Abstract

The stable and efficient photochromic and photoswitchable molecular systems designed from spirooxazines are of increasing scientific and practical interest because of their present and future applications in advanced technologies. Among these compounds, chelating spironaphthoxazines have received widespread attention due to their efficient optical response after complexation with some metal ions being of biomedical interest and environmental importance, as well as their good cycle performance and high reliability, especially by metal ion sensing. In this mini-review, we summarize our results in the design of novel photoswitchable chelating spironaphthoxazines with specific substituents in their naphthoxazine or indoline ring systems in view of recent progress in the development of such molecular systems and their applications as metal ion sensors. The design, synthesis methods, and photoresponse of such spirooxazine derivatives relevant to their applications, as well as quantum-chemical calculations for these compounds, are presented. Examples of various design concepts are discussed, such as sulfobutyl, hydroxyl, benzothiazolyl, or ester and carboxylic acid as substituents in the chelating spironaphthoxazine molecules. Further developments and improvements of this interesting and promising kind of molecular photoswitches are outlined.
1. Introduction
Spirooxazines represent an important class of organic photochromic compounds that have attracted considerable attention from researchers due to their remarkable properties, such as photofatigue resistance, strong photocoloration, and fast thermal relaxation.1 Accordingly, they have application in advanced technologies, such as, e.g., photocontrollable light filters and optical devices, photochromic plastics and liquid crystals, photochromic substances in tunable lenses, dynamic biosensors and bioelectronical materials, and smart materials for memory devices.2 Because their molecular architecture can be easily tuned (as with spiropyranes—the other large family of photochromic spiro compounds and advanced photoswitchable materials3), spirooxazines have been used to develop molecular switches and logic gates as well as to prepare optical probes, photocontrollable smart receptors, and multifunctional stimuli-responsive polymers.4 The application area of these interesting photochromic compounds includes detection of toxic substances, especially excess heavy metal ions, which can provide safety measures to solve environmental problems.5 Photochromic molecular switches engineered with spirooxazines containing suitable chromogenic or fluorogenic units are widely applied to achieve a specific response of high-performance optical devices.6
The use of spirooxazine derivatives in optical information storage devices is also attractive. This is however full of challenges because it is difficult to make the photoinduced merocyanine (MC) form of spirooxazine molecules stable enough to retain the optically recorded data over a relatively long period of time. Therefore, further improvement of these compounds and their derivatives toward more stable high-density optical storage materials is necessary. One way to achieve this is to try to trap the photomerocyanine in a metal-chelated form. This approach has been reported for spiropyranes4b,7 and in single cases for spirooxazines.8,9 Metal ion complexation has also been used to exploit this class of compounds for the development of photochromic chemosensors that allow colorimetric or fluorimetric detection of certain analytes.1f,1d,4b,7b,7d−7f Combining a photochromic moiety with an organometallic or coordination compound provides new properties arising from the combination of redox, optical, and magnetic properties of the metal complexes with the photochromic reaction. Metal complexes of photosensitive ligands are an interesting alternative to all-organic photochromic compounds. That is why the spirooxazines are extensively developed and investigated. The continuous search of photoswitchable materials with suitable photochromic, molecular switching, and light-emissive characteristics and enhanced sensing feasibility has led to the production of various spirooxazine derivatives and materials based on them, attractive for diverse applications.10
In the present mini-review, we give a brief description of the synthesis and the effect of solvent and molecular structure on the absorption properties and kinetics of thermal bleaching of selected examples of spironaphthoxazines with suitable substituents in both the naphthoxazine and indoline ring systems for complexation9c,9d,11 (Table 1). In addition, we discuss solvent and structural effects on the absorption properties and thermal bleaching kinetics of photomerocyanines and the possibility of stabilizing the colored form of MC against thermal reversion by complexation with selected metal ions in suitable solvents.9a,9b Comparison with other spironaphthoxazines is given along with the research progress on these interesting photochromic compounds. Their main application as well as the research challenges and opportunities in this scientific area are also pointed out.
Table 1. Base Spironaphthoxazine (On the Top) and the Substituted Ones That Are in the Focus of the Work.

2. Spironaphthoxazines: Design and Synthesis
Methods for the synthesis and modification of spiropyran derivatives have been well reviewed so far,1e,3b,12 but considerably less for spirooxazines.1c,12d The most commonly used method for the synthesis of spironaphthoxazines is the condensation of an alkylidene heterocycle with o-nitrosonaphthols in polar organic solvents, such as methanol, acetone, or chlorinated lower aliphatic hydrocarbons. By this method, the parent unsubstituted spiroindolinonaphthoxazine 1,3,3-trimethylspiro[indoline-2,3′-[3H]naphth[2,1-b][1,4]oxazine] (the compound 0, Scheme 1) was obtained.1a,1c,1d,11a,13 Alkylidene derivatives (a–c) are generally prepared in situ by the reactions of bases (most often triethylamine) with the corresponding quaternary salts in ethanol.
Scheme 1. Synthesis of Spironaphthoxazines 0–2.
The design and synthesis of new spironaphthoxazine derivatives are closely related to providing good photochromic conversion efficiency, taking into account the relationship between molecular structure and spectral and kinetic properties of these compounds. Some remarkable properties of spirooxazines, such as their high photofatigue resistance, excellent photostability, the lifetime of the colored MC form, and the interaction of this form with specific analytes, define the possibilities of their application. One way to control the color switching and coloration/bleaching rates of a photochromic system is the possibility of complexation with metal ions. This can be achieved by modification of the two-ring system, the naphthoxazine moiety, and the indoline ring system with appropriate substituents.4b,8d,9c,9d,10b,10c,11a
Tamaki and Ickimura8a reported the first synthesis of substituted spironaphthoxazines containing suitable groups (−SO3 or −OCH3) near the pyranyl oxygen that are capable of acting as chelating agents, leading to stabilization of the open merocyanine form. This approach has been implemented by Zhou et al. for the synthesis of 5′-carbomethoxy spirooxazine derivatives,14 whose MC does form complexes with Ni, Co, and Cu.
By introducing crown fragments, it is also possible to modify the spirooxazine molecules, which allows for the formation of complexes with metal ions. The structure and position of the crown-containing fragments are important since they affect the stability of the open merocyanine form.15a First, a spironaphthoxazine derivative incorporating a monoaza-12-crown-4 moiety at the 5′-position by condensation of 3-(monoaza-12-crown-4)ylmethyl-l-nitroso-2-naphthol with 1,3,3-trimethylindolenine was synthesized by Kimura and co-workers.8b,8c Korolev et al.8e have synthesized spironaphthoxazines by use of crown-containing dihydroisoquinolines. Later, other approaches for the synthesis of similar crown-containing spirooxazines were developed by the Russian and French research groups.15b,15d
Associated with new practical perspectives, efforts to synthesize novel spirooxazine molecular systems continue to this day. In particular, spirooxazine derivatives with additional chelating sites on the nitrogen atom of the indoline group as phenethyl substituents were recently designed by Pattaweepaiboon and co-workers for colorimetric detection of Cu2+ and Fe3+ ions.10d
The mini-review presented here is focused on two types of chelating spironaphthoxazines, ones having suitable complexable substituents in the naphthoxazine part of the molecule and the others bearing different substituents in the indoline ring system.9c,9d,11a The spironaphthoxazines containing a hydroxyl substituent in the naphthoxazine moiety (compounds 1 and 2) were synthesized by condensation of 1,3,3-trimethyl-2-methylene indoline (a) and 1-butyl-3,3-dimethyl-2-methylene indoline (b) with the corresponding o-nitrosonaphthols (c, d) (Scheme 1).11a
The spironaphthoxazines containing a benzothiazolyl substituent in the naphthoxazine moiety (compounds 3–6) were prepared according to Scheme 2 by reaction of 1-nitroso-2-hydroxy-3-(benzothiazol-2-yl)naphthalene (d), with 1,3,3-trimethyl-2-methylene indoline (e), or 1-butyl-3,3-dimethyl-2-methylene indoline (f), or the corresponding benz[c]indolium perchlorate compounds 1,3,3-trimethyl-benz[c]indolium perchlorates (g) or 1-butyl-3,3-dimethyl benz[c]indolium perchlorates (h) (Scheme 2).11a The synthesis of spirooxazine with ester 7 or carboxylic acid 8 was carried out in a four to five step sequence as depicted in Scheme 2. The compound 7 is obtained after condensation of 1-nitroso-2-hydroxy-3-(benzothiazol-2-yl)naphthalene with indolium bromide salt obtained in N-alkylation of the 2,3,3-trimethyl-3H-indole compound with methyl bromobutyrate. The compound 8 is obtained after hydrolysis of 7 with sodium hydroxide.9c Fedorov and co-workers9d obtained the spironaphthoxazine 9 bearing a sulfobutyl substituent on the indoline part of the molecule.
Scheme 2. Synthesis of Spironaphthoxazines 3–9.
3. Phototransformations of Spironaphthoxazines
The spirooxazines consist of two heterocyclic nearly planar moieties (indoline and naphthoxazine) joined by a tetrahedral spirocarbon that prevents conjugation of the π-electron systems of the two moieties. The photochromic reaction of these compounds is due to photoisomerization, reversible heterolytic cleavage, and rebinding of the Cspiro–O under UV irradiation and subsequent rotation around the C–C bond to give merocyanine (MC) (open form) (Scheme 3). When spirooxazines are irradiated with UV light, a long-wavelength spectral band in the visible spectrum appears in their absorption spectra, signifying the MC formation. The backward isomerization of MC → SP occurs by light in the same long-wavelength spectral range in the visible or spontaneously via thermal relaxation in the dark1a−1c,1e,1f,11b (designated as “Δ” in Scheme 3).
Scheme 3. Phototransformation of Spironaphthoxazines.
In order to understand the structural properties of spirooxazines and the mechanisms of their transformations as well as to establish the relationship between structure and photochemical properties, the information obtained from quantum-chemical calculations is important.1f,16 By quantum-chemical calculations, one can find optimized equilibrium structures of SP and MC forms as a necessary step to determine the transition state (TS) along the transformation of these two isomers. The obtained results can provide an opportunity to establish mechanistic features of the thermal opening reactions of the considered spirooxazines. In particular, for spirooxazine compound 9 (Table 1), quantum-chemical calculations have been performed by density functional theory (DFT).9d The SP form (the closed form) of this photochromic compound exhibits four fragment types: two cycled wings (three hexagonal connected rings C12H7NO and two hexagonal connected rings C10H6), one tail (C4H9NO3S), and one gem-dimethyl group C3H6 (two CH3 groups connected through a carbon atom) (Figure 1). These fragments are joined through a pentagonal ring consisting of one nitrogen atom and four carbon atoms (C4N). The isomer MC (open form) of the molecule of compound 9 is formed by opening of the ring—the same ring that connects the wing C12H7NO to the central pentagonal ring C4N of the isomer SP. The C–CH–N–C chain of the “broken” ring allows the rotation of the two remaining hexagonal rings. The two-connected hexagonal rings −C10H6 remain intact after isomerization. Therefore, we refer to the two wings C12H7NO and C10H6 as “rigid” and “flexible” wings, respectively.
Figure 1.
Structural regions of the SP and MC forms of compound 9. The C–O bond of the isomer SP that is broken during the transformation to MC is indicated by a green rectangle. The gray, red, blue, yellow, and white spheres represent the carbon, oxygen, nitrogen, sulfur, and hydrogen atoms, respectively.
In more detail, DFT calculations for compound 9 were performed to characterize the isolated molecules SP and MC in their optimized geometries and to investigate the transformation path of SP to MC, identifying the corresponding transition state TS by the nudged elastic band (NEB) method.17a The DFT calculations were carried out using the exchange-correlation functional B3LYP17b and the basis set 6-31+G(d,p) in the spin-unrestricted approach, with dispersion correction.17c Calculations by larger basis sets did not significantly affect the optimized geometry of the conformers considered here, but the basis set 6-31+G(d,p) allowed us to perform various calculations with reasonable computational effort. The calculations were performed using the quantum chemistry code ORCA.17d The optimized geometries of the isolated SP and MC isomers are very similar to those obtained by semiempiric HFT calculations, with parametrization PM7.9d The effect of water as a solvent was considered by means of the conductor-like polarizable continuum model (CPCM).17e In this case, the solvent is considered a polarizable continuum (Figure 2).
Figure 2.
Schematic drawings of spirooxazine compound 9 in water surroundings: (a) SP form and (b) MC form.
For compound 9, chosen as an example, the values of the free energies at the temperature of 0 K (computed as the total energy plus the zero point energy) of the two isomers indicate that the isomer SP is more stable than the MC form, with 0.282 eV in vacuum and 0.082 eV in water.17f The NEB method was used to find the minimum energy pathway between the two geometries of the SP and MC forms and to identify the geometry of the TS form of compound 9 (Figure 3). The TS configuration corresponds to the breaking of the hexagonal ring by bending. The just-opened wing is twisted so that the flexible wing is almost coplanar with the rigid wing. The tail twists during the transformation from SP to MC. The activation energy and the reaction energy for the transformation SP → MC are 1.129 and 0.262 eV, respectively.17f These values are decreased to 0.925 and 0.123 eV,17f respectively (when the effects of water as a solvent are considered in the frame of the CPCM); i.e., the water may facilitate the transformation SP → MC.
Figure 3.

Equilibrium structures of SP and MC forms of compound 9 and its transition state TS along the transformation of the two isomers, as predicted by the NEB method.
The analysis of the frontier orbitals, the highest occupied molecular orbital (HOMO), and the lowest unoccupied molecular orbital (LUMO) shows that for the isomer SP of compound 9 (Figure 4) the HOMO is localized in the wing region C10H2, and the LUMO is localized in the wing region C12H7NO. The calculated values of HOMO and LUMO orbital energies reveal that the SP form has a larger band gap than the MC form. The breaking of the ring is likely to be caused by the displacement of the HOMO toward the region C12H7NO, centering on the pentagonal ring C4N, as a result of the transfer of the π-electron from the rigid-wing region C10H6 of the broken wing.
Figure 4.

HOMO and LUMO drawings (for an isovalue of 0.03) for SP and MC isomers of compound 9. The surfaces are drawn by yellow/blue (for HOMO) and green/red (for LUMO), where the negative/positive blobs are represented by light and dark colors. Carbon, oxygen, nitrogen, sulfur, and hydrogen atoms are represented by gray, red, blue, yellow, and white spheres, respectively.
4. Optical Characterization of Spironaphthoxazines in Solutions
4.1. UV–vis Absorption
The modification of photochromic spironaphthoxazines with various substituents results in a significant change in their optical spectroscopy characteristics. Dilute solutions (1 × 10–5–1 × 10–4 M) containing spiro forms are almost colorless or pale yellow since the lowest electronic transition in the molecule occurs in the near UV region.1b,1c,11b,18 The environmental factors, such as temperature, concentration, solvent, etc., have practically no effect on the absorption spectra of the ring-closed spirooxazine, while the ring-opened forms are very sensitive.19
The spironaphthoxazines show significant changes in the position and intensity of their UV–vis absorption bands depending on the solvent polarity. The open spirooxazine ring indicates a positive solvatochromism—as the polarity of the solvent increases, there is a red shift in the MC absorption band,1e,11b,18a,20 indicating that the MC form of spirooxazines takes a keto form. From optical spectroscopy and NMR studies, it has been established that the quinoidal structure occurs in nonpolar solvents and a zwitterionic structure in high-polarity solvents.20c The quinone structure of ring-opened spirooxazines is caused by heteroatoms that participate in electron delocalization. The equilibrium states of the quinoid and zwitterionic forms also depend on the electronic properties of the substituents. When they have electron-withdrawing substituents, the main equilibrium form is the quinoid, but when the substituents are electron-donating, the zwitterion is the main equilibrium form1g,11b (Scheme 4). Minkin has distinguished two mechanisms for solvatocromic behavior of spirooxazines.1e,1f The first one is related to the displacement of equilibrium on going from one solvent to another. This is relevant to the redistribution of the intensities of the absorption bands in the spectrum of a solution containing an equilibrium mixture of the isomers or even the occurrence of new bands if only one of the isomers is present in the solution. The second mechanism is related to differences in solute–solvent interactions in solvents of different polarity.
Scheme 4. Phototransformation of Substituted Spironaphthoxazines.
The absorption spectra of the spirooxazines vary greatly according to the substituents in both the indoline and spirooxazine parts of the molecule and on the solvents due to the conjugation of both fragments occurring after the ring opening.1c,1g,9c,9d,11b,21 In particular, the absorption spectral bands close to 320 and 340 nm observed for the parent/unsubstituted spironaphthoxazine 0 in hexane are associated with the oxazine part of the molecule in its closed form.2a As reported by Minkovska et al.,11b the absorbance spectra of the substituted compounds are substantially different. The −OH derivatives 1 and 2 exhibit a broad band of absorption at about 345 nm, while benzothiazolyl-substituted compounds 3–6 have a large number of narrow absorption bands in the range of 270–340 nm formed by electronic and vibronic transitions and localized on the naphthoxazine fragment of the molecule. The UV–vis absorption spectrum of spirooxazine 9 exhibits a set of absorption bands located in the near UV region attributable to the spiro form (Figure 5). For a solution of this compound in water, the same set of absorption bands of spiro form take place as well as a more pronounced long-wavelength absorption band with a maximum at 627 nm belonging to the MC form. The presence of absorption bands belonging to both SP and MC forms is due to the thermal equilibrium between them (Scheme 4). In acetonitrile, the equilibrium between SP and MC is shifted to SP, and the absorption band with a maximum at 627 nm is almost absent (A627 nm < 0.01) (Figure 5a). These results are consistent with the known fact that the thermal equilibrium between the spiro and merocyanine forms is affected by the solvent polarity, such that polar solvents promote the formation of the colored merocyanine form at room temperature in the dark.1c,1e,11b This phenomenon is most pronounced in polar protonic solvents, such as ethanol, methanol, propanol, and water.19a In addition to its polar character, ethanol shows a tendency to form a hydrogen bond with the open form in which there is a partial negative charge on the oxygen atom.
Figure 5.

UV–vis absorption spectra of solutions of compound 9: (a) in acetonitrile, as well as in water, at a concentration of 5 × 10–5 M and (b) in ethanol, methanol, and ethylene glycol, at a concentration of 1 × 10–4 M. Temperature was 22 °C in all cases.
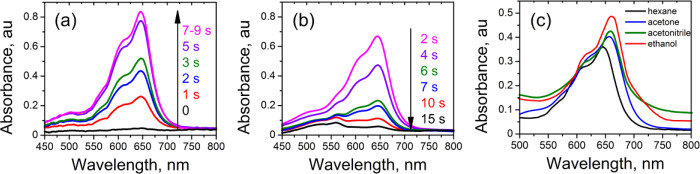
The absorption spectra corresponding to photomerocyanines 3–6 are apparently not single absorptions. They exhibit a shoulder on the shorter wavelength side of the main absorption band (Figure 6) like the spectra of other photomerocyanines of this type.1c,1f,1g,18a,19aFigure 6(a,b) illustrates the change in this absorbance following the SP → MC → SP transformation of compound 6. An example of the effect of the solvent on the absorption spectrum of the MC form of this spironaphthoxazine is shown in Figure 6(c).
Figure 6.
Absorption spectra in the visible range, as recorded for 1 × 10–5 M hexane solution of compound 6 upon continuous UV irradiation with a duration of 0, 1, 2, 3, 5, and 7–9 s (a) and on keeping the irradiated sample for 2, 4, 6, 7, 10, and 15 s in the dark (b). 0 means the start of the measurement. Reproduced with permission from ref (11b). Copyright 2004 Elsevier. (c) Absorption spectra of the MC form of spironaphthoxazine 6 in different solvents (at a concentration of 1 × 10–5 M).
By UV irradiation, the absorption spectra of the photomerocyanines containing different substituents in the oxazine part of molecules 1–6 in solvents of different polarity are characterized with an absorption band in the range of 560–660 nm. It is seen from Figure 7(a) that the addition of −OH at the 5′ position of the naphthoxazine moiety has a minor effect on the absorption band of MC forms of compounds 1 and 2, as compared to the parent compound 0. The effect of the benzothiazolyl substituent at the same position is a bathochromic shift of 30–65 nm in the spectrum of the MC derived from compound 3. The replacement of benzene in the indoline moiety with naphthalene leads to an additional shift of the absorption band of the MC forms of compounds 5 and 6 by 15–20 nm toward the longer wavelengths (Figure 7a).11b The position of the absorption band and the observed increase in its intensity correspond to the photochemical cleavage of the C–O spiro bond and the conversion of the closed spiro form into photomerocyanine.
Figure 7.
(a) Experimental values of λmax of the absorption band of a photomerocyanine form of compounds 0–6 in solvents with different polarity and (b) the colorability measured for the same compounds (temperature = 20 °C; concentration: 1 × 10–5 M). The solvents are in ascending order of their polarity (from hexane to ethanol).
UV irradiation of the compounds with selected substituents on the indoline part of molecules 7 and 8 in acetone yielded the photomerocyanine with λmax = 640 nm, while acetonitrile solutions of 9 led to the appearance of a broad absorption band in the visible region with λmax = 625 nm, which faded thermally after the irradiation was turned off. The bathochromic shift at higher solvent polarity (positive solvatochromism) (Figure 7a) indicates that these solvents better stabilize the excited state of the colored form than its ground state.11b,22 This is especially important for practical applications of spirooxazines.
As a characteristic quantity of the phototranformation (photoinduced reaction) SP → MC of the considered photochromic compounds, one can use the change in the intensity of the color of the MC form, defined by the term “colorability” (ΦC).1b,1c,9b,9d,11b,19a,23 As an example, Figure 7(b) presents ΦC for the series of synthesized spiro compounds (1–6) with substituents on the naphthoxazine part. Generally, ΦC is a combined effect of both substituents and the solvent. Their proper choice may be a way to optimize the photochromic properties.
4.2. Thermal Fading Kinetics of Photomerocyanines in Solutions
Controlling the bleaching rate of spirooxazines may open the possibility of using them for various applications. The thermal equilibrium between the colored and colorless forms and the kinetics of thermal bleaching of the photomerocyanines are affected by the solvent and substituents and are important for the application of spirooxazines.1g,9c,9d,11b,11c,18a,19a,23a Kinetic runs (absorbance strength vs time) followed immediately after irradiation of spirooxazine compounds in solutions can provide useful mechanistic information about the thermal breaking and reforming of the spiro bond. The influence of molecular structure on the recycling rate cannot be considered independently of the influence of solvent polarity. As an example, Figure 8 presents the photoresponse parameters (the rate constant (k) and the lifetime τMC–SP = 1/k) of the MC form of spironaphthoxazine compounds 0–6 in solvents of different polarity. The nonpolar aprotic solvent hexane neutralizes the effect of the substituents, and τMC–SP in hexane of all substituted compounds is very similar. The cyclization process in hexane is rapid because the solvent cannot stabilize the polar MC form. In polar solvents, the influence of substituents on the MC → SP is well observed. In solvents with a middle polarity like acetone and acetonitrile, relatively higher stabilization of the polar merocyanine compared to the nonpolar spiro compound is the reason for slower cyclization. As estimated by Minkovska et al.,11b this effect is quite pronounced in ethanol, where the decolorization reaction is up to 30 times slower than in hexane, most likely due to a combined stabilization effect of the polar merocyanine and its specific solute–solvent interaction such as hydrogen bonding with ethanol. This phenomenon also occurs in other polar solvents that readily form hydrogen bonds, such as methanol, propanol, and water.11c,19c,20b,24
Figure 8.
Rate constants k of thermal ring closure (a) as well as the lifetimes τMC–SP (b) of photomerocyanines derived from compounds 0–6 in solvents of different polarity. 1 × 10–5 M solutions, temperature 20 °C.
The solvent polarity affects the thermal equilibrium between the closed and opened forms. Polar solvents promote the formation of the colored forms at room temperature in the absence of light.18a,19a,23a The nature of the solvent has a significant effect on the rate of cyclization of the MC forms. The rate of closing of the ring after UV irradiation is directly affected by the solvent polarity. The rate of MC → SP switching has been observed to increase with decreasing solvent polarity within molecular solvents.18a,19a,23a This is due to solvent–solute interactions such as hydrogen bonding, dipole–dipole forces, polarizability of the molecule, and dipole moments, which all contribute to the stabilization of the merocyanine form.18a,19a,19c,20b,23a,24a
The rate of cyclization of photomerocyanine derived from −OH derivative 1 does not show an appreciable dependence on solvent polarity. The same behavior is observed for compound 2 and the parent unsubstituted compound 0 (Figure 8). On the contrary, the solvent nature has a significant effect on the rate of cyclization of photomerocyanine derived from benzothiazolyl-substituted compounds (3–6). The τMC–SP values increase depending on both the structural characteristics and the polarity of the solvent. As explained in Minkovska et al.,11b the combination of the effect of the benzothiazolyl substituent in the naphthoxazine fragment and the presence of a naphthalene ring fused to the benzene ring in the indoline hinders the free rotation during the recycling of the molecule and significantly extends the lifetime of its open colored form. Thus, by changing the solvent and modifying the compounds with substituents in the indoline and oxazine parts of the molecule, it was possible to control the lifetime of τMC–SP of the photomerocyanine form.
The effect of ionic liquids on the rate of cyclization of compound 3 (with the benzothiazolyl substituent) has been investigated by Coleman and co-workers.11c They observed a slower thermal relaxation in ionic liquids than in an ethanol solution of compound 3, which has similar polarity. This is an indication of a higher degree of interaction between the ions in the ionic liquid and the zwitterionic MC isomer of compound 3, leading to an increase in the lifetime of the latter.
4.3. Fluorescence Properties of Spironaphthoxazines
Under UV irradiation in the range of their absorption, the spironaphthoxazine derivatives in solutions can exhibit intense fluorescence in the blue-green region.9d,13,25 As a representative example, Figure 9 shows the fluorescence from ethanol solutions of compound 9 upon continuous broad-band excitation (with UV LEDs). The observed fluorescence spectrum was broad and with no spectral features, even when excited with the narrow line of the nitrogen laser at the wavelength of 337.1 nm (in the nanosecond pulse regime). The fluorescence spectral band was asymmetric, and its peak was at about λmax = 435 nm. Such fluorescence emission is typical for spirooxazines with electron-donating substituents.25a,25b On the other hand, the fluorescence observed from solutions of spirooxazine compounds having electron-accepting groups is located at longer wavelengths (500–600 nm).13,18c,25a,25b,26 In both cases, no fluorescence from the photoinduced MC form was present. In particular, for compound 9, the fluorescence wavelength range (Figure 9) does not overlap the absorption band of the MC form (recall Figure 5). This is of importance for the combined usage of both fluorescence- and absorbance-based detection methods using spirooxazines.
Figure 9.
Fluorescence spectra of 10–4 M ethanol solution of spirooxazine compound 9 excited with continuous broadband UV light centered at various wavelengths (λexc) under identical experimental conditions, except for the impinging UV light intensity. The spectral background recorded under the same experimental conditions with the excitation light and with a rejection long-pass edge optical filter whose transmission cutoff was at 400 nm (but without the sample-cuvette) is also shown (blue). In all cases, the temperature was kept at 22 °C.
Different kinds of photochromic spirooxazine–fluorophore conjugates were developed that utilize the photoinduced and photocontrollable fluorescence modulation.1f,1g,27 Fluorescent composites and fluorescent all-optical switches have advanced applications in optical sensing and probing technologies, as well as in a variety of optical elements and photonic devices.1f,1g,6c,18c,26,27 In particular, the change of fluorescence emitted from spirooxazines can be used in chemical sensing assays.6d For example, as demonstrated by Minkovska et al.,13 Barachevsky et al.,25a,25b and Sahoo et al.,6f the fluorescence spectra of spironaphthoxazine derivatives in solutions can be significantly changed in the presence of metal ions, such as Fe(II), Cu(II), and Cr(III), and this property can be used for their detection. In addition, Uznanski et al. demonstrated that the oxidation of spirooxazines in the presence of metal ions can result in intensively fluorescent indolinoxazole cations.28
Various photochromic molecular compounds designed with spirooxazines containing suitable chromogenic or fluorogenic units have been applied to achieve an optical response through the MC molecular form of the spirooxazines, suitable for sensitive (and most desirably selective) detection of metal ions. For instance, Huang et al.27 reported the absorption and fluorescence response of spirooxazines in ethanol solutions upon the addition of various metal ions. By excitation with UV or visible light, complexes of spirooxazines and metal ions displayed various fluorescent signals, such as fluorescence enhancement or a shift of the fluorescence spectra. In this case, selective multiplex detection of metal ions, e.g., Al3+, Cd2+, Co2+, Cr3+, Cu2+, Fe2+, Ca2+, Mg2+, Zn2+, Ni2+, and Hg2+, can be performed by analyzing the fluorescence response and its variations due to the coordination of spirooxazine with metal ions.27 Sahoo and Kumar reported a fluorescence-based sensor based on spirooxazine derivatives, for highly sensitive and selective optical detection of Fe3+ in aqueous solutions.29 The fluorescence response of dilute aqueous solutions of spirooxazines is promising for the construction of various chemosensors and fluorometric probing devices, e.g., for environment monitoring. At present, the search and development of selective photochromic fluorometric probes for metal ions are an actual task. Significant advantages of fluorescent detection by use of spirooxazines (upon appropriate excitation) are the extremely high sensitivity and quick response, which are useful for the development of reusable fluorochromic chemodosimeters and chemosensors.
5. Metal Ion Complexation Ability of Spironaphthoxazines
The reversible structural transformations between the isomer forms of spirooxazine molecules upon external stimuli (light, temperature, solvents, chemical compounds, metal ions) and, thereby, the reversible changes of the physicochemical properties of these compounds are the basis of their diverse applications. Most of the practical applications of spirooxazines are through the response of their ring-opened MC structural form. The MC isomer can form complexes with various inorganic particles, biological molecules, and organic chemicals. In particular, one of the main application areas of spirooxazines, namely, the sensing of metal (M) ions, is based on the formation of coordination complexes MC–M.1a−1f,7f,12d,30a,30b
In 1989, the first synthesized substituted spiroooxazine capable of complexing with transition metal ions was reported.8a,24a Tamaki and Ichimura8a found that spironaphthoxazines with suitable coordination substituents (−OCH3, −SO3−) near the O atom of the naphthoxazine moiety are capable of transforming into the colored form upon UV irradiation and chelated to certain metal ions, such as Ni(II), Ca(II), and Pb(II). Khairutdinov and co-workers24a have studied the photochromic behavior of some spirooxazines containing phenanthrene or phenanthroline moieties in the oxazine part and their coordination with the Ru(bpy)22+ metal center. The complexation of phenanthroline-substituted spirooxazine with metal ions leading to the formation of tris(phenanthroline) metal complexes was the object of research interest by Kopelman et al.31 They reported a strong dependence of the photochromic properties of the spirooxazine derivatives on the coordinated metal centers. This synthetic approach allows an easy way to change the photochromic behavior. The chelation reaction of some spironaphthoxazines with −COOCH3 derivatives in the presence of Co(II), Ni(II), Cu(II), and Zn(II) ions was studied by Zhou and co-workers.14 They have found different complexation behaviors of spironaphthoxazines which can form two types of chelates with certain metal ions upon UV irradiation or even in the dark: the former is unstable and decolorizes to a colorless form thermally or photochemically; the latter is stable and exhibits intense fluorescence emission.
The effect of MC–M complexation on the photochromism was established by several spironaphthoxazines synthesized with hydroxyl, benzothiazolyl, ester, carboxylic acid, and sulfobutyl substituents in a naphthoxazine and/or indoline moiety.1f,8d,9 The colorless spiro form of spironaphthoxazine derivatives did not form complexes; however, the MC form induced by the presence of the metal ions did. The ring-opening reaction of spironaphthoxazines in polar solvents can be induced in the dark by addition of metal ions that can coordinate to the chelating atom of the substituent introduced into the molecule of spironaphthoxazine derivatives.8b−8e,9 Accordingly, this leads to an increase in the absorption band, corresponding to the MC form. Regardless of whether the ring-opening reaction is induced by metal ions or by UV irradiation, the coordination of metal ions to the photochromic molecule results in a blue shift of the absorption maximum of the MC form.1f,2e,8d,9 The nature of the substituents on either the indoline or the oxazine fragment has a stronger influence on the position of the MC absorption maximum than the solvent polarity.9 Thus, substituents can provide an efficient method for tuning and designing new photochromic molecules.
From the series 0–9 of the spiroindolinonaphthoxazines considered here, the parent unsubstituted compound 0 (Table 1) does not chelate in the presence of metal ions that cause only an irreversible photodegradation. This suggests that the nitrogen atom at the 1′ position in the naphthoxazine moiety takes no part in chelation.13
In the dark or upon UV light, spironaphthoxazines containing a hydroxyl substituent at the 5′ position in the naphthoxazine moiety, compounds 1 and 2, form complexes with Al(III), Cu(II), and Fe(II) ions,9a while compounds 3–6 containing a benzothiazolyl substituent (Table 1) coordinate to Co(II), Ni(II), and Zn(II) in polar solvents9b (Scheme 5).
Scheme 5. Coordination of Spirooxazines 1–6 with Metal Ions.
The UV light-induced photomerocyanines of compounds 1 or 2 in polar solvents are characterized by an absorption band at 600–620 nm.11b UV irradiation of these compounds in the presence of Cu(II), Fe(II), or Al(III) ions leads to a photoreversible absorption band at 540–600 nm (Figure 10). The intensity of this band depends on the [M(II),(III)]/[SP] concentration ratio.9a Thermal coordination to the metal ion M also occurs, resulting in a complex MC–M that is spectroscopically identical with the photoinduced product MC–M. The intensity of the absorption band corresponding to MC–M obtained in the dark becomes more intense under UV light and returns to its initial level after the UV light is turned off.9a A blue shift of the absorption spectra of photomerocyanines in solution has been found also for benzothiazolyl-substituted spironaphthoxazines 3–6 in addition to Ni(II), Co(II), or Zn(II) (Figure 11a).9b,11b In these cases, the blue shift was found to be between 25 and 45 nm depending on both the substituent and the solvent. The wavelength of the absorption maximum λmax varies moderately depending on metal ion nature (15–45 nm). The time required to re-establish equilibrium is sensitive to the metal ion and is substantially longer than that of noncomplexed merocyanine.9b,11b
Figure 10.
Absorption maxima (λmax) of complexes obtained upon UV irradiation (λirr = 365 nm) of 5 × 10–5 M solutions of compounds 1 and 2 in the presence of 1 equiv of metal ion. The solvents: (a) EtOH/H2O (1:1) and (b) CH3COCH3/H2O (1:1). Temperature was 20 °C in all cases.
Figure 11.
Wavelength (λmax) of the maximum of the absorption bands of MC forms resulting from complexation of compounds 3–8 (a) and 9 (b) with various metal ions, M(II), by the addition of salts M(ClO4)2. In all cases, the temperature = 20 °C, [SP] = [M(ClO4)2] = 5 × 10–5 M.
It has been established that the complexing ability of the merocyanines decreases in the order 3 > 4 > 5 > 6 of the compounds considered here, and the formation constants with metal cations are changed as follows: Ni(II) > Co(II) > Zn(II).9b The complexation is favored by the higher polarity of the solvent, which results in a higher equilibrium concentration of MC.9b Minkovska and co-workers have found that the choice of substituents on the heterocyclic nitrogen has a significant effect on the kinetics of MC → SP thermal relaxation.9 In particular, the addition of Zn(II) ions to acetone solutions of compound 8 (with carboxylic acid) leads to an increase of the MC lifetime by a factor of 3 and 20, respectively.9c Hence, this substituent leads to stabilization of the MC form due to MC–Zn(II). The observed changes indicate the importance of choosing the proper substituents when designing materials containing spirooxazine molecules for the optical detection of metal ions. By studying the MC–M(II) complexation of compound 9, similar results for a blue shift of MC absorption spectrum with respect to the uncomplexed case (Figure 11b) and the stabilization of the MC form were obtained.9d
Figure 12(a) shows the change of the temporal evolution of the absorption spectrum of compound 8 in the dark,9c indicating the increase of complex concentration. In the dark, the addition of an appropriate metal ion (e.g., Zn(II)) to compounds 7 and 8 in solutions leads to a new intense photoreversible absorption band at λmax = 610 nm,9c attributed to the formation of the MC–M complex in which the concentration increases with time (Figure 12a). A similar effect for the absorption band in the visible region has been reported by other researchers, e.g., spironaphthoxazines containing hydroxyl, benzothiazolyl, or sulfobutyl substituents in the naphthoxazine or indoline moiety.9 The formation of the MC–M complex of spironaphthoxazines containing a sulfobutyl substituent on the heterocyclic nitrogen has also been evidenced by optical absorption spectroscopy. For example, compound 9 forms MC–M complexes with Mg(II), Cd(II), Ca(II), Zn(II), or Pb(II) ions.9d The spectra in Figure 12(b) showed a new intense long-wavelength absorption band centered at 605–633 nm, which is associated with the thermal opening SP → MC induced by complexation with metal ions.
Figure 12.
(a) Absorption spectra of acetone solution of compound 8, as recorded after 1, 4, 8, and 18 min in the dark after addition of ZnCl2. The temperature = 20 °C, [SP] = [ZnCl2] = 1 × 10–4 M. 0 means the start of the measurement. (b) Absorption spectra of compound 9 in acetonitrile solutions in the presence of metal salts M(ClO4)2 after 30 min to achieve equilibrium. [SP] = [M(ClO4)2] = 5 × 10–5 M; temperature = 24 °C. (c) The effect of metal ions on the coloration rate constant of the SP form of compound 9 in an acetonitrile solution at 24 °C. [SP] = [M(ClO4)2] = 5 × 10–5 M. Reproduced with permission from ref (9c) and ref (9d). Copyright 2009 and 2019 Elsevier.
A sketch of the chelation of the spironaphthoxazine derivatives considered here (1–9) and the rates corresponding to the kinetics of the processes relevant to MC–M complexation can be seen in Scheme 6. The open MC form obtained upon UV irradiation undergoes rapid complexation with the metal ions, which stabilizes the MC form. In this way, the closure of the spirooxazine ring proceeds much slower (by 2 orders of magnitude) (kr ∼ 10–3 s–1) than in uncoordinated MC.9a,9b,9d,11b An accompanying destruction process is possible, with a very low rate (kd) of the order of 10–4–10–5 s–1.9a,21b,32 The rate of the closure of the metal-ion-complexed isomer MC–M in the dark (kc) is similar to kr in polar solvents.9a,9b,9d,14 The rate constant of destruction kd is independent of whether the complexes are obtained upon UV irradiation or in the dark.9a,9b,9d,14 The thermal degradation of MC–M complexes has been described in the literature for other spirocyclic compounds and is believed to be related to a metal-ion-catalyzed oxidation process.8a,21b,32,33 The above-mentioned kinetic rates are important for all spirooxazine applications.
Scheme 6. Chelation of Spironaphthoxazines.
6. Current Application Progress of Spirooxazines
As an important class of photochromic dyes, spirooxazines present practically important properties such as intense photocoloration, high coloration contrast, fast thermal relaxation, reversibility, and good photofatigue resistance. In terms of practical use, they are in the leading positions, yielding only naphthopyran derivatives. One of the first applications of these organic photofunctional materials, in the 1990s, was their use as photosensitive dyes for plastic ophthalmic lenses.2a,2b However, they were subsequently replaced by naphthopyran dyes, which made it possible to significantly improve the properties of commercial photochromic polymer lenses.
With the progress in the synthesis of spirooxazines, their potential for application as multifunctional materials has greatly increased, including today’s areas such as mechatronics, organic electronics, wearing and printed electronics, and the engineering of flexible optoelectronic and photonic devices with various functionality that are highly responsive to distinct stimuli and well controllable. Currently, spirooxazines are finding use in specialized applications and emerging technologies such as photochromic molecular switches,34a stimuli-responsive polymers as membranes or solid films showing a high photoconversion efficiency and different colors under irradiation,19c,34b,34c as well as anticounterfeiting inks.34d,34e Other present commercial applications of spirooxazines are the photochromic inks, dyes, and various cosmetics products.
Nowadays, the spectrum of application of spirooxazines approaches that of spiropyranes. Their derivatives and modifications have diverse chemical structures that can include various substituents, thus perfectly meeting the requirements of specific sensing applications. Furthermore, the optical sensing by such photochromic molecular switches can be considerably extensified after proper chemical treatment, conjugation, and functionalization. In this way, sensitive nanoprobes, e.g., photoactivatable and reversibly photoswitchable (upon UV and visible light) fluorescent nanoparticles, are developed and practically applied.35a Clearly, such constructs have a great potential for advanced optical and photonic applications such as individually light-addressable nanoscale devices as well as fluorescent biolabeling and ultra-high-resolution imaging, as proposed several years ago.35b,35c This is an innovative area that has yet to develop.
A very promising application of spirooxazines is in the subdiffraction super-resolution fluorescence microscopy for biological imaging.36 This technique offers photoswitchable fluorescence imaging of biological samples at a resolution beyond the diffraction limit, revealing cellular structure with nanoscale spatial resolution, thereby opening new perspectives for research in biology, molecular biology, biochemistry, and biomedical diagnostics. The above-mentioned high-tech applications motivate researchers to increase their activity toward the design and synthesis of new spirooxazine-based compounds with improved properties.
One of the major application areas of spirooxazines is the detection of analytes of chemical, biological, and ecological interest. A recent example in the work on the development of sensors with high selectivity and sensitivity, are the opto-chemical probes for trace amounts of cyanide, developed by Sanjabi et al.,37a as well as the colorimetric photosensors of solvent polarity realized by photochromic spironaphthoxazine dye and its stimuli-responsive acrylic copolymer.37b Novel chemosensors based on new spirooxazines functioning as colorimetric or fluorescent probes with high selectivity and sensitivity for detection and analysis of metal ions have now been developed.38 As mentioned in the previous section, the ability to detect metal ions is due to the fact that the closed form of spirooxazines, when interacting with metal ions, transforms into an open MC form with very different optical properties. Notably, some spirooxazine derivatives can demonstrate a large contrast in their second-order nonlinear optical (NLO) properties by interaction with metal ions and serve as efficient selective sensing probes for certain metal ions, as previously found.2d
The ability of photochromic spirooxazines to largely modify the optical response of certain materials has great promise for designing multistate fluorescent photoswitches that can further have variable NLO responses. In this way, advanced multifunctional optical materials can be obtained for applications in various scientific and technological fields.6b,39 Numerous molecular designs of spironaphthoxazines are suitable for the construction of composite systems applied for fluorescence resonance energy transfer (FRET) with fluorescent materials emitting in the spectral range corresponding to the absorption of the spirooxazine MC form.1g Certainly, these research areas will continue to develop in the future.
Photochromic spirooxazine molecules immobilized on metal surfaces can provide very efficient remote control on the molecular level using light.40a As reported by Nickel et al.,40b the ring-opening molecular switching by UV light can be enhanced by orders of magnitude by a gold surface with directly adsorbed functional units consisting of spirooxazine molecules. This opens new prospects for applications by utilizing the gold surface with directly adsorbed functional units consisting of molecular photochromic switches.40b Currently, the use of spirooxazines in this field of application is challenging and has yet to be widely explored in order to be implemented in molecular electronics.
Another very interesting present-day use of spirooxazines is to produce smart photochromic textiles that can change color when exposed to UV light and reversibly restore its original color when exposed to visible light or in the dark.41a Additionally, spirooxazines are sensitive to thermal effects and reverse to a colorless state by heating. It has been shown that photochromic fabrics can produce high-resolution images capable of rapid color switching even after 20 write and erase cycles.41b However, the resistance of spirooxazines in photochromic fabrics by photoswitching is still insufficient, so their use is currently possible only in niche products.
It should be noted that for most modern applications of spirooxazines it is important that they have photochromic properties not only in solutions but also in the solid state, e.g., in the form of thin films. However, spirooxazines usually do not show photochromism in the solid state since the ring opening is hindered by intermolecular π–π stacking. To solve this problem, it was recently proposed to introduce bulky aromatic substituents into positions 4 and 7 of the skeleton of the molecules (Scheme 3), which provide them with a large volume to undergo solid-state photochromism under mild conditions. As recently reported by Zhang and co-workers,42a such spirooxazines can be successfully used for UV printing, QR codes, and photochromic fingerprints. A possible approach to the development of effective solid-state photochromic materials is the creation of hybrid systems containing photochromic molecules as well as a porous host matrix, which provides the process of their switching in the solid state. Photosensitive materials composed of a photoswitchable spirooxazine and crystalline porous metal–organic framework (MOF) have been shown to exhibit a reversible photochromic response and extremely high photostability when irradiated with UV light, which makes these materials candidates for potential applications in data storage devices.42b
At present, an innovative application of spirooxazine-based photochromic films is their building integration. As reported by Cannavale et al.,43 such novel smart windows can provide a high degree of energy saving and visual comfort. The wide-ranging application of spirooxazines also includes their use as sensitizing dyes in dye-sensitized solar cells (DSSCs). This technology area as well as the practical application of spirooxazine-based photoswitchable materials in areas such as optical information storage and optical processing/computing, for photocontrolled enzyme activity, photocontrolled release of therapeutics, and for next-generation stimuli-responsive devices, have good prospects but involve solving many challenges in the near future.
7. Conclusions and Perspectives
Photochromic molecules based on spironaphthoxazines represent an important class of compounds that, under the action of external stimuli, are capable of transitions between isomeric states with different spectral characteristics. Significant interest in spiro derivatives is due to the fact that on their basis it is possible to create intelligent materials, i.e., materials whose chemical and physical properties can be controlled using light or other external stimuli. This mini-review discusses the design and synthesis of spirooxazines aimed at modifying their spectral and kinetic characteristics as well as the chelating abilities of these molecular systems.
To ensure the efficiency of photochromic conversion, spirocyclic compounds are synthesized by taking into account the relationship between molecular structure and spectral-kinetic properties. In addition, the color properties and lifetime of the open form of MC depend on the type of medium (solution or solid matrix) and the polarity of the solvent. Complexation with metal ions has a significant effect on the spectral-kinetic properties of spirooxazines. These properties are further confirmed by studying new compounds from the group of spiroindolinonaphthoxazines modified with substituents in the indoline and naphthoxazine moiety. In polar solvents, these molecular systems partially transform into an open colored form even in the dark. As a result, the formation of complexes of spirooxazines with metal ions proceeds even without UV light irradiation.
The introduction of proper substituents at various positions of the molecular framework is an effective way to modify spirooxazines. The choice of substituent groups in the indoline or oxazine moieties of the molecule has a significant effect on the MC ↔ SP thermal relaxation kinetics in the presence of metal ions, increasing the lifetime of the open form by up to 20 times. The choice of substituent groups in the indoline or oxazine moieties of the molecule has a significant effect on the MC ↔ SP thermal relaxation kinetics in the presence of metal ions, increasing the lifetime of the open form by up to 20 times. Monovalent alkali metal cations do not affect the MC ↔ SP equilibrium, while the addition of di- or trivalent cations leads to a significant red shift of the MC absorption band and a change in its intensity. An important feature of the substituted spirooxazines discussed here is their selective optical response to certain metal ions. Spirooxazines with a hydroxyl substituent at the 5′ position in the naphthoxazine moiety are selective to Al(III), Cu(II), and Fe(II) ions. On the other hand, a high selectivity to Co(II), Ni(II), and Zn(II) ions takes place for compounds with the heterocyclic benzothiazolyl substituent in the naphthoxazine moiety, while spirooxazines with a sulfobutyl substituent in the indoline moiety are selective to Mg(II), Cd(II), Ca(II), Zn(II), and Pb(II) ions. Thus, the appropriate substituent in the indoline or oxazine moiety of the molecule allows the spirooxazine derivatives discussed here to be successfully used for the optical sensing of metal ions and the development of molecular materials with other useful functions. The naphthalene condensed with the benzene in the indoline ring of the spirooxazines influences their spectral-kinetic properties but does not affect the ability to form complexes. Replacing the −CH3 group at the −N imino atom in the indoline part with −C4H9 improves the solubility of the compounds in nonpolar solvents. As a rule, substituents have a stronger effect on the spectral-kinetic properties of the spiro compounds than the solvent. The results obtained to date on the substituents’ effects on the properties of spirooxazines and recent progress in using various synthesis strategies have shown that the variation of substituents is an efficient way to design novel photochromic molecular systems for practical use (e.g., applicable in suitable solvents), and continued efforts in this direction are promising.
Prospects for the further development of photochromic spirocyclic compounds based on spirooxazines may be associated with the engineering of molecular systems that exhibit high resistance to degradation under the action of light and are capable of multiple switching between open and closed forms and demonstrate effective photochromic conversion upon irradiation with visible light or under the influence of other factors. Further efforts in the development of photochromic spirooxazine compounds with enhanced chelating properties may lead, for example, to an expansion of the range of detectable metal ions. This can also provide attractive optical properties that meet the needs for advanced applications, such as photoswitchable high-resolution optical molecular imaging. The synthesis of hybrid systems of spirooxazines with fluorescent molecules exhibiting photoswitchable fluorescence can be of great importance in the development of optical sensors. This aspect is very challenging and deserves further studies.
Acknowledgments
Work supported by the European Regional Development Fund, as a part of the Operational Programme, “Science and Education for Smart Growth 2014-2020”, Project: CoE “National Center of Mechatronics and Clean Technologies”, BG05M2OP001-1.001-0008-C01. The authors thank Dr. Valentin Alexiev from the Institute of Catalysis, Bulgarian Academy of Sciences, for the help with quantum-chemistry calculation with the ORCA software.
Biographies

Prof. Stela Minkovska received her Ph.D. in Photocoordination Chemistry in 2006 at the Department of Analytical Chemistry, Faculty of Chemistry; Sofia University (Bulgaria), with a Doctor Thesis entitled “Photo induced complexation processes with spiroindolinonaphtoxazines”. Since 2013 she has been a professor in the Institute of Catalysis, Bulgarian Academy of Sciences, Sofia, Bulgaria. Her research experience is in the field of synthesis and study of photochromic compounds such as spiropyrans, spirooxazines, diarylethenes, fluorescent organic dyes for DNA tagging, photochromism, thermochromism, and solvatochromism. She was a postdoctoral researcher at the Politehnica University of Bucharest, Romania (FP6-Maria Curie Transfer of Knowledge for Micro-and Nanotechnologies–NANOTEC-EST), as well as in the Adaptive Information Cluster, NCSR, Dublin City University, Ireland. Also she conducted studies in the laboratory of Prof. Yasushi Yokoyama (Yokohama National University, Japan) on the project “Synthesis of new photochromic molecules and polymers containing metal complexes”.

Prof. Georgi B. Hadjichristov received his Ph.D. in Engineering Physics in 1990 at the Department of Quantum Electronics, Faculty of Physics, Sofia University, Bulgaria. In 1990, he joined the Institute of Solid State Physics (Bulgarian Academy of Sciences, Sofia, Bulgaria), where in 2020 he became Full Professor in Applied Physics, Physical Electronics and Wave Processes. He worked as a postdoctoral researcher in Wolfgang Kiefer’s Research Group (AKK) at the Institute of Physical Chemistry, University of Würzburg, Germany, where thereafter he was a postdoctoral research fellow of the Alexander-von-Humboldt Research Foundation. His research interests are in the fields of materials science, optical spectroscopy, nanobiophotonics, quantum-mechanical calculations of optical susceptibility, and nonlinear optics of organic photoactive, polar, and fluorescent media, as well as composite and nanocomposite materials. Currently, he investigates photoinduced and photocontrollable processes in luminescent metal–organic complexes and the practical applications of photoswitchable nanoprobes and biomarkers in nanobiomedicine, imaging, and sensing. Since 1991, he has been a member of the German Physical Society.

Andreea Neacsu is a permanent researcher at the Institute of Physical Chemistry “Ilie Murgulescu”, Chemical Thermodynamics Laboratory. She got a Ph.D. in Chemistry in 2018 on the supramolecular chemistry of cyclodextrins. Her research activity is focused on the physicochemical characterization of the ligand−macromolecule interactions and application of computational techniques based on the density functional theory and molecular dynamics.

Dr. Viorel Chihaia graduated from the Physics Faculty of Bucharest University in 1989 and received his Ph.D. degree in Chemistry in 1999 from the Institute of Physical Chemistry “Ilie Murgulescu”, Bucharest, Romania (IPC). Since October 1990 he has been employed at the Institute of Physical Chemistry “Ilie Murgulescu”, Romanian Academy, where he is currently a senior researcher. He works in the fields of Surface Sciences, Catalysis and High Performance Computing (HPC) for Materials Science by performing quantum chemistry and solid state calculations and molecular dynamics and Monte Carlo simulations. He developed HPC parallel algorithms and simulation methods for nano-, meso-, macro-, and multiscale phenomena. He has worked in Keimyung University, Taegu, Korea (2000), Göttingen University, Germany (2001-2004), Institute of Atomic and Molecular Sciences, Academia Sinica, Taiwan (2010), and Jülich Supercomputing Centre, Forschungszentrum Jülich, Germany (2011-2015). He was involved in several national and international research projects. He was director of the national project “Virtual Group for Atomic Scale Simulations in Materials Science” and currently leads the research program “High Performance Computing (HPC) in Physical Chemistry” at IPC. His current research interests are focused on HPC simulation methods for energy materials.

Prof. Yury V. Fedorov, D.Sc. in Chemistry, is the leading researcher of A.N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, Moscow, Russia, where he joined the laboratory of photoactive supramolecular systems in 2008. From 1993 to 2008, he was a senior researcher at the Photochemistry Center of the Russian Academy of Sciences, and since 1981 he has worked as a researcher at the Department of Chemistry at M.V. Lomonosov Moscow State University, where he received a Ph.D. in chemistry in 1986. Currently, his scientific interests are focused on the study of photoactive supramolecular systems based on crown-containing mono- and bis-styryl dyes and their application as optical and electrochemical sensors for metal ions, as well as for photocontrolled interaction with DNA.
The authors declare no competing financial interest.
Dedication
In memoriam: This work is a tribute to the memory of my supervisor, Professor DSc. Todor Deligeorgiev, from the Faculty of Chemistry and Pharmacy at St. Kliment Ohridski University of Sofia, who inspired my research activity in the field of photochromic compounds and organic dye systems (S.M.).
References
- a Chu N. Y. C. Photochromism of spiroindolinonaphthoxazine. I. Photophysical properties. Can. J. Chem. 1983, 61, 300–305. 10.1139/v83-054. [DOI] [Google Scholar]; b Maeda S.Spirooxazines. In Organic Photochromic and Thermochromic Compounds; Crano J. C., Guglielmetti R. J., Eds.; Topics in Applied Chemistry, Kluwer Academic Publ.: New York, 1999; Chapter 2, Vol. 1: Main Photochromic Families, pp 85–109. [Google Scholar]; c Lokshin V.; Samat A.; Metelitsa A. V. Spirooxazines: synthesis, structure, spectral and photochromic properties. Russ. Chem. Rev. 2002, 71, 893–916. 10.1070/RC2002v071n11ABEH000763. [DOI] [Google Scholar]; d Chu N. Y. C.4n+2 Systems: Spirooxazines. In Photochromism. Molecules and Systems; Guglielmetti R., Dürr H., Bouas-Laurent H., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; pp 493–509. [Google Scholar]; e Minkin V. I. Photo-, thermo-, solvato-, and electrochromic spiroheterocyclic compounds. Chem. Rev. 2004, 104, 2751–76. 10.1021/cr020088u. [DOI] [PubMed] [Google Scholar]; f Minkin V. I. Light-controlled molecular switches based on bistable spirocyclic organic and coordination compounds. Russ. Chem. Rev. 2013, 82, 1–26. 10.1070/RC2013v082n01ABEH004336. [DOI] [Google Scholar]; g Xia H.; Xie K.; Zou G. Advances in spiropyrans/spirooxazines and applications based on fluorescence resonance energy transfer (FRET) with fluorescent materials. Molecules 2017, 22, 2236. 10.3390/molecules22122236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Crano J. C.; Kwak W. S.; Welch C. N.. Spirooxazines and their use in photochromic lenses. In Applied Photochromic Polymer Systems; McArdle C. B., Ed.; Blackie: Glasgow, 1992; Chapter 2, pp 31–79. [Google Scholar]; b Crano J. C.; Flood T.; Knowles D.; Kumar A.; Van Gemert B. Photochromic compounds: chemistry and application in ophthalmic lenses. Pure Appl. Chem. 1996, 68, 1395–1398. 10.1351/pac199668071395. [DOI] [Google Scholar]; c Solovieva A. B.; Cherkasova A. V.; Glagolev N. N.; Kopylov A. S.; Timashev P. S.; Tsypina S. I.; Bagratashvili V. N. Stable “coloured” states of spirooxazine photochrom molecules immobilized in polymer matrixes by supercritical carbon dioxide. J. Mol. Liq. 2017, 239, 74–82. 10.1016/j.molliq.2016.12.063. [DOI] [Google Scholar]; d Ye J. T.; Wang L.; Wang H. Q.; Chen Z. Z.; Qiu Y. Q.; Xie H. M. Spirooxazine molecular switches with nonlinear optical responses as selective cation sensors. RSC Adv. 2017, 7, 642–650. 10.1039/C6RA25478K. [DOI] [Google Scholar]; e Pithan P. M.; Steup S.; Ihmels H. Cation-induced ring-opening and oxidation reaction of photoreluctant spirooxazine-quinolizinium conjugates. Beilstein J. Org. Chem. 2020, 16, 904–916. 10.3762/bjoc.16.82. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Castan A. J. M.; Mwalukuku V. M.; Riquelme A. J.; Liotier J.; Huaulme Q.; Anta J. A.; Maldivi P.; Demadrille R. Photochromic spiro-indoline naphthoxazines and naphthopyrans in dye-sensitized solar cells. Mater. Chem. Front. 2022, 6, 2994–3005. 10.1039/D2QM00375A. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Trovato V.; Sfameni S.; Rando G.; Rosace G.; Libertino S.; Ferri A.; Plutino M. S. A review of stimuli-responsive smart materials for wearable technology in healthcare: Retrospective, perspective, and prospective. Molecules 2022, 27, 5709. 10.3390/molecules27175709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Balmond E. I.; Tautges B. K.; Faulkner A. L.; Or V. W.; Hodur B. M.; Shaw J. T.; Louie A. Y. Comparative evaluation of substituent effect on the photochromic properties of spiropyrans and spirooxazines. J. Org. Chem. 2016, 81, 8744–8758. 10.1021/acs.joc.6b01193. [DOI] [PubMed] [Google Scholar]; b Keyvan Rad J.; Balzade Z.; Mahdavian A. R. Spiropyran-based advanced photoswitchable materials: A fascinating pathway to the future stimuli-responsive devices. J. Photochem. Photobiol. C: Photochem. Rev. 2022, 51, 100487. 10.1016/j.jphotochemrev.2022.100487. [DOI] [Google Scholar]
- a Crano J. C.; Guglielmetti R. J.. Organic Photochromic and Thermochromic Compounds. Topics in Appl. Chem.; Kluwer: New York, 1999; Vol. 2: Physicochemical Studies, Biological Applications, and Thermochromism. [Google Scholar]; b Natali M.; Giordani S. Interaction studies between photochromic spiropyrans and transition metal cations: the curious case of copper. Org. Biomol. Chem. 2012, 10, 1162–1171. 10.1039/C1OB06375H. [DOI] [PubMed] [Google Scholar]; c Zhao H.; Xu Y. Q.; Zhao W. K.; Gao K.; Liu D. S. Electronic transport properties of indolyl spirooxazine/merooxazine-based light-driven molecular switch: The effect of amino/nitro substituents. Phys. B 2014, 437, 41–46. 10.1016/j.physb.2013.12.027. [DOI] [Google Scholar]; d Yuan J.; Yuan Y.; Tian X.; Sun J.; Ge Y. Spirooxazine-fulgide biphotochromic molecular switches with nonlinear optical responses across four states. J. Phys. Chem. C 2016, 120, 14840–14853. 10.1021/acs.jpcc.6b04849. [DOI] [Google Scholar]; e Sun J.; Yuan Y.; Yuan J.; Zhao Y.; Yang S.; Gan T.; Xiong J. Design of single-molecular logical devices based on multistable photochromatic spirooxazine. J. Opt. Soc. Am. B 2017, 34, 837–842. 10.1364/JOSAB.34.000837. [DOI] [Google Scholar]
- a De Silva A. P.; Gunaratne H. N.; Gunnlaugsson T.; Huxley A. J.; McCoy C. P.; Rademacher J. T.; Rice T. E. Signaling recognition events with fluorescent sensors and switches. Chem. Rev. 1997, 97, 1515–1566. 10.1021/cr960386p. [DOI] [PubMed] [Google Scholar]; b Quang D. T.; Kim J. S. Fluoro- and chromogenic chemodosimeters for heavy metal ion detection in solution and biospecimens. Chem. Rev. 2010, 110, 6280–6301. 10.1021/cr100154p. [DOI] [PubMed] [Google Scholar]; c Zhu S.; Li M.; Sheng L.; Chen P.; Zhang Y.; Zhang S. X.-An. A spirooxazine derivative as a highly sensitive cyanide sensor by means of UV visible difference spectroscopy. Analyst 2012, 137, 5581–5585. 10.1039/c2an35867k. [DOI] [PubMed] [Google Scholar]
- a Berkovic G.; Krongauz V.; Weiss V. Spiropyrans and spirooxazines for memories and switches. Chem. Rev. 2000, 100, 1741–1753. 10.1021/cr9800715. [DOI] [PubMed] [Google Scholar]; b Sun H.; Tian X.; Autschbach J.; Yuan Y.; Sun J.; Liu X.; Chen C.; Cao H. Spirooxazine-based multifunctional molecular switches with tunable photochromism and nonlinear optical response. J. Mater. Chem. C 2013, 1, 5779–5790. 10.1039/c3tc31131g. [DOI] [Google Scholar]; c Xiong Y.; Vargas Jentzsch A.; Osterrieth J. W. M.; Sezgin E.; Sazanovich I. V.; Reglinski K.; Galiani S.; Parker A. W.; Eggeling C.; Anderson H. L. Spironaphthoxazine switchable dyes for biological imaging. Chem. Sci. 2018, 9, 3029–3040. 10.1039/C8SC00130H. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Sahoo P. R.; Prakash K.; Kumar S. Light controlled receptors for heavy metal ions. Coord. Chem. Rev. 2018, 357, 18–49. 10.1016/j.ccr.2017.11.010. [DOI] [Google Scholar]; e Yue C.; Liao C.; Yang Z.; Hu F. Recent advances in photoswitchable cation chemosensors. Curr. Org. Chem. 2018, 22, 1458–1467. 10.2174/1385272822666180712143730. [DOI] [Google Scholar]; f Sahoo P. R.; Kumar S. The experimental and theoretical studies of a merocyanine form based turn off fluorescent sensor for Fe3+ ions with nanomolar level sensitivity in aqueous solution. J. Lumin. 2018, 201, 203–210. 10.1016/j.jlumin.2018.04.048. [DOI] [Google Scholar]
- a Inouye M.; Ueno M.; Kitao T.; Tsuchiya K. Alkali metal recognition induced isomerization of spiropyrans. J. Am. Chem. Soc. 1990, 112, 8977–8979. 10.1021/ja00180a051. [DOI] [Google Scholar]; b Zakharova M. I.; Coudret C.; Pimienta V.; Micheau J. C.; et al. Quantitative investigations of cation complexation of photochromic 8-benzothiazole-substituted benzopyran: towards metal-ion sensors. Photochem. Photobiol. Sci. 2010, 9, 199–207. 10.1039/b9pp00112c. [DOI] [PubMed] [Google Scholar]; c Seefeldt B.; Kasper R.; Beining M.; Mattay J.; Arden-Jacob J.; Kemnitzer N.; Drexhage K. H.; Heilemann M.; Sauer M. Spiropyrans as molecular optical switches. Photochem. Photobiol. Sci. 2010, 9, 213–220. 10.1039/b9pp00118b. [DOI] [PubMed] [Google Scholar]; d Zhu J. F.; Yuan H.; Chan W. H.; Lee A. W. M. A colorimetric and fluorescent turn-on chemosensor operative in aqueous media for Zn2+ based on a multifunctionalized spirobenzopyran derivative. Org. Biomol. Chem. 2010, 8, 3957–3964. 10.1039/c004871b. [DOI] [PubMed] [Google Scholar]; e Shao N.; Wang H.; Gao X.; Yang R.; Chan W. Spiropyran-based fluorescent anion probe and its application for urinary pyrophosphate detection. Anal. Chem. 2010, 82, 4628–4636. 10.1021/ac1008089. [DOI] [PubMed] [Google Scholar]; f Paramonov S. V.; Lokshin V.; Fedorova O. A. Spiropyran, chromene or spirooxazine ligands: Insights into mutual relations between complexing and photochromic properties. J. Photochem. Photobiol. C: Photochem. Rev. 2011, 12, 209–236. 10.1016/j.jphotochemrev.2011.09.001. [DOI] [Google Scholar]; g Baldrighi M.; Locatelli G.; Desper J.; Aakeroy C. B.; Giordani S. Probing metal ion complexation of ligands with multiple metal binding sites: The case of spiropyrans. Chem. Eur. J. 2016, 22, 13976–13984. 10.1002/chem.201602608. [DOI] [PubMed] [Google Scholar]
- a Tamaki T.; Ichimura K. Photochromic chelating spironaphthoxazines. J. Chem. Soc., Chem. Commun. 1989, 19, 1477–1479. 10.1039/c39890001477. [DOI] [Google Scholar]; b Kimura K.; Yamashita T.; Kaneshige M.; Yokoyama M. Crowned spironaphthoxazine: Lithium ion-selective colouration and ion-regulated thermal stability of its coloured form. J. Chem. Soc., Chem. Commun. 1992, 969–970. 10.1039/c39920000969. [DOI] [Google Scholar]; c Kimura K.; Kaneshige M.; Yamashita T.; Yokoyama M. Cation complexation, photochromism, and reversible ion-conducting control of crowned spironaphthoxazine. J. Org. Chem. 1994, 59, 1251–1256. 10.1021/jo00085a009. [DOI] [Google Scholar]; d Fedorova O. A.; Koshkin A. V.; Gromov S. P.; Strokach Y. P.; Valova T. M.; Alfimov M. V.; Feofanov A. V.; Alaverdian I. S.; Lokshin V. A.; Samat A. Transformation of 6′- amino-substituted spiro-naphthoxazines induced by Pb(II) and Eu(III) cations. J. Phys. Org. Chem. 2005, 18, 504–512. 10.1002/poc.890. [DOI] [Google Scholar]; e Korolev V. V.; Vorobyev D. Yu.; Glebov E. M.; Grivin V. P.; Plyusnin V. F.; Koshkin A. V.; Fedorova O. A.; Gromov S. P.; Alfimov M. V.; Shklyaev Yu. V.; Vshivkova T. S.; Rozhkova Yu. S.; Tolstikov A. G.; Lokshin V. V.; Samat A. Spironaphtoxazines produced from crown-containing dihydroisoquinolines: Synthesis and spectroscopic study of cation-dependent photochromism. J. Photochem. Photobiol. A, Chem. 2007, 192, 75–83. 10.1016/j.jphotochem.2007.05.006. [DOI] [Google Scholar]; f Ko C. C.; Yam V. W. W.. Photochromic transitional metal complexes for photosensitization. In Photochromic materials: Preparation, Properties and Applications, 1st ed.; Tian H., Zhang J., Eds.; Wiley-VCH: Weinheim, Germany, 2016; Chapter 2, pp 47–70. [Google Scholar]
- a Minkovska S.; Fedieva M.; Jeliazkova B.; Deligeorgiev T. Thermally activated and light-induced metal ion complexation of 5′ -(hydroxy)spiroindolinonaphthooxazines in polar solvents. Polyhedron 2004, 23, 3147–3153. 10.1016/j.poly.2004.09.022. [DOI] [Google Scholar]; b Jeliazkova B. G.; Minkovska S.; Deligeorgiev T. Effect of complexation on the photochromism of 5′ -(benzothiazol-2-yl)spiroindolinonaphthooxazines in polar solvents. J. Photochem. Photobiol. A-Chem. 2005, 171, 153–160. 10.1016/j.jphotochem.2004.10.001. [DOI] [Google Scholar]; c Alhashimy N.; Byrne R.; Minkovska S.; Diamond D. Novel synthesis and characterisation of 3,3-dimethyl-(2-benzothiazolyl)-spironaphth(indoline-2,3-[3H]naphth[2,1-b][1,4]oxazine) derivatives. Tetrahedron Lett. 2009, 50, 2573–2576. 10.1016/j.tetlet.2009.03.080. [DOI] [Google Scholar]; d Fedorov Y. V.; Shepel N. E.; Peregudov A. S.; Fedorova O. A.; Deligeorgiev T.; Minkovska S. Modulation of photochromic properties of spirooxazine bearing sulfobutyl substituent by metal ions. J. Photochem. Photobiol. A: Chem. 2019, 371, 453–460. 10.1016/j.jphotochem.2018.10.045. [DOI] [Google Scholar]
- a Tran H. M.; Nguyen T. H.; Nguyen Q. V.; Tran P. H.; Thai L. D.; Truong T. T.; Nguyen L. T. T.; Nguyen H. T. Synthesis of a novel fluorescent cyanide chemosensor based on photoswitching poly(pyrene-1-ylmethyl-methacrylate-random-methyl methacrylate-random-methacrylate spirooxazine). Macromol. Res. 2019, 27, 25–32. 10.1007/s13233-019-7030-7. [DOI] [Google Scholar]; b Pattaweepaiboon S.; Phiromphu N.; Kaewchangwat N.; Suttisintong K.; Sirisaksoontorn W. An indolino-spironaphthooxazine probe for colorimetric detection of ferric ions in drinking water. New J. Chem. 2021, 45, 11284–11291. 10.1039/D1NJ01166A. [DOI] [Google Scholar]; c Pattaweepaiboon S.; Nanok T.; Kaewchangwat N.; Suttisintong K.; Sirisaksoontorn W. Colorimetric detection of Hg2+ and CH3Hg+ by a novel spirooxazine derivative as a highly sensitive and selective probe. Dyes Pigm. 2021, 186, 108996. 10.1016/j.dyepig.2020.108996. [DOI] [Google Scholar]; d Pattaweepaiboon S.; Foytong W.; Phiromphu N.; Nanok T.; Kaewchangwat N.; Suttisintong K.; Sirisaksoontorn W. Spirooxazine-based dual-sensing probe for colorimetric detection of Cu2+ and Fe3+ and its application in drinking water and rice quality monitoring. ACS Omega. 2022, 7, 18671–18680. 10.1021/acsomega.2c01353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Deligeorgiev T.; Minkovska S.; Jejiazkova B.; Rakovsky S. Synthesis of photochromic chelating spironaphthoxazines. Dyes Pigm. 2002, 53, 101–108. 10.1016/S0143-7208(02)00008-6. [DOI] [Google Scholar]; b Minkovska S.; Jeliazkova B.; Borisova E.; Avramov L.; Deligeorgiev T. Substituent and solvent effect on the photochromic properties of a series of spiroindolinonaphthooxazines. J. Photochem. Photobiol. A-Chem. 2004, 163, 121–126. 10.1016/S1010-6030(03)00437-4. [DOI] [Google Scholar]; c Coleman S.; Byrne R.; Minkovska S.; Diamond D. Thermal reversion of spirooxazine in ionic liquids containing the [NTf2]anion. Phys. Chem. Chem. Phys. 2009, 11, 5608–5614. 10.1039/b901417a. [DOI] [PubMed] [Google Scholar]
- a Lukyanov B. S.; Lukyanova M. B. Spiropyrans: Synthesis, properties, and application. (Review). Chem. Heterocycl. Compd. 2005, 41, 281–311. 10.1007/s10593-005-0148-x. [DOI] [Google Scholar]; b Kortekaas L.; Browne W. R. The evolution of spiropyran: fundamentals and progress of an extraordinarily versatile photochrome. Chem. Soc. Rev. 2019, 48, 3406–3424. 10.1039/C9CS00203K. [DOI] [PubMed] [Google Scholar]; c Ali A. A.; Kharbash R.; Kim Y. Chemo- and biosensing applications of spiropyran and its derivatives - A review. Anal. Chim. Acta 2020, 1110, 199–223. 10.1016/j.aca.2020.01.057. [DOI] [PubMed] [Google Scholar]; d Wang J. X.; Li C.; Tian H. Energy manipulation and metal-assisted photochromism in photochromic metal complex. Coord. Chem. Rev. 2021, 427, 213579. 10.1016/j.ccr.2020.213579. [DOI] [Google Scholar]
- Minkovska St.; Kolev K.; Jeliazkova B.; Deligeorgiev T. Photochemical properties of a photochromic naphthoxazine upon UV irradiation in the presence of transition metal ions. Dyes Pigm. 1998, 39, 25–33. 10.1016/S0143-7208(98)00004-7. [DOI] [Google Scholar]
- Zhou J.; Zhao F.; Yiting Li; Fushi Zhang; Xinqi Song Novel chelation of photochromic spironaphthoxazines to divalent metal ions. J. Photochem. Photobiol. A: Chem. 1995, 92, 193–199. 10.1016/1010-6030(95)04136-0. [DOI] [Google Scholar]
- a Alfimov M. V.; Fedorova O. A.; Gromov S. P. Photoswitchable molecular receptors. J. Photochem. Photobiol. A: Chem. 2003, 158, 183–198. 10.1016/S1010-6030(03)00033-9. [DOI] [Google Scholar]; b Fedorova O. A.; Gromov S. P.; Pershina Yu. P.; Sergeev S. S.; Strokach Yu. A.; Barachevsky V. A.; Alfimov M. V.; Pèpe C.; Samat A.; Guglielmetti R. Novel azacrown ether-containing spiro[indoline-2,3′-naphthoxazines]: design, synthesis and cation-dependent photochromism. J. Chem. Soc., Perkin Trans. 2000, 2, 56–570. [Google Scholar]; c Fedorova O. A.; Koshkin A. V.; Gromov S. P.; Avakyan V. G.; Nazarov V. B.; Brichkin S. B.; Vershinnikova T. G.; Nikolaeva T. M.; Chernych L. A.; Alfimov M. V. Crown-containing spironaphthoxazines and spiropyrans. Part 3. Synthesis and investigation of the merocyanine form of crown-containing spirobenzothiazolinonaphthoxazine. Russ. Chem. Bull. 2002, 51, 1441–1450. 10.1023/A:1020950620582. [DOI] [Google Scholar]; d Fedorova O. A.; Strokach Yu. P.; Gromov S. P.; Koshkin A. V.; Valova T. M.; Alfimov M. V.; Feofanov A. V.; Alaverdian I. S.; Lokshin V. A.; Samat A.; Guglielmetti R.; Girling R. B.; Moore J. N.; Hester R. E. Effect of metal cations on the photochromic properties of spironaphthoxazines conjugated with aza-15(18)-crown-5(6) ethers. New J. Chem. 2002, 26, 1137–1145. 10.1039/b200790h. [DOI] [Google Scholar]
- a Maurel F.; Aubard J.; Rajzmann M.; Guglielmetti R.; Samat A. A quantum chemical study of the ground state ring opening/closing of photochromic 1,3,3-trimethylspiro[indoline-2,3′-naphtho[2,1-b][1,4]oxazine]. J. Chem. Soc., Perkin Trans. 2002, 2, 1307–1315. 10.1039/b202545k. [DOI] [Google Scholar]; b Vallet J.; Micheau J. C.; Coudret C. Switching a pH indicator by a reversible photoacid: A quantitative analysis of a new two-component photochromic system. Dyes Pigm. 2016, 125, 179–184. 10.1016/j.dyepig.2015.10.025. [DOI] [Google Scholar]; c Dorogan I. V.; Minkin V. I. Theoretical modeling of electrocyclic 2H-pyran and 2H-1,4-oxazine ring opening reactions in photo- and thermochromic spiropyrans and spirooxazines. Chem. Heterocycl. Compd. 2016, 52, 730–735. 10.1007/s10593-016-1956-x. [DOI] [Google Scholar]; d Seiler V. K.; Tumanov N.; Robeyns K.; Wouters J.; Champagne B.; Leyssens T. A structural analysis of spiropyran and spirooxazine compounds and their polymorphs. Crystals 2017, 7, 84. 10.3390/cryst7030084. [DOI] [Google Scholar]; e Kortekaas L.; Chen J.; Jacquemin D.; Browne W. R. Proton-stabilized photochemically reversible E/Z isomerization of spiropyrans. J. Phys. Chem. B 2018, 122, 6423–6430. 10.1021/acs.jpcb.8b03528. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Wang K. X.; Yin B. W.; Jia P. K.; Zhang T. S.; Cui G.; Xie B. B. Electronic structure calculations and nonadiabatic dynamics simulations on reversible photochromic mechanism of spirooxazine. Dyes Pigm. 2022, 205, 110509. 10.1016/j.dyepig.2022.110509. [DOI] [Google Scholar]
- a Jonsson H.; Mills G.; Jacobsen K. W.. Nudged elastic band method for finding minimum energy paths of transitions. In Classical and Quantum Dynamics in Condensed Phase Simulations; Berne B. J., Ciccotti G., Coker D. F., Eds.; World Scientific: Singapore, 1998; pp 385–404. [Google Scholar]; b Becke A. D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. 10.1063/1.464913. [DOI] [Google Scholar]; c Rassolov V. A.; Pople J. A.; Ratner M. A.; Windus T. L. 6-31G* basis set for atoms K through Zn. J. Chem. Phys. 1998, 109, 1223–1229. 10.1063/1.476673. [DOI] [Google Scholar]; d Neese F.; Wennmohs F.; Becker U.; Riplinger C. The ORCA quantum chemistry program package. J. Chem. Phys. 2020, 152, 224108. 10.1063/5.0004608. [DOI] [PubMed] [Google Scholar]; e Barone V.; Cossi M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 1998, 102, 1995–2001. 10.1021/jp9716997. [DOI] [Google Scholar]; f Unpublished research data.
- a Favaro G.; Masetti F.; Mazzucato U.; Ottavi G.; Allegrini P.; Malatesta V. Photochromism, thermochromism and solvatochromism of some spiro[indolinoxazine]-photomerocyanine systems: Effects of structure and solvent. J. Chem. Soc. Faraday Trans. 1994, 90, 333–338. 10.1039/ft9949000333. [DOI] [Google Scholar]; b Chibisov A. K.; Görner H. Photoprocesses in spirooxazines and their merocyanines. J. Phys. Chem. A 1999, 103, 5211–5216. 10.1021/jp984822i. [DOI] [Google Scholar]; c di Nunzio M. R.; Gentili P. L.; Romani A.; Favaro G. Photochromic, Thermochromic, and fluorescent spirooxazines and naphthopyrans: A spectrokinetic and thermodynamic study. ChemPhysChem 2008, 9, 768–775. 10.1002/cphc.200700789. [DOI] [PubMed] [Google Scholar]
- a Pozzo J. L.; Samat A.; Guglielmetti R.; de Keukeleire D. Solvatochromic and photochromic characteristics of new 1,3-dihydrospiro[2H-indole-2,2-[2H]-bipyrido[3,2-f][2,3-h][1,4]benzoxazines]. J. Chem. Soc., Perkin Trans. 1993, 2, 1327–1332. 10.1039/P29930001327. [DOI] [Google Scholar]; b Metelitsa A. V.; Lokshin V.; Micheau J. C.; Samat A.; Guglielmetti R.; Minkin V. I. Photochromism and solvatochromism of push-pull or pull-push spiroindolinenaphthoxazines. Phys. Chem. Chem. Phys. 2002, 4, 4340–4345. 10.1039/B204603B. [DOI] [Google Scholar]; c Zampini G.; Ortica F.; Cannavale A.; Latterini L. Spirooxazine loading effects on the photochromism of polymer films. Dyes Pigm. 2023, 210, 111018. 10.1016/j.dyepig.2022.111018. [DOI] [Google Scholar]
- a Bohne C.; Fan M. G.; Li Z. J.; Liang Y. C.; Lusztyk J.; Scaiano J. C. Studies of photochromic processes in laser photolysis spirooxazines: solvent effect: Behavior on photomerocyanine. J. Photochem. Photobiol. A: Chem. 1992, 66, 79–90. 10.1016/1010-6030(92)85121-A. [DOI] [Google Scholar]; b Zhang C.; Zhang Z.; Fan M.; Yan W. Positive and negative photochromism of novel spiro[indoline-phenanthrolinoxazines]. Dyes Pigm. 2008, 76, 832–835. 10.1016/j.dyepig.2007.01.019. [DOI] [Google Scholar]; c Patel D. G.; Paquette M. M.; Kopelman R. A.; Kaminsky W.; Ferguson M. J.; Frank N. L. A solution- and solid-state investigation of medium effects on charge separation in metastable photomerocyanines. J. Am. Chem. Soc. 2010, 132, 12568–12586. 10.1021/ja100238h. [DOI] [PubMed] [Google Scholar]
- a Pottier E.; Dubest R.; Guglielmetti R.; Tardieu P.; Kellmann A.; Tfibel F.; Levoir P.; Aubard J. Effets de substituant, d’hétéroatome et de solvant sur les cinétiques de décoloration thermique et les spectres d’absorption de photomérocyanines en série spiro [indoline-oxazine]. Helv. Chim. Acta 1990, 73, 303–315. 10.1002/hlca.19900730210. [DOI] [Google Scholar]; b Gautron R.; Eloy D.; Escaffre P.; Guglielmetti R.; Pottier E.; Tardieu P. Étude par Photolyse à Éclairs Répétés de la Photodégradation et de la Colorabilité de Quelques Spiro [Indoline-Oxazines]. Bull. Soc. Chim. Belg. 1991, 100, 315–328. 10.1002/bscb.19911000405. [DOI] [Google Scholar]
- Zou Q.; Li X.; Zhou J.; Bai K.; Agren H. Synthesis and photochromism of a spirooxazine derivative featuring a carbazole moiety: Fast thermal bleaching and excellent fatigue resistance. Dyes Pigm. 2014, 107, 174–181. 10.1016/j.dyepig.2014.04.004. [DOI] [Google Scholar]
- a Favaro G.; Malatesta V.; Mazzucato U.; Ottavi G.; Romani A. Thermally reversible photoconversion of spiroindoline-naphthooxazines to photomerocyanines: a photochemical and kinetic study. J. Photochem. Photobiol. A: Chem. 1995, 87, 235–241. 10.1016/1010-6030(94)03991-3. [DOI] [Google Scholar]; b Wilkinson F.; Worrall D. R.; Hobley J.; Jansen L.; Williams S. L.; Langley A. J.; Matousek P. Picosecond time-resolved spectroscopy of the photocolouration reaction of photochromic naphthoxazine-spiroindolines. J. Chem. Soc. Faraday Trans. 1996, 92, 1331–1336. 10.1039/ft9969201331. [DOI] [Google Scholar]
- a Khairutdinov R. F.; Giertz K.; Hurst J. K.; Voloshina E. N.; Voloshin N. A.; Minkin V. I. Photochromism of spirooxazines in homogeneous solution and phospholipid liposomes. J. Am. Chem. Soc. 1998, 120, 12707–12713. 10.1021/ja9825985. [DOI] [Google Scholar]; b Nishikiori H.; Tanaka N.; Takagi K.; Fujii T. Solvent effect on fluorescence spectra of a spirooxazine. Res. Chem. Intermed. 2003, 29, 485–493. 10.1163/156856703322149026. [DOI] [Google Scholar]
- a Barachevsky V. A. Photofluorochromic Spirocompounds and Their Application. J.f Fluoresc. 2000, 10, 185. 10.1023/A:1009403411765. [DOI] [Google Scholar]; b Barachevsky V. A.; Strokach Y. P.; Puankov Y. A.; Kobeleva O. I.; Valova T. M.; Levchenko K. S.; Yaroshenko V. N.; Krayushkin M. M. Light-sensitive heterocyclic compounds for information nanotechnologies. Proc. 5th Eurasian Conf. Heterocyclic Chem. ARKIVOC 2008, 2009 (ix), 70–95. 10.3998/ark.5550190.0010.906. [DOI] [Google Scholar]; c Sheng X.; Peng A.; Fu H.; Liu Y.; Zhao Y.; Ma Y.; Yao J. Modulation of a fluorescence switch based on photochromic spirooxazine in composite organic nanoparticles. Nanotechnology 2007, 18, 145707. 10.1088/0957-4484/18/14/145707. [DOI] [Google Scholar]
- Meng X.; Zhu W.; Guo Z.; Wang J.; Tian H. Highly stable and fluorescent switching spirooxazines. Tetrahedron 2006, 62, 9840–9845. 10.1016/j.tet.2006.08.039. [DOI] [Google Scholar]
- Huang Y.; Li F.; Ye C.; Qin M.; Ran W.; Song Y. A photochromic sensor microchip for high-performance multiplex metal ions detection. Sci. Rep. 2015, 5, 9724. 10.1038/srep09724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Uznanski P.; Amiens C.; Donnadieu B.; Coppel Y.; Chaudret B. Oxidation of photochromic spirooxazines by coinage metal cations. Part I. Reaction with AgNO3: formation and characterisation of silver particles. New J. Chem. 2001, 25, 1486–1494. 10.1039/b105446p. [DOI] [Google Scholar]; b Uznanski P.; Amiens C.; Bardaji M.; Donnadieu B.; Coppel Y.; Chaudret B.; Laguna A. Oxidation of photochromic spirooxazines by coinage metal cations. Part II. Oxidation by gold (III) compounds and synthesis of gold colloids. New J. Chem. 2001, 25, 1495–1499. 10.1039/b105447n. [DOI] [Google Scholar]
- Sahoo P. R.; Kumar S. Photochromic spirooxazine as highly sensitive and selective probe for optical detection of Fe3+ in aqueous solution. Sens. Actuators, B 2016, 226, 548–552. 10.1016/j.snb.2015.12.039. [DOI] [Google Scholar]
- a Ko C.-C.; Wing-Wah Yam V. Transition metal complexes with photochromic ligands - photosensitization and photoswitchable properties. J. Mater. Chem. 2010, 20, 2063–2070. 10.1039/B919418E. [DOI] [Google Scholar]; b Coudret C.; Chernyshev A. V.; Metelitsa A. V.; Micheau J. C.. New Trends in spiro-compounds photochromic metals sensors: Quantitative aspects. In Photon-Working Switches; Yokoyama Y., Nakatani K., Eds.; Springer Japan KK: Tokio, 2017; Chapter 1, pp 3–35. [Google Scholar]
- Kopelman R. A.; Snyder S. M.; Frank N. L. Tunable photochromism of spirooxazines via metal complexation. J. Am. Chem. Soc. 2003, 125, 13684–13685. 10.1021/ja036306y. [DOI] [PubMed] [Google Scholar]
- Malatesta V.Photodegradation of Organic Photochromes. In Organic Photochromic and Thermochromic Compounds; Crano J. C.; Guglielmetti R. J., Eds.; Topics in Applied Chemistry. Kluwer Academic Publ.: New York, 2002; Vol. 2: Physicochemical Studies, biological Applications, and Thermochromism, Chapter 2, pp 65–166. [Google Scholar]
- a Eloy D.; Escaffre P.; Gautron R.; Jardon P. Etude par photolyse-éClair de la photodégradation sensibilisée de spiro (indoline-pyranes) et de spiro(indoline-oxazines) photochromiques. Bull. Soc. Chim. Belg. 1992, 101, 779–790. 10.1002/bscb.19921010907. [DOI] [Google Scholar]; b Baillet G.; Giusti G.; Guglielmetti R. Study of the fatigue process and the yellowing of polymeric films containing spirooxazine photochromic compounds. Bull. Chem. Soc. Jpn. 1995, 68, 1220–1225. 10.1246/bcsj.68.1220. [DOI] [Google Scholar]
- a Steen J. D.; Duijnstee D. R.; Browne W. R. Molecular switching on surfaces. Surf. Sci. Rep. 2023, 78, 100596. 10.1016/j.surfrep.2023.100596. [DOI] [Google Scholar]; b Sun B. B.; Yao B. H.; He Y. Q.; Yang B. Preparation and photochromic performance of homogeneous phase nitrocellulose membrane grafting spirooxazine moieties. Coatings 2020, 10, 569. 10.3390/coatings10060569. [DOI] [Google Scholar]; c Gao H.; Liu G.; Cui C.; Wang M.; Gao J. Preparation and properties of a polyurethane film based on novel photochromic spirooxazine chain extension. New J. Chem. 2022, 46, 9128–9137. 10.1039/D2NJ00162D. [DOI] [Google Scholar]; d Peng S.; Wen J.; Hai M.; Yang Z.; Yuan X.; Wang D.; Cao H.; He W. Synthesis and application of reversible fluorescent photochromic molecules based on tetraphenylethylene and photochromic groups. New J. Chem. 2019, 43, 617–621. 10.1039/C8NJ05388J. [DOI] [Google Scholar]; e Abdollahi A.; Roghani-Mamaqani H.; Razavi B.; Salami-Kalajahi M. Photoluminescent and chromic nanomaterials for anticounterfeiting technologies: Recent advances and future challenges. ACS Nano 2020, 14, 14417–14492. 10.1021/acsnano.0c07289. [DOI] [PubMed] [Google Scholar]
- a Rahimi S.; Stumpf S.; Grimm O.; Schacher F. H.; Schubert U. S.; Schubert S. Dual photo- and pH-responsive spirooxazine-functionalized dextran nanoparticles. Biomacromolecules 2020, 21, 3620–3630. 10.1021/acs.biomac.0c00642. [DOI] [PubMed] [Google Scholar]; b Cusido J.; Ragab S. S.; Thapaliya E. R.; Swaminathan S.; Garcia-Amorós J.; Roberti M. J.; Araoz B.; Mazza M. M. A.; Yamazaki S.; Scott A. M.; Raymo F. M.; Bossi M. L. A photochromic bioconjugate with photoactivatable fluorescence for superresolution imaging. J. Phys. Chem. C 2016, 120, 12860–12870. 10.1021/acs.jpcc.6b03135. [DOI] [Google Scholar]; c Li X.; Li C.; Wang S.; Dong H.; Ma X.; Cao D. Synthesis and properties of photochromic spirooxazine with aggregation-induced emission fluorophores polymeric nanoparticles. Dyes Pigm. 2017, 142, 481–490. 10.1016/j.dyepig.2017.03.068. [DOI] [Google Scholar]
- a Frawley A. T.; Wycisk V.; Xiong Y.; Galiani S.; Sezgin E.; Urbancic I.; Vargas Jentzsch A.; Leslie K. G.; Eggeling C.; Anderson H. L. Super-resolution RESOLFT microscopy of lipid bilayers using a fluorophore-switch dyad. Chem. Sci. 2020, 11, 8955–8960. 10.1039/D0SC02447C. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Frawley A. T.; Leslie K. G.; Wycisk V.; Galiani S.; Shrestha D.; Eggeling C.; Anderson H. L. A photoswitchable solvatochromic dye for probing membrane ordering by RESOLFT super-resolution microscopy. ChemPhysChem 2023, 24, e202300125 10.1002/cphc.202300125. [DOI] [PubMed] [Google Scholar]
- a Sanjabi S.; Keyvan Rad J.; Mahdavian A. R. Spiropyran and spironaphthoxazine based opto-chemical probes for instant ion detection with high selectivity and sensitivity to trace amounts of cyanide. J. Photochem. Photobiol. A: Chem. 2022, 424, 113626. 10.1016/j.jphotochem.2021.113626. [DOI] [Google Scholar]; b Sanjabi S.; Keyvan Rad J.; Mouraki A.; Asadi-Aghbolaghi N.; Salehi-Mobarakeh H.; Mahdavian A. R. Colorimetric photosensing of solvent polarity using photochromic spironaphthoxazine dye and its stimuli-responsive acrylic copolymer: A physico-chemical study. Opt. Mater. 2023, 142, 114083. 10.1016/j.optmat.2023.114083. [DOI] [Google Scholar]
- a Khunkhong N.; Kitchawengkul N.; Wongnongwa Y.; Jungsuttiwong S.; Keawin T.; Promarak V.; Nalaoh P.; Suttisintong K.; Chansaenpak K.; Jarujamrus P. A novel spirooxazine derivative as a colorimetric probe for Fe2+ and Pb2+ determination on microfluidic paper-based analytical device (μPAD) for maintaining in photochromic efficiency. Dyes Pigm. 2023, 208, 110869. 10.1016/j.dyepig.2022.110869. [DOI] [Google Scholar]; b Naksomboon K.; Kaewchangwat N.; Sirisaksoontorn W.; Suttisintong K. A novel indolino-spironaphthooxazine as the highly sensitive and selective probe for colorimetric detection of Au3+. Dyes Pigm. 2023, 214, 111193. 10.1016/j.dyepig.2023.111193. [DOI] [Google Scholar]; c Foytong W.; Pattaweepaiboon S.; Kaewchangwat N.; Suttisintong K.; Sirisaksoontorn W. Synthesis, structural analysis and sensing performance of a novel spirooxazine derivative as a turn-on fluorescence probe for Cu2+ detection with high selectivity and sensitivity. Supramol. Chem. 2022, 34, 46–58. 10.1080/10610278.2023.2221365. [DOI] [Google Scholar]
- Yuan J.; Yuan Y.; Tian X.; Wang H.; Liu Y.; Feng R. Photoswitchable boronic acid derived salicylidenehydrazone enabled by photochromic spirooxazine and fulgide moieties: multiple responses of optical absorption, fluorescence emission, and quadratic nonlinear optics. J. Phys. Chem. C 2019, 123, 29838–29855. 10.1021/acs.jpcc.9b08050. [DOI] [Google Scholar]
- a Nickel F.; Bernien M.; Lipowski U.; Kuch W. Optical differential reflectance spectroscopy for photochromic molecules on solid surfaces. Rev. Sci. Instrum. 2018, 89, 033113. 10.1063/1.5019415. [DOI] [PubMed] [Google Scholar]; b Nickel F.; Bernien M.; Krüger D.; Miguel J.; Britton A. J.; Arruda L. M.; Kipgen L.; Kuch W. Highly efficient and bidirectional photochromism of spirooxazine on Au(111). J. Phys. Chem. C 2018, 122, 8031–8036. 10.1021/acs.jpcc.8b02220. [DOI] [Google Scholar]
- a Gupta V. K.Photochromic dyes for smart textiles. In Dyes and Pigments - Novel Applications and Waste Treatment; Papadakis R., Ed.; IntechOpen: Rijeka, 2021, Chapter 5, pp 81–98. [Google Scholar]; b Abate M. T.; Seipel S.; Yu J.; Vikova M.; Vik M.; Ferri A.; Guan J.; Chen G.; Nierstrasz V. Supercritical CO2 dyeing of polyester fabric with photochromic dyes to fabricate UV sensing smart textiles. Dyes Pigm. 2020, 183, 108671. 10.1016/j.dyepig.2020.108671. [DOI] [Google Scholar]
- a Zhang T.; Lou X. Y.; Li X.; Tu X.; Han J.; Zhao B.; Yang Y. W. Tunable photochromism of spirooxazine in the solid state: a new design strategy based on the hypochromic effect. Adv. Mater. 2023, 35, 2210551. 10.1002/adma.202210551. [DOI] [PubMed] [Google Scholar]; b Schwartz H. A.; Werker M.; Tobeck C.; Christoffels R.; Schaniel D.; Olthof S.; Meerholz K.; Kopacka H.; Huppertz H.; Ruschewitz U. Novel photoactive spirooxazine based switch@MOF composite materials. ChemPhotoChem. 2020, 4, 195–206. 10.1002/cptc.201900193. [DOI] [Google Scholar]
- Cannavale A.; Zampini G.; Carlucci F.; Pugliese M.; Martellotta F.; Ayr U.; Maiorano V.; Ortica F.; Fiorito F.; Latterini L. Energy and daylighting performance of building integrated spirooxazine photochromic films. Sol. Energy 2022, 242, 424–434. 10.1016/j.solener.2021.10.058. [DOI] [Google Scholar]