Abstract
The incidence of peanut (PN) allergy is on the rise. As peanut allergy rates have continued to climb over the past few decades, obesity rates have increased to record highs, suggesting a link between obesity and the development of peanut allergy. While progress has been made, much remains to be learned about the mechanisms driving the development of allergic immune responses to peanut. Remaining unclear is whether consuming a Western diet, a diet characterized by overeating foods rich in saturated fat, salt, and refined sugars, supports the development of PN allergy. To address this, we fed mice a high fat diet to induce obesity. Once diet-induced obesity was established, mice were exposed to PN flour via the airways using our 4-week inhalation model. Mice were subsequently challenged with PN extract to induce anaphylaxis. Mice fed a high-fat diet developed significantly higher titers of PN-specific IgE, as well as stronger anaphylactic responses, when compared to their low-fat diet fed counterparts. These results suggest that obesity linked to eating a high-fat diet promotes the development of allergic immune responses to PN in mice. Such knowledge is critical to advance our growing understanding of the immunology of PN allergy.
Keywords: peanut allergy, obesity, high fat diet, allergic disease, anaphylaxis, Western diet
1. Introduction
The rate of obesity has risen substantially in the last three decades, culminating in approximately 40% of adults and 19% of children in the United States being obese in a recent study [1]. The prevalence of peanut (PN) allergy is also increasing rapidly [2]. The rate in which this disease has expanded over the past two decades outpaces what can be explained solely by genetics, suggesting a strong role for environmental factors. The Western diet, a diet characterized by an overindulgence of low-fiber foods rich in saturated fat, salt, refined sugars, coupled with reduced consumption of nutrient-rich foods, such as fruits and vegetables, has been implicated as a reason for the increased incidence of allergies [3-5]. Eating a Western diet has been shown to impact the microbial communities in our guts, leading to a decrease in microbial diversity and disruption in normal host-microbe interactions [6-14]. Furthermore, studies using mouse models have shown that the gut microbiomes function to induce tolerance to food allergens, including PN, and alterations in the microbial flora lead to allergic sensitization [15,16]. Collectively, these findings suggest a connection between consuming a Western diet and increasing prevalence of food allergies. In support, a recent study showed that feeding mice a high-fat diet promoted the development of allergy to a model antigen ovalbumin (OVA) [5]. Still unclear, however, is the impact diet has on PN allergy. Therefore, the goal of this study was to examine whether eating a high-fat diet promotes the development of PN allergy.
To accomplish this, we fed mice either a chow containing 45% fat (high-fat diet or HFD) or a control chow with 10% fat (low-fat diet or LFD). Once obesity was established, both cohorts of mice were exposed to PN using our established inhalation model [17]. To quantify allergic sensitization to PN, serum was tested for the presence of PN-specific antibodies and mice were challenged with PN to induce anaphylaxis. Using these approaches, we identified that mice fed a high fat diet developed higher serum levels of PN-specific IgE and stronger anaphylactic reactions. These data suggest that eating a HFD enhances the ability of immune cells to mount an allergic response to PN.
2. Materials and methods
2.1. Mice
Three-week old female BALB/c mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and placed on either a high-fat diet (HFD) to induce obesity or a control low-fat diet (LFD) (described in more detail below—see section 2.2). Male mice were not used in these experiments. Mice were housed in standard pathogen-free conditions under ad libitum feeding conditions. Mice exposed to PBS or PN were marked individually (e.g. LFD PBS-1, LFD PBS-2, LFD PN-1, HFD PBS-1, HFD PN-1, etc.) randomly on the day of retroorbitally bleeding (day 27) with a marker on their tails, and these identification markings allowed for the monitoring of rectal temperature and tracking mouse behavior following challenge with PN the next day (day 28).
2.2. Induction of diet-induced obese mouse model
Mice were split into two cohorts and either fed a HFD (45% energy by fat) or a standard LFD (10% energy by fat) for at least 29 weeks to establish diet-induced obese BALB/c mice (see Figure 2a for experimental schematic). Diets were purchased from Research Diets, Inc. (New Brunswick, NJ) and specifics about the diets can be found in Table 1. Mice were weighed weekly to track that mice fed HFD displayed significant increase in body weight, and blood was collected retroorbitally at 8- and 15-week intervals to test plasma leptin levels with ELISA (described below). Weight and leptin levels were metrics used to document that mice fed HFD have established obesity compared to mice fed standard LFD. Following induction of diet-induced obesity, mice were exposed to PN by inhalation as described below—see section 2.4.
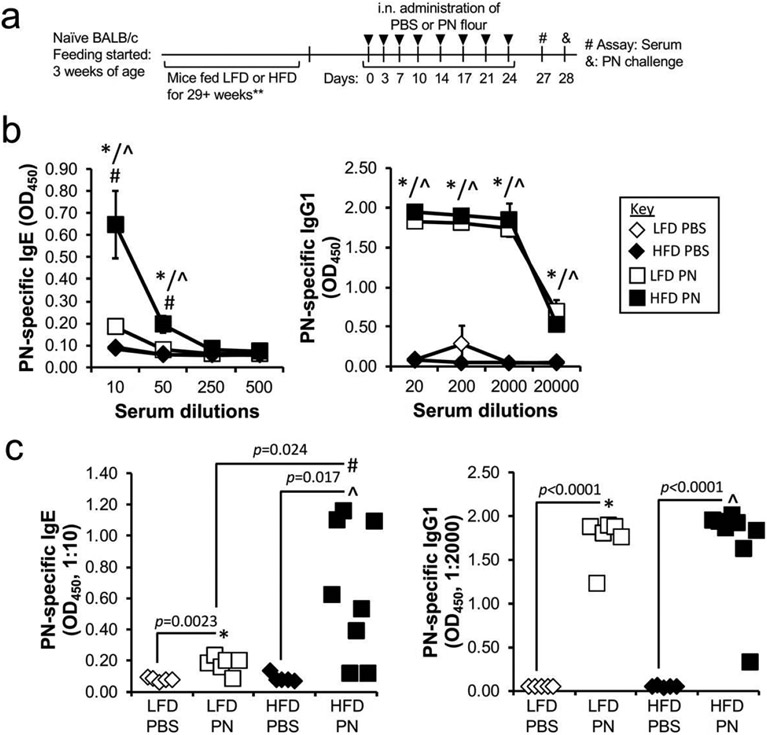
Figure 2.
Mice fed HFD produce significantly higher PN-specific IgE than those fed LFD. (a) Schematic showing timeline of experiment. Mice were fed starting at 3 weeks of age and fed for 29+ weeks (** one group of mice was fed for 29 weeks and the other group of mice were fed for 56 weeks, so the mice were 8–14 months of age at the time of sensitization by exposure to PN flour). (b) Levels of PN-specific antibodies in d27 sera were measured by ELISA. Data are pooled from 2 experiments and represented as a mean ± SEM (n = 5 in each PBS group, 6–8 mice in each PN group). (c) Scatter plots showing PN-specific antibody levels of each mouse are shown. For both (b) and (c), * reveals significance (p < 0.05) between LFD PBS and LFD PN groups, ^ indicates significance (p < 0.05) between HFD PBS and HFD PN, and # depicts significance (p < 0.05) between LFD PN and HFD PN.
Table 1.
Ingredient formulation of low-fat diet (LFD) and high-fat diet used in study.
| Macronutrient | Ingredient | LFD (control)* (g) | HFD** (g) |
|---|---|---|---|
| Protein | Casein, lactic, 30 mesh | 200.0 | 200.0 |
| Protein | Cystine, L | 3.0 | 3.0 |
| Carbohydrate | Sucrose, fine granulated | 354.0 | 176.8 |
| Carbohydrate | Starch, corn | 315.0 | 72.8 |
| Carbohydrate | Lodex 10 (maltodextrin) | 35.0 | 100.0 |
| Fat | Soybean oil, USP | 25.0 | 25.0 |
| Fat | Lard | 20.0 | 177.5 |
LFD was purchased for study from Research Diets, Inc. (D12450B). Caloric information broken down by % Kcal for LFD: protein—20% Kcal, fat—10% Kcal, Carbohydrate—70% Kcal. The energy density for LFD: 3.82 Kcal/g.
HFD was purchased for study from Research Diets, Inc. (D12451). Caloric information broken down by % Kcal for HFD: protein—20% Kcal, fat—45% Kcal, Carbohydrate—35% Kcal. The energy density for HFD: 4.7 Kcal/g.
2.3. Allergens
Peanut (PN) flour was purchased from the Golden Peanut Company (Alpharetta, GA). We tested endotoxin levels in the flour by Limulus Amebocyte Lysate assay (Lonza, Walkersville, MD) and found undetectable levels (<0.5 EU/mg flour). Crude PN extract was purchased from Greer Laboratories (Lenoir, NC) for intraperitoneal challenge.
2.4. Model to induce peanut allergy via inhalation
Naïve BALB/c mice fed either HFD to induce obesity or LFD as control were sensitized with PN flour as we have described previously [17]. In each experiment, mice were split into four groups: LFD PBS, LFD PN, HFD PBS, and HFD PN. In total across two experiments, 5 mice were in each PBS group, 6 mice were in the LFD PN group, and 8 mice were in the HFD PN group. Briefly, mice were exposed to either 100 μg peanut flour in 50 μL PBS or PBS alone twice per week for 4 weeks. On day 27, mice were retroorbitally bled to determine serum levels of PN-specific IgE and IgG1. The next day (day 28), mice were challenged with 2.5 mg PN peanut extract in 500 μL PBS via intraperitoneal injection to induce anaphylactic reaction. Rectal temperature and clinical symptoms were monitored before (0 min) and after PN challenge (every 10 min for 1 h). Rectal temperatures were recorded with an electronic thermometer (Oakton Instruments, Vernon Hills, Ill) equipped with a RET-3 rectal probe (Physitemp Instruments, Clifton, NJ). Clinical symptoms were scored based on the following published criteria [18]: 0, no symptoms; 1, scratching of ear and mouth; 2, puffiness around eyes and mouth, pilar erection, labored breathing; 3, prolonged period of motionlessness; 4, severely reduced motility, tremors, severe respiratory distress; and 5, death.
2.5. ELISA
Serum levels of PN-specific IgE and IgG1 were measured by ELISA as previously described [17]. Leptin was measured in plasma samples using a commercial mouse leptin sandwich ELISA kit (R&D Systems, Minneapolis, MN) according to manufacturer instructions.
2.6. Ethics approval of research
All animal experimental protocols and procedures were carried out with the approval of the Mayo Clinic Institutional Animal Care and Use Committee under IACUC protocol number A38914.
2.7. Statistics
Differences between the various treatment groups were deemed statistically significant using a Student t test, with p ≤ 0.05 considered statistically significant. Numerical data are presented as mean ± SEM. Error bars within figures represent SEM.
3. Results
3.1. Establishment of diet-induced obese mouse model
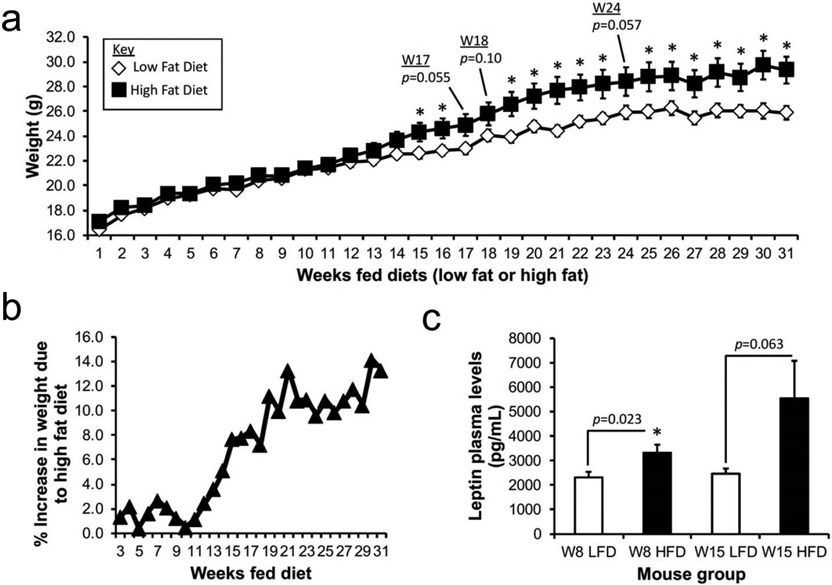
The goal of this study was to investigate whether obesity increases the susceptibility of developing PN allergy. Beginning at 3 weeks of age, BALB/c female mice were fed either a HFD to induce obesity or a control and standard LFD for at least 29 weeks prior to PN exposure. After 16 weeks of feeding a HFD, mice typically exhibit a 20–30% increase in body weight, along with other signs of obesity (e.g. adipocyte hyperplasia, mesenteric fat deposition, increased fat mass, diabetes, and hypertension) compared to mice fed control chow [19,20]. Similar to these published findings, starting after 15 weeks of eating the HFD chow, mice in our study displayed a significant increase in weight when compared to their LFD chow fed counterparts (Week 15 HFD: 24.32 ± 0.75 g; Week 15 LFD: 22.58 ± 0.39, p = 0.046 (Figure 1a). Mice fed HFD exhibited 7.82% weight gain by week 16, and from weeks 19–31, consistently showed a 10–13% higher body weight as compared to LFD-fed mice (Figure 1b). These findings are consistent with BALB/c mice being more resistant to HFD induced obesity than other mouse strains [19]. To further verify our model, leptin levels, which are known to be elevated in obese mice fed HFD [21,22], were measured in plasma at 8 and 15 weeks after feeding commenced (Figure 1c). As obesity develops, plasma leptin levels are known to increase [23]. Mice fed HFD exhibited higher leptin levels at both time points (Week 8 LFD: 2316 ± 232 pg/mL; Week 8 HFD: 3324 ± 329 pg/mL, p = 0.023; Week 15 LFD: 2468 ± 213 pg/mL; Week 15 HFD: 5549 ± 1527 pg/mL, p = 0.063). Collectively, these data strongly support that we developed diet-induced obese and appropriate control mice necessary to examine how diet impacts sensitization to PN.
Figure 1.
Establishment of a mouse model of diet-induced obesity. (a) Mice were fed with either high fat (45% energy by fat) or a standard, low fat (10% energy by fat) diet for at least 29 weeks to establish obese or control mice prior to sensitization to peanut (PN) via inhalation. Mice were weighed weekly and their weights are reported in grams. Data represents a mean ± SEM of 24 mice per a diet group. (b) Percent increase in weight of mice fed HFD as compared to mice fed LFD. Data are shown starting 3 weeks after diet commenced and is recorded weekly through week 31. (c) Leptin plasma levels were measured after 8 and 15 weeks via ELISA. Data is the mean ± SEM of 9 of the 24 mice per diet group randomly selected to test for leptin levels in plasma. For both (a) and (c), * p < 0.05 compared to control mice fed LFD, unless indicated otherwise.
3.2. HFD mice develop higher PN-specific IgE responses
HFD and LFD control mice were sensitized to PN using our 4-week inhalation model (Figure 2a) [17]. Briefly, mice were exposed to PN flour (or PBS vehicle) twice per week for 4 weeks. On day 27, blood was collected to analyze the presence of PN-specific antibodies. Regardless of diet, mice exposed to PN produced PN-specific IgE and IgG1 responses (Figure 2b,c). Strikingly, HFD mice developed 3.5-fold higher titers of PN-specific IgE following PN inhalation than their LFD PN counterparts (OD450 HFD PN: 0.648 ± 0.153; OD450 LFD PN: 0.183 ± 0.021 , p = 0.024). In contrast, such differences were not observed in the development of PN-specific IgG1 (Figure 2b,c).
3.3. HFD mice sensitized to PN undergo more severe systemic anaphylactic reactions following PN challenge
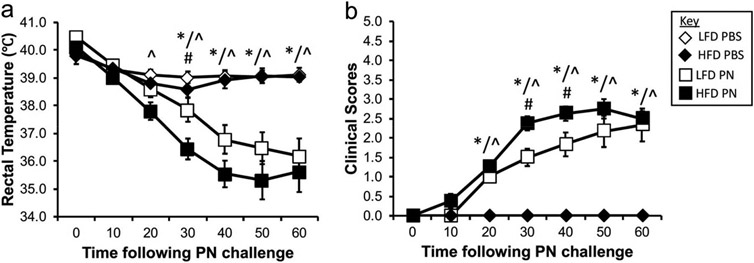
On day 28, mice were challenged intraperitoneally with PN extract to elicit an anaphylactic reaction. To track the progress of anaphylaxis, rectal temperatures and clinical scores were monitored every 10 min for 1 h following PN challenge. Both LFD and HFD mice sensitized to PN underwent anaphylaxis (Figure 3). Not surprisingly, PBS-sensitized mice did not react to PN challenge. Interestingly, HFD mice underwent a more severe anaphylactic reaction when compared to LFD control mice. Thirty minutes post-challenge, PN-sensitized HFD mice displayed a larger drop in rectal temperature (HFD PN: 36.4 ± 0.4 °C; LFD PN: 37.9 ± 0.4 °C, p = 0.029) and higher clinical score (HFD PN: 2.4 ± 0.2; LFD PN: 1.5 ± 0.2, p = 0.01) compared to LFD control mice sensitized with PN (Figure 3). Clinical scores of PN-exposed HFD mice remained significantly higher than LFD PN at the 40-minute time point. Overall, these data suggest that HFD mice sensitized to PN develop the symptoms of systemic anaphylaxis (i.e. drop in body temperature and presence of clinical signs) faster and more severely than LFD PN mice following challenge with PN.
Figure 3.
Mice fed HFD undergo more severe anaphylactic reactions upon PN challenge than LFD-fed counterparts. To track anaphylaxis to PN in mice, rectal temperature (a) and clinical scores (b) were recorded for 60 min following intraperitoneal injection with peanut extract on day 28. Additional description of clinical score values can be found in the materials and methods section. Data are pooled from 2 experiments and represent mean ± SEM (n = 5 in each PBS group, 6–8 mice in each PN group). * reveals significance (p < 0.05) between LFD PBS and LFD PN groups, ^ indicates significance (p < 0.05) between HFD PBS and HFD PN, and # depicts significance (p < 0.05) between LFD PN and HFD PN.
4. Discussion
A recent study has suggested that feeding mice a HFD promotes allergic sensitization to a model antigen OVA likely through diet-induced changes to the gut microflora [5]. Since alterations in the gut microbiome has been linked to sensitization to PN [16], we wondered what impact diet had on the development of PN allergy. To address this question, we used a straightforward approach to first develop diet-induced obese mice, along with control chow fed mice, and second, expose these mice to PN via our inhalation model [17]. We found that mice that consumed HFD developed higher titers of PN-specific IgE. The anaphylactic reactions following PN challenge were more severe than their control chow fed counterparts. These data suggest that eating HFD creates an inflammatory environment that promotes developing allergic immune responses to PN. Furthermore, these data provide additional evidence that supports the notion that eating HFD, the diet commonly associated with a Western lifestyle, is one of the reasons for an increased prevalence of allergies [3-5].
It has been reported previously that the gut microbiota changes after consuming HFD [6-14]. Microbial diversity declines and host-microbe interactions are altered. It is also known that macrophages are activated by HFD. In obese mice, macrophages accumulate in adipose tissue where they participate in driving inflammation through the release of pro-inflammatory cytokines [24,25]. Obesity also induced mast cells and eosinophils to accumulate in the trachea and lung in an eosinophilic esophagitis (EoE) mouse model [26]. Therefore, eating a high fat diet itself may induce these cellular changes in the airways, resulting in development of EoE [26]. In another study, HFD-fed female mice presented higher numbers of leukocytes in the lung tissue, attributed to higher numbers of neutrophils, macrophages, and eosinophils verses standard chow female mice [27]. While much remains to be learned about the consequences of obesity-linked cellular accumulation in the airways, these data would suggest that the increase in adipose tissue leads to an increase in inflammatory cells in the airway capable of responding to airway allergens. In agreement, genetically obese (ob/ob, db/db, and Cpefat) mice developed greater allergic responses due to inhalation of ozone, a common environmental pollutant and asthma inducer, than lean mice [28]. The susceptibility of genetically obese mice to developing PN allergy has been unknown.
While mice fed HFD displayed significantly higher levels of PN-specific IgE, no difference was observed in IgG1 titers. Intraperitoneal challenge may result in IgG1-mediated anaphylactic response via FcγRIII-expressing cells [29]. This is especially observed in IgE-deficient mice [29]. Although LFD PN mice in this study developed a significant PN-specific IgE response, the magnitude of responses was lower than we previously observed using the inhalation model [17]. Therefore, it remains possible that we failed to observe striking difference in the anaphylactic responses between HFD PN and LFD PN mice following PN challenge because IgG1 may also induce anaphylactic response in LFD PN mice. Due to lower IgE, the IgG1-mediated reaction could have been more fully manifested following challenge with PN in LFD PN mice. This finding would be consistent with data that shows both IgG1 and IgE have to be absent to fully abrogate peanut-induced anaphylaxis [29]. Future studies should examine whether in the absence of IgG1 antibody responses, more pronounced differences can be observed between LFD and HFD mice.
The specific mechanism for how HFD stimulates a more robust immune response to PN than LFD remains unclear. Recently, we have shown that a type 2 cytokine IL-13, which is secreted rapidly by group 2 innate lymphoid cells (ILC2s) following exposure to PN, is critical to drive the development of T follicular helper (Tfh) cells and production of PN-specific IgE [30]. In the same study, we also showed that IL-1α alone could induce IL-13 production from ILC2s and that the release of IL-13 caused by exposure to PN was dependent on IL-1R1 (the receptor for IL-1α and IL-1β). Furthermore, we have shown that signaling through IL-1R1 is necessary for sensitization to PN [17]. Therefore, future studies should examine whether HFD increases the presence of cells capable of secreting IL-1α into the environment to promote IL-13 production by ILC2s. Indeed, alveolar macrophages are a potential cellular source of IL-1α as they have been shown to generate IL-1α in response to inhaled fine particles [31]. Notably, mice fed HFD displayed significant increases in alveolar macrophages [32]. In agreement with the findings we describe herein, the study also showed that mice fed HFD exhibited a greater allergic response to house dust mite than control chow fed mice [32]. Taken together, it is reasonable to speculate that HFD affects one or more of the members of the immune pathway, such as alveolar macrophages, IL-1α, ILC2s, IL-13, or Tfh cells, that lead to allergic sensitization to PN via inhalation. Future studies will need to pinpoint which members of the pathway are modulated by HFD.
While obesity is well known to be linked to an increased prevalence of cardiovascular disease and type 2 diabetes, its impact on lung diseases is also well documented. Obesity is a well-known risk factor for asthma, obstructive sleep apnea, obesity hypoventilation syndrome, pulmonary hypertension, as well as affecting outcomes in acute respiratory distress syndrome and chronic obstructive pulmonary disease [33]. Obesity leads to a constant state of low-grade inflammation in pro-inflammatory macrophages that have been shown to reach up to 50% of the cellularity of subcutaneous adipose tissue in obese individuals [24,34]. In addition, obese humans and mice have been shown to display increases in mast cells in their adipose tissue [35]. Given their role as a key player in allergic responses, these data suggest that mast cells may also play a critical role in establishing the immune environment necessary to make obese individuals more susceptible to airway diseases.
We have shown previously that PN exposure through the airways elicited PN sensitization in mice, which develop systemic anaphylaxis upon PN challenge [17]. Moreover, the majority of PN-allergic children experience their first allergic reaction to PN upon first ingestion of PN [36]. The Learning Early About Peanut Allergy (LEAP) study showed early introduction of PN into the diet prevented the development of clinical PN allergy among children at high risk [37], suggesting early oral exposure may induce tolerance. Strikingly, a greater percentage of children in LEAP’S PN avoidance group developed elevated titers of PN-specific IgE antibody, suggesting sensitization to non-oral environmental PN allergens [37]. PN is readily detectable in household dust and has recently been shown to promote airway sensitization to PN in mice [38-40]. Our increased knowledge about the immunological relevance of dust to PN allergy coupled with the growing belief that young children should be orally consume PN in order to drive tolerance, strongly supports further examination of immunological pathways driving inhalation-mediated PN sensitization. Our study accomplished this task by showing that obesity linked to eating a HFD made mice more susceptible to developing allergic response to PN via the airways. Future studies will elucidate the mechanism of the response itself, but this knowledge is critical to advance our growing understanding of the immunology of PN allergy.
5. Conclusion
This project showed that mice fed high-fat diet and were sensitized to PN allergen generated significantly more PN-specific IgE and underwent more severe anaphylaxis upon PN challenge than low-fat diet fed counterparts, suggesting eating a high-fat diet promotes an immune environment more supportive to the development of PN allergy.
Acknowledgements
This work was supported by grants from the National Institutes of Health (NIH): R01 AI71106 to Hirohito Kita and Joseph Dolence was supported by a T32 Training Grant in Allergic Diseases. Additional funding was provided by the Mayo Foundation. Joseph Dolence is supported by grants from the National Center for Research Resources (NCRR; 5P20RR016469) and the National Institute for General Medical Science (NIGMS; 8P20GM103427), a component of the NIH. We would also like to thank Dr. Koji Iijima for bleeding mice, and Dr. Kimberly Carlson for editing the manuscript.
Footnotes
Conflict of interests
All authors declare no conflicts of interest in this paper.
References
- 1.Hales CM, Fryar CD, Carroll MD, et al. (2018) Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007–2008 to 2015–2016. Jama 319: 1723–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Togias A, Cooper SF, Acebal ML, et al. (2017) Addendum guidelines for the prevention of peanut allergy in the United States: Report of the National Institute of Allergy and Infectious Diseases-sponsored expert panel. J Allergy Clin Immunol 139: 29–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thorbum AN, Macia L, Mackay CR (2014) Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity 40: 833–842. [DOI] [PubMed] [Google Scholar]
- 4.Myles IA (2014) Fast food fever: reviewing the impacts of the Western diet on immunity. Nutr J 13: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hussain M, Bonilla-Rosso G, Kwong CKCK, et al. (2019) High dietary fat intake induces a microbiota signature that promotes food allergy. J Allergy Clin Immunol 144: 157–170. [DOI] [PubMed] [Google Scholar]
- 6.Ley RE, Backhed F, Turnbaugh P, et al. (2005) Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A 102: 11070–11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turnbaugh PJ, Backhed F, Fulton L, et al. (2008) Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 3: 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang EY, Leone VA, Devkota S, et al. (2013) Composition of dietary fat source shapes gut microbiota architecture and alters host inflammatory mediators in mouse adipose tissue. JPEN J Parenter Enteral Nutr 37: 746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.David LA, Maurice CF, Carmody RN, et al. (2014) Diet rapidly and reproducibly alters the human gut microbiome. Nature 505: 559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carmody RN, Gerber GK, Luevano JM, et al. (2015) Diet dominates host genotype in shaping the murine gut microbiota. Cell Host Microbe 17: 72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lam YY, Ha CW, Campbell CR, et al. (2012) Increased gut permeability and microbiota change associate with mesenteric fat inflammation and metabolic dysfunction in diet-induced obese mice. PLoS One 7: e34233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wesemann DR, Nagler CR (2016) The microbiome, timing, and barrier function in the context of allergic disease. Immunity 44: 728–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volynets V, Louis S, Pretz D, et al. (2017) Intestinal barrier function and the gut microbiome are differentially affected in mice fed a western-style diet or drinking water supplemented with fructose. J Nutr 147: 770–780. [DOI] [PubMed] [Google Scholar]
- 14.Arias-Jayo N, Abecia L, Alonso-Saez L, et al. (2018) High-fat diet consumption induces microbiota dysbiosis and intestinal inflammation in zebrafish. Microb Ecol 76: 1089–1101. [DOI] [PubMed] [Google Scholar]
- 15.Noval Rivas M, Burton OT, Wise P, et al. (2013) A microbiota signature associated with experimental food allergy promotes allergic sensitization and anaphylaxis. J Allergy Clin Immunol 131: 201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stefka AT, Feehley T, Tripathi P, et al. (2014) Commensal bacteria protect against food allergen sensitization. Proc Natl Acad Sci U S A 111: 13145–13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dolence JJ, Kobayashi T, Iijima K, et al. (2018) Airway exposure initiates peanut allergy by involving the IL-1 pathway and T follicular helper cells in mice. J Allergy Clin Immunol 142: 1144–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smarr CB, Hsu CL, Byrne AJ, et al. (2011) Antigen-fixed leukocytes tolerize Th2 responses in mouse models of allergy. J Immunol 187: 5090–5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang CY, Liao JK (2012) A mouse model of diet-induced obesity and insulin resistance. Methods Mol Biol 821: 421–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inui A (2003) Obesity—a chronic health problem in cloned mice? Trends Pharmacol Sci 24: 77–80. [DOI] [PubMed] [Google Scholar]
- 21.Frederich RC, Hamann A, Anderson S, et al. (1995) Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med 1: 1311–1314. [DOI] [PubMed] [Google Scholar]
- 22.Lin S, Thomas TC, Storlien LH, et al. (2000) Development of high fat diet-induced obesity and leptin resistance in C57Bl/6J mice. Int J Obes 24: 639–646. [DOI] [PubMed] [Google Scholar]
- 23.Van Heek M, Compton DS, France CF, et al. (1997) Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J Clin Invest 99: 385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weisberg SP, McCann D, Desai M, et al. (2003) Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112: 1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu H, Barnes GT, Yang Q, et al. (2003) Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112: 1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silva F, Oliveira EE, Ambrosio MGE, et al. (2020) High-fat diet-induced obesity worsens TH2 immune response and immunopathologic characteristics in murine model of eosinophilic oesophagitis. Clin Exp Allergy 50: 244–255. [DOI] [PubMed] [Google Scholar]
- 27.e-Lacerda, Anhe GF, Page CP, et al. (2020) Sex differences in the influence of obesity on a murine model of allergic lung inflammation. Clin Exp Allergy 50: 256–266. [DOI] [PubMed] [Google Scholar]
- 28.Johnston RA, Zhu M, Rivera-Sanchez YM, et al. (2007) Allergic airway responses in obese mice. Am J Respir Crit Care Med 176: 650–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arias K, Chu DK, Flader K, et al. (2011) Distinct immune effector pathways contribute to the full expression of peanut-induced anaphylactic reactions in mice. J Allergy Clin Immunol 127: 1552–1561. [DOI] [PubMed] [Google Scholar]
- 30.Krempski JW, Kobayashi T, Iijima K, et al. (2020) Group 2 innate lymphoid cells promote development of T follicular helper cells and initiate allergic sensitization to peanuts. J Immunol 204: 3086–3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuroda E, Ozasa K, Temizoz B, et al. (2016) Inhaled fine particles induce alveolar macrophage death and interleukin-1α release to promote inducible bronchus-associated lymphoid tissue formation. Immunity 45: 1299–1310. [DOI] [PubMed] [Google Scholar]
- 32.Tashiro H, Takahashi K, Sadamatsu H, et al. (2017) Saturated fatty acid increases lung macrophages and augments house dust mite-induced airway inflammation in mice fed with high-fat diet. Inflammation 40: 1072–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dixon AE, Peters U (2018) The effect of obesity on lung function. Expert Rev Respir Med 12: 755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Periyalil HA, Wood LG, Wright TA, et al. (2018) Obese asthmatics are characterized by altered adipose tissue macrophage activation. Clin Exp Allergy 48: 641–649. [DOI] [PubMed] [Google Scholar]
- 35.Liu J, Divoux A, Sun J, et al. (2009) Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med 15: 940–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sicherer SH, Burks AW, Sampson HA (1998) Clinical features of acute allergic reactions to peanut and tree nuts in children. Pediatrics 102: e6. [DOI] [PubMed] [Google Scholar]
- 37.Du Toit G, Roberts G, Sayre PH, et al. (2015) Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med 372: 803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trendelenburg V, Ahrens B, Wehrmann AK, et al. (2013) Peanut allergen in house dust of eating area and bed—a risk factor for peanut sensitization? Allergy 68: 1460–1462. [DOI] [PubMed] [Google Scholar]
- 39.Brough HA, Santos AF, Makinson K, et al. (2013) Peanut protein in household dust is related to household peanut consumption and is biologically active. J Allergy Clin Immunol 132: 630–638. [DOI] [PubMed] [Google Scholar]
- 40.Smeekens JM, Immormino RM, Balogh PA, et al. (2019) Indoor dust acts as an adjuvant to promote sensitization to peanut through the airway. Clin Exp Allergy 49: 1500–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]