HIGHLIGHTS
-
•
Cardiometabolic impacts on postvaccination SARS-CoV-2 infection risk are unclear.
-
•
Intermittent hypertension was associated with postvaccination infection risk.
-
•
Cardiometabolic pattern and postvaccination infection associations differed by gender.
Keywords: SARS-CoV-2, vaccine, infection, blood pressure, BMI
Abstract
Introduction
Cardiometabolic diseases are associated with greater COVID-19 severity; however, the influences of cardiometabolic health on SARS-CoV-2 infections after vaccination remain unclear. Our objective was to investigate the associations between temporal blood pressure and total cholesterol patterns and incident SARS-CoV-2 infections among those with serologic evidence of vaccination.
Methods
In this prospective cohort of blood donors, blood samples were collected in 2020–2021 and assayed for binding antibodies of SARS-CoV-2 nucleocapsid protein antibody seropositivity. We categorized participants into intraindividual pattern subgroups of blood pressure and total cholesterol (persistently, intermittently, or not elevated [systolic blood pressure <130 mmHg, diastolic blood pressure <80 mmHg, total cholesterol <200 mg/dL]) across the study time points.
Results
Among 13,930 donors with 39,736 donations representing 1,127,071 person-days, there were 221 incident SARS-CoV-2 infections among those with serologic evidence of vaccination (1.6%). Intermittent hypertension was associated with greater SARS-CoV-2 infections among those with serologic evidence of vaccination risk (adjusted incidence rate ratio=2.07; 95% CI=1.44, 2.96; p<0.01) than among participants with consistent normotension on the basis of a multivariable Poisson regression. Among men, intermittently elevated total cholesterol (adjusted incidence rate ratio=1.90; 95% CI=1.32, 2.74; p<0.01) and higher BMI at baseline (adjusted hazard ratio=1.44; 95% CI=1.07, 1.93; p=0.01; per 10 units) were associated with greater SARS-CoV-2 infections among those with serologic evidence of vaccination probability; these associations were null among women (both p>0.05).
Conclusions
Our findings underscore that the benefits of cardiometabolic health, particularly blood pressure, include a lower risk of SARS-CoV-2 infection after vaccination.
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused over 770 million confirmed cases of coronavirus disease 2019 (COVID-19) and 6 million deaths worldwide as of September 27, 2023.1 The increasing number of vaccine doses and boosters administered1 have lowered the risk of hospitalization and mortality caused by SARS-CoV-2,2 but despite increasing vaccination coverage, SARS-CoV-2 infections after vaccination have emerged as a critical issue3, 4, 5 that hinder long-term SARS-CoV-2 management strategies.2 A major challenge stems from an incomplete understanding of the key drivers, particularly modifiable factors, of interindividual variability in immune responses after COVID-19 vaccination.
Cardiometabolic diseases and conditions, particularly hypertension and elevated BMI, have been previously associated with lower vaccine immunogenicity, including lower antibody titers against SARS-CoV-2 and influenza virus.6, 7, 8 A growing body of evidence has shown that cardiometabolic diseases are independent risk factors of severe COVID-19 outcomes,9, 10, 11, 12, 13, 14, 15, 16, 17 including hospitalizations and deaths occurring after COVID-19 illness among those who had been vaccinated,18 and lower postvaccination antibody responses. These prior studies found that improved vaccine effectiveness against subsequent infection and disease burden from SARS-CoV-2 are affected by underlying cardiometabolic health.19 Thus, cardiometabolic health could represent a modifiable risk factor.
One fundamental research gap is the influence of cardiometabolic health patterns on vaccination-induced immune protection and effectiveness against SARS-CoV-2 infection. Blood pressure is homeostatically regulated to allow for dynamic responses to daily physiologic and environmental stimuli (e.g., temperature, physical activity, meal consumption, stress).20, 21, 22 High intraindividual variability in blood pressure is associated with elevated risk of cardiovascular outcomes and mortality.22, 23, 24 Interestingly, this association is relatively stronger than the associations between mean blood pressure measurements and cardiovascular disease risk.23 Previous findings support putative mechanisms explaining how dysregulated blood pressure could be further exacerbated upon SARS-CoV-2 infection.25 SARS-CoV-2 enters human host cells by binding to angiotensin-converting enzyme 2 receptors, which have key functions in blood pressure regulation.26 Separately, prior evidence has shown that greater visit-to-visit variability in circulating cholesterol and lipids could also be an independent risk factor for adverse health outcomes (e.g., all-cause mortality, myocardial infarction, stroke, coronary atheroma progression).27,28 A recent study showed that sterol regulatory element-binding protein signaling, which regulates cholesterol synthesis and affects metabolic diseases, has a key role in B-cell responses after vaccination.29 On the basis of this evidence, we hypothesized that temporal interindividual patterns of systemic blood pressure and cholesterol were associated with SARS-CoV-2 infection risk after vaccination.
With the emergence of SARS-CoV-2, several blood collection organizations conducted serosurveillance studies of previous SARS-CoV-2 infection and vaccination among a longitudinal cohort of donors.30, 31, 32, 33 Some blood donors also provide cardiometabolic health data as part of routine donor screening. Therefore, we evaluated the influences of temporal patterns of cardiometabolic indicators (blood pressure, total cholesterol) on the probability of incident SARS-CoV-2 infection among U.S. blood donors with serologic evidence of vaccination (SISV).
METHODS
This study leveraged data from 2 parent studies that focus on SARS-CoV-2 serosurveillance of allogeneic blood donors across the U.S. The National Blood Donor Seroprevalence (NBDS) study was a serial cross-sectional study conducted during 2020 and 2021.30, 31, 32, 33 The Repeat Donor Cohort (RDC) is a prospective cohort of 152,000 repeat blood donors from Vitalant (>65,000 donors) and American Red Cross (>77,000 donors), which are the 2 largest blood collection organizations in the U.S.
Study Population
This study is a secondary analysis of Vitalant RDC participants with serologic evidence of having received COVID-19 vaccination and no previous SARS-CoV-2 infection. Participants were blood donors who donated blood on multiple occasions during 2020 and 2021. Additional inclusion criteria were having (1) satisfied the U.S. Food and Drug Administration regulations and blood collection organization requirements for all blood donors (e.g., age ≥16 years; >110 lbs; healthy, including being afebrile), (2) provided ≥2 blood donations, (3) antibodies specific to SARS-CoV-2 spike protein antibodies (i.e., anti-S) seropositivity at the first study visit (defined as the first qualifying donation and referred to as baseline), and (4) antibodies specific to SARS-CoV-2 nucleocapsid protein antibodies (i.e., anti-N) seronegativity at the baseline study visit. We excluded participants with missing (1) laboratory assay results for anti-N, anti-S, or total cholesterol and (2) data for key independent variables (e.g., blood pressure [diastolic, systolic], self-reported height and weight, demographics). Donors and donations included in the final analytic data set are shown in Appendix Figure 1 (available online).
Measures
For each donation visit, every donor completed a health questionnaire, which is part of routine donor screening and used for donation eligibility criteria. Blood samples were evaluated for antibodies against SARS-CoV-2 protein antibodies using assays with emergency use authorizations from the U.S. Food and Drug Administration. Concentrations of total cholesterol (mg/dL) from nonfasting blood donation samples were assayed (Beckman Coulter AU analyzers, Brea, CA) at 5 laboratories operated by Creative Testing Solutions. Further data collection details are in the Appendix Methods (available online).
On the basis of the Centers for Disease Control and Prevention's interim guidelines for interpretation of SARS-CoV-2 antibodies among individuals without known vaccination status,34 we restricted the analytic data set to only include participants with serologic evidence of vaccination and no previous SARS-CoV-2 infection (anti-S seropositivity and anti-N seronegativity) at baseline. We defined incident SISV as the first observation of seropositivity for anti-N at any subsequent donation after the baseline donation (Appendix Table 1, available online).
In addition to SARS-CoV-2 antibodies, cardiometabolic indicators were evaluated at every donation time point in the analysis. BMI was calculated as weight (kg) divided by height squared (m2). BMI categories were <18.5 (underweight), ≥18.5 to <25 (normal weight), ≥ 25 to <30 (overweight), and ≥ 30 kg/m2 (obesity). We categorized BMI, blood pressure, and cholesterol with the cut off values recommended by the WHO,35 American College of Cardiology and American Heart Association,36 and National Cholesterol Education Program37 (Appendix Table 2, available online).
To consider temporal patterns and different measures of central tendency, we considered cardiometabolic indicators with 4 approaches: (1) baseline values, (2) maximum (peak) values, (3) median, and (4) categorization of consistently high or low. Temporal patterns are fully detailed in the Appendix Methods (available online).
Statistical Analysis
To account for missing data, a complete-case analysis approach was used. Mean (SD) or median (IQR) was used to summarize continuous variables on the basis of findings from normality assumption assessments; n (%) was reported for categorical variables. Subgroups were compared on the basis of p-values of 2-tailed test statistics (Kruskal–Wallis, Mantel–Haenszel chi-square, Fisher's). Correlations were evaluated by Spearman rank correlation coefficients. We utilized SAS (Version 9.4; SAS Institute, Cary, NC) for statistical analysis, GraphPad Prism (Version 9.3.1.; GraphPad Software, LLC, San Diego, CA), and www.BioRender.com for visualizations. Statistical significance was determined by an ɑ value of 0.05.
We used semiparametric proportional hazards regression models designed to fit interval-censored data (ICPHREG procedure in SAS) to evaluate the bivariable and multivariable associations of interest (Appendix Table 3, available online).38 For participants with incident SISV, time to event was specified as the interval between the date of observed SISV (donation during which first anti-N seropositivity is observed) and the penultimate donation date (last observed donation where anti-N remained seronegative). Participants without incident SISV were considered right censored; their time to event (end-of-study censoring) was defined with the date of their last donation as the lower value of the interval. We first assessed proportional hazard assumptions for key bivariable associations of interest on the basis of log-minus-log survival probability and Schoenfeld residual plots. Most associations did not violate the proportional hazards assumption.39 As exceptions, 2 associations (temporal pattern subgroups of blood pressure or total cholesterol as independent variables) did violate the assumption and were therefore assessed by multivariable Poisson regressions accounting for person-days at risk. On the basis of previous literature, we determined a priori a set of covariates (age, gender, race–ethnicity, geographic region); this set was utilized across multivariable regressions to facilitate comparisons between associations that were adjusted for the same potential confounders.
Because there are biological sex differences in immunity, including vaccination responses,40,41 and cardiometabolic health,42 we evaluated gender as a potential effect modifier. We evaluated 2-way interaction terms, specifically gender multiplied by the primary independent variable, in regressions; if the interaction term was significantly associated (p<0.05), we reported gender-stratified associations.
Study Ethics
All donors provided voluntary, informed consent for the use of their deidentified data and residual blood samples from routine blood donations. This protocol is approved by an IRB protocol (Advarra Number Pro00030878). In accordance with the University of California San Francisco IRB policies and guidance, NBDS was considered nonhuman subjects' research. The NBDS protocol was reviewed by the Centers for Disease Control and Prevention as nonresearch public health surveillance, given the deidentified data and routine consent for blood donation testing. The RDC study protocol was approved by an IRB (Advarra Protocol Number Pro00056783). The study methodology is also reported according to the STROBE guidelines for cohort studies.43
RESULTS
Overall, this analysis included 13,930 donors who provided 39,736 donations, representing 1,127,071 person-days from August 29, 2020 to September 30, 2021 (Table 1 and Appendix Figure 2, available online). The median number of donations per participant was 2 (IQR=2–3); donations ranged between 2 and 19 per participant. At the baseline study visit, the median age was 59 years (IQR=43–68); donors were aged between 16 and 96 years (Table 1 and Appendix Figure 3, available online). Among all donors, 49.7% were female, and 50.3% were male (Table 1). Most donors were White (non-Hispanic, 72.2%); 16.7% were Hispanic, 6.8% were Asian (non-Hispanic), 2.2% were Black (non-Hispanic), and 2.3% were of other races and ethnicities (Table 1).
Table 1.
Demographic and Clinical Characteristics of Study Participants
| Characteristics | Overall | Incident SISV statusa |
||
|---|---|---|---|---|
| Yes | No | p-value | ||
| Study participation and follow-up | ||||
| Donors, n | 13,930 | 221 | 13,709 | |
| Donations, nb | ||||
| Total | 39,736 | 796 | 38,940 | |
| Per participant | ||||
| Median (IQR) | 2 (2–3) | 3 (2–4) | 2 (2–3) | |
| Minimum | 2 | 2 | 2 | |
| Maximum | 19 | 19 | 18 | |
| Time at risk (person-days) | 1,127,071 | 19,575 | 1,107,496 | |
| Demographic characteristics | ||||
| Age, years, median (IQR) | 59 (43–68) | 52 (40–63) | 59 (43–68) | <0.01c |
| Male, n (%) | 7,012 (50.3%) | 132 (59.7%) | 6,880 (50.2%) | <0.01d |
| Female, n (%) | 6,918 (49.7%) | 89 (40.3%) | 6,829 (49.8%) | |
| Race–ethnicity, n (%) | ||||
| White, non-Hispanic | 10,057 (72.2%) | 167 (75.6%) | 9,890 (72.1%) | 0.03d |
| Hispanic | 2,320 (16.7%) | 44 (19.9%) | 2,276 (16.6%) | |
| Asian, non-Hispanic | 940 (6.8%) | 4 (1.8%) | 936 (6.8%) | |
| Black, non-Hispanic | 299 (2.2%) | 3 (1.4%) | 296 (2.2%) | |
| Other | 314 (2.3%) | 3 (1.4%) | 311 (2.3%) | |
| Cardiometabolic indicators at baseline | ||||
| Total cholesterol, n (%) | ||||
| Normal: ≤200 mg/dL | 9,592 (68.9%) | 153 (69.2%) | 9,439 (68.9%) | 0.86d |
| Borderline high: >200 to ≤239 mg/dL | 3,358 (24.1%) | 50 (22.6%) | 3,308 (24.1%) | |
| High: ≥240 mg/dL | 980 (7.0%) | 18 (8.1%) | 962 (7.0%) | |
| Blood pressure | ||||
| Systolic, mmHg, median (IQR) | 126 (116–138) | 128 (121–139) | 126 (116–138) | 0.03c |
| Diastolic, mmHg, median (IQR) | 76 (70–83) | 78 (72–84) | 76 (70–83) | <0.01c |
| Normotension, n (%) | 6,716 (48.2%) | 88 (39.8%) | 6,628 (48.4%) | 0.01d |
| Hypertension, n (%) | 7,214 (51.8%) | 133 (60.2%) | 7,081 (51.7%) | |
| BMI categories, n (%) | ||||
| Underweight: <18.5 kg/m2 | 33 (0.2%) | 0 (0.0%) | 33 (0.2%) | <0.01e |
| Normal: ≥18.5 and <25.0 kg/m2 | 3,866 (27.8%) | 38 (17.2%) | 3,828 (27.9%) | |
| Overweight: ≥25.0 and <30.0 kg/m2 | 5,317 (38.2%) | 103 (46.6%) | 5,214 (38.0%) | |
| Obesity: ≥30.0 kg/m2 | 4,714 (33.8%) | 80 (36.2%) | 4,634 (33.8%) | |
Among donors without seropositivity at baseline (first visit contributed to study), incidence was defined as the number of newly observed cases (anti-N seropositivity) during the follow-up period. At-risk time (person-days) was defined as the last visit to the first visit among participants without SISV or an interval (visit of incident SISV detection to visit immediately preceding incident SISV) among participants with SISV.
In this table, all values reflect the baseline study visit of each donor. The exceptions were the number of donations (total among all donors; median, minimum, maximum per donor).
Variable was not normally distributed on the basis of the Kolmogorov–Smirnov test statistic (p<0.05). p-value was calculated from the Kruskal–Wallis test statistic.
p-value was calculated from the Mantel–Haenszel chi-square test statistic.
p-value was calculated from Fisher's exact test statistic.
anti-N, antibodies specific to SARS-CoV-2 nucleocapsid protein antibodies; SISV, SARS-CoV-2 infection among those with serologic evidence of vaccination.
Among blood donors in this study, the cumulative incidence of SISV was 1.6% (Table 1). Donors with incident SISV (n=221) contributed a total of 796 donations and 19,575 person-days at risk; the median person-days was 66 (IQR=28–112). A total of 13,709 donors without incident SISVs contributed 38,940 donations and 1,107,496 person-days at risk; the median person-days was 70 (IQR=56–105). The incidence rate was 19.6 SISVs per 100,000 person-days.
Donors with SISVs were younger at the baseline study visit than donors without SISVs (median age of 52 years [IQR=40–63] vs 59 years [IQR=43–68]; p<0.01). There was a lower proportion of female donors among those with SISV (40.3%) than among those without SISV (49.8%; p<0.01). Percentages of participants in racial–ethnic groups differed by incident SISV (p=0.03).
At baseline, 7.0% of participants had high total cholesterol, and 24.1% had borderline elevated total cholesterol (Table 1). The percentages of donors in cholesterol categories were similar between donors with and those without incident SISVs (p=0.86).
Among all donors at baseline, 51.8% had hypertension (Table 1). A greater proportion of participants who later had SISVs were hypertensive at baseline (60.2%) than those without SISVs (51.7%; p<0.01). Median systolic blood pressure (SBP) and diastolic blood pressure (DBP) (mmHg) at baseline study visits were higher among those with SISVs than among those without (SBP=128 [IQR=121–139] vs 126 [IQR=116–138] mmHg; DBP=78 [IQR=72–84] vs 76 [IQR=70–83]; both p≤0.03).
Overall, 33.8% of donors had BMI categorized as obese, and 38.2% were overweight (Table 1). BMI categories differed by incident SISV status (p<0.01); a greater proportion of donors with incident SISV were overweight (46.6% vs 38.0%) or had obesity (36.2% vs 33.8%) than those without incident SISV.
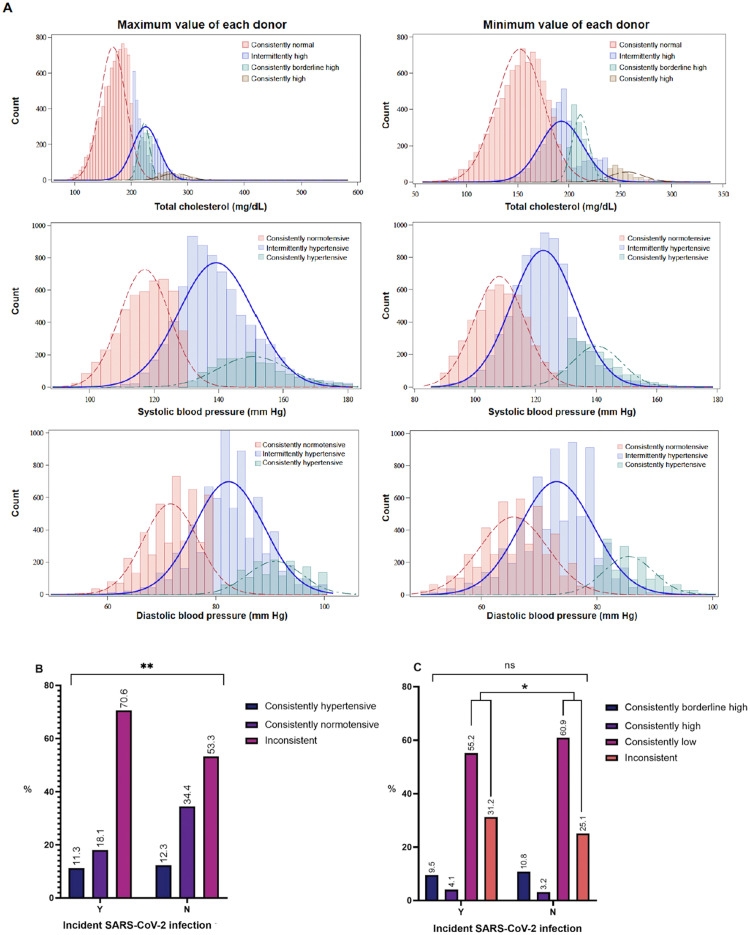
Considering all time points of each donor, median values of the minimum, median, and maximum total cholesterol (mg/dL), SBP, and DBP (mmHg) are reported in Appendix Table 4 (available online). Proportions of participants in hypertension pattern subgroups differed by incident SISV status (p<0.01) (Figure 1). Among participants without SISVs, a greater percentage (34.4% vs 18.1%) were consistently normotensive at all available donation time points, and a smaller percentage (53.3% vs 70.6%) had intermittent hypertension than those with SISVs. Among all participants, proportions in 4 cholesterol temporal pattern subgroups (consistently high, consistently borderline high, consistently normal, intermittently elevated) were similar by SISV status (p>0.05) (Figure 1).
Figure 1.
Comparison of proportion of donors in blood pressure and total cholesterol temporal pattern subgroups, stratified by incident SISV status. (A) Distribution of maximum and minimum values of total cholesterol (mg/dL) and blood pressure (mmHg) among participants (ndonors=13,930), stratified by subgroups on the basis of temporal patterns of total cholesterol and blood pressure during the study period. (B) The proportions of participants categorized as consistently hypertensive (elevated blood pressure at every study time point), consistently normotensive (normotensive blood pressure at every study time point), and intermittently hypertensive across time points were compared by incident SISV status over the study period. (C) Percentages in temporal cholesterol pattern subgroups were compared by incident SISV status. Individuals with intermittently high total cholesterol included those with high or borderline high at some but not all of their study visits (donations).
p-value of Mantel–Haenszel chi-square test statistic are reported. *p<0.05 and **p<0.01.
SISV, SARS-CoV-2 infection among those with serologic evidence of vaccination.
Bivariable associations are presented in Appendix Table 5 (available online). We adjusted for age, gender, race–ethnicity, and geographic region (Appendix Methods, available online) in the multivariable proportional hazards regression results below.
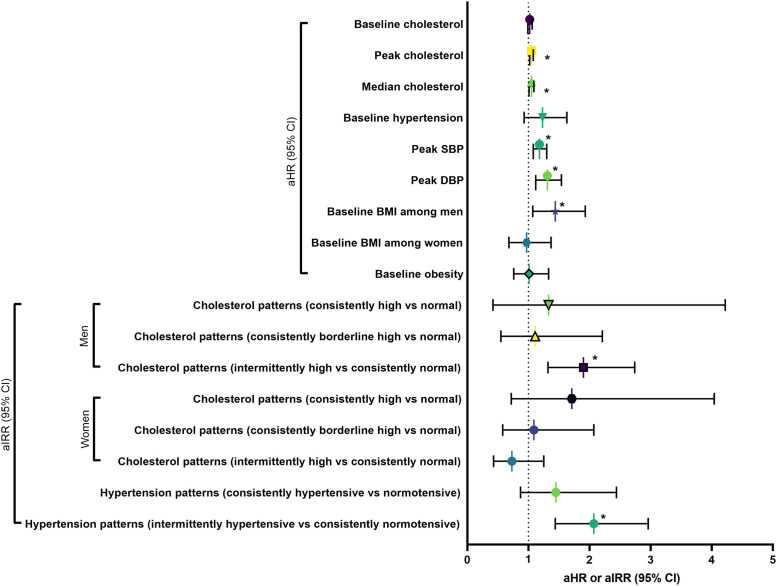
Per 10 unit increase in median total cholesterol (mg/dL) was associated with an elevated risk of incident SISV (adjusted hazard ratio [aHR]=1.05; 95% CI=1.01, 1.09; p=0.02) (Figure 2 and Appendix Table 6, available online). Among men, intermittently high cholesterol was associated with a greater risk of SISV (adjusted incidence rate ratio=1.90; 95% CI=1.32, 2.74; p<0.01) than the risk among men with consistently normal cholesterol; among women, the risk of SISV was similar across cholesterol pattern subgroups (all p>0.05). In a separate multivariable model, per 10 unit increase of the peak total cholesterol (mg/dL; maximum value during the study follow-up) was associated with a higher risk of SISV (aHR=1.05; 95% CI=1.02, 1.08; p<0.01). At baseline, per 10 unit increase of total cholesterol (mg/dL; p=0.26) and high cholesterol (≥240 mg/dL; p=0.23) were not associated with the risk of SISV.
Figure 2.
Summary of associations between blood pressure, cholesterol, BMI, and incident SISV.
Results from multivariable semiparametric proportional hazards and Poisson regression models are in Appendix Tables 6–8 (available online). Each unique symbol represents the key association of interest (aHR or aIRR) and corresponding 95% CI from a separate multivariable regression.
*p<0.05.
aHR, adjusted hazards ratio; aIRR, adjusted incidence rate ratio; DBP, diastolic blood pressure; SBP, systolic blood pressure; SISV, SARS-CoV-2 infection among those with serologic evidence of vaccination.
Per 10 unit increase (mmHg) of peak SBP (aHR=1.18; 95% CI=1.08, 1.30; p<0.01) and DBP (aHR=1.31; 95% CI=1.12, 1.54; p<0.01) was associated with greater risk of SISV (Figure 2 and Appendix Table 7, available online). Intermittent hypertension was associated with a higher risk of SISV (adjusted incidence rate ratio=2.07; 95% CI=1.44, 2.96; p<0.01) than consistent normotension as the reference; in contrast, consistent hypertension (p=0.16) or hypertension at baseline (p=0.15) was not associated with the likelihood of SISV.
Among men, per 10 unit increase in baseline BMI (kg/m2) was positively associated with incident SISV (aHR=1.44; 95% CI=1.07, 1.93; p=0.01) (Figure 2 and Appendix Table 8, available online). Among women, per 10 unit increase in baseline BMI was not associated with the risk of SISV (p=0.84). Baseline obesity status was not associated with the risk of SISV (p=0.95) among men and women.
DISCUSSION
Among 13,930 blood donors, the incidence rate of SISV was 19.6 per 100,000 person-days. Blood donors with intermittent hypertension had a greater risk of SISV than donors with consistently normotensive blood pressure. Higher baseline BMI and intermittently elevated cholesterol, respectively, were associated with a greater risk of SISV among men but not among women.
Elevated blood pressure, cholesterol, and BMI are major risk factors for cardiovascular diseases and related mortality. Our findings indicate that maintaining these cardiometabolic indicators in normal ranges could have the additional benefit of reducing the risk of SARS-CoV-2 infection after vaccination. The results from this study underscore several key research priorities. Future studies need to systematically evaluate the influences of multiple metrics of blood pressure variability on COVID-19 severity outcomes44 among people with vaccinations, particularly with emergent SARS-CoV-2 variants and vaccines currently in the pipeline. The gender-specific associations between intermittently high cholesterol or BMI and SISV risk need further elucidation (Appendix Figure 4, available online). Cholesterol-rich lipid domains in SARS-CoV-2 lipid envelope membranes are associated with SARS-CoV-2 viral entry into host cells45,46; however, biological mechanisms explaining gender and sex differences are needed.
Limitations
This study had several limitations. First, participants are not representative of the general population because they met the minimum health-related eligibility criteria for allogeneic blood donation.47 Despite this likely bias toward the null, we still found associations. Second, we were unable to account for key vaccination status details, including the number of doses, formulation, and calendar time of receipt given waning antibodies and immune protection. Previous vaccination was determined on the basis of serologic evidence. Third, we did not have exact dates of SARS-CoV-2 infections on the basis of nucleic acid amplification or antigen test results of swab samples or genome-sequencing data of SARS-CoV-2 strains. Because SARS-CoV-2 variants and subvariants have heterogeneous transmissibility and immune escape capacity, it is likely that SARS-CoV-2 subvariants are effect modifiers of the associations between cardiometabolic indicators and SISV. We were not able to account for reinfections and varying risks of infection over time, given the numerous factors affecting inter and intraindividual exposure risk. Fourth, a large proportion of blood samples were not assayed for anti-N results and were excluded, which potentially limits the generalizability of findings. We note that laboratory testing algorithms screened for serologic evidence of previous SARS-CoV-2 infection and vaccination by anti-S; many samples that were not further tested for anti-N had serologic evidence of previous infection or no COVID-19 vaccination, which are both baseline exclusion criteria in this study. Fifth, there is potential for residual confounding, despite our adjustment for covariates. As key examples, we did not evaluate models with both blood pressure and total cholesterol; donation frequency and interdonation intervals (including their respective nonlinear associations); clinical diagnoses; and treatment of cardiovascular and metabolic diseases, particularly hypertension medications (e.g., angiotensin-converting enzyme inhibitors) owing to substantial missingness of data.
One major strength of this analysis was the assessment of the risk of SISV over a large overall time at risk, including >1.1 million person-days. Another strength was the evaluation of temporal patterns in blood pressure and cholesterol as modifiable risk factors, which were based on multiple measurements of cardiometabolic health indicators; this contrasts with other studies that measured these homeostatic variables only once and therefore could contribute to the inconsistencies across previous findings. Our analysis is therefore more informative because the results are likely to reflect the individual-level cardiometabolic health profile and should aid in designing longer-term preventative approaches. In sensitivity analyses, we confirmed that key associations were similar while further adjusting for BMI or the number of donations as a continuous variable, respectively.
CONCLUSIONS
Our results indicate that cardiometabolic indicators could represent risk factors for SARS-CoV-2 infection among individuals with vaccinations.
CRediT authorship contribution statement
Elaine A. Yu: Conceptualization, Formal analysis, Investigation, Methodology, Supervision, Visualization, Writing – original draft, Writing – review & editing. Mars Stone: Data curation, Investigation, Project administration, Methodology, Writing – review & editing. Marjorie D. Bravo: Data curation, Formal analysis, Methodology, Writing – review & editing. Eduard Grebe: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. Roberta L. Bruhn: Data curation, Investigation, Project administration, Writing – review & editing. Marion C. Lanteri: Data curation, Investigation, Project administration, Resources, Writing – review & editing. Mary Townsend: Data curation, Methodology, Resources, Writing – review & editing. Hany Kamel: Data curation, Methodology, Resources, Writing – review & editing. Jefferson M. Jones: Data curation, Investigation, Project administration, Methodology, Writing – review & editing. Michael P. Busch: Data curation, Investigation, Project administration, Methodology, Supervision, Writing – original draft, Writing – review & editing. Brian Custer: Data curation, Investigation, Project administration, Methodology, Supervision, Writing – original draft, Writing – review & editing.
ACKNOWLEDGMENTS
The authors acknowledge the critical insights and feedback from Jessica K. Lerch, PhD; CareerVolt; and colleagues at the Centers for Disease Control and Prevention (CDC). Appendix Figures 1 and 5 (available online) were created with BioRender.com. MPB and BC contributed equally to this work.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
The CDC funded the parent study. Additional support was provided by the National Institute of General Medical Sciences of the NIH (R25GM143298 for EAY).
Declaration of interest: None.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.focus.2024.100186.
Appendix. Supplementary materials
REFERENCES
- 1.WHO coronavirus (COVID-19) dashboard. World Health Organization. https://covid19.who.int/. Updated December 31, 2023. Accessed January 17, 2024.
- 2.Altmann DM, Boyton RJ. COVID-19 vaccination: the road ahead. Science. 2022;375(6585):1127–1132. doi: 10.1126/science.abn1755. [DOI] [PubMed] [Google Scholar]
- 3.Iketani S, Liu L, Guo Y, et al. Antibody evasion properties of SARS-CoV-2 Omicron sublineages. Nature. 2022;604(7906):553–556. doi: 10.1038/s41586-022-04594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeGrace MM, Ghedin E, Frieman MB, et al. Defining the risk of SARS-CoV-2 variants on immune protection. Nature. 2022;605(7911):640–652. doi: 10.1038/s41586-022-04690-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu L, Iketani S, Guo Y, et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature. 2022;602(7898):676–681. doi: 10.1038/s41586-021-04388-0. [DOI] [PubMed] [Google Scholar]
- 6.Neidich SD, Green WD, Rebeles J, et al. Increased risk of influenza among vaccinated adults who are obese. Int J Obes (Lond) 2017;41(9):1324–1330. doi: 10.1038/ijo.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paich HA, Sheridan PA, Handy J, et al. Overweight and obese adult humans have a defective cellular immune response to pandemic H1N1 influenza A virus. Obesity (Silver Spring) 2013;21(11):2377–2386. doi: 10.1002/oby.20383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheridan PA, Paich HA, Handy J, et al. Obesity is associated with impaired immune response to influenza vaccination in humans. Int J Obes (Lond) 2012;36(8):1072–1077. doi: 10.1038/ijo.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stefan N, Birkenfeld AL, Schulze MB. Global pandemics interconnected — obesity, impaired metabolic health and COVID-19. Nat Rev Endocrinol. 2021;17(3):135–149. doi: 10.1038/s41574-020-00462-1. [DOI] [PubMed] [Google Scholar]
- 10.Stefan N. Metabolic disorders, COVID-19 and vaccine-breakthrough infections. Nat Rev Endocrinol. 2022;18(2):75–76. doi: 10.1038/s41574-021-00608-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drucker DJ. Diabetes, obesity, metabolism, and SARS-CoV-2 infection: the end of the beginning. Cell Metab. 2021;33(3):479–498. doi: 10.1016/j.cmet.2021.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steenblock C, Schwarz PEH, Ludwig B, et al. COVID-19 and metabolic disease: mechanisms and clinical management. Lancet Diabetes Endocrinol. 2021;9(11):786–798. doi: 10.1016/S2213-8587(21)00244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8(10):813–822. doi: 10.1016/S2213-8587(20)30272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.COVID-19 Host Genetics Initiative Mapping the human genetic architecture of COVID-19. Nature. 2021;600(7889):472–477. doi: 10.1038/s41586-021-03767-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qu HQ, Qu J, Glessner J, Hakonarson H. Mendelian randomization study of obesity and type 2 diabetes in hospitalized COVID-19 patients. Metabolism. 2022;129 doi: 10.1016/j.metabol.2022.155156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freuer D, Linseisen J, Meisinger C. Impact of body composition on COVID-19 susceptibility and severity: A two-sample multivariable Mendelian randomization study. Metabolism. 2021;118 doi: 10.1016/j.metabol.2021.154732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo S, Liang Y, Wong THT, Schooling CM, Au Yeung SL. Identifying factors contributing to increased susceptibility to COVID-19 risk: a systematic review of Mendelian randomization studies. Int J Epidemiol. 2022;51(4):1088–1105. doi: 10.1093/ije/dyac076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piernas C, Patone M, Astbury NM, et al. Associations of BMI with COVID-19 vaccine uptake, vaccine effectiveness, and risk of severe COVID-19 outcomes after vaccination in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2022;10(8):571–580. doi: 10.1016/S2213-8587(22)00158-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Westheim AJF, Bitorina AV, Theys J, Shiri-Sverdlov R. COVID-19 infection, progression, and vaccination: focus on obesity and related metabolic disturbances. Obes Rev. 2021;22(10):e13313. doi: 10.1111/obr.13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parati G, Ochoa JE, Lombardi C, Bilo G. Assessment and management of blood-pressure variability. Nat Rev Cardiol. 2013;10(3):143–155. doi: 10.1038/nrcardio.2013.1. [DOI] [PubMed] [Google Scholar]
- 21.Parati G, Torlasco C, Pengo M, Bilo G, Ochoa JE. Blood pressure variability: its relevance for cardiovascular homeostasis and cardiovascular diseases. Hypertens Res. 2020;43(7):609–620. doi: 10.1038/s41440-020-0421-5. [DOI] [PubMed] [Google Scholar]
- 22.Schutte AE, Kollias A, Stergiou GS. Blood pressure and its variability: classic and novel measurement techniques. Nat Rev Cardiol. 2022;19(10):643–654. doi: 10.1038/s41569-022-00690-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevens SL, Wood S, Koshiaris C, et al. Blood pressure variability and cardiovascular disease: systematic review and meta-analysis. BMJ. 2016;354:i4098. doi: 10.1136/bmj.i4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dasa O, Smith SM, Howard G, et al. Association of 1-year blood pressure variability with long-term mortality among adults with coronary artery disease: a post hoc analysis of a randomized clinical trial. JAMA Netw Open. 2021;4(4) doi: 10.1001/jamanetworkopen.2021.8418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Briquez PS, Rouhani SJ, Yu J, et al. Severe COVID-19 induces autoantibodies against angiotensin II that correlate with blood pressure dysregulation and disease severity. Sci Adv. 2022;8(40):eabn3777. doi: 10.1126/sciadv.abn3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bosso M, Thanaraj TA, Abu-Farha M, Alanbaei M, Abubaker J, Al-Mulla F. The two faces of ACE2: the role of ACE2 receptor and its polymorphisms in hypertension and COVID-19. Mol Ther Methods Clin Dev. 2020;18:321–327. doi: 10.1016/j.omtm.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clark D, III, Nicholls SJ, St John J, et al. Visit-to-visit cholesterol variability correlates with coronary atheroma progression and clinical outcomes. Eur Heart J. 2018;39(27):2551–2558. doi: 10.1093/eurheartj/ehy209. [DOI] [PubMed] [Google Scholar]
- 28.Kim MK, Han K, Kim HS, et al. Cholesterol variability and the risk of mortality, myocardial infarction, and stroke: a nationwide population-based study. Eur Heart J. 2017;38(48):3560–3566. doi: 10.1093/eurheartj/ehx585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pulendran B, Luo W. Lipid homeostasis mediated by cholesterol synthesis supports B cell responses to vaccination. Nat Immunol. 2023;24(2):216–217. doi: 10.1038/s41590-022-01400-1. [DOI] [PubMed] [Google Scholar]
- 30.Jones JM, Stone M, Sulaeman H, et al. Estimated US infection- and vaccine-induced SARS-CoV-2 seroprevalence based on blood donations, July 2020–May 2021. JAMA. 2021;326(14):1400–1409. doi: 10.1001/jama.2021.15161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Busch MP, Stramer SL, Stone M, et al. Population-weighted seroprevalence Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection, Vaccination, and Hybrid Immunity Among US Blood Donations From January to December 2021. Clin Infect Dis. 2022;75(suppl 2):S254–S263. doi: 10.1093/cid/ciac470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones JM, Opsomer JD, Stone M, et al. Updated US infection- and vaccine-induced SARS-CoV-2 seroprevalence estimates based on blood donations, July 2020–December 2021. JAMA. 2022;328(3):298–301. doi: 10.1001/jama.2022.9745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller MJ, Himschoot A, Fitch N, et al. Association of trends in Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Seroprevalence and State-Issued Nonpharmaceutical Interventions: United States, 1 August 2020 to 30 March 2021. Clin Infect Dis. 2022;75(suppl 2):S264–S270. doi: 10.1093/cid/ciac469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Interim guidelines for COVID-19 antibody testing. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing/antibody-tests-guidelines.html. Updated December 16, 2022. Accessed on October 3, 2023.
- 35.Obesity: preventing and managing the global epidemic. World Health Organ Tech Rep Ser. 2000;894 https://iris.who.int/handle/10665/42330 Report of a WHO consultation. i-253. [PubMed] [Google Scholar]
- 36.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13–e115. doi: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 37.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. doi: 10.1161/circ.106.25.3143. [DOI] [PubMed] [Google Scholar]
- 38.Royston P, Parmar MK. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med. 2002;21(15):2175–2197. doi: 10.1002/sim.1203. [DOI] [PubMed] [Google Scholar]
- 39.Kleinbaum DG, Klein M. In: Survival Analysis. Kleinbaum DG, Klein M, editors. Springer; New York, NY: 2012. Evaluating the proportional hazards assumption; pp. 161–200. [DOI] [Google Scholar]
- 40.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 41.Flanagan KL, Fink AL, Plebanski M, Klein SL. Sex and gender differences in the outcomes of vaccination over the life course. Annu Rev Cell Dev Biol. 2017;33(1):577–599. doi: 10.1146/annurev-cellbio-100616-060718. [DOI] [PubMed] [Google Scholar]
- 42.Gerdts E, Regitz-Zagrosek V. Sex differences in cardiometabolic disorders. Nat Med. 2019;25(11):1657–1666. doi: 10.1038/s41591-019-0643-8. [DOI] [PubMed] [Google Scholar]
- 43.Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12(12):1500–1524. doi: 10.1016/j.ijsu.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 44.Jagannatha GNP, Yasmin AAADA, Pradnyana IWAS, et al. Therapeutic target and clinical impact of day-to-day blood pressure variability in hypertensive patients with Covid-19. Hypertens Res. 2023;46(1):165–174. doi: 10.1038/s41440-022-01077-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kočar E, Režen T, Rozman D. Cholesterol, lipoproteins, and COVID-19: basic concepts and clinical applications. Biochim Biophys Acta Mol Cell Biol Lipids. 2021;1866(2) doi: 10.1016/j.bbalip.2020.158849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barrantes FJ. The constellation of cholesterol-dependent processes associated with SARS-CoV-2 infection. Prog Lipid Res. 2022;87 doi: 10.1016/j.plipres.2022.101166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Atsma F, Veldhuizen I, Verbeek A, de Kort W, de Vegt F. Healthy donor effect: its magnitude in health research among blood donors. Transfusion. 2011;51(8):1820–1828. doi: 10.1111/j.1537-2995.2010.03055.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.