Summary
Innate lymphoid cells (ILCs) have been identified as potent regulators of inflammation, cell death and wound healing, which are the main biological processes involved in the progression of chronic liver disease. Obesity and chronic alcohol consumption are the leading contributors to chronic liver diseases in developed countries, due to inappropriate lifestyles. In particular, inflammation is a key factor in these liver abnormalities and promotes the development of more severe lesions such as fibrosis, cirrhosis and hepatocellular carcinoma. Opposite roles of ILC subsets have been described in the development of chronic liver disease, depending on the stage and aetiology of the disease. The heterogeneous family of ILCs encompasses cytotoxic natural killer cells, the cytokine-producing type 1, 2 and 3 ILCs and lymphoid tissue inducer cells. Dysfunction of these immune cells provokes uncontrolled inflammation and tissue damage, which are the basis for tumour development. In this review, we provide an overview of the recent and putative roles of ILC subsets in obesity and alcohol-associated liver diseases, which are currently the major contributors to end-stage liver complications such as fibrosis/cirrhosis and hepatocellular carcinoma.
Keywords: innate lymphoid cells, MASLD, ALD, fibrosis, hepatocellular carcinoma
Key points.
-
•
Metabolic dysfunction-associated steatotic liver disease (MASLD) and alcohol-related liver disease (ALD) are the leading types of chronic liver diseases in the developed world.
-
•
Hepatocellular carcinoma is one of the most lethal cancers and patients with alcohol use disorder or obesity are at increased risk of developing it.
-
•
Natural killer (NK) cells, and innate lymphoid cell (ILC)1 and ILC2 subsets have been found to play opposite roles (protective or pathogenic) in MASLD and ALD, depending on the stage and aetiology of the disease.
-
•
NK cells and ILC1 have opposite roles in triggering steatohepatitis depending on the aetiology.
-
•
ILC2 may be protective in MASLD but deleterious in ALD and ILC3 seem to be protective in MASLD and ALD.
-
•
NK cells protect against liver fibrosis and HCC while ILC1, 2 and 3 may be deleterious.
Introduction
The liver is a central organ that performs essential functions to maintain body homeostasis in response to metabolic and immunological cues. Hepatocytes account for 80% of total liver cells and the non-parenchymal fraction constitutes the remaining 20%.1 The non-parenchymal fraction is mostly composed of sinusoidal endothelial cells and hepatic stellate cells (HSCs), which play a key role in the synthesis of extracellular matrix proteins and liver-resident immune cells. The functions of these immune cells are to maintain a state of tolerance as the liver is continuously exposed to food products, while defending against viruses, bacteria and toxins. Dysregulation of these functions may be the cause or the consequence of the development of chronic liver diseases which evolve from inflammation to fibrosis and towards more severe stages such as cirrhosis and cancer.2
Obesity is one of the main risk factors for developing the chronic liver diseases known as metabolic dysfunction-associated steatotic liver disease (MASLD).3 The worldwide prevalence of MASLD is growing in parallel with obesity, reaching nearly 33% of the population in 2022.4 MASLD has a wide pathological spectrum ranging from “simple” steatosis, characterised by lipid accumulation in hepatocytes, to metabolic dysfunction-associated steatohepatitis (MASH). MASH is a progressive and severe condition characterised by steatosis, a persistent inflammatory state, hepatocyte death and a predisposition to more severe liver abnormalities such as fibrosis/cirrhosis and hepatocellular carcinoma (HCC).2,[4], [5], [6], [7] The chronic inflammation associated with MASH is associated with the recruitment of circulating immune cells and polarization of liver innate immune cells towards a more inflammatory phenotype.2
Gut and adipose tissue are key players in the development and progression of MASLD. Alteration of the gut barrier and dysbiosis lead to increased intestinal permeability and consequently to a release of bacterial-derived products (pathogen-associated molecular patterns) that reach the liver via the portal vein.[8], [9], [10] Pathogen-associated molecular patterns bind to pattern recognition receptors and accumulate in the liver, activating local immune cells and hepatocytes.2,11 The adipose tissue also plays an important role in the progression of MASLD. When energy intake is excessive, adipocyte storage is rapidly saturated, causing a deregulation of lipid metabolism. In parallel, macrophages that produce T helper 1-type cytokines (tumour necrosis factor-α [TNF-α], interferon-γ [IFN-γ]) are recruited into the adipose tissue, leading to increased lipolysis and the development of insulin resistance associated with hyperlipidaemia.12 All these alterations are responsible for a modification in the secretion of several mediators at the hepatocyte (hepatokines) and adipocyte (adipokines) level, activating the immune system and thus contributing to the development of MASLD.13 These intra- and extrahepatic mechanisms promote the development of MASLD and increase the susceptibility of patients to infection and cancers.14,15
Alcohol-related liver disease (ALD) is the most common chronic liver disease in the world and causes more than 3.3 million deaths every year.16 A chronic and large consumption of alcohol (more than 20 g/day of alcohol for females and 30 g/day of alcohol for males) leads to a build-up of fats in the liver (steatosis) and to alcohol-related steatohepatitis, which is characterised by steatosis, hepatocyte ballooning and infiltration of neutrophils. Steatosis and steatohepatitis are critical in the development and progression of ALD. Furthermore, alcoholic hepatitis (AH) is a severe syndrome (symptoms of jaundice, fever, tachycardia, tachypnoea, hepatomegaly, leucocytosis with neutrophilia) that can occur at any stage of ALD. Like MASLD, adipose tissue and gut dysfunction trigger ALD pathogenesis. Indeed, the spectrum of liver alterations in ALD and MASLD share common mechanisms but with their own characteristics (alcohol metabolism, specific dysbiosis, insulin resistance and inflammation of adipose tissue).[17], [18], [19] Histopathological characteristics of ALD include acute portal inflammation, high neutrophil infiltration, alcoholic foamy degeneration and cholestasis.20
HCC is the sixth most common cancer in the world but the third leading cause of cancer deaths. Its incidence has tripled in developed countries over the past three decades,21,22 and is associated with the incidence of ALD and MASLD. Dysregulation of the local microenvironment and chronic inflammation associated with altered genetic/epigenetic modifications promote HCC development and progression.23,24 Advanced HCC (with portal invasion and/or extrahepatic spread) accounts for around a third of all treated cases. Recently, immune checkpoint inhibitors (ICIs), administered alone or with anti-angiogenic agents, have been shown to significantly improve survival and quality of life compared with the multi-targeted kinase inhibitors previously used in patients with advanced HCC. In fact, based on their improved efficacy and acceptable tolerability, ICIs are currently being evaluated in patients with mid-stage or even early-stage HCC.25,26 However although a significant increase has been observed in the median survival rate, not all patients respond to ICIs.27 Therefore, it is critical to better characterise the hepatic microenvironment during HCC development and to better understand the interplay between local immune cells and neighbouring cells in order to find new anti-tumour approaches and improve existing strategies.
The family of innate lymphoid cells (ILCs) has recently received attention in the field of liver diseases, as these cells regulate inflammation and fibrosis and therefore the development of cancer. Since they were first identified in 2008-2009, ILC subsets have been extensively described and discussed in many reviews.[28], [29], [30] Briefly, ILCs are non-T and non-B lymphocytes that lack rearranged antigen receptors; they form a heterogeneous group and can be divided into five subsets: cytotoxic natural killer (NK) cells, type 1, 2 and 3 ILCs (ILC1, ILC2, ILC3) and lymphoid tissue inducer (LTi) cells.28,[31], [32], [33] These cells share similarities with T helper cells and cytotoxic T cells at both the transcriptional and functional levels and most of them are cytokine producers and tissue-resident cells (gut, skin, lung, liver, adipose tissue), with the exception of NK cells which are circulating cytotoxic cells.34 ILCs regulate tissue homeostasis but also contribute to the onset of disease through their ability to produce proinflammatory and regulatory cytokines in response to local injury or infection35,36 (Fig. 1). Dysregulation of ILC activity can contribute to inflammatory disorders, thus they play a role in tumour-associated inflammation.[37], [38], [39], [40], [41], [42] LTi cells are essential for the formation of the secondary lymphoid organs during embryogenesis. In adulthood, bone marrow Lti cells replace these embryonic Lti cells and Lti-like subsets have been described in adult gut mucosa. However, their role in adult diseases is not yet well understood and needs to be studied in more detail.43 There is growing interest in the contribution of ILC subsets to the regulation of liver disease. Whether they play a pathogenic or protective role in the development of disorders is a matter of debate, which may be due to the difficulty of accurately identifying each subset.
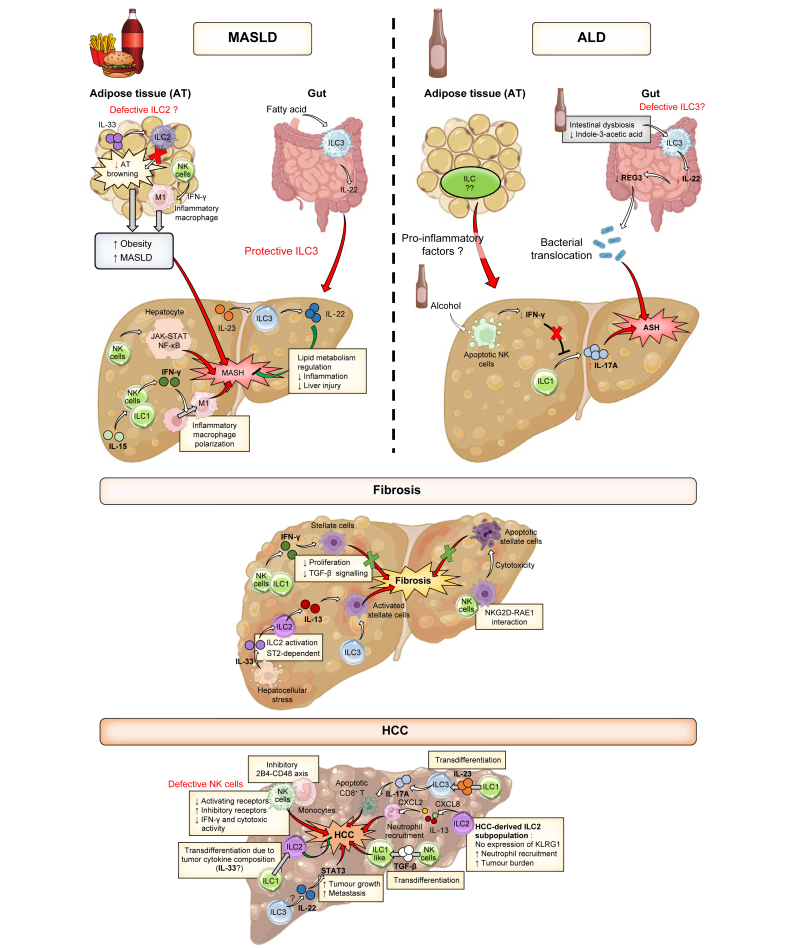
Fig. 1.
Major contributions of ILC subsets to immunity.
Historically described roles and the main recently discovered contributions of ILCs to immune responses are described. ILC(s), innate lymphoid cell(s); ILC1-3, type 1-3 ILC(s); MASH, metabolic dysfunction-associated steatohepatitis; MASLD, metabolic dysfunction-associated steatotic liver disease; NK, natural killer.
Here, we summarize current knowledge regarding the involvement of ILC subsets in regulating the development and progression of metabolic and alcohol-related liver disease in order to better understand the pathogenesis of these liver diseases and the therapeutic potential of targeting these cells.
NK cells and ILC1 in MASLD
The pathogenic or protective role of NK cells in MASH/MASLD has long been debated. This may be due to the difficulty of distinguishing conventional NK cells from tissue-resident hepatic ILC1 (also called liver-resident NK cells in some studies), which share some similarities, but which are functionally different. Conventional NK cells and ILC1 together account for approximately 30% and 10% of the total lymphocytes in the livers of humans and mice, respectively.44 NK cells have more “killing” properties, with higher expression of granzyme B and perforin than ILC1, although recent studies indicate that under certain conditions, ILC1 also exhibit cytotoxicity.45,46 However, the phenotypic and developmental properties that distinguish NK cells and ILC1 are often unclear and confusing, based on evidence from different organs and pathological conditions.47,48 RNA sequencing approaches have therefore been increasingly used to better characterise and differentiate NK cells and ILC subsets.[49], [50], [51] The purpose of our review is not to resolve these discrepancies, which are widely discussed elsewhere, but rather to outline the pathogenic or protective roles of these cells in chronic liver diseases associated with obesity or alcohol consumption.
Cytokines are known potent regulators of immune cell development, activation and functions.52 In particular, interleukin (IL)-12, IL-15 and IL-18 together control the homeostasis of NK cells and the ILC1 subset.53 Il15-knockout (KO) mice fed a high-fat diet (HFD) show a reduction of hepatic steatosis and liver expression of chemokines (CCL2, CCL5, CXCL10) known to attract NK cells. In turn, immune and NK cell infiltration into the liver are lower in diet-induced obese mice in comparison to control-diet mice.54 Albeit only correlative, these results suggest that the absence of IL-15 could affect the biology of these cells as well as other immune cells such as NKT cells and/or subsets of CD8 T cells. Consistent with this, it was recently shown that IL-15 induces auto-aggressive hepatic CXCR6+ CD8 T cells by downregulating FOXO1 (forkhead box O1) transcription factor expression during MASH, causing increased liver damage.55 The study by Cepero et al. also hints that NK cells play a pathogenic role in the onset of MASH.54 Therefore, although IL-15 is a key cytokine for NK cell development and biology at steady state, a local overproduction of IL-15 appears to be deleterious during MASH. A recent study supported this observation, showing that activated NK cells promote diet-induced MASH (methionine- and choline-deficient diet or choline-deficient HFD).56 When challenged with a MASH diet, Nfil3 (nuclear factor interleukin 3 regulated) KO mice, which lack NK cells but retain ILC1, showed reduced liver steatosis, inflammation and injury compared to control mice. This protective mechanism involves the reduction of JAK-STAT and NF-kB-p65 signalling in hepatocytes. In contrast, depletion of NKp46+ cells, which encompass NK cells and ILC1, aggravates MASH.57 These data suggest that NK cells play a pathogenic role while ILC1 possibly play a protective role during MASH development. Further, the depletion of NK cells/ILC1 is not liver specific in Nfil3-KO mice. Thus, we cannot rule out that NK cells/ILC1 act via the regulation of gut and adipose tissue function, which are also important in the development and progression of MASLD. Further investigation is needed to explore the regulation of mechanisms controlling NK cell and ILC1 functions to determine the precise role of these subsets in MASH progression.
NK cells and ILC1 in ALD
The immunosuppressive effect of alcohol has clearly been established for several years, and patients with chronic alcohol consumption are more susceptible to infection.58,59 Regarding NK cells, it has been reported that chronic alcohol consumption decreases the number and cytotoxicity of NK cells in human peripheral blood. The reduction in NK cell degranulation capacity has been related to decreased natural killer group 2, member D (NKG2D) receptor expression.[60], [61], [62], [63] Primary pre-clinical studies have provided insights into the effect of alcohol on NK cells and their contribution to the development of ALD. As observed in circulating NK cells in humans, alcohol has an immunosuppressive effect on murine hepatic NK cells, with a decrease in their number and cytotoxic functions after chronic alcohol consumption.64 Furthermore, chronic plus single-binge ethanol consumption suppressed NK cell activity, which is partly due to IL-10 secreted by NKT cells, leading to the aggravation of liver steatosis.60 In addition, the removal of HSCs by hepatic NK cells is impaired in ethanol-fed mice which is mainly related to a reduction in the expression levels of NKG2D and IFN-γ. This decrease in NK cell activity has been associated with aggravation of liver fibrosis.65 Alcohol consumption also compromises the development and maturation of NK cells due to IL-15 deficiency.66,67 This reduction in liver IL-15 levels upon ethanol feeding has been related to a decrease in IL-15-producing cells (IL15+CD11chi cells), and exogenous IL-15/IL-15 Rα supplementation is sufficient to normalise hepatic NK cell numbers.68 However, all these studies defined NK cells as CD3−NK1.1+ and did not distinguish NK cells from ILC1. The study of Zhang et al. addressed this point and demonstrated that 3 months of chronic alcohol consumption significantly decreased Eomes-expressing NK cells without affecting ILC1.66 Furthermore, the relative contributions of murine hepatic NK cells and ILC during alcoholic steatohepatitis have recently been investigated.69 Chronic (10-days chronic) plus single-binge ethanol feeding in mice induced apoptosis of NK cells resulting in ILC1 dominance and a negative outcome. Restoration of NK cells by cell transfer protected mice from alcohol-induced steatohepatitis, revealing the protective role of NK cells. In contrast, hepatic ILC1 promoted the development and aggravation of steatohepatitis through the secretion of IL-17.69 In parallel, NK-cell-derived IFN-γ, which is significantly downregulated after chronic alcohol consumption, inhibits IL-17 A production. This study highlighted the protective role of NK cells in ALD, whereas ILC1 appeared to be deleterious. It now remains to be determined whether this phenomenon also occurs in humans and thereby whether targeting the balance between NK cells and ILC1 holds therapeutic potential.
ILC2 in MASLD
Liver ILC2 represent less than 5% of all ILCs and have been little studied in the context of liver diseases. However, their role in white adipose tissue (WAT) dysfunction has been well described during obesity, which could influence the progression of associated liver diseases. In response to local IL-33, WAT ILC2 produce enkephalins and type 2 cytokines and are involved in the browning of adipose tissue via IL-4Rα signalling. Further, the activation of ILC2 by IL-33 is sufficient to promote the growth of functional beige fat.70,71 Adipose tissue browning by increasing caloric expenditure could limit the development of obesity and improve insulin sensitivity, dyslipidaemia and MASLD.72,73 It has been reported that elevated brown adipose tissue (BAT) activity is associated with improvement of metabolic disorders, and patients with MASLD displayed lower BAT activity, which could be due to defective ILC2. Specifically, it has been suggested that increased BAT ILC2 activity could ameliorate chronic liver diseases associated with obesity.74,75 However, the role of hepatic ILC2 in MASLD remains to be determined.
ILC2 in ALD
Currently there are no reports concerning the potential role of ILC2 in ALD. However, as for other ILCs, it is possible that alcohol may cause a deregulation of ILC2. In a cohort of 12 patients with cirrhosis of different aetiologies, those with alcohol-related cirrhosis (n = 4) showed the highest expression of IL-33.76 IL-33 is a potent activator of ILC2, which may facilitate the progression of fibrosis towards cirrhosis through the subsequent release of IL-13 by activated ILC2 (discussed below). Further studies are needed to evaluate the contribution of ILC2 to ALD more precisely.
ILC3 in MASLD
ILC3 are relatively rare in the liver but are present in large numbers in the intestine where they actively protect against infections. Depending on the expression of the activator receptor NKp46, ILC3 express RAR-related orphan receptor-gamma t (RORγt) and are classified into three subpopulations: NKp46+ ILC3 (NCR+ ILC3), NKp46- ILC3 (NCR- ILC3) and LTi cells. It has been shown that NCR+ ILC3 are the main source of hepatic IL-22, unlike NCR- ILC3 which produce significant quantities of IL-17 A.77,78 The role of ILC3 and IL-22 in metabolic diseases has also been addressed. It has been shown that IL-22 secreted by ILC3 plays a protective role in HFD-induced hepatic steatosis via its regulation of hepatic lipid metabolism.79 Further, in genetically obese leptin receptor-deficient (db/db) mice and HFD-fed mice, administration of exogenous IL-22 reverses many metabolic symptoms, such as insulin resistance and hyperglycaemia.80 Recently, a study described the protective role of ILC3 in mice with high-fat diet-induced steatohepatitis.81 ILC3 deficient mice (RORγtKI/KI) fed an HFD display significant fatty liver and liver fibrosis, as well as elevated hepatic and circulating palmitic acid levels, a key activator of proinflammatory macrophages. These activated macrophages are an important source of IL-23 which enhances the proliferation/activation of IL-22-producing ILC3. The secreted IL-22 in the liver then regulates lipid metabolism, decreases inflammation and has anti-apoptotic activity. Further, exogenous IL-22 administration ameliorates liver injury, inflammation, and fibrosis in diet-induced MASH mice, by correcting liver oxidative stress and attenuating inflammatory functions of hepatocyte-derived, mitochondrial DNA-enriched extracellular vesicles.82
ILC3 in ALD
To date, no study has directly described the role of hepatic ILC3 in ALD in humans and mice. However, it has been shown in a mouse model that gut ILC3 secrete decreased levels of IL-22 after chronic plus binge ethanol feeding. This reduction in IL-22 secretion was reported to be the result of ethanol-induced intestinal dysbiosis and decreased levels of indole-3-acetic acid, a microbiota-derived aryl hydrocarbon receptor ligand, which regulates IL-22 expression. This deregulation leads to reduced expression of the antimicrobial peptide REG3 (regenerating islet-derived protein 3 gamma), which promotes bacterial translocation to the liver and leads to the development of alcohol-related steatohepatitis. This study also showed that in mice fed with Lactobacillus reuteri engineered to produce IL-22, along with a chronic-binge alcohol diet, liver inflammation and injury were reduced and intestinal expression of IL-22 was upregulated.83 Thus, even if the role of hepatic ILC3 is not clearly described in ALD, intestinal ILC3 seem to play a protective role in the development of alcohol-induced hepatic complications through the gut/liver axis.
ILCs in fibrosis
Fibrosis and its advanced stage, cirrhosis, are caused by the repeated death of a critical fraction of hepatocytes due to persistent inflammation or infection, resulting in a loss of liver tissue organisation and function. HSCs, located between hepatocytes and the endothelial cells of the sinusoids in the space of Disse, are key actors in the development of fibrosis. During chronic inflammation, HSCs are activated by cytokines and transdifferentiate into myofibroblasts, resulting in the production of huge amounts of fibrous proteins (collagen, laminin, fibronectin). The balance between the production of this extracellular matrix and its degradation influences the evolution of fibrosis.84
Because ILCs have only recently been identified, few studies have investigated their role in the pathogenesis of fibrosis induced by a metabolic and/or alcoholic insult and such studies have mainly used mouse models of liver damage induced by administration of hepatotoxic compounds, such as carbon tetrachloride (CCl4). NK cells are the most well-studied ILC subset in the development of fibrosis. Several studies using mouse models of diet and/or chemical induced-liver fibrosis have reported that NK cells work against fibrosis by eliminating HSCs.57,[85], [86], [87], [88] HSCs can be “killed” by NK cells through the interaction of activating/inhibitory NK cell receptors and ligands differentially expressed by the activated HSCs during their differentiation, for example, RAE-1 (ribonucleic acid export-1) recognised by the NKG2D receptor, or a lack of MHC I expression, which triggers NK cell activation.[89], [90], [91] Other mechanisms involve the anti-fibrotic activity of IFN-γ, which inhibits HSC proliferation and attenuates pro-fibrogenic transforming growth factor-β (TGF-β) signalling.87 The source of IFN-γ might be hepatic NK cells but could also be ILC1, making both cell types important in the resolution or prevention of fibrosis in chronic liver diseases. Using mouse models of MASLD-induced hepatic fibrosis, it has been shown that the regulation of fibrotic MASH depends on the interaction between liver NK cells and macrophages. The depletion of NK cells and/or ILC1 favours the polarization of alternatively activated macrophages at the expense of the proinflammatory macrophages that lead to MASLD progression.57,92 These data provide insight into the contributions of NK cells and ILC1 during the development of fibrotic MASH in a metabolic context.93 Furthermore, ILC2 could also be involved in fibrosis associated with MASLD in an IL-33-dependent manner, as reported in a mouse model of diet-induced MASH.94 The results showed an increase in the expression of IL-33 and its receptor ST2 in the livers of mice fed a methionine- and choline-deficient diet or a HFD. While IL-33 treatment attenuated hepatic steatosis, reduced serum alanine aminotransferase activity, and improved systemic insulin resistance and glucose intolerance, liver fibrosis was aggravated in an ST2-dependent manner. Although this study does not clearly indicate the involvement of ILC2, the results are in line with previous data showing the involvement of ILC2 in liver fibrosis.95 In a mouse model of liver fibrosis induced by CCl4 treatment, IL-33 was released in response to chronic hepatocellular stress, leading to the accumulation and activation of hepatic ILC2 in a ST2 signalling-dependent manner. In turn, activated ILC2 produce IL-13, promoting the activation of HSCs. Human studies confirmed that ILC2 activation is strongly correlated with fibrosis severity.76 Since patients with fibrotic livers are at increased risk of developing cancer, this could suggest ILC2 plays a pathogenic role in HCC development. It has recently emerged that IL-22- and IL-17-producing ILC3 subsets are also important in liver fibrosis. The role of IL-22 in fibrosis development has mainly been studied in mouse models of CCl4-induced fibrosis. A decade ago, it was reported that IL-22 ameliorates hepatic fibrosis by activating the STAT2 pathway, thereby inducing HSC senescence and inhibiting HSC activation.96 However, a more recent study refutes this hypothesis and highlights the pathogenic role of ILC3 in hepatic fibrosis.97 An increase in ILC3 (Lin-CD127+RORγt+) was found in mouse fibrotic livers, which contained more subpopulations of ILC3 IL-17 A+ and ILC3 IL-22+ than ILC3 populations from control mice. Furthermore, the adoptive transfer of ILC3 into Rag1-/- ILC-depleted mice resulted in a significant increase in HSC activation. These apparently contradictory results might be explained by differences in technical approaches, such as the sorting and transfer of the very rare population of ILC3 in vivo, and thus further investigation is needed to ascertain the role of ILC3 in liver fibrosis. Recently, in human fibrotic liver, an “unconventional” ILC3-like cell producing the ILC2-related cytokine IL-13 was identified. The frequency of this subset was higher in fibrotic livers than in healthy livers, and IL-13+ ILC3-like cells can modulate HSC activation by upregulating inflammatory genes/proteins such as the monocyte-chemoattractant chemokine CXCL8.98
Together, these data strongly support that ILC subsets play a complex but important role in the pathogenesis of fibrosis and must be considered in future studies and therapeutic strategies.
ILCs in HCC
Studies examining the role of ILCs in cancer, including HCC, have yielded contradictory results, with some studies suggesting a role in tumour progression while others suggest that ILCs confer antitumor properties.99 However, the results are more consistent for the NK cell subset. NK cells represent a key weapon against tumours, as they have the unique ability to detect and eliminate malignant cells without any sensitization.91,100 It has been shown that the number of CD56+ NK cells infiltrating the liver in patients with HCC (aetiology not provided) positively correlated with survival and the elimination of cancer cells, confirming that NK cell activity protects against cancer.101 In addition, decreased IFN-γ production, upregulation of the expression of NK cell inhibitory receptors (NKG2A, TIM3, CD96) and downregulation of the expression of NK cell activating receptors (NKp46, NKG2D, TIGIT, Siglec7, CD160 and NKp30) were reported in patients with HCC.101 Furthermore, in patients with advanced HCC, NKp30-positive NK cells show reduced expression of immunostimulatory splice variants of NKp30 (NKp30a and NKp30 b) and increased expression of the inhibitory variant NKp30c.102 These studies indicate that tumour-infiltrating NK cells are deregulated in HCC and have a reduced capacity to eliminate cancer cells. This reduction in NK cell activity could be mediated by different cells and mechanisms, for example by tumour-derived monocytes/macrophages, which boost the CD48/2B4 inhibitory axis, or by myeloid-derived suppressor cells, which reduce the NKp30 receptor activating signalling pathway.[103], [104] Co-culture of NK cells with cancer-associated fibroblasts (HCC-associated fibroblasts from tumour tissues) also leads to inhibition of NK cell functions with a reduction in the expression of activating receptors and the production of granzyme B, perforin, IFN-γ and TNF-α.105 Zhang et al., uncovered the infiltration of double-negative CD11b-CD27- NK cells into the tumour tissue of patients with HCC. This population displays an immature phenotype with impaired cytotoxic capacity and IFN-γ production. These infiltrating double-negative NK cells could explain the NK cell dysfunction observed in patients with HBV-related HCC and their tumour progression.106
Currently, most findings regarding the involvement of NK cells in HCC have been observed in humans while few pre-clinical studies are available. This may be explained by the difficulty in generating mouse models that reach the HCC stage exclusively through diet-induced MASLD. Mouse models of HCC are most often transplanted tumour models where cells from human cancer cell lines are injected subcutaneously into mice. In the study of Yu et al., the Hep3B cell line, representative of the invasive and oncogenic nature of HCC, was used to investigate the role of NK cells. The NK cells infiltrating these tumours showed a non-functional phenotype associated with high expression of the transcription factor NR4A1, a regulator of antitumor immunity.107 The results thus appear to be consistent with human data, where dysfunctional NK cells in HCC are believed to promote tumour progression.
The contribution of ILC1 to the development of HCC is yet to be well documented. The tumour microenvironment could play an essential role in the orientation of the ILC1 phenotype, which may lead these cells to a variety of different fates.40,108 For example, in a mouse mammary tumour model, the abundance of IL-15 within the microenvironment leads to an increase in a specific subset of ILC1 that lacks expression of some ILC1-related proteins (CD127, TNF-α) but expresses granzyme B.109 The inflammatory environment in a mouse model of cutaneous squamous cell carcinoma also favours the increase of a peculiar subset of ILC1 that produce less IFN-γ but more of the proinflammatory cytokine IL-6 in pre-cancerous lesions, which could contribute to tumour progression.40 Furthermore, studies in other cancers (fibrosarcoma, colon carcinoma, lung carcinoma) have shown that NK cells can be converted into “ILC1-like” cells, mainly mediated by TGF-β released by the tumour microenvironment.110,111 Consistent with this, elevated concentrations of TGF-β were reported in the supernatant derived from human HCC.112 The conversion of NK cells into “ILC1-like” cells could occur in HCC and could explain the decreased NK cell numbers discussed above. Heinrich et al. showed that the cytokine profile in the tumour microenvironment shapes ILCs in patients with HCC. Single-cell RNA sequencing and flow cytometry analysis of biopsies from patients with HCC also demonstrated the presence of NK-like cells in the non-tumour tissue. These cells lose their cytotoxic capacity as they evolve towards an ILC1-like profile.113 It would be of interest to evaluate whether HCC is infiltrated by the recently described cytotoxic ILC1 subset and, if so, how the HCC environment controls the fate of these cells.45,46 Taken together, these studies suggest that the reduced ability of NK cells to clear cancer cells or their reduced frequency through possible conversion into ILC1-like cells, as well as the increase in ILC1, could lead to HCC development.
As discussed above, recent studies have demonstrated that ILC2 play a pro-fibrogenic role in the liver in mice and in humans. Therefore, they may also contribute to HCC onset in the long term. In line with this, a significant correlation has been reported between hepatic ILC2 number and poor prognosis in patients with HCC.114 The same study showed decreased expression levels of ILC2 activators (IL-33, IL-25) in HCC samples, and the loss of expression of KLRG1, a marker of ILC2 maturation. This suggests the existence of an HCC-derived ILC2 subpopulation that is functionally divergent from canonical ILC2. This population also produced elevated amounts of IL-13 and chemokines (such as CXCL2 and CXCL8) involved in neutrophil recruitment. The transfer of ILC2 into tumour-bearing mice confirmed the observation that these cells are associated with neutrophil recruitment, increased arginase expression and tumour burden.114 These hepatic ILC2 identified in mouse and human HCC could contribute to an immunosuppressive microenvironment that favours tumour development. However, the study published by Heinrich et al. reports contradictory results, suggesting that ILC2 play a protective role in human HCC. Specifically, the authors show that a high ratio of ILC2/ILC1 within patients' tumours is associated with better survival. The high ratio corresponds to an increase in tumour-associated ILC2 that could be dependent on cytokine levels, in particular IL-33, which may promote ILC2 activation.113 This discrepancy may be due to different origins of ILC2. ILC2 derived from ILC1 plasticity may not have the same phenotype as the ILC2 described by Xu et al. Furthermore, in human studies, the stages of HCC are not clearly specified and may potentially influence the type of ILC2 and their role in the tumour. The composition of the tumour microenvironment also seems to play an essential role in the behaviour of these cells and may potentially explain the divergent roles observed by the two studies.
Several studies using orthotopic models or hepatotoxic agents highlight the potential pro-tumoural role of liver ILC3 subsets in HCC development. The pro-tumorigenic role of IL-17 A, the cytokine noted above that is secreted by NCR- ILC3, T helper 17 cells and γδ T cells, is clearly described in the literature.115 It has also been reported that liver NCR- ILC3 promote the development of HCC in response to IL-23 in an orthotopic mouse model (injection of hepa1.6 cell line into the liver) and a model of diethylnitrosamine-induced HCC.116 IL-23 promotes the differentiation of ILC1 into ILC3 in the tumour microenvironment which in turn produces IL-17 A but not IL-22. IL-17 A-producing NCR- ILC3 respond to IL-23 present in the tumour microenvironment and directly promote CD8+ T apoptosis and in turn HCC progression.116 The role of NCR+ ILC3 in HCC progression has not yet been described. However, one study showed that patients with HCC had significant amounts of IL-22 in the tumour microenvironment. IL-22 inhibits apoptosis and promotes tumour growth and metastasis via STAT3 activation.117 Furthermore, a liver-specific IL-22 transgenic mouse model highlighted the involvement of IL-22 in liver carcinogenesis. IL-22 acts as a local paracrine factor capable of stimulating the proliferation of liver cancer cells.118 Thus, this study hints that IL-22-producing NCR+ ILC3 could play a pathogenic role during the development of HCC. Taken together, these studies support a potential pro-tumoural role for liver ILC3 subsets in HCC development. Further work is needed to investigate whether this is also true for metabolic and alcohol-induced HCC.
Targeting ILCs in liver diseases
Understanding the exact role of ILCs in inflammatory diseases or cancers is still the subject of intense research and the specific targeting of these cells for therapy is still in its infancy. Chronic inflammation is a key driver of MASLD and many efforts have been made recently to target inflammatory immune cells. Strategies to limit immune cell recruitment into the liver (CCR2/CCR5, CXCR2 antagonists) and/or their activation (inhibition of TLR4, NLRP3 inflammasome) in MASH have led to limited results, and they mainly target myeloid cells.119 NK cells/ILCs can also express these markers, at lower levels, thus it is possible that their functions will also be impacted by these treatments. In any case, since immune cells interact continuously and influence each other, NK cells/ILCs could also be affected indirectly by such approaches. The therapeutic manipulation of NK cells is much more advanced for oncological indications than for liver diseases. To date, over 200 clinical trials (clinicaltrials.gov) have been conducted to exploit their anti-tumoural properties. NK cell-based therapeutic approaches leading to promising results include infusion of activated and/or engineered NK cells, NK cell stimulation with cytokines and the use of agonistic and blocking antibodies that target NK cells.120 Regarding the treatment of HCC, it has been shown that injecting allogenic NK cells into patients increased progression-free survival.121,122 The use of chimeric antigen receptor (CAR)-modified NK cells also represents a promising approach in HCC treatment. CAR-NK cells have been engineered against glypican-3, c-MET oncoproteins, CD147 glycoprotein (immune cell activator) and TGF-β (inhibitor of NK cell cytotoxicity). These modified NK cells are fully functional, with increased IFN-γ production and cytotoxicity against HCC cell lines, and are able to reduce tumour growth in xenograft models.[123], [124], [125], [126] Cytokines targeting activation and expansion of NK cells (and/or other ILC subsets) are also being tested in cancer therapies, for example IL-2, IL-15 and IL-12. The strongest anti-tumoural response is induced by infusion of IL-2 but the use of this cytokine is limited by major adverse effects, such as an increase in regulatory T cells and tissue inflammation.127 Mutated IL-2, engineered to limit toxicity, represents an attractive approach to boost NK cell anti-tumoural properties in HCC.128 IL-15 is known to activate NK cell proliferation and constitutes an alternative to IL-2, as it has the capacity to recover antitumor functionality in NK cells inhibited by in vitro exposure to HCC cell lines or extracted directly from HCC.129 However, as mentioned above, the use of IL-15 could be deleterious in treating MASH as it favours the expansion of aggressive CD8 T cells and inflammatory NK cells, highlighting that the nature of the microenvironment constrains the use of this type of approach. Finally, strategies that target NK cell or ILC receptors and ICIs are also promising against chronic liver diseases. T cells were the first immune cells targeted by immunotherapy. However, NK cells and other ILC subsets have also been shown to express immune checkpoints (PD-1, TIGIT, LAG3, etc.) and thus studies focusing solely on T cells have underestimated the potential of targeting these cells as well.100 Multispecific engagers, which target one or more activating NK cell receptors (NKp46, NKG2D, etc.) and/or cytokine receptors (IL-2 receptor) designed to stimulate NK cell function, represent a new class of therapeutic molecules being developed against cancer.130 It would be of interest to evaluate the use of such molecules in chronic liver diseases and HCC.
A better characterisation of liver NK cells and ILC subsets at different stages of chronic liver diseases could lead to a better understanding of the mechanisms that regulate the local immune response and could help in the development of therapeutic strategies tailored to the liver microenvironment.
Conclusions
The involvement of ILCs in obesity- and alcohol-related liver disease is undeniable (Table 1). However, the exact nature of the protective or pathogenic roles these cells play remains difficult to elucidate. In this review we highlighted that their roles can rapidly change depending on the stage of progression of the disease due to changes in the microenvironment (Fig. 2). Currently, NK cells, due to their predominance among ILC populations in the liver, are the ILCs whose roles are best described. However, their close resemblance to ILC1 and their phenotypic heterogeneity often make their role difficult to assess. ILC2 and ILC3 are poorly represented in the liver and their involvement in this organ is not yet very well described, unlike their respective roles in adipose tissue and the gut. However, existing studies have allowed us to put forward several hypotheses that point to a pathogenic role for these cells that leads to HCC. New approaches combining single-cell RNA sequencing, flow cytometry and spatial transcriptomic technologies would help to better characterise ILC subsets in order to better understand their role within the tissue. The study of ILC metabolism could provide additional indicators of the role of these cells in different stages of obesity- and alcohol-related liver diseases, by giving insights into the functional activity of ILCs. Finally, targeting the immune system holds great promise in the fight against diseases, and in particular in cancer. Immunotherapy strategies that target ICIs and their effects on the host have mostly focused on T cells. ILCs also express inhibitory receptors and could thus be targeted by similar strategies. Recently developed cell therapy approaches that target NK cells (CAR-NK cells, multispecific NK cell engagers) to enhance their activities represent promising weapons in the fight against cancer. The development of ILC-based cell therapies that target these cells at different stages of MASLD and ALD could similarly represent a new therapeutic option that deserves further investigation.
Table 1.
Pathogenic and protective roles of ILCs in metabolic, alcohol-related.
| Metabolic dysfunction-associated steatotic liver disease | Alcohol-related liver disease | Liver fibrosis | HCC | |
|---|---|---|---|---|
| NK cells |
Pathogenic role: Responsible for inflammatory macrophage polarization93 JAK-STAT and NF-kB pathway activation in hepatocytes resulting in oxidative stress and hepatocyte damage56 |
Protective role: IFN-γ produced by NK cells inhibits IL-17 A production and consequently also liver inflammation69 |
Protective role: Leads to HSC apoptosis[57], [85], [86], [89], [90], [91] IFN-γ inhibits HSC proliferation and pro-fibrogenic TGF-β signalling87 |
Protective role: Anti-tumoural activity101 |
| ILC1 |
Pathogenic or protective role: Not yet defined |
Pathogenic role: Drives liver inflammation by producing IL-17 A69 |
Potential protective role: IFN-γ inhibits HSC proliferation and pro-fibrogenic TGF-β signalling87 |
Potential pathogenic role: NK cell conversion into ILC1-like cells by tumour microenvironment112,113 |
| ILC2 |
Potential protective role: IL-33 treatment attenuated hepatic steatosis and liver injury94 |
Pathogenic or protective role: Not yet defined |
Pathogenic role: Activated ILC2 promote HSC activation through IL-13 production76,94,95 |
Potential pathogenic role: Could contribute to an immunosuppressive microenvironment which favours tumour development114 Potential protective role: High ILC2/ILC1 ratio is associated with better survival in patients with HCC113 |
| ILC3 |
Potential protective role: IL-22, produced by ILC3, decreases metabolic syndrome79,80 |
Potential protective role: Gut ILC3 indirectly promote the development of hepatic complications through gut/liver axis83 |
Potential pathogenic role: Lead to an increase in HSC activation97 |
Potential pathogenic role: IL-22 inhibits apoptosis and promotes tumour growth via STAT3117,118 |
HCC, hepatocellular carcinoma; ILC(s), innate lymphoid cell(s); ILC1-3, type 1-3 ILC(s); NK, natural killer.
Fig. 2.
Roles of ILC subsets in chronic liver diseases.
ILC involvement in the development of: liver abnormalities after high-caloric (top left) or excessive/chronic alcohol consumption (top right); fibrosis (middle); and HCC (bottom). ALD, alcohol-related liver disease; ASH, alcohol-related steatohepatitis; AT, adipose tissue; HCC, hepatocellular carcinoma; ILC(s), innate lymphoid cell(s); ILC1-3, type 1-3 ILC(s); MASH, metabolic dysfunction-associated steatohepatitis; MASLD, metabolic dysfunction-associated steatotic liver disease; NK, natural killer.
Financial support
This work is supported by grants from Institut National de la santé et de la Recherche Médicale (INSERM), Université Côte d’Azur and various charities (Association Française pour l’Etude du Foie (AFEF) to CL, Société Nationale Française de Gastro-Entérologie (SNFGE) to RA. This work was also funded by the French Government (National Research Agency, ANR): #ANR-18-CE14-0019-02, #ANR-19-CE14-0044-01, #ANR-21-CE14-0015-03, # ANR-22-CE14-0027-01 and through the "Investments for the Future" LABEX SIGNALIFE (#ANR-11-LABX-0028-01) and the UCAJEDI Investments in the Future project (#ANR-15-IDEX-01). We would also like to thank Catherine Buchanan for her assistance with the English syntax and grammar in this manuscript. MB was successively supported by the ANR (#ANR-18-CE14-0019-02), and by doctoral grants from the French Ministry of Research and the Fondation pour la Recherche Médicale (FRM).
Authors’ contributions
Manon Bourinet and Carmelo Luci designed and wrote the manuscript. Rodolphe Anty contributed to the clinical section of the introduction. Philippe Gual gave guidance on the outline and revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors of this study declare that they do not have any conflict of interest.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2023.100962.
Contributor Information
Philippe Gual, Email: philippe.gual@inserm.fr.
Carmelo Luci, Email: carmelo.luci@inserm.fr.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Tsutsumi V., Nakamura T., Ueno T., et al. In: Liver pathophysiology [Internet] Muriel P., editor. Academic Press; Boston: 2017. Chapter 2 - structure and ultrastructure of the normal and diseased liver.https://www.sciencedirect.com/science/article/pii/B9780128042748000023 [cited 2023 Jan 27]. p. 23–44. Available from: [Google Scholar]
- 2.Luci C., Bourinet M., Leclère P.S., et al. Chronic inflammation in non-alcoholic steatohepatitis: molecular mechanisms and therapeutic strategies. Front Endocrinol. 2020 Dec 14;11 doi: 10.3389/fendo.2020.597648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rinella M.E., Neuschwander-Tetri B.A., Siddiqui M.S., et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023 May;77(5):1797. doi: 10.1097/HEP.0000000000000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riazi K., Azhari H., Charette J.H., et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022 Sep 1;7(9):851–861. doi: 10.1016/S2468-1253(22)00165-0. [DOI] [PubMed] [Google Scholar]
- 5.Anstee Q.M., Reeves H.L., Kotsiliti E., et al. From NASH to HCC: current concepts and future challenges. Nat Rev Gastroenterol Hepatol. 2019 Jul;16(7):411–428. doi: 10.1038/s41575-019-0145-7. [DOI] [PubMed] [Google Scholar]
- 6.Tran A., Gual P. Non-alcoholic steatohepatitis in morbidly obese patients. Clin Res Hepatol Gastroenterol. 2013 Feb 1;37(1):17–29. doi: 10.1016/j.clinre.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Cai J., Zhang X.J., Li H. Progress and challenges in the prevention and control of nonalcoholic fatty liver disease. Med Res Rev. 2019;39(1):328–348. doi: 10.1002/med.21515. [DOI] [PubMed] [Google Scholar]
- 8.Roy T.L., Llopis M., Lepage P., et al. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. 2013 Dec 1;62(12):1787–1794. doi: 10.1136/gutjnl-2012-303816. [DOI] [PubMed] [Google Scholar]
- 9.Wang B., Jiang X., Cao M., et al. Altered fecal microbiota correlates with liver biochemistry in nonobese patients with non-alcoholic fatty liver disease. Sci Rep. 2016 Aug 23;6(1) doi: 10.1038/srep32002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wigg A.J., Roberts-Thomson I.C., Dymock R.B., et al. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2001 Feb;48(2):206–211. doi: 10.1136/gut.48.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saltzman E.T., Palacios T., Thomsen M., et al. Intestinal microbiome shifts, dysbiosis, inflammation, and non-alcoholic fatty liver disease. Front Microbiol. 2018;9:61. doi: 10.3389/fmicb.2018.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lumeng C.N., Bodzin J.L., Saltiel A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007 Jan;117(1):175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vonghia L., Francque S. Cross talk of the immune system in the adipose tissue and the liver in non-alcoholic steatohepatitis: pathology and beyond. World J Hepatol. 2015 Jul 28;7(15):1905–1912. doi: 10.4254/wjh.v7.i15.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viel S., Besson L., Charrier E., et al. Alteration of Natural Killer cell phenotype and function in obese individuals. Clin Immunol. 2017 Apr 1;177:12–17. doi: 10.1016/j.clim.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Ringel A.E., Drijvers J.M., Baker G.J., et al. Obesity shapes metabolism in the tumor microenvironment to suppress anti-tumor immunity. Cell. 2020 Dec 23;183(7):1848–1866.e26. doi: 10.1016/j.cell.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galle P.R., Forner A., Llovet J.M., et al. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018 Jul 1;69(1):182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 17.Tang H., Sebastian B.M., Axhemi A., et al. Ethanol-induced oxidative stress via the CYP2E1 pathway disrupts adiponectin secretion from adipocytes. Alcohol Clin Exp Res. 2012 Feb;36(2):214–222. doi: 10.1111/j.1530-0277.2011.01607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Llopis M., Cassard A.M., Wrzosek L., et al. Intestinal microbiota contributes to individual susceptibility to alcoholic liver disease. Gut. 2016 May 1;65(5):830–839. doi: 10.1136/gutjnl-2015-310585. [DOI] [PubMed] [Google Scholar]
- 19.Ntandja Wandji L.C., Gnemmi V., Mathurin P., et al. Combined alcoholic and non-alcoholic steatohepatitis. JHEP Rep. 2020 May 22;2(3):100101. doi: 10.1016/j.jhepr.2020.100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tiniakos D.G. Liver biopsy in alcoholic and non-alcoholic steatohepatitis patients. Gastroentérologie Clin Biol. 2009 Oct 1;33(10):930–939. doi: 10.1016/j.gcb.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Marrero J.A., Kulik L.M., Sirlin C.B., et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology. 2018;68(2):723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 22.Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019 Apr 11;380(15):1450–1462. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 23.Llovet J.M., Kelley R.K., Villanueva A., et al. Hepatocellular carcinoma. Nat Rev Dis Primer. 2021 Jan 21;7(1):1–28. doi: 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- 24.Craig A.J., von Felden J., Garcia-Lezana T., et al. Tumour evolution in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2020 Mar;17(3):139–152. doi: 10.1038/s41575-019-0229-4. [DOI] [PubMed] [Google Scholar]
- 25.Rimassa L., Finn R.S., Sangro B. Combination immunotherapy for hepatocellular carcinoma. J Hepatol. 2023 Aug 1;79(2):506–515. doi: 10.1016/j.jhep.2023.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Finn R.S., Qin S., Ikeda M., et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020 May 14;382(20):1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 27.Giraud J., Chalopin D., Blanc J.F., et al. Hepatocellular carcinoma immune landscape and the potential of immunotherapies. Front Immunol. 2021 Mar 18;12 doi: 10.3389/fimmu.2021.655697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vivier E., Artis D., Colonna M., et al. Innate lymphoid cells: 10 Years on. Cell. 2018 Aug 23;174(5):1054–1066. doi: 10.1016/j.cell.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 29.Barrow A.D., Colonna M. Exploiting NK cell surveillance pathways for cancer therapy. Cancers. 2019 Jan 8;11(1):55. doi: 10.3390/cancers11010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korchagina A.A., Koroleva E., Tumanov A.V. Innate lymphoid cell plasticity in mucosal infections. Microorganisms. 2023 Feb 12;11(2):461. doi: 10.3390/microorganisms11020461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luci C., Reynders A., Ivanov, et al. Influence of the transcription factor RORγt on the development of NKp46+ cell populations in gut and skin. Nat Immunol. 2009 Jan;10(1):75–82. doi: 10.1038/ni.1681. [DOI] [PubMed] [Google Scholar]
- 32.Sanos S.L., Bui V.L., Mortha A., et al. RORγt and commensal microflora are required for the differentiation of mucosal interleukin 22–producing NKp46+ cells. Nat Immunol. 2009 Jan;10(1):83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cella M., Otero K., Colonna M. Expansion of human NK-22 cells with IL-7, IL-2, and IL-1β reveals intrinsic functional plasticity. Proc Natl Acad Sci. 2010 Jun 15;107(24):10961–10966. doi: 10.1073/pnas.1005641107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gasteiger G., Fan X., Dikiy S., et al. Tissue residency of innate lymphoid cells in lymphoid and non-lymphoid organs. Science. 2015 Nov 20;350(6263):981–985. doi: 10.1126/science.aac9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Korchagina A.A., Koroleva E., Tumanov A.V. Innate lymphoid cells in response to intracellular pathogens: protection versus immunopathology. Front Cel Infect Microbiol. 2021 Dec 6;11 doi: 10.3389/fcimb.2021.775554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schulz-Kuhnt A., Wirtz S., Neurath M.F., et al. Regulation of human innate lymphoid cells in the context of mucosal inflammation. Front Immunol. 2020 Jun 23;11:1062. doi: 10.3389/fimmu.2020.01062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buonocore S., Ahern P.P., Uhlig H.H., et al. Innate lymphoid cells drive IL-23 dependent innate intestinal pathology. Nature. 2010 Apr 29;464(7293):1371–1375. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halim T.Y.F., Krauß R.H., Sun A.C., et al. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012 Mar 23;36(3):451–463. doi: 10.1016/j.immuni.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 39.Kim B.S., Siracusa M.C., Saenz S.A., et al. TSLP elicits IL-33–independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med. 2013 Jan 30;5(170):170ra16. doi: 10.1126/scitranslmed.3005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luci C., Bihl F., Bourdely P., et al. Cutaneous squamous cell carcinoma development is associated with a temporal infiltration of ILC1 and NK cells with immune dysfunctions. J Invest Dermatol. 2021 Oct 1;141(10):2369–2379. doi: 10.1016/j.jid.2021.03.018. [DOI] [PubMed] [Google Scholar]
- 41.Yang Z., Tang T., Wei X., et al. Type 1 innate lymphoid cells contribute to the pathogenesis of chronic hepatitis B. Innate Immun. 2015 Aug 1;21(6):665–673. doi: 10.1177/1753425915586074. [DOI] [PubMed] [Google Scholar]
- 42.Rao Y., Le Y., Xiong J. Front Immunol. 2021 May;12:666045. doi: 10.3389/fimmu.2021.666045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van de Pavert S.A. Lymphoid Tissue inducer (LTi) cell ontogeny and functioning in embryo and adult. Biomed J. 2021 Apr;44(2):123–132. doi: 10.1016/j.bj.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi F.D., Ljunggren H.G., La Cava A., et al. Organ-specific features of natural killer cells. Nat Rev Immunol. 2011;11(10):658–671. doi: 10.1038/nri3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nixon B.G., Chou C., Krishna C., et al. Cytotoxic granzyme C–expressing ILC1s contribute to antitumor immunity and neonatal autoimmunity. Sci Immunol. 2022 Apr 8;7(70) doi: 10.1126/sciimmunol.abi8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kansler E.R., Dadi S., Krishna C., et al. Cytotoxic innate lymphoid cells sense cancer cell-expressed interleukin-15 to suppress human and murine malignancies. Nat Immunol. 2022 Jun;23(6):904–915. doi: 10.1038/s41590-022-01213-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riggan L., Freud A.G., O’Sullivan T.E. True detective: unraveling group 1 innate lymphocyte heterogeneity. Trends Immunol. 2019 Oct;40(10):909–921. doi: 10.1016/j.it.2019.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seillet C., Brossay L., Vivier E. Natural killers or ILC1s? That is the question. Curr Opin Immunol. 2021 Feb;68:48. doi: 10.1016/j.coi.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McFarland A.P., Yalin A., Wang S.Y., et al. Multi-tissue single-cell analysis deconstructs the complex programs of mouse natural killer and type 1 innate lymphoid cells in tissues and circulation. Immunity. 2021 Jun 8;54(6):1320–1337.e4. doi: 10.1016/j.immuni.2021.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crinier A., Milpied P., Escalière B., et al. High-dimensional single-cell analysis identifies organ-specific signatures and conserved NK cell subsets in humans and mice. Immunity. 2018 Nov 20;49(5):971–986.e5. doi: 10.1016/j.immuni.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krämer B., Nalin A.P., Ma F., et al. Single-cell RNA sequencing identifies a population of human liver-type ILC1s. Cell Rep. 2023 Jan 31;42(1) doi: 10.1016/j.celrep.2022.111937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang J.M. An J. Cytokines, inflammation and pain. Int Anesthesiol Clin. 2007;45(2):27–37. doi: 10.1097/AIA.0b013e318034194e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marçais A., Viel S., Grau M., et al. Regulation of mouse NK cell development and function by cytokines. Front Immunol [Internet] 2013 Dec 12;4 doi: 10.3389/fimmu.2013.00450. https://www.frontiersin.org/articles/10.3389/fimmu.2013.00450 450.1-14. [cited 2023 Mar 30]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cepero-Donates Y., Lacraz G., Ghobadi F., et al. Interleukin-15-mediated inflammation promotes non-alcoholic fatty liver disease. Cytokine. 2016 Jun 1;82:102–111. doi: 10.1016/j.cyto.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 55.Dudek M., Pfister D., Donakonda S., et al. Auto-aggressive CXCR6+ CD8 T cells cause liver immune pathology in NASH. Nature. 2021 Apr;592(7854):444–449. doi: 10.1038/s41586-021-03233-8. [DOI] [PubMed] [Google Scholar]
- 56.Wang F., Zhang X., Liu W., et al. Activated natural killer cell promotes nonalcoholic steatohepatitis through mediating JAK/STAT pathway. Cell Mol Gastroenterol Hepatol. 2022 Jan 1;13(1):257–274. doi: 10.1016/j.jcmgh.2021.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tosello-Trampont A.C., Krueger P., Narayanan S., et al. NKp46+ NK cells attenuate metabolism-induced hepatic fibrosis by regulating macrophage activation. Hepatol Baltim Md. 2016 Mar;63(3):799–812. doi: 10.1002/hep.28389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cook R.T. Alcohol abuse, alcoholism, and damage to the immune system—a review. Alcohol Clin Exp Res. 1998;22(9):1927–1942. [PubMed] [Google Scholar]
- 59.Jerrells T.R. Immunodeficiency associated with ethanol abuse. Adv Exp Med Biol. 1991;288:229–236. doi: 10.1007/978-1-4684-5925-8_26. [DOI] [PubMed] [Google Scholar]
- 60.Støy S., Dige A., Sandahl T.D., et al. Cytotoxic T lymphocytes and natural killer cells display impaired cytotoxic functions and reduced activation in patients with alcoholic hepatitis. Am J Physiol-gastrointest Liver Physiol. 2015 Feb 15;308(4):G269–G276. doi: 10.1152/ajpgi.00200.2014. [DOI] [PubMed] [Google Scholar]
- 61.Perney P., Portalès P., Corbeau P., et al. Specific alteration of peripheral cytotoxic cell perforin expression in alcoholic patients: a possible role in alcohol-related diseases. Alcohol Clin Exp Res. 2003 Nov;27(11):1825–1830. doi: 10.1097/01.ALC.0000093742.22787.30. [DOI] [PubMed] [Google Scholar]
- 62.Laso F.J., Madruga J.I., Giron J.A., et al. Decreased natural killer cytotoxic activity in chronic alcoholism is associated with alcohol liver disease but not active ethanol consumption. Hepatology. 1997;25(5):1096–1100. doi: 10.1002/hep.510250508. [DOI] [PubMed] [Google Scholar]
- 63.Li F., Cook R.T., Alber C., et al. Ethanol and natural killer cells. II. Stimulation of human natural killer activity by ethanol in vitro. Alcohol Clin Exp Res. 1997 Sep;21(6):981–987. [PubMed] [Google Scholar]
- 64.Pan H., Sun R., Jaruga B., et al. Chronic ethanol consumption inhibits hepatic natural killer cell activity and accelerates murine cytomegalovirus-induced hepatitis. Alcohol Clin Exp Res. 2006 Oct 1;30:1615–1623. doi: 10.1111/j.1530-0277.2006.00194.x. [DOI] [PubMed] [Google Scholar]
- 65.Jeong W.I., Park O., Gao B. Abrogation of the antifibrotic effects of natural killer cells/interferon-γ contributes to alcohol acceleration of liver fibrosis. Gastroenterology. 2008 Jan 1;134(1):248–258. doi: 10.1053/j.gastro.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang F., Little A., Zhang H. Chronic alcohol consumption inhibits peripheral NK cell development and maturation by decreasing the availability of IL-15. J Leukoc Biol. 2017 Apr;101(4):1015–1027. doi: 10.1189/jlb.1A0716-298RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang H., Meadows G.G. Chronic alcohol consumption perturbs the balance between thymus-derived and bone marrow-derived natural killer cells in the spleen. J Leukoc Biol. 2008 Jan 1;83(1):41–47. doi: 10.1189/jlb.0707472. [DOI] [PubMed] [Google Scholar]
- 68.Zhang H., Meadows G.G. Exogenous IL-15 in combination with IL-15rα rescues natural killer cells from apoptosis induced by chronic alcohol consumption. Alcohol Clin Exp Res. 2009 Mar;33(3):419–427. doi: 10.1111/j.1530-0277.2008.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cheng C., Zhang Q., Li Y., et al. Interplay between liver type 1 innate lymphoid cells and NK cells drives the development of alcoholic steatohepatitis. Cel Mol Gastroenterol Hepatol. 2022 Sep 27;15(1):261–274. doi: 10.1016/j.jcmgh.2022.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brestoff J.R., Kim B.S., Saenz S.A., et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015 Mar 12;519(7542):242–246. doi: 10.1038/nature14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee M.W., Odegaard J.I., Mukundan L., et al. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell. 2015 Jan 15;160(1):74–87. doi: 10.1016/j.cell.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang G.X., Zhao X.Y., Meng Z.X., et al. The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuating hepatic lipogenesis. Nat Med. 2014 Dec;20(12):1436. doi: 10.1038/nm.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Blondin D.P., Tingelstad H.C., Noll C., et al. Dietary fatty acid metabolism of brown adipose tissue in cold-acclimated men. Nat Commun [Internet] 2017 Jan 30;8 doi: 10.1038/ncomms14146. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5290270/ 14146.1-9. [cited 2023 Feb 22]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ahmed B.A., Ong F.J., Barra N.G., et al. Lower brown adipose tissue activity is associated with non-alcoholic fatty liver disease but not changes in the gut microbiota. Cell Rep Med. 2021 Sep 14;2(9) doi: 10.1016/j.xcrm.2021.100397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wibmer A.G., Becher T., Eljalby M., et al. Brown adipose tissue is associated with healthier body fat distribution and metabolic benefits independent of regional adiposity. Cel Rep Med. 2021 Jul 7;2(7) doi: 10.1016/j.xcrm.2021.100332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gonzalez-Polo V., Pucci-Molineris M., Cervera V., et al. Group 2 innate lymphoid cells exhibit progressively higher levels of activation during worsening of liver fibrosis. Ann Hepatol. 2019 Mar 1;18(2):366–372. doi: 10.1016/j.aohep.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 77.Cella M., Fuchs A., Vermi W., et al. A human NK cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009 Feb 5;457(7230):722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cupedo T., Crellin N.K., Papazian N., et al. Human fetal lymphoid tissue–inducer cells are interleukin 17–producing precursors to RORC+ CD127+ natural killer–like cells. Nat Immunol. 2009 Jan;10(1):66–74. doi: 10.1038/ni.1668. [DOI] [PubMed] [Google Scholar]
- 79.Yang L., Zhang Y., Wang L., et al. Amelioration of high fat diet induced liver lipogenesis and hepatic steatosis by interleukin-22. J Hepatol. 2010 Aug 1;53(2):339–347. doi: 10.1016/j.jhep.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 80.Wang X., Ota N., Manzanillo P., et al. Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature. 2014 Oct;514(7521):237–241. doi: 10.1038/nature13564. [DOI] [PubMed] [Google Scholar]
- 81.Hamaguchi M., Okamura T., Fukuda T., et al. Group 3 innate lymphoid cells protect steatohepatitis from high-fat diet induced toxicity. Front Immunol. 2021 Mar 15;12 doi: 10.3389/fimmu.2021.648754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hwang S., He Y., Xiang X., et al. Interleukin-22 ameliorates neutrophil-driven nonalcoholic steatohepatitis through multiple targets. Hepatol Baltim Md. 2020 Aug;72(2):412–429. doi: 10.1002/hep.31031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hendrikx T., Duan Y., Wang Y., et al. Bacteria engineered to produce IL-22 in intestine induce expression of REG3G to reduce ethanol-induced liver disease in mice. Gut. 2019 Aug 1;68(8):1504–1515. doi: 10.1136/gutjnl-2018-317232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pellicoro A., Ramachandran P., Iredale J.P., et al. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol. 2014 Mar;14(3):181–194. doi: 10.1038/nri3623. [DOI] [PubMed] [Google Scholar]
- 85.Gur C., Doron S., Kfir-Erenfeld S., et al. NKp46-mediated killing of human and mouse hepatic stellate cells attenuates liver fibrosis. Gut. 2012 Jun 1;61(6):885–893. doi: 10.1136/gutjnl-2011-301400. [DOI] [PubMed] [Google Scholar]
- 86.Radaeva S., Sun R., Jaruga B., et al. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor–related apoptosis-inducing ligand–dependent manners. Gastroenterology. 2006 Feb 1;130(2):435–452. doi: 10.1053/j.gastro.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 87.Jeong W.I., Park O., Radaeva S., et al. STAT1 inhibits liver fibrosis in mice by inhibiting stellate cell proliferation and stimulating NK cell cytotoxicity. Hepatology. 2006;44(6):1441–1451. doi: 10.1002/hep.21419. [DOI] [PubMed] [Google Scholar]
- 88.Fan Y., Zhang W., Wei H., et al. Hepatic NK cells attenuate fibrosis progression of non-alcoholic steatohepatitis in dependent of CXCL10-mediated recruitment. Liver Int. 2020;40(3):598–608. doi: 10.1111/liv.14307. [DOI] [PubMed] [Google Scholar]
- 89.Muhanna N., Tair L.A., Doron S., et al. Amelioration of hepatic fibrosis by NK cell activation. Gut. 2011 Jan 1;60(1):90–98. doi: 10.1136/gut.2010.211136. [DOI] [PubMed] [Google Scholar]
- 90.Cai X., Wang J., Wang J., et al. Intercellular crosstalk of hepatic stellate cells in liver fibrosis: new insights into therapy. Pharmacol Res. 2020 May 1;155 doi: 10.1016/j.phrs.2020.104720. [DOI] [PubMed] [Google Scholar]
- 91.Vivier E., Tomasello E., Baratin M., et al. Functions of natural killer cells. Nat Immunol. 2008 May;9(5):503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 92.Tosello-Trampont A., Surette F.A., Ewald S.E., et al. Immunoregulatory role of NK cells in tissue inflammation and regeneration. Front Immunol. 2017 Mar 20;8:301. doi: 10.3389/fimmu.2017.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Luci C., Vieira E., Perchet T., et al. Natural killer cells and type 1 innate lymphoid cells are new actors in non-alcoholic fatty liver disease. Front Immunol. 2019 May 28;10:1192. doi: 10.3389/fimmu.2019.01192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gao Y., Liu Y., Yang M., et al. IL-33 treatment attenuated diet-induced hepatic steatosis but aggravated hepatic fibrosis. Oncotarget. 2016 Jun 7;7(23):33649–33661. doi: 10.18632/oncotarget.9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McHedlidze T., Waldner M., Zopf S., et al. Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity. 2013 Aug 22;39(2):357–371. doi: 10.1016/j.immuni.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kong X., Feng D., Wang H., et al. Interleukin-22 induces hepatic stellate cell senescence and restricts liver fibrosis. Hepatol Baltim Md. 2012 Sep;56(3):1150–1159. doi: 10.1002/hep.25744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang S., Li J., Wu S., et al. Type 3 innate lymphoid cell: a new player in liver fibrosis progression. Clin Sci. 2018 Dec 13;132(24):2565–2582. doi: 10.1042/CS20180482. [DOI] [PubMed] [Google Scholar]
- 98.Raabe J., Kaiser K.M., ToVinh M., et al. Identification and characterization of a hepatic IL-13–producing ILC3-like population potentially involved in liver fibrosis. Hepatology. 2023 Sep;78(3):787. doi: 10.1097/HEP.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 99.Jacquelot N., Seillet C., Vivier E., et al. Innate lymphoid cells and cancer. Nat Immunol. 2022 Mar;23(3):371–379. doi: 10.1038/s41590-022-01127-z. [DOI] [PubMed] [Google Scholar]
- 100.Chiossone L., Dumas P.Y., Vienne M., et al. Natural killer cells and other innate lymphoid cells in cancer. Nat Rev Immunol. 2018 Nov;18(11):671–688. doi: 10.1038/s41577-018-0061-z. [DOI] [PubMed] [Google Scholar]
- 101.Cai L., Zhang Z., Zhou L., et al. Functional impairment in circulating and intrahepatic NK cells and relative mechanism in hepatocellular carcinoma patients. Clin Immunol. 2008 Dec 1;129(3):428–437. doi: 10.1016/j.clim.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 102.Mantovani S., Oliviero B., Lombardi A., et al. Deficient natural killer cell NKp30-mediated function and altered NCR3 splice variants in hepatocellular carcinoma. Hepatology. 2019;69(3):1165–1179. doi: 10.1002/hep.30235. [DOI] [PubMed] [Google Scholar]
- 103.Wu Y., Kuang D.M., Pan W.D., et al. Monocyte/macrophage-elicited natural killer cell dysfunction in hepatocellular carcinoma is mediated by CD48/2B4 interactions. Hepatology. 2013;57(3):1107–1116. doi: 10.1002/hep.26192. [DOI] [PubMed] [Google Scholar]
- 104.Hoechst B., Voigtlaender T., Ormandy L., et al. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatol Baltim Md. 2009 Sep;50(3):799–807. doi: 10.1002/hep.23054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li T., Yang Y., Hua X., et al. Hepatocellular carcinoma-associated fibroblasts trigger NK cell dysfunction via PGE2 and Ido. Cancer Lett. 2012 May 28;318(2):154–161. doi: 10.1016/j.canlet.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 106.Zhang Q.F., Yin W.W., Xia Y., et al. Liver-infiltrating CD11b−CD27− NK subsets account for NK-cell dysfunction in patients with hepatocellular carcinoma and are associated with tumor progression. Cell Mol Immunol. 2017 Oct;14(10):819–829. doi: 10.1038/cmi.2016.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yu W., He J., Wang F., et al. NR4A1 mediates NK-cell dysfunction in hepatocellular carcinoma via the IFN-γ/p-STAT1/IRF1 pathway. Immunology. 2023 May;169(1):69–82. doi: 10.1111/imm.13611. [DOI] [PubMed] [Google Scholar]
- 108.Müller N.C., Romagnani C. To kill or not to kill – the role of the tumor microenvironment in shaping group 1 ILC functions. Semin Immunol. 2022 Nov;61–64 doi: 10.1016/j.smim.2022.101670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dadi S., Chhangawala S., Whitlock B.M., et al. Cancer immunosurveillance by tissue-resident innate lymphoid cells and innate-like T cells. Cell. 2016 Jan 28;164(3):365–377. doi: 10.1016/j.cell.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ducimetière L., Lucchiari G., Litscher G., et al. Conventional NK cells and tissue-resident ILC1s join forces to control liver metastasis. Proc Natl Acad Sci U S A. 2021 Jul 6;118(27) doi: 10.1073/pnas.2026271118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gao Y., Souza-Fonseca-Guimaraes F., Bald T., et al. Tumor immunoevasion by the conversion of effector NK cells into type 1 innate lymphoid cells. Nat Immunol. 2017 Sep;18(9):1004–1015. doi: 10.1038/ni.3800. [DOI] [PubMed] [Google Scholar]
- 112.Dituri F., Mancarella S., Cigliano A., et al. TGF-β as multifaceted orchestrator in HCC progression: signaling, EMT, immune microenvironment, and novel therapeutic perspectives. Semin Liver Dis. 2019 Feb;39(1):53–69. doi: 10.1055/s-0038-1676121. [DOI] [PubMed] [Google Scholar]
- 113.Heinrich B., Gertz E.M., Schäffer A.A., et al. The tumor microenvironment shapes innate lymphoid cells in patients with hepatocellular carcinoma. Gut. 2022 Jun;71(6):1161–1175. doi: 10.1136/gutjnl-2021-325288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xu X., Ye L., Zhang Q., et al. Group-2 innate lymphoid cells promote HCC progression through CXCL2-neutrophil-induced immunosuppression. Hepatology. 2021;74(5):2526–2543. doi: 10.1002/hep.31855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhao J., Chen X., Herjan T., et al. The role of interleukin-17 in tumor development and progression. J Exp Med. 2019 Nov 14;217(1) doi: 10.1084/jem.20190297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu Y., Song Y., Lin D., et al. NCR− group 3 innate lymphoid cells orchestrate IL-23/IL-17 axis to promote hepatocellular carcinoma development. EBioMedicine. 2019 Mar 1;41:333–344. doi: 10.1016/j.ebiom.2019.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jiang R., Tan Z., Deng L., et al. Interleukin-22 promotes human hepatocellular carcinoma by activation of STAT3. Hepatology. 2011;54(3):900–909. doi: 10.1002/hep.24486. [DOI] [PubMed] [Google Scholar]
- 118.Park O., Wang H., Weng H., et al. In vivo consequences of liver-specific interleukin-22 expression: implications for human liver disease progression. Hepatol Baltim Md. 2011 Jul;54(1):252–261. doi: 10.1002/hep.24339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tacke F., Puengel T., Loomba R., et al. An integrated view of anti-inflammatory and antifibrotic targets for the treatment of NASH. J Hepatol. 2023 Aug 1;79(2):552–566. doi: 10.1016/j.jhep.2023.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chiossone L., Vivier E. Bringing natural killer cells to the clinic. J Exp Med. 2022 Sep 6;219(10) doi: 10.1084/jem.20220830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Alnaggar M., Lin M., Mesmar A., et al. Allogenic natural killer cell immunotherapy combined with irreversible electroporation for stage IV hepatocellular carcinoma: survival outcome. Cell Physiol Biochem. 2018 Aug 9;48(5):1882–1893. doi: 10.1159/000492509. [DOI] [PubMed] [Google Scholar]
- 122.Lin M., Liang S., Wang X., et al. Cryoablation combined with allogenic natural killer cell immunotherapy improves the curative effect in patients with advanced hepatocellular cancer. Oncotarget. 2017 May 11;8(47):81967–81977. doi: 10.18632/oncotarget.17804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yu M., Luo H., Fan M., et al. Development of GPC3-specific chimeric antigen receptor-engineered natural killer cells for the treatment of hepatocellular carcinoma. Mol Ther. 2018 Feb 7;26(2):366–378. doi: 10.1016/j.ymthe.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tseng H., Xiong W., Badeti S., et al. Efficacy of anti-CD147 chimeric antigen receptors targeting hepatocellular carcinoma. Nat Commun. 2020 Sep 23;11(1):4810. doi: 10.1038/s41467-020-18444-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu B., Liu Z.Z., Zhou M.L., et al. Development of c-MET-specific chimeric antigen receptor-engineered natural killer cells with cytotoxic effects on human liver cancer HepG2 cells. Mol Med Rep. 2019 Sep;20(3):2823–2831. doi: 10.3892/mmr.2019.10529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang Z., Guo L., Song Y., et al. Augmented anti-tumor activity of NK-92 cells expressing chimeric receptors of TGF-βR II and NKG2D. Cancer Immunol Immunother. 2017 Apr 1;66(4):537–548. doi: 10.1007/s00262-017-1959-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rosenberg S.A., Lotze M.T., Muul L.M., et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985 Dec 5;313(23):1485–1492. doi: 10.1056/NEJM198512053132327. [DOI] [PubMed] [Google Scholar]
- 128.Hsu E.J., Cao X., Moon B., et al. A cytokine receptor-masked IL2 prodrug selectively activates tumor-infiltrating lymphocytes for potent antitumor therapy. Nat Commun. 2021 May 13;12(1):2768. doi: 10.1038/s41467-021-22980-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Easom N.J.W., Stegmann K.A., Swadling L., et al. IL-15 overcomes hepatocellular carcinoma-induced NK cell dysfunction. Front Immunol. 2018 May 9;9:1009. doi: 10.3389/fimmu.2018.01009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Demaria O., Gauthier L., Debroas G., et al. Natural killer cell engagers in cancer immunotherapy: next generation of immuno-oncology treatments. Eur J Immunol. 2021;51(8):1934–1942. doi: 10.1002/eji.202048953. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.