Abstract
Sodium-glucose cotransporter inhibitor/inhibition (SGLTi), initially approved as a glucose-lowering therapy for type 2 diabetes, is associated with decreased risks for many of the most common conditions of aging, including heart failure, chronic kidney disease, all-cause hospitalization, atrial fibrillation, cancer, gout, emphysema, neurodegenerative disease/dementia, emphysema, non-alcoholic fatty liver disease, atherosclerotic disease, and infections. Studies also show that SGLTi improves overall life expectancy and reduces risks of cardiovascular death and cancer death. These wide-ranging health benefits are largely unexplained by the SGLTi’s modest improvements in standard risk factors. SGLTi produces upregulation of nutrient deprivation signaling and downregulation of nutrient surplus signaling. This in turn promotes autophagy, which helps to optimize cellular integrity and prevent apoptotic cell death. SGLTi decreases oxidative stress and endoplasmic reticulum stress, restores of mitochondrial health, stimulates mitochondrial biogenesis, and diminishes proinflammatory and profibrotic pathways. These actions help to revitalize senescent cells, tissues, and organs. In summary, SGLTi appears to slow aging, prevent disease, and improve life expectancy, and its mechanisms of action lend strong biological plausibility to this hypothesis. Further randomized trials are warranted to test whether SGLTi, a safe and well-tolerated, once-daily pill, might improve healthspan and lifespan.
Keywords: SGLTi, Cardiovascular cancer, Central nervous system, Aging, Longevity, Autophagy, Geroprotection
Introduction
The innate aging process is the most important risk factor for the majority of serious chronic disease and death. Aging is characterized by the progressive erosion of optimal physiological function beginning at the cellular level and culminating in musculoskeletal frailty with an increased risk for developing chronic cardiovascular (CV) disease (CVD), metabolic, neurodegenerative, infectious and neoplastic diseases. Due to the increasingly well-understood biological mechanisms of aging, this process may be theoretically modifiable using targeted therapeutic interventions. In recent decades, scientists have explored a range of nutritional and pharmacological interventions aimed at extending lifespan in lower organisms. Optimal diet and exercise appear to be effective at extending lifespan as well as healthspan—the number of years a person lives in a state of good health with full mental and physical capabilities.1,2 Aerobic exercise/cardiorespiratory fitness and calorie restriction/fasting have been shown to improve insulin sensitivity, which provides potent protection against age-related disease and premature death.2,3
Scientists are actively studying several drugs that have shown potential for slowing aging and improving lifespan and healthspan. Two of the most promising geroprotective (protective against adverse effects of aging) agents, metformin and rapamycin, are already being prescribed off-label to slow aging despite a paucity of evidence showing safety and efficacy.4–8 Sodium-glucose cotransporter inhibitor/inhibition (SGLTi) works by blocking reuptake of filtered glucose in the proximal tubule of the nephron. These agents all block the SGLT2, but sotogliflozin also blocks SGLT1, which is primarily located in the intestinal wall. Originally formulated to treat type 2 diabetes (T2D), this relatively simple mechanism of action leads to extraordinary downstream effects for preventing and treating many of the most common and serious age-related diseases regardless of T2D status. The aims of this review are a) to propose SGLTi as a potential therapy that may prevent/delay many of the common age-related diseases and improve lifespan and healthspan, and b) to discuss potential mechanisms of action whereby SGLTi might confer these benefits.
SGLTi: Effects on standard CVD risk factors
Unlike other proposed geroprotective therapies, a large body of data provides compelling evidence that SGLTi may reduce risks of many age-related diseases and improve life expectancy in humans.9 This class of medication produces a modest osmotic diuresis through inhibition of renal glucose and sodium reabsorption in patients with or without T2D—urinary glucose losses generally total 60 to 80 g/day (~300 cal/day). Randomized controlled trials (RCT) report that SGLTi used in T2D patients causes statistically significant reductions in hemoglobin A1c (HbA1c) (0.8%), weight (3.75 pounds or 2.4% of body weight) and blood pressure (BP; 4/2 mmHg) with a significant increase in high-density lipoprotein cholesterol (7%).10
However, in patients without T2D, these drugs induce small to negligent A1c reductions because the urinary glucose losses are offset by augmented hepatic gluconeogenesis.11,12 In normotensive, nonobese individuals, SGLTi-induced changes in BP and weight are minimal to absent.12 Yet, in all patients, the SGLTi-induced glucosuria and natriuresis bestow cardioprotective and renoprotective actions such as decreasing preload and afterload in the heart and suppressing sympathetic overactivity.9
SGLTi enhances utilization of body fat stores for energy production and aids transformation of white adipose tissue into brown adipose tissue (abundant mitochondria imbue these adipocytes with a brown color).13 As compared to white fat, brown fat is more metabolically active, so it burns more calories and generates less inflammation.13 SGLTi attenuates obesity-induced inflammation and insulin resistance, and decreases levels of proinflammatory mediators including tumor necrosis factor alpha (TNF-α), C-reactive protein and interleukin-6.12
SGLTi is associated with reduced risks for many of the most common conditions of aging including heart failure (HF), chronic kidney disease (CKD), atrial fibrillation (AF), cancer, gout, neurodegenerative disease, emphysema, non-alcoholic fatty liver disease, atherosclerotic CVD and infections.11,14–49 Studies also consistently report that SGLTi improves overall life expectancy with reduced risk of CVD death and cancer death.50,51 SGLTi reliably reduces hospitalizations, not only for HF but also all-cause hospitalization.11,14–16,21 These systemic benefits in healthspan and longevity, however, are not fully explained by the modest improvements in standard modifiable risk factors such as HbA1c, weight, BP and lipids.52
SGLTi and life expectancy
A meta-analysis of 21 randomized trials, mean follow-up of 2 years and inclusive of 39,593 patients in the SGLTi arm and 30,771 patients in the comparator arm, showed that SGLTi was associated with a highly significant 14% relative risk reduction in all-cause mortality (p < 0.00001).19 Another large meta-analysis of five RCTs of HF patients that included 21,947 participants found that SGLTi compared to placebo significantly reduced risk of all-cause mortality despite relatively short study durations (typically 2 to 3 years) (Fig. 1).21 Other meta-analyses also reported that SGLT2i decreases risk of death from any cause including CVD and cancer.11,14,16,21 EMPA-REG was an RCT of 7020 patients with preexisting CVD in which empagliflozin compared to placebo reduced risk of CVD death by 38% (p < 0.001).22 In a “real-world” propensity-matched observational study of ~160,000 patients with newly diagnosed T2D, the patients who were prescribed SGLTi had 45% lower risk of all-cause mortality compared with those not taking SGLT2 inhibitors (p = 0.0001) (Fig. 2).53
Fig. 1.
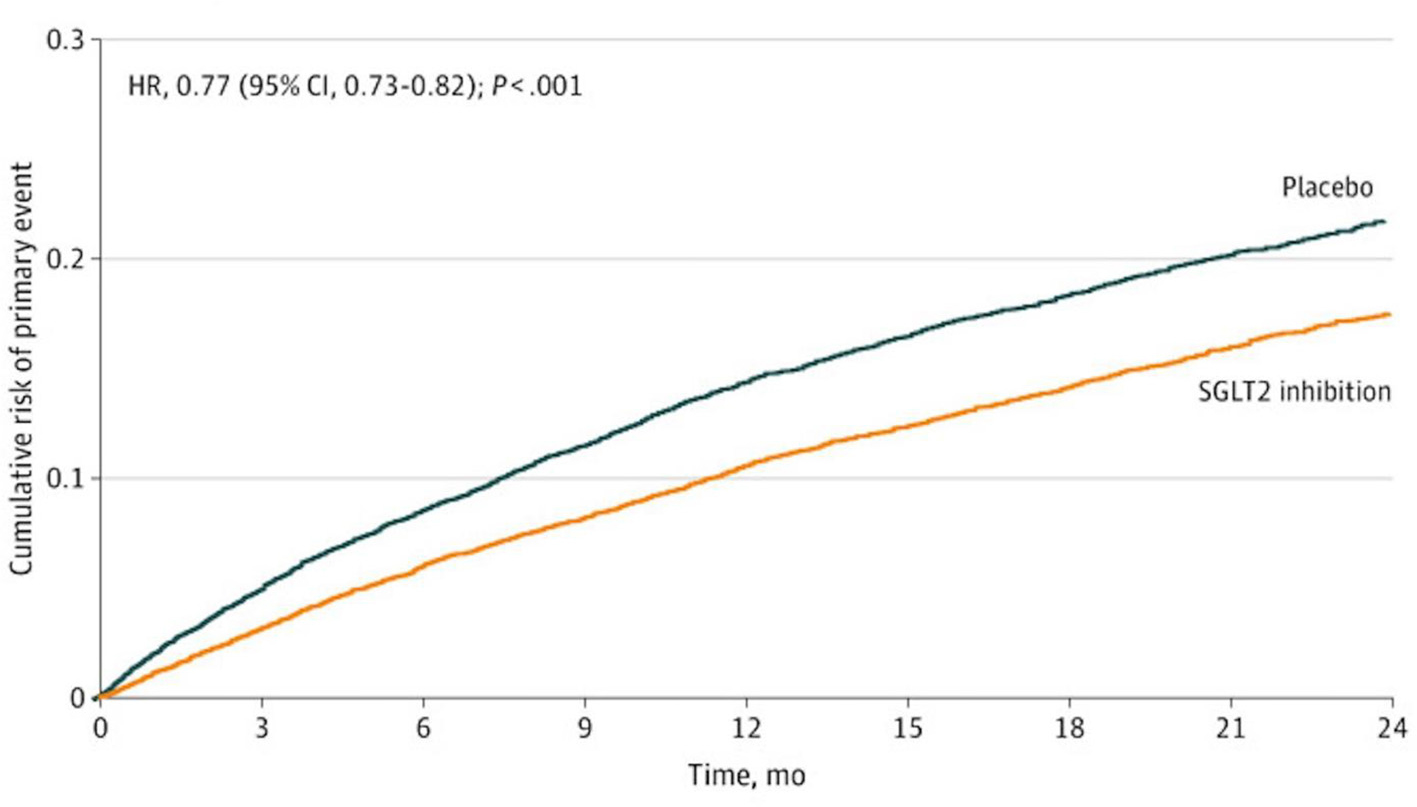
Kaplan Meier curves for primary endpoint of CV death or worsening HF in 21,947 patients in RCTs. HR hazard ratio, CI confidence interval.21
Fig. 2.
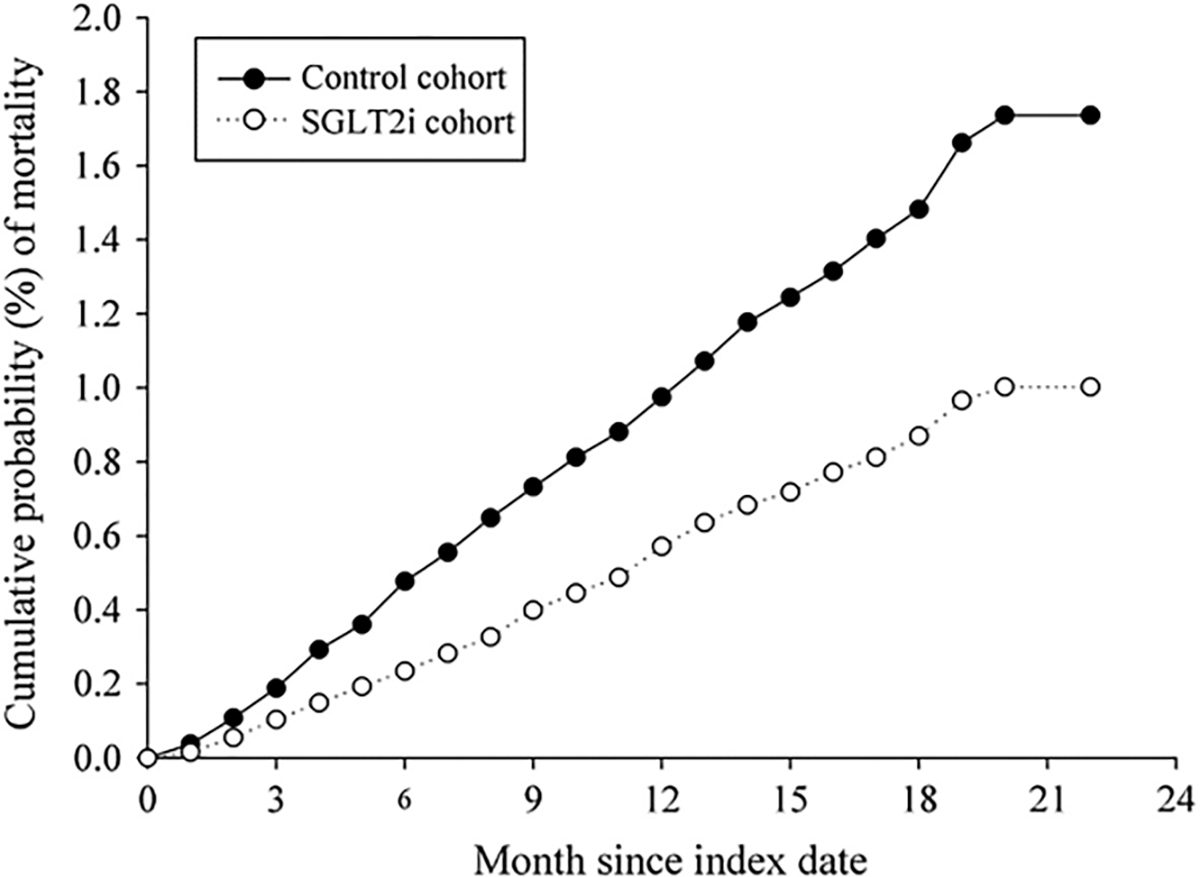
Cumulative risk curve showing all-cause mortality for the study cohort treated with SGLTi versus non-SGLTi cohort.53 Permission obtained.
An observational study of 18,500 T2D patients used propensity score matching to compare SGLTi users to dipeptidyl peptidase-4 inhibitor (DPP4i) users.20 Observational studies of SGLTi often choose DPP4i as the comparator because in RCTs the latter drug generally produces neutral effects on long term-survival and adverse CVD events. SGLTi users had significantly lower relative risk of all-cause mortality, with a hazard ratio (HR) 0.80, 95%confidence interval (CI) 0.68 to 0.94; p = 0.0057.20 Similarly, an observational study of 140,000 T2D patients from Japan, Taiwan and Korea used 70,000 matched patient pairs who used either SGLTi or DPP4i.17 SGLTi use was associated with lower risk of all-cause mortality (HR 0.60, CI 0.51 to 0.70).17 Another large, international, observational study of 388,248 T2D patients reported that initiation of SGLTi versus DPP4i was associated with a lower risk of all-cause mortality (HR 0.64, CI 0.57 to 0.72; p < 0.0001).24
In the Interventions Testing Program, SGLT2-i consistently extended median survival of male mice by 14%.18 SGLT2i led to lower fasting glucose and improved glucose tolerance in both sexes, but that study demonstrated no benefit to longevity in female mice.
Effects on CVD and respiratory diseases
Age is among the strongest risk factors for HF, and large outcome studies have consistently reported that SGLTi decreases risk of new or worsening HF for both subtypes—HF with reduced ejection fraction and HF with preserved ejection fraction.21,23,25,26,31,32 In large RCTs, SGLTi therapy demonstrates statistical superiority compared to placebo with respect to HF hospitalization and CVD death within just 12 days of drug initiation.30 A recent comprehensive meta-analysis of RCTs comprised of 17,000 HF patients reported that SGLTi significantly decreased relative risks for HF hospitalization (−29%), adverse renal outcomes (−37%), CVD mortality (−13%), and all-cause mortality (−11%).14
In patients with atherosclerotic CVD, SGLTi was shown in a large RCT to reduce risk of major adverse CVD events (myocardial infarction, ischemic stroke, or CVD death).22 A meta-analysis of nine large RCTs reported that SGLTi was associated with lower incidence of several CV disorders, including HF, AF, bradycardia, hypertension, hypertensive emergency, and varicose veins.34 AF is the most common serious arrhythmia, and large studies consistently show that SGLTi is associated with a ~25% reduction in risk of AF.53,54 Additionally, SGLTi was linked with lower rates of respiratory diseases including asthma, bronchitis, emphysema, pulmonary edema, non-small cell lung cancer, pneumonia, pleural effusion, respiratory tract infections and sleep apnea.34 SGLTi therapy, compared to DPP4i therapy, has been linked to lower risk for pneumonia and pneumonia-related mortality.34
SGLTi and CKD
Age is the most prevalent risk factor for CKD, which is present in 34% of people ≥65 years of age.29 SGLTi is the most effective drug class for preserving renal function and slowing progression of CKD.16,21,27 In large RCTs, SGLTi significantly reduced risk of kidney disease progression and acute kidney injury by 25 to 40%,16 risk of end-stage renal disease (ESRD) and risk of death from CKD.16,21,27,33 These renoprotective benefits are similar in patients with and without T2D and are present irrespective of baseline kidney function.16,21,27 A large meta-analysis of RCTs found that SGLTi reduces risk of serious hyperkalemia by 16%.28 A model based on RCT data estimated that SGLTi has the potential to delay ESRD and the need for dialysis by 15 years.41 SGLTi also acts as a functional antagonist of renal sympathetic nerve hyperactivity in the setting of HF.9 In EMPA-KIDNEY, empagliflozin decreased risk of all-cause hospitalizations by 14%.16
Elevated uric acid and gout are common age-related issues that are associated with increased risk of insulin resistance, T2D, CKD, myocardial infarction, stroke and HF. An RCT found that SGLTi therapy decreased serum uric acid level by 13% compared with placebo.44 A recent comprehensive meta-analysis reported that SGLTi was associated with a 34% lower risk of developing gout among patients with T2D.39
SGLTi and cancer
The sodium-glucose cotransporter functions as a key channel often overexpressed in cancer, which allows for glucose uptake in malignant cells and fuels tumor growth.35 Sodium-glucose cotransporters have been reported in adenocarcinomas of the pancreas, breast, kidney and prostate. In animal models, SGLTi has been shown to interfere with tumor growth and induce tumor necrosis.35 A retrospective propensity score-matched cohort study comprised of T2D patients diagnosed with cancer found that cancer patients treated with SGLTi had significantly better survival outcomes compared to patients not treated with SGLTi (Fig. 3).50 Emerging evidence suggests that SGLTi may have anticancer activity against several malignancies, including pancreatic, breast, prostate, liver, thyroid and lung cancers.35 In an observational study, SGLTi use was associated with lower risks of new-onset overall cancer, cancer-related mortality and all-cause mortality compared to DPP4i.36 Observational studies report that SGLTi is associated with improved survival among patients with non-small cell lung cancer.40
Fig. 3.
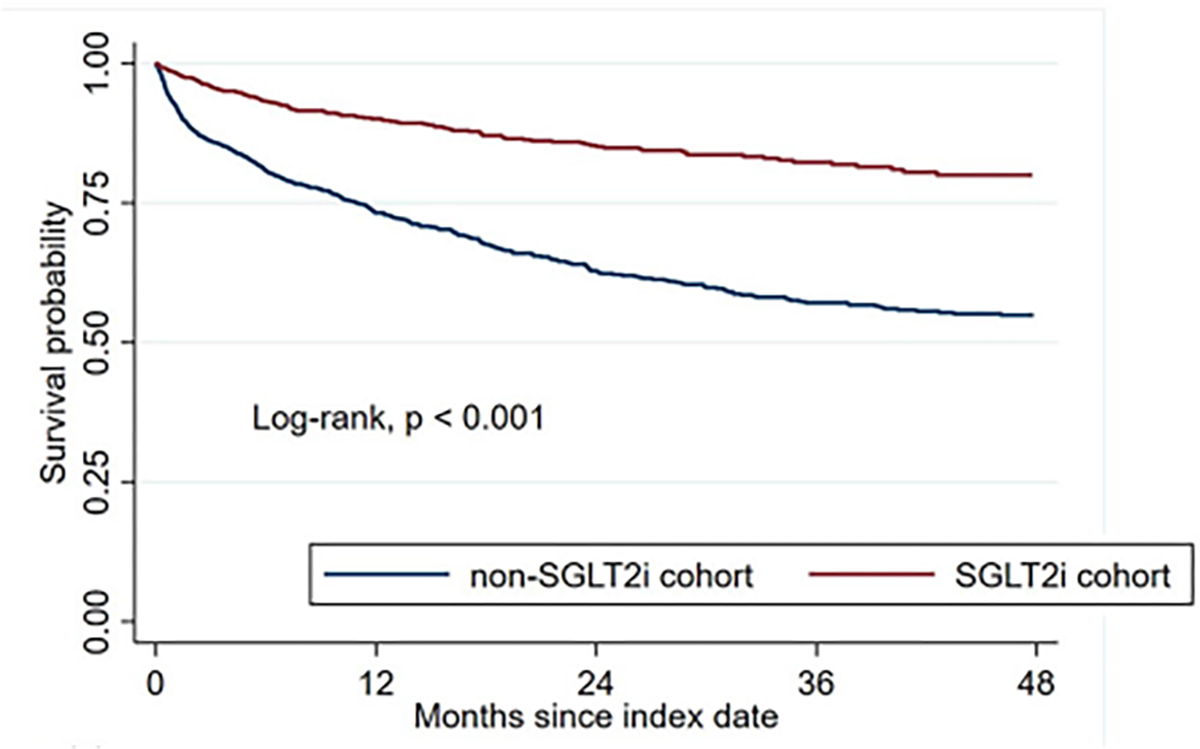
Kaplan-Meier analysis showing the overall survival for cancer patients treated with SGLTi compared to those not treated with SGLTi..50
SGLTi and dementia
Sodium-glucose cotransporters are widely present in the central nervous system (CNS). SGLTi drugs are lipid-soluble and cross the blood-brain barrier with brain/serum ratio of ~0.5; thus are being studied for their potential protective activity against neurodegenerative diseases.43 SGLTi decreases leakage of the blood-brain barrier, reduces reactive oxygen species, ameliorates microglia dysfunction and improves endothelial function.39 Empagliflozin significantly increases CNS levels of brain-derived neurotrophic factor (BDNF), which facilitates growth, survival and plasticity of neurons and favorably modulates neurotransmission.43,46 In animal models, SGLT2i reduces amyloid beta levels, senile plaque density, TNF-α and has neuroprotective effects against Parkinson’s disease.38 Observational studies of patients with T2D consistently show that SGLTi is associated with lower risks for neurodegenerative disorders, such as dementia and Parkinson’s disease.42,47,48 A meta-analysis of 10 observational studies involving 819,511 individuals with T2D reported that SGLTi users had a lower risk of all-cause dementia compared to non-SGLT2 inhibitor users (RR 0.62, CI 0.39–0.97).45
SGLTi enhances autophagy
Autophagy is an evolutionarily conserved intracellular “house-cleaning” pathway whereby senescent cellular components are encircled by a double-membrane vesicle (the autophagosome). Subsequent fusion with the lysosome allows degradative enzymes to break down and recycle the contents of the vesicle.55,56 Nutrient and/or oxygen deprivation are the fundamental stimuli that activate autophagy. Such signaling permits cellular digestion of damaged components like oxidized proteins and dysfunctional organelles for salvage and reuse, which revitalizes mitochondria and generates ATP for energy-depleted cells.57 Autophagy can be stimulated in response to stressors like fasting, calorie restriction and exercise—both aerobic and resistance training.58
Oxidative stress plays a major role in aging. Principal sources of intracellular oxidative stress are dysfunctional mitochondria and endoplasmic reticulum stress. Generally caused by excess glucose or fatty acids or accumulation of misfolded proteins, endoplasmic reticulum stress can lead to apoptosis of the cell and has been associated with neurodegenerative disorders, T2D, atherosclerosis, and cancer.9,59 Autophagic clearance of this cellular debris—dysfunctional mitochondria and endoplasmic reticuli—ameliorates intracellular oxidative stress and mitigates proinflammatory and profibrotic responses.9 The cumulative effect of enhanced autophagic flux is the preservation of cellular integrity and prevention apoptotic cell death, thereby helping to restore and maintain optimal structure and function of the cells, tissues and organs.55,56
SGLTi reliably amplifies autophagic flux throughout the body and facilitates the disposal of defective mitochondria, rejuvenates healthy mitochondrial function and stimulates mitochondrial biogenesis.39,60,61 Furthermore, SGLTi alleviates endoplasmic reticulum stress, diminishes the generation of reactive oxygen species and boosts endogenous antioxidant activity.59,62–64
Anti-aging mechanisms of action
As outlined above, SGLTi promotes cellular housekeeping by augmenting autophagic flux, which slows aging at a cellular level. These benefits appear to arise from the ability of SGLTi to simultaneously upregulate nutrient deprivation signaling and downregulate nutrient surplus signaling.65 These alterations in nutrient sensing reduce activation of mammalian target of rapamycin (mTOR) receptors and increase expression and activity of adenosine monophosphate–activated protein kinase (AMPK), sirtuin 1 (SIRT1), sirtuin 3 (SIRT3), sirtuin 6 (SIRT6) and peroxisome proliferator–activated receptor γ coactivator 1-α (PGC1-α).9,65
AMPK, sirtuins, PGC-1α and mTOR are 4 master transcription factors and enzymes that regulate numerous genes and proteins that function to maintain cellular homeostasis and allow organisms to adapt to environmental challenges and opportunities.9 During times when nutrients are plentiful, and mTOR signaling is robust, organisms prioritize the use of fuels to expand cell mass. Such activity is useful in young organisms while they are still growing, but it is potentially detrimental in mature organisms who may be at risk for cancer and disorders of nutrient excess like obesity and diabetes.66 Conversely, when nutrients are depleted, organisms shut down anabolic pathways and instead ramp up AMPK, sirtuins and PGC-1α, which are proteins synthesized in the liver as the body’s primary response to starvation.9,67 For example, AMPK is a fundamental energy sensor of the cell and, in response to energetic stress—starvation, fasting, vigorous exercise—augments energy production, mitochondrial biogenesis and insulin sensitivity.65
In the clinical setting, SGLTi-induced nutrient deprivation signaling promotes gluconeogenesis, ketogenesis, erythrocytosis and reduces uric acid levels.67 By inducing glycosuria, SGLTi mimics a state of starvation characterized by the hepatic production of ketone bodies, predominantly β-hydroxybutyrate.67 Ketogenesis and erythrocytosis are the key clinical biomarkers of the action of SGLTi because they reflect the typical physiological responses to nutrient deficit and oxygen deprivation, respectively.65 SGLTi reduces serum uric acid, a marker of oxidative stress.9 Upregulated nutrient and oxygen deprivation signaling can augment erythropoietin synthesis in the kidney and reduce oxidative stress in cardiomyocytes, neurons and other cells throughout the body.9,65 Tellingly, large RCTs have consistently identified hemoglobin increases and uric acid reductions as statistical determinants of the ability of SGLTi to decrease risks for HF hospitalizations and major adverse renal events.9
These clinical findings, in conjunction with extensive data from experimental studies, support the hypothesis that SGLTi mitigates a number of afflictions associated with aging and improves life expectancy. This ability of this class of therapy to augment healthspan and lifespan arises in part through alterations in nutrient deprivation/surplus signaling with consequent effects in promoting autophagy and restoring mitochondrial vitality, reducing reactive oxygen species, dampening inflammation and fibrosis and enhancing the viability of cells throughout the body.9 Recent studies have shown that the adaptive cellular reprogramming produced by SGLTi is also seen in isolated cell cultures. Otherwise stated, these agents have direct, glycosuria-independent, effects that decrease cellular stress and enhance cell survival.65 This finding suggests that SGLTi may bind directly to nutrient sensors to influence their function. Indeed, SGLTi has been shown to bind to mTOR in the same structural domain used by rapamycin.63
SGLTi inhibits inflammation and diminishes fibrosis by reducing proinflammatory cytokines and diminishing profibrotic pathways, fibroblast proliferation and collagen deposition.68,69 Through these actions, SGLTi helps to maintain normal tissue architecture,68,69 regress of adverse structural remodeling and rejuvenate organ function.60,70–73
Adverse effects
Any candidate compound being considered for use as a geroprotective agent to improve life expectancy should meet the Primum non nocere (first do no harm) maxim. A compound to be used for decades by healthy people must have very low risk for serious adverse effects. SGLTi are generally well tolerated, with discontinuation rates in RCTs that are similar to placebo (Fig. 4). A network meta-analysis of 47,000 patients in RCTs reported no significant changes in the risk of adverse events, including hypoglycemia, urinary tract infection, bone fractures or volume depletion.51,74 RCTs show that SGLTi users report improved quality of life metrics compared to the control group.15 Notably, SGLTi therapy does increase risk of genital yeast infection due to glycosuria. A large meta-analysis found that SGLTi use was associated with statistically significant 3.3-fold increased risk of genital yeast infections compared to controls, but the absolute risk was modest, 6.3% for SGLTi versus 1.7% for control.74
Fig. 4.
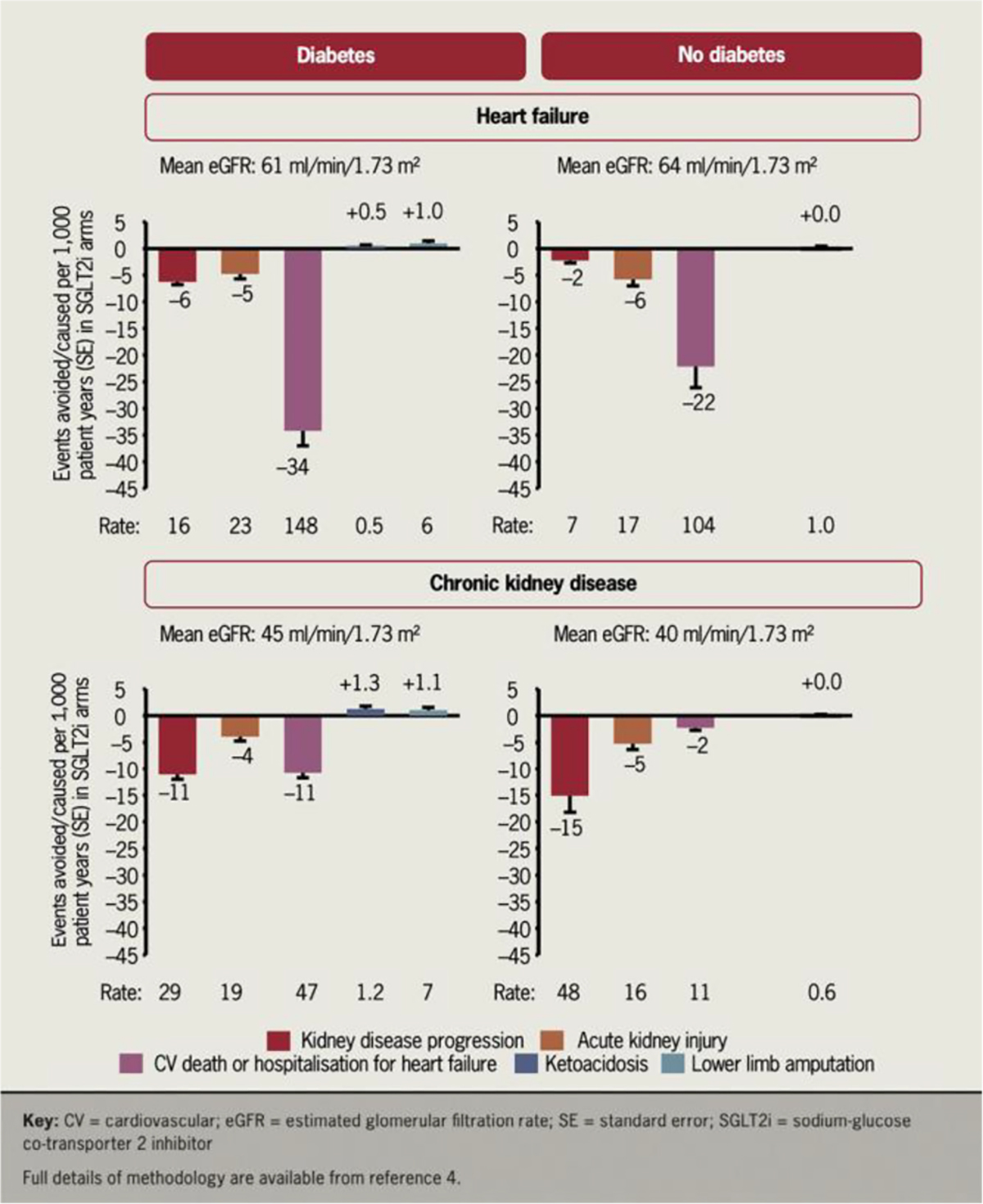
Results from two recent trials and two recent meta-analyses conclusively demonstrated the cardio-protective and kidney-protective effects of SGLTi in HF and CKD populations. The absolute benefits convincingly outweighed the potential harms.15 Permission obtained.
Although canafligozin in one study was associated with increased risk of lower limb amputation, a recent large meta-analysis showed no increased risk of amputation with any drugs in the SGLTi class.75 Fournier’s gangrene of the perineum is an exceedingly rare infection that occurs in diabetics. A total of 29 cases of Fournier’s gangrene have been reported with SGLTi.76 Additionally, SGLTi can also cause euglycemic ketoacidosis in patients with diabetes, generally in the context of serious medical illness often in and individual whose pancreas makes little to no insulin. In RCTs, ketoacidosis was a rare event despite a doubling of its risk with SGLTi.16 Only a single starvation ketoacidosis event has been reported in an individual without T2D during ~30,000 years of trial participant follow-up.16 For nondiabetic individuals, SGLT2i therapy virtually never causes ketoacidosis, lower limb amputation or Fournier’s gangrene.15,16,51 Empagliflozin, dapagliflozin, and sotagliflozin are the agents in the SGLTi drug class with the strongest safety and efficacy for improving healthspan and lifespan.51,77,78
Conclusion
SGLTi simultaneously upregulates nutrient deprivation signaling and downregulates nutrient surplus signaling. This promotes autophagy, restores mitochondrial health, reduces intracellular oxidative stress, decreases proinflammatory and profibrotic pathways, and preserves integrity and viability in cells and organs. Clinical studies show SGLTi lowers risks for premature mortality and many of the diseases of aging (Fig. 5-Central Figure). Of the candidate geroprotective agents including metformin and rapamycin, SGLTi has the most RCT data for safety and efficacy in humans. Further studies are warranted to assess SGLTi for reducing age-related disease and improving life expectancy.
Fig. 5.(Central Figure).
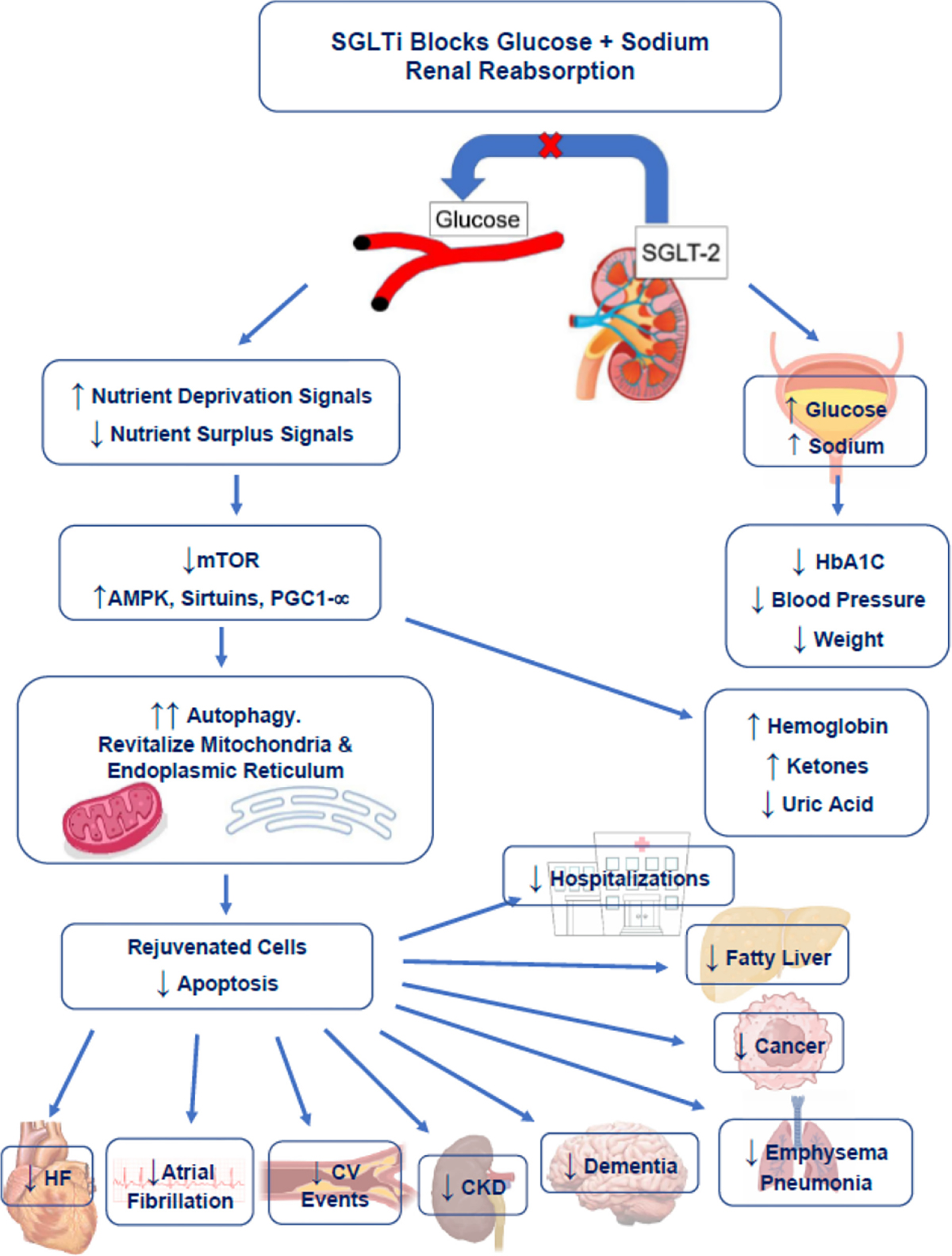
Mechanisms of action whereby SGLTi improves health and reduces risk of age-related diseases. Figure made by authors.
Abbreviations:
- AF
Atrial Fibrillation
- AMPK
Adenosine Monophosphate–activated Protein Kinase
- BDNF
Brain-Derived Neurotrophic Factor
- BP
Blood Pressure
- CKD
Chronic Kidney Disease
- CNS
Central Nervous System
- CV
Cardiovascular
- CVD
Cardiovascular Disease
- DPP4i
Dipeptidyl Peptidase-4 inhibitor
- HbA1c
Hemoglobin A1c
- HF
Heart Failure
- mTOR
Mechanistic Target of Rapamycin
- PGC1-α
Peroxisome Proliferator–activated Receptor γ Coactivator 1-alpha
- RCT
Randomized Controlled Trial
- SGLTi
Sodium-Glucose Cotransporter inhibitor
- SIRT
Sirtuin
- T2D
Type 2 Diabetes
- TNF-α
Tumor Necrosis Factor-alpha
Footnotes
Declaration of Competing Interest
JHO – Speaker for Boehringer Ingelheim.
ELO - The National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number T32HL110837 supported the research reported in this publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Remaining authors have nothing to disclose.
References
- 1.O’Keefe JH, O’Keefe EL, Eckert R, Lavie CJ. Training strategies to optimize cardiovascular durability and life expectancy. Mo Med. 2023;120:155–162. [PMC free article] [PubMed] [Google Scholar]
- 2.Kraus WE, Bhapkar M, Huffman KM, et al. 2 years of calorie restriction and cardiometabolic risk (CALERIE): exploratory outcomes of a multicentre, phase 2, randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7:673–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stens NA, Bakker EA, Manas A, et al. Relationship of daily step counts to all-cause mortality and cardiovascular events. J Am Coll Cardiol. 2023. [DOI] [PubMed] [Google Scholar]
- 4.Kaeberlein TL, Green AS, Haddad G, et al. Evaluation of off-label rapamycin use to promote healthspan in 333 adults. Online ahead of print. Geroscience. 2023;May 16:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Konopka AR, Laurin JL, Schoenberg HM, et al. Metformin inhibits mitochondrial adaptations to aerobic exercise training in older adults. Aging Cell. 2019;18, e12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selvarani R, Mohammed S, Richardson A. Effect of rapamycin on aging and age-related diseases-past and future. Geroscience. 2021;43:1135–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevenson-Hoare J, Leonenko G, Escott-Price V. Comparison of long-term effects of metformin on longevity between people with type 2 diabetes and matched non-diabetic controls. BMC Public Health. 2023;23:804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keys MT, Thinggaard M, Larsen LA, Pedersen DA, Hallas J, Christensen K. Reassessing the evidence of a survival advantage in type 2 diabetes treated with metformin compared with controls without diabetes: a retrospective cohort study. Int J Epidemiol. 2022;51:1886–1898. [DOI] [PubMed] [Google Scholar]
- 9.Packer M Critical reanalysis of the mechanisms underlying the Cardiorenal benefits of SGLT2 inhibitors and reaffirmation of the nutrient deprivation signaling/autophagy hypothesis. Circulation. 2022;146:1383–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neeland IJ, McGuire DK, Chilton R, et al. Empagliflozin reduces body weight and indices of adipose distribution in patients with type 2 diabetes mellitus. Diab Vasc Dis Res. 2016;13:119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teo YH, Teo YN, Syn NL, et al. Effects of sodium/glucose cotransporter 2 (SGLT2) inhibitors on cardiovascular and metabolic outcomes in patients without diabetes mellitus: a systematic review and meta-analysis of randomized-controlled trials. J Am Heart Assoc. 2021;10, e019463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Podesta MA, Sabiu G, Galassi A, Ciceri P, Cozzolino M. SGLT2 inhibitors in diabetic and non-diabetic chronic kidney disease. Biomedicines.. 2023:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu L, Nagata N, Nagashimada M, et al. SGLT2 inhibition by empagliflozin promotes fat utilization and browning and attenuates inflammation and insulin resistance by polarizing M2 macrophages in diet-induced obese mice. EBioMedicine. 2017;20:137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butler J, Usman MS, Khan MS, et al. Efficacy and safety of SGLT2 inhibitors in heart failure: systematic review and meta-analysis. ESC Heart Fail. 2020;7:3298–3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayne KJ, Preiss D, Herrington WG. SGLT2 inhibitors in CKD and HFpEF: two new large trials and two new meta-analyses. Br J Cardiol. 2023;30:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16..Nuffield Department of Population Health Renal Studies G, Consortium SiM-AC-RT. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet. 2022;400:1788–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim DJ, Sheu WH, Chung WJ, et al. Empagliflozin is associated with lower risk of cardiovascular events and all-cause mortality in routine care in East Asia: results from the EMPRISE study. J Diabetes Investig. 2023;14:417–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller RA, Harrison DE, Allison DB, et al. Canagliflozin extends life span in genetically heterogeneous male but not female mice. JCI Insight. 2020:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silverii GA, Monami M, Mannucci E. Sodium-glucose co-transporter-2 inhibitors and all-cause mortality: a meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2021;23:1052–1056. [DOI] [PubMed] [Google Scholar]
- 20.Suto G, Molnar GA, Rokszin G, et al. Risk of morbidity and mortality in patients with type 2 diabetes treated with sodium-glucose cotransporter-2 inhibitor and/or dipeptidyl peptidase-4 inhibitor: a nationwide study. BMJ Open Diabetes Res Care. 2021:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaduganathan M, Docherty KF, Claggett BL, et al. SGLT-2 inhibitors in patients with heart failure: a comprehensive meta-analysis of five randomised controlled trials. Lancet. 2022;400:757–767. [DOI] [PubMed] [Google Scholar]
- 22.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 23.Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–1461. [DOI] [PubMed] [Google Scholar]
- 24.Kohsaka S, Lam CSP, Kim DJ, et al. Risk of cardiovascular events and death associated with initiation of SGLT2 inhibitors compared with DPP-4 inhibitors: an analysis from the CVD-REAL 2 multinational cohort study. Lancet Diabetes Endocrinol. 2020;8:606–615. [DOI] [PubMed] [Google Scholar]
- 25.McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 26.Nassif ME, Windsor SL, Borlaug BA, et al. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: a multicenter randomized trial. Nat Med. 2021;27:1954–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heerspink HJL, Stefansson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–1446. [DOI] [PubMed] [Google Scholar]
- 28.Neuen BL, Oshima M, Agarwal R, et al. Sodium-glucose cotransporter 2 inhibitors and risk of hyperkalemia in people with type 2 diabetes: a meta-analysis of individual participant data from randomized, controlled trials. Circulation. 2022;145:1460–1470. [DOI] [PubMed] [Google Scholar]
- 29.Chronic kidney disease in the United States, 2023. In: Prevention CfDCa, ed. Prevention CfDCa. Atlanta, Georgia: US Department of Health & Human Services; 2023:; Vol 2023. [Google Scholar]
- 30.Savarese G, Sattar N, Januzzi J, et al. Empagliflozin is associated with a lower risk of post-acute heart failure Rehospitalization and mortality. Circulation. 2019;139:1458–1460. [DOI] [PubMed] [Google Scholar]
- 31.Solomon SD, McMurray JJV, Claggett B, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387:1089–1098. [DOI] [PubMed] [Google Scholar]
- 32.Talha KM, Anker SD, Butler J. SGLT-2 inhibitors in heart failure: a review of current evidence. Int J Heart Fail. 2023;5:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.The E-KCG, Herrington WG, Staplin N, et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023;388:117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yin DG, Qiu M, Duan XY. Association between SGLT2is and cardiovascular and respiratory diseases: a Meta-analysis of large trials. Front Pharmacol. 2021;12, 724405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Basak D, Gamez D, Deb S. SGLT2 inhibitors as potential anticancer agents. Biomedicines.. 2023:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chung CT, Lakhani I, Chou OHI, et al. Sodium-glucose cotransporter 2 inhibitors versus dipeptidyl peptidase 4 inhibitors on new-onset overall cancer in type 2 diabetes mellitus: a population-based study. Cancer Med. 2023;12:12299–12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cox P, Gupta S, Zhao SS, Hughes DM. The incidence and prevalence of cardiovascular diseases in gout: a systematic review and meta-analysis. Rheumatol Int. 2021;41:1209–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jasleen B, Vishal GK, Sameera M, et al. Sodium-glucose cotransporter 2 (SGLT2) inhibitors: benefits versus risk. Cureus. 2023;15, e33939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lai SW, Hwang BF, Kuo YH, Liu CS, Liao KF. Sodium-glucose cotransporter-2 inhibitors use and the risk of gout: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2023;14:1158153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luo J, Hendryx M, Dong Y. Sodium-glucose cotransporter 2 (SGLT2) inhibitors and non-small cell lung cancer survival. Br J Cancer. 2023;128:1541–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meraz-Munoz AY, Weinstein J, Wald R. eGFR decline after SGLT2 inhibitor initiation: The tortoise and the hare reimagined. Kidney360. 2021;2:1042–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mui JV, Zhou J, Lee S, et al. Sodium-glucose cotransporter 2 (SGLT2) inhibitors vs. dipeptidyl Peptidase-4 (DPP4) inhibitors for new-onset dementia: a propensity score-matched population-based study with competing risk analysis. Front Cardiovasc Med. 2021;8, 747620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pawlos A, Broncel M, Wozniak E, Gorzelak-Pabis P. Neuroprotective effect of SGLT2 inhibitors. Molecules. 2021;26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Somagutta MKR, Luvsannyam E, Jain M, et al. Sodium glucose co-transport 2 inhibitors for gout treatment. Discoveries (Craiova). 2022;10, e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang H, Shao H, Shaaban CE, et al. Newer glucose-lowering drugs and risk of dementia: a systematic review and meta-analysis of observational studies. J Am Geriatr Soc. 2023;71:2096–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tharmaraja T, Ho JSY, Sia CH, et al. Sodium-glucose cotransporter 2 inhibitors and neurological disorders: a scoping review. Ther Adv Chronic Dis. 2022;13, 20406223221086996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wium-Andersen IK, Osler M, Jorgensen MB, Rungby J, Wium-Andersen MK. Antidiabetic medication and risk of dementia in patients with type 2 diabetes: a nested case-control study. Eur J Endocrinol. 2019;181:499–507. [DOI] [PubMed] [Google Scholar]
- 48.Wu CY, Iskander C, Wang C, et al. Association of sodium-glucose cotransporter 2 inhibitors with time to dementia: a population-based cohort study. Diabetes Care. 2023;46:297–304. [DOI] [PubMed] [Google Scholar]
- 49.Zhou B, Shi Y, Fu R, et al. Relationship between SGLT-2i and ocular diseases in patients with type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Front Endocrinol (Lausanne). 2022;13, 907340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chiang CH, Chiang CH, Chiang CH, et al. Impact of sodium-glucose cotransporter-2 inhibitors on heart failure and mortality in patients with cancer. Heart. 2023;109:470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martinez-Vizcaino V, Diez-Fernandez A, Alvarez-Bueno C, Martinez-Alfonso J, Cavero-Redondo I. Safety and efficacy of SGLT2 inhibitors: a multiple-treatment meta-analysis of clinical decision indicators. J Clin Med. 2021:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lopaschuk GD, Verma S. Mechanisms of cardiovascular benefits of sodium glucose co-transporter 2 (SGLT2) inhibitors: a state-of-the-art review. JACC Basic Transl Sci. 2020;5:632–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen HY, Huang JY, Siao WZ, Jong GP. The association between SGLT2 inhibitors and new-onset arrhythmias: a nationwide population-based longitudinal cohort study. Cardiovasc Diabetol. 2020;19:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pandey AK, Okaj I, Kaur H, et al. Sodium-glucose co-transporter inhibitors and atrial fibrillation: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2021;10, e022222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Levine B, Kroemer G. Biological functions of autophagy genes: a disease perspective. Cell. 2019;176:11–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mizushima N, Levine B. Autophagy in human diseases. N Engl J Med. 2020;383:1564–1576. [DOI] [PubMed] [Google Scholar]
- 57.Gatica D, Chiong M, Lavandero S, Klionsky DJ. The role of autophagy in cardiovascular pathology. Cardiovasc Res. 2022;118:934–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Halling JF, Pilegaard H. Autophagy-dependent beneficial effects of exercise. Cold Spring Harb Perspect Med. 2017;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shibusawa R, Yamada E, Okada S, et al. Dapagliflozin rescues endoplasmic reticulum stress-mediated cell death. Sci Rep. 2019;9:9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ala M, Khoshdel MRF, Dehpour AR. Empagliflozin enhances autophagy, mitochondrial biogenesis, and antioxidant defense and ameliorates renal ischemia/reperfusion in nondiabetic rats. Oxidative Med Cell Longev. 2022;2022:1197061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cai C, Guo Z, Chang X, et al. Empagliflozin attenuates cardiac microvascular ischemia/reperfusion through activating the AMPKalpha1/ULK1/FUNDC1/mitophagy pathway. Redox Biol. 2022;52, 102288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oshima H, Miki T, Kuno A, et al. Empagliflozin, an SGLT2 inhibitor, reduced the mortality rate after acute myocardial infarction with modification of cardiac metabolomes and antioxidants in diabetic rats. J Pharmacol Exp Ther. 2019;368:524–534. [DOI] [PubMed] [Google Scholar]
- 63.Ren FF, Xie ZY, Jiang YN, et al. Dapagliflozin attenuates pressure overload-induced myocardial remodeling in mice via activating SIRT1 and inhibiting endoplasmic reticulum stress. Acta Pharmacol Sin. 2022;43:1721–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shao Q, Meng L, Lee S, et al. Empagliflozin, a sodium glucose co-transporter-2 inhibitor, alleviates atrial remodeling and improves mitochondrial function in high-fat diet/streptozotocin-induced diabetic rats. Cardiovasc Diabetol. 2019;18:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Packer M Role of deranged energy deprivation signaling in the pathogenesis of cardiac and renal disease in states of perceived nutrient overabundance. Circulation. 2020;141:2095–2105. [DOI] [PubMed] [Google Scholar]
- 66.Meijles DN, Zoumpoulidou G, Markou T, et al. The cardiomyocyte “redox rheostat”: redox signalling via the AMPK-mTOR axis and regulation of gene and protein expression balancing survival and death. J Mol Cell Cardiol. 2019;129:118–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Swe MT, Thongnak L, Jaikumkao K, Pongchaidecha A, Chatsudthipong V, Lungkaphin A. Dapagliflozin not only improves hepatic injury and pancreatic endoplasmic reticulum stress, but also induces hepatic gluconeogenic enzymes expression in obese rats. Clin Sci (Lond). 2019;133:2415–2430. [DOI] [PubMed] [Google Scholar]
- 68.Ke Q, Shi C, Lv Y, et al. SGLT2 inhibitor counteracts NLRP3 inflammasome via tubular metabolite itaconate in fibrosis kidney. FASEB J. 2022;36, e22078. [DOI] [PubMed] [Google Scholar]
- 69.Quagliariello V, De Laurentiis M, Rea D, et al. The SGLT-2 inhibitor empagliflozin improves myocardial strain, reduces cardiac fibrosis and pro-inflammatory cytokines in non-diabetic mice treated with doxorubicin. Cardiovasc Diabetol. 2021;20:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee MMY, Brooksbank KJM, Wetherall K, et al. Effect of empagliflozin on left ventricular volumes in patients with type 2 diabetes, or prediabetes, and heart failure with reduced ejection fraction (SUGAR-DM-HF). Circulation. 2021;143:516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li X, Lu Q, Qiu Y, et al. Direct cardiac actions of the sodium glucose co-transporter 2 inhibitor empagliflozin improve myocardial oxidative phosphorylation and attenuate pressure-overload heart failure. J Am Heart Assoc. 2021;10, e018298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Santos-Gallego CG, Requena-Ibanez JA, San Antonio R, et al. Empagliflozin ameliorates adverse left ventricular remodeling in nondiabetic heart failure by enhancing myocardial energetics. J Am Coll Cardiol. 2019;73:1931–1944. [DOI] [PubMed] [Google Scholar]
- 73.Tian G, Yu Y, Deng H, Yang L, Shi X, Yu B. Empagliflozin alleviates ethanol-induced cardiomyocyte injury through inhibition of mitochondrial apoptosis via a SIRT1/PTEN/Akt pathway. Clin Exp Pharmacol Physiol. 2021;48:837–845. [DOI] [PubMed] [Google Scholar]
- 74.Liu J, Li L, Li S, et al. Effects of SGLT2 inhibitors on UTIs and genital infections in type 2 diabetes mellitus: a systematic review and meta-analysis. Sci Rep. 2017;7:2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.See RM, Teo YN, Teo YH, et al. Effects of sodium-glucose cotransporter 2 on amputation events: a systematic review and meta-analysis of randomized-controlled trials. Pharmacology. 2022;107:123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bersoff-Matcha SJ, Chamberlain C, Cao C, Kortepeter C, Chong WH. Fournier gangrene associated with sodium-glucose cotransporter-2 inhibitors: a review of spontaneous Postmarketing cases. Ann Intern Med. 2019;170:764–769. [DOI] [PubMed] [Google Scholar]
- 77.Bhatt DL, Szarek M, Pitt B, et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med. 2021;384:129–139. [DOI] [PubMed] [Google Scholar]
- 78.Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021;384:117–128. [DOI] [PubMed] [Google Scholar]


