Abstract
High blood pressure (BP) is a major pathological risk factor for the development of several cardiovascular diseases. Diet is a key modifier of BP, but the underlying relationships are not clearly demonstrated. This is an umbrella review of published meta-analyses to critically evaluate the wide range of dietary evidence from bioactive compounds to dietary patterns on BP and risk of hypertension. PubMed, Embase, Web of Science, and Cochrane Central Register of Controlled Trials were searched from inception until October 31, 2021, for relevant meta-analyses of randomized controlled trials or meta-analyses of observational studies. A total of 175 publications reporting 341 meta-analyses of randomized controlled trials (145 publications) and 70 meta-analyses of observational studies (30 publications) were included in the review. The methodological quality of the included publications was assessed using Assessment of Multiple Systematic Reviews 2 and the evidence quality of each selected meta-analysis was assessed using NutriGrade. This umbrella review supports recommended public health guidelines for prevention and control of hypertension. Dietary patterns including the Dietary Approaches to Stop Hypertension and the Mediterranean-type diets that further restrict sodium, and moderate alcohol intake are advised. To produce high-quality evidence and substantiate strong recommendations, future research should address areas where the low quality of evidence was observed (for example, intake of dietary fiber, fish, egg, meat, dairy products, fruit juice, and nuts) and emphasize focus on dietary factors not yet conclusively investigated.
Keywords: blood pressure, diet, dietary patterns, hypertension, nutrients, observational studies, randomized controlled trials, umbrella review
Statement of Significance.
This umbrella review of published meta-analyses synthesized and graded the epidemiological evidence investigating the relationship between hypertension and change in blood pressure and a combination of dietary patterns, food groups, single foods, beverages, macronutrients, and micronutrients. Notably, it highlights the gap and provides a comprehensive evidence base for updating current guidelines for risk of hypertension and high blood pressure.
Introduction
High blood pressure (BP), defined as systolic BP (SBP) of ≥130 mmHg and diastolic BP (DBP) of ≥80 mmHg [1], is a major risk factor underlying the pathogenesis of multiple cardiovascular diseases, including stroke, ischemic heart disease, heart failure, hypertensive heart disease [2], and renal events [3]. High BP was identified as the leading risk factor for adult mortality according to the 2017 Global Burden of Diseases, Injuries, and Risk Factors Study comparative risk assessment [4]. In 2019, the age-standardized global prevalence of hypertension in adults aged 30–79 y was 32% in females and 34% in males, respectively [5]; less than half of those treated had achieved hypertension control, leading to global control rates of 23% for females and 18% for males with hypertension [5]. Reducing the health burden of hypertension is, therefore, a priority for public health.
Observational studies and clinical trials report that risk factors of high BP are mainly environmental, including dietary and lifestyle factors [6,7], such as sodium and potassium intake, alcohol consumption, body weight, physical activity, socioeconomic status, and genetic factors [8], and that improvement in these factors could reduce risk of hypertension. Guidelines for the management and prevention of hypertension have been published by societies such as the European Society of Cardiology/European Society of Hypertension, International Society of Hypertension [9], and American College of Cardiology/American Heart Association [1]; all providing dietary recommendations, but primarily focusing on selected nutrients and dietary patterns, such as sodium restriction, alcohol moderation, the Mediterranean diet, and the Dietary Approaches to Stop Hypertension (DASH) diet. New evidence of other nutrients and patterns related to BP has more recently emerged but have yet to be comprehensively addressed [10].
In the past decade, evidence implicating specific macronutrients and micronutrients [11,12], single food groups [[13], [14], [15]], and dietary patterns [16,17] has been evaluated in systematic reviews and meta-analyses of randomized controlled trials (RCTs) and observational studies in relation to high BP and risk of hypertension. Some major food groups and nutrients are reportedly inversely associated with risk of hypertension; including whole grains, fruits, nuts, dairy, fiber, potassium [[11], [12], [13],18], whereas those positively associated include red and processed meats, dietary sugar, and sodium [15,18,19]. Other systematic reviews of RCTs have reported lower BP in individuals who conformed to specific dietary patterns, such as the DASH, Nordic, and Mediterranean diets [16,17]. While these findings offer helpful approaches for the prevention and management of high BP, the collective quality, strength, and bias of these reviews have not been well evaluated.
The synthesis of published systematic reviews, that is, an umbrella review, offers a useful method to systematically examine and compare studies’ strength of evidence and precision of estimates, producing a high-level overview of a topic [20]. Previous umbrella reviews on the relationship between diet and BP have focused solely on dietary patterns, such as the Mediterranean [21] and other popular diets [22]; single nutrients, for example, vitamin C [23], vitamin D [24], and dietary fiber [25]; single foods, such as coffee [26], garlic [27], and chocolate [28]; and certain food groups, such as pulses and legumes [29], allium vegetables [30], nuts [31], and dairy products [32]. Furthermore, some of these umbrella reviews included only observational studies [29,32] or RCTs [22,23]. To the best of our knowledge, no single umbrella review has synthesized and graded the totality of the evidence investigating the relationship between BP and a combination of dietary patterns, food groups, single foods, beverages, macronutrients, and micronutrients, that would help update and fill the gap of current guidelines. This umbrella review of meta-analyses of RCTs and observational studies evaluates the association between these dietary factors and risk of hypertension with change in BP.
Methods
This umbrella was conducted following the Joanna Briggs Institute Umbrella Review Methodology [20]. The protocol for this umbrella review was registered on International Prospective Register of Systematic Reviews (PROSPERO) (CRD42019160516).
Literature search
A systematic literature search was conducted in the electronic bibliography databases: PubMed (MEDLINE), Embase, Web of Science, and Cochrane Central Register of Controlled Trials from inception until October 31, 2021. Meta-analyses of RCTs or observational studies were identified through predefined and tested search terms listed in Supplemental Table 1. Studies were restricted to human studies. Two authors (GA and QC) conducted independent database searches in parallel. Extracted lists were imported into Rayyan [33] for title and abstract review by GA and QC independently. Disagreements were resolved by a third author (RG).
Selection of meta-analyses
To avoid the inclusion of duplicate primary studies, when >1 meta-analysis was identified for a dietary exposure, we selected the most complete meta-analysis for RCTs and observational studies using the following stepwise process. In the first instance, we selected the one with the largest number of primary studies. If >1 meta-analysis had the same number of primary studies, we selected the one with the largest total sample size. If there was >1 study satisfying these criteria, the meta-analysis with the most information was selected. For each dietary exposure, where meta-analyses we excluded, these were reviewed to determine whether there was agreement on direction, magnitude, and significance of observed associations.
Data extraction and reporting
For each meta-analysis, the following data were extracted: first author, publication year, outcome (incidence hypertension, change in BP), intervention (any administration forms), comparison group (as defined in the RCTs), exposure, number of included primary studies, number of estimates, study design of the primary studies (RCTs, cohorts, case-control, cross-sectional), numbers of participants, number of cases (observational studies), health status of participants (normotensive, hypertensive, obesity, diabetes, or a mixture of health conditions), sex of participants, type of results (for example, high versus low or dose–response and its increment unit), heterogeneity (I2), method of analysis (random or fixed effects), estimates of effect or association and their 95% confidence interval (CI) and/or P value, risk of bias or quality score of primary studies, publication bias, and funding source and reported conflicts of interest.
The results are reported by the themes of dietary exposure: dietary patterns, foods/beverages, macronutrients, micronutrients, and other food bioactive compounds. Within each theme the results were separated into RCTs and observational studies. Summary tables were used to present characteristics of included reviews and Forest plots were constructed to visualize effect sizes using the values extracted from the meta-analyses included in the review. Individual dietary exposures were then grouped on the basis of the NutriGrade classification of evidence quality (high, moderate, low, and very low) [34] and the direction of effect on BP (decreased, increased, and no change). Finally, dietary exposures that reported a clinically significant change in BP (>3 mmHg) were identified.
Assessment of methodological quality
The methodological quality of the included publications was assessed with the validated Assessment of Multiple Systematic Reviews 2 (AMSTAR 2) tool [35]. Critical domains related to the conduct of the review, including the registration of a review protocol, adequacy of the literature search, justification of the excluded studies, risk of bias assessment of the included studies, appropriateness of meta-analytical methods, consideration of risk of bias in results interpretation, and presence and likely impact of publication bias, were evaluated. The overall confidence in the results of the included publications was then rated as high (no or 1 noncritical weakness), moderate (>1 noncritical weakness), low (1 critical flaw with or without noncritical weakness), or critically low (>1 critical flaw with or without noncritical weaknesses).
Assessment of quality of evidence
Systematic reviews serve as a valuable and reliable method that provides evidence-based dietary guidance for a wide range of research questions. To increase the confidence in the observed effects, the quality of systematic reviews is evaluated using different assessment tools. NutriGrade is a numerical scoring system that is designed to grade the evidence in nutrition research [34]. It was developed on the basis of the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) criteria [36], which grade the quality of evidence from systematic reviews for clinical practice. In addition to the GRADE criteria, the NutriGrade considers nutrition research-related data, such as risk of bias, directness of outcome (hard clinical outcome or surrogate markers), and funding bias when assessing the quality of evidence of meta-analyses of an association/effect between different dietary factors and outcomes.
Two reviewers (GA and RG) independently assessed the quality of each selected meta-analysis, and a third reviewer (DC) resolved conflicting scores. Six quality aspects were evaluated for both RCTs and observational studies: 1) risk of bias, study quality, or study limitations; 2) precision of the estimate; 3) heterogeneity; 4) directness; 5) publication bias; 6) funding bias; with 7) study design for meta-analyses of RCTs only, and 8) effect size and 9) dose–response association for observational studies only. An overall score (maximum of 10 points) of 8 points, 6 to <8 points, 4 to <6 points, and <4 points represents high, moderate, low, and very low quality of evidence, respectively. A meta-analysis could be rated as high quality even when individual items such as risk of bias, heterogeneity, or publication bias did not achieve the highest score. Where the manuscript reported multiple outcomes (for example, markers of metabolic syndrome) and the authors did not separately report risk of bias or funding bias for BP or hypertension, we scored on the basis of what the authors reported for the composite of the outcomes (for example, all outcomes of metabolic syndrome). For the meta-analyses of observational studies that reported both comparing high to low dietary intake and dose–response results, NutriGrade would be scored for the results of high to low dietary intake only.
Results
Results of the search
Figure 1 shows the PRISMA flowchart. Of the 17,099 records originally identified, 1277 publications were reviewed for its full text and 972 publications were subsequently excluded as they did not meet the selection criteria. Another 166 publications were superseded by other articles on the same topic but with a larger number of primary studies, estimates, or participants, or were replaced because they did not meet selection criteria, listed in Supplemental Table 2. A total of 175 publications reporting 341 meta-analyses of RCTs (145 publications) and 70 meta-analyses of diet exposures from observational studies (30 publications) were included (Supplemental Table 3).
FIGURE 1.
The role of diet in the prevention of hypertension and management of blood pressure: an umbrella review of meta-analyses of observational and intervention studies.
We identified the following dietary factors: 1) patterns of diet; 2) foods groups: meat, poultry, fish, and egg, milk and dairy products, fruits, vegetables, fruits and vegetable, starchy foods, legumes and pulses, nuts and seeds, cocoa, herb, spice, and condiments; 3) beverages; 4) macronutrients: carbohydrates, sugars, proteins, fats, and oils; 5) micronutrients: minerals, vitamins, probiotics, polyphenols, phytochemicals, nitrates, sweeteners (Supplemental Tables 4 and 5).
Characteristics of included meta-analyses
The primary studies in the included meta-analyses were mostly from Europe (n = 119), North America (n = 106), Asia (n = 87), a smaller number was from the Middle East (n = 61), Australia and New Zealand (n = 50), and South America (n = 36), and only a few meta-analyses from Africa (n = 10) (Supplemental Tables 4 and 5).
For RCTs, the number of primary studies included in the meta-analyses ranged from 2 [[37], [38], [39], [40], [41], [42], [43], [44], [45]] to 91 [46], total number of participants ranged from 69 [38,39] to 36,806 [47], and duration of trial ranged between 1 h [[48], [49], [50]] and 7 y [47] (Supplemental Table 4).
For the meta-analyses of observational studies, 30 publications (70 exposures) reported results comparing high to low dietary intake [13,18,[51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78]], of those 9 publications (18 exposures) also reported dose–response results [18,57,[60], [61], [62],64,72,74,75]; 3 publications (4 exposures) reported only dose–response results [13,64,67] (Supplemental Table 5). The number of primary studies included in the meta-analyses ranged from 2 [18,66,72] to 41 [68], total number of participants ranged between 5560 [76] and 640,525 [18], and the length of follow-up ranged from 1.3 [62] to 38 y [51,61,75].
Methodological quality
Overall using AMSTAR 2, 7.5% (n = 11) of the included publications for RCTs were assessed as high in methodological quality, such as very-low-carbohydrate ketogenic diet compared with (Supplemental Table 4) low-fat diet [79], Nordic diet compared with standard (usual) diet [17], and high-protein diet compared with low-protein diet [80].
About 43.2% (n = 63) were assessed as moderate, for example, fruit juice compared with controls [81], peanuts compared with standard (usual) diet [43], and cinnamon compared with controls [82]. Low methodological quality evidence was for 27.4% (n = 40) of included publications, such as curcumin compared with controls [83], decaffeinated coffee compared with controls [50], and taurine supplements compared with controls [84].
Critically low methodological quality was reported in 21.9% (n = 32) publications, for example; soy foods compared with controls [85], calcium supplements compared with controls [86], and proanthocyanidins compared with controls [87].
For observational studies, none of the publications were rated as high quality (Supplemental Table 5). About 65.5% (n = 19) were rated as moderate, for example, vegan compared with omnivore [52], legumes intake [18], and total fructose intake [59]. Low methodological quality of evidence was reported for 27.6% (n = 8), for example, adherence to Mediterranean diet [56] and dietary long-chain n-3 polyunsaturated fatty acids (PUFA) [76], whereas critically low was reported for 6.9% (n = 2), for example, selenium level and SBP [63], and dietary protein intake 2 [65] (Supplemental Table 5).
The main limitations of the low- and critically low-quality meta-analyses were no grey literature, list of excluded studies, risk of bias assessment, absence of test for publication bias, and unreported source funding.
Quality of the evidence
Using NutriGrade, 4.1% (n = 14) of the included meta-analyses of RCTs were rated as high and 11.4% (n = 39) very low and no meta-analysis of observational studies rated as high, and only (n = 4) were of moderate quality (Supplemental Tables 4 and 5). The main limitations identified were lack of tool for risk of bias assessment, selection bias, residual confounding, significant heterogeneity, small number of primary studies, absence of test for publication bias, and source of funding.
Heterogeneity
Of the included meta-analyses of RCTs, 6.5% (n = 22) did not evaluate between-study heterogeneity, whereas 35.4% (n = 121) of the meta-analyses reported significant heterogeneity defined by (P < 0.05) and 50.7% (n = 173) reported heterogeneity defined by I2 ≥ 40% (Supplemental Table 4). For observational studies, 7.1% (n = 5) did not evaluate heterogeneity, whereas 42.9% (n = 30) of the meta-analyses reported significant heterogeneity defined by (P < 0.05) and 50.0% (n = 35) reported heterogeneity defined by I2 ≥ 40% (Supplemental Table 5).
Publication bias
For RCTs, only 29.6% (n = 101) of the meta-analyses had 10 or more studies and reported no evidence of publication bias (low risk), 33.4% (n = 114) either had 5–9 studies and reported no evidence for publication bias or had 10 or more studies but reported moderate or small amount of publication bias (moderate risk), and 35.2% (n = 120) either had low number of primary studies, evidence for severe publication bias, or did not assess publication bias (unclear or severe risk).
Of the high and moderate evidence quality meta-analyses, (n = 10) and (n = 70) were of low risk of publication bias, respectively, and the interventions under reviewed were (high quality): very-low-carbohydrate ketogenic diet compared with low-fat diet (DBP) [79], DASH diet compared with controls [88], flaxseed compared with controls [89], multivitamin and multimineral compared with controls (DBP) [90], products with live bacteria compared with controls [91], grape and its products compared with controls [92], nitrates compared with controls (SBP) [93], urinary potassium compared with controls [94], and steviol glycoside compared with controls (DBP) [95].
For observational studies, 14.2% (n = 10) of the meta-analyses were of low risk, 32.9% (n = 23) moderate risk, and 57.1% (n = 40) unclear or severe risk of publication bias.
Evidence of diet, blood pressure, and hypertension
Here, we describe the findings in the meta-analyses and their evidence quality, focusing more on high- and moderate-quality evidence assessed by NutriGrade, alongside low- or very-low-quality evidence. The NutriGrade ratings for a dietary factor were often the same for SBP and DBP and the evidence was generally for populations of mixed health statuses, the exceptions were specified. We summarized the effects of diet exposure on blood pressure and risk of hypertension in Table 1 [[11], [13], [15], [17], [18], [19], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [54], [55], [56], [57], [58], [59], [60], [62], [66], [67], [71], [72], [73], [74], [75], [76], [77], [79], [80], [81], [82], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102], [103], [104], [105], [106], [107], [108], [109], [110], [111], [112], [113], [114], [115], [116], [117], [118], [120], [121], [122], [123], [124], [125], [126], [127], [128], [129], [130], [131], [132], [133], [134], [135], [136], [137], [138], [139], [141], [142], [143], [145], [146], [147], [148], [149], [150], [151], [153], [154], [155], [156], [157], [158], [159], [160], [161], [162], [163], [164], [165], [166], [167], [168], [169], [170], [171], [172], [173], [174], [175], [176], [177], [178], [179], [180], [181], [182], [183], [184], [185], [186], [187], [188], [189], [190], [191], [192], [193], [194], [195], [196], [197], [198], [199], [200], [201], [202], [203], [204], [205], [206], [207], [208], [209]]. The study characteristics, weighted mean difference, weighted mean change difference, or standardized mean difference in SBP or DBP or relative risk estimates for risk of hypertension are shown in Supplemental Table 3 for RCTs, Supplemental Table 4 for observational studies; NutriGrade and AMSTAR 2 ratings of the meta-analyses are presented in Supplemental Tables 3 and 4.
TABLE 1.
Summary of effects on blood pressure and risk of hypertension by study type and diet exposure
| (a) BP effects of diet exposure in randomized control trials by NutriGrade | ||||
|---|---|---|---|---|
| NutriGrade | Diet exposure | Decreased BP | Increased BP | No effect (NS) |
| High | Patterns of diet | Very-low-carbohydrate ketogenic diet vs. low-fat diet (DBP) [79] DASH diet1 [88] | ||
| Nuts and seeds | Flaxseed2 [89] | |||
| Minerals | Urinary potassium1 [94] | |||
| Vitamins | Multivitamin and multimineral (DBP) [90] | |||
| Probiotics | Products with live bacteria [91] | |||
| Polyphenols | Grape and its products [92] | |||
| Nitrates | Nitrates (SBP) [93] | |||
| Sweeteners | Steviol glycoside (DBP) [95] | |||
| Moderate | Patterns of diet | Low-carbohydrate diet vs. controls [102] Mediterranean diet [103] Dietary interventions1, low-calorie/fat diet, low-sodium diet, low-sodium and low-calorie/fat diet, low sodium/high potassium1, low-calorie/fat diet 1 [100] Plant-based diet [104] Vegetarian [105] High-protein diet [80] High-MUFA diet [101] |
Very-low-carbohydrate ketogenic diet (SBP) [79] Vegan diet [106] Hyperproteic diet [107] Low-carb and high-fat diet, low-carb and high-protein diet [44] Low-fat high-protein diet (DBP) [108] Low-fat high-carbohydrate diet [109] Mediterranean diet [100] |
|
| Meat, poultry, egg, and fish | Fish [117] Egg [118] |
|||
| Milk and dairy | Lactotripeptides [120] | |||
| Fruits and vegetables | Blueberry (DBP) [122] Berries3 [124] Cactus pear3 [45] Pomegranate1,2,3 [125] Beetroot1,3 [123] |
Blueberry (SBP) [122] Strawberry (SBP)2 [127] Bitter melon [126] |
||
| Legumes and pulses | Pulses (SBP) [142] | Pulses (DBP) [142] | ||
| Nuts and seeds | Sesame [143] Nigella seeds1 [143] |
Walnuts [145] | ||
| Cocoa | Cocoa products [149] | |||
| Herb, spice, and condiment | Cinnamon1 [82] | Licorice (DBP) [150] | Licorice (SBP) [150] | |
| Beverages | Alcohol reduction1 [153] Black tea [49] Green tea [96] |
Coffee [154] | ||
| Carbohydrates | Psyllium [156] Inositol [157] |
Inulin [158] | ||
| Sugars | Fructose (DBP) [161] | Free sugars (DBP) [15] | Fructose (SBP) [161] Free sugars (SBP) [15] |
|
| Proteins | L-carnitine supplements (DBP) [162] Soy protein2, soy protein isolate2 [85] Dietary peptides (SBP)1,2 [163] |
Soy foods2 [85] | ||
| Fats and oils | EPA and/or DHA supplements2 [169] | Olive oil supplements2 [170] | ||
| Minerals | Sodium or salt substitute1 [19] Magnesium supplements [176] |
Chromium supplements [177] | ||
| Vitamins | Folic acid supplements [97] Vitamin C supplements1 [184] Multivitamin and multimineral (SBP) [90] Vitamin D supplements [185] |
Vitamin D3 supplements2 [186] Vitamin D + Calcium [47] |
||
| Probiotics | Lactobacillusplantarum supplements 1 [190] | Fermented milk2 [191] | ||
| Polyphenols | Grape seed extract (SBP)2 [192] Quercetin (DBP) [194] Flavanols [46] Proanthocyanidins [87] |
Resveratrol2 [196] Anthocyanin supplements (SBP)2 [195] Grape seed extract (DBP)2 [192] |
||
| Phytochemicals | Phytosterols [202] Green coffee extract1 [203] Green tea extract (DBP) [204] Chlorogenic acid1 [205] |
Green tea extract (SBP) [204] Tea extract [204] Lycopene supplements [206] |
||
| Nitrates | Nitrates (DBP)1,2 [93] | |||
| Sweeteners | Stevioside1 [95] | Steviol glycoside (SBP), pure rebaudioside [95] | ||
| Others | Astaxanthin supplements [209] | |||
| Low | Patterns of diet | Portfolio diet [111] Paleolithic diet1 [110] Caloric restriction diet [112] Nordic diet1 [17] |
Low-fat high-protein diet (SBP) [106] High carb diet [113] |
|
| Meat, poultry, egg, and fish | ||||
| Milk and dairy | Whole-fat dairy products, low-fat dairy [121] | |||
| Fruits and vegetables | Strawberry (DBP) [127] Sour cherry (DBP) [132] Fruit juice (DBP) [81] Grapefruit (SBP)3 [131] Cranberry (SBP)1,3 [130] Ginger1,2,3 [129] Garlic1,2,3 [128] Fruit and vegetable3 [136] |
Goji berry3 [133] Sour cherry (SBP) [132] Fruit juice (SBP) [81] Orange juice [135] Grapefruit (DBP)3 [131] Cranberry (DBP)1,3 [130] Kiwi fruit3 [134] Tomato [137] |
||
| Starchy foods | Whole grains [141] | |||
| Legumes and pulses | Peanuts, soy nuts [43] | |||
| Nuts and seeds | Pistachios (SBP) [146] Almonds (DBP) [147] Cashew (SBP) [41] |
Pistachios (DBP) [146] Mixed nuts vs. standard (usual) diet, tree nuts (DBP) [37] Almonds (SBP) [147] Cashew (DBP) [41] Mixed nuts vs. controls (SBP) [43] Chia seed [148] |
||
| Herb, spice, and condiment | Curcumin (SBP) [98] | Curcumin (DBP) [98] | ||
| Beverages | Decaffeinated coffee [50] Sour tea1 [155] |
Beer [99] Tea [48] |
||
| Carbohydrates | Dietary fiber (DBP) [160] | Arabinoxylan-rich foods2 Mannans2 [11] B-glucan (DBP), Guar gum (DBP) [42] Dietary fiber (SBP) [160] |
||
| Proteins | Whey protein supplements1,2 [167] Milk protein1 [165] Dietary peptides (DBP) [163] Dietary protein2 [164] Soy milk [166] |
L-carnitine supplements (SBP) [162] L-citrulline supplements [168] Dietary animal proteins 2 [164] |
||
| Fats and oils | EPA supplements (SBP) [171] Fish oil supplements1,2 [172] |
DHA supplements, EPA supplements (DBP) [171] CLA supplements [173] |
||
| Minerals | Calcium supplements (SBP)2 [86] Dietary and supplemental calcium [179] Low vs. high urinary sodium to potassium ratio [178] Potassium supplements2 [180] |
Potassium and magnesium supplements [181] | ||
| Vitamins | Vitamin E supplements (SBP)1,2 [189] | Vitamin E supplements (DBP)1,2 [189] Active vitamin D2 [187] Folate supplements [188] |
||
| Polyphenols | Anthocyanins (DBP) [193] High-phenolic olive oil (SBP) [39] Grape polyphenols (SBP) [197] Quercetin1 [194] Flavonoid-rich cocoa (SBP)2 [199] Red wine polyphenols (SBP) [200] |
Flavonoid-rich fruits (hypertension population only) [201] Hydrobenzoic acids2 [193] High-phenolic olive oil (DBP) [39] Grape polyphenols (DBP) [197] Hesperidin [198] Flavonoid-rich cocoa (DBP)2 [199] Red wine polyphenols (SBP) [200] |
||
| Phytochemicals | Olive leaf extract (SBP) [40] | Olive leaf extract (DBP) [40] | ||
| Very low | Patterns of diet | High fruit and vegetable diet (DBP) [114] | Low AGE diet vs. high AGE diet [116] Breakfast skipping [38] Low-carb diet vs. low-fat diet2 [115] High fruit and vegetable diet (SBP) [114] |
|
| Meat, poultry, egg, and fish | Total meat | |||
| Fruits and vegetables | 100% fruit juice [138] Aronia berry (SBP) [139] |
Aronia berry (DBP) [139] | ||
| Nuts and seeds | Tree nuts (SBP) [37] Chia seed (SBP) [148] |
|||
| Herb, spice, and condiment | Saffron [151] | |||
| Carbohydrates | Chitosan [159] | B-glucan (SBP), guar gum (SBP), Konjac glucomannan, and pectin [42] | ||
| Fats and oils | Dietary and supplemental EPA and/or DHA2 [175] | ALA supplements2 [174] | ||
| Minerals | Urinary sodium2 [183] | Calcium supplements (DBP) [86] Zinc supplements [182] |
||
| Phytochemicals | Genistein (SBP) [208] | Caffeine1,2 [207] | Genistein (DBP) [208] | |
| (b) Risk of hypertension (relative risk, RR or odd ratio, OR) diet exposure in observation studies by NutriGrade | ||||
|---|---|---|---|---|
| NutriGrade | Diet exposure | Lower risk | Higher risk | No association (NS) |
| Moderate | Milk and dairy | Dairy products, low-fat dairy products, milk intake, fermented dairy intake [58] | ||
| Low | Patterns of diet | DII diet [54] | ||
| Meat, poultry, egg, and fish | Egg [77] | Total meat [77] | ||
| Milk and dairy | Whole-fat dairy products, yogurt, and cheese [58] | |||
| Fruits and vegetables | Fruits, vegetables, fruits, and vegetables [74] | |||
| Beverages | Coffee [75] | Alcohol [71] Sugar-sweetened soda [61] |
||
| Minerals | Dietary and supplemental calcium [60] | |||
| Vitamins | 25-hydroxyvitamin D [62] | |||
| Polyphenols | Flavan-3-ol2, flavanones2 [55] | |||
| Very low | Patterns of diet | Mediterranean diet [56] Healthy pattern [73] |
Acid load (PRAL) diet [67] | Acid load (NEAP) diet [67] |
| Meat, poultry, egg, and fish | Unprocessed red meat, processed red meat [18] Poultry [77] |
Fish [18] | ||
| Milk and dairy | Fluid dairy products [69] Low-fat dairy2 [13] |
|||
| Starchy foods | Whole grains [18] | French fries2 [72] | Refined grains [18] Baked/boiled/mashed potatoes2, potatoes2 [72] |
|
| Legumes and pulses | Legumes [18] | |||
| Nuts and seeds | Nuts [18] | |||
| Cocoa | Chocolate [66] | |||
| Beverages | Artificial-sweetened beverages [51,61] | |||
| Sugars | Fructose [59] | |||
| Fats and oils | Biomarker of long-chain PUFA, total long-chain PUFA [76] | Dietary long-chain PUFA [76] | ||
| Minerals | Dietary magnesium [57] | Serum magnesium [57] | ||
| Vitamins | 25-hydroxyvitamin D [62] | Dietary vitamin D [62] | ||
| Polyphenols | Anthocyanin2 [55] | Flavones2, flavonols2, total flavonoids2 [55] | ||
Abbreviations: AGE, advanced glycation end products; ALA, alpha-lipoic acid; AMSTAR, Assessment of Multiple Systematic Reviews; CLA, conjugated linoleic acid; DASH, Dietary Approaches to Stop Hypertension; DII, dietary inflammatory index; hypertension, hypertensive people only; NEAP, net endogenous acid production; PRAL, potential renal acid load.
Blood pressure change ≥3 mmHg.
Poor-quality meta-analyses (AMSTAR low or critically low).
Extract, juice, powder, paste, and supplementation.
Patterns of diet—RCTs
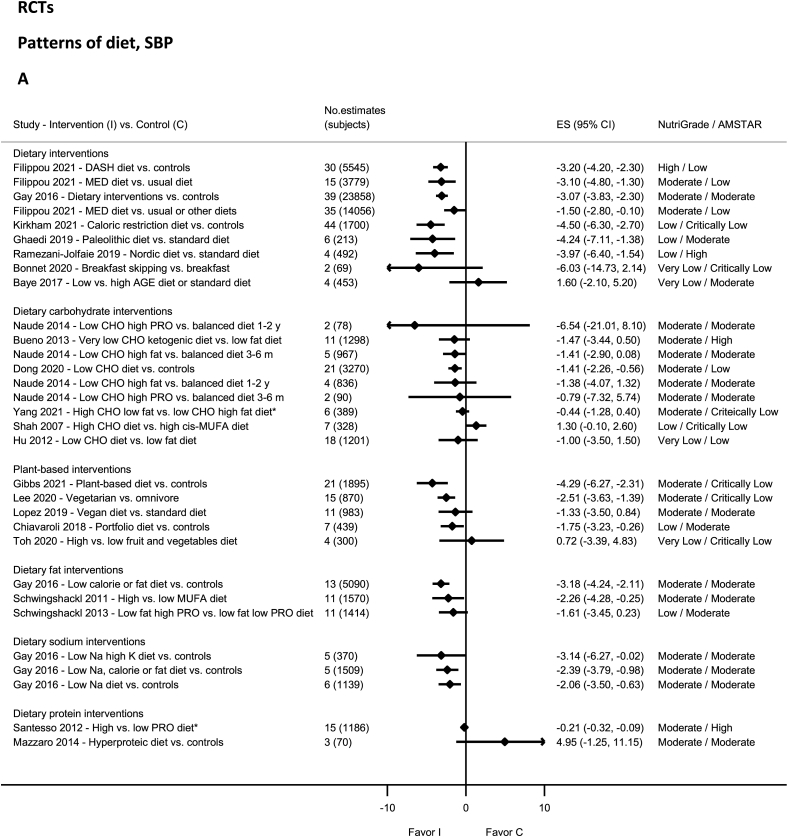
High-quality evidence on NutriGrade showed significantly decreased SBP and DBP with the DASH diet [88] and DBP with the very-low-carbohydrate ketogenic diet (Figure 2A and B) [79]. Moderate-quality evidence showed statistically significantly decreased SBP and DBP with dietary interventions [80,100,101], in particular for low-sodium diets with high-potassium [100], low-calorie or fat diets [100], low-carbohydrate diet [102], the Mediterranean diet [103], high-monounsaturated fatty acids (MUFA) diet [101], plant-based diet [104], and vegetarian diet [105]. Moderate-quality evidence showed nonsignificantly decreased SBP and DBP with other dietary patterns [44,79,[106], [107], [108], [109]] including vegan diet [106], a low-fat, high-protein diet (DBP) [108], and a low-carb, high-protein diet [44]. The evidence was rated as low quality for Paleolithic diet [110], Nordic diet [17], portfolio diet [111], caloric-restricted diet [112], low fat, high-protein diet (SBP) [108], high-carbohydrate diet [113] and very low quality for high fruit and vegetable diet [114], low-carb diet [115], breakfast skipping [38], and low advanced glycation end products [116].
FIGURE 2.
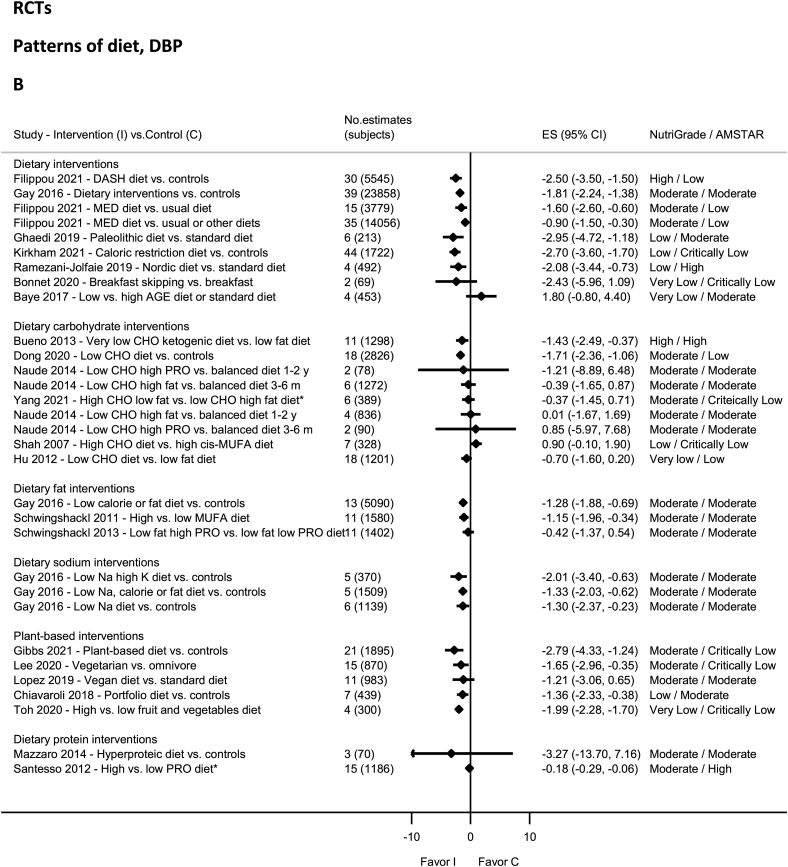
(A) Summary mean difference (ES), evidence quality (NutriGrade), and methodological quality (AMSTAR) of meta-analyses of randomized controlled trials investigating the effects of dietary patterns on systolic blood pressure. (B) Summary mean difference (ES), evidence quality (NutriGrade), and methodological quality (AMSTAR) of meta-analyses of randomized controlled trials investigating the effects of dietary patterns on diastolic blood pressure. Each solid diamond and the horizontal line across the diamond represents the summary mean difference or summary standardized mean difference (∗) and its 95% confidence interval reported by the published meta-analysis. AGE, advanced glycation end products; AMSTAR, Assessment of Multiple Systematic Reviews; CHO, carbohydrate; C, control; DASH, Dietary Approaches to Stop Hypertension; ES, mean difference estimate; I, intervention; K, potassium; MED, Mediterranean; Na, sodium; PRO, protein.
Patterns of diet—observational studies
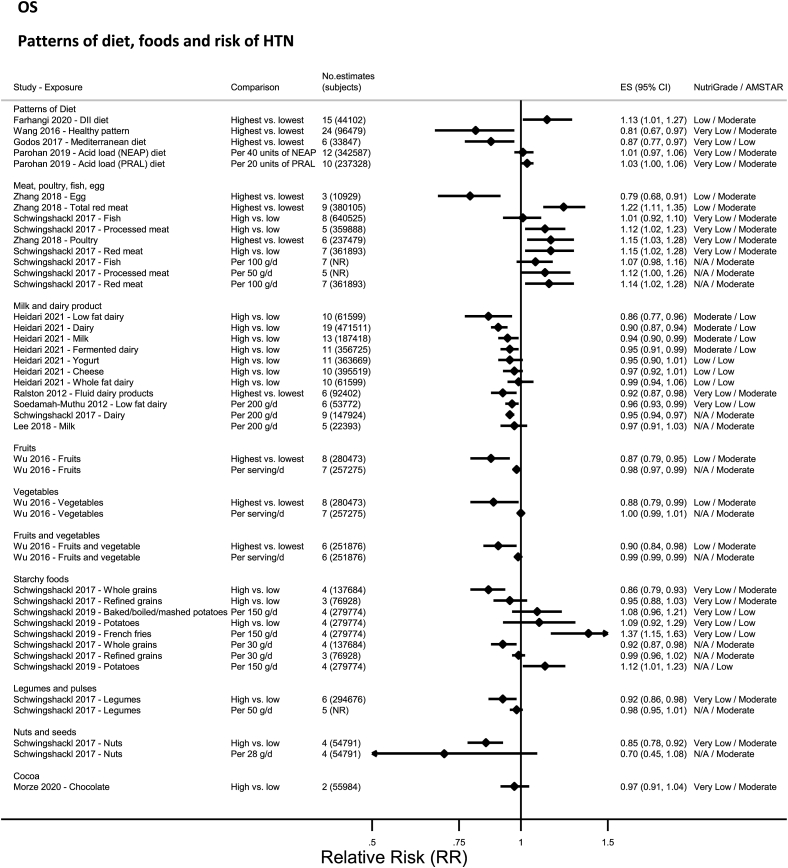
No evidence was rated as high- or moderate-quality evidence by NutriGrade (Figure 3). Only low-quality or very-low-quality evidence was available for vegan diet [52], diets characterized by the dietary inflammatory index [54], or empirically [73], Mediterranean diet [56], and acid load diets [53,67], and vegetarian diet [68].
FIGURE 3.
Summary relative risk (ES), evidence quality (NutriGrade), and methodological quality (AMSTAR) of the meta-analyses of observational studies investigating dietary patterns and foods in relation to risk of hypertension. Each solid diamond and the horizontal line across the diamond represents the summary relative risk and its 95% confidence interval for high vs. low comparison or per unit increment of exposure reported by the published meta-analysis. AMSTAR, Assessment of Multiple Systematic Reviews; DII, dietary inflammatory index; NEAP, net endogenous acid production; PRAL, potential renal acid load.
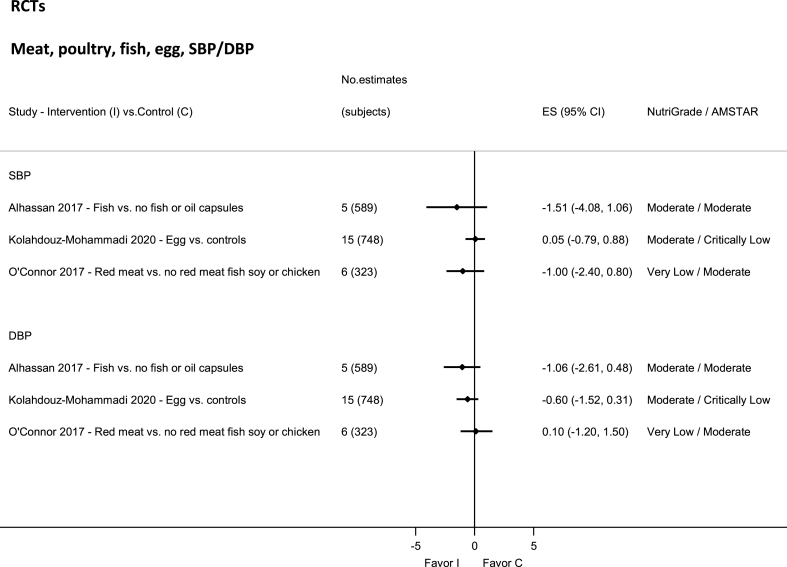
Meat, eggs, and fish—RCTs
No evidence was rated as high quality by NutriGrade (Figure 4). There was moderate-quality evidence showing nonsignificantly decreased SBP and DBP with fish intake [117], and egg [118], and very-low-quality evidence for total red meat [119].
FIGURE 4.
Summary mean difference (ES), evidence quality (NutriGrade), and methodological quality (AMSTAR) of the meta-analyses of randomized controlled trials investigating the effects of meat, poultry, fish, and egg on systolic and diastolic blood pressure. Each solid diamond and the horizontal line across the diamond represents the summary mean difference and its 95% confidence interval reported by the published meta-analysis. AMSTAR, Assessment of Multiple Systematic Reviews; C, control; ES, mean difference estimate; I, intervention.
Meat, eggs, and fish—observational studies
No evidence was rated as high or moderate quality by NutriGrade (Figure 3). There was only low-quality evidence for egg and total meat [77], and very-low-quality evidence for poultry [77], and from 1 meta-analysis on processed and unprocessed meat, red meat, and fish [18].
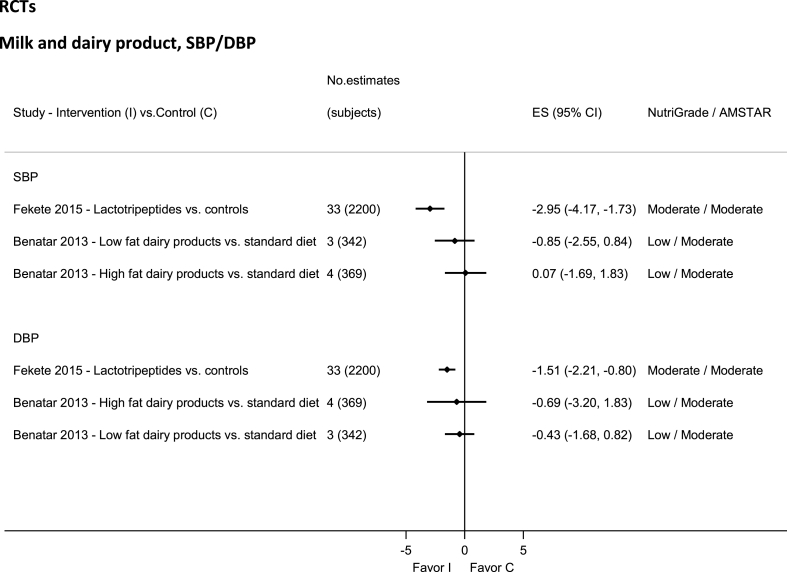
Dairy—RCTs
No evidence was rated high or very low quality by NutriGrade (Figure 5). Moderate-quality evidence showed significantly decreased SBP and DBP with lactotripeptides intake [120]. There was low-quality evidence for whole and low-fat dairy [121].
FIGURE 5.
Summary mean difference (ES), evidence quality (NutriGrade), and methodological quality (AMSTAR) of the meta-analyses of randomized controlled trials investigating the effects of milk and dairy products on systolic and diastolic blood pressure. Each solid diamond and the horizontal line across the diamond represents the summary mean difference and its 95% confidence interval reported by the published meta-analysis. AMSTAR, Assessment of Multiple Systematic Reviews; C, control; ES, mean difference estimate; I, intervention.
Dairy—observational studies
No evidence was rated as high quality by NutriGrade (Figure 3). There was moderate-quality evidence showing significantly lower HTN with total and low-fat dairy, milk, and fermented dairy [58]. There was low-quality evidence, from 1 meta-analysis, for whole-fat dairy, yogurt, cheese, and fluid dairy products [58].
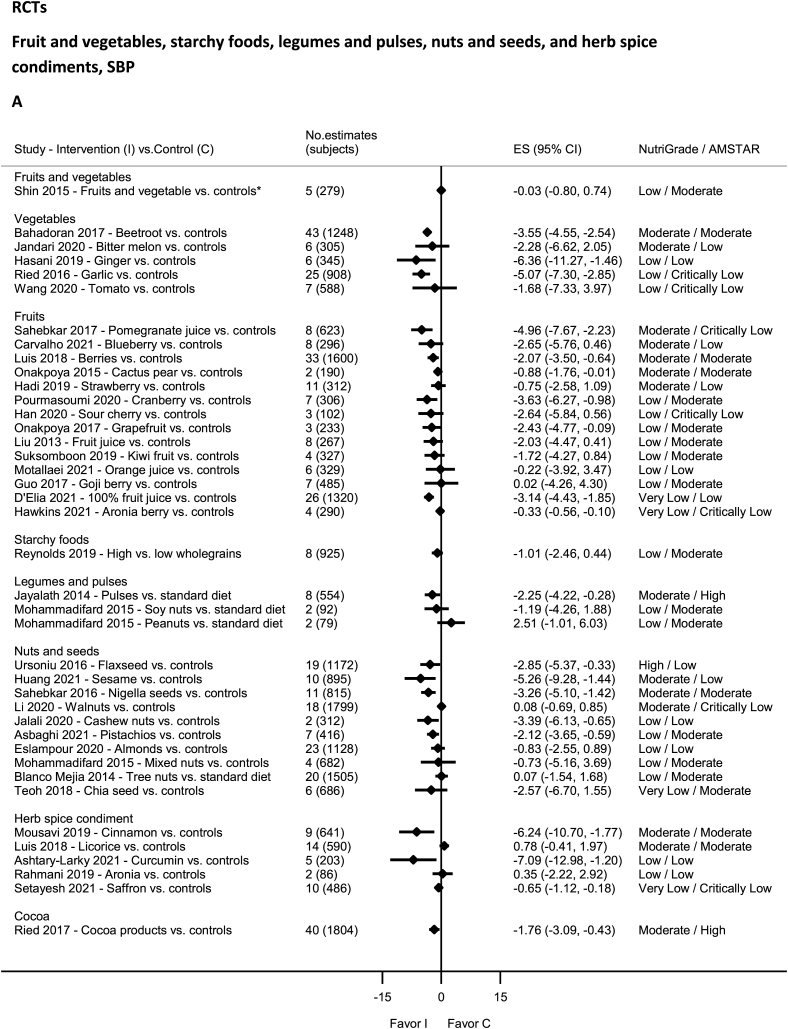
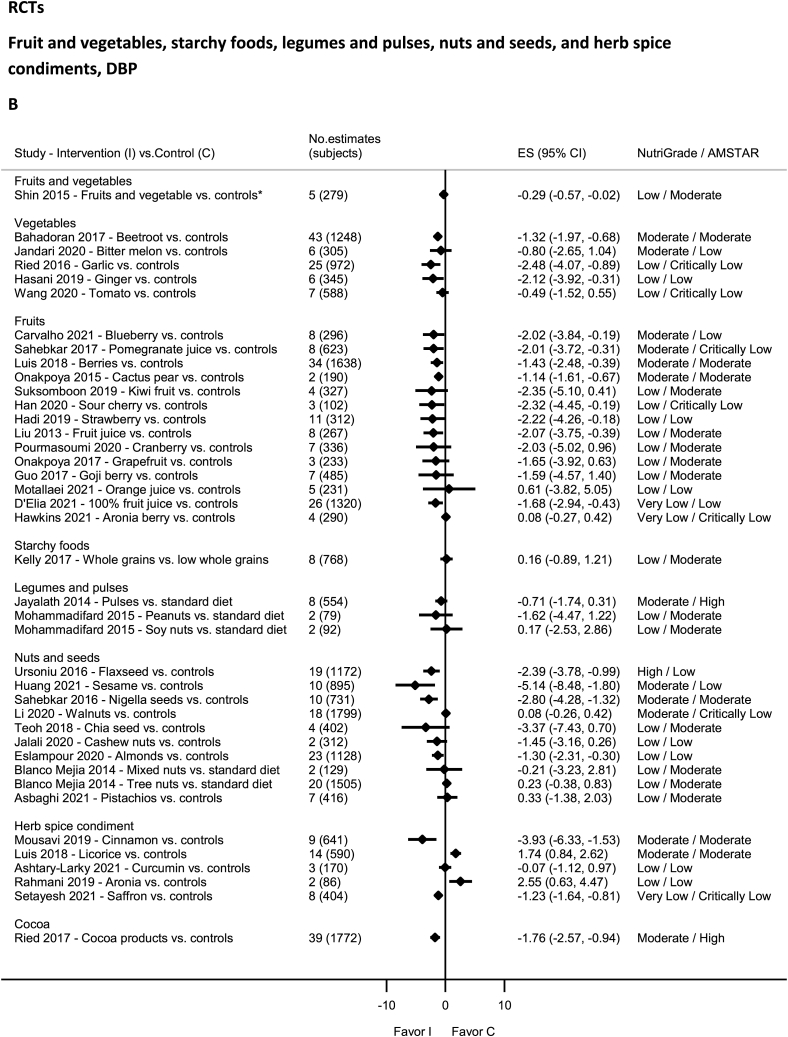
Fruit and vegetables—RCTs
No evidence was rated as high quality by NutriGrade (Figure 6A and B). There was moderate-quality evidence showing significantly decreased DBP with blueberry [122], SBP and DBP with beetroot [123], berries [124], cactus pear [45], and pomegranate [125] intakes. There was moderate-quality evidence showing nonsignificantly decreased SBP with blueberry [122], SBP and DBP with bitter melon [126] and strawberry (SBP) intakes [127]. Low-quality evidence was available for garlic [128], ginger [129], cranberry (SBP) [130], grapefruit (SBP) [131], strawberry (DBP) [127], sour cherry (DBP) [132], Goji berry [133], kiwi fruit [134], fruit juice [81], orange juice [135], fruits and vegetables [136], and tomato [137], and very-low-quality evidence for 100% fruit juice [138] and aronia berry [139].
FIGURE 6.
(A) Summary mean difference (ES), evidence quality (NutriGrade), and methodological quality (AMSTAR) of the meta-analyses of randomized controlled trials investigating the effects of fruit and vegetables, starchy foods, legumes and pulses, nuts and seeds, and herb spice condiments on systolic blood pressure. (B) Summary mean difference (ES), evidence quality (NutriGrade), and methodological quality (AMSTAR) of meta-analyses of randomized controlled trials investigating the effects of fruit and vegetables, starchy foods, legumes and pulses, nuts and seeds, and herb spice condiments on diastolic blood pressure. Each solid diamond and the horizontal line across the diamond represents the summary mean difference or summary standardized mean difference (∗) and its 95% confidence interval reported by the published meta-analysis. AMSTAR, Assessment of Multiple Systematic Reviews; C, control; ES, mean difference estimate; I, intervention.
Fruit and vegetables—observational studies
No evidence was rated as high, moderate, or very low quality (Figure 3), rather only low-quality evidence was reported by NutriGrade, for fruits, vegetables, and fruits and vegetables [74].
Starchy food: grains and potatoes—RCTs
No evidence was rated as high, moderate, or very low quality (Figure 6A and B), rather low-quality evidence by NutriGrade, for whole grains [140,141].
Starchy food: grains and potatoes—observational studies
No evidence was rated as high, moderate, or low quality (Figure 3), rather only very-low-quality evidence rated by NutriGrade was reported for whole grains [18], refined grains [18], and from 1 meta-analysis for potatoes, baked/boiled mashed potatoes, and French fries [72].
Legumes and pulses—RCTs
No evidence was rated as high or very low quality of evidence by NutriGrade (Figure 6A and B). There was moderate-quality evidence for intake of pulses (significant for SBP only) [142] and low quality of evidence for intakes of peanuts and soy nuts [43].
Legumes and pulses—observational studies
No evidence was rated as high, moderate, or low quality by NutriGrade (Figure 3), rather only very-low-quality evidence for legumes [18].
Nuts and seeds—RCTs
There was high-quality evidence by NutriGrade showing significantly decreased SBP and DBP with flaxseed intake (Figure 6A and B) [89] and moderate-quality evidence showing a significant reduction in SBP and DBP with intake of sesame [143], Nigella seeds [144], and a nonsignificant reduction in SBP and DBP with walnuts intake [145]. The evidence was rated as low quality for pistachio [146], mixed nuts (DBP) [37] (SBP) [43], tree nuts (DBP) [37], almonds [147], cashew nuts [41], chia seeds (DBP) [148]; and very low quality for chia seeds (SBP) [148] and tree nuts (SBP) [37].
Nuts and seeds—observational studies
No evidence was rated as high, moderate or low quality by NutriGrade (Figure 3), rather only very low quality evidence for nuts [18].
Cocoa—RCTs
No evidence was rated as high, low, or very low quality by NutriGrade (Figure 6A and B). There was moderate-quality evidence showing significantly decreased SBP and DBP with cocoa products intake [149].
Cocoa—observational studies
No evidence was rated as high, moderate, or low quality by NutriGrade (Figure 3). There was only very-low-quality evidence for cocoa products [66].
Herbs, spice, and condiment—RCTs
No evidence was rated as high quality by NutriGrade (Figure 6A and B). There was moderate-quality evidence showing significantly decreased SBP and DBP with intakes of cinnamon [82]; and increased DBP with licorice supplementation [150]. The evidence was rated as low quality for curcumin [83] and very low for saffron [151].
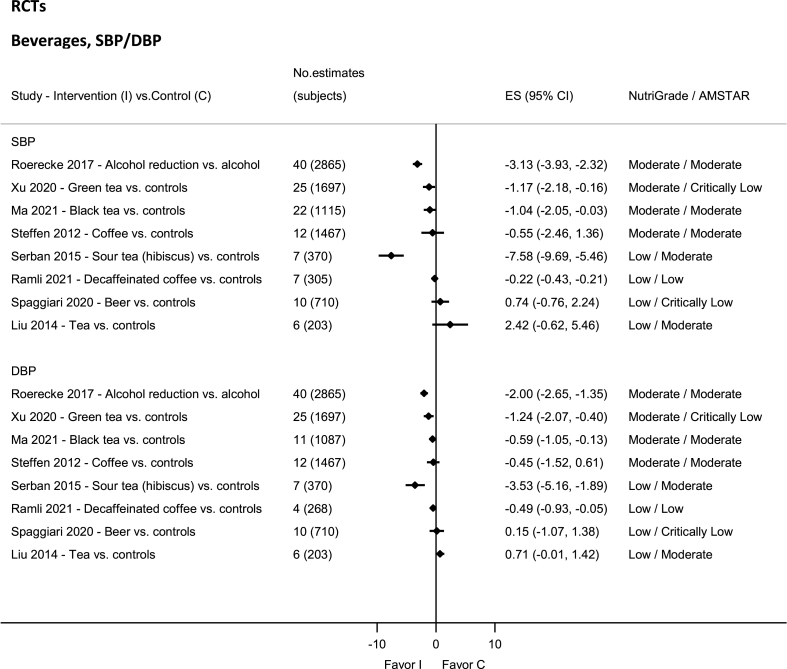
Beverages—RCTs
No evidence was rated as high or very low quality by NutriGrade (Figure 7). There was moderate-quality evidence showing significant reductions in SBP and DBP with intakes of black tea [49], green tea [152], and alcohol [153]. There was moderate-quality evidence showing a nonsignificant reduction in SBP and DBP with coffee intake [154]. The evidence was rated as low quality for tea [48], decaffeined coffee [50], and hibiscus [155].
FIGURE 7.
Summary mean difference (ES), evidence quality (NutriGrade), and methodological quality (AMSTAR) of the meta-analyses of randomized controlled trials investigating the effects of alcohol and other beverages on systolic and diastolic blood pressure. Each solid diamond and the horizontal line across the diamond represents the summary mean difference and its 95% confidence interval reported by the published meta-analysis. AMSTAR, Assessment of Multiple Systematic Reviews; C, control; ES, mean difference estimate; I, intervention.
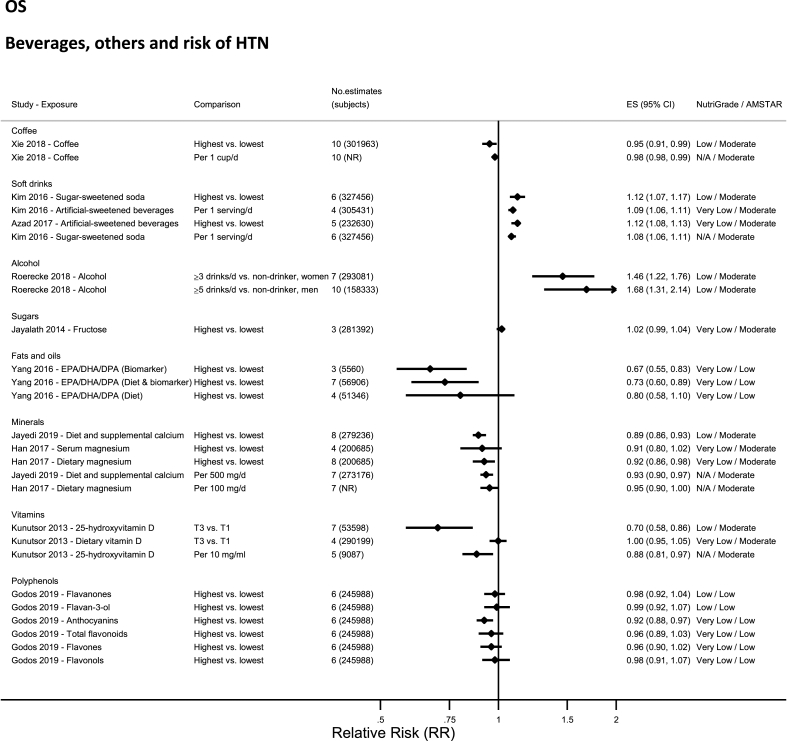
Beverages—observational studies
No evidence was rated as high or moderate quality by NutriGrade (Figure 8). There was low-quality evidence for alcohol [71], coffee [75], and soft drinks (sugar-sweetened) [61], and very-low-quality evidence for artificial-sweetened beverages [51].
FIGURE 8.
Summary relative risk (ES), evidence quality (NutriGrade), and methodological quality (AMSTAR) of the meta-analyses of observational studies investigating alcohol and other beverages in relation to risk of hypertension. Each solid diamond and the horizontal line across the diamond represents the summary relative risk and its 95% confidence interval for high vs. low comparison or per unit increment of exposure reported by the published meta-analysis. AMSTAR, Assessment of Multiple Systematic Reviews; DPA, docosapentaenoic acid; NR, not reported; T3 vs. T1, tertile 3 vs. tertile 1.
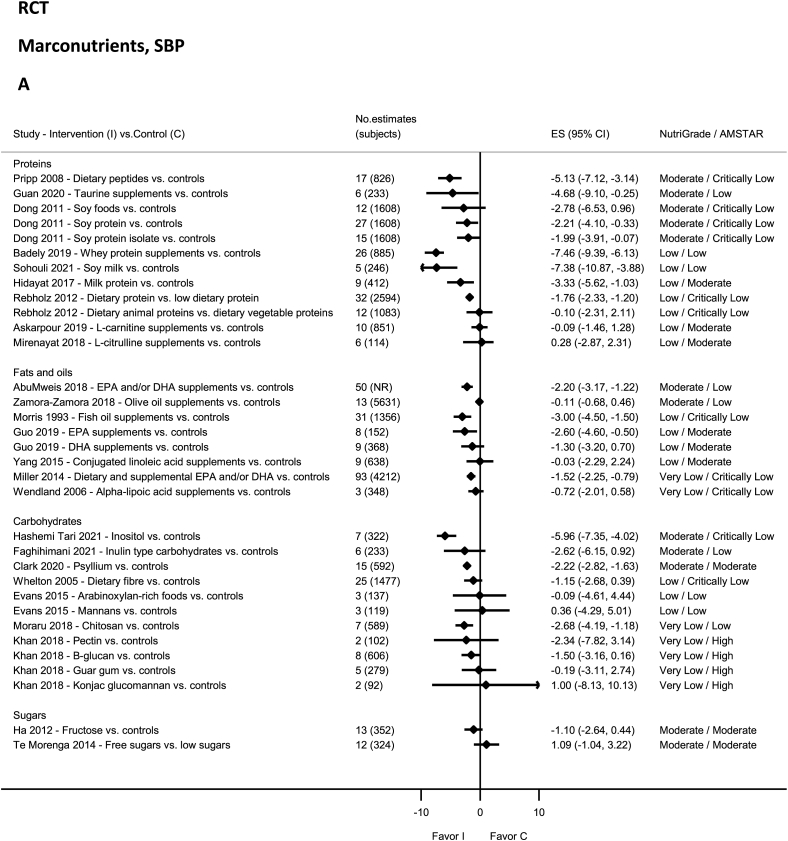
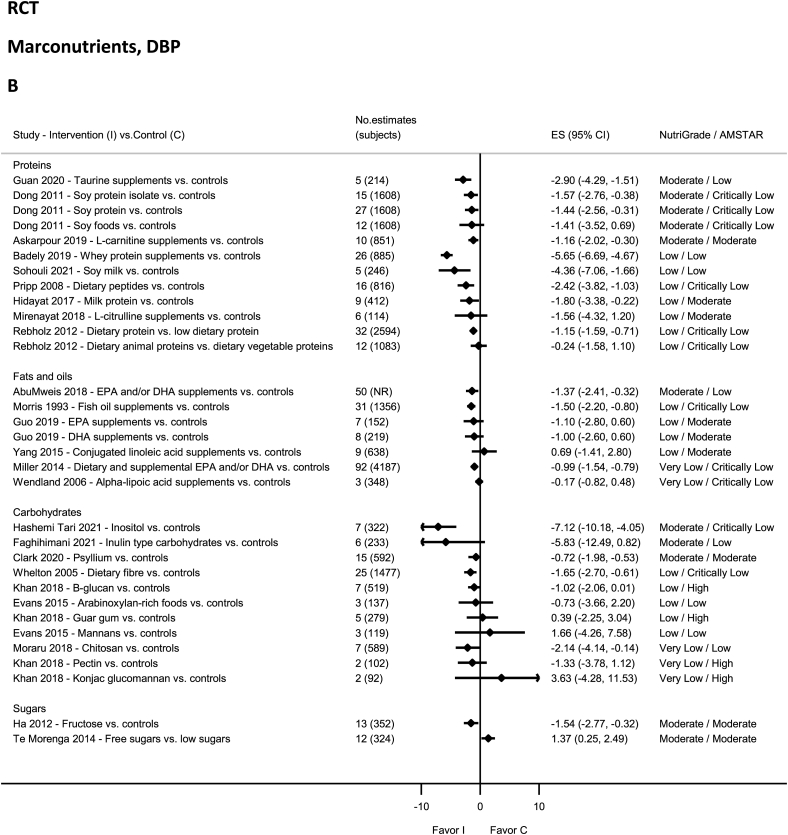
Carbohydrates, fiber, sugars—RCTs
No evidence on carbohydrates was rated as high quality by NutriGrade (Figure 9A and B). There was moderate-quality evidence showing significant reductions in SBP and DBP with psyllium intake [156] and inositol [157]. There was moderate-quality evidence showing a nonsignificant reduction in SBP and DBP with inulin [158]. The evidence was rated as low quality for Arabinoxylan-rich foods and mannans [11], guar gum (DBP), and B-glucan (DBP) [42], and very-low-quality evidence for chitosan [159], and from the same meta-analysis for Konjac glucomannan, Pectin, B-glucan (SBP), and guar gum (SBP) [42]. None of the evidence on dietary fiber was rated as high, moderate, or very low quality by NutriGrade (Figure 9A and B), rather was low-quality evidence for total fiber [160]. None of the evidence on sugars was rated as high, low, or very low quality by NutriGrade (Figure 9A and B), rather there was moderate-quality evidence showing increased SBP and DBP with free-sugars intake [15]. Moderate-quality evidence revealed decreased DBP with fructose [161].
FIGURE 9.
(A) Summary mean difference (ES), evidence quality (NutriGrade), and methodological quality (AMSTAR) of the meta-analyses of randomized controlled trials investigating the effects of macronutrients on systolic blood pressure. (B) Summary mean difference (ES), evidence quality (NutriGrade), and methodological quality (AMSTAR) of meta-analyses of randomized controlled trials investigating the effects of macronutrients on diastolic blood pressure. Each solid diamond and the horizontal line across the diamond represents the summary mean difference and its 95% confidence interval reported by the published meta-analysis. AMSTAR, Assessment of Multiple Systematic Reviews; C, control; ES, mean difference estimate; I, intervention; NR, not reported.
Carbohydrates, fiber, and sugars—observational studies
No evidence was rated as high, moderate, or low quality by NutriGrade (Supplemental Table 4), only very-low-quality evidence for fructose [59].
Proteins—RCTs
No evidence was rated as high or very low quality by NutriGrade (Figure 9A and B), but there was moderate-quality evidence showing significantly decreased SBP and DBP with intakes of L-carnitine supplements (DBP) [162], soy protein isolate [85], soy protein [85], Taurine supplement [84], and dietary peptides [163]. The evidence was rated as low quality for dietary protein and dietary animal and vegetable proteins [164], milk protein [165], soy milk [166], dietary peptides (DBP) [163], soy foods [85], whey protein supplements [167], L-carnitine supplements (SBP) [162], and L-citrulline supplementation [168].
Proteins—observational studies
No evidence was rated as high, moderate, or low quality by NutriGrade (Supplemental Table 4), only very-low-quality evidence for dietary protein [65].
Fats and oils—RCTs
No evidence was rated as high quality by NutriGrade (Figure 9A and B). There was moderate-quality evidence showing significantly decreased SBP and DBP with EPA and/or DHA supplements use [169]. There was moderate-quality evidence showing a nonsignificant reduction in SBP with olive oil supplements [170]. The evidence was rated as low quality for DHA and EPA supplements [171], fish oil supplements [172], and conjugated linoleic acid supplements [173], and very low quality for alpha-lipoic acid supplements [174], and dietary and supplemental EPA and/or DHA [175].
Fats and oils—observational studies
No evidence was rated as high, moderate, or low quality by NutriGrade (Supplemental Table 4). There was very-low-quality evidence for EPA, DHA, and docosapentaenoic acid from dietary sources, biomarkers, and both dietary sources and biomarkers [76].
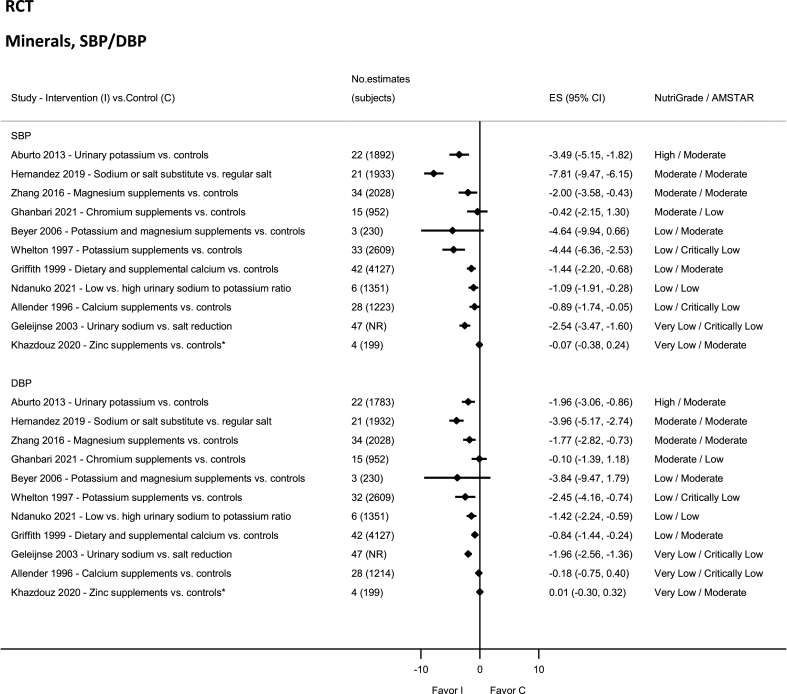
Minerals—RCTs
There was high-quality evidence showing significant reductions in SBP and DBP with high urinary potassium levels (Figure 10) [94]. Moderate-quality evidence reported significantly reduced SBP and DBP with low sodium or salt substitute [19] and magnesium supplements [176]. Moderate-quality evidence showed nonsignificant reductions in SBP and DBP with chromium intake [177]. The evidence was rated as low quality for urinary sodium and potassium ratio [178], calcium (dietary and supplement) [179], calcium (SBP) (supplement) [86], potassium (supplement) [180], and potassium and magnesium (supplement) [181] in adults with hypertension. Very low quality of evidence was reported for calcium (DBP) (supplement) [86], zinc (supplement) [182], and reduced urinary sodium [183].
FIGURE 10.
Summary mean difference (ES), evidence quality (NutriGrade), and methodological quality (AMSTAR) of the meta-analyses of randomized controlled trials investigating the effects of dietary minerals on systolic and diastolic blood pressure. Each solid diamond and the horizontal line across the diamond represents the summary mean difference or summary standardized mean difference (∗) and its 95% confidence interval reported by the published meta-analysis. AMSTAR, Assessment of Multiple Systematic Reviews; C, control; ES, mean difference estimate; I, intervention; NR, not reported.
Minerals—observational studies
No evidence was rated as high or moderate quality by NutriGrade (Figure 8). There was low-quality evidence showing significant inverse associations between dietary and supplemental calcium intake and risk of hypertension [60], and very-low-quality evidence for magnesium [57], selenium [63], and for urinary potassium [78].
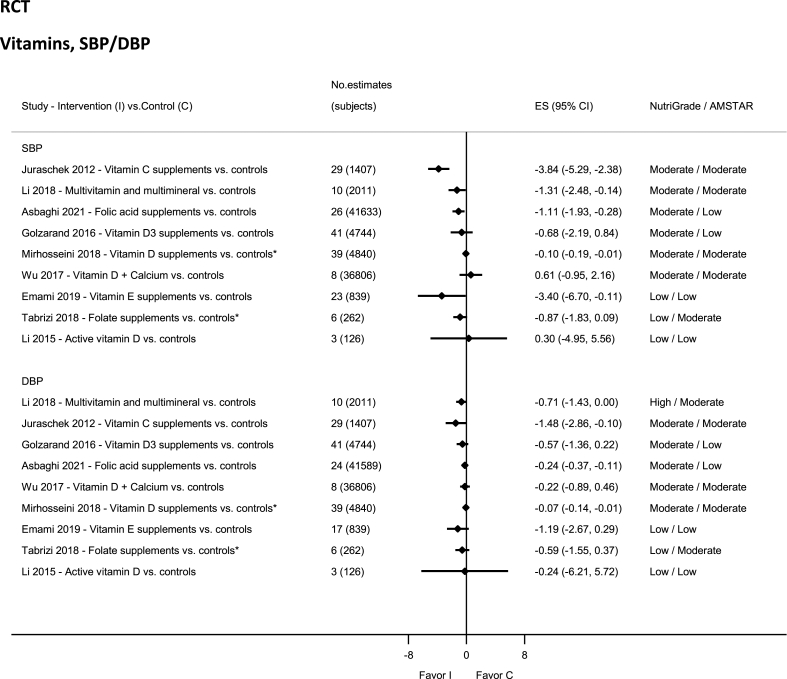
Vitamins—RCTs
There was high-quality evidence showing reduction in DBP with multivitamin intake (Figure 11) [90] and moderate-quality evidence showing significantly decreased SBP and DBP with intakes of vitamin C (supplements) [184], multivitamin intake (SBP) [90], and vitamin D (supplement) [185]. There was moderate-quality evidence showing nonsignificant results—decreased SBP and DBP with vitamin D3 supplement [186]; and decreased DBP but increased SBP with calcium plus vitamin D supplementation [47]. The evidence was rated as low quality for active vitamin D supplement [187], folate supplement [188], and vitamin E supplement [189].
FIGURE 11.
Summary mean difference (ES), evidence quality (NutriGrade), and methodological quality (AMSTAR) of the meta-analyses of randomized controlled trials investigating the effects of vitamin intakes on systolic and diastolic blood pressure. Each solid diamond and the horizontal line across the diamond represents the summary mean difference or summary standardized mean difference (∗) and its 95% confidence interval reported by the published meta-analysis. AMSTAR, Assessment of Multiple Systematic Reviews; C, control; ES, mean difference estimate; I, intervention.
Vitamins—observational studies
No evidence was rated as high or moderate quality by NutriGrade (Figure 8). There was low-quality evidence for 25-hydroxyvitamin D level [62] and serum vitamin C [70], and very low for dietary vitamin D intake [62]
Probiotics—RCTs
There was high-quality evidence showing reduction in SBP and DBP with intake of live bacteria products by NutriGrade [91] (Figure 12A and B). There was moderate-quality evidence showing significantly decreased SBP and DBP with Lactobacillusplantarum supplements [190] and nonsignificant decrease in SBP and DBP with fermented milk [191].
FIGURE 12.
(A) Summary mean difference (ES), evidence quality (NutriGrade), and methodological quality (AMSTAR) of the meta-analyses of randomized controlled trials investigating the effects of polyphenols, phytochemicals, probiotics, nitrates, sweeteners, and other dietary factors on systolic blood pressure. (B) Summary mean difference (ES), evidence quality (NutriGrade), and methodological quality (AMSTAR) of the meta-analyses of randomized controlled trials investigating the effects of polyphenols, phytochemicals, probiotics, nitrates, sweeteners, and other dietary factors on diastolic blood pressure. Each solid diamond and the horizontal line across the diamond represent the summary mean difference or summary standardized mean difference (∗) and its 95% confidence interval reported by the published meta-analysis. AMSTAR, Assessment of Multiple Systematic Reviews; C, control; ES, mean difference estimate; I, intervention; NR, not reported.
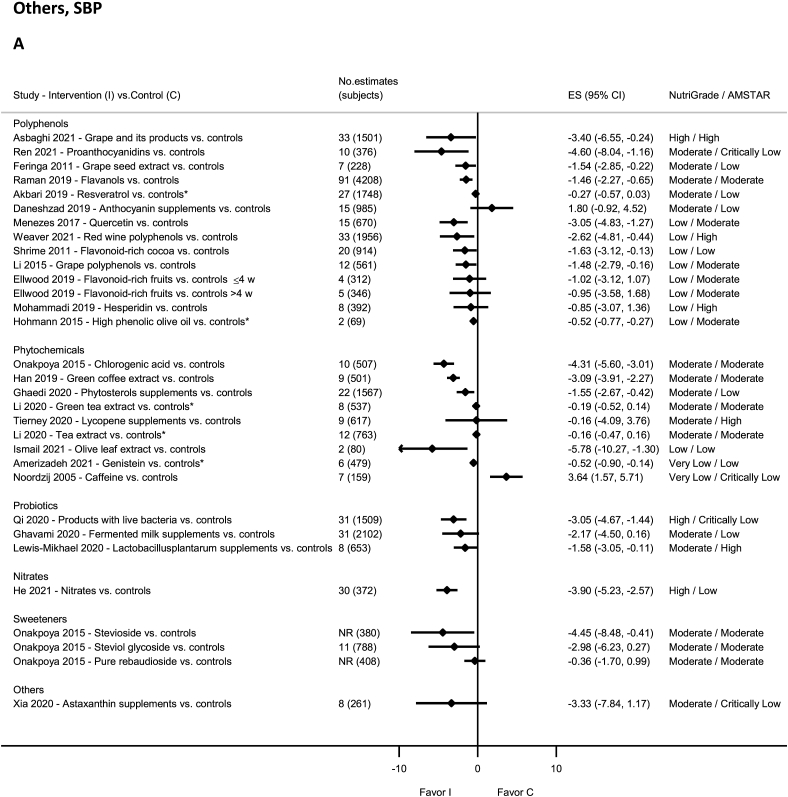
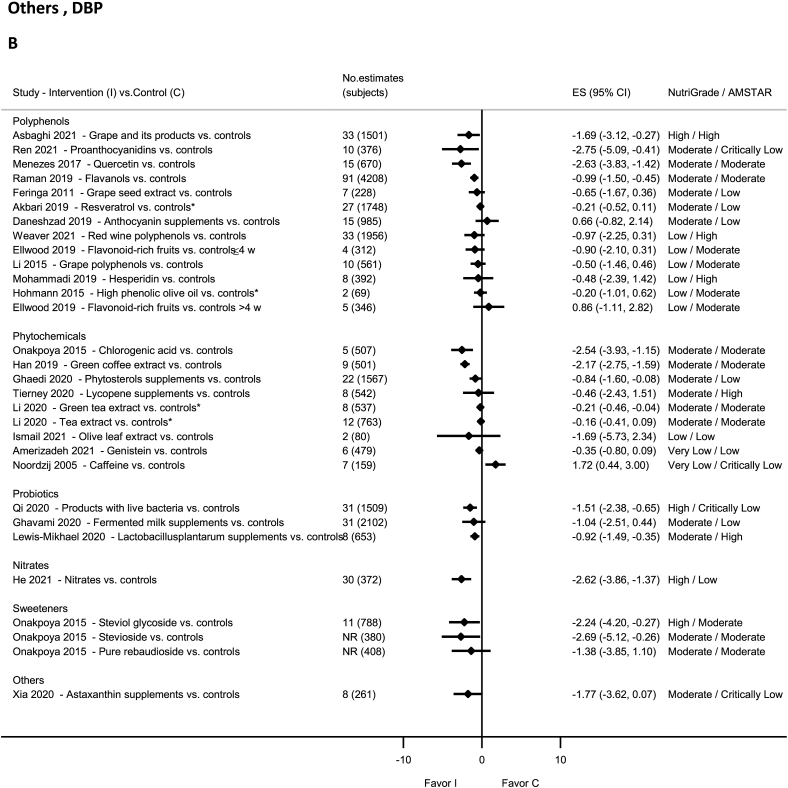
Polyphenols—RCTs
There was high-quality evidence showing reduction in SBP and DBP with grape intake and its products [92] (Figure 12A and B). There was moderate-quality evidence showing significant reductions in SBP and DBP with intakes of grape seed extract (SBP) [192], anthocyanins (berries, red grapes, and red wine) (SBP) [193], quercetin (DBP) [194], flavanols [46], and proanthocyanidins [87].
There was moderate-quality evidence showing a nonsignificant increase in SBP and DBP with anthocyanin supplements [195], and decrease in SBP and DBP with resveratrol supplementation [196] and grape seed extract (DBP) [192]. The evidence was rated as low-quality evidence for anthocyanins (berries, red grapes, and red wine) (DBP) [193], grape polyphenols [197], high-phenolic olive oil [39], hesperidin [198], hydrobenzoic acids [193], quercetin (SBP) [194], flavonoid-rich cocoa [199], red wine polyphenols [200], and flavonoid-rich fruits in adults with hypertension [201].
Polyphenols—observational studies
No evidence was rated as high or moderate quality by NutriGrade (Supplemental Table 4), rather only low- and very-low-quality evidence for anthocyanins, flavanols, flavanones, flavones, flavonols, and total flavonoids [55].
Phytochemicals—RCTs
No evidence was rated as high quality by NutriGrade (Figure 12A and B). There was moderate-quality evidence showing significantly decreased SBP and DBP with intakes of Phytosterols supplements [202], green coffee extract [203], green tea extract (DBP) [204], and chlorogenic acid [205]. There was moderate-quality evidence showing nonsignificant reductions in SBP and DBP with tea extract and green tea extract (SBP) [204], and Lycopene supplements [206]. The evidence was rated as low quality for olive leaf extract [40] and very low quality for caffeine [207] and Genistein [208].
Nitrites and nitrates—RCTs
There was high-quality evidence on NutriGrade showing significant reductions in SBP with nitrates (Figure 12A and B) and moderate-quality evidence showing significant reductions in DBP [93].
Sweeteners—RCTs
There was high-quality evidence on NutriGrade showing significantly decreased DBP with steviol glycoside intake [95] and moderate-quality evidence showing significant reductions in SBP and DBP with stevioside intake [95] (Figure 12A and B). The evidence was rated as moderate quality but showed nonsignificant reductions in SBP and DBP with intakes of pure rebaudioside and steviol glycoside (SBP) [95].
Others—RCTs
There was moderate-quality evidence on NutriGrade showing a nonsignificant decrease in SBP and DBP with Astaxanthin supplement [209] (Figure 12A and B).
Discussion
Principle findings
This umbrella review of meta-analyses of RCTs and observational studies provides the first extensive and comprehensive overview that synthesizes and grades the strength of evidence of numerous dietary factors and changes in BP and risk of hypertension. We reviewed a total of 175 publications and reported 341 meta-analyses of RCTs and 70 meta-analyses of observational studies, using NutriGrade to assess the quality of each selected meta-analysis and AMSTAR 2 for a methodological quality of included publications. Meta-analyses of DASH dietary patterns showed that it was the most effective dietary pattern for reducing BP and its effect was comparable to antihypertensive pharmacological treatment, with similar findings for the Mediterranean diet. As part of our analyses, we found that the majority of studies investigating the relationship between dietary components and hypertension and BP were of poor-quality evidence or poorly reported. Despite this, the examined evidence supports the dietary guidelines recommended by several authoritative health bodies and highlights the relationship between BP and several other dietary factors.
Meta-analyses of RCTs for DASH, very-low-carbohydrate ketogenic diet (DBP), flaxseed, nitrates (SBP), urinary potassium, multivitamins and multiminerals (DBP), steviol (DBP), products with live bacteria, nitrates (SBP), and grape and its products were graded as high confidence in the effect estimate using NutriGrade, and further research probably will not change the confidence in the effect estimate. This review shows that no meta-analyses of observational studies were graded as high, and very few were graded as moderate quality. Further research could add evidence on the confidence and may change the effect estimate of meta-analyses having low or very low quality. Likewise, the methodological quality assessed using AMSTAR 2 was graded as high in only 11 of the included publications (all RCTs), with a majority of publications graded as moderate (43% for RCTs publications and 66% for observation studies’ publications). The poor-quality assessments may be due to the lack of quantitative risk of bias assessment, selection bias, significant heterogeneity, a small number of primary studies (<5), BP considered as a surrogate marker, absence of a test for publication bias, unreported source of funding, or moderate effect sizes (for observational studies), while the poor reporting may be due to the absence of grey literature or list of excluded studies. Overall, our review found that risk of bias remains uncertain for most of the available trials owing to poor reporting. This point is particularly concerning given that the majority of the trials were conducted after the Consolidated Standards of Reporting Trials guidelines were first reported in 1993 and published in 1996 [210]. We also found high heterogeneity, which may be due to the differences in trial designs, comparison groups, study populations, and analysis methods.
This umbrella review supports current recommended guidelines for the management and prevention of hypertension [1,9] by adhering to the DASH and Mediterranean dietary patterns, and restricting sodium with moderate alcohol intake. In accordance with these recommendations, we reported that the DASH dietary pattern (high-quality RCTs) significantly lowers SBP and DBP [96], as do some of its components, including fruits and vegetables [45,74,123,124], whole grains [18], legumes and pulses [18,142], nuts and seeds [18,89,144,211], total red meat and poultry [77], and lactotripeptides intake [120]. We also found urinary potassium excretion (high-quality RCTs) [94], low-sodium diets (moderate-quality RCTs) [96] associated with lower BP, but not with low-fat dairy (not significant for RCTs, but significant in moderate-quality observational studies) [121]. Our report supports recommendations for adhering to the Mediterranean diet, on the basis of moderate-quality evidence from RCTs [100] and very-low-quality evidence from observational studies. The lowering effects of alcohol reduction were also evident on the basis of moderate-quality evidence from RCTs [153] and low-quality evidence from observational studies. However, our review did not indicate a beneficial effect of decreased BP with moderate-quality RCTs of increased vegan diet [106], hyperproteic diet [107], low-carb, high-fat diet or low-carb, high-protein diet [44], and fish [117].
Possible explanations
Biological mechanisms can be used to explain many of the findings from this study. For instance, the DASH diet and its components can reduce BP, as shown with high-quality evidence, by improving peripheral vascular function and associated metabolic improvements [212,213]. Moderate-quality evidence of restricted sodium diets can reduce BP through elevated plasma renin activity, serum aldosterone, plasma levels of noradrenaline and adrenaline, total cholesterol, and triglycerides [214]. The effect of low-calorie or fat diets on BP can be attributed to improvement in insulin resistance [215]. Furthermore, high-potassium intake decreased blood pressure in moderate-quality studies. This agrees with animal models that reported increased renin–angiotensin activity, renal injury, and raised BP in potassium-depleted rats [216,217]. For a high-protein diet and a very-low-carbohydrate ketogenic diet, the BP-lowering effect may be explained by the amino acid content, specifically arginine, a substrate for nitric oxide, that improves vasodilation, endothelial function, and insulin resistance leading to lower BP [218,219], or by the partially substituting carbohydrate with protein intake [220]. Nitric oxide may also play a role in the BP-lowering effects of other dietary components. For example, the BP-lowering effects of soy protein reported in moderate-quality evidence may be attributed to the actions of estrogen [221] and other constituents of soya, such as arginine, a precursor of nitric oxide [222]. Likewise, L-carnitine supplements demonstrated reduced DBP in moderate-quality studies, mainly due to an increase in nitric oxide [223]. Vitamin C was also found to reduce BP in moderate-quality studies, mainly related to increases in the tetrahydrobiopterin level, a cofactor that enhances the production of nitric oxide and bioavailability [224]. As for nitrates and nitrate-rich sources such as beetroot, the BP-lowering effects reported from moderate-/high-quality evidence may be related to nitric oxide production maintaining vascular health [225]. Furthermore, bioactive polyphenols and their constituents such as flavonoids, present in beetroot [226], berries, cactus pear, pomegranate, and cocoa, may be responsible for the BP reduction effects reported in moderate-quality studies through promoting nitric oxide bioactivity, reduction of some inflammatory pathways [227], and reduction of vascular resistance and arterial stiffness [228,229], which may lead to improved vascular function. Curcumin was associated with decreased BP on the basis of moderate-quality evidence, mainly through the reduction of oxidative stress and the induction of anti-inflammatory effects by suppressing factors such as adhesion molecules and nuclear factor-kappa [230], in addition to increasing nitric oxide bioavailability and inhibition of angiotensin-converting enzymes [231]. Similar explanations were suggested for the BP-lowering effects of quercetin and green tea as reported by moderate-quality evidence; in addition, green tea was shown to maintain vascular tone by balancing vasoconstricting substances, such as angiotensin II, prostaglandins, and endothelin-1 [194].
Furthermore, the BP-lowering effect by flaxseed seen in high-quality studies may be attributed to its components, such as phytoestrogens that reduced angiotensin I-induced increase of BP and alpha-linolenic acid, which reduces BP [232] through lowering the activity of soluble epoxide hydrolase [233]. EPA and/or DHA supplements reduced BP according to moderate-quality evidence. This may be related to the stimulated synthesis of prostacyclin by n-3 PUFA [234], which acts as a vasodilator, and reduces vascular resistance with n-3 PUFA [235]. Magnesium supplements showed reductions in BP as reported by moderate-quality evidence. It has been proposed that magnesium functions as a calcium channel blocker, stimulating endothelial function and vasodilation [236]. Vitamin D supplements were also found to decrease BP, as reported in moderate-quality evidence. The mechanism may be related to the association of vitamin D with reducing oxidative stress and inflammatory responses, improving endothelial function, and glucose homeostasis, and other cardioprotective effects [237]. Findings of reduced BP with green coffee extract and chlorogenic acid from moderate-quality evidence may be due to the chlorogenic acids present in green coffee that reduces the stress hormone cortisol [238], regulates glucose metabolism, and suppresses macrophage infiltration leading to blood vessel remodeling [239]. The beneficial effects of lactotripeptides and live bacteria reported in moderate-quality studies for decreasing the BP level include improved lipid levels, insulin resistance, and regulation of the renin–angiotensin system [240]. In addition, cocoa contains theobromine, linked to vasoactivity and consequently blood pressure reduction [241].
Findings of reduced BP in moderate-quality evidence with fructose intakes are inconsistent with clinical and animal studies. This may be explained by the heterogeneity in BP measures in the included studies, such as whether postprandial BP, which is most affected by fructose, was measured, rather than after an overnight fast when fructose may have been completely metabolized [161]. On the contrary, free sugars increased BP according to moderate-quality evidence, likely due to the fructose content that has been shown to stimulate triglycerides and cholesterol circulation, elevated synthesis of urate, which decreases nitric oxide synthesis resulting in vasoconstriction [242]. Licorice, used in producing candies and sweets, elevated BP according to moderate-quality evidence through its electrolyte content and effects on the renin–angiotensin–aldosterone axis [243]. Lastly, steviol glycosides were associated with decreased SBP [95], which can be explained by increased renal plasma flow and glomerular filtration rate [244].
Comparison with previous umbrella reviews
Findings from previous umbrella reviews were comparable with some of the findings presented here. For example, Dinu et al. [21] reported that greater adherence to the Mediterranean diet reduced BP in their meta-analyses of RCTs, whereas no evidence was presented for observational studies. We found moderate-quality evidence for reduced BP with the Mediterranean diet, and no evidence of an association in observational studies. Other umbrella reviews reported BP-lowering effects of high-protein diets [22], low-fat diets [22], and DASH [22,245], Nordic [245], and portfolio dietary patterns [245], consistent with our findings. Other umbrella reviews were also consistent with our report; for instance, Grosso et al. [26] reported no effects of BP with coffee intake whereas caffeine intake significantly increased BP; Schwingshackl et al. [27] and Wan et al. [30] found significant reductions in BP with garlic intake; Veronese et al. [28] reported no effects of chocolate on BP; Theodoratou et al. [24] reported no effects of supplemental vitamin D on BP; Godos et al. [32] reported lower hypertension risk and high BP with total dairy consumption; another study found the reducing effects of dietary fiber on DBP only [25]; and no effects for nuts on BP were reported in previous umbrella reviews [31]. Contrary to our findings, Ashor et al. [23] reported no effects of vitamin C on BP, which may be attributed to characteristics of included trials and large heterogeneity.
RCTs compared with observational studies
Intervention studies have known design advantages over observational studies ,including objective dietary measures, randomization including a control group. Observational studies are constrained by the scope of data relating to diet and other confounding variables, often subject to self-measurement error, residual confounding, and recall bias [246]. Despite the superiority of RCTs from a design perspective, the applicability of findings to the real world is challenging (particularly inadequate compliance in long-term dietary intervention trails) and may be limited by physiologically unacceptable levels of intake, study population, and outcomes measured [247]. Dietary recommendations and policies should be guided by rigorous systematic reviews and different study designs addressing the same dietary exposure could provide different results; any review of poor methodological quality could be misleading [248]. Therefore, the integration of evidence from both observational studies and intervention studies are required to improve dietary quality and lower disease burden.
Strengths and limitations
Our umbrella review has several strengths. We provide the first systematic, comprehensive overview of the role of dietary factors and risk of hypertension and change in BP. Furthermore, we evaluated the methodological quality and quantity of evidence using validated tools: AMSTAR 2 and NutriGrade, the latter is designed on the basis of widely used Grade but tailored for nutritional studies. This approach allowed critical identification of good-quality evidence from well-designed meta-analyses that showed statistical and more importantly clinically significant effects/associations that inform public health recommendations and policy formulation. We also highlighted topics with low-quality reviews to potentially stimulate further research efforts. Several dietary factors evaluated by RCTs are less prone to the biases inherent in observational studies, which are, for nutrition-disease research, a rare quality.
Our umbrella review also had several limitations. For example, published meta-analyses of the same dietary factor might only have few common studies because of the different inclusion criteria for the review. Interventions varied substantially (active ingredients, administrative forms, dosing, and duration) between the included RCTs, which may explain the observed heterogeneity. Furthermore, the lack of intervention blinding, a challenging aspect of nutrition-related studies, may increase risk of bias and decrease the accuracy of results. Included observational studies were of varying design, and used different analytical methods and diverse study populations, which may explain the observed heterogeneity. In addition, BP measurement methods varied across publications and may be limited by random or systematic errors [249]. Another limitation is that the use of office BP does not capture measurements during longer periods of time or in everyday activities [249].
Furthermore, evidence on other subgroups, for example, sex, age, geographical locations, race, ethnicity, diabetes or chronic kidney disease status, and weight category were not synthesized in the current umbrella review. These factors could bias the observed associations. The current umbrella review is dependent on the reporting of the published meta-analyses and could not account for any potential missing primary studies, overlapping of primary studies or study participants. Finally, we repeated the search for the time lapsed from our initial search and included publications from 1 December 2019 to 31 October 2021. We identified 3578 relevant publications. A total of 166 publications were identified as exposures covered in the current review, these were compared for the direction and magnitude of effect and where appropriate, we replaced publications from our previous search.
Conclusions and recommendations
The effect and association of dietary patterns, food groups, single foods, beverages, macronutrients, and micronutrients with risk of hypertension and change in BP were previously examined in several published meta-analyses, but this umbrella review categorically supports recommended dietary guidelines involving the DASH and Mediterranean patterns, restricting sodium, with moderate alcohol intake, as indicated by mostly moderate-quality RCTs. To achieve high-quality evidence and provide strong recommendations, future studies should consider several topics with only low quality of evidence observed (that is, small number of studies, reported bias, etc.). Future studies should focus on exposures likely to be biologically associated with risk of hypertension and change in BP but currently showing that the quality of evidence is still low. Future research should also focus on new dietary factors that have not yet been investigated (or published) so far. It is worth noting that this paper primarily focuses on dietary variables and comparisons across studies, rather than the actual differences between BP levels on the basis of baseline variability. Nonetheless, the majority of the studies examined the intervention effect on the basis of the difference of changes of BP from baseline. Hence, Wilder’s principle [250]—the expected difference in BP from the initial reading is higher if the initial BP reading is high—may be at play. In this regard, future studies should explore the potential influence of Wilder’s principle by observing the actual differences between BP levels on the basis of baseline variability.
Author contributions
The authors’ responsibilities were as follows—RG, GSA, DSMC, QC: designed research, conducted research, analyzed data, and performed analysis; RG, GSA, QC: wrote the paper; DSMC, LVH: revised the work critically for important intellectual content; QC: had primary responsibility for final content; and all authors: read and approved the final manuscript.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest
None of the authors report a conflict of interest related to research presented in this article. QC is an employee and shareholder of Amgen Inc. The work presented here was conducted while QC was an employee of Imperial College London. Amgen Inc. was not involved in this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.advnut.2023.09.011.
Contributor Information
Ghadeer S. Aljuraiban, Email: galjuraiban@ksu.edu.sa.
Rachel Gibson, Email: rachel.gibson@kcl.ac.uk.
Doris SM. Chan, Email: d.chan@imperial.ac.uk.
Linda Van Horn, Email: lvanhorn@northwestern.edu.
Queenie Chan, Email: q.chan@imperial.ac.uk.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Whelton P.K., Carey R.M., Aronow W.S., Casey D.E., Jr., Collins K.J., Dennison Himmelfarb C., et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension. 2018;71(6):1269–1324. doi: 10.1161/hyp.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 2.Lewington S., Clarke R., Qizilbash N., Peto R., Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 3.Ettehad D., Emdin C.A., Kiran A., Anderson S.G., Callender T., Emberson J., et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387(10022):957–967. doi: 10.1016/s0140-6736(15)01225-8. [DOI] [PubMed] [Google Scholar]
- 4.Stanaway J.D., Afshin A., Gakidou E., Lim S.S., Abate D., Abate K.H., et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1923–1994. doi: 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou B., Carrillo-Larco R.M., Danaei G., Riley L.M., Paciorek C.J., Stevens G.A., et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 2021;398(10304):957–980. doi: 10.1016/S0140-6736(21)01330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lichtenstein A.H., Appel L.J., Brands M., Carnethon M., Daniels S., Franch H.A., et al. Diet and lifestyle recommendations revision 2006. Circulation. 2006;114(1):82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 7.Appel L.J., Brands M.W., Daniels S.R., Karanja N., Elmer P.J., Sacks F.M. Dietary approaches to prevent and treat hypertension. Hypertension. 2006;47(2):296–308. doi: 10.1161/01.HYP.0000202568.01167.B6. [DOI] [PubMed] [Google Scholar]
- 8.Warren H.R., Evangelou E., Cabrera C.P., Gao H., Ren M., Mifsud B., et al. Genome-wide association analysis identifies novel blood pressure loci and offers biological insights into cardiovascular risk. Nat. Genet. 2017;49(3):403–415. doi: 10.1038/ng.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Unger T., Borghi C., Charchar F., Khan N.A., Poulter N.R., Prabhakaran D., et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension. 2020;75(6):1334–1357. doi: 10.1161/HYPERTENSIONAHA.120.15026. [DOI] [PubMed] [Google Scholar]
- 10.Owolabi M., Olowoyo P., Miranda J.J., Akinyemi R., Feng W., Yaria J., et al. Gaps in hypertension guidelines in low- and middle-income versus high-income countries: a systematic review. Hypertension. 2016;68(6):1328–1337. doi: 10.1161/HYPERTENSIONAHA.116.08290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans C.E., Greenwood D.C., Threapleton D.E., Cleghorn C.L., Nykjaer C., Woodhead C.E., et al. Effects of dietary fibre type on blood pressure: a systematic review and meta-analysis of randomized controlled trials of healthy individuals. J. Hypertens. 2015;33(5):897–911. doi: 10.1097/HJH.0000000000000515. [DOI] [PubMed] [Google Scholar]
- 12.Filippini T., Violi F., D'Amico R., Vinceti M. The effect of potassium supplementation on blood pressure in hypertensive subjects: a systematic review and meta-analysis. Int. J. Cardiol. 2017;230:127–135. doi: 10.1016/j.ijcard.2016.12.048. [DOI] [PubMed] [Google Scholar]
- 13.Soedamah-Muthu S.S., Verberne L.D., Ding E.L., Engberink M.F., Geleijnse J.M. Dairy consumption and incidence of hypertension: a dose-response meta-analysis of prospective cohort studies. Hypertension. 2012;60(5):1131–1137. doi: 10.1161/HYPERTENSIONAHA.112.195206. [DOI] [PubMed] [Google Scholar]
- 14.Rahmani J., Clark C., Kord Varkaneh H., Lakiang T., Vasanthan L.T., Onyeche V., et al. The effect of Aronia consumption on lipid profile, blood pressure, and biomarkers of inflammation: a systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2019;33(8):1981–1990. doi: 10.1002/ptr.6398. [DOI] [PubMed] [Google Scholar]
- 15.Te Morenga L.A., Howatson A.J., Jones R.M., Mann J. Dietary sugars and cardiometabolic risk: systematic review and meta-analyses of randomized controlled trials of the effects on blood pressure and lipids. Am. J. Clin. Nutr. 2014;100(1):65–79. doi: 10.3945/ajcn.113.081521. [DOI] [PubMed] [Google Scholar]
- 16.Ndanuko R.N., Tapsell L.C., Charlton K.E., Neale E.P., Batterham M.J. Dietary patterns and blood pressure in adults: a systematic review and meta-analysis of randomized controlled trials. Adv. Nutr. 2016;7(1):76–89. doi: 10.3945/an.115.009753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramezani-Jolfaie N., Mohammadi M., Salehi-Abargouei A. The effect of healthy Nordic diet on cardio-metabolic markers: a systematic review and meta-analysis of randomized controlled clinical trials. Eur. J. Nutr. 2019;58(6):2159–2174. doi: 10.1007/s00394-018-1804-0. [DOI] [PubMed] [Google Scholar]
- 18.Schwingshackl L., Schwedhelm C., Hoffmann G., Knuppel S., Iqbal K., Andriolo V., et al. Food groups and risk of hypertension: a systematic review and dose-response meta-analysis of prospective studies. Adv. Nutr. 2017;8(6):793–803. doi: 10.3945/an.117.017178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernandez A.V., Emonds E.E., Chen B.A., Zavala-Loayza A.J., Thota P., et al. Effect of low-sodium salt substitutes on blood pressure, detected hypertension, stroke and mortality. Heart. 2019;105(12):953–960. doi: 10.1136/heartjnl-2018-314036. [DOI] [PubMed] [Google Scholar]
- 20.Aromataris E., Fernandez R., Godfrey C.M., Holly C., Khalil H., Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int. J. Evid. Based Health. 2015;13(3):132–140. doi: 10.1097/xeb.0000000000000055. [DOI] [PubMed] [Google Scholar]
- 21.Dinu M., Pagliai G., Casini A., Sofi F. Mediterranean diet and multiple health outcomes: an umbrella review of meta-analyses of observational studies and randomised trials. Eur. J. Clin. Nutr. 2018;72(1):30–43. doi: 10.1038/ejcn.2017.58. [DOI] [PubMed] [Google Scholar]
- 22.Dinu M., Pagliai G., Angelino D., Rosi A., Dall'Asta M., Bresciani L., et al. Effects of popular diets on anthropometric and cardiometabolic parameters: an umbrella review of meta-analyses of randomized controlled trials. Adv. Nutr. 2020;11(4):815–833. doi: 10.1093/advances/nmaa006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashor A.W., Brown R., Keenan P.D., Willis N.D., Siervo M., Mathers J.C. Limited evidence for a beneficial effect of vitamin C supplementation on biomarkers of cardiovascular diseases: an umbrella review of systematic reviews and meta-analyses. Nutr. Res. 2019;61:1–12. doi: 10.1016/j.nutres.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Theodoratou E., Tzoulaki I., Zgaga L., Ioannidis J.P.A. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials, BMJ. Br. Med. J. 2014;348:g2035. doi: 10.1136/bmj.g2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McRae M.P. Dietary fiber is beneficial for the prevention of cardiovascular disease: an umbrella review of meta-analyses. J. Chiropractic Med. 2017;16(4):289–299. doi: 10.1016/j.jcm.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grosso G., Godos J., Galvano F., Giovannucci E.L. Coffee, caffeine, and health outcomes: an umbrella review. Annu. Rev. Nutr. 2017;37(1):131–156. doi: 10.1146/annurev-nutr-071816-064941. [DOI] [PubMed] [Google Scholar]
- 27.Schwingshackl L., Missbach B., Hoffmann G. An umbrella review of garlic intake and risk of cardiovascular disease. Phytomedicine. 2016;23(11):1127–1133. doi: 10.1016/j.phymed.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 28.Veronese N., Demurtas J., Celotto S., Caruso M.G., Maggi S., Bolzetta F., et al. Is chocolate consumption associated with health outcomes? An umbrella review of systematic reviews and meta-analyses. Clin. Nutr. 2019;38(3):1101–1108. doi: 10.1016/j.clnu.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 29.Viguiliouk E., Glenn A.J., Nishi S.K., Chiavaroli L., Seider M., Khan T., et al. Associations between dietary pulses alone or with other legumes and cardiometabolic disease outcomes: an umbrella review and updated systematic review and meta-analysis of prospective cohort studies. Adv. Nutr. 2019;10(Suppl_4):S308–S319. doi: 10.1093/advances/nmz113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wan Q., Li N., Du L., Zhao R., Yi M., Xu Q., et al. Allium vegetable consumption and health: an umbrella review of meta-analyses of multiple health outcomes. Food Sci. Nutr. 2019;7(8):2451–2470. doi: 10.1002/fsn3.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lukas S., Georg H., Benjamin M., Marta S.-M., Heiner B. An umbrella review of nuts intake and risk of cardiovascular disease. Curr. Pharm. Des. 2017;23(7):1016–1027. doi: 10.2174/1381612822666161010121356. [DOI] [PubMed] [Google Scholar]
- 32.Godos J., Tieri M., Ghelfi F., Titta L., Marventano S., Lafranconi A., et al. Dairy foods and health: an umbrella review of observational studies. Int. J. Food Sci. Nutr. 2020;71(2):138–151. doi: 10.1080/09637486.2019.1625035. [DOI] [PubMed] [Google Scholar]
- 33.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwingshackl L., Knüppel S., Schwedhelm C., Hoffmann G., Missbach B., Stelmach-Mardas M., et al. Perspective: NutriGrade: a scoring system to assess and judge the meta-evidence of randomized controlled trials and cohort studies in nutrition research. Adv. Nutr. 2016;7(6):994–1004. doi: 10.3945/an.116.013052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shea B.J., Reeves B.C., Wells G., Thuku M., Hamel C., Moran J., et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atkins D., Best D., Briss P.A., Eccles M., Falck-Ytter Y., Flottorp S., et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blanco Mejia S., Kendall C.W., Viguiliouk E., Augustin L.S., Ha V., Cozma A.I., et al. Effect of tree nuts on metabolic syndrome criteria: a systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2014;4(7) doi: 10.1136/bmjopen-2013-004660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonnet J.P.C., Cardel M.I., Cellini J., Hu F.B., Guasch-Ferré M. Breakfast skipping, body composition, and cardiometabolic risk: a systematic review and meta-analysis of randomized trials. Obesity. 2020;28(6):1098–1109. doi: 10.1002/oby.22791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hohmann C.D., Cramer H., Michalsen A., Kessler C., Steckhan N., Choi K., et al. Effects of high phenolic olive oil on cardiovascular risk factors: a systematic review and meta-analysis. Phytomedicine. 2015;22(6):631–640. doi: 10.1016/j.phymed.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 40.Ismail M.A.N., Norhayati M.N., Mohamad N. Olive leaf extract effect on cardiometabolic profile among adults with prehypertension and hypertension: a systematic review and meta-analysis. PeerJ. 2021;9 doi: 10.7717/peerj.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jalali M.K., Ferns M., Zare G.A., Moosavian M., Akbarzadeh S.P., M. The effects of cashew nut intake on lipid profile and blood pressure: A systematic review and meta-analysis of randomized controlled trials. Complementary therapies in medicine. 2020;50:102387. doi: 10.1016/j.ctim.2020.102387. [DOI] [PubMed] [Google Scholar]
- 42.Khan K., Jovanovski E., Ho H.V.T., Marques A.C.R., Zurbau A., Mejia S.B., et al. The effect of viscous soluble fiber on blood pressure: a systematic review and meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2018;28(1):3–13. doi: 10.1016/j.numecd.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 43.Mohammadifard N., Salehi-Abargouei A., Salas-Salvado J., Guasch-Ferre M., Humphries K., Sarrafzadegan N. The effect of tree nut, peanut, and soy nut consumption on blood pressure: a systematic review and meta-analysis of randomized controlled clinical trials. Am. J. Clin. Nutr. 2015;101(5):966–982. doi: 10.3945/ajcn.114.091595. [DOI] [PubMed] [Google Scholar]
- 44.Naude C.E., Schoonees A., Senekal M., Young T., Garner P., Volmink J. Low carbohydrate versus isoenergetic balanced diets for reducing weight and cardiovascular risk: a systematic review and meta-analysis. PLOS ONE. 2014;9(7) doi: 10.1371/journal.pone.0100652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Onakpoya I.J., O'Sullivan J., Heneghan C.J. The effect of cactus pear (Opuntia ficus-indica) on body weight and cardiovascular risk factors: a systematic review and meta-analysis of randomized clinical trials. Nutrition. 2015;31(5):640–646. doi: 10.1016/j.nut.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 46.Raman G., Avendano E.E., Chen S., Wang J., Matson J., Gayer B., et al. Dietary intakes of flavan-3-ols and cardiometabolic health: systematic review and meta-analysis of randomized trials and prospective cohort studies. Am. J. Clin. Nutr. 2019;110(5):1067–1078. doi: 10.1093/ajcn/nqz178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu L., Sun D. Effects of calcium plus vitamin D supplementation on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J. Hum. Hypertens. 2017;31(9):547–554. doi: 10.1038/jhh.2017.12. [DOI] [PubMed] [Google Scholar]
- 48.Liu G., Zheng X., Lu J., Huang X. Effects of tea intake on blood pressure: a metaanalysis of 21 randomized controlled trials, Cardiology. 2014;129:10–11. (Switzerland) [Google Scholar]
- 49.Ma C., Zheng X., Yang Y., Bu P. The effect of black tea supplementation on blood pressure: a systematic review and dose-response meta-analysis of randomized controlled trials. Food Funct. 2021;12(1):41–56. doi: 10.1039/d0fo02122a. [DOI] [PubMed] [Google Scholar]
- 50.Ramli N.N.S.A., Alkhaldy A.A., Mhd Jalil A.M. Effects of caffeinated and decaffeinated coffee consumption on metabolic syndrome parameters: a systematic review and meta-analysis of data from randomised controlled trials. Medicina. (Kaunas, Lithuania) 2021;57(9):957. doi: 10.3390/medicina57090957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Azad M.B., Abou-Setta A.M., Chauhan B.F., Rabbani R., Lys J., Copstein L., et al. Nonnutritive sweeteners and cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. CMAJ. 2017;189(28):E929–E939. doi: 10.1503/cmaj.161390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benatar J.R., Stewart R.A.H. Cardiometabolic risk factors in vegans; a meta-analysis of observational studies. PLOS ONE. 2018;13(12) doi: 10.1371/journal.pone.0209086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dehghan P., Abbasalizad Farhangi M. Dietary acid load, blood pressure, fasting blood sugar and biomarkers of insulin resistance among adults: findings from an updated systematic review and meta-analysis. Int. J. Clin. Pract. 2020;74(4) doi: 10.1111/ijcp.13471. [DOI] [PubMed] [Google Scholar]
- 54.Farhangi M.A., Nikniaz L., Nikniaz Z., Dehghan P. Dietary inflammatory index potentially increases blood pressure and markers of glucose homeostasis among adults: findings from an updated systematic review and meta-analysis. Public. Health. Nutr. 2020;23(8):1362–1380. doi: 10.1017/S1368980019003070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Godos J., Vitale M., Micek A., Ray S., Martini D., Del Rio D., Riccardi G., Galvano F., Grosso G. Dietary polyphenol intake, blood pressure, and hypertension: a systematic review and meta-analysis of observational studies. Antioxidants. (Basel). 2019;8(6):152. doi: 10.3390/antiox8060152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Godos J., Zappala G., Bernardini S., Giambini I., Bes-Rastrollo M., Martinez-Gonzalez M. Adherence to the Mediterranean diet is inversely associated with metabolic syndrome occurrence: a meta-analysis of observational studies. Int. J. Food. Sci. Nutr. 2017;68(2):138–148. doi: 10.1080/09637486.2016.1221900. [DOI] [PubMed] [Google Scholar]
- 57.Han H., Fang X., Wei X., Liu Y., Jin Z., Chen Q., et al. Dose-response relationship between dietary magnesium intake, serum magnesium concentration and risk of hypertension: a systematic review and meta-analysis of prospective cohort studies. Nutr. J. 2017;16(1):26. doi: 10.1186/s12937-017-0247-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fard Z. Heidari N.R.P., Clark C.C.T., Haghighatdoost F. Dairy products consumption and the risk of hypertension in adults: an updated systematic review and dose-response meta-analysis of prospective cohort studies. Nutr. Metab. Cardiovasc. Dis. 2021;31(7):1962–1975. doi: 10.1016/j.numecd.2021.02.033. [DOI] [PubMed] [Google Scholar]
- 59.Jayalath V.H., Sievenpiper J.L., de Souza R.J., Ha V., Mirrahimi A., Santaren I.D., et al. Total fructose intake and risk of hypertension: a systematic review and meta-analysis of prospective cohorts. J. Am. Coll. Nutr. 2014;33(4):328–339. doi: 10.1080/07315724.2014.916237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jayedi A., Zargar M.S. Dietary calcium intake and hypertension risk: a dose-response meta-analysis of prospective cohort studies. Eur. J. Clin. Nutr. 2019;73(7):969–978. doi: 10.1038/s41430-018-0275-y. [DOI] [PubMed] [Google Scholar]
- 61.Kim Y., Je Y. Prospective association of sugar-sweetened and artificially sweetened beverage intake with risk of hypertension. Arch. Cardiovasc. Dis. 2016;109(4):242–253. doi: 10.1016/j.acvd.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 62.Kunutsor S.K., Apekey T.A., Steur M. Vitamin D and risk of future hypertension: meta-analysis of 283,537 participants. Eur. J. Epidemiol. 2013;28(3):205–221. doi: 10.1007/s10654-013-9790-2. [DOI] [PubMed] [Google Scholar]
- 63.Kuruppu D., Hendrie H.C., Yang L., Gao S. Selenium levels and hypertension: a systematic review of the literature. Public Health Nutr. 2014;17(6):1342–1352. doi: 10.1017/S1368980013000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee M., Lee H., Kim J. Dairy food consumption is associated with a lower risk of the metabolic syndrome and its components: a systematic review and meta-analysis. Br. J. Nutr. 2018;120(4):373–384. doi: 10.1017/S0007114518001460. [DOI] [PubMed] [Google Scholar]
- 65.Liu L., Ikeda K., Sullivan D.H., Ling W., Yamori Y. Epidemiological evidence of the association between dietary protein intake and blood pressure: a meta-analysis of published data. Hypertens. Res. 2002;25(5):689–695. doi: 10.1291/hypres.25.689. [DOI] [PubMed] [Google Scholar]
- 66.Morze J., Schwedhelm C., Bencic A., Hoffmann G., Boeing H., Przybylowicz K., et al. Chocolate and risk of chronic disease: a systematic review and dose-response meta-analysis. Eur. J. Nutr. 2020;59(1):389–397. doi: 10.1007/s00394-019-01914-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parohan M., Sadeghi A., Nasiri M., Maleki V., Khodadost M., Pirouzi A., et al. Dietary acid load and risk of hypertension: a systematic review and dose-response meta-analysis of observational studies. Nutr. Metab. Cardiovasc. Dis. 2019;29(7):665–675. doi: 10.1016/j.numecd.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 68.Picasso M.C., Lo-Tayraco J.A., Ramos-Villanueva J.M., Pasupuleti V., Hernandez A.V. Effect of vegetarian diets on the presentation of metabolic syndrome or its components: a systematic review and meta-analysis. Clin. Nutr. 2019;38(3):1117–1132. doi: 10.1016/j.clnu.2018.05.021. [DOI] [PubMed] [Google Scholar]
- 69.Ralston R.A., Lee J.H., Truby H., Palermo C.E., Walker K.Z. A systematic review and meta-analysis of elevated blood pressure and consumption of dairy foods. J. Hum. Hypertens. 2012;26(1):3–13. doi: 10.1038/jhh.2011.3. [DOI] [PubMed] [Google Scholar]
- 70.Ran L., Zhao W., Tan X., Wang H., Mizuno K., Takagi K., et al. Association between serum vitamin C and the blood pressure: a systematic review and meta-analysis of observational studies. Cardiovascular therapeutics. 2020;2020:4940673. doi: 10.1155/2020/4940673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roerecke M., Tobe S.W., Kaczorowski J., Bacon S.L., Vafaei A., Hasan O.S.M., et al. Sex-specific associations between alcohol consumption and incidence of hypertension: a systematic review and meta-analysis of cohort studies. J. Am. Heart Assoc. 2018;7(13) doi: 10.1161/JAHA.117.008202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schwingshackl L., Schwedhelm C., Hoffmann G., Boeing H. Potatoes and risk of chronic disease: a systematic review and dose-response meta-analysis. Eur. J. Nutr. 2019;58(6):2243–2251. doi: 10.1007/s00394-018-1774-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang C.J., Shen Y.X., Liu Y. Empirically derived dietary patterns and hypertension likelihood: a meta-analysis. Kidney Blood Press Res. 2016;41(5):570–581. doi: 10.1159/000443456. [DOI] [PubMed] [Google Scholar]
- 74.Wu L., Sun D., He Y. Fruit and vegetables consumption and incident hypertension: dose-response meta-analysis of prospective cohort studies. J. Hum. Hypertens. 2016;30(10):573–580. doi: 10.1038/jhh.2016.44. [DOI] [PubMed] [Google Scholar]
- 75.Xie C., Cui L., Zhu J., Wang K., Sun N., Sun C. Coffee consumption and risk of hypertension: a systematic review and dose-response meta-analysis of cohort studies. J. Hum. Hypertens. 2018;32(2):83–93. doi: 10.1038/s41371-017-0007-0. [DOI] [PubMed] [Google Scholar]
- 76.Yang B., Shi M.Q., Li Z.H., Yang J.J., Li D. Fish, long-chain n-3 PUFA and incidence of elevated blood pressure: a meta-analysis of prospective cohort studies. Nutrients. 2016;8(1):58. doi: 10.3390/nu8010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang Y., Zhang D.-z. Red meat, poultry, and egg consumption with the risk of hypertension: a meta-analysis of prospective cohort studies. J. Hum. Hypertens. 2018;32(7):507–517. doi: 10.1038/s41371-018-0068-8. [DOI] [PubMed] [Google Scholar]
- 78.Ziaei R., Gholamreza A., Foshati S., Zolfaghari H., Clark C.C.T., Rouhani M.H. Association between urinary potassium excretion and blood pressure: a systematic review and meta-analysis of observational studies. J. Res. Med. Sci. 2020;25:116. doi: 10.4103/jrms.JRMS_167_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bueno N.B., de Melo I.S., de Oliveira S.L., da Rocha Ataide T. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br. J. Nutr. 2013;110(7):1178–1187. doi: 10.1017/S0007114513000548. [DOI] [PubMed] [Google Scholar]
- 80.Santesso N., Akl E.A., Bianchi M., Mente A., Mustafa R., Heels-Ansdell D., et al. Effects of higher- versus lower-protein diets on health outcomes: a systematic review and meta-analysis. Eur. J. Clin. Nutr. 2012;66(7):780–788. doi: 10.1038/ejcn.2012.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu K., Xing A., Chen K., Wang B., Zhou R., Chen S., et al. Effect of fruit juice on cholesterol and blood pressure in adults: a meta-analysis of 19 randomized controlled trials. PLOS ONE. 2013;8(4) doi: 10.1371/journal.pone.0061420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mousavi S.M., Karimi E., Hajishafiee M., Milajerdi A., Amini M.R., Esmaillzadeh A. Anti-hypertensive effects of cinnamon supplementation in adults: a systematic review and dose-response meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2019;1–11:3144–3154. doi: 10.1080/10408398.2019.1678012. [DOI] [PubMed] [Google Scholar]
- 83.Ashtary-Larky D., Kelishadi M.R., Bagheri R., Moosavian S.P., Wong A., Davoodi S.H., et al. The effects of nano-curcumin supplementation on risk factors for cardiovascular disease: a GRADE-assessed systematic review and meta-analysis of clinical trials. Antioxidants (Basel) 2021;10(7):1015. doi: 10.3390/antiox10071015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guan L., Miao P. The effects of taurine supplementation on obesity, blood pressure and lipid profile: a meta-analysis of randomized controlled trials. Eur. J. Pharmacol. 2020;885:173533. doi: 10.1016/j.ejphar.2020.173533. [DOI] [PubMed] [Google Scholar]
- 85.Dong J.Y., Tong X., Wu Z.W., Xun P.C., He K., Qin L.Q. Effect of soya protein on blood pressure: a meta-analysis of randomised controlled trials. Br. J. Nutr. 2011;106(3):317–326. doi: 10.1017/S0007114511000262. [DOI] [PubMed] [Google Scholar]
- 86.Allender P.S., Cutler J.A., Follmann D., Cappuccio F.P., Pryer J., Elliott P. Dietary calcium and blood pressure: a meta-analysis of randomized clinical trials. Ann. Intern. Med. 1996;124(9):825–831. doi: 10.7326/0003-4819-124-9-199605010-00007. [DOI] [PubMed] [Google Scholar]
- 87.Ren J., An J., Chen M., Yang H., Ma Y. Effect of proanthocyanidins on blood pressure: a systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2021;165:10. doi: 10.1016/j.phrs.2020.105329. [DOI] [PubMed] [Google Scholar]
- 88.Filippou C.D., Tsioufis C.P., Thomopoulos C.G., Mihas C.C., Dimitriadis K.S., Sotiropoulou L.I., et al. Dietary Approaches to Stop Hypertension (DASH) diet and blood pressure reduction in adults with and without hypertension: a systematic review and meta-analysis of randomized controlled trials. Adv. Nutr. 2020;11(5):1150–1160. doi: 10.1093/advances/nmaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ursoniu S., Sahebkar A., Andrica F., Serban C., Banach M. Lipid, Blood Pressure Meta-analysis Collaboration Group. Effects of flaxseed supplements on blood pressure: a systematic review and meta-analysis of controlled clinical trial. Clin. Nutr. 2016;35(3):615–625. doi: 10.1016/j.clnu.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 90.Li K., Liu C., Kuang X., Deng Q., Zhao F., Li D. Effects of multivitamin and multimineral supplementation on blood pressure: a meta-analysis of 12 randomized controlled trials. Nutrients. 2018;10(8):1018. doi: 10.3390/nu10081018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Qi D., Nie X.L., Zhang J.J. The effect of probiotics supplementation on blood pressure: a systemic review and meta-analysis. Lipids Health Dis. 2020;19(1):79. doi: 10.1186/s12944-020-01259-x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 92.Asbaghi O., Naeini F., Moodi V., Najafi M., Shirinbakhshmasoleh M., Kelishadi M.R., et al. Effect of grape products on blood pressure: a systematic review and meta-analysis of randomized controlled trials. Int. J. Food Prop. 2021;24(1):627–645. doi: 10.1080/10942912.2021.1901731. [DOI] [Google Scholar]
- 93.He Y., Liu J., Cai H., Zhang J., Yi J., Niu Y., Xi H., Peng X., Guo L. Effect of inorganic nitrate supplementation on blood pressure in older adults: a systematic review and meta-analysis. Nitric Oxide. 2021;113–114:13–22. doi: 10.1016/j.niox.2021.04.006. [DOI] [PubMed] [Google Scholar]
- 94.Aburto N.J., Hanson S., Gutierrez H., Hooper L., Elliott P., Cappuccio F.P. Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta-analyses. BMJ. 2013;346:f1378. doi: 10.1136/bmj.f1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Onakpoya I.J., Heneghan C.J. Effect of the natural sweetener, steviol glycoside, on cardiovascular risk factors: a systematic review and meta-analysis of randomised clinical trials. Eur. J. Prev. Cardiol. 2015;22(12):1575–1587. doi: 10.1177/2047487314560663. [DOI] [PubMed] [Google Scholar]
- 96.Peng X., Zhou R., Wang B., Yu X., Yang X., Liu K., Mi M. Effect of green tea consumption on blood pressure: a meta-analysis of 13 randomized controlled trials. Sci. Rep. 2014;4:6251. doi: 10.1038/srep06251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Asbaghi O., Salehpour S., Rezaei Kelishadi M., Bagheri R., Ashtary-Larky D., Nazarian B., et al. Folic acid supplementation and blood pressure: a GRADE-assessed systematic review and dose-response meta-analysis of 41,633 participants. Crit. Rev. Food Sci. Nutr. 2021;63:1846–1861. doi: 10.1080/10408398.2021.1968787. [DOI] [PubMed] [Google Scholar]
- 98.Azhdari M., Karandish M., Mansoori A. Metabolic benefits of curcumin supplementation in patients with metabolic syndrome: a systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2019;33(5):1289–1301. doi: 10.1002/ptr.6323. [DOI] [PubMed] [Google Scholar]
- 99.Spaggiari G., Cignarelli A., Sansone A., Baldi M., Santi D. To beer or not to beer: A meta-analysis of the effects of beer consumption on cardiovascular health. PLOS ONE. 2020;15(6) doi: 10.1371/journal.pone.0233619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gay H.C., Rao S.G., Vaccarino V., Ali M.K. Effects of different dietary interventions on blood pressure: systematic review and meta-analysis of randomized controlled trials. Hypertension. 2016;67(4):733–739. doi: 10.1161/HYPERTENSIONAHA.115.06853. [DOI] [PubMed] [Google Scholar]
- 101.Schwingshackl L., Strasser B., Hoffmann G. Effects of monounsaturated fatty acids on cardiovascular risk factors: a systematic review and meta-analysis. Ann. Nutr. Metab. 2011;59(2–4):176–186. doi: 10.1159/000334071. [DOI] [PubMed] [Google Scholar]
- 102.Dong T., Guo M., Zhang P., Sun G., Chen B. The effects of low-carbohydrate diets on cardiovascular risk factors: a meta-analysis. PLOS ONE. 2020;15(1) doi: 10.1371/journal.pone.0225348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Filippou C.D., Thomopoulos C.G., Kouremeti M.M., Sotiropoulou L.I., Nihoyannopoulos P.I., Tousoulis D.M., et al. Mediterranean diet and blood pressure reduction in adults with and without hypertension: a systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 2021;40(5):3191–3200. doi: 10.1016/j.clnu.2021.01.030. [DOI] [PubMed] [Google Scholar]
- 104.Gibbs J.G., Gaskin E., Ji C., Miller M.A., Cappuccio F.P. The effect of plant-based dietary patterns on blood pressure: a systematic review and meta-analysis of controlled intervention trials. J. Hypertens. 2021;39(1):23–37. doi: 10.1097/hjh.0000000000002604. [DOI] [PubMed] [Google Scholar]
- 105.Lee K.W.L., Loh H.C., Ching S.M., Devaraj N.K., Hoo F.K. Effects of vegetarian diets on blood pressure lowering: a systematic review with meta-analysis and trial sequential analysis. Nutrients. 2020;12(6):1604. doi: 10.3390/nu12061604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lopez P.D., Cativo E.H., Atlas S.A., Rosendorff C. The effect of vegan diets on blood pressure in adults: a meta-analysis of randomized controlled trials. Am. J. Med. 2019;132(7):875–883 e7. doi: 10.1016/j.amjmed.2019.01.044. [DOI] [PubMed] [Google Scholar]
- 107.Mazzaro C.C., Klostermann F.C., Erbano B.O., Schio N.A., Guarita-Souza L.C., Olandoski M., et al. Dietary interventions and blood pressure in Latin America – systematic review and meta-analysis. Arq. Bras. Cardiol. 2014;102(4):345–354. doi: 10.5935/abc.20140037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schwingshackl L., Hoffmann G. Long-term effects of low-fat diets either low or high in protein on cardiovascular and metabolic risk factors: a systematic review and meta-analysis. Nutr. J. 2013;12:48. doi: 10.1186/1475-2891-12-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang Q., Lang X., Li W., Liang Y. The effects of low-fat, high-carbohydrate diets vs. low-carbohydrate, high-fat diets on weight, blood pressure, serum liquids and blood glucose: a systematic review and meta-analysis. Eur. J. Clin. Nutr. 2021;76:16–27. doi: 10.1038/s41430-021-00927-0. [DOI] [PubMed] [Google Scholar]
- 110.Ghaedi E., Mohammadi M., Mohammadi H., Ramezani-Jolfaie N., Malekzadeh J., Hosseinzadeh M., et al. Effects of a Paleolithic diet on cardiovascular disease risk factors: a systematic review and meta-analysis of randomized controlled trials. Adv. Nutr. 2019;10(4):634–646. doi: 10.1093/advances/nmz007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chiavaroli L., Nishi S.K., Khan T.A., Braunstein C.R., Glenn A.J., Mejia S.B., et al. Portfolio dietary pattern and cardiovascular disease: a systematic review and meta-analysis of controlled trials. Prog. Cardiovasc. Dis. 2018;61(1):43–53. doi: 10.1016/j.pcad.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 112.Kirkham A.A.B., Beka V., Prado C.M. The effect of caloric restriction on blood pressure and cardiovascular function: a systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 2021;40(3):728–739. doi: 10.1016/j.clnu.2020.06.029. [DOI] [PubMed] [Google Scholar]
- 113.Shah M., Adams-Huet B., Garg A. Effect of high-carbohydrate or high-cis-monounsaturated fat diets on blood pressure: a meta-analysis of intervention trials. Am. J. Clin. Nutr. 2007;85(5):1251–1256. doi: 10.1093/ajcn/85.5.1251. [DOI] [PubMed] [Google Scholar]
- 114.Toh D.W.K.K., Koh E.S., Kim J.E. Incorporating healthy dietary changes in addition to an increase in fruit and vegetable intake further improves the status of cardiovascular disease risk factors: A systematic review, meta-regression, and meta-analysis of randomized controlled trials. Nutr. Rev. 2020;78(7):532–545. doi: 10.1093/nutrit/nuz104. [DOI] [PubMed] [Google Scholar]
- 115.Hu T., Mills K.T., Yao L., Demanelis K., Eloustaz M., Yancy W.S., Jr., et al. Effects of low-carbohydrate diets versus low-fat diets on metabolic risk factors: a meta-analysis of randomized controlled clinical trials. Am. J. Epidemiol. 2012;176(suppl_7):S44–S54. doi: 10.1093/aje/kws264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Baye E., Kiriakova V., Uribarri J., Moran L.J., de Courten B. Consumption of diets with low advanced glycation end products improves cardiometabolic parameters: meta-analysis of randomised controlled trials. Sci. Rep. 2017;7(1):2266. doi: 10.1038/s41598-017-02268-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Alhassan A., Young J., Lean M.E.J., Lara J. Consumption of fish and vascular risk factors: a systematic review and meta-analysis of intervention studies. Atherosclerosis. 2017;266:87–94. doi: 10.1016/j.atherosclerosis.2017.09.028. [DOI] [PubMed] [Google Scholar]
- 118.Kolahdouz-Mohammadi R.M., Malekahmadi M., Clayton Z.S., Sadat S.Z., Pahlavani N., Sikaroudi M.K., et al. Effect of egg consumption on blood pressure: a systematic review and meta-analysis of randomized clinical trials. Curr. Hypertens. Rep. 2020;22(3):24. doi: 10.1007/s11906-020-1029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.O'Connor L.E., Kim J.E., Campbell W.W. Total red meat intake of >/=0.5 servings/d does not negatively influence cardiovascular disease risk factors: a systemically searched meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2017;105(1):57–69. doi: 10.3945/ajcn.116.142521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fekete A.A., Givens D.I., Lovegrove J.A. Casein-derived lactotripeptides reduce systolic and diastolic blood pressure in a meta-analysis of randomised clinical trials. Nutrients. 2015;7(1):659–681. doi: 10.3390/nu7010659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Benatar J.R., Sidhu K., Stewart R.A. Effects of high and low fat dairy food on cardio-metabolic risk factors: a meta-analysis of randomized studies. PLOS ONE. 2013;8(10) doi: 10.1371/journal.pone.0076480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Carvalho M.F., Lucca A.B.A., Ribeiro E., Silva V.R., Macedo L.R., Silva M. Vol. 91. Nutr. Res.; New York, NY: 2021. pp. 67–80. (Blueberry intervention improves metabolic syndrome risk factors: systematic review and meta-analysis). [DOI] [PubMed] [Google Scholar]
- 123.Bahadoran Z., Mirmiran P., Kabir A., Azizi F., Ghasemi A. The nitrate-independent blood pressure-lowering effect of beetroot juice: a systematic review and meta-analysis. Adv. Nutr. 2017;8(6):830–838. doi: 10.3945/an.117.016717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Luis A., Domingues F., Pereira L. Association between berries intake and cardiovascular diseases risk factors: a systematic review with meta-analysis and trial sequential analysis of randomized controlled trials. Food Funct. 2018;9(2):740–757. doi: 10.1039/c7fo01551h. [DOI] [PubMed] [Google Scholar]
- 125.Sahebkar A., Ferri C., Giorgini P., Bo S., Nachtigal P., Grassi D. Effects of pomegranate juice on blood pressure: a systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2017;115:149–161. doi: 10.1016/j.phrs.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 126.Jandari S., Ghavami A., Ziaei R., Nattagh-Eshtivani E., Kelishadi M.R., Sharifi S., et al. Effects of Momordica charantia L on blood pressure: a systematic review and meta- analysis of randomized clinical trials. Int. J. Food Prop. 2020;23(1):1913–1924. doi: 10.1080/10942912.2020.1833916. [DOI] [Google Scholar]
- 127.Hadi A., Askarpour M., Miraghajani M., Symonds M.E., Sheikhi A., Ghaedi E. Effects of strawberry supplementation on cardiovascular risk factors: a comprehensive systematic review and meta-analysis of randomized controlled trials. Food Funct. 2019;10(11):6987–6998. doi: 10.1039/c9fo01684h. [DOI] [PubMed] [Google Scholar]
- 128.Ried K. Garlic lowers blood pressure in hypertensive individuals, regulates serum cholesterol, and stimulates immunity: an updated meta-analysis and review. J. Nutr. 2016;146(2):389S–396S. doi: 10.3945/jn.114.202192. [DOI] [PubMed] [Google Scholar]
- 129.Hasani H., Arab A., Hadi A., Pourmasoumi M., Ghavami A., Miraghajani M. Does ginger supplementation lower blood pressure? A systematic review and meta-analysis of clinical trials. Phytother. Res. 2019;33(6):1639–1647. doi: 10.1002/ptr.6362. [DOI] [PubMed] [Google Scholar]
- 130.Pourmasoumi M., Hadi A., Najafgholizadeh A., Joukar F., Mansour-Ghanaei F. The effects of cranberry on cardiovascular metabolic risk factors: a systematic review and meta-analysis. Clin. Nutr. 2020;39(3):774–788. doi: 10.1016/j.clnu.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 131.Onakpoya I., O'Sullivan J., Heneghan C., Thompson M. The effect of grapefruits (Citrus paradisi) on body weight and cardiovascular risk factors: a systematic review and meta-analysis of randomized clinical trials. Crit. Rev. Food Sci. Nutr. 2017;57(3):602–612. doi: 10.1080/10408398.2014.901292. [DOI] [PubMed] [Google Scholar]
- 132.Han B., Bhagavathula A.S., Rashid M., Chhabra M., Clark C., Abdulazeem H.M., et al. The effect of sour cherry consumption on blood pressure, IL-6, CRP, and TNF-alpha levels: A systematic review and meta-analysis of randomized controlled trials sour cherry consumption and blood pressure. J. King Saud. Univ. Sci. 2020;32(2):1687–1693. doi: 10.1016/j.jksus.2020.01.002. [DOI] [Google Scholar]
- 133.Guo X.F., Li Z.H., Cai H., Li D. The effects of Lycium barbarum L. (L. barbarum) on cardiometabolic risk factors: a meta-analysis of randomized controlled trials. Food & Funct. 2017;8(5):1741–1748. doi: 10.1039/c7fo00183e. [DOI] [PubMed] [Google Scholar]
- 134.Suksomboon N., Poolsup N., Lin W. Effect of kiwifruit on metabolic health in patients with cardiovascular risk factors: a systematic review and meta-analysis. Diabetes Metab. Syndr. Obes. 2019;12:171–180. doi: 10.2147/DMSO.S193225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Motallaei M.R.-J., Mohammadi M., Shams-Rad S.S., Jahanlou A.S., Salehi-Abargouei A.S. Effects of orange juice intake on cardiovascular risk factors: a systematic review and meta-analysis of randomized controlled clinical trials. Phytother. Res. 2021;35(10):5427–5439. doi: 10.1002/ptr.7173. [DOI] [PubMed] [Google Scholar]
- 136.Shin J.Y., Kim J.Y., Kang H.T., Han K.H., Shim J.Y. Effect of fruits and vegetables on metabolic syndrome: a systematic review and meta-analysis of randomized controlled trials. Int. J. Food Sci. Nutr. 2015;66(4):416–425. doi: 10.3109/09637486.2015.1025716. [DOI] [PubMed] [Google Scholar]
- 137.Wang Y., Li J., Zhao C., Tian H., Geng Y., Sun L., et al. The effect of tomato on weight, body mass index, blood pressure and inflammatory factors: a systematic review and dose-response meta-analysis of randomized controlled trials. J. King Saud. Univ. Sci. 2020;32(2):1619–1627. doi: 10.1016/j.jksus.2019.12.020. [DOI] [Google Scholar]
- 138.D'Elia L.D., Dinu M., Sofi F., Volpe M., Strazzullo P. 100% fruit juice intake and cardiovascular risk: a systematic review and meta-analysis of prospective and randomised controlled studies. Eur. J. Nutr. 2021;60(5):2449–2467. doi: 10.1007/s00394-020-02426-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hawkins J., Hires C., Baker C., Keenan L., Bush M. Daily supplementation with aronia melanocarpa (chokeberry) reduces blood pressure and cholesterol: a meta analysis of controlled clinical trials. J. Diet. Suppl. 2021;18(5):517–530. doi: 10.1080/19390211.2020.1800887. [DOI] [PubMed] [Google Scholar]
- 140.Kelly S.A., Hartley L., Loveman E., Colquitt J.L., Jones H.M., Al-Khudairy L., et al. Whole grain cereals for the primary or secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2017;8:CD005051. doi: 10.1002/14651858.CD005051.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Reynolds A., Mann J., Cummings J., Winter N., Mete E., Te Morenga L. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet. 2019;393(10170):434–445. doi: 10.1016/S0140-6736(18)31809-9. [DOI] [PubMed] [Google Scholar]
- 142.Jayalath V.H., de Souza R.J., Sievenpiper J.L., Ha V., Chiavaroli L., Mirrahimi A., et al. Effect of dietary pulses on blood pressure: a systematic review and meta-analysis of controlled feeding trials. Am. J. Hypertens. 2014;27(1):56–64. doi: 10.1093/ajh/hpt155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Huang H., Zhou G., Pu R., Cui Y., Liao D. Clinical evidence of dietary supplementation with sesame on cardiovascular risk factors: an updated meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2021;62:5592–5602. doi: 10.1080/10408398.2021.1888689. [DOI] [PubMed] [Google Scholar]
- 144.Sahebkar A., Soranna D., Liu X., Thomopoulos C., Simental-Mendia L.E., Derosa G., et al. A systematic review and meta-analysis of randomized controlled trials investigating the effects of supplementation with Nigella sativa (black seed) on blood pressure. J. Hypertens. 2016;34(11):2127–2135. doi: 10.1097/HJH.0000000000001049. [DOI] [PubMed] [Google Scholar]
- 145.Li J., Jian B., Santos H.O., Santos D., Singh A., Wang L. Effects of walnut intake on blood pressure: a systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2020;34(11):2921–2931. doi: 10.1002/ptr.6740. [DOI] [PubMed] [Google Scholar]
- 146.Asbaghi O., Hadi A., Campbell M.S., Venkatakrishnan K., Ghaedi E. Effects of pistachios on anthropometric indices, inflammatory markers, endothelial function and blood pressure in adults: a systematic review and meta-analysis of randomised controlled trials. Br. J. Nutr. 2021;126(5):718–729. doi: 10.1017/s0007114520004523. [DOI] [PubMed] [Google Scholar]
- 147.Eslampour E., Asbaghi O., Hadi A., Abedi S., Ghaedi E., Lazaridi A., Miraghajani M. The effect of almond intake on blood pressure: a systematic review and meta-analysis of randomized controlled trials, Complement. Ther. Med. 2020;50:102399. doi: 10.1016/j.ctim.2020.102399. [DOI] [PubMed] [Google Scholar]
- 148.Teoh S.L., Lai N.M., Vanichkulpitak P., Vuksan V., Ho H., Chaiyakunapruk N. Clinical evidence on dietary supplementation with chia seed (Salvia hispanica L.): a systematic review and meta-analysis. Nutr. Rev. 2018;76(4):219–242. doi: 10.1093/nutrit/nux071. [DOI] [PubMed] [Google Scholar]
- 149.Ried K., Fakler P., Stocks N.P. Effect of cocoa on blood pressure. Cochrane Database Syst. Rev. 2017;4:CD008893. doi: 10.1002/14651858.CD008893.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Luis A., Domingues F., Pereira L. Metabolic changes after licorice consumption: a systematic review with meta-analysis and trial sequential analysis of clinical trials. Phytomedicine. 2018;39:17–24. doi: 10.1016/j.phymed.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 151.Setayesh L., Ashtary-Larky D., Clark C.C.T., Rezaei Kelishadi M., Khalili P., Bagheri R., et al. The effect of saffron supplementation on blood pressure in adults: a systematic review and dose-response meta-analysis of randomized controlled trials. Nutrients. 2021;13(8):2736. doi: 10.3390/nu13082736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Xu R., Yang K., Ding J., Chen G. Effect of green tea supplementation on blood pressure: a systematic review and meta-analysis of randomized controlled trials. Medicine. 2020;99(6) doi: 10.1097/md.0000000000019047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Roerecke M., Kaczorowski J., Tobe S.W., Gmel G., Hasan O.S.M., Rehm J. The effect of a reduction in alcohol consumption on blood pressure: a systematic review and meta-analysis. Lancet Public Health. 2017;2(2):e108–e120. doi: 10.1016/S2468-2667(17)30003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Steffen M., Kuhle C., Hensrud D., Erwin P.J., Murad M.H. The effect of coffee consumption on blood pressure and the development of hypertension: a systematic review and meta-analysis. J. Hypertens. 2012;30(12):2245–2254. doi: 10.1097/HJH.0b013e3283588d73. [DOI] [PubMed] [Google Scholar]
- 155.Serban C., Sahebkar A., Ursoniu S., Andrica F., Banach M. Effect of sour tea (Hibiscus sabdariffa L.) on arterial hypertension: a systematic review and meta-analysis of randomized controlled trials. J. Hypertens. 2015;33(6):1119–1127. doi: 10.1097/HJH.0000000000000585. [DOI] [PubMed] [Google Scholar]
- 156.Clark C.C.T., Salek M., Aghabagheri E., Jafarnejad S. The effect of psyllium supplementation on blood pressure: a systematic review and meta-analysis of randomized controlled trials. Korean J. Intern. Med. 2020;35(6):1385–1399. doi: 10.3904/kjim.2019.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hashemi Tari S., Sohouli M.H., Lari A., Fatahi S., Rahideh S.T. The effect of inositol supplementation on blood pressure: a systematic review and meta-analysis of randomized-controlled trials. Clin. Nutr. ESPEN. 2021;44:78–84. doi: 10.1016/j.clnesp.2021.06.017. [DOI] [PubMed] [Google Scholar]
- 158.Faghihimani Z., Namazi N., Ghaffari S., Kelishadi M.R., Sharifi S., Nattagh-Eshtivani E., etal Effects of inulin type-carbohydrates on blood pressure: a systematic review and meta-analysis. Int. J. Food Prop. 2021;24(1):129–139. doi: 10.1080/10942912.2020.1858863. [DOI] [Google Scholar]
- 159.Moraru C., Mincea M.M., Frandes M., Timar B., Ostafe V. A meta-analysis on randomised controlled clinical trials evaluating the effect of the dietary supplement chitosan on weight loss, lipid parameters and blood pressure. Medicina (Kaunas, Lithuania) 2018;54(6):109. doi: 10.3390/medicina54060109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Whelton S.P., Hyre A.D., Pedersen B., Yi Y., Whelton P.K., He J. Effect of dietary fiber intake on blood pressure: a meta-analysis of randomized, controlled clinical trials. J. Hypertens. 2005;23(3):475–481. doi: 10.1097/01.hjh.0000160199.51158.cf. [DOI] [PubMed] [Google Scholar]
- 161.Ha V., Sievenpiper J.L., de Souza R.J., Chiavaroli L., Wang D.D., Cozma A.I., et al. Effect of fructose on blood pressure: a systematic review and meta-analysis of controlled feeding trials. Hypertension. 2012;59(4):787–795. doi: 10.1161/HYPERTENSIONAHA.111.182311. [DOI] [PubMed] [Google Scholar]
- 162.Askarpour M., Hadi A., Dehghani Kari Bozorg A., Sadeghi O., Sheikhi A., Kazemi M., et al. Effects of L-carnitine supplementation on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J. Hum. Hypertens. 2019;33(10):725–734. doi: 10.1038/s41371-019-0248-1. [DOI] [PubMed] [Google Scholar]
- 163.Pripp A.H. Effect of peptides derived from food proteins on blood pressure: a meta-analysis of randomized controlled trials. Food Nutr. Res. 2008;52:1641. doi: 10.3402/fnr.v52i0.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rebholz C.M., Friedman E.E., Powers L.J., Arroyave W.D., He J., Kelly T.N. Dietary protein intake and blood pressure: a meta-analysis of randomized controlled trials. Am. J. Epidemiol. 2012;176(Suppl 7):S27–S43. doi: 10.1093/aje/kws245. [DOI] [PubMed] [Google Scholar]
- 165.Hidayat K., Du H.Z., Yang J., Chen G.C., Zhang Z., Li Z.N., et al. Effects of milk proteins on blood pressure: a meta-analysis of randomized control trials. Hypertens. Res. 2017;40(3):264–270. doi: 10.1038/hr.2016.135. [DOI] [PubMed] [Google Scholar]
- 166.Sohouli M.H., Lari A., Fatahi S., Shidfar F., Gaman M.A., Guimaraes N.S., et al. Impact of soy milk consumption on cardiometabolic risk factors: a systematic review and meta-analysis of randomized controlled trials. J. Funct. Food. 2021;83:13. doi: 10.1016/j.jff.2021.104499. [DOI] [Google Scholar]
- 167.Badely M., Sepandi M., Samadi M., Parastouei K., Taghdir M. The effect of whey protein on the components of metabolic syndrome in overweight and obese individuals; a systematic review and meta-analysis. Diabetes Metab. Syndr. 2019;13(6):3121–3131. doi: 10.1016/j.dsx.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 168.Mirenayat M.S., Moradi S., Mohammadi H., Rouhani M.H. Effect of L-citrulline supplementation on blood pressure: a systematic review and meta-analysis of clinical trials. Curr. Hypertens. Reports. 2018;20(11):98. doi: 10.1007/s11906-018-0898-3. [DOI] [PubMed] [Google Scholar]
- 169.AbuMweis S., Jew S., Tayyem R., Agraib L. Eicosapentaenoic acid and docosahexaenoic acid containing supplements modulate risk factors for cardiovascular disease: a meta-analysis of randomised placebo-control human clinical trials. J. Hum. Nutr. Diet. 2018;31(1):67–84. doi: 10.1111/jhn.12493. [DOI] [PubMed] [Google Scholar]
- 170.Zamora-Zamora F., Martinez-Galiano J.M., Gaforio J.J., Delgado-Rodriguez M. Effects of olive oil on blood pressure: a systematic review and meta-analysis. Grasas Aceites. 2018;69(4):e272. doi: 10.3989/gya.0105181. [DOI] [Google Scholar]
- 171.Guo X.F., Li K.L., Li J.M., Li D. Effects of EPA and DHA on blood pressure and inflammatory factors: a meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2019;59(20):3380–3393. doi: 10.1080/10408398.2018.1492901. [DOI] [PubMed] [Google Scholar]
- 172.Morris M.C., Sacks F., Rosner B. Does fish oil lower blood pressure? a meta-analysis of controlled trials. Circulation. 1993;88(2):523–533. doi: 10.1161/01.cir.88.2.523. [DOI] [PubMed] [Google Scholar]
- 173.Yang J., Wang H.P., Zhou L.M., Zhou L., Chen T., Qin L.Q. Effect of conjugated linoleic acid on blood pressure: a meta-analysis of randomized, double-blind placebo-controlled trials. Lipids Health Disease. 2015;14:11. doi: 10.1186/s12944-015-0010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wendland E., Farmer A., Glasziou P., Neil A. Effect of alpha linolenic acid on cardiovascular risk markers: a systematic review. Heart. 2006;92(2):166–169. doi: 10.1136/hrt.2004.053538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Miller P.E., Van Elswyk M., Alexander D.D. Long-chain omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and blood pressure: a meta-analysis of randomized controlled trials. Am. J. Hypertens. 2014;27(7):885–896. doi: 10.1093/ajh/hpu024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhang X., Li Y., Del Gobbo L.C., Rosanoff A., Wang J., Zhang W., et al. Effects of magnesium supplementation on blood pressure: a meta-analysis of randomized double-blind placebo-controlled trials. Hypertension. 2016;68(2):324–333. doi: 10.1161/HYPERTENSIONAHA.116.07664. [DOI] [PubMed] [Google Scholar]
- 177.Ghanbari M.A., Amini M.R., Djafarian K., Shab-Bidar S. The effects of chromium supplementation on blood pressure: a systematic review and meta-analysis of randomized clinical trials. Eur. J. Clin. Nutr. 2021;76:340–349. doi: 10.1038/s41430-021-00973-8. [DOI] [PubMed] [Google Scholar]
- 178.Ndanuko R.N., Ibrahim R., Hapsari R.A., Neale E.P., Raubenheimer D., Charlton K.E. Association between the urinary sodium to potassium ratio and blood pressure in adults: a systematic review and meta-analysis. Adv. Nutr. 2021;12(5):1751–1767. doi: 10.1093/advances/nmab036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Griffith L.E., Guyatt G.H., Cook R.J., Bucher H.C., Cook D.J. The influence of dietary and nondietary calcium supplementation on blood pressure: an updated metaanalysis of randomized controlled trials. Am. J. Hypertens. 1999;12(1 Pt 1):84–92. doi: 10.1016/s0895-7061(98)00224-6. [DOI] [PubMed] [Google Scholar]
- 180.Whelton P.K., He J., Cutler J.A., Brancati F.L., Appel L.J., Follmann D., Klag M.J. Effects of oral potassium on blood pressure: meta-analysis of randomized controlled clinical trials. JAMA. 1997;277(20):1624–1632. doi: 10.1001/jama.1997.03540440058033. [DOI] [PubMed] [Google Scholar]
- 181.Beyer F.R., Dickinson H.O., Nicolson D., Ford G.A., Mason J. Combined calcium, magnesium and potassium supplementation for the management of primary hypertension in adults. Cochrane Database Syst. Rev. 2006;19(3):CD004805. doi: 10.1002/14651858.CD004805.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Khazdouz M., Djalalinia S., Sarrafi Zadeh S., Hasani M., Shidfar F., Ataie-Jafari A., et al. Effects of zinc supplementation on cardiometabolic risk factors: a systematic review and meta-analysis of randomized controlled trials. Biol. Trace Elem. Res. 2020;195(2):373–398. doi: 10.1007/s12011-019-01870-9. [DOI] [PubMed] [Google Scholar]
- 183.Geleijnse J.M., Kok F.J., Grobbee D.E. Blood pressure response to changes in sodium and potassium intake: a metaregression analysis of randomised trials. J. Hum. Hypertens. 2003;17(7):471–480. doi: 10.1038/sj.jhh.1001575. [DOI] [PubMed] [Google Scholar]
- 184.Juraschek S.P., Guallar E., Appel L.J., Miller E.R., 3rd. Effects of vitamin C supplementation on blood pressure: a meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2012;95(5):1079–1088. doi: 10.3945/ajcn.111.027995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mirhosseini N., Rainsbury J., Kimball S.M. Vitamin D supplementation, serum 25(OH)D concentrations and cardiovascular disease risk factors: a systematic review and meta-analysis. Front. Cardiovasc. Med. 2018;5:87. doi: 10.3389/fcvm.2018.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Golzarand M., Shab-Bidar S., Koochakpoor G., Speakman J.R., Djafarian K. Effect of vitamin D3 supplementation on blood pressure in adults: an updated meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2016;26(8):663–673. doi: 10.1016/j.numecd.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 187.Li X.H., Feng L., Yang Z.H., Liao Y.H. Effect of active vitamin D on cardiovascular outcomes in predialysis chronic kidney diseases: a systematic review and meta-analysis. Nephrol. (Carlton). 2015;20(10):706–714. doi: 10.1111/nep.12505. [DOI] [PubMed] [Google Scholar]
- 188.Tabrizi R., Lankarani K.B., Akbari M., Naghibzadeh-Tahami A., Alizadeh H., Honarvar B., et al. The effects of folate supplementation on lipid profiles among patients with metabolic diseases: a systematic review and meta-analysis of randomized controlled trials. Diabetes Metab. Syndr. 2018;12(3):423–430. doi: 10.1016/j.dsx.2017.12.022. [DOI] [PubMed] [Google Scholar]
- 189.Emami M.R., Safabakhsh M., Alizadeh S., Asbaghi O., Khosroshahi M.Z. Effect of vitamin E supplementation on blood pressure: a systematic review and meta-analysis. J. Hum. Hypertens. 2019;33(7):499–507. doi: 10.1038/s41371-019-0192-0. [DOI] [PubMed] [Google Scholar]
- 190.Lewis-Mikhael A.M.L., Davoodvandi A., Jafarnejad S. Effect of Lactobacillusplantarum containing probiotics on blood pressure: a systematic review and meta-analysis. Pharmacol. Res. 2020;153:104663. doi: 10.1016/j.phrs.2020.104663. [DOI] [PubMed] [Google Scholar]
- 191.Ghavami A., Ziaei R., Moradi S., Sharifi S., Moravejolahkami A.R., Ghaffari S., et al. Potential of favorable effects of probiotics fermented milk supplementation on blood pressure: a systematic review and meta-analysis. Int. J. Food Prop. 2020;23(1):1925–1940. doi: 10.1080/10942912.2020.1833030. [DOI] [Google Scholar]
- 192.Feringa H.H., Laskey D.A., Dickson J.E., Coleman C.I. The effect of grape seed extract on cardiovascular risk markers: a meta-analysis of randomized controlled trials. J. Am. Diet. Assoc. 2011;111(8):1173–1181. doi: 10.1016/j.jada.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 193.Garcia-Conesa M.T., Chambers K., Combet E., Pinto P., Garcia-Aloy M., Andres-Lacueva C., et al. Meta-analysis of the effects of foods and derived products containing ellagitannins and anthocyanins on cardiometabolic biomarkers: analysis of factors influencing variability of the individual responses. Int. J. Mol. Sci. 2018;19(3):694. doi: 10.3390/ijms19030694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Menezes R., Rodriguez-Mateos A., Kaltsatou A., Gonzalez-Sarrias A., Greyling A., Giannaki C., et al. Impact of flavonols on cardiometabolic biomarkers: a meta-analysis of randomized controlled human trials to explore the role of inter-individual variability. Nutrients. 2017;9(2):117. doi: 10.3390/nu9020117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Daneshzad E., Shab-Bidar S., Mohammadpour Z., Djafarian K. Effect of anthocyanin supplementation on cardio-metabolic biomarkers: a systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 2019;38(3):1153–1165. doi: 10.1016/j.clnu.2018.06.979. [DOI] [PubMed] [Google Scholar]
- 196.Akbari M., Tamtaji O.R., Lankarani K.B., Tabrizi R., Dadgostar E., Kolahdooz F., et al. The effects of resveratrol supplementation on endothelial function and blood pressures among patients with metabolic syndrome and related disorders: a systematic review and meta-analysis of randomized controlled trials. High Blood Press Cardiovasc. Prev. 2019;26(4):305–319. doi: 10.1007/s40292-019-00324-6. [DOI] [PubMed] [Google Scholar]
- 197.Li S.H., Zhao P., Tian H.B., Chen L.H., Cui L.Q. Effect of grape polyphenols on blood pressure: a meta-analysis of randomized controlled trials. PLOS ONE. 2015;10(9) doi: 10.1371/journal.pone.0137665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mohammadi M., Ramezani-Jolfaie N., Lorzadeh E., Khoshbakht Y., Salehi-Abargouei A. Hesperidin, a major flavonoid in orange juice, might not affect lipid profile and blood pressure: a systematic review and meta-analysis of randomized controlled clinical trials. Phytother. Res. 2019;33(3):534–545. doi: 10.1002/ptr.6264. [DOI] [PubMed] [Google Scholar]
- 199.Shrime M.G., Bauer S.R., McDonald A.C., Chowdhury N.H., Coltart C.E., Ding E.L. Flavonoid-rich cocoa consumption affects multiple cardiovascular risk factors in a meta-analysis of short-term studies. J. Nutr. 2011;141(11):1982–1988. doi: 10.3945/jn.111.145482. [DOI] [PubMed] [Google Scholar]
- 200.Weaver S.R., Rendeiro C., McGettrick H.M., Philp A., Lucas S.J.E. Fine wine or sour grapes? a systematic review and meta-analysis of the impact of red wine polyphenols on vascular health. Eur. J. Nutr. 2021;60(1):1–28. doi: 10.1007/s00394-020-02247-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ellwood L., Torun G., Bahar Z., Fernandez R. Effects of flavonoid-rich fruits on hypertension in adults: a systematic review. JBI Database Syst. Rev. Implement. Rep. 2019;17(10):2075–2105. doi: 10.11124/JBISRIR-D-19-00050. [DOI] [PubMed] [Google Scholar]
- 202.Ghaedi E., Foshati S., Ziaei R., Beigrezaei S., Kord-Varkaneh H., Ghavami A., et al. Effects of phytosterols supplementation on blood pressure: a systematic review and meta-analysis. Clin. Nutr. 2020;39(9):2702–2710. doi: 10.1016/j.clnu.2019.12.020. [DOI] [PubMed] [Google Scholar]
- 203.Han B., Nazary-Vannani A., Talaei S., Clark C.C.T., Rahmani J., Rasekhmagham R., et al. The effect of green coffee extract supplementation on blood pressure: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2019;33(11):2918–2926. doi: 10.1002/ptr.6481. [DOI] [PubMed] [Google Scholar]
- 204.Li X., Wang W., Hou L., Wu H., Wu Y., Xu R., et al. Does tea extract supplementation benefit metabolic syndrome and obesity? a systematic review and meta-analysis. Clin. Nutr. 2020;39(4):1049–1058. doi: 10.1016/j.clnu.2019.05.019. [DOI] [PubMed] [Google Scholar]
- 205.Onakpoya I.J., Spencer E.A., Thompson M.J., Heneghan C.J. The effect of chlorogenic acid on blood pressure: a systematic review and meta-analysis of randomized clinical trials. J. Hum. Hypertens. 2015;29(2):77–81. doi: 10.1038/jhh.2014.46. [DOI] [PubMed] [Google Scholar]
- 206.Tierney A.C., Rumble C.E., Billings L.M., George E.S. Effect of dietary and supplemental lycopene on cardiovascular risk factors: a systematic review and meta-analysis. Adv. Nutr. 2020;11(6):1453–1488. doi: 10.1093/advances/nmaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Noordzij M., Uiterwaal C.S., Arends L.R., Kok F.J., Grobbee D.E., Geleijnse J.M. Blood pressure response to chronic intake of coffee and caffeine: a meta-analysis of randomized controlled trials. J. Hypertens. 2005;23(5):921–928. doi: 10.1097/01.hjh.0000166828.94699.1d. [DOI] [PubMed] [Google Scholar]
- 208.Amerizadeh A., Asgary S., Vaseghi G., Farajzadegan Z. Effect of genistein intake on some cardiovascular risk factors: an updated systematic review and meta-analysis. Curr. Probl. Cardiol. 2021;47:100902. doi: 10.1016/j.cpcardiol.2021.100902. [DOI] [PubMed] [Google Scholar]
- 209.Xia W., Tang N., Kord-Varkaneh H., Low T.Y., Tan S.C., Wu X., et al. The effects of astaxanthin supplementation on obesity, blood pressure, CRP, glycemic biomarkers, and lipid profile: a meta-analysis of randomized controlled trials. Pharmacol. Res. 2020;161:105113. doi: 10.1016/j.phrs.2020.105113. [DOI] [PubMed] [Google Scholar]
- 210.Begg C., Cho M., Eastwood S., Horton R., Moher D., Olkin I., et al. Improving the quality of reporting of randomized controlled trials. the CONSORT statement. JAMA. 1996;276(8):637–639. doi: 10.1001/jama.276.8.637. [DOI] [PubMed] [Google Scholar]
- 211.Khosravi-Boroujeni H., Nikbakht E., Natanelov E., Khalesi S. Can sesame consumption improve blood pressure? A systematic review and meta-analysis of controlled trials. J. Sci. Food Agric. 2017;97(10):3087–3094. doi: 10.1002/jsfa.8361. [DOI] [PubMed] [Google Scholar]
- 212.Obarzanek E., Sacks F.M., Vollmer W.M., Bray G.A., Miller E.R., 3rd, Lin P.H., et al. Effects on blood lipids of a blood pressure-lowering diet: the Dietary Approaches to Stop Hypertension (DASH) trial. Am. J. Clin. Nutr. 2001;74(1):80–89. doi: 10.1093/ajcn/74.1.80. [DOI] [PubMed] [Google Scholar]
- 213.Harsha D.W., Sacks F.M., Obarzanek E., Svetkey L.P., Lin P.-H., Bray G.A., et al. Effect of dietary sodium intake on blood lipids. Hypertension. 2004;43(2):393–398. doi: 10.1161/01.HYP.0000113046.83819.a2. https://doi.org/doi: [DOI] [PubMed] [Google Scholar]
- 214.Graudal N.A., Galløe A.M., Garred P. Effects of sodium restriction on blood pressure, renin, aldosterone, catecholamines, cholesterols, and triglyceride – a meta-analysis. JAMA. 1998;279(17):1383–1391. doi: 10.1001/jama.279.17.1383. [DOI] [PubMed] [Google Scholar]
- 215.Kim M.K., Reaven G.M., Kim S.H. Dissecting the relationship between obesity and hyperinsulinemia: role of insulin secretion and insulin clearance. Obesity (Silver Spring, Md) 2017;25(2):378–383. doi: 10.1002/oby.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Suga S.-I., Phillips M.I., Ray P.E., Raleigh J.A., Vio C.P., Kim Y.-G., et al. Hypokalemia induces renal injury and alterations in vasoactive mediators that favor salt sensitivity. Am. J. Physiol.-Ren. Physiol. 2001;281(4):F620–F629. doi: 10.1152/ajprenal.2001.281.4.F620. [DOI] [PubMed] [Google Scholar]
- 217.Ray P.E., Suga S.-I., Liu X.-H., Huang X., Johnson R.J. Chronic potassium depletion induces renal injury, salt sensitivity, and hypertension in young rats. Kidney Int. 2001;59(5):1850–1858. doi: 10.1046/j.1523-1755.2001.0590051850.x. [DOI] [PubMed] [Google Scholar]
- 218.Lekakis J.P., Papathanassiou S., Papaioannou T.G., Papamichael C.M., Zakopoulos N., Kotsis V., et al. Oral L-arginine improves endothelial dysfunction in patients with essential hypertension. Int. J. Cardiol. 2002;86(2–3):317–323. doi: 10.1016/s0167-5273(02)00413-8. [DOI] [PubMed] [Google Scholar]
- 219.Palloshi A., Fragasso G., Piatti P., Monti L.D., Setola E., Valsecchi G., et al. Effect of oral L-arginine on blood pressure and symptoms and endothelial function in patients with systemic hypertension, positive exercise tests, and normal coronary arteries. Am. J. Cardiol. 2004;93(7):933–935. doi: 10.1016/j.amjcard.2003.12.040. [DOI] [PubMed] [Google Scholar]
- 220.Appel L.J., Sacks F.M., Carey V.J., Obarzanek E., Swain J.F., Miller E.R., et al. Effects of protein, Monounsaturated Fat, and Carbohydrate Intake on Blood Pressure and Serum LipidsResults of the OmniHeart Randomized Trial. JAMA. 2005;294(19):2455–2464. doi: 10.1001/jama.294.19.2455. [DOI] [PubMed] [Google Scholar]
- 221.Squadrito F., Altavilla D., Squadrito G., Saitta A., Cucinotta D., Minutoli L., et al. Genistein supplementation and estrogen replacement therapy improve endothelial dysfunction induced by ovariectomy in rats. Cardiovasc. Res. 2000;45(2):454–462. doi: 10.1016/s0008-6363(99)00359-4. [DOI] [PubMed] [Google Scholar]
- 222.Dudásová S., Grancicová E. Influence of casein and soy flour proteins on aminoacid content in the liver of experimental animals. Physiol. Res. 1992;41(6):411–416. [PubMed] [Google Scholar]
- 223.González-Ortiz M., Hernández-González S.O., Hernández-Salazar E., Martínez-Abundis E. Effect of oral L-carnitine administration on insulin sensitivity and lipid profile in type 2 diabetes mellitus patients. Ann. Nutr. Metab. 2008;52(4):335–338. doi: 10.1159/000151488. [DOI] [PubMed] [Google Scholar]
- 224.Huang A., Vita J.A., Venema R.C., Keaney J.F., Jr. Ascorbic acid enhances endothelial nitric-oxide synthase activity by increasing intracellular tetrahydrobiopterin. J. Biol. Chem. 2000;275(23):17399–17406. doi: 10.1074/jbc.M002248200. [DOI] [PubMed] [Google Scholar]
- 225.Gladwin M.T., Raat N.J.H., Shiva S., Dezfulian C., Hogg N., Kim-Shapiro D.B., et al. Nitrite as a vascular endocrine nitric oxide reservoir that contributes to hypoxic signaling, cytoprotection, and vasodilation. Am. J. Physiol.-Heart Circulat. Physiol. 2006;291(5):H2026–H2035. doi: 10.1152/ajpheart.00407.2006. [DOI] [PubMed] [Google Scholar]
- 226.Clifford T., Howatson G., West D.J., Stevenson E.J. The potential benefits of red beetroot supplementation in health and disease. Nutrients. 2015;7(4):2801–2822. doi: 10.3390/nu7042801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Grassi D., Desideri G., Ferri C. Flavonoids: antioxidants against atherosclerosis. Nutrients. 2010;2(8):889–902. doi: 10.3390/nu2080889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Loke W.M., Hodgson J.M., Proudfoot J.M., McKinley A.J., Puddey I.B., Croft K.D. Pure dietary flavonoids quercetin and (-)-epicatechin augment nitric oxide products and reduce endothelin-1 acutely in healthy men. Am. J. Clin. Nutr. 2008;88(4):1018–1025. doi: 10.1093/ajcn/88.4.1018. [DOI] [PubMed] [Google Scholar]
- 229.Schroeter H., Heiss C., Balzer J., Kleinbongard P., Keen C.L., Hollenberg N.K., et al. (-)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc. Natl. Acad. Sci. USA. 2006;103(4):1024–1029. doi: 10.1073/pnas.0510168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Panahi Y., Hosseini M.S., Khalili N., Naimi E., Majeed M., Sahebkar A. Antioxidant and anti-inflammatory effects of curcuminoid-piperine combination in subjects with metabolic syndrome: a randomized controlled trial and an updated meta-analysis. Clin. Nutr. 2015;34(6):1101–1108. doi: 10.1016/j.clnu.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 231.Santos-Parker J.R., Strahler T.R., Bassett C.J., Bispham N.Z., Chonchol M.B., Seals D.R. Curcumin supplementation improves vascular endothelial function in healthy middle-aged and older adults by increasing nitric oxide bioavailability and reducing oxidative stress. Aging. 2017;9(1):187–208. doi: 10.18632/aging.101149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Prasad K. Secoisolariciresinol diglucoside (SDG) Isolated from flaxseed, an alternative to ACE inhibitors in the treatment of hypertension. Int. J. Angiol. 2013;22(4):235–238. doi: 10.1055/s-0033-1351687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Caligiuri S.P.B., Edel A.L., Aliani M., Pierce G.N. Flaxseed for hypertension: implications for blood pressure regulation. Curr. Hypertens. Rep. 2014;16(12):499. doi: 10.1007/s11906-014-0499-8. [DOI] [PubMed] [Google Scholar]
- 234.Knapp H.R. Hypotensive effects of omega 3 fatty acids: mechanistic aspects. World Rev. Nutr. Diet. 1991;66:313–328. [PubMed] [Google Scholar]
- 235.Goodfellow J., Bellamy M.F., Ramsey M.W., Jones C.J., Lewis M.J. Dietary supplementation with marine omega-3 fatty acids improve systemic large artery endothelial function in subjects with hypercholesterolemia. J. Am. Coll. Cardiol. 2000;35(2):265–270. doi: 10.1016/s0735-1097(99)00548-3. [DOI] [PubMed] [Google Scholar]
- 236.Houston M. The role of magnesium in hypertension and cardiovascular disease. J. Clin. Hypertens. 2011;13(11):843–847. doi: 10.1111/j.1751-7176.2011.00538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Carvalho L.S., Sposito A.C. Vitamin D for the prevention of cardiovascular disease: are we ready for that? Atherosclerosis. 2015;241(2):729–740. doi: 10.1016/j.atherosclerosis.2015.06.034. [DOI] [PubMed] [Google Scholar]
- 238.Revuelta-Iniesta R., Al-Dujaili E.A.S. Consumption of green coffee reduces blood pressure and body composition by influencing 11β-HSD1 enzyme activity in healthy individuals: a pilot crossover study using green and black coffee. BioMed. Res. Int. 2014;2014:482704. doi: 10.1155/2014/482704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Roshan H., Nikpayam O., Sedaghat M., Sohrab G. Effects of green coffee extract supplementation on anthropometric indices, glycaemic control, blood pressure, lipid profile, insulin resistance and appetite in patients with the metabolic syndrome: a randomised clinical trial. Br. J. Nutr. 2018;119(3):250–258. doi: 10.1017/s0007114517003439. [DOI] [PubMed] [Google Scholar]
- 240.Khalesi S., Sun J., Buys N., Jayasinghe R. Effect of probiotics on blood pressure: a systematic review and meta-analysis of randomized, controlled trials. Hypertension. 2014;64(4):897–903. doi: 10.1161/HYPERTENSIONAHA.114.03469. [DOI] [PubMed] [Google Scholar]
- 241.Kelly C.J. Effects of theobromine should be considered in future studies. Am. J. Clin. Nutr. 2005;82(2):486–487. doi: 10.1093/ajcn.82.2.486. ; author reply 7-8, [DOI] [PubMed] [Google Scholar]
- 242.Johnson R.J., Segal M.S., Sautin Y., Nakagawa T., Feig D.I., Kang D.H., et al. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am. J. Clin. Nutr. 2007;86(4):899–906. doi: 10.1093/ajcn/86.4.899. [DOI] [PubMed] [Google Scholar]
- 243.Leskinen M.H., Hautaniemi E.J., Tahvanainen A.M., Koskela J.K., Päällysaho M., Tikkakoski A.J., et al. Daily liquorice consumption for two weeks increases augmentation index and central systolic and diastolic blood pressure. PLOS ONE. 2014;9(8) doi: 10.1371/journal.pone.0105607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Melis M.S. Stevioside effect on renal function of normal and hypertensive rats. J. Ethnopharmacol. 1992;36(3):213–217. doi: 10.1016/0378-8741(92)90046-T. [DOI] [PubMed] [Google Scholar]
- 245.Sukhato K., Akksilp K., Dellow A., Vathesatogkit P., Anothaisintawee T. Efficacy of different dietary patterns on lowering of blood pressure level: an umbrella review. Am. J. Clin. Nutr. 2020;112(6):1584–1598. doi: 10.1093/ajcn/nqaa252. [DOI] [PubMed] [Google Scholar]
- 246.Lucey A., Heneghan C., Kiely M.E. Guidance for the design and implementation of human dietary intervention studies for health claim submissions. Nutr. Bulletin. 2016;41(4):378–394. doi: 10.1111/nbu.12241. [DOI] [Google Scholar]
- 247.Satija A., Stampfer M.J., Rimm E.B., Willett W., Hu F.B. Perspective: are large, simple trials the solution for nutrition research? Adv. Nutr. 2018;9(4):378–387. doi: 10.1093/advances/nmy030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Zeraatkar D., Bhasin A., Morassut R.E., Churchill I., Gupta A., Lawson D.O., Miroshnychenko A., et al. Characteristics and quality of systematic reviews and meta-analyses of observational nutritional epidemiology: a cross-sectional study. Am. J. Clin. Nutr. 2021;113(6):1578–1592. doi: 10.1093/ajcn/nqab002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Parati G., Valentini M. Do we need out-of-office blood pressure in every patient? Curr. Opin. Cardiol. 2007;22(4):321–328. doi: 10.1097/HCO.0b013e3281bd8835. [DOI] [PubMed] [Google Scholar]
- 250.Messerli F.H., Bangalore S., Schmieder R.E. Wilder's principle: pre-treatment value determines post-treatment response. Eur. Heart J. 2014;36(9):576–579. doi: 10.1093/eurheartj/ehu467. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.