Abstract
Background
Standard management for intrauterine lesions typically involves initial imaging followed by operative hysteroscopy for suspicious findings. However, the efficacy of routine outpatient hysteroscopy in women undergoing assisted reproductive technology (ART) remains uncertain due to a lack of decisive high-quality evidence. This study aimed to determine whether outpatient hysteroscopy is beneficial for infertile women who have unremarkable imaging results prior to undergoing ART.
Methods
A systematic review and meta-analysis were conducted following PRISMA guidelines, incorporating data up to May 31, 2023, from databases such as PubMed, Embase, and the Cochrane Library. The primary outcome assessed was the live birth rate, with secondary outcomes including chemical pregnancy, clinical pregnancy rates, and miscarriage rates. Statistical analysis involved calculating risk ratios with 95% confidence intervals and assessing heterogeneity with the I2 statistic.
Results
The analysis included ten randomized control trials. Receiving outpatient hysteroscopy before undergoing ART was associated with increased live birth (RR 1.22, 95% CI 1.03–1.45, I2 61%) and clinical pregnancy rate (RR 1.27 95% CI 1.10–1.47, I2 53%). Miscarriage rates did not differ significantly (RR 1.25, CI 0.90–1.76, I2 50%). Subgroup analyses did not show a significant difference in clinical pregnancy rates when comparing normal versus abnormal hysteroscopic findings (RR 1.01, CI 0.78–1.32, I2 38%). We analyzed data using both intention-to-treat and per-protocol approaches, and our findings were consistent across both analytical methods.
Conclusions
Office hysteroscopy may enhance live birth and clinical pregnancy rates in infertile women undergoing ART, even when previous imaging studies show no apparent intrauterine lesions. Treating lesions not detected by imaging may improve ART outcomes. The most commonly missed lesions are endometrial polyps, submucosal fibroids and endometritis, which are all known to affect ART success rates. The findings suggested that hysteroscopy, given its diagnostic accuracy and patient tolerability, should be considered in the management of infertility.
Database registration
The study was registered in the International Prospective Register of Systemic Review database (CRD42023476403).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12958-024-01191-0.
Keywords: Infertility, Artificial reproductive technology, Office hysteroscopy, Outpatient hysteroscopy, Diagnostic hysteroscopy, Hysterosalpingography, Transvaginal sonography, Transvaginal ultrasound
Background
Recent global estimates suggest that roughly one in six individuals of reproductive age may encounter infertility during their lifetime [1]. In females, the causes of infertility are categorized into ovulatory, tubal/uterine, other, and unexplained [2]. Uterine cavity abnormalities are present in approximately 10% of subfertile women, and nearly half of those with recurrent implantation failure may have abnormal uterine conditions [3]. Therefore, evaluating the uterine cavity is a routine part of the initial infertility assessment in women.
Uterine factors, including endometrial polyps, fibroids, intrauterine synechiae, and congenital malformations, may distort the uterine cavity [4]. Common methods for assessing the uterine cavity in subfertile women include transvaginal sonography (TVS), saline infusion sonohysterography, and hysterosalpingography (HSG). Advanced imaging techniques such as 3D ultrasound or MRI are also options, particularly for conditions such as bicornuate uterus. More invasive procedures, such as hysteroscopy or laparoscopy, are also used [3, 5]. Hysteroscopy, being the gold standard, not only allows direct observation of lesions but also enables pathology confirmation through biopsy and potential immediate surgical correction. As such, operative hysteroscopy, which combines diagnosis and treatment, is the preferred method when suspicious lesions are detected in imaging studies.
Outpatient or office hysteroscopy has gained popularity due to advancements in endoscopic technology, which include smaller hysteroscopes and enhanced visual systems, making the procedure more feasible and acceptable [6]. Its main advantage is the avoidance of general anesthesia, reducing related risks and costs, and improving patient acceptance. Despite a reduction in instrument size, office hysteroscopes often retain a working channel, allowing biopsy or removal of small lesions. This has led more physicians to adopt outpatient hysteroscopy as a routine infertility evaluation. Nevertheless, studies have shown that outpatient hysteroscopy, saline infusion sonography, and hysterosalpingography have comparable diagnostic accuracy for uterine cavity assessment in infertile women [7]. Since outpatient hysteroscopy is more invasive and lacks evidence that it improves pregnancy rates, current guidelines recommend its use only when clinically indicated, rather than as a first-step evaluation [8, 9].
To date, compelling high-quality evidence supporting the routine use of hysteroscopy as a diagnostic tool in the evaluation of infertility—particularly in women poised for assisted reproductive technology with unremarkable transvaginal ultrasound or hysterosalpingography results—is lacking. Given the increase in assisted reproductive treatment cycles and the growing adoption of outpatient hysteroscopy, it is imperative to re-evaluate its role as an additional diagnostic tool within the infertile population. This study aims to determine whether there is a definitive benefit to routinely incorporating hysteroscopy into the diagnostic process for infertility, especially for patients with negative imaging studies who are candidates for assisted reproductive treatment.
Methods
Search methods and eligibility criteria
We performed a comprehensive search of electronic databases, including PubMed, Embase, and the Cochrane Library, up to May 31, 2023, to identify pertinent studies. Our search strategy adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. We employed the following search terms: ICSI (intracytoplasmic sperm injection), IVF (in vitro fertilization), fertilization, transfer, infertility, ART (assisted reproductive technology), reproductive technolog*, in combination with “Hysteroscop*” and “resectoscop*”.
Our analysis was confined to randomized controlled trials (RCTs) to ensure a high level of evidence and to minimize potential bias. We established predefined inclusion criteria for patient populations, radiological assessment, and control groups. Studies that satisfied all three of these criteria were considered for inclusion:
Patient population: We included infertile women slated for further assisted reproductive technology procedures, including patients either undergoing their first cycle of in vitro fertilization (IVF) or those with a history of previous IVF failure. We excluded patients pursing natural conception, undergoing dating only or those receiving intrauterine insemination (IUI).
Radiological assessment: Eligible studies were needed to have confirmed the absence of gross uterine abnormalities through normal transvaginal sonography (TVS) or hysterosalpingography (HSG) prior to the study.
Control group: Studies must have a control group that did not undergo hysteroscopy as part of their diagnostic or treatment regimen. These patients proceeded directly to further assisted reproductive technology (ART) without an outpatient hysteroscopy examination.
The primary outcome we focused on was the live birth rate, with secondary outcomes encompassing the chemical pregnancy rate, clinical pregnancy rate, and miscarriage rate. These outcomes were chosen to provide a comprehensive assessment of the reproductive results following hysteroscopy in women undergoing ART.
Study selection and data extraction
Two reviewers (Hou and Lu) independently screened titles and abstracts for relevance. Full texts of potentially eligible studies were then examined based on our criteria. Any disagreements were resolved through discussion or, if necessary, by consulting a third reviewer (Chen).
Data from the selected RCTs were extracted using a standardized form by two independent reviewers. Extracted information included study characteristics, participant demographics, intervention details, and relevant outcomes. Additionally, abnormal findings and subsequent management of hysteroscopy were recorded. In-depth details regarding infertility-associated features, ART protocols, and the hysteroscopy process are provided as supplementary data.
Appraisal of methodological quality
Three reviewers (Hou, Lu, and Huang) assessed the methodological quality of the included RCTs using the Cochrane Risk of Bias tool for randomized controlled trials 2.0 [10]. This tool evaluates biases across multiple domains such as random sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting, and other biases. Studies were classified as having low, unclear, or high risk of bias in each domain. The methodological quality assessment for each study is detailed in the supplementary data (Supplementary Fig. S1).
Statistical analysis
Data synthesis and statistical analysis were conducted in line with PRISMA guidelines [11, 12] using Review Manager version 5.3. We calculated the relative risk (RR) with 95% confidence intervals (CIs) to evaluate binary variables. A random-effects model was employed to accommodate potential study heterogeneity, which we quantified using the I2 statistic, classifying it as low (25–50%), moderate (50–75%) and high (> 75%) [13].
Analyses were conducted using both intention-to-treat (ITT) and per-protocol (PP) approaches. The ITT analysis included all RCT participants, while the PP analysis focused on those who completed the study and received ART, excluding any participants lost to follow-up. The primary data presentation is based on the PP approach, given our specific interest in ART pregnancy rates. ITT data was also presented and the further forest plot figure was illustrated in Supplementary Figure S2.
Results
Search results
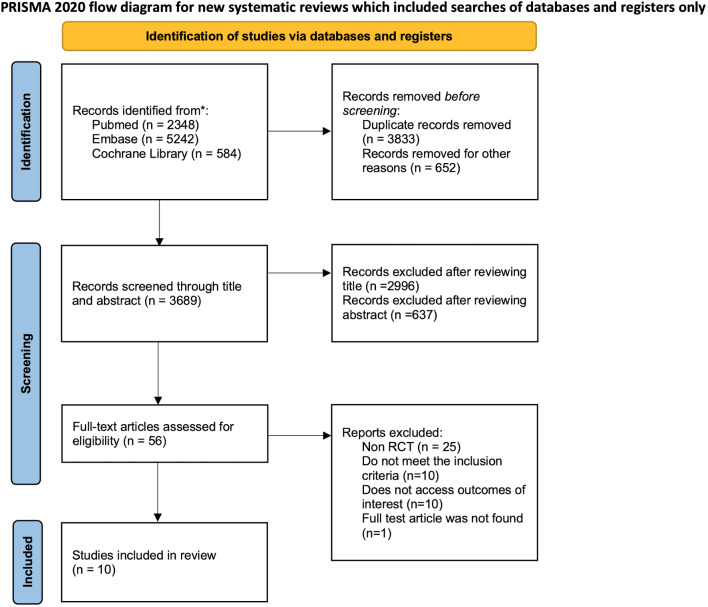
Following the PRISMA guidelines, our systematic review process is depicted in Fig. 1. Initially, 3689 records were identified for further screening. After reviewing titles and abstracts, 56 articles were assessed for eligibility. Of these, 46 articles were excluded for various reasons: 25 were non-RCTs, 20 did not meet our inclusion criteria, such as lacking a control group design, including patients with natural pregnancy or including patients undergoing intrauterine insemination, 10 did not report the outcomes of interest, 21 were not in English, and one were inaccessible in full text. Although three articles did not provide data on the primary outcome of live birth rate, they were included in this meta-analysis due to their reporting of other relevant secondary outcomes [14–16]. Ultimately, 10 RCTs were incorporated into this meta-analysis [17–23].
Fig. 1.
Study screening and inclusion process following the PRISMA guideline
Trial characteristics
Published between 2004 and 2022, these 10 RCTs comprised 3612 patients, with 1795 receiving outpatient hysteroscopy before commencing ART and 1817 proceeding directly to ART. The salient features of the included articles are summarized in Table 1. Study settings varied: seven were single-center, one spanned two centers, and two were multicenter across Europe. Patient ages ranged from 27 to 33 years. Concerning fertilization methods, two study solely used IVF, three used ICSI exclusively, and five tailored the use of IVF or ICSI to the clinical situation. Regarding embryo transfer methods, seven studies utilized fresh embryo transfer, two reported frozen embryo transfer, and one did not document this information. Detailed characteristics of the infertility conditions and treatment protocols are presented in supplementary table S1.
Table 1.
Characteristics of the included randomized control trials
| Author, publication year | Study area | Main inclusion criteria | Main exclusion criteria | Intervention | Control | Live birth rate (HSC/Control) |
Clinical pregnancy rate (HSC/Control) |
Conclusion |
|---|---|---|---|---|---|---|---|---|
| Demirol et al. (2004) | Single center in Turkey | ≥ 2 previous implantation failure | Outpatient HSC (N = 210) | Immediate IVF (N = 211) | NA | 32.1%/21.5% | HSC improves PR. | |
| Raju et al. (2006) | Single clinic in India | ≥ 2 previous implantation failure | Outpatient HSC (N = 265) | Immediate IVF (N = 255) | 28.5%/16.8% | 43.1%/26.3% | HSC improves LBR and PR. | |
| Shawki et al. (2012) | Single center in Egypt | For first or further ICSI | Outpatient HSC (N = 105) | Immediate IVF (N = 110) | 37.8%/28.0% | 44.4%/30.0% | HSC improves PR but does not improve LBR. | |
| Elsetohy et al. (2015) | Single center in Egypt | For first ICSI | Recurrent miscarriage | Outpatient HSC (N = 97) | Immediate IVF (N = 96) | 59.8%/34.4% | 70.1%/45.8% | HSC improves LBR and PR. |
| Alleyassin et al. (2015) | Single center in Iran | For first ICSI | Recurrent miscarriage | Outpatient HSC (N = 110) | Immediate IVF (N = 110) | NA | 48.2%/38.2% | HSC improves PR. |
| Smit et al. (2016) | Multicenter in Netherlands | For first IVF or ICSI | ≥ 2 miscarriages | Outpatient HSC (N = 369) | Immediate IVF (N = 373) | 54.5%/52.3% | NA | HSC does not improve LBR. |
| El-Toukhy et al. (2016) | Multicenter in UK, Belgium, Italy, Czech Republic |
Age < 38 y/o 2–4 times previous implantation failure |
BMI > 35 kg/m² ≥ 37 y/o with < 8 oocytes retrieved previously |
Outpatient HSC (N = 350) | Immediate IVF (N = 352) | 31.6%/33.1% | 37.9%/37.9% | HSC does not improve LBR or PR. |
| Abid et al. (2021) | Single center in Tunisia |
Age < 40 y/o BMI ≤ 30 kg/m² For first IVF |
Outpatient HSC (N = 84) | Immediate IVF (N = 87) | 25.0%/19.3% | 32.4%/28.9% | HSC does not improve LBR or PR. | |
| Pounikar et al. (2022) | Two centers in India |
Age < 45 y/o ≥ 1 previous implantation failure |
First time IVF | Outpatient HSC (N = 90) | Immediate IVF (N = 90) | NA | 30.0%/23.3% | HSC improves PR. |
| Ghasemi et al. (2022) | Single center in Iran |
Age ≤ 40 year For first IVF |
≥ 3 miscarriage | Outpatient HSC and irritation of uterine cavity (N = 109) | Immediate IVF (N = 119) | 57.8%/47.9% | 63.3%/58.0% | HSC improves cumulative LBR. |
HSC, hysteroscopy; PR, clinical pregnancy rate; LBR, live birth rate; ICSI, intracytoplasmic sperm injection; IVF, in vitro fertilization
Quality of included studies
Four studies were assessed as having low risk of bias, while five exhibited some concerns, primarily due to insufficient details about the randomization process and when patients were informed of their allocation. Blinding is challenging in invasive procedures such as hysteroscopy; however, one study was deemed at high risk of bias due to a significant disparity in the number of patients with only one prior IVF failure between the hysteroscopy and control groups. Such disparities could influence the live birth rate, our primary outcome of interest.
Primary outcome
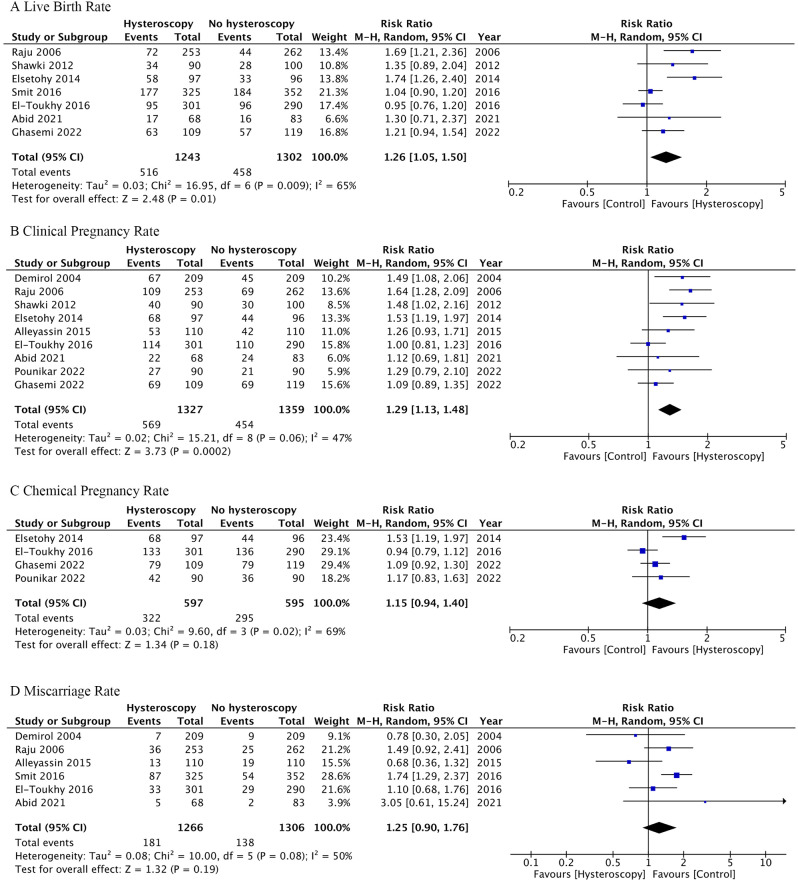
Seven studies reported live birth rates, defined as the delivery of a viable baby after 24 weeks of gestation. For patients with prior negative transvaginal ultrasound or hysterosalpingography, outpatient hysteroscopy was associated with a significant increase in live birth rates (RR 1.26, CI 1.05–1.50, I2 65%, analyzed by the per-protocol approach; RR 1.22, CI 1.03–1.45, I2 61%, analyzed by the intention-to-treat approach) (Fig. 2A and Fig. S2A). The degree of heterogeneity was moderate (I2 = 65% and 61%, by per-protocol and intention-to-treat approach, respectively).
Fig. 2.
Forest plot comparing outcomes between the hysteroscopy and control groups. There was a significant increase in (A) live birth rate and (B) clinical pregnancy rate among the outpatient hysteroscopy group. There was no significant difference in (C) chemical pregnancy rate or (D) miscarriage rate between the two groups. These results were analyzed through per-protocol approach. The results analyzed through intention-to-treat approach was showed in Fig. S2
Secondary outcome
Clinical pregnancy rates were documented in nine studies, with clinical pregnancy defined as the visualization of fetal heartbeat via ultrasound. The hysteroscopy group demonstrated a significantly higher clinical pregnancy rate than the control group (RR 1.29, CI 1.13–1.48, I2 47%, analyzed by the per-protocol approach; RR 1.27, CI 1.10–1.47, I2 53%, analyzed by the intention-to-treat approach) (Fig. 2B and Fig. S2B), indicating low heterogeneity.
Four studies examined chemical pregnancy rates, typically defined as a positive beta-human chorionic gonadotropin blood test 14 days post-embryo transfer. There was no significant difference in chemical pregnancy rates between the groups (RR 1.15, CI 0.94–1.40, I2 69%, analyzed by the per-protocol approach; RR 1.13, CI 0.92–1.38, I2 68%, analyzed by the intention-to-treat approach) (Fig. 2C and Fig S2C), with moderate heterogeneity observed. Similarly, six studies reported miscarriage rates, finding no significant difference between the groups (RR 1.25, CI 0.90–1.76, I2 50%, analyzed by the per-protocol approach; RR 1.25, CI 0.93–1.69, I2 38%, analyzed by the intention-to-treat approach) (Fig. 2D and Fig S2D), accompanied by low heterogeneity.
Subgroup analysis according to the hysteroscopic findings
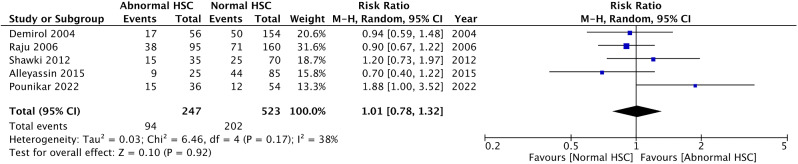
A subgroup analysis within the hysteroscopy cohort explored the impact of normal versus abnormal findings on ART outcomes. Five studies compared clinical pregnancy rates between patients with normal and abnormal findings. Common practice among these studies was to biopsy and treat any detected abnormalities. The analysis revealed no significant difference in clinical pregnancy rates based on hysteroscopy findings (RR 1.01, CI 0.78–1.32, I2 38%) (Fig. 3).
Fig. 3.
Forest plot depicting subgroup analysis of clinical pregnancy rates among the hysteroscopy group. Comparing outcomes between the normal hysteroscopic finding and abnormal hysteroscopic finding groups, there was no significant difference in the clinical pregnancy rate
Abnormal hysteroscopy findings
Nine studies provided details on the diagnosis of abnormal hysteroscopic findings. These lesions, not identified by transvaginal sonography or hysterosalpingography, were detected through outpatient hysteroscopy. The studies indicated a false-negative rate for imaging studies between 13% and 43%. The most commonly identified new lesions via outpatient hysteroscopy included endometrial polyps, found in 3–15.7% of cases; intrauterine adhesions, in 1–13%; submucosal fibroids, in 0.4–7.2%; endometritis, in 1.9–8.3%; septate or arcuate uterus, in 0.9–13.6%; and endometrial hyperplasia, in 1.8–4.8%. Additionally, cervical adhesion or stenosis was noted in 2.3–12.0% of procedures. The comprehensive findings from hysteroscopy are presented in Table 2.
Table 2.
Characteristics and findings of hysteroscopy in included RCTs
| Author, publication year | Abnormal hysteroscopy |
Etiology of unsuspected lesions1 | Instrument | Anesthesia/ Sedation |
Timing of procedure/subsequent ART |
|---|---|---|---|---|---|
| Demirol et al. (2004) | 26.7% |
EM polyps 15.7% IUA 8.5% Cervix stenosis 2.3% |
5 mm diameter with 5 Fr working channel, 30° view (Bettocchi) | Midazolam 0.1 mg/kg IV when needed | Early follicular phase/NA |
| Raju et al. (2006) | 38.0% |
EM polyps 12.8% Abn Cervix 12% IUA 4.8% EM hyperplasia 4.8% Fibroids 0.4% Anomalies2 3.2% |
5 mm diameter with 5 Fr working channel, 30° view (Krishna) | Midazolam 0.1 mg/kg IV when needed | NA/NA |
| Shawki et al. (2012) | 33.4% |
EM polyps 10.8% IUA 3.8% Fibroids 3.8% Endometritis 1.9% Anomalies2 0.9% EM hyperplasia 2.8% |
3.5 mm diameter, 0° view (Versascope, Gynecare, Ethicon, Sommerville, NJ, USA) | None | NA/NA |
| Elsetohy et al. (2014) | 43.3% |
EM polyps 13.4% Anomalies2 10.4% Fibroids 7.2% IUA 6.2% |
4.3 mm diameter with 5 Fr working channel, 30° view | NA | Early to mid-follicular phase/Start ICSI cycle within 3 months |
| Alleyassin et al. (2015) | 22.7% |
Anomalies2 13.6% EM polyps 5.4% IUA 0.9% EM hyperplasia 1.8% |
4 mm diameter rigid hysteroscopy, 30°view | None | Mid-luteal phase/Start ICSI next cycle |
| Smit et al. (2016) | 13.0% |
EM polyps 9.5% Anomalies2 2.3% Fibroids 1.3% IUA 1% |
5 mm diameter with 5 Fr working channel, 30° view | Paracervical block when needed | Early to mid-follicular phase/Start ICSI cycle within 3 months |
| El-Toukhy et al. (2016) | 26.0% |
Anomalies2 8% Cx stenosis 4% EM polyps 3%3 IUA 0.9% |
2.9 mm diameter rigid hysteroscopy, 30° view (TROPHYscope; Karl Storz, Tuttlingen) | None | Follicular phase/Started IVF in the following month |
| Abid et al. (2021) | 31.0% |
IUA 13% EM polyps 8.3% Endometritis 8.3% Fibroids 1.2% |
2.9 mm diameter (26,120 BA STORZ) | Analgesic treatment level 2 (Paracetamol/Codeine 500 mg/30 mg) 1 h before the procedure | Mid-follicular phase/Started IVF in the following month |
| Pounikar et al. (2022) | 40.0% | NA | NA | NA | NA/Started IVF in the following month |
| Ghasemi et al. (2022) | NA | NA | 5 Fr working channel, 30° view4 | NA | Early to mid-follicular phase/NA |
ART, artificial reproductive treatment; EM, endometrial; IUA, intrauterine adhesion; Cx, cervix; IV, intravenous; NA, not available; ICSI, intracytoplasmic sperm injection
1 The percentage was calculated based on the number of patients who received hysteroscopy
2 The anomalies include septate, arcuate, or bicornuate uterus
3 The incidence included 1% micropolyps
4 The study did not report the diameter of the hysteroscopy
Discussion
The analysis of live birth and clinical pregnancy rates both showed a significant improvement in patients who underwent outpatient hysteroscopy compared to those who did not. This beneficial effect persisted across both intention-to-treat and per-protocol analyses. The finding that outpatient hysteroscopy can positively influence live birth and clinical pregnancy rates is promising, suggesting that the treatment of subtle intrauterine pathologies, potentially missed by conventional imaging, can substantially enhance reproductive outcomes.
Our study examined the impact of outpatient hysteroscopy, detailing the procedural specifics in Table 2. Ben Abid et al. (2021) preemptively administered level 2 analgesic treatment (paracetamol with codeine, 500 mg/30 mg) one hour before the procedure [23]. Other studies did not routinely prescribe anesthesia or sedation but provided midazolam (0.1 mg/kg IV) or a paracervical block as needed. The majority of patients allocated to the hysteroscopy group underwent the examination successfully. Smit et al. (2016) reported a procedural failure rate of 8.9%, which might be overestimated since a 5-mm outer diameter continuous flow hysteroscope was employed [17, 24]. Utilizing a 3.5-mm mini hysteroscope can reduce discomfort, enhance visualization, and increase the success rate [25]. Notably, the included studies reported no adverse events associated with outpatient hysteroscopy.
Operative findings and final diagnoses from hysteroscopy in the included studies indicated a false-negative rate between 13% and 43%. This rate of abnormal findings is in line with Karayalcin et al. (2010), who reported a 22.9% prevalence of endometrial pathology in 2,500 cases undergoing hysteroscopy before IVF [26]. Variability in incidence rates across studies can be attributed to differences in patient selection. For instance, the prevalence of uterine fibroids or endometrial polyps tends to increase with age [27, 28]. Additionally, many studies did not stratify by symptomatic indications for anomalies or differentiate patients with normal sonography.
Emerging evidence suggests that intrauterine lesions can decrease pregnancy likelihood, and their treatment may enhance pregnancy rates and ART success. Bosteels et al. (2018) assessed the effect of hysteroscopic removal of endometrial polyps, submucous fibroids, uterine septum, or intrauterine adhesions in subfertile women or those undergoing IUI, IVF, or ICSI. Despite the evidence being of low to moderate quality, their findings suggest that removing submucous fibroids and endometrial polyps can improve the chances of clinical pregnancy [29]. In our study’s subgroup analysis, the clinical pregnancy rate showed no significant difference between normal and abnormal hysteroscopic findings. Also, the physicians of the included studies would manage the abnormal lesions found through hysteroscopy as their medical routine. Due to the above reasons, we assumed that treating lesions identified by outpatient hysteroscopy might contribute to improved clinical pregnancy rates.
Hysteroscopy can detect smaller lesions and ascertain their precise location more accurately than imaging studies. The impact of lesion size on subsequent pregnancy remains unclear; however, evidence indicates that even small lesions can disrupt implantation. For instance, Perez-Medina et al. (2005) reported improved pregnancy rates following the removal of polyps smaller than 1 cm [30]. Additionally, micropolyps are now considered indicative of chronic endometritis [31], and lesion location is crucial, particularly those at the uterotubal junction [32]. Therefore, hysteroscopy may uncover small or critically located lesions that require appropriate management.
Compared to major abnormal intrauterine lesions associated with infertility, diagnosing chronic endometritis (CE) through transvaginal sonography or hysterosalpingography is challenging. CE, characterized by persistent local inflammation, can significantly impede reproductive success [33]. Often asymptomatic, CE frequently goes undetected by affected women and clinicians alike. The hallmark of CE is the presence of plasma cells within the endometrial stroma [34], necessitating histological diagnosis through hematoxylin and eosin (H&E) staining or immunohistochemistry (IHC) for Syndecan-1 (CD138) following endometrial sampling or biopsy. CD138 has proven more sensitive for diagnosing CE, with a reported prevalence of 10.4% in infertile women [35]. Additionally, hysteroscopic observation of the endometrium without biopsy serves as an alternative diagnostic method. Typical hysteroscopic hallmarks of CE include hyperemia, edema, and micropolyps [31], with a diagnostic accuracy of 93.4% when these criteria are combined. Hence, hysteroscopy, with or without biopsy, is effective for diagnosing CE, potentially enhancing the pregnancy rate among infertile patients.
Beyond detecting and treating abnormal lesions, outpatient hysteroscopy itself may have a beneficial impact on pregnancy outcomes. Instrument passage through the cervical canal allows for the early detection and resolution of cervical issues prior to ART. The fluid infusion and irrigation during hysteroscopy could mechanically cleanse the endometrium, potentially eliminating anti-adhesive glycoproteins and fostering a more implantation-conducive environment [36, 37]. Increased pregnancy and live birth rates after hysterosalpingography have been documented [38, 39], likely due to uterine flushing, which removes tubal debris and modulates macrophage secretion of interleukins and prostaglandins [40–42]. While hysteroscopy employs a gentler flushing force and utilizes fluid instead of contrast media, its pregnancy rate improvement may share a similar mechanism with HSG. Additionally, the included studies reported no major complications. The reported complication rate for diagnostic hysteroscopy was 0.13%, significantly lower than that for operative hysteroscopy. Perforation was the most commonly reported complication in diagnostic hysteroscopy [43].
The consensus on whether to perform outpatient hysteroscopy in patients without intrauterine lesions on imaging is still evolving. Two thorough systematic reviews and meta-analyses by Di Spiezio Sardo et al. (2016) and Kamath et al. (2019) reported improved live birth and clinical pregnancy rates in hysteroscopy groups but stopped short of endorsing routine screening hysteroscopy due to the quality of evidence [44, 45]. Notably, our study population and inclusion criteria differed from these analyses. Di Spiezio Sardo et al. (2016) included studies that compared “operative” hysteroscopy with the absence of the procedure, which might potentially overemphasize the benefits of hysteroscopy. Conversely, Kamath et al. (2019) included studies examining the impact of outpatient hysteroscopy on intrauterine insemination. We excluded patients undergoing IUI for two main reasons: First, our goal is to provide more precise insights for patients considering invasive ART, such as IVF or ICSI, who typically deliberate more frequently on whether to undergo various procedures than those opting for natural conception or IUI. Second, cost-efficiency plays a crucial role in deciding to proceed with a procedure. Given the significantly higher costs of IVF or ICSI compared to IUI, we believe it is justifiable to focus our study exclusively on patients undergoing IVF or ICSI. Also, our inclusion was strictly for studies confirming normal transvaginal sonography or hysterosalpingography results, which was different from other studies. Although doing so will reduce the patient population that our research can be applied to, we hope that such results can provide clearer answers to the questions of this smaller group of patients. Furthermore, we analyzed data using both intention-to-treat and per-protocol approaches. Considering that ART is elective and many factors can influence the decision to proceed with it, per-protocol analysis—based on patients who eventually received ART rather than all those included in the trial—is more reflective of real-world scenarios. Our findings were consistent across both analytical methods.
The ESHRE add-ons working group recently concluded that screening hysteroscopy does not substantially improve live birth rates when performed prior to initiating IVF treatments. However, they acknowledged the potential benefits for patients with recurrent implantation failure [8, 46]. This conclusion was primarily drawn from findings by Kamath et al. (2019), who observed improved live birth and clinical pregnancy rates in the hysteroscopy group [45]. Despite our results indicating a statistical improvement in these rates, we concur with the ESHRE add-ons working group’s cautious recommendation. The decision to perform an add-on procedure such as hysteroscopy requires a comprehensive assessment that considers not only pregnancy outcomes but also cost-effectiveness.
The prevalence of undetected intrauterine abnormalities in asymptomatic patients varies widely, with reports as low as 11% [47]. Conversely, our findings suggested that office hysteroscopy could increase the chance of live birth by 1.2 times. When combining the prevalence of undetected lesions with the relative risk improvement, hysteroscopy resulted in six additional live births per 100 procedures. This simplistic calculation offers a preliminary cost‒benefit analysis adaptable to varying IVF and hysteroscopy costs across regions. A more sophisticated cost-effectiveness evaluation was undertaken by Kasius et al. (2013), suggesting that routine hysteroscopy before IVF could be economically viable. Their model, based on data from the Netherlands in 2013, estimated the costs per IVF cycle and per screening hysteroscopy at $2726 and $134, respectively [48]. However, an updated, region-specific analysis is warranted, considering that diagnostic rates, live birth rates from IVF, and associated costs are subject to change over time and differ by location.
In our analysis, we refined the research question to focus on a more specific subset of the population than previous studies. Nonetheless, this study has limitations. The quality of the included RCTs varied, with some being small-scale and having bias concerns, including one study with a high risk of bias [14]. Sensitivity analyses, excluding the study with high risk, did not alter our results. The invasive nature of hysteroscopy precludes the possibility of double-blinded RCTs, contributing to moderate to high heterogeneity in our results. Another limitation of our study is that various patient characteristics, such as recurrent implantation failure, the cause of infertility, or the indications for choosing ICSI/IVF, could affect the outcomes. Conducting subgroup analyses for these characteristics might not be feasible due to the limited number of studies available. This meta-analysis underscores the need for further high-quality randomized control trials that address the everyday clinical decisions faced by infertility specialists. Additionally, there is a call for comprehensive studies on the cost-effectiveness of such interventions.
Conclusion
Our findings indicate that for infertile women, undergoing office hysteroscopy prior to artificial reproductive technology, despite previous imaging studies such as transvaginal sonography or hysterosalpingography showing no apparent intrauterine lesions, could enhance live birth and clinical pregnancy rates. Addressing lesions that imaging studies may have falsely missed—particularly endometrial polyps, submucosal fibroids, and endometritis—appears to positively influence ART outcomes. These lesions are known to adversely affect pregnancy rates, and thus, their identification and treatment through hysteroscopy should be considered.
Office hysteroscopy stands out for its safety, ease of use, diagnostic accuracy, and high patient tolerability. While current evidence indicates an increase in clinical pregnancy and live birth rates following outpatient hysteroscopy, prevailing guidelines and our perspective do not support routine use of outpatient hysteroscopy solely for enhancing live birth rates. The current evidence highlights a pressing need for further research to determine the value of hysteroscopy as a routine screening method for all women undergoing assisted reproductive technologies. Future studies should focus not only on the efficacy of hysteroscopy in improving fertility outcomes but also on its cost-effectiveness and impact on patient care pathways.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material: Supplementary Figure S1. Quality assessment using the Cochrane Risk of Bias tool for randomized controlled trials
Supplementary Material: Supplementary Figure S2. Forest plot comparing outcomes between the hysteroscopy and control groups using intention-to-treat approach
Supplementary Material: Supplementary Figure S3. Forest plots after sensitivity testing by excluding Elsetohy study comparing outcomes between the hysteroscopy and control groups
Supplementary Material: Supplementary Table S1. Characteristics of the infertility condition and artificial reproductive treatment of the included RCTs
Supplementary Material: Supplementary Table S2. Comparative analysis of data pre- and post-sensitivity testing by excluding Elsetohy study
Acknowledgements
The authors would like to thank Professor Chyi-Huey Bai and Associate Professor Yuan-Hung Wang for providing statistical expertise.
Abbreviations
- ART
Artificial reproductive technology
- CE
Chronic endometritis
- CI
Confidence intervals
- HSG
Hysterosalpingography
- H&E
Hematoxylin and eosin
- ICSI
Intracytoplasmic sperm injection
- IHC
Immunohistochemistry
- ITT
Intention-to-treat
- IUI
Intrauterine insemination
- IVF
In vitro fertilization
- PP
Per-protocol
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RCT
Randomized controlled trials
- RR
Risk ratios
- TVS
Transvaginal sonography
Author contributions
JH Hou and Chi-Huang Chen devised and designed the study. JH Hou drafted the initial protocol and manuscript. JH Hou and BJ Lu conducted the search, study selection, data extraction and data analysis. YL Huang and Chih-Heng Chen checked the process if it fit reporting guidelines and checked statistical methods. All authors discussed and interpreted the results together. JH Hou drafted the final manuscript. All authors approved the final version of the manuscript.
Funding
This research received no external funding.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO. : Infertility Prevalence Estimates, 1990–2021. (Organization WH ed. Geneva; 2023.
- 2.Practice Committee of the American Society for Reproductive M Effectiveness and treatment for unexplained infertility. Fertil Steril. 2006;86:111–4. doi: 10.1016/j.fertnstert.2006.07.1475. [DOI] [Google Scholar]
- 3.Pundir J, El Toukhy T. Uterine cavity assessment prior to IVF. Womens Health (Lond) 2010;6:841–7. doi: 10.2217/WHE.10.61. [DOI] [PubMed] [Google Scholar]
- 4.Carson SA, Kallen AN. Diagnosis and management of infertility: a review. JAMA. 2021;326:65–76. doi: 10.1001/jama.2021.4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Domali E, Kyriakopoulos K, Antsaklis A. Ultrasonography: the Main Diagnostic Tool in Subfertile Women. Donald School Journal of Ultrasound in Obstetrics and Gynecology. 2012;6:270–85. doi: 10.5005/jp-journals-10009-1250. [DOI] [Google Scholar]
- 6.Pluchino N, Ninni F, Angioni S, Artini P, Araujo VG, Massimetti G, Genazzani AR, Cela V. Office vaginoscopic hysteroscopy in infertile women: effects of gynecologist experience, instrument size, and distention medium on patient discomfort. J Minim Invasive Gynecol. 2010;17:344–50. doi: 10.1016/j.jmig.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 7.Brown SE, Coddington CC, Schnorr J, Toner JP, Gibbons W, Oehninger S. Evaluation of outpatient hysteroscopy, saline infusion hysterosonography, and hysterosalpingography in infertile women: a prospective, randomized study. Fertil Steril. 2000;74:1029–34. doi: 10.1016/S0015-0282(00)01541-7. [DOI] [PubMed] [Google Scholar]
- 8.Lundin K, Bentzen JG, Bozdag G, Ebner T, Harper J, Le Clef N, Moffett A, Norcross S, Polyzos NP, Rautakallio-Hokkanen S et al. Good practice recommendations on add-ons in reproductive medicinedagger. Hum Reprod 2023. [DOI] [PMC free article] [PubMed]
- 9.NICE. : In Fertility: Evidence Update March 2015: A summary of selected new evidence relevant to NICE clinical guideline 156 ‘Assessment and treatment for people with fertility problems’ (2013) London; 2015. [PubMed]
- 10.Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, et al. RoB 2: a revised tool for assessing risk of bias in randomized trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 11.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pounikar M, Shrivastava D, Sharma S, Tadghare J. Role of Hysteroscopy in patients with previous in Vitro Fertilization failure: an institutional experience in Rural Population. J Obstet Gynaecol India. 2023;73:77–82. doi: 10.1007/s13224-022-01729-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demirol A, Gurgan T. Effect of treatment of intrauterine pathologies with office hysteroscopy in patients with recurrent IVF failure. Reprod Biomed Online. 2004;8:590–4. doi: 10.1016/S1472-6483(10)61108-X. [DOI] [PubMed] [Google Scholar]
- 16.Alleyassin A, Abiri A, Agha-Hosseini M, Sarvi F. The Value of Routine Hysteroscopy before the first intracytoplasmic sperm injection treatment cycle. Gynecol Obstet Invest. 2017;82:125–30. doi: 10.1159/000445801. [DOI] [PubMed] [Google Scholar]
- 17.Smit JG, Kasius JC, Eijkemans MJC, Koks CAM, van Golde R, Nap AW, Scheffer GJ, Manger PAP, Hoek A, Schoot BC, et al. Hysteroscopy before in-vitro fertilisation (inSIGHT): a multicentre, randomised controlled trial. Lancet. 2016;387:2622–9. doi: 10.1016/S0140-6736(16)00231-2. [DOI] [PubMed] [Google Scholar]
- 18.Shawki HE. Routine office hysteroscopy prior to ICSI and its impact on assisted reproduction program outcome: a randomized controlled trial. Middle East Fertility Society Journal 2011, 17.
- 19.Rama Raju GA, Shashi Kumari G, Krishna KM, Prakash GJ, Madan K. Assessment of uterine cavity by hysteroscopy in assisted reproduction programme and its influence on pregnancy outcome. Arch Gynecol Obstet. 2006;274:160–4. doi: 10.1007/s00404-006-0174-7. [DOI] [PubMed] [Google Scholar]
- 20.Ghasemi M, Aleyasin A, Fatemi HM, Ghaemdoust F, Shahrakipour M. Uterine cavity irrigation with Office Hysteroscopy during Ovarian Stimulation for IVF: a Randomized Controlled Trial. Front Endocrinol (Lausanne) 2022;13:778988. doi: 10.3389/fendo.2022.778988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elsetohy KA, Askalany AH, Hassan M, Dawood Z. Routine office hysteroscopy prior to ICSI vs. ICSI alone in patients with normal transvaginal ultrasound: a randomized controlled trial. Arch Gynecol Obstet. 2015;291:193–9. doi: 10.1007/s00404-014-3397-z. [DOI] [PubMed] [Google Scholar]
- 22.El-Toukhy T, Campo R, Khalaf Y, Tabanelli C, Gianaroli L, Gordts SS, Gordts S, Mestdagh G, Mardesic T, Voboril J, et al. Hysteroscopy in recurrent in-vitro fertilisation failure (TROPHY): a multicentre, randomised controlled trial. Lancet. 2016;387:2614–21. doi: 10.1016/S0140-6736(16)00258-0. [DOI] [PubMed] [Google Scholar]
- 23.Ben Abid H, Fekih M, Fathallah K, Chachia S, Bibi M, Khairi H. Office hysteroscopy before first in vitro fertilization. A randomized controlled trial. J Gynecol Obstet Hum Reprod. 2021;50:102109. doi: 10.1016/j.jogoh.2021.102109. [DOI] [PubMed] [Google Scholar]
- 24.Smit JG, Kasius JC, Eijkemans MJ, Koks CA, Van Golde R, Oosterhuis JG, Nap AW, Scheffer GJ, Manger PA, Hoek A, et al. The inSIGHT study: costs and effects of routine hysteroscopy prior to a first IVF treatment cycle. A randomised controlled trial. BMC Womens Health. 2012;12:22. doi: 10.1186/1472-6874-12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campo R, Molinas CR, Rombauts L, Mestdagh G, Lauwers M, Braekmans P, Brosens I, Van Belle Y, Gordts S. Prospective multicentre randomized controlled trial to evaluate factors influencing the success rate of office diagnostic hysteroscopy. Hum Reprod. 2005;20:258–63. doi: 10.1093/humrep/deh559. [DOI] [PubMed] [Google Scholar]
- 26.Karayalcin R, Ozcan S, Moraloglu O, Ozyer S, Mollamahmutoglu L, Batioglu S. Results of 2500 office-based diagnostic hysteroscopies before IVF. Reprod Biomed Online. 2010;20:689–93. doi: 10.1016/j.rbmo.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 27.Marshall LM, Spiegelman D, Barbieri RL, Goldman MB, Manson JE, Colditz GA, Willett WC, Hunter DJ. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967–73. doi: 10.1016/S0029-7844(97)00534-6. [DOI] [PubMed] [Google Scholar]
- 28.Hileeto D, Fadare O, Martel M, Zheng W. Age dependent association of endometrial polyps with increased risk of cancer involvement. World J Surg Oncol. 2005;3:8. doi: 10.1186/1477-7819-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bosteels J, van Wessel S, Weyers S, Broekmans FJ, D’Hooghe TM, Bongers MY, Mol BWJ. Hysteroscopy for treating subfertility associated with suspected major uterine cavity abnormalities. Cochrane Database Syst Rev. 2018;12:Cd009461. doi: 10.1002/14651858.CD009461.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perez-Medina T, Bajo-Arenas J, Salazar F, Redondo T, Sanfrutos L, Alvarez P, Engels V. Endometrial polyps and their implication in the pregnancy rates of patients undergoing intrauterine insemination: a prospective, randomized study. Hum Reprod. 2005;20:1632–5. doi: 10.1093/humrep/deh822. [DOI] [PubMed] [Google Scholar]
- 31.Cicinelli E, Resta L, Nicoletti R, Zappimbulso V, Tartagni M, Saliani N. Endometrial micropolyps at fluid hysteroscopy suggest the existence of chronic endometritis. Hum Reprod. 2005;20:1386–9. doi: 10.1093/humrep/deh779. [DOI] [PubMed] [Google Scholar]
- 32.Yanaihara A, Yorimitsu T, Motoyama H, Iwasaki S, Kawamura T. Location of endometrial polyp and pregnancy rate in infertility patients. Fertil Steril. 2008;90:180–2. doi: 10.1016/j.fertnstert.2007.05.072. [DOI] [PubMed] [Google Scholar]
- 33.Kitaya K, Matsubayashi H, Yamaguchi K, Nishiyama R, Takaya Y, Ishikawa T, Yasuo T, Yamada H. Chronic endometritis: potential cause of infertility and obstetric and neonatal complications. Am J Reprod Immunol. 2016;75:13–22. doi: 10.1111/aji.12438. [DOI] [PubMed] [Google Scholar]
- 34.Greenwood SM, Moran JJ. Chronic endometritis: morphologic and clinical observations. Obstet Gynecol. 1981;58:176–84. [PubMed] [Google Scholar]
- 35.Liu Y, Chen X, Huang J, Wang CC, Yu MY, Laird S, Li TC. Comparison of the prevalence of chronic endometritis as determined by means of different diagnostic methods in women with and without reproductive failure. Fertil Steril. 2018;109:832–9. doi: 10.1016/j.fertnstert.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi K, Tomiyama TMT, Oka C. High pregnancy rate after Hysteroscopy with Irrigation in Uterine Cavity prior to blastocyst transfer in patients who have failed to conceive after blastocyst transfer. Fertil Steril. 2000;74:206. doi: 10.1016/S0015-0282(00)01328-5. [DOI] [Google Scholar]
- 37.O’Connor BB, Pope BD, Peters MM, Ris-Stalpers C, Parker KK. The role of extracellular matrix in normal and pathological pregnancy: future applications of microphysiological systems in reproductive medicine. Exp Biol Med (Maywood) 2020;245:1163–74. doi: 10.1177/1535370220938741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caglayan E, Okyay E, Bodur T, Ertugrul C, Koyun E, Kovali M, Dogan E, Gulekli B. Hysterosalpingography prior to the gonadotropin stimulated intrauterine insemination improves clinical pregnancy rates in women with unexplained infertility. Clin Exp Obstet Gynecol. 2017;44:65–9. doi: 10.12891/ceog3217.2017. [DOI] [PubMed] [Google Scholar]
- 39.Dreyer K, van Rijswijk J, Mijatovic V, Goddijn M, Verhoeve HR, van Rooij IAJ, Hoek A, Bourdrez P, Nap AW, Rijnsaardt-Lukassen HGM, et al. Oil-based or Water-Based contrast for Hysterosalpingography in Infertile Women. N Engl J Med. 2017;376:2043–52. doi: 10.1056/NEJMoa1612337. [DOI] [PubMed] [Google Scholar]
- 40.Johnson JV, Montoya IA, Olive DL. Ethiodol oil contrast medium inhibits macrophage phagocytosis and adherence by altering membrane electronegativity and microviscosity. Fertil Steril. 1992;58:511–7. doi: 10.1016/S0015-0282(16)55254-6. [DOI] [PubMed] [Google Scholar]
- 41.Wang R, Watson A, Johnson N, Cheung K, Fitzgerald C, Mol BWJ, Mohiyiddeen L. Tubal flushing for subfertility. Cochrane Database Syst Rev. 2020;10:CD003718. doi: 10.1002/14651858.CD003718.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maheux-Lacroix S, Dodin S, Moore L, Bujold E, Lefebvre J, Bergeron ME. Preovulatory uterine flushing with saline as a treatment for unexplained infertility: a randomised controlled trial protocol. BMJ Open. 2016;6:e009897. doi: 10.1136/bmjopen-2015-009897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jansen FW, Vredevoogd CB, van Ulzen K, Hermans J, Trimbos JB, Trimbos-Kemper TC. Complications of hysteroscopy: a prospective, multicenter study. Obstet Gynecol. 2000;96:266–70. doi: 10.1016/s0029-7844(00)00865-6. [DOI] [PubMed] [Google Scholar]
- 44.Di Spiezio Sardo A, Di Carlo C, Minozzi S, Spinelli M, Pistotti V, Alviggi C, De Placido G, Nappi C, Bifulco G. Efficacy of hysteroscopy in improving reproductive outcomes of infertile couples: a systematic review and meta-analysis. Hum Reprod Update. 2016;22:479–96. doi: 10.1093/humupd/dmw008. [DOI] [PubMed] [Google Scholar]
- 45.Kamath MS, Bosteels J, D’Hooghe TM, Seshadri S, Weyers S, Mol BWJ, Broekmans FJ, Sunkara SK. Screening hysteroscopy in subfertile women and women undergoing assisted reproduction. Cochrane Database Syst Rev. 2019;4:CD012856. doi: 10.1002/14651858.CD012856.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.RPL. EGGo B, Atik R, Christiansen OB, Elson J, Kolte AM, Lewis S, Middeldorp S, McHeik S, Peramo B, Quenby S, et al. ESHRE guideline: recurrent pregnancy loss: an update in 2022. Hum Reprod Open. 2023;2023:hoad002. doi: 10.1093/hropen/hoad002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fatemi HM, Kasius JC, Timmermans A, van Disseldorp J, Fauser BC, Devroey P, Broekmans FJ. Prevalence of unsuspected uterine cavity abnormalities diagnosed by office hysteroscopy prior to in vitro fertilization. Hum Reprod. 2010;25:1959–65. doi: 10.1093/humrep/deq150. [DOI] [PubMed] [Google Scholar]
- 48.Kasius JC, Eijkemans RJ, Mol BW, Fauser BC, Fatemi HM, Broekmans FJ. Cost-effectiveness of hysteroscopy screening for infertile women. Reprod Biomed Online. 2013;26:619–26. doi: 10.1016/j.rbmo.2013.02.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material: Supplementary Figure S1. Quality assessment using the Cochrane Risk of Bias tool for randomized controlled trials
Supplementary Material: Supplementary Figure S2. Forest plot comparing outcomes between the hysteroscopy and control groups using intention-to-treat approach
Supplementary Material: Supplementary Figure S3. Forest plots after sensitivity testing by excluding Elsetohy study comparing outcomes between the hysteroscopy and control groups
Supplementary Material: Supplementary Table S1. Characteristics of the infertility condition and artificial reproductive treatment of the included RCTs
Supplementary Material: Supplementary Table S2. Comparative analysis of data pre- and post-sensitivity testing by excluding Elsetohy study
Data Availability Statement
No datasets were generated or analysed during the current study.