Abstract
Background
Rearranged during transfection (RET) gene fusion is a target for non-small cell lung cancer (NSCLC) treatment, and RET inhibitors are approved for advanced NSCLC. The role of immune checkpoint inhibitors (ICIs) in RET fusion-positive NSCLC remains controversial. This retrospective study analyzed the efficacy of ICIs and RET inhibitors in Chinese patients with RET fusion-positive NSCLC.
Methods
Data from patients diagnosed with advanced NSCLC harboring RET fusion from Jan 2017 to Sep 2021 were analyzed. Clinicopathological characteristics and outcomes of ICIs and RET inhibitors treatments were collected.
Results
Seventy-five patients with RET fusion-positive advanced NSCLC were identified. The median age of patients was 57 years, half of the patients were female (50.3%), and most were non-smokers or light smokers (72%). Of the cancer types diagnosed in study patients, the KIF5B-RET fusion subtype accounted for 73.3% (55/75), twelve patients (16%) had CCDC6-RET fusion, and three (4%) had NCOA4-RET fusion. Sixteen patients were treated with ICIs. In previously untreated patients, we observed an objective response rate (ORR) of 71.4% and median progression free survival (PFS) of 7.5 months in seven assessable patients. Of four patients with PD-L1 overexpression (>50%) one received pembrolizumab and the other three patients received pemetrexed, carboplatin, and pembrolizumab or camrelizumab. In these patients, the ORR was 75% and disease control rate was 100%. Fifteen patients received selective RET inhibitors (pralsetinib and selpercatinib), resulting in an ORR of 53.3% (8/15) and median PFS of 10.0 months (95% CI 5.2–14.9).
Conclusions
ICIs for PD-L overexpression and treatment naive patients offer comparable benefits for RET fusion-positive NSCLC, warranting further investigation.
Keywords: RET fusion, Lung cancer, Immunotherapy, Targeted therapy, RET inhibitors
Highlights
-
•
Relatively high proportion of PD-L1 expression in RET fusion-positve lung cancer.
-
•
RET fusion-positve patients with PD-L1 overexpression may benefit from chemoimmunotherapy.
-
•
Selective RET inhibitors displayed superior efficacy compared to cabozantinib.
-
•
KRAS mutation and EGFR amplification may mediates RET inhibitors resistance.
1. Introduction
Targeted therapy for driver mutation-positive metastatic non-small cell lung cancer (NSCLC) has dramatically improved overall survival. Approximately 1%–2% of patients with NSCLC are diagnosed with rearranged during transfection (RET) gene fusion especially younger patients, non-smokers, and patients diagnosed with adenocarcinoma [1,2]. Multiple fusion partners have been identified, the most common RET fusion partners include KIF5B, CCDC6, and NCOA4 [3,4].
RET fusion is defined as a unique subtype of NSCLC that may benefit from precision medicine. Multi-kinase inhibitors (MKIs) such as cabozantinib and vandetanib showed modest clinical activity but are associated with off-target toxicity [5,6]. The limitations of MKIs prompted the development of more selective target therapy for RET fusion. Selpercatinib and pralsetinib are highly selective RET inhibitors. In early phase studies involving RET fusion-positive NSCLC, both agents demonstrated robust efficacy and favorable tolerability [7,8]. Selpercatinib and pralsetinib were later approved for patients with RET fusion-positive advanced NSCLC.
Immune checkpoint inhibitors (ICIs) have become the standard of care for patients with advanced NSCLC without driver mutations [[9], [10], [11]]. The use of ICIs in RET fusion-positive advanced NSCLC remains controversial. The IMMUNOTARGET study reported a poor response of RET fusion-positive NSCLC to ICIs, with a 6.3% overall response rate (ORR) and a median of 2.1 months progression-free survival (PFS) [12]. However, data from real-world databases in the US showed good efficacy in patients with RET fusion-positive NSCLC who received ICI-based therapy, with a 53.8% ORR and 4.2 months median PFS [13].
Owing to the limited and conflicting reports on the efficacy of ICIs in RET fusion-positive NSCLC, the use of ICIs is still uncertain. However, the low prevalence of this subtype (approximately 1%–2% in NSCLC) means data pertaining to ICI therapy in this cancer type is scarce. In this study, we collected and analyzed the clinicopathological data of Chinese patients with RET fusion-positive NSCLC and examined the clinical efficacy of their treatment with RET inhibitors.
2. Materials and methods
2.1. Patients
In this retrospective study, patients diagnosed with RET fusion-positive lung cancer in our center between Jan 2017 and Sep 2021 were identified. Clinical, pathological, and molecular data were extract from electronic health records. Patients receiving RET inhibitors or ICIs were included in the efficacy analysis. Clinical response was evaluated according to the Response Evaluation Criteria in Solid Tumors version (RECIST) 1.1. Progression -free survival was measured from initiation of the therapy to disease progression or death. Patients who continued therapy were censored at the last follow-up. All data were updated as of 3 Dec 2021. The study was approved by the Institutional Review Board of the Cancer Hospital, Chinese Academy of Medical Sciences. Written informed consent was obtained from every patient.
2.2. Detection of RET fusion partners
RET fusion was identified using DNA based targeted next generation sequencing (NGS). A 56-gene panel sequencing was performed with genomic DNA as previously reported [14]. For some uncommon RET fusion variants, targeted RNA NGS was performed as previously reported [15].
2.3. PD-L1 immunohistochemistry
Tumor tissue sections were stained with the 22C3 pharmDx kit (Agilent, Santa Clara, CA, USA) using the Dako Autostainer Link 48 platform (Agilent). Expression of PD-L1 was evaluated by tumor proportion score (TPS) which was defined as the percentage of tumor cells with partial or complete membrane staining.
2.4. Statistical analysis
Descriptive statistics were used to summarize patient characteristics. RECIST 1.1 guidelines were used to evaluate treatment efficacy. Patients who were alive without disease progression were censored at the initiation of a new therapy or the last follow-up. Survival curves were calculated using the Kaplan–Meier method and compared using the log-rank test. Statistical significance was set at p < 0.05. All analyses were performed using the IBM SPSS® Statistics version 23.
3. Results
3.1. Patient characteristics
From Jan 2017 to Sep 2021, data from seventy-five patients with RET fusion-positive advanced lung cancer were retrospectively analyzed. Patient baseline characteristics are summarized in Table 1. The median age of patients was 57 years (range 51–65), half of the patients were female (50.7%), and most patients were non-smokers or light smokers (72%). Most patients were also diagnosed with adenocarcinoma (94.6%), whereas two patients were diagnosed with adenosquamous carcinomas, one with large cell neuroendocrine carcinoma and the other with sarcomatoid carcinoma. Seventeen patients (22.7%) had brain metastases and twenty-seven patients (36%) had accompanying pleural metastasis.
Table 1.
Patients baseline characteristics.
| Characteristic | N (%) |
|---|---|
| Age, median (years) | 57 (51, 65) |
| Age (years) | |
| ≤65 | 56 (74.7%) |
| >65 | 19 (25.3%) |
| Sex | |
| male | 37 (49.3%) |
| female | 38 (50.7%) |
| Smoking status | |
| never or light smoking | 54 (72%) |
| heavy smoking | 21 (28%) |
| ECOG | |
| 0 | 36 (48%) |
| 1 | 39 (52%) |
| Histological subtype | |
| adenocarcinoma | 71 (94.6%) |
| non-adenocarcinoma | 4 (5.4%) |
| Brain metastasis | |
| no | 58 (77.3%) |
| yes | 17 (22.7%) |
| Liver metastasis | |
| no | 67 (89.3%) |
| yes | 8 (10.3%) |
| Pleural metastasis | |
| no | 48 (64%) |
| yes | 27 (36%) |
| Immune checkpoint inhibitors (ICIs) | |
| Pembrolizumab | 1 (1.3%) |
| chemotherapy and ICIs | 15 (20%) |
| RET inhibitors | |
| Pralsetinib | 11 (14.7%) |
| Selpercatinib | 4 (5.3%) |
| Cabozantinib | 6 (8%) |
3.2. Genomic alterations and PD-L1 expression
Targeted DNA-based NGS was used to identify RET fusion subtypes. The KIF5B-RET subtype was identified for 73% (55/75) of patients, twelve patients (16%) had CCDC6-RET fusion and three patients (4%) had NCOA4-RET fusion. We identified several rare RET fusion partners, including JMJD1C-RET, NRG3-RET, FXYD4-RET, CELF2-RET, and ARHGAP12-RET (Fig. 1A). The most common concurrent mutations were TP53 (17, 22.7%) and PTEN (3, 4%) (Fig. 2A). Two patients with EGFR-sensitive mutations acquired RET fusion after EGFR-TKI resistance. The PD-L1 expression assay was performed in twenty patients, of these five patients exhibited PD-L1 overexpression (≥50%), ten patients exhibited intermediate (1–49%) expression and five patients were PD-L1 negative (<1%) (Fig. 1B).
Fig. 1.
Genotype and PD-L1 expression in RET fusion NSCLC. A: RET upstream fusion partners. B: PD-L1 expression in RET fusion NSCLC. Abbreviations: PD-L1: programmed cell death ligand 1; RET: rearranged during transfection; NSCLC: non-small cell lung cancer.
Fig. 2.
Co-mutations in RET fusion NSCLC. Abbreviations: RET: rearranged during transfection; NSCLC: non-small cell lung cancer.
3.3. Clinical outcomes of RET inhibitor treatment
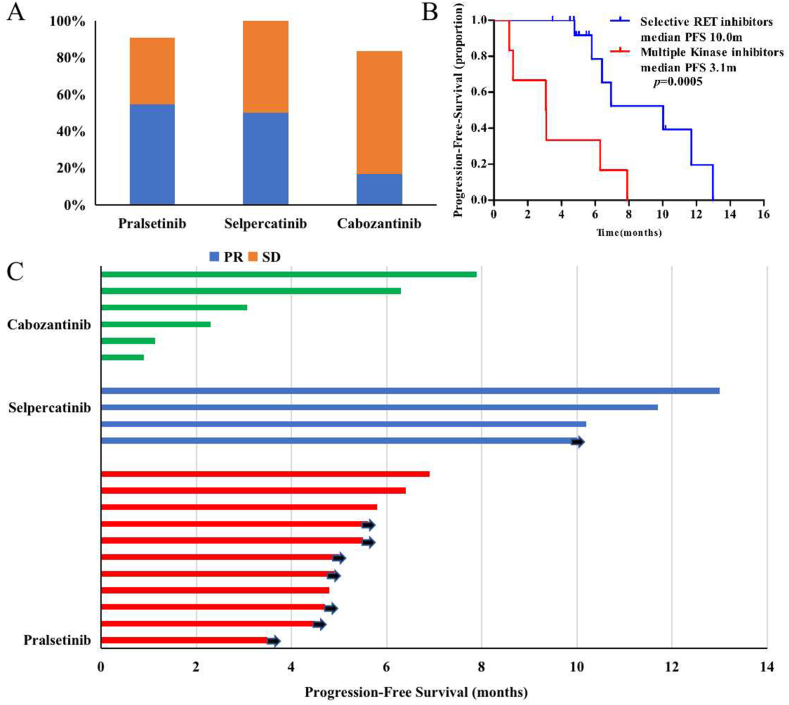
Twenty-one patients received either cabozantinib or selective RET inhibitors. Pralsetinib, the only approved selective RET inhibitor in China, was administered to eleven patients (14.7%) and ten patients (90.9%) received pralsetinib as second or later line therapy. The ORR was 54.5% and disease control rate (DCR) was 90.9% (Fig. 3A). Median PFS was not available on the date of data cutoff (3 Dec 2021). Four patients received another selective RET inhibitor, selpercatinib, in clinical trials, with an ORR of 50% and DCR of 100%. The PFS was 13.0, 10.0 and 11.7 months (Fig. 3C). Cabozantinib, the most common MKIs for RET fusion, was administered to six patients after failure of standard therapy. Only one patient had a partial response, ORR and DCR were 16.7% and 83.3%, respectively, median PFS was 3.1 months. Selective RET inhibitors displayed superior efficacy compared to cabozantinib for RET fusion-positive NSCLC (Fig. 3B).
Fig. 3.
Clinical efficacy of patients with advanced NSCLC received RET inhibitors. A: Clinical response types. B: Progression free survival by RET inhibitors. C: Swimmer plot of PFS across cabozantinib, selpercatinib and pralsetinib cohorts. Abbreviations: RET: rearranged during transfection; PFS - progression-free survival, PR - partial response, SD - stable disease.
3.4. Clinical outcomes of ICI treatment
Fifteen patients received chemoimmunotherapy, one patient received mono-immunotherapy. Four patients were treated with RET inhibitors after failure of immunotherapy. Patient characteristics and ICI responses are shown in Table 2. Seven patients received ICIs as the first-line therapy, including one pembrolizumab monotherapy and six chemoimmunotherapy. In previously untreated patients undergoing ICI treatment, we observed an ORR of 71.4% and a DCR of 100%. One patient (P 1) had objective response to mono-immunotherapy and one patient (P 4) had durable remission after first-line chemo-immunotherapy with PFS over 12 months. After failure of first line chemotherapy, the ORR to ICIs was lower, only one of nine (11%) patients achieved partial response (Table 3). Median PFS was 7.5 months (95% CI 4.5–10.5) and 4.7 (months (95% CI 1.1–8.3) for first- and subsequent-line treatments, respectively. Four patients had PD-L1 overexpression (>50%), one received pembrolizumab monotherapy, and the other three patients received pemetrexed, carboplatin, and pembrolizumab or camrelizumab. The ORR was 75% and the DCR was 100%.
Table 2.
Patient characteristics and immune checkpoint inhibitors response.
| Case | Age/Sex | Histology | Stage | PD-L1 | RET Fusion | Immunotherapy | Treatment Lines | Before or after RET-TKI | Response | PFS (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| P1 | 63/F | Ade | IV | 90% | KIF5B-RET | Pembrolizumab | 1 | Before | PR | 5.6 |
| P2 | 66/M | Ade | IV | NA* | JMJD1C-RET | Pemetrexed + Camrelizumab | 3 | – | SD | 7.9 |
| P3 | 55/M | Ade | IV | 20% | KIF5B-RET | Pemetrexed + Carboplatin + Bevacizumab + Pembrolizumab | 2 | – | SD | 7.2 |
| P4 | 54/F | Ade | IV | NA* | KIF5B-RET | Pemetrexed + Carboplatin + Camrelizumab | 1 | – | PR | 12.6 |
| P5 | 48/M | Ade | IV | 5% | KIF5B-RET | Pemetrexed + Carboplatin + Pembrolizumab | 3 | Before | SD | 6.6 |
| P6 | 43/M | Ade | IV | 0 | FXYD4-RET | Nab-paclitaxe + Bevacizumab + Tislelizumab | 3 | – | PR | 6.6 |
| P7 | 65/M | Ade | IV | 80% | KIF5B-RET | Pemetrexed + Carboplatin + Pembrolizumab | 1 | – | SD | 7.5 |
| P8 | 63/M | Ade | IV | 1% | KIF5B-RET | Pemetrexed + Carboplatin + Bevacizumab + Camrelizumab | 2 | Before | PD | 1.5 |
| P9 | 54/M | Ade | IV | NA* | KIF5B-RET | Pemetrexed + Carboplatin + Tislelizumab | 1 | – | PR | 6.1 |
| P10 | 47/F | LCNC | IV | 0 | KIF5B-RET | Nab-paclitaxe + Durvalumab | 4 | Before | SD | 3.5 |
| P11 | 56/F | Ade | IV | NA* | KIF5B-RET | Paclitaxe + Sintilimab | 2 | – | SD | 4.7 |
| P12 | 49/M | Ade | IV | 35% | KIF5B-RET | Docetaxe + Nedaplatin + Pembrolizumab | 2 | – | PD | 0.8 |
| P13 | 79/M | Ade | IV | NA* | KIF5B-RET | Pembrolizumab + Anlotinib | 2 | – | PD | 1.3 |
| P14 | 63/M | Ade | IV | NA* | CCDC6-RET | Pemetrexed + Carboplatin + Bevacizumab + Toripalimab | 1 | – | SD | 9.4 |
| P15 | 65/F | Ade | IV | 95% | KIF5B-RET | Pemetrexed + Carboplatin + Pembrolizumab | 1 | – | PR | 5.6 |
| P16 | 49/F | Ade | IV | 90% | KIF5B-RET | Pemetrexed + Carboplatin + Camrelizumab | 1 | – | PR | 5.1 |
Note: *NA defined as PD-L1 expression unknown.
Table 3.
Clinical response of immune checkpoint inhibitors.
| ORR | DCR | Median PFS (months) | |
|---|---|---|---|
| First line (n = 7) | 5 (71.4%) | 7 (100%) | 7.5 |
| Subsequent line (n = 9) | 1 (11%) | 6 (66.7%) | 4.7 |
| PD-L1 positive (n = 8) | 3 (37.5%) | 6 (75%) | 6.6 |
| PD-L1 negative or unknown (n = 8) | 3 (37.5%) | 7 (87.5%) | 6.1 |
| PD-L1 high (≥50%) (n = 4) |
3 (75%) | 4 (100%) | 5.6 |
Abbreviations: ORR: objective response rate. DCR: disease control rate. PFS: progression-free survival.
Patient with KIF5B-RET fusion and PD-L1 overexpression sequentially responds to pembrolizumab and selpercatinib
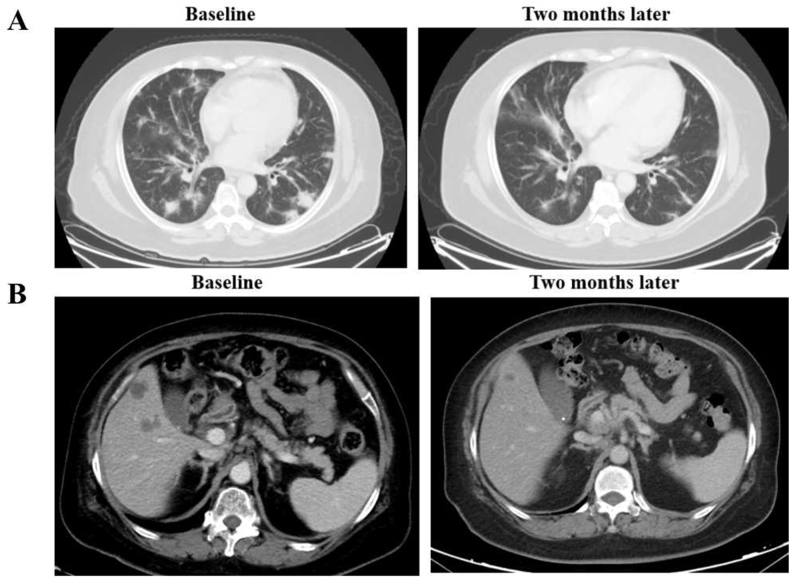
A 63-year-old woman presented with cough and multiple lung masses. Bronchoscopic biopsy revealed lung adenocarcinoma. Tests for common driver mutations such as EGFR, KRAS, ALK, ROS1, MET, and BRAF were negative. Tumor tissues showed high levels of PD-L1 expression (TPS 90%). She was administrated pembrolizumab monotherapy as first line therapy and achieved a confirmed partial response. Five months later, clinical evaluation showed disease progression with an enlarged lung mass. A repeat biopsy of the lung mass showed poorly differentiated adenocarcinoma and PD-L1 overexpression (TPS 70%). We performed NGS with a 1021-gene panel and detected KIF5B-RET fusion. Owing to the unavailability of selective RET inhibitors in China between 2018 and 2020, she received five lines of therapy, including chemoimmunotherapy and anlotinib. She was subsequently treated with the MKI cabozantinib as the seventh-line treatment, which was discontinued a month after initiation because of disease progression in the liver. She took part in the selpercatinib study in China, and imaging indicated a partial response. Thirteen months after selpercatinib treatment, computed tomography showed pleural effusion and enlarged liver lesions. Pleural cytology revealed the presence of adenocarcinoma cells. Molecular testing of the pleural effusion cell-free DNA (cfDNA) revealed KIF5B-RET fusion, KRAS p.A146V mutation, and EGFR amplification with a copy number of 2.1 (Fig. 4).
Fig. 4.
Clinical response of pembrolizumab and selpercatinib for the case with RET fusion and PD-L1 over-expression. A: CT scan revealing the clinical response to pembrolizumab. B: CT scan revealing the clinical response to selpercatinib. Abbreviations: PD-L1: programmed cell death ligand 1; RET: rearranged during transfection; CT: computed tomography.
4. Discussion
Platinum-based chemotherapies are the standard care for treatment of naïve RET fusion-positive NSCLC in China. Selective RET inhibitors are recommended after failure of standard chemotherapy. The efficacy of ICIs in these subtype of patients remains uncertain. In this study, we found a relatively high proportion of PD-L1 expression in RET fusion-positive NSCLC. Efficacy associated with ICIs, especially for patients with high PD-L1 expression levels and treatment naive patients, were comparable to those reported for unselected populations. The selective RET inhibitors, selpercatinib and pralsetinib, displayed superior outcomes compared with the MKIs cabozantinib.
In this study, half of the RET fusion patients were female, non-smokers, and diagnosed with adenocarcinoma. Patients with RET fusion subtypes had clinical characteristics that were consistent with previous studies [2]. We identified RET fusion in one large-cell neuroendocrine carcinoma and one sarcomatoid carcinoma. To our knowledge, this is the first report of sarcomatoid carcinoma accompanied by RET fusion. We found that 75% of RET fusion-positive lung cancers exhibited positive (>1%) PD-L1 expression. This is consistent with a previous report that RET fusion-positive NSCLC was associated with high PD-L1 expression [16]. In our study, TP53 co-mutations occurred more frequently compared with other genes. Previous studies have shown that concurrent mutations in tumor suppression genes, such as TP53 and PTEN, contribute to inferior outcomes for EGFR-TKI treatments when compared to the pure EGFR mutation subgroup [17]. Owing to the limited number of patients receiving selective RET inhibitors, we did not find influence of TP53 co-mutation on the clinical outcomes. Further research is warranted to evaluate the impact of TP53 co-mutation on selective RET inhibitors.
Before the approval of selective target agents, some patients with RET fusion-positive lung cancer received ICIs as the first-line therapy, either as monotherapy or in combination with chemotherapy. Previous retrospective studies reported a poor response to ICIs with a 6.3% ORR and median PFS of approximately 2.1–3.4 months [12,18]. Given the small sample size and that most patients had low PD-L1 expression, efficacy of ICI treatment in patients with RET fusion-positive cancer remains unclear. In this study, approximately 75% of patients exhibited positive PD-L1 expression. Four patients with PD-L1 overexpression (>50%) were administrated pembrolizumab or pembrolizumab plus pemetrexed and carboplatin as first-line treatment. Three patients obtained a partial response and one had stable disease. Lu et al. reported a satisfactory response to ICIs in two patients with RET fusion and high PD-L1 expression [19]. Marinello et al. reported that ICIs as monotherapy and combined with chemotherapy showed durable clinical response, with an ORR of 31% and 43%, and median PFS of 5.0 months and 9.4 months, respectively. Biomarker analysis showed relatively high PD-L1 expression in ICI responders [20]. In the randomized phase III trial LIBRETTO-431, Zhou et al. reported patients receiving platinum based chemotherapy with pembrolizumab had better PFS than those previously reported in Keynote189 trial [21]. These results suggested that ICIs, especially for patients with PD-L1 overexpression, combined with chemotherapy, may offer benefits for RET fusion-positive NSCLC. Our study contributes information on real-world outcomes of ICIs in patients with RET fusion-positive lung cancers.
As a member of tyrosine kinase receptor family, RET shares a similar kinase domain structure to other tyrosine kinase receptors [22]. Multi-kinase inhibitors such as cabozantinib, vandetanib, sunitinib, and alectinib have demonstrated activity against RET fusion-positive cancers [5,6,23]. The ORR (18%–37%) and median PFS (2.3 months) were lower compared to those in patients with other driver gene-positive cancers receiving targeted agents [24]. Selective RET inhibitors were then developed, and results from clinical trials such as ARROW (NCT03037385), LIBRETTO-001 (NCT03157128) and LIBRETTO-431(NCT04194944) demonstrated superior activity and tolerability [7,8,21]. Our real-world data also showed that selective RET inhibitors had favorable efficacy compared with cabozantinib for RET fusion NSCLC cancers.
Here, we report a case with KIF5B-RET fusion and PD-L1 overexpression that sequentially responded to pembrolizumab, chemoimmunotherapy, and later-line selpercatinib. Following resistance to selpercatinib, pleural effusion cfDNA testing revealed KIF5B-RET fusion, KRAS p.A146V mutation, and EGFR amplification. Currently, limited reports describe resistance mechanisms to selective RET inhibitors. Solomon et al. reported that three patients with RET fusion-positive NSCLC acquired RET G810 solvent-front mutations after selpercatinib resistance [25]. Lin et al. found three patients acquired MET amplification and one acquired KRAS amplification [26]. Preclinical studies showed that activation of the EGFR pathway mediated cancer cells resistance to RET inhibitors [27,28]. Mechanisms of resistance to selective RET inhibitors still need to be explored.
This study evaluated the real-world efficacy of ICIs and RET inhibitors in RET fusion-positive NSCLC, however, the study has several limitations. As this was a retrospective analysis, it was exposed to potential bias. The number of patients receiving selective RET inhibitors or ICIs was relatively small, despite it being one of the larger cohorts reported in China. Different combinations of chemotherapy and ICIs may also influence the efficacy of immunotherapy. Future analyses of ICIs, particularly in PD-L1 overexpression populations and use in combination with chemoimmunotherapy, are warranted.
5. Conclusions
This study observed that selective RET inhibitors had superior efficacy compared to cabozantinib. We also found a relatively high proportion of PD-L1 expression in RET fusion-positive NSCLC. Efficacy of ICIs, especially for PD-L overexpression and treatment naive patients, were comparable to those reported for unselected populations, warranting further investigation.
List of abbreviations: DCR, disease control rate. EGFR, epidermal growth factor receptor. ICIs, immune checkpoint inhibitors. MKIs, multi-kinase inhibitors. NGS, next generation sequencing. NSCLC, non-small-cell lung cancer. ORR, objective response rate. PD-L1, programmed death ligand 1. PFS, progression free survival. RET, Rearranged during transfection. TPS, tumor proportion score.
Declarations
Ethics statement
This study was reviewed and approved by the Ethics Committee of the Cancer Hospital, Chinese Academy of Medical Sciences, with the approval number NCCC2020C-499. All patients provided informed consent to participate in the study. All patients provided informed consent for the publication of their anonymised case details and images.
Consent for publication
Written informed consent for publication was obtained from all participants.
Funding
This work was supported by the National Key R&D program of China (2022YFC2505000); NSFC general program (82272796); NSFC special program (82241229); CAMS Innovation Fund for Medical Sciences (CIFMS 2022-I2M-1-009); CAMS Key Laboratory of Translational Research on Lung Cancer (2018PT31035); Beijing Hope Run Special Fund of the Cancer Foundation of China (LC2020B10); Cultivation project of Medical Oncology Key Foundation of Cancer Hospital Chinese Academy of Medical Sciences (CICAMS-MOCP2022008); Beijing Natural Science Foundation (7214249).
Data availability statement
Data will be made available on request.
CRediT authorship contribution statement
Rui Wan: Writing – review & editing, Writing – original draft, Formal analysis, Data curation, Conceptualization. Weihua Li: Writing – review & editing, Methodology, Formal analysis, Data curation, Conceptualization. Zhijie Wang: Writing – review & editing, Project administration. Jia Zhong: Writing – review & editing, Project administration. Lin Lin: Writing – review & editing, Project administration. Jianchun Duan: Writing – review & editing, Project administration. Jie Wang: Writing – review & editing, Writing – original draft, Project administration, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank editage’ excellent work in text editing.
References
- 1.Cancer Genome Atlas Research Network Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511:543–550. doi: 10.1038/nature13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang K., Chen H., Wang Y., Yang L., Zhou C., Yin W., Wang G., Mao X., Xiang J., Li B., Zhang T., Fei S. Clinical characteristics and molecular patterns of RET-rearranged lung cancer in Chinese patients. Oncol. Res. 2019;27:575–582. doi: 10.3727/096504018X15344979253618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li A.Y., McCusker M.G., Russo A., Scilla K.A., Gittens A., Arensmeyer K., Mehra R., Adamo V., Rolfo C. RET fusions in solid tumors. Cancer Treat Rev. 2019;81 doi: 10.1016/j.ctrv.2019.101911. [DOI] [PubMed] [Google Scholar]
- 4.Shi M., Wang W., Zhang J., Li B., Lv D., Wang D., Wang S., Cheng D., Ma T. Identification of RET fusions in a Chinese multicancer retrospective analysis by next-generation sequencing. Cancer Sci. 2022;113:308–318. doi: 10.1111/cas.15181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drilon A., Rekhtman N., Arcila M., Wang L., Ni A., Albano M., Voorthuysen M.V., Somwar R., Smith R.S., Montecalvo J., Plodkowski A., Ginsberg M.S., Riely G.J., Rudin C.M., Ladanyi M., Kris M.G. Cabozantinib in patients with advanced RET-rearranged non-small-cell lung cancer: an open-label, single-centre, phase 2, single-arm trial. Lancet Oncol. 2016;17:1653–1660. doi: 10.1016/S1470-2045(16)30562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee S.H., Lee J.K., Ahn M.J., Kim D.W., Sun J.M., Keam B., Kim T.M., Heo D.S., Ahn J.S., Choi Y.L., Min H.S., Jeon Y.K., Park K. Vandetanib in pretreated patients with advanced non-small cell lung cancer-harboring RET rearrangement: a phase II clinical trial. Ann. Oncol. 2017;28(2):292–297. doi: 10.1093/annonc/mdw559. [DOI] [PubMed] [Google Scholar]
- 7.Drilon A., Subbiah V., Gautschi O., Tomasini P., de Braud F., Solomon B.J., Tan D.S.W., Alonso G., Wolf J., Park K., Goto K., Soldatenkova V., Szymczak S., Barker S.S., Puri T., Lin A.B., Loong H., Besse B. Selpercatinib in patients with RET fusion-positive non-small-cell lung cancer: updated safety and efficacy from the registrational LIBRETTO-001 Phase I/II trial. J. Clin. Oncol. 2023;41:385–394. doi: 10.1200/JCO.22.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gainor J.F., Curigliano G., Kim D.W., Lee D.H., Besse B., Baik C.S., Doebele R.C., Cassier P.A., Lopes G., Tan D.S.W., Garralda E., Paz-Ares L.G., Cho B.C., Gadgeel S.M., Thomas M., Liu S.V., Taylor M.H., Mansfield A.S., Zhu V.W., Clifford C., Zhang H., Palmer M., Green J., Turner C.D., Subbiah V. Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): a multi-cohort, open-label, phase 1/2 study. Lancet Oncol. 2021;22:959–969. doi: 10.1016/S1470-2045(21)00247-3. [DOI] [PubMed] [Google Scholar]
- 9.Gandhi L., Rodríguez-Abreu D., Gadgeel S., Esteban E., Felip E., Angelis F.D., Domine M., Clingan P., Hochmair M.J., Powell S.F., Cheng S.Y.-S., Bischoff H.G., Peled N., Grossi F., Jennens R.R., Reck M., Hui R., Garon E.B., Boyer M., Rubio-Viqueira B., Novello S., Kurata T., Gray J.E., Vida J., Wei Z., Yang J., Raftopoulos H., Pietanza M.C., C Garassin M. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N. Engl. J. Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 10.Herbst R.S., Baas P., Kim D.W., Felip E., Pérez-Gracia J.L., Han J.Y., Molina J., Kim J.H., Arvis C.D., Ahn M.J., Majem M., Fidler M.J., de Castro G., Jr., Garrido M., Lubiniecki G.M., Shentu Y., Im E., Dolled-Filhart M., Garon E.B. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 11.Borghaei H., Paz-Ares L., Horn L., Spigel D.R., Steins M., Ready N.E., Chow L.Q., Vokes E.E., Felip E., Holgado E., Barlesi F., Kohlhäufl M., Arrieta O., Burgio M.A., Fayette J., Lena H., Poddubskaya E., Gerber D.E., Gettinger S.N., Rudin C.M., Rizvi N., Crinò L., Jr G.R.B., Antonia S.J., Dorange C., Harbison C.T., Finckenstein F.G., Brahmer J.R. Nivolumab versus docetaxel in advanced nonsquamous non-small-Cell lung cancer. N. Engl. J. Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazieres J., Drilon A., Lusque A., Mhanna L., Cortot A.B., Mezquita L., Thai A.A., Mascaux C., Couraud S., Veillon R., Van den Heuvel M., Neal J., Peled N., Früh M., Ng T.L., Gounant V., Popat S., Diebold J., Sabari J., Zhu V.W., Rothschild S.I., Bironzo P., Martinez-Marti A., Curioni-Fontecedro A., Rosell R., Lattuca-Truc M., Wiesweg M., Besse B., Solomon B., Barlesi F., Schouten R.D., Wakelee H., Camidge D.R., Zalcman G., Novello S., Ou S.I., Milia J., Gautschi O. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the immunotarget registry. Ann. Oncol. 2019;30:1321–1328. doi: 10.1093/annonc/mdz167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhandari N.R., Hess L.M., Han Y., Zhu Y.E., Sireci A.N. Efficacy of immune checkpoint inhibitor therapy in patients with RET fusion-positive non-small-cell lung cancer. Immunotherapy. 2021;13:893–904. doi: 10.2217/imt-2021-0035. [DOI] [PubMed] [Google Scholar]
- 14.Li W., Liu Y., Li W., Chen L., Ying J. Intergenic breakpoints identified by DNA sequencing confound targetable kinase fusion detection in NSCLC. J. Thorac. Oncol. 2020;15:1223–1231. doi: 10.1016/j.jtho.2020.02.023. [DOI] [PubMed] [Google Scholar]
- 15.Li W., Guo L., Liu Y., Dong L., Yang L., Chen L., Liu K., Shao Y., Ying J. Potential unreliability of uncommon ALK, ROS1, and RET genomic breakpoints in predicting the efficacy of targeted therapy in NSCLC. J. Thorac. Oncol. 2021;16:404–418. doi: 10.1016/j.jtho.2020.10.156. [DOI] [PubMed] [Google Scholar]
- 16.Jin Y., Xue Q., Shen X., Zheng Q., Chen H., Zhou X., Li Y. PD-L1 expression and comprehensive molecular profiling predict survival in nonsmall cell lung cancer: a real-world study of a Large Chinese cohort. Clin. Lung Cancer. 2022;23:43–51. doi: 10.1016/j.cllc.2021.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z., Cheng Y., An T., Gao H., Wang K., Zhou Q., Hu Y., Song Y., Ding C., Peng F., Liang L., Hu Y., Huang C., Zhou C., Shi Y., Zhang L., Ye X., Zhang M., Chuai S., Zhu G., Hu J., Wu Y.L., Wang J. Detection of EGFR mutations in plasma circulating tumour DNA as a selection criterion for first-line gefitinib treatment in patients with advanced lung adenocarcinoma (BENEFIT): a phase 2, single-arm, multicentre clinical trial. Lancet Respir. Med. 2018;6:681–690. doi: 10.1016/S2213-2600(18)30264-9. [DOI] [PubMed] [Google Scholar]
- 18.Offin M., Guo R., Wu S.L., Sabari J., Land J.D., Ni A., Montecalvo J., Halpenny D.F., Buie L.W., Pak T., Liu D., Riely G.J., Hellmann M.D., Benayed R., Arcila M., Kris M.G., Rudin C.M., Li B.T., Ladanyi M., Rekhtman N., Drilon A. Immunophenotype and response to immunotherapy of RET-rearranged lung cancers. JCO Precis. Oncol. 2019;3 doi: 10.1200/PO.18.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu C., Dong X.R., Zhao J., Zhang X.C., Chen H.J., Zhou Q., Tu H.Y., Ai X.H., Chen X.F., An G.L., Bai J., Shan J.L., Wang Y.N., Yang S.Y., Liu X., Zhuang W., Wu H.T., Zhu B., Xia X.F., Chen R.R., Gu D.J., Xu H.M., Wu Y.L., Yang J.J. Association of genetic and immuno-characteristics with clinical outcomes in patients with RET-rearranged non-small cell lung cancer: a retrospective multicenter study. J. Hematol. Oncol. 2020;13:37. doi: 10.1186/s13045-020-00866-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aldea M., Marinello A., Duruisseaux M., Zrafi W., Conci N., Massa G., Metro G., Monnet I., Iranzo P.G., Tabbo F., Bria E., Guisier F., Vasseur D., Lindsay C.R., Ponce-Aix S., Cousin S., Citarella F., Fallet V., Minatta J.N., Eisert A., de Saint Basile H., Audigier-Valette C., Mezquita L., Calles A., Mountzios G., Tagliament M., Masip J.R., Raimbourg J., Terrisse S., Russo A., Cortinovis D., Rochigneux P., Pinato D.J., Cortellini A., Leonce C., Gazzah A., Ghigna M.R., Ferrara R., Dall’Olio F.G., Passiglia F., Ludovini V., Barlesi F., Felip E., Planchard D., Besse B. RET-MAP: an international multicenter study on Clinicobiologic features and treatment response in patients with lung cancer harboring a RET fusion. J. Thorac. Oncol. 2021;18:576–586. doi: 10.1016/j.jtho.2022.12.018. [DOI] [PubMed] [Google Scholar]
- 21.Zhou C., Solomon B., Loong H.H., Park K., Perol M., Arriola E., Novello S., Han B., Zhou J., Ardizzoni A., Mak M.P., Santini F.C., Elamin Y.Y., Drilon A., Wolf J., Payakachat N., Uh M.K., Rajakumar D., Han H., Puri T., Soldatenkova V., Lin A.B., Lin B.K., Goto K. First-line selpercatinib or chemotherapy and pembrolizumab in RET fusion–positive NSCLC. N. Engl. J. Med. 2023;389(20):1839–1850. doi: 10.1056/NEJMoa2309457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plaza-Menacho I. Structure and function of RET in multiple endocrine neoplasia type 2. Endocr. Relat. Cancer. 2018;25 doi: 10.1530/ERC-17-0354. https://10.1530/ERC-17-0354 T79–T90. [DOI] [PubMed] [Google Scholar]
- 23.Lin J.J., Kennedy E., Sequist L.V., Brastianos P.K., Goodwin K.E., Stevens S., Wanat A.C., Stober L.L., Digumarthy S.R., Engelman J.A., Shaw A.T., Gainor J.F. Clinical activity of alectinib in advanced RET-rearranged non-small cell lung cancer. J. Thorac. Oncol. 2016;11:2027–2032. doi: 10.1016/j.jtho.2016.08.126. https://10.1016/j.jtho.2016.08.126 [DOI] [PubMed] [Google Scholar]
- 24.Gautschi O., Milia J., Filleron T., Wolf J., Carbone D.P., Owen D., Camidge R., Narayanan V., Doebele R.C., Besse B., Remon-Masip J., Janne P.A., Awad M.M., Peled N., Byoung C.C., Karp D.D., Heuvel M.V.D., Wakelee H.A., Neal J.W., K Mok T.S., Yang J.C.H., Ou S.H.I., Pall G., Froesch P., Zalcman G., Gandara D.R., Riess J.W., Velcheti V., Zeidler K., Diebold J., Früh M., Michels S., Monnet I., Popat S., Rosell R., Karachaliou N., Rothschild S.I., Shih J.Y., Warth A., Muley T., Cabillic F., Mazières J., Drilon A. Targeting RET in patients with RET-rearranged lung cancers: results from the global, multicenter RET registry. J. Clin. Oncol. 2017;35:1403–1410. doi: 10.1200/JCO.2016.70.9352. https://10.1200/JCO.2016.70.9352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solomon B.J., Tan L., Lin J.J., Wong S.Q., Hollizeck S., Ebata K., Tuch B.B., Yoda S., Gainor J.F., Sequist L.V., Oxnard G.R., Gautschi O., Drilon A., Subbiah V., Khoo C., Zhu E.Y., Nguyen M., Henry D., Condroski K.R., Kolakowski G.R., Gomez E., Ballard J., Metcalf A.T., Blake J.F., Dawson S.J., Blosser W., Stancato L.F., Brandhuber B.J., Andrews S., Robinson B.G., Rothenberg S.M. RET solvent front mutations mediate acquired resistance to selective RET inhibition in RET-driven malignancies. J. Thorac. Oncol. 2020;15:541–549. doi: 10.1016/j.jtho.2020.01.006. https://10.1016/j.jtho.2020.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin J.J., Liu S.V., McCoach C.E., Zhu V.W., Tan A.C., Yoda S., Peterson J., Do A., Prutisto-Chang K., Dagogo-Jack I., Sequist L.V., Wirth L.J., Lennerz J.K., Hata A.N., Mino-Kenudson M., Nardi V., Ou S.H.I., Tan D.S.W., Gainor J.F. Mechanisms of resistance to selective RET tyrosine kinase inhibitors in RET fusion-positive non-small-cell lung cancer. Ann. Oncol. 2020;31:1725–1733. doi: 10.1016/j.annonc.2020.09.015. https://10.1016/j.annonc.2020.09.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang H., Sung J.H., Moon S.U., Kim H.S., Kim J.W., Lee J.S. EGF induced RET inhibitor resistance in CCDC6-RET lung cancer cells. Yonsei Med. J. 2017;58:9–18. doi: 10.3349/ymj.2017.58.1.9. https://10.3349/ymj.2017.58.1.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaishnavi A., Schubert L., Rix U., Marek L.A., Le A.T., Keysar S.B., Glogowska M.J., Smith M.A., Kako S., Sumi N.J., Davies K.D., Ware K.E., Varella-Garcia M., Haura E.B., Jimeno A., Heasley L.E., Aisner D.L., Doebele R.C. EGFR mediates responses to small-molecule drugs targeting oncogenic fusion kinases. Cancer Res. 2017;77:3551–3563. doi: 10.1158/0008-5472.CAN-17-0109. https://10.1158/0008-5472.CAN-17-0109 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.