Abstract
This report discusses the occurrence of tumor-to-tumor metastasis—an atypical phenomenon in oncology where a secondary malignancy develops within an existing primary tumor. The case of a 64-year-old woman is presented, who, with a history of stage II invasive ductal carcinoma of the breast treated with mastectomy and chemoradiotherapy, developed neurological symptoms indicative of a secondary brain tumor. MRI and subsequent histopathological analysis post-craniotomy confirmed a meningioma with a metastatic breast carcinoma, demonstrating the clinical importance of considering tumor-to-tumor metastasis in similar patient histories.
Keywords: Breast neoplasms, Meningeal neoplasms, Neoplasm metastasis, Carcinoma, Ductal
Introduction
Tumor-to-tumor metastasis represents a unique and complex clinical scenario characterized by a metastatic tumor emerging within another primary neoplasm [1]. Among recipient tumors, meningiomas are the most common, with breast carcinoma being a frequent metastasizing primary tumor. Although exceedingly rare, this phenomenon is documented sparingly within the medical literature.
Case presentation
A 64-year-old female with a background of stage II Left breast invasive ductal carcinoma that was Estrogen and Progesterone receptors negative and HER-2 receptor positive, had undergone left modified radical mastectomy and adjuvant chemoradiotherapy with a good clinical response (Fig. 1). After 2 years, she developed new onset headaches and two episodes of generalized tonic-clonic seizures. Neurological examination revealed subtle Rt sided weakness with hyperreflexia and no other deficits. An MRI of the brain demonstrated a left temporal dural-based contrast-enhancing brain tumor, indicative of meningioma (Fig. 2).
Fig. 1.
Histopathology of left breast intraductal carcinoma (IDC). (XA) Primary carcinoma of the breast, Grade 2 (x100*) HE (yB) estrogen receptor negative(x100*). (YC) Progesterone Receptor negative (x100*) (yD) Strong HER2 membrane staining (3+) ID (x100*). x-H&E Staining, y-Immunohistochemistry, * -magnification.
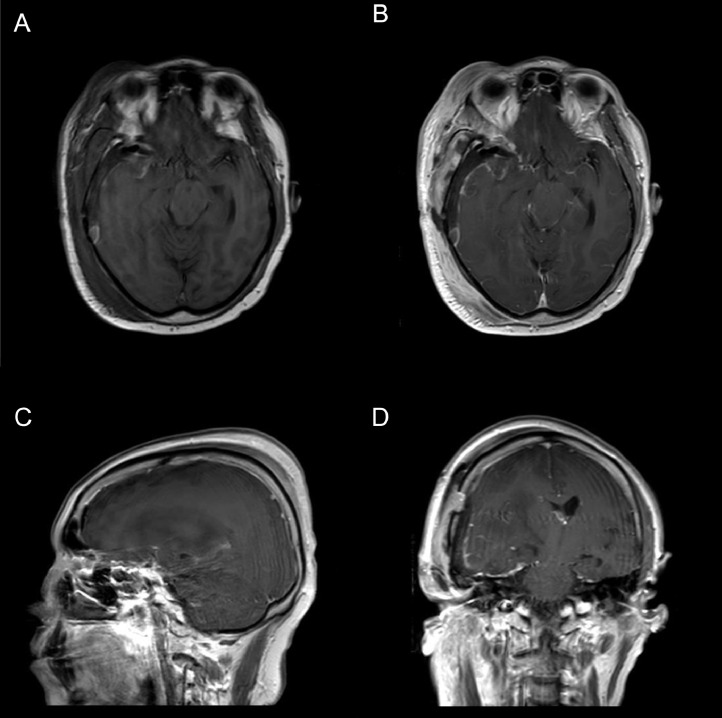
Fig. 2.
Preoperative MRI images with gadolinium. (A) MRI: Pre-Operative Axial T1 SE Non-Contrasted (B) MRI: Pre-Operative Axial T1 SE + Gadolinium Contrast (C) MRI: Pre-Operative Sagittal T1 SE + Gadolinium Contrast (D) MRI: Pre-Operative CoronalT1 SE + Gadolinium Contrast.
The histopathology, following a gross total resection of the tumor via craniotomy, revealed two adjacent tumor types without a distinct demarcation. On immunohistochemistry, the predominant tumor was GATA3 positive, aligning with a breast primary, and exhibited the same biomarker status as the initial breast tumor. The secondary tumor displayed characteristics consistent with a meningioma.
On immunohistochemistry, the predominant tumor was GATA3 positive, in keeping with a breast primary. It also showed ER/PR negative and HER2 positive (3+) biomarker status, as was the case with the original breast tumor. The second tumor was positive for PR and S100, in a pattern consistent with a meningioma, World Health Organization (WHO) grade I meningioma (Fig. 3).
Fig. 3.
Histopathology left temporal brain tumor. (xA) Comedonecrosis of the ductal carcinoma (circled) (x40*). (xB) Meningothelial cells (arrowhead), IDC cells bottom left (arrow) (x100*). (xC) Meningothelial cells, with few ductal carcinoma cells in between (circled) (x100*). (yD) Strong HER2 membrane staining (3+) in IDC; negative in meningothelial cells (x100*). (yE) GATA3 nuclear positivity (brown nuclear staining) in IDC component (x100*). (yF) PR positivity in meningothelial cells, (brown nuclear staining), whorling pattern. Surrounding IDC negative (x100*). x-H&E Staining, y-Immunohistochemistry, * -magnification; IDC, intraductal carcinoma.
The patient's recovery was uneventful, and she was discharged on antiepileptic medications. She underwent adjuvant whole-brain radiotherapy as part of her multidisciplinary team follow-up and remains recurrence-free 22 months postcraniotomy as shown in Fig. 4.
Fig. 4.
Postoperative MRI images with gadolinium. (A) MRI: Post-Operative Axial T1 SENon-Contrasted (B) MRI: Post-Operative Axial T1 SE + Gadolinium Contrast. (C) MRI: Post-Operative Sagittal T1 SE + Gadolinium Contrast (D) MRI: Post-Operative Coronal T1 SE + Gadolinium Contrast.
Discussion
Tumor-to-tumor metastasis, while exceptional, has substantial implications for disease management strategies. In this particular case, the detection of metastasis in the meningioma potentially escalated disease staging and influenced the surgical and adjuvant treatment plan [1,2].
In our case, for instance, the disease staging could have been escalated to stage IV if metastasis was detected at the initial diagnosis. Additionally, meningiomas that harbor metastasis and extend to juxtaposed tissues may necessitate more resection margins, with adjuvant radiotherapy, as evidenced in this case [3].
Further treatment relies on the extent of resection, tumor biology and clinical variables such as location [4,5]. Meningiomas are outside the blood-brain barrier, hence may be more susceptible to chemotherapy even in partially resected or inoperable meningioma with metastasis differing from that of brain parenchymal metastasis. This noteworthy characteristic underlines the importance of personalized treatment approaches based on the metastatic patterns [2,3].
Epidemiologically the most implicated primary neoplasm in tumor-to-tumor metastasis is breast carcinoma followed by lung and squamous cell carcinomas. Approximately one fifth of solid tumors metastasize to the central nervous system with a minuscule percentage metastasizing to leptomeningeal meningiomas. Literature reveals about seventy documented cases of metastasis to meningiomas [1], [2], [3].
Histopathological examination often plays a pivotal role in disease management. In our case, the unclear demarcation between the metastasis and the meningeal parenchyma made recognition challenging, underscoring the importance of careful histopathological assessment for appropriate therapeutic planning [3].
The molecular mechanisms driving metastatic anchoring and spread along the meninges remain largely elusive. While no meningioma subtypes have increased risk of tumor-to-tumor metastasis [1,6], specific features of metastasis may facilitate the process. A common putative feature could be hormone receptor expression and in particularly progesterone receptors that seems to play a significant role in meningiomas harboring metastatic breast carcinoma. Notably our patient had a progesterone receptor-negative primary intraductal carcinoma of the breast with a progesterone receptor-positive meningioma [7,8].
Meningiomas exhibit high vascularity due to secretion of vascular endothelial growth factor (VEGF) that enhance vascular proliferation and vasogenic edema both thought to facilitates metastasis [1,9]. Multiple cell adhesion molecules like E-cadherin,P-selectin,CXCL12, ICAM,PECAM-1 and SDF-1 are implicated in aiding breast carcinoma metastases [10], [11], [12], [13]. Additionally, the expression of mesothelin by leptomeninges and by extension meningiomas, which in itself acts as an anchoring protein that binds mucin-16 [14,15] seems to facilitate metastasis. Transmembrane mucins such as MUC1 and MUC16 ubiquitous in carcinomas are thought to facilitate metastasis of numerous carcinomas including lung adenocarcinomas [14,15]
Tumor-to-tumor metastasis have significant implications to therapeutic approaches. Clinicians must be vigilant to its possibility in patients with a known history of malignancy presenting with new neurological symptoms and have radiological evidence of meningiomas. Molecular analytical tools that are gaining traction in oncology, aiming for tailored personalized therapy depending on patient-specific factors such as genetic and molecular characteristics are likely to be the next frontier in treating these cases. Therefore, understanding the molecular basis of tumor-to-tumor metastasis may open avenues to effective personalized treatments.
A multidisciplinary team with neurosurgeons, oncologists and pathologists is mandatory for timely diagnosis and accurate treatment planning. This collaboration helps elucidate appropriate histopathological analyses with the appropriate management plan aligning with the biological features of the neoplasms.
Lastly, we must continue to broaden our insights on the mechanisms governing tumor-to-tumor metastasis. These insights will illuminate the most effective therapeutic interventions ultimately with better patient outcomes.
Conclusion
Addressing tumor-to-tumor metastasis is crucial for optimal management of cancer patients. Clinicians should maintain a high degree of vigilance for this rare occurrence, and a multidisciplinary approach is paramount in managing these complex presentations.
Patient consent
We would like to state that we have obtained written informed consent from the patient before submitting the manuscript entitled “A Rare Case of Breast Carcinoma Metastasis into a Meningioma in a 64-Year-Old Female Patient” for consideration of publication in Radiology Case Reports.
Footnotes
Acknowledgments: There was no funding for this study.
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Papadakis B.K, Vorrias E, Brautigam K, Chochlidakis N, Koutsopoulos A, Mavroudis D, et al. Intrameningioma metastasis: a case-based literature review. J Clin Neurosci. 2021;93:168–173. doi: 10.1016/j.jocn.2021.08.028. [DOI] [PubMed] [Google Scholar]
- 2.Doron Y, Gruszkiewicz J. Metastasis of invasive carcinoma of the breast to an extradural meningioma of the cranial vault. Cancer. 1987;60:1081–1084. doi: 10.1002/1097-0142(19870901)60:5<1081::aid-cncr2820600526>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez C, Cappelli L, Chapin S, Kenyon L, Farrell CJ, Shi W. Breast carcinoma metastasis in a resected meningioma with early diagnosis of oligometastatic disease: a case report. Chin Clin Oncol. 2020;9:71–76. doi: 10.21037/cco-20-122. [DOI] [PubMed] [Google Scholar]
- 4.Takei H, Powell SZ. Tumor-to tumor metastasis to the central nervous system. Neuropathology. 2009;29:303–308. doi: 10.1111/j.1440-1789.2008.00952.x. [DOI] [PubMed] [Google Scholar]
- 5.Scott BJ, Oberheim-Bush NA, Kesari S. Leptomeningeal metastasis in breast cancer—a systematic review. Oncotarget. 2016;7:3740–3747. doi: 10.18632/oncotarget.5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sayegh ET, Burch EA, Henderson GA, Oh T, Bloch O, Parsa AT. Tumor-to-tumor metastasis: breast carcinoma to meningioma. J Clin Neurosci. 2015;22:268–274. doi: 10.1016/j.jocn.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Hsu DW, Efird JT, Hedley-Whyte ET. Progesterone and estrogen receptors in meningiomas. Prognostic considerations. J Neurosurg. 1997;86:113–120. doi: 10.3171/jns.1997.86.1.0113. [DOI] [PubMed] [Google Scholar]
- 8.Blankenstein MA, Verheijen FM, Jacobs JM, Donker TH, van Duijnhoven WF, Thijssen JHH. Occurrence, regulation and significance or progesterone receptors in human meningioma. Steroids. 2000;63:795–800. doi: 10.1016/s0039-128x(00)00193-8. [DOI] [PubMed] [Google Scholar]
- 9.Lewy-Trenda I, Omulecka A, Janczukowicz J, Papierz W. The morphological analysis of vasculature and antigenic potential in meningiomas: immunoexpression of CD31 and VEGF antibodies. Folia Neuropathol. 2003;4:149–153. [PubMed] [Google Scholar]
- 10.Lanotte M, Benech F, Panciani PP, Cassoni P, Ducati A. Systemic cancer metastasis in a meningioma: report of two cases and review of the literature. Clin Neurol Neurosurg. 2009;111:87–93. doi: 10.1016/j.clineuro.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Shimada S, Ishizawa K, Hirose T. Expression of E cadherin and catenins in meningiomas: ubiquitous expression and irrelevance to malignancy. Pathol Int. 2005;55:1–7. doi: 10.1111/j.1440-1827.2005.01786.x. [DOI] [PubMed] [Google Scholar]
- 12.Aghi M, Kiehl TR, Brisman JL. Breast adenocarcinoma metastatic to epidural cervical spine meningioma. J Neurooncol. 2005;75:149–155. doi: 10.1007/s11060-005-1408-4. [DOI] [PubMed] [Google Scholar]
- 13.Pedrosa RMSM, Mustafa DA, Soffietti R, Kros JM. Breast cancer brain metastasis: molecular mechanisms and directions for treatment. Neuro Oncol. 2018;20:1439–1449. doi: 10.1093/neuonc/noy044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gubbels JA, Belisle J, Onda M, Rancourt C, Migneault CM, Ho M, et al. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol Cancer. 2006;5:50–65. doi: 10.1186/1476-4598-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rump A, Morikawa Y, Tanaka M, Minami S, Umesaki N, Takeuchi M, et al. Binding of ovarian cancer cell antigen CA125/MUC16 to mesothelin mediates cell adhesion. J Biol Chem. 2004;279:9190–9198. doi: 10.1074/jbc.M312372200. [DOI] [PubMed] [Google Scholar]