HIGHLIGHTS
-
•
Program achieved significant weight loss for women with obesity and low income.
-
•
Program was cost-effective in achieving weight loss for women with low income.
-
•
Program was delivered through a telewellness platform to improve outreach.
Keywords: Cost-effectiveness, weight loss, obesity, women, low-income, telewellness
Abstract
Introduction
The purpose of this study was to perform a cost-effectiveness analysis of the Koa Family Program, a community-based telewellness weight reduction intervention for overweight and obese women aged 21–45 years with low income. The Koa Family Program resulted in an approximately 8-pound weight loss as demonstrated in an RCT published previously.
Methods
Estimates for the cost-effectiveness were derived from the prospective 25-week RCT including 70 women (25 kg/m2≤BMI<40 kg/m2). The analysis was from a program-funder perspective. Base case costs, as well as low and high scenario costs, were estimated from the services provided to intervention participants. The incremental costs were compared with the incremental effectiveness, with weight loss being the outcome of interest. Costs were in 2021 U.S. dollars. Cost-effectiveness was assessed using the incremental cost-effectiveness ratio and the incremental net benefit. The statistical uncertainty was characterized using an incremental net benefit by willingness-to-pay plot and a cost-effectiveness acceptability curve.
Results
The base case average cost per participant was $564.39. The low and high scenario average costs per participant were $407.34 and $726.22, respectively. Over the 25-week study timeframe, participants lost an average 7.7 pounds, yielding a base case incremental cost-effectiveness ratio of approximately $73 per extra pound lost. The probability that the Koa Family Program is cost-effective is 90%, assuming a willingness-to-pay of $115 for a 1-pound reduction, and is 95%, assuming a willingness-to-pay of $140.
Conclusions
The Koa Family Program provides good value with cost-effectiveness in line with other weight-loss interventions. This is a striking finding given that the Koa Family Program serves a more vulnerable population than is typically engaged in weight loss research studies.
INTRODUCTION
Obesity elevates morbidity and mortality risks of several chronic diseases including coronary heart disease, Type 2 diabetes, and several cancers.1,2 Excess medical costs associated with obesity have been estimated to be $173 billion per year.3 In addition, obesity rates have been rising in the U.S. since around 1980.4 The most recent data (2017–2018) from the National Health and Nutrition Examination Surveys show an adult obesity prevalence rate of 42.4% and an overweight prevalence rate of 27.5%.4 Due to the ongoing need to develop effective interventions to address the adverse impact of obesity, we conducted an RCT testing the weight-reducing effect of a 17-week community-based telewellness obesity intervention called the Koa Family Program (KFP) for women of reproductive age with low income. This population has elevated rates of obesity and chronic disease risk.5,6 The KFP resulted in an approximately 8-pound weight loss among the study population of overweight and obese women aged 21–45 years.7 Although the KFP had a positive impact on weight loss, dissemination of the program would likely depend on decision-makers in government or the private sector committing resources and investing in the provision of KFP services. Such funding determinations are increasingly dependent on cost-effectiveness analysis (CEA) of interventions.8, 9, 10 Thus, the purpose of this study was to perform a CEA of the KFP from the perspective of a program funder.
METHODS
Study Intervention
Data for the CEA were derived from a prospective RCT designed to determine the effect of the KFP on study participants.7 The core of the KFP was the Whole Health Program (WHP), which was delivered to 5 groups of 4–10 women over 17 weeks, starting in March 2021. Each group met for 90 minutes weekly on Zoom with 1 of 3 lay health coaches trained by 2 investigators (DB and NK). Two health coaches were assigned to 2 groups each, and the remaining coach facilitated 1 group. Session topics included nutrition, physical activity, and related lifestyle and environmental factors conducive to weight loss. Each participant had up to 3, 15-minute personal check-ins with her coach over the course of the intervention. The participants received booklets and other WHP support materials at the beginning and midpoint of the program. The use of social media was intended to support WHP, with each participant assigned to a private Facebook group. The groups received WHP content posted by program staff and health coaches 3 to 5 times per week, and they had an opportunity to post and share their ideas for behavior change. The participants also received a weekly text message reminder to weigh themselves and a weekly morning motivational quote for encouragement.
A tree planting and stewardship campaign was the third component of the KFS intervention. However, only 10 unique participants were involved in the 5 tree plantings, which were voluntary for intervention group participants. Because of this low turnout and because tree plantings would not be part of telewellness programs in practice, costs for tree planting events were excluded from this CEA.
Study Sample
Seventy women with overweight or obesity (25 kg/m2≤BMI<40 kg/m2), aged 21–45 years, and who were eligible for Supplemental Nutrition Assistance Program Education (household income ≤185% of the Federal Poverty Level)11 at study entry were recruited from the Sacramento, California region from December 2020 to February 2021. In the study population, 10% identified as Asian; 37% as Black or African American; 44% as Hispanic; and 9% as White, non-Hispanic/non-Latina; 16% were multiracial. Participants could identify with >1 category, so percentages do not add to 100%. Participants were randomly assigned to the KFP intervention group (n=34) or control group (n=36). The study protocol was approved by the University of California, Davis IRB. The study was registered with www.ClinicalTrials.gov (NCT04662593).
Measures
Surveys at weeks 18 and 25 had evaluation questions assessing diet, physical activity, and related psychosocial measures.7 Study participants also received a remote monitoring scale that transmitted weight data via cellular network and the internet to a secure server.12 Participants were trained on how to use the scale and were asked to weigh themselves weekly. Although the intervention concluded by week 18, the investigators collected week 25 data to determine whether there was weight gain among intervention participants after the study's end.
Intervention Costs
All costs were in 2021 U.S. dollars. Base case costs, as well as low and high case costs, were estimated from the services provided to intervention participants. The cost for the KFP was computed as the sum of the costs for personnel and materials, which were sourced from study records. The equation below expresses the main areas in which costs were accumulated:
where Costpersonnel equals the sum of costs for coaches, community liaison, communication specialist, and project director, and costmaterials equals the sum of costs for coaches' WHP curriculum and participant materials. Base case and low and high estimates were computed to provide greater insights into the value of the KFP. Table 1 contains specific values for the low and high scenarios (arrived at by assuming 25% lower and 25% higher unit costs for each category). Average cost per participant was calculated as the total cost divided by the number of study participants (n=34).
Table 1.
Cost Assumptions for Key Inputs
| Cost category: Personnel | ||||||
|---|---|---|---|---|---|---|
| Cost variable | Unit costa | Effort | Duration | Total Cost | Comments | |
| Coaches | High | $45.69/hour | 6.74 hours/week/coach | 17 weeks | $15,693.66 | 343.5 hours (all 3 coaches)
|
| Mid | $36.55/hour | $12,554.93 | ||||
| Low | $27.41/hour | $9,416.19 | ||||
| Community liaison | High Mid Low |
$45.69/hour $36.55/hour $27.41/hour |
1.24 hours/week | 17 weeks | $959.44 $767.55 $575.66 |
21 hours total
|
| Communication specialist | High | $45.69/hour | 0.75 hours/week | 17 weeks | $582.52 | 12.75 hours total
|
| Mid | $36.55/hour | $466.01 | ||||
| Low | $27.41/hour | $349.51 | ||||
| Project director | High Mid Low |
$87.50/hour $70.00/hour $52.50/hour |
3.47 hours/week | 17 weeks | $5,162.50 $4,130.00 $3,097.50 |
59 hours total
|
| Coaches’ Whole Health Program guide | High Mid Low |
$66.56/guide $33.35/guide $9.99/guide |
1 guide/coach | $66.56/coach $199.68 for 3 coaches $33.35/coach $100.05 for 3 coaches $9.99/coach $29.97 for 3 coaches |
High: Subcontracted to Marketing by Design Mid: No subcontractor, lower cost items except for participant booklets Low: No subcontractor, all lower cost items including in-office printing |
|
| Participant booklets and support materials | High Mid Low |
$61.58/kit $34.43/kit $11.20/kit |
1 kit/participant | $61.58/participant $2,093.72 for 34 participants $34.43/participant $1,170.62 for 34 participants $11.20/participant $380.80 for 34 participants |
High: Subcontracted to Marketing by Design Mid: No subcontractor, lower cost items except for participant booklets Low: No subcontractor, all lower cost items including in-office printing |
|
Note: Key assumptions: 17-week program; 34 participants distributed among 5 groups; 3 coaches, 1 working with 1 group and 2 with 2 groups.
All personnel hourly rates include salary, benefits, vacation accrual.
Control Costs
Control group participants were directed to the U.S. Department of Agriculture's MyPlate website.13 Providing access to the online resources at this website constituted usual care for the control participants. The costing exercise focused on incremental cost components only.
KFS Effectiveness
The overall treatment effect of the KFP was a 7.7-pound weight loss at both week 18 and week 25 of the study. KFP-associated improvements in diet, physical activity, stages of change, and self-efficacy were also observed.7
Statistical Analysis
Analyses were conducted using Stata 17 software.14 The analysis was from the perspective of a program funder. To estimate the incremental effectiveness of the KFP in terms of weight loss, we used results from Backman et al.,7 which focused on measuring the average treatment effect, conditional on treatment, or the average treatment effect on the treated. The additional effect of the KFP (compared with usual care) was constructed by weighting the control group such that the distribution of covariates matched the intervention group. The coefficients were estimated by ordinary least squares using entropy weights and cluster-robust standard errors, clustered at the individual level.7
The CEA used the estimates of incremental cost (ΔC) and incremental effect (ΔE) to compute the incremental cost-effectiveness ratio (ICER; ICER=ΔC/ΔE) and the incremental net benefit (INB; INB=WTP × ΔE–ΔC). To compute INB, the decision-maker's willingness-to-pay (WTP) was varied from a WTP value of $0 to $200 for 1 pound of weight loss. This range reflects costs per 1 pound of weight lost for several popular commercial weight-loss programs.15 The statistical uncertainty surrounding the ICER and the INB were characterized using an INB by WTP plot and a cost-effectiveness acceptability curve. Statistical uncertainty for the ΔE estimate comes from the trial's treatment effect data,7 and uncertainty for ΔC comes from sensitivity analysis using low and high scenario costs. This is congruent with assuming people in the same intervention group have the same intervention cost but have different weight loss results (as was observed in the RCT). This approach was necessary given the available data (i.e., person-level outcome data and intervention-level cost data). Therefore, when we use the 95% CIs for the INB estimate to indicate the 95% CIs for the ICER, this is driven by the statistical uncertainty in the intervention's effectiveness, conditional on a particular cost scenario (e.g., high costs). To be clear, the low case and high case scenarios relate to the intervention's cost (not effect).
RESULTS
Costs
Table 1 shows the cost assumptions for various key inputs. Unit costs for high, base case, and low scenarios are presented in the Unit Cost column. The Total Cost column shows the results of multiplying the unit cost estimates by the hours per week (in the Effort column) and the total weeks (in the Duration column). Table 2 summarizes the subtotal, total, and average cost per participant for the base case scenario. The Personnel subtotal was estimated to be $17,918.49 with an additional Materials subtotal of $1,270.67. Total cost of $19,189.16 was calculated as the sum of these 2 subtotals. The total cost for the low- and high-cost scenarios added up to $13,849.64 and $24,691.51, respectively. The vast majority (>90%) of total cost is composed of personnel costs.
Table 2.
Base Case Subtotal, Total, and Average Cost Estimates of the KFP
| Cost category | |
|---|---|
| Personnel | |
| Coaches | $12,554.93 |
| Community Liaison | $767.55 |
| Communication Specialist | $466.01 |
| Project Director | $4,130.00 |
| Subtotal | $17,918.49 |
| Materials | |
| Coaches’ Whole Health Program guide | $100.05 |
| Participant booklets and other support materials | $1,170.62 |
| Subtotal | $1,270.67 |
| Total | $19,189.16 |
| Sample Size | 34 |
| Average cost per participant | $564.39 |
KFP, Koa Family Program.
The base case average cost per participant was $564.39. The low and high scenario average cost per participant were $407.34 and $726.22, respectively. These are about 28% lower and higher than the base case scenario. Over a 17-week timeframe, the base case average cost per participant is approximately $33 per week per participant.
Cost-Effectiveness Estimates
Table 3 shows the incremental cost and incremental effect estimates as well as their ratio, the ICER. Dividing KFP's incremental cost of $564.39 by its incremental effectiveness (∼7.7 pounds lost) produces an ICER of $73.30. This ICER estimate means that the KFP reduces weight at a cost of about $73 per pound lost. Although the KFP formally ended in week 17, making week 18’s weight the final weight measurement during active treatment, by week 25, the weight loss improvements continued (having increased from 7.69 pounds lost to 7.72 pounds lost). Thus, the ICERs at week 18 and week 25 are both approximately $73. The ICER estimates using the low- and high-cost estimates are about $53 and $94 per additional pound lost, respectively.
Table 3.
Incremental Cost-Effectiveness and ICER at Weeks 18 and 25
| Incremental | |
|---|---|
| Cost | |
| Base case | $564.39 |
| Llow | $407.34 |
| High | $726.22 |
| Effect | |
| At 18 weeks | 7.69 |
| At 25 weeks | 7.72 |
| Cost-effectiveness ratio (rounded) | |
| Base case | $73.30 |
| Low | $52.90 |
| High | $94.31 |
Note: Calculations based on 34 participants. Incremental is the difference between the average for the KFP and the control participants. Effect measured as pounds lost. Rounded ICER is computed as rounded incremental cost divided by 7.7.
ICER, incremental cost-effectiveness ration; KFP, Koa Family Program.
Cost-Effectiveness Uncertainty
Figure 1 shows both the ICER and INB estimates and characterizes statistical uncertainty. The solid line indicates the INB estimate. When this line intersects the horizontal axis, it indicates the ICER estimate; this occurs at a WTP of $73, and this is the ICER estimate. For WTP values >$73, the KFP appears to be cost-effective because the INB estimate line is above the horizontal axis (i.e., INB>0). The dashed lines indicate the 95% CIs for the INB estimate. When these dashed lines intersect the horizontal axis, they indicate the 95% CIs for the ICER. For the base case, the 95% CI for the ICER is 48 to 160. The 95% CIs for the low and high cases, not shown in Figure 1, are 34 to 115 and 61 to 206, respectively. The 95% CI for the KFP treatment effect of –7.69 pounds was –11.97 to –3.41, as determined in an RCT published previously.7
Figure 1.
Incremental net benefit by WTP plot showing estimates and statistical uncertainty in the form of 95% CIs.
Note: The dashed lines indicate the 95% CIs for the INB estimate. When these dashed lines intersect the horizontal axis, they indicate the 95% CIs for the ICER at 48 to 160. The solid line indicates the INB estimate. When this line intersects the horizontal axis at 73, it indicates the ICER estimate is 73.
ICER, incremental cost-effectiveness ratio; INB, incremental net benefit; WTP, willingness to pay.
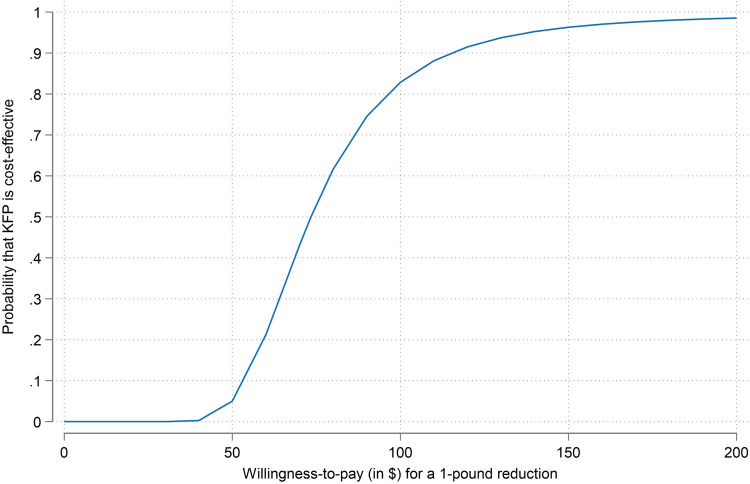
Figure 2 is a cost-effectiveness acceptability curve illustrating the probability that the KFP is cost-effective as a function of the assumed WTP value for a 1-pound reduction. There is a marked gain in the probability of cost-effectiveness between a WTP of $50 and a WTP of $150. If decision-makers are willing to pay $50 for a 1-pound reduction in weight, the probability of the KFP being cost effective is 5%; if they are willing to pay $150 for a 1-pound reduction in weight, the probability of cost-effectiveness increases to >95%.
Figure 2.
Cost-effectiveness acceptability curve showing the probability that KFP is cost-effective as a function of a decision maker's willingness-to-pay.
Note: The cost-effectiveness acceptability curve (CEAC) above characterizes the statistical uncertainty about the probability that the KFP is cost-effective as a function of the unknown WTP value. The probability that the intervention is cost-effective is most sensitive to WTP values between 50 and 150 for a 1-pound reduction. WTP values >$150 (or <$50) inform easy yes (and easy no) decisions.
CEAC, cost-effectiveness acceptability curve; KFP, Koa Family Program; WTP, willingness-to-pay.
DISCUSSION
The KFP resulted in a statistically significant 7.7-pound weight loss for the intervention group compared with controls, at both week 18 and week 25 of the study. The extra cost from the KFP was estimated to be $564.39 in the base case scenario. Based on these estimates, weight loss was achieved at approximately $73 per pound lost. Sensitivity analysis indicated a range from $53 per pound lost to $94 per pound lost. Characterizing the statistical uncertainty, the probability that the KFP is cost-effective is 90% assuming a WTP of $115 for a 1-pound reduction and is 95% assuming a WTP of $140. Our conclusions are most sensitive to WTP values between $50 and $150, where the probability that KFP is cost-effective jumps from 1% to 96%. WTP values >$150 (or <$50) inform easy yes (and easy no) decisions.
To put our cost-effectiveness results in context, the cost-effectiveness of KFP with a base case of $73 per pound lost is in the same range of commercial weight loss interventions that Finkelstein and Kruger reported: $70.45 per pound lost (Weight Watchers), $92.7 per pound lost (Qsymia, a prescription weight loss drug), and $192.7 per pound lost (Jenny Craig).15 Other outcomes in cost per pound, described by Gustafson et al.10 fall in the range of $10 to $133 per pound lost.10,16,17 This is notable because the KFP participants were recruited from populations with low income, which typically have higher obesity prevalence rates than those with higher income.18 In addition, the KFP was designed to serve women of reproductive age, a population that has seen significant increases in the rates of prediabetes mellitus and undiagnosed diabetes mellitus, contributing to a significant cardiovascular risk burden for women in the U.S.6 In a recent systematic review of telehealth-delivered diet and exercise interventions, 12 of 24 controlled studies were found to be cost-effective.19 A number of other weight loss interventions have been shown to be cost-effective, based on a wide range of outcomes including cost per quality adjusted life year,20 cost per participant achieving at least 5% loss in body weight,21 and cost per discounted life year gained.10 Although the wide variety of summary measures used in some cost-effectiveness analyses makes direct comparison of interventions challenging, our findings provide evidence that an underserved population can receive the benefits of weight loss at a reasonable cost.
Our cost-effectiveness study of the KFP contributes to the field because individuals from low-income communities and from racial/ethnic minority populations are underrepresented in studies of obesity treatment.22 In fact, we only found 1 RCT of weight loss in an intervention for women with low income that had a subsequent CEA conducted.10,23 The program was described as cost-effective, with an incremental cost per life year gained from decreased obesity of $1,862. However, the intervention was conducted in an older study population than the KFP, among participants already enrolled in an overall cardiovascular risk-reduction program.10 Another notable aspect of our study is that our recent search of the literature found no comparable telehealth-based group weight-loss program designed for women with low income.7
Limitations
Limitations of this study include the relatively small size of the study population and the unavailability of long-term outcomes. Because weight regain is common following weight loss,24 cost-effectiveness of the program would likely diminish over time without sustained intervention. Strengths of the study include the ethnic and racial diversity of the study population and the recruitment of participants with low income,7 who are at greater risk of obesity and often face environmental conditions contributing to adverse health outcomes.25
CONCLUSIONS
Our analysis demonstrated that the KFP is economically attractive with a reasonable cost-effectiveness profile. The findings provide potential payers with key information to guide potential investment and implementation of programs similar to the KFP. Thus, the study contributes to addressing the continuing need for effective, affordable interventions to reduce the significant threats to population health associated with high rates of obesity. Programs that are effective in populations with low income are particularly important because these groups bear a disproportionately high morbidity and mortality burden, in part because of higher prevalence rates of overweight and obesity.25,26 In addition, telehealth programs in particular, such as the KFP, have the potential to effectively serve hard-to-reach populations such as rural and low income.27
CRediT authorship contribution statement
Jeffrey S. Hoch: Conceptualization, Formal analysis, Writing – review & editing. Neal D. Kohatsu: Conceptualization, Writing – review & editing. Julia Fleuret: Data curation, Writing – review & editing. Desiree R. Backman: Conceptualization, Writing – review & editing.
ACKNOWLEDGMENTS
Funding for the KFP was provided by the California Department of Social Services (Agreement Number 18-3110) and the California Department of Forestry and Fire Protection (Agreement Number 8GA19400).
Declarations of interest: none.
REFERENCES
- 1.NIH, HHS, National Heart, Lung, and Blood Institute. Managing overweight and obesity in adults: Systematic Evidence Review from the Obesity Expert Panel. Bethesda, MD: NIH, HHS, National Heart, Lung, and Blood Institute. https://www.nhlbi.nih.gov/health-topics/managing-overweight-obesity-in-adults. Published November 2013. Accessed May 5, 2023.
- 2.Pi-Sunyer X. The medical risks of obesity. Postgrad Med. 2009;121(6):21–33. doi: 10.3810/pgm.2009.11.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ward ZJ, Bleich SN, Long MW, Gortmaker SL. Association of body mass index with health care expenditures in the United States by age and sex. PLoS One. 2021;16(3) doi: 10.1371/journal.pone.0247307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fryar CD, Carroll MD, Afful J. Centers for Disease Control and Prevention; Atlanta, GA: Published December 2020. Prevalence of overweight, obesity, and severe obesity among adults aged 20 and over: United States, 1963-1965 through 2017–2018.https://www.cdc.gov/nchs/data/hestat/obesity-child-17-18/overweight-obesity-child-H.pdf Accessed September 19, 2023. [Google Scholar]
- 5.Fan JX, Wen M, Li K. Associations between obesity and neighborhood socioeconomic status: variations by gender and family income status. SSM Popul Health. 2020;10 doi: 10.1016/j.ssmph.2019.100529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshida Y, Wang J, Zu Y, Fonseca VA, Mauvais-Jarvis F. Rising prediabetes, undiagnosed diabetes, and risk factors in young women. Am J Prev Med. 2023;64(3):423–427. doi: 10.1016/j.amepre.2022.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Backman DR, Kohatsu ND, Padovani AJ, et al. Achieving weight loss through a community-based, telewellness programme: a randomized controlled trial. Health Educ J. 2023;82(1):82–94. doi: 10.1177/00178969221139234. [DOI] [Google Scholar]
- 8.Gold MB, Stirling Ginsburg M, Sofaer S. California Healthcare Foundation; Oakland, CA: 2017. Value proposition: the role of cost-effectiveness in coverage decisions, Published December.https://www.chcf.org/wp-content/uploads/2017/12/PDF-CostEffectivenessAnalysis.pdf Accessed December 14, 2023. Accessed December 14, 2023. [Google Scholar]
- 9.Kim DD, Basu A. How does cost-effectiveness analysis inform health care decisions? AMA J Ethics. 2021;23(8):E639–E647. doi: 10.1001/amajethics.2021.639. [DOI] [PubMed] [Google Scholar]
- 10.Gustafson A, Khavjou O, Stearns SC, et al. Cost-effectiveness of a behavioral weight loss intervention for low-income women: the Weight-Wise Program. Prev Med. 2009;49(5):390–395. doi: 10.1016/j.ypmed.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Supplemental nutrition assistance program education plan guidance (fiscal year 2021). U.S. Department of Agriculture. https://snaped.fns.usda.gov/sites/default/files/documents/Introduction%20and%20Section%201%20Overview_0.pdf. Accessed December 17, 2023.
- 12.Vitally Health. https://www.vitally.health/. Accessed April 30, 2023.
- 13.MyPlate. U.S. Department of Agriculture. https://www.myplate.gov/. Accessed December 17, 2023.
- 14.Stata Statistical Software, Release 17. https://www.stat.com. Accessed January 18, 2024.
- 15.Finkelstein EA, Kruger E. Meta- and cost-effectiveness analysis of commercial weight loss strategies. Obesity (Silver Spring) 2014;22(9):1942–1951. doi: 10.1002/oby.20824. [DOI] [PubMed] [Google Scholar]
- 16.Pavlovich WD, Waters H, Weller W, Bass EB. Systematic review of literature on the cost-effectiveness of nutrition services. J Am Diet Assoc. 2004;104(2):226–232. doi: 10.1016/j.jada.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 17.Sherwood NE, Jeffery RW, Pronk NP, et al. Mail and phone interventions for weight loss in a managed-care setting: weigh-to-be 2-year outcomes. Int J Obes (Lond) 2006;30(10):1565–1573. doi: 10.1038/sj.ijo.0803295. [DOI] [PubMed] [Google Scholar]
- 18.Kim TJ, von dem Knesebeck O. Income and obesity: what is the direction of the relationship? A systematic review and meta-analysis. BMJ Open. 2018;8(1) doi: 10.1136/bmjopen-2017-019862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Law L, Kelly JT, Savill H, et al. Cost-effectiveness of telehealth-delivered diet and exercise interventions: a systematic review. J Telemed Telecare. In press. Online February 2, 2022. 10.1177/1357633X211070721. [DOI] [PubMed]
- 20.Corso PS, Ingels JB, Padilla HM, et al. Cost effectiveness of a weight management program implemented in the worksite: translation of fuel your life. J Occup Environ Med. 2018;60(8):683–687. doi: 10.1097/JOM.0000000000001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson A, Maiberger M, Donegan S, Kaplan NC, Kinner P. Cost effectiveness of two lifestyle interventions in the Vermont WISEWOMAN program. Prev Chronic Dis. 2019;16:E31. doi: 10.5888/pcd16.180417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harvey JR, Ogden DE. Obesity treatment in disadvantaged population groups: where do we stand and what can we do? Prev Med. 2014;68:71–75. doi: 10.1016/j.ypmed.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samuel-Hodge CD, Johnston LF, Gizlice Z, et al. Randomized trial of a behavioral weight loss intervention for low-income women: the Weight Wise Program. Obesity (Silver Spring) 2009;17(10):1891–1899. doi: 10.1038/oby.2009.128. [DOI] [PubMed] [Google Scholar]
- 24.Hall KD, Kahan S. Maintenance of lost weight and long-term management of obesity. Med Clin North Am. 2018;102(1):183–197. doi: 10.1016/j.mcna.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galea S, Tracy M, Hoggatt KJ, Dimaggio C, Karpati A. Estimated deaths attributable to social factors in the United States. Am J Public Health. 2011;101(8):1456–1465. doi: 10.2105/AJPH.2010.300086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anekwe CV, Jarrell AR, Townsend MJ, Gaudier GI, Hiserodt JM, Stanford FC. Socioeconomics of obesity. Curr Obes Rep. 2020;9(3):272–279. doi: 10.1007/s13679-020-00398-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perri MG, Shankar MN, Daniels MJ, et al. Effect of telehealth extended care for maintenance of weight loss in rural U.S. communities: a randomized clinical trial. JAMA Netw Open. 2020;3(6) doi: 10.1001/jamanetworkopen.2020.6764. [DOI] [PMC free article] [PubMed] [Google Scholar]