Abstract
RGD peptide can be found in cell adhesion and signaling proteins, such as fibronectin, vitronectin, and fibrinogen. RGD peptides' principal function is to facilitate cell adhesion by interacting with integrin receptors on the cell surface. They have been intensively researched for use in biotechnology and medicine, including incorporation into biomaterials, conjugation to medicinal molecules or nanoparticles, and labeling with imaging agents. RGD peptides can be utilized to specifically target cancer cells and the tumor vasculature by engaging with these integrins, improving drug delivery efficiency and minimizing adverse effects on healthy tissues. RGD‐functionalized drug carriers are a viable option for cancer therapy as this focused approach has demonstrated promise in the future. Writing a review on the RGD peptide can significantly influence how drugs are developed in the future by improving our understanding of the peptide, finding knowledge gaps, fostering innovation, and making drug design easier.
Keywords: cancer targeting, challenges, conjugation process, RGD peptides
1. INTRODUCTION
Three amino acids compose the structure of the RGD peptide: arginine (Arg), glycine (Gly), and aspartic acid (Asp). The RGD motif is formed by a linear arrangement of these amino acids. 1 , 2 This peptide can be found in cell adhesion and signaling proteins, such as fibronectin, vitronectin, and fibrinogen. 3 , 4 , 5 RGD peptides' principal role is to facilitate cell adhesion by binding to the integrin receptors on the cell's surface. 1 Integrins are proteins located in the transmembrane that are essential for cell signaling, motility, and survival. They participate in a variety of biological processes, such as immune response, wound healing, and angiogenesis. 5 , 6 , 7 , 8 In the timeline of RGD peptide, the RGD sequence was first identified as a crucial motif for cell adhesion in the extracellular matrix in 1984. In the 1990s, the RGD peptide began to be modified by scientists to enhance its binding affinity and selectivity to specific integrin receptors. By 1997, biomaterials were incorporated with RGD peptide for tissue engineering, promoting cell adhesion and tissue regeneration. In the early 2000s, the use of RGD peptide as a targeting ligand for drug delivery systems was explored by researchers, enabling specific delivery to cells expressing integrin receptors. In 2010, RGD peptide‐conjugated nanoparticles were developed for targeted delivery of anticancer drugs to tumor cells, showing promising results in preclinical studies. Since 2015, RGD peptide has also been utilized in the development of scaffolds and hydrogels for tissue engineering applications, facilitating cell adhesion and promoting tissue regeneration. In 2020, RGD peptide was incorporated into bioinks used in 3D bioprinting, enabling precise deposition of cells and promoting their attachment to the printed structures. They found that these integrins bind to their native ligands that contain the RGD sequence. This discovery opened up new possibilities for utilizing RGD peptides as targeting motifs in cancer treatment for both therapeutic and diagnostic purposes. The absence of intellectual property protection further fueled the development of various RGD‐based agents, including fluorescence markers, radiopharmaceuticals, drug conjugates, nanoparticles, and micelles. While RGD applications related to angiogenesis and αvβ3‐integrin have been prominent in the field of radiopharmacy, it is important to note that RGD is not limited to these specific targets. Researchers have also explored the affinity and selectivity of RGD peptides for other integrins, such as αvβ6‐integrin. The development of radiolabeled ligands targeting αvβ3‐integrin has been a significant breakthrough in radiotheranostics for cancer therapy. The future of RGD in targeted cancer therapy holds great promise. Ongoing research is focused on the continuous development of novel RGD‐based ligands and exploring the potential of integrins in therapy. RGD peptides may find applications beyond cancer therapy, including in the field of cancer immunotherapy. The complexity of integrin functions suggests that future research on RGD‐related molecules will encompass diverse areas of molecular medicine and life science. 9 , 10 , 11 , 12 , 13 , 14 , 15 RGD peptides can be modified to enhance its anticancer properties. A few Examples of RGD analogues that were used for the targeting cancer were depicted in Figure 1.
FIGURE 1.
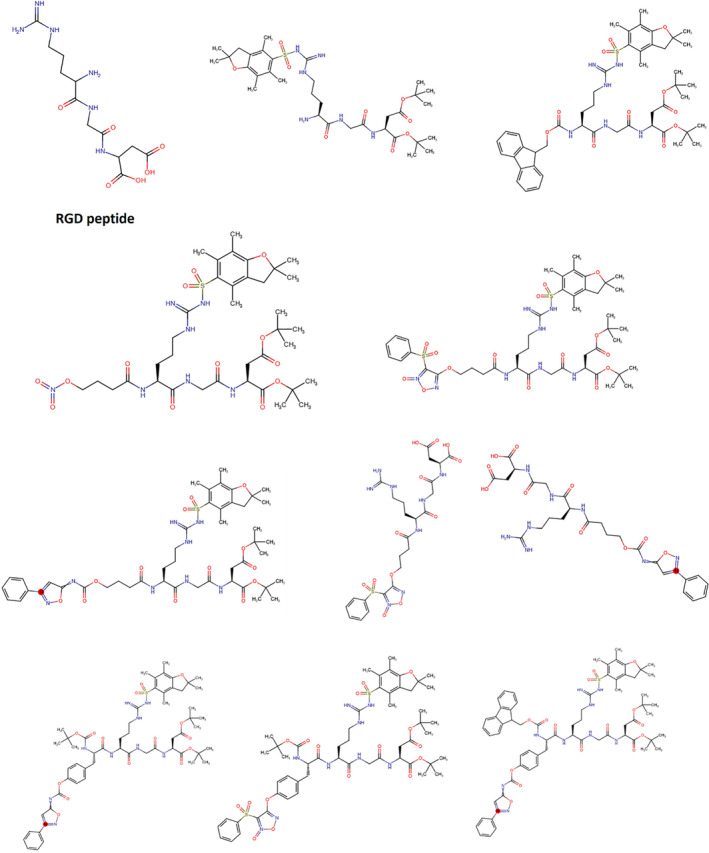
Examples of RGD analouges.
Specific integrins identify the RGD motif within proteins and bind to this peptide, allowing cell adhesion and communication. RGD peptides have been widely explored for diverse uses in medicine and biotechnology due to their ability to improve cell adhesion and target particular integrin receptors. 10 , 16 , 17 , 18 , 19 To increase cell adhesion and growth, RGD peptides can be introduced into biomaterials such as hydrogels and scaffolds. This may enhance these materials' biocompatibility and efficacy in the regeneration and repair of tissues. 20 , 21 , 22 , 23
To target particular integrin receptors that are overexpressed in illnesses such as cancer, RGD peptides can be coupled to therapeutic molecules or nanoparticles. 5 , 24 , 25 , 26 , 27 Integrins, notably αvβ3 and αvβ5, are elevated in malignant cells and vasculature. The medicine may be administered selectively to the tumor location by connecting RGD peptides to drug carriers, limiting the impact on normal cells and tissues. RGD peptides can help drug carriers get into cells by engaging with integrin receptors, which are found on the cell's surface. This receptor‐dependent endocytosis can boost the absorption of drugs in the cell, resulting in greater therapeutic effectiveness (Figure 2). 28 , 29 , 30 , 31 This is especially effective for delivering drugs with low cell permeability or that breakdown quickly in the extracellular environment. RGD‐functionalized drug carriers can be engineered to carry numerous therapeutic agents to the target area, such as chemotherapeutic medicines and genes. This can aid in the treatment of drug resistance and improve the overall therapeutic impact.
FIGURE 2.
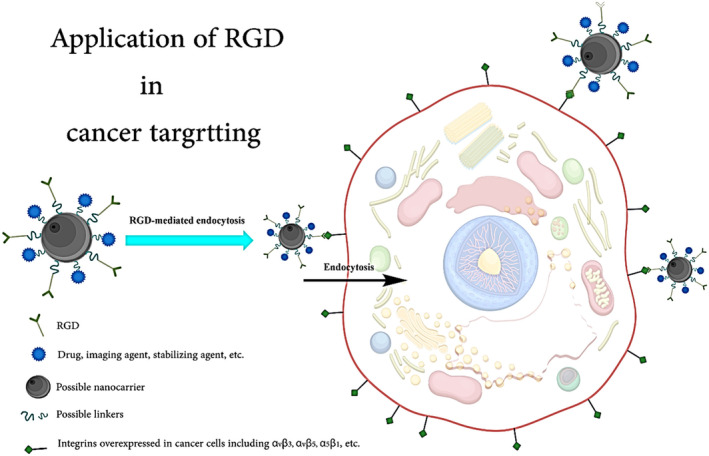
Receptor‐mediated endocytosis using RGD‐peptide drug, or carrier, etc. conjugates.
RGD‐functionalized drug carriers can be programmed to release their payload in response to external stimuli like as pH, temperature, or the presence of certain enzymes. 32 , 33 , 34 This can guarantee that the medicine is only delivered at the intended spot, reducing adverse effects, and boosting therapeutic efficacy. To observe integrin expression in animal models, RGD peptides can be tagged with imaging agents like fluorescent dyes or radiotracers. 35 , 36 , 37 This can aid in identifying and monitoring illnesses caused by aberrant integrin expressions, such as cancer and cardiovascular disease. Furthermore, RGD peptides can be combined with imaging agents to allow for real‐time monitoring of drug distribution and treatment response. This can aid in the optimization of treatment procedures and give crucial information on the efficacy of the therapy. 38 , 39 , 40 , 41 , 42
By inhibiting the interaction between integrins and their ligands, RGD peptides can be utilized alone to limit tumor development and angiogenesis. This has the potential to alter signaling pathways involved in cancer cell survival, migration, and invasion. Angiogenesis inhibition is a viable treatment option for cancer and other disorders characterized by excessive blood vessel formation. RGD peptide‐conjugated medicines can preferentially target angiogenesis‐related integrins, resulting in more effective anti‐angiogenic therapy. 43 , 44 , 45 , 46
RGD peptides, RGD peptide‐conjugated medicines, and RGD peptide‐conjugated nanoparticles/nanocarriers have considerable potential for targeted drug delivery and imaging. Their capacity to selectively target integrin‐expressing cells may result in better treatment results, fewer side effects, and enhanced disease identification and monitoring. New medications targeting the RGD peptide have the potential to enhance the lives of millions of individuals suffering from illnesses such as cancer, cardiovascular disease, and inflammatory ailments. A thorough review can aid in the consolidation of existing information regarding RGD peptides, their interactions with integrins, and their significance in many biological processes. This can serve as a good basis for researchers to find new therapeutic targets. A review of RGD peptides can have a substantial influence on future drug development by improving our understanding of the peptide, revealing knowledge gaps, promoting innovation, and aiding drug design.
2. OVEREXPRESSED INTEGRINS FOR RGD PEPTIDE‐BASED CANCER THERAPIES
A class of cell surface receptors known as integrins is essential for cell adhesion, migration, and signaling. They are heterodimeric proteins composed of two subunits, α and β, which come together to create different integrin pairs. Certain integrins are particularly appealing targets for RGD peptide‐based therapeutics because they are overexpressed on the surfaces of cancer cells and the tumor vasculature in malignancies. 15 , 47 , 48 , 49 Integrins that recognize the RGD motif particularly interact with the RGD peptide. Several cancer cell types, including melanoma, glioblastoma, and breast, prostate, and ovarian malignancies, overexpress the αvβ3 integrin. 50 , 51 , 52 , 53 , 54 Additionally, it supports angiogenesis by being abundantly expressed in the tumor vasculature. 55 , 56 , 57 RGD peptides that target the αvβ3 integrin can aid in preventing tumor angiogenesis, invasion, and proliferation. Similar to αvβ3, αvβ5 integrin is overexpressed in a variety of cancer types and contributes to tumor angiogenesis. 58 , 59 , 60 , 61 To stop the creation of new blood vessels and restrain tumor growth, RGD peptides can interfere with this integrin's function. α5β1 integrin, which is overexpressed in several malignancies including breast, lung, and colon cancers, is involved in cell adhesion and migration. 62 , 63 , 64 , 65 RGD peptides can help stop cancer cells from migrating and invading by targeting the α5β1 integrin. Numerous malignancies, such as pancreatic, lung, and colon tumors, have elevated levels of the integrin αvβ6. 36 , 66 , 67 , 68 Cancer cell invasion and metastasis include the αvβ6 integrin. It is possible to stop the spread of cancer by targeting this integrin using RGD peptides. The surface of platelets expresses the αIIbβ3 integrin, which is essential for platelet aggregation. Even while platelet aggregation is not directly connected to tumor cells, tumor cells can use it to encourage metastasis. Inhibiting platelet aggregation and lowering the risk of metastasis can be accomplished by targeting αIIbβ3 integrin with RGD peptides. 69 , 70 , 71 , 72 RGD peptides can be utilized to specifically target cancer cells and the tumor vasculature by engaging with these integrins, improving drug delivery efficiency, and minimizing adverse effects on healthy tissues. RGD‐functionalized drug carriers are a viable option for cancer therapy since this focused approach has demonstrated promise in studies.
3. RGD PEPTIDES: CHALLENGES
The potential applications of the RGD peptide in cancer targeting have garnered significant attention within the field of cancer research. The discovery of the RGD peptide's cancer targeting properties has opened up novel avenues for the precise delivery of drugs and imaging agents in cancer therapy. By linking anticancer drugs or imaging agents to the RGD peptide, cancer cells can be specifically targeted while minimizing harm to healthy cells. This targeted approach holds promise for improving the effectiveness of cancer treatments and reducing undesirable side effects. Additionally, the RGD peptide can be employed in the development of imaging agents that are specific to cancer. By attaching a radioactive or fluorescent label to the RGD peptide, it becomes feasible to visualize and detect tumors using diverse imaging techniques such as positron emission tomography (PET) or fluorescence imaging. 9 , 10 , 15 , 25 , 73 , 74 , 75 , 76
RGD peptides are derived from natural proteins, which may trigger an immune response in some patients (Table 1, Figure 3). This could lead to the production of antibodies against the RGD peptide, potentially reducing the effectiveness of the therapy or causing adverse reactions. 77 , 78 , 79 , 80 Peptides, including RGD peptides, can be susceptible to degradation by proteases in the body. This may limit their stability and reduce their effectiveness in drug delivery. 81 , 82 , 83 To overcome this issue, researchers often use modified or cyclic RGD peptides, which exhibit increased stability and resistance to protease degradation. The synthesis of RGD‐functionalized drug carriers can be complex and costly, particularly when multiple components, such as drugs, imaging agents, and stimuli‐responsive materials, are involved. 25 , 84 , 85 , 86 This may limit the widespread adoption of RGD peptide‐based drug delivery systems. Integrin expression can vary between different types of cancer and even between individual tumors of the same type. 66 , 87 This heterogeneity may affect the effectiveness of RGD peptide‐based drug delivery, as the therapy may be more effective in some patients than in others.
TABLE 1.
The possible issues, effects, and possible related solutions.
| RGD peptides | ||
|---|---|---|
| Issues | Effect | Possible solutions |
| Induction of an immune response through T‐cell activation | Production of anti‐RGD antibodies | Using modified versions of the RGD peptide that cannot activate T‐cells or are less immunogenic. |
| The immune response may affect the efficacy of RGD peptide‐based therapies | Pretreatment of patients with immunosuppressive drugs. | |
| Repeated exposure to RGD peptide | Chronic immune responses and severe adverse reactions | Functionalizing RGD peptide with biocompatible polymers or coatings to reduce immune recognition and increase the lifespan of the peptide. |
| Altered conformation of the RGD peptide | The induction of new antigenic epitopes that can provoke an immune response | Choosing a suitable delivery system that can protect the RGD peptide from immune recognition. |
| Purity and quality of the RGD peptide | Presence of contaminating antigens that can trigger an immune response | Using high‐quality RGD peptide products that meet the regulatory standards for purity and absence of contaminants. |
| Short half‐life | Rapid degradation by proteases | Conjugation to protein carriers or nanoparticles to increase stability and half‐life. |
| Low solubility | Poor bioavailability | Chemical modifications to improve solubility and reduce protease susceptibility. |
| Poor stability in biological environments | Rapid degradation | Encapsulation in liposomes or other drug delivery systems to protect against degradation. |
| Lack of target specificity | Use of site‐specific targeting approaches. | |
| High toxicity in some cases | Modification of the peptide sequence to reduce toxicity. | |
| The complexity of the peptide structure and synthesis | Develop more efficient synthetic methods using specialized reagents and devices. | |
| High synthesis cost and low yields. | Optimize the reaction parameters such as temperature, pH, and concentration to improve yields and reduce cost. | |
| Difficulties in developing scalable synthesis methods. | Implement automation techniques to reduce handling errors and increase throughput. | |
| Limited availability of amino acid building blocks. | Improve the availability and cost of amino acid building blocks by developing new synthetic routes or using alternative sources. | |
| The need for multiple purification steps to obtain high purity. | Develop novel purification techniques such as immobilized‐metal affinity chromatography or reverse‐phase high‐performance liquid chromatography to reduce the number of steps required for purification. | |
| Heterogeneity of integrin expression on different cell types and within a single cell type. | Using integrin‐specific antibodies or aptamers to capture or target specific integrin‐expressing cells. | |
| Variable affinity and selectivity of RGD peptides for different integrin subtypes. | Design and optimize RGD peptides with higher affinity and selectivity for a specific integrin subtype by modifying the peptide structure. | |
| RGD peptide internalization and degradation by cells. | Use modified RGD peptides or peptide conjugates that are resistant to degradation and can prolong integrin binding. | |
| Competition for integrin binding by other ligands in the extracellular matrix. | Combine the RGD peptide with other proteins or peptides that can selectively compete for the binding sites of integrins and enhance RGD peptide binding. | |
| Poor penetration of RGD peptides into solid tumors or tissues. | Combine the RGD peptide with nanocarriers, such as liposomes or nanoparticles, to improve delivery and penetration into target tissues. | |
FIGURE 3.
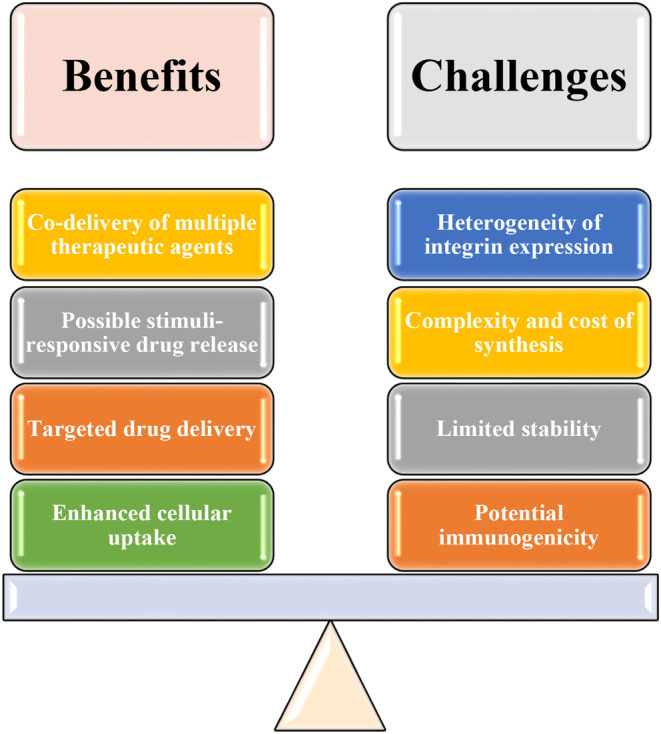
Possible benefits and challenges of RGD peptides.
4. POTENTIAL IMMUNOGENICITY
To overcome the potential immunogenicity of RGD peptides, altering the peptide structure can help reduce immunogenicity. For example, enlarging the peptide ring of c(RGDyK) by introducing an amino sequence serine‐glycine‐serine (SGS) has been shown to reduce the incidence of anaphylaxis after repeated intravenous c(RGDyKSGS)‐liposome stimulation. 79 This modification was effective in reducing the incidence of anaphylaxis post the repeated intravenous c(RGDyKSGS)‐liposome stimulation. Therefore, the introduction of the SGS sequence into c(RGDyK)‐liposomes serves as a strategy to mitigate the immunogenicity‐associated issue and enhance the safety profile of these drug delivery systems. Incorporating RGD peptides into stealth lipids, such as polyethylene glycol (PEG)‐lipids, can help reduce immunogenicity by shielding the peptide from the immune system. 88 , 89 , 90 This approach can also prolong the circulation time of the RGD peptide in the bloodstream, allowing for more effective delivery to the target site. A promising strategy for attenuating the immune response is presented when RGD peptide‐based lipids are combined with immunosuppressive agents. This approach was exemplified by the study mentioned in the provided document, where RGD‐modified lipid nanoparticles (LNPs), specifically RGD‐PEG‐lipid modified through the post‐insertion method, were utilized. This modification allowed the nanocarrier system to be preferentially targeted toward integrin‐expressing tumor endothelial cells (TECs). In a recent study, an immunosuppressive agent, aPD‐1 monoclonal antibody (mAb), was employed. The programmed cell death protein 1 (PD‐1) receptor, a key regulator of T‐cell responses, was inhibited by this antibody, thereby enhancing antitumor immune responses. By combining RGD‐modified LNPs with aPD‐1 mAb and siVegfr2 (small interfering RNA against Vegfr2), a reduction in tumor‐infiltrating lymphocytes (TILs), indicative of immune response mitigation, was achieved. Furthermore, vascular normalization was induced, and tumor growth was suppressed through this combination approach. It is worth noting that RGD‐PEG‐lipid was the lipid utilized in the study, and aPD‐1 mAb was the specific immunosuppressive agent employed. This strategy, which involves the utilization of RGD peptide‐based lipids and immunosuppressive agents, aligns with the broader goal of improving the efficacy of gene therapy while mitigating potential adverse immune reactions. It thus represents a promising avenue for further exploration in cancer treatment. 91 , 92 This approach can be particularly useful when the RGD peptide is used in combination with other therapeutic agents, such as small molecules or antibodies. 10 , 93 , 94 , 95 The choice of delivery system can have a significant impact on the immunogenicity of RGD peptides, which may include liposomes, nanoparticles, and micelles, etc. It is needed to find the most suitable option for reducing immunogenicity while maintaining therapeutic efficacy. 96 , 97 , 98 , 99 Considering the diversity of major histocompatibility complex (MHC) alleles in different populations and races, designing personalized peptides based on an individual's MHC profile can help reduce the risk of immunogenicity. 100 It is essential to carefully evaluate each approach's benefits and drawbacks to determine the most suitable method for a specific application.
5. LIMITED STABILITY
The RGD peptide's poor solution stability can be overcome by cyclizing the linear RGD peptide. 101 , 102 It can improve its stability greatly by lowering conformational flexibility and making it less sensitive to proteolysis. A covalent link is formed between two amino acids in the linear sequence to accomplish cyclization. Multiple copies of RGD sequences incorporated into a single molecule can boost their affinity for integrin receptors, compensating for lower individual binding affinities. Multimeric structures incorporating surface‐bound RGD peptides, such as dendrimers or nanoparticles, have shown increased biological activity. The addition of polyethylene glycol (PEG) chains to the RGD peptide improves its solubility, decreases immunogenicity, and extends its circulation duration in vivo. 103 , 104 , 105 , 106 PEGylation protects peptides from enzymatic breakdown while retaining bioactivity. By embedding or conjugating RGD peptides onto biomaterial surfaces such as scaffolds, controlled release over time can be achieved which could enhance local concentration at local sites. 105 , 106 , 107 Co‐administration or conjugation with serpins may protect the peptide against early hydrolysis by inhibiting the breakdown of enzymes. 108 , 109 Serpins, also known as serine protease inhibitors, are a superfamily of proteins that play a crucial role in regulating protease activity. They are characterized by a conserved structure and mechanism of action. Serpins inhibit serine proteases by forming a covalent complex with the protease, leading to its inactivation. This interaction involves a reactive center loop (RCL) within the serpin molecule that acts as a bait for the protease. Serpins are involved in various physiological processes, including blood clotting, immune response, and inflammation. They also have implications in diseases such as cancer, thrombosis, and neurodegenerative disorders. 109 Chemical changes such as N‐methylation or D‐amino acid substitution to particular amino acids within an RGD motif may assist increase resistance to enzymatic cleavage without reducing receptor affinity. Non‐peptide analogs or peptidomimetics that replicate the structure and function of the RGD peptide can improve stability, bioavailability, and potency. When compared to endogenous peptides, these molecules are generally less vulnerable to proteolysis. 110 , 111 , 112 It is feasible to overcome the restricted stability of RGD peptides while retaining their biological activity for diverse therapeutic purposes by using these tactics singly or in combination.
6. COMPLEXITY AND COST OF SYNTHESIS CHALLENGES OF RGD PEPTIDE
Due to the necessity for specialized modifications or multimeric structures, the synthesis of RGD peptides can be complicated, time‐consuming, and expensive. To address these obstacles, new RGD motifs with simpler structures or more accessible synthetic methods may provide equal biological activity while lowering synthesis complexity and expense. 4 , 25 , 113 , 114
Solid‐phase peptide synthesis (SPPS) provides for the effective step‐by‐step assembly of amino acids on a solid platform, eliminating purification stages and enhancing total yield. Automated SPPS synthesizers reduce manual effort and human error even further. Adjusting reaction parameters such as temperature, solvent choice, coupling reagents, or protecting groups may increase peptide synthesis efficiency and prevent side reactions that cause contaminants. Continuous improvement in production procedures through optimization studies will increase process efficiency, resulting in less waste, higher product quality, and lower cost per unit. Scaling up production processes by investing in larger reactors/fermenters will result in lower cost per unit product as fixed expenses are dispersed over a larger number of units produced. 115 , 116 , 117 , 118
For longer or more complex RGD‐containing peptides or proteins, chemical ligation techniques like native chemical ligation (NCL) enable the convergent assembly of multiple smaller peptide fragments with greater synthetic accessibility. 119 , 120 , 121 , 122 Producing RGD peptides using recombinant DNA technology in bacterial (e.g., E.coli), yeast (e.g., Pichia pastoris), insect cells (e.g., baculovirus system), or mammalian cell cultures can provide higher yields compared to traditional chemical synthesis methods at lower costs per batch. 123 , 124 , 125 , 126 , 127 Efficient bioconjugation strategies such as click chemistry allow for site‐specific attachment of functional moieties like PEG chains without requiring additional protection/deprotection steps during peptide synthesis. 38 , 128 , 129 , 130
By adopting these strategies individually or in combination, it is possible to overcome the complexity and cost challenges associated with the synthesis of RGD peptides without compromising their therapeutic potential.
7. HETEROGENEITY OF INTEGRIN EXPRESSION CHALLENGE OF RGD PEPTIDE
Heterogeneous expression of integrins on different cell types and tissues can impact the targeting specificity and therapeutic efficacy of RGD peptides. To overcome this challenge, design RGD peptide derivatives that selectively target specific integrin subtypes overexpressed in pathological conditions such as cancer or inflammation. 131 This can be achieved by modifying amino acid sequences, incorporating additional functional groups, or using peptidomimetics.
The application of targeted extracellular matrix (ECM)‐derived peptides for promoting neovascularization in a rodent model of myocardial infarction is the focus of the document. One specific approach mentioned in the document is the conjugation of targeting moieties, such as antibodies or aptamers, to RGD peptide constructs. The interaction of the RGD peptide sequence with integrin receptors on the surface of cells is well‐known. By conjugating targeting moieties, such as antibodies or aptamers, to RGD peptide constructs, the specificity of the peptides toward cells with co‐expression patterns related to disease conditions is increased. This results in an increased likelihood of the peptides binding to and interacting with cells specifically involved in the disease process, such as cells in the infarcted area of the heart. The reduction of off‐target effects on normal cells is achieved by increasing the specificity of the peptides. This is important as it minimizes any potential negative impact on healthy cells and tissues. The conjugation of targeting moieties to RGD peptide constructs enables a more precise and targeted delivery of the peptides to the desired cells, thereby enhancing their therapeutic potential. Hence, a promising strategy for improving the specificity and effectiveness of ECM‐derived peptides in promoting neovascularization in the context of myocardial infarction is offered by the approach of conjugating targeting moieties to RGD peptide constructs. Conjugating other targeting moieties (e.g., antibodies, aptamers) to RGD peptide construct increases specificity toward cells with co‐expression patterns related to disease conditions while reducing off‐target effects on normal cells. 132 Developing stimuli‐responsive drug delivery systems is also helpful because they can release their cargo only under certain physiological/pathological conditions like acidic tumor microenvironments or enzymatic cleavage by proteases specifically upregulated in diseased tissue. 31 , 133 , 134 , 135 Nanoparticles and nanocarriers could utilize engineered surface‐bound RGD motifs for enhanced cellular uptake via receptor‐mediated endocytosis while carrying a therapeutic payload within the core/shell matrix. 134 , 136 Designing prodrugs that are activated upon binding to a specific integrin subtype through enzymatic cleavage or conformational changes, etc. resulting from receptor‐ligand interaction can release an active RGD molecule at targeted sites. 137 , 138 , 139 A combination of RGD peptide‐based therapies with other treatments like chemotherapy agents or immune checkpoint inhibitors could enhance treatment efficacy while potentially mitigating resistance mechanisms associated with single‐agent therapy. 26 , 140 , 141 , 142
Identifying biomarkers indicative of high integrin expression levels corresponding to likely responders allows for better patient stratification and personalized treatment approaches. Determining the optimal dosing, frequency, and routes of administration would maximize therapeutic efficacy while minimizing off‐target effects on normal tissues with lower integrin expression levels. 142 , 143 , 144 , 145 Utilizing imaging techniques like PET or MRI in conjunction with RGD‐based probes for visualizing changes in integrin expression during therapy allows early identification of responders and facilitates timely adjustments to the treatment plan if necessary. 53 , 146 , 147 , 148 By employing these strategies individually or in combination, it is possible to overcome challenges posed by heterogeneous integrin expression when using RGD peptides for targeted therapies while maximizing their clinical potential.
8. SOME AVAILABLE RGD‐BASED DRUGS
RGD peptides are short peptide fragments derived from the amino acid sequence of several extracellular matrix proteins, such as fibrinogen, fibronectin, vitronectin, collagen, and laminin. They are widely present in fibronectin, laminin, fibrinogen, osteopontin, and vitronectin.
RGD can be divided into two types:
RGD: This is a tripeptide sequence of RGD.
RGD polypeptide: This is a functional peptide containing RGD.
Depending on the application and the integrin targeted, RGD can be chemically modified or replaced by a similar peptide which promotes cell adhesion. For example, RGD peptides can be cyclized, or made into a cyclic compound, via disulfide, thioether, or rigid aromatic ring linkers. This leads to an increase in binding affinity and selectivity for integrin αVβ3 relative to αIIBβ3.
Another type of RGD‐derived peptide is the “internalizing RGD” or “iRGD.” This peptide has the ability to recognize the integrin receptor on the cancer cell surface like its ancestor with an additional outstanding feature to penetrate to extravascular space of tumor and ability to penetrate to cancer cells unlike the original peptide. 149 , 150 , 151 , 152 , 153 , 154
Cilengitide is a cyclic RGD peptide that targets αvβ3 and αvβ5 integrins (Figure 4). It has been investigated as a potential treatment for glioblastoma, a type of brain cancer. Cilengitide has shown promising results in preclinical studies (Table 2), but its efficacy in clinical trials has been limited. One disadvantage of cilengitide is its potential immunogenicity, which may reduce its effectiveness in some patients. 71 , 155 , 156 , 157 Eptifibatide is also a cyclic RGD peptide that targets αIIbβ3 integrin, which is involved in platelet aggregation. Eptifibatide is used as an antiplatelet agent to prevent blood clots in patients with acute coronary syndrome or undergoing percutaneous coronary intervention. One advantage of eptifibatide is its rapid onset of action, which can help prevent thrombotic events. 158 , 159 , 160 , 161 Abegrin is a cyclic RGD peptide that αvβ3 integrin, which is overexpressed in several types of cancer. Abegrin has been investigated as a potential treatment for various cancers, including breast cancer and pancreatic cancer. The associated clinical trials can be found in Table 3. One advantage of Abegrin is its high specificity for αvβ3 integrin, which can improve the efficacy of the therapy. 9 , 162 , 163 , 164
FIGURE 4.
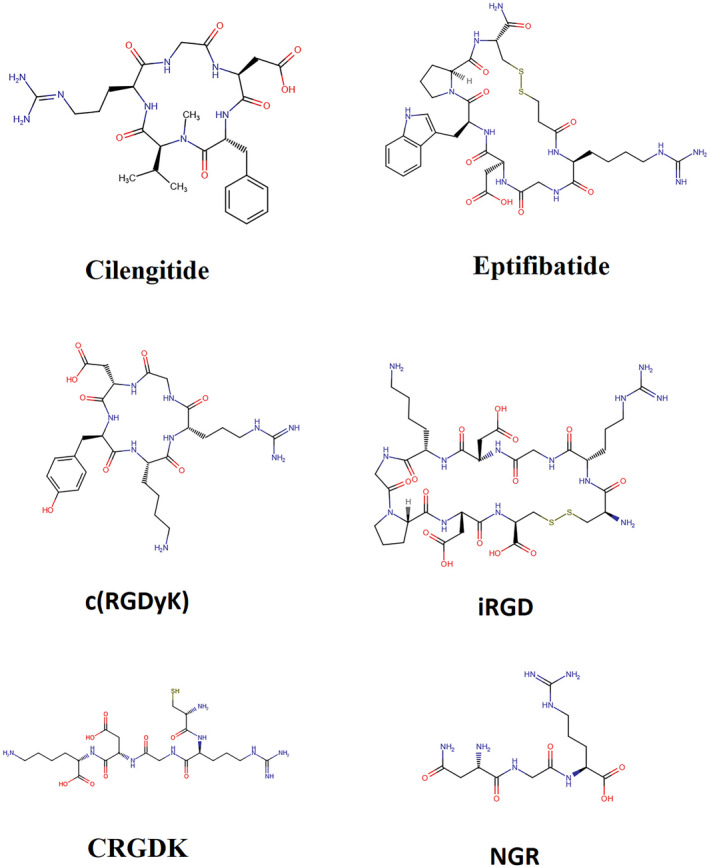
Stucture of some RGD‐peptides.
TABLE 2.
Cilengitide in preclinical studies.
| NCT number | Study title | Study status | Brief summary | Study Results | Phases | Start Date | Completion Date |
|---|---|---|---|---|---|---|---|
| NCT00842712 | Cilengitide and Cetuximab in Combination With Platinum‐based Chemotherapy as First‐line Treatment for Subjects With Advanced Non Small Cell Lung Cancer (NSCLC) | Completed |
Primary objective of the study's safety run‐in: ‐ To determine the maximum tolerated dose (MTD) of cilengitide in combination with cetuximab, and platinum‐based chemotherapy (cisplatin/vinorelbine or cisplatin/gemcitabine). Primary objective of the study's randomization part: ‐ To assess the efficacy of cilengitide in combination with cetuximab and platinum‐based chemotherapy (cisplatin/vinorelbine or cisplatin/gemcitabine) compared to cetuximab and platinum‐based chemotherapy alone in terms of progression‐free survival (PFS) time. Study design and plan: This is a multicenter, open‐label, randomized, and controlled phase II study with a safety run‐in part in subjects with advanced non‐small cell lung cancer (NSCLC). During the safety run‐in, the regimen was intensified stepwise by cohort (cilengitide intravenous \[iv\] 1000 milligram \[mg\] to 2000 mg twice a week) in a classical 3 + 3 subjects (for each platinum‐based chemotherapy regimens separately) approach with predefined dose‐ and schedule reduction rules. In the safety run‐in 12 subjects were included and evaluated for safety and feasibility of different escalating doses of cilengitide administered twice weekly in combination with cetuximab, cisplatin and vinorelbine or gemcitabine. After completion of the safety run‐in, the randomized part will be started, during which all subjects will receive cetuximab and platinum‐based chemotherapy (cisplatin/vinorelbine or cisplatin/gemcitabine). Subjects will be centrally randomized on a 1:1 basis to either Group A or C; Group B will be closed with implementation of Amendment No. 4 (dated 20 December 2010): • Group A: Cilengitide 2000 mg once weekly (Days 1, 8, and 15 of every 3‐week chemotherapy cycle) in combination with cetuximab and platinum‐based chemotherapy that will consist of the following: * Cetuximab once weekly (Days 1, 8, and 15), plus cisplatin on Day 1 and vinorelbine on Days 1 and 8 of every 3‐week chemotherapy cycle, or * Cetuximab once weekly (Days 1, 8, and 15), plus cisplatin on Day 1 and gemcitabine on Days 1 and 8 of every 3‐week chemotherapy cycle. The decision which of the 2 chemotherapy regimens will be applied for a given subject is at the discretion of the treating investigator. • Group B: Cilengitide 2000 mg twice weekly (Days 1, 4, 8, 11, 15, and 18 of every 3‐week chemotherapy cycle) in combination with cetuximab and platinum‐based chemotherapy as described for Group A. Group B will be closed with implementation of Amendment No. 4 (global, dated December 20, 2010). Subjects randomized to Group B before implementation of Amendment No 4 will continue to be treated as planned. • Group C: cetuximab and platinum‐based chemotherapy as described for Group A Chemotherapy will be given until radiographically documented progressive disease (PD) or unacceptable toxicity but for no more than 6 cycles. Cilengitide and cetuximab will be given until radiographically documented PD or unacceptable toxicity. Randomization will be performed centrally using an interactive voice/web response system (IXRS). A stratified block randomization procedure will be employed using chosen first‐line chemotherapy (cisplatin/vinorelbine vs. cisplatin/gemcitabine) as stratification criterion. |
YES | PHASE2 | 2009–02 | 2013–07 |
| NCT00077155 | Cilengitide (EMD 121974) in Treating Patients With Advanced Solid Tumors or Lymphoma | Completed | This Phase I trial is studying the side effects and best dose of EMD 121974 in treating patients with solid tumors or lymphoma. Cilengitide (EMD 121974) may stop the growth of cancer cells by stopping blood flow to the cancer. | NO | PHASE1 | 2003–12 | |
| NCT00112866 | Cilengitide in Treating Patients Who Are Undergoing Surgery for Recurrent or Progressive Glioblastoma Multiforme | Terminated | Cilengitide may stop the growth of glioblastoma multiforme by blocking blood flow to the tumor. Giving cilengitide before and after surgery may be an effective treatment for glioblastoma multiforme. This Phase II trial is studying how well cilengitide works in treating patients who are undergoing surgery for recurrent or progressive glioblastoma multiforme. | YES | PHASE2 | 2005–01 | 2009–03 |
| NCT01122888 | Cilengitide and Sunitinib Malate in Treating Patients With Advanced Solid Tumors or Glioblastoma Multiforme | Terminated | This clinical trial is studying how well giving cilengitide together with sunitinib malate works in treating patients with advanced solid tumors or glioblastoma multiforme. Cilengitide and sunitinib malate may stop the growth of tumor cells by blocking blood flow to the tumor. Giving cilengitide together with sunitinib malate may kill more tumor cells. Studying samples of blood in the laboratory from patients receiving cilengitide and sunitinib malate may help doctors understand the effect of these drugs on biomarkers. | NO | PHASE1 | 2009–12 | 2015–04 |
| NCT00085254 | Cilengitide, Temozolomide, and Radiation Therapy in Treating Patients With Newly Diagnosed Glioblastoma Multiforme | Completed | Cilengitide may stop the growth of cancer by stopping blood flow to the tumor. Drugs used in chemotherapy, such as temozolomide, work in different ways to stop the growth of tumor cells, either by killing the cells or by stopping them from dividing. Radiation therapy uses high‐energy x‐rays to damage tumor cells. Giving cilengitide together with temozolomide and radiation therapy may kill more tumor cells. This randomized phase I/II trial is studying the side effects and best dose of cilengitide when given together with temozolomide and radiation therapy and to compare how well they work in treating patients with newly diagnosed glioblastoma multiforme. | YES | PHASE1|PHASE2 | 2005–04 | 2012–11 |
| NCT00082875 | Cilengitide in Treating Patients With Unresectable or Metastatic Melanoma | Terminated | This randomized phase II trial is studying how well cilengitide works in treating patients with unresectable Stage III or Stage IV melanoma. Cilengitide may stop the growth of melanoma by stopping blood flow to the tumor. | NO | PHASE2 | 2004–03 | |
| NCT00813943 | Cilengitide, Temozolomide, and Radiation Therapy in Treating Patients With Newly Diagnosed Glioblastoma and Unmethylated Gene Promoter Status | Completed |
CORE is a Phase 2 clinical trial in newly diagnosed glioblastoma in subjects with an unmethylated O6‐methylguanine‐deoxyribonucleic acid methyltransferase (MGMT) gene promoter in the tumor tissue. The MGMT gene promoter is a section of deoxyribonucleic acid (DNA) that acts as a controlling element in the expression of MGMT. Methylation of the MGMT gene promoter has been found to appear to be a predictive marker for benefit from temozolomide (TMZ) treatment. In a safety run‐in period in dedicated study centers, the safety and tolerability of cilengitide given as an intense treatment in combination with the first part of standard therapy will be assessed. Thereafter the trial will investigate the overall survival and progression‐free survival in subjects receiving two different regimens of cilengitide in combination with standard treatment versus standard treatment alone. |
YES | PHASE2 | 2009–03 | 2013–08 |
| NCT00103337 | Cilengitide in Treating Patients With Metastatic Prostate Cancer | Completed | This randomized phase II trial is studying how well cilengitide works in treating patients with metastatic prostate cancer. Cilengitide may stop the growth of prostate cancer by blocking blood flow to the tumor. | NO | PHASE2 | 2005–01 | |
| NCT00006222 | EMD 121974 in Treating Patients With HIV‐Related Kaposi's Sarcoma | Terminated | Phase I trial to study the effectiveness of EMD 121974 in treating patients who have HIV‐related Kaposi's sarcoma. EMD 121974 may stop the growth of Kaposi's sarcoma by stopping blood flow to the tumor. | NO | PHASE1 | 2000–09 | 2001–03 |
| NCT01558687 | Cilengitide Imaging Trial in Glioblastoma | Terminated |
The main purpose of this clinical trial is to find out if cilengitide has an effect on brain tumor cells but also particularly on the blood vessels supplying the tumor with nutrient and oxygen in patients newly diagnosed with non‐resectable (inoperable) glioblastoma. In addition, this clinical trial will investigate if the addition of cilengitide in combination with standard treatment prolongs life in patients with non‐resectable glioblastoma. Similarly, the duration of response of the cancer to this treatment and the side effects of the therapy will be analyzed. Furthermore, additional data on how the body deals with this substance will be collected (this is called pharmacokinetics or pharmacokinetic (PK) analysis). In this clinical trial the investigators would also like to learn more about the disease and the response to the experimental medication by measuring certain “markers.” This imaging trial will investigate the biological effects of cilengitide monotherapy on the tumor microvascular function and tumor viability in a homogenous non‐pretreated subject population with newly diagnosed gliobastoma (GBM). The purpose of this clinical trial is to study the effect that cilengitide may have on certain markers of cancer in your tumor and/or blood and to learn if there are any disease‐related markers that could help in predicting how subjects respond to the administration of cilengitide. The investigators anticipate that approximately 30 subjects will participate in this clinical trial. The clinical trial will be conducted in approximately 4 medical centers in the following countries: Germany, Poland, and Switzerland. The investigators anticipate the clinical trial will last until the end of 2013. Your participation in the trial may last up to 86 weeks. |
NO | PHASE1 | 2012–08 | 2013–02 |
| NCT01165333 | Cilengitide in Combination With Irradiation in Children With Diffuse Intrinsic Pontine Glioma | Completed | The aim of the study is to determine the safety of cilengitide in combination with radiation therapy. | NO | PHASE1 | 2010–08 | 2015–03 |
| NCT00705016 | Cilengitide in Recurrent and/or Metastatic Squamous Cell Carcinoma of the Head and Neck (SCCHN) | Completed |
The purpose of this open‐label, randomized, controlled, Phase 1/2 study of the integrin inhibitor cilengitide is to evaluate the safety and efficacy of the combination of different regimens of cilengitide added to cisplatin, 5‐fluorouracil (5‐FU), and cetuximab in participants with recurrent/metastatic squamous cell carcinoma of the head and neck (SCCHN). The Phase 1 part was conducted in dedicated study centers. In the Phase 2 part of this trial, cilengitide is administered at two different doses to two experimental groups. The third group will only receive cisplatin, 5‐FU and cetuximab. In the Phase 1 part of this trial, the dose of cilengitide in combination with cisplatin, 5‐FU, and cetuximab was determined. Cilengitide is an experimental anticancer substance interacting with so‐called integrins. Integrins are protein molecules that are known to be present on the surface of certain cancer cells. Integrins are also found on certain cells that belong to growing blood vessels (endothelial cells). Integrins potentially facilitate the blood vessels' support of the tumor (angiogenesis) as well as the tumor's growth and further spread throughout the body (metastasis). By inhibiting integrins on the tumor cell surface, cilengitide potentially kills cancer cells, and potentially sensitizes cancer cells to other co‐administered therapeutics. By inhibiting integrins on the endothelial cell surface, it potentially inhibits the ingrowth of additional blood vessels toward the tumor. Cilengitide is given as an intravenous infusion (given by a drip in one vein of your arm). If any unacceptable side effect occurs, treatment with the study drug will be stopped. |
YES | PHASE1|PHASE2 | 2008–10 | 2013–06 |
| NCT01044225 | Effect of Radiation Therapy Plus Temozolomide Combined With Cilengitide or Cetuximab on the 1‐year Overall Survival of Patients With Newly Diagnosed MGMT‐promoter Unmethylated Glioblastoma | Terminated | The investigators propose to conduct a multicenter, open‐label, randomized, phase II study in patients with newly diagnosed glioblastoma (CeCil). Patients should meet all eligibility criteria for the CENTRIC phase III trial at the exception that no MGMT‐promoter methylation could be demonstrated. The treatment backbone in both study arms will consist of postoperative radiation therapy with concomitant daily temozolomide, followed by 6 cycles of temozolomide according to a 21 out of 28 days regimen (as in the experimental arm of the RTOG 0525/EORTC 26052–22,053 phase III study). In study arm (A) cilengitide (at a dose of 2000 mg by iv administration, 2x/week) will be added to this backbone while in the second study arm (B), cetuximab will be added (at an initial dose of 400 mg/mÂ2 administered by intravenous infusion over 2 hours and followed by a weekly dose of 250 mg/mÂ2 iv over 1 h). In both study arms, treatment will be administered for 52 consecutive treatment weeks. The 1 year overall survival (1y‐OS) following randomization will serve as the primary endpoint in both study arms. | NO | PHASE2 | 2009–09 | 2011–09 |
| NCT00689221 | Cilengitide, Temozolomide, and Radiation Therapy in Treating Patients With Newly Diagnosed Glioblastoma and Methylated Gene Promoter Status | Completed |
CENTRIC is a Phase 3 clinical trial assessing efficacy and safety of the investigational integrin inhibitor, cilengitide, in combination with standard treatment versus standard treatment alone in newly diagnosed glioblastoma subjects with a methylated O6‐methylguanine‐deoxyribonucleic acid methyltransferase (MGMT) gene promoter in the tumor tissue. The MGMT gene promoter is a section of deoxyribonucleic acid (DNA) that acts as a controlling element in the expression of MGMT. Methylation of the MGMT gene promoter has been found to be a predictive marker for benefit from temozolomide (TMZ) treatment. |
YES | PHASE3 | 2008–09 | 2013–08 |
| NCT00121238 | Cilengitide in Treating Patients With Prostate Cancer | Completed | This phase II trial is studying how well cilengitide works in treating patients with prostate cancer. Cilengitide may stop the growth of prostate cancer by blocking blood flow to the tumor | YES | PHASE2 | 2005–01 | 2015–11 |
| NCT00089388 | Cilengitide in Treating Patients With Acute Myeloid Leukemia | Terminated | This randomized phase II trial is studying how well cilengitide works in treating patients with acute myeloid leukemia. Cilengitide may stop the growth of cancer cells by blocking the enzymes necessary for their growth | NO | PHASE2 | 2004–07 | |
| NCT00979862 | Cediranib Maleate and Cilengitide in Treating Patients With Progressive or Recurrent Glioblastoma | Completed | This Phase I trial is studying the side effects and best dose of cediranib maleate when given together with cilengitide in treating patients with progressive or recurrent glioblastoma. Cediranib maleate and cilengitide may stop the growth of tumor cells by blocking blood flow to the tumor. Giving cediranib maleate together with cilengitide may kill more tumor cells. | NO | PHASE1 | 2010–03 | 2014–02 |
| NCT00679354 | Cilengitide in Treating Younger Patients With Recurrent or Progressive High‐Grade Glioma That Has Not Responded to Standard Therapy | Completed | This Phase II trial studies how well cilengitide works in treating younger patients with recurrent or progressive high‐grade glioma that has not responded to standard therapy. Cilengitide may stop the growth of tumor cells by blocking blood flow to the tumor. | YES | PHASE2 | 2008–06 | 2011–07 |
| NCT00006093 | EMD 121974 in Treating Patients With Progressive or Recurrent Glioma | Completed |
RATIONALE: EMD 121974 may stop the growth of cancer by stopping blood flow to the tumor. PURPOSE: Phase I/II trial to study the effectiveness of EMD 121974 in treating patients who have progressive or recurrent malignant glioma. |
NO | PHASE1|PHASE2 | 2000–09 | 2006–10 |
| NCT00063973 | Cilengitide in Treating Children With Refractory Primary Brain Tumors | Completed | This phase I trial is studying the side effects and best dose of cilengitide in treating children with recurrent, progressive, or refractory primary CNS tumors. Cilengitide may slow the growth of brain cancer cells by stopping blood flow to the tumor. | NO | PHASE1 | 2003–07 | |
| NCT00022113 | EMD 121974 in Treating Patients With Advanced Solid Tumors | Completed | Phase I trial to study the effectiveness of EMD 121974 in treating patients who have advanced solid tumors. EMD 121974 may slow the growth of solid tumors by stopping blood flow to the tumor | NO | PHASE1 | 2001–05 | |
| NCT01276496 | Weekly Doses of Cilengitide and Paclitaxel in Treating Patients With Advanced Solid Tumors That Cannot Be Removed by Surgery | Completed | This Phase I trial studies the side effects and the best dose of cilengitide when given together with paclitaxel weekly in treating patients with solid tumors that have spread nearby or to other areas of the body and cannot be removed by surgery. Cilengitide may stop the growth of tumor cells by blocking blood flow to the tumor. Drugs used in chemotherapy, such as paclitaxel, work in different ways to the stop the growth of tumor cells, either by killing the cells or by stopping them from dividing. Giving cilengitide together with paclitaxel may kill more tumor cells. | NO | PHASE1 | 2010–12 | |
| NCT00093964 | Cilengitide (EMD 121974) for Recurrent Glioblastoma Multiforme (Brain Tumor) | Completed | This study will investigate clinical activity, safety, and tolerability of the antiangiogenic compound cilengitide (EMD 121974) in the treatment of first recurrence of glioblastoma multiforme (GBM). | YES | PHASE2 | ######## | ######## |
| NCT01118676 | Cilengitide Together With Radiochemotherapy in Patients With Locally Advanced Non Small Cell Lung Cancer | Completed |
This is a two‐center study which includes 24 patients maximum on 36 months: 24 months accrual ‐ 12 months follow‐up. Eligible patients are included according to a standard 3 + 3 design. Patients included in the trial will be treated with a combination of radiochemotherapy (standard radiotherapy of 66 Gy, 2 Gy per daily fraction, and cisplatin and vinorelbine‐based chemotherapy). Cilengitide will be administered alone as continuous infusion two weeks before the radiochemotherapy and will then be continued during radiochemotherapy as continuous infusion. The dose levels investigated will be applied to the continuous administration (a maximum of 4 dose levels). After the end of concomitant radiochemotherapy, cilengitide will be administered i.v. at a dose of 2000 mg twice weekly until the end of chemotherapy. The dose of Cilengitide administered after radiotherapy will not be increased. 4 dose levels are defined:12, 18, 27 et 40 mg/hour. |
NO | PHASE1 | 2010–03 | 2015–04 |
| NCT00884598 | Cilengitide and Whole‐Brain Radiation Therapy in Treating Patients With Brain Metastases From Lung Cancer | Unknown |
RATIONALE: Cilengitide may stop the growth of brain metastases by blocking blood flow to the tumor. Radiation therapy uses high energy x‐rays to kill tumor cells. Giving cilengitide together with radiation therapy may kill more tumor cells. PURPOSE: This Phase I trial is studying the side effects and best dose of cilengitide when given together with whole‐brain radiation therapy in treating patients with brain metastases from lung cancer. |
NO | PHASE1 | 2008–12 | 2011–12 |
| NCT01124240 | Temozolomide and Procarbazine With Cilengitide for Patients With Glioblastoma Multiforme Without Methylation of the MGMT Promoter Gene | Unknown |
Cilengitide 2000 mg flat iv twice weekly is administered over a period of 18 months without interruption. Starting one week after the initiation of Cilengitide, RTX (60 Gy, 2 Gy per fraction) with concurrent daily temozolomide (60 mg/m2 p.o.) and daily procarbazine (PCB, 50 mg p.o. if BSA\ < 1.7; 100 mg p.o. if BSA 1.7) is given over a period of 6 weeks (RTX Monday to Friday, both TMZ and PCB 7 days a week). After a break of 4 weeks, adjuvant TMZ (50 mg/m2 p.o in first cycle, 60 mg/m2 p.o. in subsequent cycles) and PCB (50 mg p.o. if BSA \ < 1.7; 100 mg p.o. if BSA 1.7) are then given daily D1 to 20. This TMZ/PCB cycle is repeated every 28 days over a total period of 6 cycles. |
NO | PHASE2 | 2009–11 | 2014–01 |
| NCT00004258 | EMD 121974 in Treating Patients With Locally Advanced or Metastatic Cancer | Completed |
RATIONALE: EMD 121974 may stop the growth of cancer by stopping blood flow to the tumor. PURPOSE: Phase I trial to study the effectiveness of EMD 121974 in treating patients who have locally advanced or metastatic cancer. |
NO | PHASE1 | 1999–12 | 2001–09 |
| NCT01782976 | Ph II Cilengitide Plus Bevacizumab for Recurrent Glioblastoma (GBM) | Withdrawn |
The goal of this clinical research study is to learn if cilengitide given in combination with bevacizumab can help to control glioblastoma. The safety of this drug combination will also be studied. Cilengitide is designed to block the flow of blood to cancer cells, which may help to slow or block the growth of cancer. Bevacizumab is designed to block the growth of new blood vessels, which may help to slow or block the growth of cancer. |
NO | PHASE2 | 2013–06 | |
| NCT01517776 | Cilengitide and Metronomic Temozolomide for Relapsed or Refractory High Grade Gliomas or Diffuse Intrinsic Pontine Gliomas in Children and Adolescents | Terminated |
The primary objective of this study is to evaluate the efficacy of a combined treatment with cilengitide and metronomic oral temozolomide as measured by 6 months overall survival (OS) after diagnosis of relapse or tumor progression in children and adolescents with relapsed or refractory high‐grade malignant glioma and diffuse intrinsic pontine glioma. Secondary objectives include: 1. To evaluate the safety and toxicity of the study treatment by common toxicity criteria (CTC; version 4.0). 2. To assess * the response rates at 6 months (continuous complete response = CCR, complete response = CR, partial response = PR, stable disease = SD, progressive disease = PD) and * progression‐free survival (PFS) at 6 months, and * response rates, OS, and PFS at 12 months after relapse diagnosis or diagnosis of tumor progression. Response will be presented including histopathological variants. 3. To assess the pharmacokinetics of cilengitide administered as part of the study treatment. Indication and study population for this trial: Treatment of relapsed or refractory high grade gliomas and diffuse intrinsic pontine gliomas in pediatric patients 3 years and\<18 years of age. Patients included in the study receive * Cilengitide 1800 mg/mÂ2 iv twice weekly * Temozolomide 75 mg/mÂ2/d p.o. for 6 weeks, followed by 1 week rest with a mandatory platelet‐count dependent dose adaptation rule: mandatory blood counts twice weekly: Platelets stop temozolomide until platelet recovery * Study treatment in the individual patient is scheduled for 1 year unless tumor progression or excessive toxicity occurs. However, study treatment may be extended beyond 1 year upon individual decision. |
NO | PHASE2 | 2012–01 | 2014–04 |
TABLE 3.
Abegrin in clinical trials.
| NCT number | Study title | Study status | Brief summary | Study Results | Phases | Start Date | Completion Date |
|---|---|---|---|---|---|---|---|
| NCT00049712 | Monoclonal Antibody Therapy in Treating Patients With Refractory Advanced Solid Tumors or Lymphoma | Completed |
RATIONALE: Monoclonal antibodies can locate cancer cells and either kill them or deliver cancer‐killing substances to them without harming normal cells. PURPOSE: Phase I trial to study the effectiveness of monoclonal antibody therapy in treating patients who have refractory advanced solid tumors or lymphoma. |
NO | PHASE1 | 2002–10 | 2006–02 |
| NCT00263783 | Phase I Trial of Weekly MEDI‐522 in Patients With Refractory Solid Tumors | Completed | To determine the safety profile of single and multiple doses of MEDI522 in patients with refractory solid tumors. | NO | PHASE1 | 2001–03 | 2002–06 |
| NCT00284817 | Phase I Study of MEDI522 in Patients With Irinotecan‐Refractory Advanced Colorectal Cancer | Completed | #NAME? | NO | PHASE1|PHASE2 | 2001–07 | 2005–05 |
| NCT00684996 | Bevacizumab With or Without MEDI‐522 in Treating Patients With Unresectable or Metastatic Kidney Cancer | Terminated | This Phase I/randomized Phase II trial is studying the side effects and best dose of bevacizumab and to see how well it works when given together with or without MEDI‐522 in treating patients with unresectable or metastatic kidney cancer. Monoclonal antibodies, such as bevacizumab and MEDI‐522, can block tumor growth in different ways. Some block the ability of tumor cells to grow and spread. Others find tumor cells and help kill them or carry tumor‐killing substances to them. Bevacizumab and MEDI‐522 may also stop the growth of tumor cells by blocking blood flow to the tumor. It is not yet known whether bevacizumab is more effective when given together with or without MEDI‐522 in treating kidney cancer. | YES | PHASE1|PHASE2 | 2008–06 | 2010–10 |
| NCT00111696 | Study of the Tumor Saturation and Biological Activity of MEDI‐522 (Abergrin) in Patients With Advanced Malignant Melanoma | Completed | To describe the tumor tissue saturation by MEDI‐522 in patients with advanced malignant melanoma. | NO | PHASE1 | 2005–05 | 2007–11 |
| NCT00066196 | Evaluating The Antitumor Activity Of MEDI‐522 With Or Without Dacarbazine In Patients With Metastatic Melanoma | Completed |
The primary objectives of this study are: * To explore the antitumor activity of MEDI‐522 Â ± DTIC in patients with metastatic melanoma. * To determine the safety of MEDI‐522 Â ± DTIC in this patient population. |
NO | PHASE2 | 2003–08 | 2007–06 |
| NCT00027729 | Monoclonal Antibody Therapy in Treating Patients With Advanced Colorectal Cancer | Completed |
RATIONALE: Monoclonal antibodies can locate tumor cells and either kill them or deliver tumor‐killing substances to them without harming normal cells. PURPOSE: Phase I/II trial to study the effectiveness of monoclonal antibody therapy in treating patients who have advanced colorectal cancer that has not responded to irinotecan. |
NO | PHASE1|PHASE2 | 2001–06 | 2004–11 |
| NCT00072930 | MEDI‐522 in the Treatment of Patients With Metastatic Androgen‐Independent Prostate Cancer | Completed |
The primary objectives of this study are: 1. To explore the antitumor activity of MEDI‐522 in combination with docetaxel, prednisone, and zoledronic acid in patients with metastatic Androgen‐Independent Prostate Cancer (AIPC); and 2. To summarize the safety of MEDI‐522 in combination with docetaxel, prednisone, and zoledronic acid in this patient population. |
NO | PHASE2 | 2003–12 | 2007–06 |
9. RGD‐BASED DRUGS IN CLINICAL TRIALS
The clinical studies focus on various types of cancer, including ovarian cancer, lung cancer, head and neck cancer, glioblastoma, non‐small cell lung cancer, cervical cancer, and others. Each study aims to evaluate the specific application of the drugs in the context of these cancer types. The studies utilize different imaging techniques, such as PET/CT (positron emission tomography/computed tomography) and PET/MRI (positron emission tomography/magnetic resonance imaging), to assess tumor characteristics, angiogenesis, and response to treatment. These imaging techniques provide valuable information about the distribution and activity of the drugs within the body. Some studies have secondary objectives, such as evaluating immunologic responses, determining potential toxicities, assessing the predictive value of imaging techniques, and correlating imaging parameters with clinical treatment response (Table 4).
TABLE 4.
RGD peptides in clinical trials.
| NCT number | Study title | Study status | Brief summary | Study Results | Phases | Start Date | Completion Date |
|---|---|---|---|---|---|---|---|
| NCT03393689 | RGD PET/MRI in Sporadic Vestibular Schwannoma | Unknown | The aim of this non‐randomized, prospective study is to investigate the applicability and prognostic value of angiogenesis PET/MR with the radioligand 68Ga‐NODAGA‐ E\[c(RGDyK)\]2 in patients with sporadic vestibular schwannomas. | NO | PHASE2 | 1/2/2018 | 1/2/2021 |
| NCT00562003 | Safety Study of a Genetically Modified Adenovirus in Ovarian Cancer Patients | Completed |
The primary purpose of this study is to determine the maximally tolerated dose and spectrum of toxicities encountered with intraperitoneal delivery of a RGD modified conditionally replicative adenovirus (Ad5‐Delta 24RGD) in patients with recurrent ovarian cancer. Secondary objectives: * To determine the biologic effects encountered with intraperitoneal delivery of Ad5‐Delta 24RGD in patients with recurrent ovarian cancer cells. * To determine immunologic response generated against Ad5‐Delta 24RGD when administered intraperitoneally to patients with recurrent ovarian adenocarcinoma. * To determine potential clinical activity of Ad5‐Delta 24RGD when administered intraperitoneally to patients with recurrent ovarian adenocarcinoma. |
NO | PHASE1 | 2007–06 | 2010–06 |
| NCT01806675 | 18F‐FPPRGD2 PET/CT or PET/MRI in Predicting Early Response in Patients With Cancer Receiving Anti‐Angiogenesis Therapy | Completed | The purpose of the study is to conduct research of a new PET radiopharmaceutical in cancer patients. The uptake of the novel radiopharmaceutical 18F‐FPPRGD2 will be assessed in study participants with glioblastoma multiforme (GBM), gynecological cancers, and renal cell carcinoma (RCC) who are receiving antiangiogenesis treatment. | YES | PHASE1|PHASE2 | 3/4/2013 | 2019–04 |
| NCT05515783 | 68Ga‐FAP‐RGD PET/CT: Dosimetry and Preliminary Clinical Translational Studies | Recruiting | As an new dual targeting PET radiotracer, 68Ga‐FAP‐RGD is promising as an excellent imaging agent applicable to various cancers. In this study, we observed the safety, biodistribution and radiation dosimetry of 68Ga‐FAP‐RGD in patients with various types of cancer and compared them with the results of 68Ga‐FAPI‐02 or 18F‐FDG imaging to evaluate the dosimetric characteristics and diagnostic efficacy of 68Ga‐FAP‐RGD. | NO | PHASE1|PHASE2 | 5/1/2022 | 5/1/2024 |
| NCT05976607 | Clinical Study of 18F ‐FAPI‐RGD in Renal Tumor | Not yet recruiting | The goal of this observational study is to learn about the value of 18F‐FAPI‐RGD PET/CT imaging in renal tumor. Participants will undergo clinical evaluation and 18F‐FAPI‐RGD PET/CT examination. | NO | 2023–08 | 2024–02 | |
| NCT04222543 | Imaging of Tumor Microenvironment in Patients With Oropharyngeal Head and Neck Squamous Cell Carcinoma Using RGD PET/CT Imaging | Unknown | Known risk factors inducing squamous cell carcinomas of the head and neck are tobacco and alcohol intake. However, the incidence of human papillomavirus (HPV)‐related oropharyngeal carcinomas is increasing. It is known that HPV+ and HPV‐ tumors have a different reaction to (chemo)radiotherapy. The exact mechanisms underlying these differences is not yet known but might be caused by changes in vascularity. Therefore the vasculature is imaged with the help of a study specific Gallium‐68‐DOTA‐(RGD)2 PET/CT scan and a CT perfusion scan. | NO | PHASE2 | 11/22/2019 | 9/1/2023 |
| NCT05543954 | 68Ga‐FAPI‐RGD PET/CT Imaging in the Lung Cancer Patients | Recruiting | Based on the high expression of specific receptors on the surface of diseased tissues and neovascularization, noninvasive targeted molecular imaging can be used to visualize lesions in vitro by combining specific ligands labeled with short half‐life isotopes. Lung cancer tissues express fibroblast activating protein FAP, and also have high expression of integrin Î ± VÎ23 receptor on the surface of blood vessels. In this study, a novel dual‐target imaging agent 68Ga‐FAPI‐RGD was used for PET/CT imaging of lung cancer. | NO | EARLY_PHASE1 | 9/3/2022 | 12/31/2023 |
| NCT02749019 | Dual Integrin Î ± vÎ23 and GRPR Targeting PET Imaging in Breast Cancer Patients | Unknown | This is an open‐label positron emission tomography/computed tomography (PET/CT) study to investigate the diagnostic performance and evaluation efficacy of 68Ga‐NOTA‐BBN‐RGD in breast cancer patients. A single dose of 111–148 Mega‐Becquerel (MBq) 68Ga‐NOTA‐BBN‐RGD will be injected intravenously. Visual and semiquantitative method will be used to assess the PET/CT images. | NO | PHASE1 | 2015–07 | |
| NCT05013086 | 177Lu‐AB‐3PRGD2 in Patients With Non Small Cell Lung Cancer | Unknown | This is an open‐label, non‐controlled, non‐randomized study to assess the safety and measure image‐based absorbed dose of 177Lu‐AB‐3PRGD2 in patients with non‐small cell lung cancer (NSCLC) who will undergo radioligand therapy using 177Lu‐AB‐3PRGD. | NO | EARLY_PHASE1 | 10/1/2021 | 6/1/2023 |
| NCT00565721 | A Proof‐of‐concept Study to Assess the Ability of [18F]AH‐111585 PET Imaging to Detect Tumours and Angiogenesis | Completed |
This proof‐of‐concept study is designed to assess the ability of \[18F\]AH‐111585 PET imaging to detect tumors and angiogenesis. Up to 30 evaluable subjects are planned to be included at up to two study centers in the United States. Subjects are considered evaluable if they undergo administration of AH‐111585 (18F) Injection, dynamic and static PET imaging, and tumor tissue acquisition. The targeted population is adult subjects at initial diagnosis or recurrence with tumors ≥2.5 cm in diameter who are scheduled to undergo resection or biopsy of the tumor as a result of routine clinical treatment. The tumors must belong to one of the following five types: * High‐grade glioma, including glioblastoma multiforme, anaplastic astrocytoma, and anaplastic oligodendroglioma * Lung cancer, including small cell lung cancer and non‐small cell lung cancer * Head and neck (H\&N) tumors, including laryngeal squamous cell carcinoma, well‐differentiated thyroid and oral cavity carcinoma * Sarcoma * Melanoma Safety will be assessed from the rates of adverse events, changes in vital signs, changes in electrocardiogram (ECG) parameters, changes in physical examination findings, and changes in clinical laboratory findings. Efficacy will be assessed as the correlations between parameters derived from the PET images and the reference standards. The reference standards will be immunohistology for Î ± vÎ23 integrins and other biomarkers specific for oncology and angiogenesis and from the standard of care imaging. Measures obtained from optional DCE‐CT imaging may also be used to compare the uptake and retention of \[18F\]AH‐111585 in tumors obtained from the dynamic PET to assess functional status of the vascular system of the tumor. |
YES | PHASE2 | 2007–11 | 2012–09 |
| NCT01492192 | RGD‐PET‐CT in Cancer Angiogenesis | Terminated | The goal of this clinical research study is to look at two new methods of scanning and see whether they can help researchers predict which tumors will respond to drugs that attack tumor blood supply. | NO | PHASE2 | 2013–05 | 2015–09 |
| NCT02325349 | PET/CT Imaging of Angiogenesis in Lung or Head and Neck Cancers Prior or During Chemotherapy With Antiangiogenic Agents | Terminated |
The primary objective of this Phase II study is to evaluate the use of labeled RGD ligand in PET/CT to predict and/or to early assess the efficacy of chemotherapy including an agent with antiangiogenic effect. The predictive value of this approach will be determined by independent assessors on basis of data at the end of the treatment: RECIST 1.1 criteria for CT or MRI, PERCIST criteria for FDG PET/CT, clinical, endoscopic and histological findings. |
NO | PHASE2 | 3/20/2015 | 9/20/2018 |
| NCT02481726 | 68Ga‐AlfatideII for the Differential Diagnosis of Lung Cancer and Lung Tuberculosis by PET/CT | Completed | Comparison of 68Ga‐AlfatideII and 18F‐FDG in differential diagnosis effectiveness toward the solitary pulmonary nodules of lung cancer or tuberculosis. | NO | PHASE1|PHASE2 | 2014–03 | |
| NCT01582516 | Safety Study of Replication‐competent Adenovirus (Delta‐24‐rgd) in Patients With Recurrent Glioblastoma | Completed |
In the Netherlands a two‐center investigator‐driven phase I/II clinical trial is initiated in June 2010 testing the oncolytic adenovirus Delta24‐RGD to treat glioblastoma patients. The virus is administrated using convection‐enhanced delivery by four catheters as delivery technique, targeting solid tumor as well as infiltrated tumor cells within the peri‐tumoral brain. Patients will be enrolled in cohorts of 3 per dose‐level. The dose levels to be explored are: 10\^7, 10\^8, 10\^9, 10\^10, 3\*10\^10 and 10\^11 viral particles (vp). Once the MTD has been determined, or the study has reached the highest dose cohort, a further 6 or 9 patients will be enrolled at the MTD and evaluated for safety and preliminary signs of efficacy, such that in total at least 12 patients have received the MTD. The primary objective is to determine the safety and tolerability of Delta‐24‐RGD administered by CED to the tumor and the surrounding infiltrated brain in patients with recurrent GBM. Secondary objectives are to determine the progression‐free survival (PFS), overall survival (OS), and tumor response rate in patients with recurring tumors amenable for surgical resection and treated at the MTD. Cerebrospinal fluid as well as brain interstitial fluid by microdialysis next to the routinely collected samples of blood at various timepoints before, during and after virus infusion. Various neurodegenerative biomarkers as well as markers of immune response will be assessed in these samples. Furthermore extensive sampling and PCR analyses will be performed to evaluate distribution and shedding of the virus. |
NO | PHASE1|PHASE2 | 2010–06 | 2014–12 |
| NCT05543317 | 68Ga‐FAPI‐RGD PET/CT for Dual Integrin Î ± vÎ23 and FAP‐targeted Imaging in Patients With Various Types of Cancer and Compared With 18F‐FDG | Completed | As a new dual receptor (integrin Î ± vÎ23 and FAP) targeting PET radiotracer, 68Ga‐FAPI‐RGD is promising as an excellent imaging agent applicable to various cancers. In this research, we investigate the safety, biodistribution and radiation dosimetry of 68Ga‐FAPI‐RGD in healthy volunteers. Moreover, we evaluate the potential usefulness of 68Ga‐FAPI‐RGD positron emission tomography/computed tomography (PET/CT) for the diagnosis of primary and metastatic lesions in various types of cancer, and compared with 18F‐FDG PET/CT. | NO | NA | 7/1/2022 | 12/31/2022 |
| NCT02798406 | Combination Adenovirus + Pembrolizumab to Trigger Immune Virus Effects | Completed |
Glioblastoma (GBM) and gliosarcoma (GS) are the most common and aggressive forms of malignant brain tumor in adults and can be resistant to conventional therapies. The purpose of this Phase II study is to evaluate how well a recurrent glioblastoma or gliosarcoma tumor responds to one injection of DNX‐2401, a genetically modified oncolytic adenovirus, when delivered directly into the tumor followed by the administration of intravenous pembrolizumab (an immune checkpoint inhibitor) given every 3 weeks for up to 2 years or until disease progression. Funding Source‐FDA OOPD |
NO | PHASE2 | 10/6/2016 | 6/30/2021 |
| NCT02747290 | 68Ga‐NOTA‐BBN‐RGD PET/CT in Prostate Cancer Patients | Unknown | This is an open‐label positron emission tomography/computed tomography (PET/CT) study to investigate the diagnostic performance and evaluation efficacy of 68Ga‐NOTA‐BBN‐RGD in prostate cancer patients. A single dose of 111–148 Mega‐Becquerel (MBq) 68Ga‐NOTA‐BBN‐RGD will be injected intravenously. Visual and semiquantitative method will be used to assess the PET/CT images. | NO | EARLY_PHASE1 | 2014–07 | |
| NCT02817945 | Dual SSTR2 and Integrin Î ± vÎ23 Targeting PET/CT Imaging | Recruiting | This is an open‐label positron emission tomography/computed tomography (PET/CT) study to investigate the diagnostic performance and evaluate the efficacy of 68Ga‐NOTA‐3PTATE‐RGD in lung cancer patients and neuroendocrine neoplam patients. A single dose of 111–185 Mega‐Becquerel (MBq) 68Ga‐NOTA‐3P‐TATE‐RGD will be injected intravenously. Visual and semiquantitative method will be used to assess the PET/CT images. | NO | EARLY_PHASE1 | 6/1/2016 | 12/31/2022 |
| NCT05976620 | Clinical Study of 18F‐FAPI‐RGD in Breast Tumors | Not yet recruiting | The goal of this observational study is to learn about the value of 18F‐FAPI‐RGD PET/CT imaging in breast tumors. Participants will undergo clinical evaluation and 18F‐FAPI‐RGD PET/CT examination. | NO | 2023–08 | 2024–02 | |
| NCT02490891 | Study of the Angiogenesis by PET/CT in Patients With Lymphoma | Unknown | The aim of the study is to measure tumoral angiogenesis modifications by RGD‐K5 PET/CT before and after two cycles of chemotherapy in patients with lymphoma and a large tumoral mass | NO | PHASE2 | 2015–11 | 2020–05 |
| NCT02317393 | Contribution of the Imaging to the Expression of intégrines Î ± vÎ23 for the Characterization of Residual Masses of Non‐seminoma Tumors at the End of Chemotherapy | Completed |
The purpose of this study is to evaluate the contribution of the imaging to the expression of intégrines Î ± vÎ23 for the characterization of the residual masses of non‐seminoma tumors at the end of chemotherapy. The investigators hope that the results of this first stage of the clinical trial come to consolidate the preclinical results obtained by the investigators team to characterizing the interest and the strong contribution of the use of a tracer resting on the expression of Î ± vÎ23 integrine for the diagnosis of simple necrosed mass at the end of the treatment of a non‐seminoma tumor, so allowing to defer a surgery to about 40% of the patients. |
NO | PHASE2 | 2014–12 | 2020–03 |
| NCT03655977 | Radiation Therapy Planning by Multi‐parametric PET/MRI Imaging in Patients With Cervical Cancer | Unknown | The main goal of this project is to evaluate the potential and feasibility of hybrid PET/MRI functional imaging to non‐invasively measure tumor characteristics for radiation therapy planning (RT) for cervical cancer. It will be assessed how the complementary information of tumor characteristics can contributed to better understanding of tumor delineation. Another endpoint of this study is to evaluate a new PET‐tracer (68Ga‐NODAGA‐ E\[c(RGDyK)\]2) enabling imaging of tumor‐angiogenesis. | NO | NA | 9/1/2018 | 9/1/2021 |
| NCT02197169 | DNX‐2401 With Interferon Gamma (IFN‐Î3) for Recurrent Glioblastoma or Gliosarcoma Brain Tumors | Completed | Glioblastoma (GBM) and gliosarcoma (GS) are the most common and aggressive forms of malignant primary brain tumor in adults and can be resistant to conventional therapies. The purpose of this Phase Ib study is to evaluate how well a recurrent glioblastoma or gliosarcoma tumor responds to one injection of DNX‐2401, a genetically modified, conditionally replicative and oncolytic human‐derived adenovirus. DNX‐2401 is delivered directly into the tumor where it may establish an active infection by replicating in and killing tumor cells. | NO | PHASE1 | 9/11/2014 | 3/15/2018 |
| NCT03384511 | The Use of 18F‐ALF‐NOTA‐PRGD2 PET/CT Scan to Predict the Efficacy and Adverse Events of Apatinib in Malignancies. | Completed |
This is an open‐label, single‐arm study to explore whether 18F‐ALF‐NOTA‐PRGD2 PET/CT scan can predict the efficacy and adverse events of apatinib in patients with malignancies. Integrin Î ± vÎ23 has been shown to play an important role in angiogenesis and up‐regulated obviously in various types of tumor cells and activated endothelial cells. The arginine‐glycine‐aspartic acid (RGD) tripeptide sequence can bind to integrin Î ± vÎ23 with high affinity and specificity. The 18F‐ALF‐NOTA‐PRGD2 will highly combine with Î ± vÎ23, and thus will monitor the antiangiogenic status. In the current study, investigators propose to evaluate the feasibility of 18F‐RGD PET/CT in monitoring efficacy and adverse events of apatinib in malignancies. |
NO | PHASE4 | 9/30/2016 | 1/28/2018 |
| NCT00805376 | DNX‐2401 (Formerly Known as Delta‐24‐RGD‐4C) for Recurrent Malignant Gliomas | Completed | The goal of this clinical research study is to find the highest tolerable dose of DNX‐2401 that can be injected directly into brain tumors and into the surrounding brain tissue where tumor cells can multiply. A second goal is to study how the new drug DNX‐2401 affects brain tumor cells and the body in general. | NO | PHASE1 | 2009–02 | 2015–02 |
| NCT00743353 | Exploratory, Phase 0 Study of Positron Emission Tomography (PET) Imaging Agent, F‐18 RGD‐K5 | Completed | The purpose of this research study is to get information from volunteers without cancer and patients with cancer who have received a new investigational study agent called, “\[F‐18\] RGDK5,” to evaluate biodistribution and dosimetry for the study agent and determine F‐18 RGD‐K5 uptake in angiogenic tumor. the system. | NO | EARLY_PHASE1 | 2008–08 | 2009–01 |
| NCT04191460 | Fluorescence‐guided Surgery Using cRGD‐ZW800‐1 in Oral Cancer | Recruiting | This is a two‐staged clinical trial to investigate the feasibility of intraoperative fluorescence imaging (FLI) to adequately assess tumor margins in patients with oral cancer using cRGD‐ZW800‐1. | NO | PHASE2 | 7/12/2022 | 3/1/2025 |
| NCT01956734 | Virus DNX2401 and Temozolomide in Recurrent Glioblastoma | Completed |
Phase I trial, unicentric, uncontrolled. Intratumoral injection or intramural (into the resected tumor cavity) of DNX2401 into brain tissue will be followed by up to two 28days cycles of oral temozolomide (TMZ) in schedule of 7 days on/7 days off to evaluate safety of the combination. Completion of two full cycles of TMZ will be dependent upon tolerance and toxicity. The rationale in using the virus with chemotherapy begins with the lessons learned in many clinical trials in glioblastoma (GBM) about both the great difficulty of treating this disease with monotherapy and the limitations of the therapeutic virus. The best clinical results in recent years have been achieved with combinations of multiple therapeutics efforts, including, maximum resection and chemotherapy, immunotherapy, and targeted therapies. There are very strong preclinical data about the synergy of DNX‐2401 and TMZ proposed in our trial design. The dose‐dense schemes of TMZ like the one we will use, have been developed with the aim to saturate o6‐methylguanine‐DNA‐methyltransferase (MGMT). The published results to date have shown reasonable toxicity albeit with modest efficacy’ these schemes are now in Phase III trials. In addition, autophagy triggered by TMZ could help viral replication in the tumor cells 11. The last argument in favor of this virus + TMZ combination is the proved efficacy in killing GBM tumor stem cells. In vitro and animals models have shown this combination is much more effective that any of the treatments alone against GBM stem cells and the tumors derived from them. |
NO | PHASE1 | 2013–09 | 2017–03 |
| NCT05549024 | 68Ga‐RM26‐RGD PET/CT Imaging in the GRPR and Î ± vÎ23 Positive Tumor Patients | Recruiting | Based on the high expression of specific receptors on the surface of diseased tissues and neovascularization, noninvasive targeted molecular imaging can be used to visualize lesions in vitro by combining specific ligands labeled with short half‐life isotopes. In this study, a novel dual‐target imaging agent 68Ga‐RM26‐RGD was used for clinical study of tumor PET/CT imaging to further verify its clinical application value. | NO | EARLY_PHASE1 | 8/16/2022 | 12/31/2023 |
| NCT04233476 | A Study of 99mTc‐3PRGD2 Injection in Lung Cancer Patient | Completed |
The study drug Technetium \[99mTc\] Hydrazinonicotinamide PEGylated Bicyclic RGD Peptide Injection(99mTc‐3PRGD2) of this study is a novel radioactive diagnostic preparation for clinical use as a nuclear medicine molecular probe for tumor SPECT/CT imaging. After 99mTc‐3PRGD2 is injected into the body, it is specifically taken up by integrin receptor‐positive tumor tissue, and the image of tumor tissue can be obtained by SPECT/CT, This can be used for molecular imaging diagnosis and individualized treatment of common tumors. The primary objective of this study was to evaluate the efficacy of 99mTc‐3PRGD2 for the diagnosis of lymph node metastasis in lung tumors. The minor objective was to evaluate the efficacy of 99mTc‐3PRGD2 in the differential diagnosis of benign and malignant lung tumors and the safety of 99mTc‐3PRGD2 in vivo of humans. |
NO | PHASE3 | 10/12/2019 | 5/8/2021 |
| NCT01447134 | RGD‐K5 in Head and Neck Cancer Patients | Unknown |
1. Primary endpoint(s): To determine the relationship between the drug distribution and angiogenesis in head and neck cancer patients. 2. Secondary endpoint(s): To expand the safety database of \[F‐18\]RGD‐K5 and to correlate the parameters from the image study to clinical treatment response and prognosis. |
NO | 2011–06 | 2014–06 | |
| NCT00988936 | Efficacy Study of [F‐18]RGD‐K5 Positron Emission Tomography (PET) as a Tool to Monitor Response to an Anti‐angiogenic Drug | COMPLETED |
A pilot Phase II study The primary objective for this study is * To explore the usefulness of \[F‐18\]RGD‐K5 PET/CT to predict efficacy or early response to Avastin® (the anti‐angiogenesis drug) plus chemotherapy treatment before the full course of treatment is completed The secondary objectives for this study are: * To continue safety evaluation by collection of safety data from all patients * To gain experience with \[F‐18\]RGD‐K5 PET/CT in order to improve the study design and conduct of future studies Design: An open label, non‐randomized, uncontrolled, single group assignment, pilot efficacy study Duration: Screening visit (3–4 h), pre‐treatment imaging visit of \[F‐18\]RGD‐K5 PET/CT (\ ~ 3–4 h) and the standard \[F‐18\]FDG PET/CT (\ ~ 3–4 h) or diagnostic CT, followed by two \[F‐18\]RGD‐K5 PET/CT scans, one after the second but before the third Avastin® treatment, and one after the fourth but before the fifth Avastin® treatment, and a follow‐up standard \[F‐18\]FDG PET (\ ~ 3–4 h or diagnostic CT. Procedures: Informed consent, collection of demographic information, medical history, blood labs, physical examination, vital signs, ECGs, three sets of \[F‐18\]RGD‐K5 dosing and imaging scans including pretreatment, early mid‐treatment, and later mid‐treatment, concomitant medication collection, adverse event monitoring, and assessment of tumor response to treatment Patients: Approximately forty 38 patients with non‐squamous non‐small cell lung cancer, metastatic breast cancer, metastatic colon or rectum cancer who will receive chemotherapy plus Avastin®. This allows for approximately 30 evaluable patients to complete this study at approximately four to eight sites internationally |
NO | PHASE2 | 2009–09 | 2012–05 |
RGD‐based radiopharmaceuticals are designed to specifically target integrin αvβ3 receptors. These receptors are proteins found on the surface of cells, including cancer cells and activated endothelial cells involved in angiogenesis. The RGD sequence has a specific structure that allows it to bind to these receptors. To enable imaging of tumor angiogenesis, the RGD‐based radiopharmaceuticals are labeled with short half‐life isotopes like 68Ga and 99mTc (Table 4). These isotopes emit positrons (in the case of 68Ga) or gamma rays (in the case of 99mTc). PET/CT or SPECT/CT imaging techniques are used to detect and capture these emissions. By administering the radiopharmaceuticals to patients and performing PET/CT or SPECT/CT scans, it becomes possible to visualize and quantify the extent and characteristics of tumor angiogenesis. This imaging approach provides valuable information for various aspects of cancer management. First, it aids in cancer diagnosis by providing detailed images that can help identify the presence and location of tumors. It can also assist in staging, which involves determining the extent and spread of the disease. Furthermore, this imaging technique is useful in treatment planning. By visualizing tumor angiogenesis, healthcare professionals can better understand the blood supply to the tumor and make informed decisions regarding treatment strategies. It can help determine the most appropriate treatment approach, such as the use of chemotherapy, radiation therapy, or targeted therapies. Additionally, molecular imaging of tumor angiogenesis using RGD‐based radiopharmaceuticals allows for the monitoring of therapeutic response. By comparing images taken before and after treatment, healthcare professionals can assess the effectiveness of the chosen treatment and make adjustments if necessary. Hence, RGD‐based radiopharmaceuticals, labeled with short half‐life isotopes like 68Ga and 99mTc, target integrin αvβ3 receptors. This enables molecular imaging of tumor angiogenesis using PET/CT or SPECT/CT imaging techniques. By visualizing and quantifying the extent and characteristics of tumor angiogenesis, this imaging approach provides valuable information for cancer diagnosis, staging, treatment planning, and monitoring of therapeutic response. It offers insights into the biology and behavior of tumors, aiding in cancer management and treatment decision‐making.
Additionally, conditionally replicative adenoviruses (CRAds) are a type of adenovirus that have been genetically modified to selectively replicate in cancer cells while sparing normal cells. This targeted replication within cancer cells can lead to their destruction and potentially provide a therapeutic benefit. One specific type of CRAd mentioned in Table 4 is Ad5‐Delta 24RGD. This CRAd is designed to infect and replicate within cancer cells that overexpress integrin αvβ3 receptors, which are commonly found on the surface of tumor cells. By targeting these receptors, Ad5‐Delta 24RGD can selectively infect and replicate within cancer cells, leading to their destruction. Another CRAd mentioned is DNX‐2401. This CRAd is being evaluated in clinical trials for the treatment of glioblastoma, a type of aggressive brain cancer. DNX‐2401 is delivered directly into the tumor, either through intratumoral injection or into the resected tumor cavity. Once inside the tumor, DNX‐2401 can replicate and spread, targeting and destroying cancer cells. In addition to glioblastoma, CRAds are also being studied in various types of cancer, including ovarian cancer. Clinical trials are being conducted to evaluate the safety, tolerability, and efficacy of CRAds in these cancer types. These trials aim to determine the appropriate dosing, administration routes, and potential side effects of CRAds in order to assess their therapeutic potential. By selectively targeting cancer cells and replicating within them, CRAds have the potential to provide a targeted and effective treatment approach for various types of cancer. However, it is important to note that clinical trials are still ongoing, and further research is needed to fully understand the safety and efficacy of CRAds in different cancer types.
Apatinib is an antiangiogenic drug that selectively inhibits vascular endothelial growth factor receptor 2 (VEGFR‐2). VEGFR‐2 is a protein that plays a crucial role in angiogenesis, the process by which new blood vessels are formed to supply nutrients and oxygen to tumors.
By specifically targeting and blocking VEGFR‐2, apatinib disrupts the signaling pathway that promotes angiogenesis. This inhibition of angiogenesis can help to starve tumors of their blood supply, potentially slowing down their growth and progression. Clinical studies are currently being conducted to evaluate the efficacy and safety of apatinib in patients with different malignancies. These studies aim to assess the drug's ability to inhibit angiogenesis and its impact on tumor growth and patient outcomes. In the context of molecular imaging, 18F‐ALF‐NOTA‐PRGD2 PET/CT scans are being used in these clinical studies to predict the response to apatinib treatment. PRGD2 is a peptide that specifically binds to integrin αvβ3 receptors, which are overexpressed in tumor‐associated blood vessels. By labeling PRGD2 with the radioactive isotope 18F and performing PET/CT scans, researchers can visualize and quantify the expression of integrin αvβ3 receptors in tumors. These PET/CT scans provide valuable information about the extent and characteristics of tumor angiogenesis, which can help predict the response to apatinib treatment. By assessing the level of integrin αvβ3 receptor expression, researchers can gain insights into the tumor's angiogenic activity and its potential sensitivity to apatinib therapy (Table 4).
10. OTHER PEPTIDES THAT CAN COMPETE WITH RGD
The RGD peptide is one of the most commonly used peptides for targeted cancer therapy. The RGD peptide has a high affinity for integrin receptors, which are overexpressed on the surface of many cells. Other peptides can compete with RGD for integrin receptor binding and have been investigated for targeted cancer therapy. examples of peptides that have been investigated for targeted cancer therapy include the iRGD peptide, which can penetrate tumor tissue and enhance drug delivery, 165 , 166 , 167 and the CRGDK homing peptide, which has a high affinity for αvβ3 integrin. 168 , 169 , 170 However, the RGD peptide remains one of the most widely studied and utilized peptides for targeted cancer therapy due to its high affinity for integrin receptors and its ability to selectively target tumor cells (Table 5).
TABLE 5.
The application of different pedtides in therapies.
| Peptide's type | Applications | Explanation |
|---|---|---|
| RGD peptide | ||
| Specificity | RGD peptides have high specificity toward fibronectin receptors and can selectively bind to them. | |
| Biocompatibility | RGD peptides are biocompatible and do not cause any adverse immune response in the body. | |
| Tissue regeneration | RGD peptides promote tissue regeneration by stimulating cell growth, differentiation, and migration. | |
| Wound healing | RGD peptides stimulate angiogenesis and improve wound healing by increasing the supply of oxygen and nutrients to the wound site. | |
| Anti‐inflammatory | RGD peptides possess anti‐inflammatory properties and can mitigate the inflammatory response in the body. | |
| Antitumor | RGD peptides inhibit tumor growth and metastasis by preventing the formation of new blood vessels. | |
| Drug delivery | RGD peptides can be used as a targeted drug delivery system to deliver drugs specifically to the site of interest. | |
| Imaging | RGD peptides can be used for imaging purposes to detect cancer cells and other abnormalities in the body. | |
| Safe | RGD peptides are safe and have been extensively studied for their use in various medical applications. | |
| Noninvasive | RGD peptides are non‐invasive and can be administered topically or orally, making treatment less painful and more convenient. | |
| NGR peptide | ||
| Tumor‐targeting | NGR peptides specifically bind to overexpressed CD13 or aminopeptidase N on tumor cells, which can help target the delivery of drugs to these cells. | |
| Low toxicity | NGR peptides have been shown to be safe and well‐tolerated in both animal and human studies. | |
| Enhanced drug delivery | NGR peptides have been shown to enhance the delivery of a variety of drugs to tumor cells, including chemotherapy agents, radioisotopes, and gene therapy. | |
| Antiangiogenic effects | NGR peptides have been shown to inhibit angiogenesis or the formation of new blood vessels, which can help prevent tumor growth and metastasis. | |
| Immunostimulatory effects | NGR peptides have been shown to activate the immune system and induce antitumor immune responses, which can help eradicate tumors and prevent recurrence. | |
| Increased therapeutic efficacy | NGR peptides can improve the efficacy of various cancer treatments and increase patient survival rates. | |
| iRGD peptide | ||
| Enhanced tumor penetration | iRGD peptides improve the penetration of therapeutic agents into tumors by enhancing their binding to tumor‐specific receptors and enabling them to diffuse more deeply into the tumor microenvironment. | |
| Lower toxicity | The improved tumor penetration of iRGD peptides allows for lower doses of therapeutic agents to be used, reducing the risk of toxicity and side effects. | |
| Increased efficacy | RGD peptides improve the delivery of therapeutic agents to tumor cells, increasing their efficacy in killing cancer cells and reducing the potential for drug resistance. | |
| Targeted delivery | iRGD peptides specifically bind to tumor cells, allowing for targeted delivery of therapeutic agents to the site of the tumor, reducing the potential for off‐target effects on healthy cells. | |
| Improved imaging | iRGD peptides can be used in imaging studies to improve the detection and localization of tumors, facilitating more accurate diagnosis and treatment planning. | |
| Improved prognosis | The increased efficacy of iRGD peptides in delivering therapeutic agents to tumors can potentially improve patient outcomes and prognosis. | |
| CRGDK peptide | ||
| Anti‐inflammatory properties | CRGDK peptide has been shown to have anti‐inflammatory properties, which can be beneficial in the treatment of a variety of inflammatory diseases. | |
| Wound healing | CRGDK peptide has also been found to promote wound healing by stimulating angiogenesis and collagen synthesis. | |
| Bone regeneration | CRGDK peptide has been shown to promote bone regeneration, making it a potential treatment option for conditions such as osteoporosis and bone fractures. | |
| Neuroprotection | CRGDK peptide has been found to have neuroprotective properties, which can be beneficial in the treatment of neurological disorders such as Alzheimer's disease and Parkinson's disease. | |
| Anti‐cancer effects | CRGDK peptide has been shown to have anti‐cancer effects by inducing apoptosis (cell death) in cancer cells. | |
| Cardiovascular health | CRGDK peptide has also been found to have cardio‐protective properties, which can be beneficial in the treatment of cardiovascular diseases such as hypertension and heart failure. | |
11. iRGD PEPTIDE
The iRGD (internalizing RGD) peptide is a modified version of the traditional RGD peptide with an amino acid sequence: CRGDKGPDC. It has been developed to improve tumor targeting and penetration abilities. 165 , 166 , 167 The RGD motif within the iRGD peptide binds to αvβ3 and αvβ5 integrins overexpressed on tumor endothelial cells, facilitating initial accumulation at the tumor site. 39 , 171 , 172 , 173 Following proteolytic cleavage of the iRGD peptide after binding to integrins, a CendR motif (C‐terminal arginine or lysine residue) is exposed which interacts with neuropilin‐1 (NRP‐1) receptors present on tumor cells and vasculature, leading to enhanced tissue penetration and cellular uptake via receptor‐mediated endocytosis. 174 , 175 , 176 , 177 Potential applications of iRGD peptides in cancer treatment include conjugating chemotherapy agents or cytotoxic drugs for targeted delivery, coupling with nanoparticles or nanocarriers for efficient drug transport, enhancing gene therapy approaches by conjugating viral vectors or gene‐editing tools and boosting immunotherapy efficacy through targeted delivery of immune checkpoint inhibitors.
12. CRGDK PEPTIDE
The CRGDK peptide is a pentapeptide containing an RGD sequence flanked by cysteine residues at both ends, resulting in improved stability due to cyclization via disulfide bond formation. Its applications are similar to those associated with other RGD‐based peptides but may offer better resistance against enzymatic degradation due to its cyclic structure. 168 , 169 , 170 Some potential applications include tumor targeting via selective binding to αvβ3/αvβ5 integrins overexpressed on cancerous tissues, conjugation with chemotherapeutic or cytotoxic agents for targeted treatments, development of nanoparticles or nanocarriers to improve drug delivery and reduce off‐target effects. 178 , 179 , 180
Both iRGD and CRGDK peptides are derived from the RGD peptide family but possess unique properties that can be exploited for various treatment purposes. The iRGD peptide demonstrates improved tumor targeting and penetration abilities via dual receptor binding (integrins and NRP‐1), while the cyclic structure of CRGDK offers enhanced stability against proteolytic degradation. Each peptide has potential applications in targeted chemotherapy, nanoparticle‐based drug delivery systems, gene therapy, and immunotherapy approaches for cancer treatment.
13. INTERACTION BETWEEN THE RGD MOTIF AND INTEGRIN
Integrins are heterodimeric proteins composed of α and β subunits. In humans, there are 18 α subunits and 8 β subunits, which can combine to form different integrin receptors. The extracellular domain of integrins contains a ligand‐binding head region, a single‐pass transmembrane domain, and a cytoplasmic tail that interacts with intracellular signaling molecules. The ligand‐binding head region consists of a β‐propeller domain and a βI‐like domain, which together form a binding pocket for the RGD motif. The RGD motif is a short peptide sequence found in various ECM proteins, such as fibronectin, vitronectin, and collagen. It plays a crucial role in cell adhesion and migration by interacting with integrin receptors. The arginine residue in the RGD motif forms a salt bridge with a conserved glutamic acid residue in the integrin receptor, while the glycine and aspartic acid residues contribute to the overall conformation and stability of the motif. 73 , 150 , 181 , 182 , 183
The interaction between the RGD motif and integrin receptors is a multistep process. Initially, the RGD motif binds to the ligand‐binding head region of the integrin receptor, primarily through interactions with the α subunit. This binding induces conformational changes in the integrin, leading to the exposure of a high‐affinity state. This conformational change allows the integrin to bind to other ECM proteins, leading to the formation of focal adhesions and initiation of downstream signaling events. The interaction between the RGD motif and integrin receptors triggers various intracellular signaling pathways. These pathways regulate cell survival, proliferation, migration, and differentiation. One of the well‐studied signaling pathways is the focal adhesion kinase (FAK) pathway, which activates downstream signaling molecules, including mitogen‐activated protein kinases (MAPKs) and phosphatidylinositol 3‐kinase (PI3K). These signaling events ultimately regulate cellular processes such as cytoskeletal rearrangement, gene expression, and cell cycle progression. The RGD motif‐integrin interaction is essential for numerous physiological processes, including embryonic development, tissue homeostasis, and immune response. It is involved in cell adhesion and migration during wound healing, angiogenesis, and tissue regeneration. Dysregulation of the RGD motif‐integrin interaction has been implicated in various pathological conditions, including cancer metastasis, fibrosis, and autoimmune diseases. Targeting this interaction has emerged as a potential therapeutic strategy for these diseases. 10 , 15 , 25 , 184 , 185 , 186 , 187 , 188
The interaction between the RGD motif and integrin receptors is a complex and highly regulated process that plays a crucial role in cell adhesion, migration, and signaling. Understanding the detailed information about this interaction provides insights into the mechanisms underlying physiological and pathological processes. Further research in this field may lead to the development of novel therapeutic approaches targeting the RGD motif‐integrin interaction for the treatment of various diseases.
14. THE RGD PEPTIDE IN CANCER TARGETING
14.1. Breast cancer
Breast cancer is a complex illness with several subtypes that have diverse genomic profiles, clinical characteristics, and therapy responses. 189 Integrins, a kind of cell surface receptor, are important in cell adhesion, migration, and signaling and are frequently dysregulated in breast cancer. The RGD peptide is known to interact with numerous integrins, making it an attractive option for breast cancer‐targeted therapy and diagnostics. 190 , 191 , 192 Understanding the particular integrin expression patterns in various breast cancer subtypes can aid in the development of RGD peptide‐based techniques for more effective and tailored breast cancer therapy. 10 , 73 , 193
14.2. Triple‐negative breast cancer (TNBC)
TNBC is distinguished by the lack of expression of the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). 194 It is a dangerous subtype with few therapeutic choices and a dismal prognosis. Integrins αvβ3, αvβ5, and α5β1 are widely overexpressed in TNBC, 195 , 196 and their interaction with RGD peptides has been studied for targeted drug delivery, imaging, and treatment. 197 , 198 , 199 , 200 MDA‐MB‐231 is a breast cancer cell line generated from a patient with triple‐negative/basal‐like breast cancer, which is distinguished by the lack of ER, PR, and HER2 expression and has a poor prognosis. Another breast cancer cell line produced by a patient with triple‐negative/basal‐like breast cancer is MDA‐MB‐468. It has been found that MDA‐MB‐231 and MDA‐MB‐468 cells have integrins αvβ3, αvβ5, and α5β1, which can bind with RGD peptides (Table 6). 201 , 202 , 203 , 204 , 205
TABLE 6.
The probable integrins in different cancers which could interact with RGD peptide.
| Cancer | Cell line | Integrin |
|---|---|---|
| Breast cancer | ||
| Triple‐negative breast cancer (TNBC) | MDA‐MB‐231 and MDA‐MB‐468 | αvβ3, αvβ5, and α5β1 |
| Luminal breast cancer | MCF‐7, and T47D | αvβ3, αvβ5, and α5β1 |
| Lung cancer | ||
| Non‐small cell lung cancer (NSCLC) | A549, H1299, H460, HCC827, PC‐9 and H1975 | αvβ3 and αvβ5 |
| Small cell lung cancer (SCLC) | H69 | αvβ3 and αvβ5 |
| Cervical cancer | ||
| Squamous cell carcinoma (SCC) | HeLa, and SiHa | αvβ3 and αvβ5 |
| Colorectal cancer (CRC) | ||
| HCT116, HT‐29, SW620, Caco‐2, LoVo, LS174T, DLD‐1, SW480 and Colo205 | αvβ3 | |
| RKO | αvβ5 | |
| Liver cancer | ||
| Hepatocellular carcinoma (HCC) | HepG2 and Huh7 | αvβ3, αvβ5, and α5β1 |
| Pancreatic cancer | ||
| Pancreatic ductal adenocarcinoma (PDAC) | PANC‐1, MIA PaCa‐2, BxPC‐3 and AsPC‐1 | αvβ3, αvβ5, and α5β1 |
| Renal cell carcinoma (RCC) | ||
| Clear cell RCC (ccRCC) | 786‐O and A498 | αvβ3, αvβ5, and α5β1 |
14.3. Luminal breast cancer
Luminal breast cancer is distinguished by the presence of hormone receptors (ER and/or PR) and has a better prognosis than TNBC and HER2‐positive breast cancer. Integrins αvβ3, αvβ5, and α5β1 can also be overexpressed in luminal breast cancer, and RGD peptide‐based techniques for targeted therapeutics and diagnostics have been investigated. 206 , 207 , 208 , 209 MCF‐7 is a frequently used breast cancer cell line obtained from a patient with luminal A breast cancer, which is distinguished by the presence of estrogen receptor (ER) and/or progesterone receptor (PR) expression and low levels of human epidermal growth factor receptor 2 (HER2). T47D is another breast cancer cell line generated from a luminal A breast cancer patient. MCF‐7 and T47D cells have been shown to express integrins αvβ5, and α5β1, which can bind RGD peptides. 151 , 210 , 211 MCF‐7 cells are typically employed as a model for low integrin αvβ3 expression, and similarly, T47D cells exhibit a low level of integrin expression. 52 , 62 , 212 , 213
14.4. Lung cancer
Lung cancer is a diverse collection of cancers that may be divided into two types: non‐small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). NSCLC is further classified into three major subtypes: adenocarcinoma, squamous cell carcinoma, and giant cell carcinoma. SCLC is distinguished by its fast development and early metastasis. 214 , 215 RGD peptides have been demonstrated in multiple lung cancer cell lines to bind with integrins αvβ3 and αvβ5. 216 , 217 , 218 These interactions could be used in targeted drug delivery and imaging applications, highlighting the promise of RGD peptides for lung cancer targeted treatment. HCC827 (NSCLC, adenocarcinoma), PC‐9 (NSCLC, adenocarcinoma), H1975 (NSCLC, adenocarcinoma), and H69 (SCLC) are the most often utilized lung cancer cell lines. 73 , 219 , 220 , 221 These cells have been shown to express integrins αvβ3 and αvβ5, which can bind to RGD peptides.
14.5. Cervical cancer
Cervical cancer is the fourth most frequent cancer in women globally, with HPV infection being the leading risk factor. Squamous cell carcinoma (SCC) and adenocarcinoma (AC) are the two primary histological subtypes of cervical cancer. 222 , 223 Several integrins have been shown to interact with RGD peptides, adding to the complexity and heterogeneity of cervical cancer. In cervical cancer, integrins that engage with RGD peptides typically bind to fibronectin (α5β1, αvβ3, and αvβ6) and vitronectin (αvβ3 and αvβ5). 224 , 225 , 226 , 227 Integrin α5β1 overexpression has been found in several cervical cancer cell lines (e.g., HeLa), where it contributes to cell adhesion and migration via interactions with its natural ligand‐fibronectin with an RGD sequence. 224 , 225 , 226 , 228 Due to its different binding specificity from the other mentioned integrins, Integrin α2β1 does not directly interact with classical linear/cyclic‐RGD peptides, but studies suggest that it may be involved indirectly through crosstalk/signaling pathways related to cervical cancer progression mediated by other RGD‐binding integrins such as α5β1. These integrins' expression and function differ depending on the stage and severity of cervical cancer. 229 , 230 , 231 The use of RGD‐based inhibitors to target these integrins provides a promising treatment option for cervical cancer, with the potential to enhance patient outcomes and lessen the burden of this deadly illness. RGD peptides, on the other hand, have been demonstrated to interact with integrins αvβ3 and αvβ5, particularly binding to fibronectin in cervical cancer cell lines such as HeLa (SCC) and SiHa (SCC). 217 , 232
14.6. Colorectal cancer
Colorectal cancer (CRC) is a complex disease with several molecular subgroups that can interact with RGD peptides. 10 , 233 , 234 The interaction of integrins with RGD peptides is important in the development, metastasis, and angiogenesis of colorectal cancer and colon cancer. Several integrins, including αvβ3, αvβ5, αvβ6, α5β1, and α2β1, can interact with RGD peptides in CRC. 234 , 235 , 236 , 237 Accordingly, targeting these integrins and their interactions with RGD peptides may constitute a viable therapeutic method for the treatment of CRC. Integrin αvβ3 is significantly expressed in CRC and has been linked to tumor growth, angiogenesis, and metastasis. The interaction between αvβ3 and RGD peptides increases cell adhesion, migration, and invasion, which contributes to CRC cell aggression. Inhibiting the αvβ3‐RGD connection has been demonstrated in preclinical models to inhibit tumor development and angiogenesis, indicating that targeting this integrin may be a potential treatment approach for CRC. Another RGD‐binding integrin that is increased in CRC is integrin αvβ5. It has been associated with cell adhesion, migration, and invasion regulation, and with the activation of growth factor signaling pathways such as the VEGF pathway. Targeting the αvβ5‐RGD connection might aid in the inhibition of tumor development and angiogenesis in CRC. Integrin expression and interactions with RGD peptides may change between cell lines and subtypes. HCT116, HT‐29, SW620, Caco‐2, LoVo, LS174T, DLD‐1, SW480, and Colo205 colorectal cancer cell lines contain integrin αvβ3, which can interact with RGD peptides. 234 , 238 The RKO cell line has been demonstrated to interact with RGD peptides, which can attach to integrin αvβ5. 239 , 240
14.7. Liver cancer
Hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma (ICC), and combination hepatocellular‐cholangiocarcinoma (cHCC‐CC) are all subtypes of liver cancer. The interaction between integrins and RGD peptides is critical in the development, metastasis, and angiogenesis of liver cancer. Several integrins, including αvβ3, αvβ5, α5β1, and α6β1, have been found to interact with RGD peptides in liver cancer. 208 , 241 , 242 , 243 , 244
These integrins have been shown to interact with RGD peptides in several liver cancer cell lines. These interactions contribute to tumor growth, metastasis, angiogenesis, and therapy resistance, making them attractive therapeutic targets for the treatment of liver cancer. Integrins αvβ3, αvβ5, and α5β1 are expressed by HepG2, and Huh7 (well‐established HCC cell lines). 245 , 246 , 247
14.8. Pancreatic cancer
Pancreatic cancer is a formidable adversary in the world of oncology, characterized by its aggressive nature, limited treatment options, and poor prognosis. Pancreatic cancer is a very aggressive and diverse disease, with pancreatic ductal adenocarcinoma (PDAC) being the most frequent subtype. The search for innovative approaches to combat this deadly disease has led researchers to explore the application of RGD peptides, which have shown promise in targeting integrins—a group of cell surface receptors that play pivotal roles in tumor growth, invasion, angiogenesis, metastasis, and therapy resistance. 248 In pancreatic cancer, the dysregulation of integrin expression on cancer cells has been extensively studied. Specifically, integrins αvβ3, αvβ5, and αvβ6 have been implicated in pancreatic cancer progression, making them attractive therapeutic targets. 13 Several other integrins, including α5β1, and α6β1, may also interact with RGD peptides in pancreatic cancer. 249 , 250 , 251 , 252 Integrins αvβ3, αvβ5, and α5β1 are expressed by PANC‐1, MIA PaCa‐2, BxPC‐3, and AsPC‐1 (all well‐established PDAC cell lines). 253 , 254 , 255 , 256 , 257 , 258
One promising approach involves utilizing RGD peptides with high binding affinity for integrins, particularly αvβ3, to enhance drug delivery specificity. RGD peptides have been incorporated into liposomal drug carriers, allowing the targeted delivery of anticancer therapeutics directly to pancreatic cancer cells. This modification enhances the tumor specificity of vesicles, increasing the precision of treatment. 13 RGD‐conjugated albumin nanoparticles have shown promise as delivery vehicles for therapeutic agents in pancreatic cancer. These nanoparticles, by targeting integrin avβ3 receptors on pancreatic cancer cells, enhance drug penetration, improve antitumor efficacy, and inhibit tumor growth and metastasis. 259 RGD peptides have also been employed in conjunction with gemcitabine, a standard drug for pancreatic cancer treatment, to enhance drug penetration into tumors. The coadministration of GEM and RGD peptides has demonstrated effectiveness in reducing tumor size, particularly in cell line‐based xenografts, emphasizing the potential clinical utility of this approach 260 In the realm of diagnostic imaging, RGD peptides have been utilized to improve the labeling and uptake of superparamagnetic iron oxide (SPIO) nanoparticles in pancreatic cancer cells. This approach enhances the sensitivity of pancreatic cancer imaging, aiding in early detection and precise evaluation. 261 Another innovative application of RGD in pancreatic cancer involves using a quantum dots‐RGD probe as a photosensitizer in photodynamic therapy (PDT). This approach inhibits cell proliferation and induces apoptosis in pancreatic carcinoma cells, offering a promising avenue for clinical treatment. 262
The application of RGD peptide in pancreatic cancer has emerged as a multifaceted approach encompassing targeted drug delivery, tumor‐selective immunotherapies, photodynamic therapy, diagnostic imaging, and more. By targeting integrins, RGD peptides offer a promising avenue to enhance the specificity and efficacy of pancreatic cancer diagnosis and therapy. However, continued research is imperative to fully realize the potential of RGD‐based approaches in combating this challenging disease.
14.9. Kidney cancer
Kidney cancer, commonly known as renal cell carcinoma (RCC), is a diverse group of malignancies that develop from renal tubular epithelial cells. RCC is classified into four subtypes: clear cell RCC (ccRCC), papillary RCC (pRCC), chromophobe RCC (chRCC), and collecting duct carcinoma (CDC). Each subtype has different histological characteristics, molecular changes, and clinical consequences. 263 , 264
The most prevalent subtype of RCC is ccRCC. It is distinguished by transparent cytoplasm and a well‐defined cell membrane. The deletion of the von Hippel–Lindau (VHL) tumor suppressor gene, which leads to the stability of hypoxia‐inducible factors (HIFs) and the stimulation of angiogenesis, is the most prevalent genetic change in ccRCC. Integrins such as αvβ3, αvβ5, and α5β1 may interact with RGD peptides in ccRCC. 265 , 266 , 267 RGD peptides may reduce cell adhesion, migration, and invasion in ccRCC cell lines such as 786‐O and A498, by targeting these integrins. The second most prevalent kind of RCC is pRCC. The presence of papillary structures bordered by cuboidal or columnar cells marks it. pRCC is classified into two subtypes: Type 1, which has a better prognosis, and Type 2, which is more aggressive and has a worse prognosis. 263 , 264 The prognosis for CDC is poor, with a high prevalence of metastasis and resistance to traditional therapy. 263 , 264 There is little known about the roles of integrins and RGD peptides in chRCC, and CDC. Integrins such as αvβ3 and αvβ5 may, however, have a role in both pRCC, chRCC and CDC, as they are implicated in cell adhesion, migration, and invasion in other RCC subtypes. 9 , 268
15. CONCLUSION
Integrins are a kind of cell adhesion molecule that is required for the growth and spread of cancer. By targeting these molecules with RGD peptides, researchers were able to selectively deliver drugs to cancer cells and the tumor vasculature while preventing injury to healthy organs. This technique has recently shown a lot of promise, with RGD‐functionalized drug carriers demonstrating increased efficacy and lower toxicity when compared to traditional chemotherapy. RGD‐functionalized drug carriers have also been shown to improve therapeutic efficacy by increasing medicine absorption and retention inside the tumor microenvironment. Because different cancer types may require different treatments or dosages, the precision of this method allows for the development of individualized treatment strategies. RGD peptides and their conjugates can help detect and track the progression of cancer by serving as imaging agents and improving therapy administration. RGD‐conjugates are expected to become a more important weapon in the fight against cancer as research in this field advances.
AUTHOR CONTRIBUTIONS
Hossein Javid: Conceptualization (equal); data curation (equal); formal analysis (equal); methodology (equal); project administration (equal); writing – original draft (equal). Mahsa Akbari Oryani: Data curation (equal); formal analysis (equal); validation (equal); writing – original draft (equal). Nastaran Rezagholinejad: Conceptualization (equal); resources (equal); software (equal). Ali Esparham: Conceptualization (equal); formal analysis (equal); writing – original draft (equal). Mahboubeh Tajaldini: Formal analysis (equal); methodology (equal); software (equal). Mehdi Karimi‐Shahri: Project administration (equal); supervision (equal); validation (equal); visualization (equal); writing – review and editing (equal).
FUNDING INFORMATION
Funding information is not applicable/no funding was received.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest.
ETHICAL APPROVAL
Ethics approval for this type of article (A review) is not applicable.
CODE AVAILABILITY
Not applicable.
Javid H, Oryani MA, Rezagholinejad N, Esparham A, Tajaldini M, Karimi‐Shahri M. RGD peptide in cancer targeting: Benefits, challenges, solutions, and possible integrin–RGD interactions. Cancer Med. 2024;13:e6800. doi: 10.1002/cam4.6800
Contributor Information
Hossein Javid, Email: javidh@varastegan.ac.ir.
Mehdi Karimi‐Shahri, Email: labratorylabratory@yahoo.com.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1. Bellis SL. Advantages of RGD peptides for directing cell association with biomaterials. Biomaterials. 2011;32(18):4205‐4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sarin V, Gaffin RD, Meininger GA, Muthuchamy M. Arginine‐glycine‐aspartic acid (RGD)‐containing peptides inhibit the force production of mouse papillary muscle bundles via alpha 5 beta 1 integrin. J Physiol. 2005;564(Pt 2):603‐617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ruoslahti E, Pierschbacher MD. New perspectives in cell adhesion: RGD and Integrins. Science. 1987;238(4826):491‐497. [DOI] [PubMed] [Google Scholar]
- 4. Yamada Y, Onda T, Wada Y, Hamada K, Kikkawa Y, Nomizu M. Structure–activity relationships of RGD‐containing peptides in integrin αvβ5‐mediated cell adhesion. ACS Omega. 2023;8(5):4687‐4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pang X, He X, Qiu Z, et al. Targeting integrin pathways: mechanisms and advances in therapy. Signal Transduct Target Ther. 2023;8(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schnittert J, Bansal R, Storm G, Prakash J. Integrins in wound healing, fibrosis and tumor stroma: high potential targets for therapeutics and drug delivery. Adv Drug Deliv Rev. 2018;129:37‐53. [DOI] [PubMed] [Google Scholar]
- 7. Koivisto L, Heino J, Häkkinen L, Larjava H. Integrins in wound healing. Adv Wound Care (New Rochelle). 2014;3(12):762‐783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang Y, Wang H. Integrin signalling and function in immune cells. Immunology. 2012;135(4):268‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Danhier F, Le Breton A, Préat V. RGD‐based strategies to target alpha(v) Beta(3) integrin in cancer therapy and diagnosis. Mol Pharm. 2012;9(11):2961‐2973. [DOI] [PubMed] [Google Scholar]
- 10. Nieberler M, Reuning U, Reichart F, et al. Exploring the role of RGD‐recognizing Integrins in cancer. Cancer. 2017;9(9):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alipour M, Baneshi M, Hosseinkhani S, et al. Recent progress in biomedical applications of RGD‐based ligand: from precise cancer theranostics to biomaterial engineering: a systematic review. J Biomed Mater Res A. 2020;108(4):839‐850. [DOI] [PubMed] [Google Scholar]
- 12. Pirooznia N, Abdi K, Beiki D, et al. 177Lu‐labeled cyclic RGD peptide as an imaging and targeted radionuclide therapeutic agent in non‐small cell lung cancer: biological evaluation and preclinical study. Bioorg Chem. 2020;102:104100. [DOI] [PubMed] [Google Scholar]
- 13. Bergonzini C, Kroese K, Zweemer AJM, Danen EHJ. Targeting Integrins for cancer therapy ‐ disappointments and opportunities. Front Cell Dev Biol. 2022;10:863850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Notni J. RGD forever!‐past, present, and future of a 3‐letter‐code in radiopharmacy and life sciences. Pharmaceuticals (Basel). 2022;16(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sheikh A, Alhakamy NA, Md S, Kesharwani P. Recent Progress of RGD modified liposomes as multistage rocket against cancer. Front Pharmacol. 2022;12:803304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shepard JA, Huang A, Shikanov A, Shea LD. Balancing cell migration with matrix degradation enhances gene delivery to cells cultured three‐dimensionally within hydrogels. J Control Release. 2010;146(1):128‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dai J, Xu Z, Xu J, et al. Improving crossing of multiple bio‐delivery barriers by a novel bio‐interface design based on hydrophobic nanoparticle surfaces. J Mater Chem B. 2023;11(6):1344‐1355. [DOI] [PubMed] [Google Scholar]
- 18. Bodenberger N, Kubiczek D, Paul P, et al. Beyond bread and beer: whole cell protein extracts from baker's yeast as a bulk source for 3D cell culture matrices. Appl Microbiol Biotechnol. 2017;101(5):1907‐1917. [DOI] [PubMed] [Google Scholar]
- 19. Wang X, Li S, Shi Y, et al. The development of site‐specific drug delivery nanocarriers based on receptor mediation. J Control Release. 2014;193:139‐153. [DOI] [PubMed] [Google Scholar]
- 20. Mndlovu H, du Toit LC, Kumar P, Choonara YE. Tannic acid‐loaded chitosan‐RGD‐alginate scaffolds for wound healing and skin regeneration. Biomed Mater. 2023;18(4):45009. [DOI] [PubMed] [Google Scholar]
- 21. Marić I, Yang L, Li X, et al. Tailorable and biocompatible supramolecular‐based hydrogels featuring two dynamic covalent chemistries. Angew Chem Int Ed. 2023;62(14):e202216475. [DOI] [PubMed] [Google Scholar]
- 22. Mansur AAP, Rodrigues MA, Capanema NSV, Carvalho SM, Gomes DA, Mansur HS. Functionalized bioadhesion‐enhanced carboxymethyl cellulose/polyvinyl alcohol hybrid hydrogels for chronic wound dressing applications. RSC Adv. 2023;13(19):13156‐13168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gila‐Vilchez C, Mañas‐Torres MC, García‐García OD, et al. Biocompatible short‐peptides fibrin Co‐assembled hydrogels. ACS Appl Polym Mater. 2023;5(3):2154‐2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang Y, Mo Y, Zhang Y, Yuan J, Zhang Q. MMP‐3‐mediated cleavage of OPN is involved in copper oxide nanoparticle‐induced activation of fibroblasts. Part Fibre Toxicol. 2023;20(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yin L, Li X, Wang R, Zeng Y, Zeng Z, Xie T. Recent research Progress of RGD peptide–modified Nanodrug delivery Systems in Tumor Therapy. Int J Pept Res Ther. 2023;29(4):53. [Google Scholar]
- 26. Sanati M, Afshari AR, Aminyavari S, Kesharwani P, Jamialahmadi T, Sahebkar A. RGD‐engineered nanoparticles as an innovative drug delivery system in cancer therapy. J Drug Deliv Sci Technol. 2023;84:104562. [Google Scholar]
- 27. Qin W, Chandra J, Abourehab MAS, et al. New opportunities for RGD‐engineered metal nanoparticles in cancer. Mol Cancer. 2023;22(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu Z, Huang J, Zhang T, et al. RGD peptide modified RBC membrane functionalized biomimetic nanoparticles for thrombolytic therapy. J Mater Sci Mater Med. 2023;34(4):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thakur R, Suri CR, Kaur IP, Rishi P. Peptides as diagnostic, therapeutic, and Theranostic tools: Progress and future challenges. Crit Rev Ther Drug Carrier Syst. 2023;40(1):49‐100. [DOI] [PubMed] [Google Scholar]
- 30. Raut SY, Fu K, Taichun H, et al. Engineered Nano‐carrier systems for the oral targeted delivery of follicle stimulating hormone: development, characterization, and, assessment of in vitro and in vivo performance and targetability. Int J Pharm. 2023;637:122868. [DOI] [PubMed] [Google Scholar]
- 31. Manjusha V, Reshma LR, Anirudhan TS. Mesoporous silica gated mixed micelle for the targeted co‐delivery of doxorubicin and paclitaxel. J Drug Delivery Sci Technol. 2023;79:104032. [Google Scholar]
- 32. Diaferia C, Rosa E, Accardo A, Morelli G. Peptide‐based hydrogels as delivery systems for doxorubicin. J Pept Sci. 2022;28(1):e3301. [DOI] [PubMed] [Google Scholar]
- 33. He T, He J, Younis MR, et al. Dual‐stimuli‐responsive Nanotheranostics for dual‐targeting Photothermal‐enhanced chemotherapy of tumor. ACS Appl Mater Interfaces. 2021;13(19):22204‐22212. [DOI] [PubMed] [Google Scholar]
- 34. Juan HF, Wang IH, Huang TC, Li JJ, Chen ST, Huang HC. Proteomics analysis of a novel compound: cyclic RGD in breast carcinoma cell line MCF‐7. Proteomics. 2006;6(10):2991‐3000. [DOI] [PubMed] [Google Scholar]
- 35. Zhang J, Su X, Weng L, et al. Gadolinium‐hybridized mesoporous organosilica nanoparticles with high magnetic resonance imaging performance for targeted drug delivery. J Colloid Interface Sci. 2023;633:102‐112. [DOI] [PubMed] [Google Scholar]
- 36. Monieri M, Rainone P, Sacchi A, et al. A stapled chromogranin A‐derived peptide homes in on tumors that express αvβ6 or αvβ8 integrins. Int J Biol Sci. 2023;19(1):156‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zang J, Wen X, Lin R, et al. Synthesis, preclinical evaluation and radiation dosimetry of a dual targeting PET tracer [68Ga]Ga‐FAPI‐RGD. Theranostics. 2022;12(16):7180‐7190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Qin J, Liang Q, Wang G, et al. Targeted delivery of nuclear targeting probe for bladder cancer using cyclic pentapeptide c(RGDfK) and acridine orange. Clin Transl Oncol. 2023;25(2):375‐383. [DOI] [PubMed] [Google Scholar]
- 39. Qian J, Zhou S, Lin P, et al. Recent advances in the tumor‐penetrating peptide internalizing RGD for cancer treatment and diagnosis. Drug development. Research. 2023;84:654‐670. [DOI] [PubMed] [Google Scholar]
- 40. Liu X, Meng L, Wang Z, et al. Novel construction of multifunctional photo‐responsive and nucleic acid‐triggered doxorubicin‐releasing liposomes for cancer therapy. Eur J Med Chem. 2023;250:115207. [DOI] [PubMed] [Google Scholar]
- 41. Li Y, Dang G, Rizwan Younis M, et al. Peptide functionalized actively targeted MoS2 nanospheres for fluorescence imaging‐guided controllable pH‐responsive drug delivery and collaborative chemo/photodynamic therapy. J Colloid Interface Sci. 2023;639:302‐313. [DOI] [PubMed] [Google Scholar]
- 42. Shetake NG, Ali M, Kumar A, Bellare J, Pandey BN. Theranostic magnetic nanoparticles enhance DNA damage and mitigate doxorubicin‐induced cardio‐toxicity for effective multi‐modal tumor therapy. Biomater Adv. 2022;142:213147. [DOI] [PubMed] [Google Scholar]
- 43. Jia P, Zhao X, Liu Y, et al. The RGD‐modified self‐assembling D‐form peptide hydrogel enhances the therapeutic effects of mesenchymal stem cells (MSC) for hindlimb ischemia by promoting angiogenesis. Chem Eng J. 2022;450:138004. [Google Scholar]
- 44. Moyon A, Garrigue P, Fernandez S, et al. Comparison of a new68ga‐radiolabelled pet imaging agent scd146 and rgd peptide for in vivo evaluation of angiogenesis in mouse model of myocardial infarction. Cell. 2021;10(9):2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jung KH, Lee YJ, Kim JY, Lee KC, Park JA, Choi JY. Preparing a 68Ga‐labeled arginine glycine aspartate (RGD)‐peptide for angiogenesis. J Vis Exp. 2019;143;e58218, doi: 10.3791/58218. [DOI] [PubMed] [Google Scholar]
- 46. Xin MH, Xiang H, Si WB, Zhao W, Xiao H, You QD. Synthesis and antiangiogenic properties of 2‐methoxestradiol‐RGD peptide conjugates. J China Pharm Univ. 2011;42(3):198‐205. [Google Scholar]
- 47. Pirooznia N, Abdi K, Beiki D, et al. Radiosynthesis, biological evaluation, and preclinical study of a 68Ga‐labeled cyclic RGD peptide as an early diagnostic agent for overexpressed α v β 3 integrin receptors in non‐small‐cell lung cancer. Contrast Media Mol Imaging. 2020;2020:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Katsamakas S, Chatzisideri T, Thysiadis S, Sarli V. RGD‐mediated delivery of small‐molecule drugs. Future Med Chem. 2017;9(6):579‐604. [DOI] [PubMed] [Google Scholar]
- 49. Yu HM, Chen JH, Lin KL, Lin WJ. Synthesis of 68Ga‐labeled NOTA‐RGD‐GE11 heterodimeric peptide for dual integrin and epidermal growth factor receptor‐targeted tumor imaging. J Label Compd Radiopharm. 2015;58(7):299‐303. [DOI] [PubMed] [Google Scholar]
- 50. Vats K, Sharma R, Sharma AK, Sarma HD, Satpati D. Assessment of 177Lu‐labeled carboxyl‐terminated polyamidoamine (PAMAM) dendrimer‐RGD peptide conjugate. J Pept Sci. 2022;28(2):e3366. [DOI] [PubMed] [Google Scholar]
- 51. Gyuricza B, Szűcs Á, Szabó JP, et al. The synthesis and preclinical investigation of Lactosamine‐based radiopharmaceuticals for the detection of Galectin‐3‐expressing melanoma cells. Pharmaceutics. 2022;14(11):2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang L, Shan X, Meng X, et al. Novel integrin αvβ3‐specific ligand for the sensitive diagnosis of glioblastoma. Mol Pharm. 2019;16(9):3977‐3984. [DOI] [PubMed] [Google Scholar]
- 53. Zhang J, Mao F, Niu G, et al. 68Ga‐BBN‐RGD PET/CT for GRPR and integrin αvβ3 imaging in patients with breast cancer. Theranostics. 2018;8(4):1121‐1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Long Y, Shao F, Ji H, et al. Evaluation of a CD13 and integrin αvβ3 dual‐receptor targeted tracer 68Ga‐NGR‐RGD for ovarian tumor imaging: comparison with 18F‐FDG. Front Oncol. 2022;12:884554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fu S, Xu X, Ma Y, Zhang S, Zhang S. RGD peptide‐based non‐viral gene delivery vectors targeting integrin αvβ3 for cancer therapy. J Drug Target. 2019;27(1):1‐11. [DOI] [PubMed] [Google Scholar]
- 56. Knetsch PA, Zhai C, Rangger C, et al. [68Ga]FSC‐(RGD)3 a trimeric RGD peptide for imaging αvβ3 integrin expression based on a novel siderophore derived chelating scaffold‐synthesis and evaluation. Nucl Med Biol. 2015;42(2):115‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li J, Ji J, Holmes LM, et al. Fusion protein from RGD peptide and fc fragment of mouse immunoglobulin G inhibits angiogenesis in tumor. Cancer Gene Ther. 2004;11(5):363‐370. [DOI] [PubMed] [Google Scholar]
- 58. Zamora A, Gandioso A, Massaguer A, et al. Toward angiogenesis inhibitors based on the conjugation of organometallic platinum(II) complexes to RGD peptides. ChemMedChem. 2018;13(17):1755‐1762. [DOI] [PubMed] [Google Scholar]
- 59. Oba M, Fukushima S, Kanayama N, et al. Cyclic RGD peptide‐conjugated polyplex micelles as a targetable gene delivery system directed to cells possessing αvβ 3 and αvβ5 integrins. Bioconjug Chem. 2007;18(5):1415‐1423. [DOI] [PubMed] [Google Scholar]
- 60. Indrevoll B, Kindberg GM, Solbakken M, et al. NC‐100717: a versatile RGD peptide scaffold for angiogenesis imaging. Bioorg Med Chem Lett. 2006;16(24):6190‐6193. [DOI] [PubMed] [Google Scholar]
- 61. Buerkle MA, Pahernik SA, Sutter A, Jonczyk A, Messmer K, Dellian M. Inhibition of the alpha‐ν integrins with a cyclic RGD peptide impairs angiogenesis, growth and metastasis of solid tumours in vivo. Br J Cancer. 2002;86(5):788‐795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Van Der Pluijm G, Vloedgraven H, Papapoulos S, et al. Attachment characteristics and involvement of integrins in adhesion of breast cancer cell lines to extracellular bone matrix components. Lab Invest. 1997;77(6):665‐675. [PubMed] [Google Scholar]
- 63. Jiang Q, Pan Y, Cheng Y, Li H, Liu D, Li H. Lunasin suppresses the migration and invasion of breast cancer cells by inhibiting matrix metalloproteinase‐2/−9 via the FAK/Akt/ERK and NF‐κB signaling pathways. Oncol Rep. 2016;36(1):253‐262. [DOI] [PubMed] [Google Scholar]
- 64. Petersen MA, Hillmyer MA, Kokkoli E. Bioresorbable polymersomes for targeted delivery of cisplatin. Bioconjug Chem. 2013;24(4):533‐543. [DOI] [PubMed] [Google Scholar]
- 65. Garg A, Kokkoli E. PH‐sensitive PEGylated liposomes functionalized with a fibronectin‐mimetic peptide show enhanced intracellular delivery to colon cancer cells. Curr Pharm Biotechnol. 2011;12(8):1135‐1143. [DOI] [PubMed] [Google Scholar]
- 66. Steiger K, Quigley NG, Groll T, et al. There is a world beyond αvβ3‐integrin: Multimeric ligands for imaging of the integrin subtypes αvβ6, αvβ8, αvβ3, and α5β1 by positron emission tomography. EJNMMI Res. 2021;11(1):106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Quigley NG, Tomassi S, Saverio Di Leva F, et al. Click‐chemistry (CuAAC) Trimerization of an αvβ6 integrin targeting Ga‐68‐peptide: enhanced contrast for in‐vivo PET imaging of human lung adenocarcinoma xenografts. Chembiochem. 2020;21(19):2836‐2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Muller M, Altmann A, Sauter M, et al. Preclinical evaluation of peptide‐based radiotracers for integrin αvβ6‐positive pancreatic carcinoma. Nuklearmedizin. 2019;58(4):309‐318. [DOI] [PubMed] [Google Scholar]
- 69. Guzzetti I, Civera M, Vasile F, et al. Determination of the binding epitope of RGD‐peptidomimetics to αvβ3 and αiIbβ 3 integrin‐rich intact cells by NMR and computational studies. Org Biomol Chem. 2013;11(23):3886‐3893. [DOI] [PubMed] [Google Scholar]
- 70. Arosio D, Casagrande C, Manzoni L. Integrin‐mediated drug delivery in cancer and cardiovascular diseases with peptide‐functionalized nanoparticles. Curr Med Chem. 2012;19(19):3128‐3151. [DOI] [PubMed] [Google Scholar]
- 71. Mas‐Moruno C, Rechenmacher F, Kessler H. Cilengitide: the first anti‐Angiogenic small molecule drug candidate. Design, synthesis and clinical evaluation. Anticancer Agents Med Chem. 2010;10(10):753‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Meyer A, Auernheimer J, Modlinger A, Kessler H. Targeting RGD recognizing integrins: drug development, biomaterial research, tumor imaging and targeting. Curr Pharm Des. 2006;12(22):2723‐2747. [DOI] [PubMed] [Google Scholar]
- 73. Ludwig BS, Kessler H, Kossatz S, Reuning U. RGD‐binding Integrins revisited: how recently discovered functions and novel synthetic ligands (Re‐)shape an ever‐evolving field. Cancers (Basel). 2021;13(7):1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zitzmann S, Ehemann V, Schwab M. Arginine‐glycine‐aspartic acid (RGD)‐peptide binds to both tumor and tumor‐endothelial cells in vivo. Cancer Res. 2002;62(18):5139‐5143. [PubMed] [Google Scholar]
- 75. Sheikh A, Md S, Kesharwani P. RGD engineered dendrimer nanotherapeutic as an emerging targeted approach in cancer therapy. J Control Release. 2021;340:221‐242. [DOI] [PubMed] [Google Scholar]
- 76. Battistini L, Bugatti K, Sartori A, Curti C, Zanardi F. RGD peptide‐drug conjugates as effective dual targeting platforms: recent advances. Eur J Org Chem. 2021;2021(17):2506‐2528. [Google Scholar]
- 77. Xin L, Yuan YW, Liu C, et al. Preparation of internalizing RGD‐modified recombinant Methioninase exosome active targeting vector and antitumor effect evaluation. Dig Dis Sci. 2021;66(4):1045‐1053. [DOI] [PubMed] [Google Scholar]
- 78. Liao L, Cen B, Li G, et al. A bivalent cyclic RGD–siRNA conjugate enhances the antitumor effect of apatinib via co‐inhibiting VEGFR2 in non‐small cell lung cancer xenografts. Drug Deliv. 2021;28(1):1432‐1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wang X, Meng N, Wang S, et al. Factors influencing the immunogenicity and Immunotoxicity of cyclic RGD peptide‐modified Nanodrug delivery systems. Mol Pharm. 2020;17(9):3281‐3290. [DOI] [PubMed] [Google Scholar]
- 80. Qiu M, Wang X, Sun H, Zhang J, Deng C, Zhong Z. Cyclic RGD‐peptide‐functionalized Polylipopeptide micelles for enhanced loading and targeted delivery of monomethyl Auristatin e. Mol Pharm. 2018;15(11):4854‐4861. [DOI] [PubMed] [Google Scholar]
- 81. Lin W, Ma G, Yuan Z, et al. Development of Zwitterionic polypeptide Nanoformulation with high doxorubicin loading content for targeted drug delivery. Langmuir. 2019;35(5):1273‐1283. [DOI] [PubMed] [Google Scholar]
- 82. Palanikumar L, Kim HY, Oh JY, et al. Noncovalent surface locking of mesoporous silica nanoparticles for exceptionally high hydrophobic drug loading and enhanced colloidal stability. Biomacromolecules. 2015;16(9):2701‐2714. [DOI] [PubMed] [Google Scholar]
- 83. Psimadas D, Fani M, Gourni E, et al. Synthesis and comparative assessment of a labeled RGD peptide bearing two different 99mTc‐tricarbonyl chelators for potential use as targeted radiopharmaceutical. Bioorg Med Chem. 2012;20(8):2549‐2557. [DOI] [PubMed] [Google Scholar]
- 84. Cossu J, Thoreau F, Boturyn D. Multimeric RGD‐based strategies for selective drug delivery to tumor tissues. Pharmaceutics. 2023;15(2):525. doi: 10.3390/pharmaceutics15020525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Yang X, Zhang C, Li A, Wang J, Cai X. Red fluorescent ZnO nanoparticle grafted with polyglycerol and conjugated RGD peptide as drug delivery vehicles for efficient target cancer therapy. Mater Sci Eng C. 2019;95:104‐113. [DOI] [PubMed] [Google Scholar]
- 86. Sun Y, Kang C, Liu F, Zhou Y, Luo L, Qiao H. RGD peptide‐based target drug delivery of doxorubicin Nanomedicine. Drug Dev Res. 2017;78(6):283‐291. [DOI] [PubMed] [Google Scholar]
- 87. Lü H, Jin DD, Bie M. Relationship between the expression of alpha integrin and the malignant invasion of osteosarcoma. Chin J Clin Rehab. 2005;9(46):86‐88. [Google Scholar]
- 88. Noiri M, Kushiro K, Togo S, et al. Influence of cell adhesive molecules attached onto PEG‐lipid‐modified fluid surfaces on cell adhesion. Colloids Surf B Biointerfaces. 2019;175:375‐383. [DOI] [PubMed] [Google Scholar]
- 89. Wang HJ, Shrestha R, Zhang Y. Encapsulation of photosensitizers and upconversion nanocrystals in lipid micelles for photodynamic therapy. Part Part Syst Charact. 2014;31(2):228‐235. [Google Scholar]
- 90. Kibria G, Hatakeyama H, Ohga N, Hida K, Harashima H. Dual‐ligand modification of PEGylated liposomes shows better cell selectivity and efficient gene delivery. J Control Release. 2011;153(2):141‐148. [DOI] [PubMed] [Google Scholar]
- 91. Cho R, Sakurai Y, Jones HS, Akita H, Hisaka A, Hatakeyama H. Silencing of VEGFR2 by RGD‐modified lipid nanoparticles enhanced the efficacy of anti‐PD‐1 antibody by accelerating vascular normalization and infiltration of T cells in tumors. Cancer. 2020;12(12):1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Aghamiri S, Talaei S, Ghavidel AA, et al. Nanoparticles‐mediated CRISPR/Cas9 delivery: recent advances in cancer treatment. J Drug Deli Sci Technol. 2020;56:101533. [Google Scholar]
- 93. Yamaguchi A, Anami Y, Ha SYY, et al. Chemical generation of small molecule‐based bispecific antibody‐drug conjugates for broadening the target scope. Bioorg Med Chem. 2021;32:116013. [DOI] [PubMed] [Google Scholar]
- 94. Zhao J, Li S, Jin Y, et al. Multimerization increases tumor enrichment of peptide–photosensitizer conjugates. Molecules. 2019;24(4):817. doi: 10.3390/molecules24040817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Duro‐Castano A, Gallon E, Decker C, Vicent MJ. Modulating angiogenesis with integrin‐targeted nanomedicines. Adv Drug Deliv Rev. 2017;119:101‐119. [DOI] [PubMed] [Google Scholar]
- 96. Khabazian E, Vakhshiteh F, Norouzi P, Fatahi Y, Dinarvand R, Atyabi F. Cationic liposome decorated with cyclic RGD peptide for targeted delivery of anti‐STAT3 siRNA to melanoma cancer cells. J Drug Target. 2022;30(5):522‐533. [DOI] [PubMed] [Google Scholar]
- 97. Wang X, Wang H, Jiang K, et al. Liposomes with cyclic RGD peptide motif triggers acute immune response in mice. J Control Release. 2019;293:201‐214. [DOI] [PubMed] [Google Scholar]
- 98. Gong Z, Liu X, Zhou B, et al. Tumor acidic microenvironment‐induced drug release of RGD peptide nanoparticles for cellular uptake and cancer therapy. Colloid Surf B. 2021;202:111673. [DOI] [PubMed] [Google Scholar]
- 99. Qin J, Xue L, Gong N, et al. RGD peptide‐based lipids for targeted mRNA delivery and gene editing applications. RSC Adv. 2022;12(39):25397‐25404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Naeimi R, Bahmani A, Afshar S. Investigating the role of peptides in effective therapies against cancer. Cancer Cell Int. 2022;22(1):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Bogdanowich‐Knipp SJ, Jois DSS, Siahaan TJ. The effect of conformation on the solution stability of linear vs. cyclic RGD peptides. J Pept Res. 1999;53(5):523‐529. [DOI] [PubMed] [Google Scholar]
- 102. Bogdanowich‐Knipp SJ, Chakrabarti S, Williams TD, Dillman RK, Siahaan TJ. Solution stability of linear vs. cyclic RGD peptides. J Pept Res. 1999;53(5):530‐541. [DOI] [PubMed] [Google Scholar]
- 103. Sorolla A, Wang E, Clemons TD, et al. Triple‐hit therapeutic approach for triple negative breast cancers using docetaxel nanoparticles, EN1‐iPeps and RGD peptides. Nanomedicine. 2019;20:102003. [DOI] [PubMed] [Google Scholar]
- 104. Chakravarty R, Chakraborty S, Guleria A, et al. Clinical scale synthesis of intrinsically radiolabeled and cyclic RGD peptide functionalized 198Au nanoparticles for targeted cancer therapy. Nucl Med Biol. 2019;72‐73:1‐10. [DOI] [PubMed] [Google Scholar]
- 105. Yang X, Wang L, Li L, et al. A novel dendrimer‐based complex co‐modified with cyclic RGD hexapeptide and penetratin for noninvasive targeting and penetration of the ocular posterior segment. Drug Deliv. 2019;26(1):989‐1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Suga T, Kato N, Hagimori M, et al. Development of high‐functionality and ‐quality lipids with RGD peptide ligands: application for PEGylated liposomes and analysis of Intratumoral distribution in a murine colon cancer model. Mol Pharm. 2018;15(10):4481‐4490. [DOI] [PubMed] [Google Scholar]
- 107. Lee JW, Lee YJ, Shin UC, et al. Improved pharmacokinetics following PEGylation and dimerization of a c(RGD‐ACH‐K) conjugate used for tumor positron emission tomography imaging. Cancer Biother Radiopharm. 2016;31(8):295‐301. [DOI] [PubMed] [Google Scholar]
- 108. Mi X, Du H, Guo X, et al. Asparagine endopeptidase‐targeted ultrasound‐responsive Nanobubbles alleviate tau cleavage and amyloid‐β deposition in an Alzheimer's disease model. Acta Biomater. 2022;141:388‐397. [DOI] [PubMed] [Google Scholar]
- 109. Yin R, Guo L, Zhang J, et al. RGD and polyhistidine tumor homing peptides potentiates the action of human Maspin as an antineoplastic candidate. Appl Microbiol Biotechnol. 2016;100(14):6209‐6218. [DOI] [PubMed] [Google Scholar]
- 110. Pina A, Kadri M, Arosio D, et al. Multimeric presentation of RGD Peptidomimetics enhances integrin binding and tumor cell uptake. Chem A Eur J. 2020;26(33):7492‐7496. [DOI] [PubMed] [Google Scholar]
- 111. Civera M, Arosio D, Bonato F, et al. Investigating the interaction of cyclic RGD peptidomimetics with αVβ6 integrin by biochemical and molecular docking studies. Cancer. 2017;9(10):128. doi: 10.3390/cancers9100128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Sernissi L, Petrovic M, Scarpi D, et al. Cyclopropane pipecolic acids as templates for linear and cyclic peptidomimetics: application in the synthesis of an arg‐gly‐asp (RGD)‐containing peptide as an αvβ3 integrin ligand. Chem A Eur J. 2014;20(35):11187‐11203. [DOI] [PubMed] [Google Scholar]
- 113. Siemion IZ, Kluczyk A, Cebrat M. CHAPTER 82 ‐ RGD‐peptides and some immunological problems. In: Kastin AJ, ed. Handbook of Biologically Active Peptides. Academic Press; 2006:573‐578. [Google Scholar]
- 114. Kumar VB, Tiwari OS, Finkelstein‐Zuta G, Rencus‐Lazar S, Gazit E. Design of Functional RGD peptide‐based biomaterials for tissue engineering. Pharmaceutics. 2023;15(2):345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Krishnamoorthy R, Singh M, Anaikutti P, Paul LE, Dhanasekaran S, Sathiah T. Design and synthesis of novel N‐terminal peptides of integrin and aminopeptidase are new finding for anticancer activity. Bioorg Chem. 2023;134:106434. [DOI] [PubMed] [Google Scholar]
- 116. van den Kerkhof DL, Nagy M, Wichapong K, et al. Inhibition of platelet adhesion, thrombus formation, and fibrin formation by a potent αIIbβ3 integrin inhibitor from ticks. Res Pract Thromb Haemost. 2021;5(1):231‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ge F, Qiao R, Song P, et al. Construction of the targeted and pH‐sensitive paclitaxel drug delivery system RGD/PTX@ZIF‐90 and anti‐tumor activity research. Mater Res Exp. 2021;8(4): 045012. doi: 10.1088/2053-1591/abed89 [DOI] [Google Scholar]
- 118. Mohammadi R, Shokri B, Shamshirian D, Zarghi A, Shahhosseini S. Synthesis and biological evaluation of RGD conjugated with Ketoprofen/naproxen and radiolabeled with [99mTc] via N4(GGAG) for αVβ3 integrin‐targeted drug delivery. DARU, J Pharma Sci. 2020;28(1):87‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Giesler RJ, Erickson PW, Kay MS. Enhancing native chemical ligation for challenging chemical protein syntheses. Curr Opin Chem Biol. 2020;58:37‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Liu M, Li C, Pazgier M, et al. D‐peptide inhibitors of the p53‐MDM2 interaction for targeted molecular therapy of malignant neoplasms. Proc Natl Acad Sci U S A. 2010;107(32):14321‐14326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Xiao J, Chen R, Pawlicki MA, Tolbert TJ. Targeting a homogeneously glycosylated antibody fc to bind cancer cells using a synthetic receptor ligand. J Am Chem Soc. 2009;131(38):13616‐13618. [DOI] [PubMed] [Google Scholar]
- 122. Jung JP, Jones JL, Cronier SA, Collier JH. Modulating the mechanical properties of self‐assembled peptide hydrogels via native chemical ligation. Biomaterials. 2008;29(13):2143‐2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Walsh G. Biopharmaceutical benchmarks 2014. Nat Biotechnol. 2014;32(10):992‐1000. [DOI] [PubMed] [Google Scholar]
- 124. Abla AB, Boeuf G, Elmarjou A, et al. Engineering of bio‐adhesive ligand containing recombinant RGD and PHSRN fibronectin cell‐binding domains in fusion with a colored multi affinity tag: simple approach for fragment study from expression to adsorption. Int J Mol Sci. 2021;22(14):7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Wang S, Jiang J, Wang Y, et al. rLj‐RGD3, a novel recombinant toxin protein from Lampetra japonica, prevents coronary thrombosis‐induced acute myocardial infarction by inhibiting platelet functions in rats. Biochem Biophys Res Commun. 2018;498(1):240‐245. [DOI] [PubMed] [Google Scholar]
- 126. Lu Q, Wang J, Jiang J, et al. RLj‐RGD3, a novel recombinant toxin protein from Lampetra japonica, protects against cerebral reperfusion injury following middle cerebral artery occlusion involving the integrin‐PI3K/Akt pathway in rats. PloS One. 2016;11(10):e0165093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Javid H, Sharbaf Mashhad A, Yazdani S, et al. The role of viruses in cancer development versus cancer therapy: an oncological perspective. Cancer Med. 2023;12:11127‐11148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Bock N, Forouz F, Hipwood L, et al. GelMA, click‐chemistry gelatin and bioprinted polyethylene glycol‐based hydrogels as 3D ex vivo drug testing platforms for patient‐derived breast cancer organoids. Pharmaceutics. 2023;15(1):261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Lei L, Hu Y, Shi H, et al. Biofunctional peptide‐click PEG‐based hydrogels as 3D cell scaffolds for corneal epithelial regeneration. J Mater Chem B. 2022;10(31):5938‐5945. [DOI] [PubMed] [Google Scholar]
- 130. Kato N, Sato T, Fuchigami Y, et al. Synthesis and evaluation of a novel adapter lipid derivative for preparation of cyclic peptide‐modified PEGylated liposomes: application of cyclic RGD peptide. Eur J Pharm Sci. 2022;176:106239. [DOI] [PubMed] [Google Scholar]
- 131. Höltke C. isoDGR‐peptides for integrin targeting: is the time up for RGD? J Med Chem. 2018;61(17):7471‐7473. [DOI] [PubMed] [Google Scholar]
- 132. Mihardja SS, Gao D, Sievers RE, et al. Targeted in vivo extracellular matrix formation promotes neovascularization in a rodent model of myocardial infarction. PloS One. 2010;5(4):e10384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Wang F, Huang Q, Wang Y, et al. Rational design of multimodal therapeutic nanosystems for effective inhibition of tumor growth and metastasis. Acta Biomater. 2018;77:240‐254. [DOI] [PubMed] [Google Scholar]
- 134. Zhao J, Santino F, Giacomini D, Gentilucci L. Integrin‐targeting peptides for the design of functional cell‐responsive biomaterials. Biomedicine. 2020;8(9):307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Zhao J, Shi J, Meng X, et al. ROS‐activated nanoscale coordination polymers for enhanced ultrasound‐mediated therapy for the treatment of cancer. Acta Biomater. 2022;143:372‐380. [DOI] [PubMed] [Google Scholar]
- 136. Cheng YJ, Qin SY, Liu WL, et al. Dual‐targeting photosensitizer‐peptide Amphiphile conjugate for enzyme‐triggered drug delivery and synergistic chemo‐photodynamic tumor therapy. Adv Mater Interfaces. 2020;7(19):2000935. [Google Scholar]
- 137. Dal Corso A, Pignataro L, Belvisi L, Gennari C. αvβ3 integrin‐targeted peptide/peptidomimetic‐drug conjugates: in‐depth analysis of the linker technology. Curr Top Med Chem. 2016;16(3):314‐329. [DOI] [PubMed] [Google Scholar]
- 138. Dal Corso A, Caruso M, Belvisi L, et al. Synthesis and biological evaluation of RGD peptidomimetic‐paclitaxel conjugates bearing lysosomally cleavable linkers. Chem A Eur J. 2015;21(18):6921‐6929. [DOI] [PubMed] [Google Scholar]
- 139. Peng M, Qin S, Jia H, Zheng D, Rong L, Zhang X. Self‐delivery of a peptide‐based prodrug for tumor‐targeting therapy. Nano Research. 2016;9(3):663‐673. [Google Scholar]
- 140. Du M, Gu J, Liu C, et al. Genome‐wide CRISPR screen identified Rad18 as a determinant of doxorubicin sensitivity in osteosarcoma. J Exp Clin Cancer Res. 2022;41(1):154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Wang Z, Liu Z, Wang S, et al. Implantation of hydrogel‐liposome nanoplatform inhibits glioblastoma relapse by inducing ferroptosis. Asian J Pharma Sci. 2023;18(3):100800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Zuppone S, Assalini C, Minici C, et al. A novel RGD‐4C‐Saporin conjugate inhibits tumor growth in mouse models of bladder cancer. Front Oncol. 2022;12:846958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Brockmueller A, Shayan P, Shakibaei M. Evidence that β1‐integrin is required for the anti‐viability and anti‐proliferative effect of resveratrol in CRC cells. Int J Mol Sci. 2022;23(9). doi: 10.3390/ijms23094714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Fu J, Xie Y, Fu T, et al. [99mTc]Tc‐Galacto‐RGD2 integrin αvβ3‐targeted imaging as a surrogate for molecular phenotyping in lung cancer: real‐world data. EJNMMI Res. 2021;11(1). doi: 10.1186/s13550-021-00801-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Wei X, Lv R, Wang X, He J, He J. RGD peptide inhibits tumor growth by affecting angiogenesis related signaling pathway of laryngeal cancer stem cells. Int J Morphol. 2022;40(6):1587‐1593. [Google Scholar]
- 146. Li L, Chen X, Yu J, Yuan S. Preliminary clinical application of RGD‐containing peptides as PET radiotracers for imaging tumors. Front Oncol. 2022;12:837952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Liolios C, Sachpekidis C, Kolocouris A, Dimitrakopoulou‐Strauss A, Bouziotis P. Pet diagnostic molecules utilizing multimeric cyclic rgd peptide analogs for imaging integrin αvβ3 receptors. Molecules. 2021;26(6):1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Liu X, Wang F, Liu L, et al. Functionalized polydopamine nanospheres as in situ spray for photothermal image‐guided tumor precise surgical resection. Biosens Bioelectron. 2023;222:114995. [DOI] [PubMed] [Google Scholar]
- 149. Yang M, Zhang Z‐C, Liu Y, et al. Function and mechanism of RGD in bone and cartilage tissue engineering. Front Bioeng Biotechnol. 2021;9:773636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Davoodi Z, Shafiee F. Internalizing RGD, a great motif for targeted peptide and protein delivery: a review article. Drug Deliv Transl Res. 2022;12(10):2261‐2274. [DOI] [PubMed] [Google Scholar]
- 151. Yan H, You Y, Li X, et al. Preparation of RGD peptide/folate acid double‐targeted mesoporous silica nanoparticles and its application in human breast cancer MCF‐7 cells. Front Pharmacol. 2020;11:898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Greenspoon N, Hershkoviz R, Alon R, et al. Structural analysis of integrin recognition and the inhibition of integrin‐mediated cell functions by novel nonpeptidic surrogates of the Arg‐Gly‐asp sequence. Biochemistry. 1993;32(4):1001‐1008. [DOI] [PubMed] [Google Scholar]
- 153. Reed J, Hull WE, Von Der Lieth CW, Kübler D, Suhai S, Kinzel V. Secondary structure of the Arg‐Gly‐asp recognition site in proteins involved in cell‐surface adhesion: evidence for the occurrence of nested β‐bends in the model hexapeptide GRGDSP. Eur J Biochem. 1988;178(1):141‐154. [DOI] [PubMed] [Google Scholar]
- 154. Cheresh DA, Spiro RC. Biosynthetic and functional properties of an Arg‐Gly‐asp‐directed receptor involved in human melanoma cell attachment to vitronectin, fibrinogen, and von Willebrand factor. J Biol Chem. 1987;262(36):17703‐17711. [PubMed] [Google Scholar]
- 155. Chillà A, Bianconi D, Geetha N, et al. Effects of cilengitide in osteoclast maturation and behavior. Exp Cell Res. 2015;337(1):68‐75. [DOI] [PubMed] [Google Scholar]
- 156. Meena CL, Singh D, Weinmüller M, et al. Novel cilengitide‐based cyclic RGD peptides as αvβ3 integrin inhibitors. Bioorg Med Chem Lett. 2020;30(8):127039. [DOI] [PubMed] [Google Scholar]
- 157. Vilaҫa H, Ferreira PMT, Micaelo NM. New cyclic RGD peptides: synthesis, characterization, and theoretical activity towards α v β 3 integrin. Tetrahedron. 2014;70(35):5420‐5427. [Google Scholar]
- 158. Bardania H, Shojaosadati SA, Kobarfard F, et al. Encapsulation of eptifibatide in RGD‐modified nanoliposomes improves platelet aggregation inhibitory activity. J Thromb Thrombolysis. 2017;43(2):184‐193. [DOI] [PubMed] [Google Scholar]
- 159. Hantgan RR, Rocco M, Nagaswami C, Weisel JW. Binding of a fibrinogen mimetic stabilizes integrin αIIbβ3's open conformation. Protein Sci. 2001;10(8):1614‐1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Pathak A, Zhao R, Huang J, Stouffer GA. Eptifibatide and abciximab inhibit insulin‐induced focal adhesion formation and proliferative responses in human aortic smooth muscle cells. Cardiovasc Diabetol. 2008;7:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Roussa VD, Stathopoulou EM, Papamichael ND, et al. A highly constrained cyclic (S,S)‐CDC‐ peptide is a potent inhibitor of carotid artery thrombosis in rabbits. Platelets. 2011;22(5):361‐370. [DOI] [PubMed] [Google Scholar]
- 162. Liu Z, Jia B, Zhao H, Chen X, Wang F. Specific targeting of human integrin α(v)β (3) with (111)in‐labeled Abegrin™ in nude mouse models. Mol Imaging Biol. 2011;13(1):112‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Delbaldo C, Raymond E, Vera K, et al. Phase I and pharmacokinetic study of etaracizumab (Abegrin), a humanized monoclonal antibody against alphavbeta3 integrin receptor, in patients with advanced solid tumors. Invest New Drugs. 2008;26(1):35‐43. [DOI] [PubMed] [Google Scholar]
- 164. Mulgrew K, Kinneer K, Yao XT, et al. Direct targeting of alphavbeta3 integrin on tumor cells with a monoclonal antibody. Abegrin Mol Cancer Ther. 2006;5(12):3122‐3129. [DOI] [PubMed] [Google Scholar]
- 165. Diaz Bessone MI, Simón‐Gracia L, Scodeller P, et al. IRGD‐guided tamoxifen polymersomes inhibit estrogen receptor transcriptional activity and decrease the number of breast cancer cells with self‐renewing capacity. J Nanobiotechnol. 2019;17(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Hamilton AM, Aidoudi‐Ahmed S, Sharma S, et al. Nanoparticles coated with the tumor‐penetrating peptide iRGD reduce experimental breast cancer metastasis in the brain. J Mol Med. 2015;93(9):991‐1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Zhang L, Xing Y, Gao Q, Sun X, Zhang D, Cao G. Combination of NRP1‐mediated iRGD with 5‐fluorouracil suppresses proliferation, migration and invasion of gastric cancer cells. Biomed Pharmacother. 2017;93:1136‐1143. [DOI] [PubMed] [Google Scholar]
- 168. Cano‐Cortes MV, Navarro‐Marchal SA, Ruiz‐Blas MP, Diaz‐Mochon JJ, Marchal JA, Sanchez‐Martin RM. A versatile theranostic nanodevice based on an orthogonal bioconjugation strategy for efficient targeted treatment and monitoring of triple negative breast cancer. Nanomedicine. 2020;24:102120. [DOI] [PubMed] [Google Scholar]
- 169. Mao XL, Zhang H, Yu Y, et al. Preparation and properties of salinomycin micelles modified by tumor‐penetrating peptide. Chinese J N Drugs. 2014;23(23):2812‐2816. [Google Scholar]
- 170. Yin XC, Xie PF, Liu L. Sunitinib‐loaded tumor penetrating peptide‐functionalized nanomicelles in oral squamous cell carcinoma. J Biomater Tissue Eng. 2016;6(7):509‐515. [Google Scholar]
- 171. Chen J, Cao F, Cao Y, Wei S, Zhu X, Xing W. Targeted therapy of lung adenocarcinoma by the Nanoplatform based on Milk exosomes loaded with paclitaxel. J Biomed Nanotechnol. 2022;18(4):1075‐1083. [DOI] [PubMed] [Google Scholar]
- 172. Zhang Q, Zhang Y, Li K, Wang H, Li H, Zheng J. A novel strategy to improve the therapeutic efficacy of gemcitabine for non‐small cell lung cancer by the tumor‐penetrating peptide iRGD. PloS One. 2015;10(6). doi: 10.1371/journal.pone.0129865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Zuo H. IRGD: a promising peptide for cancer imaging and a potential therapeutic agent for various cancers. J Oncol. 2019;2019:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Cho HJ, Lee SJ, Park SJ, et al. Activatable iRGD‐based peptide monolith: targeting, internalization, and fluorescence activation for precise tumor imaging. J Control Release. 2016;237:177‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Teesalu T, Sugahara KN, Ruoslahti E. Tumor‐penetrating peptides. Front Oncol. 2013;3:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Du R, Zhong T, Zhang WQ, et al. Antitumor effect of iRGD‐modified liposomes containing conjugated linoleic acid‐paclitaxel (CLA‐PTX) on B16‐F10 melanoma. Int J Nanomedicine. 2014;9(1):3091‐3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Hu H, Wang B, Lai C, et al. iRGD‐paclitaxel conjugate nanoparticles for targeted paclitaxel delivery. Drug Dev Res. 2019;80(8):1080‐1088. [DOI] [PubMed] [Google Scholar]
- 178. Kumar A, Huo S, Zhang X, et al. Neuropilin‐1‐targeted gold nanoparticles enhance therapeutic efficacy of platinum(IV) drug for prostate cancer treatment. ACS Nano. 2014;8(5):4205‐4220. [DOI] [PubMed] [Google Scholar]
- 179. Liu D, Wang C, Yang J, An Y, Yang R, Teng G. CRGDK‐functionalized PAMAM‐based drug‐delivery system with high permeability. ACS Omega. 2020;5(16):9316‐9323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Liu H, Shi X, Wu D, et al. Injectable, biodegradable, Thermosensitive nanoparticles‐aggregated hydrogel with tumor‐specific targeting, penetration, and release for efficient postsurgical prevention of tumor recurrence. ACS Appl Mater Interfaces. 2019;11(22):19700‐19711. [DOI] [PubMed] [Google Scholar]
- 181. Srichai MB, Zent R. Integrin structure and function. In: Zent R, Pozzi A, eds. Cell‐Extracellular Matrix Interactions in Cancer. Springer New York; 2010:19‐41. [Google Scholar]
- 182. Anderson LR, Owens TW, Naylor MJ. Structural and mechanical functions of integrins. Biophys Rev. 2014;6(2):203‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Pan L, Zhao Y, Yuan Z, Qin G. Research advances on structure and biological functions of integrins. SpringerPlus. 2016;5(1):1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Tvaroška I, Kozmon S, Kóňa J. Molecular modeling insights into the structure and behavior of Integrins: a review. Cell. 2023;12(2):324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Bartolomé RA, Peláez‐García A, Gomez I, et al. An RGD motif present in cadherin 17 induces integrin activation and tumor growth. J Biol Chem. 2014;289(50):34801‐34814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Tselepis VH, Green LJ, Humphries MJ. An RGD to LDV motif conversion within the disintegrin kistrin generates an integrin antagonist that retains potency but exhibits altered receptor specificity: evidence for a functional equivalence of acidic integrin‐binding motifs. J Biol Chem. 1997;272(34):21341‐21348. [DOI] [PubMed] [Google Scholar]
- 187. Schwab EH, Halbig M, Glenske K, Wagner A‐S, Wenisch S, Cavalcanti‐Adam EA. Distinct effects of RGD‐glycoproteins on integrin‐mediated adhesion and osteogenic differentiation of human mesenchymal stem cells. Int J Med Sci. 2013;10(13):1846‐1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. D'Onofrio PM, Shabanzadeh AP, Choi BK, Bähr M, Koeberle PD. MMP inhibition preserves integrin ligation and FAK activation to induce survival and regeneration in RGCs following optic nerve damage. Invest Ophthalmol Vis Sci. 2019;60(2):634‐649. [DOI] [PubMed] [Google Scholar]
- 189. Dumitrescu RG. Interplay between genetic and epigenetic changes in breast cancer subtypes. Methods Mol Biol. 2018;18562018:19‐34. [DOI] [PubMed] [Google Scholar]
- 190. Beer AJ, Niemeyer M, Carlsen J, Sarbia M, Nahrig J, Watzlowik P, et al. Patterns of αvβ3 expression in primary and metastatic human breast cancer as shown by 18F‐galacto‐RGD PET. J Nucl Med 2008;49(2):255–259. [DOI] [PubMed] [Google Scholar]
- 191. Gaertner FC, Kessler H, Wester HJ, Schwaiger M, Beer AJ. Radiolabelled RGD peptides for imaging and therapy. Eur J Nucl Med Mol Imaging. 2012;39(SUPPL.1):S126‐S138. [DOI] [PubMed] [Google Scholar]
- 192. Zako T, Nagata H, Terada N, et al. Cyclic RGD peptide‐labeled upconversion nanophosphors for tumor cell‐targeted imaging. Biochem Biophys Res Commun. 2009;381(1):54‐58. [DOI] [PubMed] [Google Scholar]
- 193. Worm DJ, Els‐Heindl S, Beck‐Sickinger AG. Targeting of peptide‐binding receptors on cancer cells with peptide‐drug conjugates. Peptide Science. 2020;112(3):e24171. [Google Scholar]
- 194. Park HJ, Helfman DM. Up‐regulated fibronectin in 3D culture facilitates spreading of triple negative breast cancer cells on 2D through integrin β‐5 and Src. Sci Rep. 2019;9(1):19950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Miskin RP, Warren JSA, Ndoye A, Wu L, Lamar JM, DiPersio CM. Integrin α3β1 promotes invasive and metastatic properties of breast cancer cells through induction of the Brn‐2 transcription factor. Cancers (Basel). 2021;13(3):480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Lahlou H, Muller WJ. β1‐integrins signaling and mammary tumor progression in transgenic mouse models: implications for human breast cancer. Breast Cancer Res. 2011;13(6):229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Chen R, Ni S, Chen W, Liu M, Feng J, Hu K. Improved anti‐triple negative breast cancer effects of docetaxel by RGD‐modified lipid‐core micelles. Int J Nanomedicine. 2021;16:5265‐5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Cheng Y, Chen Q, Qian Z, et al. Versatile red blood cells for triple‐negative breast cancer treatment via stepwise Photoactivations. Adv Healthc Mater. 2023;12(3):e2201690. [DOI] [PubMed] [Google Scholar]
- 199. Fan J, Liu B, Long Y, et al. Sequentially‐targeted biomimetic nano drug system for triple‐negative breast cancer ablation and lung metastasis inhibition. Acta Biomater. 2020;113:554‐569. [DOI] [PubMed] [Google Scholar]
- 200. Vaidya AM, Sun Z, Ayat N, et al. Systemic delivery of tumor‐targeting siRNA nanoparticles against an oncogenic LncRNA facilitates effective triple‐negative breast cancer therapy. Bioconjug Chem. 2019;30(3):907‐919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Chen Z, Fu H, Wu H, et al. Syntheses and preliminary evaluation of dual target pet probe [18f]‐nota‐gly3‐e (2peg4‐rgd‐wh701) for pet imaging of breast cancer. Anticancer Agents Med Chem. 2020;20(13):1548‐1557. [DOI] [PubMed] [Google Scholar]
- 202. Georgieva MG, Balacheva A, Detcheva R, Pajpanova T. Chemical modifications and anti‐proliferative activity of a Bi‐functional peptide against MDA‐MB‐231 breast cancer cells. J Chem Technol Metall. 2020;55(5):955‐958. [Google Scholar]
- 203. Goyal R, Jerath G, Akhil R, et al. Geometry encoded functional programming of tumor homing peptides for targeted drug delivery. J Control Release. 2021;333:16‐27. [DOI] [PubMed] [Google Scholar]
- 204. Gai Y, Jiang Y, Long Y, et al. Evaluation of an integrin αvβ3 and aminopeptidase N dual‐receptor targeting tracer for breast cancer imaging. Mol Pharm. 2020;17(1):349‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Torcasio SM, Oliva R, Montesi M, et al. Three‐armed RGD‐decorated starPLA‐PEG nanoshuttle for docetaxel delivery. Biomater Adv. 2022;140:213043. [DOI] [PubMed] [Google Scholar]
- 206. Abu‐Tayeh H, Weidenfeld K, Zhilin‐Roth A, et al. 'Normalizing' the malignant phenotype of luminal breast cancer cells via alpha(v)beta(3)‐integrin. Cell Death Dis. 2016;7(12):e2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Campbell PS, Mavingire N, Khan S, et al. AhR ligand aminoflavone suppresses α6‐integrin–Src–Akt signaling to attenuate tamoxifen resistance in breast cancer cells. J Cell Physiol. 2018;234(1):108‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Grigoryeva ES, Tashireva LA, Savelieva OE, et al. The Association of Integrins β3, β4, and αVβ5 on exosomes, CTCs and tumor cells with localization of distant metastasis in breast cancer patients. Int J Mol Sci. 2023;24(3):2929. doi: 10.3390/ijms24032929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Wu J, Tian J, Zhang Y, et al. 18F‐Alfatide II for the evaluation of axillary lymph nodes in breast cancer patients: comparison with 18F‐FDG. Eur J Nucl Med Mol Imaging. 2022;49(8):2869‐2876. [DOI] [PubMed] [Google Scholar]
- 210. Lv X, Zhang C, Shuaizhen Q, Yu R, Zheng Y. Design of integrin αvβ3 targeting self‐assembled protein nanoparticles with RGD peptide. Biomed Pharmacother. 2020;128:110236. [DOI] [PubMed] [Google Scholar]
- 211. Bolzati C, Salvarese N, Carpanese D, et al. [Tc(N)PNP43]‐labeled RGD peptides as new probes for a selective detection of αvβ3 integrin: synthesis, structure‐activity and pharmacokinetic studies. J Med Chem. 2018;61(21):9596‐9610. [DOI] [PubMed] [Google Scholar]
- 212. Antonow MB, Franco C, Prado W, et al. Arginylglycylaspartic acid‐surface‐functionalized doxorubicin‐loaded lipid‐Core Nanocapsules as a strategy to target alpha(V) Beta(3) integrin expressed on tumor cells. Nanomaterials. 2018;8(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Mohri K, Nhat KPH, Zouda M, et al. Lasso peptide microcin J25 variant containing RGD motif as a PET probe for integrin a v ß 3 in tumor imaging. Eur J Pharm Sci. 2023;180:106339. [DOI] [PubMed] [Google Scholar]
- 214. Lv J, Gao D, Zhang Y, Wu D, Shen L, Wang X. Heterogeneity of lipidomic profiles among lung cancer subtypes of patients. J Cell Mol Med. 2018;22(10):5155‐5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Zhang Y, Wang DC, Shi L, Zhu B, Min Z, Jin J. Genome analyses identify the genetic modification of lung cancer subtypes. Semin Cancer Biol. 2017;42:20‐30. [DOI] [PubMed] [Google Scholar]
- 216. Bernhagen D, De Laporte L, Timmerman P. High‐affinity RGD‐Knottin peptide as a new tool for rapid evaluation of the binding strength of unlabeled RGD‐peptides to αvβ3, αvβ5, and α5β1 integrin receptors. Anal Chem. 2017;89(11):5991‐5997. [DOI] [PubMed] [Google Scholar]
- 217. Misra SK, Kondaiah P, Bhattacharya S, Boturyn D, Dumy P. Co‐liposomes comprising a lipidated multivalent RGD‐peptide and a cationic gemini cholesterol induce selective gene transfection in αvβ3 and αvβ5 integrin receptor‐rich cancer cells. J Mater Chem B. 2014;2(35):5758‐5767. [DOI] [PubMed] [Google Scholar]
- 218. Zheng Y, Ji S, Czerwinski A, Valenzuela F, Pennington M, Liu S. FITC‐conjugated cyclic RGD peptides as fluorescent probes for staining integrin αvβ3/αvβ5 in tumor tissues. Bioconjug Chem. 2014;25(11):1925‐1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Park KY, Jeong J, Kim J. Synthesis and biological evaluation of cilengitide derivatives on TGF‐β1‐induced epithelial‐to‐mesenchymal transition in human non‐small cell lung cancer cells. Pept Sci. 2022;114(6):e24285. doi: 10.1002/pep2.24285 [DOI] [Google Scholar]
- 220. Monti M, De Rosa V, Iommelli F, et al. Neutrophil extracellular traps as an adhesion substrate for different tumor cells expressing RGD‐binding integrins. Int J Mol Sci. 2018;19(8):2350. doi: 10.3390/ijms19082350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Liu B, Zhang Z, Wang H, Yao S. Preclinical evaluation of a dual sstr2 and integrin αvβ3‐targeted heterodimer [68Ga]‐NOTA‐3PEG4‐TATE‐RGD. Bioorg Med Chem. 2019;27:115094. [DOI] [PubMed] [Google Scholar]
- 222. Kori M, Arga KY, Mardinoglu A, Turanli B. Repositioning of anti‐inflammatory drugs for the treatment of cervical cancer sub‐types. Front Pharmacol. 2022;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Sirvent JJ, Blázquez S. Uterine cervical cancer. Anatomopathologic Types Ciencia Ginecologika. 2003;7(5):317‐327. [Google Scholar]
- 224. Goyal R, Jerath G, Chandrasekharan A, Kumar TRS, Ramakrishnan V. Peptide‐based delivery vectors with pre‐defined geometrical locks. RSC Med Chem. 2020;11(11):1303‐1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Lin Y, Zhong Y, Chen Y, et al. Ligand‐modified erythrocyte membrane‐cloaked metal‐organic framework nanoparticles for targeted antitumor therapy. Mol Pharm. 2020;17(9):3328‐3341. [DOI] [PubMed] [Google Scholar]
- 226. Zhang G, Gao R, Wang Y, et al. Hyperplastic thymus with increased angiogenesis is correlated with elevated serum thyroglobulin level in differentiated thyroid cancer patients with TENIS syndrome. Oncotarget. 2018;9(3):3406‐3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Zhang Y, Xiu W, Sun Y, et al. RGD‐QD‐MoS2 nanosheets for targeted fluorescent imaging and photothermal therapy of cancer. Nanoscale. 2017;9(41):15835‐15845. [DOI] [PubMed] [Google Scholar]
- 228. Li W, Sun D, Li N, Shen Y, Hu Y, Tan J. Therapy of cervical cancer using 131 I‐labeled nanoparticles. J Int Med Res. 2018;46(6):2359‐2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Jeffers MD, Paxton J, Bolger B, Richmond JA, Kennedy JH, McNicol AM. E‐cadherin and integrin cell adhesion molecule expression in invasive and in situ carcinoma of the cervix. Gynecol Oncol. 1997;64(3):481‐486. [DOI] [PubMed] [Google Scholar]
- 230. Kim JE, Lee SK, Park J, et al. Buddlejasaponin IV induces apoptotic cell death by activating the mitochondrial‐dependent apoptotic pathway and reducing α2β1 integrin‐mediated adhesion in HT‐29 human colorectal cancer cells. Oncol Rep. 2023;49(3):58. doi: 10.3892/or.2023.8495 [DOI] [PubMed] [Google Scholar]
- 231. Soares MQS, Mendonça JA, Morais MO, Leles CR, Batista AC, Mendonça EF. E‐cadherin, β‐catenin, and α2βl and α3βl integrin expression in primary oral squamous cell carcinoma and its regional metastasis. Histol Histopathol. 2015;30(10):1213‐1222. [DOI] [PubMed] [Google Scholar]
- 232. Chatterjee A, Chattopadhyay N. Role of αvβ3 integrin receptor in the invasive potential of human cervical cancer (SiHa) cells. J Environ Pathol Toxicol Oncol. 2001;20(3):211‐221. [PubMed] [Google Scholar]
- 233. Huang C‐C, Liu F‐R, Feng Q, Pan X‐Y, Song S‐L, Yang J‐L. RGD4C peptide mediates anti‐p21Ras scFv entry into tumor cells and produces an inhibitory effect on the human colon cancer cell line SW480. BMC Cancer. 2021;21(1):321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Chin Y‐T, He Z‐R, Chen C‐L, et al. Tetrac and NDAT induce anti‐proliferation via integrin αvβ3 in colorectal cancers with different K‐RAS status. Front Endocrinol. 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Böger C, Kalthoff H, Goodman SL, Röcken C. Validation and comparison of anti‐αvβ3 and anti‐αvβ5 rabbit monoclonal versus murine monoclonal antibodies in four different tumor entities. Appl Immunohistochem Mol Morphol. 2013;21(6):553‐560. [DOI] [PubMed] [Google Scholar]
- 236. Pelillo C, Bergamo A, Mollica H, Bestagno M, Sava G. Colorectal cancer metastases settle in the hepatic microenvironment through α5β1 integrin. J Cell Biochem. 2015;116(10):2385‐2396. [DOI] [PubMed] [Google Scholar]
- 237. Yang B, Gao J, Rao Z, Shen Q. Clinicopathological and prognostic significance of α5β1‐integrin and MMP‐14 expressions in colorectal cancer. Neoplasma. 2013;60(3):254‐261. [DOI] [PubMed] [Google Scholar]
- 238. Bourn MD, Mohajerani S, Mavria G, Ingram N, Coletta PL, Evans SD, et al., Tumour associated vasculature‐on‐a‐chip for the evaluation of microbubble‐mediated delivery of targeted liposomes. Lab Chip. 2023;23:1674‐1693 doi: 10.1039/D2LC00963C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Kerr JS, Wexler RS, Mousa SA, et al. Novel small molecule αv integrin antagonists: comparative anti‐cancer efficacy with known angiogenesis inhibitors. Anticancer Res. 1999;19(2 A):959‐968. [PubMed] [Google Scholar]
- 240. Lee YM, Yoon YS, Roh SA, Cho DH, Kim JC. Invasiveness of and drug sensitivity to various anti‐cancer regimens in five colorectal cancer cell lines. J Korean Soc Coloproctol. 2010;26(2):98‐104. [Google Scholar]
- 241. Zhou YQ, Lv XP, Li S, Bai B, Zhan LL. Synergy of urokinase‐type plasminogen activator receptor isomer (D1D2) and integrin α5β1 causes malignant transformation of hepatic cells and the occurrence of liver cancer. Mol Med Rep. 2014;10(5):2568‐2574. [DOI] [PubMed] [Google Scholar]
- 242. Yan C, Yang Q, Gong Z. Activation of hepatic stellate cells during liver carcinogenesis requires fibrinogen/integrin αvβ5 in zebrafish. Neoplasia. 2018;20(5):533‐542. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 243. Ji P, Li Z, Dong J, Yi H. SO2 derivatives and As co‐exposure promote liver cancer metastasis through integrin αvβ3 activation. Ecotoxicol Environ Saf. 2019;181:572‐578. [DOI] [PubMed] [Google Scholar]
- 244. Popova NA, Nikolin VP, Kaledin VI, et al. Experimental study of antitumor activity of Pefagtal addressed to αvβ3 Integrins. Bull Exp Biol Med. 2022;173(1):105‐109. [DOI] [PubMed] [Google Scholar]
- 245. Li J, Zhou P, Xu H, et al. Antitumor activity of integrin αVβ3 antibody conjugated‐cationic microbubbles in liver cancer. Transl Cancer Res. 2019;8(3):899‐908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Capasso D, Del Gatto A, Comegna D, et al. Selective targeting of αvβ5 integrin in HepG2 cell line by RGDechi15D peptide. Molecules. 2020;25(18):4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Schmohl KA, Han Y, Tutter M, et al. Integrin αvβ3‐dependent thyroid hormone effects on tumour proliferation and vascularisation. Endocr Relat Cancer. 2020;27(12):685‐697. [DOI] [PubMed] [Google Scholar]
- 248. Collisson EA, Bailey P, Chang DK, Biankin AV. Molecular subtypes of pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2019;16(4):207‐220. [DOI] [PubMed] [Google Scholar]
- 249. Cao J, Li J, Sun L, et al. Hypoxia‐driven paracrine osteopontin/integrin αvβ3 signaling promotes pancreatic cancer cell epithelial–mesenchymal transition and cancer stem cell‐like properties by modulating forkhead box protein M1. Mol Oncol. 2019;13(2):228‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Debreli Coskun M, Sudha T, Bharali DJ, Celikler S, Davis PJ, Mousa SA. αvβ3 integrin antagonists enhance chemotherapy response in an Orthotopic pancreatic cancer model. Front Pharmacol. 2020;11:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Sudha T, Godugu K, Glinsky GV, Mousa SA. Triazole modified Tetraiodothyroacetic acid conjugated to polyethylene glycol, a Thyrointegrin αvβ3 antagonist as a radio‐and chemo‐sensitizer in pancreatic cancer. Biomedicine. 2022;10(4):795. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 252. Kanemaru M, Maehara N, Iwamura T, Chijiiwa K. Thrombin stimulates integrin β1‐dependent adhesion of human pancreatic cancer cells to vitronectin through protease‐activated receptor (PAR)‐1. Hepatogastroenterology. 2012;59(117):1614‐1620. [DOI] [PubMed] [Google Scholar]
- 253. Al Faruque H, Choi ES, Kim JH, Kim E. Enhanced effect of autologous EVs delivering paclitaxel in pancreatic cancer. J Control Release. 2022;347:330‐346. [DOI] [PubMed] [Google Scholar]
- 254. Chen YC, Chang YT, Chen CY, et al. Structural insight into integrin recognition and anticancer activity of Echistatin. Toxins. 2020;12(11):709. doi: 10.3390/toxins12110709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Geng T, Leung E, Chamley LW, Wu Z. Functionalisation of extracellular vesicles with cyclic‐RGDyC potentially for glioblastoma targeted intracellular drug delivery. Biomater Adv. 2023;149:213388. [DOI] [PubMed] [Google Scholar]
- 256. Mitsuyuki K, Watabe T, Naka S, et al. Evaluation of integrin αvβ3 expression in murine xenograft models: [68ga]ga‐dota‐c(rgdfk) pet study with immunohistochemical confirmation. Diagnostics. 2021;11(7):1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Jiang Y, Long Y, Ji H, et al. Development and evaluation of a peptide heterodimeric tracer targeting CXCR4 and integrin αvβ3 for pancreatic cancer imaging. Pharmaceutics. 2022;14(9):1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Zhao H, Gao H, Luo C, et al. An integrin‐αvβ6/α5β1‐Bitargeted probe for the SPECT imaging of pancreatic adenocarcinoma in preclinical and primary clinical studies. Bioconjug Chem. 2021;32(7):1298‐1305. [DOI] [PubMed] [Google Scholar]
- 259. Ji S, Xu J, Zhang B, et al. RGD‐conjugated albumin nanoparticles as a novel delivery vehicle in pancreatic cancer therapy. Cancer Biol Ther. 2012;13(4):206‐215. [DOI] [PubMed] [Google Scholar]
- 260. Akashi Y, Oda T, Ohara Y, et al. Anticancer effects of gemcitabine are enhanced by co‐administered iRGD peptide in murine pancreatic cancer models that overexpressed neuropilin‐1. Br J Cancer. 2014;110(6):1481‐1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Zuo HD, Yao WW, Chen TW, et al. The effect of superparamagnetic iron oxide with iRGD peptide on the labeling of pancreatic cancer cells in vitro: a preliminary study. Biomed Res Int. 2014;2014:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Zhou M, Ni Q‐W, Yang S‐Y, et al. Effects of integrin‐targeted photodynamic therapy on pancreatic carcinoma cell. World J Gastroenterol: WJG. 2013;19(39):6559‐6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Ikuemonisan J, Togun A, Oyejinmi I, Bakare A, Adejoro O. Influence of histologic types and subtypes on survival outcomes of intermediate‐high and high‐risk renal cell carcinoma following nephrectomy: findings from the SEER database. Urology. 2022;159:146‐151. [DOI] [PubMed] [Google Scholar]
- 264. Serie DJ, Joseph RW, Cheville JC, et al. Clear cell type a and B molecular subtypes in metastatic clear cell renal cell carcinoma: tumor heterogeneity and aggressiveness. Eur Urol. 2017;71(6):979‐985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Wechsel HW, Petri E, Feil G, Nelde HJ, Bichler KH, Loesr W. Renal Cell Carcinoma: Immunohistological Investigation of Expression of the Integrin αvβ3. Anticancer Research; 1999. [PubMed] [Google Scholar]
- 266. Mierke CT, Frey B, Fellner M, Herrmann M, Fabry B. Integrin α5β1 facilitates cancer cell invasion through enhanced contractile forces. J Cell Sci. 2011;124(3):369‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Lee EJ, Ahmad K, Pathak S, et al. Identification of novel fnin2 and fnin3 fibronectin‐derived peptides that promote cell adhesion, proliferation and differentiation in primary cells and stem cells. Int J Mol Sci. 2021;22(6):1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Xiong J, Yan L, Zou C, et al. Integrins regulate stemness in solid tumor: an emerging therapeutic target. J Hematol Oncol. 2021;14(1):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.


