Abstract

BaZrS3 chalcogenide perovskites have emerged as a promising absorber due to their exceptional properties. However, there are no experimental reports on the applicability of BaZrS3 in photovoltaics. Thus, theoretical knowledge of device structure engineering is essential for its successful fabrication. In this regard, we have proposed various BaZrS3 device configurations by altering 12 electron transport layers (ETLs) in combination with 13 hole transport layers (HTLs) using SCAPS-1D, wherein a total of 782 devices are simulated by tuning the thickness, carrier concentration, and defect density of BaZrS3, ETLs, and HTLs. Interestingly, the absorber’s thickness optimization enhanced the absorption in the device by 2.31 times, elevating the generation rate of charge carriers, while the increase in its carrier concentration boosted the built-in potential from 0.8 to 1.68 V, reducing the accumulation of charge carriers at the interfaces. Notably, on further optimization of ETL and HTL combinations, the best power conversion efficiency (PCE) of 28.08% is achieved for FTO/ZrS2/BaZrS3/SnS/Au, occurring due to the suppressed barrier height of 0.1 eV at the ZrS2/BaZrS3 interface and degenerate behavior of SnS, which increased charge carrier transportation and conductivity of the devices. Upon optimizing the work function, an ohmic contact is achieved for Pt, boosting the PCE to 28.17%. Finally, the impact of Ti alloying on BaZrS3 properties is examined on the champion FTO/ZrS2/BaZrS3/SnS/Pt device where the maximum PCE of 32.58% is obtained for Ba(Zr0.96,Ti0.04)S3 at a thickness of 700 nm due to extended absorption in the NIR region. Thus, this work opens doors to researchers for the experimental realization of high PCE in BaZrS3 devices.
1. Introduction
With the increase in global energy demand, extensive efforts have been made to address the depletion of fossil fuels like coal, oil, and natural gas. Thus, the quest for enduring eco-friendly energy solutions to meet the escalating demand has become crucial. Amidst these pursuits, solar energy conversion employing photovoltaic (PV) technology is a sustainable and environmentally conscious approach to generating electricity.1 Over the past decade, lead halide perovskites (MAPbX3 where X = I, Br, Cl) have revolutionized the development of PV materials. They demonstrated a power conversion efficiency (PCE) of 3.8% in 20092 by using liquid electrolytes as a hole transport layer (HTL).3 The PCE was improved to 9.7% in 2012 by replacing the HTL with the solid-state spiro-OMeTAD. Within a few years, the lead halide perovskites emerged as a promising optoelectronic material with its unpreceded rate of increase in PCE up to 26.1% in 2023.4 Despite the enhanced performance, the toxicity of lead and instability of halides in humidity, heat, and light hinder their pace of commercialization. As a result, researchers have been exploring lead-free perovskite materials to address these environmental concerns. Various materials are being developed as alternatives to lead-based perovskites. To be specific, perovskites based on tin (Sn) and germanium (Ge) such as MASnI3, CsSnI3, FASnI3, CsGeI3, MAGeI3, FAGeI3, and CsSn0.5Ge0.5I3have emerged as promising alternatives due to their similar isoelectronic (s2p2) configuration, high carrier mobility, nontoxicity, and narrow band gaps. Although there are some challenges when it comes to stability, such as Sn2+ and Ge2+ ions being easily oxidized and converting to Sn4+ and Ge4+ when exposed to ambient atmosphere, the high cost and lack of Ge in the earth’s crust also limit its applicability.5 Certainly, researchers have found some alternative solutions. For instance, they have introduced trivalent cations like Bi3+ and Sb3+ instead of Pb2+ ions. However, the PCE achieved is still much lower than lead halide perovskites due to their low-dimensional structure, which results in unfavorable optoelectronic properties. To overcome these challenges, halide double perovskites (HDPs) have been developed with a structure similar to A2B+B3+X6, replacing Pb2+ with eco-friendly Bi3+ while maintaining a three-dimensional (3D) structure. This innovative solution involves replacing two Pb2+ ions with one B+ ion and one B3+ ion.6 Some notable examples of these HDPs are Cs2AgBiBr6, Cs2NaBiCl6, (CH3NH3)2AgBiBr6, and (CH3NH3)2KBiCl6, which have recently emerged as highly promising materials. While HDPs have great potential, their efficiency is limited by large charge carrier effective masses, high band gap (>2 eV), and weak charge carrier transport capabilities. Research has been focused on exploring antimony-based perovskite materials (such as Cs2SbCuCl6, Cs4SbCuCl12, Cs2SbAgCl6, Cs2SbAgI6, and Cs2SbAgBr6) as they offer unique attributes like stability at high temperatures and exceptional charge carrier conductivity. However, their wide band gaps, high binding energies, and high carrier effective mass limit their performance.7 Apart from perovskites, researchers are also exploring other light absorbers such as ternary II–IV–N2 nitrides (ZnSnN2),8,9 binary halides (InI10 and BiI311), AbZ2 chalcogenides (AgBiS2,12 NaBiS2,13 and NaSbS214), Bi-based chalcohalides (BiOI,15 BiSI,16 Bi19S27Br3, and Bi13S18I2), Sb-based chalcohalides (SbSI,18,19 SbSeI,20 and SbBiISI21), Pb-based chalcohalides (Pb4S3Br222 and Pb2SbS2I323), Ag-based chalcohalides (Ag3BiI6–2xSx),24 Sn-based chalcohalides (Sn2SbS2I3),25 hybrid organic–inorganic metal chalcogenides (MASbSI226 and MA3Bi2I9–2xSx27), I2–II–IV–VI4 semiconductors (Cu2BaSnSe4,28 Cu2SrSnS429), and II–V semiconductors (Zn3P2).30−32 Despite their promise, these emerging absorbers have exhibited PCEs below 8%. Therefore, we are urged to discover new materials that can retain the extraordinary properties of lead halide perovskites while overcoming the aforementioned limitations.
In this concern, chalcogenide perovskites emerged as an alternative novel material due to their nontoxicity and high stability. They grabbed the limelight in 2015 when Sun et al. theoretically screened 18 ABX3 (A = Ba, Sr, Ca; B = Ti, Zr, Hf; X = S, Se) compounds to be promising for optoelectronic applications.33 Among them, BaZrS3 is considered to be ideal from the standpoint of the direct band gap of 1.7 eV,34 high absorption coefficient >105 cm–1 at photon energy larger than 1.97 eV (<700 nm),35 elevated carrier mobility of 30 cm2/(V s) with p-type conductivity,36 and exceptional stability toward moisture, light, and temperature.37 Prior research indicates that the performance of BaZrS3 can be improved further by alloying with both titanium (Ti) and selenium (Se). So far, Ti alloying has proven to be more productive due to its effective band gap reduction with less atom %. Specifically, Meng et al. reported that the band gap obtained for 0.1 atom % Ti alloying can only be accomplished at 1 atom % of Se alloying.38 However, no experimental reports related to the applicability of BaZrS3 and Ba(Zr,Ti)S3 in PV exist due to its challenging synthesis temperatures exceeding 900 °C, limiting its feasibility and exploration of chalcogenide perovskites.39
Nevertheless, recent advancements have resulted in a reduction in the operating temperature required to synthesize BaZrS3. For example, Comparotto et al. reported the synthesis of BaZrS3 thin films, employing a sputter-sulfurization approach with a process temperature below 600 °C.40 Similarly, Nag et al. detailed their work on BaZrS3 nanocrystals (powder) synthesis at 600 °C, followed by the formulation of an ink and film-coating process, while Yu et al. demonstrated the synthesis of BaZrS3 thin films at an even lower temperature of 500 °C by modifying the chemical reaction pathway.41 Interestingly, Yang et al. and Zilevu et al. reported colloidal synthesis of BaZrS3 nanopowders at an impressive temperature below 350 °C, underscoring the potential of solution-based processing of BaZrS3.42,112 Most recently, in 2023, Vincent et al. introduced a groundbreaking approach for the solution processing of direct BaZrS3 thin films. This method involves heat treatment of precursor thin films in a sulfur-containing atmosphere at modest temperatures ranging from 500 to 575 °C.43 These reports are a major accomplishment in producing BaZrS3. They used processing temperatures similar to those of traditional absorbers, which is a significant advancement. These developments have opened new opportunities for creating effective BaZrS3 devices. Therefore, to predict the device performance, we utilized a solar cell capacitance simulator in a one dimension (SCAPS-1D) theoretical tool to study the influence of each layer’s parameters, interfacial defects, stability, and band alignment of the device by saving the time and cost before investing into fabrication.44 Moreover, SCAPS-1D is a highly reliable software as the simulation results are consistent with the experimental work.45
Researchers recently employed BaZrS3 and Ba(Zr,Ti)S3 as absorbers in thin-film devices and studied their influence using SCAPS-1D.37,46,47 However, a comprehensive study on the device engineering and properties of BaZrS3 and Ba(Zr,Ti)S3 is scarce, creating more room to discover and understand the potential of these absorbers in thin-film devices. Also, choosing the right electron transport layers (ETLs) and HTLs is crucial in creating efficient devices. ETLs are particularly important as they help extract and transfer photogenerated electrons while blocking holes. An ideal ETL should possess several vital attributes, including a wide band gap that facilitates high light transmission, perfect energy levels, cost-effectiveness, high electron mobility for efficient electron injection and transport, and photochemical and thermal stability. Organic materials and polymers are lead halide perovskites, the most widely used ETLs. However, these materials tend to be expensive and less stable. Interestingly, inorganic ETLs are a promising alternative due to their high stability, easy preparation process, cost-effectiveness, and excellent electronic properties.48,49 Considering these advantages, we have carefully curated a list of 12 promising inorganic ETLs, including Bi2S3,50,51 ZrS2,52,53 SnS2,54 In2S3,55 ZnS,55 Zn2SnO4,56 BaSnO3,57 Al2O3,58,59 WO3,58 TiO2,60 ZnO,60 and SnO260 for our simulation. On the other hand, the choice of HTLs significantly affects the device’s performance, durability, and production cost. While small-molecule HTLs can enhance the device’s performance, they often suffer from stability issues. In contrast, polymeric HTLs offer stability at high temperatures and compatibility with other materials, but their poor optoelectronic properties hinder efficiency. Additionally, commonly used organic HTLs like Spiro-MeOTAD are expensive and unstable in ambient conditions. Interestingly, inorganic HTLs offer numerous advantages, such as outstanding stability, high hole mobility, low cost, and easy preparation techniques, and have proven to be promising for achieving stable and efficient device performance.6,61 In this context, we have selected 13 HTLs, including MoS2,62 CuO,63 Cu2S,64 FeS2,65 Sb2S3,66 SnS,67 Cu3BiS3,68 Cu2SnS3,69 CuSbS2,70 Cu2BaSnS4,71 CuInGaS2,72 Cu2ZnSnS4,73 and Cu2ZnSn1–xGexS4.73 Thus, our work embarks on modeling 782 thin-film devices employing BaZrS3 as the absorber, varying 13 HTLs and 12 ETLs to elucidate an ideal device structure. The material properties have been studied extensively by varying the thickness, carrier concentration, and defect density of each ETL with 13 HTLs to investigate their suitability for emerging BaZrS3-based devices. Moreover, the role of back contacts is meticulously examined by tuning the metal electrode work functions. Furthermore, we studied the influence of Ti alloying, with its concentration ranging from 1 to 4 atom % in the BaZrS3 absorber for the device that attained the highest PCE.74 To this end, an array of analyses, including current density–voltage (J–V), capacitance–voltage (C–V), quantum efficiency (QE), electric field distributions, generation profiles, and recombination profiles, have been utilized to gain a broader knowledge of the functioning of devices. On top of that, the role of band alignment between the absorber and transporting layers was also examined using the energy band diagram extracted from SCAPS-1D. Thus, we firmly believe that our work creates a significant impact among researchers worldwide by providing a better direction for understanding the performance of BaZrS3 and Ba(Zr,Ti)S3 and facilitates the research community in selecting cost-effective and efficient devices for commercialization as the wheel of development rolls on.
2. Computational Strategy and Device Description
One-dimensional Solar Cell Capacitance Simulator (SCAPS-1D) is a well-known software program developed by Mark Burgelmann at the University of Ghent in Belgium to predict the device performance concerning the characteristics of each layer and interface. This software offers numerous advantages, such as conducting performance analysis at up to seven levels to perform comprehensive and batch analyses.75 The theoretical calculation relies on three fundamental equations, namely, Poisson’s equation, the carrier continuity equation, and the drift-diffusion equation, which are as follows76
| 1 |
| 2 |
| 3 |
where q is the charge of electrons (C), p is the concentration of holes (cm–3), ε is the dielectric constant, n is the concentration of electrons (cm–3), φ is the electric potential (V), R is the carrier recombination rate (cm–3 s–1), G is the carrier generation rate (cm–3 s–1), Jp is the current density of holes (mA/cm2), Jn is the current density of electrons (mA/cm2). Dn and Dp refer to coefficients of electrons and hole diffusion (cm2/s), respectively, while μn and μp are the mobilities of electrons and holes (cm2/(V s)).76,77
In this work, we initially designed a base device with superstrate configuration FTO/SnO2/BaZrS3/Cu2S/Au, as shown in Figure 1a, using SCAPS-1D (version 3.3.10). The light enters through fluorine-doped tin oxide (FTO) and gets transmitted through the SnO2 ETL to the absorber. Due to the p–n junction’s built-in potential, charge carriers are generated, separated, and collected at the respective contacts.78 A summary of the simulation’s initial device parameters for FTO, SnO2, BaZrS3, and Cu2S is shown in Table 1. All of the analyses were performed under AM 1.5G (100 W cm–2, one sun) spectral irradiance, and the operational temperature of devices was maintained at 300 K.
Figure 1.
(a) Initial device structure and (b) J–V characteristics of the emerging BaZrS3 chalcogenide perovskite device.
Table 1. SCAPS-1D Input Parameters of the Initial Device Structure.
| parameter | FTO | SnO2 | BaZrS3 | Cu2S |
|---|---|---|---|---|
| thickness (nm) | 10 | 30 | 500 | 50 |
| band gap Eg (eV) | 3.50 | 3.50 | 1.70 | 1.21 |
| affinity χ (eV) | 4.00 | 4.00 | 4.10 | 4.45 |
| dielectric permittivity εr | 9.0 | 9.0 | 9.6 | 30 |
| conduction band effective density of states NC (cm–3) | 2.2 × 1018 | 2.20 × 1017 | 2.2 × 1018 | 1.00 × 1019 |
| valence band effective density of states NV (cm–3) | 1.8 × 1019 | 2.20 × 1016 | 1.8 × 1019 | 6.15 × 1019 |
| electron thermal velocity (cm/s) | 1 × 107 | 1 × 107 | 1 × 107 | 1 × 107 |
| hole thermal velocity (cm/s) | 1 × 107 | 1 × 107 | 1 × 107 | 1 × 107 |
| electron mobility (cm2/(V s)) | 2 × 101 | 2.00 × 1001 | 1.10 × 10–02 | 5.00 × 101 |
| hole mobility (cm2/(V s)) | 1 × 101 | 1.00 × 1001 | 3.90 × 10–02 | 4.00 × 100 |
| donor density ND (cm–3) | 1 × 1018 | 1 × 1018 | 0 | 0 |
| acceptor density NA (cm–3) | 0 | 0 | 1 × 1015 | 7 × 1016 |
| defect density NT (cm–3) | 1 × 1015 | 1 × 1015 | 1 × 1010 | 1 × 1013 |
| references | (79) | (80) | (46,47) | (81) |
To begin with, the initial device performance (Section 3.1) is represented in the J–V curve (Figure 1b). After that, the influence of varying the thickness, carrier concentration, and defect density of ETL, absorber, and HTL is studied and reported in Section 3.2. The corresponding device parameters, such as open-circuit voltage (Voc), short-circuit current density (Jsc), fill factor (FF), and PCE, are obtained. After optimizing each layer in the initial device, the impact of different ETLs and HTLs on the device’s PCE is explained in Section 3.3. A schematic representation of energy band diagrams and the 156 devices that achieved the highest PCE for each ETL and HTL combination is shown in Figure 4 and Table 2, respectively. In addition, the influence of metal contacts with different work functions is studied in Section 3.4. Then, the effect of Ti alloying for the device with the highest PCE is briefly investigated in Section 3.5. Finally, devices with the highest PCE are selected from the simulations above, and their performances are investigated by varying series resistance (RS), shunt resistance (RSh), and working temperature from 1 to 10 Ω cm2, 10 to 107 Ω cm2, and 300 to 400 K, respectively (Section 3.6). Subsequently, the material properties are elucidated using J–V, C–V, QE, electric field distributions, generation profiles, and recombination profiles from SCAPS-1D.
Figure 4.
Schematic representation of energy-level diagram for different ETLs, HTLs, and BaZrS3 chalcogenide perovskite absorbers.
Table 2. Influence of Metal Work Function on PV Parameters.
| metal contacts | work function (eV) | Voc (V) | Jsc (mA/cm2) | FF (%) | PCE (%) |
|---|---|---|---|---|---|
| Cu/Mo | 4.6 | 0.87 | 23.81 | 73.14 | 15.16 |
| Ag | 4.7 | 0.88 | 26.22 | 70.81 | 16.36 |
| Fe | 4.8 | 0.94 | 29.55 | 74.73 | 20.78 |
| Cu-doped C | 5.0 | 1.11 | 29.74 | 81.95 | 27.09 |
| Au | 5.1 | 1.17 | 29.74 | 80.42 | 28.08 |
| W | 5.2 | 1.18 | 29.74 | 80.16 | 28.17 |
| Ni | 5.5 | 1.18 | 29.74 | 80.15 | 28.17 |
| Pd | 5.6 | 1.18 | 29.74 | 80.15 | 28.17 |
| Pt | 5.7 | 1.18 | 29.74 | 80.15 | 28.17 |
3. Results and Discussion
3.1. Initial Device Performance
To acquire a suitable device configuration for enhanced performance of chalcogenide perovskites, we designed a base device structure (FTO/SnO2/BaZrS3/Cu2S/Au) as illustrated in Figure 1a. The simulations were performed according to the parameters listed in Table 1, and the resulting J–V characteristics of the initial device and its respective PV parameters are provided in Figure 1b. Afterward, ETL, absorber, and HTL properties were optimized to enhance performance by varying their thickness, carrier concentration, and defect density. The results of the simulations are shown in Section 3.2.
3.2. Optimization of ETL, Absorber, and HTL Parameters
To begin with, SnO2 ETL was optimized by varying the parameters such as thickness, carrier concentration, and defect density. However, no significant changes in the PV parameters were observed with the ETL optimization, as shown in Figure S1. Interestingly, significant improvements in the device performance have been achieved by optimizing the absorber and HTL, which are comprehensively discussed in the following sections.
3.2.1. Impact of Absorbers Thickness, Carrier Concentration, and Defect Density
In this study, we varied the absorber
thickness from 100 to 2000 nm. Figure 2a demonstrates that the Voc reaches an optimum value of 1.37 V at 1000 nm and remains insignificant
with an increase in thickness beyond 1000 nm. In contrast, the Jsc surges from 13.90 to 21.95 mA/cm2 as the thickness increases from 100 to 2000 nm. This increase in Jsc is attributed to the enhanced spectral response
at longer wavelengths.82 Conversely, the Voc saturates as the absorber becomes thick enough
to absorb almost all incident light. This saturation could be linked
to nonradiative losses associated with point defects, carrier lifetime,
and interface recombination.83 Examining Figure 2b, we observe that
the FF increases from 48.64 to 79.12% as the thickness increases from
100 to 1000 nm and then diminishes. Conversely, the PCE experiences
a substantial increase from 7.96 to 24.43% as the thickness increases
from 100 to 2000 nm. Notably, the PCE increases by three times when
the thickness extends from 100 to 1000 nm, while it improves by 0.94
times when the thickness is doubled from 1000 to 2000 nm. FF is the
ratio of the maximum power output (Pmax) to the product of Jsc and 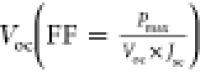 , while PCE is the ratio of Pmax to incident
power of light i.e.,
, while PCE is the ratio of Pmax to incident
power of light i.e., 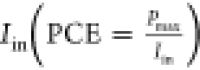 .83 The
increased Jsc and Voc at an
optimum thickness result in higher FF. However, charge carrier depletion
accelerates at an absorber thickness beyond 1000 nm, and the series
resistance increases, leading to a subtle decrease in the fill factor.84 Furthermore, the PCE of the device is influenced
by both light absorption and carrier transport. When the absorber
is thin, it absorbs a small portion of light and then transmits the
unreflected remaining incident light, resulting in major absorption
in the HTL. Subsequently, charge carriers accumulate near the back
contact, enhancing recombination in the device. When the thickness
increases, more light is absorbed in the absorber layer, reducing
the buildup of charge carriers in the back contact.82 This behavior is documented in Figure 2c, illustrating the charge carrier generation
rate at two distinct thicknesses, 100 and 1000 nm. At 100 nm, the
generation rate is higher in the HTL region than in the absorber region,
indicating a large accumulation of charge carriers, while at 1000
nm, it is more prominent in the absorber region, revealing increased
photon absorption in the absorber, resulting in enhanced device performance.85 Additionally, QE measurements in Figure 2d show that the integral area
of QE absorption increases from 27.2 to 62.9% when the thickness increases
from 100 to 1000 nm, whereas it improves by only 1% with further increments
to 2000 nm. Considering the device’s generation rate, QE measurements,
and manufacturing cost, 1000 nm is the optimum thickness.
.83 The
increased Jsc and Voc at an
optimum thickness result in higher FF. However, charge carrier depletion
accelerates at an absorber thickness beyond 1000 nm, and the series
resistance increases, leading to a subtle decrease in the fill factor.84 Furthermore, the PCE of the device is influenced
by both light absorption and carrier transport. When the absorber
is thin, it absorbs a small portion of light and then transmits the
unreflected remaining incident light, resulting in major absorption
in the HTL. Subsequently, charge carriers accumulate near the back
contact, enhancing recombination in the device. When the thickness
increases, more light is absorbed in the absorber layer, reducing
the buildup of charge carriers in the back contact.82 This behavior is documented in Figure 2c, illustrating the charge carrier generation
rate at two distinct thicknesses, 100 and 1000 nm. At 100 nm, the
generation rate is higher in the HTL region than in the absorber region,
indicating a large accumulation of charge carriers, while at 1000
nm, it is more prominent in the absorber region, revealing increased
photon absorption in the absorber, resulting in enhanced device performance.85 Additionally, QE measurements in Figure 2d show that the integral area
of QE absorption increases from 27.2 to 62.9% when the thickness increases
from 100 to 1000 nm, whereas it improves by only 1% with further increments
to 2000 nm. Considering the device’s generation rate, QE measurements,
and manufacturing cost, 1000 nm is the optimum thickness.
Figure 2.
Changes in (a) Voc, Jsc, (b) FF, PCE, (c) generation rate, and (d) QE as a function of absorber thickness. Changes in (e) Voc, Jsc (f) FF, PCE, and (g) Mott–Schottky as a function of the absorber carrier concentration. Changes in (h) Voc, Jsc (i) FF, PCE, and (j) recombination rate as a function of absorber defect density.
Higher carrier concentration can lead to Auger recombination and hinder hole transportation from the absorber to the HTL due to increased recombination rate and impurity scattering in the absorber.86 To investigate this, we conducted simulations with carrier concentrations ranging from 1012 to 1017 cm–3, as shown in Figure 2e,f. It can be noted that Jsc decreases with increasing carrier concentration, which can be attributed to the reduction in the diffusion length of minority carriers and the depletion width.6 Conversely, Voc and FF remain relatively stable from 1012 to 1014 cm–3 and increase beyond 1014 cm–3. The increase in Voc originates from the elevating built-in potential (Vbi) at the interfaces for higher carrier concentrations, which is evident in Figure 2g, where Vbi increases from 0.80 to 1.68 V when the carrier concentration is varied from 1012 to 1017 cm–3. This subsequently improves the separation and collection of charge carriers at the respective contacts without recombination. However, the increase in FF is linked to the reduction in series resistance in the absorber layer.87 Overall, when the carrier concentration is increased from 1012 to 1017 cm–3, PCE is enhanced from 22.85 to 24.27% due to the increase in Voc and FF, while the decrease in Jsc does not affect the device performance. However, Meng et al. reported that only a weakly p-type BaZrS3 film is possible under stoichiometric (S-rich/Zr-poor) conditions. Based on this understanding, we performed further simulations by selecting a carrier concentration of 1015 cm–3 instead of 1017 cm–3, even though the latter achieved a higher efficiency.37
Defects play a significant role in regulating interfacial recombination, carrier lifetime, and material doping levels. They can arise from uncoordinated atoms, nonstoichiometry, etc., and are predominantly found at the interface, surface, or grain boundaries. Bulk defects, such as interstitial and vacancy defects, and Schottky and Frenkel defects, also adversely affect the absorber’s properties.37 Therefore, the influence of defect density is studied and reported in Figure 2h,i. As the defect density increases, a decrease in all of the PV parameters is observed. This can be attributed to the increased recombination rate (Figure 2g) at higher defect densities.88 Thus, a defect density of 1012 cm–3 is selected as an optimum value for the BaZrS3 absorber. Generally, BaZrS3 synthesized under S-rich/Zr-poor conditions is identified to be defect-tolerant owing to its high formation energy, which surpasses deep-level defects, revealing the possibility of achieving lower defect density experimentally.38
3.2.2. Impact of HTL Thickness, Carrier Concentration, and Defect Density
The thickness of the HTL is varied from 10 to 100 nm (Figure 3a,b), where a slight decrease in Voc from 1.13 to 1.06 V is observed, indicating a surging probability of recombination with HTL thickness.82 In contrast, Jsc improves from 22.01 to 27.22 mA/cm2 with increasing HTL thickness due to the enhanced light absorption in the device.89 This can also be witnessed in Figure 3c, where the absorption increases from 54 to 61% when the HTL thickness is extended from 10 to 100 nm. In addition, FF improves marginally from 79.02 to 81.56%, resulting from the enriched charge transportation from the absorber.90 As a result, PCE increases from 19.81 to 23.60%, primarily driven by increased Jsc and FF. Generally, the HTL should be thicker than the ETL to ensure an equal number of charge carriers reach the terminals simultaneously, minimizing the likelihood of recombination. Additionally, a thicker HTL creates room for more photons to be absorbed.6 Based on these considerations, an optimal thickness of 100 nm is selected for further optimization.
Figure 3.
Changes in (a) Voc, Jsc, (b) FF, PCE, and (c) QE as a function of HTL thickness. Changes in (d) Voc, Jsc (e) FF, PCE, and (f) electric field (E) along the HTL region as a function of HTL carrier concentration. Changes in (g) Voc, Jsc (h) FF, and PCE as a function of HTL defect density.
We varied the carrier concentration from 1012 to 1020 cm–3, as displayed in Figure 3d,e. It can be observed that all of the PV parameters exhibit insignificant changes until a concentration of 1015 cm–3. This indicates that the carrier concentration of the HTL does not influence the PV parameters when it is lower than that of the absorber. However, when the carrier concentration exceeds 1015 cm–3, the variation in Jsc remains insignificant, while Voc demonstrates an increase from 1.01 to 1.16 V, which together with the improvement in the FF from 77.61 to 82.21%, enhances the PCE from 21.35 to 26.10%. This elevation in PCE is attributed to the upward shift in energy bands that occurs with an increase in the carrier concentration. Consequently, a strong built-in electrical field is formed at the interface, as displayed in Figure 3f. This intense electric field promotes the transportation of holes from BaZrS3 to the back contact through Cu2S by raising the charge transport velocity and conductivity91 while restricting the flow of minority carriers, thereby reducing the carrier recombination rate. Overall, it is evident that the carrier concentration of the HTL must be higher than that of the absorber to achieve high device performance. As a result, a carrier concentration of 1020 cm–3 is selected for further studies.
Defects create additional nonradiative recombination pathways, converting light into heat instead of electricity. In particular, the recombination centers trap the photogenerated carriers before they can reach the terminals of the device, thus reducing the carrier’s lifetime.92 Therefore, it is crucial to minimize defects to enhance the performance of devices. To analyze the influence of defect density, we varied the defect density of the HTL from 1012 to 1020 cm–3, as displayed in Figure 3g,h. There was a noticeable reduction in the Voc, Jsc, FF, and PCE from 1.19 to 0.91 V, 27.22 to 21.40 mA/cm2, 81.46 to 76.58%, and 26.46 to 15.02%, respectively. This significant decrease in the PV parameters can be attributed to decreased carrier diffusion length at higher defect densities, resulting in an increased recombination rate at the BaZrS3/Cu2S interface.93 Therefore, a HTL defect density of 1012 cm–3 is required for highly efficient chalcogenide perovskite devices.
3.3. Influence of Different ETL and HTL
We systematically examined 12 ETLs (Bi2S3,50,51 ZrS2,52,53 SnS2,54 In2S3,55 ZnS,55 Zn2SnO4,56 BaSnO3,57 Al2O3,58,59 WO3,58 TiO2,60 ZnO,60 SnO260) and 13 HTLs (MoS2,62 CuO,63 Cu2S,64 FeS2,65 Sb2S3,66 SnS,67 Cu3BiS3,68 Cu2SnS3,69 CuSbS2,70 Cu2BaSnS4,71 CuInGaS2,72 Cu2ZnSnS4,73 Cu2ZnSn1–xGexS473) to identify suitable transport layers for emerging BaZrS3 chalcogenide perovskite devices (Figure 4) and their simulation parameters are given in Table S1. As previously discussed, we initially optimized SnO2 (ETL), BaZrS3 (absorber), and Cu2S (HTL) in our base device by varying the thickness, carrier concentration, and defect density. We altered the ETL and changed the abovementioned parameters in this optimized configuration. Then, while keeping the optimized ETL and absorber, we varied 13 HTLs and optimized their parameters to determine the most suitable device structure. We applied this approach to all of the remaining ETLs and HTLs, resulting in 782 devices (Table S1). Among them, 156 devices demonstrated the highest PCE for each ETL and HTL combination (Table S2). Interestingly, 139 devices showed a PCE > 20%, and 50 devices exhibited a PCE over 25%. Notably, 10 of these devices displayed an impressive PCE above 27%. To be specific, the devices that are represented as SC11, SC24, SC37, SC50, SC63, SC76, SC89, SC102, and SC128 in Table S2 demonstrated efficiencies of 27.34, 27.44, 27.44, 27.35, 27.43, 27.44, 27.35, 27.34, and 27.47%, respectively, and the highest PCE of 28.08% was accomplished for the champion device SC141 with structure ZrS2/BaZrS3/SnS. Figure 5 displays the QE of the aforementioned 10 devices with PCE greater than 27% in the wavelength range of 300–900 nm. An average QE > 70% was obtained for all device structures, wherein the highest QE of 76.23% was achieved for the champion SC141, which reveals that the light absorption in this device is considerably increased. The generated charge carriers are efficiently separated and collected at the contacts with less recombination due to the proper band alignment at the ZrS2/BaZrS3 and BaZrS3/SnS interface, leading to its superior device performance.
Figure 5.
QE measurements of 10 devices obtained a PCE of >27%. (a) SC11, (b) SC24, (c) SC37, (d) SC50, (e) SC63, (f) SC76, (g) SC89, (h) SC102, (i) SC128, and (j) SC141.
The critical factor behind the device’s overall performance is the band alignment between the absorber, ETL, and HTL. This alignment guarantees the effective separation and transportation of charge carriers. Conversely, any disparity in energy band positioning could lead to elevated carrier recombination and increased interface resistance. To understand the obtained PCE as a function of their energy band alignment, we selected three devices among 156 devices, demonstrating low (In2S3/BaZrS3/Cu2SnS3, 15.96%), intermediate (TiO2/BaZrS3/Cu2BaSnS4, 21.30%), and high (ZrS2/BaZrS3/SnS, 28.08%) PCEs, and their corresponding energy band diagrams were extracted from SCAPS-1D (Figure 6b–d) to perform a comparative analysis. It can be seen in Figure 6b that a spike-like barrier is observed at the BaZrS3/In2S3 and In2S3/FTO interface, respectively. This leads to the accumulation of electrons at both interfaces, which impedes flow to the front contact, resulting in high interface recombination.94 Considering the Cu2SnS3/BaZrS3 interface, a VBO of 0.2 eV indicates a smaller barrier for holes. However, a cliff at the CBO reveals that most holes will be collected in the HTL instead of blocking the electrons, leading to high recombination at the interface.95 A similar kind of alignment is observed for TiO2/BaZrS3/Cu2BaSnS4 (Figure 6c) with a difference that the CBO is comparatively low at the TiO2/BaZrS3 interface, reducing the accumulation of electrons and leading to improved PCE. Interestingly, for ZrS2/BaZrS3/SnS devices (Figure 6d), we can see that the cliff-like barrier at ZrS2/BaZrS3 is reduced to 0.1 eV. Also, the barrier height at the ZrS2/FTO interface is minute, which minimizes the electron accumulation in the ETL and facilitates the transportation of electrons to the front contact. In addition, CBO at the BaZrS3/SnS interface is high (0.3 eV), which blocks the flow of electrons to SnS, preventing the recombination at the interface. However, we can see that VBO at the BaZrS3/SnS interface is 0.6 eV, which is comparatively larger than the VBO of Cu2SnS3 (0.2 eV) and Cu2BaSnS4 (0.1 eV)-based devices used for the comparison. Nevertheless, SnS portrays degenerate behavior, i.e., the quasi-hole Fermi level is within the valence band. This implies that the conductivity is substantial at the BaZrS3/SnS interface, which enhances the flow of holes from the absorber to HTL, irrespective of the large barrier.96 Consequently, it enhances the collection of holes at the back contact. Thus, the high conductivity of SnS, in combination with less barrier for electrons at the ETL/absorber interface and high barrier for electrons at the HTL/absorber interface, resulted in the highest PCE of the ZrS2/BaZrS3/SnS device. Hence, achieving a suitable alignment enhances charge separation and transport, reduces recombination losses, and boosts device efficiency, which underscores the importance of understanding band alignment to craft high-performance devices.
Figure 6.
(a) Histogram of PCE for 156 devices detailed in Table S2. Energy band diagram for device structures, (b) In2S3/BaZrS3/Cu2SnS3, (c) TiO2/BaZrS3/Cu2BaSnS4, and (d) ZrS2/BaZrS3/SnS.
3.4. Influence of Different Metal Contacts
To study the influence of metal contacts on the high-performing device ZrS2/BaZrS3/SnS (PCE 28.08%), we varied different metal contacts, namely, Cu/Mo, Ag, Fe, Cu-doped C, Au, W, Ni, Pd, and Pt with work functions of 4.6, 4.7, 4.8, 5.0, 5.1, 5.2, 5.5, 5.6, and 5.7 eV, respectively. As displayed in Figure 7a,b, when the work function is low (4.6), the Fermi level of the metal contact approaches the conduction band of the HTL, leading to a significant barrier for hole transfer from the HTL to the metal contact. In addition, due to a low barrier (1.1 eV) for electrons, there is easy transfer between the conduction band of the HTL and the metal contact, leading to increased recombination in devices. Interestingly, a higher metal work function induces an inverse bending of the energy bands, facilitating the transfer of photogenerated holes to the metal contacts while creating a Schottky barrier (2.2 eV) for electron transfer.97 This behavior is observed in Pt contacts, where the metal’s Fermi level moves toward the valence band of the HTL, resulting in effortless hole transportation and improved device performance. However, the PCE of devices with Ni, Pd, and Pt metal contacts remains constant (Table 2). This phenomenon is attributed to the saturation of the Vbi when the metal work function increased beyond 5.2 eV, primarily caused by increased ohmic resistance at the HTL/metal contact interface.100 Consequently, we achieved the highest PCE of 28.17% with the ZrS2/BaZrS3/SnS/Pt device structure.
Figure 7.
Influence of (a) lowest and (b) highest metal work functions in the device performance.
3.5. Investigating the Influence of Ti Alloying in the BaZrS3 Absorber
Alloying is a widely employed technique to attain the desired optoelectronic properties that enhance the device’s overall performance. Thus, alloying BaZrS3 with a suitable metal can further enhance its optical properties. Notably, the band gap of BaZrS3 (1.7 eV) is slightly larger than the optimal band gap for single-junction devices, as governed by the Shockley–Quiesser limit.38,98 To reduce BaZrS3’s band gap through alloying, Ti and Se are ideal choices due to their favorable energy states, atomic size, and electronegativity. In a first-principles study, Meng et al. demonstrated that Ti alloying lowers the conduction band edge, making Ti suitable for facilitating the n-type character in BaZrS3, while Se alloying raises the valence band edge, making it ideal for lowering the band gap and enabling p-type doping by shallowing the acceptors.38 However, Se alloying exhibits a bowling effect, familiar in anion alloying, and requires approximately 1 atom % of Se to achieve the same band gap reduction, which is significantly higher than the 0.1 atom % estimated for Ti.38 Considering these factors, we focused on Ti alloying for our study. The alloying process was performed on the best device structure, incorporating ZrS2 as the ETL, SnS as the HTL, and Ba(Zr(1–x)Tix)S3 as an absorber with x values ranging from 0.01 to 0.04. This small concentration of alloying effectively reduced the band gap from 1.71 to 1.51 eV and resulted in a performance boost.74 Therefore, we investigated the influence of alloying with four different concentrations: Ba(Zr0.99,Ti0.01)S3 (Device T1), Ba(Zr0.98,Ti0.02)S3 (Device T2), Ba(Zr0.97,Ti0.03)S3 (Device T3), and Ba(Zr0.96,Ti0.04)S3 (Device T4) on the PCE as shown in the following subsections.
3.5.1. Impact of Ti Alloying in Absorbers Thickness, Carrier Concentration, and Defect Density
The thickness of the absorber is varied from 100 to 1000 nm to study its influence on the PV parameters for devices T1, T2, T3, and T4 (Figure S2). A modest increase in Voc is noticed due to improved quasi-Fermi-level splitting, while a significant increase in Jsc values results from increased absorption.99 Conversely, a subtle reduction in FF is linked to increased internal resistance associated with thickness. Notably, the PCE demonstrates an initial increase with thickness up to 700 nm, followed by a marginal decline. This dip aligns with increased recombination rates at higher thicknesses due to the extended travel path for photogenerated charge carriers.93 Interestingly, for absorber thickness ranging from 100 to 700 nm, devices TI, T2, T3, and T4 exhibit escalated PCEs from 26.64 to 30.20%, 27.20 to 31.43%, 27.53 to 32.09%, and 27.59 to 32.57%, respectively. This trend is further confirmed by QE measurements (Figure 8a–d). The QE drops for devices with less thickness, while a consistent spectral response occurs from 348 to 763 nm, 358 to 790 nm, 355 to 808 nm, and 350 to 840 nm, accompanied by an absorption increase of 79.02, 82.14, 83.85, and 85.24% for devices T1, T2, T3, and T4, respectively. Notably, a reduced band gap expands the absorption range into the NIR range (750–840 nm), fostering increased absorption and boosting performance.
Figure 8.
Changes in the QE % for devices (a) T1, (b) T2, (c) T3, and (d) T4 as a function of absorber thickness.
Analyzing the impact of carrier concentration (1012 to 1020 cm–3) on devices T1, T2, T3, and T4 (Figure S3) unveils a clear trend. It can be observed that Jsc and PCE remain stable until 1014 cm–3, beyond which a slight decrease occurs. The root cause of this behavior is the escalated recombination rate observed at higher carrier concentrations (Figure 9a–d). Within 1012 to 1014 cm–3, recombination in the HTL region remains the same, attributing to the unaltered device performance. However, beyond 1014 cm–3, recombination in the HTL region increases significantly, resulting in a rapid decrease in Jsc and PCE. While a slight increase in Voc and FF with higher carrier concentration may occur due to elevated electric fields at the interfaces, this can also intensify the possibility of recombination.87 Thus, increasing the carrier concentration of the absorber beyond an optimum value led to a dramatic increase in HTL recombination, adversely affecting the device performance. Therefore, an optimal carrier concentration of 1014 cm–3 is chosen for further simulations.
Figure 9.
Changes in the recombination rate at the HTL/absorber region in (a) T1, (b) T2, (c) T3, and (d) T4 as a function of the absorber carrier concentration.
Likewise, the influence of defect density for the devices T1, T2, T3, and T4 is analyzed by varying it from 1012 to 1020 cm–3. A steep increase in the Voc is observed for unknown reasons, while Jsc, FF, and PCE decrease (Figure S4) with defect density. This is attributed to higher defects acting as traps for photogenerated charge carriers, hindering their separation as they have lower diffusion lengths and carrier lifetime.93 As a result, a high PCE is observed at 1012 cm–3, chosen as the optimal defect density for further studies.
3.5.2. Output Performance of BaZrS3 and Ba(Zr0.96,Ti0.04)S3 Devices
The J–V characteristics of devices utilizing BaZrS3 and Ba(Zr0.96,Ti0.04)S3 (T4) are shown in Figure 10a. Strikingly, the Ba(Zr,Ti)S3 absorber yields a higher PCE of 32.58% compared to that of BaZrS3 (PCE of 28.17%). This enhancement is primarily attributed to the improved Jsc arising from more light absorption as it has a lower band gap of 1.51 eV in Ba(Zr,Ti)S3 than 1.7 eV in BaZrS3. Additionally, optimal band alignment contributes to reduced recombination at the interface. Moreover, the low band gap of Ba(Zr,Ti)S3 enables it to absorb a large fraction of solar spectrum incident upon it, resulting in a high PCE.
Figure 10.
Changes in the (a) J–V curve and (b) QE measurements as a function of band gap in BaZrS3 and Ba(Zr,Ti)S3 devices.
The obtained QE plots for BaZrS3 and Ba(Zr,Ti)S3 are displayed in Figure 10b. It can be noticed that the absorption edge is at 729 nm for BaZrS3 and 858 nm for Ba(Zr,Ti)S3. This is due to alloying, which shifts the absorption to the NIR region as it has a lower band gap. This increased absorption from 76 to 85% in the NIR region contributes to the enhancement in the PCE in association with an increase in Jsc, significantly impacting the device performance.
Furthermore, the improvement in PCE can be justified by calculating the lattice mismatch. In general, the lattice mismatch between layers and surface roughness causes interfacial defects, which act as recombination centers and negatively impact the performance of the devices. Additionally, it affects the symmetry at the ETL/absorber and absorber/HTL interfaces, causing dislocations, point defects, and lattice inhomogeneities.46 Therefore, we assessed the lattice mismatch (δ) between the transport layers and the absorber for device structures that achieved the best PCE of 32.58% (FTO/ZrS2/Ba(Zr0.96,Ti0.04)S3/SnS/Pt) and 28.17% (FTO/ZrS2/BaZrS3/SnS/Pt) using the formula
| 4 |
Here, “as” represents the lattice constant of the substrate, while “ae” stands for the lattice constant of the epitaxial layer.93
Table 3 displays the lattice constants46,100,101 and compares the δ values for BaZrS3 and Ba(Zr0.96Ti0.04)S3 absorbers concerning the ETL and HTL layers. The ZrS2/BaZrS3 and BaZrS3/SnS interface exhibits δ values of 19.17 and 55.09%, respectively. On the other hand, slightly lesser δ values of 19.08 and 45.60% are observed for the ZrS2/Ba(Zr0.96, Ti0.04)S3 and Ba(Zr0.96, Ti0.04)S3/SnS interface, respectively. It is evident that the reduction in mismatch is closely linked to the concentration of Ti alloying at the Zr site.46 This reduces the carrier recombination rate and minimizes the occurrence of dislocation or defects at the Ba(Zr0.96Ti0.04)S3 interface, leading to enhanced performance.
Table 3. Lattice Mismatch of BaZrS3 and Ba(Zr0.96Ti0.04)S3 with ETL and HTL.
| lattice
parameters (Å) |
lattice
mismatch (%) |
||||
|---|---|---|---|---|---|
| layers | a | b | c | ETL/absorber | absorber/HTL |
| BaZrS3 | 7.06 | 9.98 | 7.02 | 19.17 | 55.09 |
| Ba(Zr0.96Ti0.04)S3 | 7.05 | 9.90 | 7.02 | 19.08 | 45.60 |
| ZrS2 (ETL) | 3.65 | 5.80 | |||
| SnS (HTL) | 3.64 | 11.21 | 3.99 | ||
3.6. Influence of Rs, Rsh, and Working Temperature
Parasitic resistances, such as RS and RSh, play a significant role in the PV performance of the device. These resistances dictate the characteristics of J–V curves and reflect the considerable loss mechanisms within the device. In general, devices should exhibit low RS and high RSh values. However, in practical applications, RS depends on the contacts between charge transfer layers (ETL and HTL) and the metal contact layer, which affects charge transfer. Likewise, RSh may be reduced due to factors like morphologies or unavoidable leakage currents.46 Hence, this section explores the influence of varying RS and RSh of the final device T4 (FTO/ZrS2/Ba(Zr0.96,Ti0.04)S3/SnS/Pt).
Initially, the RS of the device is varied from 1 to 10 Ω cm2, as displayed in Figure 11a,b. It can be noticed that Voc and Jsc remain unaltered across the entire range. However, a decrease in FF is evident, dropping from 84.94 to 70.52%, with an increase in RS attributed to the substantial power loss within the device. Since power loss is directly proportional to RS, increasing RS leads to higher power loss, impacting FF. Consequently, the PCE of the device reduced from 32.58 to 27.05%. This underscores the importance of maintaining a low RS of 1 Ω cm2 for efficient device functioning. Thereafter, we varied the RSh from 10 to 107 Ω cm2 to study its influence on the performance of the devices (Figure 11c,d). The findings reveal a slight increase in Voc up to 103 Ω cm2; after that, it remains unchanged. Likewise, Jsc remains constant. On the other hand, when the RSh is increased from 10 to 107 Ω cm2, an increase in FF and PCE from 25 to 84.94% and 2.60 to 32.58% is observed. Therefore, with higher values of RSh, the device shows improved performance.37,46
Figure 11.
Changes in the PV parameters as a function of (a, b) RS, (c, d) RSh, and (e, f) working temperature.
The impact of temperature has been investigated by varying it from 300 to 400 K. Figure 11e,f illustrates the changes in the PV parameters as a function of temperature. The Jsc and Voc remain unaltered throughout the entire range, whereas the FF and PCE experience a slight decrease from 84.94 to 82.95% and 32.58 to 31.79%, respectively. This can be attributed to the increased RS and charge carrier recombination within the layers at elevated temperatures. Yet, it is noteworthy that the PCE dropped by just 0.8%, underscoring the stability of Ba(Zr,Ti)S3 compared with lead halide perovskites. Therefore, these findings highlight the potential of Ba(Zr,Ti)S3 as a stable PV device.37,47
4. Comparison of SCAPS-1D Results with BaZrS3 and Ba(Zr,Ti)S3 Chalcogenide Perovskite Devices in the Literature
Table 4 summarizes theoretical works on chalcogenide perovskite absorbers (BaZrS3, BaZrSe3, Ba(Zr,Ti)S3) using SCAPS-1D. Notably, the highest PCE of 30% emerged from the (a-Si/Ba(Zr0.95,Ti0.05)S3/CdS/i-ZnO/AZO) device. However, its inclusion of CdS has a buffer layer containing the toxic element Cd, which limits its commercialization due to environmental toxicity concerns.102 Following closely, the (FTO/TiO2/BaZrSe3/Spiro-OMeTAD/Au) device achieved the second highest PCE of 25% by employing Spiro-OMeTAD. Although it is a prominently used HTL, its stability and high cost remain a major barrier.52 Nevertheless, so far in the literature, only oxide ETLs have been studied for BaZrS3-based chalcogenide perovskites, which sets the research gap. In recent years, sulfide ETLs emerged as a promising material owing to their exceptional photoelectric properties stemming from the valence electron shell structure within the 3d orbitals. Compared with oxide ETLs, metal sulfides offer many compelling advantages. To begin with, metal sulfides exhibit exceptional electron transport mobility, which serves as a catalyst for efficient charge extraction and transportation, effectively mitigating the risk of charge accumulation and recombination at the ETL/absorber interface. Moreover, they can be synthesized using low-temperature methods, underscoring their energy efficiency and compatibility with flexible substrates. Furthermore, metal sulfides distinguish themselves as wide band gap materials without oxygen vacancies, enhancing the device’s optical stability. Indeed, the substantial binding energy between metal sulfides and absorbers can effectively eliminate the ionic migration within the absorber, offering the pathway to elevate the overall performance and durability of devices.48,,104
Table 4. Summary of BaZrS3 and Ba(Zr,Ti)S3 Chalcogenide Perovskite Devices Reported in the Literature.
| device structure | VOC (V) | JSC (mA/cm2) | FF (%) | PCE (%) | ref |
|---|---|---|---|---|---|
| FTO/TiO2/BaZrS3/Spiro-OMeTAD/Au | 1.21 | 16.54 | 86.26 | 17.29 | (88) |
| FTO/TiO2/BaZrS3/Cu2O/Au | 1.16 | 12.24 | 87.13 | 12.42 | (46) |
| FTO/TiO2/BaZrS3/CuSbS2/W | 1.00 | 22.57 | 73.7 | 17.13 | (105) |
| FTO/TiO2/BaZrS3/Spiro-OMeTAD/Au | 0.70 | 22.00 | 79.40 | 12.12 | (106) |
| AZO/i-ZnO/CdS/BaZrS3/a-Si | 1.31 | 19.08 | 78.88 | 19.72 | (37) |
| FTO/TiO2/BaZrSe3/Spiro-OMeTAD/Au | 0.72 | 46.65 | 77.32 | 25.84 | (106) |
| FTO/TiO2/Ba(Zr0.87,Ti0.12)S3/Cu2O/back contact | 1.09 | 26.57 | 85.78 | 24.86 | (107) |
| AZO/i-ZnO/CdS/Ba(Zr0.95,Ti0.05)S3/a-Si | 1.26 | 27.06 | 88.47 | 30.06 | (37) |
| FTO/ZrS2/BaZrS3/SnS/Pt | 1.18 | 29.74 | 80.15 | 28.17 | this work |
| FTO/ZrS2/Ba(Zr0.96,Ti0.04)S3/SnS/Pt | 1.18 | 32.26 | 84.94 | 32.58 | this work |
To date, various sulfide ETLs such as MoS2, WS2, TiS2, TaS2, SnS2, SnS, In2S3, Bi2S3, ZnS, and Sb2S3 have been investigated for lead halide perovskites, revealing their dominant characteristics over oxides.104 Hence, it is interesting to investigate the suitability of new sulfide ETLs. In this concern, we have meticulously selected ZrS2 ETL and examined its applicability for emerging BaZrS3 chalcogenide perovskite devices, which creates opportunities for experimental scientists to explore the effectiveness of novel ZrS2 ETLs in practice. Hence, we have performed comprehensive device engineering and accomplished PCEs of 32.58% (FTO/ZrS2/Ba(Zr0.96,Ti0.04)S3/SnS/Pt) and 28.17% (FTO/ZrS2/BaZrS3/SnS/Pt) which is the highest among the reported works. Thus, we firmly believe that our work will ignite the interest in the scientific community and provide a roadmap for experimentalists to fabricate highly efficient BaZrS3 and Ba(Zr,Ti)S3 chalcogenide perovskite devices.
4.1. Experimental Suggestions to Improve the Device Performance in Practice Based on Simulation Outcomes
Our study employed SCAPS-1D theoretical simulation to identify the most suitable device configuration for the new BaZrS3 device. We propose the most effective device configurations of ZrS2/BaZrS3/SnS and ZrS2/Ba(Zr,Ti)S3/SnS, which offer impressive PCE of 28.17 and 32.58%, respectively. The optimization of essential parameters such as thickness, carrier concentration, and defect density of the ETL, absorber, and HTL are the key factors that led to high PCE values. However, it is essential to note that practical guidance for the successful fabrication of the devices is still necessary. In view of this, we have outlined various strategies to optimize the key parameters experimentally.
To start with, we found that ZrS2, a transition-metal dichalcogenide semiconductor (TMDS), performs best when its influential parameters, such as carrier concentration (1019 cm–3) and defect density (1016 cm–3), are within the optimal range. Substitutional and chemical doping can increase the carrier concentration. External dopants like transition metals and halogens can replace metal or chalcogen sites in substitutional doping to boost the carrier concentration. Chemical doping, on the other hand, involves the adsorption of dopants onto the semiconductor surface without altering its structure. This process stimulates the surface charge transfer between the dopants and TMDS, improving carrier concentration and charge transfer properties. It is noteworthy that annealing with continuous electron beam irradiation has been explored as an alternative technique to improve the carrier concentration.52 ZrS2 is generally prone to sulfur defects, which can be rectified by a sulfur vapor annealing process to minimize defects and improve crystallinity.108
As stated in Section 3.2, the BaZrS3 absorber showed its best performance at an optimum thickness of 1000 nm. This can be achieved using various techniques such as vapor deposition, sputtering, and electrodeposition, which allow for better control over factors like film thickness, composition, purity, and uniformity by changing deposition conditions such as time, temperature, pressure, and potential.109 The film thickness can be increased by repeating the deposition time and annealing steps. Furthermore, Meng et al. reported that BaZrS3 and Ba(Zr,Ti)S3 films synthesized under S-rich/Zr-poor conditions exhibit strong p-type behavior with an optimum carrier concentration of 1015 cm–3 and surpass the deep-level defects due to their high formation energy that results in lower defect density. However, films synthesized under S-poor/Zr-rich conditions possess n-type behavior with a high carrier concentration of >1017 cm–3, making them unsuitable for the absorber. Thus, it is necessary to adjust the elemental composition while synthesizing BaZrS3 and Ba(Zr,Ti)S3 films to obtain an optimum carrier concentration and reduce the formation of deep-level defects.38
Interestingly, the highest PCEs of 28.17 and 32.58% were achieved with SnS as the HTL, indicating its suitability and appropriate band alignment with the absorber. However, the formation of impurity phases such as Sn2S3 and SnS2 in SnS remains a significant drawback. To overcome this, Steinmann et al. reported that annealing SnS for 1 h under vacuum conditions (15 m Torr) at 500 °C or in an Ar or H2 gas atmosphere at ambient temperature prevents the formation of impurity phases.110 Moreover, introducing Sn excess composition (Sn1.05S) during the synthesis process significantly reduces the formation of Sn2S3 without additional thermal processing.111 In summary, the methods and strategies proposed have the potential to significantly benefit the PV community by enabling them to fabricate highly efficient BaZrS3-based devices as technology advances.
5. Conclusions
In this work, we have comprehensively shed light on the device engineering of BaZrS3 solar cells using SCAPS-1D numerical simulation. Initially, we optimized each layer (FTO/SnO2/BaZrS3/Cu2S/Au) of the base device structure by varying its thickness, carrier concentration, and defect density. Thereafter, we altered the ETL and optimized it by keeping other layers constant. In the next step, by keeping the optimized conditions of ETL and absorber, we varied 13 HTLs and optimized it. This stepwise process was adopted to find the suitable device structure for the BaZrS3 absorber. We modeled 782 devices by this approach, and 156 devices showed the best PCE for each ETL and HTL combination. Interestingly, the FTO/ZrS2/BaZrS3/SnS/Pt device demonstrated the highest PCE of 28.08%. This rotational selection of ETLs and HTLs resulted in the efficient collection of electrons and holes and gave desirable band offset, restricting the interfacial recombination and ideal band alignment, resulting in enhanced performance. Furthermore, the PCE of the device was enhanced to 28.17% after optimizing the metal contacts. In addition, the performance of BaZrS3 was further improved by Ti alloying with 4 atom % and achieved a maximum theoretical PCE of 32.58% due to its extended absorption to the NIR region and less lattice mismatch compared with BaZrS3. Our study provides a guiding rule for understanding the material properties and selecting cost-effective and efficient pin structures.
Acknowledgments
The authors thank Marc Burgelman, ELSI, University of Gent, Belgium, for providing SCAPS-1D simulation software.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c06627.
Changes in the PV parameters as a function of ETL thickness, carrier concentration, and defect density; changes in the PV parameters for devices TI, T2, T3, and T4 as a function of absorber thickness, carrier concentration, and defect density; simulation parameters for 12 ETLs and 13 HTLs; simulation parameters for devices TI, T2, T3, and T4; and device engineering of 782 chalcogenide perovskite devices (PDF)
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The authors declare no competing financial interest.
Supplementary Material
References
- Zhang J.; Gao X.; Deng Y.; Zha Y.; Yuan C. Comparison of Life Cycle Environmental Impacts of Different Perovskite Solar Cell Systems. Sol. Energy Mater. Sol. Cells 2017, 166, 9–17. 10.1016/j.solmat.2017.03.008. [DOI] [Google Scholar]
- Hoefler S. F.; Trimmel G.; Rath T. Progress on Lead-Free Metal Halide Perovskites for Photovoltaic Applications: A Review. Monatsh. Chem. 2017, 148, 795–826. 10.1007/s00706-017-1933-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair S.; Patel S. B.; Gohel J. V. Recent Trends in Efficiency-Stability Improvement in Perovskite Solar Cells. Mater. Today Energy 2020, 17, 100449 10.1016/j.mtener.2020.100449. [DOI] [Google Scholar]
- https://www.nrel.gov/pv/cell-efficiency.html.
- Wang X.; Zhang T.; Lou Y.; Zhao Y. All-Inorganic Lead-Free Perovskites for Optoelectronic Applications. Mater. Chem. Front. 2019, 3 (3), 365–375. 10.1039/C8QM00611C. [DOI] [Google Scholar]
- Hossain M. K.; Samajdar D. P.; Das R. C.; Arnab A. A.; Rahman M. F.; Rubel M. H. K.; Islam M. R.; Bencherif H.; Pandey R.; Madan J.; Mohammed M. K. A. Design and Simulation of Cs2BiAgI6 Double Perovskite Solar Cells with Different Electron Transport Layers for Efficiency Enhancement. Energy Fuels 2023, 37 (5), 3957–3979. 10.1021/acs.energyfuels.3c00181. [DOI] [Google Scholar]
- Kalaph K. A.; Jafar A. M.; Abdalameer N. K.; Hmood A. M. A Review on Recent Advances in Materials of Hybrid Organic–Inorganic Perovskite Solar Cells. IJOIR 2022, 9 (2), 148–158. 10.53523/ijoirVol9I2ID181. [DOI] [Google Scholar]
- Javaid K.; Yu J.; Wu W.; Wang J.; Zhang H.; Gao J.; Zhuge F.; Liang L.; Cao H. Thin Film Solar Cell Based on ZnSnN2/SnO Heterojunction. Phys. Status Solidi RRL 2018, 12 (1), 1700332 10.1002/pssr.201700332. [DOI] [Google Scholar]
- Javaid K.; Wu W.; Wang J.; Fang J.; Zhang H.; Gao J.; Zhuge F.; Liang L.; Cao H. Band Offset Engineering in ZnSnN2 -Based Heterojunction for Low-Cost Solar Cells. ACS Photonics 2018, 5 (6), 2094–2099. 10.1021/acsphotonics.8b00427. [DOI] [Google Scholar]
- Dunlap-Shohl W. A.; Hill I. G.; Yan Y.; Mitzi D. B. Photovoltaic Effect in Indium(I) Iodide Thin Films. Chem. Mater. 2018, 30 (22), 8226–8232. 10.1021/acs.chemmater.8b03593. [DOI] [Google Scholar]
- Tiwari D.; Alibhai D.; Fermin D. J. Above 600 MV Open-Circuit Voltage BiI3 Solar Cells. ACS Energy Lett. 2018, 3 (8), 1882–1886. 10.1021/acsenergylett.8b01182. [DOI] [Google Scholar]
- Huang P.-C.; Yang W.-C.; Lee M.-W. AgBiS2 Semiconductor-Sensitized Solar Cells. J. Phys. Chem. C 2013, 117 (36), 18308–18314. 10.1021/jp4046337. [DOI] [Google Scholar]
- Patterson R. J.; Xia H.; Hu L.; Zhang Z.; Yuan L.; Yang J.; Chen W.; Chen Z.; Gao Y.; Hu Y.; Veettil B. P.; Stride J. A.; Conibeer G.; Huang S.. Oxygen Substitution and Sulfur Vacancies in NaBiS2: A Pb-Free Candidate for Solution Processable Solar Cells. In 2017 IEEE 44th Photovoltaic Specialist Conference (PVSC); IEEE, 2017; pp 2392–2394.
- Xia Z.; Yu F.-X.; Lu S.-C.; Xue D.-J.; He Y.-S.; Yang B.; Wang C.; Ding R.-Q.; Zhong J.; Tang J. Synthesis and Characterization of NaSbS2 Thin Film for Potential Photodetector and Photovoltaic Application. Chin. Chem. Lett. 2017, 28 (4), 881–887. 10.1016/j.cclet.2017.03.003. [DOI] [Google Scholar]
- Hoye R. L. Z.; Lee L. C.; Kurchin R. C.; Huq T. N.; Zhang K. H. L.; Sponseller M.; Nienhaus L.; Brandt R. E.; Jean J.; Polizzotti J. A.; Kursumović A.; Bawendi M. G.; Bulović V.; Stevanović V.; Buonassisi T.; MacManus-Driscoll J. L. Strongly Enhanced Photovoltaic Performance and Defect Physics of Air-Stable Bismuth Oxyiodide (BiOI). Adv. Mater. 2017, 29 (36), 1702176 10.1002/adma.201702176. [DOI] [PubMed] [Google Scholar]
- Tiwari D.; Cardoso-Delgado F.; Alibhai D.; Mombrú M.; Fermín D. J. Photovoltaic Performance of Phase-Pure Orthorhombic BiSI Thin-Films. ACS Appl. Energy Mater. 2019, 2 (5), 3878–3885. 10.1021/acsaem.9b00544. [DOI] [Google Scholar]
- Yoo B.; Ding D.; Marin-Beloqui J. M.; Lanzetta L.; Bu X.; Rath T.; Haque S. A. Improved Charge Separation and Photovoltaic Performance of BiI3 Absorber Layers by Use of an In Situ Formed BiSI Interlayer. ACS Appl. Energy Mater. 2019, 2 (10), 7056–7061. 10.1021/acsaem.9b00838. [DOI] [Google Scholar]
- Choi Y. C.; Hwang E.; Kim D.-H. Controlled Growth of SbSI Thin Films from Amorphous Sb2S3 for Low-Temperature Solution Processed Chalcohalide Solar Cells. APL Mater. 2018, 6 (12), 121108 10.1063/1.5058166. [DOI] [Google Scholar]
- Nie R.; Seok S. Il Efficient Antimony-Based Solar Cells by Enhanced Charge Transfer. Small Methods 2020, 4 (2), 1900698 10.1002/smtd.201900698. [DOI] [Google Scholar]
- Nie R.; Hu M.; Risqi A. M.; Li Z.; Seok S. Il Efficient and Stable Antimony Selenoiodide Solar Cells. Adv. Sci. 2021, 8 (8), 2003172 10.1002/advs.202003172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie R.; Im J.; Seok S. I. Efficient Solar Cells Employing Light-Harvesting Sb0.67Bi0.33SI. Adv. Mater. 2019, 31 (18), 1808344 10.1002/adma.201808344. [DOI] [PubMed] [Google Scholar]
- Toso S.; Akkerman Q. A.; Martín-García B.; Prato M.; Zito J.; Infante I.; Dang Z.; Moliterni A.; Giannini C.; Bladt E.; Lobato I.; Ramade J.; Bals S.; Buha J.; Spirito D.; Mugnaioli E.; Gemmi M.; Manna L. Nanocrystals of Lead Chalcohalides: A Series of Kinetically Trapped Metastable Nanostructures. J. Am. Chem. Soc. 2020, 142 (22), 10198–10211. 10.1021/jacs.0c03577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie R.; Kim B.; Hong S.-T.; Seok S. Il Nanostructured Heterojunction Solar Cells Based on Pb2SbS2I3: Linking Lead Halide Perovskites and Metal Chalcogenides. ACS Energy Lett. 2018, 3 (10), 2376–2382. 10.1021/acsenergylett.8b01332. [DOI] [Google Scholar]
- Pai N.; Lu J.; Gengenbach T. R.; Seeber A.; Chesman A. S. R.; Jiang L.; Senevirathna D. C.; Andrews P. C.; Bach U.; Cheng Y.; Simonov A. N. Silver Bismuth Sulfoiodide Solar Cells: Tuning Optoelectronic Properties by Sulfide Modification for Enhanced Photovoltaic Performance. Adv. Energy Mater. 2019, 9 (5), 1803396 10.1002/aenm.201803396. [DOI] [Google Scholar]
- Nie R.; Lee K. S.; Hu M.; Paik M. J.; Seok S. Il Heteroleptic Tin-Antimony Sulfoiodide for Stable and Lead-Free Solar Cells. Matter 2020, 3 (5), 1701–1713. 10.1016/j.matt.2020.08.020. [DOI] [Google Scholar]
- Liu H.; Xin Z.; Cao B.; Xu Z.; Xu B.; Zhu Q.; Yang J.; Zhang B.; Fan H. J. Polyhydroxylated Organic Molecular Additives for Durable Aqueous Zinc Battery. Adv. Funct. Mater. 2023, 2309840 10.1002/adfm.202309840. [DOI] [Google Scholar]
- Zhang C.; Teo S.; Guo Z.; Gao L.; Kamata Y.; Xu Z.; Ma T. Development of a Mixed Halide-Chalcogenide Bismuth-Based Perovskite MABiI2S with Small Bandgap and Wide Absorption Range. Chem. Lett. 2019, 48 (3), 249–252. 10.1246/cl.180919. [DOI] [Google Scholar]
- Shin D.; Zhu T.; Huang X.; Gunawan O.; Blum V.; Mitzi D. B. Earth-Abundant Chalcogenide Photovoltaic Devices with over 5% Efficiency Based on a Cu2BaSn(S,Se)4 Absorber. Adv. Mater. 2017, 29 (24), 1606945 10.1002/adma.201606945. [DOI] [PubMed] [Google Scholar]
- Crovetto A.; Nielsen R.; Stamate E.; Hansen O.; Seger B.; Chorkendorff I.; Vesborg P. C. K. Wide Band Gap Cu2SrSnS4 Solar Cells from Oxide Precursors. ACS Appl. Energy Mater. 2019, 2 (10), 7340–7344. 10.1021/acsaem.9b01322. [DOI] [Google Scholar]
- Spadaro M. C.; Escobar Steinvall S.; Dzade N. Y.; Martí-Sánchez S.; Torres-Vila P.; Stutz E. Z.; Zamani M.; Paul R.; Leran J. B.; Fontcuberta I Morral A.; Arbiol J. Rotated Domains in Selective Area Epitaxy Grown Zn3P2: Formation Mechanism and Functionality. Nanoscale 2021, 13 (44), 18441–18450. 10.1039/D1NR06190A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R.; Tabernig S. W.; Reñé Sapera J.; Hurni J.; Tiede A.; Liu X.; Damry D. A.; Conti V.; Zamani M.; Escobar Steinvall S.; Dimitrievska M.; Alarcon-Lladó E.; Piazza V.; Boland J.; Haug F. J.; Polman A.; Fontcuberta i Morral A. Carrier Generation and Collection in Zn3P2/InP Heterojunction Solar Cells. Sol. Energy Mater. Sol. Cells 2023, 256, 112349 10.1016/j.solmat.2023.112349. [DOI] [Google Scholar]
- Stutz E. Z.; Ramanandan S. P.; Flór M.; Paul R.; Zamani M.; Escobar Steinvall S.; Sandoval Salaiza D. A.; Xifra Montesinos C.; Spadaro M. C.; Leran J. B.; Litvinchuk A. P.; Arbiol J.; Fontcuberta i Morral A.; Dimitrievska M. Stoichiometry Modulates the Optoelectronic Functionality of Zinc Phosphide (Zn3–xP2+x). Faraday Discuss. 2022, 239, 202–218. 10.1039/D2FD00055E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y. Y.; Agiorgousis M. L.; Zhang P.; Zhang S. Chalcogenide Perovskites for Photovoltaics. Nano Lett. 2015, 15 (1), 581–585. 10.1021/nl504046x. [DOI] [PubMed] [Google Scholar]
- Sharma S.; Ward Z. D.; Bhimani K.; Sharma M.; Quinton J.; Rhone T. D.; Shi S.-F.; Terrones H.; Koratkar N. Machine Learning-Aided Band Gap Engineering of BaZrS3 Chalcogenide Perovskite. ACS Appl. Mater. Interfaces 2023, 15 (15), 18962–18972. 10.1021/acsami.3c00618. [DOI] [PubMed] [Google Scholar]
- Wei X.; Hui H.; Zhao C.; Deng C.; Han M.; Yu Z.; Sheng A.; Roy P.; Chen A.; Lin J.; Watson D. F.; Sun Y.-Y.; Thomay T.; Yang S.; Jia Q.; Zhang S.; Zeng H. Realization of BaZrS3 Chalcogenide Perovskite Thin Films for Optoelectronics. Nano Energy 2020, 68, 104317 10.1016/j.nanoen.2019.104317. [DOI] [Google Scholar]
- Han Y.; Xu J.; Liang Y.; Chen X.; Jia M.; Zhang J.; Lian L.; Liu Y.; Li X.; Shi Z. P-Type Conductive BaZrS3 Thin Film and Its Band Gap Tunning via Ruddlesden-Popper Ba3Zr2S7 and Titanium Alloying. Chem. Eng. J. 2023, 473, 145351 10.1016/j.cej.2023.145351. [DOI] [Google Scholar]
- Barman B.; Ingole S. Analysis of Si Back-Contact for Chalcogenide Perovskite Solar Cells Based on BaZrS3 Using SCAPS-1D. Adv. Theory Simul. 2023, 6, 2200820 10.1002/adts.202200820. [DOI] [Google Scholar]
- Meng W.; Saparov B.; Hong F.; Wang J.; Mitzi D. B.; Yan Y. Alloying and Defect Control within Chalcogenide Perovskites for Optimized Photovoltaic Application. Chem. Mater. 2016, 28 (3), 821–829. 10.1021/acs.chemmater.5b04213. [DOI] [Google Scholar]
- Panikar Ramanandan S.; Giunto A.; Stutz E. Z.; Xavier Marie Reyner B.; Tiphaine Françoise Marie Lefevre I.; Rusu M.; Schorr S.; Unold T.; Fontcuberta Morral A.; Márquez Prieto J.; Dimitrievska M. Understanding the Growth Mechanism of BaZrS3 Chalcogenide Perovskite Thin Films from Sulfurized Oxide Precursors. J. Phys. Energy 2023, 014013 10.1088/2515-7655/aca9fe. [DOI] [Google Scholar]
- Comparotto C.; Ström P.; Donzel-Gargand O.; Kubart T.; Scragg J. J. S. Synthesis of BaZrS3 Perovskite Thin Films at a Moderate Temperature on Conductive Substrates. ACS Appl. Energy Mater. 2022, 5, 6335–6343. 10.1021/acsaem.2c00704. [DOI] [Google Scholar]
- Yu Z.; Wei X.; Zheng Y.; Hui H.; Bian M.; Dhole S.; Seo J. H.; Sun Y. Y.; Jia Q.; Zhang S.; Yang S.; Zeng H. Chalcogenide Perovskite BaZrS3 Thin-Film Electronic and Optoelectronic Devices by Low Temperature Processing. Nano Energy 2021, 85, 105959 10.1016/j.nanoen.2021.105959. [DOI] [Google Scholar]
- Yang R.; Jess A. D.; Fai C.; Hages C. J. Low-Temperature, Solution-Based Synthesis of Luminescent Chalcogenide Perovskite BaZrS3 Nanoparticles. J. Am. Chem. Soc. 2022, 144, 15928–15931. 10.1021/jacs.2c06168. [DOI] [PubMed] [Google Scholar]
- Zilevu D.; Parks O. O.; Creutz S. E. Solution-Phase Synthesis of the Chalcogenide Perovskite Barium Zirconium Sulfide as Colloidal Nanomaterials. Chemical Communications 2022, 58 (75), 10512–10515. 10.1039/D2CC03494H. [DOI] [PubMed] [Google Scholar]
- Vincent K. C.; Agarwal S.; Turnley J. W.; Agrawal R. Liquid Flux–Assisted Mechanism for Modest Temperature Synthesis of Large-Grain BaZrS3 and BaHfS3 Chalcogenide Perovskites. Adv. Energy Sustainability Res. 2023, 4 (5), 2300010 10.1002/aesr.202300010. [DOI] [Google Scholar]
- Khattak Y. H.; Baig F.; Shuja A.; Atourki L.; Riaz K.; Soucase B. M. Device Optimization of PIN Structured Perovskite Solar Cells: Impact of Design Variants. ACS Appl. Electron Mater. 2021, 3 (8), 3509–3520. 10.1021/acsaelm.1c00460. [DOI] [Google Scholar]
- Karthick S.; Velumani S.; Bouclé J. Experimental and SCAPS Simulated Formamidinium Perovskite Solar Cells: A Comparison of Device Performance. Sol. Energy 2020, 205, 349–357. 10.1016/j.solener.2020.05.041. [DOI] [Google Scholar]
- Karthick S.; Velumani S.; Bouclé J. Chalcogenide BaZrS3 Perovskite Solar Cells: A Numerical Simulation and Analysis Using SCAPS-1D. Opt Mater. 2022, 126, 112250 10.1016/j.optmat.2022.112250. [DOI] [Google Scholar]
- Chawki N.; Rouchdi M.; Fares B.. Numerical Study of BaZrS3 Based Chalcogenide Perovskite Solar Cell Using SCAPS-1D Device Simulation 2022 10.21203/rs.3.rs-1251663/v1. [DOI] [Google Scholar]
- Mohamad Noh M. F.; Teh C. H.; Daik R.; Lim E. L.; Yap C. C.; Ibrahim M. A.; Ahmad Ludin N.; Mohd Yusoff A. R. B.; Jang J.; Mat Teridi M. A. The Architecture of the Electron Transport Layer for a Perovskite Solar Cell. J. Mater. Chem. C 2018, 6, 682–712. 10.1039/C7TC04649A. [DOI] [Google Scholar]
- Cheng M.; Zuo C.; Wu Y.; Li Z.; Xu B.; Hua Y.; Ding L. Charge-Transport Layer Engineering in Perovskite Solar Cells. Sci. Bull. 2020, 65, 1237–1241. 10.1016/j.scib.2020.04.021. [DOI] [PubMed] [Google Scholar]
- Capistrán-Martínez J.; Loeza-Díaz D.; Mora-Herrera D.; Pérez-Rodríguez F.; Pal M. Theoretical Evaluation of Emerging Cd-Free Cu3BiS3 Based Solar Cells Using Experimental Data of Chemically Deposited Cu3BiS3 Thin Films. J. Alloys Compd. 2021, 867, 159156 10.1016/j.jallcom.2021.159156. [DOI] [Google Scholar]
- Linhart W. M.; Zelewski S. J.; Scharoch P.; Dybała F.; Kudrawiec R. Nesting-like Band Gap in Bismuth Sulfide Bi2S3. J. Mater. Chem. C 2021, 9 (39), 13733–13738. 10.1039/D1TC03625D. [DOI] [Google Scholar]
- Arockiya-Dass K. T.; Sekar K.; Marasamy L. Theoretical Insights of Degenerate ZrS2 as a New Buffer for Highly Efficient Emerging Thin-Film Solar Cells. Energy Technol. 2023, 11 (9), 2300333 10.1002/ente.202300333. [DOI] [Google Scholar]
- Li Y.; Kang J.; Li J. Indirect-to-Direct Band Gap Transition of the ZrS2 Monolayer by Strain: First-Principles Calculations. RSC Adv. 2014, 4 (15), 7396–7401. 10.1039/c3ra46090h. [DOI] [Google Scholar]
- Pansuriya T.; Malani R.; Kheraj V. Investigations on the Effect of Buffer Layer on CMTS Based Thin Film Solar Cell Using SCAPS 1-D. Opt Mater. 2022, 126, 112150 10.1016/j.optmat.2022.112150. [DOI] [Google Scholar]
- Oyedele S. O.; Aka B. Numerical Simulation of Varied Buffer Layer of Solar Cells Based on CIGS. Model. Numer. Simul. Mater. Sci. 2017, 07 (03), 33–45. 10.4236/mnsms.2017.73003. [DOI] [Google Scholar]
- Rassol R. A.; Hasan R. F.; Ahmed S. M. Numerical Analysis of SnO2/Zn2SnO4/n-CdS/p-CdTe Solar Cell Using the SCAPS-1D Simulation Software. Iraqi J. Sci. 2021, 505–516. 10.24996/ijs.2021.62.2.17. [DOI] [Google Scholar]
- Rahman A.Design and Simulation of High-Performance Planar Npp+ Heterojunction CH3NH3PbI3 Based Perovskite Solar Cells Using BaSnO3 ETM and Cu2O HTM. 2021. 10.21203/rs.3.rs-441744/v1. [DOI] [Google Scholar]
- Chihi A.; Boujmil M. F.; Bessais B. Investigation on the Performance of CIGS/TiO2 Heterojunction Using SCAPS Software for Highly Efficient Solar Cells. J. Electron. Mater. 2017, 46 (8), 5270–5277. 10.1007/s11664-017-5547-0. [DOI] [Google Scholar]
- Filatova E. O.; Konashuk A. S. Interpretation of the Changing the Band Gap of Al2O3 Depending on Its Crystalline Form: Connection with Different Local Symmetries. J. Phys. Chem. C 2015, 119 (35), 20755–20761. 10.1021/acs.jpcc.5b06843. [DOI] [Google Scholar]
- Ahmed S.; Jannat F.; Khan Md. A. K.; Alim M. A. Numerical Development of Eco-Friendly Cs2TiBr6 Based Perovskite Solar Cell with All-Inorganic Charge Transport Materials via SCAPS-1D. Optik 2021, 225, 165765 10.1016/j.ijleo.2020.165765. [DOI] [Google Scholar]
- Elseman A. M.; Sajid S.; Shalan A. E.; Mohamed S. A.; Rashad M. M. Recent Progress Concerning Inorganic Hole Transport Layers for Efficient Perovskite Solar Cells. Appl. Phys. A: 2019, 125, 476 10.1007/s00339-019-2766-7. [DOI] [Google Scholar]
- Kohnehpoushi S.; Nazari P.; Nejand B. A.; Eskandari M. MoS2: A Two-Dimensional Hole-Transporting Material for High-Efficiency, Low-Cost Perovskite Solar Cells. Nanotechnology 2018, 29 (20), 205201 10.1088/1361-6528/aab1d4. [DOI] [PubMed] [Google Scholar]
- Sawicka-Chudy P.; Sibiński M.; Wisz G.; Rybak-Wilusz E.; Cholewa M. Numerical Analysis and Optimization of Cu2O/TiO2,CuO/TiO2, Heterojunction Solar Cells Using SCAPS. J. Phys. Conf. Ser. 2018, 1033, 012002 10.1088/1742-6596/1033/1/012002. [DOI] [Google Scholar]
- Wells B.Nanostructured Device Designs for Enhanced Performance in CdS/Cu2S Heterojunction Solar Cells 2020 10.13023/etd.2020.166. [DOI] [Google Scholar]
- Livingston L. M. M.; Raj A. G. S.; Prabu R. T.; Kumar A. Computational Analysis of FeS2 Material for Solar Cell Application. Opt. Quantum Electron. 2023, 55 (3), 244 10.1007/s11082-022-04531-9. [DOI] [Google Scholar]
- Basak A.; Singh U. P. Numerical Modelling and Analysis of Earth Abundant Sb2S3 and Sb2Se3 Based Solar Cells Using SCAPS-1D. Sol. Energy Mater. Sol. Cells 2021, 230, 111184 10.1016/j.solmat.2021.111184. [DOI] [Google Scholar]
- Minbashi M.; Ghobadi A.; Ehsani M. H.; Rezagholipour Dizaji H.; Memarian N. Simulation of High Efficiency SnS-Based Solar Cells with SCAPS. Sol. Energy 2018, 176, 520–525. 10.1016/j.solener.2018.10.058. [DOI] [Google Scholar]
- Capistrán-Martínez J.; Loeza-Díaz D.; Mora-Herrera D.; Pérez-Rodríguez F.; Pal M. Theoretical Evaluation of Emerging Cd-Free Cu3BiS3 Based Solar Cells Using Experimental Data of Chemically Deposited Cu3BiS3 Thin Films. J. Alloys Compd. 2021, 867, 159156 10.1016/j.jallcom.2021.159156. [DOI] [Google Scholar]
- Amiri I. S.; Ahmad H.; Ariannejad M. M.; Ismail M. F.; Thambiratnam K.; Yasin M.; Nik abdul-aziz N. M. A. Performance Analysis Of Copper Tin Sulfide, Cu2SnS3 (CTS) with Various Buffer Layers By Using SCAPS in Solar Cells. Surf. Rev. Lett. 2017, 24 (06), 1750073 10.1142/S0218625X17500731. [DOI] [Google Scholar]
- Devi C.; Mehra R. Device Simulation of Lead-Free MASnI3 Solar Cell with CuSbS2 (Copper Antimony Sulfide). J. Mater. Sci. 2019, 54 (7), 5615–5624. 10.1007/s10853-018-03265-y. [DOI] [Google Scholar]
- Ghobadi A.; Yousefi M.; Minbashi M.; Kordbacheh A. H. A.; Abdolvahab A. R. H.; Gorji N. E. Simulating the Effect of Adding BSF Layers on Cu2BaSnSSe3 Thin Film Solar Cells. Opt Mater. 2020, 107, 109927 10.1016/j.optmat.2020.109927. [DOI] [Google Scholar]
- Ashraf M. A.; Alam I. Numerical Simulation of CIGS, CISSe and CZTS-Based Solar Cells with In2S3 as Buffer Layer and Au as Back Contact Using SCAPS 1D. Eng. Res. Express. 2020, 2 (3), 035015 10.1088/2631-8695/abade6. [DOI] [Google Scholar]
- Adewoyin A. D.; Olopade M. A.; Oyebola O. O.; Chendo M. A. Development of CZTGS/CZTS Tandem Thin Film Solar Cell Using SCAPS-1D. Optik 2019, 176, 132–142. 10.1016/j.ijleo.2018.09.033. [DOI] [Google Scholar]
- Wei X.; Hui H.; Perera S.; Sheng A.; Watson D. F.; Sun Y. Y.; Jia Q.; Zhang S.; Zeng H. Ti-Alloying of BaZrS3 Chalcogenide Perovskite for Photovoltaics. ACS Omega 2020, 5 (30), 18579–18583. 10.1021/acsomega.0c00740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobayel K.; Shahinuzzaman M.; Amin N.; Karim M. R.; Dar M. A.; Gul R.; Alghoul M. A.; Sopian K.; Hasan A. K. M.; Akhtaruzzaman M. Efficiency Enhancement of CIGS Solar Cell by WS2 as Window Layer through Numerical Modelling Tool. Sol. Energy 2020, 207, 479–485. 10.1016/j.solener.2020.07.007. [DOI] [Google Scholar]
- Islam M. S.; Islam M. T.; Sarker S.; Jame H. Al; Nishat S. S.; Jani M. R.; Rauf A.; Ahsan S.; Shorowordi K. M.; Efstathiadis H.; Carbonara J.; Ahmed S. Machine Learning Approach to Delineate the Impact of Material Properties on Solar Cell Device Physics. ACS Omega 2022, 7 (26), 22263–22278. 10.1021/acsomega.2c01076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabvuer F. T.; Nya F. T.; Dzifack Kenfack G. M.; Laref A. Lowering Cost Approach for CIGS-Based Solar Cell Through Optimizing Band Gap Profile and Doping of Stacked Active Layers–SCAPS Modeling. ACS Omega 2023, 8 (4), 3917–3928. 10.1021/acsomega.2c06501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regmi G.; Ashok A.; Chawla P.; Semalti P.; Velumani S.; Sharma S. N.; Castaneda H. Perspectives of Chalcopyrite-Based CIGSe Thin-Film Solar Cell: A Review. J. Mater. Sci. Mater. Electron 2020, 31, 7286–7314. 10.1007/s10854-020-03338-2. [DOI] [Google Scholar]
- Alipour H.; Ghadimi A. Optimization of Lead-Free Perovskite Solar Cells in Normal-Structure with WO3 and Water-Free PEDOT: PSS Composite for Hole Transport Layer by SCAPS-1D Simulation. Opt Mater. 2021, 120, 111432 10.1016/j.optmat.2021.111432. [DOI] [Google Scholar]
- Rassol R. A.; Hasan R. F.; Ahmed S. M. Numerical Analysis of SnO2/Zn2SnO4/n-CdS/p-CdTe Solar Cell Using the SCAPS-1D Simulation Software. Iraqi J. Sci. 2021, 62 (2), 505–516. 10.24996/ijs.2021.62.2.17. [DOI] [Google Scholar]
- Tousif M. N.; Mohamma S.; Ferdous A. A.; Hoque M. A. Investigation of Different Materials as Buffer Layer in CZTS Solar Cells Using SCAPS. JOCET 2018, 6 (4), 293–296. 10.18178/JOCET.2018.6.4.477. [DOI] [Google Scholar]
- Mushtaq S.; Tahir S.; Ashfaq A.; Sebastian Bonilla R.; Haneef M.; Saeed R.; Ahmad W.; Amin N. Performance Optimization of Lead-Free MASnBr3 Based Perovskite Solar Cells by SCAPS-1D Device Simulation. Sol. Energy 2023, 249, 401–413. 10.1016/j.solener.2022.11.050. [DOI] [Google Scholar]
- Pandey R.; Bhattarai S.; Sharma K.; Madan J.; Al-Mousoi A. K.; Mohammed M. K. A.; Hossain M. K. Halide Composition Engineered a Non-Toxic Perovskite–Silicon Tandem Solar Cell with 30.7% Conversion Efficiency. ACS Appl. Electron Mater. 2023, 5, 5303–5315. 10.1021/acsaelm.2c01574. [DOI] [Google Scholar]
- Hossain M. K.; Toki G. F. I.; Alam I.; Pandey R.; Samajdar D. P.; Rahman Md. F.; Islam Md. R.; Rubel M. H. K.; Bencherif H.; Madan J.; Mohammed M. K. A. Numerical Simulation and Optimization of a CsPbI3 -Based Perovskite Solar Cell to Enhance the Power Conversion Efficiency. New J. Chem. 2023, 47 (10), 4801–4817. 10.1039/D2NJ06206B. [DOI] [Google Scholar]
- Hossain M. K.; Rubel M. H. K.; Toki G. F. I.; Alam I.; Rahman Md. F.; Bencherif H. Effect of Various Electron and Hole Transport Layers on the Performance of CsPbI3 -Based Perovskite Solar Cells: A Numerical Investigation in DFT, SCAPS-1D, and WxAMPS Frameworks. ACS Omega 2022, 7 (47), 43210–43230. 10.1021/acsomega.2c05912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bag A.; Radhakrishnan R.; Nekovei R.; Jeyakumar R. Effect of Absorber Layer, Hole Transport Layer Thicknesses, and Its Doping Density on the Performance of Perovskite Solar Cells by Device Simulation. Sol. Energy 2020, 196, 177–182. 10.1016/j.solener.2019.12.014. [DOI] [Google Scholar]
- Ali M. H.; Al Mamun M. A.; Haque M. D.; Rahman M. F.; Hossain M. K.; Md. Touhidul Islam A. Z. Performance Enhancement of an MoS2 -Based Heterojunction Solar Cell with an In2Te3 Back Surface Field: A Numerical Simulation Approach. ACS Omega 2023, 8 (7), 7017–7029. 10.1021/acsomega.2c07846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawki N.; Rouchdi M.; Fares B.. Numerical Study of BaZrS3 Based Chalcogenide Perovskite Solar Cell Using SCAPS-1D Device Simulation. 2022. 10.21203/rs.3.rs-1251663/v1. [DOI] [Google Scholar]
- Kim G.-W.; Shinde D. V.; Park T. Thickness of the Hole Transport Layer in Perovskite Solar Cells: Performance versus Reproducibility. RSC Adv. 2015, 5 (120), 99356–99360. 10.1039/C5RA18648J. [DOI] [Google Scholar]
- Jannat F.; Ahmed S.; Alim M. A. Performance Analysis of Cesium Formamidinium Lead Mixed Halide Based Perovskite Solar Cell with MoOx as Hole Transport Material via SCAPS-1D. Optik 2021, 228, 166202 10.1016/j.ijleo.2020.166202. [DOI] [Google Scholar]
- Ahmad O.; Rashid A.; Ahmed M. W.; Nasir M. F.; Qasim I. Performance Evaluation of Au/p-CdTe/Cs2TiI6/n-TiO2/ITO Solar Cell Using SCAPS-1D. Opt Mater. 2021, 117, 111105 10.1016/j.optmat.2021.111105. [DOI] [Google Scholar]
- Gamal N.; Sedky S. H.; Shaker A.; Fedawy M. Design of Lead-Free Perovskite Solar Cell Using Zn1-XMgxO as ETL: SCAPS Device Simulation. Optik 2021, 242, 167306 10.1016/j.ijleo.2021.167306. [DOI] [Google Scholar]
- Ali M. H.; Al Mamun M. A.; Haque M. D.; Rahman M. F.; Hossain M. K.; Abu A. Z. Performance Enhancement of an MoS2-Based Heterojunction Solar Cell with an In2Te3 Back Surface Field: A Numerical Simulation Approach. ACS Omega 2023, 8 (7), 7017–7029. 10.1021/acsomega.2c07846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffari N.; Walter D.; White T. P.; Bui A. D.; Tabi G. D.; Weber K.; Catchpole K. R. Unraveling the Role of Energy Band Alignment and Mobile Ions on Interfacial Recombination in Perovskite Solar Cells. Sol RRL 2022, 6 (6), 2101087 10.1002/solr.202101087. [DOI] [Google Scholar]
- Ding C.; Zhang Y.; Liu F.; Kitabatake Y.; Hayase S.; Toyoda T.; Yoshino K.; Minemoto T.; Katayama K.; Shen Q. Effect of the Conduction Band Offset on Interfacial Recombination Behavior of the Planar Perovskite Solar Cells. Nano Energy 2018, 53, 17–26. 10.1016/j.nanoen.2018.08.031. [DOI] [Google Scholar]
- Orazem M. E. Electron and Hole Transport in Degenerate Semiconductors. AIChE J. 1986, 32 (5), 765–772. 10.1002/aic.690320506. [DOI] [Google Scholar]
- Sandberg O. J.; Kurpiers J.; Stolterfoht M.; Neher D.; Meredith P.; Shoaee S.; Armin A. On the Question of the Need for a Built-In Potential in Perovskite Solar Cells. Adv. Mater. Interfaces 2020, 7 (10), 2000041 10.1002/admi.202000041. [DOI] [Google Scholar]
- Sharma S.; Ward Z.; Bhimani K.; Li K.; Lakhnot A.; Jain R.; Shi S. F.; Terrones H.; Koratkar N. Bandgap Tuning in BaZrS3 Perovskite Thin Films. ACS Appl. Electron Mater. 2021, 3 (8), 3306–3312. 10.1021/acsaelm.1c00575. [DOI] [Google Scholar]
- Welch A. W.; Baranowski L. L.; Peng H.; Hempel H.; Eichberger R.; Unold T.; Lany S.; Wolden C.; Zakutayev A. Trade-Offs in Thin Film Solar Cells with Layered Chalcostibite Photovoltaic Absorbers. Adv. Energy Mater. 2017, 7 (11), 1601935 10.1002/aenm.201601935. [DOI] [Google Scholar]
- Ghafari A.; Janowitz C. Electronic and Thermoelectric Properties of ZrSxSe2-x. Comput. Mater. Sci. 2019, 169, 109109 10.1016/j.commatsci.2019.109109. [DOI] [Google Scholar]
- Andrade-Arvizu J. A.; Courel-Piedrahita M.; Vigil-Galán O. SnS-Based Thin Film Solar Cells: Perspectives over the Last 25 Years. J. Mater. Sci. Mater. Electron 2015, 26 (7), 4541–4556. 10.1007/s10854-015-3050-z. [DOI] [Google Scholar]
- Rombach F. M.; Haque S. A.; Macdonald T. J.. Lessons Learned from Spiro-OMeTAD and PTAA in Perovskite Solar Cells. Energy Environ. Sci. 2021145161–5190. 10.1039/D1EE02095A. [DOI] [Google Scholar]
- Kakavelakis G.; Gouda L.; Tischler Y.; Kaliakatsos I.; Petridis K.. 2D Transition Metal Dichalcogenides for Solution-Processed Organic and Perovskite Solar Cells. In Two Dimensional Transition Metal Dichalcogenides; Springer: Singapore, 2019; pp 203–239. [Google Scholar]
- He Z.; Zhou Y.; Liu A.; Gao L.; Zhang C.; Wei G.; Ma T. Recent Progress in Metal Sulfide-Based Electron Transport Layers in Perovskite Solar Cells. Nanoscale 2021, 13, 17272–17289. 10.1039/D1NR04170C. [DOI] [PubMed] [Google Scholar]
- Goutham Kumar S.; Pramod A.; Prashanth C. R.; Honnavar G. V. Proposal for a Novel Perovskite Solar Cell Based on BaZrS3 with Optimized Electron and Hole Transport Layer Using SCAPS-1D. Eur. Chem. Bull. 2023, 12 (10), 10289–10307. 10.48047/ecb/2023.12.10.728. [DOI] [Google Scholar]
- Thakur N.; Kumar P.; Neffati R.; Sharma P. Design and Simulation of Chalcogenide Perovskite BaZr(S,Se)3 Compositions for Photovoltaic Applications. Phys. Scr. 2023, 98 (6), 065921 10.1088/1402-4896/accfc6. [DOI] [Google Scholar]
- Kanoun M. B.; Ul Haq B.; Kanoun A.-A.; Goumri-Said S. Ti Alloying as a Route to BaZrS3 Chalcogenide Perovskite with Enhanced Photovoltaic Performance. Energy Fuels 2023, 37 (13), 9548–9556. 10.1021/acs.energyfuels.3c01272. [DOI] [Google Scholar]
- Otomo M.; Hamada M.; Ono R.; Muneta I.; Kakushima K.; Tsutsui K.; Wakabayashi H. Chemical States of PVD-ZrS2 Film underneath Scaled High-k Film with Self-Oxidized ZrO2 Film as Interfacial Layer. Jpn. J. Appl. Phys. 2023, 62, SC1015 10.35848/1347-4065/aca7cf. [DOI] [Google Scholar]
- Thakur N.; Aly K. A.; Mohery M.; Ebrahium M. M.; Kumar P.; Sharma P. Recent Advances in BaZrS3 Perovskites: Synthesis, Properties, and Future Trends. J. Alloys Compd. 2023, 957, 170457 10.1016/j.jallcom.2023.170457. [DOI] [Google Scholar]
- Spalatu N.; Hiie J.; Kaupmees R.; Volobujeva O.; Krustok J.; Acik I. O.; Krunks M. Postdeposition Processing of SnS Thin Films and Solar Cells: Prospective Strategy to Obtain Large, Sintered, and Doped SnS Grains by Recrystallization in the Presence of a Metal Halide Flux. ACS Appl. Mater. Interfaces 2019, 11 (19), 17539–17554. 10.1021/acsami.9b03213. [DOI] [PubMed] [Google Scholar]
- Park B. I.; Jang Y. H.; Lee S. Y.; Lee D. K. Mechanochemically Synthesized SnS Nanocrystals: Impact of Nonstoichiometry on Phase Purity and Solar Cell Performance. ACS Sustainable Chem. Eng. 2018, 6 (3), 3002–3009. 10.1021/acssuschemeng.7b02711. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.













