Abstract
Introduction.
Data on the effect of body mass index (BMI) on laparoscopic liver resections are conflicting. We performed this study to investigate the association between BMI and postoperative outcomes following laparoscopic major hepatectomies (LMH).
Methods:
This is a retrospective review of 4,348 laparoscopic major hepatectomies at 58 centers between 2005 and 2021, of which 3,383 met the study inclusion criteria. Concomitant major operations, vascular resections, and previous liver resections were excluded Associations between BMI and perioperative outcomes were analyzed using restricted cubic splines. Modeled effect sizes were visually rendered and summarized.
Results.
1810 patients (53.5%) had normal weight while 1057 (31.2%) were overweight and 392 (11.6%) were obese. One hundred and twenty-four patients (3.6%) were underweight. Most perioperative outcomes showed a linear worsening trend with increasing BMI. There was a statistically significant increase in open conversion rate (16.3%, 10.8%, 9.2%, and 5.6%, p<0.001), longer operation time (320 vs. 305 vs. 300 and 266 minutes, p<0.001), increasing blood loss (300 vs. 300 vs. 295 vs. 250 ml, p=0.022) and higher postoperative morbidity (33.4% vs. 26.3% vs. 25.0% vs. 25.0%, p=0.009) in obese, overweight, normal weight, and underweight patients respectively (p<0.001). However, postoperative major morbidity demonstrated a “U” shaped association with BMI whereby the highest major morbidity rates were observed in underweight and obese patients.
Conclusion:
LMH was associated with poorer outcomes with increasing BMI for most perioperative outcome measures.
Keywords: laparoscopic liver, laparoscopic hepatectomy, minimally invasive liver, minimally invasive hepatectomy, body mass index, difficulty score
Short abstract:
LMH was associated with poorer outcomes with increasing BMI for most perioperative outcome measures. Postoperative major morbidity demonstrated a “U” shaped association with BMI with the worst outcomes observed in underweight and obese patients.
INTRODUCTION
Obesity is one of the major global health problems.1 Approximately 1.5 billion people worldwide are overweight and more than 670 million are obese. Western countries have the highest prevalence of obesity, and the incidence is still on the rise in the United States.2, 3 A high body mass index (BMI) is frequently associated with metabolic syndrome (MS) and multiple comorbidities such as diabetes, cardiovascular diseases, and chronic obstructive pulmonary disease.4 Furthermore, obese individuals more commonly develop non-alcoholic fatty liver disease (NAFLD), which encompasses significant changes in the liver’s tissue architecture from simple steatosis to steatohepatitis, possibly leading to fibrosis and eventually cirrhosis.5 Surgery in obese patients is associated with increased risks of postoperative complications across a wide range of specialties and procedures.6–10 As a matter of fact, despite mortality and morbidity rates having significantly decreased in high volume centers over the last two decades, liver resections in patients with obesity and MS are associated with a two-fold risk of postoperative morbidity considering patients’ comorbidities and parenchymal changes.11–14 The extent of liver resection is also a major risk factor for postoperative complications. Indeed, major hepatectomies have shown to be associated with increased risks of overall complications as compared to minor resections.15
Increased BMI in otherwise healthy patients have been shown to be an independent predictive factor for mortality after hepatectomy in a large nationwide United States cohort.16 Obesity limits surgical exposure and increases technical challenges because of the abundance of intra-abdominal fatty tissue: dissection and isolation of structures are demanding and time-consuming, as well as mobilization and parenchymal transection which are much more difficult with a large and steatotic liver. 17–20
Laparoscopic liver resections (LLRs) have gained widespread popularity due to the decreased morbidity, length of hospital stays, pain, and more rapid recovery compared with the standard open approach. More than 9000 LLRs have been reported for both benign and malignant diseases, with significant improvements over time.21, 22 However, given the technical challenges, LLR is still limited to minor resections in most centers worldwide and only a minority of major hepatic resections are performed by laparoscopy.23 Patients with high BMI could potentially benefit the most from a mini-invasive approach but results are so far conflicting. Small and heterogenous studies have reported safe postoperative outcomes but long operative time, high blood loss and conversions rates, as well as an increased risk for postoperative infections.24–27 To date, no studies has focused on major liver resections. This study aimed to investigate the association between BMI and postoperative outcomes following laparoscopic major hepatectomies (LMH).
MATERIALS AND METHODS
This was a retrospective review of 4348 patients who underwent pure laparoscopic major LMH at 58 international centers between 2005 and 2021. There were 37 centers from the West and 21 centers from the East. All institutions obtained approvals according to their local center’s requirements. The study was approved by the Singapore General Hospital Institution Review Board and the need for patient consent was waived. The de-identified data were collected by the individual centers. These were collected and analyzed centrally at the Singapore General Hospital. Patients who underwent concomitant major operations such as bilio-enteric anastomoses, lymph node dissections, colectomies, stoma reversals, gastrectomies, splenectomies, and vascular resections were excluded. Patients with previous liver resections were also excluded. Patients who underwent concomitant minor operations such as hernia repairs and ablations were included. After exclusion, there were 3637 LMH. Two-hundred and fifty-four cases with missing data on BMI were excluded and finally, 3383 patients were included in the analysis.
Definitions
Body mass index was divided into four categories: underweight (<18.5 kg/m2), normal BMI (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), and obese (≥30.0 kg/m2). Liver resections were defined according to the 2000 Brisbane classification.28 Formal major resections were classified as resections of 3 or more segments. Additionally, right anterior, and right posterior sectionectomies were considered technically major resections in this study.29–30 Diameter of the largest lesion was used for tumor size in cases with multiple tumors. The difficulty of resections was graded according to the Iwate score and Institut Mutualiste Montsouris (IMM) system.31–34 Postoperative complications were classified according to the Clavien-Dindo classification and recorded for up to 30 days or during the same hospitalization.35
Statistical analyses
Continuous and categorical variables were compared between the BMI groups using Kruskal Wallis and Fisher’s exact tests respectively, and omnibus p-values <0.05 were interpreted to indicate statistically significant differences between categories. As it is conceivable that the dose-response relationships between BMI and perioperative outcomes could be non-linear or even non-monotonic, we also analyzed associations using restricted cubic splines. This approach makes fewer a priori assumptions regarding model specification compared to standard regression approaches which typically assume linear or log-linear relationships. The number of knots, between 3 to 7, was chosen based on minimizing the Akaike information criterion (AIC) of quantile regression or modified Poisson regression models for continuous and binary endpoints respectively, adjusted for all baseline characteristics and centered at their means. Knot locations were placed at the recommended percentiles according to Harrell and colleagues. Modeled effect sizes (median differences or relative risks for continuous and binary outcomes respectively) were visually rendered and summarized.
RESULTS
Three thousand three hundred and eighty-three patients from January 2005 to December 2021 were included in this study of which 1728 cases were performed in Eastern centers and 1655 in Western centers. Baseline patient characteristics are summarized in Table 1. Most procedures were performed for malignant diseases (82.9%) with a median tumor size of 42 mm (IQR 25–70) and 28.6% multiple tumors. Seventy-three percent of patients underwent a formal major hepatectomy (right, right extended, left or left extended) while 27.3% underwent a technically major operation. The IWATE score of the entire cohort was “expert level” in 59.4% and 70% of the procedures had the highest grade of the IMM score (level III).
Table 1.
Baseline characteristics stratified by BMI.
| All | Underweight | Normal BMI | Overweight | Obese | ||
|---|---|---|---|---|---|---|
| n=3383 | (BMI < 18.5 kg/m2) | (BMI 18.5–24.9 kg/m2) | (BMI 25.0–29.9kg/m2) | (BMI ≥ 30.0 kg/m2) | p-value | |
| n=124 | n=1810 | n=1057 | n=392 | |||
|
| ||||||
| Median age (IQR), yrs | 62 (52–71) | 61 (48–71) | 62 (52–71) | 63 (54–71) | 62 (53–71) | 0.519 |
| Male gender, n (%) | 2093/3381 (61.9%) | 64/124 (51.6%) | 1104/1809 (61.0%) | 710/1056 (67.2%) | 215/392 (54.8%) | <0.001 |
| Previous abdominal surgery, n (%) | 1097/3360 (32.6%) | 44/124 (35.5%) | 524/1801 (29.1%) | 362/1048 (34.5%) | 167/387 (43.2%) | <0.001 |
| ASA score, n (%) | <0.001 | |||||
| 1–2 | 2555 (75.5%) | 106 (85.5%) | 1413 (78.0%) | 778 (73.6%) | 258 (65.8%) | |
| 3–4 | 828 (24.5%) | 18 (14.5%) | 397 (22.0%) | 279 (26.4%) | 134 (34.2%) | |
| Malignant neoplasm, n (%) | 2803/3382 (82.9%) | 106/124 (85.5%) | 1503/1810 (83.0%) | 885/1056 (83.8%) | 309/392 (78.8%) | 0.134 |
| Disease | 0.006 | |||||
| HCC/ICC | 1767/3382 (52.2%) | 63/124 (50.8%) | 985/1810 (54.4%) | 553/1056 (52.4%) | 166/392 (42.3% | |
| CRLM | 848/3382 (25.1%) | 35/124 (28.2%) | 424/1810 (23.4%) | 275/1056 (26.0%) | 114/392 (29.1%) | |
| Other metastases | 143/3382 (4.2%) | 7/124 (5.6%) | 72/1810 (4.0%) | 41/1056 (3.9%) | 23/392 (5.9%) | |
| Benign | 624 (18.5%) | 19/124 (15.3%) | 329 (18.2%) | 187/1056 (17.7%) | 89/392 (22.7%) | |
| Median tumor size, mm (IQR) | 42 (25–70) | 40 (28–67) | 40 (25–70) | 44 (25–70) | 46 (30–70) | 0.332 |
| Multiple tumors, n (%) | 968/3382 (28.6%) | 41/124 (33.1%) | 501/1810 (27.7%) | 295/1056 (27.9%) | 131/392 (33.4%) | 0.085 |
| Formal major (>3 segments), n (%) | 2459 (72.7%) | 93 (75.0%) | 1305 (72.1%) | 770 (72.8%) | 291 (74.2%) | 0.785 |
| Technical major, n (%) | 923 (27.3%) | 31 (25.0%) | 505 (27.9%) | 286 (27.1%) | 101 (25.8%) | 0.884 |
| Difficult segments (I, IVa, VII, VIII), n (%) | 2503 (74.0%) | 94 (75.8%) | 1346 (74.4%) | 775 (73.3%) | 288 (73.5%) | 0.891 |
| Multiple resections, n (%) | 269 (8.0%) | 12 (9.7%) | 150 (8.3%) | 77 (7.3%) | 30 (7.7%) | 0.664 |
| Median Iwate difficulty score, (IQR) | 10 (9–11) | 10 (9–11) | 10 (9–11) | 10 (9–11) | 10 (9–10) | 0.759 |
| Iwate difficulty score, n (%) | 0.412 | |||||
| Low | 0/3380 (0.0%) | 0/124 (0.0%) | 0/1810 (0.0%) | 0/1056 (0.0%) | 0/390 (0.0%) | |
| Intermediate | 122/3380 (3.6%) | 5/124 (4.0%) | 68/1810 (3.8%) | 40/1056 (3.8%) | 9/390 (2.3%) | |
| High | 1250/3380 (37.0%) | 36/124 (29.0%) | 666/1810 (36.8%) | 402/1056 (38.1%) | 146/390 (37.4%) | |
| Expert | 2008/3380 (59.4%) | 83/124 (66.9%) | 1076/1810 (59.4%) | 614/1056 (58.1%) | 235/390 (60.3%) | |
| IMM difficulty, n (%) | 0.453 | |||||
| I | 1/3383 (0.0%) | 0 (0.0%) | 1 (0.1%) | 0 (0.0%) | 0 (0.0%) | |
| II | 1013 (29.9%) | 45 (36.3%) | 526 (29.1%) | 318 (30.1%) | 124 (31.6%) | |
| III | 2369 (70.0%) | 79 (63.7%) | 1283 (70.9%) | 739 (69.9%) | 268 (68.4%) | |
IQR, interquartile range; ASA, American Society of Anesthesiology; HCC, hepatocellular carcinoma; ICC, Intrahepatic cholangiocarcinoma; CRLM, colorectal liver metastases; IMM, Institut Mutualiste Montsouris.
One thousand eight hundred and ten patients (53.5%) had normal weight while 1057 (31.2%) were overweight and 392 (11.6%) were obese. One hundred and twenty-four patients (3.6%) were underweight. There were no major clinical differences between the groups except for gender, previous abdominal surgery, disease, and ASA score (Table 1). Not unexpectedly, in the underweight and normal weight groups there was a higher proportion of cases performed at Eastern centers [88/124 (71.0%) and 1092/1810 (60.3%)] whereas in the overweight and obese groups there was a higher proportion of cases in Western centers [585/1057 (55.3%) and 316/392 (80.6%)].
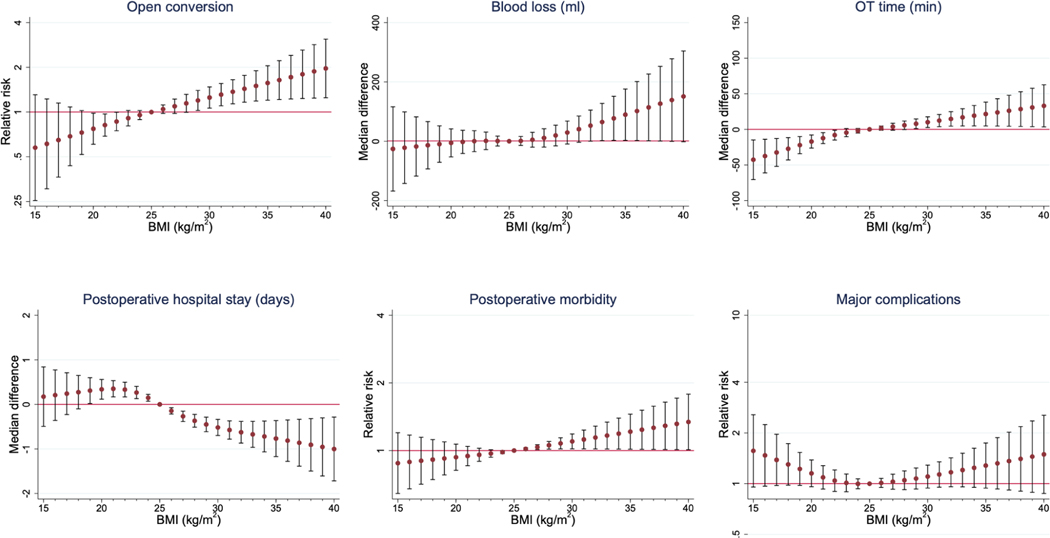
Of note, overweight and obese patients had overall more comorbidities, with a statistically significant higher rate of ASA 3–4 score (34.2% vs. 26.3% vs. 21.9% vs. 14.5% in obese, overweight, normal weight, and underweight respectively; p<0.001). Conversion to open was reported in 16.3%, 10.8%, 9.2%, and 5.6% of obese, overweight, normal weight, and underweight patients respectively (p<0.001). Median operative time was statistically significantly longer in obese patients (320 vs. 305 vs. 300 and 266 minutes in obese, overweight, normal weight, and underweight respectively; p<0.001, Table 2). Intraoperative blood loss was statistically significantly more in obese and overweight patients (300 vs. 300 vs. 295 and 250 ml in obese, overweight, normal weight, and underweight respectively; p=0.022). Interestingly, median postoperative hospital stay was shorter in obese patients (6 vs. 7 vs. 7 and 7 days in obese, overweight, normal weight, and underweight respectively; p<0.001). Finally, postoperative overall morbidity was statistically significantly higher in obese patients (33.4% vs. 26.3% vs. 25.0% vs. 25.0%, obese, overweight, normal weight, and underweight respectively; p=0.009) while no differences were observed in terms of major complications (p=0.262). A linear association between higher BMI and longer operative time was shown from the restricted cubic splines analysis (Table 3). A similar linear association was also shown between BMI and blood loss, conversion to open, and postoperative morbidity. The above-mentioned linear associations are visually rendered in Figure 1. A “U” shaped association was observed between major morbidity and BMI. The detailed description of type of major morbidity (Clavien Dindo grade ≥3) stratified by the 4 BMI groups is summarized in Table 4.
Table 2.
Perioperative data stratified by BMI.
| All | Underweight | Normal BMI | Overweight | Obese | ||
|---|---|---|---|---|---|---|
| n=3383 | (BMI < 18.5 kg/m2) | (BMI 18.5–24.9 kg/m2) | (BMI 25.0–29.9kg/m2) | (BMI ≥ 30.0 kg/m2) | p-value | |
| n=124 | n=1810 | n=1057 | n=392 | |||
|
| ||||||
| Conversion to open, n (%) | 352 (10.4%) | 7 (5.6%) | 167 (9.2%) | 114 (10.8%) | 64 (16.3%) | <0.001 |
| Median operative time (IQR), min | 300 (235–390) | 266 (195–365) | 300 (225–380) | 305 (240–390) | 320 (250–416) | <0.001 |
| Median blood loss (IQR), ml | 300 (130–500) | 250 (100–500) | 295 (100–500) | 300 (150–540) | 300 (200–500) | 0.022 |
| Median postoperative stay (IQR), days | 7 (5–10) | 7(5–10) | 7(5–10) | 7 (5–9) | 6 (4–9) | <0.001 |
| Postoperative morbidity, n (%) | 892/3382 (26.4%) | 31/124 (25.0%) | 452/1810 (25.0%) | 278/1056 (26.3%) | 131/392 (33.4%) | 0.009 |
| Major morbidity (Clavien-Dindo grade> 2) | 347/3382 (10.3%) | 14/124 (11.3%) | 180/1810 (9.9%) | 102/1056 (9.7%) | 51/392 (13.0%) | 0.262 |
| 30-day mortality, n (%) | 28 (0.8%) | 2 (1.6%) | 16 (0.9%) | 7 (0.7%) | 3 (0.8%) | 0.576 |
| 90-day mortality, n (%) | 47 (1.4%) | 1 (0.8%) | 31 (1.7%) | 11 (1.0%) | 4 (1.0%) | 0.478 |
Table 3.
Modelled effect sizes from restricted cubic splines (RCS) analyses, depicting adjusted association between BMI and perioperative outcomes. Reference BMI is set as 25kg/m2.
| BMI | Operative time | Blood loss | Pringle maneuver | Blood transfusion | Open conversion | Morbidity | Major morbidity | Length of stay | 90-day mortality |
|---|---|---|---|---|---|---|---|---|---|
| (kg/m2) | MD (95% CI) | MD (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | MD (95% CI) | RR (95% CI) |
| 15 | −43 (−71 to −15) | −26 (−168 to 116) | 0.94 (0.78 to 1.14) | 1.53 (1.03 to 2.25) | 0.58 (0.25 to 1.31) | 0.88 (0.64 to 1.2) | 1.57 (0.96 to 2.57) | 0.2 (−0.5 to 0.8) | 3.72 (1.7 to 8.12) |
| 16 | −38 (−61 to −14) | −22 (−142 to 99) | 0.96 (0.82 to 1.12) | 1.44 (1.03 to 2.01) | 0.61 (0.3 to 1.23) | 0.89 (0.68 to 1.17) | 1.47 (0.97 to 2.24) | 0.2 (−0.4 to 0.8) | 3.21 (1.61 to 6.41) |
| 17 | −32 (−52 to −13) | −18 (−117 to 82) | 0.97 (0.85 to 1.11) | 1.36 (1.03 to 1.8) | 0.65 (0.36 to 1.15) | 0.9 (0.71 to 1.15) | 1.38 (0.98 to 1.97) | 0.2 (−0.2 to 0.7) | 2.77 (1.52 to 5.06) |
| 18 | −27 (−43 to −12) | −14 (−93 to 66) | 0.99 (0.89 to 1.09) | 1.29 (1.02 to 1.62) | 0.69 (0.44 to 1.08) | 0.91 (0.74 to 1.12) | 1.3 (0.98 to 1.73) | 0.3 (−0.1 to 0.7) | 2.39 (1.44 to 3.99) |
| 19 | −22 (−34 to −10) | −10 (−71 to 52) | 1 (0.92 to 1.08) | 1.22 (1.01 to 1.46) | 0.73 (0.52 to 1.02) | 0.92 (0.78 to 1.09) | 1.22 (0.98 to 1.53) | 0.3 (0 to 0.6) | 2.07 (1.36 to 3.15) |
| 20 | −17 (−26 to −8) | −6 (−53 to 42) | 1.01 (0.95 to 1.08) | 1.15 (0.99 to 1.34) | 0.77 (0.61 to 0.98) | 0.93 (0.82 to 1.07) | 1.15 (0.96 to 1.38) | 0.3 (0.1 to 0.6) | 1.79 (1.28 to 2.49) |
| 21 | −12 (−20 to −5) | −2 (−41 to 37) | 1.02 (0.98 to 1.08) | 1.09 (0.95 to 1.25) | 0.82 (0.69 to 0.97) | 0.95 (0.86 to 1.04) | 1.09 (0.93 to 1.28) | 0.4 (0.2 to 0.5) | 1.54 (1.2 to 1.98) |
| 22 | −8 (−15 to −1) | 0 (−35 to 36) | 1.03 (0.99 to 1.08) | 1.05 (0.93 to 1.18) | 0.86 (0.75 to 1) | 0.96 (0.9 to 1.02) | 1.04 (0.9 to 1.21) | 0.3 (0.2 to 0.5) | 1.35 (1.13 to 1.6) |
| 23 | −5 (−10 to 1) | 1 (−28 to 31) | 1.03 (0.99 to 1.07) | 1.02 (0.92 to 1.12) | 0.91 (0.81 to 1.03) | 0.97 (0.93 to 1.01) | 1.01 (0.9 to 1.14) | 0.3 (0.1 to 0.4) | 1.19 (1.07 to 1.33) |
| 24 | −2 (−5 to 1) | 1 (−16 to 17) | 1.02 (0.99 to 1.04) | 1 (0.95 to 1.06) | 0.95 (0.89 to 1.02) | 0.98 (0.97 to 1) | 1 (0.93 to 1.07) | 0.2 (0.1 to 0.2) | 1.08 (1.02 to 1.14) |
| 25 | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| 26 | 2 (−1 to 5) | 1 (−14 to 16) | 0.99 (0.97 to 1.01) | 1 (0.95 to 1.05) | 1.05 (0.98 to 1.12) | 1.02 (1 to 1.03) | 1.01 (0.95 to 1.07) | −0.2 (−0.2 to −0.1) | 0.96 (0.89 to 1.02) |
| 27 | 4 (−1 to 9) | 5 (−20 to 30) | 0.98 (0.95 to 1.01) | 0.99 (0.91 to 1.08) | 1.09 (0.98 to 1.22) | 1.04 (1.01 to 1.06) | 1.03 (0.93 to 1.14) | −0.3 (−0.4 to −0.2) | 0.93 (0.8 to 1.09) |
| 28 | 6 (0 to 12) | 11 (−20 to 42) | 0.98 (0.94 to 1.02) | 0.98 (0.88 to 1.09) | 1.14 (1 to 1.32) | 1.06 (1.01 to 1.1) | 1.05 (0.92 to 1.19) | −0.4 (−0.5 to −0.2) | 0.93 (0.72 to 1.19) |
| 29 | 8 (1 to 15) | 19 (−15 to 54) | 0.99 (0.94 to 1.03) | 0.97 (0.86 to 1.09) | 1.2 (1.03 to 1.4) | 1.08 (1.02 to 1.14) | 1.07 (0.93 to 1.24) | −0.5 (−0.6 to −0.3) | 0.93 (0.65 to 1.34) |
| 30 | 10 (3 to 18) | 29 (−9 to 68) | 0.99 (0.95 to 1.04) | 0.95 (0.83 to 1.09) | 1.25 (1.06 to 1.48) | 1.1 (1.02 to 1.19) | 1.1 (0.94 to 1.29) | −0.5 (−0.7 to −0.3) | 0.94 (0.58 to 1.52) |
| 31 | 12 (4 to 21) | 41 (−4 to 85) | 1.01 (0.95 to 1.06) | 0.94 (0.79 to 1.1) | 1.31 (1.1 to 1.56) | 1.12 (1.02 to 1.23) | 1.13 (0.95 to 1.35) | −0.6 (−0.8 to −0.4) | 0.96 (0.52 to 1.74) |
| 32 | 15 (4 to 25) | 53 (0 to 105) | 1.02 (0.96 to 1.08) | 0.92 (0.75 to 1.12) | 1.37 (1.14 to 1.65) | 1.14 (1.02 to 1.28) | 1.17 (0.96 to 1.42) | −0.6 (−0.9 to −0.4) | 0.97 (0.47 to 1.99) |
| 33 | 17 (5 to 29) | 65 (2 to 128) | 1.03 (0.96 to 1.11) | 0.9 (0.71 to 1.14) | 1.43 (1.16 to 1.76) | 1.17 (1.02 to 1.34) | 1.21 (0.96 to 1.52) | −0.7 (−1 to −0.4) | 0.98 (0.42 to 2.28) |
| 34 | 19 (5 to 34) | 77 (3 to 151) | 1.05 (0.97 to 1.14) | 0.88 (0.66 to 1.17) | 1.5 (1.19 to 1.9) | 1.19 (1.02 to 1.39) | 1.24 (0.95 to 1.62) | −0.7 (−1.1 to −0.4) | 1 (0.38 to 2.61) |
| 35 | 22 (5 to 38) | 89 (3 to 176) | 1.06 (0.97 to 1.17) | 0.87 (0.62 to 1.2) | 1.57 (1.2 to 2.05) | 1.21 (1.01 to 1.45) | 1.28 (0.94 to 1.74) | −0.8 (−1.2 to −0.4) | 1.01 (0.34 to 2.99) |
| 36 | 24 (5 to 43) | 102 (2 to 201) | 1.08 (0.97 to 1.2) | 0.85 (0.58 to 1.24) | 1.64 (1.21 to 2.22) | 1.24 (1.01 to 1.51) | 1.32 (0.93 to 1.88) | −0.8 (−1.3 to −0.4) | 1.03 (0.31 to 3.42) |
| 37 | 26 (4 to 48) | 114 (2 to 227) | 1.09 (0.97 to 1.24) | 0.83 (0.54 to 1.28) | 1.72 (1.23 to 2.41) | 1.26 (1.01 to 1.58) | 1.36 (0.92 to 2.02) | −0.9 (−1.4 to −0.3) | 1.04 (0.28 to 3.92) |
| 38 | 28 (4 to 53) | 126 (1 to 252) | 1.11 (0.97 to 1.28) | 0.82 (0.51 to 1.32) | 1.8 (1.23 to 2.61) | 1.29 (1.01 to 1.64) | 1.4 (0.9 to 2.18) | −0.9 (−1.5 to −0.3) | 1.06 (0.25 to 4.49) |
| 39 | 31 (4 to 58) | 139 (−1 to 278) | 1.13 (0.96 to 1.31) | 0.8 (0.47 to 1.36) | 1.88 (1.24 to 2.84) | 1.31 (1.01 to 1.71) | 1.45 (0.89 to 2.36) | −1 (−1.6 to −0.3) | 1.07 (0.22 to 5.14) |
| 40 | 33 (3 to 63) | 151 (−2 to 304) | 1.14 (0.96 to 1.35) | 0.78 (0.44 to 1.4) | 1.97 (1.25 to 3.1) | 1.34 (1.01 to 1.78) | 1.49 (0.87 to 2.55) | −1 (−1.7 to −0.3) | 1.09 (0.2 to 5.89) |
Figure 1.
Graphical representation of the modelled effect sizes from restricted cubic splines (RCS) analyses, depicting adjusted association between BMI and perioperative outcomes.
Table 4.
Summary of major complications in the 4 cohorts stratified by BMI.
| Major morbidity type | N (%) | Total, n (%) | |
|---|---|---|---|
| Underweight | 14/124(11.3) | ||
| Bleeding/ hematoma | 2 (0.2) | ||
| Bile leak/ infected collection | 8 (6.5) | ||
| Pulmonary | 2 (1.6) | ||
| Sepsis/ infection | 1 (0.8) | ||
| Others | 1 (0.8) | ||
|
| |||
| Normal weight | 180/1810 (9.9) | ||
| Bleeding/hematoma | 7 (0.4) | ||
| Bile leak/ infected collection | 95 (5.2) | ||
| Pulmonary | 29 (1.6) | ||
| Sepsis/ infection | 4 (0.2) | ||
| Liver failure | 13 (0.7) | ||
| Wound complications | 4 (0.2) | ||
| Ascites | 6 (0.3) | ||
| Others | 32 (1.8) | ||
|
| |||
| Overweight | 102/1056 (9.7) | ||
| Bleeding/hematoma | 8 (0.8) | ||
| Bile leak/ infected collection | 60 (5.7) | ||
| Pulmonary | 16 (1.5) | ||
| Sepsis/ infection | 1 (0.1) | ||
| Liver failure | 6 (0.6) | ||
| Wound complications | 6 (0.6) | ||
| Ascites | 5 (0.5) | ||
| Others | 9 (0.9) | ||
|
| |||
| Obese | 51/341 (13.0) | ||
| Bleeding/hematoma | 5 (1.5) | ||
| Bile leak/ infected collection | 29 (8.5) | ||
| Pulmonary | 8 (2.3) | ||
| Liver failure | 1 (0.3) | ||
| Wound complications | 7 (2.1) | ||
| Others | 3 (0.9) | ||
At a subgroup analysis, results were similar between male and female patients with no significant differences between both cohorts (Supplemental Figures 1 and 2).
Subset analyses was also performed to determine the association between BMI and perioperative outcomes in Eastern and Western centers. In Eastern centers, we observed the same linear association between BMI and operation time, blood loss and open conversions with BMI (Supplemental Figures 3–5). There was no significant correlation between BMI and postoperative hospital stay (Supplemental Figure 6). Similarly in Western centers, we observed a linear association between BMI and blood loss, blood transfusion, open conversions, operation time and postoperative morbidity (Supplemental Figures 7–11). There was no significant correlation between BMI and postoperative hospital stay (Supplemental Figure 12).
DISCUSSION
In this study, we have shown that for patients undergoing minimally invasive major hepatectomies, BMI demonstrated a linear trend with most perioperative outcomes. Increasing BMI was associated with increasing operative time, blood loss, conversion to open, and postoperative morbidity.
Obesity and its associated body shape and composition create perceived technical difficulty in both open and laparoscopic surgery. Indeed, abdominal organs and structures such as blood vessels, are surrounded by an extra layer of adipose tissue that needs to be dissected off, adding extra surgical time, and increasing the chance of unintentional injuries during surgery. High BMI is well-known to be associated with poorer perioperative outcomes for different operative procedures across various surgical specialities. 6–10 In the past, obesity was even considered a contraindication to laparoscopy. However, thanks to the learning curve and the worldwide implementation, minimally invasive surgery in this setting is now performed with safe outcomes across different surgical fields and operations, from cholecystectomies to colectomies and gastrectomies.36 However, for obese patients undergoing liver resections, further considerations are needed. Indeed, the histological changes associated with BMI (steatosis, steatohepatitis, fibrosis), increase the risk of bleeding during mobilization and parenchymal transection.37,38,39 In this setting, laparoscopic hepatectomies in obese patients require an accurate selection of candidates, referral centers, and surgical proficiency, to ensure optimal postoperative outcomes. Indeed, it has been previously shown that BMI is a useful measure in predicting postoperative outcomes following minimally invasive liver resections.37,38 Data are however limited. A recent systematic review pooling the existing evidence on LLRs for patients with high BMI included only seven retrospective studies gathering a total of 481 obese patients.37 Only 25% of these underwent a major hepatectomy. The authors conclude that LLR in obesity was safe, but that evidence remained scarce. Indeed, most of the included studies were from Eastern countries with obesity being defined with a BMI ≥25.0 kg/m2, which is a threshold that can be applied only to specific populations in Asia. Most Western patients indeed, are defined as obese with a BMI ≥30 kg/m2. These differences between East and West limit the generalizability of the results among global populations. Furthermore, there is probably a difference in body shape and composition as at the same BMI values, there might be higher percentages of visceral rather than subcutaneous fat in Eastern compared to Western patients.25, 40
Reports in the literature from centers in the West are also conflicting. Nomi et al. analyzed 228 patients undergoing LLR and found that higher BMI did not negatively affect the short-term outcomes.26 However, overweight, and obese patients had longer operative time and increased blood loss in a North American study, with severely obese patients being associated with increased complications.41 Similarly, obesity was associated with failure to achieve textbook outcomes in the recent French nationwide study.42 Despite this, the laparoscopic approach was found to be associated with fewer postoperative complications and reduced hospital stay compared to open hepatectomy for both overweight and obese patients in a recent report from Germany, therefore favoring the minimally invasive approach for the treatment of such patients.43 Similarly, another recent multicenter study performed in Europe confirmed the advantages of minimally-invasive over the open approach in both obese and non-obese patients.44
None of these studies, however, focused on major hepatectomies which are the most challenging procedure to perform minimally invasively, especially in obese patients. Indeed, while only the parenchymal transection phase is required for most minor hepatectomies, during major resections the surgeon must deal with multiple steps which all have their technical challenges, that are even more demanding in obese patients. For instance, the hilar dissection is more challenging because of the extra fat surrounding the inflow structures, and the mobilization of the liver is limited by the large dimensions of the organ and because of the fragile parenchyma that can easily bleed during maneuvers. Finally, depending on the degree of the histopathological changes, the parenchymal transection phase can bleed as well, increasing the possibility of conversion to open. Yoon et al. recently described the outcomes of 120 overweight patients undergoing laparoscopic major hepatectomies compared to the open approach. Laparoscopy was associated with lower blood loss and shorter hospital stay. Furthermore, BMI≥30 kg/m2 was an independent predictor of morbidity.45 The authors concluded that when applying the laparoscopic approach to patients with a BMI higher than 30, special attention should be paid to the possibility of complications.
It is also important to note that with the recent increase in adoption of robotic liver resections and its reported advantages over the conventional laparoscopic approach 46–48, the use of robotic assistance may be also be advantageous over conventional laparoscopy in obese patients. Indeed, a recent single center study concluded that BMI had minimal impact on the outcomes of robotic liver resection although the study had a small sample size of only 38 patients.49 However, with limited data in the literature addressing this topic, further studies are needed to determine the role of robotic liver surgery in this subset of obese patients.
Our study pooled the data from 58 centers around the world, representing the largest series available evaluating the outcomes of 1057 overweight and 392 obese patients undergoing minimally invasive major hepatectomies. Blood loss and operative time increased linearly with increasing BMI. Obese patients had the longest procedures with the highest median blood losses. Conversion to open was also more frequent in overweight and obese patients. These findings confirm what has been described in previous studies in different settings.25 Postoperative complications were also more frequent in obese patients. Obese patients have a slower return to ambulation and to functional recovery which might increase the risk of developing wound complications, pleural effusions, pneumonia, and urinary tract infections.
Interestingly, we observed that major morbidity unlike overall complications did not increase linearly with increasing BMI. Instead, unlike the other perioperative outcome measures, there was a “U” shaped association between major morbidity with BMI. Both underweight and obese patients experienced the highest rates of major complications after LMH. This might be related to the intrinsic limitation of the BMI variable which does not assess for body composition. Indeed, it has been recently shown that muscle mass and function are more reliable than BMI in predicting postoperative results.50 Lower BMI (underweight patients) is also associated with cachexia and higher BMI with sarcopenic obesity, thereby explaining the “U” shaped association with major morbidity demonstrated in this study.37,38 Furthermore, as opposed to technical outcomes such as blood loss and conversion rates which demonstrated a linear association with BMI, postoperative major morbidity is also influenced by multiple other factors such as patients’ characteristics and comorbidities. However notably, a higher ASA score was not observed in the low BMI cohort in the present study. Hence, further studies are needed to determine the exact reasons behind this finding.
Another interesting observation from this study was that postoperative hospital stay was significantly longer among patients with higher BMI values. This result, although counterintuitive, finds its explanation in the global and multicenter nature of this study. Due to general differences among global healthcare systems, the length of hospital stay is highly variable worldwide, with an overall tendency to be longer in Asian countries due to the reimbursement policies. On the contrary, in the West, there is a general focus on functional recovery and early discharge to minimize healthcare costs. For the above-mentioned differences in populations, as reported in our results, patients with lower BMIs were more likely gathered from Eastern centers while more obese/ overweight patients were collected from Western countries. This confounding factor likely accounted for the shorter hospital stay observed in higher BMI patients. This hypothesis was further supported upon subset analyses where by there was no significant correlation between BMI and postoperative stay either in Eastern or Western centers.
This study has several limitations. The retrospective and multicenter fashion might have introduced some selection bias that has already been discussed. Furthermore, as an international multicenter study, there was wide variability in the surgical technique, center experience, patient characteristics and perioperative management of patients. Nonetheless, these results would be more reflective of real-world data and increased the generalizability of our findings. Larger studies with homogeneous populations are warranted to confirm our results and validate our findings among different geographic areas. As above-mentioned, BMI is a validated measure that however does not discriminate between muscle mass or fat, and between central or subcutaneous obesity, and is therefore considered limited to precisely estimating and predicting surgical outcomes. Accurate analysis of body composition including muscle mass and strength, and sarcopenia, should be included in the categorization of these patients to evaluate changes in outcomes.38,51
CONCLUSIONS
LMH was associated with poorer outcomes with increasing BMI for most perioperative outcome measures. Postoperative major morbidity demonstrated a “U” shaped association with BMI with the worst outcomes observed in underweight and obese patients.
Supplementary Material
There was a linear association with worsening outcomes after laparoscopic major hepatectomy for most perioperative outcome measures such as operation time, blood loss and open conversion rate with increasing body mass index (BMI). However, postoperative major morbidity demonstrated a “U” shaped association with BMI whereby the highest major morbidity rates were observed in underweight and obese patients.
Funding/Support:
Dr T. P. Kingham was partially supported by the US National Cancer Institute MSKCC Core Grant number P30 CA008748 for this study
Dr M. Yin was partially funded by the Research Project of Zhejiang Provincial Public Welfare Fund project in the Field of Social development (LGF20H160028)
Dr Brian Goh was partially supported by the Intuitive Foundation Grant for this study.
i) Dr Goh BK has received travel grants and honorarium from Johnson and Johnson, Olympus and Transmedic the local distributor for the Da Vinci Robot.
ii) Dr Marino MV is a consultant for CAVA robotics LLC.
iii) Johann Pratschke reports a research grant from Intuitive Surgical Deutschland GmbH and personal fees or non-financial support from Johnson & Johnson, Medtronic, AFS Medical, Astellas, CHG Meridian, Chiesi, Falk Foundation, La Fource Group, Merck, Neovii, NOGGO, pharma-consult Peterson, and Promedicis.
iv) Moritz Schmelzle reports personal fees or other support outside of the submitted work from Merck, Bayer, ERBE, Amgen, Johnson & Johnson, Takeda, Olympus, Medtronic, Intuitive.
v) Asmund Fretland reports receiving speaker fees from Bayer.
vi) Fernando Rotellar reports speaker fees and support outside the submitted work from Integra, Medtronic, Olympus, Corza, Sirtex and Johnson & Johnson.
Footnotes
Conflict of Interest/Disclosure: We confirm all the authors are accountable for all aspects of the work
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
International robotic and laparoscopic liver resection study group investigators are coauthors of this study:
Mikel Prieto, Celine De Meyere, Kit-Fai Lee, Kelvin K. Ng, Diana Salimgereeva, Ruslan Alikhanov, Lip-Seng Lee, Jae Young Jang, Masayuki Kojima, Jaime Arthur Pirola Kruger, Victor Lopez-Lopez, Margarida Casellas I Robert, Roberto Montalti, Mariano Giglio, Boram Lee, Hao-Ping Wang, Mansour Saleh, Shian Yu, Simone Vani, Francesco Ardito, Ugo Giustizieri, Davide Citterio, Federico Mocchegiani, Marco Colasanti, Yoelimar Guzmán, Kevin P. Labadie, Maria Conticchio, Epameinondas Dogeas, Emanuele F. Kauffmann, Mario Giuffrida, Daniele Sommacale, Alexis Laurent, Paolo Magistri, Kohei Mishima, Felix Krenzien, Prashant Kadam, Eric C. H. Lai, Jacob Ghotbi, Åsmund Avdem Fretland, Fabio Forchino, and Alessandro Mazzotta
Data access:
Data will be available from the corresponding author on reasonable request. It is not available publicly due to ethical and privacy concerns.
REFERENCES
- 1.Wise J. Obesity rates rise substantially worldwide. BMJ 2014; 348:g3582. [DOI] [PubMed] [Google Scholar]
- 2.Berghofer A, Pischon T, Reinhold T, et al. Obesity prevalence from a European perspective: a systematic review. BMC Public Health 2008; 8:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lissau I, Overpeck MD, Ruan WJ, et al. Body mass index and overweight in adolescents in 13 European countries, Israel, and the United States. Arch Pediatr Adolesc Med 2004; 158(1):27–33. [DOI] [PubMed] [Google Scholar]
- 4.Zogg CK, Mungo B, Lidor AO, et al. Influence of body mass index on outcomes after major resection for cancer. Surgery 2015; 158(2):472–85. [DOI] [PubMed] [Google Scholar]
- 5.Agopian VG, Kaldas FM, Hong JC, et al. Liver transplantation for nonalcoholic steatohepatitis: the new epidemic. Ann Surg 2012; 256(4):624–33. [DOI] [PubMed] [Google Scholar]
- 6.Barco-Castillo C, Plata M, Zuluaga L, et al. Obesity as a risk factor for poor outcomes after sling surgery in women with stress urinary incontinence: A systematic review and meta-analysis. Neurourol Urodyn 2020. [DOI] [PubMed] [Google Scholar]
- 7.Gurunathan U, Ramsay S, Mitric G, et al. Association Between Obesity and Wound Infection Following Colorectal Surgery: Systematic Review and Meta-Analysis. J Gastrointest Surg 2017; 21(10):1700–1712. [DOI] [PubMed] [Google Scholar]
- 8.Onggo JR, Onggo JD, de Steiger R, et al. Greater risks of complications, infections, and revisions in the obese versus non-obese total hip arthroplasty population of 2,190,824 patients: a meta-analysis and systematic review. Osteoarthritis Cartilage 2020; 28(1):31–44. [DOI] [PubMed] [Google Scholar]
- 9.Ratnayake CB, Loveday BP, Shrikhande SV, et al. Impact of preoperative sarcopenia on postoperative outcomes following pancreatic resection: A systematic review and meta-analysis. Pancreatology 2018; 18(8):996–1004. [DOI] [PubMed] [Google Scholar]
- 10.Williams T, Gulack BC, Kim S, et al. Operative Risk for Major Lung Resection Increases at Extremes of Body Mass Index . Ann Thorac Surg 2017; 103(1):296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cauchy F, Zalinski S, Dokmak S, et al. Surgical treatment of hepatocellular carcinoma associated with the metabolic syndrome. Br J Surg 2013; 100(1):113–21. [DOI] [PubMed] [Google Scholar]
- 12.de Meijer VE, Kalish BT, Puder M, et al. Systematic review and meta-analysis of steatosis as a risk factor in major hepatic resection. Br J Surg 2010; 97(9):1331–9. [DOI] [PubMed] [Google Scholar]
- 13.Koh YX, Tan HJ, Liew YX, et al. Liver Resection for Nonalcoholic Fatty Liver Disease-Associated Hepatocellular Carcinoma. J Am Coll Surg 2019; 229(5):467–478 e1. [DOI] [PubMed] [Google Scholar]
- 14.Wakai T, Shirai Y, Sakata J, et al. Surgical outcomes for hepatocellular carcinoma in nonalcoholic fatty liver disease. J Gastrointest Surg 2011; 15(8):1450–8. [DOI] [PubMed] [Google Scholar]
- 15.Bagante F, Ruzzenente A, Beal EW, et al. Complications after liver surgery: a benchmark analysis. HPB (Oxford) 2019; 21(9):1139–1149. [DOI] [PubMed] [Google Scholar]
- 16.Urdaneta Perez MG, Garwe T, Stewart K, et al. Obesity Is an Independent Risk Factor for Mortality in Otherwise Healthy Patients After Hepatectomy. J Surg Res 2020; 255:50–57. [DOI] [PubMed] [Google Scholar]
- 17.Acosta LF, Garcia CR, Dugan A, et al. Impact of super obesity on perioperative outcomes after hepatectomy: The weight of the risk. Surgery 2017; 162(5):1026–1031. [DOI] [PubMed] [Google Scholar]
- 18.Cucchetti A, Cescon M, Ercolani G, et al. Safety of hepatic resection in overweight and obese patients with cirrhosis. Br J Surg 2011; 98(8):1147–54. [DOI] [PubMed] [Google Scholar]
- 19.Langella S, Russolillo N, Forchino F, et al. Impact of obesity on postoperative outcome of hepatic resection for colorectal metastases. Surgery 2015; 158(6):1521–9. [DOI] [PubMed] [Google Scholar]
- 20.Mathur AK, Ghaferi AA, Osborne NH, et al. Body mass index and adverse perioperative outcomes following hepatic resection. J Gastrointest Surg 2010; 14(8):1285–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ciria R, Cherqui D, Geller DA, et al. Comparative Short-term Benefits of Laparoscopic Liver Resection: 9000 Cases and Climbing. Ann Surg 2016; 263(4):761–77. [DOI] [PubMed] [Google Scholar]
- 22.Berardi G, Van Cleven S, Fretland AA, et al. Evolution of Laparoscopic Liver Surgery from Innovation to Implementation to Mastery: Perioperative and Oncologic Outcomes of 2,238 Patients from 4 European Specialized Centers. J Am Coll Surg 2017; 225(5):639–649. [DOI] [PubMed] [Google Scholar]
- 23.Tozzi F, Berardi G, Vierstraete M, et al. Laparoscopic Versus Open Approach for Formal Right and Left Hepatectomy: A Propensity Score Matching Analysis. World J Surg 2018; 42(8):2627–2634. [DOI] [PubMed] [Google Scholar]
- 24.Toriguchi K, Hatano E, Sakurai T, et al. Laparoscopic liver resection in obese patients. World J Surg 2015; 39(5):1210–5. [DOI] [PubMed] [Google Scholar]
- 25.Yu X, Yu H, Fang X. The impact of body mass index on short-term surgical outcomes after laparoscopic hepatectomy, a retrospective study. BMC Anesthesiol 2016; 16(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nomi T, Fuks D, Ferraz JM, et al. Influence of body mass index on postoperative outcomes after laparoscopic liver resection. Surg Endosc 2015; 29(12):3647–54. [DOI] [PubMed] [Google Scholar]
- 27.Ome Y, Hashida K, Yokota M, et al. The safety and efficacy of laparoscopic hepatectomy in obese patients. Asian J Surg 2019; 42(1):180–188. [DOI] [PubMed] [Google Scholar]
- 28.Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg 2005; 12(5):351–5. [DOI] [PubMed] [Google Scholar]
- 29.Goh BKP, Prieto M, Syn N, et al. Validation and comparison of the Iwate, IMM, Southampton and Hasegawa difficulty scoring systems for primary laparoscopic hepatectomies. HPB (Oxford) 2021; 23(5):770–776. [DOI] [PubMed] [Google Scholar]
- 30.Chin KM, Linn YL, Cheong CK et al. Minimally-invasive vs open major hepatectomies fo liver malignancies:a propensity score-matched analysis. J Gastrointes Surg 2022;26:1041–53. [DOI] [PubMed] [Google Scholar]
- 31.Kawaguchi Y, Fuks D, Kokudo N, et al. Difficulty of Laparoscopic Liver Resection: Proposal for a New Classification. Ann Surg 2018; 267(1):13–17. [DOI] [PubMed] [Google Scholar]
- 32.Wakabayshi G. What has changed after the Morioka consensus conference 2014 on laparoscopic liver resection? HBSN 2016;5(4):281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanaka S, Kawaguchi Y, Kubo S, et al. Validation of index-based IWATE criteria as an improved difficulty scoring system for laparoscopic liver resection. Surgery 2019; 165(4):731–740. [DOI] [PubMed] [Google Scholar]
- 34.Linn YL, Wu AG, Han HS, et al. Systematic review and meta-analysis of difficulty scoring systems for laparoscopic and robotic liver resections. J Hepatobiliary Pancreat Sci 2023;30:36–59. [DOI] [PubMed] [Google Scholar]
- 35.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240(2):205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buia A, Stockhausen F, Hanisch E. Laparoscopic surgery: a qualfiied systematic review. World J Methodol 2015;5:238–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwan B, Waters PS, Keogh C, et al. Body mass index and surgical outcomes in laparoscopic liver resections: a systematic review. ANZ J Surg 2021; 91(11):2296–2307. [DOI] [PubMed] [Google Scholar]
- 38.Chua DW, Syn N, Koh Y, et al. Association of standardized liver volume and body mass index with outcomes of minimally-invasive liver resections. Surg Endosc 2023;37:456–465. [DOI] [PubMed] [Google Scholar]
- 39.Tan HL, Goh BK. The effect of preoperative low-calorie diets on liver resecstion outcomes. Transl Gastroenterol Hepatol 2019;13:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Consultation WHOE. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004; 363(9403):157–63. [DOI] [PubMed] [Google Scholar]
- 41.Lee JL, Hauch A, E. K, et al. Effect of obesity on perioperative outcomes after laparoscopic hepatectomy. Hepatoma Res 2016(2):323–7. [Google Scholar]
- 42.Genser L, Lim C, Barbier L, et al. Assessment of factors associated with morbidity and textbook outcomes of laparoscopic liver resection in obese patients: a French nationwide study. [DOI] [PubMed] [Google Scholar]
- 43.Heise D, Bednarsch J, Kroh A, et al. Laparoscopic hepatectomy reduces postoperative complications and hospital stay in overweight and obese patients. World J Gastrointest Surg 2021; 13(1):19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zimitti G, Sijberden JP, Osei-Bordom D, et al. Indications, trends, and perioperative outcomes of minimally invasive and open liver surgery in non-obese and obese patients: An international multicentre popensity score matched retrospective cohort study of 9963 patients. Int J Surg 2022;107:106957. [DOI] [PubMed] [Google Scholar]
- 45.Yoon YI, Kim KH, Cho HD, et al. Operative and long-term oncologic outcomes of laparoscopic versus open major liver resection in patients with a high body mass index (> 25 kg/m(2)): a propensity score matching analysis. Surg Endosc 2022. [DOI] [PubMed] [Google Scholar]
- 46.D’Silva M, Han HS, Liu R et al. Limited liver resections in the posterosuperior segments: international multicentre propensity score-matched and coarsened exact-matched analysis comparing the laparoscopic and robotic approaces. Br J Surg 2022;109:1140–40. [DOI] [PubMed] [Google Scholar]
- 47.Chong CC, Fuks D, Lee KF, et al. Propensity score-matched analysis comparing robotic and laparoscopic right and extended right hepatectomy. JAMA Surg 2022;157:436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chiow AK, Fuks D, Choi GH, et al. International multicentre propensity score-matched analysis comparing robotic versus laparoscopic right posterior sectionectomy. Br J Surg 2021;108:1513–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sucandy I, Attilli A, Spence J, et al. The impact of body mass index on perioperative outcomes after robotic liver resection. J Robot Surg 2020;14:41–46. [DOI] [PubMed] [Google Scholar]
- 50.Simonsen C, de Heer P, Bjerre ED, et al. Sarcopenia and Postoperative Complication Risk in Gastrointestinal Surgical Oncology: A Meta-analysis. Ann Surg 2018; 268(1):58–69. [DOI] [PubMed] [Google Scholar]
- 51.Berardi G, Antonelli G, Colasanti M, et al. Association of Sarcopenia and Body Composition With Short-term Outcomes After Liver Resection for Malignant Tumors. JAMA Surg 2020; 155(11):e203336. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be available from the corresponding author on reasonable request. It is not available publicly due to ethical and privacy concerns.