Abstract
Ehrlichia sennetsu is the causative agent of human Sennetsu ehrlichiosis. Heat shock protein 60 (HSP60) and HSP70 (DnaK) are two major bacterial HSPs, and their interaction modulates the stress response. Previously, we cloned and sequenced groE and expressed groEL of E. sennetsu. HSP60 (GroEL) was immunogenic and cross-reactive in Ehrlichia spp. The present study was designed to (i) characterize the HSP70 gene of this organism and (ii) determine whether the expression of these two HSPs is inducible upon exposure to heat stress. A gene encoding an HSP70 homolog was isolated and sequenced from a gene library. The ehrlichial HSP70 gene encoded a 637-amino-acid protein, which had an approximate molecular mass of 68,354 Da and which was homologous to DnaK of Escherichia coli. A DNA sequence resembling −35 and −10 promoter sequences of E. coli dnaK was observed upstream of the ehrlichial HSP70 gene. Alignment of the predicted amino acid sequence with that of E. coli DnaK and Brucella, Salmonella, Borrelia, Chlamydia, and Mycobacterium HSP70s showed 63, 67, 63, 62, 58, and 53% identity, respectively. By reverse transcription-PCR analysis, the mRNA levels of ehrlichial HSP70 and HSP60 were examined after temperature shifts from 28 to 37°C and from 37 to 40°C. HSP70 mRNA induction levels were greater than those of HSP60 mRNA after a 37-to-40°C temperature shift, whereas the reverse was true after a 28-to-37°C temperature shift. Our data suggest that HSP60 and HSP70 may play different roles during transfer from vector temperature to human body temperature and during a febrile condition characteristic of ehrlichial disease. This study also provides a useful model system for examining mRNA expression in obligatory intracellular bacteria.
Ehrlichia sennetsu, which belongs to the family Rickettsiaceae, is an obligate intracellular bacterium of monocytes and macrophages. E. sennetsu is the etiologic agent of human Sennetsu ehrlichiosis, a febrile illness with lymphadenopathy, cases of which have occurred in western Japan and recently in Malaysia (10, 28). The term heat shock protein (HSP) refers to the evolutionarily highly conserved stress-inducible or constitutive proteins that maintain homeostasis in eukaryotic and prokaryotic cells (16, 23). The immunology of HSP has been studied extensively. For example, HSP60 (GroEL) and HSP70 (DnaK) of a number of bacteria, including Mycobacterium, Borrelia, Chlamydia, and Legionella spp., have been recognized as common antigens in the immune response to bacterial infection and in autoimmune diseases (4, 8, 11, 13, 15, 25, 30, 35). Recent studies have revealed that bacterial HSP60 and HSP70 modulate immunity by directly inducing cytokine mRNA production in macrophages (25). In addition, studies of the prokaryotic cell also revealed that an HSP70 homolog might play a role in the recognition or binding between a pathogen and the host cell, both of which are believed to be critical for Ehrlichia infection. To date, these data suggest that HSP70 may be present on the bacterial surface and that the heat shock response appears to mediate adhesion to the host cell (12, 14, 22, 24). It is reported that bacterial HSP70 contributes to the pathogenesis of Mycobacterium spp., which infect and replicate in macrophages (25). The role of bacterial HSP70 in Ehrlichia-infected macrophages has not been established.
Bacterial HSPs are regulated by heat shock promoters that can be recognized by the ς32 factor of RNA polymerase holoenzyme. It is reported that Escherichia coli and chlamydial HSP70s have −35 promoter regions which are similar to the heat shock promoter of HSP60 (5, 9). Genetic data show that the interaction between HSP60 and HSP70 modulates the heat shock response (7, 20, 22, 33). In a sense, HSP70 plays the role of chaperone by primarily preventing aggregation or premature folding until the substrate protein can assemble into the appropriate multisubunit complex and be translocated across a membrane or passed on to a different chaperone HSP60 (1). It is also believed that HSP70 acts as a negative modulator of the heat shock response via interaction with a ς32 homolog (21, 22). The interaction between ehrlichial HSPs and host immunity has not been established. In our laboratory, the HSP60 homolog of E. sennetsu was characterized, expressed, and immunologically analyzed (34). So far, the HSP60 genes and proteins of several Ehrlichia spp. have been characterized (16, 31, 32, 34). Although a DNA sequence of a small fragment of the DnaK gene from Rickettsia prowazekii was reported (3), a complete HSP70 base or amino acid sequence has not been reported for any Rickettsia or Ehrlichia spp.
We have been interested in the role of HSPs in ehrlichial pathogenesis. The present study was designed to examine whether HSP60 and HSP70 expression in E. sennetsu is inducible following a temperature shift, which may occur in Ehrlichia when it infects a human host. For obligate intracellular bacteria, it is difficult to investigate the heat shock responses of their HSPs at the protein level because purification of the organisms is difficult, purification itself may cause a stress response, and the presence of homologous HSPs in the host cell may not be easy to distinguish. There is no report on HSP70 or HSP60 mRNA expression in Ehrlichia or Rickettsia spp. Therefore, we developed a reverse transcription-PCR (RT-PCR) method with the 16S rRNA of E. sennetsu as the internal control and investigated the thermoinducibility of HSP60 and HSP70 mRNA. Since the complete HSP70 gene has never been isolated in Ehrlichia spp., it was necessary to sequence the HSP70 gene of E. sennetsu in order to conduct our experiment. In this study, therefore, the entire HSP70 gene of E. sennetsu was cloned and sequenced.
MATERIALS AND METHODS
Bacterial strains, vectors, and reagents.
λ phage, Bluescript plasmid, and E. coli XL1-blue MRF′ and SOLR strains were purchased from Stratagene (La Jolla, Calif.). E. coli DH5α competent cells and restriction enzymes were purchased from GIBCO-BRL (Grand Island, N.Y.).
Ehrlichial cultivation and purification.
E. sennetsu was cultivated in a P388D1 murine macrophage-like cell line (26). DNA was extracted from the organisms, which had been purified by Sephacryl S-1000 chromatography (27).
Cloning the partial HSP70 gene from an E. sennetsu gene library.
All procedures were carried out by using the λZAPII/CIAP cloning kit (Stratagene), according to the manufacturer’s instructions. Briefly, genomic DNA was prepared from purified E. sennetsu by sodium dodecyl sulfate (SDS) lysis, phenol extraction, and ethanol precipitation and digested with the XbaI restriction enzyme. The digested fragments were ligated into the XbaI site of the λZAPII vector. The gene library was constructed by infecting the E. coli XL1-blue MRF′ strain with the recombinant phage. Clones expressing ehrlichial antigens were identified by using the rabbit anti-E. sennetsu serum (34), which was preabsorbed with E. coli lysate and the recombinant HSP60 of E. sennetsu (34). This absorbed serum specifically reacted with the 70-kDa protein and some other proteins, but not with the HSP60 of E. sennetsu, in Western blotting. The 70-kDa protein is one of the major protein components in a Coomassie blue-stained E. sennetsu SDS-polyacrylamide gel electrophoresis gel. When the intact organism was mildly treated with Sarkosyl, this 70-kDa protein appeared to be predominant in the soluble fraction of the organism. We believe that this 70-kDa protein is an HSP70 homolog. A recombinant pBluescript phagemid (pES70X) was excised from the positive λZAPII phage in the presence of helper phage f1 and was used to transform E. coli SOLR cells, according to the manufacturer’s instructions. The transformed E. coli cells were cultured at 37°C overnight in Luria broth (LB) medium containing 50 μg of ampicillin per ml. The phagemids were isolated by an alkaline method (29).
DNA sequence analysis.
The DNA sequence was determined by the dideoxy-termination method with an Applied Biosystems (Foster City, Calif.) 373A DNA sequencer. The DNA sequence reaction was conducted with suitable synthetic oligonucleotides as primers. Translation of the nucleotide sequence and alignment of the amino acid sequence were performed by using DNASIS computer software (Hitachi Software Engineering Co., Ltd., Yokohama, Japan). A homology search of the GenBank database (National Center for Biotechnology Information, Bethesda, Md.) was conducted by using a software basic local alignment search tool (2).
Southern blot analysis of genomic DNA of E. sennetsu.
A restriction enzyme map was constructed based on the base sequence of the cloned partial HSP70 gene. The phagemid (pES70X) containing the HSP70 gene truncated at the 5′ end (32 μg) was digested with restriction enzyme XbaI (30 U) at 37°C for 1 h; then the same amount of EcoRI (30 U) was added (the reaction buffer was adjusted by following the instructions of the manufacturer), and the mixture was incubated at 37°C for an additional 1 h. The reaction mixture was immediately applied to a 1% agarose gel and electrophoresed at 90 mA for about 2 h. A DNA ladder (HaeIII-digested φ174 phage; GIBCO-BRL) was used to identify the molecular range of the fragments. An approximately 0.4-kb restriction fragment was recovered from the gel by using a QIAEX II agarose gel extraction kit (Qiagen, Chatsworth, Calif.). The fragment was labeled with 32P by using a multipriming DNA fragment labeling kit (Amersham, Arlington Heights, Ill.). The labeled fragments were used as probes for Southern blot analysis and colony hybridization. Genomic DNA of E. sennetsu was digested with one of various restriction enzymes, either ClaI, BamHI, EcoRI, HindIII, or KpnI (1 U of enzyme per μg of DNA), at 37°C for 90 min. The digested fragments were electrophoresed in a 0.7% agarose gel and transferred onto a nylon membrane (Amersham); then the membrane was baked at 80°C for 2 h. Southern blotting was conducted as recommended by the nylon transfer membrane supplier. Hybridization was carried out at 65°C overnight, and washing was conducted at 65°C with 0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) containing 1% SDS. Autoradiography was conducted at −80°C overnight with high-performance autoradiography film (Hyperfilm; Amersham).
Cloning the 5′ end of the HSP70 gene from an E. sennetsu gene library.
The genomic DNA of E. sennetsu was digested with restriction enzyme HindIII at 37°C for 2 h and electrophoresed in a 0.7% agarose gel immediately. The 1- to 2-kb fragments were recovered from the gel as described above. About 3 μg of Bluescript/+SK (PB/+SK) plasmids (Strategene) was digested with 5 μl of HindIII at 37°C for 4 h, and then 0.17 U of calf intestinal alkaline phosphatase (GIBCO-BRL) was added and the mixture was incubated for an additional 30 min at 37°C. The dephosphorylated plasmids were electrophoresed in a 0.7% agarose gel and were recovered from the gel. About 10 ng of the digested PB/+SK vector and 7 ng of the genomic DNA (1- to 2-kb range) were mixed to carry out a ligation reaction in the presence of T4 ligase (GIBCO-BRL) at 14°C overnight, by following the manufacturer’s instructions. The ligation mixture was used to transform E. coli DH5α competent cells as described by the manufacturer. The transformed cells were spread on an LB plate (100 mm in diameter) containing 50 μg of ampicillin and 0.2 mg of methicillin per ml in the presence of isopropyl-β-d-thiogalactopyranoside (IPTG; 1 mM) and 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal). The plates were incubated at 37°C overnight. The visible colonies were transferred to nitrocellulose membranes (Schleicher & Schuell, Inc., Keene, N.H.). The colonies on the membrane were lysed by using an alkaline method (29), and DNA was fixed onto the membrane by baking the membrane at 80°C under vacuum. The colony hybridization was carried out under the conditions described above. The positive colonies were picked up from the master plate and cultured overnight in LB in the presence of 50 μg of ampicillin per ml. The recombinant plasmids, designated pES70H, were isolated in accordance with the alkaline minipreparation method of Sambrook et al. (29), and the insert was confirmed by Southern blot analysis and sequenced as described above.
Analysis of E. sennetsu heat stress response.
E. sennetsu-infected P388D1 cells were grown in RPMI medium with 10% fetal bovine serum and 2 mM l-glutamine (26) at either 28 or 37°C until they reached about 70% infectivity. To cause heat stress, either fresh medium prewarmed at 37°C was added to cells which had been cultivated at 28°C and which were continuously cultivated at 37°C for up to 12 h after the addition or fresh medium prewarmed at 40°C was added to cells which had been cultivated at 37°C and which were continuously cultivated at 40°C for up to 12 h after the addition. The cells (107 cells per time point) were harvested for RNA isolation at 0, 0.5, 1, 2, 4, 6, and 12 h after the temperature shift.
Time course analysis of HSP70 and HSP60 transcription under heat stress by RT-PCR.
RNA was prepared from 107 E. sennetsu-infected P388D1 cells by the guanidine thiocyanate method with TRIzol reagent (Life Technologies, Gaithersburg, Md.). The isolated RNA (2 μg) was heated at 75°C for 3 min and reverse transcribed in a 30-μl reaction mixture containing 1× reaction buffer (50 mM Tris-HCl [pH 8.3], 75 mM KCl, 3 mM MgCl2), 0.5 mM deoxynucleotide triphosphate mixture, 1 U of RNase inhibitor per μl, 10 U of reverse transcriptase (GIBCO-BRL), and 1.5 μM concentrations of primers which were complementary to the sequence of the HSP70 mRNA (5′-TTGTGGTGTTGCGGTCTATG-3′) or the groEL mRNA (5′-TTCACCCTCAACATCCTCAGCAAT-3′) of E. sennetsu (34) at 42°C for 1 h. The reaction was terminated by incubating the mixture at 94°C for 2 min. The cDNA product (1 to 2 μl) was amplified in a 50-μl reaction mixture containing 1× PCR buffer (10 mM Tris-HCl [pH 8.3], 50 mM KCl, 1.5 mM MgCl2), a 0.2 mM deoxynucleotide triphosphate mixture, 2.0 U of Taq DNA polymerase (Life Technologies, Inc.), and 0.4 μM concentrations of 3′ and 5′ primers (5′-CCAGGGAAAGTGGTGTGACGTC-3′ and 5′-ACTGCTGATGCTGCAGGTCCT-3′, respectively, based on the E. sennetsu HSP70 gene sequence, for the partial E. sennetsu HSP70 gene or 5′-ATTGGTTGTATGCTAGAGAGT-3′ and 5′-CGGAAGTAACCAAGGATGGTTATAA-3′, respectively, based on the E. sennetsu HSP60 gene, for the partial E. sennetsu groEL gene) in a DNA thermal cycler (model 480; The Perkin-Elmer Corp., Norwalk, Conn.). Each PCR cycle consisted of denaturation at 94°C for 45 s, annealing at 60°C for 45 s, and extension at 72°C for 2 min and was repeated for 28 cycles. To rule out contamination of DNA during RNA preparation, a negative control was prepared by assembling a reaction mixture that contained RNA and all reagents except for reverse transcriptase. To monitor the influence of ehrlichial growth during the incubation period, and as an internal control, 16S rRNA primers based on the 16S rRNA gene of E. sennetsu (5′-AGAACGAACGCTAGCGGTAGGC-3′ and 5′-CGTATTACCGCGGCTGCTGGCA-3′) were added to the reaction mixture with HSP70 or HSP60 primers. The different primers made the RT-PCR products distinguishable based on the sizes of their amplified products (approximately 300 bp for HSP70 RNA, 500 bp for HSP60 RNA, and 400 bp for 16S rRNA). To make sure that the primers used were specific to ehrlichiae and did not cross-react with the host HSP60 or HSP70 gene, RNA of the uninfected P388D1 cells was isolated and used as a template as well.
Determination of relative HSP70 and HSP60 mRNA levels under heat stress.
To compare relative levels of HSP70 and HSP60 mRNA, RT-PCR products were electrophoresed in a 1.8% agarose gel. HaeIII-digested φX174 replicative-form DNA fragments (GIBCO-BRL) were used as molecular size markers (72 to 1353 bp). The amount of PCR products was analyzed by using a gel video system (Gel Print 2000i; BioPhotonics Corp., Ann Arbor, Mich.) and image analysis software (ImageQuaNT; Molecular Dynamics, Sunnyvale, Calif.).
Nucleotide sequence accession numbers.
The nucleotide sequences of the HSP70 genes of Brucella, E. coli, Salmonella, Borrelia, Chlamydia, and Mycobacterium have been assigned GenBank accession number as follows: Brucella, M95799; E. coli, D10765; Salmonella, U58360; Borrelia, M97912; Chlamydia, M69227; and Mycobacterium, X58406. The nucleotide sequence of E. sennetsu HSP70 has been assigned GenBank accession no. AF060197.
RESULTS
Cloning of the E. sennetsu HSP70 gene.
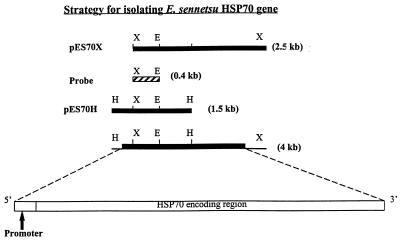
An XbaI fragment (2.5 kb) of E. sennetsu genomic DNA was cloned in λ phage. The phagemid excised from the recombinant phage was designated pES70X and consisted of a 1.3-kb open reading frame (ORF) at one end of the insert (Fig. 1). The nucleotide sequence of the ORF and the corresponding predicted amino acid sequence indicated that the ORF was the partial HSP70 gene. Based on the restriction enzyme map of the ORF (data not shown), XbaI and EcoRI sites (at nucleotide positions 701 and 1140, respectively) were used to make an approximately 0.4-kb probe for cloning the 5′ end of the ehrlichial HSP70 gene (Fig. 1). Southern blot analysis revealed that genomic DNA digestion with HindIII resulted in a 1.5-kb fragment which contained the 5′ end of the HSP70 gene (Fig. 2). The fragment was isolated and ligated into a HindIII-digested pBluescript plasmid, referred to as pES70H. The sequence of the insert indicated that there was 1,342 bp of ORF at one end of the fragment. The nucleotide sequence at the 3′ end of the ORF in the pES70H insert overlapped with that of the 5′ end of the ORF in the pES70X insert. The overlapped region contained the sequence of the 0.4-kb probe (Fig. 1). These two ORFs represent the full length of the region encoding the ehrlichial HSP70 (Fig. 1).
FIG. 1.
Restriction maps and cloning strategy for the E. sennetsu HSP70 gene. The ehrlichial DNA fragment was cloned in the pBluescript vector. pES70X containing the truncated 5′ end of the HSP70 gene was used to make a probe to identify pES70X containing the 5′ end of the HSP70 gene. Both plasmids, which had overlapping sequences, were used to identify the full-length HSP70 gene (1,911 bp). X, E, and H: XbaI, EcoRI, and HindIII restriction sites, respectively. Open and solid bars represent the ehrlichial inserts in the vectors. The hatched bar represents the probe. The arrow indicates a promoter.
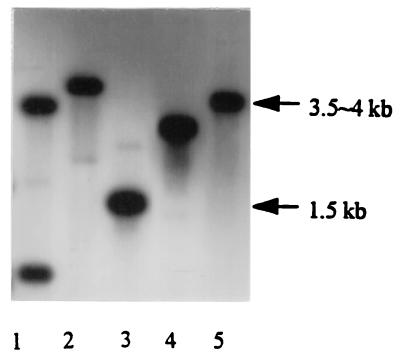
FIG. 2.
Southern blot analysis of E. sennetsu genomic DNA digested with various restriction enzymes. Lanes: 1, ClaI; 2, EcoRI; 3, HindIII; 4, XbaI; 5, BamHI. A 32P-labeled 0.4-kb fragment of the ehrlichial HSP70 gene was used as the probe. Arrows indicate molecular sizes.
Characterization of the E. sennetsu HSP70 gene.
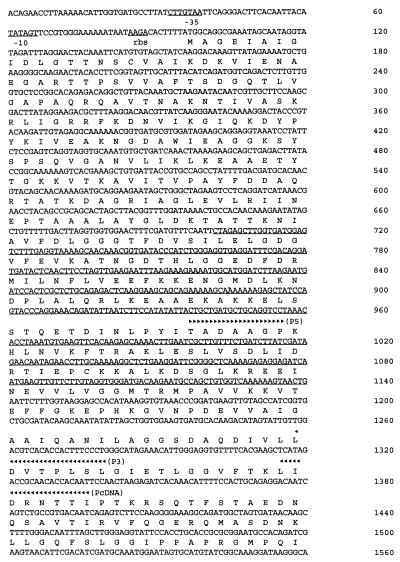
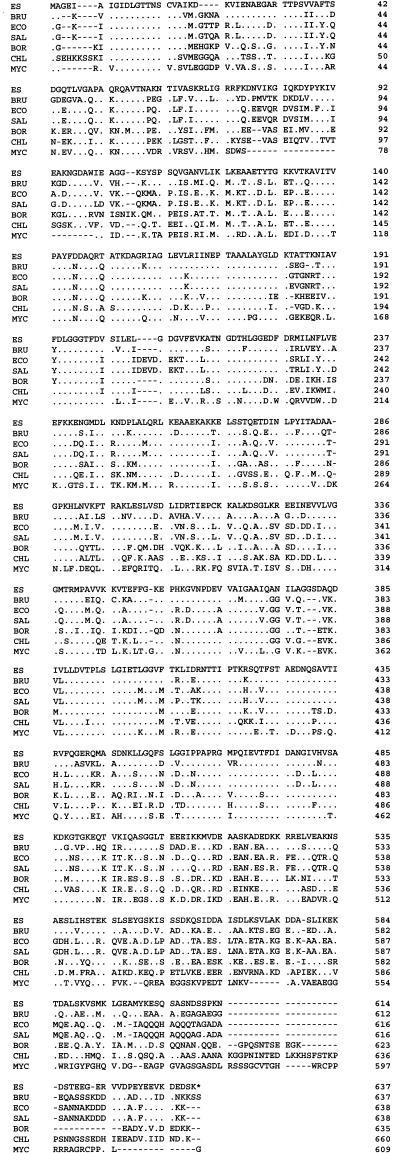
The nucleotide sequence of the gene encoding the E. sennetsu HSP70 homolog and the predicted amino acid sequence are shown in Fig. 3. A 1,911-bp ORF commences with a methionine codon (ATG) and terminates with a stop codon; it encodes a 637-amino-acid protein with an approximate molecular mass of 68,354 Da. A purine-rich putative ribosome-binding site (Shine-Dalgarno sequence) is located 7 nucleotides ahead of the ATG initial codon. DNA sequences resembling −35 and −10 promoter sequences of ς70 precede the start codon by 55 and 28 bp, respectively (Fig. 3). The −35 promoter region was also similar to the consensus heat shock promoter recognized by ς32, the ς32 subunit of E. coli RNA polymerase (Fig. 3). Alignment of the predicted amino acid sequence with the protein sequences of E. coli DnaK indicates that the encoded protein is an HSP70 homolog (Fig. 4). Figure 4 shows that the ehrlichial HSP70 has 67, 63, 63, 62, 58, and 53% identity with that of Brucella, E. coli, Salmonella, Borrelia, Chlamydia, and Mycobacterium, respectively. The pattern of hybridization between a 0.4-kb HSP70 probe and ehrlichial genomic DNA digested with various restriction enzymes is presented in Fig. 2. The hybridization result indicates that the ehrlichial chromosome contains a single copy of the HSP70 gene. Based on the sequence data, there were no BamHI sites within the HSP70 gene, only one EcoRI site (at nucleotide position 1140), two ClaI sites (at nucleotide positions 1014 and 1515), and two HindIII sites (at nucleotide positions 1437 and 1703) (Fig. 3). Since there is a ClaI site in the 0.4-kb probe sequence (at nucleotide position 1014), there were two bands for ClaI-digested DNA in the Southern blot result (Fig. 2). The results of restriction enzyme digestion and Southern blot analysis (Fig. 2) corresponded to the nucleotide sequence information.
FIG. 3.
DNA sequence of the E. sennetsu HSP70 gene and corresponding deduced amino acid sequence. Putative promoter and ribosome-binding sites (rbs) are underlined. The sequence of the probe used to carry out colony hybridization and Southern blotting is also underlined. The sequences of RT-PCR primers are underlined with arrowheads (P5 and P3 represent the 5′- and 3′-end primers for PCR, respectively; PcDNA represents the primer for reverse transcription).
FIG. 4.
Alignment of the E. sennetsu (ES) HSP70 protein sequence with that of E. coli (ECO) DnaK and Brucella (BRU), Borrelia (BOR), Salmonella (SAL), Chlamydia (CHL), and Mycobacterium (MYC) HSP70. A dot represents an amino acid identical to that of ehrlichial HSP70; a dash represents a gap introduced into the sequence.
Observation of relative mRNA levels of the ehrlichial HSP60 and HSP70 genes under heat stress.
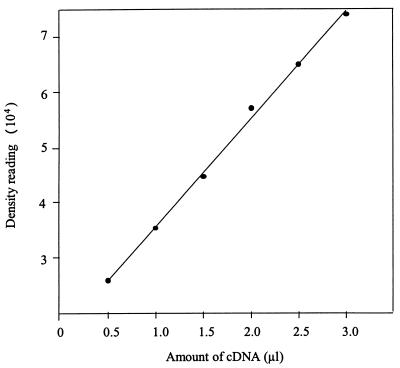
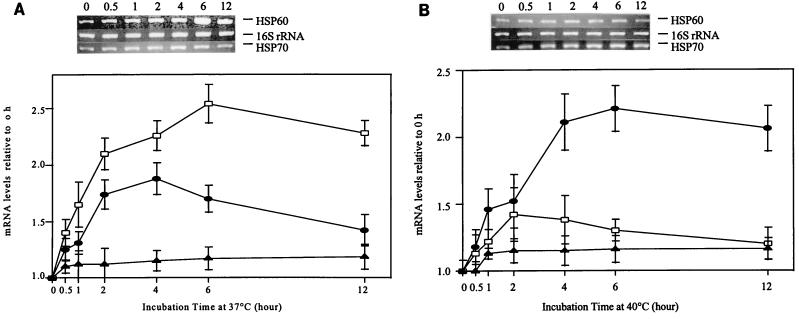
To examine the patterns of HSP60 and HSP70 mRNA levels under heat stress, a time course analysis was performed by using RT-PCR. The linearity of the RT-PCR assay was verified by using various amounts of target cDNA (HSP70 cDNA) (Fig. 5). Figure 5 shows that the intensities of the PCR products as measured by densitometry, when plotted against the amount of cDNA, yielded a linear relationship (r = 0.99). A photograph of an ethidium bromide-agarose gel was obtained under optimal exposure conditions to avoid saturating strong bands while exposing weak bands. Additional PCR experiments performed with the target cDNA (16S rRNA) in the same manner showed similar results (data not shown). No PCR product was generated in the control lacking reverse transcriptase for each sample (data not shown). No PCR product was generated when RNA of uninfected P388D1 cells was used as the template (data not shown). For the 28-to-37°C temperature transition, levels of ehrlichial HSP60 mRNA increased 1.5-fold 1 h after the temperature shift, reached a peak (2.5-fold) at 6 h, and remained at a higher level (about 2-fold) at 12 h (Table 1 and Fig. 6A). The response of HSP70 mRNA was slightly slower than that of HSP60 mRNA. The HSP70 mRNA response started 1 h after the temperature shift (Table 1 and Fig. 6A). The HSP70 mRNA levels increased by 1.7-fold at 2 h and stayed at the same level until they declined to 1.4-fold 12 h after the transition. The internal control, 16S rRNA levels, did not significantly increase by 6 h after the transition to 37°C (Table 1). For the 37-to-40°C transition, the levels of HSP60 mRNA were less influenced (Fig. 6B) than they were by the 28-to-37°C transition. The levels of HSP70 mRNA were relatively more upregulated by the 37-to-40°C temperature shift (by 1.5-fold from ∼1 to 2 h after the shift and by greater than 2-fold from 2 to 12 h) (Table 1 and Fig. 6B). The 16S rRNA levels did not significantly increase after the transition to 40°C (Table 1). The 16S rRNA results in this study demonstrated that most changes of HSP mRNA were caused by heat stress rather than ehrlichial growth. The 16S rRNA data also suggest that E. sennetsu was not killed at 40°C.
FIG. 5.
The linearity of RT-PCR assays with various amounts of target HSP70 cDNA. The intensities of the PCR products on an ethidium bromide-agarose gel were measured by using a gel video system and image analysis software. The intensities were plotted against the amounts of cDNA in the PCR mixture. The line was drawn from a linear regression analysis of all data points (r = 0.99). PCR experiments performed with different target cDNA showed similar results.
TABLE 1.
Statistical analysis of mRNA expression based on densitometry of RT-PCR products
| Time under heat stress (h) | Mean RNA expression level relative to level at 0 h ± SD for indicated temp shifta
|
|||||
|---|---|---|---|---|---|---|
| 28 to 37°C
|
37 to 40°C
|
|||||
| HSP60 mRNA | HSP70 mRNA | 16S rRNA | HSP60 mRNA | HSP70 mRNA | 16S rRNA | |
| 0 | 1A | 1A | 1A | 1A | 1A | 1A |
| 0.5 | 1.4 ± 0.12B | 1.25 ± 0.10B | 1.1 ± 0.06A | 1.13 ± 0.14A | 1.18 ± 0.13A | 1 ± 0.10A |
| 1 | 1.65 ± 0.20B | 1.31 ± 0.10B | 1.12 ± 0.12A | 1.22 ± 0.09A+B | 1.46 ± 0.15B | 1.13 ± 0.04A |
| 2 | 2.1 ± 0.14C | 1.74 ± 0.13C | 1.15 ± 0.14A | 1.42 ± 0.15B | 1.52 ± 0.20B | 1.15 ± 0.09A |
| 4 | 2.26 ± 0.13C+D | 1.88 ± 0.14C | 1.15 ± 0.09A | 1.38 ± 0.16B | 2.11 ± 0.21C | 1.15 ± 0.11A |
| 6 | 2.54 ± 0.17D | 1.7 ± 0.12C | 1.17 ± 0.10A | 1.3 ± 0.08B | 2.21 ± 0.17C | 1.16 ± 0.10A |
| 12 | 2.28 ± 0.11D+C | 1.42 ± 0.14B+C | 1.17 ± 0.11A | 1.2 ± 0.12A+B | 2.04 ± 0.16C | 1.16 ± 0.08A |
Results are from triplicate assays. Data with the same superscript are not significantly different.
FIG. 6.
Relative levels of HSP60 and HSP70 mRNA at different temperature transitions. (A) Relative levels of HSP60 and HSP70 mRNA at the transition from 28 to 37°C determined by using RT-PCR. The top part of the panel shows RT-PCR agarose gel results for the 37°C transition; the bottom part of the panel shows mRNA levels relative to that at 0 h based on the densities of the PCR products. The y axis represents the ratios of the intensities of PCR products, with the mRNA level at 0 h defined as 1. Symbols: □, HSP60 mRNA; •, HSP70 mRNA; ▴, 16S rRNA (internal control). The values are means ± standard deviations (SD) and were determined based on three independent experiments. (B) Relative levels of HSP60 and HSP70 mRNA at the transition from 37 to 40°C determined by using RT-PCR. The top part of the panel shows the RT-PCR agarose gel result for the 40°C transition; the bottom part of the panel shows mRNA levels relative to that at 0 h based on the densities of the PCR products. The y axis represents the ratios of the intensities of PCR products, with the mRNA level at 0 h defined as 1. Symbols: □, HSP60 mRNA; •, HSP70 mRNA; ▴, 16S rRNA (internal control). The values are means ± SD and were determined based on three independent experiments.
DISCUSSION
This is the first report of HSP mRNA expression in ehrlichiae and rickettsiae. Although the physiological mechanisms of the heat shock response in prokaryotic cells are yet to be investigated, upregulation of the HSP level under stress is critical for survival under unfavorable circumstances. Regulation of the heat shock response in prokaryotic cells has been extensively investigated for E. coli. High levels of both GroEL and DnaK synthesis have protective roles for E. coli growth between 20 and 40°C. Our data demonstrated that HSP60 and HSP70 mRNA of E. sennetsu were induced in different patterns by thermal stress, which may occur in ehrlichial infection. In this study, both ehrlichial HSP70 and HSP60 (GroEL) mRNA expression levels increased after 1 to 6 h of a heat shock consisting of a temperature shift from 28 to 37°C. Higher levels remained after 12 h. This indicated that both HSP60 and HSP70 are important for ehrlichial adaptation to ideal growth conditions (37°C) when they are transmitted from the tick to the mammalian host. It is unclear how the induced HSPs enhance ehrlichial viability in vivo during growth at the core temperatures of the human body. It is possible that HSPs facilitate cell adhesion between the organisms and host cells or stabilize ς32 for rapid growth under ideal conditions. McCarty and Walker (19) indicated that a DnaK mutant impairs E. coli growth only at temperatures above 39°C. Our results for ehrlichial HSP70 showed a pattern similar to that found in the E. coli DnaK study. Ehrlichial HSP70 mRNA increased more than HSP60 mRNA when the temperature was raised from 37 to 40°C. The level of HSP70 mRNA increased significantly at 40°C and remained more than twice the level at 37°C at 12 h after the transition. In contrast, the level of HSP60 mRNA increased less after 2 h at 40°C and returned to the basal level after 12 h. These results suggest that HSP70 may play a more active role than HSP60 in ehrlichial survival during the febrile stage (40°C) in patients.
The interaction between HSP60 and HSP70 plays a critical role in the heat shock response. For instance, the dissociation of the GroEL (HSP60)-DnaK (HSP70) complex in cytosol upregulates HSP60 expression in E. coli (19). Binding of ς32 at the −35 region (heat shock promoter) is thought to upregulate both GroEL and DnaK (5, 9, 33), which regulate the heat shock response. Like the HSP70 genes of E. coli and Chlamydia (5, 9), the ehrlichial HSP70 gene had a −35 region that is similar to the consensus ς32 promoter. However, previous studies also showed that the interaction between HSP60 and HSP70 under stress varies among bacteria. As in E. coli, HSP70 acts as a negative modulator for HSP60 expression in Haemophilus ducreyi in response to heat shock (22). However, Mogk et al. (20) reported that, in stressed Bacillus subtilis, GroEL acted as the modulator of the heat shock response instead of HSP70. They observed that the overproduction of GroEL decreased the expression of DnaK and that decreased expression of GroEL activated the expression of DnaK. The present ehrlichial study produced results similar to those of the B. subtilis study (20). After 2 h of heat stress at 40°C, the level of HSP70 mRNA of E. sennetsu kept increasing while the level of HSP60 mRNA declined. Although the trend at the 37°C transition was less remarkable than the trend at the 40°C transition, the transition results showed that increased HSP60 mRNA accompanied a stabilized HSP70 mRNA level. Our results suggest that HSP60 and HSP70 may play different roles under normal growth conditions and under febrile conditions. To understand the interaction between these major bacterial HSPs will require further investigation.
The role of HSP70 in ehrlichial pathogenesis is still unclear. Based on studies of other bacteria, HSP70 is not only involved in protein synthesis as a chaperone but also associated with the function of the bacterial outer membrane protein (12, 14, 24). Recent investigations of Haemophilus spp., Borrelia spp., and Chlamydia spp. show that thermoinduced or cell surface HSP70 may facilitate bacterial growth and survival by enhancing the binding between bacterial surface components and the membrane receptors of host cells (12, 14, 22, 24). It also has been reported that Mycobacterium HSP70 directly and rapidly induced cytokine mRNA production including interleukin-1α (IL-1α), IL-1β, IL-6, tumor necrosis factor alpha, and granulocyte-macrophage colony-stimulating factor mRNA in macrophages (25). Since the interaction of ehrlichiae with host cells influences proinflammatory cytokine mRNA expression (17, 18), the first isolation of ehrlichial HSP genes and the determination of the mRNA levels of ehrlichial HSP genes will give us a better understanding of pathogenesis in ehrlichiosis, an emerging disease. Cloning ehrlichial HSP70 might provide an additional tool for the investigation of Ehrlichia spp. and the host interaction.
This is the first report on cloning, sequencing, and expression of the ehrlichial HSP70 gene. Based on E. sennetsu HSP70 amino acid sequence data, the ehrlichial HSP70 has 67, 63, 63, 62, 58, and 53% identity with HSP70 of Brucella, E. coli, Salmonella, Borrelia, Chlamydia, and Mycobacterium, respectively. The heat shock response and HSPs have been evolutionarily conserved. Therefore, when more data are available, a phylogenetic analysis of HSP70 may provide us another tool to investigate molecular evolution among prokaryotic cells. The system may serve as a model for studying mRNA expression and regulation of intracellular bacteria.
ACKNOWLEDGMENT
This work was supported by grant R01 AI 30010 from the National Institutes of Health.
REFERENCES
- 1.Agad D A. To fold or not to fold. Science. 1993;260:1903–1904. doi: 10.1126/science.8100365. [DOI] [PubMed] [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Anderson S G E, Eriksson A, Naeslund A K, Anderson M S, Karland C G. The Rickettsia prowazekii genome. A random sequence analysis. Microb Comp Genom. 1996;1:293–315. [PubMed] [Google Scholar]
- 4.Anzola J, Luft B, Gorgone G, Dattwyler R J, Soderberg C, Lahesmaa R, Peltz G. Borrelia burgdorferi HSP 70 homolog: characterization of an immunoreactive stress protein. Infect Immun. 1992;60:3704–3713. doi: 10.1128/iai.60.9.3704-3713.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birkelund S, Lundemose A G, Christiansen G. The 75-kilodalton cytoplasmic Chlamydia trachomatis L2 polypeptide is a DnaK-like protein. Infect Immun. 1990;58:2098–2104. doi: 10.1128/iai.58.7.2098-2104.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chappell T G, Welch W J, Schlossmann D M, Palter K B, Schlesinger M J, Rothman J E. Uncoating ATPase is a member of the 70 kilodalton family of stress proteins. Cell. 1986;45:3–13. doi: 10.1016/0092-8674(86)90532-5. [DOI] [PubMed] [Google Scholar]
- 7.Cluss R G, Goel A S, Rehm H L, Schoenecker J G, Boothby J T. Coordinate synthesis and turnover of heat shock proteins in Borrelia burgdorferi: degradation of DnaK during recovery from heat shock. Infect Immun. 1996;64:1736–1743. doi: 10.1128/iai.64.5.1736-1743.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen I R, Young D B. Autoimmunity, microbial immunity and the immunological homunculus. Immunol Today. 1991;12:105–110. doi: 10.1016/0167-5699(91)90093-9. [DOI] [PubMed] [Google Scholar]
- 9.Cowing D W, Bardwell J C, Craig E A, Woolford C, Hendrix R W, Gross C A. Consensus sequence for Escherichia coli heat shock gene promoters. Proc Natl Acad Sci USA. 1985;82:2679–2683. doi: 10.1073/pnas.82.9.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukuda T, Kitao T, Keida Y. Studies on the causative agent of “Hyuganetsu” diseases. I. Isolation of the agent and its inoculation trial in human beings. Med Biol. 1954;32:200–209. [Google Scholar]
- 11.Haregewoin A, Soman G, Hom R C, Finberg R W. Human τδ+T cells respond to mycobacterial heat-shock protein. Nature (London) 1989;340:309–312. doi: 10.1038/340309a0. [DOI] [PubMed] [Google Scholar]
- 12.Hartmann E, Lingwood C. Brief heat shock treatment induces a long-lasting alteration in the glycolipid receptor binding specificity and growth rate of Haemophilus influenzae. Infect Immun. 1997;65:1729–1733. doi: 10.1128/iai.65.5.1729-1733.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffman P S, Houston L, Butler C A. Legionella pneumophila htpAB heat shock operon: nucleotide sequence and expression of the 60-kilodalton antigen in L. pneumophila-infected HeLa cells. Infect Immun. 1990;58:3380–3387. doi: 10.1128/iai.58.10.3380-3387.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaneda K, Masuzawa T, Yasugami K, Suzuki T, Suzuki Y, Yanagihara Y. Glycosphingolipid-binding protein of Borrelia burgdorferi sensu lato. Infect Immun. 1997;65:3180–3185. doi: 10.1128/iai.65.8.3180-3185.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koga T, Wand-Wuerttenberger A, DeBruyn J, Munk M E, Schoel B, Kauffmann S H E. T cells against bacterial heat shock protein recognize stressed macrophages. Science. 1989;245:1112–1115. doi: 10.1126/science.2788923. [DOI] [PubMed] [Google Scholar]
- 16.Kolbert C P, Bruinsma E S, Abdulkarim A S, Hofmeister E K, Tompkins R B, Telford III S R, Mitchell P D, Adams-Stich J, Persing D H. Characterization of an immunoreactive protein from the agent of human granulocytic ehrlichiosis. J Clin Microbiol. 1997;35:1172–1178. doi: 10.1128/jcm.35.5.1172-1178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee E H, Rikihisa Y. Absence of tumor necrosis factor alpha, interleukin-6 (IL-6), and granulocyte-macrophage colony-stimulating factor expression but presence of IL-1β, IL-8, and IL-10 expression in human monocytes exposed to viable or killed Ehrlichia chaffeensis. Infect Immun. 1996;64:4211–4219. doi: 10.1128/iai.64.10.4211-4219.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee E H, Rikihisa Y. Anti-Ehrlichia chaffeensis antibody complexed with E. chaffeensis induces potent proinflammatory cytokine mRNA expression in human monocytes through sustained reduction of IκB-α and activation of NF-κB. Infect Immun. 1997;65:2890–2897. doi: 10.1128/iai.65.7.2890-2897.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCarty J S, Walker G C. DnaK mutants defective in ATPase activity are defective in negative regulation of the heat shock response: expression of mutant DnaK proteins results in filamentation. J Bacteriol. 1994;176:764–780. doi: 10.1128/jb.176.3.764-780.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mogk A, Homuth G, Scholz C, Kim L, Schmid F X, Schumann W. The GroE chaperonin machine is a major modulator of the CIRCE heat shock regulon of Bacillus subtilis. EMBO J. 1997;16:4579–4590. doi: 10.1093/emboj/16.15.4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paek K-H, Walker G C. Escherichia coli dnaK null mutants are inviable at high temperature. J Bacteriol. 1987;169:283–290. doi: 10.1128/jb.169.1.283-290.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parsons L M, Limberger R J, Shayegani M. Alterations of levels of DnaK and GroEL result in diminished survival and adherence of stressed Haemophilus ducreyi. Infect Immun. 1997;65:2413–2419. doi: 10.1128/iai.65.6.2413-2419.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rassow J, von Ahsen O, Boamer U, Pfanner N. Molecular chaperones: towards a characterization of the heat-shock protein 70 family. Trends Cell Biol. 1997;7:129–133. doi: 10.1016/S0962-8924(96)10056-8. [DOI] [PubMed] [Google Scholar]
- 24.Raulston J E, Davis C H, Schmiel D H, Morgan M W, Wyrick P B. Molecular characterization and outer membrane association of a Chlamydia trachomatis protein related to the HSP 70 family of proteins. J Biol Chem. 1993;268:23139–23147. [PubMed] [Google Scholar]
- 25.Retzlaff C, Yamamoto Y, Hoffman P S, Friedman H, Klein T W. Bacterial heat shock proteins directly induce cytokine mRNA and interleukin-1 secretion in macrophage cultures. Infect Immun. 1994;62:5689–5693. doi: 10.1128/iai.62.12.5689-5693.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rikihisa Y. Clinical and immunological responses of ponies to Ehrlichia sennetsu and subsequent Ehrlichia risticii challenge. Infect Immun. 1988;56:2960–2966. doi: 10.1128/iai.56.11.2960-2966.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rikihisa Y, Zhang Y, Park J. Role of Ca2+ and calmodulin in ehrlichial infection in macrophages. Infect Immun. 1995;63:2310–2316. doi: 10.1128/iai.63.6.2310-2316.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ristic M. Current strategies in research on ehrlichiosis. In: Williams J C, Kakoma I, editors. Ehrlichiosis: a vector-borne disease of animals and humans. Boston, Mass: Kluwer Academic Publishers; 1990. pp. 136–153. [Google Scholar]
- 29.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 30.Shanafelt M-C, Hinderson P, Soderberg C, Mensi N, Turck C W, Webb D, Yssel H, Peltz G. T cell and antibody reactivity with the Borrelia burgdorferi 60-kDa heat shock protein in Lyme arthritis. J Immunol. 1991;146:3985–3992. [PubMed] [Google Scholar]
- 31.Sumner J W, Sims K G, Jones D C, Anderson B E. Ehrlichia chaffeensis expresses an immunoreactive protein homologous to the Escherichia coli GroEL protein. Infect Immun. 1993;61:3536–3539. doi: 10.1128/iai.61.8.3536-3539.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sumner J W, Nicholson W L, Massung R F. PCR amplification and comparison of nucleotide sequences from the groESL heat shock operon of Ehrlichia species. J Clin Microbiol. 1997;35:2087–2092. doi: 10.1128/jcm.35.8.2087-2092.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tilly K, McKittrick N, Zylicz M, Georgopoulos C. The dnaK protein modulates the heat shock response of Escherichia coli. Cell. 1983;34:641–646. doi: 10.1016/0092-8674(83)90396-3. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Ohashi N, Lee E, Tamura A, Rikihisa Y. Ehrlichia sennetsu groE operon and antigenic properties of the GroEL homolog. FEMS Immunol Med Microbiol. 1997;18:39–46. doi: 10.1111/j.1574-695X.1997.tb01025.x. [DOI] [PubMed] [Google Scholar]
- 35.Zhong G, Brunham R C. Antigenic analysis of the chlamydial 75-kilodalton protein. Infect Immun. 1992;60:1221–1224. doi: 10.1128/iai.60.3.1221-1224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]