Summary
The late Ediacaran Jiangchuan biota, from the Dengying Formation in eastern Yunnan, is well-known for its diverse macroalgal fossils, opening a window onto eukaryotic-dominated ecosystems from the late Neoproterozoic of South China. Although multiple lines of evidence suggest that metazoans had already evolved by the late Ediacaran, animal fossils have not yet been formally described from this locality. Here, we report a putative disc-shaped macrofossil from the Jiangchuan biota, Lobodiscus tribrachialis gen. et sp. nov. This specimen shows the triradial symmetry characteristic of trilobozoans, a group of Ediacaran macrofossils previously documented in Australia and Russia. Lobodiscus could record the youngest known occurrence of trilobozoans, strengthening taxonomic and ecological continuities between the Ediacaran “White Sea” and “Nama” assemblages. Our findings may expand the known paleogeographical distribution of trilobozoans and provide data for Ediacaran biostratigraphic correlations across the Yangtze block and globally, helping to track the diversification of early metazoan-grade organisms.
Subject areas: Fossil geochemistry, Ecology, Evolutionary biology
Graphical abstract

Highlights
-
•
The Jiangchuan biota of Yunnan preserves a late-Ediacaran eukaryotic assemblage
-
•
We describe the first potential animal fossil from the Jiangchuan biota
-
•
This new triradial fossil might represent the youngest known trilobozoan
-
•
Our finding may strengthen taxonomic overlap among late-Ediacaran faunas
Fossil geochemistry; Ecology; Evolutionary biology
Introduction
At the end of the last century, a taxonomically diverse assemblage of carbonaceous compression macrofossils was discovered in the Jiucheng Member of the upper Ediacaran Dengying Formation, in the Jiangchuan area of eastern Yunnan, China (Figures 1A and 1B). This fossil assemblage came to be known as the Jiangchuan Biota,1,2 and has tentatively been assigned a ∼546 Ma age based on U-Pb dating.3 The described macrofossil record of the Jiangchuan Biota is limited to macroalgae,1,4,5,6 and convincing metazoan fossils have so far been conspicuously absent from this locality.
Figure 1.
Locations, stratigraphic column of the macrofossil sections, Jiangchuan Biota in Yuxi area, Eastern Yunnan, and Schematic of Ediacaran assemblages
(A) Geographical location of the fossil-bearing sections of the Jiangchuan Biota.7 1X: Houjiashan, 2X: Gugeng Village, 3X: Wangjiawan, Liujie; (B) stratigraphic column of the Dengying Formation, East Yunnan.7 DTL Fm: Donglongtan Formation, BYS Mb: Baiyanshao Member; Zone a—Shaanxilithes, zone b—Chuaria-Tawuia-Pumilibaxa, zone c—Shouhsienia-Vendotaenia-Houjiashania, zone d—Longfengshani-Cycliomedusa-Lobodiscus, and zone e—Vendotaenia-Tyrasotaenia. (C). Schematic of Ediacaran assemblages showing major deposits of each and the temporal ranges of key taxonomic constituents of the Ediacaran Biota; yellow denotes Nama assemblage localities, blue White Sea, and purple Avalon. The Jiangchuan Biota is highlighted in red. Age and faunal data after Mussini and Dunn,8 and references therein, and Xiao and Laflamme,9 Xiao et al.,10 Bowyer et al.,11,12 and Uahengo et al.13
However, over the past decades Ediacaran soft-bodied macrofossils of probable animal affinities14,15 have increasingly been reported from most continents, spanning a succession of three biotic assemblages ranging from about 571 to 539 million years ago (Ma): the deepwater “Avalon” (∼571-558 Ma), the diverse, shallow-water “White Sea” (∼558–550 Ma), and the relatively depauperate shallow-water “Nama” (∼550–539 Ma)16,17,18,19,20,21 (Figure 1C). Faunas from these assemblages have illuminated the tempo and mode of early animal evolution, contributing to reconcile molecular clock chronologies with the fossil record22 and shedding light on the early assembly of metazoan developmental programs14,15 and animal-dominated ecologies.9,23,24
Among putative Ediacaran animals are a group of macrofossils with a distinctive triradial, discoidal bodyplan, known from the White Sea assemblage of Australia and Russia25,26,27,28,29,30,31 and potentially from late Ediacaran sections of the Doushantuo Formation from Guizhou, China.32 This group comprises the genera Tribrachidium, Anfesta, Albumares, Hallidaya, Skinnera, and Rugoconites,25,33,34,35,36,37 and might also include several other problematica in need of restudy (see16).
Fedonkin assigned this group of soft-bodied triradial organisms to the newly erected class Trilobozoa together with the tube-dwelling, biomineralized anabaritids,38,39 and suggested that they were related to cnidarians. However, the triradial symmetry and discoidal, lobate bodyplan of the soft-bodied trilobozoans, such as Tribrachidium, differ from those of cnidarians or any other extant phylum.37,40 For this reason, these Ediacaran organisms are now considered to fall outside of crown-group Cnidaria,40,41 and are alternatively referred to as “Tribrachiomorpha”42,43 or “Triradialomorpha”.18,37 Their taxonomy31 and broader placement within Metazoa are unclear,9,41,44 and the group remains one of the most enigmatic among Ediacaran macrofossils due to its rarity and poorly understood anatomy.31,40 Nonetheless, a total-group metazoan affinity for triradialomorphs is strongly supported by the evidence for developmentally constrained symmetry, tissue-grade differentiation,37,45 and possible motility40 in this group. At the same time, their unique extinct bodyplan strengthens the hypothesis that Ediacaran and early Phanerozoic animal evolution was defined by early morphospace expansion followed by taxonomic diversification, with major implications for our understanding of the developmental and macroevolutionary assembly of living phyla.9,44
Here, we report a putative triradial macrofossil from the Jiangchuan Biota, Lobodiscus tribrachialis gen. et sp. nov., from the Houjiashan section of the Dengying Formation. Lobodiscus, the first trilobozoan-type fossil to be reported in the Dengying Formation, may expand the known diversity, disparity, and paleogeographical distribution of Ediacaran triradial animals, suggesting possible scenarios for the evolution of their distinctive bodyplan and pointing to a previously undocumented presence of metazoan-grade organisms in the Jiangchuan Biota. Lobodiscus may represent the youngest documented occurrence of trilobozoans globally, pointing to their survival into the “Nama” interval20 and suggesting that the Jiucheng Member may record a transitional biota intermediate between classic White Sea and Nama-type localities.
Results
Systematic palaeontology
Genus Lobodiscus gen. nov.
Type species Lobodiscus tribrachialis sp. nov.
Parainariajiangchengensis Tang In Tang et al.,46 (nom. nud.), p. 2154, figs. 3a , 3b.
Etymology
Lobo-from the Latin lobus, meaning lobe, -discus from the Latin discus, meaning disk, denoting the disc-like main body with radiating lobes; the species name combines the prefix tri- (from the Latin tres, three) and brachium, the Latin for “arm”, with reference to its 3-fold symmetry.
Diagnosis
Macroscopic, triradially symmetric discoidal fossil characterized by three raised branches radiating outward from the center of the upper body surface and meeting at approximately 120° angles. The bases of the branches are slightly offset, resulting in a weak helical arrangement. The branches partition the disc into three lobes, each comprising a series of densely spaced finger-like ridges radiating outwards from the center of the disc.
Holotype
No. KGS00228 (part and counterpart; Figure 2).
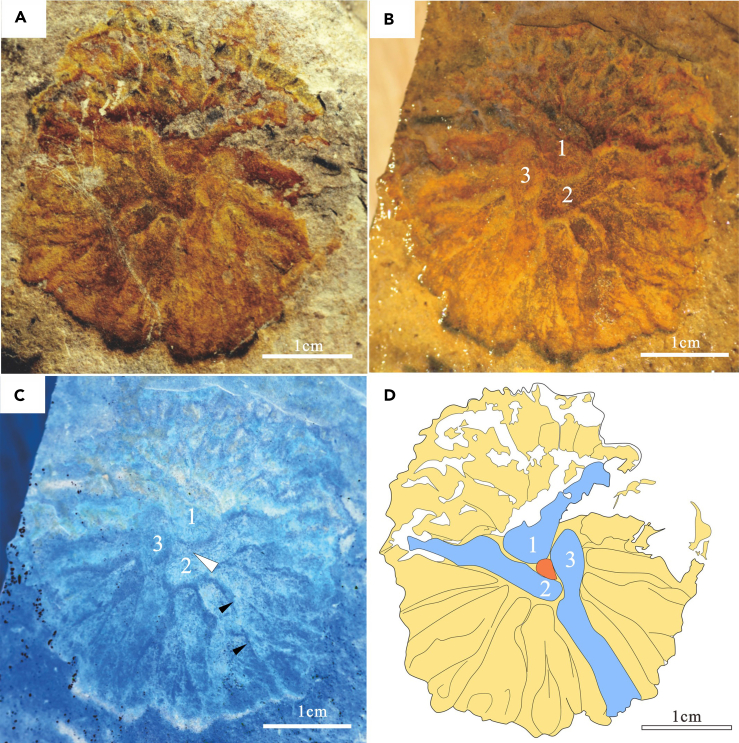
Figure 2.
Lobodiscus tribrachialis (KGS00228)
(A) part, (B) counterpart, (C) inverse color photograph of b; white arrow indicates the position of the apical pit, black arrows consecutive branching orders); (D) Interpretative drawing. Red denotes the central depression, blue the main branches (numbered), yellow the disc surface with thinner branching ridges.
Type locality and geologic setting
Jiucheng Member, Dengying Formation, cropping out in the Houjiashan, Jiangchuan area, Yuxi City, Yunnan Province, Southern China.
Description
The holotype of L. tribrachialis (Figure 2) measures approximately 37 mm in diameter. The body takes the shape of an approximately circular disc, preserved as part (in positive epirelief) and counterpart and showing faint overlying reddish-colored iron films and dark, potentially carbonaceous films (Figures 2A and 2B). The disc shows a shield-like profile, with the center slightly raised. Its three main radiating branches (“main ridges” sensu Ivantsov and Zakrevskaya40) are characterized by more prominent relief than the rest of the disc surface and meet at approximately 120° angles around its center (Figure 2). Their bases are rounded and somewhat thicker than the rest of the branch, and surround a small, circular depression that may represent an apical pit (Figures 2B–2D). The three lobes defined by the branches have a distinctive surface morphology, characterized by a dense mesh of digitiform ridges radiating outwards from the center of the disc. The ridges differ in length and show occasionalbifurcations, with at least two visible branching orders (Figure 2C). Their occasional bending suggests the same degree of rigidity as the branches, but they are thinner, less distinct, and characterized by lower relief. Unlike in Albumares and Tribrachidium (e.g.40), the lobes show no signs of distal separation. However, the distal extensions of the ridges yield an irregular body margin (Figure 2). No tentacles fringe the body margin, consistent with the notion that reports of these structures in other trilobozoans47 stem from anatomical misinterpretation.40
Discussion
Lobodiscus and the evolution of the trilobozoan bodyplan
The Lobodiscus holotype (Figure 2) can be interpreted as a macroorganismal fossil based on standard biogenicity criteria, including shape, degree of morphological differentiation, biologically plausible size range, and topological correspondence to well-established biogenic counterparts.48,49 The degree of morphological differentiation of Lobodiscus, which shows at least two orders of bifurcating surface ridges, regularly spaced branches, and a potential apical pit in addition to well-defined lobes, contrasts with that of simpler radially symmetric sedimentary concretions erroneously interpreted as macrofossils.50 Lobate discs with undulate outer edges may occur in pyrite concretions forming in fluid mixtures with different viscosities,51 but the preservation of the specimen as siltstone is at odds with the physical and mineralogical demands of this process. Similarly, septarian concretions may present radial or concentric features, but these consist of cracks in a clay matrix typically infilled with crystallized quarts or calcite, rather than curved lobes or ridges, and differ in mineralogical composition to the Lobodiscus specimen.52 Lobodiscus also shows chromatic and textural differences from the surrounding matrix, reflected in the presence of overlying reddish-colored iron and dark, potentially carbonaceous films suggestive of an originally organic structure (Figures 2A and 2B). In the absence of a more compelling alternative explanation for its morphology, these characteristics argue against interpretations of the specimen as an abiotic sedimentary structure.
In contrast, L. tribrachialis shows a morphology reminiscent of better-characterized Ediacaran trilobozoans from Australia and Russia,31,34,37,40 and at about 37 mm of diameter it falls neatly within the upper size range of these organisms.40 Like Lobodiscus, all trilobozoans share a discoidal shield-like body often partitioned into three equal lobes, radial branches and/or bifurcating ridges, and three-radial or rotational symmetry.16,17,40 These distinctive shared features tentatively suggest that trilobozoans form a monophyletic group.37 However, trilobozoan taxa differ with respect to the shape, geometric arrangement, prominence, and degree of twisting of the lobes and branches, as well as their degree of integration into the disc’s structure.
Lobodiscus’s prominent main branches differ from their subdued or morphologically indistinct counterparts in Hallidaya and Skinnera (Figures 3A, 3B, 4A, and 4B) linking the new taxon to the trilobozoan morphogroup comprising Rugoconites, Anfesta, Albumares, and Tribrachidium37,40 (Figures 3C–3F, 4C, 4D, 4F, and 4G). In Lobodiscus, the branches radiate from the center at 120-degree angles in a clockwise direction, surrounding the rounded central depression and reaching the outer edge of the disc (Figure 2). The branches are morphologically similar to those of Anfesta38,40 (Figure 3D), known from the White Sea of Russia38 and South Australia,17 that also shows three approximately straight, elongated branches with rounded ends, radiating from the center of the body at 120-degree angles. As in Anfesta, the branches of Lobodiscus are also surrounded by regions of thinner bifurcating ridges and grooves extending to the disc’s outer margin (Figure 2C). Similar branching grooves also occur in Albumares (Figures 3E and 4F), where they radiate from the center of the shield and bifurcate at least four times toward the periphery.40,47 However, unlike those of Lobodiscus the branches of both Anfesta and Albumares do not reach the edge of the discoidal body.27,40
Figure 3.
Representative Ediacaran trilobozoans from the White Sea assemblage of South Australia
After Hall et al.37
(A) Skinnera (SAM F16473); (B) Hallidaya (SAM F16464a); (C) Rugoconites enigmaticus (STC-J 560/493); (D) Anfesta (SAM P36588c); (E) Albumares (SAM P42554); (F) Tribrachidium (SAM P42662). All scale bars represent 1 cm.
Figure 4.
Summary table with proposed homology scheme, morphological features, size range, preservational mode and geographical occurrence of trilobozoans
(A) Skinnera; (B) Hallidaya; (C) Rugoconites; (D) Anfesta; (E) Lobodiscus; (F) Albumares; (G) Tribrachidium. Central depressions or pits are noted in red, hypothetical digestive cavities in blue, and disc surface with branching ridges or grooves in yellow. Colored cells denote the presence of morphological features listed in the first column to the left. Differentiated lobes are scored as a question mark in Lobodiscus to account for the possibility of taphonomic absence. White bars in the cell denoting “Single apical depression” in Hallidaya reflect the presence of three fused apical pits (B), potentially intermediate between a single pit (C‒G) and the condition in Skinnera (A). NT, Northern Territories (Australia); OP, Onega Peninsula, Russia; PO, Podolia (Ukraine); SA, South Australia; SC; southern China; WS, White Sea region (Russia). Schematic drawings, size ranges, and sedimentological and occurrence data after Ivantsov and Zakrevsaya,40 for D, F, and G, Hall et al.37 and references therein for a-c.
Lobodiscus also differs from Anfesta in the geometry of its three putative body lobes. In Anfesta (Figures 3D and 4D), each represents a 120° sector of a circle, with the branches meeting apically to define a clear Y-shape.40 This results in a simple radially symmetrical configuration of the lobes. In contrast, in Lobodiscus the basal offset between branches confers glide symmetry to the lobes (Figure 2). Glide symmetry is also clearly observed in Albumares and Tribrachidium. In these taxa, each lobe is elongated and bent clockwise, giving the body a helical aspect. As a consequence, the lobes are separated distally by asymmetrical notches.31,37,40 However, it is worth noting that the asymmetrical marginal notches are less pronounced in Tribrachidium, where they are occasionally not preserved, compared to Albumares.37,40 Likewise, distal notches are not observed in the Lobodiscus holotype, where the helical arrangement seems confined to the radiating branches and the lobes do not appear bent and twisted clockwise (Figure 2). However, the lack of additional specimens leaves open the possibility that the absence of notches may be taphonomic.
While Tribrachidium (Figures 3F and 4G) has been characterized as most distantly related to other trilobozoans,37 this hypothesis is contradicted by morphological comparisons with Lobodiscus and previously described taxa. The hypotheses that Tribrachidium is the only trilobozoans with features that bend instead of extending straight out, and that it lacks branching structures37 are contradicted by the evidence from Russian White Sea specimens.40 Grooves with multiple orders of branching, akin to those of Anfesta, Albumares, and most likely Lobodiscus (Figure 2C) occur distally on the shield of Tribrachidium (Ivantsov and Zakrevskaya,40 plates 3–4). Moreover, the recurved, helically arranged branches of Tribrachidium are reminiscent of those of Lobodiscus (Figure 2) and Albumares, although the lobes of Tribrachidium display the most extreme asymmetric bending.
Comparisons of lobe shapes, branching patterns, and symmetry across trilobozoans (Figure 4) suggest possible scenarios for the evolution of their bodyplan. If trilobozoans are monophyletic, with their rotational glide symmetry not observed in any other known group,31,41 we tentatively suggest that their common ancestor possessed simple radial symmetry and a discoidal body shape. Hypothetically, this condition may be exemplified by Rugoconites, that lacks separate lobes and possesses inconspicuous branches meeting apically to define a Y-shape, or by Hallidaya and Skinnera. The latter two taxa lack distinct main branches altogether and are united by the presence of rings of multiple central depressions, that are apparently absent in other trilobozoans (Figure 4).37,40 In contrast, Anfesta, Lobodiscus, Albumares, and Tribrachidium40 seemingly possessed main branches surrounding a single apical pit. In lieu of simple radial symmetry, Albumares and Tribrachidium also show a clear helical arrangement not found in any living phylum,40 and which might thus represent a derived condition.
Speculatively, over the course of trilobozoan evolution the main branches of a Rugoconites-like ancestor may have become increasingly prominent and the bifurcating patterns of the radial grooves increasingly complex, possibly to maximize the ciliary surface and enhance feeding efficiency.40 This would have yielded a configuration akin to that of Anfesta (Figure 4). Under this scenario, this morphology may have been further elaborated upon in Lobodiscus, where the beginnings of a helical bodyplan may manifest in the offset between the three main radiating branches (Figure 4). From this baseline condition, increased clockwise torsion of the branches and distal separation of the lobes to varying degrees might have resulted in the configurations observed in Albumares and Tribrachidium (Figures 4F and 4G). However, it remains possible that the non-helical, radially symmetrical bodyplans of Rugoconites, Hallidaya, and Skinnera reflect secondary simplification of an originally helical morphology. Alternatively, the distinct branching patterns and bodyplan symmetries of different trilobozoans might record an exclusively ecological rather than phylogenetic signal. For instance, these morphological features may have been shaped to optimize feeding efficiency under distinct local hydrodynamic flow regimes (e.g., Rahman et al.41). A conclusive test of these alternatives, which remain speculative, will require at once disentangling the placement of trilobozoans among other Ediacaran macrobionts and extant phyla31,40 and systematic comparative analyses of their paleoenvironmental settings of occurrence.
Feeding process
Although most metazoans exhibit some form of symmetry defining the whole body or some of its parts, trilobozoans are characterized by triradial symmetry, which is not present in living phyla. By comparison, most extant echinoderms exhibit pentameral symmetry, with the extinct Edrioasteroidea showing superimposed twisting distantly reminiscent of the trilobozoan condition.53 Edrioasteroids and other fossil echinoderms were most likely filter feeders, and their twisted ambulacra may have helped to enhance feeding efficiency.54 However, the fundamentally distinct bodyplan and putative epibenthic habits of trilobozoans suggest that this resemblance is superficial, and that their lifestyle may lack any clear Phanerozoic analog.
No direct evidence for motility is observed in Lobodiscus. However, putative fossil trackways suggest that at least some trilobozoans, including Tribrachidium, may have had modest mobility, presumably allowing the organism to evade negative stimuli and track suitable feeding spots.40 Based on their rigidity, reflected by the well-delineated contours of putative trackways and their consistent trajectory in body fossils, it seems likely that the lobes of trilobozoans could not fold or bend to assist food capture. Instead, food capture may have been achieved mainly through the system of bifurcating grooves covering the disc surface. As suggested by Ivantsov and Zakrevskaya,40 the grooves may have been covered by a ciliary epithelium, with food particles carried to a digestive cavity located in the middle of the disc by ciliary activity. This active feeding mode would have enabled transport of nutrients from organic-rich benthic detritus to the apical depression, where the putative mouth was located. Moreover, it would have helped to prevent wastage caused by food particles sliding down over the shield-like body. Hypothetically, this active feeding mode may have contributed to the evolution of trilobozoans with more complex surface ornamentation, which would have increased the surface area of the ciliary epithelium for more efficient feeding.40 Selective pressures to maximize the surface area of this ciliary “conveyor belt” may conceivably have driven a trend toward increasingly twisted and elongated body lobes in trilobozoans, as tentatively suggested by the morphology of Lobodiscus (Figures 2 and 4). Instead, cilia on the underside of the trilobozoan body might have played an important role in aiding movement, as in placozoans55 and many living aquatic invertebrate larvae.40 While the absence of preserved cilia in Lobodiscus makes this hypothesis speculative, its probable branching ridges and grooves (Figure 2) tentatively suggest that the feeding strategy proposed by by Ivantsov and Zakrevskaya40 may have been universal across trilobozoans.
Sedimentary environment and ecology
Lobodiscus (Figure 2) occurs in dolomitic siltstones recording a warm, low-energy shallow-marine environment. In line with the limited or absent motility, benthic habits, and low-energy depositional context proposed for Lobodiscus, the fossil shows no signs of transport. The discovery of Longfengshania5 and Cycliomedusa7 in the same facies of the Jiangchuan Biota suggest considerable potential for the discovery of additional Ediacaran macrofossils, and may reflect high levels of local primary productivity supplying Lobodiscus with abundant food sources, including organic particulate from co-occurring algae.5
Our findings suggest that Lobodiscus may be reconstructed as a benthic suspension or filter-feeding organism (Figure 5) akin to other triradial animals,40,41 that it was benthic, sessile to slow-moving,40 and likely of a more complex grade of anatomical organization than poriferans: by comparison with Russian White Sea specimens, its branches tentatively point to the presence of internal digestive cavities.40
Figure 5.
Artistic reconstruction of L. tribrachialis as a benthic metazoan-grade organism
Globally, trilobozoans occur in depositionally and paleoenvironmentally diverse facies, suggesting that they represented a highly adaptable group thriving in varied benthic habitats.37 These triradial animals probably became extinct by the beginning of the Paleozoic leaving no extant descendants, but in the late Ediacaran they show a wide distribution and considerable diversity relative to other contemporary metazoan-grade groups.37,40 For the first time, our findings from the Jiangchuan Biota tentatively extend their paleogeographical record to South China, suggesting stratigraphic correlations between the Jiucheng Member of the Ediacaran Dengying Formation and late Ediacaran assemblages in South Australia37 and globally. In particular, the absence of trilobozoans and a relative scarcity of other benthic, prostrate Ediacaran macrobionts from known Nama-aged intervals worldwide, which may reflect their extinction due to the escalating effects of bilaterian ecosystem engineering,20,56 would suggest an age equivalent to that of the White Sea assemblage (∼558–550 Ma) for the middle Jiucheng Member. Alternatively, like the Shibantan Lagerstätte10 the Jiangchuan Biota may record a “transitional” biota temporally and ecologically intermediate between White Sea and Nama communities, in accord with U-Pb dating of ash beds suggesting a ∼546 Ma age for the middle Jiucheng Member.3 Under this scenario, which appears most likely given the available geochronological evidence, Lobodiscus could represent the youngest recorded occurrence of trilobozoans globally, strengthening the taxonomic overlap between White Sea and Nama communities (e.g.20). Therefore, the occurrence of a trilobozoan in the Jiangchuan Biota may corroborate the hypothesis of a gradual, protracted phase-out of White Sea-type communities (e.g.8,24), contradicting scenarios centered on geologically rapid, environmentally driven mass extinctions between the White Sea and Nama assemblages.57 Testing these hypotheses will require, crucially, enhanced sampling of the Jiucheng Member across the full spectrum of its macrofossil and trace fossil records.
Conclusions
We describe the first putative triradial animal (Trilobozoa) from the late Ediacaran Jiucheng Member of the Dengying Formation in Yunnan province, southwest China. This specimen records the first plausible occurrence of soft-bodied, metazoan-grade macroorganisms in the Jiangchuan Biota, and might be morphologically intermediate between simple discoidal triradial forms and trilobozoans with a helical bodyplan. Its occurrence suggests a productive late Ediacaran ecosystem hosting an undocumented fauna in the Jiangchuan Biota. Moreover, it may contribute to expand the known record of trilobozoans beyond the Ediacaran fossil localities of Australia and Russia to South China, elucidating Ediacaran metazoan evolution and paleobiogeography in the southwest Yangtze margin and globally. Its occurrence in the Jiangchuan Biota, a probable Nama-interval locality, suggests that Lobodiscus could represent the youngest known trilobozoan, and by implication that ecological communities in the Jiangchuan Biota may have retained taxa typical of earlier White Sea-type faunas.
Limitations of the study
Our diagnosis, description, and assessment of the biogenicity and putative biological affinities of Lobodiscus are based on a single specimen preserving part and counterpart. Pending the discovery of additional fossil material, our conclusions must be regarded as tentative. In the absence of more convincing abiotic or biotic explanations for the morphology of the Lobodiscus holotype, we provisionally regard the hypothesis of a close affinity to other Ediacaran trilobozoans as the most plausible.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| Lobodiscus tribrachialis | Kunming General Survey of Natural Resources Center, China Geological Survey | KGS00228 |
Resource availability
Lead contact
Further information and requests for materials should be directed to and will be fulfilled by the lead contacts, Feng Tang (tangfeng65@qq.com).
Materials availability
All materials examined in this study, including part and counterpart of the Lobodiscus holotype (KGS00228), are deposited in the Kunming General Survey of Natural Resources Center, China Geological Survey.
Data and code availability
This paper analyzed a fossil specimen. The repository and accession number of this specimen are listed in the key resources table.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental model and subject details
No experimental model organism was used in this study.
Method details
Geological setting
The study region is in Jiangchuan, Yuxi City of eastern Yunnan, in the Neoproterozoic-Mesozoic fracture belt on the upper Yangtze block. It belongs to the Kunming district of Kangdian in the upper Yangtze sequence area of South China. Neoproterozoic to Paleozoic strata are widely exposed in the region, comprising (from oldest to youngest) the Sinian Dengying Formation, the Cambrian Yuhucun Formation, Qiongzhusi Formation, Canglangpu Formation, Longwangmiao Formation, Xiwangmiao Formation, and Loushanguan Formation, and the Permian Yangxin Formation.3,58,59
The fossil-bearing stratigraphic sequences comprising the Jiangchuan Biota are mainly located in the middle and upper parts of the Dengying Formation, which crops out from Houjiashan to the north side of the mine road in the town of Jiangcheng, Jiangchuan District, Yuxi City, Yunnan Province (Figure 1A), and from Taoxi Village to Gugeng Village (Figure 1A), as well as in the continuous outcrop section of the Dengying Formation adjacent to the county road from Liujie to Wangjiawan in the Jinning District, Kunming City (Figure 1A). Locally, the Dengying Formation is subdivided into the Algal Dolomite (the original Donglongtan Formation), Jiucheng, and Baiyanshao members in ascending order.1,60,61 Its sequence of fossil-rich exposures is well zoned, and after intensive excavation over recent years, five macrofossil assemblages have been identified from the base upward.6,46,62,63,64 These comprise assemblages A-D in the Jiucheng Member of the middle Dengying Formation, characterized by layers of fine-grained dolomite interbedded with muddy siltstone and shale, and assemblage E in the Baiyanshao Member in the upper part of the Dengying Formation: Zone a–Shaanxilithes, zone b–Chuaria-Tawuia-Pumilibaxa, zone c–Shouhsienia-Vendotaenia-Houjiashania, zone d–Longfengshani-Cycliomedusa-Lobodiscus, and zone e–Vendotaenia-Tyrasotaenia (Figure 1B).
Fossil materials and imaging methods
The fossil described in this study was preserved in situ in thin layers of grey-black dolomite-bearing siltstone in the central part (D assemblage) of the Jiucheng Member (Figure 1B), and it was exposed by mechanical cleaving of the bedding planes without further preparation. The outcrop in the fossil pit preserving the B-D assemblages (Figure 1B) is located approximately 900 meters southwest of Houjiashan Village, at an elevation of H = 2106 m, latitude N: 24°27′39.3″, longitude E: 102°46′48.4" (data sourced from the Beidou Navigation Satellite System). Due to weathering, the fossil-bearing layers are often yellowish-grey with a flat lying microstratigraphy, deposited in an intertidal sandy and muddy environment.1 Carbonaceous compression fossils from this site are exposed at the weathered levels and easy to identify.
The fossil examined for this study was visualized using a stereomicroscope (JSZ6D) both under normal light and using a cold light illuminator (XZ-150W) to enhance the visibility of low-relief features and better characterize its morphology in detail. All photographs were taken using a Canon EOS-50D camera. All images were made in CorelDRAW 2018 and Adobe Photoshop 2022.
Acknowledgments
This study was supported by National Natural Science Foundation of China (42172035, 41572024).
Author contributions
M.Z. performed data analysis and wrote this manuscript; G.M. proposed substantial edits and made modifications to the manuscript; Y.L. and M.L. carried out data analysis and drew relevant maps; F.T. and P.V.-R. proposed some suggestions for the manuscript; F.T. and A.C. collected specimens and geological data through fieldwork. All authors read and approved the final manuscript.
Declaration of interests
The authors declare no competing interests.
Published: January 9, 2024
Contributor Information
Feng Tang, Email: tangfeng65@qq.com.
Patricia Vickers-Rich, Email: pat.rich@monash.edu.
References
- 1.Luo H.L., Wu X.C., Ou Y.L., Jiang Z.W., Song X.L. New correlation opinions on the sections of Sinian-Cambrian Boundary in the Yangtze Platform. Yunnan Geol. 1988;7:13–27. [Google Scholar]
- 2.Luo H.L., Tao Y.H., Gao S.M. Early Cambrian trace fossils near Kunming, Yunnan. Acta Palaeontol. Sin. 1994;33:676–685. [Google Scholar]
- 3.Yang C., Li X.H., Zhu M., Condon D.J. SIMS U–Pb zircon geochronological constraints on upper Ediacaran stratigraphic correlations, South China. Geol. Mag. 2017;154:1202–1216. [Google Scholar]
- 4.Xing Y.S., Duan C.H., Liang Y.Z., Cao R.G. Geological Publishing House; 1985. Pre-Cambrian Paleontology of China; pp. 1–243. [Google Scholar]
- 5.Tang F., Song X., Yin C., Liu P., Awramik S.M., Wang Z., Gao L. Discoveries of new Longfengshaniaceae from the uppermost Ediacaran in eastern Yunnan, South China and the significance. Front. Earth Sci. China. 2007;1:142–149. [Google Scholar]
- 6.Tang F., Yin C.Y., Liu P.J., Gao L.Z., Zhang W.Y. A New Diverse Macrofossil Lagerstätte from the Uppermost Ediacaran of Southwestern China. Acta Geol. Sin. 2008;82:1095–1103. [Google Scholar]
- 7.Li Y.L., Wang H., Liu A.R., Li M., Liang Y.Z., Zhou Y., Tang F., Ren L.D. Sausage-like macrofossils from the Ediacaran Jiangchuan Biota in eastern Yunnan—New phylogenetic interpretation of Tawuia. Geol. Rev. 2022;68:1585–1603. [Google Scholar]
- 8.Mussini G., Dunn F.S. Decline and fall of the Ediacarans: late-Neoproterozoic extinctions and the rise of the modern biosphere. Biol. Rev. 2024;99:110–130. doi: 10.1111/brv.13014. [DOI] [PubMed] [Google Scholar]
- 9.Xiao S., Laflamme M. On the eve of animal radiation: phylogeny, ecology and evolution of the Ediacara biota. Trends Ecol. Evol. 2009;24:31–40. doi: 10.1016/j.tree.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Xiao S., Chen Z., Pang K., Zhou C., Yuan X. The Shibantan Lagerstätte: insights into the Proterozoic–Phanerozoic transition. J. Geol. Soc. 2021;178 jgs2020-j2135. [Google Scholar]
- 11.Bowyer F.T., Uahengo C.I., Kaputuaza K., Ndeunyema J., Yilales M., Alexander R.D., Curtis A., Wood R.A. Constraining the onset and environmental setting of metazoan biomineralization: The Ediacaran Nama Group of the Tsaus Mountains, Namibia. Earth Planet Sci. Lett. 2023;620 [Google Scholar]
- 12.Bowyer F.T., Zhuravlev A.Y., Wood R., Shields G.A., Zhou Y., Curtis A., Poulton S.W., Condon D.J., Yang C., Zhu M. Calibrating the temporal and spatial dynamics of the Ediacaran-Cambrian radiation of animals. Earth Sci. Rev. 2022;225 [Google Scholar]
- 13.Uahengo C.I., Bowyer F. The oldest Nama Group exposed: Insights from the Tsaus Mountains (Tsau Khaeb National Park) EGU General Assembly; 2023. p. EGU-3841. [Google Scholar]
- 14.Dunn F.S., Liu A.G., Grazhdankin D.V., Vixseboxse P., Flannery-Sutherland J., Green E., Harris S., Wilby P.R., Donoghue P.C.J. The developmental biology of Charnia and the eumetazoan affinity of the Ediacaran rangeomorphs. Sci. Adv. 2021;7:eabe0291. doi: 10.1126/sciadv.abe0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunn F.S., Liu A.G., Donoghue P.C.J. Ediacaran developmental biology. Biol. Rev. 2018;93:914–932. doi: 10.1111/brv.12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fedonkin M.A., Gehling J.G., Grey K., Narbonne G.M., Vickers-Rich P. Johns Hopkins University Press; 2007. The Rise of Animals. Evolution and Diversification of the Kingdom Animalia; p. 326. [Google Scholar]
- 17.Fedonkin M.A., Ivantsov A.Y., Leonov M.V., Serezhnikova E.A. In: The Rise and Fall of the Vendian (Ediacaran) Biota. Origin of the Modern Biosphere. Semikhatov M.A., editor. 2007. Dynamics of evolution and biodiversity in the late Vendian: a view from the White Sea; pp. 6–9. [Google Scholar]
- 18.Laflamme M., Darroch S.A., Tweedt S.M., Peterson K.J., Erwin D.H. The end of the Ediacara: Extinction, biotic replacement, or Cheshire Cat? Gondwana Res. 2013;23:558–573. [Google Scholar]
- 19.Boag T.H., Darroch S.A.F., Laflamme M. Ediacaran distributions in space and time: testing assemblage concepts of earliest macroscopic body fossils. Paleobiology. 2016;42:574–594. [Google Scholar]
- 20.Darroch S.A.F., Smith E.F., Laflamme M., Erwin D.H. Ediacaran extinction and Cambrian explosion. Trends Ecol. Evol. 2018;33:653–663. doi: 10.1016/j.tree.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Muscente A.D., Bykova N., Boag T.H., Buatois L.A., Mángano M.G., Eleish A., Prabhu A., Pan F., Meyer M.B., Schiffbauer J.D., et al. Ediacaran biozones identified with network analysis provide evidence for pulsed extinctions of early complex life. Nat. Commun. 2019;10:911. doi: 10.1038/s41467-019-08837-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cunningham J.A., Liu A.G., Bengtson S., Donoghue P.C.J. The origin of animals: can molecular clocks and the fossil record be reconciled? Bioessays. 2017;39:1–12. doi: 10.1002/bies.201600120. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell E.G., Bobkov N., Bykova N., Dhungana A., Kolesnikov A.V., Hogarth I.R.P., Liu A.G., Mustill T.M.R., Sozonov N., Rogov V.I., et al. The influence of environmental setting on the community ecology of Ediacaran organisms. Interface Focus. 2020;10 doi: 10.1098/rsfs.2019.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eden R., Manica A., Mitchell E.G. Metacommunity analyses show an increase in ecological specialisation throughout the Ediacaran period. PLoS Biol. 2022;20 doi: 10.1371/journal.pbio.3001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glaessner M.F., Daily B. The geology and late Precambrian fauna of the Ediacara fossil reserve. Rec. South Austr. Mus. 1959;13:369–401. [Google Scholar]
- 26.Glaessner M.F. The late precambrian fossils from Ediacara, South Australia. Palaeontology. 1966;9:599–628. [Google Scholar]
- 27.Fedonkin M.A. In: Vendskaya Sistema, Vol. 1. Paleontology. Sokolov B.S., Ivanovski A.B., editors. Nauka; 1985. Systematic description of Vendian Metazoa; pp. 70–106. [Google Scholar]
- 28.Seilacher A. Biomat-related lifestyle in the Precambrian. Palaios. 1999;14:83–96. [Google Scholar]
- 29.Ivantsov A.Y., Fedonkin M.A. Conulariid-like fossil from the Vendian of Russia: a metazoan clade across the Proterozoic/Palaeozoic boundary. Palaeontol. 2002;45:1219–1229. [Google Scholar]
- 30.Mccall G.J.H. The Vendian (Ediacaran) in the geological record: Enigmas in geology's prelude to the Cambrian explosion. Earth Sci. Rev. 2006;77:1–229. [Google Scholar]
- 31.Hall C.M., Droser M.L., Gehling J.G., Dzaugis M.E. Paleoecology of the enigmatic Tribrachidium: New data from the Ediacaran of South Australia. Precambrian Res. 2015;269:183–194. [Google Scholar]
- 32.Wang Y., Du W., Komiya T., Wang X.L., Wang Y. Macroorganism paleoecosystems during the Middle-Late Ediacaran Period in the Yangtze Block, South China. Paleontol. Res. 2015;19:237–250. [Google Scholar]
- 33.Jenkins R.J.F. In: Origins and Early Evolution of Metazoa. Lipps J.H., Signor P.W., editors. Plenum Press; 1992. Functional and ecological aspects of Ediacaran assemblages; pp. 131–176. [Google Scholar]
- 34.Fedonkin M.A., Ivantsov A.Y. Ventogyrus, a possible siphonophore-like trilobozoan coelenterate from the Vendian Sequence (late Neoproterozoic), northern Russia. Geol. Soc. Lond. Spec. Pub. 2007;286:187–194. [Google Scholar]
- 35.Vickers-Rich P., Ivantsov A.Y., Trusler P.W., Narbonne G.M., Hall M., Wilson S.A., Greentree C., Fedonkin M.A., Elliott D.A., Hoffmann K.H., Schneider G.I.C. Reconstructing Rangea: new discoveries from the Ediacaran of southern Namibia. J. Paleontol. 2013;87:1–15. [Google Scholar]
- 36.Ivantsov A.Y., Gritsenko V.P., Paliy V.M., Velikanov V.A., Konstantinenko L.I., Menasova A.S., Fedonkin M.A., Zakrevskaya M.A., Serezhnikova E.I. Russian Academy of Sciences; 2015. Makrofossilii Verkhnego Venda Vostochnoy Yevropy. Sredneye Prid-Nestrov’yei Volyn (Upper Vendian Macrofossils of Eastern Europe. Middle Dniester Area and Volhynia) pp. 1–143. [Google Scholar]
- 37.Hall C.M.S., Droser M.L., Clites E.C., Gehling J.G. The short-lived but successful triradial body plan: a view from the Ediacaran of Australia. Aust. J. Earth Sci. 2020;67:885–895. [Google Scholar]
- 38.Fedonkin M.A. In: Stratigraphy and Paleontology of the Earliest Phanerozoic. Sokolov B.S., Ivanovskiy A.B., editors. Nauka; 1984. Promorphology of the Vendian Radialia; pp. 30–58. [Google Scholar]
- 39.Fedonkin M.A. Non-skeletal fauna of the Vendian and its place in the evolution of Metazoa: Trudy of the Paleontological Institute. Akad. Nauk SSSR. 1987;22:1–174. [Google Scholar]
- 40.Ivantsov A.Y., Zakrevskaya M.A. Trilobozoa, Precambrian Triradial Organisms. Paleontol. J. 2021;55:727–741. [Google Scholar]
- 41.Rahman I.A., Darroch S.A.F., Racicot R.A., Laflamme M. Suspension feeding in the enigmatic Ediacaran organism Tribrachidium demonstrates complexity of Neoproterozoic ecosystems. Sci. Adv. 2015;1 doi: 10.1126/sciadv.1500800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grazhdankin D.V. In: Encyclopedia of Geobiology. Reitner J., Thiel V., Kappler A., Konhauser K.O., Nunez A.E., Reid P., Zhang X.L., editors. Springer Science; 2011. Ediacaran biota; pp. 342–348. [Google Scholar]
- 43.Grazhdankin D. Patterns of evolution of the Ediacaran soft-bodied Biota. J. Paleontol. 2014;88:269–283. [Google Scholar]
- 44.Erwin D.H., Laflamme M., Tweedt S.M., Sperling E.A., Pisani D., Peterson K.J. The Cambrian conundrum: early divergence and later ecological success in the early history of animals. Sci. 2011;334:1091–1097. doi: 10.1126/science.1206375. [DOI] [PubMed] [Google Scholar]
- 45.Evans S.D., Droser M.L., Erwin D.H. Developmental processes in Ediacara macrofossils. Proc. Biol. Sci. 2021;288 doi: 10.1098/rspb.2020.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang F., Gao L.Z., Yin C.Y., Wan Y., Gu P. Macrofossil biotas in the Late Ediacaran-Cambrian boundary interval of South China and stratotype correlation. Geol. Bull. China. 2015;34:2150–2162. [Google Scholar]
- 47.Keller B.M., Fedonkin M.A. New organic finds in the Precambrian Valday series along the Syuz’ma River. Int. Geol. Rev. 1977;19:924–930. [Google Scholar]
- 48.Baucon A., Neto De Carvalho C., Felletti F., Cabella R. Ichnofossils, cracks or crystals? A test for biogenicity of stick-like structures from Vera Rubin Ridge, Mars. Geosciences. 2020;10:39. [Google Scholar]
- 49.Rouillard J., van Zuilen M., Pisapia C., Garcia-Ruiz J.M. An alternative approach for assessing biogenicity. Astrobiology. 2021;21:151–164. doi: 10.1089/ast.2020.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nolan M.R., Walker S.E., Selly T., Schiffbauer J. Is the middle Cambrian Brooksella a hexactinellid sponge, trace fossil or pseudofossil? PeerJ. 2023;11 doi: 10.7717/peerj.14796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.García-Ruiz J. In: Garcia-Ruiz J., Louis E., Meakin P., Sander L., editors. Vol. 304. Springer US; 1993. Natural viscous fingering; pp. 183–189. (Growth Patterns in Physical Sciences and Biology. NATO ASI Series). [Google Scholar]
- 52.Potter P.E., Maynard J.B., Pryor W.A. Springer-Verlag; 1980. Sedimentology of Shale : Study Guide and Reference Source; pp. 23–36. [Google Scholar]
- 53.Rozhnov S.V. Development of symmetry and asymmetry in the early evolution of the echinoderms. Paleontol. J. 2012;46:780–792. [Google Scholar]
- 54.Dudit L., Rahman I., Cunnigham J. 2018. Investigating the Evolution of Symmetry in Cambrian Echinoderms. Progressive Palaeontology Conference. [Google Scholar]
- 55.Smith C.L., Pivovarova N., Reese T.S. Coordinated feeding behavior in Trichoplax, an animal without synapses. PLoS One. 2015;10 doi: 10.1371/journal.pone.0136098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Darroch S.A., Cribb A.T., Buatois L.A., Germs G.J., Kenchington C.G., Smith E.F., Mocke H., O’Neil G.R., Schiffbauer J.D., Maloney K.M., et al. The trace fossil record of the Nama Group, Namibia: exploring the terminal Ediacaran roots of the Cambrian explosion. Earth Sci. Rev. 2021;212 [Google Scholar]
- 57.Evans S.D., Tu C., Rizzo A., Surprenant R.L., Boan P.C., McCandless H., Marshall N., Xiao S., Droser M.L. Environmental drivers of the first major animal extinction across the Ediacaran White Sea-Nama transition. Proc. Natl. Acad. Sci. 2022;119 doi: 10.1073/pnas.2207475119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gao Y., Qin Y., Lin S., Ling W. Nd isotopic stratigraphy of Paleoproterozoic to late Paleozoic sedimentary strata of the southwestern Yangtze Block and implications for its tectonic evolution. Int. Geol. Rev. 2020;62:740–753. [Google Scholar]
- 59.Liu P., Yin C., Tang F. In: Meso-Neoproterozoic Geology and Petroleum Resources in China. Wang T., editor. Springer Nature; 2022. Research Progress of Ediacaran (Sinian) Biostratigraphy in South China; pp. 155–179. [Google Scholar]
- 60.Luo H.L., Jiang Z.W., Wu X.C., Song X.L., Ou Y.L. Kunming: Yunnan People's Publishing House; 1982. The Sinian-Cambrian Boundary in Eastern Yunnan, China; pp. 1–265. [Google Scholar]
- 61.Xing Y.S., Ding Q.X., Luo H.L., He T.G., Wang Y.G. The Sinian-Cambrian boundary of China. Bull. Chin. Acad. Geol. Sci. 1984;10:1–262. [Google Scholar]
- 62.Tang F., Yin C.Y., Liu P.J., Wang Z.Q., Gao L.Z. Discovery of diverse macrofossil assemblages from the Jiucheng Member of uppermost Ediacaran in eastern Yunnan. J. Palaeogeogr. 2007;9:533–540. [Google Scholar]
- 63.Tang F., Chen J.S., Ren L.D., Gao L.Z., Hua H. Yunnan Science and Technology Press; 2020. The Trailblazer of Animals—The Fossil Documents and Comparative Study of the First Candidate GSSP Meishucun Section in China; pp. 1–498. [Google Scholar]
- 64.Gu P., Zhong L., Zhang G.D., Song S.C., Tang F., Ling M.Q., Gao L.Z. The division of the Late Ediacaran-Cambrian boundary interval stratigraphy and new options of Index Fossil FAD in South China. Acta Geol. Sin. 2018;92:449–465. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This paper analyzed a fossil specimen. The repository and accession number of this specimen are listed in the key resources table.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.