Abstract
Aldehydes are important synthons for DNA-encoded library (DEL) construction, but the development of a DNA-compatible method for the oxidation of alcohols to aldehydes remains a significant challenge in the field of DEL chemistry. We report that a copper/TEMPO catalyst system enables the solution-phase, DNA-compatible oxidation of DNA-linked primary activated alcohols to aldehydes. The semi-aqueous, room-temperature reaction conditions afford oxidation of benzylic, heterobenzylic and allylic alcohols in high yield, with DNA compatibility verified by mass spectrometry, qPCR, Sanger sequencing, and ligation assays. Subsequent transformations of the resulting aldehydes demonstrate the potential of the method for robust library diversification.
Keywords: DNA-encoded libraries, Oxidation, DNA-compatible, Solution-phase, Alcohols
Graphical Abstract
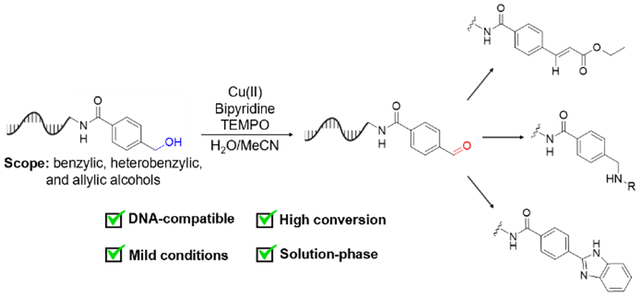
DNA encoded libraries (DELs) are a powerful tool for drug discovery that have recently led to the identification of therapeutic precursors which have entered clinical trials1,2,3,4,5,6. A DEL is a library of DNA-linked small molecules in which each DNA sequence (built through the attachment of a series of DNA barcodes) describes the synthetic steps used to prepare each covalently linked, small molecule library member. This library can then be screened for bioactivity or affinity for a given target, and small molecules with promising activity can be identified through PCR amplification and DNA sequencing of the attached barcodes. DELs accelerate drug discovery by enabling simultaneous screening of large libraries, so designing a diverse library that covers a wide chemical space is critical to the success of the screen. Constructing diverse DELs requires DNA-compatible methods for the incorporation of diversity-enabling functional groups7.
Aldehydes are important synthons for DEL diversification because they react with a wide variety of building blocks to form multiple functional groups using existing DNA-compatible methods7. This wide scope of reactivity has been demonstrated by Paciaroni et al. in an “explosion” strategy for DEL synthesis using DNA-linked aldehydes to access nine different diversification reactions8. Despite this utility, the use of aldehydes in DELs is limited by the accessibility of multifunctional aldehyde building blocks that can be attached to DNA. A commercial availability search revealed that there are 3,031 commercially available aldehydes bearing a carboxylic acid handle (for attachment to DNA) compared to 12,749 similarly functionalized primary alcohol derivatives (see Figure 1A). While bifunctional aldehyde building blocks could be pre-synthesized prior to attachment to DNA, the individual oxidation and purification of each compound creates a heavy synthetic burden. This burden could be bypassed with a DNA-compatible oxidation method allowing for simultaneous oxidation of all library members in a growing split-and-pool DEL.
Figure 1a.
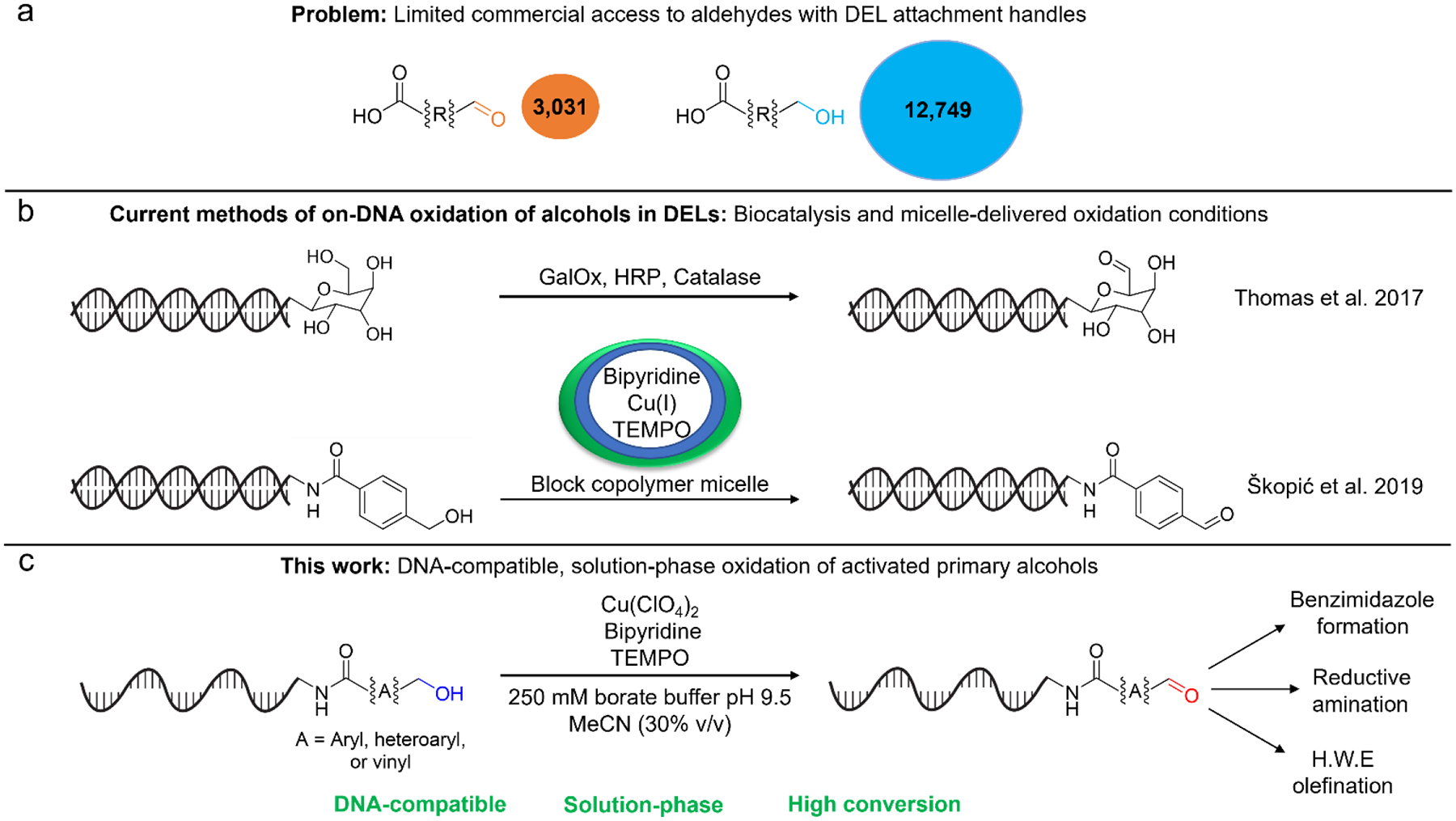
Comparison of commercial availability of carboxylic acid bearing bifunctional alcohols and aldehydes. b. Current literature methods for DNA-compatible oxidation of alcohols in DNA-encoded libraries (DELs). c. New DNA-compatible method for oxidation of primary activated alcohols to aldehydes as a precursor to DNA-compatible aldehyde transformations (Benzimidazole formation, Reductive amination, Horner-Wadsworth-Emmons Olefination).
The formation of aldehydes on DNA from alcohol precursors poses a substantial challenge because DNA is notoriously sensitive to oxidation,9–13 and established oxidation reactions lead to significant oxidative damage14,15 interfering with the PCR amplification16 and the sequencing readout17 of a DEL. The susceptibility of DNA to damage severely limits the scope of oxidation reactions that can be used in a DEL14,15.
To date, only two methods for alcohol oxidation in a DEL context have been developed. In the first demonstration of biocatalysis for DEL synthesis, Thomas et al. used galactose oxidase variants to oxidize the C6 alcohols of DNA-linked hexoses (Figure 1B)18. However, the substrate specificity of enzymes is problematic in the context of most DELs where maximizing substrate diversity is critical. Škopić et al. explored the use of Cu/TEMPO alcohol oxidation reaction for three DNA-linked alcohols but were only able to achieve DNA-compatible oxidation in the presence of a block-copolymer micelle, introducing procedural complexity and deviating from the solution-phase chemistry commonly used in split-and-pool DEL synthesis19. Thus, there is a need for a more robust and procedurally simple DNA-compatible transformation of alcohols to aldehydes.
Copper/TEMPO alcohol oxidations are exceptionally mild and allow alcohol oxidation without intermediacy of highly oxidizing oxoammonium intermediates commonly invoked in TEMPO-catalyzed oxidations20,21. Although traditionally run under anhydrous conditions, the reaction works in semi-aqueous conditions and in the presence of DNA22,23,24. While DNA is known to be damaged under oxidizing conditions and in the presence of copper25, copper is commonly used for DNA-compatible click reactions26,27 and oxidative DEL methods involving copper have been reported for inverse-electron-demand Diels-Alder reactions and oxidative amidation methods28,29, with reports of minimal DNA damage. Motivated by this literature precedent and our experience with Cu/TEMPO oxidations in the presence of DNA24, we sought to identify high-yielding, solution phase, DNA-compatible conditions for alcohol oxidation for use in DELs.
Here we report a mild, solution-phase Cu/TEMPO oxidation of benzylic, hetero-benzylic, and allylic alcohols to aldehydes (Figure 1C), demonstrate its DNA-compatibility by qPCR and high-resolution and accurate-mass mass spectrometry, and explore its utility for DEL synthesis in a model workflow by introducing diversity elements through solution-phase, DNA-compatible reductive amination, olefination, and heterocyclization.
To determine the optimal conditions for alcohol oxidation on DNA, we assessed a variety of reaction conditions using a model benzylic alcohol substrate covalently linked to a 26-nucleotide single-stranded DNA oligomer (without HPLC purification following bioconjugation). We evaluated reaction outcomes using two criteria: reaction yield (determined by ion-pair reversed-phase-HPLC, with formation of the intended product confirmed via high-resolution mass spectrometry; see SI) and DNA-compatibility (determined by qPCR measurement of DNA amplifiability after reaction, see SI). Yields were calculated using peak integration of starting material and product peaks on HPLC chromatograms (excluding impurities from bioconjugation reactions) (See S3). Impurities were identified via HPLC and characterized via high-resolution MS. (See S35). We began by investigating catalyst concentration and found that with 500 μM catalyst loading (500 μM Cu(ClO4)2, 500 μM TEMPO, and 500 μM bipyridine, condition 5), the aldehyde product formed in 90% yield after 12 hours of reaction. A 2.02 Da mass decrease was observed via mass spectrometry, indicating the formation of the aldehyde (Figure 2c.-2d.). Deviating from this loading led to poor reaction outcomes: increasing catalyst concentration 10-fold (condition 1) led to extensive damage and loss of DNA, while lowering the catalyst concentration 10-fold (condition 11) decreased the yield to 5%. In assessing DNA-compatibility, the 5 mM catalyst loading led to <1% amplifiability after reaction, while the 50 μM and 500 μM loadings led to greater than 60% amplifiability (exceeding the 30% benchmark for DNA-compatibility proposed by Malone and Paegel)16. We next explored the effect of changing the solvent composition. Decreasing the water content below 50 % or increasing the water content above 70 % decreased the yield, while decreasing water content below 50% led to significant decreases in amplifiability. Taking both the yield and DNA-compatibility into account, we determined 500 μM catalyst loading in a 70% aqueous solvent system to be the optimal conditions for this reaction. Comparison of our optimized conditions with stoichiometric oxoammonium salt oxidations (using TEMPO+ and Bobbitt’s Salt) confirmed that the co-catalytic copper/TEMPO system is milder: both oxoammonium salts led to lower DNA amplifiability after reaction. To further ensure that our conditions would be acceptable for a DEL, we demonstrated the retention of a 5′ phosphoryl group, successful ligation, and sequence integrity post-oxidation (S38–S41).
Figure 2a.
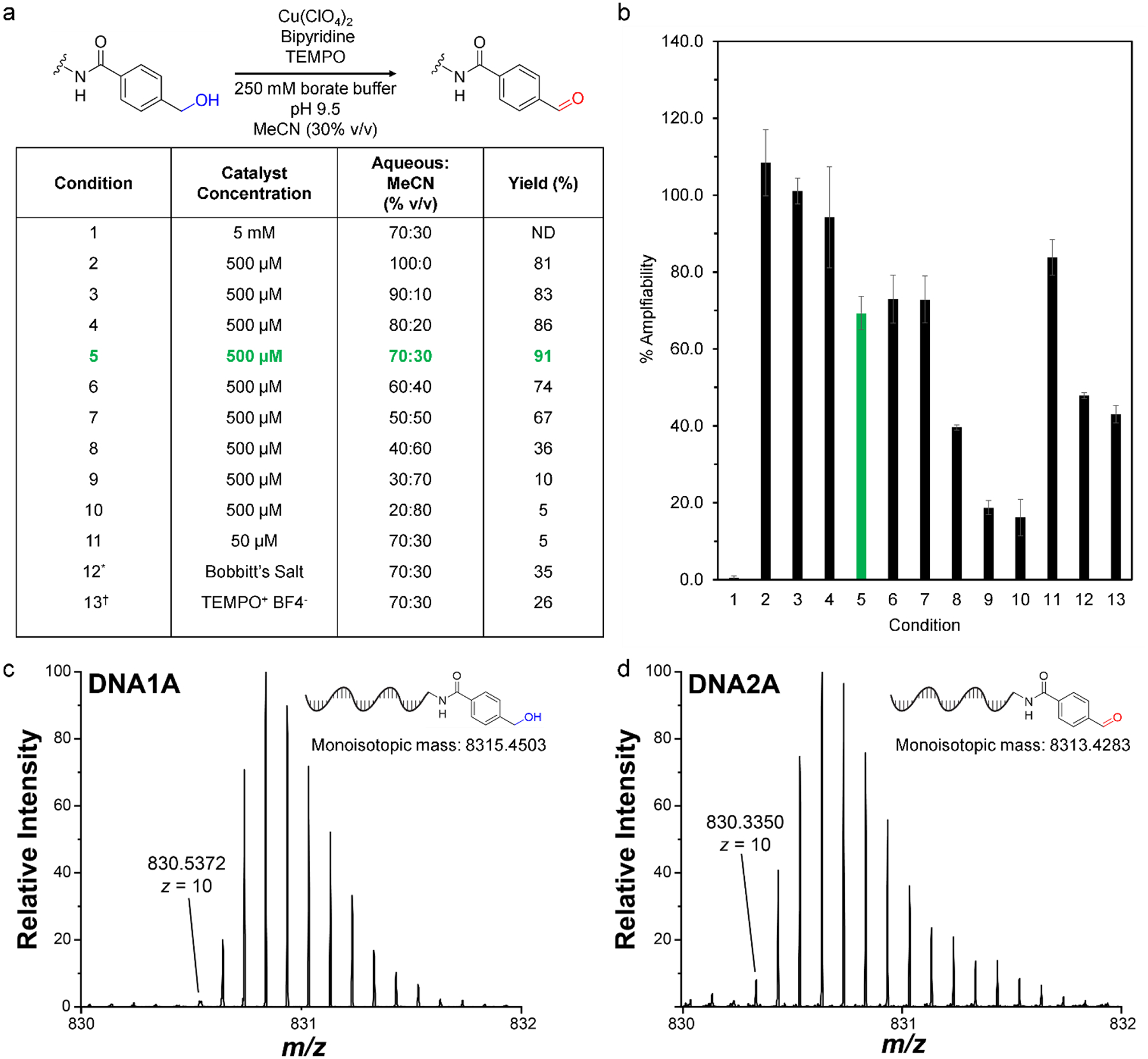
Optimization table for oxidation of DNA-linked benzylic alcohol using Cu/TEMPO/bipyridine catalyst system. Standard Reaction Conditions: 100 equiv. Cu(ClO4)2, 2,2′-bipyridine, TEMPO (20 μL, 5 mM stock in MeCN), 1 equiv. DNA conjugate (1 nmol), 250 mM borate buffer pH 9.5 (16 μL, 16.5% v/v), nuclease-free water (52.5% v/v). *100 equiv. Bobbitt’s salt (10 μL, 5 mM in MeCN), MeCN (20 μL, 20% v/v), 250 mM borate buffer pH 9.5 (16 μL, 16.5% v/v), nuclease-free water (51 μL, 52.5% v/v). †100 equiv TEMPO+ BF4− salt (10 μL, 5 mM in MeCN), MeCN (20 μL, 20% v/v), 250 mM borate buffer pH 9.5 (16 μL, 16.5% volume), nuclease-free water (51 μL, 52.5% v/v). 2b. qPCR-determined amplifiability of an unmodified DNA oligo after exposure to varied oxidation conditions. 2c. Partial ESI-MS of DNA1A showing predominant species; full spectra in SI. 2d. Partial ESI-MS of DNA2A showing predominant species; full spectra in SI
With optimized conditions in hand, we then investigated the substrate scope of activated primary alcohol oxidation. Formation of desired products was confirmed via high-resolution mass spectrometry (see SI). We successfully demonstrated the oxidation of a variety of DNA-linked primary benzylic alcohols containing halogens, electron-donating groups, and electron-withdrawing groups. Notably, the nitro-functionalized alcohol had a lower conversion compared to non-functionalized and differently functionalized alcohols. To demonstrate that the reaction is applicable to substrates beyond benzylic alcohols, we exemplified the high-yielding oxidation of several heterobenzylic alcohols (including a pyridine-containing substrate) and an allylic alcohol. Several of these substrates also showed further reactivity: oxidation of an allylic alcohol under these conditions resulted in addition of water (presumably at the beta position, Figure 3 Panel b). Furthermore, while overoxidation to the carboxylic acid was generally not observed, the carboxylic acid was observed to be a minor product for the 3-hydroxymethyl pyridine substrate (Figure 3 Panel c).
Figure 3.
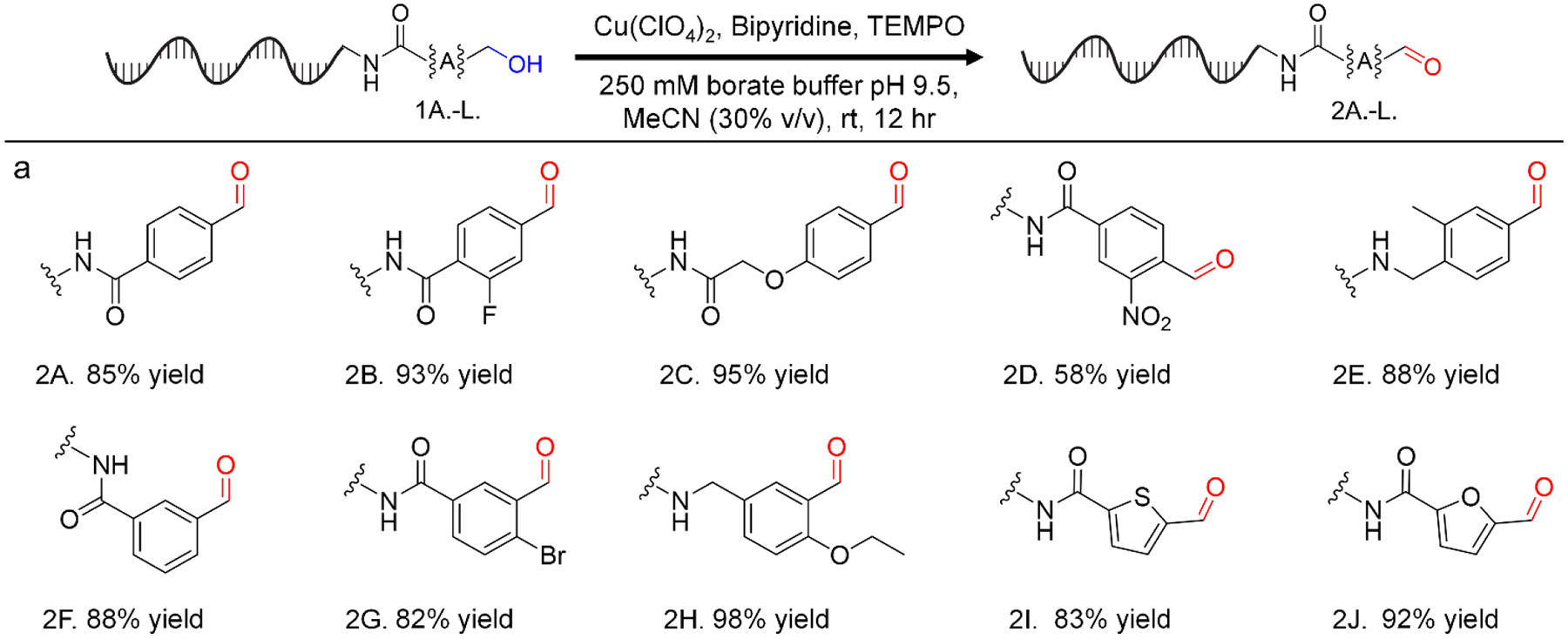
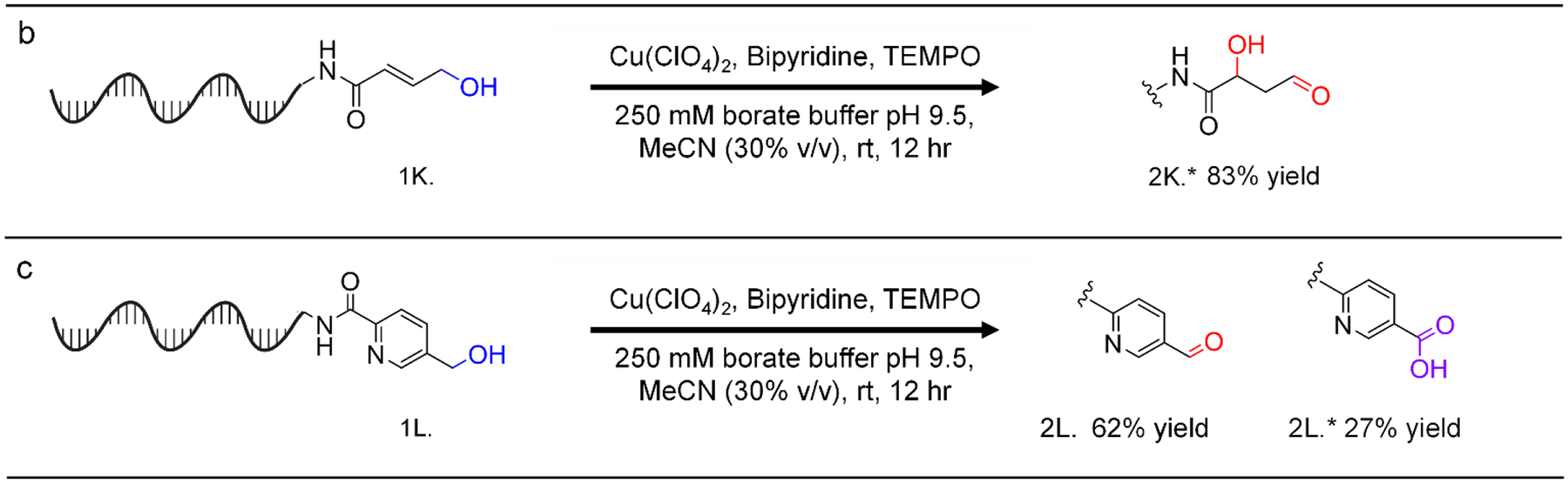
Oxidation of diverse, DNA-linked, primary activated alcohols to the corresponding aldehydes. Reaction conditions: DNA (1 equiv, 1 nmol), Cu(ClO4)2 (100 equiv, 20 μL of 5 mM stock in MeCN), TEMPO (100 equiv, 20 μL of 5 mM stock in MeCN), bipyridine (100 equiv, 20 μL of 5 mM in MeCN), H2O/250 mM borate buffer pH 9.5/MeCN (v/v/v 52.5/16.5/31 −194 μL total volume), rt, 12 hours.
Copper/TEMPO catalyst systems have been successfully used for oxidation of primary aliphatic alcohols. However, when we exposed a DNA-linked primary aliphatic alcohol to our optimized reaction conditions, we did not observe the oxidation product. Replacing TEMPO with a less-hindered nitroxyl radical co-catalyst (ABNO) known to accelerate primary aliphatic alcohol oxidations30 led to oxidation of the DNA-linked primary alcohol (to the carboxylic acid) as well as the unprotected primary alcohol on the 5′ end of the oligonucleotide (S44). Control experiments using unmodified and 5′-phosphorylated oligonucleotides showed that the presence of a 5′-phosphate group can prevent Cu/ABNO-catalyzed oxidation of the 5′-alcohol (S44). These results suggest that it may be possible in the future to develop alternative copper/nitroxyl radical catalyst systems that enable aliphatic alcohol oxidations in DEL workflows.
To demonstrate that this alcohol oxidation could fit into a synthetic sequence with known DEL chemistry, we converted a DNA-linked benzyl alcohol to a variety of amines using a two-step sequence: our optimized alcohol oxidation followed by a DNA-compatible reductive amination31. We observed moderate to excellent reductive amination yields using a variety of amines, including primary and secondary amines, aliphatic and aromatic amines, and amines bearing secondary synthons such as another benzylic alcohol and an aryl chloride (Figure 4.). Formation of desired products was confirmed via high-resolution mass spectrometry (see SI).
Figure 4.
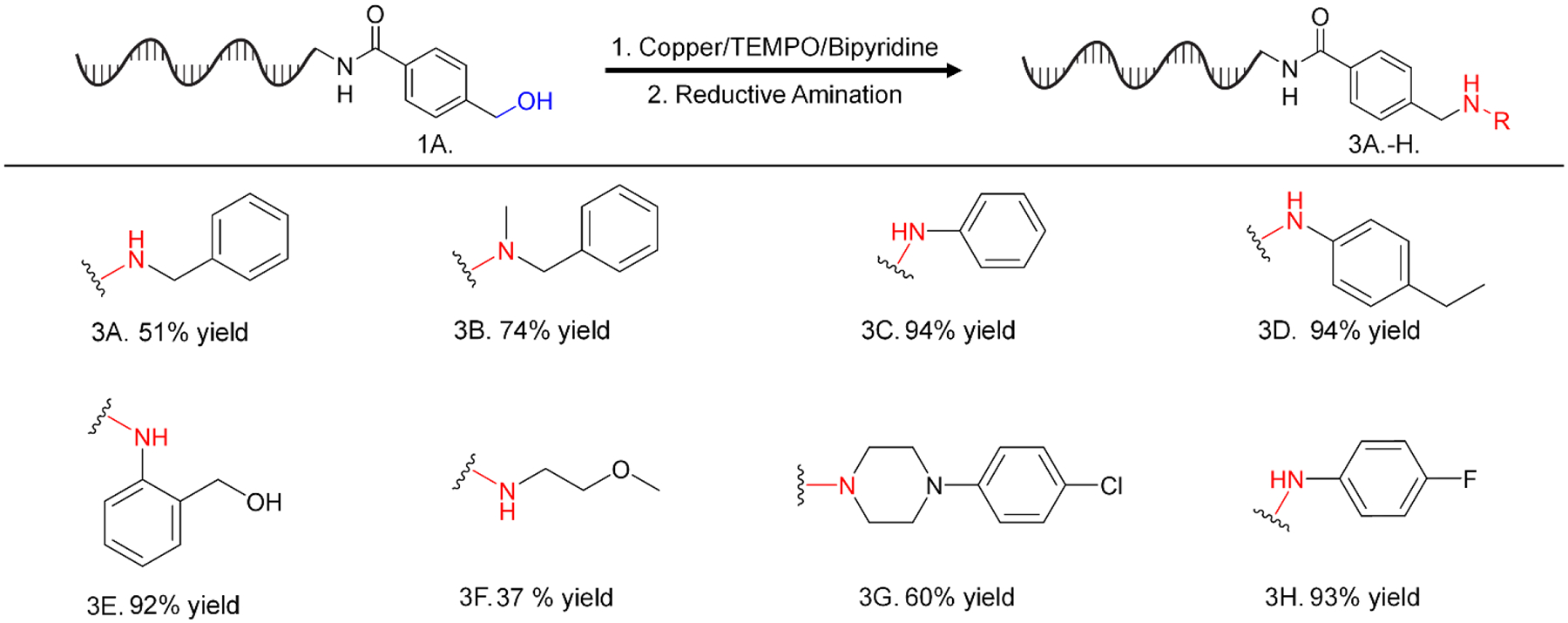
Reductive amination using a DNA-linked aldehyde produced using our optimized oxidation conditions. Reaction conditions: DNA (1 equiv, 1 nmol), 250 mM sodium phosphate buffer pH 5.5 (25 μL, 33% v/v), amine (5,000 equiv, 25 μL of 200 mM stock in DMA (33% v/v), sodium cyanoborohydride (5,000 equiv, 25 μL of 200 mM stock in MeCN (33% v/v), rt, 15 hr. Yields reported above are for the reductive amination step.
We envisioned that DNA-appended aldehydes generated through alcohol oxidation could act as a starting point for an “explosive” diversification of a DNA-encoded library using solution-phase methods compatible with standard split-and-pool library construction. Towards this end, we adapted on-bead reactions8 into solution-phase conditions for the Horner-Wadsworth-Emmons olefination32 33 and benzimidazole heterocyclization of DNA-linked aldehydes. We performed each reaction in sequence with our optimized copper/TEMPO-catalyzed alcohol oxidation reaction (see Scheme 1). The reactions were both high-yielding, and the heterocyclization afforded the benzimidazole adduct without need for a photosensitizer34. Thus, in addition to demonstrating reductive amination with a variety of amines, we realized high-yielding solution-phase olefin formation and benzimidazole formation from aldehydes produced from on-DNA alcohol oxidation.
Scheme 1.
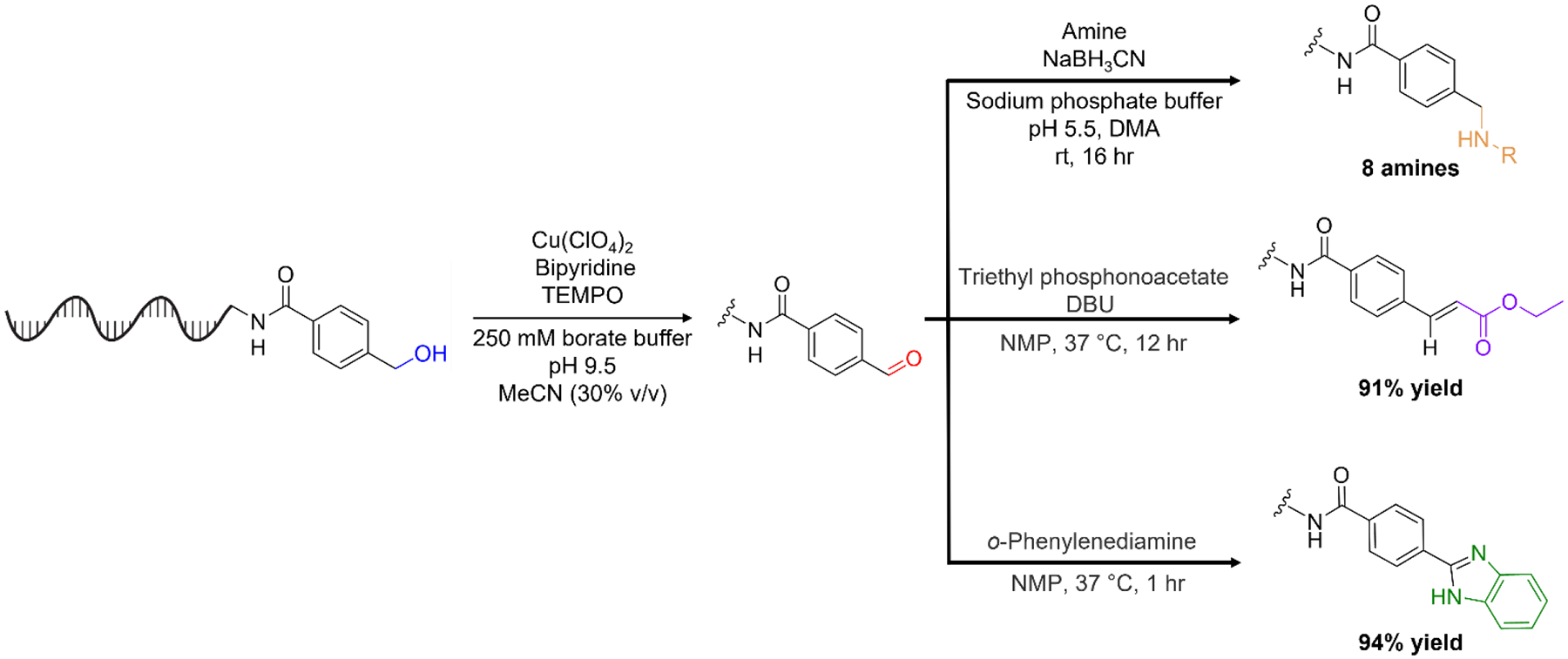
Oxidation of an activated alcohol to an aldehyde followed by solution phase reductive amination, Horner-Wadsworth-Emmons olefination, and benzimidazole formation.
In summary, we have addressed the outstanding challenge of alcohol oxidation in the DEL chemical space through the development of a mild, DNA-compatible method for the oxidation of activated alcohols to aldehydes using a copper/TEMPO catalyst system. These conditions successfully oxidized activated alcohols including benzylic, heterobenzylic, and allylic alcohols, and tolerated both electron-donating and -withdrawing substituents. This preliminary scope demonstrates the possible application of this method for the oxidation of a wide scope of activated alcohols. We also obtained promising results suggesting that, in the future, related catalyst systems may enable the challenging oxidation of aliphatic alcohols in a DEL context.
The DNA-compatibility of the reaction was demonstrated extensively through qPCR, Sanger sequencing, ligation assays, and mass spectrometry. These results confirm that alcohol oxidations can be DNA-compatible and open the door for further exploration of oxidative DEL methods. Furthermore, the mildness of these conditions suggests that these oxidations may find broader applications in biomolecular modification methods.
Finally, we explored how this alcohol oxidation could be incorporated into an “explosion” strategy for DEL diversification by performing the reaction in sequence with three different solution-phase coupling reactions. This showcases the key role that alcohol oxidation can play as an intermediate step in DEL synthesis, and we hope that this method will find ready application in pharmaceutical and agrochemical discovery chemistry.
Supplementary Material
ACKNOWLEDGMENT
The authors would like to thank the Shannon Stahl group for sharing TEMPO+ BF4, and Prof. Daniel Weix for helpful discussions. The authors would like to thank Prof. Jennifer Schomaker and Yun Hu for the use of their HILIC column and for the ESI-LC-MS method developed by Yun Hu. J.D.M. thanks the Office of Naval Research for a Young Investigator Award (N00014-23-1-2039). This work was in part supported by the National Institutes of General Medical Sciences of the National Institutes of Health (P41GM108538 and RM118110 to J.J.C) and the National Human Genome Research Institution through a training grant to the Genomic Sciences Training Program (T32HG002760 to D.J.N.). T.M.P.C. acknowledges the ACS Division of Analytical Chemistry and Agilent for support through a graduate fellowship. E. B. P thanks the Department of Chemistry for support through a William B. Dickinson Annual Fellowship.The qPCR in this work was accomplished using a BioRad CFX96 RealTime PCR provided by the University of Wisconsin Carbone Cancer Center Support Grant P30 CA014520 as well as at the University of Wisconsin-Madison Biophysics Instrumentation Facility, which was established with support from the University of Wisconsin-Madison and grants BIR-9512577 (NSF) and S10RR13790 (National Institutes of Health). The Thermo Q Exactive™ FOCUS used in this work was funded by a UW2020 WARF Discovery Initiative Award.
ABBREVIATIONS
- TEMPO
2,2,6,6-tetramethyl-1-piperidyloxy radical
- TEMPO BF4
2,2,6,6-tetramethyl-1-piperidyloxy tetrafluoroborate
- DEL
DNA Encoded Library
- DNA
Deoxyribose Nucleic Acid
- ABNO-9
Azabicyclo[3.3.1] nonane N-oxyl radical
- qPCR
Quantitative polymerase chain reaction
- NaBH3CN
Sodium Cyanoborohydride
- MeCN
Acetonitrile
- NMP
N-Methylpyrrolidine
- DBU
1,8-Diazabicyclo[5.4.0]undec-7-ene
- DMA
Dimethylacetamide
- Cu(ClO4)2
Copper Perchlorate
- GalOx
Galactose Oxidase
- HRP
Horse Radish Peroxidase
- HWE Olefination
Horner-Wadsworth-Emmons
Footnotes
Supporting information: Experimental procedures, HPLC and ESI-MS of DNA conjugates, qPCR example calculation, supplementary experiments (damage analysis, aliphatic alcohol oxidation).
The authors declare the following competing financial interest(s): J.J.C. is a consultant for Thermo Fisher Scientific.
REFERENCES
- (1).Franzini RM; Neri D; Scheuermann J DNA-Encoded Chemical Libraries: Advancing beyond Conventional Small-Molecule Libraries. Acc. Chem. Res 2014, 47 (4), 1247–1255. 10.1021/ar400284t. [DOI] [PubMed] [Google Scholar]
- (2).Neri D; Lerner RA DNA-Encoded Chemical Libraries: A Selection System Based On Endowing Organic Compounds With Amplifiable Information. Annu. Rev. Biochem 2018, 87, 479–502. 10.1146/annurev-biochem-062917-012550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Belyanskaya SL; Ding Y; Callahan JF; Lazaar AL; Israel DI Discovering Drugs with DNA-Encoded Library Technology: From Concept to Clinic with an Inhibitor of Soluble Epoxide Hydrolase. ChemBioChem 2017, 18 (9), 837–842. 10.1002/cbic.201700014. [DOI] [PubMed] [Google Scholar]
- (4).Scheuermann J; Dumelin CE; Melkko S; Zhang Y; Mannocci L; Jaggi M; Sobek J; Neri D DNA-Encoded Chemical Libraries for the Discovery of MMP-3 Inhibitors. Bioconjug. Chem 2008, 19 (3), 778–785. 10.1021/bc7004347. [DOI] [PubMed] [Google Scholar]
- (5).Peterson JT The Importance of Estimating the Therapeutic Index in the Development of Matrix Metalloproteinase Inhibitors. Cardiovasc. Res 2006, 69 (3), 677–687. 10.1016/j.cardiores.2005.11.032. [DOI] [PubMed] [Google Scholar]
- (6).Brown DG; Boström J Where Do Recent Small Molecule Clinical Development Candidates Come From? J. Med. Chem 2018, 61 (21), 9442–9468. 10.1021/acs.jmedchem.8b00675. [DOI] [PubMed] [Google Scholar]
- (7).Fair RJ; Walsh RT; Hupp CD The Expanding Reaction Toolkit for DNA-Encoded Libraries. Bioorg. Med. Chem. Lett 2021, 51, 128339. 10.1016/j.bmcl.2021.128339. [DOI] [PubMed] [Google Scholar]
- (8).Paciaroni NG; Ndungu JM; Kodadek T Solid-Phase Synthesis of DNA-Encoded Libraries Via An “Aldehyde Explosion” Strategy. Chem. Commun. Camb. Engl 2020, 56 (34), 4656–4659. 10.1039/d0cc01474e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Burrows CJ; Muller JG Oxidative Nucleobase Modifications Leading to Strand Scission. Chem. Rev 1998, 98 (3), 1109–1152. 10.1021/cr960421s. [DOI] [PubMed] [Google Scholar]
- (10).Gates KS An Overview of Chemical Processes That Damage Cellular DNA: Spontaneous Hydrolysis, Alkylation, and Reactions with Radicals. Chem. Res. Toxicol 2009, 22 (11), 1747–1760. 10.1021/tx900242k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Steenken S; Jovanovic SV How Easily Oxidizable Is DNA? One-Electron Reduction Potentials of Adenosine and Guanosine Radicals in Aqueous Solution. J. Am. Chem. Soc 1997, 119 (3), 617–618. 10.1021/ja962255b. [DOI] [Google Scholar]
- (12).Lewis FD; Letsinger RL; Wasielewski MR Dynamics of Photoinduced Charge Transfer and Hole Transport in Synthetic DNA Hairpins. Acc. Chem. Res 2001, 34 (2), 159–170. 10.1021/ar0000197. [DOI] [PubMed] [Google Scholar]
- (13).Kanvah S; Joseph J; Schuster GB; Barnett RN; Cleveland CL; Landman U Oxidation of DNA: Damage to Nucleobases. Acc. Chem. Res 2010, 43 (2), 280–287. 10.1021/ar900175a. [DOI] [PubMed] [Google Scholar]
- (14).Flood DT; Asai S; Zhang X; Wang J; Yoon L; Adams ZC; Dillingham BC; Sanchez BB; Vantourout JC; Flanagan ME; Piotrowski DW; Richardson P; Green SA; Shenvi RA; Chen JS; Baran PS; Dawson PE Expanding Reactivity in DNA-Encoded Library Synthesis via Reversible Binding of DNA to an Inert Quaternary Ammonium Support. J. Am. Chem. Soc 2019, 141 (25), 9998–10006. 10.1021/jacs.9b03774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Dickson P; Kodadek T Chemical Composition of DNA-Encoded Libraries, Past Present and Future. Org. Biomol. Chem 2019, 17 (19), 4676–4688. 10.1039/c9ob00581a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Malone ML; Paegel BM What Is a “DNA-Compatible” Reaction? ACS Comb. Sci 2016, 18 (4), 182–187. 10.1021/acscombsci.5b00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Sauter B; Schneider L; Stress C; Gillingham D An Assessment of the Mutational Load Caused by Various Reactions Used in DNA Encoded Libraries. Bioorg. Med. Chem 2021, 52, 116508. 10.1016/j.bmc.2021.116508. [DOI] [PubMed] [Google Scholar]
- (18).Thomas B; Lu X; Birmingham WR; Huang K; Both P; Reyes Martinez JE; Young RJ; Davie CP; Flitsch SL Application of Biocatalysis to On-DNA Carbohydrate Library Synthesis. ChemBioChem 2017, 18 (9), 858–863. 10.1002/cbic.201600678. [DOI] [PubMed] [Google Scholar]
- (19).Škopić MK; Götte K; Gramse C; Dieter M; Pospich S; Raunser S; Weberskirch R; Brunschweiger A Micellar Brønsted Acid Mediated Synthesis of DNA-Tagged Heterocycles. J. Am. Chem. Soc 2019, 141 (26), 10546–10555. 10.1021/jacs.9b05696. [DOI] [PubMed] [Google Scholar]
- (20).Highly Practical Copper(I)/TEMPO Catalyst System for Chemoselective Aerobic Oxidation of Primary Alcohols | Journal of the American Chemical Society. https://pubs.acs.org/doi/full/10.1021/ja206230h (accessed 2023-04-24). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Hoover JM; Ryland BL; Stahl SS Mechanism of Copper(I)/TEMPO-Catalyzed Aerobic Alcohol Oxidation. J. Am. Chem. Soc 2013, 135 (6), 2357–2367. 10.1021/ja3117203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Matsushita T; Moriyama Y; Nagae G; Aburatani H; Okamoto A DNA-Friendly Cu(Ii)/TEMPO-Catalyzed 5-Hydroxymethylcytosine-Specific Oxidation. Chem. Commun 2017, 53 (42), 5756–5759. 10.1039/C7CC02814H. [DOI] [PubMed] [Google Scholar]
- (23).Gamez P; E. Arends IWC; Reedijk J; A. Sheldon R Copper(Ii)-Catalysed Aerobic Oxidation of Primary Alcohols to Aldehydes. Chem. Commun 2003, 0 (19), 2414–2415. 10.1039/B308668B. [DOI] [PubMed] [Google Scholar]
- (24).Pimentel EB; Peters-Clarke TM; Coon JJ; Martell JD DNA-Scaffolded Synergistic Catalysis. J. Am. Chem. Soc 2021, 143 (50), 21402–21409. 10.1021/jacs.1c10757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Kennedy LJ; Moore K; Caulfield JL; Tannenbaum SR; Dedon PC Quantitation of 8-Oxoguanine and Strand Breaks Produced by Four Oxidizing Agents. Chem. Res. Toxicol 1997, 10 (4), 386–392. 10.1021/tx960102w. [DOI] [PubMed] [Google Scholar]
- (26).Favalli N; Bassi G; Zanetti T; Scheuermann J; Neri D Screening of Copper and Palladium-Mediated Reactions Compatible with DNA-Encoded Chemical Libraries. Helv. Chim. Acta 2019, 102 (4), e1900033. 10.1002/hlca.201900033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Scott SK On-DNA-1,2,3-Triazole Formation via Click Reaction. In DNA-Encoded Chemical Libraries: Methods and Protocols; Israel D, Ding Y, Eds.; Methods in Molecular Biology; Springer US: New York, NY, 2022; pp 39–43. 10.1007/978-1-0716-2545-3_6. [DOI] [PubMed] [Google Scholar]
- (28).Li H; Sun Z; Wu W; Wang X; Zhang M; Lu X; Zhong W; Dai D Inverse-Electron-Demand Diels–Alder Reactions for the Synthesis of Pyridazines on DNA. Org. Lett 2018, 20 (22), 7186–7191. 10.1021/acs.orglett.8b03114. [DOI] [PubMed] [Google Scholar]
- (29).Li K; Qu Y; An Y; Breinlinger E; Webster MP; Wen H; Ding D; Zhao M; Shi X; Wang J; Su W; Cui W; Satz AL; Yang H; Kuai L; Little A; Peng X DNA-Compatible Copper-Catalyzed Oxidative Amidation of Aldehydes with Non-Nucleophilic Arylamines. Bioconjug. Chem 2020, 31 (9), 2092–2097. 10.1021/acs.bioconjchem.0c00392. [DOI] [PubMed] [Google Scholar]
- (30).Steves JE; Stahl SS Copper(I)/ABNO-Catalyzed Aerobic Alcohol Oxidation: Alleviating Steric and Electronic Constraints of Cu/TEMPO Catalyst Systems. J. Am. Chem. Soc 2013, 135 (42), 15742–15745. 10.1021/ja409241h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Ding Y; Franklin GJ; DeLorey JL; Centrella PA; Mataruse S; Clark MA; Skinner SR; Belyanskaya S Design and Synthesis of Biaryl DNA-Encoded Libraries. ACS Comb. Sci 2016, 18 (10), 625–629. 10.1021/acscombsci.6b00078. [DOI] [PubMed] [Google Scholar]
- (32).Solution-phase conditions for on-DNA HWE olefination have been reported previously:; Satz AL; Cai J; Chen Y; Goodnow R; Gruber F; Kowalczyk A; Petersen A; Naderi-Oboodi G; Orzechowski L; Strebel Q DNA Compatible Multistep Synthesis and Applications to DNA Encoded Libraries. Bioconjug. Chem 2015, 26 (8), 1623–1632. 10.1021/acs.bioconjchem.5b00239. [DOI] [PubMed] [Google Scholar]
- (33).Fang X; Wang Y; He P; Liao H; Zhang G; Li Y; Li Y Visible Light-Promoted Divergent Benzoheterocyclization from Aldehydes for DNA-Encoded Chemical Libraries. Org. Lett 2022, 24 (17), 3291–3296. 10.1021/acs.orglett.2c01187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


