Abstract
In 1905, Robert Behrend reported the preparation of a substance that remained a dry powder despite absorbing water and that had the ability to absorb dyes from solution. Over seven decades later, William L. Mock repeated the Behrend synthesis and discovered cucurbituril, a remarkably symmetric molecule with a central cavity welcoming diverse guests. Mock et al. ploughed a lonely furrow in the 1980s, establishing the versatility of cucurbituril in the field of supramolecular chemistry, including a demonstration of the Huisgen click reaction in a molecular cavity.
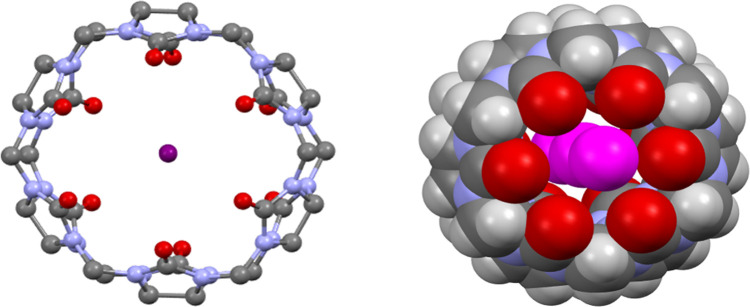
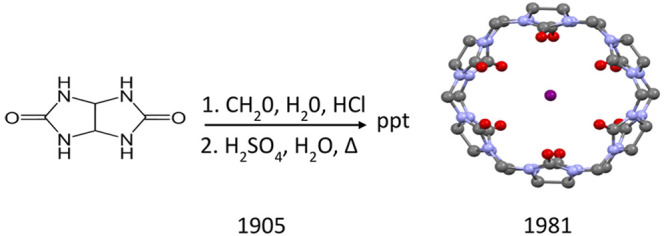
In 1905, Robert Behrend noted that, occasionally, synthetic attempts do not lead to the desired goals.1 He was reporting his analysis of products obtained by the condensation of glycoluril with formaldehyde under acidic conditions. The products they described appeared to have unusual properties. The substances he struggled to characterize had the ability to absorb large amounts of water without losing the property of being a dusty powder. They also had the remarkable property of being able to associate with all of the salts investigated and to completely absorb dyes from solutions. Behrend’s starting material, glycoluril, was itself derived from simple precursors, urea and glyoxal. His observations, described in great detail, remained buried in the vast literature of 19th and early 20th century chemistry. William L. Mock resurrected the Behrend paper in 1981 in a remarkable communication in JACS, which must be read as much for its content as its brevity.2 Mock and his colleagues had little difficulty in reproducing Behrend’s procedures, obtaining a substance that yielded an IR band at 1720 cm–1 and a surprisingly simple proton NMR spectrum, consisting of three signals of equal intensity, a singlet, and two doublets in the region 4.4–6.0 ppm, indicative of a highly symmetrical structure. In the early 1980s, fast atom bombardment mass spectrometry did provide a means of measuring masses for poorly ionizing organic molecules. The Behrend–Mock substance was unyielding, refusing to be coaxed into the gas phase. Single crystals provided diffraction data, amenable to structure solution using the direct methods program, MULTAN, which was in vogue in the mid-1970s and early 1980s. The structure, determined by Wade Freeman, revealed an organic macrocycle described as a cyclic hexamer of dimethanoglycoluril, a symmetric, rotund molecule, with a large internal cavity and an array of carbonyl groups at both ends ready to complex cations (Figure 1).2,3
Figure 1.
Two views of cucurbituril-6 (CB6) in crystals as reported by Freeman and Mock, drawn from the coordinate set for the entry BEBDOB10 in the CCDC database. (left) View down the 6-fold axis. (right) Space filling view, tilted to illustrate the three entrapped water molecules that lie along the 6-fold axis.
The forbiddingly complex name prescribed by IUPAC for this new macrocycle (footnote in ref (2)) and its striking similarity to the pumpkin (family Cucurbitaceae), prompted Mock to christen the new entrant to the field of supramolecular chemistry as cucurbituril. Behrend’s paper of 1905 had already pointed to the possibility that the condensation product of glycoluril and formaldehyde had the ability to soak up both water and organic dyes. The initial Freeman–Mock communication in 1981 notes: “Aside from its enigmatic spontaneous synthesis, the most captivating feature of 2 is the presence of an internal cavity of approximately 5.5-Å diameter within the relatively rigid macrocyclic structure, to which access is provided by 4-Å diameter portals situated among the carbonyl groups”. Selective host–guest complexation was reported for cycloalkylmethylamines with a surprisingly high affinity for cyclopentylmethylamine. By 2015, the area of cucurbituril research had exploded, and a comprehensive survey lists as many as 416 references,4 beginning with a citation to the Ph.D. thesis of E. Meyer in Behrend’s laboratory in 1904.5
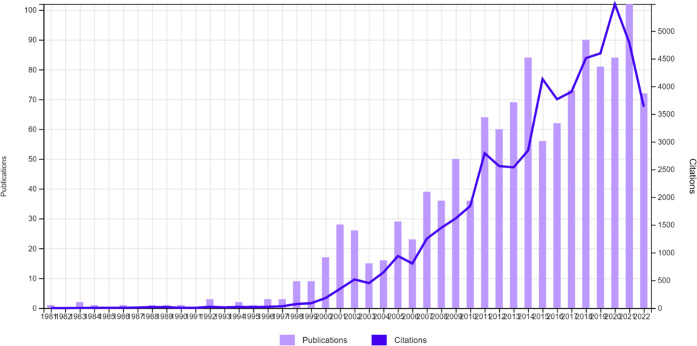
In all 1250 papers indexed in the Web of Science (WoS) have “Cucurbituril” in the title or abstract or author keywords (data as seen on 10 October 2022). The growth of papers, represented in Figure 2 and Table 1, illustrates the very slow rise in over a decade and a half after the Mock 1981 communication.
Figure 2.
Growth of papers having cucurbituril in the title or abstract or author keywords and citations over the period 1981–2022.
Table 1. Papers Listed in the Historiography (Figure 2).
| paper | local citation score | global citation score | |
|---|---|---|---|
| 9 | Freeman et al., 19812 | 1116 | 1156 |
| 10 | Mock et al., 19836 | 209 | 216 |
| 11 | Mock et al., 198310 | 199 | 208 |
| 21 | Mock et al., 19867 | 510 | 525 |
| 33 | Mock et al., 19888 | 173 | 180 |
| 39 | Mock et al., 19899 | 175 | 181 |
| 72 | Mock et al., 199516 | 179 | 317 |
| 78 | Jeon et al., 199622 | 193 | 322 |
| 108 | Kim et al., 200023 | 708 | 1424 |
| 149 | Day et al., 200124 | 429 | 876 |
| 151 | Day et al., 200225 | 358 | 529 |
| 189 | Lee et al., 200326 | 545 | 1603 |
| 211 | Marquez et al., 200427 | 202 | 399 |
| 236 | Lagona et al., 200528 | 706 | 2035 |
| 281 | Liu et al., 200529 | 277 | 692 |
| 283 | Liu et al., 200530 | 166 | 264 |
| 347 | Kim et al., 200731 | 244 | 748 |
| 738 | Masson et al., 201232 | 242 | 748 |
| 984 | Assaf and Nau, 201533 | 188 | 875 |
| 1062 | Barrow et al., 20154 | 172 | 1102 |
In this period, it is work exclusively originating from Mock’s laboratory at the University of Illinois, Chicago, which set the tone for what was to follow. Working with the characterized cucurbituril, containing six glycouril units (cucurbit[6]uril, CB6), Mock and his colleagues demonstrated selective and high affinity complexation of alkylammonium ions, dissecting the factors contributing to binding selectivity.6−8 Dissociation constants as high as 7.8 × 10–8 M were reported for spermidine.7 Mock’s analysis of the dynamics of molecular recognition by cucurbituril emphasized the advantages of working with a “relatively inflexible polycyclic receptor”.9 Most importantly, in 1983, Mock demonstrated the 5.5 × 104 rate enhancement of the Huisgen 1,3-dipolar cycloaddition reaction of alkynes and azides by cucurbiturils,10 a forerunner of the field of “click chemistry”, recognized by the 2022 Nobel Prize. In 1989, Mock presented an incisive analysis of the issues involved in mimicking the “Pauling principle” of enzymic catalysis by synthetic receptors.11 In a 2002 interview with Chemical and Engineering News, for a feature highlighting his use of click chemistry at a binding cavity in the enzyme acetylcholinesterase,9−12 Barry Sharpless is quoted as saying: “I cannot get over the nearly perfect match between Mock’s cucurbituril experiments and our acetylcholinesterase experiments done 20 years later. If cucurbituril had been a small enzyme, then the two systems would be indistinguishable”.13
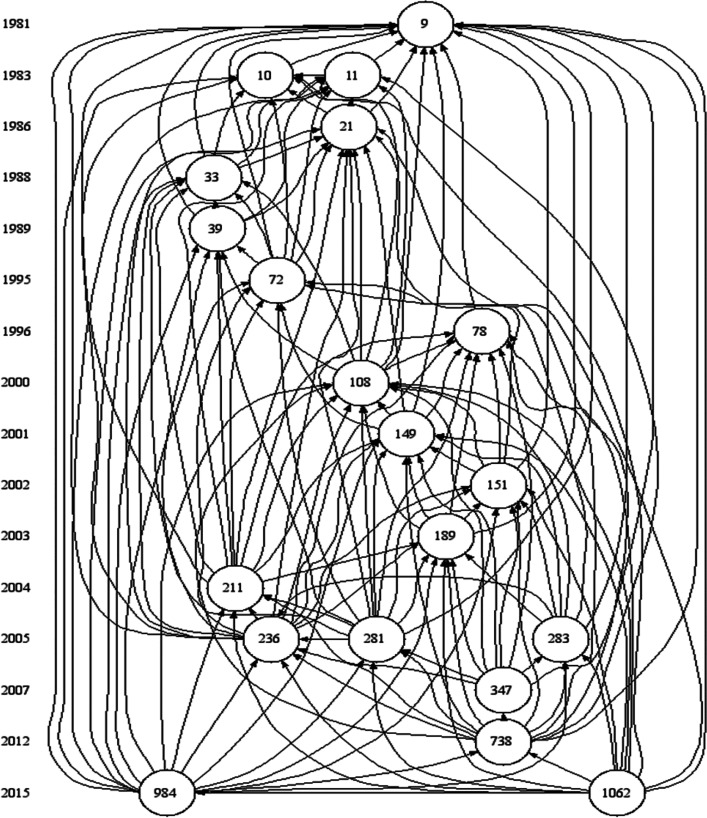
Cucurbituril was noticed, and a derivatized pentameric cage was reported from the Stoddart group in England in 1992.14 Buschmann et al. reported that its complexation abilities for cations exceeded that of the crown ether 18-crown-6.15 Mock reviewed the cucurbituril area in 1995 just before the flood. This article has only 17 references, of which 14 pertain directly to cucurbituril.16Figure 3 presents a Garfield historiograph,17 which clearly highlights the seminal nature of Mock’s contributions to the development of cucurbiturils as synthetic receptors, which now have many diverse applications in chemistry and biochemistry.
Figure 3.
Garfield historiograph based on the top 20 most cited papers (Table 1) in the collection of 1595 papers that cite six of Mock’s works published during 1981–1989 (Data as seen in WoS on 22nd September 2022). Histcite was used to draw this historiograph.
How well was the Behrend paper of 1905 recognized in the literature? A search of the WoS database revealed 403 citations in the journals covered during the period 1945–2022. All of them were in the period after the Mock–Freeman paper of 1981. A sole citation appeared in the Journal of Organic Chemistry in 1963, which referred to Behrend’s work on the condensation of urea with glyoxal, the first step on the way to cucurbituril.19 Clearly, the Behrend paper was a “sleeping beauty”,20,21 which rose to scientific prominence only after resurrection by Mock.
In an overview of the area in 2011, introducing a special issue of the Israel Journal of Chemistry, the editors Nau and Scherman pay a rich tribute to Robert Behrend, calling the synthesis of cucurbituril his finest achievement. Using the hindsight of history, they suggest that the production of the uncharacterized product of the condensation of glycouril with formaldehyde dwarfs Behrend’s synthesis of uric acid and the organic reaction that bears his name.18
For over a decade after the resurrection of the Behrend 1905 paper, the entire corpus of work on cucurbituril available in the literature emerged from Mock’s laboratory at the University of Illinois at Chicago. The remarkable ability of cucurbituril to encapsulate guests and the use of the molecular cavity to perform the cycloaddition reaction were forerunners of click chemistry in protein cavities. The subsequent explosion of cucurbituril research has been fueled by the separation of larger oligomers from the Behrend reaction mixture, and many new applications in chemistry and biochemistry will undoubtedly appear in the future.
Acknowledgments
I am deeply grateful to Madhan Muthu for the scientometric analyses presented here and to Dr. Prema Vasudev for drawing the structures of Mock’s CB6 and to Professor Tim Keiderling of the University of Illinois, Chicago, for providing copies of departmental brochures. My interest in cucurbiturils was stimulated by discussions with Prof. V. Ramamurthy, University of Miami. This essay was catalyzed by the fact that I had met Bill Mock over 50 years ago, as a graduate student in search of a mentor, at Carnegie-Mellon University.
Biography
P. Balaram retired as a Professor in the Molecular Biophysics Unit, Indian Institute of Science, Bangalore, India, and is currently a Visiting Professor at the National Centre for Biological Sciences, Tata Institute of Fundamental Research, Bangalore.
The author declares no competing financial interest.
Special Issue
Published as part of ACS Omegavirtual special issue “Nucleic Acids: A 70th Anniversary Celebration of DNA”.
References
- Behrend R.; Meyer E.; Rusche F. Ueber Condensationsproducte aus Glycoluril mit Formaldehyd. Liebigs Ann.Chem. 1905, 339, 1–37. 10.1002/jlac.19053390102. [DOI] [Google Scholar]
- Freeman W. A.; Mock W. L.; Shih N.-Y. Cucurbituril. J. Am. Chem. Soc. 1981, 103, 7367–7368. 10.1021/ja00414a070. [DOI] [Google Scholar]
- Freeman W. A. Structures of the p-Xylylenediammonium Chloride and Calcium Hydrogensulfate Adducts of the Cavitand ’Cucurbituril’, C36H36N24O12. Acta Crystallogr. 1984, B40, 382–387. 10.1107/S0108768184002354. [DOI] [Google Scholar]
- Barrow S. J.; Kasera S.; Rowland M. J.; del Barrio J.; Scherman O. A. Cucurbituril-Based Molecular Recognition. Chem. Rev. 2015, 115, 12320–12406. 10.1021/acs.chemrev.5b00341. [DOI] [PubMed] [Google Scholar]
- Meyer E.Uber die Condensation des Harnstoffs mit Glyoxal und des Glykolurils mit Formaldehyd. Ph.D. Thesis, Heidelberg University, Heidelberg, 1904. [Google Scholar]
- Mock W. L.; Shih N.-Y. Host-Guest Binding Capacity of Cucurbituril. J. Org. Chem. 1983, 48, 3618–3619. 10.1021/jo00168a069. [DOI] [Google Scholar]
- Mock W. L.; Shih N.-Y. Structure and Selectivity in Host-Guest Complexes of Cucurbituril. J. Org. Chem. 1986, 51, 4440–4446. 10.1021/jo00373a018. [DOI] [Google Scholar]
- Mock W. L.; Shih N.-Y. Organic Ligand-Receptor Interactions between Cucurbituril and Alkylammonium Ions. J. Am. Chem. Soc. 1988, 110, 4706–4710. 10.1021/ja00222a031. [DOI] [Google Scholar]
- Mock W. L.; Shih N.-Y. Dynamics of Molecular Recognition Involving Cucurbituril. J. Am. Chem. Soc. 1989, 111, 2697–2699. 10.1021/ja00189a053. [DOI] [Google Scholar]
- Mock W. L.; Irra T. A.; Wepsiec J. P.; Manimaran T. L. Cycloaddition Induced by Cucurbituril. A Case of Pauling Principle Catalysis. J. Org. Chem. 1983, 48, 3619–3620. 10.1021/jo00168a070. [DOI] [Google Scholar]
- Mock W. L.; Irra T. A.; Wepsiec J. P.; Adhya M. Catalysis by Cucurbituril. The Significance of Bound-Substrate Destabilization for Induced Triazole Formation. J. Org. Chem. 1989, 54, 5302–5308. 10.1021/jo00283a024. [DOI] [Google Scholar]
- Lewis W. G.; Green L. G.; Grynszpan F.; Radic Z.; Carlier P. R.; Taylor P.; Finn M. G.; Sharpless K. B. Click Chemistry In Situ: Acetylcholinesterase as a Reaction Vessel for the Selective Assembly of a Femtomolar Inhibitor from an Array of Building Blocks. Angew. Chem., Int. Ed. 2002, 41, 1053–1057. . [DOI] [PubMed] [Google Scholar]
- Borman S. In situ Click Chemistry. Chem. Eng. News 2002, 80, 29–34. 10.1021/cen-v080n006.p029. [DOI] [Google Scholar]
- Flinn A.; Hough G. C.; Stoddart J. F.; Williams D. J. Decamethylcucurbit[5]uril. Angew. Chem., Int. Ed. Engl. 1992, 31, 1475–1477. 10.1002/anie.199214751. [DOI] [Google Scholar]
- Buschmann H.-J.; Cleve E.; Schollmeyer E. Cucurbituril as a ligand for the complexation of cations in aqueous solutions. Inorg. Chim. Acta 1992, 193, 93–97. 10.1016/S0020-1693(00)83800-1. [DOI] [Google Scholar]
- Mock W. L. Cucurbituril. Topics in Current Chem. 1995, 175, 1–24. 10.1007/3-540-58800-0_16. [DOI] [Google Scholar]
- Garfield E. Historiographic mapping of knowledge domains literature. J. Information Sci. 2004, 30, 119–145. 10.1177/0165551504042802. [DOI] [Google Scholar]
- Nau W. M.; Sherman O. A. The World of Cucurbiturils — From Peculiarity to Commodity. Israel J.Chem. 2011, 51, 492–494. 10.1002/ijch.201100052. [DOI] [Google Scholar]
- Nematollahi J.; Ketcham R. Imidazoimidazoles. I. The Reaction of Ureas With Glyoxal. Tetrahydroimidazo[4,5-d]imidazole-2,5-diones. J. Org. Chem. 1963, 28, 2378–2380. 10.1021/jo01044a055. [DOI] [Google Scholar]
- Van Raan A. F. J. Sleeping Beauties in Science. Scientometrics 2004, 59, 467–472. 10.1023/B:SCIE.0000018543.82441.f1. [DOI] [Google Scholar]
- Ke Q.; Ferrara E.; Radicchi F.; Flammini A. Defining and Identifying Sleeping Beauties in Science. Proc.Natl.Acad.Sci. USA 2015, 112, 7426–7431. 10.1073/pnas.1424329112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon Y.-M.; Kim J.; Whang D.; Kim K. J. Am. Chem. Soc. 1996, 118, 9790–9791. 10.1021/ja962071x. [DOI] [Google Scholar]
- Kim J.; Jung I.-S.; Kim S.-Y.; Lee E.; Kang J.-K.; Sakamoto S.; Yamaguchi K.; Kim K. J. Am. Chem. Soc. 2000, 122, 540–541. 10.1021/ja993376p. [DOI] [Google Scholar]
- Day A.; Arnold A. P.; Blanch R. J.; Snushall B. J. Org. Chem. 2001, 66, 8094–8100. 10.1021/jo015897c. [DOI] [PubMed] [Google Scholar]
- Day A. I.; Blanch R. J.; Arnold A. P.; Lorenzo S.; Lewis G. R.; Dance I. Angew Chem Int Ed 2002, 41, 275.. [DOI] [PubMed] [Google Scholar]
- Lee J. W.; Samal S.; Selvapalam N.; Kim H.-J.; Kim K. Acc. Chem. Res. 2003, 36, 621. 10.1021/ar020254k. [DOI] [PubMed] [Google Scholar]
- Marquez C.; Hudgins R. R.; Nau W. M. J. Am. Chem. Soc. 2004, 126, 5806. 10.1021/ja0319846. [DOI] [PubMed] [Google Scholar]
- Lagona J.; Mukhopadhyay P.; Chakrabarti S.; Isaacs L. Angew Chem Int Ed 2005, 44, 4844. 10.1002/anie.200460675. [DOI] [PubMed] [Google Scholar]
- Liu S.; Ruspic C.; Mukhopadhyay P.; Chakrabarti S.; Zavalij P. Y.; Isaacs L. J. Am. Chem. Soc. 2005, 127, 15959. 10.1021/ja055013x. [DOI] [PubMed] [Google Scholar]
- Liu S.; Zavalij P. Y.; Isaacs L. J. Am. Chem. Soc. 2005, 127, 16798. 10.1021/ja056287n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.; Selvapalam N.; Ko Y. H.; Park K. M.; Kim D.; Kim J. Chem Soc Rev 2007, 36, 267. 10.1039/B603088M. [DOI] [PubMed] [Google Scholar]
- Masson E.; Ling X.; Joseph R.; Kyeremeh-Mensah L.; Lu X. RSC Adv 2012, 2, 1213. 10.1039/C1RA00768H. [DOI] [Google Scholar]
- Assaf K. I.; Nau W. M. Chem Soc Rev 2015, 44, 394. 10.1039/C4CS00273C. [DOI] [PubMed] [Google Scholar]