Abstract
Rationale & Objective
This study describes the epidemiology, characteristics, and clinical outcomes of patients with focal segmental glomerulosclerosis (FSGS)-attributed kidney failure in the US Renal Data System (USRDS) during 2008-2018, and health care resource utilization and costs among those with Medicare-linked data.
Study Design
This was a retrospective cohort study.
Setting & Population
Patients with FSGS-attributed kidney failure in the USRDS were enrolled in the study.
Outcomes
The outcomes were as follows: Prevalence and incidence, clinical and demographic characteristics, time to kidney transplant or death, health care resource utilization, and direct health care costs.
Analytical Approach
Patients with FSGS as the primary cause of kidney failure were followed from USRDS registration (index date) until death or data end. Prevalence and incidence were calculated per 1,000,000 US persons. Patient characteristics at index and treatment modalities during follow-up were described. Time to kidney transplant or death was assessed with Kaplan-Meier and competing risk analyses. Health care resource utilization and costs were reported among patients with 1 year Medicare Part A+B coverage postindex, including (Medicare Coverage subgroup) or excluding (1-year Medicare Coverage subgroup) those who died.
Results
The FSGS cohort and Medicare Coverage and 1-year Medicare Coverage subgroups included 25,699, 6,340, and 5,575 patients, respectively. Mean annual period prevalence and incidence rates of FSGS-attributed kidney failure were 87.6 and 7.5 per 1,000,000 US persons, respectively. Initial treatment for most patients was in-center hemodialysis (72.1%), whereas 7.3% received kidney transplant. Accounting for competing risk of death, year 1 and 5 kidney transplant rates were 15% and 34%, respectively. In the Medicare Coverage and 1-year Medicare Coverage subgroups, 76.6% and 74.2% required inpatient admission, 69.9% and 67.3% visited the emergency room, and mean monthly health care costs were $6,752 and $5,575 in the year postindex, respectively.
Limitations
Drug costs may be underestimated because Medicare Part D coverage was not required; kidney acquisition costs were not available.
Conclusions
FSGS-attributed kidney failure is associated with substantial clinical and economic burden, prompting the need for novel therapies for FSGS to delay kidney failure.
Index Words: Focal segmental glomerulosclerosis, health care costs, health care resource utilization, kidney failure, United States Renal Data System
Plain-Language Summary
This study of patients in the US Renal Data System observed increasing prevalence and fluctuating incidence of focal segmental glomerulosclerosis (FSGS)-attributed kidney failure from 2008 to 2018. Patients experienced a high clinical burden, including more than 3 years of treatment with dialysis, one-third receiving a kidney transplant, and one-third dying during follow-up. In the first year after US Renal Data System registration, three-quarters of patients with Medicare coverage required hospitalization, and more than two-thirds visited the emergency room. The total annual health care costs were >$68,000 per patient with FSGS-attributed kidney failure, underscoring the high economic burden of this disorder and the treatments required to sustain life. Novel therapies for FSGS are needed to delay or ideally prevent the need dialysis and transplantation after kidney failure.
Focal segmental glomerulosclerosis (FSGS), a kidney lesion characterized by scattered scarring in at least one glomerulus, accounts for ∼35% of nephrotic syndrome cases and is a common glomerular cause of kidney failure.1, 2, 3 FSGS can be caused by a variety of factors, including diabetes, sickle cell disease, genetics, drugs, and infections, and occurs in both adults and children.1,3 The prevalence and incidence of FSGS-associated kidney failure have increased dramatically over the past 3 decades but vary across geographic and ethnic populations, with higher risk among Black compared to White or Asian patients.3,4 In the United States, the incidence of FSGS is approximately 3.2 per 100,000 person-years (2004-2013), a 41% increase over the prior decade (1994-2003).5
Given the diverse origins of FSGS, treatment depends on its classification (ie, primary, secondary, or uncertain cause) and the presence of nephrotic syndrome, although the goal of therapy remains the reduction or remission of proteinuria.6 However, there is no cure for FSGS, and there are currently limited treatment options, with no therapy specifically approved for FSGS in the United States. Patients with primary FSGS typically receive immunosuppressive agents (ie, glucocorticoids or calcineurin inhibitors) and supportive measures, such as renin-angiotensin inhibitors.7, 8, 9 Patients with secondary, genetic, or unknown forms of FSGS receive treatment directed at the underlying disorder (ie, cessation of nephrotoxic drugs) or supportive therapy alone. Medical therapy may be used in combination with dietary sodium restriction or diuretics to help control proteinuria, blood pressure, and edema. However, the mainstay therapy, namely, glucocorticoids, are associated with problematic adverse events and high rates of treatment failure.10 Although other immunosuppressive agents, such as cyclosporine and tacrolimus, may be used to improve the possibility of long-term remission, they are also accompanied by adverse events, such as kidney damage or opportunistic infection.6 There are several emerging or investigational therapies for primary FSGS, including rituximab, sparsentan, and voclosporin, although their efficacy remains unclear.
Ultimately, over 50% of patients with FSGS will progress to kidney failure within 5-10 years, necessitating more intensive intervention such as kidney replacement treatment.11 Additionally, the 7-year kidney survival rate for FSGS is lower than for other primary glomerular diseases (69% vs 88% for membranous nephropathy and 82% for IgA nephropathy).11 FSGS contributes a substantial economic burden to global health care systems because of the high costs of dialysis and transplantation for kidney failure.12, 13, 14, 15 In the United States alone, kidney failure affects approximately 786,000 people, leading to more than $51 billion in associated health care costs for Medicare beneficiaries in 2019.16,17
Although a detailed understanding of the epidemiology and treatment landscape of FSGS-attributed kidney failure is crucial to meeting the unmet medical needs of this patient population, there is a lack of real-world evidence in the United States. This retrospective observational study used the US Renal Data System (USRDS) database to characterize patients with FSGS-attributed kidney failure, including estimates of prevalence and incidence and descriptions of patient characteristics, treatment modalities, and clinical outcomes. Additionally, health care resource utilization (HRU) and direct health care costs were described for subgroups of patients with FSGS-attributed kidney failure with linked Medicare claims data.
Methods
Data Source
This study used data from the USRDS (2008-2018), the national data registry that collects, analyzes, and distributes information on US patients with kidney failure (see additional methods in Item S1 for more details).18 The USRDS defines kidney failure as chronic kidney failure requiring kidney replacement treatment (ie, dialysis or transplant) to sustain life. All kidney failure patients in the United States are included in the USRDS database, regardless of insurance coverage and age. Kidney failure patients with Medicare coverage have linked Medicare claims data, permitting analysis of HRU and direct health care costs.
This study received an exemption from ethical review by the WCG Institutional Review Board on March 20, 2021 (IRB number: 17-1409204-1).
Study Design and Sample Selection
This retrospective cohort study identified patients with FSGS-attributed kidney failure (FSGS cohort) as those whose primary cause of kidney failure, as recorded in the Medical Evidence Report (MER) form at USRDS registration, was FSGS. The MER form includes a field for primary cause of kidney failure using International Classification of Diseases, ninth edition (ICD-9-CM; 1995-2014) or 10th edition (ICD-10-CM; 2015 onward) diagnosis codes. Patients with FSGS as the primary cause of kidney failure were identified using ICD-9 or 10-CM codes listed in Table S1.
The “index date” was defined as the date of registration in the USRDS data (ie, the earliest date of first dialysis or transplant). The “follow-up period” was defined as the period from the index date until the first date of death, loss to follow-up, or end of data availability.
Among patients in the FSGS cohort, a “Medicare Coverage subgroup” was constructed of patients with ≥1 year of continuous Medicare Part A and B coverage following the index date or until death if it occurred within 1 year postindex. A “1-Year Medicare Coverage subgroup” was constructed to include only patients with ≥1 year of continuous Medicare Part A and B coverage after index (ie, excluding patients who died within 1 year postindex). For both Medicare subgroups, cardiovascular events, HRU, and direct health care costs during the 1-year period after index were reported because they had the linked Medicare claims required for these analyses.
Study Measures
Epidemiology
Estimates of the prevalence and incidence of FSGS-attributed kidney failure in the United States were calculated from 2008 to 2018 on a per 1,000,000 persons per year basis, overall, and by age groups (≥18 and <18 years). Incidence in each year was calculated as the number of FSGS-attributed kidney failure patients with index dates occurred during the year, divided by the size of the US population in that year.19,20 Prevalence in each year was calculated as the number of living FSGS-attributed kidney failure patients whose index dates occurred during or before the year, divided by the size of the US population during that year.19,20
Patient Characteristics
Demographics, laboratory values of estimated glomerular filtration rate (eGFR), and selected comorbid conditions as reported at registration were summarized overall and for the Medicare subgroups.
Treatment Modalities
Treatment modalities, including types of dialysis and/or kidney transplant received by the FSGS cohort as of the index date, during the first year after index and during the entire follow-up period were described. Time of treatment with dialysis during the follow-up period was reported.
Clinical Outcomes
The time to kidney transplant, time to death, and the 10 most common causes of death were assessed for the FSGS cohort. Rates of cardiovascular events were summarized for the Medicare subgroups during the one-year period postindex.
HRU and Health Care Costs
HRU and direct health care costs were summarized among the Medicare subgroups in the 1-year postindex period, stratified by place of service [outpatient, inpatient, emergency room (ER), home health agency, skilled nursing facility (SNF), hospice, or other]. “Other” costs comprised those incurred outside of the other categories and includes some kidney failure treatment facilities. The total number of inpatient days was summarized for patients with ≥1 inpatient stay. Prescription drug costs were summarized. Direct health care costs were separately reported for 4 mutually exclusive subgroups defined by treatment received: kidney transplant, in-center hemodialysis only, peritoneal dialysis only, and other or mixed dialysis.
Statistical Analyses
Incidence and prevalence of FSGS-associated kidney failure were reported using counts and rates per 1,000,000 persons in the US population. Categorical variables were described using frequencies and percentages, whereas continuous variables were described using means, medians, and standard deviations (SD).
Kaplan-Meier (KM) analyses were used to assess the times from the index date to kidney transplant and death, with patients censored at the earliest of death, loss to follow-up, or data end. The times to kidney transplant and death were reported as medians with 95% confidence intervals (CIs) and 1-, 3-, 5-, and 10-year rates. Times to kidney transplant and death were also assessed using competing risk estimators, with death and kidney transplant as the respective competing risks.
HRU and health care costs for the Medicare subgroups were estimated per patient per month (PPPM) to account for different follow-up times across patients. Health care costs were adjusted to 2020 US dollars using the Consumer Price Index. Annual results for the 1-year Medicare Coverage subgroup are presented in Tables S1-S5.
All analyses were conducted using SAS Enterprise Guide Software v7.1 and R software v4.0.3, including the cmprsk package for competing risk analysis.21,22
Results
Sample Selection
Of the 3,135,443 patients in the USRDS database, 25,699 met the criteria for inclusion in the FSGS cohort, whereas 6,340 and 5,575 met the criteria for the Medicare Coverage and 1-year Medicare Coverage subgroups, respectively (Fig 1).
Figure 1.
Sample selection. Abbreviations: FSGS, focal segmental glomerulosclerosis; ICD-9-CM, International Classification of Diseases, ninth Revision, Clinical Modification; ICD-10-CM, International Classification of Diseases, 10th Revision, Clinical Modification; USRDS, United States Renal Data System. aIncluded patients with a record in both the patient’s file and in the medical evidence form file. The patient’s file was used to collect their USRDS registration date. The medical evidence form file was used to collect their demographics and additional information from their kidney failure registration. bThe index date was defined as the date of first USRDS registration.
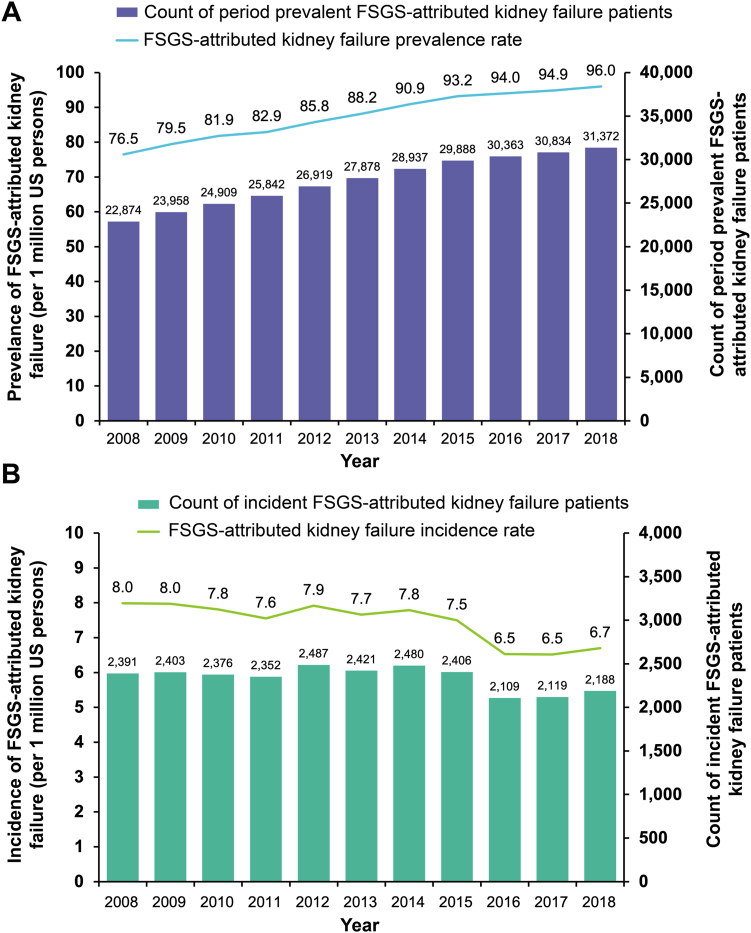
Incidence and Prevalence of FSGS-Attributed Kidney Failure
The mean annual period prevalence and incidence rates of FSGS-attributed kidney failure from 2008 to 2018 were 87.6 and 7.5 per million in the US population, respectively. The prevalence of FSGS-attributed kidney failure steadily increased over time from 76.5 per million US persons in 2008 to 96.0 per million in 2018 (Fig 2A) while the incidence of FSGS-attributed kidney failure remained stable from 2008 to 2015 before declining in 2016 (Fig 2B). The mean period prevalence of FSGS-attributed kidney failure was lower among patients aged <18 years compared to adults aged ≥18 years (31.4 vs 104.9 per million, respectively) (Table S1), higher among males than females (108.0 vs 67.9 per million), and highest among Native Hawaiian or Pacific Islanders (262.9 per million) and Black or African Americans (256.3 per million) (Table S2).
Figure 2.
(A) Prevalence and (B) incidence of FSGS-attributed kidney failure. Abbreviations: FSGS, focal segmental glomerulosclerosis.
Patient Baseline Characteristics
Demographic and clinical characteristics of the FSGS cohort and Medicare subgroups as of the index date are summarized in Table 1.23 For the FSGS cohort, the mean (SD) age at USRDS registration was 51.0 (18.9) years, and 61.4% were male. The majority were White (61.8%) or Black (32.6%). The most common comorbid condition was history of hypertension (87.7%) followed by diabetes (17.9%).
Table 1.
Patient Characteristics
| FSGS-Attributed Kidney Failure Patients |
Medicare Coverage Subgroup |
1-year Medicare Coverage Subgroup |
|
|---|---|---|---|
| (n = 25,699) | (n = 6,340) | (n = 5,575) | |
| Demographic Characteristics | |||
| Age at index (y), mean (SD) [median] | 51.0 (18.9) [52.6] | 63.0 (17.2) [67.6] | 61.8 (17.5) [66.8] |
| Male, n (%) | 15,788 (61.4%) | 3,937 (62.1%) | 3,467 (62.2%) |
| Race, n (%) | |||
| White | 15,886 (61.8%) | 4,532 (71.5%) | 3,920 (70.3%) |
| Black | 8,373 (32.6%) | 1,537 (24.2%) | 1,407 (25.2%) |
| Asian | 994 (3.9%) | 182 (2.9%) | 164 (2.9%) |
| Native Hawaiian or Pacific Islander | 201 (0.8%) | 29 (0.5%) | 28 (0.5%) |
| Other | 101 (0.4%) | 60 (0.9%) | 20 (0.4%) |
| Medical coverage at indexa(%) | |||
| Employer group health insurance | 9,666 (37.6%) | 916 (14.4%) | 818 (14.7%) |
| Medicare | 9,008 (35.1%) | 5,278 (83.2%) | 4,545 (81.5%) |
| Medicaid | 5,794 (22.5%) | 1,483 (23.4%) | 1,347 (24.2%) |
| Other medical insurance | 4,479 (17.4%) | 1,943 (30.6%) | 1,676 (30.1%) |
| No medical insurance | 1,840 (7.2%) | 238 (3.8%) | 234 (4.2%) |
| Medicare Advantage | 1,377 (5.4%) | 107 (1.7%) | 86 (1.5%) |
| Clinical Characteristics | |||
| eGFR (mL/min/1.73 m2)b, mean (SD) [median] | 9.0 (6.4) [8.1] | 9.5 (5.4) [8.7] | 9.4 (5.5) [8.6] |
| Comorbid conditions, n (%)c | |||
| History of hypertension | 22,539 (87.7%) | 5,709 (90.0%) | 5,041 (90.4%) |
| Diabetes | 4,604 (17.9%) | 1,524 (24.0%) | 1,256 (22.5%) |
| Congestive heart failure | 3,142 (12.2%) | 1,262 (19.9%) | 968 (17.4%) |
| Total follow-up duration (mo)d, mean (SD) [median] | 54.4 (36.8) [49.4] | 49.3 (33.8) [43.4] | 55.3 (31.5) [49.4] |
Abbreviations: eGFR, estimated glomerular filtration rate; FSGS, focal segmental glomerulosclerosis; SD, standard deviation; USRDS, United States Renal Data System.
Patients may have had multiple types of medical coverage.
eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation and reported directly in the Medical Evidence file of the USRDS data.28
The top 3 comorbid conditions recorded in the Medical Evidence file are shown.
Follow-up duration was calculated as the time from the index date until the earliest of death, loss to follow-up, or end of data availability.
Among the Medicare Coverage subgroup, the mean age at USRDS registration was 63.0 (SD, 17.2) years; 62.1% were male. The majority of patients were White (71.5%) and, similar to the FSGS cohort, the most common comorbid conditions were history of hypertension (90.0%) and diabetes (24.0%). Among the 1-year Medicare Coverage subgroup, the mean age was 61.8 (SD, 17.5) years; 62.2% were males. Most patients (70.3%) were White, and the most common comorbid condition was history of hypertension (90.4%).
Treatment Modalities
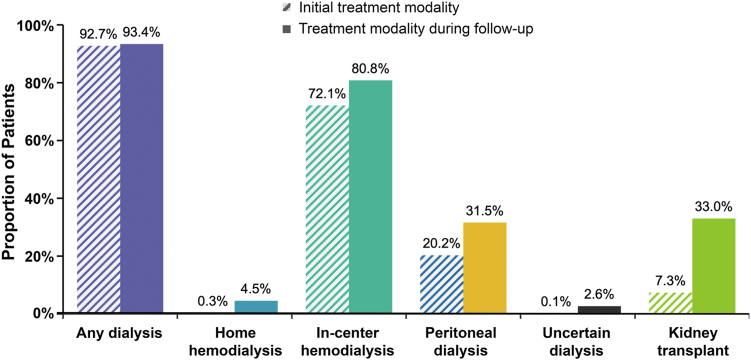
The mean follow-up time for the FSGS cohort was 54.4 (SD, 36.8) months. In-center hemodialysis (72.1%) was the most common initial treatment (ie, treatment at USRDS registration), followed by peritoneal dialysis (20.2%). In total, 7.3% of patients received a kidney transplant as initial treatment, and 0.3% received home hemodialysis. For 0.1% of patients, the type of dialysis was not specified (Fig 3). During the first year after index, the proportion of the FSGS cohort receiving a kidney transplant doubled to 14.7%. Across the entire study period, one-third (33.0%) of patients received a kidney transplant. Among patients with any time of treatment with dialysis, the average time spent was 3.3 (2.6) years, with patients spending on average almost 3 years on in-center dialysis [2.9 (SD, 2.6) years] and 2 years on peritoneal dialysis [2.0 (1.9) years].
Figure 3.
Initial and follow-up period treatment modalities among patients with FSGS- attributed kidney failure (N = 25,699). During the follow-up period, patients may have received more than 1treatment modality. The initial and follow-up treatment modalities were not mutually exclusive, and the treatments summarized for the follow-up period include the initial treatment. Abbreviation: FSGS, focal segmental glomerulosclerosis.
Clinical Outcomes
Kidney Transplant and Death
A total of 8,480 patients had a kidney transplant during the follow-up period, with median time to transplant of 7.2 years (95% CI, 7.0-7.5). Kidney transplant rates at 1 year and 5 years from KM analysis were 15% and 39%, respectively (Fig 4). Overall, 8,278 (32.2%) patients died during follow-up. Median time to death was 9.7 years (95% CI, 9.4-9.9), with mortality rates of 7% at 1 year and 29% at 5 years. Considering death as a competing risk, transplant rates decreased from 59%-43% at 10 years postindex (Fig 4). The interpretation of these results is that 59% of patients would receive a kidney transplant within 10 years after USRDS registration if they lived long enough, based on the KM analysis in which death is considered as censored. Because some patients died before the chance of transplant within 10 years after index, actually, 43% patients would get a kidney transplant within 10 years post-USRDS registration. This estimate is based on the cause-specific cumulative incidence estimate in which death is considered a competing risk of transplant. The most common specified causes of death were cardiac arrest (8.0%) and withdrawal from dialysis or uremia (4.3%); the second most common cause of death overall was undefined or unknown (5.5%) (Table 2).
Figure 4.
Cumulative incidence curves of death and kidney transplant in the FSGS-attributed kidney failure cohort using Kaplan-Meier and competing risk estimators. In the Kaplan-Meier analysis, patients were censored at the earliest of date of death (for the time to transplant analysis only), loss to follow-up, or end of data availability. In the competing risk analysis of time to transplant, patients were censored at the earliest of lost to follow-up or end of data availability; death was considered a competing risk. In the competing risk analysis of time to death before transplant, patients were censored at the earliest of loss to follow-up or end of data availability; transplant was considered a competing risk. Abbreviations: CI, confidence interval; FSGS, focal segmental glomerulosclerosis.
Table 2.
The 10 Most Common Causes of Death Among Patients With FSGS-Attributed Kidney Failure
| FSGS-Attributed Kidney Failure |
|
|---|---|
| N = 25,699 | |
| Cause of death, n (%) | |
| Patients who died (any cause) | 8,278 (32.2%) |
| Cardiac arrest, cause unknown | 2,047 (8.0%) |
| Withdrawal from dialysis or uremia | 1,096 (4.3%) |
| Other identified cause of death, not specified | 375 (1.5%) |
| Septicemia, other | 344 (1.3%) |
| Myocardial infarction, acute | 263 (1.0%) |
| Malignant disease | 204 (0.8%) |
| Cardiac arrhythmia | 171 (0.7%) |
| Cerebrovascular accident (including intracranial hemorrhage) | 145 (0.6%) |
| Cachexia | 141 (0.5%) |
| Unknown | 1,401 (5.5%) |
Abbreviation: FSGS, focal segmental glomerulosclerosis.
Rates of Cardiac Events
Among the Medicare Coverage subgroup, 49.0% had any cardiovascular event during the 1-year postindex period (Table S3). The most common cardiovascular events were heart failure (32.1%) and atrial fibrillation (19.7%). Among the 1-year Medicare Coverage subgroup, 44.9% experienced a cardiovascular event during the 1-year postindex period, most commonly heart failure (28.2%) and atrial fibrillation (16.6%).
HRU
During the year after kidney failure registration, 76.6% of the Medicare Coverage subgroup had an inpatient admission and 69.9% visited the ER (Table 3). On average, hospitalized patients spent 3.8 days in the hospital PPPM. Patients had on average 2.6 outpatient visits PPPM. Over half (56.6%) of patients received home health visits, and 14.7% required visits to SNFs. Among the 1-year Medicare Coverage subgroup, 74.2% required inpatient admission, and 67.3% visited the ER. Among hospitalized patients, the mean stay was 2.5 days PPPM. The mean number of outpatient visits was 2.6 PPPM.
Table 3.
Monthly HRU for the Medicare Coverage and 1-Year Medicare Coverage Subgroups During the 1-Year Period Following the Index Date
| HRU | Proportion With ≥1 Visit, n (%) | Number of Visits PPPMa, Mean (SD) [median] |
|---|---|---|
| Medicare Coverage subgroup | N = 6,340 | N = 6,340 |
| Inpatient admissions | 4,854 (76.6%) | 0.2 (0.4) [0.2] |
| Inpatient length of stay (d)b | — | 3.8 (6.1) [1.7] |
| Outpatient visits | 6,191 (97.6%) | 2.6 (2.1) [2.4] |
| Emergency room visits | 4,431 (69.9%) | 0.3 (0.9) [0.2] |
| Home health agency visits | 3,589 (56.6%) | 0.4 (1.0) [0.1] |
| Skilled nursing facility visits | 935 (14.7%) | 0.7 (2.8) [0.0] |
| Hospice | 147 (2.3%) | 0.1 (1.1) [0.0] |
| 1-year Medicare Coverage subgroup | N = 5,575 | N = 5,575 |
| Inpatient admissions | 4,139 (74.2%) | 0.2 (0.2) [0.1] |
| Inpatient length of stay (d)b | — | 2.5 (3.7) [1.2] |
| Outpatient visits | 5,475 (98.2%) | 2.6 (1.5) [2.4] |
| Emergency room visits | 3,753 (67.3%) | 0.2 (0.3) [0.1] |
| Home health agency visits | 3,113 (55.8%) | 0.4 (0.6) [0.1] |
| Skilled nursing facility visits | 641 (11.5%) | 0.4 (2.1) [0.0] |
| Hospice | 26 (0.5%) | 0.0 (0.8) [0.0] |
Abbreviations: FSGS, focal segmental glomerulosclerosis; HRU, health care resource utilization; PPPM, per patient per month; SD, standard deviation.
The number of visits was calculated among all patients in the Medicare subgroup.
Inpatient length of stay was calculated among patients with at least 1 inpatient admission.
Health Care Costs
In the Medicare Coverage subgroup, mean monthly health care costs were $6,752 (SD: $7,272), driven by inpatient [$2,674 ($6,767)] and outpatient costs [$2,372 ($1,777)] (Table 4). Analyses of health care costs by treatment type during the 1 year postindex indicated that patients with in-center hemodialysis incurred the highest mean costs [$7,428 (SD: $8,211) PPPM], followed by other or mixed dialysis [$6,918 ($5,785) PPPM].
Table 4.
Health Care Costs for the Medicare Coverage and 1-Year Medicare Coverage Subgroups During the 1-Year Period Following the Index Date, Overall and by Treatment Categories
| PPPM Health Care Costsa, Mean (SD) Median [25th-75th Percentile] |
Overall | By Treatment Receivedb |
|||
|---|---|---|---|---|---|
| In-Center Hemodialysis | Peritoneal | Other or Mixed Dialysisc | Kidney Transplant | ||
| Medicare Coverage subgroup, N | 6,340 | 3,958 | 1,026 | 803 | 553 |
| Total health care costs | 6,752 (7,272) 5,181 [3,847-7,596] |
7,428 (8,211) 5,548 [4,137-8,176] |
4,538 (3,871) 3,903 [3,139-5,010] |
6,918 (5,785) 5,595 [4,186-7,792] |
5,778 (5,842) 5,181 [3,173-7,675] |
| Pharmacy | 356 (1,511) 112 [0-303] |
372 (1,349) 118 [0-317] |
227 (649) 87 [0-235] |
403 (2,806) 96 [0-269] |
418 (948) 157 [0-482] |
| Medical | 6,395 (7,047) 4,869 [3,682-7,158] |
7,056 (8,032) 5,214 [3,942-7,727] |
4,310 (3,781) 3,722 [2,995-4,744] |
6,515 (5,026) 5,298 [3,966-7,415] |
5,360 (5,688) 4,770 [2,945-7,040] |
| Inpatient | 2,674 (6,767) 727 [11-3,000] |
3,014 (7,813) 787 [45-3,096] |
1,053 (3,546) 0 [0-899] |
2,536 (4,638) 936 [52-3,116] |
3,449 (5,222) 2,869 [2,113-4,013] |
| Outpatient | 2,372 (1,777) 2,517 [681-3,695] |
2,488 (1,820) 2,651 [755-3,816] |
2,259 (1,564) 2,780 [515-3,524] |
2,754 (1,740) 3,155 [1,060-3,891] |
1,198 (1,357) 779 [423-1,581] |
| ER | 49 (235) 16 [0-53] |
63 (294) 23 [0-68] |
20 (42) 0 [0-24] |
41 (69) 21 [0-55] |
17 (37) 0 [0-18] |
| HHA | 119 (313) 0 [0-88] |
111 (328) 0 [0-66] |
63 (221) 0 [0-24] |
74 (186) 0 [0-66] |
349 (386) 229 [22-544] |
| Hospice | 6 (129) 0 [0-0] |
9 (143) 0 [0-0] |
6 (155) 0 [0-0] |
1 (10) 0 [0-0] |
0 (0) 0 [0-0] |
| SNF | 70 (414) 0 [0-0] |
101 (500) 0 [0-0] |
7 (110) 0 [0-0] |
41 (306) 0 [0-0] |
1 (19) 0 [0-0] |
| Otherd | 1,104 (1,333) 397 [176-2,220] |
1,270 (1,446) 500 [228-2,516] |
903 (1,055) 291 [126-1,811] |
1,067 (1,172) 430 [207-2,059] |
345 (691) 27 [0-285] |
| 1-Year Medicare Coverage subgroup, N | 5,575 | 3,319 | 978 | 733 | 545 |
| Total health care costs | 5,699 (3,697) 4,933 [3,766-6,891] |
6,050 (3,715) 5,183 [4,011-7,154] |
4,214 (2,418) 3,883 [3,139-4,865] |
6,207 (4,420) 5,423 [4,088-7,306] |
5,539 (3,741) 5,169 [3,257-7,601] |
| Pharmacy | 353 (1,419) 124 [0-312] |
369 (1,129) 139 [0-333] |
215 (471) 90 [0-239] |
410 (2,925) 96 [0-275] |
423 (954) 162 [0-490] |
| Medical | 5,346 (3,319) 4,636 [3,587-6,427] |
5,681 (3,458) 4,869 [3,812-6,680] |
4,000 (2,307) 3,704 [2,998-4,632] |
5,797 (3,236) 5,109 [3,940-7,036] |
5,115 (3,464) 4,750 [2,950-6,974] |
| Inpatient | 1,680 (2,774) 437 [0-2,436] |
1,666 (2,893) 454 [24-2,093] |
736 (1,915) 0 [0-766] |
1,878 (2,835) 757 [41-2,687] |
3,191 (2,545) 2,867 [2,182-3,977] |
| Outpatient | 2,415 (1,735) 2,657 [721-3,714] |
2,579 (1,764) 2,864 [854-3,897] |
2,256 (1,553) 2,780 [521-3,521] |
2,784 (1,685) 3,202 [1,138-3,930] |
1,206 (1,362) 3,202 [1,138-3,930] |
| ER | 32 (58) 13 [0-41] |
38 (64) 17 [0-50] |
18 (37) 0 [0-23] |
35 (57) 18 [0-46] |
17 (36) 0 [0-18] |
| HHA | 112 (283) 0 [0-87] |
99 (285) 0 [0-61] |
62 (214) 0 [0-25] |
62 (145) 0 [0-55] |
353 (387) 235 [23-549] |
| Hospice | 2 (93) 0 [0-0] |
2 (85) 0 [0-0] |
5 (158) 0 [0-0] |
0 (0) 0 [0-0] |
0 (0) 0 [0-0] |
| SNF | 41 (255) 0 [0-0] |
63 (315) 0 [0-0] |
7 (111) 0 [0-0] |
17 (155) 0 [0-0] |
1 (15) 0 [0-0] |
| Otherd | 1,063 (1,238) 358 [168-2,185] |
1,233 (1,324) 442 [224-2,525] |
915 (1,064) 292 [132-1,835] |
1,021 (1,148) 401 [205-1,988] |
347 (694) 29 [0-287] |
Abbreviations: ER, emergency room; HHA, home health agency; PPPM, per patient per month; SD, standard deviation; SNF, skilled nursing facility.
Health care costs were evaluated during the 12-month period immediately following the index date, or until death. All health care costs were inflated to 2020 US dollars and presented on a PPPM basis to account for varying follow-up times.
Patients were categorized into mutually exclusive groups based on the treatment modality received during the 12 months following the index date.
Other or mixed dialysis includes home hemodialysis, uncertain dialysis, or multiple types of dialysis during the follow-up period.
Other medical services include claims in which the place of service is not inpatient, outpatient, ER, HHA, hospice, or SNF. This includes but is not limited to kidney failure treatment facilities, assisted living facilities, community mental health centers, inpatient psychiatric facilities, independent care, ambulatory surgical centers, nursing facilities, state or local public health clinics, and urgent care facilities.
Among the 1-year Medicare Coverage subgroup, which excluded patients who died in the 1-year postindex period, the mean total health care costs PPPM during the 1 year following kidney failure registration were $5,699 (SD: $3,697), comprised medical costs [$5,346 ($3,319)] and pharmacy costs [$353 ($1,419)] (Table 4). Outpatient visits were the primary cost driver [$2,415 ($1,735)], followed by inpatient visits [$1,680 ($2,774)]. In this subgroup, patients with other or mixed dialysis and in-center hemodialysis incurred the highest mean total PPPM health care costs [$6,207 (SD: $4,420) and $6,050 ($3,715), respectively]. Patients with kidney transplant incurred the highest mean inpatient costs [$3,191 (SD: $2,545)]. Because all patients in the 1-year Medicare Coverage subgroup had a full year of follow-up postindex, annual costs are presented in Table S4. The mean total annual health care costs were $68,384 (SD: $44,364), driven by outpatient [$28,980 ($20,822)] and inpatient [$20,159 ($33,289)] costs. Total health care costs were estimated at $381.2 million for the 1-year Medicare Coverage subgroup during the 1-year follow-up period.
Discussion
This large retrospective cohort study of patients in the USRDS observed fluctuating incidence of FSGS-attributed kidney failure from 2008 to 2018, decreasing slightly from 8.0 to 6.7 persons per million, whereas the prevalence increased steadily from 76.5 to 96.0 persons per million throughout the same timeframe. Incidence was relatively stable between 2008 and 2015 but dropped beginning in 2016 (to a low of 6.5 per million) before appearing to increase again. This reduction in incidence may be associated with a change in the MER forms used at USRDS patient registration. The forms used until mid-2015 provided choices of disease names and ICD-9-CM codes, whereas those used from mid-2015 to 2018 required ICD-10-CM codes but did not provide a list of disease names or codes. This may have led to coding uncertainty regarding the cause of kidney failure and potential undercounting of FSGS-attributed kidney failure starting in mid-2015. Nevertheless, the USRDS data exhibited a 25% increase in the overall prevalence of FSGS-attributed kidney failure from 2008 to 2018. Of note, the prevalence increased among both pediatric and adult patients and was particularly high among Blacks and Native Hawaiian or Pacific Islanders.
The clinical burden observed for this study’s cohort was high, emphasized by a reliance on kidney transplantation, which was similar (33% during follow-up) to that of the general prevalent kidney failure population (30%).18 Transplantation was the initial treatment for 7% of patients, and nearly all (93.4%) received some form of dialysis during follow-up. Nearly one-third of the cohort (32.2%) died during follow-up. HRU and health care costs were substantial among the subset of patients with Medicare-linked data, with almost three-quarters requiring hospitalization and more than two-thirds visiting the ER in the first year after USRDS registration. Furthermore, inpatient stays were lengthy for patients who required them, totaling over 4 weeks per year on average. Outpatient visits were a major driver of HRU, and patients had approximately 31 annual outpatient visits on average. Home health care services were also common, used by more than half of patients.
Total annual health care costs were over $68,000 per patient, contributing to a total burden of over $381 million to Medicare annually across the entire 1-year Medicare Coverage subgroup. This is similar to the annual cost of kidney failure due to glomerulonephritis ($58,000−$66,000) included in the 2022 USRDS Annual Report and slightly lower than the overall annual per person per year spending for patients with kidney failure ($75,000−$82,000).24 However, their methodology differed in that this study examined costs in the first year after kidney failure. Despite this high burden, the true health care costs are likely much higher because the cost of organ acquisition during kidney transplant could not be assessed. Considering the high rate of kidney transplant in this cohort and that the average cost of kidney transplant in the United States was $442,500 in 2020,25 organ acquisition costs are assumed to be substantial. This study also provides evidence of the high costs associated with mortality in patients with FSGS-attributed kidney failure. The PPPM total health care costs increased from $5,699 to $6,752 when patients who died were included in the analyses, an increase of approximately 19%. Although outpatient costs were relatively similar, inpatient costs increased by 60% when patients who died were included in the sample.
In the past 30 years, the overall prevalence of kidney failure has risen steadily, along with increasing health care expenditures for kidney failure due to the chronic and progressive nature of the disease and costly treatment modalities required to sustain life (ie, dialysis and kidney transplant). This increase in prevalence was also observed in the current cohort FSGS-attributed kidney failure patients, underscoring the urgent need for therapies to prevent progression. Kidney failure accounted for 7.1%-7.2% of all Medicare expenditures throughout the past decade, increasing from $49.2 billion in 2018 to $51 billion in 2019.18 The available therapies for kidney failure not only impose a heavy economic burden on patients and health care systems, but also contribute to greater morbidity due to surgical complications, transplant failure, lengthy inpatient stays, and extensive time devoted to dialysis sessions. Transplants impose a substantial upfront burden on patients and health systems; although a successful transplant may lead to lower costs later on, the progressive nature of FSGS and its high recurrence rate in transplanted kidneys can often lead to transplant failure.26,27 Novel therapies which target the pathophysiology of FSGS-attributed kidney failure are needed to help assuage this large health care burden and improve clinical outcomes by delaying or ideally preventing the need for transplant or dialysis during a patient’s lifetime.
This study benefits from several strengths, including the use of longitudinal USRDS data, a comprehensive and contemporary data source on the kidney failure population in the United States, allowing for a large cohort with FSGS-attributed kidney failure. The ability to link USRDS data to Medicare claims for a subset of patients allowed for the analysis of HRU and health care costs. The results of this study should be interpreted in consideration of several limitations. First, inclusion in the USRDS registry is conditional on receiving kidney failure treatment (dialysis or transplant), so kidney failure should be interpreted as “treated kidney failure” for this study and all studies using USRDS data. Second, FSGS was identified with diagnostic codes, and it was not possible to distinguish between primary or secondary FSGS. Third, the HRU and cost analyses are limited to patients with Medicare coverage as of the index date. Although patients who progress to kidney failure typically become eligible for Medicare, the timing of eligibility and enrollment may vary.28 Therefore, patients with Medicare coverage as of the index date are more likely to be aged ≥65 years, and their HRU and health care costs may not be representative of the general population with FSGS-attributed kidney failure. Fourth, organ acquisition costs were not available in the Medicare claims data and were therefore not included in the health care costs associated with kidney transplants, but are assumed to be substantial. Fifth, prescription drug costs may be underestimated in this study because Medicare Part D coverage was not required for cohort inclusion. However, a sensitivity analysis among a sample of patients with required Medicare Part D coverage illustrated that, although the sample size was reduced by almost 50%, pharmacy costs represented only approximately 9% of total costs. Thus, prescription drugs were not a large driver of the total health care costs for patients with FSGS-attributed kidney failure.
In conclusion, FSGS-attributed kidney failure is associated with a substantial clinical and economic burden to patients and health care systems in the United States as well as a steeply rising prevalence. There is a high unmet need for treatments that delay progression to kidney failure to minimize or prevent the need for dialysis and transplantation, lower the economic burden, and reduce the risk of death and morbidity among this patient population.
Article Information
Authors’ Full Names and Academic Degrees
Mark E. Bensink, PhD, Deborah Goldschmidt, PhD, Zheng-Yi Zhou, PhD, Kaijun Wang, PhD, Richard Lieblich, BS, and C. Martin Bunke, MD
Authors’ Contributions
Research idea and study design: MEB, DG, ZZ, KW, CMB; Data acquisition: DG, ZZ; Data analysis/interpretation: MEB, DG, ZZ, KW, EL, CMB; Statistical analysis: DG, ZZ, KW; Supervision: MEB, ZZ, KW, RL, CMB. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
This work was supported by Travere Therapeutics, Inc. The funder had a role in the study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication.
Financial Disclosure
Dr Wang was an employee of Travere Therapeutics, Inc at the time of the study and held stock/options. Dr Bensink is an employee of Benofit Consulting, which received consulting fees from Travere Therapeutics, Inc. Drs Goldschmidt and Zhou are employees of Analysis Group, Inc, which has received consulting fees from Travere Therapeutics, Inc. Dr Lieblich is an employee of VJA Consulting, which received consulting fees from Travere Therapeutics, Inc. Dr Bunke is an employee of CM Bunke Consulting, which received consulting fees from Travere Therapeutics, Inc.
Acknowledgments
Medical writing was provided by Shelley Batts, PhD, an independent contractor of Analysis Group, Inc, and funded by Travere Therapeutics, Inc.
Disclaimer
The data reported here have been supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the US government.
Peer Review
Received April 18, 2023. Evaluated by 2 external peer reviewers, with direct editorial input by the Statistical Editor and the Editor-in-Chief. Accepted in revised form September 10, 2023.
Footnotes
Complete author and article information provided before references.
Item S1: Supplemental Methods.
Table S1: Diagnostic Codes Used to Identify FSGS.
Table S2: Prevalence and Incidence of FSGS-Attributed Kidney Failure by Year and Age Groups.
Table S3: US Prevalence and Incidence of FSGS-Attributed Kidney Failure by Racial or Ethnic Group.
Table S4: Patients With Cardiovascular Events During the 1-Year Period Following the Index Date Among the Medicare Coverage and 1-Year Medicare Coverage Subgroups.
Table S5: Annual Costs for the 1-Year Medicare Coverage Subgroup During the 1-Year Period Following the Index Date, Overall and by Treatment Category.
Supplementary Material
Item S1; Tables S1-S5.
References
- 1.Rosenberg A.Z., Kopp J.B. Focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2017;12(3):502–517. doi: 10.2215/CJN.05960616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haas M., Meehan S.M., Karrison T.G., Spargo B.H. Changing etiologies of unexplained adult nephrotic syndrome: a comparison of renal biopsy findings from 1976-1979 and 1995-1997. Am J Kidney Dis. 1997;30(5):621–631. doi: 10.1016/s0272-6386(97)90485-6. [DOI] [PubMed] [Google Scholar]
- 3.Kitiyakara C., Eggers P., Kopp J.B. Twenty-one-year trend in ESRD due to focal segmental glomerulosclerosis in the United States. Am J Kidney Dis. 2004;44(5):815–825. [PubMed] [Google Scholar]
- 4.Chun M.J., Korbet S.M., Schwartz M.M., Lewis E.J. Focal segmental glomerulosclerosis in nephrotic adults: presentation, prognosis, and response to therapy of the histologic variants. J Am Soc Nephrol. 2004;15(8):2169–2177. doi: 10.1097/01.ASN.0000135051.62500.97. [DOI] [PubMed] [Google Scholar]
- 5.Hommos M.S., De Vriese A.S., Alexander M.P., et al. The incidence of primary vs secondary focal segmental glomerulosclerosis: a clinicopathologic study. Mayo Clin Proc. 2017;92(12):1772–1781. doi: 10.1016/j.mayocp.2017.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rovin B.H., Adler S.G., Barratt J., et al. KDIGO 2021 Clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100(4):S1–S276. doi: 10.1016/j.kint.2021.05.021. [DOI] [PubMed] [Google Scholar]
- 7.Laurin L.P., Gasim A.M., Poulton C.J., et al. Treatment with glucocorticoids or calcineurin inhibitors in primary FSGS. Clin J Am Soc Nephrol. 2016;11(3):386–394. doi: 10.2215/CJN.07110615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braun N., Schmutzler F., Lange C., et al. Immunosuppressive treatment for focal segmental glomerulosclerosis in adults. Cochrane Dat Syst Rev. 2008;2008(3):CD003233. doi: 10.1002/14651858.CD003233.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell K.N., Pennese N., Zaffalon A., et al. Efficacy and safety of ACE inhibitor and angiotensin receptor blocker therapies in primary focal segmental glomerulosclerosis treatment: a systematic review and meta-analysis. Kidney Med. 2022;4(5) doi: 10.1016/j.xkme.2022.100457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kveder R. Therapy-resistant focal and segmental glomerulosclerosis. Nephrol Dial Transplant. 2003;18(suppl 5):v34–v37. doi: 10.1093/ndt/gfg1042. [DOI] [PubMed] [Google Scholar]
- 11.Moranne O., Watier L., Rossert J., Stengel B., GN-Progress Study Group Primary glomerulonephritis: an update on renal survival and determinants of progression. Qjm. 2008;101(3):215–224. doi: 10.1093/qjmed/hcm142. [DOI] [PubMed] [Google Scholar]
- 12.Zelmer J.L. The economic burden of end-stage renal disease in Canada. Kidney Int. 2007;72(9):1122–1129. doi: 10.1038/sj.ki.5002459. [DOI] [PubMed] [Google Scholar]
- 13.Ismail H., Manaf M.R., Gafor A.H., Zaher Z.M., Ibrahim A.I. Economic burden of ESRD to the Malaysian health care system. Kidney Int Rep. 2019;4(9):1261–1270. doi: 10.1016/j.ekir.2019.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwon C.S., Daniele P., Forsythe A., Ngai C. A systematic literature review of the epidemiology, health-related quality of life impact, and economic burden of immunoglobulin A nephropathy. J Health Econ Outcomes Res. 2021;8(2):36–45. doi: 10.36469/001c.26129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalantar-Zadeh K., Baker C.L., Copley J.B., et al. A retrospective study of clinical and economic burden of focal segmental glomerulosclerosis (FSGS) in the United States. Kidney Int Rep. 2021;6(10):2679–2688. doi: 10.1016/j.ekir.2021.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kidney disease statistics for the United States 2021. National Institute of Diabetes and Digestive and Kidney Disorders. https://www.niddk.nih.gov/health-information/health-statistics/kidney-disease
- 17.Healthcare expenditures for persons with ESRD 2022. United States Renal Disease System. https://adr.usrds.org/2021/end-stage-renal-disease/9-healthcare-expenditures-for-persons-with-esrd
- 18.US Renal Data System . National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2021. 2021 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. [Google Scholar]
- 19.Annual estimates of the resident population for selected age groups by sex for the United States: April 1, 2010 to July 1, 2019 (NC-EST2019-AGESEX). United States Census Bureau. https://www.census.gov/data/tables/time-series/demo/popest/2010s-national-detail.html
- 20.Annual estimates of the resident population by sex, race, and Hispanic origin for the United States: April 1, 2010 to July 1, 2019 (NC-EST2019-SR11H). United States Census Bureau. https://www2.census.gov/programs-surveys/popest/tables/2010-2019/national/asrh/nc-est2019-sr11h.xlsx
- 21.R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R-project.org/
- 22.Gray B. cmprsk: subdistribution analysis of competing risks. R package version 2.2-11 2022. https://CRAN.R-project.org/package=cmprsk
- 23.Levey A.S., Stevens L.A., Schmid C.H., et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.2022 USRDS annual report. United States Renal Disease System. https://usrds-adr.niddk.nih.gov/2022/reference-tables
- 25.Wang J.H., Hart A. Global perspective on kidney transplantation: United States. Kidney360. 2021;2(11):1836–1839. doi: 10.34067/KID.0002472021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trachtman R., Sran S.S., Trachtman H. Recurrent focal segmental glomerulosclerosis after kidney transplantation. Pediatr Nephrol. 2015;30(10):1793–1802. doi: 10.1007/s00467-015-3062-1. [DOI] [PubMed] [Google Scholar]
- 27.Uffing A., Pérez-Sáez M.J., Mazzali M., et al. Recurrence of FSGS after kidney transplantation in adults. Clin J Am Soc Nephrol. 2020;15(2):247–256. doi: 10.2215/CJN.08970719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foley R.N., Collins A.J. The USRDS: what you need to know about what it can and can't tell us about ESRD. Clin J Am Soc Nephrol. 2013;8(5):845–851. doi: 10.2215/CJN.06840712. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Item S1; Tables S1-S5.