Abstract
In experimental murine cutaneous leishmaniasis, the purified Leishmania pifanoi amastigote protein P-4 has been shown to induce significant protection against infection. Further, recent studies examining the response of peripheral blood mononuclear cells (PBMC) from Leishmania braziliensis-infected human patients have demonstrated that the P-4 protein selectively elicits a significant TH1-like response. Because a TH1-like response is associated with cure, epitope studies were conducted to further evaluate the human response to P-4. PBMC from confirmed cutaneous leishmaniasis patients infected with L. braziliensis in Rio de Janeiro, Brazil, an area where the disease is endemic, were examined for T-cell proliferation and/or cytokine production in response to whole-parasite homogenate, isolated P-4 protein, and/or P-4 peptides. Twenty of the 22 patients (91%) examined responded to the native P-4 protein by proliferation and/or gamma interferon (IFN-γ) production. According to the proliferation data, PBMC from 14 patients (64%) were found to respond to the intact P-4 protein (stimulation index of ≥2.5). Fifty-seven percent of the P-4-responsive patients studied responded to at least one of the P-4 peptides; 11 individual peptides were found to elicit a proliferative response. Of 17 patients examined for cytokine production, no PBMC produced detectable interleukin-4 in response to P-4 protein or peptides. However, PBMC from 14 patients (82%) produced significant levels of IFN-γ (≥20 pg/ml) in response to native P-4 protein. Nineteen of the 23 peptides were found to elicit an IFN-γ response from at least two patients. These data indicate that multiple epitopes spanning the entire P-4 molecule are responsible for the TH1-like immune response observed, indicating that the intact P-4 amastigote molecule, rather than selected peptides, may prove to be the most useful for leishmaniasis vaccine development.
Leishmania species are dimorphic, obligate intracellular protozoa that cause a spectrum of cutaneous, mucocutaneous, and visceral diseases that affect millions of people worldwide (25). The flagellated promastigotes replicate and differentiate within the gut of the sandfly vector and are transmitted to a vertebrate host when the sandfly takes a blood meal. Survival of the parasite within the mammalian host requires successful entry into a macrophage and transformation into the amastigote form, which lives and multiplies within the phagolysosome. The ability to culture the promastigote form of Leishmania has allowed the detailed study of this developmental stage. Moreover, the recent development and availability of axenic cultures of several species and strains (3, 12, 13, 24) have facilitated the study of the amastigote form. The amastigote stage is responsible for disease and pathology in the mammalian host and is thus implicated as the source of the antigens responsible for inducing the apparent self-healing that occurs in most cases of cutaneous leishmaniasis (25). These amastigote studies may therefore hold promise for the development of a vaccine.
Both animal model studies and human research have been conducted in efforts directed toward the development of a vaccine against leishmaniasis. In animal model studies, two general approaches have been employed. The first involves immunization with whole parasites; these studies have employed virulent organisms, attenuated or auxotropic mutant parasites, or organisms that have been killed or disrupted (1, 2, 14, 16, 18, 21, 23, 39). The second approach is to induce immunoprotection by using purified and/or recombinant antigens or DNA (6, 22, 26, 34, 40). These studies indicate that a principal means for evaluating the effects of a potential vaccine is the specific T-cell immune response induced by the parasite. The particular cytokines produced by stimulated T-cell subsets appear to cause opposing effects associated with either the cure or the aggravation of disease (4, 5, 20, 27, 38). Cytokines, such as gamma interferon (IFN-γ) and tumor necrosis factor (TNF), produced by the TH1 subset of CD4+ T cells have been shown to be vital in the process of macrophage activation and parasite destruction. Conversely cytokines, such as interleukin-4 (IL-4), IL-10, and transforming growth factor β, produced in part by the TH2 subset of CD4+ T cells have been shown to down-regulate the TH1 response, hinder macrophage activation, and consequently aggravate disease. Additionally, evidence from murine models and studies of human patients suggests that CD8+ T cells may also play a role in the curative process by modulating CD4+-T-cell activity and/or directly interacting with or activating parasitized macrophages via cytokines (11, 21, 35). Specifically, a subpopulation of CD8+ T cells (Tc1), similar to CD4+ TH1 cells, selectively produces IFN-γ and TNF (9) and is capable of lytic activity towards parasitized macrophages (11).
Several defined parasite proteins that appear to induce beneficial human T-cell responses or protection against infection in a murine model system have been identified. Such proteins include dp72, gp46, gp63, Leishmania eukaryotic initiation factor, P-4, P-8, and lipophosphoglycan-associated proteins and may constitute potential vaccine candidates (6, 17, 26, 28, 29, 34). The protein evaluated in this study, P-4, is an internal membrane-associated molecule purified from in vitro-cultured Leishmania pifanoi amastigotes (Leishmania mexicana complex). Previous studies indicate that immunization with P-4, together with Corynebacterium parvum as an adjuvant, provides partial to complete protection of BALB/c mice against infection (36). The protectively immunized mice exhibited profound T-cell proliferation, as well as increased levels of IFN-γ and no IL-4 production, in response to P-4. These results suggest that a type 1-like cell-mediated immune reaction is associated with resistance in these mice. Subsequently, 32 human patients with cutaneous leishmaniasis due to infection with Leishmania braziliensis were studied for their responses to the P-4 protein (7). The T-cell phenotypes and cytokines produced in response to the P-4 protein both before and after antimonial therapy were very similar to the responses to whole L. braziliensis promastigote antigens characteristic of cured patients. In those studies, the activation of similar numbers of CD4+ and CD8+ T cells and a selective type 1-like cytokine response were noted.
Because peptide-based vaccines potentially provide the advantage of directing an immune response towards specific epitopes (32), we conducted the present study, aiming at evaluating the antigenic response induced by various peptides of the P-4 protein. Twenty-three peptides were synthesized and tested for their ability to elicit proliferative responses, as well as IFN-γ and IL-4 production, in peripheral blood mononuclear cells (PBMC) of 22 cutaneous leishmaniasis patients in Rio de Janeiro, Brazil, an area where the disease is endemic.
MATERIALS AND METHODS
Protein analysis and peptide synthesis.
The P-4 protein is a single-strand-specific nuclease. The gene encoding P-4 has been cloned (GenBank accession no. AF057351), and the characterization of the gene and its expression, phylogenetic distribution, and sequence, as well as the enzymatic properties of its product, will be described elsewhere (37). The protein sequence of the P-4 molecule was determined by using cDNA-derived amino acid sequences and confirmed by internal peptide sequences. The mature P-4 protein is 215 amino acids in length; the 30-amino-acid signal sequence of the protein was not included in the analysis. Of note, no repetitive sequences were found in the protein. The peptides were synthesized on an ABIMED AMS42 multiple-peptide synthesizer. Resins, PyBOP (benzotriazole-1-yl-oxy-tris-pyrrolidino-phosphonium-hexafluorophosphate), and amino acid derivatives were purchased from Novabiochem (Laufelfingen, Switzerland), while solvents were purchased from Scharlau (La Jota, Spain) and Fluka (Buchs, Switzerland). The C-terminal amidated peptides were synthesized on Rink amide 4-methyl-benzhydrylamine resin by successive additions of the corresponding amino acids at a 10 μM scale by using 9-fluorenylmethoxycarbonyl chemistry. Couplings were performed by using PyBOP with N-methylmorpholine, and 9-fluorenylmethoxycarbonyl deprotection was accomplished with 20% piperidine in dimethylformamide. Appropriate residues were protected, and cleavage and deprotection were carried out by incubation of the resin with trifluoroacetic acid in the presence of scavengers. The peptides were precipitated with cold diethyl ether and lyophilized. Peptides were analyzed by mass spectroscopy with a Brüker Byflex mass spectrophotometer; purity was always greater than 85%. The lyophilized peptides were reconstituted in phosphate-buffered saline, and the protein concentration was determined spectrophotometrically by A215.
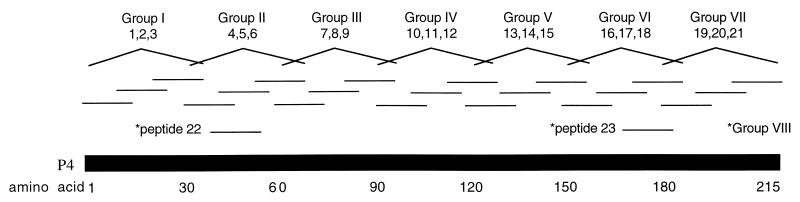
A systematic approach was used as a first approximation to determine the potential epitopes of the P-4 protein. Sequential segments of 15 amino acids with overlapping edges of 5 amino acids were synthesized based on the 215-amino-acid sequence of the mature protein (Fig. 1). A length of 15 amino acids was chosen for each peptide because major histocompatibility complex (MHC) class II restriction molecules are known to bind to 12-amino-acid peptide fragments. Additional amino acids on either side of the 12 amino acids were added to increase the likelihood of finding the immunogenic epitopes. An experimental computer simulation, Predictor 4.0, was employed to assess peptide sequence and structure and to predict which areas of the P-4 sequence would bind most strongly to an arbitrarily chosen MHC class II allele (15); this method has been used to successfully identify a cross-reactive, immunodominant epitope of influenza virus hemagglutinin. Although the results obtained are not necessarily applicable to all MHC alleles, two additional 15-amino-acid peptides (no. 22 and 23) of the P-4 protein that were indicated to potentially have strong MHC class II binding potentials were selected for synthesis (Fig. 1). In addition, it should be noted that the Predictor 4.0 analysis indicated that other P-4-derived peptides (no. 2, 4, 10, 11, and 21) potentially have strong MHC class II binding potentials.
FIG. 1.
Peptide design for evaluation of epitopes of the L. pifanoi P-4 amastigote protein. Twenty-one peptides were synthesized based on the sequence of the native P-4 protein; each peptide was 15 amino acids in length and overlapped its neighboring peptides by 5 amino acids. In addition, peptides 22 and 23 were synthesized based on computer-based prediction of potential for MHC class II binding (14). Groups of three overlapping peptides employed are indicated; in addition, peptides 22 and 23 were tested together.
Subjects.
Twenty-two patients (10 male and 12 female) with localized cutaneous leishmaniasis were studied. All had been infected in the state of Rio de Janeiro, an area where only L. braziliensis is endemic (11). The diagnoses were confirmed by clinical appearance, as well as a delayed-type hypersensitivity response to leishmanin antigen and/or demonstration of the parasite in biopsy samples. Although MHC class II typing was not performed, the racial heterogeneity of the patient population and the lack of subject selection in the study suggest that a diversity in MHC class II is present in the patient group. Additionally, six subjects (two in Brazil and four in the United States) who were not infected with any form of leishmaniasis were also tested as controls. Each subject was examined one time during the study. Because of the difficulty in reliably contacting patients, which made subsequent experiments very difficult to arrange, none of the patients were initially screened for response to the intact P-4 protein before testing of the grouped or individual peptides. All subjects were studied between June and September 1996.
Proliferation assays.
As described previously (7), approximately 30 ml of human blood was collected into heparinized tubes and processed within 24 h. After the blood was diluted 1:1 with RPMI 1640 medium (Sigma), PBMC were separated by centrifugation over a gradient of Ficoll-Hypaque (Histopaque 1077; Sigma). Mononuclear cells were resuspended in RPMI 1640 supplemented with 10% heat-activated human AB Rh+ serum, 10 mM HEPES, 1.5 mM l-glutamine, 0.04 mM 2-mercaptoethanol, 200 IU of penicillin per ml, and 200 μg of streptomycin per ml and were adjusted to 3 × 106 cells/ml. Cells (3 × 105−) were distributed in triplicate into 96-well, round-bottom plates containing various concentrations and/or combinations of the peptides in a final volume of 200 μl. Controls included medium alone and cells incubated with concanavalin A (Pharmacia, Uppsala, Sweden) (20 μg/ml), L. braziliensis antigen (∼106 disrupted promastigotes; 5 μg/ml), or isolated P-4 protein (5 μg/ml). The peptides were initially tested in groups of three (i.e., group I consisted of peptides 1, 2, and 3, and group II consisted of peptides 4, 5, and 6, etc.) to facilitate the process of identifying immunodominant regions with a limited number of PBMC; grouped peptides were tested for patients 1 through 16. Individual peptides in addition to the grouped peptides were assessed for induction of PBMC proliferation for patients 9 through 16. Individual peptides alone were tested for patients 17 through 22.
PBMC were stimulated with the peptides and controls for 5 days, and 1 μCi of [3H]thymidine (Amersham International, Amersham, United Kingdom) was added to each well 16 h before harvest. Cells were harvested onto fiber filters by using a Titertek cell harvester, and radioactivity was measured in a Packard 1600 CA liquid scintillation beta counter. Results are expressed as the stimulation index (SI), which indicates the mean counts per minute in wells containing antigen divided by background counts per minute. An SI of greater than or equal to 2.5 times the background was considered significant. This value is based on results of previous experiments comparing leishmaniasis patients to healthy controls (7, 8). Initially, the peptides were tested at concentrations ranging from 1 to 100 μg/ml. Although some variation was observed, optimal proliferation was generally observed at a peptide concentration of 1 or 5 μg/ml.
Cytokine ELISAs.
Cytokine assays were performed with grouped peptides to stimulate the PBMC of patients 6 through 16; the responses to individual peptides were examined for patients 10 through 22. Supernatants from each well of PBMC (100 μl) were collected after 3 days of incubation for detecting IL-4 and after 5 days for detecting IFN-γ and were stored at −20°C until analysis. These collection points were determined as optimal in preliminary experiments in which supernatants from 16 h and 1 to 5 days were tested for cytokine production (7, 8). All samples were tested in duplicate and compared to standard curves to determine the cytokine concentration. IL-4 was assessed by enzyme-linked immunosorbent assay (ELISA) for cytokine quantification by using Intertest kits (Genzyme, Cambridge, Mass.). The sensitivity of IL-4 detection by ELISA has been compared to that of detection by intracellular staining assays, and no difference was found (30). The minimum level of IL-4 detected was 4 pg/ml.
IFN-γ was measured by ELISA with reagents from Endogen (Cambridge, Mass.) according to a modified manufacturer’s protocol. The minimum level detected reproducibly was 4 pg/ml, as assessed with a standard curve. A significant level of IFN-γ production was set at greater than or equal to 20 pg/ml. This cutoff was based on the fact that peptides inducing at least 20 pg of IFN-γ per ml consistently induced IFN-γ production at all peptide concentrations tested (1, 5, and/or 50 μg/ml). Briefly, plates were coated with human recombinant IFN-γ antibodies overnight at room temperature. After blocking with a bovine serum albumin buffer for 1 h and washing with a 50 mM Tris–0.1% Tween–phosphate-buffered saline buffer (pH 7.0 to 7.5), plates were incubated with samples or standards overnight at 4°C. After washing, biotinylated IFN-γ antibody was added and left for 1 h at 34°C. The plates were developed by reaction of streptavidin-alkaline phosphatase with a p-nitrophenol phosphate substrate (Pierce, Rockford, Ill.) and analyzed on a Vmax microplate reader (Molecular Devices, Sunnyvale, Calif.) at 405 nm.
RESULTS
Immune responses to leishmanial and P-4 antigens.
PBMC from 22 patients with confirmed cutaneous leishmaniasis caused by L. braziliensis infection were separated and stimulated with the P-4 protein and peptides, as well as concanavalin A and L. braziliensis promastigote homogenate. The proliferative responses and the levels of IFN-γ detected after stimulation with L. braziliensis antigens, concanavalin A, or native P-4 protein are presented in Table 1. In proliferation assays, the PBMC from 21 of the 22 patients (96%) responded to L. braziliensis antigens, while 14 patients (64%) responded to the P-4 protein. The IFN-γ levels produced by PBMC in response to the P-4 protein and L. braziliensis antigens were determined for patients 6 through 22. All of the infected patients produced IFN-γ in response to L. braziliensis antigens, with levels ranging from 108 to 14,182 pg/ml (average, 4,466 pg/ml). Fourteen of the patients (82%) produced IFN-γ in response to the P-4 protein, with levels ranging from 36 to 4,096 pg/ml (average, 849 pg/ml). After stimulation with the P-4 protein, a higher percentage of patient PBMC produced IFN-γ than proliferated, consistent with earlier observations (7); these data suggest that cytokine production may be a more sensitive measure of antigenic responsiveness in infected individuals. Overall, 20 of the 22 patients (91%) responded to the P-4 protein, as judged by proliferation and/or IFN-γ production. None of the six uninfected individuals studied responded to the native P-4 protein; neither proliferation (i.e., an SI of ≥2.5) nor production of IFN-γ (i.e., a detectable IFN-γ level [≥4 pg/ml]) was observed.
TABLE 1.
Summary of proliferation and IFN-γ production from PBMC of leishmaniasis patients infected with L. braziliensis
| Patient | SI with:
|
IFN-γ (pg/ml) with:
|
|||
|---|---|---|---|---|---|
| ConA | L. braziliensis | P-4 | L. braziliensis | P-4 | |
| 1 | 17.2 | 5.0 | 6.8 | NDb | ND |
| 2 | 16.7 | 36.6 | 21.8 | ND | ND |
| 3 | 4.9 | 6.8 | 3.7 | ND | ND |
| 4 | 14.2 | 23.3 | 17.1 | ND | ND |
| 5 | 11.4 | 7.7 | 5.1 | ND | ND |
| 6 | 3.5 | 1.5 | 1.5 | 108 | 91 |
| 7 | 28.4 | 60.2 | 59.9 | 2,400 | 212 |
| 8 | 39.4 | 45.1 | 6.5 | 14,182 | 645 |
| 9 | 5.2 | 21.5 | 6.8 | 8,658 | 4,096 |
| 10 | 16.9 | 11.6 | 5.1 | 5,414 | 1,016 |
| 11 | 21.5 | 19.5 | 7.7 | 1,822 | 61 |
| 12 | 22.4 | 7.1 | 0.6 | 284 | <4 |
| 13 | 22.0 | 56.2 | 3.7 | 1,741 | <4 |
| 14 | 15.7 | 141.0 | 23.4 | 2,400 | 1,785 |
| 15 | 5.0 | 27.6 | 2.4 | 9,274 | 1,107 |
| 16 | 10.0 | 3.4 | 1.8 | 3,126 | 163 |
| 17 | 11.9 | 8.4 | 1.0 | 4,008 | 14 |
| 18 | 30.2 | 11.8 | 1.6 | 7,857 | 580 |
| 19 | 35.5 | 12.5 | 4.1 | 6,440 | 235 |
| 20 | 47.0 | 49.9 | 2.4 | 2,331 | 36 |
| 21 | 6.6 | 24.3 | 5.6 | 3,011 | 1,684 |
| 22 | 24.6 | 35.9 | 1.6 | 2,875 | 172 |
PBMC were stimulated with concanavalin A (ConA), L. braziliensis, and native P-4 protein.
ND, not determined.
T-cell proliferation to P-4 peptides.
In addition to L. braziliensis antigens and the native P-4 protein (as indicated above), the response of patient PBMC to the synthetic P-4 peptides was examined. Initially, eight sets of three sequential or grouped peptides (i.e., group I consisted of peptides 1, 2, and 3, and group II consisted of peptides 4, 5, and 6, etc.) were employed to minimize the number of PBMC required; subsequently, the responses to individual peptides were examined. The PBMC from patients 1 through 8 were stimulated with the groups of P-4 peptides only, while PBMC from patients 9 through 16 were stimulated with both the groups of sequential peptides and individual peptides. PBMC from patients 17 through 22 were stimulated with the individual peptides alone. Proliferation induced by the P-4 peptides was assessed. Each group of peptides (spanning a total of 30 amino acids of sequence) was found to stimulate PBMC proliferation in two to five of the patients (Table 2). Significant proliferation in response to three or more peptide groups was observed for five patients (patients 2, 4, 7, 8, and 14). The SI in all patients ranged from 2.5 to 7.6 (average of 3.9); background counts ranged from 166 to 1,674 cpm (average of 604 cpm).
TABLE 2.
Summary of proliferation results for PBMC stimulated with groups of P-4 peptidesa
| Peptide group | SI for patient:
|
Total no. of responsive patients | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | ||
| I | —b | 6.8 | — | 2.8 | — | — | — | 2.6 | — | — | — | — | 2.6 | 3.4 | — | — | 5 |
| II | — | 9.2 | 5.2 | 2.6 | — | — | — | 2.6 | — | — | — | — | — | — | — | — | 4 |
| III | — | 7.7 | — | — | — | — | — | 3.0 | — | — | — | — | — | 5.0 | — | — | 3 |
| IV | — | 8.1 | — | — | — | — | 2.5 | — | — | — | — | — | 3.1 | 3.0 | — | — | 4 |
| V | — | 4.5 | — | 2.6 | — | — | 3.5 | — | — | — | — | — | — | 2.9 | — | — | 4 |
| VI | — | 6.5 | — | — | — | — | 3.1 | — | — | — | — | — | — | 4.3 | — | — | 3 |
| VII | — | — | — | — | — | — | 2.5 | — | — | — | — | — | — | 7.6 | — | — | 2 |
| VIII | — | 4.2 | — | — | — | — | 2.8 | 2.9 | — | — | 2.5 | — | — | 5.5 | — | — | 5 |
PBMC from patients 1 through 16 were stimulated with groups of peptides as described in Material and Methods. After 5 days of antigenic stimulation, [3H]thymidine incorporation was measured as a marker of cell proliferation.
—, SI of <2.5.
Eleven individual peptides (no. 2, 7, 9, 11, 12, 15, 16, 19, 20, 22, and 23) were observed to induce significant proliferation in at least one of the patients. Moreover, the PBMC from four patients, consistent with results for the grouped peptides, proliferated in response to three or more of the individual peptides. None of the individual peptides, however, induced a proliferative response in more than 30% of the patients (data not shown). Additionally, none of the grouped or individual peptides elicited a proliferative response in the six uninfected individuals examined as controls. Together, these results suggest the presence of multiple P-4 epitopes.
Cytokine production in response to P-4 peptides.
As with the proliferation experiments, the PBMC from patients 6 through 9 were stimulated with the groups of sequential peptides, those from patients 10 through 16 were stimulated with grouped peptides and individual peptides, and those from patients 17 through 22 were stimulated with individual peptides alone. PBMC supernatants were collected after 5 days and tested for IFN-γ levels by ELISA; the minimum level for a positive response, as indicated in Materials and Methods, was 20 pg of IFN-γ per ml. Each group of peptides was found to induce significant levels of IFN-γ in 3 to 6 of the 11 patients examined (Table 3). The level of IFN-γ induced by the grouped peptides ranged from 21 to 310 pg/ml (average, 108 pg/ml).
TABLE 3.
Summary of IFN-γ production in PBMC stimulated with groups of P-4 peptidesa
| Peptide group | IFN-γ (pg/ml) for patient:
|
Total no. of responsive patients | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | ||
| I | 108 | 310 | 83 | 67 | —b | — | 95 | — | 171 | — | — | 6 |
| II | — | 141 | 86 | 41 | — | — | 71 | — | 195 | — | — | 5 |
| III | — | 38 | 61 | — | — | — | 28 | — | 208 | 26 | 21 | 6 |
| IV | 60 | 98 | — | 49 | — | — | 101 | — | 131 | — | — | 5 |
| V | — | 301 | — | 56 | — | — | 28 | — | 176 | — | — | 4 |
| VI | 217 | 161 | — | 27 | — | — | 30 | — | 149 | — | — | 5 |
| VII | — | 262 | — | 38 | — | — | — | — | 123 | — | — | 3 |
| VIII | — | 124 | 138 | 45 | — | — | — | — | 131 | — | 29 | 5 |
PBMC from patients 6 through 16 were stimulated with groups of peptides as described in Material and Methods. After 5 days of antigenic stimulation, supernatants were harvested and IFN-γ levels were measured by ELISA.
—, <20 pg/ml.
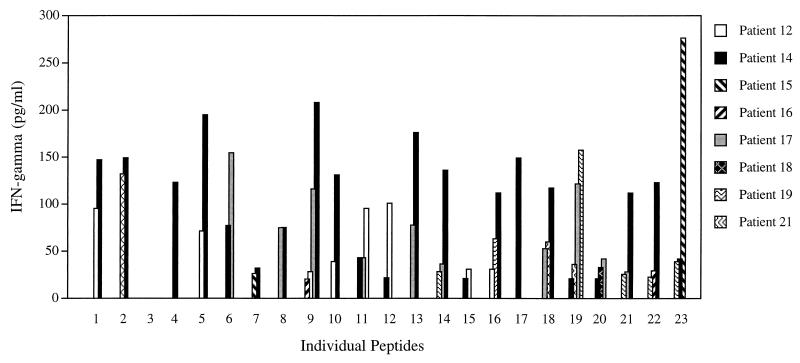
Twenty-two of the 23 individual peptides (all but peptide 3) stimulated IFN-γ production in at least one patient (Fig. 2); 10 peptides (no. 9, 11, 14, 16, 18, 19, 20, 21, 22, and 23) elicited an IFN-γ response in three or more patients (Fig. 2). Significant IFN-γ production in response to at least three individual peptides was observed for 5 of the 14 patients tested (patients 12, 14, 17, 19, and 21). The levels of IFN-γ stimulated by the individual peptides were comparable to those found for the grouped peptides and ranged from 21 to 277 pg/ml (average, 82 pg/ml). PBMC from the uninfected individuals examined did not produce IFN-γ in response to either the grouped or individual P-4 peptides.
FIG. 2.
IFN-γ production for PBMC stimulated with individual P-4 peptides. PBMC from patients 10 through 22 were stimulated with individual P-4 peptides for 5 days. Supernatants were harvested and IFN-γ levels were measured by ELISA, as indicated in Materials and Methods. The total IFN-γ produced is presented for cases where significant levels (≥20 pg/ml) were observed in response to the indicated individual peptide.
PBMC supernatants from patients 6 through 22 were also collected after 3 days of incubation with the P-4 peptides and assessed for the production of IL-4 by ELISA. The minimum level of detectable IL-4 was 4 pg/ml. No IL-4 was detected in any of the supernatants in response to the intact P-4 protein or to any of the individual or grouped P-4 peptides.
Additionally, the T-cell phenotypes of PBMC stimulated with several of the P-4 peptides were assessed in the current study for two patients (for one patient before and after treatment and for the other after treatment only). An overall dominance of CD4+ T cells was found, although CD8+ T cells were clearly present; the proportion of CD4+ T cells compared to CD8+ T cells ranged from 1.5:1 to 32:1 (data not shown). Thus, the tested epitopes (as expected) appeared to preferentially stimulate the CD4+-T-cell subpopulation.
DISCUSSION
Peptide-based vaccines are of interest, as the techniques for peptide synthesis are both readily available and economical. Further, studies of peptide specificity among HLA allelic families have suggested that certain epitopes (pan-DR class II and MHC class I supertype) may be able to elicit antigenic responses in a relatively large portion of a given population (31–33). The determination of such epitopes conserved among the Leishmania species could potentially prove useful in the development of a peptide-based vaccine. Given the significant phylogenetic distance between L. braziliensis and other leishmanial species (10, 19), it is possible that a cross-reactive response to protein homologes (of L. braziliensis and another leishmanial species) could represent a limited number of conserved areas of sequence. Consequently, the immune response of individuals infected with L. braziliensis to the L. pifanoi (L. mexicana complex) P-4 protein (7) potentially might reflect a limited number of epitopes capable of eliciting the preferential TH1 response found for the native P-4 protein (7). However, analysis of the P-4 protein sequence by using an experimental computer simulator (15, 33) to predict immunogenic areas suggested that seven areas distributed across the P-4 molecule may contribute to the observed immune response. Although the computation-based predictions are not applicable to the presumed variation of MHC alleles in the study population, the presence of multiple epitopes was of interest. Consequently, the T-cell response to the P-4 protein was investigated to determine if it was due to a limited span or area of protein sequence or to multiple epitopes.
The development of a successful vaccine depends on the ability of the given antigen to stimulate a beneficial T-cell response. In this study, PBMC proliferation and IFN-γ production as elicited by the P-4 peptides were used as markers of this type of T-cell response. Each group of three peptides (representing a 30-amino-acid span of protein sequence) stimulated significant proliferation of PBMC. Similar to the proliferation data, each group of three peptides and the majority (22 of 23) of the individual peptides were found to stimulate an IFN-γ response in at least one patient. Therefore, these data indicate that the epitopes responsible for the immune response to the P-4 protein are distributed throughout the molecule. However, it should be noted that certain peptides (peptides 9, 11, 14, 16, 18, and 19 through 23) elicited an IFN-γ response from 23 to 31% of the patients examined. Consequently, although the number of patients studied is small, further evaluation of the response to these peptides and combinations of these peptides (e.g., peptides 9 and 19) by cutaneous leishmaniasis patients may be of interest.
The data for both the grouped and individual peptides indicate that the responses to the P-4 protein were due to a number of epitopes distributed across the P-4 molecule at nonclustered sites. The peptides chosen for this study, however, are only a sample of possible epitopes on the P-4 protein and represent a combination of the use of empirically selected overlapping peptides and peptides selected by computational analysis based upon known MHC class II motifs (15). Of the seven peptides predicted to elicit a T-cell response (peptides 2, 4, 10, 11, 21, 22, and 23), four were found to stimulate a proliferative response in at least one patient, and all seven peptides elicited an IFN-γ response. Further, peptides 22 and 23 would not have been identified as epitopes had the empirical approach alone been employed. However, other peptides tested (but not computationally predicted to have a high potential for MHC class II binding) were, in fact, found to stimulate a T-cell response in comparable or higher numbers of patients. Consequently, a combination of both approaches appears to be optimal for determining specific T-cell epitopes. However, it should be noted that the PBMC from a small subgroup of the patients examined, although clearly responsive to the intact P-4 protein, failed to respond in either assay (proliferation or IFN-γ production) to any of the synthetic peptides. Consequently, it is likely that other epitopes, not included in this study, exist within the P-4 protein. A further refinement of the approaches employed (perhaps with a larger sequence overlap between peptides) may therefore need to be considered in future epitope studies.
The production of IFN-γ in this study suggests that the majority of the P-4 peptides elicit a beneficial TH1-type response. Furthermore, the level of IFN-γ produced is comparable to that observed in a study of Leishmania eukaryotic initiation factor protein, as well as to that seen in an epitope mapping study of another vaccine candidate, the Leishmania protein gp63 (28, 34). In the latter study as well as the present study, variation occurred among the individual patient responses to the various gp63 peptides; however, in contrast to the current study, one gp63 peptide (PT7) stimulated the T cells of all six patients examined (28). Additionally, the lack of detectable IL-4 production following stimulation with the P-4 peptides supports the presence of a TH1-type response. However, further characterization of the T-cell response is of interest, including the production of the cytokines TNF, IL-5, and IL-10. Further, earlier Leishmania studies have shown that CD8+ T cells are capable of producing a curative response to infection (11, 21, 35). The intact P-4 protein has been observed to stimulate both CD4+- and CD8+-T-cell proliferation, resulting in a Th1-like Tc1 cytokine response (7). The average CD4+/CD8+ ratio found for P-4-responsive T cells was approximately 1:1; although this ratio varied somewhat from patient to patient, the data clearly indicated that both subpopulations of T cells were consistently activated. Consequently, the cytokines produced by CD8+ T cells in response to stimulation by the P-4 epitopes may warrant investigation.
In summary, the variation in the immune response observed for the P-4 peptides in leishmaniasis patients reflects the existence of multiple epitopes, as well as MHC heterogeneity of the patient population. Given these results, it does not appear that a peptide vaccine based on the P-4 molecule that might provide optimal protection against Leishmania infection could readily be constructed. However, PBMC from 91% of the patients studied did produce a proliferative and/or IFN-γ response to the native P-4 protein. The TH1-type cytokine profile (presence of IFN-γ and absence of IL-4) suggests that P-4 and possibly other amastigote proteins may ultimately prove useful in the development of a recombinant and/or DNA vaccine against leishmaniasis.
ACKNOWLEDGMENTS
This study was made possible by funding from the National Institutes of Health (grant AI27811), the Brazilian National Council for Scientific and Technologic Development (grant CNPq), CICYT grant BI092-0936-C02-01, and the Yale University School of Medicine Office of Student Research.
REFERENCES
- 1.Antunes C M F, Mayrink W, Magalhaes P A, Costa C A, Melo M N, Dias M, Michalick M S M, Williams P, Lima A O, Vieira J B F, Schettini A P M. Controlled field trials of a vaccine against New World cutaneous leishmaniasis. Int J Epidemol. 1986;15:572–580. doi: 10.1093/ije/15.4.572. [DOI] [PubMed] [Google Scholar]
- 2.Barral-Netto M, Reed S G, Sadigursky M, Sonnenfeld G. Specific immunization of mice against Leishmania mexicana amazonensis using solubilized promastigotes. Clin Exp Immunol. 1987;67:11–19. [PMC free article] [PubMed] [Google Scholar]
- 3.Bates P A. Axenic culture of Leishmania amastigotes. Parasitol Today. 1993;9:143–146. doi: 10.1016/0169-4758(93)90181-e. [DOI] [PubMed] [Google Scholar]
- 4.Carvalho E M, Correia-Filho D, Bacellar O, Almeida R P, Lessa H, Rocha H. Characterization of the immune response in subjects with self-healing cutaneous leishmaniasis. Am J Trop Med Hyg. 1995;53:273–277. doi: 10.4269/ajtmh.1995.53.273. [DOI] [PubMed] [Google Scholar]
- 5.Castés M, Maros Z, Martinez A, Trujillo D, Castellanos P L, Rondon A J, Convit J. Cell-mediated immunity in localized cutaneous leishmaniasis patients before and after treatment with immunotherapy or chemotherapy. Parasite Immunol. 1989;11:211–222. doi: 10.1111/j.1365-3024.1989.tb00660.x. [DOI] [PubMed] [Google Scholar]
- 6.Champsi J, McMahon-Pratt D. Membrane glycoprotein M-2 protects against Leishmania amazonensis infection. Infect Immun. 1988;56:3272–3279. doi: 10.1128/iai.56.12.3272-3279.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coutinho S G, Oliveira M P, Da-Cruz A M, De Luca P M, Mendonça S C, Bertho A L, Soong L, McMahon-Pratt D. T-cell responsiveness of American cutaneous leishmaniasis patients to purified Leishmania pifanoi amastigote antigens and Leishmania braziliensis promastigote antigen: immunologic patterns associated with cure. Exp Parasitol. 1996;84:144–155. doi: 10.1006/expr.1996.0100. [DOI] [PubMed] [Google Scholar]
- 8.Coutinho S G, Da-Cruz A M, Bertho A L, Santiago M A, De-Luca P. Immunologic patterns associated with cure in American cutaneous leishmaniasis. Braz J Med Biol Res. 1998;31:139–142. doi: 10.1590/s0100-879x1998000100019. [DOI] [PubMed] [Google Scholar]
- 9.Croft M, Carter L, Swain S L, Dutton R W. Generation of polarized antigen-specific CD8 effector populations: reciprocal action of interleukin IL-4 and IL-12 in promoting type 2 versus type 1 cytokine profiles. J Exp Med. 1994;180:1715–1728. doi: 10.1084/jem.180.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cupilillo E, Grimaldi G, Momen H, Beverley S M. Intergenic region typing (IRT): a rapid molecular approach to the characterization and evolution of Leishmania. Mol Biochem Parasitol. 1995;73:145–155. doi: 10.1016/0166-6851(95)00108-d. [DOI] [PubMed] [Google Scholar]
- 11.Da-Cruz A M, Conceição-Silva F, Bertho A L, Coutinho S G. Leishmania-reactive CD4+ and CD8+ T cells associated with cure of human cutaneous leishmaniasis. Infect Immun. 1994;62:2614–2618. doi: 10.1128/iai.62.6.2614-2618.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doyle P S, Engel J C, Pimenta P F, de Silva P P, Dwyer D M. Leishmania donovani: long-term culture of axenic amastigotes at 37°C. Exp Parasitol. 1991;73:326–334. doi: 10.1016/0014-4894(91)90104-5. [DOI] [PubMed] [Google Scholar]
- 13.Eperon S, McMahon-Pratt D. I. Extracellular cultivation and morphological characterization of amastigote-like forms of Leishmania panamensis and L. braziliensis. J Protozool. 1989;36:502–510. doi: 10.1111/j.1550-7408.1989.tb01086.x. [DOI] [PubMed] [Google Scholar]
- 14.Greenblatt C L. The present and future of vaccination for cutaneous leishmaniasis. In: Mezrahi A, Hertman I, Klingberg M A, Kohn A, editors. New development with human and veterinary vaccines. New York, N.Y: Alan R. Liss, Inc.; 1980. pp. 259–285. [PubMed] [Google Scholar]
- 15.Hammer J, Bono E, Gallazzi F, Belunis C, Nagy Z, Sinigaglia F. Precise prediction of major histocompatibility complex class II-peptide interaction based on peptide side chain scanning. J Exp Med. 1994;180:2353–2358. doi: 10.1084/jem.180.6.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howard J G, Nicklin S, Hale C, Liew F Y. Prophylactic immunization against experimental leishmaniasis. I. Protection induced in mice genetically vulnerable to fatal Leishmania tropica infection. J Immunol. 1982;129:2206–2212. [PubMed] [Google Scholar]
- 17.Kemp M, Hey A S, Bendtzen K, Kharazmi A, Theander T G. Th1-like human T-cell clones recognizing Leishmania gp63 inhibit Leishmania major in human macrophages. Scand J Immunol. 1994;40:629–635. doi: 10.1111/j.1365-3083.1994.tb03515.x. [DOI] [PubMed] [Google Scholar]
- 18.Kimsey P B, Theodos C M, Mitchen T K, Turco S J, Titus R G. An avirulent lipophosphoglycan-deficient Leishmania major clone induces CD4+ T cells which protect susceptible BALB/c mice against infection with virulent L. major. Infect Immun. 1993;61:5205–5213. doi: 10.1128/iai.61.12.5205-5213.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kreutzer R D, Souraty N, Semko M E. Biochemical identities and differences among Leishmania species and subspecies. Am J Trop Med Hyg. 1987;36:22–32. doi: 10.4269/ajtmh.1987.36.22. [DOI] [PubMed] [Google Scholar]
- 20.Liew F Y, Millot S, Li Y, Lelchuk R, Chan W L, Zitner H. Macrophage activation by interferon-gamma from host protective T cells is inhibited by interleukin (IL-3 and IL-4) produced by disease-promoting T cells in leishmaniasis. Eur J Immunol. 1989;19:1227–1232. doi: 10.1002/eji.1830190712. [DOI] [PubMed] [Google Scholar]
- 21.Mendonça S C, De Luca P M, Mayrink W, Restom T G, Conceição-Silva F, Da-Cruz A M, Bertho A L, Da Costa C A, Genaro O, Toledo V P, Coutinho S G. Characterization of human T lymphocyte-mediated immune responses induced by a vaccine against American tegumentary leishmaniasis. Am J Trop Med Hyg. 1995;53:195–201. doi: 10.4269/ajtmh.1995.53.195. [DOI] [PubMed] [Google Scholar]
- 22.Murray P J, Spithill T W, Handman E. Characterization of integral membrane proteins of Leishmania major by Triton X-114 fractionation and analysis of vaccination effects in mice. Infect Immun. 1989;57:2203–2209. doi: 10.1128/iai.57.7.2203-2209.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nascimento E, Mayrink W, da Costa C A, Michalick M S, Melo M N, Barros G C, Dias M, Atunes C M, Lima M S, Taboada D C, Liu T Y. Vaccination of humans against cutaneous leishmaniasis: cellular and humoral immune responses. Infect Immun. 1990;58:2198–2203. doi: 10.1128/iai.58.7.2198-2203.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan A A, Duboise S M, Eperon S, Rivas L, Hodgkinson V, Traub-Cseko Y, McMahon-Pratt D. Developmental life cycle of Leishmania: cultivation and characterization of cultured extracellular amastigotes. J Eukaryot Microbiol. 1993;40:213–223. doi: 10.1111/j.1550-7408.1993.tb04906.x. [DOI] [PubMed] [Google Scholar]
- 25.Peters E, Killick-Kendrick R. The leishmaniases in biology and medicine. London, United Kingdom: Academic Press; 1987. [Google Scholar]
- 26.Rachmim N, Jaffe C L. Pure protein from Leishmania donovani protects mice against both cutaneous and visceral leishmaniasis. J Immunol. 1993;150:2322–2331. [PubMed] [Google Scholar]
- 27.Reiner S L, Locksley R M. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 28.Russo D M, Jardim A, Carvalho E M, Sleath P R, Armitage R J, Olafson R W, Reed S G. Mapping human T cell epitopes in Leishmania gp63. J Immunol. 1993;150:932–939. [PubMed] [Google Scholar]
- 29.Russo D M, Turco S J, Burns J M, Reed S G. Stimulation of human T lymphocytes by Leishmania lipophosphoglycan-associated proteins. J Immunol. 1992;148:202–207. [PubMed] [Google Scholar]
- 30.Santiago, M., P. M. De-Luca, A. L. Bertho, R. B. G. Azeredo-Coutinho, and S. G. Coutinho. 1997. Analysis of intracellular cytokines in peripheral blood mononuclear cells from patients with American tegumentary leishmaniasis (ATL) by flow cytometry. Mem. Inst. Oswaldo Cruz 92(Suppl. 1):215.
- 31.Sidney J, del Gercio M-F, Southwood S, Engelhard V H, Appella E, Rammensee H-G, Falk K, Rotzschke O, Takiguchi M, Kubo R T, Grey H M, Sette A. Several HLA alleles share overlapping peptide specificities. J Immunol. 1995;154:247–259. [PubMed] [Google Scholar]
- 32.Sidney J R, Kubo T, Wentworth P A, Alexander J, Chesnut R W, Grey H M, Sette A. Broadly reactive HLA restricted T cell epitopes and their implications for vaccine design. In: Stefan H, Kaufmann E, editors. Concepts in vaccine development. Berlin, Germany: Walter de Gruyter Publishing; 1996. pp. 169–186. [Google Scholar]
- 33.Sinigaglia F, Hammer J. Motifs and supermotifs for MHC class II binding proteins. J Exp Med. 1995;181:449–451. doi: 10.1084/jem.181.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skeiky Y A, Guderian J A, Benson D R, Bacelar O, Carvalho E M, Kubin M, Badaro R, Trinchieri G, Reed S G. A recombinant Leishmania antigen that stimulates human peripheral blood mononuclear cells to express a Th1-type cytokine profile and to produce interleukin-12. J Exp Med. 1995;181:1527–1537. doi: 10.1084/jem.181.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith L E, Rodrigues M, Russell D G. The interaction between CD8+ cytotoxic T cells and Leishmania-infected macrophages. J Exp Med. 1991;174:499–505. doi: 10.1084/jem.174.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soong L, Duboise S M, Kima P, McMahon-Pratt D. Leishmania pifanoi amastigote antigens protect mice against cutaneous leishmaniasis. Infect Immun. 1995;63:3559–3566. doi: 10.1128/iai.63.9.3559-3566.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soong, L., K. Goldsmith, and D. McMahon-Pratt. Unpublished results.
- 38.Squires K E, Schreiber R D, McElrath M J, Rubin B Y, Anderson S L, Murray H W. Experimental visceral leishmaniasis: role of endogenous IFN-gamma in host defense and tissue granulomatous response. J Immunol. 1989;143:4244–4249. [PubMed] [Google Scholar]
- 39.Titus R G, Gueiros-Filho F J, de Freitas L A, Beverley S M. Development of a safe live Leishmania vaccine line by gene replacement. Proc Natl Acad Sci USA. 1995;92:10267–10271. doi: 10.1073/pnas.92.22.10267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu D, Liew F Y. Protection against leishmaniasis by injection of DNA encoding a major surface glycoprotein, gp63, of L. major. Immunology. 1995;84:173–176. [PMC free article] [PubMed] [Google Scholar]