Abstract
Aims:
Antisocial behavior (ASB) is characterized by frequent violations of the rights and properties of others, as well as aggressive conduct. While ample evidence points to a critical role of serotonin in the emotional modulation of social responses, the implication of this neurotransmitter in ASB is unclear. Here, we performed the first-ever postmortem analysis of serotonergic markers in the orbitofrontal cortex (OFC) of male subjects with ASB (n=9). We focused on this brain region, given its well-recognized role in ASB pathophysiology. Given that all subjects also had a diagnosis of substance use disorder (SUD), we used two age-matched control groups: SUD-only and unaffected controls.
Methods:
Tissues were processed for immunoblotting analyses on eight key serotonergic targets: tryptophan hydroxylase 2 (TPH2), the rate-limiting enzyme of brain serotonin synthesis; serotonin transporter (SERT), the main carrier for serotonin uptake; monoamine oxidase A (MAOA), the primary enzyme for serotonin catabolism; and five serotonin receptors previously documented to influence social behavior: 5-HT1A, 5-HT1B, 5-HT2A, 5-HT2C, and 5-HT4. Analyses were performed by nested t-test.
Results:
We found a significant increase of 5-HT2A receptor levels in the ASB+SUD group compared with SUD-only controls. Furthermore, TPH2 levels were significantly reduced in the SUD group (including SUD-only and ASB+SUD) in comparison with unaffected controls. No difference was detected in the expression of any other serotonergic target.
Conclusions:
These results are in keeping with previous evidence showing high 5-HT2A receptor binding in the OFC of pathologically aggressive individuals and point to this molecule as a potential target for ASB treatment.
Keywords: Antisocial behavior, orbitofrontal cortex, serotonin, postmortem samples
INTRODUCTION
Antisocial behavior (ASB) is a persistent pattern of violation of societal norms, often entailing damage to the basic rights and properties of others as well as covert and overt hostility1. Multiple disorders in the DSM-5 are characterized by pervasive ASB, such as conduct disorder (CD) in children and adolescents and antisocial personality disorder (ASPD) in adults. The prevalence of CD and ASPD are respectively estimated at 9.5%2 and 1–4%3–4. Both disorders are characterized by a marked male predominance, with gender ratios ranging from 3 to 7:15–7.
One of the major features of ASB is the impairment of self- and interpersonal functioning with specific symptoms of antagonism and disinhibition. Although ASB is not limited to aggressive manifestations, both verbal and physical aggression are common symptoms of both CD and ASPD8,9. Furthermore, these disorders are highly comorbid with several major mental disorders10–12, and particularly with substance use disorders (SUD), which are estimated to be present in more than half of adolescent CD patients13 and nearly 90% of ASPD patients14.
The socio-economic impact of ASB is staggering, in consideration of its association with high rates of violent behavior and criminal offending15,16. Making matters worse, no therapies have been approved by the Food and Drug Administration (FDA) for the treatment of CD and ASPD, partially reflecting our limited knowledge of the biological and neurochemical basis of these disorders. In particular, most available evidence on the neurobiology of ASB is based on neuroimaging and genetic findings, but very little is known about the molecular foundation of antisociality. For example, to the best of our knowledge, no studies have ever been performed on postmortem samples of antisocial individuals.
Ample evidence has shown that ASB is associated with deficits in facial affect processing17, which lead to maladaptive social responses18–20. The main brain area that regulates the interpretation of social cues and the enactment of appropriate emotional response is the orbitofrontal cortex (OFC)21,22. In fact, this region is activated by exposure to negative emotional expression, including anger, social disapproval, guilt-related emotion, shame, and embarrassment23,24, and its lesions reduce social expression recognition and guilt experience22,25. In line with this evidence, several studies have shown a key implication of the OFC in prosocial responses. For example, the results of a recent prospective study have shown a significant thinning of the OFC in affected individuals with life-course-persistent ASB 26. These data are consistent with previous reports showing that the gray matter of the OFC is reduced in relation to aggression and antisocial traits27–29.
Previous studies have shown that the regulation of aggression and social responses by the OFC is directly shaped by the serotonergic system30. Indeed, serotonin dysfunctions are a major risk factor for aggressive behavior and other key components of antisocial behavior, such as punishment sensitivity and loss of behavioral control31. Furthermore, alterations of serotonin metabolism have been shown in ASPD32. Despite these important premises, only little is known about the alterations of the serotonergic system in the OFC of antisocial individuals.
Building on these premises, here we conducted the first-ever study of postmortem OFC samples from male individuals affected by ASPD, to assess the levels of key serotonin targets in this brain region. In particular, we used immunoblotting to test the levels of the following targets: 1) tryptophan hydroxylase 2 (TPH2), the enzyme catalyzing the rate-limiting step of serotonin biosynthesis; 2) serotonin transporter (SERT), the main carrier of serotonin synaptic uptake; 3) monoamine oxidase A, the main serotonin-catabolic enzyme, and 4–8) the serotonin receptors 5-HT1A, 5-HT1B, 5-HT2A, 5-HT2C, and 5-HT4, in consideration of their extensive association with aggression and ASB-related characteristics33. Given the high comorbidity of ASPD with SUD, we used two sets of controls: age-matched controls with a SUD diagnosis as well as non-affected controls.
MATERIALS AND METHODS
Human Brain Collection and Donor Information.
OFC tissues were obtained from the NIH NeuroBioBank (NBB) Brain and Tissue Repository (BTR) at the University of Pittsburgh. The right hemisphere of each brain was dissected in the coronal plane, immediately frozen and stored at −80°C in accordance with the policies and procedures utilized by the BTRs participating in the NIH NBB. Consent was obtained according to legal provisions from the next of kin. DSM5 diagnoses were made by experienced research clinicians using structured interviews with family members and/or review of prior medical records. The absence of a psychiatric diagnosis was confirmed in unaffected control subjects using the same approach. The analysis was performed in post-mortem OFC tissue and associated clinical data including age, sex, brain pH and post-mortem interval (PMI) for age- and sex-matched cohorts of 9 individuals receiving SUD diagnoses, 9 individuals receiving diagnoses of ASB+SUD and 9 individuals with no history of psychiatric disease (Table 1). As evidenced in Table 2, all subjects were males, and no significant differences were found between groups with respect to either demographic characteristics (age and race distribution) or indices of tissue quality (PMI, pH, and RIN).
TABLE 1.
Demographic characteristics of subjects
| Group | Manner of Death | SUD diagnoses | ASB diagnoses | Other comorbid disorders |
|---|---|---|---|---|
| Unaffected Controls | ACCIDENTAL | / | / | / |
| NATURAL | / | / | / | |
| NATURAL | / | / | / | |
| ACCIDENTAL | / | / | / | |
| NATURAL | / | / | / | |
| ACCIDENTAL | / | / | / | |
| NATURAL | / | / | / | |
| NATURAL | / | / | / | |
| NATURAL | / | / | / | |
| SUD only | NATURAL | Alcohol (S); tobacco (M); Opioids (M); Cocaine (M) | / | / |
| NATURAL | Alcohol (S); Sedatives (Mi) | / | Anxiety disorder | |
| NATURAL | Alcohol (Mi) | / | Seizure disorder | |
| ACCIDENTAL | Alcohol (Mi); Cocaïne (Mi); Opioids (Mi) | / | / | |
| PENDING | Alcohol (Mi); Cocaine (S); Cannabis (M) | / | / | |
| ACCIDENTAL | Alcohol (M); Tobacco (M); opioids (Mi) | / | / | |
| NATURAL | Alcohol (M); Cannabis (M); Tobacco (M) | / | / | |
| ACCIDENTAL | Alcohol (Mi); Cocaïne (Mi); Hallucinogens (Mi) | / | / | |
| ACCIDENTAL | Amphetamines (M); Tobacco (M); Cannabis (Mi) | / | / | |
| SUD+ASB | NATURAL | Alcohol (S); Opioids (M); Sedatives (M) | ASPD | / |
| ACCIDENTAL | Alcohol (M); Cocaine (Mi); Cannabis (Mi) | CD (childhood onset) | Dysthymia | |
| ACCIDENTAL | Alcohol (S); Opioids (Mi) | CD (adolescence onset); | Intermittent explosive disorder | |
| NATURAL | Alcohol (S), Tobacco (S); Sedatives (M); Opioids (M); Amphetamines (Mi) | ASPD | Unspecified depressive disorder Borderline personality disorder | |
| ACCIDENTAL | Alcohol (S); Cocaine (S); Cannabis (M); Opioids (Mi) | ASPD | Major depressive disorder, Single episode | |
| ACCIDENTAL | Cannabis (M); Alcohol (Mi); Opioids (Mi); Sedatives (Mi); Cocaine (Mi) | ASPD | / | |
| SUICIDE | Cannabis (Mi) | CD (adolescence onset); ASPD; | Major depressive disorder, Single episode; learning disorders; Borderline personality disorder | |
| ACCIDENTAL | Tobacco, opioids (S); Cannabis (M); Alcohol (Mi) | CD (unspecified onset); ASPD | Unspecified depressive disorder; Gambling disorder; ADHD; trauma and stress-reated disorder | |
| ACCIDENTAL | Opioids, Tobacco (S); Alcohol (M); Cocaine (Mi) | CD (unspecified onset); | Disruptive, impulse-control disorder |
S: severe; M: moderate; Mi: mild
TABLE 2.
Statistical comparisons of demographic and sample characteristics across diagnostic groups. Data are represented as means ± SEM.
| Unaffected Controls | SUD only | SUD+ASB | p value | |
|---|---|---|---|---|
| Race (White:Black) | 7W:2B | 7W:2B | 6W:3B | 0.8 |
| Sex (Male: Female) | 9M:0F | 9M:0F | 9M:0F | 1.0 |
| Age at death | 42.7 ± 2.8 | 42.7 ± 3.5 | 43.67 ± 3.8 | 0.9 |
| PMI (hr) | 17.5 ± 1,9 | 19.0 ± 2.2 | 17.5 ± 1.9 | 0.5 |
| RIN | 8.2 ± 0.4 | 7.3 ± 0.3 | 7.8 ± 0.2 | 0.1 |
| pH | 6.6 ± 0.1 | 6.4 ± 0.07 | 6.4 ± 0.08 | 0.4 |
Immunoblotting.
Tissues were stored at −80°C until assayed. To analyze the expression levels of MAOA, SERT, TPH2 and 5-HT receptors, tissues were weighted and diluted (1 mg/10 μl) in RIPA buffer containing 20mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA, 1mM EGTA, 1% NP-40, 1% sodium deoxycholate, 2.5 mM sodium pyrophosphate, 1mM beta-glycerophosphate, 1mM Na3VO4, 1 μg/ml leupeptin and protease inhibitor cocktail.
Small aliquots of the homogenate were used for protein determination using a modified Lowry protein assay method (DC protein assay, Bio-Rad Laboratories, Hercules, CA). Samples containing 30 μg of total proteins were run onto 4–15% Criterion™ TGX Stain-free™ precast gels (Bio-Rad Laboratories) and transferred to nitrocellulose membranes (Bio-Rad Laboratories). Stain-free™ gel formulation includes a trihalo compound that, when exposed to ultraviolet (UV) irradiation, generates a covalent reaction with tryptophan residues of proteins and allows them to be visualized within the gel or after transfer to a blotting membrane. Following protein transfer, the membrane was detected by UV and blot image was collected for total protein.
Primary antibodies for anti-5-HT2A (rabbit polyclonal #ab66049 Abcam, Cambridge, UK; dilution 1:100), anti-MAOA (mouse monoclonal #sc271123 Santa Cruz Biotechnology, Dallas, TX; dilution 1:750); anti-SERT (rabbit polyclonal #ab272912 Abcam; dilution 1:1000); anti-5-HT1A (rabbit polyclonal #ab85615 Abcam; dilution 1:1000); anti-5-HT1B (rabbit polyclonal #NB56350 Novus Biologicals, Centennial, CO; dilution 1:1000); anti-5-HT2C (goat polyclonal #NB1524 Novus Biologicals; dilution 1:750); anti-5-HT4 (rabbit polyclonal #ab60359 Abcam; dilution 1:1000); anti-TPH2 (rabbit polyclonal #PA1–778 Thermo Fisher Scientific, Waltham, MA; dilution 1:750) were incubated in TBS-T containing 3% (w/v) BSA buffer overnight at 4 °C. Next, blots were washed in TBS-T, and then incubated in TBS-T containing goat anti-rabbit HRP-conjugated (#31462, Thermo Fisher Scientific; dilution 1:10000), goat anti-mouse HRP-conjugated (#31430, Thermo Fisher Scientific; dilution 1:5000) or rabbit anti-goat HRP-conjugated (#5160–2504, Bio-Rad laboratories; dilution 1:10000) secondary antibodies, for 90 minutes at room temperature. Chemiluminescence was detected with the ChemiDoc™ XRS+ Imaging System using the Clarity Western ECL substrate (Bio-Rad Laboratories). Bands were quantified in arbitrary units and normalized using the software Image Lab (Bio-Rad Laboratories). Samples containing the same amounts of total proteins in each experimental group were run on the same immunoblots and then analyzed together.
To accurately compare western blot signal in normalization procedure and control for potential losses of gray matter in ASB brain samples, stain-free total proteins signals were used34,35. Indeed, normalization based on housekeeping protein (e.g. b actin) may be suboptimal in this context since reduced gray matter, surface area and thickness of prefrontal cortex have been described in individuals with ASB26,27,29,36,37. However, since actin is a crucial component of cellular machinery to maintain cell morphology and trafficking, membranes were stripped and re-probed with primary antibody anti-β-actin (mouse monoclonal #sc47778 Santa Cruz Biotechnology) in order to determine its expression as index of cytoarchitectural changes. No significant changes in b actin levels were found across samples (F1, 25= 0.14, P= 0.708).
Statistical analysis.
Demographic and neurochemical data were analyzed by Nested T-Test using GraphPad Prism package (GraphPad, San Diego, CA, USA) and expressed as means ± SEM. Race and sex distribution were compared across group by chi-square statistics. Univariable regression analysis was used to test whether PMI or age modifies significantly brain proteins levels. Whenever PMI was found to be associated with protein expression, this index was used as a covariate in multiple regression analyses to assess its effect on potential differences in protein levels across diagnostic groups. To control for multiple comparisons, Bonferroni’s correction was used, and significance was set at α < 0.00625.
RESULTS
TPH2.
We evaluated TPH2 expression since reduced TPH2 levels and function have been associated with aggression-related personality traits in humans38 and tph2 null mice exhibited impulsive and hyperaggressive behavior39. Analyses showed that subjects with a history of SUD (irrespective of ASB) exhibited a significant decrease in TPH2 levels in comparison with unaffected controls (F1,25 = 18.26; P=0.0002) (Figs. 1A–B). However, no differences were shown between the ASB + SUD and SUD-only groups (Fig. 1A). Regression analyses showed no statistically significant association between TPH2 levels and either PMI (F1,25=1.64; R2=0.06, P=0.21) (Fig. 1C) or age (F1,25=2.19; R2=0.08, P=0.15) (Fig. 1D).
Fig. 1. Analysis of TPH2 expression levels in the OFC of antisocial individuals.
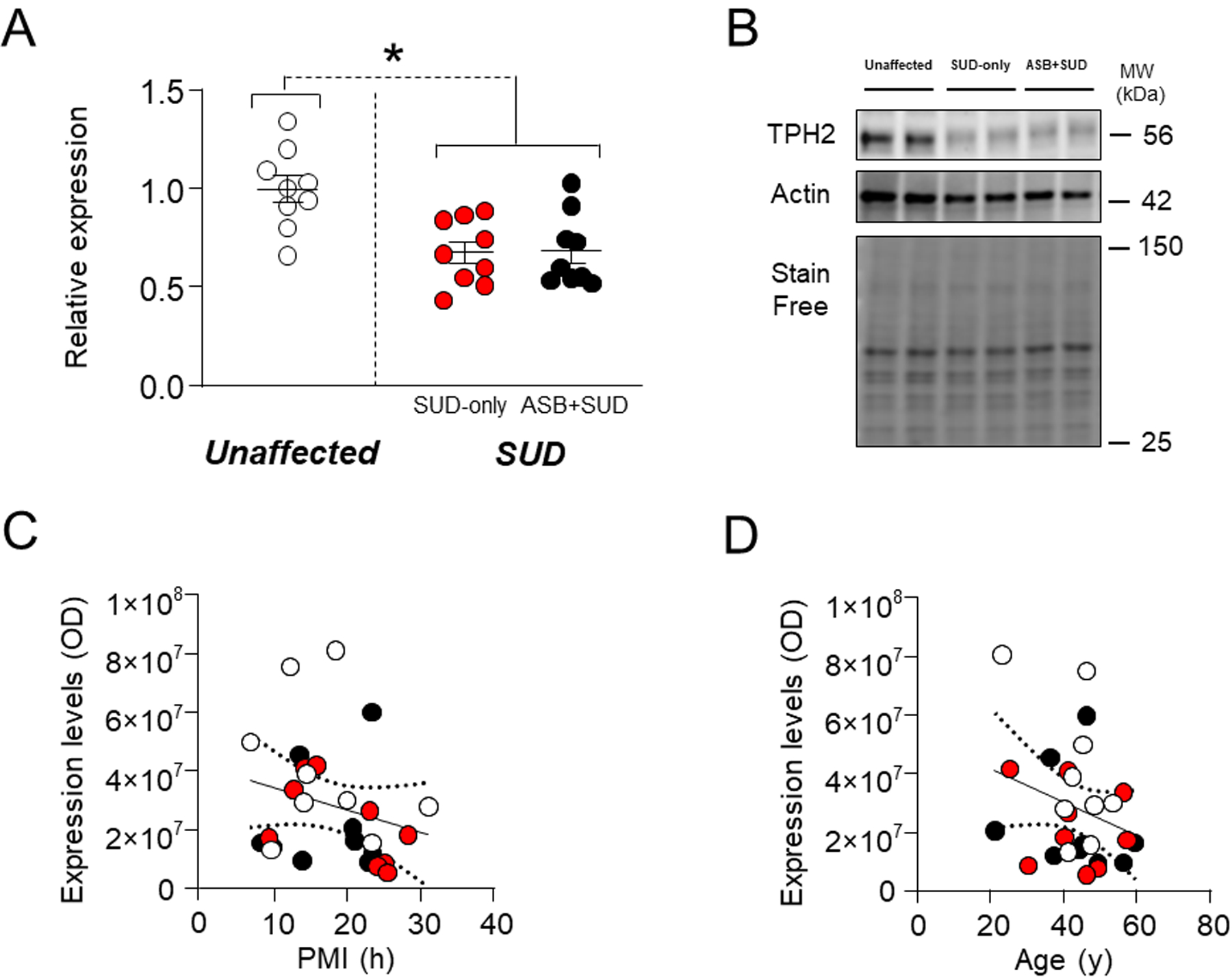
Irrespective of ASB, individuals with a history of SUD exhibited a significant decrease in TPH2 levels in comparison with unaffected controls. A. Protein expression levels. B. Representative immunoblotting. C. Simple linear regression analysis representing the association of PMI with TPH2 levels. D. Simple linear regression analysis representing the association of age with TPH2 levels. * P= 0.0002 compared to unaffected control (Nested T-Test). ASB: Antisocial behavior; SUD: Substance use disorder; PMI: post-mortem interval
SERT.
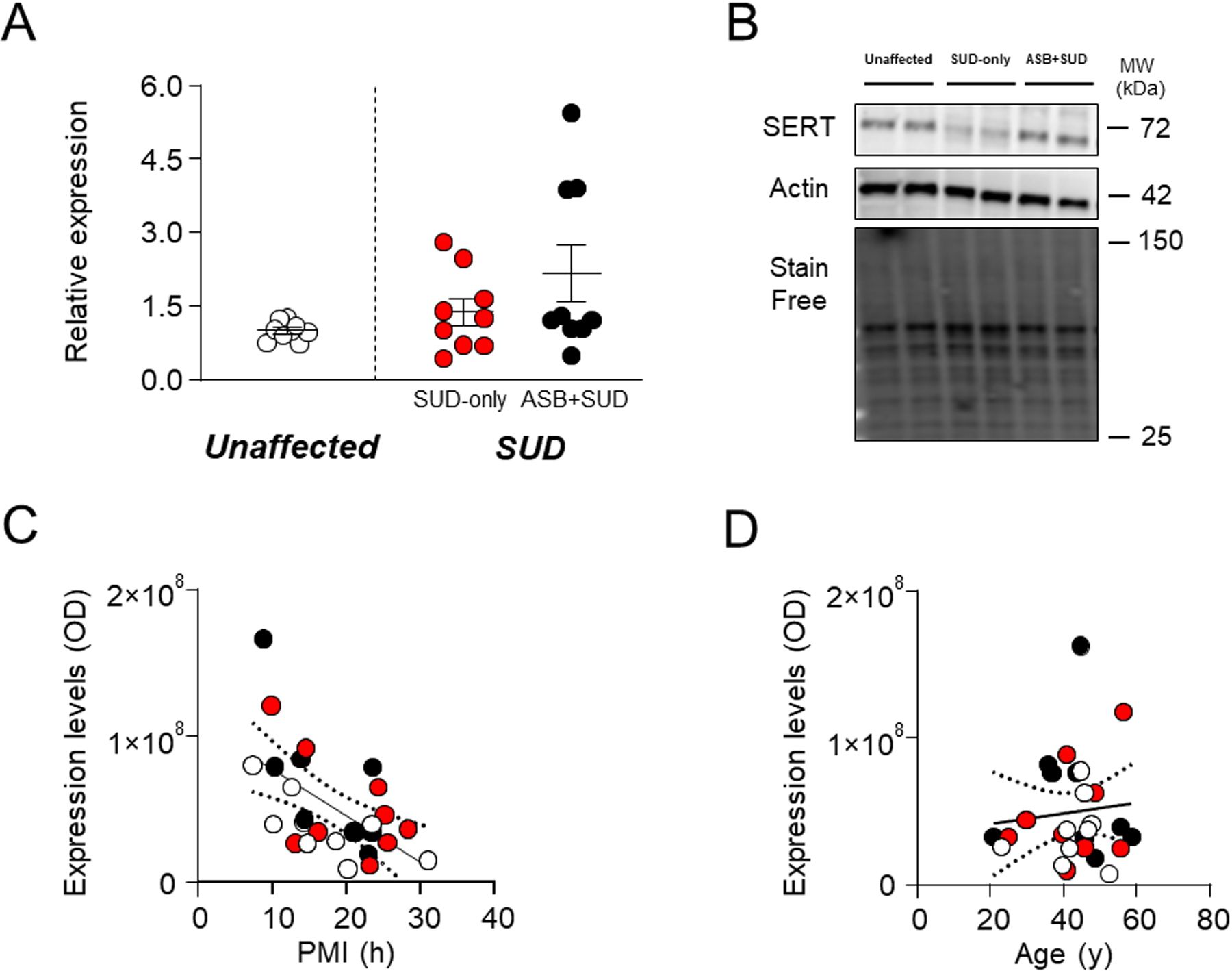
We next measured SERT levels, since genetic polymorphisms of this carrier have been associated with phenotypes related to ASB, including negative emotionality, aggression, violent behavior, impulsivity, and disinhibition40–43. Our results revealed no significant differences between samples from SUD-affected subjects and unaffected controls (F1,25 = 1.31; P=0.47) or between SUD-only and ASB +SUD groups (Fig. 2A–B). The analysis of regression showed that PMI was negatively associated with SERT levels [F1,25=12.04; R2=0.33, P=0.002] (Fig.2C); however, using PMI as a covariate in ANCOVA to compare for potential differences between groups confirmed the lack of significant differences in protein levels across groups (P = 0.144). Finally, no relation was found between age and SERT protein levels (F1,25=0.24; R2=0.009, P=0.63) (Fig. 2D).
Fig. 2. Analysis of MAOA expression levels in the OFC of antisocial individuals.
No differences were detected between groups. A. Protein expression levels. B. Representative immunoblotting. C. Simple linear regression analysis representing the association of PMI with MAOA levels. D. Simple linear regression analysis representing the association of age with MAOA levels. ASB: Antisocial behavior; SUD: Substance use disorder; PMI: post-mortem interval
MAOA.
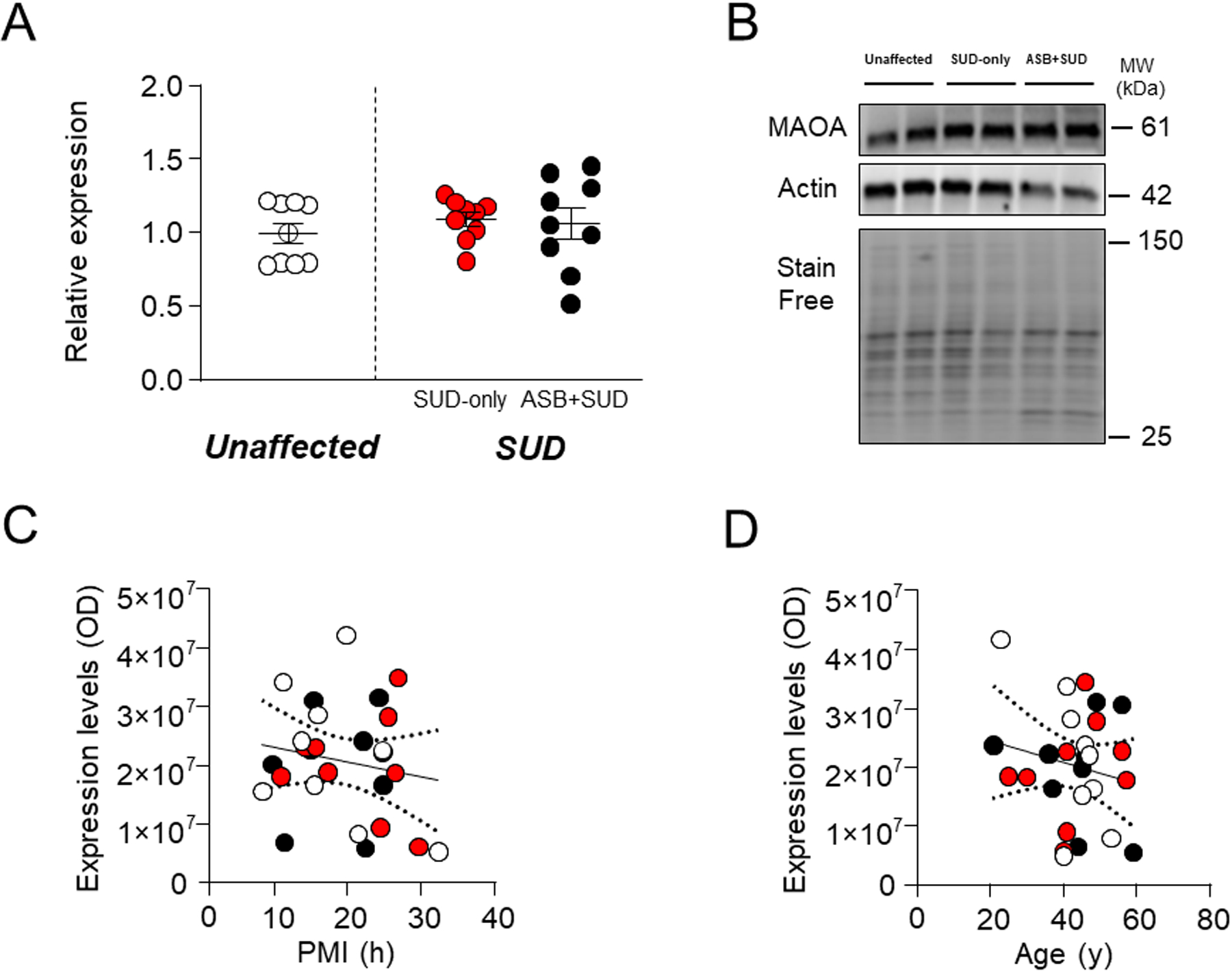
MAOA is the main enzyme catalyzing the degradation of 5-HT44 and is the best-known molecular target implicated in the biology of ASB and aggression45,46. Surprisingly, our analyses revealed no significant differences in MAOA protein expression levels between samples from SUD-affected subjects and unaffected controls (F1,25 =0.71; P=0.405) or between SUD-only and ASB+SUD groups (Fig. 3A–B). Furthermore, regression analyses showed no significant associations between MAOA levels and either PMI (F1,25=0.78; R2=0.03, P=0.39) (Fig. 3C) or age (F1,25=0.89; R2=0.03, P=0.35) and MAOA levels (Fig.3D).
Fig. 3. Analysis of SERT expression levels in the OFC of antisocial individuals.
No significant alteration was reported between groups. A. Protein expression levels. B. Representative immunoblotting. C. Simple linear regression analysis representing the association of PMI with SERT levels (F1,25=12.04; R2=0.33, p=0.002) D. Simple linear regression analysis representing the association of age with SERT levels. ASB: Antisocial behavior; SUD: Substance use disorder; PMI: post-mortem interval
5-HT1A.
We then measured 5HT1A receptors, given that pharmacological activation of this receptor has been shown to reduce aggression in patients47–49 and animal50,51 (but also see Ref. 52 for contrasting results). No significant differences were found between samples from SUD-affected subjects and unaffected controls (F1,25 =0.33; P=0.57) or between SUD-only and ASB+SUD groups (Fig. 4A). Regression analyses of 5-HT1A receptors revealed no significant association of 5-HT1A levels with either PMI (F1,25=0.47; R2=0.02, P=0.49) (Fig. 4C) or age (F1,25=2.13; R2=0.08, P=0.16) (Fig. 4D).
Fig. 4. Analysis of 5HT1A receptor levels in the OFC of antisocial individuals.
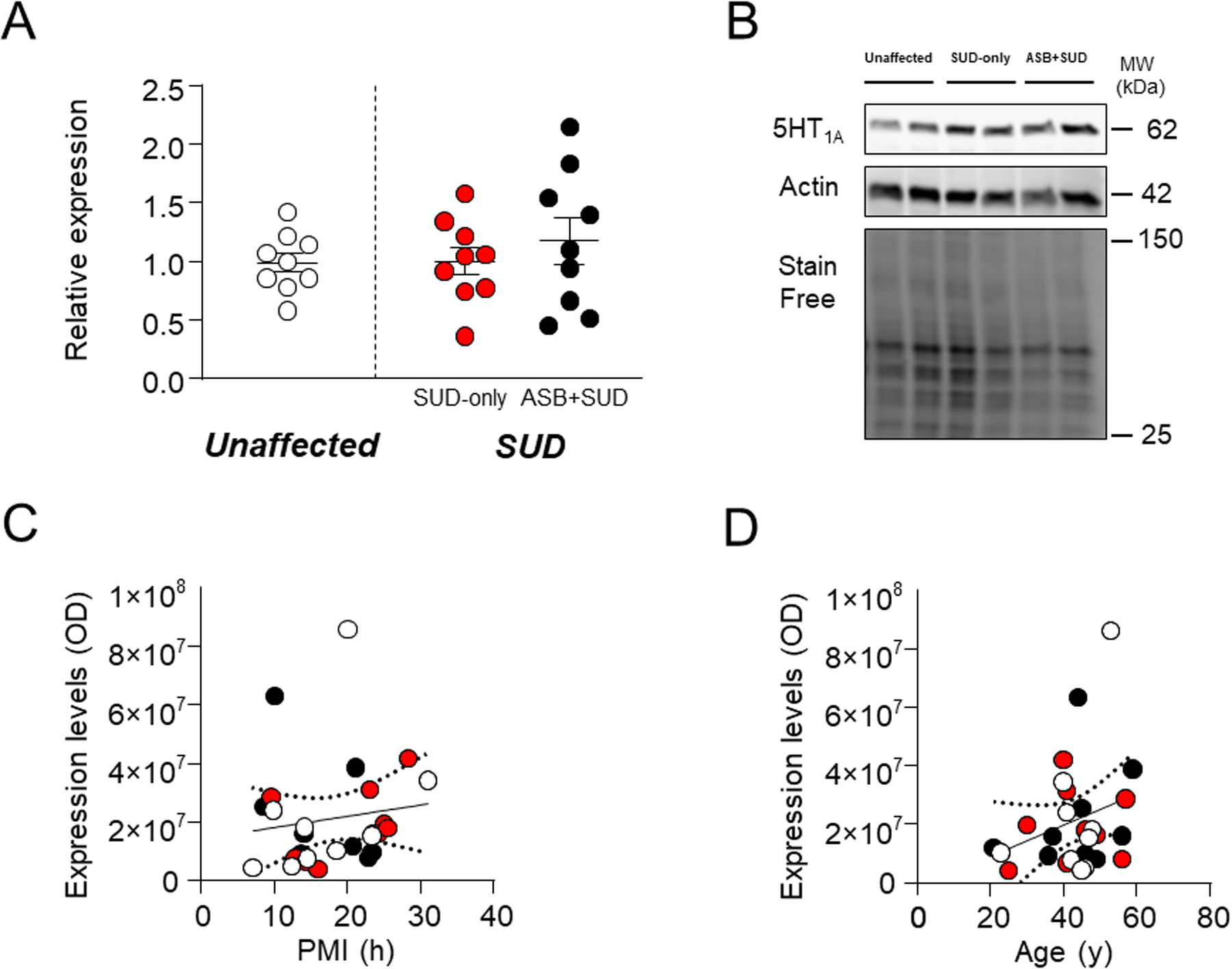
No significant change was reported between groups. A. Protein expression levels. B. Representative immunoblotting. C. Simple linear regression analysis representing the association of PMI with 5HT1A receptor levels D. Simple linear regression analysis representing the association of age with 5HT1A receptor levels. ASB: Antisocial behavior; SUD: Substance use disorder; PMI: post-mortem interval
5-HT1B.
Ample evidence points to a direct implication of 5-HT1B receptors in the neurobiology of aggression and ASB53–56. Similar to what observed for 5-HT1A receptors, no significant differences were found between samples from SUD-affected subjects and unaffected controls (F1,25 =0.008; P=0.93) or between SUD-only and ASB+SUD groups (Fig. 5A–B). 5-HT1B levels were not associated with either PMI (F1,25=0.68; R2=0.0265, P=0.42) (Fig. 5C) or age (F1,25=0.97; R2=0.0374, P=0.333) (Fig. 5D).
Fig. 5. Analysis of 5HT1B receptor levels in the OFC of antisocial individuals.
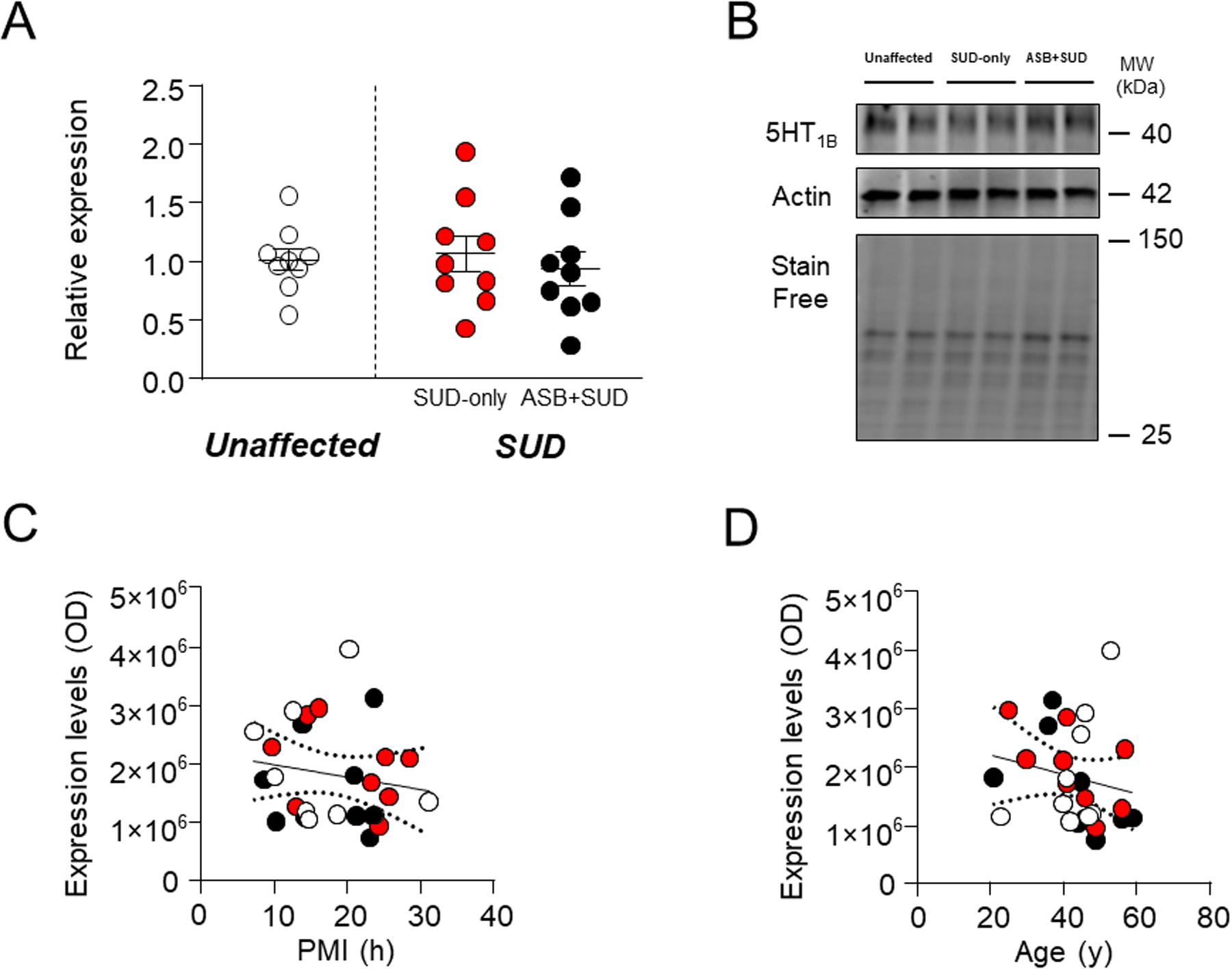
No significant change was reported between groups. A. Protein expression levels. B. Representative immunoblotting. C. Simple linear regression analysis representing the association of PMI with 5HT1B receptor levels D. Simple linear regression analysis representing the association of age with 5HT1B receptor levels. ASB: Antisocial behavior; SUD: Substance use disorder; PMI: post-mortem interval
5-HT2A.
Previous studies have shown that 5-HT2A receptors availability in OFC is increased in individuals with aggressive personality57. Consistently with these data, we found a significant elevation of 5-HT2A receptor in the ASB+SUD group compared with SUD-only controls (χ2 =8.43; P=0.004); in contrast, no significant differences between SUD-affected individuals and unaffected controls (F1,25 =0.006; P=0.95) were found (Fig.6A-B). No association was observed between 5-HT2A levels and either PMI (F1,25=0.34; R2=0.01, P=0.56) (Fig. 6C) or age (F1,25=0.76; R2=0.03, P=0.40; Fig.6D). These results suggest that enhanced 5-HT2A receptor expression in the OFC may be a marker of ASB.
Fig. 6. Analysis of 5HT2A receptor in the OFC of antisocial individuals.
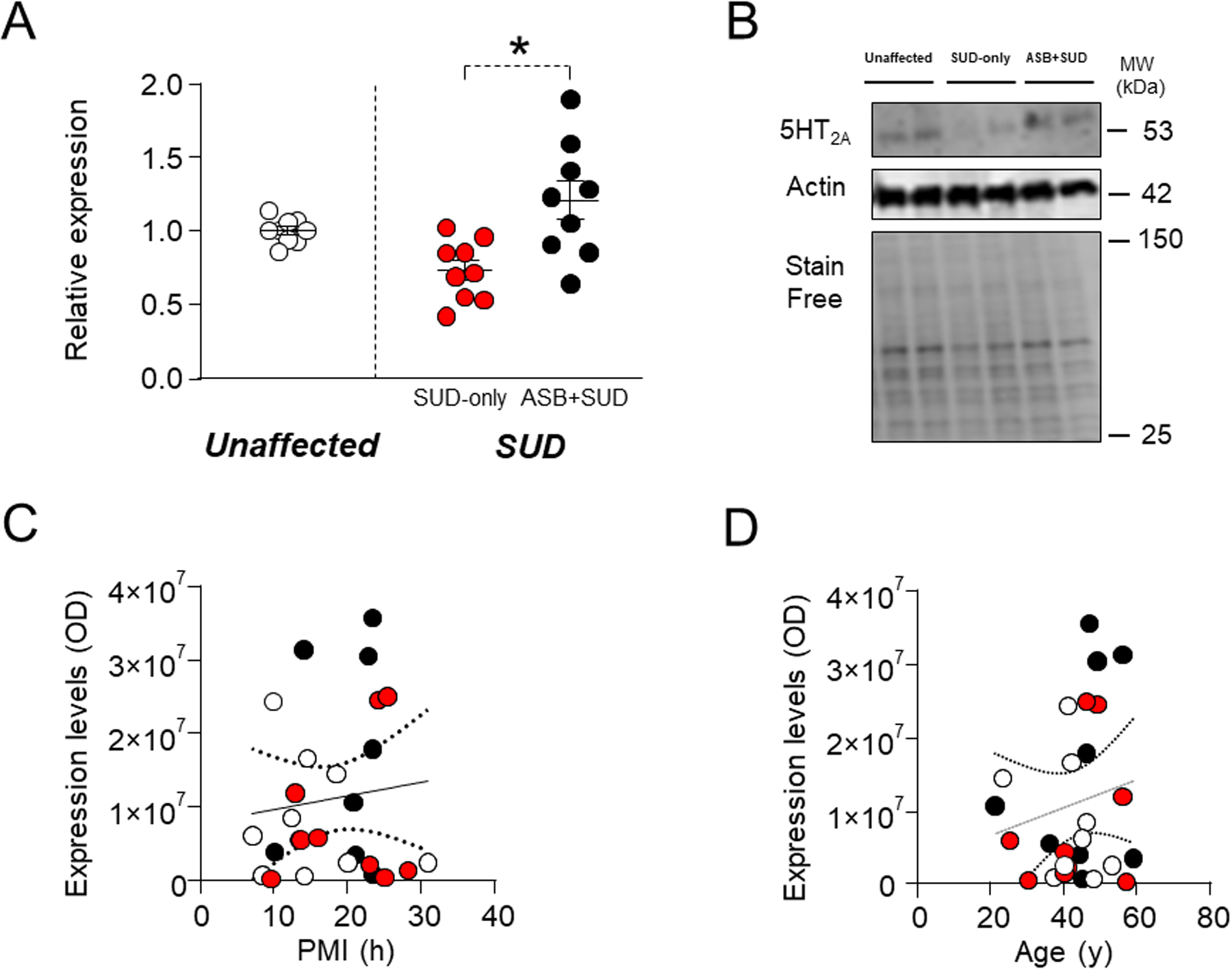
Individuals with ASPD or CD and a history of SUD exhibited a significant increase in 5HT2A levels in comparison with SUD-only subjects. A. Protein expression levels. B. Representative immunoblotting. C. Simple linear regression analysis representing the association of PMI with 5HT2A receptor levels. D. Simple linear regression analysis representing the association of age with 5HT2A receptor levels.
* P= 0.004 compared to SUD-only group (χ2 =8.43). ASB: Antisocial behavior; SUD: Substance use disorder; PMI: post-mortem interval
5-HT2C.
Selective 5-HT2C activation reduces impulsive aggression58, and its polymorphism rs3618 has been associated with criminal behavior59. These premises notwithstanding, no significant differences were found between samples from SUD-affected subjects and unaffected controls (F1,25 =1.01; P=0.32) or between SUD-only and ASB+SUD groups (Fig. 7A–B). Finally, 5-HT2C levels were not significantly associated to either PMI (F1,25=0.04; R2=0.001, P=0.85) (Fig. 7C) or age (F1,25=1.87; R2=0.07, P=0.18) (Fig. 7D).
Fig. 7. Analysis of 5HT2C receptor levels in the OFC of antisocial individuals.
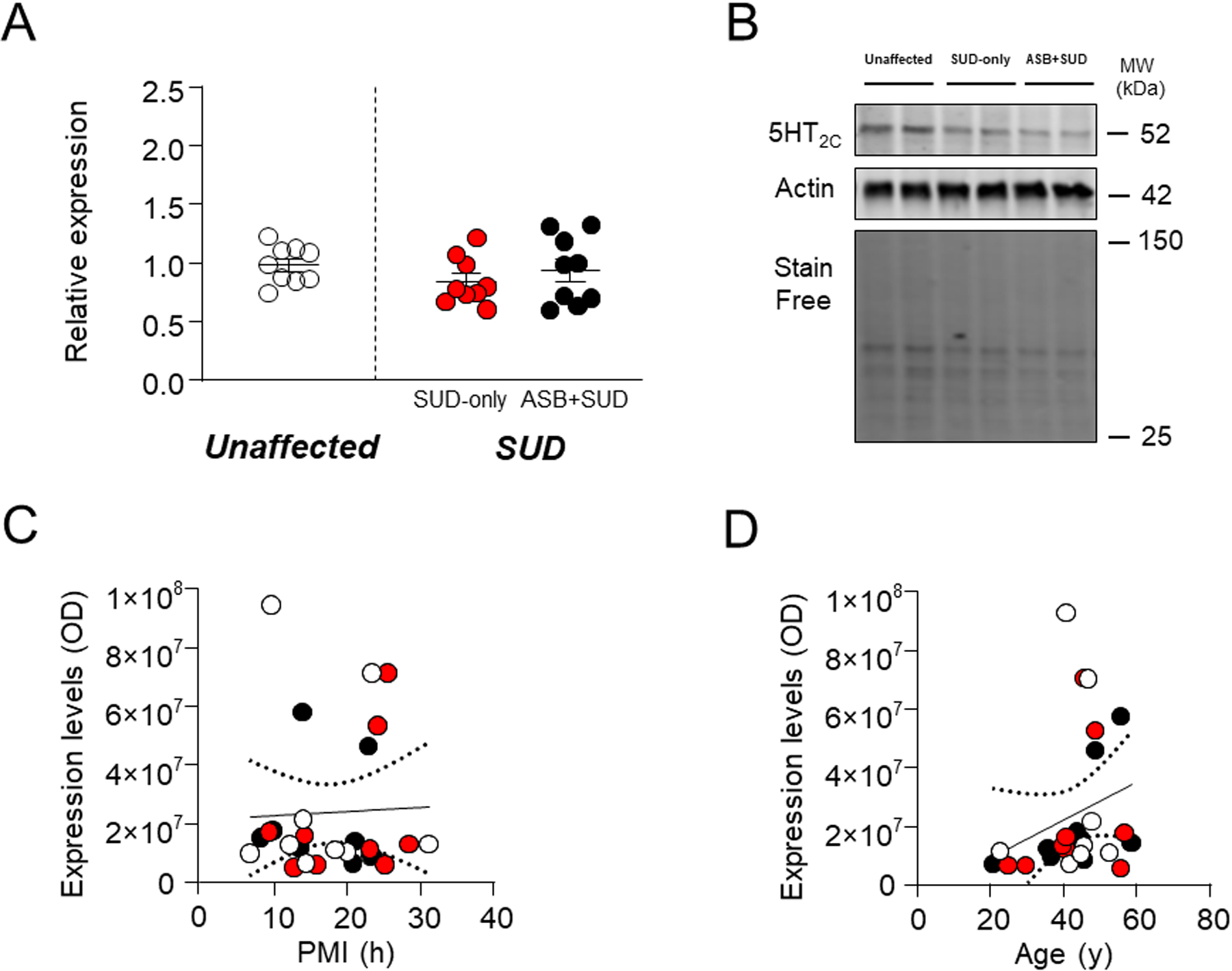
No significant change was reported between groups. A. Protein expression levels. B. Representative immunoblotting. C. Simple linear regression analysis representing the association of PMI with 5HT2C receptor levels D. Simple linear regression analysis representing the association of age with 5HT2C receptor levels. ASB: Antisocial behavior; SUD: Substance use disorder; PMI: post-mortem interval
5HT4.
Finally, we evaluated 5-HT4 expression levels, since neuroimaging studies reported an association between 5-HT4 receptor binding and trait aggression in the whole brain, in a sex- and age-dependent fashion60. However, no significant changes were found in 5-HT4 levels between SUD-affected and unaffected controls (F1,25 = 0.36; P=0.0002) and between the ASB + SUD and SUD-only groups (Fig. 8A–B). The regression analysis of 5-HT4 levels in relation to PMI (Fig. 8C) or age (Fig. 8D) revealed no statistically significant effects [PMI: F1,25=0.37; R2=0.01, P=0.54; age: F1,25=0.007; R2=0.0001, P=0.93].
Fig. 8. Analysis of 5HT4 receptor levels in the OFC of antisocial individuals.
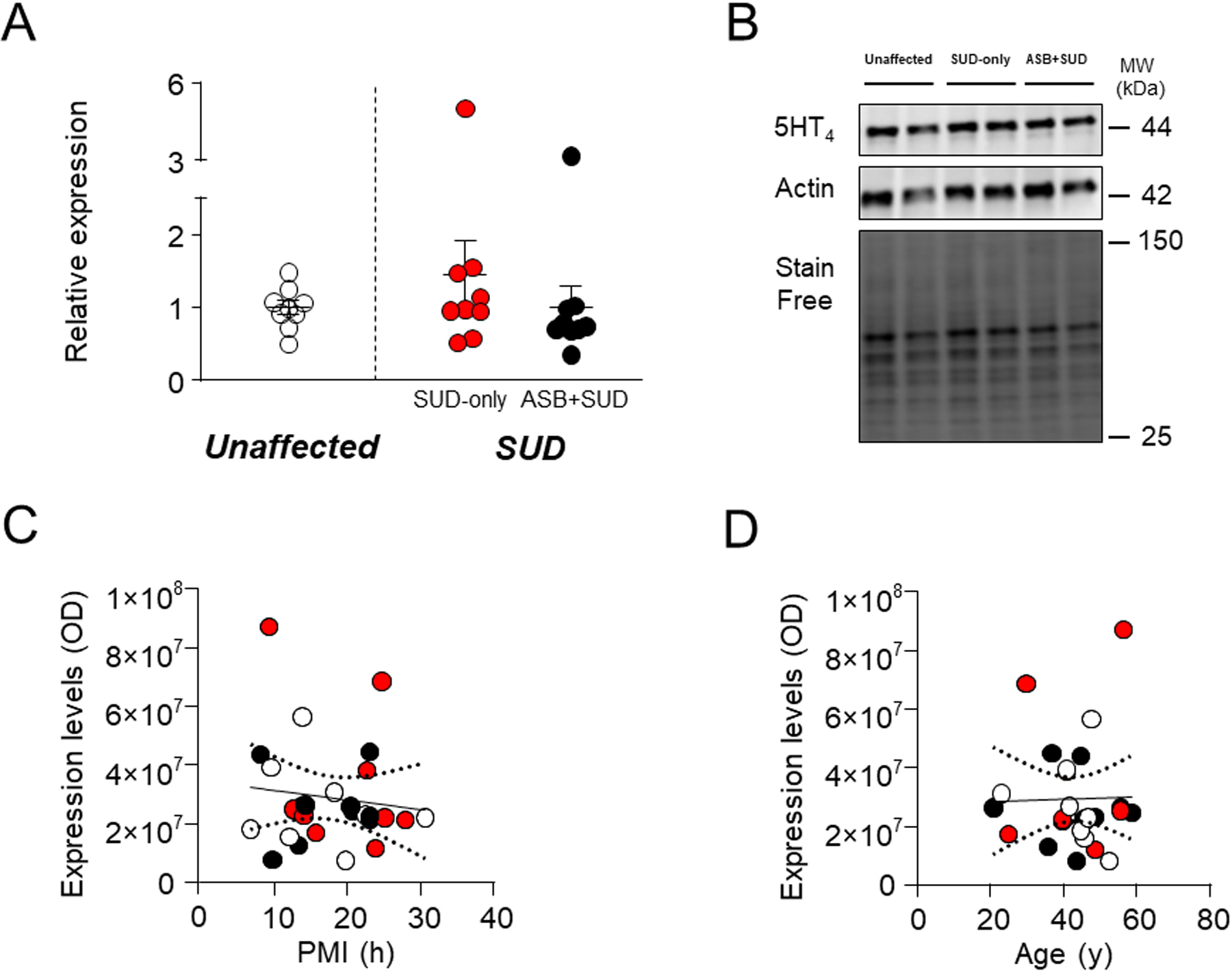
No significant change was reported between groups. A. Protein expression levels. B. Representative immunoblotting. C. Simple linear regression analysis representing the association of PMI with 5HT4 receptor levels D. Simple linear regression analysis representing the association of age with 5HT4 receptor levels. ASB: Antisocial behavior; SUD: Substance use disorder; PMI: post-mortem interval.
DISCUSSION
The main results of this study show that, in postmortem OFC samples of subjects with a diagnosis of SUD in combination with either CD or ASPD, the protein levels of 5-HT2A receptors were significantly elevated in comparison with age-matched controls with SUD only. Conversely, no differences were observed in the OFC expression of other serotonergic molecules, including the biosynthetic and catalytic enzymes TPH2 and MAOA, the reuptake carrier SERT, and key serotonin receptors implicated in the modulation of aggression.
These findings are in substantial evidence with previous neuroimaging data indicating high binding density of 5-HT2A receptors in the OFC of aggressive individuals57; furthermore, previous reports documented that the expression of these receptors was increased in the OFC of young suicide victims (who typically manifest high levels of aggression)61 and was correlated with lifetime aggression scores62. Finally, impulsive aggression levels were found to be correlated with 5-HT2A platelet binding63, even though this index is not representative of 5-HT2A binding in the cortex64. As mentioned above, aggression is a frequent pathognomonic characteristic of CD and ASPD, and, particularly in early life, it serves as a robust predictor of ASB65, generally suggesting that 5-HT2A receptor in the OFC may also be an important biomarker of antisociality. In partial support of this conclusion, genetic data have also shown associations between 5-HT2A genetic polymorphisms and aggression in the general population66–68 as well as in antisocial individuals69.
As previously mentioned, the OFC attaches emotional valence to social and environmental cues, and codes the motivational attributes of responses to these stimuli by regulating the activation of the amygdala70,71. For example, several studies have shown that the OFC integrates facial affective information of others21,72, and this role is likely due to an inhibitory control over amygdaloid activation73. From this perspective, it is worth noting that 5-HT2A receptors play a critical role in processing emotionally salient information74 by modulating the connectivity between OFC and amygdala. For example, inter-individual variations in 5-HT2A receptor density in the prefrontal cortex are inversely correlated with the activation of the amygdala by angry or fearful faces75, and the OFC response to these facial expressions is reduced by antagonism of these receptors76.
The function of 5-HT2A receptors in the neural processing of emotionally salient facial expression is in line with previous evidence on the role of serotonin in this function77,78. These data suggest that the upregulation of 5-HT2A receptors in the OFC may alter the connectivity of this region with the amygdala and impair the recognition of facial affect, thereby predisposing to aggression and ASB.
The results of our analyses did not reveal any other difference in the levels of any other serotonergic molecule with respect to the comparison between SUD + ASB and SUD-only subjects. These findings are surprising, given the strong implication of serotonin in the function of the OFC in the neural processing of emotional salient facial expressions77,78. Serotonin is also involved in the ontogeny of ASB79 and aggression33, 80–82. Particularly striking is the lack of differences in MAOA levels, considering the critical role of this enzyme in the ontogeny of ASB (for a comprehensive review, see Ref. 46). An ample body of evidence has particularly indicated that low activity of MAOA in the OFC and other brain region is a critical predictor of trait aggression83. In contrast with these results, our present findings documented no differences in enzyme expression between different antisocial individuals and their controls. Notably, the best-documented involvement of MAOA in ASB is related to the interaction of low-activity MAOA genotypes and child maltreatment84,85. We recently reproduced this gene x environment interaction in mice and found that this construct leads to increased aggression via an upregulation of 5-HT2A receptors in the OFC and other portions of the prefrontal cortex86. These data point to the possibility that, even though MAOA may be only one of the mechanisms underlying ASB, the upregulation of 5-HT2A receptors in the OFC is the key downstream mechanism whereby low MAOA predisposes to ASB.
We found a significant reduction in TPH2 levels in the OFC samples from SUD-affected individuals, in comparison with age-matched healthy controls. Given that TPH2 is the enzyme catalyzing the rate-limiting step of serotonin synthesis in the brain87,88, these data suggest that the OFC in SUD may exhibit low levels of serotonin. Previous findings showed that TPH2 genetic variants predict serotonin synthesis in human OFC, again pointing to the critical influence of this enzyme in serotonin synthesis in this region89. In correspondence of this idea, a reduction in serotonin synthesis has been shown to predispose to some components of impulsivity90,91, a key trait in SUD predisposition. In line with this concept, serotonin stimulation in the OFC increases the patience for a reward in mice92. These data complement recent reports on the critical role of serotonin in the modulation of the role of the OFC in decision-making related to drug seeking93,94. Nevertheless, it should be noted that our analysis was not specifically focused on one type of SUD but used this diagnostic category only as an internal control for ASB, given the high comorbidity of CD and ASPD with drug misuse. Furthermore, our study did not specifically address any of the heterogeneous constructs encompassed by SUD, such as abuse, addiction, or abstinence. Thus, extreme caution should be advocated on drawing conclusions from the present findings on the lower expression of TPH2 in SUD-affected individuals. Even so, these studies highlight the need for future analyses on TPH2 expression in the OFC of individuals affected by alcohol use disorder as well as other SUDs.
Our analyses on the potential influence of PMI and age on the expression of serotonin receptors pointed to a significant inverse association between this index and SERT levels, which was not paralleled by similar findings on any other serotonergic target. While our analyses showed that this effect did not interfere with the comparisons across different diagnostic groups, these results are interesting, as they appear to suggest a specific sensitivity of the serotonin carrier to post-mortem degradative processes, at least in the OFC. The degradation of SERT in the brain is a relatively fast process95 requiring PKC-dependent phosphorylation and internalization96,97. Thus, our findings may be in line with previous data showing that PMI has a major impact on the levels of proteins targeted by phosphorylation, as detected by western blotting98. Alternatively, the observed decrease in SERT levels may reflect a PMI-dependent degradation in serotonergic axon (given the presynaptic nature of SERT); however, this possibility is challenged by the fact that, in our dataset, only SERT, and not other (mainly) presynaptic targets were affected by PMI (such as 5- HT1B receptors). Furthermore, no correlation was found between PMI and the length of SERT-immunoreactive of the OFC99.
Several limitations should be acknowledged. First, our analysis only encompassed a limited number of samples, and our diagnostic criteria were not accompanied by any data on specific psychological domains related to ASB, such as aggression, psychopathy, etc. Second, while we studied total protein levels, it is possible that changes in receptor and enzyme concentrations may not correspond to changes in binding and signaling. Third, due to the specific predominance of ASB in males and in SUD-affected subjects, we could not verify the specific impact of these targets in antisocial women and/or without a comorbid SUD diagnosis. Even with these important limitations, this is the first study performed on postmortem brain samples of individuals with ASB. As mentioned above, these data are strikingly aligned with previous evidence from our group on the upregulation of 5-HT2A in the OFC and other areas of the prefrontal cortex of mouse models reproducing gene x environment interactions in ASB86. Furthermore, we and others have documented that 5-HT2A receptor blockade prevents and blocks aggressive behaviors in several rodent models of aggression86, 100–103. Taken together, our data and this background strongly suggest that the upregulation of 5-HT2A receptors in the OFC may be a biomarker of antisociality and that selective 5-HT2A receptor antagonists approved for clinical use - such as the recently approved drug pimavanserin - may have therapeutic potential in the treatment of CD and ASPD. Future studies are warranted to fully evaluate these intriguing possibilities.
Key points:
We performed the first-ever postmortem analysis of serotonergic brain markers in antisocial individuals
Comparisons were drawn with age-matched subjects with similar substance use disorder diagnoses
The orbitofrontal cortex of antisocial individuals exhibits increased 5-HT2A receptor level
ACKNOWLEDGMENTS
This work was partially supported by National Institute of Mental Health (NIH R01 MH104603) and by a Skaggs Foundation Grant (to M.B.). Post-mortem human samples were obtained through the NIH NeuroBioBank from the University of Pittsburgh Brain Tissue Repository. The authors would like to thank Jill Glausier, Karen Odeh, and Roberto Cadeddu for their help in obtaining the brain samples and editorial suggestions.
Footnotes
CONFLICT OF INTEREST
The authors report no conflict of interest.
ETHICS STATEMENT
Tissues were obtained from the Brain Tissue Repository at the University of Pittsburgh, through the NIH NeuroBioBank program. All brain tissues were procured, stored, and distributed according to state and federal guidelines, and regulation involving consent, protection of human subjects, and donor anonymity.
REFERENCES
- 1.Vitiello B, Stoff DM. Subtypes of aggression and their relevance to child psychiatry. J Am Acad Child Adolesc Psychiatry. 1997;36(3):307–315. doi: 10.1097/00004583-199703000-00008 [DOI] [PubMed] [Google Scholar]
- 2.Nock MK, Kazdin A, Hiripi E, Kessler RC. Prevalence, subtypes, and correlates of DSM-IV conduct disorder in the National Comorbidity Survey Replication. Psychol Med. 2006; 36(5):699–710. doi: 10.1017/S0033291706007082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lenzenweger MF, Lane MC, Loranger AW, Kessler RC. DSM-IV Personality Disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;62(6):553–564. doi: 10.1016/j.biopsych.2006.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trull TJ, Jahng S, Tomko RL, Wood PK, Sher KJ. Revised NESARC personality disorder diagnoses: gender, prevalence, and comorbidity with substance dependence disorders. J Pers Disord. 2010; 24(4): 412–426. doi: 10.1521/pedi.2010.24.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grant BF, Hasin DS, Stinson FS, et al. Prevalence, correlates, and disability of personality disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. J Clin Psychiatry. 2004;65(7):948–958. doi: 10.4088/JCP.V65N0711 [DOI] [PubMed] [Google Scholar]
- 6.Maughan B, Rowe R, Messer J, Goodman R, Meltzer H. Conduct disorder and oppositional defiant disorder in a national sample: developmental epidemiology. J Child Psychol Psychiatry. 2004;45(3):609–621. doi: 10.1111/J.1469-7610.2004.00250.X [DOI] [PubMed] [Google Scholar]
- 7.Hamdi NR, Iacono WG. Lifetime prevalence and co-morbidity of externalizing disorders and depression in prospective assessment. Psychol Med. 2014;44(2):315–324. doi: 10.1017/S0033291713000627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nouvion SO, Cherek DR, Lane SD, Tcheremissine OV, Lieving LM. Human proactive aggression: association with personality disorders and psychopathy. Aggress Behav. 2007;33(6):552–562. doi: 10.1002/AB.20220 [DOI] [PubMed] [Google Scholar]
- 9.Lobbestael J, Cima M, Arntz A. The relationship between adult reactive and proactive aggression, hostile interpretation bias, and antisocial personality disorder. J Pers Disord. 2013;27(1):53–66. doi: 10.1521/PEDI.2013.27.1.53 [DOI] [PubMed] [Google Scholar]
- 10.Connor DF, Ford JD, Albert DB, Doerfler LA. Conduct disorder subtype and comorbidity. Ann Clin Psychiatry. 2007;19(3):161–168. doi: 10.1080/10401230701465269 [DOI] [PubMed] [Google Scholar]
- 11.Black DW. The Natural History of Antisocial Personality Disorder. Can J Psychiatry. 2015;60(7):309–314. doi: 10.1177/070674371506000703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nichita EC, Buckley PF. Comorbidities of Antisocial Personality Disorder. The Wiley International Handbook on Psychopathic Disorders and the Law. Published online August 17, 2020:645–670. doi: 10.1002/9781119159322.CH28 [DOI] [Google Scholar]
- 13.Reebye P, Moretti MM, Lessard JC. Conduct disorder and substance use disorder: comorbidity in a clinical sample of preadolescents and adolescents. Can J Psychiatry. 1995;40(6):313–319. doi: 10.1177/070674379504000606 [DOI] [PubMed] [Google Scholar]
- 14.Messina NP, Wish ED, Nemes S. Therapeutic community treatment for substance abusers with antisocial personality disorder. J Subst Abuse Treat. 1999;17(1–2):121–128. doi: 10.1016/S0740-5472(98)00066-X [DOI] [PubMed] [Google Scholar]
- 15.Robins LN, Regier DA. Psychiatric Disorders in America : The Epidemiologic Catchment Area Study. Free Press ;;Collier Macmillan Canada ;;Maxwell Macmillan International; 1991. [Google Scholar]
- 16.Coid J, Yang M, Roberts A, et al. Violence and psychiatric morbidity in a national household population--a report from the British Household Survey. Am J Epidemiol. 2006;164(12):1199–1208. doi: 10.1093/AJE/KWJ339 [DOI] [PubMed] [Google Scholar]
- 17.Marsh AA, Blair RJR. Deficits in facial affect recognition among antisocial populations: a meta-analysis. Neurosci Biobehav Rev. 2008;32(3):454–465. doi: 10.1016/J.NEUBIOREV.2007.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blair RJR. Facial expressions, their communicatory functions and neuro-cognitive substrates. Philos Trans R Soc Lond B Biol Sci. 2003;358(1431):561–572. doi: 10.1098/RSTB.2002.1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montagne B, Kessels RPC, Frigerio E, de Haan EHF, Perrett DI. Sex differences in the perception of affective facial expressions: do men really lack emotional sensitivity? Cogn Process. 2005;6(2):136–141. doi: 10.1007/S10339-005-0050-6 [DOI] [PubMed] [Google Scholar]
- 20.Walker DW, Leister C. Recognition of Facial Affect Cues by Adolescents with Emotional and Behavioral Disorders. Behav Disorders. 1994; 19(4):269–276. doi: 10.1177/019874299401900408 [DOI] [Google Scholar]
- 21.Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex. 2000;10(3):295–307. doi: 10.1093/CERCOR/10.3.295 [DOI] [PubMed] [Google Scholar]
- 22.Blair RJR, Cipolotti L. Impaired social response reversal. A case of “acquired sociopathy.” Brain. 2000;123 (Pt 6)(6):1122–1141. doi: 10.1093/BRAIN/123.6.1122 [DOI] [PubMed] [Google Scholar]
- 23.Blair RJR. The roles of orbital frontal cortex in the modulation of antisocial behavior. Brain Cogn. 2004;55(1):198–208. doi: 10.1016/S0278-2626(03)00276-8 [DOI] [PubMed] [Google Scholar]
- 24.Lavarco A, Ahmad N, Archer Q, et al. Self-Conscious Emotions and the Right Fronto-Temporal and Right Temporal Parietal Junction. Brain Sci. 2022;12(2). doi: 10.3390/BRAINSCI12020138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zald DH. Orbitofrontal cortex contributions to food selection and decision making. Ann Behav Med. 2009;38 Suppl 1(SUPPL.). doi: 10.1007/S12160-009-9117-4 [DOI] [PubMed] [Google Scholar]
- 26.Carlisi CO, Moffitt TE, Knodt AR, et al. Associations between life-course-persistent antisocial behaviour and brain structure in a population-representative longitudinal birth cohort. Lancet Psychiatry. 2020;7(3):245–253. doi: 10.1016/S2215-0366(20)30002-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P. Reduced prefrontal gray matter volume and reduced autonomic activity in antisocial personality disorder. Arch Gen Psychiatry. 2000;57(2):119–127. doi: 10.1001/ARCHPSYC.57.2.119 [DOI] [PubMed] [Google Scholar]
- 28.Gansler DA, McLaughlin NCR, Iguchi L, et al. A multivariate approach to aggression and the orbital frontal cortex in psychiatric patients. Psychiatry Res. 2009;171(3):145–154. doi: 10.1016/J.PSCYCHRESNS.2008.03.007 [DOI] [PubMed] [Google Scholar]
- 29.Hofhansel L, Weidler C, Votinov M, Clemens B, Raine A, Habel U. Morphology of the criminal brain: gray matter reductions are linked to antisocial behavior in offenders. Brain Struct Funct. 2020;225(7):2017–2028. doi: 10.1007/S00429-020-02106-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ranganath C, Johnson MK, D’Esposito M. Prefrontal activity associated with working memory and episodic long-term memory. Neuropsychologia. 2003;41(3):378–89. doi: 10.1016/s0028-3932(02)00169-0. [DOI] [PubMed] [Google Scholar]
- 31.Rygula R, Clarke HF, Cardinal RN, et al. Role of Central Serotonin in Anticipation of Rewarding and Punishing Outcomes: Effects of Selective Amygdala or Orbitofrontal 5-HT Depletion. Cereb Cortex. 2015;25(9):3064–3076. doi: 10.1093/CERCOR/BHU102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Åsberg M Neurotransmitters and suicidal behavior. The evidence from cerebrospinal fluid studies. Ann N Y Acad Sci. 1997;836:158–181. doi: 10.1111/J.1749-6632.1997.TB52359.X [DOI] [PubMed] [Google Scholar]
- 33.Bortolato M, Pivac N, Muck Seler D, Nikolac Perkovic M, Pessia M, di Giovanni G. The role of the serotonergic system at the interface of aggression and suicide. Neuroscience. 2013;236:160–185. doi: 10.1016/J.NEUROSCIENCE.2013.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gürtler A, Kunz N, Gomolka M, et al. Stain-Free technology as a normalization tool in Western blot analysis. Anal Biochem. 2013;433(2):105–111. doi: 10.1016/J.AB.2012.10.010 [DOI] [PubMed] [Google Scholar]
- 35.Rivero-Gutiérrez B, Anzola A, Martínez-Augustin O, de Medina FS. Stain-free detection as loading control alternative to Ponceau and housekeeping protein immunodetection in Western blotting. Anal Biochem. 2014;467:1–3. doi: 10.1016/J.AB.2014.08.027 [DOI] [PubMed] [Google Scholar]
- 36.Yang Y, Raine A. Prefrontal Structural and Functional Brain Imaging findings in Antisocial, Violent, and Psychopathic Individuals: A Meta-Analysis. Psychiatry Res. 2009;174(2):81. doi: 10.1016/J.PSCYCHRESNS.2009.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rogers JC, de Brito SA. Cortical and Subcortical Gray Matter Volume in Youths With Conduct Problems: A Meta-analysis. JAMA Psychiatry. 2016;73(1):64–72. doi: 10.1001/JAMAPSYCHIATRY.2015.2423 [DOI] [PubMed] [Google Scholar]
- 38.Waider J, Araragi N, Gutknecht L, Lesch KP. Tryptophan hydroxylase-2 (TPH2) in disorders of cognitive control and emotion regulation: a perspective. Psychoneuroendocrinology. 2011;36(3):393–405. doi: 10.1016/J.PSYNEUEN.2010.12.012 [DOI] [PubMed] [Google Scholar]
- 39.Gutknecht L, Popp S, Waider J, et al. Interaction of brain 5-HT synthesis deficiency, chronic stress and sex differentially impact emotional behavior in Tph2 knockout mice. Psychopharmacology (Berl). 2015;232(14):2429. doi: 10.1007/S00213-015-3879-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haberstick BC, Smolen A, Hewitt JK. Family-based association test of the 5HTTLPR and aggressive behavior in a general population sample of children. Biol Psychiatry. 2006;59(9):836–843. doi: 10.1016/J.BIOPSYCH.2005.10.008 [DOI] [PubMed] [Google Scholar]
- 41.Beitchman JH, Baldassarra L, Mik H, et al. Serotonin transporter polymorphisms and persistent, pervasive childhood aggression. Am J Psychiatry. 2006;163(6):1103–1105. doi: 10.1176/AJP.2006.163.6.1103 [DOI] [PubMed] [Google Scholar]
- 42.Oades RD, Lasky-Su J, Christiansen H, et al. The influence of serotonin- and other genes on impulsive behavioral aggression and cognitive impulsivity in children with attention-deficit/hyperactivity disorder (ADHD): Findings from a family-based association test (FBAT) analysis. Behav Brain Funct. 2008;4. doi: 10.1186/1744-9081-4-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Payer DE, Nurmi EL, Wilson SA, McCracken JT, London ED. Effects of methamphetamine abuse and serotonin transporter gene variants on aggression and emotion-processing neurocircuitry. Transl Psychiatry. 2012;2(2). doi: 10.1038/TP.2011.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bortolato M, Chen K, Shih JC. Monoamine oxidase inactivation: from pathophysiology to therapeutics. Adv Drug Deliv Rev. 2008;60(13–14):1527–1533. doi: 10.1016/J.ADDR.2008.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang-James Y, Fernàndez-Castillo N, Hess JL, et al. An integrated analysis of genes and functional pathways for aggression in human and rodent models. Mol Psychiatry. 2019;24(11):1655–1667. doi: 10.1038/S41380-018-0068-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kolla NJ, Bortolato M. The role of monoamine oxidase A in the neurobiology of aggressive, antisocial, and violent behavior: A tale of mice and men. Prog Neurobiol. 2020;194. doi: 10.1016/J.PNEUROBIO.2020.101875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ratey J, Sovner R, Parks A, Rogentine K. Buspirone treatment of aggression and anxiety in mentally retarded patients: a multiple-baseline, placebo lead-in study. J Clin Psychiatry. 1991;52(4):159–162. Accessed April 4, 2022. https://pubmed.ncbi.nlm.nih.gov/2016248/ [PubMed] [Google Scholar]
- 48.Connor DF, Steingard RJ. A clinical approach to the pharmacotherapy of aggression in children and adolescents. Ann N Y Acad Sci. 1996;794:290–307. doi: 10.1111/J.1749-6632.1996.TB32529.X [DOI] [PubMed] [Google Scholar]
- 49.Pabis DJ, Stanislav SW. Pharmacotherapy of aggressive behavior. Ann Pharmacother. 1996;30(3):278–287. doi: 10.1177/106002809603000312 [DOI] [PubMed] [Google Scholar]
- 50.de Boer SF, Koolhaas JM. Defensive burying in rodents: ethology, neurobiology and psychopharmacology. European Journal of Pharmacology. 2003;463(1–3):145–161. doi: 10.1016/S0014-2999(03)01278-0 [DOI] [PubMed] [Google Scholar]
- 51.Miczek KA, Hussain S, Faccidomo S. Alcohol-heightened aggression in mice: attenuation by 5-HT1A receptor agonists. Psychopharmacology (Berl). 1998;139(1–2):160–168. doi: 10.1007/S002130050701 [DOI] [PubMed] [Google Scholar]
- 52.de Almeida RMM, Lucion AB. 8-OH-DPAT in the median raphe, dorsal periaqueductal gray and corticomedial amygdala nucleus decreases, but in the medial septal area it can increase maternal aggressive behavior in rats. Psychopharmacology (Berl). 1997;134(4):392–400. doi: 10.1007/S002130050476 [DOI] [PubMed] [Google Scholar]
- 53.Saudou F, Amara DA, Dierich A, et al. Enhanced aggressive behavior in mice lacking 5-HT1B receptor. Science. 1994;265(5180):1875–1878. doi: 10.1126/SCIENCE.8091214 [DOI] [PubMed] [Google Scholar]
- 54.Lappalainen J, Long JC, Eggert M, et al. Linkage of antisocial alcoholism to the serotonin 5-HT1B receptor gene in 2 populations. Arch Gen Psychiatry. 1998;55(11):989–994. doi: 10.1001/ARCHPSYC.55.11.989 [DOI] [PubMed] [Google Scholar]
- 55.Soyka M, Preuss UW, Koller G, Zill P, Bondy B. Association of 5-HT1B receptor gene and antisocial behavior in alcoholism. J Neural Transm (Vienna). 2004;111(1):101–109. doi: 10.1007/S00702-003-0064-0 [DOI] [PubMed] [Google Scholar]
- 56.Zouk H, McGirr A, Lebel V, Benkelfat C, Rouleau G, Turecki G. The effect of genetic variation of the serotonin 1B receptor gene on impulsive aggressive behavior and suicide. Am J Med Genet B Neuropsychiatr Genet. 2007;144B(8):996–1002. doi: 10.1002/AJMG.B.30521 [DOI] [PubMed] [Google Scholar]
- 57.Rosell DR, Thompson JL, Slifstein M, et al. Increased serotonin 2A receptor availability in the orbitofrontal cortex of physically aggressive personality disordered patients. Biol Psychiatry. 2010;67(12):1154–1162. doi: 10.1016/J.BIOPSYCH.2010.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Coccaro EF, Lee RJ. 5-HT 2c agonist, lorcaserin, reduces aggressive responding in intermittent explosive disorder: A pilot study. Hum Psychopharmacol. 2019;34(6). doi: 10.1002/HUP.2714 [DOI] [PubMed] [Google Scholar]
- 59.Toshchakova VA, Bakhtiari Y, Kulikov A v., et al. Association of Polymorphisms of Serotonin Transporter (5HTTLPR) and 5-HT2C Receptor Genes with Criminal Behavior in Russian Criminal Offenders. Neuropsychobiology. 2018;75(4):200. doi: 10.1159/000487484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.da Cunha-Bang S, McMahon B, MacDonald Fisher P, Steen Jensen P, Svarer C, Knudsen GM. High trait aggression in men is associated with low 5-HT levels, as indexed by 5-HT4 receptor binding. Social Cognitive and Affective Neuroscience. 2016;11(4):548. doi: 10.1093/SCAN/NSV140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pandey GN, Dwivedi Y, Rizavi HS, et al. Higher expression of serotonin 5-HT(2A) receptors in the postmortem brains of teenage suicide victims. Am J Psychiatry. 2002;159(3):419–429. doi: 10.1176/APPI.AJP.159.3.419 [DOI] [PubMed] [Google Scholar]
- 62.Oquendo MA, Russo SA, Underwood MD, et al. Higher postmortem prefrontal 5-HT2A receptor binding correlates with lifetime aggression in suicide. Biol Psychiatry. 2006;59(3):235–243. doi: 10.1016/J.BIOPSYCH.2005.06.037 [DOI] [PubMed] [Google Scholar]
- 63.Coccaro EF, Kavoussi RJ, Sheline YI, Berman ME, Csernansky JG. Impulsive aggression in personality disorder correlates with platelet 5-HT2A receptor binding. Neuropsychopharmacology. 1997;16(3):211–216. doi: 10.1016/S0893-133X(96)00194-7 [DOI] [PubMed] [Google Scholar]
- 64.Yatham LN, Liddle PF, Shiah IS, Lam RW, Zis AP. A PET study of brain 5-HT2 receptors and their correlation with platelet 5-HT2 receptors in healthy humans. Psychopharmacology (Berl). 2000;151(4):424–427. doi: 10.1007/S002130000522 [DOI] [PubMed] [Google Scholar]
- 65.Whipp AM, Korhonen T, Raevuori A, et al. Early adolescent aggression predicts antisocial personality disorder in young adults: a population-based study. Eur Child Adolesc Psychiatry. 2019;28(3):341–350. doi: 10.1007/S00787-018-1198-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Giegling I, Hartmann AM, Möller HJ, Rujescu D. Anger- and aggression-related traits are associated with polymorphisms in the 5-HT-2A gene. J Affect Disord. 2006;96(1–2):75–81. doi: 10.1016/J.JAD.2006.05.016 [DOI] [PubMed] [Google Scholar]
- 67.Banlaki Z, Elek Z, Nanasi T, et al. Polymorphism in the serotonin receptor 2a (HTR2A) gene as possible predisposal factor for aggressive traits. PLoS One. 2015;10(2). doi: 10.1371/JOURNAL.PONE.0117792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Butovskaya ML, Butovskaya PR, Vasilyev VA, et al. Serotonergic gene polymorphisms (5-HTTLPR, 5HTR1A, 5HTR2A), and population differences in aggression: traditional (Hadza and Datoga) and industrial (Russians) populations compared. J Physiol Anthropol. 2018;37(1). doi: 10.1186/S40101-018-0171-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nedic Erjavec G, Tudor L, Nikolac Perkovic M, et al. Serotonin 5-HT2A receptor polymorphisms are associated with irritability and aggression in conduct disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2022;117:110542. doi: 10.1016/J.PNPBP.2022.110542 [DOI] [PubMed] [Google Scholar]
- 70.Barbas H, de Olmos J. Projections from the amygdala to basoventral and mediodorsal prefrontal regions in the rhesus monkey. J Comp Neurol. 1990;300(4):549–571. doi: 10.1002/CNE.903000409 [DOI] [PubMed] [Google Scholar]
- 71.Morecraft RJ, Geula C, Mesulam M ‐ M. Cytoarchitecture and neural afferents of orbitofrontal cortex in the brain of the monkey. J Comp Neurol. 1992;323(3):341–358. doi: 10.1002/CNE.903230304 [DOI] [PubMed] [Google Scholar]
- 72.Salzman CD, Fusi S. Emotion, cognition, and mental state representation in amygdala and prefrontal cortex. Annu Rev Neurosci. 2010;33:173–202. doi: 10.1146/ANNUREV.NEURO.051508.135256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stein JL, Wiedholz LM, Bassett DS, et al. A validated network of effective amygdala connectivity. Neuroimage. 2007;36(3):736–745. doi: 10.1016/J.NEUROIMAGE.2007.03.022 [DOI] [PubMed] [Google Scholar]
- 74.Frokjaer VG, Mortensen EL, Nielsen FÅ, et al. Frontolimbic serotonin 2A receptor binding in healthy subjects is associated with personality risk factors for affective disorder. Biol Psychiatry. 2008;63(6):569–576. doi: 10.1016/J.BIOPSYCH.2007.07.009 [DOI] [PubMed] [Google Scholar]
- 75.Fisher PM, Meltzer CC, Price JC, et al. Medial prefrontal cortex 5-HT(2A) density is correlated with amygdala reactivity, response habituation, and functional coupling. Cereb Cortex. 2009;19(11):2499–2507. doi: 10.1093/CERCOR/BHP022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hornboll B, MacOveanu J, Rowe J, et al. Acute serotonin 2A receptor blocking alters the processing of fearful faces in the orbitofrontal cortex and amygdala. J Psychopharmacol. 2013;27(10):903–914. doi: 10.1177/0269881113494106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Norbury R, Taylor MJ, Selvaraj S, Murphy SE, Harmer CJ, Cowen PJ. Short-term antidepressant treatment modulates amygdala response to happy faces. Psychopharmacology (Berl). 2009;206(2):197–204. doi: 10.1007/S00213-009-1597-1 [DOI] [PubMed] [Google Scholar]
- 78.Passamonti L, Crockett MJ, Apergis-Schoute AM, et al. Effects of acute tryptophan depletion on prefrontal-amygdala connectivity while viewing facial signals of aggression. Biol Psychiatry. 2012;71(1):36–43. doi: 10.1016/J.BIOPSYCH.2011.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yildirim BO, Derksen JJL. Systematic review, structural analysis, and new theoretical perspectives on the role of serotonin and associated genes in the etiology of psychopathy and sociopathy. Neurosci Biobehav Rev. 2013;37(7):1254–1296. doi: 10.1016/J.NEUBIOREV.2013.04.009 [DOI] [PubMed] [Google Scholar]
- 80.Krakowski M Violence and serotonin: influence of impulse control, affect regulation, and social functioning. J Neuropsychiatry Clin Neurosci. 2003;15(3):294–305. doi: 10.1176/JNP.15.3.294 [DOI] [PubMed] [Google Scholar]
- 81.Coccaro EF, Fanning JR, Phan KL, Lee R. Serotonin and impulsive aggression. CNS Spectr. 2015;20(3):295–302. doi: 10.1017/S1092852915000310 [DOI] [PubMed] [Google Scholar]
- 82.da Cunha-Bang S, Knudsen GM. The Modulatory Role of Serotonin on Human Impulsive Aggression. Biol Psychiatry. 2021;90(7):447–457. doi: 10.1016/J.BIOPSYCH.2021.05.016 [DOI] [PubMed] [Google Scholar]
- 83.Alia-Klein N, Goldstein RZ, Kriplani A, et al. Brain monoamine oxidase A activity predicts trait aggression. J Neurosci. 2008;28(19):5099–5104. doi: 10.1523/JNEUROSCI.0925-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Caspi A, McCray J, Moffitt TE, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297(5582):851–854. doi: 10.1126/SCIENCE.1072290 [DOI] [PubMed] [Google Scholar]
- 85.Byrd AL, Manuck SB. MAOA, childhood maltreatment, and antisocial behavior: meta-analysis of a gene-environment interaction. Biol Psychiatry. 2014;75(1):9–17. doi: 10.1016/J.BIOPSYCH.2013.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Godar SC, Mosher LJ, Scheggi S, et al. Gene-environment interactions in antisocial behavior are mediated by early-life 5-HT 2A receptor activation. Neuropharmacology. 2019;159. doi: 10.1016/J.NEUROPHARM.2019.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Walther DJ, Peter JU, Bashammakh S, et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299(5603):76. doi: 10.1126/SCIENCE.1078197 [DOI] [PubMed] [Google Scholar]
- 88.Zhang X, Beaulieu JM, Sotnikova TD, Gainetdinov RR, Caron MG. Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science. 2004;305(5681):217. doi: 10.1126/SCIENCE.1097540 [DOI] [PubMed] [Google Scholar]
- 89.Booij L, Turecki G, Leyton M, et al. Tryptophan hydroxylase(2) gene polymorphisms predict brain serotonin synthesis in the orbitofrontal cortex in humans. Mol Psychiatry. 2012;17(8):809–817. doi: 10.1038/MP.2011.79 [DOI] [PubMed] [Google Scholar]
- 90.Cools R, Blackwell A, Clark L, Menzies L, Cox S, Robbins TW. Tryptophan depletion disrupts the motivational guidance of goal-directed behavior as a function of trait impulsivity. Neuropsychopharmacology. 2005;30(7):1362–1373. doi: 10.1038/SJ.NPP.1300704 [DOI] [PubMed] [Google Scholar]
- 91.Dougherty DM, Richard DM, James LM, Mathias CW. Effects of acute tryptophan depletion on three different types of behavioral impulsivity. Int J Tryptophan Res. 2010;3(1):99–111. doi: 10.4137/IJTR.S4317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Miyazaki K, Miyazaki KW, Sivori G, Yamanaka A, Tanaka KF, Doya K. Serotonergic projections to the orbitofrontal and medial prefrontal cortices differentially modulate waiting for future rewards. Sci Adv. 2020;6(48). doi: 10.1126/SCIADV.ABC7246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wright AM, Zapata A, Baumann MH, Elmore JS, Hoffman AF, Lupica CR. Enduring Loss of Serotonergic Control of Orbitofrontal Cortex Function Following Contingent and Noncontingent Cocaine Exposure. Cereb Cortex. 2017;27(12):5463–5476. doi: 10.1093/CERCOR/BHW312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li A, Jing D, Dellarco D v., et al. Role of BDNF in the development of an OFC-amygdala circuit regulating sociability in mouse and human. Mol Psychiatry. 2021;26(3):955–973. doi: 10.1038/S41380-019-0422-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vicentic A, Battaglia G, Ivy Carroll F, Kuhar MJ. Serotonin transporter production and degradation rates: studies with RTI-76. Brain Res. 1999;841(1–2):1–10. doi: 10.1016/S0006-8993(99)01761-8 [DOI] [PubMed] [Google Scholar]
- 96.Ramamoorthy S, Shippenberg TS, Jayanthi LD. Regulation of monoamine transporters: Role of transporter phosphorylation. Pharmacol Ther. 2011;129(2):220–238. doi: 10.1016/J.PHARMTHERA.2010.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rahbek-Clemmensen T, Bay T, Eriksen J, Gether U, Jrøgensen TN. The serotonin transporter undergoes constitutive internalization and is primarily sorted to late endosomes and lysosomal degradation. J Biol Chem. 2014;289(33):23004–23019. doi: 10.1074/JBC.M113.495754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li J, Gould TD, Yuan P, Manji HK, Chen G. Post-mortem interval effects on the phosphorylation of signaling proteins. Neuropsychopharmacology. 2003;28(6):1017–1025. doi: 10.1038/SJ.NPP.1300112 [DOI] [PubMed] [Google Scholar]
- 99.Rajkowska G, Mahajan G, Legutko B, et al. Length of axons expressing the serotonin transporter in orbitofrontal cortex is lower with age in depression. Neuroscience. 2017;359:30. doi: 10.1016/J.NEUROSCIENCE.2017.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shih JC, Ridd MJ, Chen K, et al. Ketanserin and tetrabenazine abolish aggression in mice lacking monoamine oxidase A. Brain Res. 1999;835(2):104–112. doi: 10.1016/S0006-8993(99)01478-X [DOI] [PubMed] [Google Scholar]
- 101.Sakaue M, Ago Y, Sowa C, et al. Modulation by 5-hT2A receptors of aggressive behavior in isolated mice. Jpn J Pharmacol. 2002;89(1):89–92. doi: 10.1254/JJP.89.89 [DOI] [PubMed] [Google Scholar]
- 102.Schwartzer JJ, Ricci LA, Melloni RH. Adolescent anabolic-androgenic steroid exposure alters lateral anterior hypothalamic serotonin-2A receptors in aggressive male hamsters. Behavioural brain research. 2009;199(2):257–262. doi: 10.1016/J.BBR.2008.11.048 [DOI] [PubMed] [Google Scholar]
- 103.Frau R, Pardu A, Godar S, Bini V, Bortolato M. Combined Antagonism of 5-HT 2 and NMDA Receptors Reduces the Aggression of Monoamine Oxidase a Knockout Mice. Pharmaceuticals (Basel). 2022;15(2). doi: 10.3390/PH15020213 [DOI] [PMC free article] [PubMed] [Google Scholar]


