Abstract
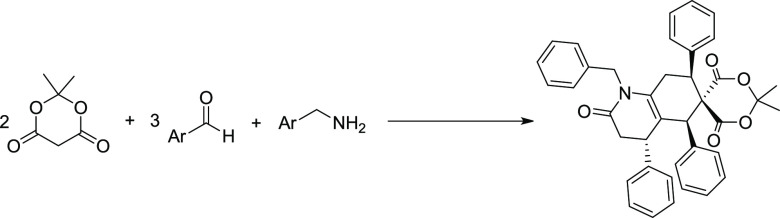
In this study, a homogeneous acid-catalyzed reaction of a series of benzaldehydes, benzylamines, and Meldrum’s acid was presented, allowing the novel one-pot and multicomponent synthesis of hexahydroquinolines with high stereoselectivity. The current strategy has advantages including high regioselectivity, good efficiency, reasonable diversity, utilization of an inexpensive and safe catalyst, and easy purification of products by simple recrystallization. The current reaction utilizes 2 equiv of Meldrum’s acid, 3 equiv of benzaldehyde derivatives, and one equiv of amine derivatives to yield (4’S,5′S,7’S)-1′-benzyl-2,2-dimethyl-4′,5′,7′-triphenyl-3′,4′,7′,8′-tetrahydro-1′H-spiro[[1,3]dioxane-5,6′-quinoline]-2′,4,6(5′H)-trione derivatives.
Introduction
Multicomponent reactions (MCRs) are efficient synthetic strategies that have been utilized in delivering a single product from merging three or more starting materials in a one-pot fashion tandem transformation.1−3 They are frequently employed as prominent tools to design and discover novel and diverse libraries of biologically important compounds.4−6 Their popularity in academia and industrial laboratories is based on the simplicity and versatility of experimental approaches.7,8 The lack of need for the isolation and purification of intermediates saves energy, time, and resources.9
Diversity-oriented synthesis (DOS) has been developing as an area of significance in the fields of medicinal and organic chemistry. Multifarious arrays of molecules with biologically active properties are the prominent purpose of DOS. It is noteworthy that DOS is able to provide various heterocyclic scaffolds, themselves of importance as potential new drugs or as analogues of natural products. The emergence of new MCRs has been a great asset for DOS.10−13
Heterocycles consist of a cyclic system with one or more (same or different) heteroatoms in their rings.14−16 Heterocyclic compounds were shown to be a specific category of compounds with natural origin as well as chemical, medicinal, and industrial significance.17,18 They seem to be significant and useful agents against different types of medical disorders. As previously reported in the literature,19 approximately 60% of the drugs employed for cancer therapy contain heterocyclic scaffolds. Nowadays, synthetic derivatives of organic products incorporating a heterocyclic ring have attracted scientists’ attention due to exhibiting a wide spectrum of significant pharmaceutical activities. Among them, nitrogen-based compounds have been found to be highly effective against fungi, bacteria, and cancer.20,21 They are also applied in the structure of vitamins and herbicides.22,23 On the other hand, highly functionalized heterocycles have attracted a great deal of attention in drug discovery and organic chemistry.24−27 Thus, it has been a high-priority research area for scientists in recent years.
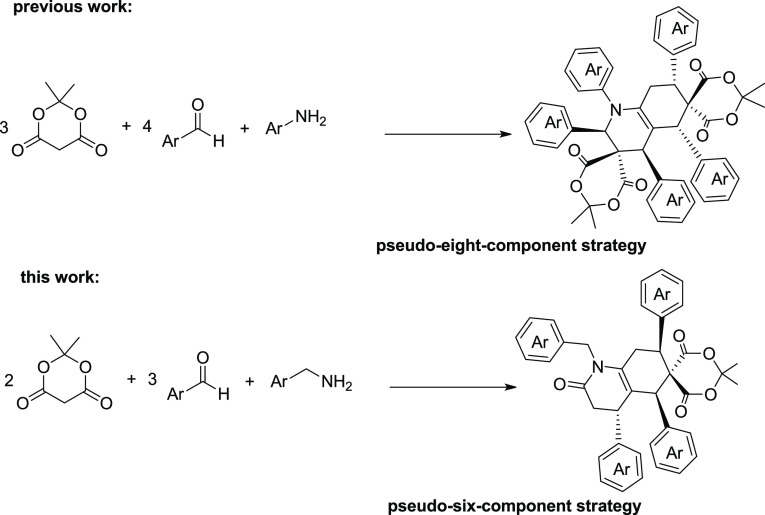
Lately, highly functionalized heterocycle synthesis is one of the most significant research subjects in organic chemistry owing to the ubiquity of these products in molecules of biological priority.28−31 Significant work has been done by research groups including Guo and co-workers,32 Lee and co-workers,33 Doyle and co-workers,34 and Xu and co-workers35 who reported their contributions using different catalytic systems. Recent works by Maghsoodlou and co-workers exhibited that phthalic acid was also capable of catalyzing the pseudo-six-component reaction of Meldrum’s acid, benzaldehyde derivatives, and aniline derivatives to afford polysubstituted hydroquinoline derivatives under mild reaction conditions (Scheme 1).36,37 The difference between the current research and the prominent work presented by Maghsoodlou et al. is that the current reaction utilizes 2 equiv of Meldrum’s acid, 3 equiv of benzaldehyde derivatives, and 1 equivalent of amine derivatives to yield new structures.
Scheme 1. Comparison between the Current Pseudo-Six-Component Strategy and Previously Reported Pseudo-Eight-Component Strategy.
In recent years, our research group has been actively engaged in advancing multicomponent reactions.38−40 In this context, our current focus is on the development of a one-pot and pseudo-six-component approach for synthesizing hexahydroquinoline derivatives. The benefits of this protocol include using an inexpensive catalyst, mild reaction conditions, high selectivity, and high diversity.
Results and Discussion
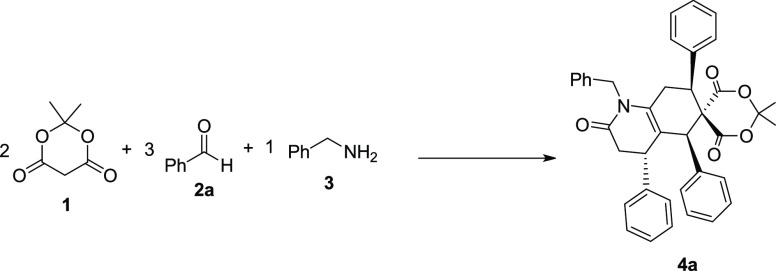
Meldrum’s acid (1), benzaldehyde (2a), and benzylamine (3) were selected as model substrates to start our investigations in the presence of TFA or CH3CO2H using a variety of solvents at 60 °C. As illustrated in Table 1, entries 4 and 8, no target product 4a was detected in the presence of TFA or CH3CO2H in aqueous media. To achieve the product 4a, utilization of other solvents such as MeOH, CH3CN, and EtOH was then explored (Table 1). However, satisfying results were not observed, except for CH3CN, in which the starting materials were efficiently transferred to the desired product 4a in the presence of CH3CO2H as an inexpensive catalyst (Table 1, entry 6).
Table 1. Optimization of One-Pot and Pseudo-Six-Component Synthesis of Hexahydroquinoline (4a).
| entry | catalyst | solvent | isolated yield (%) |
|---|---|---|---|
| 1 | TFA | MeOH | 23 |
| 2 | TFA | CH3CN | 28 |
| 3 | TFA | EtOH | 25 |
| 4 | TFA | H2O | NR |
| 5 | CH3CO2H | MeOH | 30 |
| 6 | CH3CO2H | CH3CN | 78 |
| 7 | CH3CO2H | EtOH | 33 |
| 8 | CH3CO2H | H2O | NR |
Reaction conditions: 1 (2.0 mmol), 2a (3.0 mmol), 3a (1.0 mmol), catalyst (0.08 mmol), solvent (5 mL), under air.
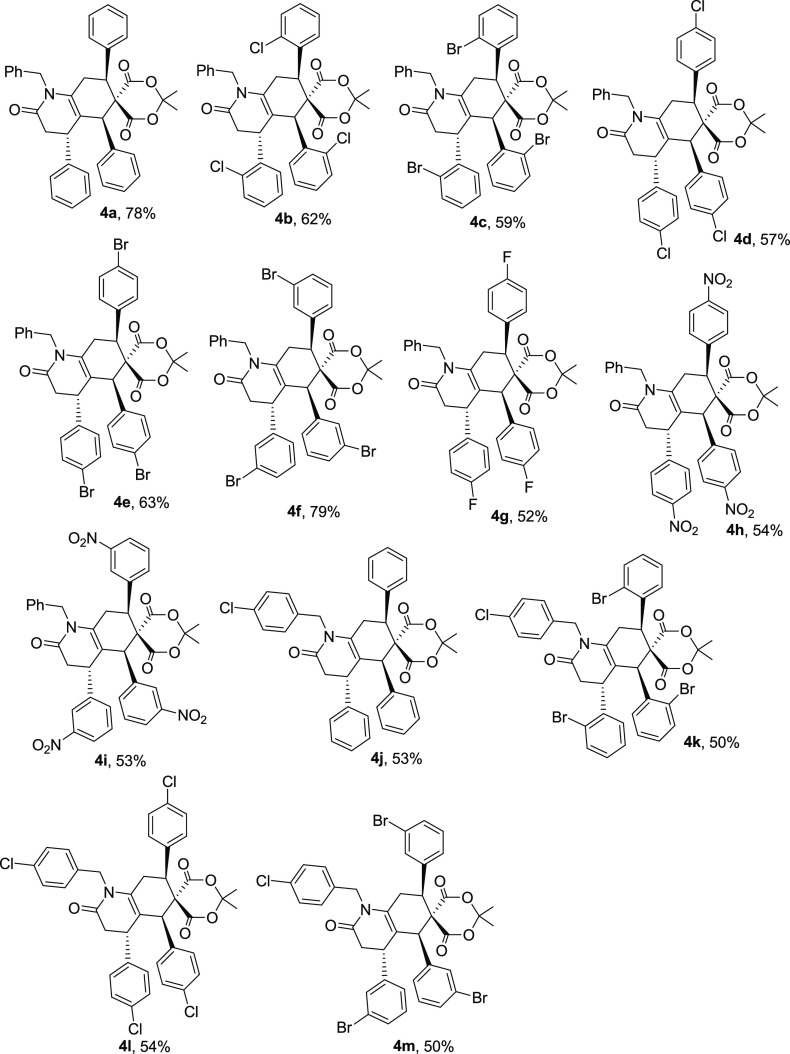
The scope and limitations of the reaction concerning benzaldehyde derivatives (2) and benzylamine derivatives (3) were subsequently explored under optimized reaction conditions (Scheme 2). A broad spectrum of aromatic aldehydes (2) and benzylamines (3) containing electron-poor, electron-rich, and halogen substitutions was tested. All transformations occurred in moderate-to-good yields. When the reaction of benzaldehyde (2a) and Meldrum’s acid (1) with 2-chlorobenzaldehyde (4b), 4-bromobenzaldehyde (4e), or 3-bromobenzaldehyde (4f) was performed, good yields of products were obtained (Scheme 1). It is worth noting that a wide range of substitutions such as NO2, I, Br, Cl, and F were applied to this approach to give the corresponding products in moderate-to-good yields under optimized reaction conditions. Ortho-substitution at the benzaldehydes does not seem to have a significant effect on the yields (Scheme 2).
Scheme 2. Substrate Scope of One-Pot and Pseudo-Six-Component Synthesis of Hexahydroquinoline Derivatives (4).
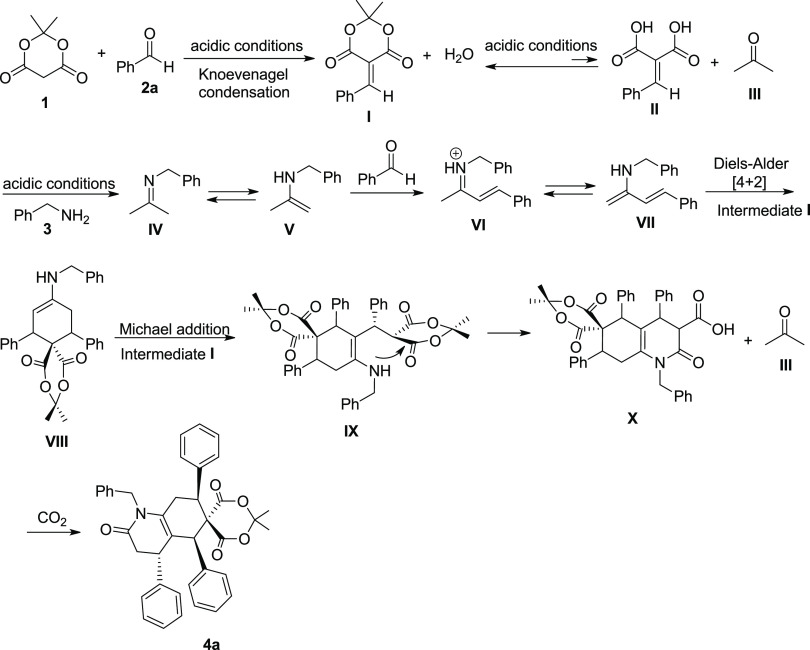
Herein, a rational mechanism for the tandem/cyclization preparation of hexahydroquinoline (4a) is offered (Scheme 3), which is in analogy to the previously reported articles.36,41,42 The tandem Knoevenagel and Michael reactions are the key steps of this transformation. Initially, the benzylidene derivative of Meldrum’s acid I is produced via the Knoevenagel transformation, followed by reverting the intermediate I to acetone (III) and intermediate II in an equilibrium reaction. In the next step, acetone is condensed with benzylamine to deliver imine IV. This intermediate is tautomerized to enamine V. In continuation, Barbas dienamine VII (2-amino-1,3-butadiene) is formed via the condensation of benzaldehyde with enamine V. The resulting intermediate VII is transformed to enamine VIII by reacting with the Knoevenagel product I. The intermediate VIII is then added to Knoevenagel product I to give intermediate IX. In the last step, intermediate IX cyclized to afford intermediate X and acetone (III), followed by the release of a carbon dioxide molecule to arrive at the final product 4a. In this reaction, acetone (III) could be formed directly from Meldrum’s acid and in the reaction from IX. Indeed, acetone could be present in a small amount via hydrolysis of Meldrum’s acid or any of the condensation products. It can be regenerated in the step before the last step, as well.
Scheme 3. Proposed Reaction Mechanism for One-Pot and Pseudo-Six-Component Synthesis of Hexahydroquinoline (4a).
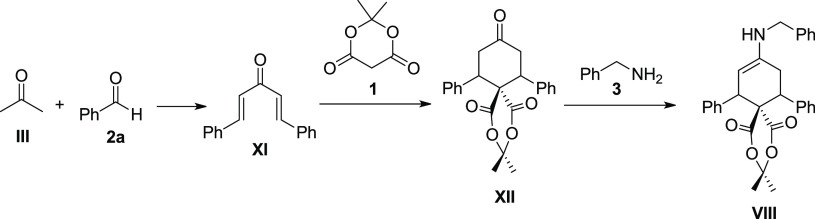
We also propose an alternative mechanism possible not involving the Barbas dienamine for intermediate VIII. In the presence of a base (amine) and excess benzaldehyde 2a, bis benzalacetone XI could be formed, and this has been approved to form a spiro compound XII with Meldrum’s acid in high yield [42]. It can be surmised that intermediate VIII (Scheme 4) is formed from spirocyclohexanone XII and benzylamine 3.
Scheme 4. Alternative Mechanism, Not Involving the Barbas Dienamine, for the Formation of Intermediate VIII.
The data collected from nuclear magnetic resonance (NMR), infrared (IR) spectroscopy, and X-ray crystallographic analysis were interpreted to confirm the structure of the product 4. The IR spectrum of 4a displayed two absorption bands at 1678 and 1730 cm–1 which belong to the carbonyl groups of Meldrum’s acid and benzylamine ring. The structure was also confirmed by NMR and 2D NMR. The 1H NMR signals at 0.43 and 0.51 ppm integrate for three hydrogens and do not show coupling to other hydrogens. These signals, therefore, belong either to the three hydrogens on carbon 22 or 23. The 1H NMR signals at 4.96 and 5.05 ppm belong to the hydrogens on carbon 33. According to the HSQC and COSY analyses, these hydrogens are geminal and only couple to each other. This can only be in accordance with the proposed assignment. The 1H NMR signals at 6.72, 7.05, 7.15, 7.27, and 7.44 ppm all belong to aromatic hydrogens. This assignment is in accordance with the observed integration values and is supported by the HSQC analysis. The 1H NMR signals at 2.72 and 3.06 ppm were assigned to the hydrogens on carbon 9. According to HSQC, these signals belong to geminal hydrogens. The 1H NMR signals at 2.79 and 3.22 ppm also belong to geminal hydrogens. However, the 2.79 and 3.22 ppm signals do not show any coupling to carbonyl carbons in the HMBC spectrum, whereas the 2.72 and 3.06 ppm signals do. This is in line with the proposed assignment. The 13C NMR signals at 163.0, 168.2, and 168.5 ppm were assigned to carbonyl carbons based on their chemical shift and the observation in the HSQC spectrum that these are quaternary carbons. Subsequently, the 1H NMR signals at 2.79 and 3.22 ppm can be assigned to the hydrogens on carbon 3. At first, only two 1H NMR signals were found by HSQC analysis that could accord to the hydrogens of a nonaromatic CH (3.80 and 4.49 ppm). However, three signals were expected. Upon close inspection of the HSQC spectrum, it became clear that one CH signal was hidden underneath the water peak, as a positive peak showed coupling to the 38.1 ppm of 13C NMR signal. The 1H NMR signal at 3.32 ppm (underneath the water peak) was assigned to the hydrogen on carbon 10. Both the other CH signals (3.80 and 4.49 ppm) show coupling to the same carbonyl carbon (163.0 ppm) in the HMBC spectrum. This is only possible if these signals belong to the hydrogens on carbons 2 and 6. The 1H NMR signal at 3.80 ppm was assigned to the hydrogen on carbon 2. In the HSQC spectrum, it was seen that the 13C NMR signal of carbon that directly bonded to this hydrogen appeared at 46.6 ppm. This 13C NMR signal showed coupling to the hydrogens on carbon 3 in the HMBC spectrum. On the other hand, the 13C NMR signal belonging to the carbon that directly bonded to the hydrogen of signal 4.49 ppm was found to be 52.7 ppm by HSQC. This 13C NMR signal did not show coupling to the hydrogens of carbon 3 in the HMBC spectrum and only showed coupling to the 3.80 ppm signal. The assignment of hydrogen on carbon 2 and on carbon 6 could thus be made. The 1H NMR signal at 4.49 ppm was assigned to the hydrogen on carbon 6. Having assigned all aliphatic 1H NMR signals, a NOESY analysis made it clear that the stereochemistry of the phenyl rings connected to positions 2 and 6 is cis, as proposed at the beginning of this section. It could be seen that the hydrogens of carbon 2 and carbon 6 couple in the NOESY spectrum. These hydrogens are, therefore, within 5 Å of each other. This does not correspond with the alternating stereochemistry of the phenyl rings. Yet, it is in accordance with the proposed stereochemistry. Hydrogens 6 and 10, as expected, do not give coupling in NOESY as expected from the X-ray analysis, confirming trans-stereochemistry of the connected phenyl groups (all NMR and 2D NMR data are available in the Supporting Information). Finally, the structure of compound 4a was further confirmed by X-ray crystallographic analysis (Figure 1).
Figure 1.
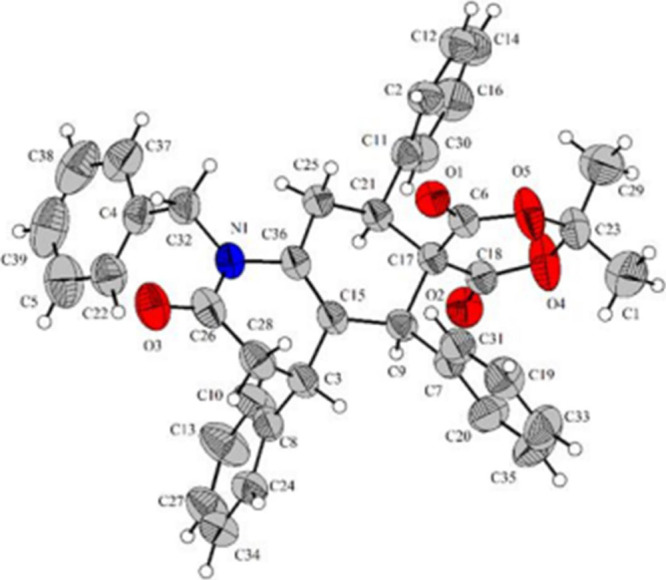
ORTEP diagram of 4a.
An efficient and ecofriendly strategy has been presented for the pseudo-six-component diastereoselective synthesis of hexahydroquinoline under homogeneous acidic conditions. This catalytic system starts from commercially available starting materials and involves a highly innovative pseudo-six-component reaction, conducted under mild reaction conditions and allowing for the simple purification of products. It is particularly noteworthy that four stereocenters and ten new bonds were constructed with excellent diastereoselectivity within this transformation. Additional research is currently underway in our laboratory to expand the scope of this reaction and study the reaction mechanism.
Experimental Section
General Information
All reagents and substrates were commercial and used without further purification unless otherwise indicated. All reactions were monitored by TLC, which was performed on precoated aluminum sheets of silica gel 60 (F254) and visualized by exposure to UV light (254 nm). Melting points were measured by using a melting point instrument and were uncorrected.
1H NMR spectra were recorded on a Bruker spectrometer (at 500 MHz) and reported relative to tetramethylsilane as the internal standard. Data for 1H NMR spectra were reported as follows: chemical shift (d/ppm), multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet), coupling constant (J/Hz), and integration. 13C NMR spectra were recorded on a Bruker spectrometer (126 MHz).
Benzaldehyde derivative (3.0 mmol, 2) was added to a solution of Meldrum’s acid (2.0 mmol, 1) and benzylamine derivative (1.0 mmol, 3) in acetonitrile (5 mL), followed by the addition of acetic acid (0.08 mmol). Further, the mixture was stirred at 60 °C for 24 h. The reaction progress was monitored by TLC. After completing the reaction, water as an antisolvent was added to the mixture to obtain the desired precipitate. The resulting precipitate was filtered and dried to produce the final powder.
(4’S,5′S,7’S)-1′-Benzyl-2,2-dimethyl-4′,5′,7′-triphenyl-3′,4′,7′,8′-tetrahydro-1′H-spiro[[1,3]dioxane-5,6′-quinoline]-2′,4,6(5′H)-trione (4a)
Powder white. mp: 210 °C; IR (KBr, cm–1): ν 3467, 3014, 2922, 1767, 1730, 1678, 1573. 1H NMR (500 MHz, DMSO) δ 7.43 (td, J = 7.6, 1.5 Hz, 1H, H–Ar), 7.37–7.17 (m, 13H, H–Ar), 7.17–7.10 (m, 2H, H–Ar), 7.09–6.99 (m, 4H, H–Ar), 6.79–6.65 (m, 1H, CH), 5.10–4.91 (m, 2H, CH2), 4.46 (d, J = 3.0 Hz, 1H, CH), 3.77 (dd, J = 11.8, 5.1 Hz, 1H, CH), 3.26–2.99 (m, 2H, CH2), 2.81–2.62 (m, 2H, CH2), 0.50 (s, 3H, CH3), 0.42 (s, 3H, CH3). 13C NMR (126 MHz, DMSO-d6) δ (ppm): 168.51, 168.15, 162.97, 140.63, 138.37, 137.47, 137.24, 134.88, 130.54, 129.21, 128.83, 128.56, 128.41, 128.09, 126.94, 126.72, 126.69, 126.16, 111.57, 105.39, 60.11, 52.70, 46.63, 43.72, 38.09, 29.31, 27.84, 27.47. Anal. Calcd for C39H35NO5 (597.70): C, 78.37; H, 5.90; N, 2.34. Found: C, 78.41; H, 6.01; N, 2.39
Benzyl-4,5,7-Tris(2-chlorophenyl)-2′,2′-dimethyl-1,3,4,5,7,8-hexahydro-2H-spiro[quinoline-6,5′-[1,3]dioxane]-2,4′,6′-trione (4b)
White powder (yield 62%, mp: 224 °C), IR (KBr, cm–1): 3421, 3063, 3002, 2922, 1739,1674, 1567. 1H NMR (500 MHz, DMSO-d6) δ (ppm) 7.50 (dd, J = 7.9, 1.5 Hz, 1H, H–Ar), 7.47–7.40 (m, 1H, HAr),7.40–7.25 (m, 13H, H–Ar), 7.25–7.15 (m, 2H, CH2), 5.10–4.96 (m, 2H, CH2), 4.95–4.89 (m,1H, CH), 4.48 (dd, J = 12.0, 5.1 Hz, 1H, CH), 3.69–3.60 (m, 1H, CH), 3.19–3.10 (m, 1H, CH), 2.91 (ddd, J = 17.0, 6.9, 3.8 Hz, 2H, CH2), 2.63 (dd, J = 16.1, 6.2 Hz, 1H, CH), 0.99 (s, 3H, Me), 0.62 (s, 3H, Me).13C NMR (126 MHz, DMSO-d6) δ (ppm) 167.77, 166.86, 164.46, 138.66, 137.35, 137.24, 136.16, 135.40, 134.33, 133.44, 130.99, 130.59, 130.39, 129.43, 128.97, 128.54, 128.36, 128.25, 127.50, 126.95, 111.87, 106.34, 56.50, 48.37, 40.06, 39.90, 28.95, 28.10. Anal. Calcd for C39H32Cl3NO5 (701.03): C, 66.82; H, 4.60; N, 2.00. Found: C, 66.87; H, 4.63; N, 2.09.
1-Benzyl-4,5,7-tris(2-bromophenyl)-2′,2′-dimethyl-1,3,4,5,7,8-hexahydro-2H-spiro[quinoline-6,5′-[1,3]dioxane]-2,4′,6′-trione (4c)
White powder (yield 59%, mp: 235 °C), IR (KBr, cm–1): 3430, 3063, 2992, 2925,1751, 1690, 1585. 1H NMR (500 MHz, DMSO-d6) δ (ppm) 7.67 (dd, J = 8.1, 1.3 Hz, 1H, H–Ar), 7.58–7.50 (m, 2H, HAr), 7.45–7.27 (m, 7H, H–Ar), 7.27–7.17 (m, 7H, H–Ar), 5.16 (d, J = 16.3 Hz, 1H, CH), 4.95–4.82 (m, 2H, CH2), 4.43 (dd, J = 12.0, 5.0 Hz, 1H, CH), 3.58–3.51 (m, 1H, CH), 3.25–3.10 (m, 1H, CH), 3.01 (d, J = 8.1 Hz, 1H, CH), 2.99–2.91 (m, 2H, CH2), 1.10 (s, 3H, Me), 0.61 (s, 3H, Me). 13C NMR (126 MHz, DMSO-d6) δ (ppm): 167.60, 166.62, 164.67, 138.58, 137.88, 137.11, 136.41, 134.37, 134.12, 133.91, 132.18, 130.94, 130.63, 129.86, 129.27, 128.98, 128.85, 128.79, 128.20, 127.56, 127.13, 126.59, 125.66, 124.52, 111.77, 106.52, 56.25, 51.21, 46.5344.48, 39.10, 37.62, 31.48, 29.10, 28.01. Anal. Calcd for C39H32Br3NO5 (834.39): C, 56.14; H, 3.87; N, 1.68. Found: C, 56.16; H, 3.82; N, 1.62.
1-Benzyl-4,5,7-tris(4-chlorophenyl)-2′,2′-dimethyl-1,3,4,5,7,8-hexahydro-2H-spiro[quinolone-6,5′-[1,3]dioxane]-2,4′,6′-trione (4d)
White powder (yield 57%, mp: 242 °C), IR (KBr, cm–1): 3442, 3069, 2992, 2934, 1893, 1724, 1678, 1585. 1H NMR (500 MHz, DMSO-d6) δ (ppm) 7.50 (dd, J = 8.4, 2.4 Hz, 1H, H–Ar), 7.46–7.40 (m, 2H, H–Ar), 7.34–7.21 (m, 8H, H–Ar), 7.21–7.10 (m, 3H, H–Ar), 7.06 (p, J = 2.5 Hz, 3H, H–Ar), 6.67 (dd, J = 8.3, 2.4 Hz, 1H, CH), 4.99 (d, J = 4.6 Hz, 2H, CH2), 4.43 (d, J = 3.3 Hz, 1H, CH), 3.88 (dd, J = 11.8, 5.2 Hz, 1H, CH), 3.19–3.06 (m, 1H, CH), 2.99 (dd, J = 16.0, 6.7 Hz, 1H, CH), 2.84–2.63 (m, 2H, CH2), 0.63 (s, 3H, Me), 0.55 (s, 3H, Me). 13C NMR (126 MHz, DMSO-d6) δ (ppm) 28.11,28.58, 29.80, 38.32, 39.34, 39.55,39.75, 39.96, 40.17, 40.38, 40.59, 44.29, 46.48, 52.30, 60.42, 106.13, 111.41, 126.72, 127.32, 128.95, 129.25, 129.44, 129.60, 129.88, 130.82, 131.32, 131.86, 132.71, 133.43, 133.68, 136.03, 136.42, 138.80, 140.13, 163.39, 168.49, 168.42. Anal. Calcd for C39H32Cl3NO5 (701.03): C, 66.82; H, 4.60; N, 2.00. Found: C, 66.77; H, 4.67; N, 1.96.
1-Benzyl-4,5,7-tris(4-bromophenyl)-2′,2′-dimethyl-1,3,4,5,7,8-hexahydro-2H-spiro[quinolone-6,5′-[1,3]dioxane]-2,4′,6′-trione (4e)
White powder (yield 63%, mp: 257 °C), IR (KBr, cm–1): 3439, 2989, 2931, 1902, 1727, 1674, 1573. 1H NMR (500 MHz, DMSO-d6) δ (ppm) 7.63 (dd, J = 8.4, 2.3 Hz, 1H, H–Ar), 7.59–7.55 (m, 2H, H–Ar), 7.44–7.40 (m, 3H, H–Ar), 7.32–7.29 (m, 2H, H–Ar), 7.27–7.22 (m, 1H, H–Ar), 7.19–7.13 (m, 3H, H–Ar), 7.01 (ddd, J = 8.6, 5.0, 2.4 Hz, 5H, H–Ar), 6.63 (dd, J = 8.3, 2.4 Hz, 1H, CH), 4.98 (d, J = 3.2 Hz, 2H, CH2), 4.41 (d, J = 3.1 Hz, 1H, CH), 3.85 (dd, J = 11.8, 5.2 Hz, 1H, CH), 3.12 (t, J = 14.5 Hz, 1H, CH), 3.00 (dd, J = 16.1, 6.8 Hz, 1H, CH), 2.81–2.65 (m, 2H, CH2), 0.63 (s, 3H, Me), 0.55 (s, 3H, Me). 13C NMR (126 MHz, DMSO-d6) δ (ppm): 168.80, 168.49, 163.38, 140.56, 138.80, 137.23, 136.83, 136.05, 133.03, 132.81, 132.38, 132.23, 131.90, 131.65, 131.13, 129.97, 128.90, 127.33, 126.73, 122.21, 121.97, 120.37, 111.28, 106.15, 60.30, 52.42, 46.58, 44.32, 38.35, 29.76, 28.57, 28.11. Anal. Calcd for C39H32Br3NO5 (834.39): C, 56.14; H, 3.87; N, 1.68. Found: C, 56.11; H, 3.90; N, 1.64.
(5S,7R)-1-Benzyl-4,5,7-tris(3-bromophenyl)-2′,2′-dimethyl-1,3,4,5,7,8-hexahydro-2H-spiro [quinoline-6,5′-[1,3]dioxane]-2,4′,6′-trione (4f)
White powder (yield 79%, mp: 205 °C), IR (KBr, cm–1): 3417, 3057, 2992, 2940, 1773, 1739, 1668. 1576, 1H NMR (500 MHz, DMSO-d6) δ (ppm) 7.55 (d, J = 8.5 Hz, 1H, H–Ar), 7.51–7.47 (m, 1H, H–Ar), 7.34 (td, J = 15.2, 8.0 Hz, 5H, H–Ar), 7.28–7.20 (m, 5H, H–Ar), 7.18 (t, J = 9.7 Hz, 1H, H–Ar), 7.14–7.08 (m, 3H, H–Ar), 7.01 (d, J = 2.2 Hz, 1H, H–Ar), 6.53 (d, J = 6.2 Hz, 1H, CH), 5.11–4.93 (m, 2H, CH2), 4.47–4.35 (m, 1H, CH), 3.89 (ddd, J = 11.9, 5.1, 2.7 Hz, 1H, CH), 3.52 (t, J = 7.5 Hz, 1H, CH), 3.28–3.14 (m, 1H, CH), 2.84 (d, J = 7.3 Hz, 1H, CH), 2.72 (dt, J = 17.2, 5.9 Hz, 1H, CH), 0.67 (s, 3H, Me), 0.54 (d, J = 5.2 Hz, 3H, Me). 13C NMR (126 MHz, DMSO-d6) δ (ppm): 168.81, 168.66, 168.55, 163.24, 144.06, 140.54, 139.71, 138.85, 136.19, 133.18, 131.63, 131.21, 130.90, 129.96, 128.95, 128.18, 127.26, 126.76, 126.72, 123.00, 122.56, 122.34, 122.17, 111.49, 106.23, 106.18, 60.44, 52.44, 52.38, 46.61, 40.50, 39.10, 28.54, 28.37, 27.98, 27.89. Anal. Calcd for C39H32Br3NO5 (834.39): C, 56.14; H, 3.87; N, 1.68. Found: C, 56.17; H, 3.91; N, 1.63.
1-Benzyl-4,5,7-tris(4-fluorophenyl)-2′,2′-dimethyl-1,3,4,5,7,8-hexahydro-2H-spiro[quinoline-6,5′-[1,3]dioxane]-2,4′,6′-trione (4g)
White powder (yield 52%, mp: 200 °C), IR (KBr, cm–1): 3461, 3103, 3073, 2999, 2934, 1733, 1665, 1H NMR (500 MHz, DMSO-d6) δ (ppm) 7.46–7.29 (m, 2H, H–Ar), 7.29–7.14 (m, 7H, H–Ar), 7.14–7.01 (m, 8H, H–Ar), 6.65 (ddd, J = 8.2, 5.5, 2.3 Hz, 1H, CH), 5.08–4.96 (m, 2H, CH2), 4.45 (d, J = 2.0 Hz, 1H, CH), 3.87 (dd, J = 11.8, 5.2 Hz, 1H, CH), 3.23–3.10 (m, 1H, CH), 2.96 (dd, J = 16.0, 6.6 Hz, 1H, CH), 2.83–2.68 (m, 2H, CH2), 0.62 (s, 3H, Me), 0.53 (s, 3H, Me). 13C NMR (126 MHz, DMSO-d6) δ (ppm): 28.10, 28.57, 39.34, 39.55, 39.75, 39.96, 40.17, 40.38, 40.59, 60.76, 106.02, 115.62, 115.82, 116.18, 126.70, 127.30, 128.90, 129.51, 129.59, 138.84, 163.51, 168.57, 169.01. Anal. Calcd for C39H32F3NO5 (651.67): C, 71.88; H, 4.95; N, 2.15. Found: C, 71.90; H, 4.99; N, 2.18.
(5S,7R)-1-Benzyl-2′,2′-dimethyl-4,5,7-tris(4-nitrophenyl)-1,3,4,5,7,8-hexahydro-2H-spiro[quinoline-6,5′-[1,3]dioxane]-2,4′,6′-trione (4h)
White powder (yield 54%, mp: 243 °C), IR (KBr, cm–1): 3424, 2992, 1779, 1742, 1671, 1567. 1H NMR (500 MHz, DMSO-d6) δ (ppm) 8.28 (dd, J = 8.5, 4.8 Hz, 3H, H–Ar), 8.06 (d, J = 8.3 Hz, 2H, H–Ar), 8.01 (dd, J = 8.5, 2.6 Hz, 1H, H–Ar), 7.53 (dd, J = 8.7, 2.1 Hz, 1H, H–Ar), 7.45–7.30 (m, 7H, H–Ar), 7.25 (dd, J = 14.3, 7.4 Hz, 3H, H–Ar), 6.81 (dd, J = 8.5, 2.1 Hz, 1H, CH), 5.13–4.94 (m, 2H, CH2), 4.64 (s, 1H, CH), 4.24–4.19 (m, 1H, CH), 3.64–3.51 (m, 2H, CH2), 3.00 (dd, J = 16.0, 6.4 Hz, 1H, CH), 2.85 (d, J = 7.1 Hz, 1H, CH), 0.61 (s, 3H, Me), 0.49 (s, 3H, Me). 13C NMR (126 MHz, DMSO-d6) δ (ppm): 168.37, 168.26, 163.14, 149.19, 148.00, 147.66, 146.81, 144.56, 138.66, 136.96, 130.60, 129.42, 128.96, 126.78, 124.58, 124.02, 110.47, 106.45, 59.95, 28.85, 28.17. Anal. Calcd for C39H32N4O11 (732.69): C, 63.93; H, 4.40; N, 7.65. Found: C, 63.98; H, 4.47; N, 7.66.
1-Benzyl-2′,2′-dimethyl-4,5,7-tris(3-nitrophenyl)-1,3,4,5,7,8-hexahydro-2H-spiro[quinoline-6,5′-[1,3]dioxane]-2,4′,6′-trione (4i)
White powder (yield 53%, mp: 193 °C), IR (KBr, cm–1): 3445, 3069, 2992, 2925, 2777, 1736, 1674, 1573. 1H NMR (500 MHz, DMSO-d6) δ (ppm) 8.24–7.95 (m, 5H, H–Ar), 7.73–7.54 (m, 6H, H–Ar), 7.38–7.27 (m, 6H, H–Ar), 5.20–4.90 (m, 2H, CH2), 4.61 (t, J = 2.0 Hz, 1H, CH), 4.36 (t, J = 5.1 Hz, 1H, CH), 4.24 (ddd, J = 11.8, 5.0, 2.3 Hz, 1H, CH), 3.45 (qd, J = 7.0, 5.1 Hz, 1H, CH), 2.94 (td, J = 15.7, 10.1 Hz, 1H, CH), 2.88–2.73 (m, 2H, CH2), 0.59 (d, J = 1.8 Hz, 3H, Me), 0.47–0.42 (m, 3H, Me). Anal. Calcd for C39H32N4O11 (732.69): C, 63.93; H, 4.40; N, 7.65. Found: C, 63.90; H, 4.45; N, 7.62.
1-(4-Chlorobenzyl)-2′,2′-dimethyl-4,5,7-triphenyl-1,3,4,5,7,8-hexahydro-2H-spiro[quinoline-6,5′-[1,3]dioxane]-2,4′,6′-trione (4j)
White powder (yield 53%, mp: 253 °C), IR (KBr, cm–1): 3445, 3045, 2934, 2879, 1958, 1890, 1730, 1656, 1585. 1H NMR (500 MHz, DMSO-d6) δ (ppm) 7.45 (td, J = 7.6, 1.5 Hz, 1H, H–Ar), 7.39–7.16 (m, 15H, H–Ar), 7.09–7.02 (m, 3H, H–Ar), 6.70 (d, J = 7.6 Hz, 1H, CH), 5.00 (d, J = 3.8 Hz, 1H, CH), 4.48 (s, 1H, CH), 3.84 (dd, J = 11.8, 5.2 Hz, 1H, CH), 3.30–3.00 (m, 2H, CH2), 2.88–2.58 (m, 2H, CH2), 2.10 (s, 1H, CH), 0.53 (s, 3H, Me), 0.44 (s, 3H, Me).13C NMR (126 MHz, DMSO-d6) δ (ppm) 28.00, 28.37, 29.85, 38.64, 39.33, 39.75, 39.96, 40.17, 40.37, 40.58, 43.65, 47.07, 53.20, 60.63, 105.95, 112.43, 127.24, 127.49, 128.62, 128.72, 128.83, 128.87, 128.99, 129.11, 129.39, 129.75, 131.06, 131.80, 135.27, 137.71, 137.97, 138.00, 141.10, 163.54, 168.79, 169.03. Anal. Calcd for C39H34ClNO5 (632.14): C, 74.10; H, 5.42; N, 2.22. Found: C, 63.90; H, 4.45; N, 2.27.
4,5,7-Tris(2-bromophenyl)-1-(4-chlorobenzyl)-2′,2′-dimethyl-1,3,4,5,7,8-hexahydro-2H-spiro[quinoline-6,5′-[1,3]dioxane]-2,4′,6′-trione (4k)
White powder (yield 50%, mp: 215 °C), IR (KBr, cm–1): 3461, 3060, 2999, 2931, 2728, 2641, 1770, 1742, 1681, 1576. 1H NMR (500 MHz, DMSO-d6) δ 7.85 (s, 1H, H–Ar), 7.68–7.66 (m, 1H, H–Ar), 7.60 (dd, J = 8.0, 1.2 Hz, 1H, H–Ar), 7.56–7.50 (m, 3H, H–Ar), 7.48–7.44 (m, 3H, H–Ar), 7.43–7.34 (m, 2H, H–Ar), 7.31–7.25 (m, 3H, H–Ar), 7.23–7.20 (m, 1H, H–Ar), 7.16 (td, J = 5.1, 2.5 Hz, 1H, H–Ar), 5.08 (d, J = 16.6 Hz, 1H, CH), 4.93–4.84 (m, 2H, CH2), 4.43 (dd, J = 12.0, 5.0 Hz, 1H, CH), 4.04 (s, 1H, CH), 3.53 (d, J = 6.4 Hz, 1H, CH), 3.12 (t, J = 14.8 Hz, 1H, CH), 2.99–2.87 (m, 2H, CH2), 1.09 (s, 3H, Me), 0.60 (s, 3H, Me). 13C NMR (126 MHz, DMSO-d6) δ (ppm): 167.69, 166.60, 164.65, 137.86, 137.69, 136.95, 133.52, 131.33, 130.64, 129.88, 129.11, 129.02, 128.98, 127.06, 125.69, 124.48, 112.03, 106.54, 56.22, 46.49, 42.08, 29.11, 28.00. Anal. Calcd for C39H31Br3NO5 (868.83): C, 53.91; H, 3.60; N, 1.61. Found: C, 53.98; H, 3.57; N, 1.67.
1-(4-Chlorobenzyl)-4,5,7-tris(4-chlorophenyl)-2′,2′-dimethyl-1,3,4,5,7,8-hexahydro-2H-spiro[quinoline-6,5′-[1,3]dioxane]-2,4′,6′-trione (4l)
White powder (yield 54%, mp: 248 °C), IR (KBr, cm–1): 3451, 3057, 2999, 2925, 1899, 1724, 1674, 1576, 1H NMR (500 MHz, DMSO-d6) δ (ppm) 7.51–7.42 (m, 3H, H–Ar), 7.38 (d, J = 8.1 Hz, 2H, H–Ar), 7.27 (dd, J = 7.9, 4.3 Hz, 3H, H–Ar), 7.20 (d, J = 7.8 Hz, 3H, H–Ar), 7.10–7.03 (m, 5H, H–Ar), 6.63 (dd, J = 8.4, 2.4 Hz, 1H, CH), 5.08–4.77 (m, 2H, CH2), 4.42 (s, 1H, CH), 3.91 (dd, J = 11.8, 5.2 Hz, 1H, CH), 3.10 (t, J = 14.6 Hz, 1H, CH), 2.96 (dd, J = 16.1, 6.6 Hz, 1H, CH), 2.83–2.64 (m, 2H, CH2), 0.63 (s, 3H, Me), 0.54 (s, 3H, Me). 13C NMR (126 MHz, DMSO-d6) δ (ppm) 28.10,28.58, 29.80, 38.38, 39.33, 39.54, 39.75, 39.96, 40.17, 40.38, 40.59, 43.68, 46.41, 52.26, 60.41, 106.14, 111.74, 128.74, 128.95, 129.22, 129.45, 129.60, 130.85, 131.32, 131.85, 132.68, 133.40, 135.86, 136.33, 136.83, 137.87, 140.12, 163.40, 168.57, 168.81. Anal. Calcd for C39H31Cl4NO5 (735.48): C, 63.69; H, 4.25; N, 1.90. Found: C, 63.72; H, 4.23; N, 1.88.
4,5,7-Tris(3-bromophenyl)-1-(4-chlorobenzyl)-2′,2′-dimethyl-1,3,4,5,7,8-hexahydro-2H-spiro[quinoline-6,5′-[1,3]dioxane]-2,4′,6′-trione (4m)
White powder (yield 50%, mp: 233 °C), IR (KBr, cm–1): 3439, 3060, 3005, 2925,1881, 1764, 1733, 1668, 1576. 1H NMR (500 MHz, DMSO-d6) δ (ppm) 7.55–7.45 (m, 2H, H–Ar), 7.42–7.30 (m, 5H, H–Ar), 7.30–7.23 (m, 4H, H -Ar), 7.20 (tdd, J = 7.2, 5.5, 2.8 Hz, 1H, H–Ar), 7.16–7.04 (m, 4H, H–Ar), 5.05–4.90 (m, 2H, CH2), 4.36 (s, 1H, CH), 3.91 (dd, J = 11.8, 5.1 Hz, 1H,CH), 3.53–3.43 (m, 1H, CH), 3.15 (t, J = 13.7 Hz, 1H, CH), 2.82 (h, J = 9.5 Hz, 2H, CH2), 2.69 (dd, J = 17.2, 5.8 Hz, 1H, CH), 0.66 (s, 3H, Me), 0.52 (d, J = 7.9 Hz, 3H, Me). 13C NMR (126 MHz, DMSO-d6) δ (ppm): 168.79, 168.63, 163.26, 144.00, 143.95, 140.53, 139.61, 137.88, 136.00, 133.16, 131.87, 131.70, 131.27, 130.88, 129.94, 128.88, 128.81, 128.78, 128.56, 128.19, 126.76, 122.55, 122.32, 122.13, 106.25, 106.19, 60.43, 52.41, 46.56, 39.33, 28.53, 28.37, 27.97, 27.89. Anal. Calcd for C39H31Br3ClNO5 (868.83): C, 53.91; H, 3.60; N, 1.61. Found: C, 53.87; H, 3.56; N, 1.57.
Acknowledgments
The K. N. Toosi University of Technology and KU Leuven (grant C14/19/78) are acknowledged for the financial support of this synthetic work.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c06264.
The authors declare no competing financial interest.
Supplementary Material
References
- Alizadeh A.; Roosta A.; Rezaiyehrad R.; Halvagar M. Efficient one pot and chemoselective synthesis of functionalized 3-bromo-4, 5-dihydroisoxazole derivatives via 1, 3-dipolar cycloaddition reactions of nitrile oxides. Tetrahedron 2017, 73, 6706. 10.1016/j.tet.2017.10.003. [DOI] [Google Scholar]
- Zhi S.; Ma X.; Zhang W. Consecutive multicomponent reactions for the synthesis of complex molecules. Org. Biomol. Chem. 2019, 17, 7632. 10.1039/C9OB00772E. [DOI] [PubMed] [Google Scholar]
- Ibarra I. A.; Islas-Jácome A.; González-Zamora E. Synthesis of polyheterocycles via multicomponent reactions. Org. Biomol. Chem. 2018, 16, 1402. 10.1039/C7OB02305G. [DOI] [PubMed] [Google Scholar]
- Younus H. A.; Al-Rashida M.; Hameed A.; Uroos M.; Salar U.; Rana S.; Khan K. M. Multicomponent reactions (MCR) in medicinal chemistry: a patent review (2010–2020). Expert Opin. Ther. Pat. 2021, 31, 267. 10.1080/13543776.2021.1858797. [DOI] [PubMed] [Google Scholar]
- Graebin C. S.; Ribeiro F. V.; Rogério K. R.; Kümmerle A. E. Multicomponent reactions for the synthesis of bioactive compounds: A review. Curr. Org. Synth. 2019, 16, 855. 10.2174/1570179416666190718153703. [DOI] [PubMed] [Google Scholar]
- Brandão P.; Marques C.; Burke A. J.; Pineiro M. The application of isatin-based multicomponent-reactions in the quest for new bioactive and druglike molecules. Eur. J. Med. Chem. 2021, 211, 113102 10.1016/j.ejmech.2020.113102. [DOI] [PubMed] [Google Scholar]
- Afshari R.; Shaabani A. Materials functionalization with multicomponent reactions: state of the art. ACS Comb. Sci. 2018, 20, 499. 10.1021/acscombsci.8b00072. [DOI] [PubMed] [Google Scholar]
- Ghashghaei O.; Caputo S.; Sintes M.; Revés M.; Kielland N.; Estarellas C.; Luque F. J.; Aviñó A.; Eritja R.; Serna-Gallego A. Multiple multicomponent reactions: unexplored substrates, selective processes, and versatile chemotypes in biomedicine. Chem. – Eur. J. 2018, 24, 14513. 10.1002/chem.201802877. [DOI] [PubMed] [Google Scholar]
- Farhid H.; Khodkari V.; Nazeri M. T.; Javanbakht S.; Shaabani A. Multicomponent reactions as a potent tool for the synthesis of benzodiazepines. Org. Biomol. Chem. 2021, 19, 3318. 10.1039/D0OB02600J. [DOI] [PubMed] [Google Scholar]
- Gerry C. J.; Schreiber S. L. Recent achievements and current trajectories of diversity-oriented synthesis. Curr. Opin. Chem. Biol. 2020, 56, 1–9. 10.1016/j.cbpa.2019.08.008. [DOI] [PubMed] [Google Scholar]
- Pavlinov I.; Gerlach E. M.; Aldrich L. N. Next generation diversity-oriented synthesis: a paradigm shift from chemical diversity to biological diversity. Org. Biomol. Chem. 2019, 17, 1608. 10.1039/C8OB02327A. [DOI] [PubMed] [Google Scholar]
- Murlykina M. V.; Morozova A. D.; Zviagin I. M.; Sakhno Y. I.; Desenko S. M.; Chebanov V. A. Aminoazole-based diversity-oriented synthesis of heterocycles. Front. Chem. 2018, 6, 527. 10.3389/fchem.2018.00527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss L.; Benke Z.; Remete A. M.; Fülöp F. Diversity-oriented Functionalization of Cyclodienes Through Selective Cycloaddition/Ring-opening/Cross-metathesis Protocols; Transformation of a “Flatland” into Three-dimensional Scaffolds With Stereo- and Regiocontrol. Chem. Rec. 2020, 20, 1129. 10.1002/tcr.202000070. [DOI] [PubMed] [Google Scholar]
- Shiri P. An overview on the copper-promoted synthesis of five-membered heterocyclic systems. Appl. Organomet. Chem. 2020, 34, e5600 10.1002/aoc.5600. [DOI] [Google Scholar]
- Aboonajmi J.; Panahi F.; Sharghi H. One-pot multicomponent coupling reaction of catechols, benzyl alcohols/benzyl methyl ethers, and ammonium acetate toward synthesis of benzoxazoles. ACS omega 2021, 6, 22395. 10.1021/acsomega.1c03207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiri P. Novel hybrid molecules based on triazole-β-lactam as potential biological agents. Mini Rev. Med. Chem. 2021, 21, 536. 10.2174/1389557520666201027160436. [DOI] [PubMed] [Google Scholar]
- Bur S. K.; Padwa A. The Pummerer reaction: methodology and strategy for the synthesis of heterocyclic compounds. Chem. Rev. 2004, 104, 2401. 10.1021/cr020090l. [DOI] [PubMed] [Google Scholar]
- Esmaili S.; Moosavi-Zare A. R.; Khazaei A.; Najafi Z. Synthesis of novel pyrimido [4, 5-b] quinolines containing benzyloxy and 1, 2, 3-triazole moieties by DABCO as a basic catalyst. ACS omega. 2022, 7, 45314. 10.1021/acsomega.2c05896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali I.; Mukhtar S. D.; Hsieh M. F.; Alothman Z. A.; Alwarthan A. Facile synthesis of indole heterocyclic compounds based micellar nano anti-cancer drugs. RSC Adv. 2018, 8, 37905. 10.1039/C8RA07060A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henary M.; Kananda C.; Rotolo L.; Savino B.; Owens E. A.; Cravotto G. Benefits and applications of microwave-assisted synthesis of nitrogen containing heterocycles in medicinal chemistry. RSC Adv. 2020, 10, 14170. 10.1039/D0RA01378A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerru N.; Gummidi L.; Maddila S.; Gangu K. K.; Jonnalagadda S. B. A review on recent advances in nitrogen-containing molecules and their biological applications. Molecules 2020, 25, 1909. 10.3390/molecules25081909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C. T. Nature loves nitrogen heterocycles, Nature loves nitrogen heterocycles. Tetrahedron Lett. 2015, 56, 3075. 10.1016/j.tetlet.2014.11.046. [DOI] [Google Scholar]
- Dua R.; Shrivastava S.; Sonwane S.; Srivastava S. Pharmacological significance of synthetic heterocycles scaffold: a review. Adv. Biol. Res. 2011, 5, 120. [Google Scholar]
- Fuentes N.; Kong W.; Fernandez-Sanchez L.; Merino E.; Nevado C. Cyclization cascades via N-amidyl radicals toward highly functionalized heterocyclic scaffold. J. Am. Chem. Soc. 2015, 137, 964. 10.1021/ja5115858. [DOI] [PubMed] [Google Scholar]
- Uno H.; Imai T.; Harada K.; Shibata N. Synthesis of highly functionalized 12-membered trifluoromethyl heterocycles via a nondecarboxylative Pd-catalyzed [6+ 6] annulation. ACS Catal. 2020, 10, 1454. 10.1021/acscatal.9b05377. [DOI] [Google Scholar]
- Kumar R. S.; Almansour A. I.; Arumugam N.; Kotresha D.; Manohar T. S.; Venketesh S. Cholinesterase inhibitory activity of highly functionalized fluorinated spiropyrrolidine heterocyclic hybrids. Saudi J. Biol. Sci. 2021, 28, 754. 10.1016/j.sjbs.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharghi H.; Aberi M.; Doroodmand M. M.; Shiri P. Chromium(III)-salen complex nanoparticles on AlPO4: as an efficient heterogeneous and reusable nanocatalyst for mild synthesis of highly functionalized piperidines, 2-arylbenzimidazoles, and 2-arylbenzothiazoles. J. Iran. Chem. Soc. 2017, 14, 1557. 10.1007/s13738-017-1097-x. [DOI] [Google Scholar]
- Del Corte X.; López-Francés A.; Maestro A.; Villate-Beitia I.; Sainz-Ramos M.; Martínez de Marigorta E.; Pedraz J. L.; Palacios F.; Vicario J. A multicomponent protocol for the synthesis of highly functionalized γ-lactam derivatives and their applications as antiproliferative agents. Pharmaceuticals 2021, 14, 782. 10.3390/ph14080782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiri P.; Amani A. M.; Mayer-Gall T. A recent overview on the synthesis of 1, 4, 5-trisubstituted 1, 2, 3-triazoles. Beilstein J. Org. Chem. 2021, 17, 1600. 10.3762/bjoc.17.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazeri N.; Lashkari M.; Fatahpour M.; Sheikhveisi M. Synthesis of Pyrazolopyranopyrimidine and Dihydropyrano [2, 3-c] pyrazole Derivatives using Vitamin D as an Efficient Catalyst under Green Condition. Bull. Korean Chem. Soc. 2020, 41, 786. 10.1002/bkcs.12067. [DOI] [Google Scholar]
- Rui P.; Xu Z.; Liu J.; Huangc Q. L-Ascorbic acid as an efficient organocatalyst for the synthesis of dispiro [tetrahydroquinoline-bis (1, 3-dioxane-4, 6-dione)] derivatives. ARKIVOC 2021, 2021, 96. 10.24820/ark.5550190.p011.463. [DOI] [Google Scholar]
- Zuo L.; Yang Y.; Guo W. Modular domino process toward highly functionalized pyrroles via Pd-catalyzed [4+ 1] annulation under mild conditions. Org. Lett. 2013, 2021, 23. 10.1021/acs.orglett.1c00148. [DOI] [PubMed] [Google Scholar]
- Nale S. D.; Maiti D.; Lee Y. R. Construction of Highly Functionalized Xanthones via Rh-Catalyzed Cascade C–H Activation/O-Annulation. Org. Lett. 2021, 23, 2465. 10.1021/acs.orglett.1c00391. [DOI] [PubMed] [Google Scholar]
- Dong K.; Humeidi A.; Griffith W.; Arman H.; Xu X.; Doyle M. P. AgI-catalyzed reaction of enol diazoacetates and imino ethers: synthesis of highly functionalized pyrroles. Angew. Chem., Int. Ed. 2021, 60, 13394. 10.1002/anie.202101641. [DOI] [PubMed] [Google Scholar]
- Dong J.; Feng W.; Wang L.; Li M.; Chen Z.; Xu X. Cu/base co-catalyzed [3+ 3] cycloaddition for the synthesis of highly functionalized 4-fluoropyridines. Chem. Commun. 2021, 57, 12635. 10.1039/D1CC05412K. [DOI] [PubMed] [Google Scholar]
- Fatahpour M.; Lashkari M.; Hazeri N.; Sadeh F. N.; Maghsoodlou M. T. Stereoselective synthesis of polysubstituted hydroquinolines in a one-pot, pseudo-eight-component strategy. Org. Prep. Proced. Int. 2019, 51, 576. 10.1080/00304948.2019.1677992. [DOI] [Google Scholar]
- Hazeri N.; Lashkari M.; García-Granda S.; Torre-Fernández L. Novel synthesis, molecular structure, and theoretical Studies of dispiro compounds via pseudo-eight-component reaction. Aust. J. Chem. 2014, 67, 1656. 10.1071/CH13713. [DOI] [Google Scholar]
- Seyrani H.; Ramezanpour S.; Vaezghaemi A.; Kobarfard F. A sequential Ugi–Smiles/transition-metal-free endo-dig Conia–ene cyclization: the selective synthesis of saccharin substituted 2, 5-dihydropyrroles. New J. Chem. 2021, 45, 15647. 10.1039/D1NJ01159F. [DOI] [Google Scholar]
- Ramezanpour S.; Bigdeli Z.; Rominger F. Saccharin as a new organocatalyzed: a fast, highly efficient and environmentally friendly protocol for synthesis of imidazo[1,2-α]pyridine derivatives via a one-three component reaction. Asian J. Green Chem. 2019, 4, 87. 10.22034/AJGC/2020.1.7. [DOI] [Google Scholar]
- Ramezanpour S.; Panahi A.; Rominger F. Diastereoselective synthesis of peptidomimetics in one-pot Ugi reaction using trans-4-isopropylcyclohexanecarboxylic acid. Monatsh. Chem. 2018, 149, 625. 10.1007/s00706-017-2086-6. [DOI] [Google Scholar]
- Salahi S.; Maghsoodlou M. T.; Hazeri N.; Lashkari M.; Torbati N. A.; Kazemian M. A.; Garcia-Granda S.; Torre-Fernandez L. Bro̷nsted acidic ionic liquid catalyzed synthesis of poly-substituted hydroquinolines through diastereoselective, one-pot and pseudo-eight-component reaction. J. Saudi Chem. Soc. 2016, 20, 349. 10.1016/j.jscs.2014.11.002. [DOI] [Google Scholar]
- Chande M. S.; Khanwelkar R. R. Michael addition approach for the synthesis of novel spiro compounds and 2-substituted malonic acid derivatives from Meldrum’s acid. Tetrahedron Lett. 2005, 46, 7787. 10.1016/j.tetlet.2005.09.030. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.