Summary
Background
Some locally advanced (IIIA/IIIB) non-small cell lung cancers (NSCLCs) might have surgical options available. However, information regarding the effectiveness of neoadjuvant immunotherapy for potentially resectable IIIA/IIIB NSCLC is limited. The intent of this investigation was to offer a more favourable alternative to the standard approach of chemoradiotherapy (concurrent or sequential chemoradiotherapy) followed by immunotherapy for potentially resectable stage III NSCLC.
Methods
This prospective, single-arm, phase 2 clinical trial (NCT04326153) enrolled treatment-naïve patients with ‘potentially resectable’ IIIA/IIIB NSCLC who were deemed unsuitable for complete (R0) resection upon initial diagnosis. The study period was between March 20, 2020, and August 20, 2021. Patients underwent neoadjuvant chemoimmunotherapy (sintilimab combined with nab-paclitaxel and carboplatin) for two to three cycles prior to surgical resection of the lung carcinoma and systematic nodal dissection within 30–45 days. The primary endpoint was the 2-year disease-free survival (DFS) rate, with secondary endpoints encompassing major pathological response (MPR) rate, pathological complete response (pCR) rate, overall survival, objective response rate (ORR), downstaging rate, and adverse events (AEs). Tumour immune cell infiltrates, identified via immunohistochemistry, were assessed as biomarkers at baseline and after surgery.
Findings
Among 30 patients who received neoadjuvant chemoimmunotherapy, 20 underwent complete resection. The disease control rate was 96.7% (95% CI: 90.3%–99.99%), with an ORR of 55% (95% CI: 37.2%–72.8%) and a downstaging rate of 80% (95% CI: 65.7%–94.3%). In the subgroup of 20 patients who underwent surgery, the MPR rate was 65% (95% CI: 43.3%–82.9%), and the pCR rate was 40% (95% CI: 21.2%–46.3%). The 2-year DFS rate in the surgical group was 75% (95% CI 56%–94%). Notably, the MPR group demonstrated significantly prolonged DFS compared with the non-MPR group (p = 0.00024). A significant increase in pretreatment CD8 expression correlated with improved DFS (p = 0.00019). Three patients (10%) experienced grade 3 or higher immune-related AEs—one case of grade 3 elevated myocardial enzymes, one case of grade 3 interstitial pneumonia, and one case of grade 5 bronchopleural fistula.
Interpretation
Neoadjuvant immunotherapy markedly enhanced the rate of pathological response and 2-year DFS in patients with potentially resectable IIIA/IIIB NSCLC. Overexpression of CD8 before treatment (H score≥3) may serve as a potential predictive biomarker for DFS. Consequently, the treatment landscape for potentially resectable IIIA/IIIB NSCLC could undergo changes.
Funding
This study did not receive any financial support.
Keywords: Non-small cell lung cancer, Potentially resectable, Locally advanced, Neoadjuvant, Chemoimmunotherapy
Research in context.
Evidence before this study
Notably, various large-scale clinical trials involving the combination of neoadjuvant chemotherapy with PD-1 and PD-L1 inhibitors for resectable stage I–III non-small cell lung cancer (NSCLC) reported an 36.9%–62% MPR rate and led to substantial improvements in survival time. Despite favourable outcomes, rigorous evidence supporting neoadjuvant chemotherapy with PD-1 and PD-L1 inhibitors in the treatment of potentially resectable stage IIIA/IIIB NSCLC is limited. Additionally, there is a lack of DFS data for periods of ≥2 years to evaluate the effect of neoadjuvant chemoimmunotherapy in potentially resectable stage IIIA/IIIB NSCLC.
Added value of this study
Our prospective clinical trial aimed to assess the effectiveness and safety of combining PD-1 inhibitors with chemotherapy in the context of locally advanced NSCLC cases; the emphasis on extended follow-up duration with the primary endpoint of a 2-year DFS rate was distinguished from other published clinical trials. Our findings showed that neoadjuvant immunotherapy for patients with potentially resectable stage IIIA/IIIB NSCLC expanded the opportunities for achieving R0 resection in initially unresectable cases and significantly extended the 2-year DFS rate. Furthermore, this study identified pretreatment overexpression of CD8 as a potential predictor of DFS.
Implications of all the available evidence
The intent of this investigation was to ascertain whether neoadjuvant chemoimmunotherapy offers a more favourable alternative to the standard approach of concurrent or sequential chemoradiotherapy (CRT/SRT) followed by immunotherapy for potentially resectable stage III NSCLC. However, confident conclusions cannot be drawn based on the results of our study or other available evidence. Another randomized controlled trial of neoadjuvant chemoimmunotherapy followed by surgical treatment and CRT followed by immune consolidation therapy in patients with potentially resectable stage III NSCLC is needed to confirm these results.
Introduction
Non-small cell lung cancer (NSCLC) constitutes 80%–85% of all lung cancer cases globally. Approximately 20% of patients with NSCLC are diagnosed with potentially resectable T4N1-2 or T1-3N2 (stage IIIA or IIIB) disease owing to direct organ invasion (T4) or mediastinal lymph node spread (N2).1,2 Managing these cases presents a challenge, as various treatment approaches result in a 5-year overall survival (OS) rate of 41% for stage IIIA and 24% for stage IIIB.3 Over the past decades, neoadjuvant chemotherapy before surgery, concurrent or sequential chemoradiotherapy (CRT/SRT), neoadjuvant or adjuvant radiotherapy, and surgery after CRT have been attempted in patients with potentially resectable stage IIIA/IIIB NSCLC.4, 5, 6 Unfortunately, these efforts have yielded minimal advancements, thus warranting the exploration of alternative treatments.
Recent advancements in programmed cell death 1 (PD-1) and programmed cell death-ligand 1 (PD-L1) inhibitors have reshaped metastatic NSCLC therapy.7 Improved outcomes have also been noted in unresectable stage III lung cancers when immunotherapy is administered after chemoradiotherapy. The median progression-free survival (PFS) in Phase II clinical trials, such as the DETERRED and LUN 14-179 studies, was 18.7 and 18.1 months, respectively8,9; however, the 2-year PFS of these two studies was not available. Two significant randomized clinical trials, the PACIFIC and GEMSTONE-301 studies, have demonstrated that immune checkpoint inhibitor (ICI) incorporation markedly enhances PFS and OS in patients with unresectable stage III NSCLC who undergo CRT/SRT, in contrast to conventional standard chemoradiotherapy. Currently, post-definitive CRT/SRT with durvalumab and sugemalimab represent established therapeutic options for patients with unresectable stage III NSCLC. However, the median PFS in the PACIFIC and GEMSTONE-301 studies stood at 16.8 and 14.3 months, respectively, with a 2-year PFS rate around 40%,10,11 which remains suboptimal. Exploring ways to improve PFS in cases of locally advanced NSCLC and determining whether the potential for extended survival or recovery exists presents a substantial challenge.
To the best of our knowledge, employing neoadjuvant chemotherapy before surgery has demonstrated the potential to enhance disease-free survival (DFS) in resectable stage III NSCLC compared to surgery alone (20 months vs. 5 months). Nevertheless, the 2-year and 5-year DFS rates remain modest at approximately 40%–50% and 30%–40%, respectively (Supplementary Table S1).12, 13, 14, 15 Within the cohort subjected to chemotherapy, only 30%–40% achieved complete resection, with a major pathological response (MPR) of 7%–20% and a complete pathological response (pCR) of 2.2% at the time of surgery.12,16 Notably, recent research involving the combination of neoadjuvant chemotherapy with PD-1 and PD-L1 inhibitors for resectable stage III NSCLC reported an 80% downstaging rate and 78%–89% complete resection rate.17 MPR ranging from 36.9% to 62% and pCR rates of 18%–24% were observed in various large-scale clinical trials.18,19 The incorporation of neoadjuvant chemoimmunotherapy also led to substantial improvements in survival time, with 2-year DFS rates ranging from 63.8% to 68%, and a 3-year OS rate exceeding 80%.20,21 The correlation among high MPR, pCR, and extended survival has been documented in cases where neoadjuvant chemoimmunotherapy was administered.16,20 The conceptual basis for neoadjuvant immunotherapy is the premise that the tumour antigen load might amplify immune recognition, thereby promoting the reactivation and expansion of CD8+ T cells within the macroscopic tumour microenvironment. This combination chemotherapy can enhance anti-tumour activation. Additionally, it remains an open question whether neoadjuvant chemoimmunotherapy can be used for potentially resectable stage IIIA/IIIB NSCLC, leading to downstaging from unresectable to resectable disease. This, in turn, may reduce recurrence rates and improve survival by increasing MPR and pCR rates during surgery. Achieving the correct balance between efficacy and safety in this innovative approach for potentially resectable stage IIIA/IIIB NSCLC remains a complex challenge. Therefore, further validation is required.
Although promising outcomes in terms of MPR and pCR have been demonstrated in patients with potentially resectable stage III disease, there is an absence of comprehensive data for those with potentially resectable stage IIIA/IIIB NSCLC who undergo neoadjuvant chemoimmunotherapy followed by surgery, particularly with respect to long-term follow-up. Therefore, we conducted a prospective clinical trial to assess the effectiveness and safety of combining the PD-1 inhibitor (sintilimab) with chemotherapy (nab-paclitaxel and carboplatin) in patients with locally advanced NSCLC. This study emphasises an extended follow-up duration, with the primary endpoint centred on the observation of the 2-year DFS rate, distinguishing it from other published clinical trials. The intent of this investigation was to ascertain whether neoadjuvant chemoimmunotherapy offers a more favourable alternative to the standard approach of CRT/SRT followed by immunotherapy for potentially resectable stage III NSCLC. We anticipate that these findings will shed new light on the management of patients with locally advanced NSCLC.
Methods
Study design and participants
This phase 2 clinical trial (Registration Number: NCT04326153) was conducted as a prospective, single-centre, single-arm study at The First Bethune Hospital of Jilin University in Changchun, China. This study aimed to evaluate the efficacy and safety of sintilimab combined with nab-paclitaxel and carboplatin in patients diagnosed with potentially resectable stage IIIA/IIIB NSCLC.
Patients aged ≥18 years with histologically or cytologically confirmed, treatment-naive, potentially resectable stage IIIA/IIIB NSCLC were eligible for participation. The detailed criteria for inclusion, exclusion, and withdrawal are provided in the Supplementary Methods. The pTNM stages of all the patients were determined according to the Eighth Edition of The Lung Cancer Staging Classification. The initial assessment and staging procedures included enhanced chest computed tomography (CT), brain magnetic resonance imaging, bone scans, and abdominal CT scans, which were conducted as routine practice. We used the term ‘sex’ based on the biological factors of each participant. We determined the sex of each participant through information of their identification cards, which are confirmed by the public security bureau of the People's Republic of China to verify participants' birth details.
The term ‘potentially resectable' was defined as follows: Detection of T4 invasion, such as involvement with the pericardium, large blood vessels, trachea, or the presence of multiple enlarged ipsilateral mediastinal lymph nodes. Although R0 resection was unfeasible at the time of initial diagnosis, the possibility of achieving such a resection arose after neoadjuvant treatment. All assessments were conducted by a multidisciplinary team (MDT) consultations that involved collaboration among the oncology, thoracic surgery, imaging, and radiation therapy departments.
Ethics
This study was performed in accordance with the guidelines of the Declaration of Helsinki and Good Clinical Practice. The protocol was reviewed and approved by the Ethics Committee of Jilin University First Hospital (reference number 19K112-001). All patients provided oral and written informed consent.
Procedures
The treatment regimens are shown in Supplementary Figure S1. Patients received intravenous administration of sintilimab (200 mg) on day 1, nab-paclitaxel (260 mg/m2) on day 1, and carboplatin with an area under the curve (AUC) of 5 (dosed at 5 mg/mL per min) on day 2 of a 21-day cycle. This regimen was repeated for two or three cycles before surgery, contingent on the remission status. Surgical resection of the lung carcinoma and systematic nodal dissection were scheduled 30–45 days post-neoadjuvant treatment. Adjuvant treatment commenced 28–60 days after surgery and involved two cycles of intravenous nab-paclitaxel (260 mg/m2) on day 1 and carboplatin (AUC 5; 5 mg/mL per min) on day 2. Additionally, continuation of sintilimab (200 mg) for 6 months was recommended, although patients could choose to discontinue treatment based on their individual preferences and physical condition. Follow-up assessments, including chest CT scans and safety tests for immune markers, were conducted every 3 months.
The determination of patient suitability for surgery was based on clinical remission and was carried out through MDT consultations involving thoracic surgeons, radiologists, imagists, and oncologists, both before and after neoadjuvant chemoimmunotherapy. Experienced thoracic surgeons performed the surgical procedures. Intraoperative exploration was initially conducted to reassess the resectability and evaluate potential adhesions and tumour invasion in the fissures and nodal stations. Surgical interventions included lobectomy or total pneumonectomy, coupled with comprehensive hilar and mediastinal lymphadenectomy, encompassing dissecting stations 2, 4, 7, 8, 9, and 10 on the right side and stations 4, 5, 6, 7, 8, 9, and 10 on the left side. The frozen sections were evaluated to exclude residual disease.
Pretreatment and postoperative specimens were subjected to immunohistochemistry (IHC) testing for each patient following the manufacturer's guidelines (see Supplementary assessments). Tumour immune cell infiltrates were analysed using a comprehensive set of biomarkers (CD4, CD8, CD20, CD56, FoxP3, CD86, CD163, and CD11b). Slides were scanned using an Olympus microscope (Olympus, BX53, Japan), and an experienced pathologist evaluated pathological remission by assigning scores based on the staining intensity and percentage of positive cells in the tumour area for each biomarker. The tumour area encompasses the tumour cells, interstitial tissue within the tumour, and contiguous interstitial tissue surrounding the tumour. Intravascular immune cells exhibited positive staining but were excluded from the score range. Staining intensity was graded as follows: 0, no positive staining (negative); 1, light yellow (weakly positive); 2, brown-yellow (positive); and 3, brown (strongly positive). Depending on the percentage of positive cells, the scores were assigned as follows: 1 point, ≤25%; 2, 26%–50%; 3, 51%–75%; and 4, >75%; these two scores were multiplied to calculate the final Histochemistry score (H-score).
Morbidity, mortality, and surgical complications were monitored for 90 days post-surgery. Subsequent follow-up visits were scheduled 1 month after surgery and every 3 months thereafter. Tumour assessments were conducted according to RECIST version 1.1, and the sum of longest diameters was calculated by a radiologist at the hospital. Adverse events (AEs) and abnormal laboratory findings were graded based on the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0.
Outcomes
The primary endpoint was the 2-year DFS rate for surgical patients, defined as the duration from the date of surgery to the first occurrence of local or distant recurrence or death. Secondary endpoints included the rate of MPR for surgical patients, characterised by 10% or less residual viable tumour post-surgery; the rate of pCR for surgical patients; the proportion of patients undergoing R0 resection; the proportion of intention-to-treat (ITT) patients achieving tumour downstaging; OS for surgical patients; objective response rate (ORR) for ITT patients; AEs for ITT patients; and quality of life for ITT patients, calculated from the date of enrolment date until disease recurrence or death.
Statistics
The primary endpoint was the 2-year DFS rate; however, because of the extended observation time required for this endpoint, it was not suitable for the prompt validation of treatment response. Given the established correlation between the MPR rate and DFS, as reported in prior investigations,17 the MPR rate was employed to calculate the necessary sample size and assess treatment response. For this purpose, Simon's admissible two-stage design was employed.22 Therefore, we assumed that the addition of sintilimab to chemotherapy would increase the MPR rate from 8.9% to 35% (Supplementary Table S1). The sample size was calculated to be 29 (17 patients in the first stage and 12 in the second stage). In the first stage, the study would be concluded if ≤ 2 patients achieved MPR, indicating a negative outcome. Conversely, if > 2 patients achieved MPR, the study proceeded by enrolling additional 12 patients. Considering the sample size calculation results and sufficient funding, one additional patient was enrolled to increase the sample size, resulting in a final enrolment of 30 patients. Moreover, when eight people were enrolled, the number of MPR exceeded two. Therefore, the inclusion of the enrolled patients did not affect the efficacy measures in the first stage. The type 1 error rate was set to 0.05. The outlined protocol yielded 95% statistical power to detect an MPR rate of 35% under the alternative hypotheses.
The intention-to-treat population was also analysed. Data are expressed through descriptive statistics such as mean, standard deviation, median, maximum, and minimum interquartile range (IQR) values. Enumeration and ranked data are presented as counts (proportions) and corresponding percentages. Subsequent post-hoc comparisons involved categorising patients based on factors such as pathological remission, PD-L1 expression, and adjuvant therapy. Spearman's correlation coefficient was used to analyse the correlation between radiological and pathological remission. The exact two-sided 95% confidence intervals (CIs) were computed using the Clopper–Pearson method. The Kaplan–Meier method was employed to estimate DFS and OS rates. The median follow-up duration and corresponding interquartile range (IQR) were determined through reverse Kaplan–Meier method. Statistical analyses were performed using the R software (ver. 3.6.1 and 4.3.0, Vienna, Austria). The 'waterfalls' package (ver. 1.0.0) was used to draw waterfall plots; the' ggplot2' package (ver. 3.4.2) was used to draw scatter plots and fit straight lines, and the ‘survival’ package (ver. 3.5.5) and ‘survminer’ package (ver. 0.4.9) were used to construct the survival functions. The GraphPad PRISM (ver. 8, GraphPad Software, Inc., California, USA) was used to draw the swim lane diagrams and box plots. Statistical significance was set at p < 0.05.
Role of funding source
This study did not receive any financial support.
Results
Patients and treatment
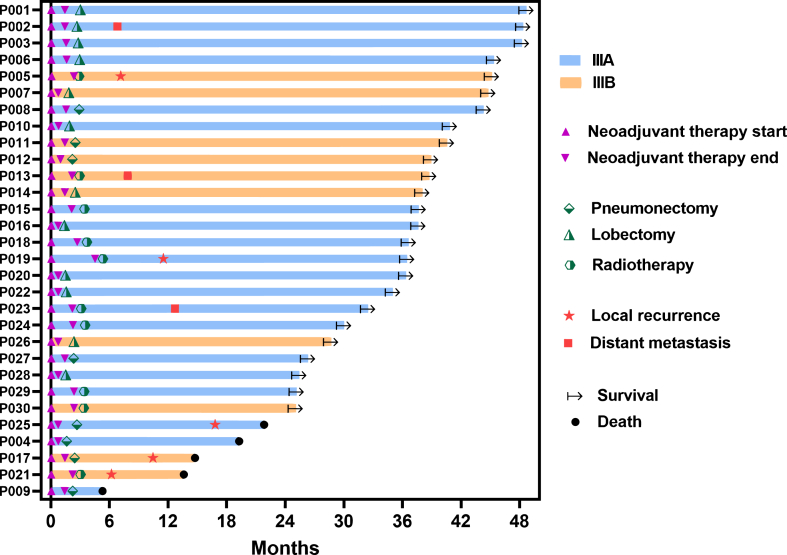
Between March 20, 2020, and August 20, 2021, 30 patients diagnosed with stage IIIA/IIIB NSCLC were enrolled in the study (see Table 1 and Fig. 1). Among these patients, 26 (86.7%) had squamous cell lung cancer, while four were diagnosed with adenocarcinoma. Additionally, 20 patients (66.7%) were categorized as potential stage IIIA, as per the definition provided by the eighth edition of the TNM classification for Lung Cancer-IASLC. The remaining patients were classified as having a potentially resectable stage IIIB disease. Furthermore, 18 patients (60.0%) had T4 disease, and an equal number presented with N2 involvement. Of the 30 patients, 20 underwent successful surgery and all achieved R0 resection status. Within this group, 12 patients (60%) underwent lobectomy, while the remaining eight (40%) underwent left total pneumonectomy. Notably, there were no instances of treatment-related surgical delays, surgery-related deaths, or conversion to open surgery. The average time between diagnosis and surgery was 82.6 days, and the average interval between surgery and adjuvant therapy was 56.9 days. The remaining ten patients did not undergo surgery (see Supplementary Table S2); seven fulfilled the surgical criteria but three declined owing to personal reasons and four missed the surgical window because of COVID-19 epidemic isolation. Three of the ten patients did not meet the surgical criteria because their lesions were deemed unresectable. The subset of ten patients who did not undergo surgery received chemoimmunotherapy and radiotherapy (Fig. 2).
Table 1.
Baseline demographic characteristics.
| Characteristic | All Patients (%) (N = 30) |
|---|---|
| Age, y | |
| Mean ± SD | 58.6 ± 8.1 |
| Median (IQR) | 60.5 (53.5–64.0) |
| Sex, n (%) | |
| Female | 1 (3.3) |
| Male | 29 (96.7) |
| Smoking History, n (%) | |
| No | 2 (6.7) |
| Yes | 28 (93.3) |
| Histological Type, n (%) | |
| Adenocarcinoma | 4 (13.3) |
| Squamous cell | 26 (86.7) |
| Staging, n (%) | |
| IIIA | 20 (66.7) |
| IIIB | 10 (33.3) |
| T staging, n (%) | |
| T1-3 | 12 (40.0) |
| T4 | 18 (60.0) |
| N staging, n (%) | |
| N1 | 12 (40.0) |
| N2 | 18 (60.0) |
| PD-L1 expression, n (%) | |
| <1 | 14 (46.7) |
| 1–49 | 8 (26.7) |
| ≥50 | 8 (26.7) |
| Neoadjuvant cycles, n (%) | |
| 2 | 14 (46.7) |
| 3 | 16 (53.3) |
Fig. 1.
Consort flow chart ofparticipants.
Fig. 2.
Follow-up, recurrence, type of surgery, and staging details for the 30 enrolledpatients.
Efficacy
Radiological and pathological remission
Based on the RECIST 1.1 criteria, 29 of the 30 patients (96.7%) achieved disease control (complete response [CR]+partial response [PR]+stable disease [SD]). Among them, 16 (53.3%) achieved a PR, and 13 (43.3%) maintained SD (Fig. 3a). The disease control rate was 96.7% (95% CI: 90.3%–99.99%), while the ORR was 55% (95% CI: 37.2%–72.8%). No CR was observed. Notably, the downstaging rate was 80% (95% CI: 65.7%–94.3%).
Fig. 3.
Radiological and pathological remission. a. Pathological remission. b. Radiological remission. c. Correlation between pathological remission and radiological remission. No correlation was found between pathological remission and radiological remission. Confidence band (the gray area) was 95% CI.
Regarding pathological response rates in the subgroup of 20 patients who underwent surgery, 13 individuals (65.0%; 95% CI: 43.3%–82.9%) demonstrated a significant MPR. Of these 13 patients, eight (40.0%; 95% CI: 21.2%–46.3%) achieved pCR (Fig. 3b). All patients with pCR had squamous cell NSCLC. Differentiating between patients who received two and three cycles of neoadjuvant therapy, the MPR rates were 30.0% (3 out of 10) and 50.0% (5 out of 10), respectively. The MPR and pCR rates were 43.3% and 26.7%, respectively, for the entire ITT patient population. Discrepancies between radiological and pathological remission were observed (Fig. 3c and Supplementary Table S3).
DFS and OS
As of 31 August, 2023, the median follow-up duration was 39.7 months. Of the 20 patients who underwent surgery, 15 (75%) remained recurrence-free, and neither the median DFS nor OS was reached. Up to this point, 25 of the 30 patients survived, with one patient (who did not undergo surgery) passing away owing to an unrelated car accident. Among the four deceased patients who underwent surgery, one succumbed to infection resulting from postoperative bronchopleural fistula (BPF) 3 months after left pneumonectomy, while the remaining three patients died owing to lung cancer. The 2-year DFS rate in the surgery group and the 2-year EFS rate in the ITT population were recorded at 75% (95% CI 56%–94%) and 67% (95% CI 50%–84%), respectively. Furthermore, the 2-year OS rates for the surgery group and ITT population were 80% (95% CI 63%–97%) and 83% (95% CI 70%–97%), respectively (Fig. 4).
Fig. 4.
Survival data. a. 2-year DFS rate for patients who underwent surgery is 75%. b. 2-year EFS rate for ITT patients. c. OS for patients who underwent surgery. d. OS for ITT patients. The imaginal line indicated the 2-year survival rate.
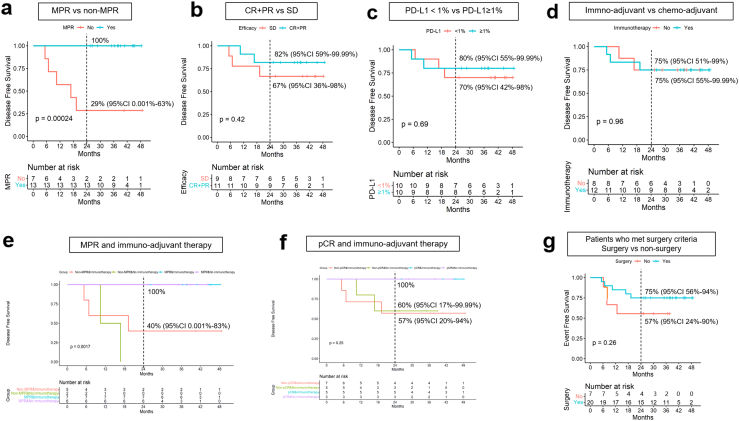
In the subgroup analysis, no instances of recurrence were observed among the MPR group. Notably, the MPR group exhibited significantly prolonged DFS than that of the non-MPR group (p = 0.00024) (Fig. 5a). Conversely, there was no significant correlation between radiological remission and DFS (Fig. 5b). Similar findings were noted between PD-L1 negative and PD-L1 ≥ 1% subgroups, although a statistically significant correlation between PD-L1 expression and DFS was not observed (refer to Fig. 5c). Of these patients, 12 underwent adjuvant chemoimmunotherapy, while eight underwent adjuvant chemotherapy alone; both groups displayed a two-year DFS rate of 75% (Fig. 5d). To account for the potential impact of MPR or pCR ratios, separate analyses were conducted for the MPR, non-MPR, immune-adjuvant therapy, pCR, non-pCR, and immune-adjuvant therapy subgroups (Fig. 5e–f). Immune adjuvant therapy had a notable impact on DFS in the non-MPR group but not in the non-pCR group. Ten patients underwent two cycles of chemoimmunotherapy, and 10 patients underwent three cycles of chemoimmunotherapy. There was no statistically significant difference between the two DFS groups (Supplementary Figure S2). Notably, seven patients who met the surgical criteria did not undergo surgery and hence were treated with two additional cycles of immunochemotherapy and radiotherapy after radiological remission evaluation. Additional details are provided in Supplementary Table S2. The 2-year EFS rate was 57%. In comparison to surgical patients, the benefits of chemoimmunotherapy combined with radiotherapy for EFS were somewhat less pronounced, although the difference was not statistically significant (Fig. 5g). Demographic variables, such as age, sex, smoking history, and histological type, were not significantly related to the 2-year DFS rate (Supplementary Table S4).
Fig. 5.
Subgroup analysis for DFS. a. There was a significant difference in DFS between MPR and non-MPR patients. b. No substantial correlation was found between radiological remission and DFS. c. There was no substantial correlation between PD-L1 expression and DFS. d. There was no increase in the use of adjuvant immunotherapy, compared with the use of adjuvant chemotherapy, in all surgical patients. e. Immune adjuvant therapy exhibited a notable impact on DFS in the non-MPR group. f. Immune adjuvant therapy exhibited no statistical impact on DFS in the non-pCR group. g. In patients meeting the surgery criteria, the benefits of combining chemoimmunotherapy with radiotherapy for EFS were somewhat less pronounced, although the difference did not attain statistical significance. The imaginal line indicated the 2-year survival rate.
Safety
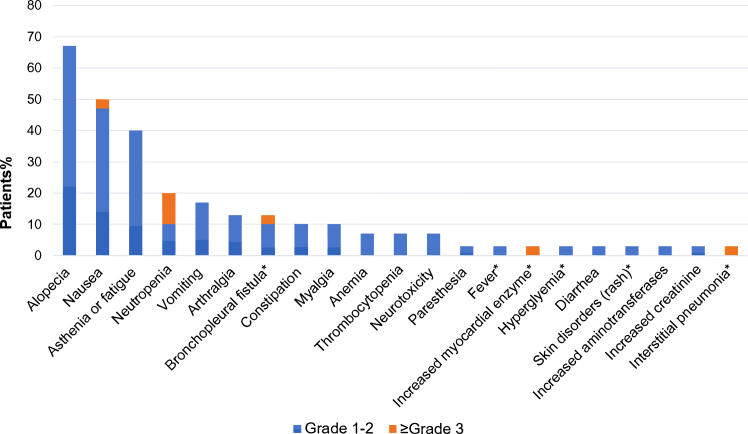
Among the 30 enrolled patients, 22 (73%) experienced treatment-related adverse events (TRAEs) during neoadjuvant treatment (Supplementary Table S5). Half of these TRAEs were of grade 1 or 2 severity (15 out of 30, 50%), encompassing conditions such as alopecia (67%), nausea (47%), and asthenia or fatigue (40%) (Fig. 6 and Supplementary Table S5). Immune-related adverse events (irAEs) of any grade were observed in nine patients (30%), including six (20%) at grade 1 or 2. No AE led to dose reduction, treatment discontinuation, surgical delay, or death during the neoadjuvant treatment. Furthermore, seven of the 30 patients (23%) experienced grade 3 or higher treatment-related AEs during adjuvant therapy, which included three irAEs. These grade 3 or higher irAEs included an instance of increased myocardial enzyme levels at grade 3, one case of interstitial pneumonia at grade 3, and one instance of BPF at grade 5. Increased myocardial enzyme levels and interstitial pneumonia necessitated the discontinuation of adjuvant sintilimab. After discontinuation and symptomatic management, all patients recovered fully without any lingering effects. Notably, four patients (13%) encountered postoperative BPF, a rare occurrence in previous studies. Among these cases, three were of grade 1–2 severity, and one was classified as grade 5. Adjuvant therapy was delayed in conjunction with the management of BPF, and no further instances of BPF were reported. No other grade 4 or 5 treatment-related AEs were observed. Generally, the combination of neoadjuvant sintilimab and chemotherapy was well-tolerated.
Fig. 6.
Adverseeffects.
Exploration of potential biomarkers
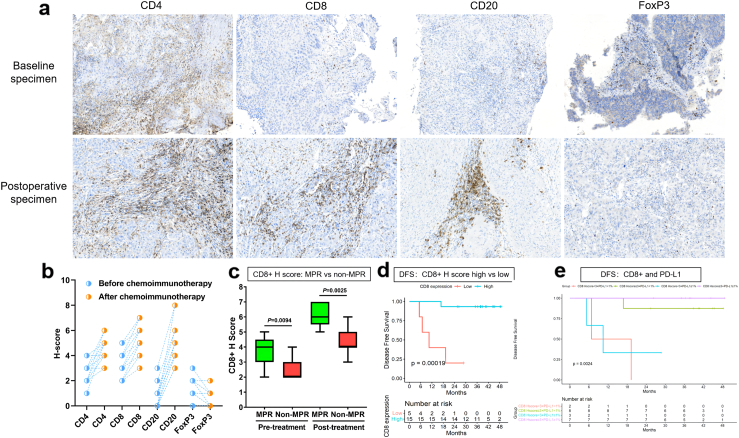
A noteworthy increase in the counts of infiltrating CD4+ (T cells), CD8+ (T cells), and CD20+ (B cells) cells was observed following neoadjuvant chemoimmunotherapy, accompanied by a significant reduction in the number of infiltrating FoxP3+ (Treg cells) cells (Fig. 7a–b). Additionally, a statistically significant difference in the H-score of CD8 between the MPR and non-MPR groups was observed in both the pretreatment and postoperative specimens (Fig. 7c). There was a visible increase in the counts of infiltrating CD86+ (M1 macrophages) and CD56+ (NK cells) cells, along with a declining trend in CD163+ (M2 macrophages) and CD11b+ (myeloid-derived suppressor cells, MDSC); however, these trends were not statistically significant (Supplementary Figure S3). IHC score analysis and their relationship with DFS revealed that pretreatment overexpression of CD8 was significantly correlated with DFS (p = 0.00019) (Fig. 7d). Conversely, no significant correlations were observed between DFS and biomarkers of CD4, CD20, and FoxP3 (Supplementary Figure S4). We investigated the association between DFS and the dual factors CD8 and PD-L1. The outcomes indicated that optimal DFS was associated with both PD-L1 positivity and a pretreatment CD8 H score of ≥3; however, pretreatment CD8 expression remained the predominant factor influencing this relationship (refer to Fig. 7e). Considering that some clinical factors might also jointly affect DFS, a multivariate analysis was conducted using pretreatment CD8 expression, staging, and the number of neoadjuvant cycles. The results showed that only pre-treatment CD8 expression had predictive significance for DFS (Supplementary Table S4).
Fig. 7.
IHC of biomarkers and DFS rate. a-b. A noteworthy increase in the counts of infiltrating CD4+, CD8+, and CD20+ cells was observed subsequent to neoadjuvant chemoimmunotherapy, accompanied by a significant reduction in the number of infiltrating FoxP3+ cells. c. There was a statistically significant distinction in the H score of CD8 between the MPR (green) and non-MPR (red) groups. d. A statistically significant distinction was noted between pretreatment overexpression of CD8 and DFS (p = 0.00019). e. Optimal DFS was associated with PD-L1 positivity and pretreatment CD8 H score ≥3 concurrently.
Discussion
To the best of our knowledge, the Neo-Pre-IC trial is the first phase 2 study evaluating neoadjuvant chemoimmunotherapy (sintilimab plus chemotherapy) in patients with potentially resectable stage III NSCLC, utilising a 2-year DFS endpoint. Additionally, this study investigated the role of PD-L1 expression and tumour immune microenvironment (TIME) subtype as predictive biomarkers in neoadjuvant chemoimmunotherapy. The results demonstrated that neoadjuvant sintilimab, in conjunction with chemotherapy, effectively transformed potentially resectable NSCLC into resectable NSCLC, with a conversion rate of 80% and a resection rate of 66.7%. Moreover, the achieved 2-year DFS was 75% (with a median follow-up period of 39.7 months), aligning closely with outcomes from other neoadjuvant chemoimmunotherapy trials17, 18, 19 such as NADIM (stage IIIA with N2 positive, 2-year EFS 77.1%), Checkmate 816 (stage IB-IIIA, 2-year EFS 63.8%), and SAKK 16/14 (stage IIIA with N2 positivity, 2-year EFS 68%). The management of potentially resectable stage III NSCLC remains controversial. Neoadjuvant chemotherapy followed by surgery and concurrent chemoradiotherapy are established treatment options. For potentially resectable NSCLC, neoadjuvant chemoimmunotherapy seems to offer a substantial 2-year DFS advantage over neoadjuvant chemotherapy alone (2-year DFS 40%–50%).14,15 Similarly, the inclusion of durvalumab (2-year PFS, 46.0% vs. 26.1%) or sugemalimab (2-year PFS, 42.5% vs. 34.0%) as consolidation therapy following chemoradiation significantly improved PFS compared to placebo for patients with unresectable stage III NSCLC.10,11 Despite the efficacy of consolidation immunotherapy in patients with locally advanced NSCLC, its effectiveness remains unsatisfactory, particularly when compared to patients who experienced downstaging and had surgical opportunities. Therefore, further investigation into the effect of neoadjuvant chemoimmunotherapy on downstaging is warranted for potentially resectable NSCLC. We plan to continue follow-ups to confirm the durability of long-term survival.
Published trials23,24 have reported MPR rates ranging from 19% to 45% in monotherapy with neoadjuvant immunotherapy and 50% in dual ICI therapy. The combination of chemotherapy and immunotherapy as neoadjuvant treatment has further elevated MPR rates. Additionally, neoadjuvant chemoimmunotherapy has yielded pCR rates ranging from 9% to 63% in localised NSCLC.17,25,26 In our trial, the MPR rate was 65%, inclusive of a 40% pCR rate. This finding is consistent with those of previously published trials that investigated neoadjuvant chemoimmunotherapy.17, 18, 19,27 Moreover, patients achieving MPR or pCR displayed an improved DFS or EFS in previous studies.18,19,27 Consistent with these findings, our trial showed a robust association between MPR and DFS, contributing to a significant 2-year DFS benefit (100% vs. 29%, p = 0.00024). This pronounced association between MPR and clinical benefit underscores that, in potentially resectable NSCLC, surgery followed by neoadjuvant sintilimab combined with chemotherapy confers a significant advantage over consolidation immunotherapy followed by chemoradiotherapy. The association between the pathological response and survival benefits warrants further evaluation in ongoing neoadjuvant therapy trials involving patients with NSCLC.
Studies exploring the utilisation of neoadjuvant immunotherapy or combinations of chemoimmunotherapies have demonstrated the viability of this approach and encouraging outcomes in terms of pathological response rates. However, concerns about the immune-mediated toxicities associated with checkpoint inhibition are significant when employing this novel treatment strategy.
Thirty patients were enrolled in our trial, of which 20 underwent surgery. Among the remaining 10 patients who did not undergo surgery, seven met the surgical criteria but three declined surgery for personal reasons and four were unable to undergo surgery owing to COVID-19 pandemic isolation. Three of the ten patients did not meet the surgical criteria because of unresectable lesions. This trial reaffirms the safety of administering pre-treatment chemotherapy and sintilimab. In this study, 13% (4/30) of patients developed BPF, which led to the death of one patient. An Interim analysis had previously addressed this issue,28 underscoring the importance of reinforcing the bronchial stump, particularly for neoadjuvant immunochemotherapy, where the risk of BPF is elevated. Following an increased focus on preventing BPF, no other cases of BPF were observed among the operated patients with stage IIIA/IIIB disease during the subsequent period. Moreover, there were no indications of heightened surgical complications such as infections, wound dehiscence, or prolonged hospital stay.
Unlike the NADIM and SAKK 16/14 trials17,19 where patients received adjuvant immunotherapy for 1 year postoperatively, other neoadjuvant trials did not follow the same pattern. Additionally, the duration and necessity of adjuvant immunotherapy in neoadjuvant chemoimmunotherapy trials for NSCLC remain unclear. Our analyses indicated that the inclusion of adjuvant immunotherapy may extend DFS, although statistical significance was not achieved. Further analysis was performed to explore the relationship between DFS and adjuvant immunotherapy based on MPR status. Interestingly, immune adjuvant therapy did not affect DFS in the MPR or pCR groups but did affect the non-MPR group. This prompts the question of whether patients, particularly those achieving a pCR or MPR, can benefit from adjuvant immunotherapy, which warrants further investigation. This highlights the need to identify suitable predictive biomarkers for early patient selection.
Currently, there are no established biomarkers for predicting therapeutic response in the context of neoadjuvant chemoimmunotherapy. Biomarkers play crucial roles in clinical trials. PD-L1 expression has been extensively studied as a potential marker, primarily in advanced stage NSCLC cases.29,30 However, our analyses revealed that DFS was not influenced by PD-L1 expression in patients undergoing neoadjuvant chemoimmunotherapy. The TIME has a significant influence on the immunotherapy response owing to its central role in complex interactions between tumour cells and the immune system. Few studies have investigated the TIME of neoadjuvant chemotherapy, and its prognostic value in neoadjuvant chemoimmunotherapy for NSCLC has received less attention. Given that various immune cell components are associated with the clinical benefits of immunotherapy, we examined the infiltration levels of CD4+ TIL, CD8+ TIL, CD20+ B cells, FoxP3+ Treg cells, CD56+ NK cells, CD86+ M1 macrophages, CD163+ M2 macrophages, and CD11b+ MDSCs in tumour tissue specimens before and after neoadjuvant chemoimmunotherapy. Therefore, this study aimed to explore their prognostic significance. As anticipated, we observed significant increases in CD4+ TIL, CD8+ TIL, and CD20+ B cells, along with a decrease in FoxP3+ Treg cells, following neoadjuvant chemoimmunotherapy. This suggests that this treatment approach reshapes the TIME, reduces immunosuppression, and enhances immune cell attacks against tumours. Notably, high expression of naïve CD8+ TIL was associated with longer DFS (p = 0.00019), which was not observed for other immune cell types. This indicates that naïve CD8+ TIL expression could potentially serve as a predictive biomarker for DFS in patients with potentially resectable NSCLC who are undergoing neoadjuvant chemoimmunotherapy. Further investigations are required to explore whether this correlates with OS. Moreover, patients with high PD-L1 expression and high naïve CD8+ TIL expression exhibited the best DFS outcomes in our study. Notably, some subgroups in this analysis were small, thus limiting the statistical power of the findings. The potential impact of a TIME classification model that integrates PD-L1 and CD8+ TIL expression requires further validation.
The present study had some limitations. First, it was a single-arm study with a small sample size and a limited follow-up period. Consequently, the results of the reported comparisons are exploratory and hypothesis generating. Another limitation is that the number of pre- and post-therapy samples and the number of tissues derived from resected tumours were limited. Therefore, some of the subgroups in the analysis of potential biomarkers were small, thus limiting the statistical power of the findings. Additionally, four of the 30 patients (13.3%) who participated in this study could not undergo surgery owing to the city lockdown during the COVID-19 pandemic. Furthermore, in our study, most participants (96.7%) were male, while only 3.3% were female. This bias was considered to be associated with the inclusion criteria. We only enrolled patients with ‘potentially resectable’ locally advanced stage III NSCLC, the majority of whom had higher-stage central lung cancers with pericardium, large blood vessels, or tracheal invasion. Male patients account for a high percentage of these locally advanced stage III patients, a trend consistent with findings in other studies, such as GEMSTONE-301 and a real-world study.11 Therefore, more male patients with locally advanced stage III lung cancer were enrolled in our study, aligning with findings from previous reports. This represents another limitation of our data, making it challenging to conduct thorough subgroup analyses based on sex in our results. Future studies should aim to enrol more participants to better assess the influence of sex on the findings.
Neoadjuvant chemoimmunotherapy for patients with potentially resectable stage IIIA/IIIB NSCLC has expanded the opportunities for achieving R0 resection in initially unresectable cases. Similar pathological response rates were observed in the NADIM II trial (neoadjuvant chemoimmunotherapy for resectable IIIA/IIIB NSCLC), which extended the 2-year DFS rate (75%) compared with the rate achieved via concurrent chemoradiotherapy and consolidation immunotherapy for unresectable IIIA/IIIB NSCLC (2-year PFS rate, 42.5%–46.0%). Notably, this study reported fewer grade 3 TRAEs than those in the NADIM trial. Furthermore, the study identified pretreatment overexpression of CD8 (H score≥3) as a potential predictor of DFS. Collectively, these findings suggest that the treatment paradigm for potentially resectable stage IIIA/IIIB NSCLC may transform in the future.
Contributors
Chao Sun: Managed neoadjuvant data (efficiency) and contributed to original writing.
Xu Wang: Managed the neoadjuvant data (adverse events) and contributed to the original writing.
Yinghui Xu: Managed biomarkers data and contributed to original writing.
Guoguang Shao: Managed surgical procedures.
Xi Chen: Assisted with data curation.
Yunpeng Liu: Managed postoperative data.
Peng Zhang: Managed postoperative data.
Xingyu Lin: Assisted with follow-up.
Xiaobo Ma: Interpreted pathological effects.
Hua He: Conducted statistical analysis.
Shi Qiu: Assisted with data curation.
Zhiguang Yang: Contributed to writing and editing.
Kewei Ma: Managed the project and contributed to writing and editing.
All the authors have read and approved the final version of the manuscript. Chao Sun, Xu Wang, and Yinghui Xu have verified the underlying data.
Data sharing statement
Data supporting the findings of this study are available from the corresponding author upon reasonable request. These data are not publicly available because of privacy concerns.
Declaration of interests
The authors declare that they have no conflicts of interest or competing interests.
Acknowledgements
We thank all the patients, their families, and caregivers for their participation in this study.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102422.
Contributor Information
Zhiguang Yang, Email: zgyang@jlu.edu.cn.
Kewei Ma, Email: makw@jlu.edu.cn.
Appendix A. Supplementary data
References
- 1.Aupérin A., Le Péchoux C., Rolland E., et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28(13):2181–2190. doi: 10.1200/JCO.2009.26.2543. [DOI] [PubMed] [Google Scholar]
- 2.Deng H., Liu J., Cai X., et al. Radical minimally invasive surgery after immuno-chemotherapy in initially-unresectable stage IIIB non-small cell lung cancer. Ann Surg. 2022;275(3):e600–e602. doi: 10.1097/SLA.0000000000005233. [DOI] [PubMed] [Google Scholar]
- 3.Stefani D., Plönes T., Viehof J., et al. Lung cancer surgery after neoadjuvant immunotherapy. Cancers. 2021;13(16):4033. doi: 10.3390/cancers13164033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pless M., Stupp R., Ris H.-B., et al. Induction chemoradiation in stage IIIA/N2 non-small-cell lung cancer: a phase 3 randomised trial. Lancet. 2015;386(9998):1049–1056. doi: 10.1016/S0140-6736(15)60294-X. [DOI] [PubMed] [Google Scholar]
- 5.Albain K.S., Swann R.S., Rusch V.W., et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet. 2009;374(9687):379–386. doi: 10.1016/S0140-6736(09)60737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pechoux C.L., Pourel N., Barlesi F., et al. Postoperative radiotherapy versus no postoperative radiotherapy in patients with completely resected non-small-cell lung cancer and proven mediastinal N2 involvement (Lung ART): an open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23(1):104–114. doi: 10.1016/S1470-2045(21)00606-9. [DOI] [PubMed] [Google Scholar]
- 7.Remon J., Passiglia F., Ahn M.J., et al. Immune checkpoint inhibitors in thoracic malignancies: review of the existing evidence by an IASLC expert panel and recommendations. J Thorac Oncol. 2020;15(6):914–947. doi: 10.1016/j.jtho.2020.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Yufei L., Luyang Y., Neda K., et al. Final efficacy outcomes of atezolizumab with chemoradiation for unresectable NSCLC: the phase II DETERRED trial. Lung Cancer. 2022;174:112–117. doi: 10.1016/j.lungcan.2022.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Greg A., Salma K., Sandra K., et al. A phase 2 trial of consolidation pembrolizumab following concurrent chemoradiation for patients with unresectable stage III non-small cell lung cancer: hoosier cancer research network LUN 14-179. Cancer. 2020;19:4353–4361. doi: 10.1002/cncr.33083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antonia S.J., Villegas A., Daniel D., et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Q., Chen M., Jiang Q., et al. Sugemalimab versus placebo after concurrent or sequential chemoradiotherapy in patients with locally advanced, unresectable, stage III non-small-cell lung cancer in China (GEMSTONE-301): interim results of a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2022;23(2):209–219. doi: 10.1016/S1470-2045(21)00630-6. [DOI] [PubMed] [Google Scholar]
- 12.Milroy R., Macbeth F. Neoadjuvant chemotherapy in stage IIIa non-small cell lung cancer. Thorax. 1995;50 Suppl 1(Suppl 1):S25–S30. doi: 10.1136/thx.50.suppl_1.s25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sinn K., Mosleh B., Steindl A., et al. Neoadjuvant chemoradiotherapy is superior to chemotherapy alone in surgically treated stage III/N2 non-small-cell lung cancer: a retrospective single-center cohort study. ESMO Open. 2022;7(2) doi: 10.1016/j.esmoop.2022.100466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajaram R., Correa A.M., Xu T., et al. Locoregional control, overall survival, and disease-free survival in stage IIIA (N2) non-small-cell lung cancer: analysis of resected and unresected patients. Clin Lung Cancer. 2020;21(4):e294–e301. doi: 10.1016/j.cllc.2020.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Gilligan D., Nicolson M., Smith I., et al. Pretreatment chemotherapy in patients with resectable non-small cell lung cancer: results of the MRC LU22/NVALT 2/EORTC 08012 multicentre randomised trial and update of systematic review. Lancet. 2007;369(9577):1929–1937. doi: 10.1016/S0140-6736(07)60714-4. [DOI] [PubMed] [Google Scholar]
- 16.Hellmann M.D., Chaft J.E., William W.N., Jr., et al. Pathologic response after neoadjuvant chemotherapy in resectable non-small cell lung cancers: proposal for the use of “major pathologic response” as a surrogate endpoint. Lancet Oncol. 2014;15(1):e42–e50. doi: 10.1016/S1470-2045(13)70334-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Provencio M., Nadal E., Insa A., et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;21(11):1413–1422. doi: 10.1016/S1470-2045(20)30453-8. [DOI] [PubMed] [Google Scholar]
- 18.Forde P.M., Spicer J., Lu S., et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386(21):1973–1985. doi: 10.1056/NEJMoa2202170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rotschild S.I., Zippelius A., Eboulet E.I., et al. SAKK 16/14: durvalumab in addition to neoadjuvant chemotherapy in patients with stage IIIA(N2) non–small-cell lung cancer—a multicenter single-arm phase II trial. J Clin Oncol. 2021;39(26):2872–2880. doi: 10.1200/JCO.21.00276. [DOI] [PubMed] [Google Scholar]
- 20.Ahern E., Solomon B.J., Hui R., et al. Neoadjuvant immunotherapy for non-small cell lung cancer: right drugs, right patient, right time? J Immunother Cancer. 2021;9(6) doi: 10.1136/jitc-2020-002248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yujia C., Jianjun Q., Yajing W., et al. Does major pathological response after neoadjuvant Immunotherapy in resectable non-small-cell lung cancers predict prognosis? a systematic review and meta-analysis. Int J Surg. 2023;109(9):2794–2807. doi: 10.1097/JS9.0000000000000496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10(1):1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 23.Forde P.M., Chaft J.E., Smith K.N., et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. 2018;378:1976–1986. doi: 10.1056/NEJMoa1716078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jamie E.C., Filiz O., Mark G.K., et al. Neoadjuvant atezolizumab for resectable non-small cell lung cancer: an open-label, single-arm phase II trial. Nat Med. 2022;28(10):2155–2161. doi: 10.1038/s41591-022-01962-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tfayli A., Al Assaad M., Fakhri G., et al. Neoadjuvant chemotherapy and avelumab in early stage resectable nonsmall cell lung cancer. Cancer Med. 2020;9(22):8406–8411. doi: 10.1002/cam4.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Provencio M., Serna R., Nadal E., et al. 2022. Progression free survival and overall survival in NADIM II study. Presented at 2022 world conference on lung cancer; August 6-9. Vienna, Austria. abstract PL03.12. [Google Scholar]
- 27.Shu C.A., Gainor J.F., Awad M.M., et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;21:786–795. doi: 10.1016/S1470-2045(20)30140-6. [DOI] [PubMed] [Google Scholar]
- 28.Sun C., Liu Y., Zhang P., et al. Interim analysis of the efficiency and safety of neoadjuvant PD-1 inhibitor (sintilimab) combined with chemotherapy (nab-paclitaxel and carboplatin) in potentially resectable stage IIIA/IIIB non-small cell lung cancer: a single-arm, phase 2 trial. J Cancer Res Clin Oncol. 2023;149(2):819–831. doi: 10.1007/s00432-021-03896-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herbst R.S., Giaccone G., de Marinis F., et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med. 2020;383:1328–1339. doi: 10.1056/NEJMoa1917346. [DOI] [PubMed] [Google Scholar]
- 30.Mok T.S.K., Wu Y.L., Kudaba I., et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced ormetastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.