered the use of a balanced lethal system to overcome the problem of plasmid instability in vivo. This approach relies on the expression plasmid carrying a gene that complements an otherwise lethal mutation in the chromosomal copy of the same gene, which leads to strong positive selection for plasmid maintenance in vivo. The approach taken by our group has been to use regulated promoters that have low activity during in vitro growth but which are optimally expressed in vivo (8).
Most of our work has focused on the nirB promoter (PnirB) (6–8, 22, 27), which exhibits low activity during aerobic growth but is upregulated in anaerobic environments (32). The promoter is also induced by entry of salmonella into cells (17). We and others have found that Salmonella strains expressing a variety of antigens from PnirB have immunogenicity superior to that of similar strains in which expression of the same antigens is controlled by a constitutive promoter (8). This is manifest with respect to both the magnitude and the consistency of the immune response. For example, all mice immunized once orally with a Salmonella typhimurium aroA aroD strain (BRD509) possessing a plasmid that carries the gene for the nontoxic C-terminal 50 kDa of tetanus toxin (fragment C [Frg C]) under the control of PnirB developed serum anti-tetanus toxin antibodies and were protected from tetanus (8). In contrast, only 20% of mice similarly immunized with BRD509 constitutively expressing Frg C were immune to tetanus (8), despite the fact that in vitro equivalent or higher levels of Frg C were expressed by the Ptac-Frg C strain than by the strain with the PnirB-Frg C construct (8). However, the PnirB-Frg C plasmid was stable in BRD509 in the tissues of mice, whereas the Ptac-Frg C plasmid was rapidly lost from BRD509 (8). This finding indicates that unregulated, high-level expression of Frg C in salmonella in murine tissue is detrimental. It is possible that PnirB is activated only once dissemination has occurred or that expression of Frg C is not maximal. It has recently been established that PnirB is active in salmonella found in liver abscesses in mice infected with a purE mutant of S. typhimurium (16).
It is hoped that a variety of antigens can be expressed and effectively delivered to the vertebrate immune system by using a single salmonella vector. Expressing multiple antigens from the same regulated promoter is likely to be problematic. This is because multiple copies of the promoter sequences would titrate the regulatory protein(s), for example, FNR in the case of PnirB, dysregulating antigen expression and possibly affecting immunogenicity of the protein. Because of this, we have been looking for other in vivo-regulated promoters that can be used for controlling antigen expression in salmonella. It is possible that different promoters are active at different stages in the life cycle of salmonella and therefore may give rise to different immune responses to the antigen. For example, a promoter that is primarily active in the gut-associated lymphoid tissue (GALT) may stimulate a stronger mucosal response than another promoter that is active in tissues distal to the gut. It is also possible that the quality of the immune response is altered by using different promoters.
To determine the effects of different promoters on the immune response to the salmonella-delivered antigen, it is important that the only variable is the promoter sequences themselves. To this end, we have devised vectors and a strategy that allows the Frg C gene to be placed under the control of different promoters but that maintains other important aspects of the plasmid such as the Shine-Dalgarno sequence. We illustrate the utility of this approach by comparing the immune responses to Frg C in mice immunized orally with an S. typhimurium double aro mutant possessing a PnirB-Frg C plasmid or an identical plasmid on which expression of Frg C is controlled by the promoter for the stress response gene htrA (20). We also analyze how the immune response to salmonella-delivered Frg C differs from that elicited by parenteral immunization with purified Frg C absorbed to alhydrogel.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
SL1344 is a wild-type mouse-virulent strain of S. typhimurium (19). BRD509 (SL1344 aroA aroD), BRD847 (BRD509/pTETnir15), and plasmid pTETnir15 have all been described previously (8, 42). The construction of plasmid pTEThtrA1 and strain BRD937 (BRD509/pTEThtrA1) is described below. For routine culture bacteria, strains were grown aerobically in L broth or on L agar (LA).
Western blotting.
Bacteria were grown overnight on LA at different temperatures. Bacterial cells were harvested into phosphate-buffered saline (PBS), and the cell density was adjusted to 1.7 × 109 CFU/ml. One milliliter of cell suspension was pelleted in a microcentrifuge, resuspended in 1× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer, and boiled to lyse cells. Eight microliters of bacterial lysate from each sample was subjected to SDS-PAGE, and the separated polypeptides were transferred to nitrocellulose by electroblotting. Frg C was visualized by using a rabbit polyclonal anti-Frg C antiserum as previously described (43).
Immunization.
For immunization of mice, salmonellae were grown statically overnight in L broth (15). The bacteria were recovered by centrifugation and resuspended in sterile PBS (pH 7.2) to approximately 1 × 1010 to 5 × 1010 CFU/ml. Female BALB/c mice (6 to 8 weeks old; Charles River, Margate, United Kingdom) were orally immunized with salmonella suspension (0.2 ml) administered by gavage tube as described previously (28). Viable counts were performed on all inocula. For subcutaneous (s.c.) immunization with Frg C, a 100-μl sample (containing 10 μg of Frg C) was injected into the skin folds in the back of the neck. Frg C was adsorbed overnight (4°C) to alhydrogel (0.25%). Recombinant Frg C was produced from Pichia pastoris as previously described (11).
Anti-Frg C serum response.
Anti-Frg C-specific antibodies were measured by enzyme-linked immunosorbent assay (ELISA) as previously described (34). Briefly, 96-well EIA/RIA plates (Costar, High Wycombe, Buckinghamshire, United Kingdom) were coated with recombinant Frg C (50 μl; 2.5 μg/ml in PBS, overnight, 4°C), washed three times with PBS containing 0.05% (vol/vol) Tween 20 (PBST; Sigma), and then blocked with PBS–1% bovine serum albumin (BSA). After being washed, plates were incubated with serial dilutions of serum for 2 h at 37°C. All samples and reagents were diluted in PBST–0.1% BSA. Plates were washed and incubated with biotin-conjugated goat anti-mouse immunoglobulin G (IgG) (Sigma) or, to determine the anti-Frg C IgG subclass response, biotin-conjugated rat anti-mouse IgG1, IgG2a, IgG2b, or IgG3 (Pharmingen, AMS Biotech, Witney, Oxon, United Kingdom) for 1 h at 37°C. Following washing, horseradish peroxidase (HRP)-conjugated streptavidin (Dako, High Wycombe, Buckinghamshire, United Kingdom) was added, and bound antibodies were visualized by adding o-phenylenediamine (OPD) substrate (0.04% OPD in citrate-phosphate buffer [pH 5] containing 0.01% H2O2). After color development, reaction was stopped with 3 M H2SO4 and absorbance was read as optical density (OD) at 490 nm. Absorbance values were plotted against dilutions and titers were determined as the reciprocal of the highest sample dilution giving an OD of 0.3.
Measurement of the anti-Frg C fecal IgA responses.
Fresh fecal pellets (two to four/mouse) were collected into microcentrifuge tubes. One milliliter of a solution consisting of 1% (wt/vol) BSA (Sigma) and 1 mM phenylmethylsulfonyl fluoride (Sigma) in PBS was added to each tube, and the tubes were then incubated overnight at 4°C. Subsequently, the tubes were vortexed to disrupt all solid material and then centrifuged at 16,000 × g for 5 min. The supernatant was recovered and stored at −20°C until analysis.
The fecal extracts were assayed by ELISA for Frg C-specific IgA. The method was essentially that for the serum ELISA with the following modifications. Ninety-six-well EIA/RIA plates were coated overnight with 50 μl of a 10-μg/ml solution of Frg C; 100 μl of neat or diluted fecal extract was added to each well, and the plates were incubated at 4°C overnight. The Frg C-specific IgA was detected by using an anti-mouse IgA (α chain specific)-biotin conjugated antibody (Sigma) followed by a streptavidin-HRP conjugate (Dako), and finally OPD was used as the substrate. Endpoint titers were calculated as the reciprocal of the highest sample dilution giving an OD of 0.5 after background subtraction.
To correct for variation in IgA present in each sample, the total IgA concentration of each of the fecal samples was determined by an IgA-specific capture ELISA. Ninety-six-well EIA/RIA plates were coated overnight with α-chain-specific goat anti-mouse IgA (0.1 μg/well; Sigma). Samples were added and incubated for 1 h at room temperature. Captured IgA was detected by the sequential addition of goat anti-mouse IgA (α chain)-biotin conjugate (Sigma), streptavidin-HRP (Dako), and OPD substrate. The concentrations of IgA in the samples were calculated from an IgA standard curve prepared by using a mouse myeloma IgA standard (ICN, Oxford, United Kingdom). Final results were calculated by dividing the Frg C-specific IgA endpoint titer by the total IgA (milligrams) in the fecal sample.
Tetanus toxin challenge.
Mice were challenged s.c. with 0.01 μg (50 50% lethal doses [LD50]) of purified tetanus toxin as previously described (8), and fatalities were recorded for 4 days.
Salmonella challenge.
Mice were challenged orally with 0.2 ml of a suspension of S. typhimurium SL1344 (4 × 109 CFU/ml in PBS). Mice were observed for 28 days, and fatalities were recorded.
RESULTS
Construction of the PhtrA-Frg C expression plasmid pTEThtrA1.
pTETnir15 is a plasmid that specifies the production of Frg C from the nirB promoter (8). The promoter region of pTETnir15 is flanked by EcoRI and BglII sites (Fig. 1). The effects of different promoter sequences on the expression of Frg C and the immunogenicity of salmonella-Frg C constructs can be investigated by replacing the small EcoRI-BglII fragment of pTETnir15 that contains PnirB with oligonucleotides carrying the nucleotide sequence of alternative promoters (Fig. 1). Unfortunately, the presence of a second EcoRI site within the coding sequence of the Frg C gene of pTETnir15 makes this strategy problematic. To overcome this problem, an intermediate plasmid, pBD907, was created (Fig. 1). This was achieved by replacing the 2.8-kb SacII-BamHI fragment of pTETnir15, which contains the EcoRI site, with a irrelevant SacII-BamHI fragment that lacks EcoRI sites—specifically, a 1.8-kb fragment from pFHA-5, a plasmid possessing a portion of the Bordetella pertussis fhaB gene (Fig. 1). pBD907 can then be used as the starting plasmid for the construction of Frg C expression plasmids that differ only in the control sequences regulating Frg C expression. To bring Frg C expression under the control of the htrA promoter, pBD907 was digested with EcoRI and BglII, gel purified, and ligated with a previously annealed 55-bp pair of oligonucleotides (5′AATTCTATTCCGGAACTTCGCGTTATAAAATGAATGTGACGTACACAGCAATTTA3′ and 5′GATCTAAATTGCTGTGTACGTCACATTCATTCATTTTATAACGCGAAGTTCCGGAATAG 3′) that contain the promoter region of the htrA gene. The ligated plasmid was transformed into Escherichia coli, and the sequence of each of a number of the clones was checked by double-stranded sequencing of plasmid DNA (data not shown). A plasmid with the correct sequence at the 5′ end of the Frg C gene was digested with SacII and BamHI to liberate the fragment of fhaB DNA; following gel purification, the 2.8-kb SacII-BamHI fragment was ligated with the 0.9-kb SacII-BamHI fragment from pTETnir15 which contains the 3′ end of the Frg C gene. This allows the regeneration of a plasmid encoding the full-length Frg C gene; the resulting plasmid was called pTEThtrA1. This plasmid was transformed into E. coli, and Frg C expression was checked by colony blotting. The plasmid from a clone positive for Frg C expression was isolated and introduced into S. typhimurium BRD509 to create strain BRD937.
FIG. 1.
Construction of plasmid pTEThtrA1. The steps involved in the replacement of the nirB promoter of pTETnir15 with the htrA promoter to create pTEThtrA1 are shown schematically. Full details are given in the text.
Expression of Frg C in vitro.
Regulation of Frg C expression in E. coli and S. typhimurium was determined by growing the bacteria at different temperatures and performing immunoblotting on total cell lysates of bacteria separated by SDS-PAGE. The results of one such analysis of BRD937 are shown in Fig. 2. Frg C expression increases as the growth temperature is increased from 30 to 42°C. Frg C expression was similarly regulated in the S. typhimurium htrA strain BRD915 and E. coli transformed with pTEThtrA1 (data not shown).
FIG. 2.
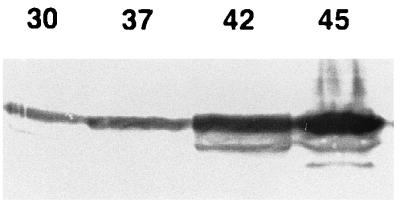
Effect of temperature on the expression of fragment C in S. typhimurium BRD915. BRD915 was grown overnight at 30, 37, 42, or 45°C (as indicated above the lanes) on LA. Lysates of bacterial cells were subjected to SDS-PAGE, and the separated proteins were transferred to nitrocellulose. Frg C was visualized by using a rabbit polyclonal anti-fragment C antibody and an appropriate antibody conjugate.
Immunization.
We compared the immunogenicities and kinetics of the serum and fecal immune responses to Frg C in mice immunized either orally with a single dose of BRD847 (1.23 × 1010 CFU), BRD937 (1.14 × 1010 CFU), or BRD509 (2 × 1010 CFU; negative control) or s.c. with a single dose of purified Frg C (10 μg) absorbed to alhydrogel. Anti-Frg C antibodies were measured in the serum and in fecal extracts at periods after immunization by ELISA.
Serum anti-Frg C response.
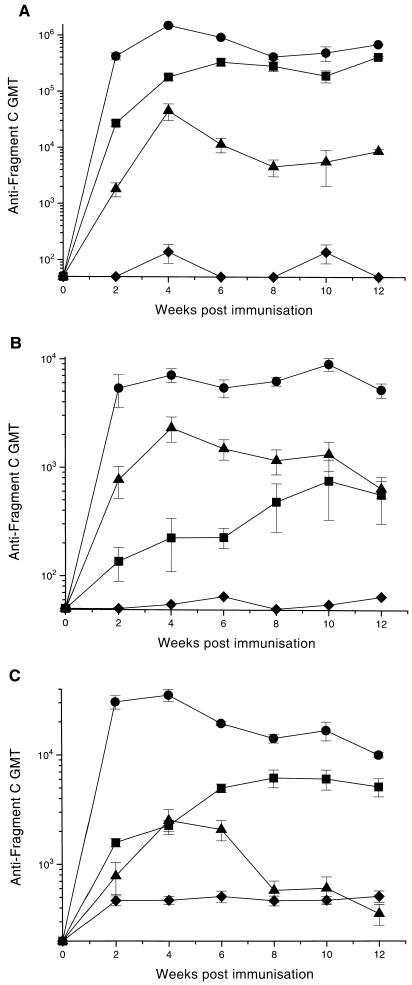
Mice immunized with BRD937 developed very high serum antibody titers to Frg C (Fig. 3). Two weeks postimmunization, the IgG anti-Frg C titer was greater than that seen in mice immunized with BRD847 or Frg C s.c. (Fig. 3). In fact, throughout the experiment the anti-Frg C IgG titers were higher in the BRD937 group than in the BRD847 and Frg C s.c. groups. The kinetics of the anti-Frg C IgG response were similar in the salmonella-immunized mice, with peak response occurring at 4 weeks postimmunization (Fig. 3). In both groups of salmonella-immunized mice there was a decline in the IgG anti-Frg C between weeks 4 and 8, and thereafter the titer rose gently during the remainder of the experiment. The major difference in the patterns of the IgG anti-Frg C responses between the salmonella groups is the titer at 2 weeks, which was already high for the BRD937 group but was relatively low for the BRD847 group but then climbed rapidly between weeks 2 and 4 (Fig. 3). There was a more gradual rise in the IgG response in the s.c. group, and the peak occurred at 12 weeks postimmunization (Fig. 3).
FIG. 3.
Serum Frg C response. Serum samples were taken from mice at periods after immunization orally with BRD847 (•), BRD915 (▪), or BRD509 (▴) or s.c. with fragment C plus alhydrogel (⧫). The IgG anti-Frg C response is shown in panel A, and the specific IgA and IgM responses are shown in panels B and C, respectively. Each point represents the geometric mean of the titer (GMT) of five sera ± the standard error of the mean.
The IgA anti-Frg C response was greater in mice immunized orally with BRD937 or BRD847 than in mice immunized s.c. with purified Frg C (Fig. 3). This probably reflects stimulation of the GALT, which is rich in IgA-producing B cells. As with the IgG titer, high levels of IgA anti-Frg C were present in the BRD937-immunized mice at 2 weeks postimmunization, and the titer changed little for the duration of the experiment, although the peak titer was seen at week 10. The kinetics of the serum IgA anti-Frg C response in the BRD847 group paralleled that of the IgG response.
The kinetics of the IgM responses in the BRD937 and BRD847 groups were different (Fig. 3). As with the other immunoglobulin classes, there were high IgM titers present at the first time point (2 weeks) in the BRD937 group. After 4 weeks, the IgM titer gradually declined. In contrast, at 2 weeks the IgM anti-Frg C titer was very low in sera of BRD847-immunized mice, and after peaking around 6 weeks the titer rapidly dropped to background levels by week 8. In the s.c. group, the Frg C-specific IgM titer rose gradually, reaching a plateau after 8 weeks. For all immunoglobulin classes and at all time points, the anti-Frg C titers were higher in the sera of mice immunized with BRD937 than in the sera of mice immunized with BRD847 or Frg C s.c. (Fig. 3). Apart from the IgA response, the s.c. immunized mice had higher anti-Frg C titers than mice in the BRD847 group.
Serum IgG anti-Frg C subclasses.
We analyzed the serum anti-Frg C IgG subclasses in the different groups to determine if there were any differences in the subclasses of the anti-Frg C elicited by the different vaccines and whether the response changes over time. In both salmonella groups, the anti-Frg C response was dominated by the IgG2 subclasses, particularly IgG2a (Table 1). This indicated that the mice in these groups had developed a predominantly type 1 helper (Th1) T-cell response to Frg C. The largest difference in the ratio between the IgG2a and the IgG1 subclasses was seen in the sera of the BRD937-immunized mice (Table 1). In the mice immunized s.c. with Frg C plus alhydrogel, IgG1 was the predominant subtype; however, the differences in the IgG1 and IgG2a titers were less in this group than in the salmonella-immunized mice.
TABLE 1.
Ratios of IgG1 to IgG2a anti-Frg C titers in sera of mice at periods after immunizationa
| Group | IgG1/IgG2a at indicated wk postimmunization
|
||
|---|---|---|---|
| 2 | 4 | 12 | |
| BRD847 | 52 | 193 | 108 |
| BRD937 | 1,657 | 956 | 483 |
| Frg C s.c. | 0.11 | 0.28 | 0.14 |
The specific anti-Frg C IgG1 and IgG2a titers were determined on pools of sera taken at the indicated times after immunization.
Intestinal anti-Frg C IgA response.
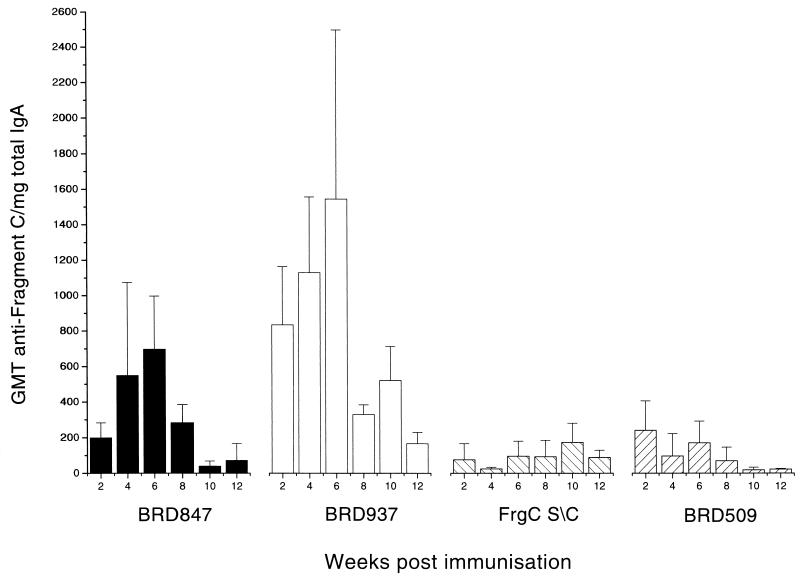
The local gut anti-Frg C IgA response was measured by analysis of extracts of fecal pellets (Fig. 4). Not unexpectedly, the response was quite variable. A measurable response was seen in both the BRD937- and BRD847-immunized mice. Only a very small response was seen in the s.c. group, and this was not above that seen in the control mice. Again, titers were higher in the BRD937 group than in the BRD847 group. However, the kinetics of the responses were the same in both groups, with a steady increase in titer from weeks 2 to 6 and a rapid drop in titer thereafter.
FIG. 4.
Intestinal IgA Frg C response. Extracts were prepared from fecal pellets collected from mice at periods after immunization orally with BRD847, BRD915, or BRD509 or s.c. with fragment C plus alhydrogel. The specific IgA titer was determined. To correct for variations in the amount feces collected and in the amount of IgA extracted, the titer was divided by the total amount of IgA. Each bar represents the geometric mean of the corrected titer (GMT) ± the standard error of the mean.
Immunity to tetanus and salmonella.
At 15 weeks mice were challenged with 50 LD50 of tetanus toxin and monitored for signs of tetanus for 4 days (Table 2). All mice in the BRD937, BRD847, and Frg C s.c. groups were completely immune to tetanus toxin, whereas control mice all died within 4 days. Two weeks later, mice were orally challenged with wild-type S. typhimurium SL1344. All mice in the BRD937 and BRD847 groups were solidly immune to S. typhimurium.
TABLE 2.
Immunity of mice to tetanus and salmonellaea
| Immunogen | No. survivors/5 challenged
|
|
|---|---|---|
| Tetanus challenge | Salmonella challenge | |
| BRD847 | 5 | 5 |
| BRD937 | 5 | 5 |
| Frg C + alhydrogel | 5 | 0 |
| BRD509 | 0 | ND |
| None | 0 | 0 |
Fifteen weeks after immunization, mice were challenged s.c. with 50 LD50 of tetanus toxin. Animals were monitored for signs of tetanus for 4 days. Two weeks after tetanus challenge, surviving and naive control mice were orally challenged with 8 × 108 CFU of wild-type S. typhimurium SL1344. Mice were observed for 28 days, and fatalities were recorded. ND, not determined.
DISCUSSION
We have compared the immune responses to Frg C in mice immunized with a live salmonella vector expressing Frg C from two different regulated promoters, PhtrA and PnirB, and mice immunized parenterally with Frg C plus adjuvant. The purpose was to determine if PhtrA has utility for expressing foreign antigens in salmonella and also if there were advantages of using PhtrA rather than PnirB. We found that BRD937 (BRD509 pTEThtrA1) was superior, in terms of the anti-Frg C response, to BRD847 (BRD509 pTETnir15). The difference was manifest in terms of the magnitude of the antibody response and also in the speed with which anti-Frg C antibodies developed. Surprisingly, over the 12-week course of the experiment, the anti-Frg C titers in the sera of the BRD937-immunized mice were also superior to those in the mice parenterally immunized with Frg C s.c. plus alhydrogel. This applied to all antibody classes. Also, the peak serum anti-Frg C response occurred earlier in the mice immunized with BRD937 than in mice immunized parenterally. In contrast, apart from the serum IgA anti-Frg C titers, the serum anti-Frg C response was greater in the s.c. group than in mice immunized with BRD847. It is possible that increased amounts of Frg C administered parenterally would lead to an improved immune response. The dose used, 10 μg, was arbitrarily chosen; however, submicrogram doses of Frg C are immunogenic in BALB/c mice, and immunization with 1 μg will protect all vaccinated mice from tetanus (18). The amount of Frg C produced in vivo by the Salmonella strain is unknown, as is the amount of Frg C that the murine immune system is exposed to.
As expected, s.c. immunization with Frg C did not stimulate an intestinal IgA response. Intestinal IgA anti-Frg C antibodies were induced by oral immunization of mice with either of the Salmonella strains expressing Frg C. The fecal IgA anti-Frg C titers were high in mice in the BRD937 group, but the kinetics of the responses were similar in both groups, with the peak response occurring at 6 weeks after immunization. This is later than the peak IgG serum responses (4 weeks in both salmonella-Frg C groups).
The peak anti-Frg C IgA titer occurred around week 10 in the BRD937 group. The superior IgA anti-Frg C response in the salmonella-Frg C groups over that in the Frg C s.c. groups probably arises because of systemic seeding of lymphoid tissue by GALT-derived IgA-producing B cells.
The major difference in the kinetics of the serum Frg C responses in the mice immunized with the two Salmonella strains is the much higher levels of anti-Frg C antibodies, particularly of the IgG and IgM classes, at 2 weeks postimmunization in the BRD937 group. For example, the mean 2-week serum IgG anti-Frg C titer of BRD937-immunized mice is 232-fold higher than in BRD847-immunized mice. This difference drops to 33-fold by week 4. We do not know the reason why the anti-Frg C immune response develops more rapidly in BRD937-immunized mice. The only difference between BRD937 and BRD847 is the promoter controlling Frg C expression. It is possible that Frg C expression is switched on earlier in vivo in BRD937 than in BRD847 or that in vivo expression of Frg C is higher in BRD937 than in BRD847. Preliminary studies indicate that there is no difference in vivo in the growth kinetics of BRD847 and BRD937 or in the stability of pTETnir15 and pTEThtrA1 in S. typhimurium BRD509 (unpublished observation). Also, there was no significant difference in the serum antibody responses to S. typhimurium lipopolysaccharide in mice immunized with BRD847 and BRD937 (data not shown). Another difference in the kinetics of the Frg C responses is the decline in the IgM anti-Frg C titers to background levels by 8 weeks in the BRD847 group, while in the BRD937 group they remained higher than in the s.c. group.
The difference in the IgA anti-Frg C titers (both systemically and locally) elicited by immunization with BRD937 or BRD847 is much lower than the difference in the serum IgG and IgM anti-Frg C titers. The average fold difference in anti-Frg C titers in BRD937- and BRD847-immunized mice are as follows: serum IgG, ∼1,000; serum IgM, 24; serum IgA, 6; and fecal IgA, 4. If we assume that stimulation of the GALT gives rise to the local anti-Frg C IgA and in part to the serum IgA anti-Frg C antibodies, then these results may indicate that Frg C is expressed to similar levels or in similar cells or tissues by BRD937 and BRD847 in the GALT. It would then follow that the difference in the immune responses elicited by BRD937 and BRD847 arises once the organisms have disseminated systemically.
Everest et al. (17) used lacZ as a reporter gene to study the activity of different promoters, including PhtrA and PnirB, in S. typhimurium under different environmental conditions. They found (i) that the activities of PhtrA and PnirB were very similar upon temperature increase from 30 to 45°C, (ii) entry of S. typhimurium into epithelial and macrophage-like cells, and (iii) that PhtrA was activated by anaerobiosis (17). However, we observed that growth temperature does not greatly affect expression of Frg C from PnirB (unpublished observation) whereas Frg C expression from PhtrA is greatly increased by increasing the growth temperature. We have also not noticed an effect of low oxygen tension on Frg C expression in BRD937. These differences may result from the different proteins expressed or from differences in the background strain: Everest et al. (17) used an htrA mutant, BRD915, whereas BRD509 is an aroA aroD mutant. With green fluorescent protein as a reporter protein, it is possible to study the effects of different microenvironments in vivo on the activity of salmonella promoters by examination of tissue sections in a fluorescence microscope or fluorescence-activated cell sorter. Such studies have already revealed that PnirB is switched on in an S. typhimurium purE mutant present in liver abscesses (16). By using such constructs, it should prove possible to determine if there are differences in the activities of PnirB and PhtrA in salmonella in mucosal and systemic tissues.
The serum response to Frg C is long-lived even following a single oral immunization. At 12 weeks postimmunization, serum IgG anti-Frg C titers were not declining in any of the groups. All mice in the BRD937, BRD847, and Frg C s.c. groups were protected from tetanus challenge. Also, all mice in the BRD937 and BRD847 groups were immune to salmonella challenge. We have found that immunity to tetanus is still solid in mice immunized orally with a single dose of BRD937 or BRD847 25 weeks after vaccination (data not shown).
With any vaccination scheme, it is important to determine the number of animals that respond appropriately to the vaccine (in this case, the seroconversion rate) as well as the magnitude of the immune response. In the case of tetanus vaccines, challenge of experimental animals will reveal how many animals have developed a protective immune response, but similar assays are not possible for all infectious agents. At all time points examined, all mice in the BRD937, BRD847, and Frg C s.c. groups had significant IgG anti-Frg C titers (data not shown). It is important to measure the responses of individual animals rather than pools of serum because we have found that a single animal that has a high response can mask poorer responses in other mice if pooled sera are analyzed (unpublished observation).
HtrA (also known as DegP) is a serine protease active in the periplasm (24–26, 38, 41). HtrA is essential for survival of E. coli but not S. typhimurium at elevated temperatures (20, 24–26, 38, 41). Salmonella htrA mutants are highly attenuated in mice and are defective in intramacrophage survival (3, 10, 20). The mechanisms controlling expression of HtrA have been studied in E. coli in vitro (30). Expression of HtrA is controlled at the transcriptional level by two regulatory systems: the alternative sigma factor ςE and the Cpx two-component regulator (14, 30, 33). Both of these systems are responsive to changes in protein composition of the cell envelope (30). The ςE regulon is also induced by general stresses such as heat shock or exposure to ethanol (30). Overexpression of a number of outer membrane proteins in E. coli upregulates ςE activity and increases expression of htrA (29). Overexpression of a specific lipoprotein, NplE, also induces htrA expression, and this is dependent on the Cpx system, which consists of CpxA, a sensor kinase, and CpxR, its cognate response regulator (39). Pathways that lead to induction of the ςE and Cpx regulons are independent: increased expression of NlpE does not upregulate ςE-regulated genes other than htrA, and increased expression of outer membrane proteins does not enhance expression of members of the Cpx regulon other than htrA (14, 33). Null mutations in the cpx genes do not affect temperature regulation of htrA (33). Genes regulated by ςE have characteristic promoter sequences with defined −35 and −10 regions (29, 36, 37). Recently two binding sites for CpxR have been mapped upstream of the ςE-dependent promoter of htrA (33). These Cpx binding sites were not present in the oligonucleotides used to construct pTEThtrA1. It will be interesting to see if a construct that includes the CpxR binding sites has properties different from those of pTEThtrA1 used in this study. Likewise, it will be interesting to see if non-ςE-dependent promoters that are regulated by Cpx, such as ppiA, are useful for expressing heterologous antigens in salmonella (33).
We and others have shown that antigens expressed in salmonella stimulate predominately a Th1 T-cell response (1, 4, 35, 45, 46). T-cell and antibody analysis has shown that this is also the case for Frg C in mice immunized with BRD847 (21, 45). Interestingly, from the anti-Frg C IgG1/IgG2a ratio it appears that BRD937 may induce a stronger Th1 response than BRD847. It will be necessary to perform more in-depth studies analyzing the T-cell cytokine response to determine if this is in fact the case. If it is, then the htrA promoter may be more appropriate than the nirB promoter for expressing antigens when a polarized Th1 response is required for immunity, such as with Leishmania sp. (46).
Despite the existence of an effective tetanus vaccine, half a million people die from tetanus annually, most in the developing world (40). The reason for this is the current vaccine, tetanus toxoid, is not ideal for use in the developing world. The drawbacks of the current vaccine are that it needs a cold chain for storage and that multiple doses need to be administered to stimulate full immunity. The need to inject the vaccine is also a drawback to the use of tetanus toxoid in developing countries because of the reuse of needles, which is prevalent and exposes the vaccinee to infection with blood-borne agents such as hepatitis B and human immunodeficiency viruses. Strains of S. typhi expressing Frg C are being developed for use as oral tetanus vaccines. We have constructed two PhtrA-Frg C-expressing S. typhi strains based on the double aro mutant CVD908 (44). We have introduced pTEThtrA1 into CVD908 and also integrated a single copy of PhtrA-Frg C into the aroA gene (data not shown). In both cases, Frg C expression is upregulated when the growth temperature is increased (data not shown). These strains or similar strains expressing Frg C from PhtrA will, we hope, be useful in the development of noninjectable tetanus vaccines for humans.
ACKNOWLEDGMENTS
We thank Susan Humphreys and Andrew Stevenson for critical reading of the manuscript.
This work was supported by grant P05639 from the BBSRC.
REFERENCES
- 1.Anderson R, Dougan G, Roberts M. Delivery of the pertactin/P.69 polypeptide of Bordetella pertussis using an attenuated Salmonella typhimurium vaccine strain: expression levels and immune response. Vaccine. 1996;14:1384–1390. doi: 10.1016/s0264-410x(96)00036-9. [DOI] [PubMed] [Google Scholar]
- 2.Bajaj V, Lucas R L, Hwang C, Lee C A. Coordinate regulation of Salmonella typhimurium invasion genes by environmental and regulatory factors is mediated by control of hila expression. Mol Microbiol. 1996;22:703–714. doi: 10.1046/j.1365-2958.1996.d01-1718.x. [DOI] [PubMed] [Google Scholar]
- 3.Baumler A J, Kusters J G, Stojiljkovic I, Heffron F. Salmonella typhimurium loci involved in survival within macrophages. Infect Immun. 1994;62:1623–1630. doi: 10.1128/iai.62.5.1623-1630.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brett S J, Dunlop L, Liew F Y, Tite J P. Influence of the antigen delivery system on immunoglobulin isotype selection and cytokine production in response to influenza a nucleoprotein. Immunology. 1993;80:306–312. [PMC free article] [PubMed] [Google Scholar]
- 5.Cardenas L, Clements J D. Oral immunization using live attenuated Salmonella spp. as carriers of foreign antigens. Clin Microbiol Rev. 1992;5:328–342. doi: 10.1128/cmr.5.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chabalgoity J A, Khan C M A, Nash A A, Hormaeche C E. A Salmonella typhimurium htrA live vaccine expressing multiple copies of a peptide comprising amino acids 8-23 of herpes simplex virus glycoprotein D as a genetic fusion to tetanus toxin fragment c protects mice from herpes simplex virus infection. Mol Microbiol. 1996;19:791–801. doi: 10.1046/j.1365-2958.1996.426965.x. [DOI] [PubMed] [Google Scholar]
- 7.Chacon M R, Londono P, Dougan G, Selkirk M E. Heterologous expression of the cuticular glutathione-peroxidase of lymphatic filariae in an attenuated vaccine strain of Salmonella typhimurium abrogates H-2-restriction of specific antibody-responses. Parasite Immunol. 1996;18:307–316. doi: 10.1046/j.1365-3024.1996.d01-105.x. [DOI] [PubMed] [Google Scholar]
- 8.Chatfield S N, Charles I G, Makoff A J, Oxer M D, Dougan G, Pickard D, Slater D, Fairweather N F. Use of the nirB promoter to direct the stable expression of heterologous antigens in salmonella oral vaccine strains: development of a single-dose oral tetanus vaccine. Bio/Technology. 1992;10:888–892. doi: 10.1038/nbt0892-888. [DOI] [PubMed] [Google Scholar]
- 9.Chatfield S N, Roberts M, Dougan G, Hormaeche C, Khan C M A. The development of oral vaccines against parasitic diseases utilizing live attenuated salmonella. Parasitology. 1995;110:S17–S24. doi: 10.1017/s0031182000001451. [DOI] [PubMed] [Google Scholar]
- 10.Chatfield S N, Strahan K, Pickard D, Charles I G, Hormaeche C E, Dougan G. Evaluation of Salmonella typhimurium strains harboring defined mutations in htrA and aroA in the murine salmonellosis model. Microb Pathog. 1992;12:145–151. doi: 10.1016/0882-4010(92)90117-7. [DOI] [PubMed] [Google Scholar]
- 11.Clare J J, Rayment F B, Ballantine S P, Sreekrishna K, Romanos M A. High-level expression of tetanus toxin fragment C in Pichia pastoris strains containing multiple tandem integrations of the gene. Bio/Technology. 1991;9:455–460. doi: 10.1038/nbt0591-455. [DOI] [PubMed] [Google Scholar]
- 12.Coulson N M, Fulop M, Titball R W. Effect of different plasmids on colonization of mouse tissues by the aromatic amino acid dependent Salmonella typhimurium SL3261. Microb Pathog. 1994;16:305–311. doi: 10.1006/mpat.1994.1031. [DOI] [PubMed] [Google Scholar]
- 13.Curtiss R, III, Galan J E, Nakayama K, Kelly S M. Stabilization of recombinant avirulent vaccine strains in vivo. Res Microbiol. 1990;141:797–805. doi: 10.1016/0923-2508(90)90113-5. [DOI] [PubMed] [Google Scholar]
- 14.Danese P N, Silhavy T J. The sigma factor E and the Cpx signal transduction systems control the synthesis of periplasmic protein-folding enzymes in Escherichia coli. Genes Dev. 1997;11:1183–1193. doi: 10.1101/gad.11.9.1183. [DOI] [PubMed] [Google Scholar]
- 15.Davis R W, Botstein D, Roth J R. Advanced bacterial genetics: a manual for genetic engineering. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1980. [Google Scholar]
- 16.Everest P, Allen J, Papakonstantinopoulou A, Mastroeni P, Roberts M, Dougan G. Salmonella typhimurium infections in mice deficient in interleukin-4 production—role of IL-4 in infection-associated pathology. J Immunol. 1997;159:1820–1827. [PubMed] [Google Scholar]
- 17.Everest P, Frankel G, Li J, Lund P, Chatfield S, Dougan G. Expression of LacZ from the htrA, nirB and groE promoters in a salmonella vaccine strain: influence of growth in mammalian cells. FEMS Microbiol Lett. 1995;126:97–101. doi: 10.1111/j.1574-6968.1995.tb07398.x. [DOI] [PubMed] [Google Scholar]
- 18.Fairweather N F, Lyness V A, Maskell D J. Immunization of mice against tetanus with fragments of tetanus toxin synthesized in Escherichia coli. Infect Immun. 1987;55:2541–2545. doi: 10.1128/iai.55.11.2541-2545.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoiseth S K, Stocker B A D. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 20.Johnson K, Charles I, Dougan G, Pickard D, Ogaora P, Costa G, Ali T, Miller I, Hormaeche C. The role of a stress-response protein in Salmonella typhimurium virulence. Mol Microbiol. 1991;5:401–407. doi: 10.1111/j.1365-2958.1991.tb02122.x. [DOI] [PubMed] [Google Scholar]
- 21.Karem K L, Chatfield S, Kuklin N, Rouse B T. Differential induction of carrier antigen-specific immunity by Salmonella typhimurium live-vaccine strains after single mucosal or intravenous immunization of BALB/c mice. Infect Immun. 1995;63:4557–4563. doi: 10.1128/iai.63.12.4557-4563.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan C M A, VillarrealRamos B, Pierce R J, De Hormaeche R D, McNeill H, Ali T, Chatfield S, Capron A, Dougan G, Hormaeche C E. Construction, expression, and immunogenicity of multiple tandem copies of the Schistosoma mansoni peptide 115-131 of the P28 glutathione S-transferase expressed as C-terminal fusions to tetanus toxin fragment C in a live aro- attenuated vaccine strain of salmonella. J Immunol. 1994;153:5634–5642. [PubMed] [Google Scholar]
- 23.Lee C A, Falkow S. The ability of salmonella to enter mammalian cells is affected by bacterial growth state. Proc Natl Acad Sci USA. 1990;87:4304–4308. doi: 10.1073/pnas.87.11.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lipinska B, Fayet O, Baird L, Georgopoulos C. Identification, characterization, and mapping of the Escherichia coli htrA gene, whose product is essential for bacterial growth only at elevated temperatures. J Bacteriol. 1989;171:1574–1584. doi: 10.1128/jb.171.3.1574-1584.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lipinska B, Sharma S, Georgopoulos C. Sequence-analysis and regulation of the htrA gene of Escherichia coli—a sigma-32-independent mechanism of heat-inducible transcription. Nucleic Acids Res. 1988;16:10053–10067. doi: 10.1093/nar/16.21.10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipinska B, Zylicz M, Georgopoulos C. The HtrA (DegP) protein, essential for Escherichia coli survival at high temperatures, is an endopeptidase. J Bacteriol. 1990;172:1791–1797. doi: 10.1128/jb.172.4.1791-1797.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Londono L P, Chatfield S, Tindle R W, Herd K, Gao X M, Frazer I, Dougan G. Immunization of mice using Salmonella typhimurium expressing human papillomavirus type-16 E7 epitopes inserted into hepatitis-b virus core antigen. Vaccine. 1996;14:545–552. doi: 10.1016/0264-410x(95)00216-n. [DOI] [PubMed] [Google Scholar]
- 28.Maskell D J, Sweeney K J, Ocallaghan D, Hormaeche C E, Liew F Y, Dougan G. Salmonella typhimurium aroA mutants as carriers of the Escherichia coli heat-labile enterotoxin-b subunit to the murine secretory and systemic immune systems. Microb Pathog. 1987;2:211–221. doi: 10.1016/0882-4010(87)90022-2. [DOI] [PubMed] [Google Scholar]
- 29.Mecsas J, Rouviere P E, Erickson J W, Donohue T J, Gross C A. The activity of sigma factor E, an Escherichia coli heat-inducible sigma factor, is modulated by expression of outer-membrane proteins. Genes Dev. 1993;7:2618–2628. doi: 10.1101/gad.7.12b.2618. [DOI] [PubMed] [Google Scholar]
- 30.Missiakas D, Raina S. Protein misfolding in the cell envelope of Escherichia coli: new signaling pathways. Trends Biochem Sci. 1997;22:59–63. doi: 10.1016/s0968-0004(96)10072-4. [DOI] [PubMed] [Google Scholar]
- 31.Nakayama K, Kelly S M, Curtiss R., III Construction of an asd+ expression-cloning vector: stable maintenance and high level expression of cloned genes in a salmonella vaccine strain. Bio/Technology. 1988;6:693–697. [Google Scholar]
- 32.Oxer M D, Bentley C M, Doyle J G, Peakman T C, Charles I G, Makoff A J. High-level heterologous expression in Escherichia coli using the anaerobically-activated nirB promoter. Nucleic Acids Res. 1991;19:2889–2892. doi: 10.1093/nar/19.11.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pogliano J, Lynch A S, Belin D, Lin E C C, Beckwith J. Regulation of Escherichia coli cell envelope proteins involved in protein folding and degradation by the Cpx two-component system. Genes Dev. 1997;11:1169–1182. doi: 10.1101/gad.11.9.1169. [DOI] [PubMed] [Google Scholar]
- 34.Roberts M, Bacon A, Rappuoli R, Pizza M, Cropley I, Douce G, Dougan G, Marinaro M, McGhee J, Chatfield S. A mutant pertussis toxin molecule that lacks ADP-ribosyltransferase activity, PT-9K/129G, is an effective mucosal adjuvant for intranasally delivered proteins. Infect Immun. 1995;63:2100–2108. doi: 10.1128/iai.63.6.2100-2108.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts M, Chatfield S N, Dougan G. Salmonella as carriers of heterologous antigens. In: O’Hagan D T, editor. Novel delivery systems for oral vaccines. Boca Raton, Fla: CRC Press; 1994. pp. 27–58. [Google Scholar]
- 36.Rouviere P E, Penas A D L, Mecsas J, Lu C Z, Rudd K E, Gross C A. rpoE, the gene encoding the 2nd heat-shock sigma-factor, sigma factor E, in Escherichia coli. EMBO J. 1995;14:1032–1042. doi: 10.1002/j.1460-2075.1995.tb07084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schurr M J, Yu H, Boucher J C, Hibler N S, Deretic V. Multiple promoters and induction by heat shock of the gene encoding the alternative sigma factor AlgU (ςE) which controls mucoidy in cystic fibrosis isolates of Pseudomonas aeruginosa. J Bacteriol. 1995;177:5670–5679. doi: 10.1128/jb.177.19.5670-5679.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seol J H, Woo S K, Jung E M, Yoo S J, Lee C S, Kim K J, Tanaka K, Ichihara A, Ha D B, Chung C H. Protease Do is essential for survival of Escherichia coli at high temperatures—its identity with the htrA gene-product. Biochem Biophys Res Commun. 1991;176:730–736. doi: 10.1016/s0006-291x(05)80245-1. [DOI] [PubMed] [Google Scholar]
- 39.Snyder W B, Davis L J B, Danese P N, Cosma C L, Silhavy T J. Overproduction of NlpE, a new outer membrane lipoprotein, suppresses the toxicity of periplasmic LacZ by activation of the Cpx signal transduction pathway. J Bacteriol. 1995;177:4216–4223. doi: 10.1128/jb.177.15.4216-4223.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stanfield J P, Galazka A. Neonatal tetanus in the world today. Bull W H O. 1984;62:647–669. [PMC free article] [PubMed] [Google Scholar]
- 41.Strauch K L, Johnson K, Beckwith J. Characterization of degP, a gene required for proteolysis in the cell envelope and essential for growth of Escherichia coli at high temperature. J Bacteriol. 1989;171:2689–2696. doi: 10.1128/jb.171.5.2689-2696.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strugnell R, Dougan G, Chatfield S, Charles I, Fairweather N, Tite J, Jing Li L, Beesley J, Roberts M. Characterization of a Salmonella typhimurium aro vaccine strain expressing the P.69 antigen of Bordetella pertussis. Infect Immun. 1992;60:3994–4002. doi: 10.1128/iai.60.10.3994-4002.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strugnell R A, Maskell D, Fairweather N, Pickard D, Cockayne A, Penn C, Dougan G. Stable expression of foreign antigens from the chromosome of Salmonella typhimurium vaccine strains. Gene. 1990;88:57–63. doi: 10.1016/0378-1119(90)90059-z. [DOI] [PubMed] [Google Scholar]
- 44.Tacket C O, Hone D M, Losonsky G A, Guers L, Edelman R, Levine M M. Clinical acceptability and immunogenicity of CVD908 Salmonella typhi vaccine strain. Vaccine. 1992;10:443–446. doi: 10.1016/0264-410x(92)90392-w. [DOI] [PubMed] [Google Scholar]
- 45.Vancott J L, Staats H F, Pascual D W, Roberts M, Chatfield S N, Yamamoto M, Coste M, Carter P B, Kiyono H, McGhee J R. Regulation of mucosal and systemic antibody-responses by T-helper cell subsets, macrophages, and derived cytokines following oral immunization with live recombinant salmonella. J Immunol. 1996;156:1504–1514. [PubMed] [Google Scholar]
- 46.Yang D M, Fairweather N, Button L L, McMaster W R, Kahl L P, Liew F Y. Oral Salmonella typhimurium (aroA-) vaccine expressing a major leishmanial surface protein (gp63) preferentially induces T helper 1 cells and protective immunity against leishmaniasis. J Immunol. 1990;145:2281–2285. [PubMed] [Google Scholar]