Abstract
Background
Numerous prior studies have extensively highlighted the significance of the microbiome in association with asthma. While several studies have concentrated on the asthma microbiome in previous research, there is currently a lack of publications that employ bibliometric methods to assess this area.
Methods
In this study, the Web of Science Core Collection database was utilized as the data source, and the SCI-EXPANDED index was employed to ensure that the retrieved data were comprehensive and accurate. All original research articles and review articles related to the correlation between asthma and the microbiome were systematically searched from the inception of the database until June 20, 2023. These articles were subsequently visualized and analyzed using VOSviewer and CiteSpace software.
Results
A total of 1366 relevant publications were acquired, indicating a consistent annual increase in global publications in the field. The United States and China emerged as the top two contributors to international publications. Among prolific authors, Susan V. Lynch achieved the highest publication record, with Hans Bisgaard and Jakob Stokholm sharing the second position. The majority of publications concentrated on allergy-related and microbiome areas, with a few comprehensive journals standing out. Journals with 40 or more publications included the Journal of Allergy and Clinical Immunology, Allergy, Frontiers in Immunology, and PLOS One. The top 5 cited journals were the Journal of Allergy and Clinical Immunology, PLOS One, American Journal of Respiratory and Critical Care Medicine, Clinical and Experimental Allergy, and Nature. Upon analyzing keywords, high-frequency terms, such as asthma, gut microbiota, microbiome, children, childhood asthma, allergy, risk, exposure, inflammation, diversity, and chain fatty acids emerged as representative terms in the field.
Conclusion
This study systematically presented a comprehensive overview of the literature regarding the association between asthma and the microbiome over the last two decades. Through a bibliometric perspective, the findings may assist researchers with a better understanding of the essential information in the field.
Keywords: Asthma, Microbiome, Bibliometric analysis, CiteSpace, VOSviewer
1. Introduction
Asthma is one of the most common chronic respiratory diseases. The prevalent symptoms of asthma, including coughing, wheezing, shortness of breath, and chest tightness, cause noticeable distress to the daily lives and professional activities of both children and adults, with substantial implications for public health. Despite encouraging progress in human scientific research on asthma in recent decades, the global prevalence of asthma is on the rise, while regional variations are noteworthy. Data indicate that around 300 million individuals worldwide are currently dealing with asthma, and an additional 100 million are projected to be affected by 2025 [1]. Among them, the prevalence of asthma is greater in high-income countries compared with low-income countries [2]. In recent decades, there has been continuous research on the pathogenesis of asthma, revealing that asthma is not a condition with a singular cause. Extensive studies indicated that asthma is a complex, multifactorial disease influenced by various factors, including genetic susceptibility, environmental exposures, and host factors, contributing to the initiation and advancement of asthma [3]. The initiation and progression of asthma involve intricate mechanisms, including while not limited to toll-like receptors and various subpopulations of regulatory T-cells. This pathway may be influenced, in part, by changes in microbiota composition resulting from diverse lifestyle factors. In the late 1980s, Strachan introduced the “hygiene hypothesis,” the first theory proposing a correlation between microbes and allergies [4]. It suggests that childhood exposure to specific microbes plays a crucial role in the development of the immune system. The original “hygiene hypothesis” has been further substantiated and refined considering contemporary lifestyle changes, such as increased rates of cesarean section, elevated utilization of antibiotics, widespread adoption of formula feeding, and alterations in modern dietary patterns, all of which have significantly impacted the components of the intestinal microbiome [5,6]. Both gut flora dysbiosis and respiratory flora dysbiosis have been reported to be linked to the development of the immune system, and their association with the progression of asthma is also noteworthy [7]. In recent years, the integration of interdisciplinary approaches and advanced scientific tools into the asthma research has enhanced our comprehension of the condition. This integration is expected to provide fresh perspectives on the intricate etiopathogenesis of asthma, highlighting the multifaceted interactions among genetics, the environment, and the host.
With advancements in science and technology, our comprehension of the microbiome is deepening, and the relationship between the microbiome and human health and disease is becoming a focal point of attention. The healthy human microbiota is abundant [8], making early microbiome studies challenging. In contrast to traditional microbial culture methods, the application of multiomics techniques (e.g., metabolomics, metagenomics, metatranscriptomics, and metaproteomics) for studying the human microbiome has been resulted in a more in-depth investigation, leading to a qualitative leap in understanding microbiome diversity and abundance [[9], [10], [11], [12]]. Among these techniques, sequence analysis of cloned microbial small-subunit ribosomal RNA genes [16S ribosomal DNA (rDNA)] is the most commonly utilized method for microbiome studies. Usually, the terms microbiome and microbiota are interchangeably utilized in the literature [13,14]. In recent years, it has been consistently shown that the intestinal microbiota, respiratory microbiota, and skin microbiota are closely associated with the progression of asthma [7,13,[15], [16], [17], [18], [19], [20]]. The microbiomes of different anatomical sites do not affect the progression of asthma independently, while the microbiomes across various anatomical sites are interconnected and engage in mutual interactions. The concept of the gut-lung axis has significantly influenced the exploration of the association between asthma and the microbiome. It has been found that the gut-lung axis plays a substantial role in the initiation and progression of asthma [16,21]. Dysregulation of the gut microbiota not only modulates the immune response in the gastrointestinal tract, but also affects the immunity of distal organs, such as lung and airways, which may increase the risk of wheezing disorders caused by inflammation in children [22]. A growing body of evidence demonstrated that the microbiome of asthmatics is different from that of healthy individuals, and investigating the interactions between beneficial and pathogenic microbes, along with the associated immune-inflammatory responses, is pivotal for comprehending the mechanisms underlying their pathogenesis. Such exploration provides valuable insights for developing targeted interventions and innovative therapies. Over the past decades, the field of asthma microbiome research has witnessed numerous publications on the relationships among microbiome variations, therapeutic strategies, and different disease states. Despite the extensive literature reviews within this field, to date, no bibliometric study has concentrated on the microbiomes of asthmatics to analyze the relevant trends.
Bibliometrics originated in the early 20th century and evolved into an independent discipline in 1969 [23], and it is widely utilized for literature analysis [24]. Bibliometric analysis supports quantitative methods to systematically review and explore existing literature within a specific domain [25]. This analysis allows for the extraction of detailed information, such as authors, keywords, journals, countries, institutions, and references. Consequently, bibliometric analysis, including performance analysis, provides insights into the development of a field [26]. Ninkov, A., underscores the significance of a visual approach to co-citation analysis in bibliometrics for effective data interpretation, enhancing the comprehensiveness of the results [27]. Moreover, various elements within an article, such as authors, keywords, institutions, and countries, can be subjected to this analysis. The application of bibliometrics is particularly crucial in the medical field, where it has been implemented early on [28]. Hence, bibliometrics as a method enables the development of a comprehensive knowledge map for the asthma and microbiome field. This approach also has the potential to predict, to some extent, the emerging trends and focal points within this domain.
Therefore, a bibliometric and visual analysis was conducted on literature related to the association between asthma and the microbiome. This analysis included the examination of the number of documents, references, and trends in research based on country, author, and keyword, using VOSviewer and CiteSpace software. The study utilized the Web of Science Core Collection (WOSCC) database. The present study aimed to identify current research hotspots and predict trends in this area. The findings of this research may provide a reliable reference for researchers in this field and contribute to its further development.
2. Methods
2.1. Data source and search strategy
In this study, the WOSCC was selected as the data source, with the index set to SCI-EXPANDED to ensure comprehensive and accurate data retrieval. The study initiated the search process in The National Library of Medicine/MeSH database, generating a list of Medical Subject Headings (MeSHs). Subsequently, a search strategy was formulated as follows: TS = (“microbiota” OR “microbiome” OR “microbial community composition” OR “microbial community” OR “human microbiome” OR “microbial community structure”) AND (“Asthma” OR “Asthmas” OR “Bronchial Asthma” OR “Asthma, Bronchial”). The search spanned from the inception of the database to the date of the search (2023-06-20). To mitigate bias resulting from continuous database updates, the extraction and export of all files were completed on the same date (2023-06-20).
2.2. Inclusion and exclusion criteria
The inclusion criteria for document selection in this study comprised articles concentrating on asthma and the microbiome, specifically including original research articles and review articles. On the other hand, exclusion criteria encompassed types of literature, such as conference abstracts, letters, editorial materials, books, chapters, withdrawn publications, etc. Unpublished articles, duplicates, and any articles unrelated to the subject matter of this study were excluded.
2.3. Data analysis and visualization
Knowledge mapping in this study was conducted by the utilization of CiteSpace and VOSviewer software, each contributing its unique strengths. CiteSpace employs a data standardization method grounded in set theory for measuring the similarity of knowledge units. This enables the generation of Time zone and Timeline views, providing a clear depiction of knowledge evolution, historical literature spans, and field development trends within specific clusters over time [29]. On the other hand, VOSviewer employs a probabilistic-based data normalization approach and provides diverse visualization views in areas, such as keywords, co-institutions, and co-authors. This includes network visualization, overlay visualization, and density visualization, featuring user-friendly mapping and aesthetically pleasing imagery [30].
3. Results
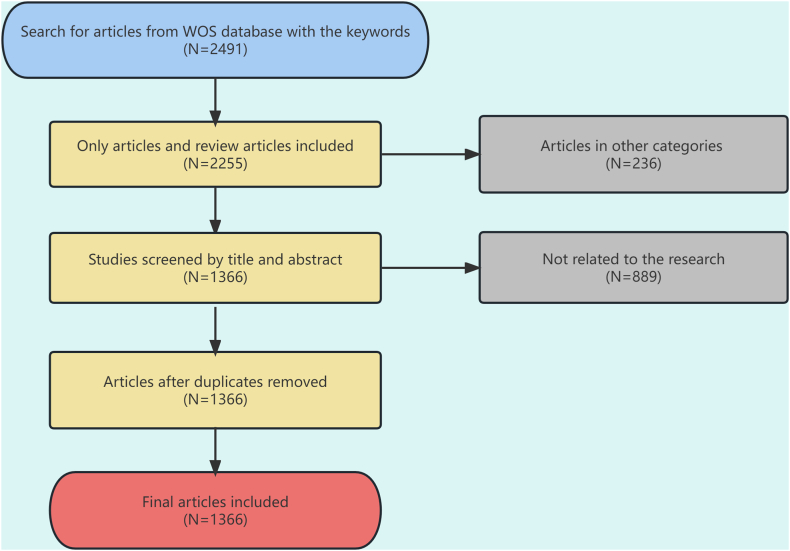
Initially, 2491 articles were retrieved from the WOSCC database, and they were subsequently screened by two physicians independently in strict accordance with the inclusion and exclusion criteria. In cases of disagreement, the final decision regarding study inclusion was reached through discussion. A total of 1366 articles were ultimately involved in the analysis. Fig. 1 illustrates the specific literature screening process.
Fig. 1.
Flow chart of literature screening process.
The analysis encompassed 1366 articles involving 6569 authors affiliated to 1892 organizations across 77 countries. These articles were published in 473 journals, and they were cited 47,138 times in 5681 journals.
3.1. Annual growth trend
Fig. 2 shows the temporal distribution of articles published in the research area of the relationship between bronchial asthma and the microbiome. Overall, the number of relevant published articles has increased; especially after 2011, the number of published relevant articles has rapidly increased, and the number of published articles was stabilized at more than 147 from 2017 to 2022. There is evidence of a rising interest among researchers in recent years, signifying the establishment of a novel and significant research domain in asthma and microbiome studies. Nevertheless, a marginal decline in publication number in 2022 was found, which could be attributed to the coronavirus disease 2019 (COVID-19) pandemic.
Fig. 2.
Trends in the number of publications from 2004 to 2023.
3.2. Authors’ analysis
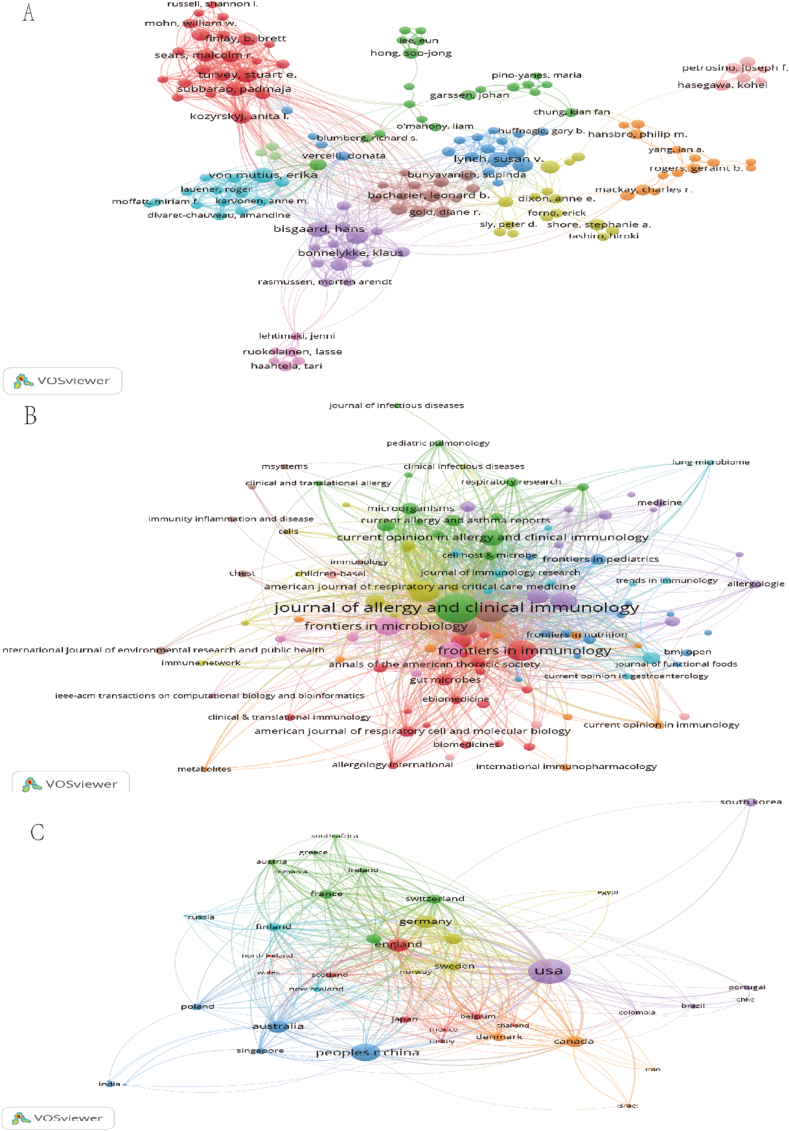
Through an analysis of document authors, data related to the typical authors and the central influence of studies in the research domain could be obtained. VOSviewer outputs revealed that the most productive authors had an article count represented by Nmax = 23. The minimum number of articles from typical authors in a specific area was calculated as M = Nmax1/2 × 0.749 ≈ 3.59. Accordingly, authors with equal to or more than 4 articles were acknowledged as typical authors in the area. In total, there were 74 core authors contributing to 1308 publications, constituting 95.75% of the overall publications. This meets the half (50%) criterion proposed by Price, signifying the formation of a relatively stable collaborative group of authors in the field of bronchial asthma and microbiome-related research. Table 1 lists the most productive authors who published more than 15 articles in the area, and highlights the top 11 authors based on their publication count. Utilizing VOSviewer software, authors with 5 or more publications were visually represented, as illustrated in Fig. 3A.
Table 1.
The top 11 authors with the highest number of publications.
| Rank | First author’s name | Number of articles | Number of citations | Average citation/publication |
|---|---|---|---|---|
| 1 | Lynch, Susan V. | 23 | 2656 | 115.48 |
| 2 | Bisgaard, Hans | 21 | 1867 | 88.90 |
| 3 | Stokholm, Jakob | 21 | 1773 | 84.43 |
| 4 | Turvey, Stuart e. | 20 | 1977 | 98.85 |
| 5 | Von Mutius, Erika | 20 | 1584 | 79.20 |
| 6 | Huang, Yvonne J. | 18 | 1778 | 98.78 |
| 7 | Subbarao, Padmaja | 17 | 1668 | 98.12 |
| 8 | Finlay, B. Brett | 16 | 2031 | 126.94 |
| 9 | Kozyrskyj, Anita L. | 16 | 947 | 59.19 |
| 10 | Marsland, Benjamin J. | 15 | 1521 | 101.40 |
| 11 | Sears, Malcolm R. | 15 | 1742 | 116.13 |
Fig. 3.
(A) Visualization map of authors. Each node represents an author, the size of the node is proportional to the number of documents published and the lines between nodes represent cooperation between authors. (B) Visualization map of journals. Each node represents a journal, the size of the node is proportional to the number of documents published and the lines between nodes represent links between journals. (C) Visualization map of countries. Each node represents a country, the size of the node is proportional to the number of documents published and the lines between nodes represent cooperation between countries.
The most productive author was Susan V. Lynch, with a total of 23 publications from 2004 to June 2023, accounting for 2656 citations and an average of 115.48 citations per article. Susan V. Lynch is affiliated to the Department of Medicine at the University of California, San Francisco (San Francisco, CA, USA). Her research concentrates on investigating the role of gut flora and respiratory flora in the pathogenesis, prevention, and treatment of asthma. Additionally, she explores the relevance of the neonatal microbiome to the onset and progression of asthma. Hans Bisgaard and Jakob Stokholm ranked second, both with 21 publications. Hans Bisgaard received 1867 citations, averaging 88.90 citations per article, while Jakob Stokholm received 1773 citations, averaging 84.43 citations per article. Both Bisgaard and Stokholm are affiliated to Herlev and Gentofte Hospital, University of Copenhagen (Denmark), maintaining a close collaborative effort. They study the microbiome of perinatal infants in association with asthma prevalence and treatment outcomes.
3.3. Analysis of journals
Table 2 presents statistics on the journals featuring literature in this field. The top 10 journals with 3 or more articles were visualized using VOSviewer software (Fig. 3B). The analysis revealed that, over the past two decades, journals primarily centered around allergy-related and microbiome topics have been prolific in publishing articles on this subject. Journals with a publication volume exceeding or equal to 40 articles included the Journal of Allergy and Clinical Immunology, Allergy, Frontiers in Immunology, and PLOS ONE. The Journal of Allergy and Clinical Immunology, with 85 articles, emerged as the leading journal in this field. Notably, Frontiers in Immunology and PLOS ONE were both open-access journals. The analysis of journal citations, detailed in Table 2, identified the top 10 journals. The Journal of Allergy and Clinical Immunology, with 85 articles, was found as the most cited journal, accumulating 7874 citations and an average of 92.64 citations per article. This underscores the high quality and substantial interest garnered by this journal regarding the association between bronchial asthma and the microbiome. An examination of the literature published in this journal revealed a dedicated concentration on the roles of the intestinal and respiratory microbiome in the initiation, progression, and prevention of asthma.
Table 2.
The top 10 journals in the field.
| Rank | Source | Number of publications | Number of citations | Average citation/publication |
|---|---|---|---|---|
| 1 | Journal of Allergy and Clinical Immunology | 85 | 7874 | 92.64 |
| 2 | Allergy | 43 | 2084 | 48.47 |
| 3 | Frontiers in Immunology | 40 | 2144 | 53.60 |
| 4 | PLOS one | 40 | 2784 | 69.60 |
| 5 | Frontiers in Microbiology | 33 | 1284 | 38.91 |
| 6 | Clinical and Experimental Allergy | 30 | 1717 | 57.23 |
| 7 | Scientific reports | 26 | 1171 | 45.04 |
| 8 | Pediatric allergy and immunology | 25 | 764 | 30.56 |
| 9 | Current Opinion in Allergy and Clinical Immunology | 23 | 822 | 35.74 |
| 10 | Annals of Allergy Asthma & Immunology | 20 | 509 | 25.45 |
3.4. Analysis of countries/regions
This study conducted an analysis of the number of articles from 77 countries to assess the contributions made by different nations in the field of bronchial asthma and microbiome research. VOSviewer software was utilized to visualize countries with 4 or more publications, and the results are illustrated in Fig. 3C. The size of the nodes in the circle correlates with the number of articles issued; thicker connecting lines signify stronger collaboration, indicating joint publications between two countries. The varied colors of the nodes represent different clusters. Fig. 3C displays an imbalanced distribution of publications, demonstrating a pronounced top-effect, with few countries contributing the majority of articles. For a more in-depth analysis of highly productive countries, Table 3 outlines the top 10 nations based on the number of publications in the field. Examining the data in Table 3 revealed that scholars from the United States had the highest number of research articles in this area (447 articles in total), accounting for 32.72% of the total publications in the field. These articles received 30,589 citations, with an impressive average of 68.43 citations. Following closely is China, with a total of 224 articles and 4840 citations. Australia ranked as the highest average citations per article, with 105 articles receiving a total of 9538 citations and an impressive average of 90.84 citations per article.
Table 3.
The top 10 countries in the field.
| Rank | Country | Number of publications | Number of citations | Average citation/publication |
|---|---|---|---|---|
| 1 | USA | 447 | 30,589 | 68.43 |
| 2 | China | 224 | 4840 | 21.61 |
| 3 | UK | 121 | 7841 | 64.80 |
| 4 | Germany | 112 | 7778 | 69.45 |
| 5 | Australia | 105 | 9538 | 90.84 |
| 6 | Canada | 93 | 7478 | 80.41 |
| 7 | Netherlands | 78 | 5184 | 66.46 |
| 8 | Italy | 62 | 3366 | 54.29 |
| 9 | Switzerland | 62 | 4199 | 67.73 |
| 10 | France | 59 | 1793 | 30.39 |
3.5. Analysis of keywords
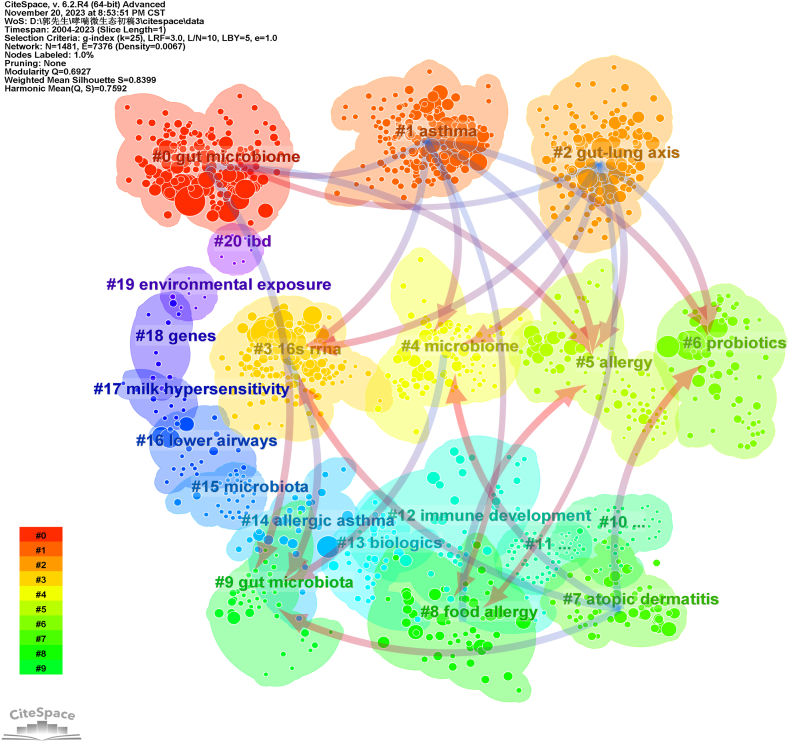
Keywords encapsulate the core essence of an article, and analyzing keyword co-occurrence can reveal research hotspots in a scientific field. Utilizing VOSviewer, a keyword co-occurrence network view for 1366 articles was generated, highlighting 276 keywords with a frequency of ≥10 for visualization (Fig. 4). As illustrated in Fig. 4A, larger nodes indicate more frequent appearances, representing key field hotspots. The connecting lines between nodes reflect the strength of association, and thicker lines denote more frequent co-occurrence. Colors of nodes and lines indicate different clusters, reflecting distinct research themes. Fig. 4B depicts the hotness of the research field, and brighter colors indicate higher prominence. Table 4 showcases high-frequency keywords with a frequency of more than 95, revealing that terms, such as asthma, gut microbiota (or intestinal microbiota), microbiome (or microbiota), children, childhood asthma, allergy, risk, exposure, inflammation, diversity, and short-chain fatty acids are representative in the field. The keyword clustering network diagram, generated using CiteSpace software for 1366 articles, is displayed in Fig. 5. The diagram revealed 10 clusters, including lung microbiome, gut microbiome (microbiota), allergic diseases, allergic asthma, metabolic dysfunction, and preterm infants. Over the past two decades, research has primarily concentrated on investigating the role of the microbiome in the onset, development, and treatment of asthma. The gut microbiome, as a primary focal point, has noticeably attracted scholars’ attention. There has been a growing interest in exploring the influence of the lung microbiome on asthma, particularly concerning pediatric populations. This suggests a sustained concentration on the relationship between the gut microbiome and asthma in children over the past two decades.
Fig. 4.
Network visualization of keywords.
Table 4.
List of high-frequency keywords.
| Keyword | Occurrences | Total link strength | Keyword | Occurrences | Total link strength |
|---|---|---|---|---|---|
| Asthma | 779 | 6123 | early-life | 192 | 1664 |
| Gut microbiota | 483 | 3663 | disease | 165 | 1224 |
| Microbiome | 309 | 2577 | exposure | 164 | 1376 |
| Microbiota | 276 | 2190 | inflammation | 141 | 1078 |
| Children | 243 | 2056 | diversity | 139 | 1081 |
| Intestinal microbiota | 235 | 1932 | bacteria | 121 | 947 |
| Childhood asthma | 214 | 1786 | health | 118 | 939 |
| Allergy | 210 | 1935 | chain fatty-acids | 102 | 741 |
| Risk | 204 | 1675 | airway inflammation | 95 | 723 |
Fig. 5.
Clustering network of keywords.
3.6. Keyword timeline analysis
To explore the evolution of the asthma microbiome field over time, the keyword co-occurrence network mapping was depicted using the Timeline diagram by CiteSpace software (Fig. 6). This diagram illustrates the progressive changes in research hotspots within this field in recent years. It was revealed that the retrieved articles could be classified into 13 major clusters, with each cluster corresponding to the research hotspot of a specific period outlined in the timeline (Fig. 6). The timeline was divided into two phases: from 2004 to 2010, the concentration was on the correlation between asthma and the microbiome, encompassing the gut microbiome, lung microbiome, and gut-lung axis, studied consistently across the timeline. Subsequently, between 2010 and 2023, various research areas emerged, expanding on the original topics and incorporating areas, such as microbiome diversity, food allergy, and inflammation.
Fig. 6.
Timeline view of keywords.
3.7. Outbreak keyword analysis
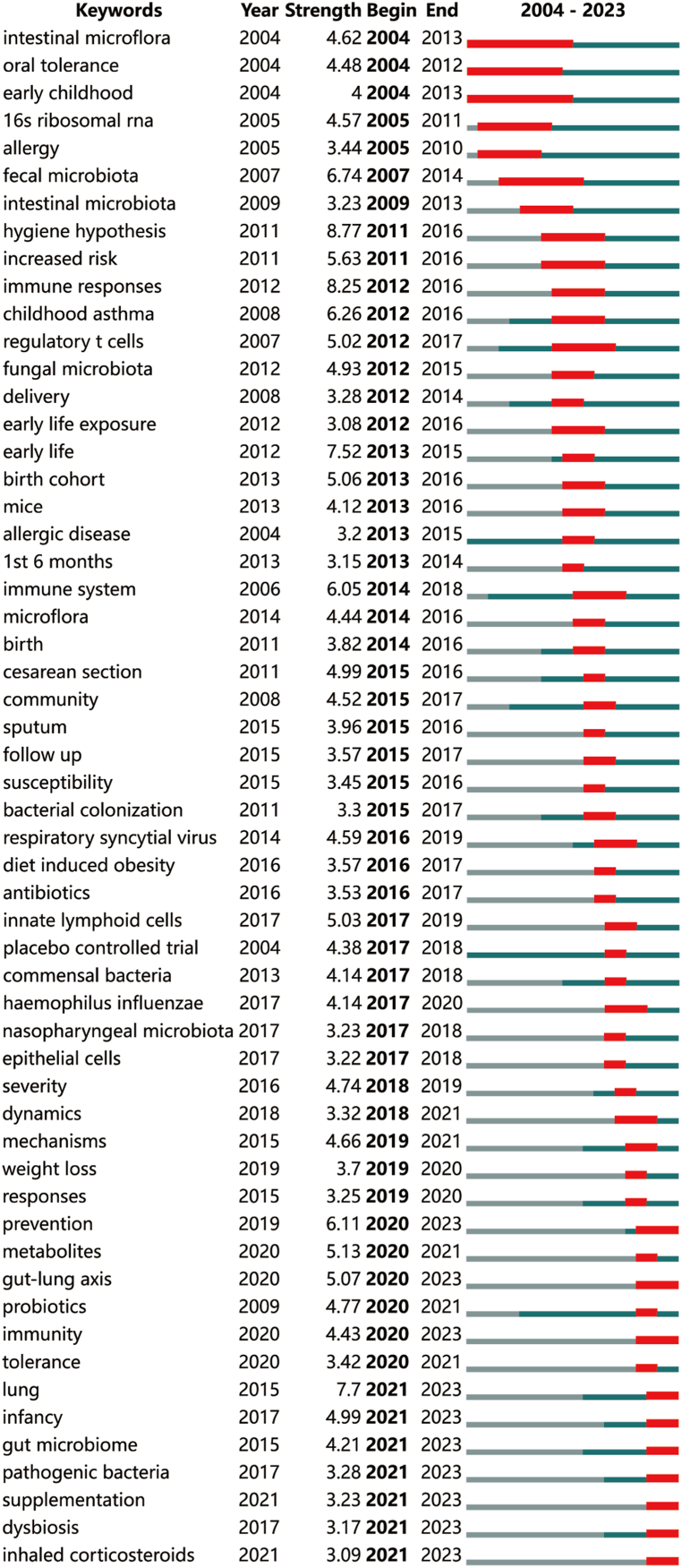
In order to achieve a clearer understanding of the abrupt emergence of research hotspots in the correlation between bronchial asthma and the microbiome, the Bursts analysis function of CiteSpace was utilized to examine outbreak keywords (Fig. 7). Notably, there were relatively few outbreak keywords between 2004 and 2009, likely reflecting the nascent stage of research in the field of asthma and microbiome correlation. Subsequently, from 2011 to 2021, as research deepened, stable outbreak keywords annually emerged. Analysis of the outbreak keywords indicated that between 2011 and 2015, the field primarily concentrated on the microbiome’s impact on asthma during childbirth and early childhood. From 2015 to 2018, research in the field shifted toward a more specific concentration on the influence of particular microbial species. Since 2018, there has been an elevated emphasis on holistic studies and mechanisms, concentrating on the microbiome’s role in preventing and treating asthma. Moreover, there has been an increased emphasis on in-depth investigations into the correlation between microbial diversity and asthma, adopting a comprehensive perspective (e.g., the gut-lung axis).
Fig. 7.
The top 56 keywords with the strongest citation bursts.
3.8. Analysis of co-cited journals
The purpose of co-citation analysis is to identify commonly referenced publications and journals in the field. A minimum threshold of 367 was set for journal co-citations and VOSviewer software was utilized to generate a co-citation map of cited journals. The analysis retained 50 journals for co-citation analysis, and Fig. 8 illustrates the resulting co-citation maps. The co-citation network of journals was classified into three clusters, corresponding to the three colors in Fig. 8. The top 5 journals based on citation count were the Journal of Allergy and Clinical Immunology (8478 citations), PLOS ONE (3234 citations), American Journal of Respiratory and Critical Care Medicine (3150 citations), Clinical and Experimental Allergy (2827 citations), and Nature (2635 citations). Four of these journals were outstanding JCR Q1 journals, with PLOS ONE being a JCR Q2 journal. All the five are prominent journals in the allergy and respiratory discipline, excluding Nature. In the blue cluster, journals primarily concentrated on allergy-related fields, emphasizing the microbiome’s impact on asthma as one of the allergic diseases. These journals concentrated on intrinsic immune-regulatory and inflammatory response mechanisms. Journals in the green cluster were predominantly in the field of respiratory-related diseases, highlighting a greater interest in the microbiome’s impact on clinical symptoms and treatment of asthma. The red cluster comprises journals in the microbiome-related field, placing a greater emphasis on variations in the microbiome composition in patients with asthma.
Fig. 8.
Visualization map of cited journals.
3.9. Analysis of co-cited articles
The co-citations of the literature were further analyzed by VOSviewer software to find out the top 5 cited articles in this field between 2004 and 2023, as illustrated in Table 5. According to Table 5, among the 5 highly co-cited articles, 2 articles were published in the excellent medical journal, New England Journal of Medicine, concentrating on the effects of neonatal airway bacteria and exposure to environmental microbiome on asthma; another article also investigated the effects of the microbiome on the risk of asthma during infancy; an article from PLOS ONE concentrated on the disordered microbial communities in the airways of asthmatics, while an article from Nature Medicine assessed the impact of dietary fiber gut microbes on allergic airway disease.
Table 5.
Top 5 Co-cited articles.
| Rank | Title | Year | Number of citations |
|---|---|---|---|
| 1 | Disordered microbial communities in asthmatic airways | 2010 | 292 |
| 2 | Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis | 2014 | 286 |
| 3 | Early infancy microbial and metabolic alterations affect risk of childhood asthma | 2015 | 276 |
| 4 | Childhood asthma after bacterial colonization of the airway in neonates | 2007 | 215 |
| 5 | Exposure to environmental microorganisms and childhood asthma | 2011 | 212 |
3.10. Analysis of co-cited references
Finally, VOSviewer software was employed to visualize the co-citation relationships among document references, using a minimum citation threshold of 100. A total of 36 articles were selected for co-citation analysis, and the resulting co-citation relationships are illustrated in Fig. 9. It was revealed that the co-citation network for highly cited literature could be categorized into four main clusters, corresponding to the 4 colors (Fig. 9). The red clusters primarily concentrate on the effects of different microbiome compositions on asthma, with a particular concentration on the composition of the intestinal flora. The green clusters concentrate on the effects of the respiratory microbiome on asthma. The blue cluster is associated with the impact of microbiome metabolites on immunomodulation and inflammatory responses in asthma, placing greater emphasis on exploring intrinsic mechanisms. The yellow clusters concentrate on the microbiome and its metabolites in the pathogenesis of asthma in children. To achieve a more detailed understanding of the research preoccupations and hotspots in this field, the cluster dependencies function of CiteSpace was utilized to conduct a more in-depth analysis of the information provided by these co-cited references (Fig. 10). Through the clustered network view of co-cited references generated by the software, it was evident that, based on terms, such as 16s rRNA, microbiome, and allergy, studies related to the gut microbiome (microbiota), asthma, gut-lung axis, probiotics, and similar topics have noticeably attracted researchers’ attention.
Fig. 9.
Visualization map of cited references.
Fig. 10.
Clustering network of cited references.
4. Discussion
The study is based on articles retrieved from the WOSCC database, involving the bibliometric and visual analysis of 1366 publications exploring the relationship between asthma and the microbiome. These publications, spanning from 2004 to 2023 (as of 2023-06-20), were examined using VOSviewer and CiteSpace software. The aim was to comprehend the current state of research in this field globally, identify prevalent research topics, and predict future trends.
Publications investigating the correlation between asthma and the microbiome exhibit a notable upward trajectory from 2004 to 2023. Between 2004 and 2010, the number of publications concentrated on asthma-microbiome correlations remained relatively low and exhibited erratic trends. A consistent increase in publications occurred from 2011 to 2021, surpassing 140 publications annually since 2017. However, there was a minor decline in 2022, likely influenced by the global COVID-19 pandemic. These findings indicate a rapid expansion of research on the correlation between asthma and the microbiome between 2011 and 2022.
The number of articles published by a country serves as a meaningful indicator of its scholarly capacity. Table 3 highlights the top 5 countries with the highest publication number in the field of asthma-microbiome correlation, namely the United States, China, the United Kingdom, Germany, and Australia. Each of these countries has contributed to the publication of over 100 articles, and the United States was the leading country with 447 publications, signifying its dominant position. Together, these 5 countries contribute to over 70% of the published articles, emphasizing their substantial research efforts in this domain. The United States holds the highest total citations, while Australia leads in the average number of citations per article. These outcomes underscore the substantial contributions made by the United States and Australia, establishing them as key players in the asthma-microbiome correlation field. Regarding international collaboration, Fig. 3C illustrates that the United Kingdom and the United States exhibit the closest cooperation with other countries. European nations, particularly the United Kingdom and Germany, alongside the United States, form the core of the international cooperation network in asthma-microbiome correlation research, fostering extensive collaborative relationships.
Table 1 lists productive authors with more than 15 articles in the area of asthma-microbiome correlation, as well as the top 11 authors with the highest number of publications. Among the top 10 authors with the highest number of publications, Lynch Susan V. ranked the first. Bisgaard Hans and Stokholm Jakob ranked the second. Notably, B. Brett Finlay, despite sharing the eighth position with 16 publications, achieved the highest average number of citations per article (126.94), highlighting his significant influence in the field. Susan V. Lynch has consistently published numerous articles each year since her inaugural article published in 2010, featuring in top-tier journals, such as Nature Medicine, Nature Microbiology, Nature Communications, Cell Host & Microbe, and Cell Reports Medicine, as well as other leading generalist and specialty journals in allergy, respiratory disease, and microbiology-related fields. Lynch’s research primarily concentrated on the role of intestinal and respiratory flora in asthma’s pathogenesis, prevention, and treatment, targeting the correlation between neonatal microbiome and asthma occurrence and development. Similarly, Hans Bisgaard and Jakob Stokholm have contributed significantly to major comprehensive and specialty journals, concentrating on the microbiome of perinatal infants and its association with asthma prevalence and treatment outcomes.
An analysis of international peer-reviewed journals that published articles related to asthma and the microbiome was conducted to enhance understanding of current research trends in the field and assist scholars in identifying crucial journals. Notably, the Journal of Allergy and Clinical Immunology and PLOS ONE were among the top 10 journals with the highest number of publications and ranked among the top 5 co-cited journals with the highest number of citations. This underscores their pivotal importance in the field, making it essential for researchers to stay informed about the latest developments in these journals.
In Fig. 6, key outbreak words that persisted until 2023, including “prevention,” “gut-lung axis,” “immunity,” “lung,” “infancy,” “gut microbiome,” “pathogenic bacteria,” “supplementation,” “dysbiosis,” and “inhaled corticosteroids,” were filtered out. The top five words with the highest outbreak strength were “lung,” “prevention,” “gut-lung axis,” “infancy,” and “immunity.” These findings reveal recent research hotspots in the correlation between asthma and the microbiome, concentrating on the microbiome’s role in asthma prevention, the relationship between the gut-lung axis and asthma onset and development, the connection between asthma in infancy and the microbiome, and the involvement of the microbiome in the immunomodulation of asthma.
Consequently, we outline research advances and trends in the field of asthma-microbiome correlation studies in the following five directions.
4.1. Gut microbiome and asthma
The intestinal microbiome is comprised of 1000–5000 bacterial species, yet an individual's gut typically harbors only around 160 species. Notably, the gut microbiota boasts approximately 3.3 million genes [13]. The involvement of the intestinal microbiome and its metabolites in the pathogenesis, diagnosis, and treatment of asthma has been a focal point of research for several decades. In a state of health, the gut microbiome predominantly consists of Proteobacteria, Bacteroidetes, Actinobacteria, and Firmicutes. Among them, Bacteroidetes and Firmicutes hold particular significance, with the class Clostridia occupying a dominant position within the Firmicutes sequences [13]. It is important to acknowledge that the microbiome encompasses more than just bacteria, including fungi and viruses [6,[31], [32], [33], [34]]. Disturbances in the microbiome and metabolic disorders elevate the risk of asthma in infancy, and these effects may persist into the preschool years. The microbiome plays a crucial role in modulating immune function, and its temporal and dynamic interactions with immunity are essential for the onset and chronicity of asthma [35]. Research cohorts have identified a reduction in the diversity of the intestinal microbiome in the early postpartum phase. Additionally, a higher abundance of Clostridium difficile (phylum Firmicutes) or Candida and Rhodotorula fungi, along with a lower abundance of Dialister, taxa Lachnobacterium, and Lachnospira, has been strongly linked to asthma in later life [[36], [37], [38], [39], [40], [41]]. A study by Lee-Sarwar et al. found a strong correlation between infant fecal microbial taxa and asthma phenotypes. The study revealed that longitudinal gut microbiome characterization was associated with early asthma, and that cesarean section led to reduced levels of Lactobacillus and microbial sphingolipids in infant feces, increasing susceptibility to early asthma [42]. In a prospective birth cohort, the deposition of specific Mycobacterium fragilis or Clostridium perfringens subgroups at 3 weeks of age was identified as an early indicator of potential asthma later in life [43]. Colonization by Clostridium difficile at age of 1 month was associated with wheeze throughout the first 6–7 years of life and with asthma at age 6–7 years [37]. In a study by J. Stokholm et al., gut microbial composition in the first year of life was linked to subsequent asthma risk. The researchers sequenced 16S rRNA gene amplicons from 690 participants and found that 1-year-old with an immature microbial composition had an increased risk of asthma at age 5 years [44]. A prospective study in Copenhagen followed 700 children from birth, investigating the impact of cesarean section on gut microbiome composition in the first year of life through 16S rRNA gene amplicon sequencing. The study revealed marked changes in the gut microbiome of cesarean section infants at one week and one month of age. However, by one year of age, the differences were not significant compared to vaginal delivery. Children born by cesarean section had an elevated risk of asthma only if their gut microbiome composition retained the microbial signature of the cesarean section at one year of age, suggesting that proper maturation of the gut microbiome attenuates the increased asthma risk associated with changes in gut microbiology resulting from cesarean section [39]. Another study, while noting that the mode of delivery was not directly related to asthma, found that cesarean section reduced fecal Lactobacillus spp. and microbial sphingolipids, thereby increasing susceptibility to early asthma [42]. A population-based and prospective cohort analysis published in Lancet Respiratory Medicine, a leading journal in the field of respiratory medicine, revealed that antibiotic use in infancy was associated with an increased risk of asthma. Reduced morbidity during the research was linked to decreased antibiotic use in infant life, and an increase in gut microbiome alpha-diversity was associated with a decreased risk of asthma at 5 years of age [45]. Vancomycin exposure-altered gut microbiome was found to upregulate type 2 innate lymphocytes and exacerbate respiratory tract inflammation in mice [17]. In a cohort study involving 662 Danish children, antibiotic load was also linked to the maturation and composition of gut microbiome bacteria, correlating with an elevated risk of asthma [46]. Recognizing the pivotal role of the microbiome in asthma, studies have explored its potential in asthma prevention. The Study in Rural Environments (PASTURE) birth cohort, led by Martin Depner et al., developed a maturation model using human gut microbiome 16S rRNA sequence data from 2- to 12-month-old infants. This model suggested that the gut microbiome may contribute to asthma protection through metabolites, supporting the concept of a gut-lung axis in humans [47]. Collectively, significant research remains to be conducted to fully understand the correlation between asthma and the intestinal microbiome. Additional in-depth studies are required to achieve a deeper and more detailed understanding of the intrinsic mechanisms and to elucidate the associations between them.
4.2. Microbiome of the airways and asthma
The advent of “next-generation sequencing” technology has revolutionized research on the microbial composition of healthy human airways through 16S rRNA gene sequencing, dispelling the notion that the “airways are sterile.” The airway microbiome can be explored by collecting samples from the upper airway (nasal, nasopharyngeal, oropharyngeal, and saliva) and/or lower airway (deep sputum, bronchial aspirate, or bronchoalveolar lavage). In the nasopharynx, the bacterial flora stabilizes, with Moraxella spp. and Dolosigranulu emerging as predominant bacterial groups. In the lungs of healthy individuals, six phyla are predominantly detected: Actinobacteria, Firmicutes, Bacteroidetes, Proteobacteria, Eusobacteria, and Acidobacteri. Notably, the diversity of the Proteobacteria phylum increases in asthma patients [48,49]. Various factors, both internal and external, such as age, nutrition, lifestyle, genetics, cigarette smoke, exposure to pollution, and potential diseases, can influence the typology and quantity of the microbiome in the lungs, potentially leading to dysbiosis of the respiratory flora. The respiratory microbiome serves as a crucial regulator of immunity, cellular function, and metabolism, responding to inflammatory responses and immune regulation associated with host asthma. It may mediate host asthma susceptibility, influence its severity, and modulate asthma phenotypes [50]. In healthy children and adults, the respiratory microbiome is characterized by a predominance of Mycobacterium-like organisms, whereas asthmatics exhibit a predominance of Aspergillus spp. [49]. Subsequent studies have demonstrated a positive correlation between increased nasal Aspergillus spp. in asthmatics and their frequent emergency department visits due to acute asthma exacerbations [51]. Upper respiratory colonization with pneumococcus, Haemophilus influenzae, and/or Katachnerella at the age of 4 weeks is associated with elevated blood levels of eosinophil IgE and an increased incidence of asthma and wheezing at 5 years of age [52]. Mite-sensitized children were found to have significantly lower bacterial species richness and diversity, while food- or IgE-sensitized children did not exhibit a significant reduction [53]. An assay of nasal microbiological samples revealed that an increased risk of worsening inflammatory asthma was predicted when the Corynebacterium Dolosigranulum flora shifted to Moraxella in the early stages of uncontrolled asthma [54]. Further studies have found that the sputum microbiome of patients with severe asthma was characterized by the presence of streptococci and eosinophilia that differed from healthy controls and non-severe asthmatics [55]. Both bacterial and fungal members of the respiratory microbiome are associated with and likely drivers of specific asthmatic molecular phenotypes in adults [2]. The airway microbiome, with its newfound accessibility through advanced sequencing techniques, provides novel ideas for understanding asthma. It suggests insights into the diagnosis, clinical processes, and therapy of asthma [31,[56], [57], [58]].
Neonatal mice exposed to room-dust mite allergens exhibited a higher likelihood of increased airway eosinophilia, elevated type 2 helper T-cell cytokine production, and greater airway hyperreactivity compared with older mice. Alterations in the microbiome were associated with reduced responsiveness to airborne allergens, accompanied by the emergence of a subpopulation of Helios(-) Treg cells dependent on engagement with procedural death ligand 1 (PD-L1) for their development [59]. Certain bacterial species, such as Dolosigranulum spp. and Prevotella spp., appear to have asthma-protective properties, while an abundance of Streptococcus spp., Aspergillus spp., and Fungi spp. in asthma patients may influence the progression and severity of asthma [60]. Distinct levels of inflammation and microbiome compositions have been found in studies comparing neutrophilic and eosinophilic asthmatics. Further investigations have indicated that the microbiome may influence the degree of bronchial obstruction and the duration of symptoms through a Th-17-driven mechanism [2,61,62]. Stein et al. discovered that nasal instillation of dust from different environments reduced ovalbumin-induced allergic inflammation in the airways of mice, possibly because dust from different environments has different bacterial species, especially Bartonellaceae [63], which may enhance the possibilities for future studies exploring the modulation of the respiratory microbiome as a potential target for controlling asthma.
4.3. The gut-lung axis and asthma
Dysbiosis in gut and lungs appears as a key contributor to the increased prevalence of asthma [64]. In recent years, as confirmation of the gut-lung axis hypothesis persists, there is accumulating evidence of potential interactions between the intestinal and pulmonary mucosa. However, the precise mechanism by which this axis activates the innate immune system should be elucidated [5,65,66]. Although exploring the effects of the airway, gut, and environmental microbiomes on the onset, manifestation, and remission of asthma and allergic diseases is a comparatively new field of investigation, the available evidence suggests that the airway and/or gut microbiome may be a fertile target for the prevention or treatment of allergic asthma and other diseases that are prominently characterized by adaptive immune dysfunction [67]. The gut-lung axis is likely to be important for maintaining a normal microbiome in the gut and lungs, influencing the immune responses of both systems, and maintaining homeostasis in vivo [62]. It has been demonstrated that the intestinal microbiome is capable of generating regional and generalized mediators, which may in turn modulate the function of Treg lymphocytes and ultimately mediate the development of asthma by the gut-lung axis [68,69]. In addition, both metabolites and bacterial ligands, as well as biologically active ligands derived from the intestinal tract, also reach the blood, which may in turn modulate the translocation of immunocytes in the airways and participate in the airway immune response in patients with asthma [70]. The gut-lung axis may play a crucial role in maintaining a normal microbiome in the gut and lungs, as well as maintaining homeostasis in the body.
The gut microbiome may affect pulmonary immune response by different strategies. A possible mechanism is binding of molecular shapes related to pathological agents (e.g., lipopolysaccharides) that can be stimulated by toll-like receptors, thereby setting off genes that modulate the reactions of both innate immune system and inflammation [13]. Another mechanism is associated with metabolites produced by microorganisms, such as the fermentation of dietary fiber by the microbiome to short-chain fatty acids (SCFA), especially propionate, butyrate, and acetate, acting as a microbial metabolite that may inhibit processes, such as histone deacetylases, chemokine and cytokine production, cell proliferation, differentiation, and apoptosis, and thereby altering gene expression. Finally, the microbiome may also undergo epigenetic modification of DNA via SCFA [[71], [72], [73]]. The interaction of dendritic cells, Treg cells, and alveolar macrophages plays a pivotal role in the immune pathway of the respiratory system, ensuring the maintenance of its immune function [74].
Bifidobacterium longum can secrete an exopolysaccharide to inhibit Th17 in lung and gut [75]. Gut bacteria can produce oxylipins, including metabolites that reduce the productivity and frequency of IL-10 by pulmonary Treg cells, thereby enhancing allergic inflammation and potentially increasing the risk of asthma development. Dysbiosis-induced natural killer T cells may induce Th2 cell differentiation and IgE isotype switching through the release of IL-4 and IL-13. Furthermore, dysbiosis can contribute to increased circulating basophils, mast cells, inflammation, and the degradation of IgA antibodies, representing mechanisms by which it can induce or exacerbate asthma [76]. Flora dysbiosis and decreased microbial diversity in the gut and lungs can lead to dysregulation of the two-way gut-lung axis crosstalk, resulting in hypersensitization and hyperresponsiveness to airway and dietary allergens. A study by Herbst et al. observed differences between germ-free mice and microbiome-colonized mice upon simultaneous ovalbumin stimulation. Germ-free mice developed airway hyperresponsiveness and Th2-type inflammatory responses, while the recolonization of the microbiome in mice showed a decrease in the level of allergic inflammation. This strongly indicates that the airway and gut-colonized microbiome has a protective effect against asthma [77]. In summary, there is an expectation that, in the near future, asthma could be controlled by modifying the components of the intestinal and respiratory microbiome in asthmatic patients. Exploration of mechanisms involved from the perspective of the gut-lung axis is anticipated to yield significant breakthroughs in asthma control and prevention. Utilizing research results in clinical practice holds the potential to provide greater benefits to asthmatic patients and will likely be a key concentration of future research.
4.4. Possibility of controlling asthma by modulating the microbiome
Having uncovered the inseparable association between asthma and the microbiome, the question arises: how can asthma be controlled by manipulating the microbiome? The existing research concentrates on treating asthma through probiotics and bacterial lysates.
Probiotics, as defined by the World Health Organization (WHO), are active microorganisms beneficial to the host, capable of colonizing the body and altering the flora composition in specific regions. They contribute to maintaining homeostasis and health by regulating the host’s mucous membranes and systemic immune function, with lactobacilli as their primary component. Probiotics produce metabolites, such as SCFA, particularly butyrate, inhibiting allergic inflammation in the airways. Ongoing research is exploring their potential benefits in asthma prevention and management. In a randomized, double-blind, placebo-controlled study by Chen et al., school-aged children (6–12 years old) with asthma and allergic rhinitis underwent pulmonary function tests and assessments of asthma severity, clinical parameters, and immune physiological markers, including total IgE and cytokines produced from peripheral blood mononuclear cells (PBMCs). Post-probiotic treatment, a significant decrease in IFN-γ, TNF-α, IL-13, and IL-12 production by PBMCs was observed. Lung function, spirometry, clinical symptoms of asthma, and wheezing scores also exhibited substantial improvement [78]. Huang et al. evaluated influences of Lactobacillus fermentum (LF), Lactobacillus paracasei (LP), or their combination (LF + LP) on the immune biomarkers, clinical severity, and quality of life in asthmatic children. Treated children demonstrated lower asthma severity compared with the placebo group, along with the elevated peak expiratory flow rate (PEFR) and decreased IgE level [79]. Another study highlighted the therapeutic effects of Bifidobacterium infantis (B. infantis) on allergic asthma by promoting Th1 and inhibiting Th2 immune responses [80]. These findings facilitate the control of asthma in the future by exploring the modulation of intestinal flora. Diverse probiotic formulations will be expectedly developed for the prevention and management of asthma.
An increasing number of studies have explored the utilization of bacterial lysates to control the onset and progression of asthma due to their nonspecific immunomodulatory effects, including strains of bacteria that have been described to cause dysbiosis of the microflora and have been associated with the onset of wheezing and asthma, such as Cattaramorpha, Haemophilus influenzae, and Streptococcus pneumoniae. A randomized, double-blind, placebo-controlled, parallel-group study was conducted on 152 patients with allergic asthma, assigning patients to receive either placebo or polyvalent mechanical bacterial lysate (PMBL®) tablets, a chemical bacterial lysate that has been successfully utilized to reduce wheezing exacerbations in preschool children. The study evaluated the effects of the PMBL® tablets on the clinical course and asthma control of children who aged 6–16 years with partially controlled or uncontrolled asthma and found that the average number of asthma exacerbations was markedly less in the PMBL® tablet group compared with the placebo group, confirming that administration of PMBL® tablets could significantly reduce exacerbation rates, and it emerged safe and effective in children with school-age allergic asthma [81]. A similar study found that administration of PMBL® tablets altered the composition of T-lymphocytes in asthmatics, accompanying by a significant reduction in CD69+, CD3+, and CD25+ subsets, and an increase in CD8+, CD4+CD25+FOXP3+, and CD3−CD16+CD56+, as well as an increase in Th-cell count [82]. Nieto A. et al. examined the efficacy and safety of sublingual mucosal immune therapy using fully inactivated bacterial lysate (MV130) and observed a significant reduction in wheezing episodes, along with decreases in episode duration, patients’ symptoms, and medication needs, all without reported side effects [83].
Upon recognizing the correlation between asthma and the microbiome, the aim is to regulate the microbiota with the ultimate goal of preventing or controlling asthma. Specific probiotics and bacterial lysates, as mentioned earlier, may play a role in asthma control, and there is potential for the development of more tailored biologics for asthma patients in the future. The diversity of the microbiota to which infants and young children are exposed early in life has been found to significantly impact asthma development. Modifying environmental and host risk factors to influence the composition of the microbiota in individuals at risk for asthma may ultimately lead to asthma prevention. Existing research evidence suggests that reducing the risk of asthma in the next generation can be achieved through promoting a more rational maternal diet, appropriate supplementation of nutritional supplements, such as vitamin D during pregnancy, a high-fiber diet, and suitable antioxidants. Additionally, based on available research, advocating for normal delivery over cesarean section is encouraged, as the microbiome of normal delivery differs from that of cesarean section, and babies born through normal delivery are less likely to develop asthma. Similarly, breastfeeding is preferred over formula feeding based on existing research evidence. Recognizing the impact of antibiotic misuse on the human microbiome, there is a strong recommendation for the rational use of antibiotics, control of respiratory infections (e.g., rhinovirus and RSV), and other preventive measures. Collectively, adopting an appellate approach to modify the microbiota composition in infants holds promise for the prevention and/or treatment of asthma [84]. Shifting from the treatment of disease to the cornerstone of primary prevention through the modification of risk factors and microbiota composition is a burgeoning research area.
The available evidence underscores the pivotal role of changes in the human microbiome in influencing asthma risk, etiopathogenesis, and clinical manifestations, necessitating further exploration through sophisticated analytical tools and well-constructed experimental clinical investigations. These efforts should consider the intricate interdependencies among the human host, its microbiome, and the impact of environmental and lifestyle elements across the lifespan on these interactions.
5. Limitations
Utilizing VOSviewer and CiteSpace software for analysis provides a more comprehensive and purposeful examination of data compared to traditional reviews, yet the study design has inherent limitations. Firstly, the search was confined to the WOSCC database, which, while encompassing the majority of high-quality research papers, may omit relevant studies exclusive to other databases like Embase, PubMed, and Scopus. This potential bias and fragmentation in included studies should be acknowledged. Secondly, the inclusion criteria concentrated solely on original research articles and review articles, excluding other formats, such as letters, conference papers, and book chapters, possibly resulting in oversights. Furthermore, the analyses included in this article have a cut-off date of June 20, 2023. While this date offers a representative snapshot of the current state and focal points in the field, the continuous updating of the WOS core collection indicates that emerging studies beyond this date might be overlooked. Consequently, the present article may not fully capture the evolving situation in 2023. Nevertheless, given the credibility of the WOSCC database and the substantial number of publications analyzed, this study can effectively illustrate prevailing trends and research centers in the field. Future studies with similar objectives should address and eliminate these inherent limitations.
6. Conclusions
This study employed bibliometric methods, specifically leveraging VOSviewer and Citespace software for visualization and analysis, to systematically summarize the knowledge structure and research frontiers regarding the association between asthma and the microbiome. The analysis concentrated on the key elements of current global research, providing insights into the future trajectory of the field. The consistent year-on-year increase in the number of articles indicated a growing interest in this area over recent years. The existing investigations primarily aimed to reveal the mechanisms through which the microbiome influences immunomodulation and inflammatory responses in asthmatic patients, as well as exploring pathways for preventing and controlling asthma symptoms through microbiome modulation. This study provides a comprehensive overview of the existing basic scientific understanding of the association between the microbiome and asthma, elucidating various interrelationships and providing crucial observations about emerging research trends and cutting-edge insights. This study may assist researchers in attaining a deeper understanding of the ongoing trends in this field, providing a more comprehensive perspective on the subject as a whole.
Ethical declaration
Informed consent was not required for this study because it was an analysis of previously published literature.
Data availability statement
The original data presented in the study is included in the article. For further inquiries, please contact the corresponding author.
Funding
This study was supported by the Startup Fund for Scientific Research of Fujian Medical University (Grant No. 2021QH1115).
CRediT authorship contribution statement
ZhiFeng Guo: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. LingHong Huang: Writing – review & editing, Writing – original draft, Visualization, Software, Resources, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. SuMei Lai: Writing – review & editing, Visualization, Software, Methodology, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Dharmage S.C., Perret J.L., Custovic A. Epidemiology of asthma in children and adults. Front. Pediatr. 2019;7:246. doi: 10.3389/fped.2019.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kozik A.J., Huang Y.J. The microbiome in asthma: role in pathogenesis, phenotype, and response to treatment. Ann. Allergy Asthma Immunol. 2019;122(3):270–275. doi: 10.1016/j.anai.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eder W., Ege M.J., von Mutius E. The asthma epidemic. N. Engl. J. Med. 2006;355(21):2226–2235. doi: 10.1056/NEJMra054308. [DOI] [PubMed] [Google Scholar]
- 4.Strachan D.P. Hay fever, hygiene, and household size. Bmj. 1989;299(6710):1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frati F., et al. The role of the microbiome in asthma: the gut-lung Axis. Int. J. Mol. Sci. 2019;20(1):12. doi: 10.3390/ijms20010123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daley D. The evolution of the hygiene hypothesis: the role of early-life exposures to viruses and microbes and their relationship to asthma and allergic diseases. Curr. Opin. Allergy Clin. Immunol. 2014;14(5):390–396. doi: 10.1097/ACI.0000000000000101. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann A., et al. Compositional differences between gut microbiota of adult patients with asthma and healthy controls. Postępy Dermatologii i Alergologii. 2023;40(1):142–149. doi: 10.5114/ada.2022.117998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckburg P.B., et al. Diversity of the human intestinal microbial flora. Science. 2005;308(5728):1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hold G.L., et al. Assessment of microbial diversity in human colonic samples by 16S rDNA sequence analysis. FEMS Microbiol. Ecol. 2002;39(1):33–39. doi: 10.1111/j.1574-6941.2002.tb00904.x. [DOI] [PubMed] [Google Scholar]
- 10.Wang X., et al. Molecular characterization of the microbial species that colonize human ileal and colonic mucosa by using 16S rDNA sequence analysis. J. Appl. Microbiol. 2003;95(3):508–520. doi: 10.1046/j.1365-2672.2003.02005.x. [DOI] [PubMed] [Google Scholar]
- 11.Suau A., et al. Direct analysis of genes encoding 16S rRNA from complex communities reveals many novel molecular species within the human gut. Appl. Environ. Microbiol. 1999;65(11):4799–4807. doi: 10.1128/aem.65.11.4799-4807.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayashi H., Sakamoto M., Benno Y. Phylogenetic analysis of the human gut microbiota using 16S rDNA clone libraries and strictly anaerobic culture-based methods. Microbiol. Immunol. 2002;46(8):535–548. doi: 10.1111/j.1348-0421.2002.tb02731.x. [DOI] [PubMed] [Google Scholar]
- 13.Valverde-Molina J., Garcia-Marcos L. Microbiome and asthma: microbial dysbiosis and the Origins, phenotypes, persistence, and severity of asthma. Nutrients. 2023;15(3):25. doi: 10.3390/nu15030486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sokolowska M., et al. Microbiome and asthma. Asthma Res. Pract. 2018;4:1. doi: 10.1186/s40733-017-0037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Losol P., Sokolowska M., Chang Y.S. Interactions between microbiome and underlying mechanisms in asthma. Respir. Med. 2023;208:9. doi: 10.1016/j.rmed.2023.107118. [DOI] [PubMed] [Google Scholar]
- 16.Xue S.C., et al. Gut microecological regulation on bronchiolitis and asthma in children: a review. Clin. Respirat. J. 2023:11. doi: 10.1111/crj.13622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiang Q.W., et al. Intestinal microflora altered by vancomycin exposure in early life up-regulates type 2 innate lymphocyte and aggravates airway inflammation in asthmatic mice. Inflammation. 2023;46(2):509–521. doi: 10.1007/s10753-022-01748-4. [DOI] [PubMed] [Google Scholar]
- 18.Xu X.Y., et al. Upper respiratory tract mycobiome alterations in different kinds of pulmonary disease. Front. Microbiol. 2023;14:13. doi: 10.3389/fmicb.2023.1117779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y., et al. Upper respiratory tract microbiota is associated with small airway function and asthma severity. BMC Microbiol. 2023;23(1):11. doi: 10.1186/s12866-023-02757-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu B.H., et al. Adult asthma with symptomatic eosinophilic inflammation is accompanied by alteration in gut microbiome. Allergy. 2023:13. doi: 10.1111/all.15691. [DOI] [PubMed] [Google Scholar]
- 21.Xie Y., et al. Ablation of CD226 on CD4+ T cells modulates asthma progress associated with altered IL-10 response and gut microbiota. Int. Immunopharm. 2023;118:12. doi: 10.1016/j.intimp.2023.110051. [DOI] [PubMed] [Google Scholar]
- 22.Chunxi L., et al. The gut microbiota and respiratory diseases: new evidence. J Immunol Res. 2020;2020 doi: 10.1155/2020/2340670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pritchard A. Statistical bibliography or bibliometrics? J. Doc. 1969;25:348–349. [Google Scholar]
- 24.Diem A., Wolter S.C. The use of bibliometrics to measure research performance in education sciences. Res. High. Educ. 2013;54(1):86–114. [Google Scholar]
- 25.Mayr P., Scharnhorst A. Scientometrics and information retrieval: weak-links revitalized. Scientometrics. 2015;102(3):2193–2199. [Google Scholar]
- 26.Abramo G., D’Angelo C.A., Viel F. The field-standardized average impact of national research systems compared to world average: the case of Italy. Scientometrics. 2011;88:599–615. [Google Scholar]
- 27.Ninkov A., Frank J.R., Maggio L.A. Bibliometrics: methods for studying academic publishing. Perspect. Med. Educ. 2022;11(3):173–176. doi: 10.1007/s40037-021-00695-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kokol P., Blažun Vošner H., Završnik J. Application of bibliometrics in medicine: a historical bibliometrics analysis. Health Inf. Libr. J. 2021;38(2):125–138. doi: 10.1111/hir.12295. [DOI] [PubMed] [Google Scholar]
- 29.Chen C. CiteSpace II: detecting and visualizing emerging trends and transient patterns in scientific literature. J. Am. Soc. Inf. Sci. Technol. 2006;57(3):359–377. [Google Scholar]
- 30.van Eck N.J., Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84(2):523–538. doi: 10.1007/s11192-009-0146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang R., et al. Different airway inflammatory phenotypes correlate with specific fungal and bacterial microbiota in asthma and chronic obstructive pulmonary disease. J. Immunol. Res. 2022;2022:10. doi: 10.1155/2022/2177884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinez F.D. Childhood asthma inception and progression role of microbial exposures, susceptibility to viruses and early allergic sensitization. Immunol. Allergy Clin. 2019;39(2):141–+. doi: 10.1016/j.iac.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carpagnano G.E., et al. Analysis of the fungal microbiome in exhaled breath condensate of patients with asthma. Allergy Asthma Proc. 2016;37(3):E41–E46. doi: 10.2500/aap.2016.37.3943. [DOI] [PubMed] [Google Scholar]
- 34.Sharma A., et al. Associations between fungal and bacterial microbiota of airways and asthma endotypes. J. Allergy Clin. Immunol. 2019;144(5):1214–+. doi: 10.1016/j.jaci.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang Y.J., et al. Microbiome-immune interactions in allergy and asthma. J. Allergy Clin. Immunol. Pract. 2022;10(9):2244–2251. doi: 10.1016/j.jaip.2022.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abrahamsson T.R., et al. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin. Exp. Allergy. 2014;44(6):842–850. doi: 10.1111/cea.12253. [DOI] [PubMed] [Google Scholar]
- 37.van Nimwegen F.A., et al. Mode and place of delivery, gastrointestinal microbiota, and their influence on asthma and atopy. J. Allergy Clin. Immunol. 2011;128(5) doi: 10.1016/j.jaci.2011.07.027. 948-U371. [DOI] [PubMed] [Google Scholar]
- 38.Fujimura K.E., et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat. Med. 2016;22(10):1187–1191. doi: 10.1038/nm.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stokholm J., et al. Delivery mode and gut microbial changes correlate with an increased risk of childhood asthma. Sci. Transl. Med. 2020;12(569):14. doi: 10.1126/scitranslmed.aax9929. [DOI] [PubMed] [Google Scholar]
- 40.Galazzo G., et al. Development of the microbiota and associations with birth mode, diet, and atopic disorders in a longitudinal analysis of stool samples, collected from infancy through early childhood. Gastroenterology. 2020;158(6):1584–1596. doi: 10.1053/j.gastro.2020.01.024. [DOI] [PubMed] [Google Scholar]
- 41.Zimmermann P., et al. Association between the intestinal microbiota and allergic sensitization, eczema, and asthma: a systematic review. J. Allergy Clin. Immunol. 2019;143(2):467–485. doi: 10.1016/j.jaci.2018.09.025. [DOI] [PubMed] [Google Scholar]
- 42.Lee-Sarwar K.A., et al. The maternal prenatal and offspring early-life gut microbiome of childhood asthma phenotypes. Allergy. 2023;78(2):418–428. doi: 10.1111/all.15516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vael C., et al. Denaturing gradient gel electrophoresis of neonatal intestinal microbiota in relation to the development of asthma. BMC Microbiol. 2011;11:7. doi: 10.1186/1471-2180-11-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stokholm J., et al. Maturation of the gut microbiome and risk of asthma in childhood. Nat. Commun. 2018;9:10. doi: 10.1038/s41467-017-02573-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patrick D.M., et al. Decreasing antibiotic use, the gut microbiota, and asthma incidence in children: evidence from population-based and prospective cohort studies. Lancet Respir. Med. 2020;8(11):1094–1105. doi: 10.1016/S2213-2600(20)30052-7. [DOI] [PubMed] [Google Scholar]
- 46.Li X.J., et al. The infant gut resistome associates with E. coli, environmental exposures, gut microbiome maturity, and asthma-associated bacterial composition. Cell Host Microbe. 2021;29(6):975–+. doi: 10.1016/j.chom.2021.03.017. [DOI] [PubMed] [Google Scholar]
- 47.Depner M., et al. Maturation of the gut microbiome during the first year of life contributes to the protective farm effect on childhood asthma. Nat. Med. 2020;26(11):1766–+. doi: 10.1038/s41591-020-1095-x. [DOI] [PubMed] [Google Scholar]
- 48.Huang Y.J., et al. The microbiome in allergic disease: current understanding and future opportunities-2017 PRACTALL document of the American academy of allergy, asthma & Immunology and the European academy of allergy and clinical Immunology. J. Allergy Clin. Immunol. 2017;139(4):1099–1110. doi: 10.1016/j.jaci.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hilty M., et al. Disordered microbial communities in asthmatic airways. PLoS One. 2010;5(1):9. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heul A.V., Planer J., Kau A.L. The human microbiota and asthma. Clin. Rev. Allergy Immunol. 2019;57(3):350–363. doi: 10.1007/s12016-018-8719-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang H.J., et al. Association of nasal microbiome and asthma control in patients with chronic rhinosinusitis. Clin. Exp. Allergy. 2018;48(12):1744–1747. doi: 10.1111/cea.13255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bisgaard H., et al. Childhood asthma after bacterial colonization of the airway in neonates. N. Engl. J. Med. 2007;357(15):1487–1495. doi: 10.1056/NEJMoa052632. [DOI] [PubMed] [Google Scholar]
- 53.Chiu C.Y., et al. Airway microbial diversity is inversely associated with mite-sensitized rhinitis and asthma in early childhood. Sci. Rep. 2017;7:8. doi: 10.1038/s41598-017-02067-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou Y.J., et al. The upper-airway microbiota and loss of asthma control among asthmatic children. Nat. Commun. 2019;10:10. doi: 10.1038/s41467-019-13698-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Q.L., et al. Airway microbiota in severe asthma and relationship to asthma severity and phenotypes. PLoS One. 2016;11(4):16. doi: 10.1371/journal.pone.0152724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shah R., Bunyavanich S. The airway microbiome and pediatric asthma. Curr. Opin. Pediatr. 2021;33(6):639–647. doi: 10.1097/MOP.0000000000001054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alamri A. Diversity of microbial signatures in asthmatic airways. Int. J. Gen. Med. 2021;14:1367–1378. doi: 10.2147/IJGM.S304339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rick E.M., et al. The airway fungal microbiome in asthma. Clin. Exp. Allergy. 2020;50(12):1325–1341. doi: 10.1111/cea.13722. [DOI] [PubMed] [Google Scholar]
- 59.Gollwitzer E.S., et al. Lung microbiota promotes tolerance to allergens in neonates via PD-L1. Nat. Med. 2014;20(6):642–647. doi: 10.1038/nm.3568. [DOI] [PubMed] [Google Scholar]
- 60.Losol P., et al. The role of upper airway microbiome in the development of adult asthma. Immune Network. 2021;21(3):18. doi: 10.4110/in.2021.21.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mannion J.M., McLoughlin R.M., Lalor S.J. The airway microbiome-IL-17 Axis: a critical regulator of chronic inflammatory disease. Clin. Rev. Allergy Immunol. 2023;64(2):161–178. doi: 10.1007/s12016-022-08928-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chung K.F. Airway microbial dysbiosis in asthmatic patients: a target for prevention and treatment? J. Allergy Clin. Immunol. 2017;139(4):1071–1083. doi: 10.1016/j.jaci.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 63.Stein M.M., et al. Innate immunity and asthma risk in amish and hutterite farm children. N. Engl. J. Med. 2016;375(5):411–421. doi: 10.1056/NEJMoa1508749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hufnagl K., et al. Dysbiosis of the gut and lung microbiome has a role in asthma. Semin. Immunopathol. 2020;42(1):75–93. doi: 10.1007/s00281-019-00775-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim Y.J., et al. The gut/lung microbiome Axis in obesity, asthma, and bariatric surgery: a literature review. Obesity. 2021;29(4):636–644. doi: 10.1002/oby.23107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schuijt T.J., et al. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut. 2016;65(4):575–583. doi: 10.1136/gutjnl-2015-309728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fujimura K.E., Lynch S.V. Microbiota in allergy and asthma and the emerging relationship with the gut microbiome. Cell Host Microbe. 2015;17(5):592–602. doi: 10.1016/j.chom.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cereta A.D., et al. Early life microbial exposure and immunity training effects on asthma development and progression. Front. Med. 2021;8:6. doi: 10.3389/fmed.2021.662262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barcik W., et al. The role of lung and gut microbiota in the pathology of asthma. Immunity. 2020;52(2):241–255. doi: 10.1016/j.immuni.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alcazar C.G., et al. The association between early-life gut microbiota and childhood respiratory diseases: a systematic review. Lancet Microbe. 2022;3(11):e867–e880. doi: 10.1016/S2666-5247(22)00184-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McKenzie C., et al. The nutrition-gut microbiome-physiology axis and allergic diseases. Immunol. Rev. 2017;278(1):277–295. doi: 10.1111/imr.12556. [DOI] [PubMed] [Google Scholar]
- 72.Tang H.H.F., et al. The intersect of genetics, environment, and microbiota in asthma-perspectives and challenges. J. Allergy Clin. Immunol. 2021;147(3):781–793. doi: 10.1016/j.jaci.2020.08.026. [DOI] [PubMed] [Google Scholar]
- 73.Samuelson D.R., Welsh D.A., Shellito J.E. Regulation of lung immunity and host defense by the intestinal microbiota. Front. Microbiol. 2015;6:1085. doi: 10.3389/fmicb.2015.01085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Magryś A. Microbiota: a missing link in the pathogenesis of chronic lung inflammatory diseases. Pol. J. Microbiol. 2021;70(1):25–32. doi: 10.33073/pjm-2021-013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jeong J., Lee H.K. The role of CD4(+) T cells and microbiota in the pathogenesis of asthma. Int. J. Mol. Sci. 2021;22(21) doi: 10.3390/ijms222111822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Salameh M., et al. The role of gut microbiota in atopic asthma and allergy, implications in the understanding of disease pathogenesis. Scand. J. Immunol. 2020;91(3):8. doi: 10.1111/sji.12855. [DOI] [PubMed] [Google Scholar]
- 77.Herbst T., et al. Dysregulation of allergic airway inflammation in the absence of microbial colonization. Am. J. Respir. Crit. Care Med. 2011;184(2):198–205. doi: 10.1164/rccm.201010-1574OC. [DOI] [PubMed] [Google Scholar]
- 78.Chen Y.S., et al. Randomized placebo-controlled trial of lactobacillus on asthmatic children with allergic rhinitis. Pediatr. Pulmonol. 2010;45(11):1111–1120. doi: 10.1002/ppul.21296. [DOI] [PubMed] [Google Scholar]
- 79.Huang C.F., Chie W.C., Wang I.J. Efficacy of lactobacillus administration in school-age children with asthma: a randomized, placebo-controlled trial. Nutrients. 2018;10(11) doi: 10.3390/nu10111678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang W., et al. Bifidobacterium infantis relieves allergic asthma in mice by regulating Th1/Th2. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2020;26 doi: 10.12659/MSM.920583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Emeryk A., et al. Mechanical bacterial lysate administration prevents exacerbation in allergic asthmatic children-The EOLIA study. Pediatr. Allergy Immunol. 2018;29(4):394–401. doi: 10.1111/pai.12894. [DOI] [PubMed] [Google Scholar]
- 82.Bartkowiak-Emeryk M., et al. Impact of Polyvalent Mechanical Bacterial Lysate on lymphocyte number and activity in asthmatic children: a randomized controlled trial. Allergy Asthma Clin. Immunol. 2021;17(1):10. doi: 10.1186/s13223-020-00503-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nieto A., et al. Bacterial mucosal immunotherapy with MV130 prevents recurrent wheezing in children: a randomized, double-blind, placebo-controlled clinical trial. Am. J. Respir. Crit. Care Med. 2021;204(4):462–472. doi: 10.1164/rccm.202003-0520OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Polk B.I., Bacharier L.B. Potential strategies and targets for the prevention of pediatric asthma. Immunol. Allergy Clin. 2019;39(2):151–+. doi: 10.1016/j.iac.2018.12.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original data presented in the study is included in the article. For further inquiries, please contact the corresponding author.