Abstract
Objectives:
Compare lung parenchymal and pulmonary artery (PA) growth and hemodynamics following early and delayed PA stent interventions for treatment of unilateral branch PA stenosis (PAS) in swine.
Background:
How the pulmonary circulation remodels in response to different durations of hypoperfusion and how much growth and function can be recovered with catheter directed interventions at differing time periods of lung development is not understood.
Methods:
18 swine were assigned to four groups: sham (n=4), untreated left PAS (LPAS) (n=4), early intervention (EI) (n=5) and delayed intervention (DI) (n=5). EI had LPA stenting at 6 weeks (6 Kg) with re-dilation at 10 weeks. DI had stenting at 10 weeks. All underwent right heart catheterization, CT, MRI and histology at 20 weeks (55 Kg).
Results:
EI decreased the extent of histologic changes in the left lung as DI had marked alveolar septal and bronchovascular abnormalities (p=0.05 and p<0.05 vs sham) that were less prevalent in EI. EI also increased left lung volumes and alveolar counts compared to DI. EI and DI equally restored LPA pulsatility, R heart pressures and distal LPA growth. EI and DI improved, but did not normalize LPA stenosis diameter (LPA/DAo ratio: sham 1.27±0.11mm/mm, DI 0.88±0.10mm/mm, EI 1.01±0.09mm/mm) and pulmonary blood flow distributions (LPA-Flow%: sham 52±5%, LPAS 7±2%, DI 44±3%, EI 40±2%).
Conclusion:
In this surgically created PAS model, EI was associated with improved lung parenchymal development compared to DI. Longer durations of L lung hypoperfusion did not detrimentally affect PA growth and R heart hemodynamics. Functional and anatomical discrepancies persist despite successful stent interventions that warrant additional investigation.
Keywords: Congenital heart disease, pediatric interventions, pulmonary artery stenting
INTRODUCTION
From birth until 3–5 years of age in healthy individuals there is rapid increase in alveoli number and concurrent expansion of both the pulmonary vasculature branching and cross sectional arterial area (1,2). Subsequent to this rapid developmental period, the pulmonary vasculature continues a gradual increase in size corresponding with somatic growth. Branch pulmonary artery stenosis (PAS) occurring in the first years of life alters pulmonary blood flow (PBF) during these critical periods of development and growth. Over subsequent years, chronic PAS is associated with PBF mal-distribution that may cause PA hypertension, pulmonary valve insufficiency and increased right ventricular afterload (3,4) all contributing to long term morbidity and mortality (5–7). Given the fact that severely stenotic or occluded branch pulmonary arteries do not grow normally (8,9), it is assumed that normal pulsatile PBF is essential for lung and PA development and growth. Yet, it is unknown how the pulmonary circulation remodels in response to different durations of hypoperfusion and how much growth and function can be recovered with interventions at differing time periods of lung development.
PAS is associated with many forms of congenital heart disease (CHD) and is a frequent indication for intervention (10–17). Operations to address PAS in infants and children during the alveolar and PA developmental phase have varying outcomes and re-stenosis rates (18–20) and in several centers first line therapy for PAS is accomplished using the catheter based techniques of angioplasty and intravascular stenting. Evidence supports the use of PA stenting for older CHD patients with PAS (21–23). PA stent intervention early in life can be effective at relieving stenosis, however this advantage is limited by increased procedural risk (24), and technical limitations imposed by the fixed diameter of small stents implanted in growing patients (25–27). The development of new stent technologies (28,29) with low profile delivery systems and capability to expand from small implant diameters to adult PA size, has presented an opportunity to perform earlier stent interventions for PAS. Additionally, ongoing interest and support for the development of stent technologies designed specifically for smaller patients depends upon demonstration that earlier interventions improve the anatomy and function of the (30,31) lungs and pulmonary vasculature. To date, these data do not exist and thus the ability to judge the risk: benefit ratio of an earlier stent intervention for PAS is not feasible. The optimal timing for PAS stent intervention to minimize complications and maximize lung development and PA growth remains unclear and no published studies directly compare outcomes of early versus delayed stent intervention for PAS, highlighting a significant gap in knowledge.
Our previous work with a swine model of unilateral branch PAS demonstrated that early stent interventions followed by serial dilations with deliberate stent fracturing was safe and resulted in improved growth of the distal PA vasculature (32). Also, morphometric analysis of the postnatal development of the swine lung show similar patterns to man, but occurring at a much faster rate with alveolar and arterial multiplication complete by 10–12 weeks of age (33). Based on this information, a swine model of unilateral branch PAS allows us to test our hypothesize that early stent interventions with small diameter stents with capacity for dilation to adult diameters that restore pulsatile PBF during the phase of alveolar and PA developmental is superior to stent interventions performed later when PA multiplication is no longer possible. The results presented here define the anatomic and physiologic consequences of untreated PA stenosis in a swine model of left PAS (32) and compares lung and PA development and growth as well as hemodynamics following early and delayed PA stent interventions.
MATERIALS AND METHODS
Eighteen domestic male pigs were obtained from the University of Wisconsin Swine Research and Teaching Center (2 weeks old, 5±1 kg). In 14 animals, discrete proximal left PA stenosis (LPAS) was surgically created. Five animals with LPAS underwent early stent intervention (EI group) at 5 weeks of age in the alveolar multiplication period with subsequent stent dilation at 10 weeks of age. Five animals with LPAS underwent delayed stent intervention (DI group) at 10 weeks of age near the end of the alveolar multiplication period. Four animals each served as LPAS and sham controls. All groups had a single experimental catheterization with imaging and histology at 20 weeks of age. The Institutional Animal Care and Use Committee of the University of Wisconsin reviewed and approved this protocol and the overall experimental timeline is shown in Figure 1.
Figure 1:
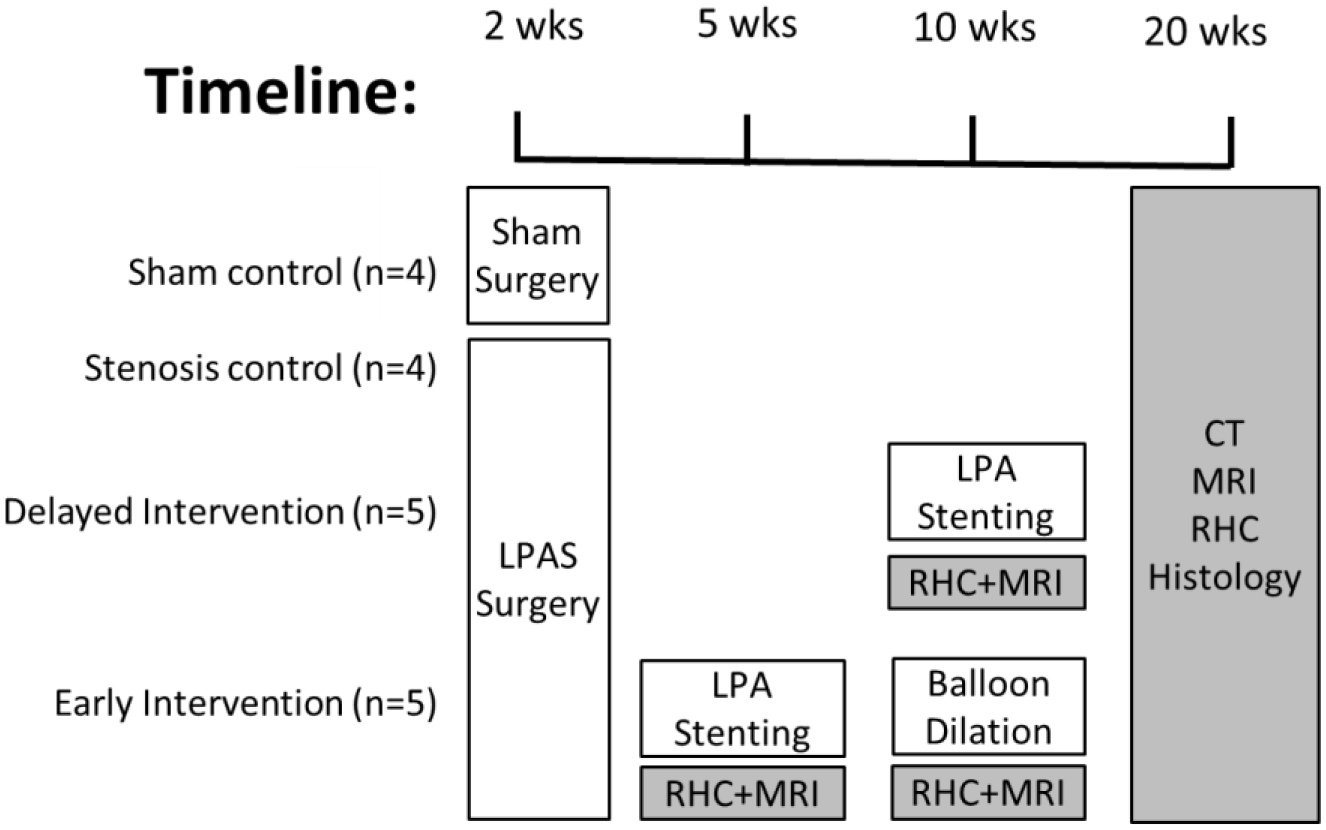
Study timeline
Surgical creation of LPAS:
Piglets were anesthetized with isoflurane (2–5%), intubated, given a single dose of intravenous cefazolin (25 mg/kg) and a left thoracotomy was performed. The LPA was isolated and a short segment of 4 mm Gore-Tex shunt was sutured around the proximal LPA as previously described (32,34). At the conclusion of the operation, local anesthesia was administered into the surgical site and the animals were given intramuscular analgesia for an additional 48 hours.
Interventional sequence:
EI animals had LPA stenting at 5 weeks (5.8 ± 1.4 Kg) with low profile 6–7 mm diameter × 18 mm length premounted (Valeo) stents with stent dilation at 10 weeks. The aim of the 10-week catheterization for the EI animals (20 ± 2 Kg) was to dilate the LPA stent to a diameter equal to the maximal distal LPA diameter that ranged from 8.5–11 mm, thus Evercross 10–12 mm balloons were used for 10 week stent dilation. DI animals had LPA stenting at 10 weeks (30 ± 6 Kg) with large diameter stents (EV3 1610 DS) implanted on 10–12 mm Powerflex balloons, implant diameter again matching the diameter of the distal LPA that ranged from 9–11.7 mm.
Catheterization hemodynamic measurements:
Following induction (telazol and xylazine IM) and anesthesia (isoflurane 2–5%), venous access was obtained percutaneously and intravenous heparin was given. Beat-by-beat pressure waveforms were obtained in the right atrium (RA), right ventricle (RV), main (MPA), right (RPA), LPA, and bilateral capillary wedge positions before and after each intervention using end-hole fluid filled catheters attached to a pressure transducer. In addition at the 20-week catheterization all animals had hemodynamic recording at rest and following a dobutamine stress challenge (5 mcg/kg/min infusion).
Computed tomography:
Multidetector-row computed tomography (MSCT) was performed using a 64-slice CT scanner (GE 750 CT, GE Healthcare, Waukesha, WI) with retrospective cardiac gating. Contrast enhancement was achieved with intravenous Iopamidol-370 (Bracco Diagnostics Inc., Monroe Township, NJ). PA measures were performed independently by two investigators (RP, LL) using a McKesson Technology Solutions workstation (Alpharetta, GA). Maximum orthogonal dimensions were recorded from 3D data sets of the proximal LPA diameters measured immediately distal to the MPA bifurcation in the sham group, at the LPAS in the stenosis group, and within the stent in the DI and EI groups. Six RPA and five LPA comparable, 1st order/segmental branches were consistently identified from the 3D data sets as previously defined (32,35) and the 1st order branch artery origin and adjacent main RPA/LPA diameters were manually measured and recorded for each individual location. To account for differing body weights, all PA dimensions were indexed to diameter of aorta at the diaphragm. PA measurement locations are shown in Appendix Figure A1. Left and right lung volumes were also segmented from the 20 week CT images and the left lung volume ratio was calculated as the left lung volume/total lung volume as previously described (36).
Magnetic resonance imaging:
Magnetic resonance imaging (MRI) was performed before and after each intervention and at 20 weeks in all animals on a 3T imaging system (Discovery MR750, GE Healthcare, Waukesha, WI) without a contrast agent. A balanced steady state free precession (bSSFP) sequence was used for cardiac imaging. Short axis images were segmented to calculate cardiac output (CO). Cardiac index (CI) is CO normalized by body surface area and values reported are averages of LV and RV CI. Perfusion of the lungs was quantified using four dimensional flow sensitive MRI – PC-VIPR sequence as previously described (37,38) using flow visualization software (EnSight, Ansys, Canonsburg, PA). As MR signal is lost in a metal stent, left lung flow rate for stented animals was calculated as MPA flow rate - RPA flow rate.
Histology:
After the final procedures, all animals were euthanized with IV pentobarbital. The lungs were inflated to 25 cm H2O and fixed with 10% buffered formalin infused into the endotracheal tube for 25 minutes while simultaneously irrigating the MPA with heparinized saline. The heart and lungs were harvested for gross inspection and histology. The RV was dissected free of the intraventricular septum (IVS) and LV and weighed separately to calculate a Fulton index (RV/(LV+IVS)). Five left and eight right corresponding lower lung segment tissue blocks were collected and embedded in paraffin and stained with hematoxylin and eosin for histologic analysis that included alveolar counts (33), percent medial wall thickness of 50–300μm diameter arteries (9), and semi-quantitative histologic analysis (see Appendix for grading scales) by an experienced lung pulmonary vascular pathologist (SA).
Statistics.
Data are reported as mean ± standard error (SE) unless otherwise indicated. Statistical analysis of the early and delayed intervention time courses was performed using the non-parametric, repeated-measures Friedman test. Post-hoc comparisons only considered subsequent time-points. Statistical analysis of the untreated LPAS time course used the pre-stenting EI group data from 5 weeks of age, the pre-stenting DI group data from 10 weeks of age, and the LPAS group data from 20 weeks of age. LPAS time course statistical analysis was performed with the non-parametric Kruskal-Wallis test. Statistical comparison of the DI and EI groups at 10 weeks of age both before and after intervention uses the non-parametric Mann-Whitney U test. Statistical comparison between groups at the 20-week time point was performed with the non-parametric Kruskal-Wallis test. All post-hoc testing used a Bonferroni correction. Response to dobutamine was quantified as the difference between pre and post dobutamine measurements.
RESULTS
Somatic growth
At the initial surgical intervention, bodyweights were similar between groups (sham 5.4±0.3kg, LPAS 5.3±0.3kg, DI 5.2±0.2kg, EI 5.0±0.3kg). At 10 weeks the EI group was smaller than the DI group (21±1kg vs 32±3kg). At 20 weeks the EI group still trended towards a decreased bodyweight compared to DI group (59±3kg vs 48±5kg) due to two swine with weights of 36kg and 37kg. The other three EI swine weighed 55±1 kg that is comparable to the other groups.
Untreated LPAS time course
The time course of untreated LPAS in Table 1 is created from pre-intervention data from the EI group at 5 weeks, pre-intervention data from the DI group at 10 weeks and the LPAS group at 20 weeks of age. The stenosis diameter was comparable for all three groups. The largest distal LPA diameter was measured at 10 weeks of age (10.3±0.5mm). Left lung perfusion decreased from 18±3% at 5 weeks, to 6–7±2% at the 10 and 20-week time points. RV, MPA, and RPA systolic pressures increased between the 10 to the 20-week time points and LPA systolic pressure with limited pulsatility was similar for all time points.
Table 1:
Untreated LPAS group time course
| EI Pre-Cath #1 (n=5) | DI Pre-Cath #2 (n=5) | LPAS Cath #3 (n=4) | |
|---|---|---|---|
| Weight (kg) | 6.7±1.1 | 32±7 | 57±2 |
| Proximal LPA Diameter (mm) | 1.7±0.2 | 2.2±0.3 | 1.4±0.1 |
| Distal LPA Diameter (mm) | 6.5±0.3 | 10.3±0.5* | 6.9±1.9 |
| HR (BPM) | 120±8 | 92±4 | 83±2* |
| CI (L/min/m2) | 2.0±0.2 | 4.2±0.3 (n=4) | 3.2±0.1 |
| L Lung Perfusion (%) | 18±3 | 6±2 | 7±2 |
| Mean RA Pressure (mmHg) | 6±1 | 8±1 | 10±1 |
| RV Systolic Pressure (mmHg) | 29±3 | 29±1 | 38±3 |
| MPA Pressure (sys / dia, mmHg) | 27±2 / 13±2 | 29±2 / 14±1 | 38±3* / 17±2 |
| RPA Pressure (sys / dia, mmHg) | 25±2 / 10±1 | 28±1 / 14±1 | 37±2* / 18±2 |
| LPA Pressure (sys / dia, mmHg) | 13±3 / 13±3 | 13±1 / 13±1 | 15±4 / 13±4 |
| Stenosis/Stent Pressure Gradient (mmHg) | 14±3 | 16±1 | 23±3 |
| PCWP (mmHg) | 8±2 | 10±0 (n=4) | 11±3 |
p<0.05 vs 5 week,
p<0.05 vs 10 week
Delayed intervention time course
Longitudinal data for the delayed intervention group is shown in Table 2. Proximal LPA diameter increased with stenting but no distal LPA growth occurred from 10 to 20 weeks. Left lung perfusion increased both acutely with stenting and from 10–20 weeks. RA, RV, MPA and RPA pressures were at the upper limits of normal before and after the 10-week intervention and decreased to normal at 20 weeks. LPA systolic pressure acutely increased with DI stenting that also decreased the proximal LPA pressure gradient that remained low from 10–20 weeks.
Table 2:
Delayed intervention group time course
| Pre-Cath #1 (n-5) | Post-Cath #1 (n=5) | Cath #2 (n=2) | |
|---|---|---|---|
| Weight (kg) | 32±7 | 59±3 | |
| Proximal LPA Diameter (mm) | 2.2±0.3 | 10.7±0.4* | 10.0±0.8 |
| Distal LPA Diameter (mm) | 10.3±0.5 | 10.7±0.4 | 10.3±0.3 |
| HR (BPM) | 92±4 | 83±2 | 105±6 |
| CI (L/min/m2) | 4.2±0.3 (n=4) | 3.2±0.3 (n=3) | 3.7±0.4 |
| L Lung Perfusion (%) | 6±2# | 26±9 | 44±3 |
| Mean RA Pressure (mmHg) | 8±1 | 10±1# | 6±1 |
| RV Systolic Pressure (mmHg) | 29±1 | 30±2# | 27±2 / 7±1 |
| MPA Pressure (sys / dia, mmHg) | 29±2# / 14±1 | 29±1 / 16±1 | 25±1 / 13±0 |
| RPA Pressure (sys / dia, mmHg) | 28±1 / 14±1 | 28±1 / 15±1 | 25±2 / 14±1 |
| LPA Pressure (sys / dia, mmHg) | 13±1 / 13±1 | 28±1* / 15±1 | 23±2 / 14±1 |
| Stenosis/Stent Pressure Gradient (mmHg) | 16±1 | 1±0* | 1±1 |
| PCWP (mmHg) | 10±0 (n=4) | 11±0 (n=4) | 8±1* |
p<0.05 vs the previous time-point from post-hoc testing,
p<0.05 vs EI at corresponding catheterization
Early intervention time course
Longitudinal data for the EI group are shown in Table 3. As expected, initial stenting and subsequent balloon dilation increased proximal LPA diameters. The distal LPA diameter increased from 5–10 weeks with little growth occurring from 10–20 weeks. Left lung perfusion increased acutely with stenting and then remained constant for the 10 and 20-week time points. RA, RV, MPA and RPA pressures were normal over the intervention sequence. LPA systolic pressure and the proximal LPA pressure gradient responded favorably to 5-week stenting and 10-week balloon dilation.
Table 3:
Early intervention group time course
| Pre-Cath #1 (n=5) | Post-Cath #1 (n=5) | Pre-Cath #2 (n=5) | Post-Cath #2 (n=5) | Cath #3 (n=5) | |
|---|---|---|---|---|---|
| Weight (kg) | 6.7±1.1 | 21±1 | 48±5 | ||
| Proximal LPA Diameter (mm) | 1.7±0.2 | 6.0±0.2 | 5.8±0.3 | 9.6±0.5 | 10.4.±0.5 |
| Distal LPA Diameter (mm) | 6.5±0.3 | 6.9±0.3 | 9.4±0.5 | 9.0±0 | 10.3±0.9 |
| HR (BPM) | 120±8 | 133±8 | 112±7 | 90±7 | 96±8 |
| CI (L/min/m2) | 2.0±0.2 | 3.2±0.1 (n=4) | 3.5±0.2 | 2.7±0.4 | 3.6±0.3 |
| L Lung Perfusion (%) | 18±3 | 35±3 | 39±3# | 41±4 | 40±2 |
| Mean RA Pressure (mmHg) | 6±1 | 8±1 | 5±0 | 5±1# | 6±1# (n=4) |
| RV Systolic Pressure (mmHg) | 29±3 | 29±4 | 26±3 | 26±1# | 29±1 / 6±0 (n=4) |
| MPA Pressure (sys / dia, mmHg) | 27±2 / 13±2 | 27±3 / 12±2 | 25±1# / 10±0 | 26±0 / 13±1 | 28±1 / 13±2 (n=4) |
| RPA Pressure (sys / dia, mmHg) | 25±2 / 10±1 | 26±3 / 14±2 | 22±1 / 10±0 | 24±1 / 12±1 | 24±2# / 13±1 (n=4) |
| LPA Pressure (sys / dia, mmHg) | 13±3 / 13±3 | 25±3 / 14±2 | 15±2 / 9±1 (n=3) | 24±2 / 13±1 | 22±2 / 13±2 (n=4) |
| Stenosis/Stent Pressure Gradient (mmHg) | 14±3 | 2±1 | 10±3 (n=3) | 2±1 | 6±1 (n=4) |
| PCWP (mmHg) | 8±2 | 8±2 (4) | 7±1 | 7±1 | 7±1 (n=4) |
p<0.05 vs the previous time-point,
p<0.05 vs DI at corresponding catheterization
EI vs DI hemodynamics & PBF at 10 weeks:
Pre-intervention the EI group demonstrated a trend towards decreased right heart pressures with mean RA (5±0 mmHg vs 8±1 mmHg, p=0.15), systolic RV (26±3 mmHg vs 29±1 mmHg, p=0.15), MPA (25±1 mmHg vs 29±2 mmHg, p=0.02) and RPA (22±1 mmHg vs 28±1 mmHg, p=0.06) respectively. The DI group had a LPAS pressure gradient of 16±1 mmHg vs the EI LPA stent pressure gradient of 10±3 mmHg (p=0.14). EI had greater PBF to the left lung (39±3% vs 6±2%, p=0.04).
Immediately following the 10-week intervention the EI group had continued trends toward lower right heart pressures with mean RA (5±1 mmHg vs 10±1 mmHg, p<0.01), RV systolic (26±1 mmHg vs 30±2 mmHg, p=0.03), MPA (26±0 mmHg vs 29±1 mmHg, p=0.22), and RPA (24±1 mmHg vs 28±1 mmHg, p=0.41) respectively. The EI and DI groups had comparable stent pressure gradients (2±1 mmHg vs 1±0 mmHg, p=0.15). EI continued to have greater PBF to the left lung (41±4% vs 26±9%, p=0.38). Cardiac index pre and post 10 week interventions were lower for EI than DI (pre: 3.5±0.2 vs 4.2±0.3 L/min/m2 p=0.11, post: 2.7±0.4 vs 3.2±0.3 p=0.57).
Hemodynamics & PBF at 20 weeks:
At the 20 week catheterization, the LPAS group also had increased mean RA and systolic RV, MPA and RPA pressures with a LPAS stenosis gradient of 23±3 mmHg and negligible LPA pulse pressures (2±2 mmHg) (Table 4). Both DI and EI normalized all measured right heart pressures with no significant differences noted between the two intervention groups. Left lung PBF was significantly decreased from 52±5% in the shams to 7±2% in the LPAS group (p<0.01). Both interventions improved left lung PBF compared to LPAS but values did not reach normal as DI and EI left lung PBF measured 44±3% and 40±2% (EI vs DI p=1.0) respectively. DI and EI had trends toward increased measured CI compared to sham and LPAS although these differences did not reach significance. At rest and with dobutamine (5 mcg/kg/min) there was not a significant difference in HR between groups (Figure 2). With dobutamine (5 mcg/kg/min), the LPAS group had the greatest increase in RV systolic pressure (p=0.07 vs DI, Figure 2).
Table 4:
Hemodynamics and PBF at 20-weeks for all groups
| Sham (n=4) | LPAS (n=4) | Delayed Intervention (n=5) | Early Intervention (n=5) | |
|---|---|---|---|---|
| BW (kg) | 56±3 | 57±2 | 59±3 | 48±5 |
| HR (BPM) | 84±4 | 83±2 | 105±6 | 96±8 |
| Fulton Index (g/g) | 0.42±0.02 | 0.40±0.02 | 0.44±0.02 | 0.40±0.02 |
| CI (L/min/m2) | 2.8±0.2 | 3.2±0.1 | 3.7±0.4 | 3.6±0.3 |
| L Lung Perfusion (%) | 52±5 | 7±2* | 44±3 (n=4) | 40±2 |
| Mean RA Pressure (mmHg) | 7±2 | 10±1 | 6±1 | 6±1# (n=4) |
| RV Systolic Pressure (mmHg) | 28±3 / 7±2 | 38±3 / 10±1 | 27±2# / 7±1 | 29±1 / 6±0 (n=4) |
| MPA Pressure (sys / dia, mmHg) | 29±2 / 14±2 | 38±3 / 17±2 | 25±1# / 13±0 | 28±1 / 13±2 (n=4) |
| RPA Pressure (sys / dia, mmHg) | 27±2 / 15±2 | 37±2 / 18±2 | 25±2 / 14±1 | 24±2# / 13±1 (n=4) |
| LPA Pressure (sys / dia, mmHg) | 28±1 / 17±1 | 15±4* / 13±4 | 23±2 / 14±1 | 22±2 / 13±2 (n=4) |
| Stenosis/Stent Pressure Gradient (mmHg) | 1±2 | 23±3* | 1±1# | 6±1 (n=4) |
| PCWP (mmHg) | 10±1 | 11±3 | 8±1 | 7±1 (n=4) |
p<0.05 vs sham control,
p<0.05 vs LPAS control
Figure 2:
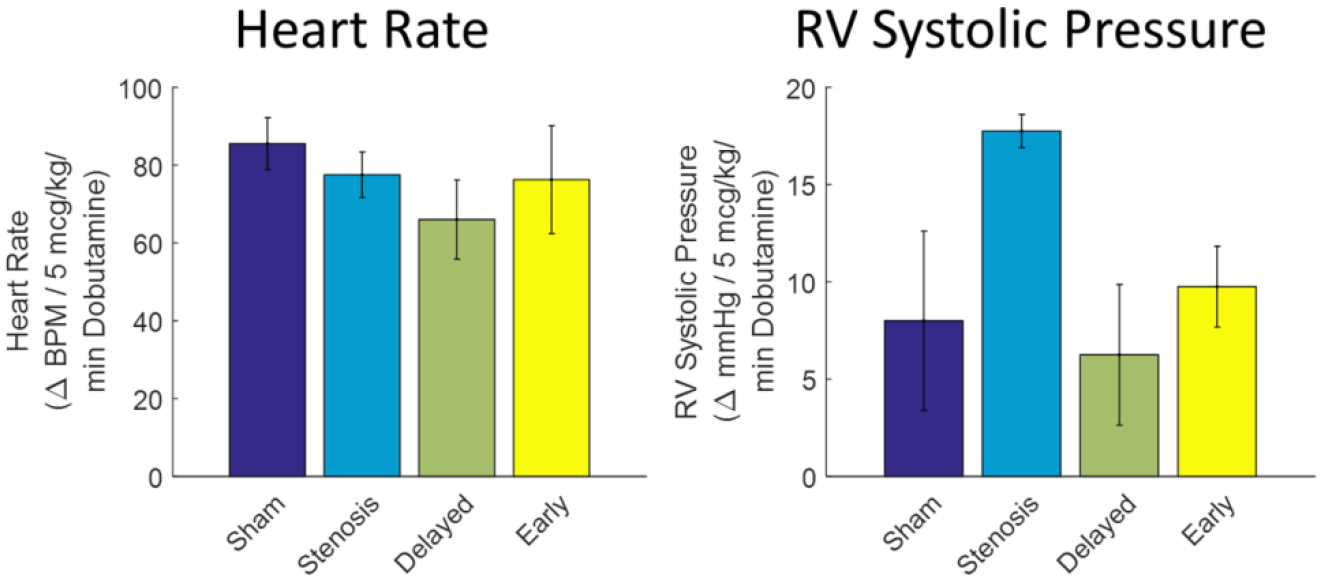
Response of HR and RV systolic pressure to 5mcg/kg/min dobutamine infusion. Sham (n=4), LPAS (n=4), DI (n=4), EI (n=4).
20-week CT imaging of PA and lung anatomy:
Representative 3D reconstructions of the PA vasculature are shown in Figure 3. Both DI and EI improved the proximal LPA-to-aorta diameter ratio (the stented segment) compared to LPAS. The proximal LPA-to-aorta diameter ratio was decreased compared to sham (1.27±0.11 mm/mm) for DI (0.88±0.10 mm/mm – vs sham p=0.71) and EI (1.01±0.09 mm/mm - vs sham p=1.0). At LPA measurement locations 1–5, DI and EI diameters were similar for the two intervention groups, comparable to shams and increased compared to LPAS. Similar results were seen for LPA 1st order branch artery diameters. No statistical differences were observed for the main RPA or RPA 1st order branch diameters indexed to aortic dimensions, however; at each RPA measurement point dimensions not indexed to aortic diameters were greatest for the LPAS group (Appendix Figure A3 - PA diameters not indexed to aortic diameter).
Figure 3:

3D reconstructions of PA anatomy, proximal LPA diameter, main LPA diameters and LPA 1st order branch diameters. Sham (n=4), LPAS (n=4), DI (n=5) and EI (n=5). * p<0.05 vs sham control, # p<0.05 vs LPAS control
The ratio of the left lung to total lung volume measured from CT imaging was 39±2% in the shams decreasing to 30±1% in the LPAS group (p<0.01). There was a trend towards increased total left lung volume in EI group at 36±3% (p=0.07 vs LPAS) compared to 34±2% in the DI group (p=0.87 vs LPAS).
Histopathology:
The Fulton index, RV/(LV+IVS), was similar for all groups indicating no RV hypertrophy in response to varying durations of LPAS over 20 week time frame (Table 4). Left lung alveoli density was similar between LPAS and DI (1.36±0.14 and 1.43±0.11 1,000/mm3). Although not statistically significant, EI trended towards greater left lung alveoli density (1.86±0.34 1,000/mm3) closer to sham controls (2.10±0.43 1,000/mm3). Right lung alveoli density was similar between groups. Percent medial wall thickness trended towards increased in the left lung of the LPAS group and was not different between sham, DI and EI groups.
In the left lung, semi-quantitative histopathology assessment (Table 5) showed alveolar septal abnormalities in LPAS (p<0.01 vs sham), DI (p=0.05 vs sham) which was less prevalent in the EI group (p=0.5 vs sham). Normal left lung bronchovascular anatomy was observed in the sham group while LPAS and DI had marked infiltration of bronchiolar arterioles (p<0.05 vs sham). EI had only minor abnormalities in left lung bronchovascular anatomy (Figure 5). No left lung interstitial inflammation was observed in any group and LPAS had focal interstitial smooth muscle metaplasia that was not present in sham, DI or EI. Right lung histopathology assessment was similar for all groups (Appendix Table A1).
Table 5:
Left lung histopathology
| Sham (n=4) | LPAS (n=4) | Delayed Intervention (n=5) | Early Intervention (n=5) | |
|---|---|---|---|---|
| Medial Wall Thickness (%) | 15±5 | 23±2 | 15±3 | 15±2 |
| Alveolar Density (1,000 / mm3) | 2.10±0.43 | 1.36±0.14 | 1.43±0.11 | 1.86±0.34 |
| Septal Abnormalities (1–4) | 1.0±0.0 | 3.5±0.1* | 3.2±0.2 | 2.6±0.3 |
| Bronchovascular Abnormalities (1–4) | 2.0±0.0 | 3.2±0.0* | 3.1±0.2* | 2.5±0.2 |
| Interstitial Inflammation (1–4) | 1.5±0.2 | 2.2±0.3 | 1.5±0.2 | 1.4±0.2 |
| Alveolar Metaplasia/Attenuation (1–4) | 1.0±0.0 | 1.6±0.2* | 1.1±0.1 | 1.2±0.1 |
p<0.05 vs sham control,
p<0.05 vs LPAS control
Figure 5:

Representative left lung bronchiole with associated pulmonary arteriole for (A) sham, (B) LPA stenosis control demonstrating increased bronchiolar arterioles (black arrows) and a small pulmonary arteriole with significant medial hypertrophy (white arrow) and (C) EI, demonstrating relatively normal bronchiolar arteries with normal appearing pulmonary arterioles.
Discussion
To our knowledge, the current study is the first to compare the consequences of timing of stent intervention to relieve branch PAS on pulmonary vasculature anatomy, right heart hemodynamics and lung parenchyma growth. This is a relevant clinical question as indications for and optimal timing of PAS interventions are not defined and it is unknown how the pulmonary circulation remodels with different durations of hypoperfusion and responds to interventions at different time periods of lung development. Our hypothesis that earlier stent intervention with restoration of pulsatile pulmonary blood flow during the rapid lung developmental phase would enhance PA and lung parenchyma growth is supported by the results of this study mainly in terms of improved alveolar and lung parenchymal anatomy. Compared to DI, the EI group had favorable trends toward larger left lung volumes with greater left lung alveolar density and less detrimental lung parenchymal remodeling. Bronchiolar artery infiltration and parenchymal septal abnormalities, seen with severe untreated stenosis (8), were more prevalent with greater durations of PAS and limited when pulsatile PBF is reestablished earlier in lung development. Comparison between intervention groups at the 10-week catheterization, which is the equivalent lung developmental stage to a 3–5 year old human, demonstrated lower R heart pressures with EI and significant differences in PBF and increased RV afterload for the DI group for early periods of life that is likely detrimental to long term cardiopulmonary health. In contrast, measure of PA growth, hemodynamics and lung perfusion at 20-weeks were independent of intervention timing. These results suggest that lung parenchymal growth is more sensitive to hypoperfusion while PA growth and hemodynamics can be normalized with relief of stenosis at later stages of lung development.
Stent implantation for treatment of PAS in CHD has been performed for the past thirty years with favorable long-term results (26). Initial PA stent interventions were restricted to older and larger patients due mainly to limitations in technology. With the development of small low profile delivery systems, stents are now deployed in the pulmonary vasculature of the smallest of children (28,29) who theoretically have better lung parenchymal and PA growth potential. Implantation of small stents can be an effective short term solution but this therapeutic strategy is associated with the expected need for catheter reinterventions to accommodate somatic PA growth and with high rates of need for surgical stent interventions (39). Unlike existing long-term data when adult PA diameters can be achieved through stenting (26), there is limited data describing longitudinal outcomes following small diameter stent interventions in the PAs of young children. We attempted to address this issue with a prior PAS study where small diameter stents were implanted in the pulmonary vasculature of piglets (6 Kg) and serially dilated to adult (60 Kg) PA diameters using intentional stent fracture techniques (32). With this previous study, we observed similar anatomic outcomes to our current findings, with relative restoration of distal PA growth following serial catheterizations with intentional stent fracture. In a related clinical study assessing distal PA growth following early PAS stent interventions, Takao et al (40) described PA lobar growth following stent implant for unilateral branch PAS in a group of 39 young children, 3.5–4.8 years at initial intervention. At five-year follow-up PA stenting resulted in lobar growth distal to the stent similar to lobar growth from the contralateral nonstenotic PA and patients with lower weight at the time of stent implantation showed greater lobar growth prompting the authors to advocate for earlier aggressive stent intervention for PAS. This study did not include infants and 37/39 patients received large diameter stents, thus the outcomes do not represent PAS interventions with small premounted stents performed very early in life.
Despite our findings of improved distal PA anatomy in intervention animals, abnormalities in individual lung perfusion remained (52±5% L lung perfusion in the shams vs 44±3% and 40±2% for DI and EI) which can be attributed to the smaller proximal LPA stents and the relatively smaller distal LPA compared to shams. Of note for both intervention groups the distal LPA did not increase in size between the 10–20 week catheterizations and further stent dilation to improve LPA flow would not be indicated given the distal PA dimension limitations. Why the distal LPA did not grow to dimensions equal to shams in response to improved L lung PBF following 10 weeks is unclear suggesting changes in the pulmonary vasculature persist that stent interventions do not completely address.
Both early and delayed interventions normalized the trend of increased medial wall thickness in the vascular bed of 50–300μm diameter arteries distal to the stenosis (Figure 5) and there were no differences in medial wall thickness in the right lung despite significant differences in pulmonary blood flow. In swine, it appears that medial wall thickening in response to PAS occurs over time and that stenting prevents these vascular changes. This increased medial wall thickness is contrary to unilateral PAS studies in neonatal rats (9) where decreased pulmonary blood flow led to smaller PAs in a constant state of vasodilation that histologically manifests as medial atrophy proposed to reduce PVR. Distal to the PAS in the developing rat pulmonary blood flow, PA pressures and pulsatility are reduced leading to abnormal lung development with a reduced number of vessels and a loss of vascular organization, both of which effected alveolar growth. In our PAS controls, we observed reduced number, size, and organization of the distal vasculature.
To compensate for the deleterious vascular effects of PAS early in life, the bronchial circulation is recruited to provide the pulmonary microvasculature at the precapillary level with oxygenated blood from the systemic circulation to ensure normal vascular development at the site of gas exchange (9,41) and reverse ischemic changes to lung mechanics (42). It is possible this compensatory remodeling is detrimental to long-term cardiopulmonary function due to a combination of increases in LV volume load, PA pressure, and RV afterload. In the current study, the PAS controls and DI groups had smaller left lungs, abnormal bronchiolar arteriole infiltration of the left lung and increased lung parenchymal septal abnormalities. These results are consistent with the effects of complete LPA ligation in swine (8). Early stent interventions were associated with improved left lung size and bronchiolar artery infiltration; however, the left lung volume was still smaller and there were still more bronchiolar arteries seen in early intervention animals than shams. This would suggest that stent interventions, regardless of timing, do not completely address the lung remodeling that occurs in response to PAS. Detailed ultrastructural and molecular studies defining the pulmonary microvascular remodeling (42,43) that occurs in association with PAS and stent interventions are needed to inform future alternative approaches to targeted PAS therapies.
Limitations:
This study used an animal model of PAS created by an external band in swine with no other cardiovascular disease, and thus may be physiologically different from post-operative PAS that is frequently the results of scarring, folds or stretching of the surgical anastomosis and may have associated diffuse PA hypoplasia. In addition, the number of animals is relatively small, there were differences in somatic growth between groups, and the experimental time course is short compared to humans where PAS and PA stents may be present for years. The amount of scar tissue development, in-stent stenosis and their subsequent effects on PA and lung parenchymal anatomy could be underestimated. How these findings relate to patients with PAS and CHD is uncertain and corollary clinical studies assessing long-term outcomes of early and delayed PA stent interventions are warranted.
CONCLUSION
In this swine PAS model, early stent interventions were associated with improved lung parenchyma anatomy but not PA growth, hemodynamics or lung perfusion compared to delayed stent interventions. These data would suggest that stenosis relief and lung reperfusion during the rapid alveolar multiplication period improves lung parenchyma growth. While improvements were observed with both stent intervention timings, persistent functional and anatomical discrepancies between intervention groups and sham controls exist. These differences include impaired perfusion of the stented lung and abnormal lung histopathology. Further research is required to understand both how these results relate to human patients and the underlying pathophysiology of the lung response to PAS and current interventional therapies.
Figure 4:

3D reconstructions of the right and left lung volumes from CT and the left lung volume ratios for sham (n=4), LPAS (n=4), DI (n=5) and EI (n=5). * p<0.05 vs sham control, # p<0.05 vs LPAS control
ACKNOWLEDGMENTS
This investigation was supported by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR002373 (AR, LL and CF) and under the NIH Ruth L. Kirschstein National Research Service Award T32 HL 007936 from the National Heart Lung and Blood Institute to the University of Wisconsin-Madison Cardiovascular Research Center (RP). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
ABBREVIATIONS
- BSA
Body surface area
- CHD
Congenital heart disease
- CO
Cardiac output
- CI
Cardiac Index
- DI
Delayed intervention
- EI
Early intervention
- HR
Heart rate
- IVS
Interventricular septum
- LPA
Left pulmonary artery
- LPAS
Left pulmonary artery stenosis
- LV
Left ventricle
- MPA
Main pulmonary artery
- PA
Pulmonary artery
- PAS
Pulmonary artery stenosis
- PCWP
Pulmonary capillary wedge pressure
- PVRI
Pulmonary vascular resistance index
- RPA
Right pulmonary artery
- RV
Right ventricle
APPENDIX
MSCT Angiography Measurements:
Figure A1:

A. Measurement of RPA, LPA and 1st order branch PA diameters were made at 6 consistently identified right side 1st order branches and 5 consistently identified left side 1st order branches. B-C. RPA/LPA diameters were measured adjacent to the first order branch and the 1st order branch diameters were measured at the branch artery origins.
Semi-quantitative histology:
Histology slides were graded on the below scale by an experienced lung pulmonary vascular pathologist.
Septal Abnormalities
Normal septal anatomy without edema or prominent muscular vessels
One or more small muscular vessels with a combined intimal and medial thickness less than or equal to the luminal diameter, essentially normal septal anatomy
One or more small muscular vessels with a combined intimal and medial thickness greater than the luminal diameter and without luminal occlusion
One or more small muscular vessels with intimal or medial thickening resulting in complete luminal occlusion
Bronchovascular Abnormalities
Absence of small interstitial muscular vessels (bronchial arterioles)
Normal bronchovascular anatomy
One or more small muscular vessels with a combined intimal and medial thickness greater than the luminal diameter and without luminal occlusion
One or more small muscular vessels with intimal or medial thickening resulting in complete luminal occlusion.
Interstitial Inflammation
None
One to two small foci of interstitial/perivascular mononuclear inflammation or small non-necrotizing interstitial granulomas
Three of more foci of interstitial/perivascular mononuclear inflammation or extensive non-necrotizing interstitial granulomatous inflammation
Diffuse interstitial mononuclear inflammation
Alveolar Metaplasia/Attenuation
Normal alveolar anatomy
Focal interstitial smooth muscle metaplasia with or without septal attenuation
Diffuse interstitial smooth muscle metaplasia with extensive septal attenuation (i.e. emphysematous change)
Severe with multiple affected lung segments
RPA and RPA 1st Order Branch Diameters Indexed to Aortic Diameter
Figure A2:

Main RPA diameter and RPA 1st order branch diameters were not different between groups.
PA Diameters in mm
Figure A3:

Proximal LPA, main LPA, LPA 1st order branch, main RPA, RPA 1st order branch diameters in mm (i.e. not indexed to Ao diameter). * p<0.05 vs sham, #p<0.05 vs LPAS
Right lung semi-quantitative histopathology
Table A1:
Right lung semi-quantitative histopathology was not different between groups
| Sham | LPAS | Delayed Intervention | Early Intervention | |
|---|---|---|---|---|
| Medial Wall Thickness (%) | 12±2 | 12±1 | 14±2 | 17±2 |
| Alveoli Count (# / 2mm) | 32±2 | 31±2 | 27±1 | 31±1 |
| Septal Abnormalities (1–4) | 1.5±0.1 | 1.8±0.4 | 1.3±0.1 | 1.4±0.1 |
| Bronchovascular Abnormalities (1–4) | 2.0±0.0 | 2.1±0.1 | 2.0±0.0 | 2.0±0.0 |
| Interstitial Inflammation (1–4) | 1.5±0.2 | 1.4±0.1 | 1.4±0.1 | 1.2±0.1 |
| Alveolar Metaplasia/Attenuation (1–4) | 1.0±0.0 | 1.0±0.0 | 1.1±0.0 | 1.0±0.0 |
p<0.05 vs sham control,
p<0.05 vs LPAS control
REFERENCES
- 1.Hislop AA, Pierce CM Growth of the vascular tree. Paediatr Respir Rev 2000;1(4):321–8. Doi: 10.1053/PRRV.2000.0071. [DOI] [PubMed] [Google Scholar]
- 2.Hislop A Developmental biology of the pulmonary circulation. Paediatr Respir Rev 2005;6(1):35–43. Doi: 10.1016/J.PRRV.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Gatzoulis MA, Balaji S, Webber SA, et al. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet 2000;356(9234):975–81. Doi: 10.1016/S0140-6736(00)02714-8. [DOI] [PubMed] [Google Scholar]
- 4.Harrison DA, Harris L, Siu SC, et al. Sustained Ventricular Tachycardia in Adult Patients Late After Repair of Tetralogy of Fallot. J Am Coll Cardiol 1997;30(5):1368–73. Doi: 10.1016/S0735-1097(97)00316-1. [DOI] [PubMed] [Google Scholar]
- 5.Rhodes J, Dave A, Pulling MC, et al. Effect of Pulmonary Artery Stenoses on the Cardiopulmonary Response to Exercise Following Repair of Tetralogy of Fallot. Am J Cardiol 1998;81(10):1217–9. Doi: 10.1016/S0002-9149(98)00095-2. [DOI] [PubMed] [Google Scholar]
- 6.Hislop A, Reid L Pulmonary arterial development during childhood: branching pattern and structure. Thorax 1973;28(2):129. Doi: 10.1136/thx.28.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies G, Reid L Growth of the alveoli and pulmonary arteries in childhood. Thorax 1970;25(6):669. Doi: 10.1136/thx.25.6.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haworth SG, McKenzie SA, Fitzpatrick ML Alveolar development after ligation of left pulmonary artery in newborn pig: clinical relevance to unilateral pulmonary artery. Thorax 1981;36(12):938. Doi: 10.1136/thx.36.12.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Razavi H, Stewart SE, Xu C, et al. Chronic effects of pulmonary artery stenosis on hemodynamic and structural development of the lungs. Am J Physiol - Lung Cell Mol Physiol 2013;304(1). Doi: 10.1152/ajplung.00412.2011. [DOI] [PubMed] [Google Scholar]
- 10.Hirsch JC, Mosca RS, Bove EL Complete Repair of Tetralogy of Fallot in the Neonate Results in the Modern Era. vol. 232. n.d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parry AJ, Mcelhinney DB, Kung GC, Reddy VM, Brook MM, Hanley FL Elective Primary Repair of Acyanotic Tetralogy of Fallot in Early Infancy: Overall Outcome and Impact on the Pulmonary Valve. J Am Coll Cardiol 2000;36(7):2279–83. Doi: 10.1016/S0735-1097(00)00989-X. [DOI] [PubMed] [Google Scholar]
- 12.Groh MA, Meliones JN, Bove EL, et al. Repair of tetralogy of Fallot in infancy. Effect of pulmonary artery size on outcome. Circulation 1991;84(5 Suppl):III206–12. [PubMed] [Google Scholar]
- 13.Kim H, Sung SC, Chang YH, Lee HD, Park JA Early and midterm outcomes of left pulmonary artery angioplasty using an anterior wall flap of the main pulmonary artery in tetralogy of Fallot repair. J Thorac Cardiovasc Surg C 2014;148(6). Doi: 10.1016/j.jtcvs.2013.10.060. [DOI] [PubMed] [Google Scholar]
- 14.Wilder TJ, Van Arsdell GS, Pham-Hung E, et al. Aggressive Patch Augmentation May Reduce Growth Potential of Hypoplastic Branch Pulmonary Arteries After Tetralogy of Fallot Repair. Ann Thorac Surg 2016;101:996–1004. Doi: 10.1016/j.athoracsur.2015.11.040. [DOI] [PubMed] [Google Scholar]
- 15.Mavroudis C, Stewart RD, Backer CL, Rudra H, Vargo P, Jacobs ML Reoperative Techniques for Complications After Arterial Switch. Ann Thorac Surg 2011;92(5):1747–55. Doi: 10.1016/J.ATHORACSUR.2011.04.102. [DOI] [PubMed] [Google Scholar]
- 16.Raju V, Burkhart HM, Durham LA, et al. Reoperation After Arterial Switch: A 27-Year Experience. Ann Thorac Surg 2013;95(6):2105–13. Doi: 10.1016/J.ATHORACSUR.2013.02.040. [DOI] [PubMed] [Google Scholar]
- 17.Pruetz JD, Badran S, Dorey F, Starnes VA, Lewis AB Differential branch pulmonary artery growth after the Norwood procedure with right ventricle–pulmonary artery conduit versus modified Blalock–Taussig shunt in hypoplastic left heart syndrome. J Thorac Cardiovasc Surg 2009;137(6):1342–8. Doi: 10.1016/J.JTCVS.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 18.Vida VL, Rito M Lo., Zucchetta F, et al. Pulmonary Artery Branch Stenosis in Patients with Congenital Heart Disease. J Card Surg 2013;28(4):439–45. Doi: 10.1111/jocs.12121. [DOI] [PubMed] [Google Scholar]
- 19.Mainwaring RD, Patrick WL, Roth SJ, et al. Surgical algorithm and results for repair of pulmonary atresia with ventricular septal defect and major aortopulmonary collaterals. J Thorac Cardiovasc Surg 2018;156(3):1194–204. Doi: 10.1016/j.jtcvs.2018.03.153. [DOI] [PubMed] [Google Scholar]
- 20.Cresalia NM, Armstrong AK, Romano JC, et al. Long-Term Outcomes After Surgical Pulmonary Arterioplasty and Risk Factors for Reintervention. Ann Thorac Surg 2018;105(2):622–8. Doi: 10.1016/j.athoracsur.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 21.O’laughlin MP, Perry SB, Lock JE, Mullins CE Use of Endovascular Stents in Congenital Heart Disease. Circulation 1991;83:1923–39. [DOI] [PubMed] [Google Scholar]
- 22.Lewis MJ, Kennedy KF, Ginns J, et al. Procedural Success and Adverse Events in Pulmonary Artery Stenting Insights From the NCDR. vol. 67. 2016. [DOI] [PubMed] [Google Scholar]
- 23.Law MA, Shamszad P, Nugent AW, et al. Pulmonary artery stents: Long-term follow-up. Catheter Cardiovasc Interv 2010;75(5):757–64. Doi: 10.1002/ccd.22356. [DOI] [PubMed] [Google Scholar]
- 24.Holzer RJ, Gauvreau K, Kreutzer J, et al. Balloon angioplasty and stenting of branch pulmonary arteries: adverse events and procedural characteristics: results of a multi-institutional registry. Circ Cardiovasc Interv 2011;4(3):287–96. Doi: 10.1161/CIRCINTERVENTIONS.110.961029. [DOI] [PubMed] [Google Scholar]
- 25.Hallbergson A, Lock JE, Marshall AC Frequency and Risk of In-Stent Stenosis Following Pulmonary Artery Stenting. Am J Cardiol 2014;113(3):541–5. Doi: 10.1016/J.AMJCARD.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 26.McElhinney DB, Bergersen L, Marshall AC In situ fracture of stents implanted for relief of pulmonary arterial stenosis in patients with congenitally malformed hearts. Cardiol Young 2008;18(4):405–14. Doi: 10.1017/S1047951108002424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baerlocher L, Kretschmar O, Harpes P, Arbenz U, Berger F, Knirsch W Stent implantation and balloon angioplasty for treatment of branch pulmonary artery stenosis in children. Clin Res Cardiol 2008;97(5):310–7. Doi: 10.1007/s00392-007-0631-8. [DOI] [PubMed] [Google Scholar]
- 28.Kudumula V, Noonan P, Taliotis D, Duke C Implantation and preliminary follow-up of the Bard Valeo stent in pulmonary artery stenosis. Catheter Cardiovasc Interv 2014;84(2):197–203. Doi: 10.1002/ccd.25443. [DOI] [PubMed] [Google Scholar]
- 29.Travelli FC, Sullivan PM, Takao C, Ing FF The Valeo stent: A pre-mounted, open-cell, large stent for use in small children with CHD. Cardiol Young 2016;26(6):1187–93. Doi: 10.1017/S104795111500219X. [DOI] [PubMed] [Google Scholar]
- 30.McCrossan BA, McMahon CJ, Walsh KP First reported use of drug-eluting bioabsorbable vascular scaffold in congenital heart disease. Catheter Cardiovasc Interv 2016;87(2):324–8. Doi: 10.1002/ccd.25768. [DOI] [PubMed] [Google Scholar]
- 31.Alexy RD, Levi DS Materials and Manufacturing Technologies Available for Production of a Pediatric Bioabsorbable Stent. Biomed Res Int 2013;2013:11. Doi: 10.1155/2013/137985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bates ML, Anagnostopoulos PV, Nygard C, et al. Consequences of an early catheter-based intervention on pulmonary artery growth and right ventricular myocardial function in a pig model of pulmonary artery stenosis. Catheter Cardiovasc Interv 2018. Doi: 10.1002/ccd.27593. [DOI] [PubMed] [Google Scholar]
- 33.Rendas A, Branthwaite M, Reid L Growth of pulmonary circulation in normal pig-structural analysis and cardiopulmonary function. n.d. [DOI] [PubMed] [Google Scholar]
- 34.Petit CJ, Gillespie MJ, Harris MA, et al. Relief of branch pulmonary artery stenosis reduces pulmonary valve insufficiency in a swine model. J Thorac Cardiovasc Surg 2009;138(2):382–9. Doi: 10.1016/j.jtcvs.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 35.Lee YC, Clark AR, Fuld MK, et al. MDCT-based quantification of porcine pulmonary arterial morphometry and self-similarity of arterial branching geometry. J Appl Physiol 2013;114(9):1191–201. Doi: 10.1152/japplphysiol.00868.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarwahi V, Sugarman EP, Wollowick AL, Amaral TD, Harmon ED, Thornhill B Scoliosis Surgery in Patients With Adolescent Idiopathic Scoliosis Does Not Alter Lung Volume. Spine (Phila Pa 1976) 2014;39(6):E399–405. Doi: 10.1097/BRS.0000000000000203. [DOI] [PubMed] [Google Scholar]
- 37.Wieben O, Francois C, Reeder SB Cardiac MRI of ischemic heart disease at 3 T: Potential and challenges. Eur J Radiol 2008;65(1):15–28. Doi: 10.1016/j.ejrad.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 38.François CJ, Srinivasan S, Schiebler ML, et al. 4D cardiovascular magnetic resonance velocity mapping of alterations of right heart flow patterns and main pulmonary artery hemodynamics in tetralogy of Fallot. J Cardiovasc Magn Reson 2012;14:16. Doi: 10.1186/1532-429X-14-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ooi YK, Kim SIH, Gillespie SE, Kim DW, Vincent RN, Petit CJ Premounted stents for branch pulmonary artery stenosis in children: A short term solution. Catheter Cardiovasc Interv 2018;92(7):1315–22. Doi: 10.1002/ccd.27800. [DOI] [PubMed] [Google Scholar]
- 40.Takao CM, El Said H, Connolly D, Hamzeh RK, Ing FF Impact of stent implantation on pulmonary artery growth. Catheter Cardiovasc Interv 2013;82(3):445–52. Doi: 10.1002/ccd.24710. [DOI] [PubMed] [Google Scholar]
- 41.Wagner EM, Petrache I, Schofield B, Mitzner W, Wagner EM Pulmonary ischemia induces lung remodeling and angiogenesis. J Appl Physiol 2006;100:587–93. Doi: 10.1152/japplphysiol.00029.2005.-Cellular. [DOI] [PubMed] [Google Scholar]
- 42.Fields MJ, Bishai JM, Mitzner W, Wagner EM Effects of pulmonary ischemia on lung morphology. Am J Physiol Lung Cell Mol Physiol 2007;293:254–8. Doi: 10.1152/ajplung.00398.2006.-Pulmonary. [DOI] [PubMed] [Google Scholar]
- 43.Eldridge L, Wagner EM Angiogenesis in the lung. J Physiol 2019;597(4):1023–32. Doi: 10.1113/JP275860. [DOI] [PMC free article] [PubMed] [Google Scholar]


