Graphical abstract
Keywords: Learning and memory, Egocentric memory, Procedural memory, Spatial memory, Cognitive flexibility, Cognitive tests for safety studies, Neurobehavioral regulatory studies, Developmental neurotoxicity guideline studies, Rats
Highlights
-
•
Why rodent tests for learning and memory in safety studies are important.
-
•
Tests of spatial navigation/hippocampus are recommended (Morris water maze).
-
•
Tests of egocentric navigation/striatum are recommended (Cincinnati water maze).
-
•
Why different tests of two types of learning and memory are better than one.
-
•
Recommendations for how the Morris and Cincinnati water mazes should be used.
Abstract
For decades, regulatory guidelines for safety assessment in rodents for drugs, chemicals, pesticides, and food additives with developmental neurotoxic potential have recommended a single test of learning and memory (L&M). In recent years some agencies have requested two such tests. Given the importance of higher cognitive function to health, and the fact that different types of L&M are mediated by different brain regions assessing higher functions represents a step forward in providing better evidence-based protection against adverse brain effects. Given the myriad of tests available for assessing L&M in rodents this leads to the question of which tests best fit regulatory guidelines. To address this question, we begin by describing the central role of two types of L&M essential to all mammalian species and the regions/networks that mediate them. We suggest that the tests recommended possess characteristics that make them well suited to the needs in regulatory safety studies. By brain region, these are (1) the hippocampus and entorhinal cortex for spatial navigation, which assesses explicit L&M for reference and episodic memory and (2) the striatum and related structures for egocentric navigation, which assesses implicit or procedural memory and path integration. Of the tests available, we suggest that in this context, the evidence supports the use of water mazes, specifically, the Morris water maze (MWM) for spatial L&M and the Cincinnati water maze (CWM) for egocentric/procedural L&M. We review the evidentiary basis for these tests, describe their use, and explain procedures that optimize their sensitivity.
Introduction
The brain is organized into a collection of networks that makes attributing specific functions to specific regions challenging. Nevertheless, it is possible to identify regions that primarily mediate the brain systems responsible for different types of learning and memory (L&M). Fortunately, these relationships have become better defined over the last 50 years by significant advances in neuroscience with known circuits for spatial and egocentric learning and memory, path integration, and episodic memory (Bjerknes et al., 2018, Gofman et al., 2019, Obenhaus et al., 2022, Nagelhus et al., 2023, Ulsaker-Janke et al., 2023).
Learning is a change in behavior that is the result of experience. Memory is the storage and retrieval of learned information. There are two general types of learning: associative and non-associative. Associative learning includes priming, procedural (skills and habits), classical/Pavlovian, and instrumental. Non-associative learning includes habituation and sensitization. Memory is categorized as short-term or long-term. Short-term or working memory is retention of new information that requires attention and rehearsal and has a limited capacity, whereas long-term memory retains information relatively permanently. Long-term memory is categorized as explicit (declarative) and implicit (procedural). Declarative memory is memory for facts, location, and events (episodic memory); implicit memory is for skills, habits, conditioned responses, and priming.
It is impractical to assess all these in safety studies. The specific tests used depends on the regulatory agency, whether it is for regulation of chemicals, conditions of exposure (e.g., workplace, consumer, fence-line [exposure to those living near plants that use or manufacture a given chemical], agricultural, household, commons [parks and recreational areas], drugs, foods, food additives, or dietary supplements).
Given the complexity of L&M systems, the imprimatur in guidelines of the past was to require “a test of learning and memory”. But this is very limited and does not provide protection of the brain given the centrality of L&M to neurocognitive health. Today, our understanding of brain structure–function relationships is better than when safety guidelines were written. Some regulatory agencies recognize this and have begun to request two tests for L&M. Those that make such requests sometimes ask for two different tests or one test given at different ages.
Factors important in the selection of such tests are how translatable they are to humans and how practical they are to perform. Some tests meet these goals better than others. Spatial learning, for example, is an essential function in rodents and humans and is assessable in both species in analogous ways. For rodents, there are water mazes that assess spatial navigation, whereas in humans there are virtual spatial mazes (Cornwell et al., 2008, Suthana et al., 2009, Baumann and Mattingley, 2010, Brown et al., 2014, Guderian et al., 2015, Kolarik et al., 2016). There are limitations to water mazes, as there are with any test, but they have advantages too. In basic research when modeling human neuropsychiatric disorders, schedule-controlled operant methods are suggested to be preferable to water mazes (Silverman et al., 2020), but operant methods have limitations for regulatory studies. For example, (1) they require food restriction which can be problematic if the test compound affects appetite, body weight, reward salience, or reinforcement value, (2) teaching rodents the response requirements (nose-poking, touch screen, or lever press) to obtain reinforcement requires training before the animals can perform complex discriminations that mimic higher human abilities. Although more time consuming than water mazes, there is an extensive literature on operant methods used in neurotoxicity that are primarily focused on in-depth analyses of a prior screening test finding rather than for initial safety evaluation. Operant methods have the advantage that they are flexible and many different cognitive functions can be assessed in the same apparatus.
Regulatory studies must consider multiple factors such as (1) the capacity, costs, and time to conduct large scale studies, (2) test efficiency, (3) simplicity of the task when running large numbers of animals, (4) standardization, (5) test–retest and interlaboratory reliability, (6) interpretability, (7) construct, predictive, and face validity, and (8) the scientific literature underlying the test, which for the water mazes is extensive. Based on these considerations we suggest two different water mazes that meet these regulatory requirements for both hazard identification and determining dose-relationships.
Tests of learning and memory
Many tests of L&M are used in regulatory studies, including delayed matching to sample, olfactory conditioning, schedule controlled operant behavior, active and passive shock avoidance, the Biel water maze (BWM), Cincinnati water maze (CWM), radial arm maze (RAM), Morris water maze (MWM), and two-choice T, E, and M-shaped mazes (Tsuji and Crofton, 2012). Active and passive (or inhibitory) avoidance tests are straightforward, commercially available, automated, and have a long history of use in behavioral neuroscience. Both avoidance tests use foot-shock to motivate performance, and some strains learn well on these tasks and others do not, but all strains have a percentage of non-performers that must be accounted for, something that rarely occurs in water mazes. Moreover, there are data indicating avoidance tests are not sensitive to neurotoxicity. For example, passive avoidance was unaffected in rats treated prior to weaning with methamphetamine whereas clear learning impairments were found in the MWM and in the CWM from this treatment in the same rats (Jablonski et al., 2017, Jablonski et al., 2019). In addition, shock tests are influenced by strain-specific activity levels and some strains freeze in response to shock, whereas locomotor activity levels do not affect swimming speed (Cravens, 1974).
It is beyond our scope to review all the L&M tests in current use except to note that water mazes are widely used, including the BWM, and to a lesser extent the CWM. The CWM is more sensitive than the BWM, but this increase in sensitivity comes at a cost, in that the CWM takes longer to run. How much longer depends on the test procedure. In general, it takes 5 days to run the CWM with standard room lighting but 3–4-times longer under infrared light. The upside to testing under infrared light is that it increases its sensitivity in part by reducing access to distal cues rodents could use for spatial navigation if lights are on. Infrared lighting forces the rat to use internal self-movement (egocentric) cues to find its way (Fouquet et al., 2013). But consistent with our emphasis on L&M tests with established brain region underpinnings, we provide an overview of these associations for the CWM and several other widely used tests.
Brain–behavior relationships
-
1.
Prefrontal cortex (PFC): Working memory: RAM, water radial arm maze (RWM), spontaneous alternation, operant methods.
-
2.
PFC: Social learning: Crawley social preference test, social interaction test.
-
3.
Frontal and parietal cortices: Executive function: MWM reversal learning (cognitive flexibility), operant conditioning, 5-choice serial reaction time test (5-CSRTT).
-
4.
Striatum, thalamus, and hippocampus: Egocentric L&M: BWM, CWM, Stone maze, Whishaw test, figure-eight-maze (not to be confused with the figure-eight locomotor activity test).
-
5.
Hippocampus and entorhinal cortex: Spatial L&M: MWM, Barnes maze, hole poke/cheese-board maze.
-
6.
Dorsal hippocampus: Incidental learning: Novel object recognition (NOR), novel place recognition (NPR), and variations thereof.
-
7.
Amygdala: Emotional learning: Cued conditioned freezing, resident-intruder test.
-
8.
Cerebellum and other regions: Classical conditioning: Eyeblink conditioning, shock avoidance.
Pros and cons to these tests
-
1.
Working memory: the RAM is a test of trial-dependent memory, however there are procedures that permit it to be used for both trial-dependent and trial-independent memory by either baiting all arms or baiting some arms and not others. There are 8, 12, 16, and 17-arm versions of the RAM, although the 8-arm is the most common. It is an appetitive task; therefore, it requires food deprivation to incentivize rats to search for food located at the end of each arm. In the fully baited version, food is placed in all arms. In the combined working/reference memory version some arms are baited and some are not. The number of potential errors with this test is small. In an 8-arm maze, if run with 4 baited and 4 un-baited arms the range of possible errors of each type is 0–4. Even in a 16-arm RAM with 12 baited and 4 un-baited arms, control rats typically make 8–10 working memory errors at the beginning and 2–3 by the end, and 6–7 reference memory errors at the beginning and 4–5 at the end (Levin et al., 2001, Levin et al., 2002). In mice in an 8-arm RAM with 4 baited and 4 un-baited arms, controls typically make 6–7 working memory errors at the beginning and ∼1 by day 15, and ∼12 reference memory errors at the beginning and 1–2 by day 15 (Zhou et al., 2009). Low error rates limit detection sensitivity because the dynamic range is narrow and results in treatment effects of 2–4 error differences between groups. The narrow dynamic range, combined with food restriction, and test times of 10 min per trial, make the RAM slow to run which limits its practicality. In addition, one must carefully observe the pattern of arm visits as rats can obtain all rewards if they visit each arm serially (chaining), which defeats the test as an index of working memory. Chaining can be prevented by closing un-chosen arms on each trial until the rat returns to the center before opening all arms before the rat makes a new choice. The RAM has been used in both pharmacological (Olton, 1987) and neurotoxicity studies (Tsuji and Crofton, 2012) and many studies show treatment effects, e.g., (Buresova and Bures, 1982, Gibbs and Johnson, 2008, Kenney and Gould, 2008, Zlomuzica et al., 2009, Conrad, 2010,Levin, 1988, Safaei et al., 2023) but it requires food restriction to maintain equivalent motivation in all rats, typically to 85 % of their free feeding weight.
-
2.
Egocentric L&M: One test that is used for egocentric learning in safety studies is the BWM and to a lesser extent the CWM (Vorhees, 1987, Vorhees et al., 1991). Water mazes have large dynamic response ranges in terms of the number of errors made and latencies to escape and require less time to run than appetitive mazes and operant tasks. In the BWM or CWM in visible light, the task is learned in 5–6 days with 2 trials/day for rats. Rats typically make 15–50 errors in the CWM rather than 15 or less as in the RAM and other mazes. Alternatively, the CWM, when run under infrared light takes 18–21 days, 2 trials/day, and rats also make large numbers of errors anywhere from 50 to 125. Under infrared light, rats cannot even find the goal on the first few trials because of the complexity of the task, but once they find it, trial times decrease to under 1 min and errors decrease to as few as 5. Hence, water mazes are well suited for regulatory studies. The BWM and CWM have almost no non-performers when tested in the light and very few even when tested under infrared light. Examples of other egocentric mazes are the Stone maze (Knowlton et al., 1985, Ingram, 1988), the Hebb-Williams maze (Hebb and Williams, 1946), the Lashley III maze (Lashley, 1929, Nasello and Ramirez, 1978), the Star maze (Fouquet et al., 2013), and others, none of which are used in safety studies.
-
3.
Whishaw Maze: Wishaw developed a test of dead reckoning or path integration, a type of egocentric navigation. Thus far, this test has only been used in Whishaw’s lab (Whishaw et al., 1997, Whishaw and Tomie, 1997, Whishaw, 1998b, Whishaw, 1998a). How sensitive it is to neurotoxic agents is unknown, but it is an interesting test and is discussed here because it assesses a form of navigation related to that assessed by the CWM. The Whishaw maze uses a large circular platform. The rat is food deprived and placed near the edge with food scattered across the area opposite the rat under standard lighting. After the rat explores and finds the food, the lights are turned off and the rat must navigate its way back to the start. There are several variations as well, but the concept is that the rat visits multiple locations outbound and therefore cannot retrace its path returning to the start especially when distal cues are removed when the lights are turned off and must use vector addition (path integration) to find a more direct path home.
-
4.
Spatial L&M: The MWM is the most widely used L&M test in the world (Morris, 1981, Morris, 1984, Stewart and Morris, 1993, Jeffery and Morris, 1993, Vorhees and Williams, 2006, Able et al., 2006). Although physical specifications and testing procedures vary, the test is sensitive, reliable, and valid in many contexts and has appeared in thousands of articles. No other test in neuroscience, neurotoxicology, or experimental psychology comes close to the MWM in prevalence of use. This is because it is sensitive to hippocampal/entorhinal dysfunction (Morris et al., 1986a, Morris et al., 1986b, Brandeis et al., 1989, Bach et al., 1995, Burgess et al., 2002, Buzsaki and Moser, 2013, Eichenbaum, 2017, Moser et al., 2017), is straightforward to setup, efficient to run, and inexpensive. The next most frequently used spatial test is the Barnes maze (Barnes, 1979). The Barnes maze is a valid spatial test but can produce variable data, has non-performer issues, and takes longer to run per trial than water mazes. However, its efficiency can be improved with modifications, for example, by placing a fan over the maze which rodents find aversive which motivates them to find the escape (Inman-Wood et al., 2000). The Barnes maze is a circular platform with holes at regular intervals around the perimeter with one of the holes leading to an escape box. Finding the goal relies on the rodent’s aversion to open spaces and/or blowing air. With or without blowing air, what slows the test is that rats engage in off-task behaviors, such as grooming, sniffing, urinating, defecating, and the general slow pace of a rat’s exploration of a new environment, behaviors not seen in water mazes. Even a fan above the maze which improves performance still does not make trials as efficient as water mazes (Inman-Wood et al., 2000). In a comparison of spatial L&M in the MWM and RAM (Hodges, 1996), there were minor differences when used repetitively in favor of the RAM, which makes sense given that in the RAM there is no transfer of learning from day to day unlike the MWM. However, in rats with ischemic brain injury, rats tested in the MWM had large deficits in latency, whereas the same rats in the RAM showed no significant deficits in either working or reference memory errors; latency was not assessed in the RAM. They (Hodges, 1996) also compared rats with ischemic brain lesions of the hippocampus in a working memory version of the MWM compared with operant delayed non-matching to position and found large latency effects in the MWM as a function of lesion size but no effects on percent correct performance in the operant task. These results support the view that the MWM is more sensitive to hippocampal mediated injury than RAM or operant delayed non-matching to position, reinforcing the value of the MWM as a screening method to detect spatial L&M deficits.
-
5.
Classical conditioning: Eyeblink conditioning is a well-established test of classical conditioning. However, it is not simple, but is sensitive to some drugs (Skosnik et al., 2008); it has not found use in guideline studies thus far. Active and passive avoidance are mixed methods with classical and instrumental components. For these procedures an initially neutral stimulus, such as a light, is paired with an unconditioned stimulus (foot-shock) that elicits an unconditioned response (running). When this pairing is repeated, eventually the light (conditioned stimulus) will elicit running as a conditioned response which in passive avoidance reinforces staying on the lighted side, and in active shuttle-box avoidance reinforces running to the lighted side in order to avoid shock.
-
6.
Emotional learning: Conditioned freezing/fear is a common method to assess emotional L&M. There are two common types of conditioned freezing: Contextual and cued conditioning. Contextual conditioning can be done with or without cues and uses a single test environment along with foot-shock. For this test, rats are placed in a new environment and allowed to explore after which they are given several foot-shocks. Contextual memory is assessed 1–24 h later by placing rats back in the same environment and measuring time spent immobile, i.e., freezing. A reduction in movement/increase in freezing reflects how well the rat remembers its previous surroundings. A variation of this is to give rats a pre-exposure day in which rats are allowed to explore the environment the first day and are returned to the same environment the second day and conditioned using foot-shock. The following day the rats are tested for contextual freezing in the same environment (Jablonski et al., 2012). Contextual memory is dependent on the hippocampus. For cued freezing, a light, tone, or both are paired with foot-shock on the conditioning day. Later, the rat is placed in a different compartment with different floor, walls, and ceiling and allowed to explore and then presented once again with the conditioning cues. Rats freeze to the extent that they recall the association between the cues and foot-shock. This part of the test is sensitive to stress and emotion mediated by the amygdala. Conditioned freezing has seldom been used in regulatory studies.
An alternate way to assess emotional (amygdala mediated) conditioning is by using delayed versus trace cued fear conditioning. In delayed conditioning the unconditioned stimulus (foot-shock) is delivered immediately after the conditioned stimulus (light) whereas in trace conditioning there is a no-stimulus interval between the conditioned and unconditioned stimulus (Kong et al., 2023). The entorhinal cortex is essential for trace but not delay fear conditioning. If a distractor stimulus is added, the method can be used to test anterior cingulate cortex mediated attention (Han et al., 2003).
-
1.
Social learning: The Crawley social preference test (Crawley, 2007b) and social interaction test (Crawley, 2007a) have not been used in regulatory studies to our knowledge, although FDA has indicated that social tests may be appropriate in juvenile toxicity studies. The Crawley test has three connected compartments. The rat is placed in the center compartment and allowed to explore. One of the end compartments contains a small cage that has a conspecific in it, a so-called stranger, and the other has an identical small empty cage or has an inanimate object in it (Rein et al., 2020). Control rats spend more time on the side with the stranger than the side without a conspecific and this preference is taken as its social preference. There are variations of this test as well. A related method is for direct social interaction. For this, two rats are put in the same cage and their interactions are scored by hand from a list of behaviors related to how they behave toward one another. Both tests are time-consuming, variable, and have not seen use in safety studies.
-
2.
Incidental/recognition L&M: NOR and its variants (Bevins and Besheer, 2006, Barker et al., 2007, Heyser et al., 2013) are widely used in neuroscience, although their suitability for safety assessment is unclear. In these tests, rats are placed in a test chamber and exposed to 2 or 4 identical objects. Later, from 1 to 24 h later depending on whether testing short to long-term memory, rats are placed back in the chamber with one new object and the same original object(s). Most control rats will spend more time investigating the new object versus the old one, a preference disrupted by lesions of the dorsal hippocampus (Clark et al., 2000). Object recognition tests can be finicky and not always replicable because of variability. Some rats spend little time attending to the objects from the outset which requires an inclusion/exclusion criterion to use only rats that reach a minimum performance level. These tests also have a narrow dynamic range, meaning that rats show preference in the range of 62–67 % versus chance (50 %). This narrow range limits the ability of the test to detect effects. Moreover, these tests are based on an inherently transient phenomenon. Whether rodent or human, seeing a new object attracts attention only briefly; as soon as the novelty wears off so does the preference. The fleeting nature of the behavior limits its reliability and validity. In addition, many studies do not control time spent with the objects during familiarization, which can lead to erroneous interpretations of retention data.
-
3.
Executive function: There are 12 generally recognized executive functions in humans: self-restraint, working memory, emotional control, focus, task initiation, planning/prioritization, organization, time management, defining and achieving goals, flexibility, observation, and stress tolerance. Many of these can also be evaluated in rats using tests that assess discrimination, cognitive flexibility, delayed discounting, impulse control, delayed alternation, differential responding to low rates of reinforcement (DRL), probabilistic learning, etc. In rats, executive function usually requires operant conditioning using nose-poke, lever-pressing, or touch-screen responses [see (Schindler, 1993)]. The 5-choice operant test uses schedule-controlled conditioning requiring extended training and food restriction. The 5-choice test is, however, well suited for assessing PFC-mediated behavior. These methods are valuable but because of their complexity have seen limited use in safety studies. These tests are best reserved for follow-up experiments when an effect is found that requires more detailed analyses.
Regulatory guidelines for learning and memory
As mentioned, regulatory guidelines have referred to “a test of L&M” but no single test provides general protection from adverse CNS effects, but if two tests measuring different types of L&M and different brain regions are used it significantly enhances neurotoxicity coverage.
So far there is little guidance on how a two-test requirement should be implemented. One approach is to have one test of hippocampal/entorhinal cortex function, i.e., spatial navigation and one test of striatal/hippocampal function, i.e., egocentric navigation. Another is to have one test given at two different ages or have two tests but give one early and the other later.
Recommended test for hippocampal dependent spatial learning and memory
-
A.
Spatial L&M: The optimal choice for this form of L&M is the MWM (Morris, 1984, Stewart and Morris, 1993, Jeffery and Morris, 1993, Vorhees and Williams, 2006, Able et al., 2006, Vorhees and Williams, 2014a). However, not all test procedures or apparatus are equal. Important factors are tank size, tank characteristics, platform size, room cues, water temperature, trials/day, number of test days, training, control trials, test phases, lighting, trial length, intertrial interval (ITI), and experimenter effects. Spatial L&M is largely mediated by the hippocampus. The hippocampus has a dorsal to ventral representation of an organism’s surroundings mediated by place cells, i.e., cells that respond to the size and features of the space surrounding it. This organization of the place cells in the hippocampus has implications for tank size; smaller mazes activate dorsal place cells whereas larger mazes activate ventral place cells (Kjelstrup et al., 2008, Moser et al., 2017). It is important to use appropriately sized mazes. If mazes are too small, rats are able to find platforms using non-spatial strategies in small mazes by swimming away from the wall in concentric circles until they touch the platform. The recommendations below are based on our experience of having published 60 papers using the MWM.
-
B.
Maze Size: We conducted experiments on the neurotoxicity of developmental methamphetamine that impairs spatial L&M. We tested rats in a MWM that was 183 cm (6 ft.) in diameter, one that was 210 cm (∼7 ft.), and one that was 244 cm (8 ft.) in diameter, all with 10 cm diameter platforms. Comparing control rats in a 122 cm versus 210 cm diameter maze, it took rats in the larger maze significantly longer per trial per day than those in the smaller maze, however, by day 4 the learning curves converged significantly but not completely (Vorhees and Williams, 2006, Able et al., 2006). Later, we compared control rats in a 210 cm versus 244 cm diameter maze and showed that in the even larger maze rats took significantly longer to find the platform compared with those in the 210 cm maze on days 1–4 and although only small differences remained on days 5 and 6 they were not completely convergent (Vorhees and Williams, 2014a). We found significant differences in adult methamphetamine-treated rats as a function of maze size, see (Fig. 1). There were no significant methamphetamine-induced spatial learning deficits in a 210 cm diameter maze (Herring et al., 2008, Vorhees et al., 2008), whereas there were methamphetamine-induced deficits using a 244 cm maze (Gutierrez et al., 2018). Notice that the slope of the learning curves is also different between the two mazes and this parameter can be calculated. These data show that commonly used MWMs 210 cm in diameter and smaller are less sensitive than larger ones. Therefore, we recommend mazes larger than 210 cm in diameter. Morris himself, after his first paper using a small maze, switched to a 200 cm diameter tank when testing drug or lesion effects. Adult or developmental neurotoxicity studies, where more subtle effects can occur, would significantly benefit from a larger maze, and this is why we recommend a 244 cm tank to detect a larger range of treatment effects. However, we have found effects in 210 cm MWMs. Examples include after exposure to kaolin that induces hydrocephalus (Williams et al., 2014), developmental exposure to some antidepressants, particularly citalopram (Sprowles et al., 2016, Sprowles et al., 2017), developmental exposure to manganese (Amos-Kroohs et al., 2017), developmental exposure to the pesticide deltamethrin (Pitzer et al., 2019), developmental exposure to the novel street drug 5-methoxy-N,N-diisopropyltrypamine (“Foxy”) and methylenedioxymethamphetamine (MDMA) (Skelton et al., 2009, Vorhees et al., 2009), developmental exposure to d-fenfluramine (Morford et al., 2002) and ionizing protons (Williams et al., 2020, Williams et al., 2022). The 244 cm maze size is not size prohibitive since it can be moved into a laboratory in sections and chemically welded by a fabricator. Moreover, when custom made it can be constructed on legs with a conical bottom and center drainpipe to a floor drain for efficient water changes to keep the maze clean. Since tank size determines what regions of the hippocampus are activated, larger spaces (tanks) activate more place cells than smaller spaces (tanks) (Strange et al., 2014). But tank size is also relevant to the age of rats being tested. For young rats around postnatal (P) day 25 (P25), a 152 cm in diameter (5 ft) maze may suffice. We have data showing that P30 rats can learn well in 183 or 210 cm diameter mazes; rats as young as P21-25 may be able to learn in these size mazes, we simply have not tested rats of this age. It is important to appreciate that the hippocampus is not fully developed in rats until 4 weeks of age in terms of precision of spatial mapping (Ramsaran et al., 2023). Therefore, testing rats younger than P28 could create interpretational issues given that hippocampal cells needed for spatial learning are not all present prior to P28. In mice, tank sizes of 150 or 120 cm in diameter work well, however, tanks 75 cm in diameter make the task too easy and likely not a test of spatial L&M (Van Dam et al., 2006).
-
C.
Platform size: Platform size is a significant determinant of the rate of spatial learning. In a tank of a given size, when rats were compared using 5, 10, or 14 cm2 platforms, rats learned faster with progressively larger platforms as one might expect. The unexpected finding was that on the probe trial at the end of the hidden platform trials, rats that learned with the smallest platform more accurately swam to the location of where the platform used to be than those that learned on the larger platform, indicating better reference memory in rats that learned on the small platform. Hence, large platforms are not advisable. Platforms should be 10 cm or smaller to best assess spatial navigation (Mactutus and Booze, 1994).
-
D.
Tank characteristics: It is important that there are no cues inside the tank. Hence, the interior walls must be featureless without seams or other imperfections that a rat might use for orientation. In addition, the water level should be far enough below the edge that rats are not trying to jump out nor so low that the walls of the tank interfere with the rat’s ability to see the room cues on the walls.
-
E.
Water conditions: Some labs put milk powder or tempura paint in the water to make it opaque. This stems from what Richard Morris did in early experiments as a precaution to ensure that the submerged platform could not be seen. However, since then this precaution has proved unnecessary if attention is given to camouflaging the platform. We use a black tank with black or transparent platform. This makes the platform invisible to the rat at water level. We have shown that rats cannot see the platform as demonstrated by the fact that they do not notice it in early trials even if they swim close or bump it but ignore it and keep swimming. After finding the platform a few times, rats still sometimes swim pass it very closely, which if they could see it they would not hesitate to climb on it to escape.
-
F.
Water temperature: Early experiments used warm water (24–26 °C) under the assumption that this would prevent hypothermia that might interfere with performance. However, we and others find that warm water slows swimming and delays learning (Sandi et al., 1997). The opposite is also true, that cold water slows learning. Average vivarium room temperatures around 19–21 °C work well for adult rats. These water temperatures motivate rats to search but do not cause hypothermia when given 4 back-to-back trials per day. However, young rats are different, they do benefit from spaced trials, as do mice, or their performance will deteriorate from heat loss.
-
G.
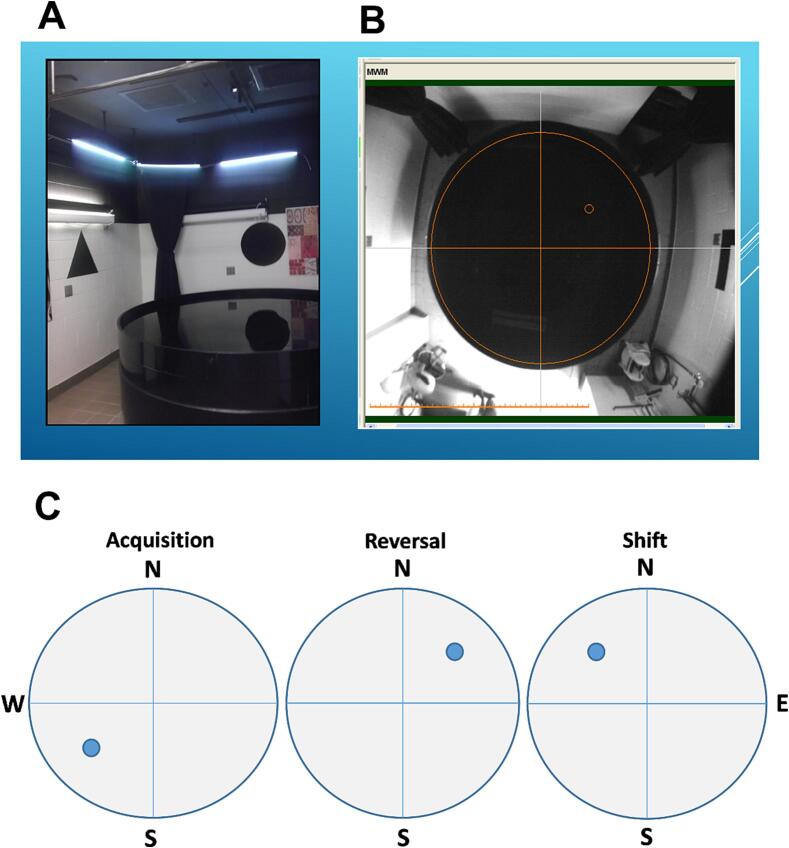
Escape Platform: We use a 10 cm diameter platform as do most labs, but in some procedures we change the size of the platform since a smaller platform increases the search area to target ratio thereby making the task more difficult (Vorhees and Williams, 2006, Able et al., 2006). Many labs use the same size platform during initial learning (acquisition) and on reversal trials, i.e., moving the platform to the opposite quadrant. We typically do spatial learning to a hidden platform in three phases with three different platform sizes. The phases are acquisition, reversal, and shift, with shift placing the platform in one of the previously unused quadrants. We use a 10 cm platform for acquisition, a 7 cm diameter platform on reversal, and a 5 cm platform on shift.
-
H.
Start positions and time limits: If the maze is conceptually divided into four equal quadrants, it lends itself to four equidistant cardinal locations around the perimeter used as start positions such that if each one of these is used once per day it results in 4 unique trials/day. Many other numbers of trials/day have been used, but 4 trials/day is the most widely used method. Similarly, there is nothing sacred about dividing the maze into four equal quadrants. It could be divided into 6 or 8 sectors just as easily, but what one gains with more divisions can be lost in the distinctiveness of the positions if they are close together. Nor is there anything requiring the four start positions to be only cardinal positions. In fact, using cardinal positions results in two start positions that are closer to the platform and two that are farther away. There is no complete solution to this near vs far problem, but it can be lessened by using two cardinal and two ordinal start positions such that all starts are distal to the platform. In terms of timing, we use a 2 min time limit per trial. Some labs use 1.5 min, and a few labs use longer times.
-
I.
Time per Trial: We use 4 trials/day but there is no agreement in the literature on the optimal number and there are no studies we could find comparing different numbers to determine if there are numbers of trials per day that make the test more sensitive than others. The range of trials per day is large, with studies using as few as 1 trial/day (Fleischmann, 2003), 2 trials/day (Rudy and Paylor, 1987, Rudy et al., 1987, Rudy and Paylor, 1988, Tees et al., 1990, Teixeira et al., 2006, Possin et al., 2016), 4 trials on day-1 and 2 trials/day for the remaining days (During et al., 2003), 3 trials/day (Burwell et al., 2004, Jo et al., 2007, Dupret et al., 2008), the most widely used method of 4 trials/day (Schenk, 1985, Upchurch and Wehner, 1990, Lamberty and Gower, 1991, Conrad and Roy, 1995, Mogensen et al., 1995, van Rijzingen et al., 1995, Kraemer et al., 1996, Whishaw and Tomie, 1997, Whishaw et al., 1997, Wolfer et al., 1998, Otnaess et al., 1999, Miyakawa et al., 2001, Commins et al., 2003, Iivonen et al., 2003, Sircar, 2003, Topic et al., 2007, Bannerman et al., 2008, Zhang et al., 2008), 6 trials/day (Tonkiss et al., 1992, Guzowski et al., 2001), 8 trials/day (Devan et al., 1992, Packard and McGaugh, 1992, Carman and Mactutus, 2001, Bekinschtein et al., 2007), two day methods with 10 trials on day-1 and 12 trials on day-2 (Rudy et al., 1987, Rudy and Paylor, 1987), 10 trials/day for 2 days and 4 trials/day for 10 days (Deacon et al., 2002), 10 trials on only one day (Hoh et al., 1999), and 12 trials on one day (Wesierska et al., 1990). Unfortunately, one cannot compare these different procedures across studies because of differences in tank size, platform size, intertrial interval, strain, water temperature, use of assisted versus unassisted escape on time limit trials, lighting, number of days of testing, whether pretraining was given or not, distal cues, etc. In terms of intertrial interval, the general principle that distributed trials increase learning compared with massed trials has been shown when comparing learning in the MWM with intertrial intervals of 1-min vs. 10-min (Commins et al., 2003). However, even though this effect was significant it was small. We find that rats learn well given four back-to-back trials/day. However, if one prefers distributed trials, rotating through the group giving each rat trial-1 until all rats complete trial-1 before starting over and giving each rat trial-2, also works well.
-
J.
Trial failures: There will be rats that fail to find the platform within 2 min. There are two approaches to this situation: (a) assisted escape and (b) unassisted escape. We use unassisted escape for the following reasons: First, it is a premise of research that intervention by the experimenter should be avoided because it runs the risk of biasing the outcome. Bias can be unintentional but still significant. Since it is easily avoided, there is no reason to use assisted escape. Another reason assisted escape is ill-advised is that no two experimenters and no two rats can be guided or pushed to the platform in the same way, thereby producing uneven and potentially unequal assistance. Furthermore, if a higher percentage of experimental rats reach the time limit, then a higher percentage of experimental rats will require assistance, and that could result in reduced group differences, the opposite of what the experiment is designed to test. Moreover, assisted escape carries the implicit assumption that rats need help or they will not learn. This is easily disproven by the many published studies that get good learning without using assisted escape (Sutherland et al., 1982, Rudy et al., 1987, Rudy and Paylor, 1987, Upchurch and Wehner, 1988b, Upchurch and Wehner, 1988a, Annett et al., 1989, Mandel et al., 1989, Morris, 1989, Eichenbaum et al., 1990, Tees et al., 1990, Upchurch and Wehner, 1990, Grant et al., 1992, McNaughton and Morris, 1992, McDonald and White, 1994, Holscher, 1999, Williams et al., 2002, Williams et al., 2003b, Williams et al., 2003a, Williams et al., 2003c, Vorhees et al., 2004, Able et al., 2006, Vorhees and Williams, 2006, Vorhees and Williams, 2006, Able et al., 2006, Vorhees et al., 2008, Herring et al., 2008, Vorhees et al., 2009, Skelton et al., 2009, Braun et al., 2012, Braun et al., 2015, Vorhees et al., 2015, Braun et al., 2016a, Braun et al., 2016b) compared with those that do use assisted escape (Wade and Maier, 1986, Rudy et al., 1987, Rudy and Paylor, 1987, Rudy and Paylor, 1988, Wesierska et al., 1990, Devan et al., 1992, Packard and McGaugh, 1992, Tonkiss et al., 1992, Conrad and Roy, 1995, van Rijzingen et al., 1995, Guzowski et al., 2001, Carman and Mactutus, 2002, Commins et al., 2003, Blokland et al., 2004, Burwell et al., 2004, Kubik and Fenton, 2005, Teixeira et al., 2006, Bekinschtein et al., 2007, Jo et al., 2007, Zhang et al., 2008). To ensure that there are no experimenter effects and to prevent reducing group differences, assisted escape should be avoided. In cases where the rat reaches the time limit, it should be retrieved from wherever it is when time runs out and (a) placed briefly on the platform or (b) placed in a holding cage. If placed on the platform some labs leave the rat on for 30 s so it can ‘familiarize’ itself with distal cues. We find no evidence that this improves learning, therefore, we leave the rat on the platform for 5–10 s, long enough for the experimenter to set the software program for the next trial, pick the rat up, place it in the pool at the next start location, and start the trial.
-
K.
Intertrial intervals: Some labs towel dry rats after each trial, others put them under a heat lamp. These procedures apply when there is a significant intertrial interval, not when trials are run back-to-back. With massed trials, rats get four trials in a row and are then dried. While rats learn slightly faster using distributed rather than massed trials, this effect is modest in rats but significant in mice (Vorhees and Williams, 2014b). Giving trials in rotation while testing a cohort of rats yields good learning curves as does giving only one trial per day. The goal is to obtain learning curves that are neither too shallow (indicating the task is too difficult) nor too steep (indicating the task is too easy). Intermediate slopes provide the best evidence that one will not encounter ceiling or floor effects.
-
L.
Strain: We use Charles River Sprague Dawley (strain 001) rats in most experiments, but we have used other strains, including F344 (Vorhees, 1983, Schaefer et al., 2012), Dark Agouti (Vorhees et al., 1999), and Long-Evans as have others (Tonkiss et al., 1992). Other common rat strains are Wistar, Brown Norway, Lister, etc. All rat strains tested learn the MWM. Several mouse strains have also been tested, and in mice strain differences are larger than in rats (Upchurch and Wehner, 1988b, Wahlsten et al., 2005).
-
M.
Days of Testing: We use 5 or 6 days of platform trials and both work well. Even in a 244 cm maze, by the fifth day rats are performing well. We find that the sixth day does not show much improvement over the fifth day. Over-training is not useful. There can be effects of over-training on reversal learning where overlearning facilitates reversal learning in simple two-choice tasks (Dhawan et al., 2019), but we see no evidence of this in the MWM. In most experiments, we find that rats improve across test phases, i.e., they do better on reversal than on acquisition, and better on shift than on reversal, presumably from transfer of training effects. To partially offset this training effect, we make each phase more difficult by reducing platform size.
-
N.
Test phases and Cognitive Flexibility: We test in 4 phases: acquisition, reversal, shift, and cued. Reversal and shift phases test cognitive flexibility an executive function. For reversal, the platform is moved to the quadrant opposite the one used for acquisition. For shift, the platform is moved to one of the adjacent quadrants. One can continue this process and do a second shift to the remaining quadrant and then start over (Williams et al., 2003c). We showed that developmental methamphetamine exposure results in MWM deficits on acquisition, reversal, shift, and on a second shift with the magnitude of the effect largest on acquisition, shift, and second reversal with a smaller platform but not as large as on reversal. The cognitive flexibility required on shift trials appears more difficult perhaps because there is greater retroactive interference from having had two previous positions to learn. Adding the shift phase adds some time to the task, but the addition of a few days, given the large investment in treating and testing rats in a large safety experiment, adds little to overall investment of resources and time. The fourth phase is cued and is a control procedure to ensure that rats do not have a sensorimotor or proximal cue learning deficit that might compromise interpretation of hidden platform effects. For the cued phase, curtains are closed around the maze to block the rat from seeing room cues, and the start and platform positions are changed on every trial to discourage use of any residual room cues not covered by the curtains (e.g., the ceiling). The platform is made visible on these trials, so the rat can use direct line-of-sight to swim to the platform without needing distal cues. We use a 10 cm platform for this phase with a hole in the center of the platform into which a stainless-steel rod is inserted with a plastic ball mounted on top that is 10–12 cm above the water to make the location visible. This phase consists of 4 trials per day for 2 days with no probe trial.
Fig. 1.
Comparison of MWMs that are either 210 cm in diameter, panel A, or 244 cm in diameter, panel B, in rats treated with equivalent doses of methamphetamine (Meth). Data are for latency to the goal for acquisition and reversal, 4 trials/day for 5 days for each phase. Meth induced no significant effects in the 210 cm maze but significant effects in the 244 cm maze.
Transfer of training: It is important to appreciate that if there is a deficit in performance on acquisition in which the experimental group lags behind controls and fails to catch up by the last day, the experimental group will start reversal having unequal learning compared with the control group. The same applies if a shift phase is used and the experimental group never caught up with controls during reversal. How serious this is when screening for neurotoxicity of test agents is not known but it is important in the interpretation of the results in terms of drawing inferences about whether these later phases reflect cognitive flexibility or a mixture of incomplete acquisition combined with compromised cognitive flexibility.
-
O.
Reference memory: In all MWM procedures, learning trials with the platform are followed by a probe trial for reference memory given at least 24 h after the last learning day. Reference memory for spatial learning is analogous to declarative memory in people, i.e., in people memory for faces, places, things, and events. Obviously, memory for people does not apply to rodents, but the MWM tests place memory, therefore it assesses the homologous function to declarative memory in people. If acquisition is 5 days, the probe trial is on day 6. For the probe trial there is no platform. Rather it is an unreinforced trial to measure where the rat spends the most time. Average distance from the former platform site, number of times the rat crosses the spot where the platform used to be, and time and distance in the target quadrant are assessed. The start position for the rat on a probe trial is a point around the perimeter distal to the former platform location that was not used on any of the platform trials during that phase. For example, if the platform is in the SW quadrant during acquisition with start positions of N, E, SE, and NW used as start positions in different orders on each day, the probe trial 24 h after the last acquisition trial would be from NE since this position was not used on any of the acquisition trials. The same would be done for reversal and shift, with the probe position being from none of the positions used on platform trials. Probe trials can be informative, but they are limited since they provide data from only a single, short trial. A common time limit on probe trials is 60 s, but we find that 45 s is sufficient. There are few data on the optimal probe trial length, but preference for the target quadrant in control rats drops precipitously after 30 s (Blokland et al., 2004) but some target quadrant preference continues after that. The reason is that it does not take long for rats to figure out that the platform is not where it used to be, and they start searching other places diluting the assessment of reference memory.
-
P.
Dependent variables: The classic measure on this test is latency (time) from the start of a trial until the rat reaches the platform. However, latency has the drawback that if a treatment affects swim speed, there might be an effect based on performance rather than learning. A measure that helps is path length. Path length is not affected by speed except at extremes of slow or fast swimming and path length is captured by commercial video tracking software. A measure we find helpful is path efficiency. Path efficiency is unitless because it is the ratio of the length of a straight-line from the start to the goal divided by the path the rat takes on each trial. Path efficiency is low on early trials, 0.1–0.2 and improves to 0.3–0.4 on later acquisition trials. Path efficiency often reaches 0.6 on late reversal trials. We find path efficiency a useful gauge of learning compared with latency or path length in part because speed does not affect it. Many tracking programs provide swim speed separately. Speed can be used to determine if there are rate differences between groups and if there are such differences, this may temper the interpretation of L&M effects. We have seen cases where experimental groups swim slower or faster than controls and in both cases path efficiency showed impaired learning. When the experimental group shows faster swimming than controls the interpretation is straightforward, but when the experimental group swims slower than controls it raises concerns about whether the L&M effects are secondary to speed. Most tracking programs calculate speed by dividing path length by trial time to give average swim speed, they do not provide second by second speed. Swimming speed is generally consistent across trials and phases and minor speed differences seldom correlate with learning measures and have seldom been found to be determinative.
-
Q.
Trial order: Choosing start positions for each trial is best done by making sure one does not inadvertently introduce orientation bias. It is advisable to use pseudo-random, balanced start positions relative to the position of the platform. If one labels the four cardinal positions as N, S, E, and W, and puts the platform in one quadrant, there are two long paths and two short paths to the platform from the far and near cardinal points. We reduce this difference by using two cardinal and two ordinal start positions. This makes the distances more similar. With 4 trials per day, we use four different start positions each day and arrange the starts so two start positions have the platform to the right of the rat and two to the left, i.e., one far right, one near right, one far left, and one near left but the sequence of these is quasi-randomized and balanced.
-
R.
Day-1: An issue that becomes relevant if a treatment causes a L&M deficit is whether the impairment is a learning or performance deficit. One way to help resolve this is to analyze the Day-1 data trial-by-trial. If rats have a performance deficit, they perform worse than controls from the outset even on the first and second trials. If, however, the rats have a learning deficit but no performance deficit, the groups all start out the same and gradually diverge. We typically see groups being comparable on trials 1 and 2, and sometimes trial 3 before controls improve more than the experimental group. Showing such data assures the reader that the experimental group does not have a pre-existing performance impairment.
-
S.
Stress effects: The most frequent criticism of the MWM and all water mazes is that putting rats or mice in water is stressful and since stress can interfere with learning, water mazes may impair the rate of learning or interact with the independent variable causing effects that would be different in a test that was less stressful. Examination of learning curves in MWM experiments where the parameters of tank and platform size are appropriate show that rats display graded learning curves that are neither too steep nor too shallow. Part of this concern may arise because people know the forced swim test (FST) is a method designed to induce stress, but FST effects are not comparable to water mazes where the subject can escape. It is well known that (a) moderate stress facilitates learning and (b) stress responses are influenced by locus of control. A stressor from which there is no escape raises corticosterone levels far more than the same stressor where the subject can escape.
One study compared serum corticosterone levels in two groups of C57BL/6J mice tested in the MWM versus the Barnes maze (Harrison et al., 2009). After 5 days of testing, 30 min after the last trial, serum corticosterone levels were elevated in both groups but were higher in the MWM group by approximately 35 % compared with the Barnes maze group. But is this difference enough to interfere with learning in the MWM? Apparently not because these authors report that the performance of the mice on the last day of testing showed equal escape latencies between mice in the Barnes and MWM, hence, the higher corticosterone levels in the MWM group did not affect the rate of learning. This study is often cited as a criticism of the MWM invoking the idea that this maze is stressful, but the study actually shows that learning in the two mazes is equivalent. We also reported increases in corticosterone in rats immediately after the last trial in the MWM that decreased over time to normal levels by 60 min after the last trial (Skelton et al., 2007). In the Skelton (2007) study, even though there were no treatment differences in corticosterone levels after MWM testing, the rats treated neonatally with methamphetamine and tested as adults had deficits compared with controls and by 30 min levels were lower than in C57BL/6J mice in the Harrison et al. study (Harrison et al., 2009).
MWM Summary. The MWM is a valuable test of spatial learning and reference memory that is suitable for regulatory studies. However, getting the apparatus and testing procedure optimized makes a significant difference in maximizing its sensitivity, reliability, and validity.
Recommendations
≥210 cm diameter tank with a 10 cm platform is recommended for rats and larger tanks have significant advantages, e.g., up to 244 cm in diameter. The platform should be clear or match the color of the inside of the tank (black for albino rats, white for black or hooded rats, or beige if using both). Rats do not need training prior to hidden platform trials and should begin with acquisition with the platform in a fixed position and with start positions in a pseudo-randomized balanced order on each trial for 4 trials/day for 5 days with two starts from cardinal positions and two from ordinal positions or from four cardinal positions. Each trial should have a 2 min limit and unassisted escape when the time limit is reached. Rats should be given 5–10 s on the platform between trials. On day-6 rats should be given a single 45 s probe trial with no platform starting from a novel location distal to the target zone. After acquisition, rats should be given reversal trials with the platform in the opposite quadrant for the same number of trials/day and same number of days with a single probe trial 24 h after the last platform trial. Adding a shift phase increases test sensitivity for assessing cognitive flexibility. Use room temperature water and change it once or twice a week depending on how many rats are tested. When cleaning, drain the maze at the end of one day and refill it the same day so the water has time to equilibrate to room temperature overnight. Record water temperature each day. Rats should be given 2 days of cued trials, 4 trials/day, at the end of the shift phase with obscured distal cues, and random start and random goal positions. Rats as young as P30 can learn the MWM in a tank 183–210 cm in diameter or even larger. There are limited data in rats younger than P30 making it unclear if spatial L&M or some other strategy is being evaluated at these ages. If an early L&M test is needed, the MWM starting on P30 works (Vorhees et al., 2007).
MWM Example
Fig. 2A shows our MWM, Fig. 2B has superimposed lines marking the cardinal points, and Fig. 2C shows the platform positions for the three phases of testing. Table 1 lists all recommended parameters of the task.
Fig. 2.
A,B, Photographs of our MWM. A, at this angle one can see some of the wall cues. B, a top-down view showing a demarcation for the position of a platform in the NE quadrant. C, Schematic showing platform positions for acquisition, reversal, and shift.
Table 1.
Morris water maze (MWM)).
| Factor | Specification |
|---|---|
| Tank size-adults | 183 (min.) 200–210 (OK) 244 (best) cm internal diameter |
| Tank size-juveniles | 150–210 cm internal diameter |
| Tank depth | 50 cm measured near tank wall, internal |
| Construction | Sloped bottom to drain, laminated polypropylene |
| Curtains | Ceiling mounted on tracks |
| Water depth | 25 cm |
| Interior | Opposite coat color of rats, or neutral (beige) |
| Interior surface | Uniform, no detectable seams or marks |
| Platform size | 10 cm diameter (or smaller, not larger) |
| Platform color | Clear or matching interior |
| Water | Tap water (nothing added) |
| Water temperature | 19–21 °C, allow at least 12 h to equilibrate to room temp |
| Platform depth | 1–1.5 cm below water surface |
| Platform position | Halfway between wall and center |
| Starts during acquisition | Platform SW, then starts N,W,E,S or N, NW, S, SE in pseudo-random order, 1 from each position/day |
| Time limit | 90 or 120 s per trial |
| Intertrial interval | If ≤4 trials/day then do back-to-back trials; alternatively test in rotation (mice always in rotation) |
| Trial failures | Remove rat and place on platform for ITI (unassisted escape) |
| Training | None for rats, 1 day for mice to visible platform |
| Trials/day | Usually 4, but can range from 1 to 12/day |
| Number of learning days | 4–5 per phase |
| Learning phases | Acquisition, Reversal, Shift |
| Cued phase | Close curtains, 4 trials/day, 2 days, visible cue on platform; platform and starts changed on every trial |
| Memory | Probe trial, no platform, 24 h after each phase |
| Probe length | 45–60 s |
| Rat strain | Sprague Dawley, Long Evans, Lister, F344, Wistar, Dark Agouti and more |
| Learning trial measures | Latency, path length, path efficiency, speed (best to use camera and tracking software) |
| Probe trial measures | Cumulative distance from platform, time to first platform zone crossing, number of zone crossings (optional: quadrant time) |
| Data analysis on learning trials | Mixed general linear ANOVA-RM, Group × Sex × Day; if developmental include litter as a random factor |
| Day-1 | Analyze trial by trial; same ANOVA model |
| Distal cues | Prominent cues on walls |
| Lighting | Uniform and at a level that minimizes reflections |
Young rats: Adult rats do not need rest between trials, but young rats do. One approach is to test young rats in rotation so that for a given cohort, all rats receive trial-1, then trial-2 in rotation thereby providing approximately 10 min between trials. At young ages, place paper towels in the bottom of each cage and/or use a heat lamp or space heater to prevent hypothermia.
Recommendation for a striatal dependent test of egocentric learning and memory
-
A.
Egocentric L&M: There are two types of egocentric or dead reckoning L&M, (a) wayfinding/pathfinding and (b) path integration. Mazes with channels such as the BWM, CWM, Stone maze, etc. assess pathfinding because what is learned is a set of sequential turns. Path integration is where an animal leaves their home base (burrow or nest) to forage for food and move to different locations and when they return home, they do not retrace their steps but navigate a more direct route back. The ability to chart a more direct return requires the integration of previous locations relative to where they started to determine a shorter way back. Whishaw developed a test for path integration as noted above (Whishaw et al., 2001).
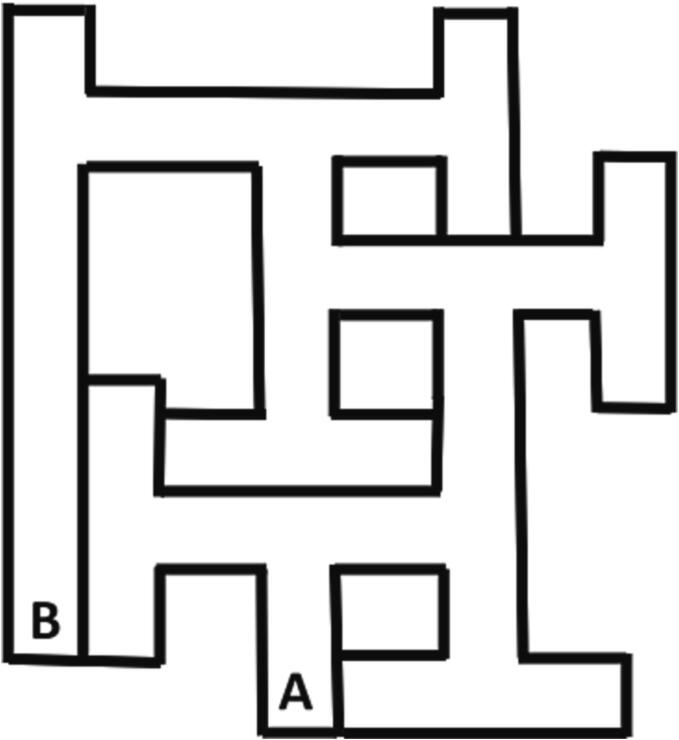
The predecessor to the CWM is the BWM (Fig. 3). Biel began by training rats to escape from a simple straight channel by blocking off the long corridor from the rest of the maze and recorded transit time to ensure that all rats had swimming experience, found the escape, and swam at comparable speeds. He then gave rats maze trials by starting them at point A and counted errors and time until they reached point B (Biel, 1940). No one used the BWM after that until Polidora et al. 20 years later (Polidora et al., 1963, Polidora et al., 1966b, Polidora et al., 1966a). At first Palidora et al. used the maze as Biel had, giving rats pre-maze trials in the long arm followed by learning trials from point A to B (Polidora et al., 1963). In subsequent experiments they started the same way but after rats learned the path from A to B, they gave them additional trials to learn to swim from B to A (Polidora et al., 1966b, Polidora et al., 1966a). What they found was that rats made more errors swimming B to A than from A to B. Later the maze was used by Butcher and colleagues (Butcher, 1970, Butcher et al., 1970). Butcher also gave pre-maze trials in the long channel, then trials from A to B, followed by trials from B to A. Whereas Polidora used Long Evans rats, Butcher used Sprague Dawley rats and both strains readily learned the task. However, the task could only be used in younger rats. To test older rats, the maze had to be resized, therefore, one of us (CVV) redesigned the apparatus.
-
B.
Maze design: Today, there are two CWM designs, one has 9 multiple Ts (CWM9) and the other has 10 multiple Ts (CWM10). These are in contrast with the BWM that has 6 Ts. The first limitation of the BWM was that it had narrow channels. This limited the size of rats that could be tested and caused secondary issues. Adult rats could put their legs against the side walls at intersections and brace themselves to avoid swimming. Once rats started this behavior, they often continued doing it. Even when not bracing, rats often perseverated on corners and edges rather than search. This behavior, in turn, led to attempts to jump out or dive to try to find an escape. While these attempts failed, they represent off-task behaviors that disrupt learning. Widening the channels and changing the material from galvanized steel to acrylic eliminated these problems. The BWM cul-de-sacs are arranged such that there is a long channel at the end that runs the length of the maze (Fig. 3). Biel used this channel for pre-maze training. However, during maze trials a rat’s prior exposure with this part of the maze had the effect of cueing the location of the goal before the rat got there, essentially making the task a 5-unit T maze because the arm opposite the long arm was ignored. In the redesign, the long arm was eliminated, and a separate training channel was constructed and placed in a separate room so that room cues were also different.
-
C.
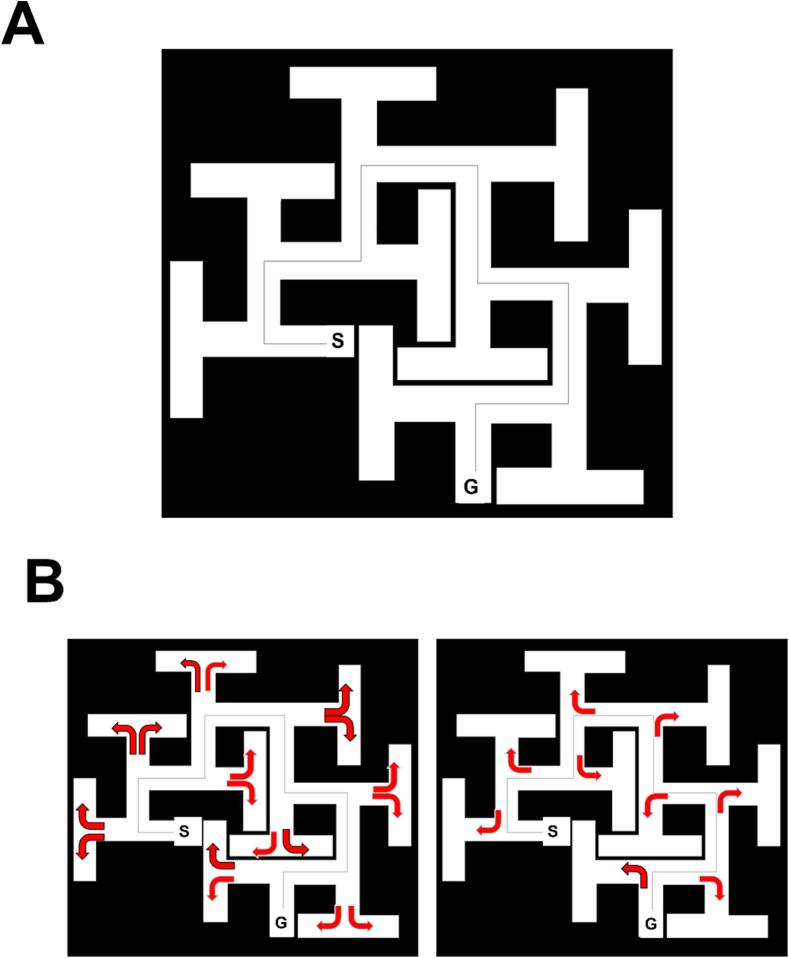
Maze asymmetry. As currently used, rats swim through the maze from point S to G (Fig. 4A). Fig. 4B shows T errors on the left and stem errors on the right. When rats are tested in path G to S and swim straight ahead, at each decision point there is a 50:50 chance of making a correct turn. By contrast, that same strategy when tested in path S to G when they reach a perpendicular wall, it is certain they will make an error regardless of whether they turn right or left. Between the two types of errors, there are 27 unique errors possible plus repeat errors. However, the probability of making an error is greater in the reverse path because of the way the cul-de-sacs are arranged in relation to how rats learn the maze. The S to G path increases the difficulty of the maze because rats swim straight until forced to turn and this strategy leads to far more errors than on path G to S. To reach the goal in path S to G, the rat must learn to backtrack and search for an alternate way out. The alternate way is to turn down an opening located halfway along the wall. Rats find this difficult and hence make many errors before learning to turn before reaching the end of a channel. Once rats enter a side channel for the first time, they confront the same difficulty a second time, then a third time, et cetera, until they reach the goal.
-
D.
Paths: Early users of the BWM ran A to B and B to A paths sequentially. When the CWM9 was developed this sequence was retained. But then one of us (CVV) tested which configuration was the most sensitive to experimental perturbation. For this, prenatal exposure to the antiepileptic drug phenytoin was used. To compare the CWM9 with the BWM, rats were run in the CWM9 or in the BWM. There were 3 doses of phenytoin (100, 150, and 200 mg/kg) and vehicle controls gavaged daily from embryonic day (E)7–18. All rats were given the A to B or G to S path first and the B to A or S to G path second. The high dose group had impaired learning on the A to B or G to S path in the mazes, but there were no effects in the lower two dose groups. In the B to A and S to G path, the effects were large at all doses in both mazes but were larger in the CWM9 than in the BWM. Thus, comparison of littermates run in the two mazes in parallel showed that the CWM better differentiated the groups than the BWM (Vorhees, 1987).
-
E.
Path Order: Next CWM9 and BWM performance were tested by splitting offspring from multiple litters into different sets with each set tested differently. Rather than two different mazes, arms in the CWM9 were blocked off to create a BWM without the long arm. Two doses of phenytoin (100 and 200 mg/kg) dosed as above were used in this study (Vorhees et al., 1991). The first experiment confirmed that the CWM was more sensitive than the BWM. With the second set of offspring, path order was tested in the CWM9. Half the rats in this set were tested in the G to S path followed by the S to G path and the other half received the S to G path first followed by the G to S path. The data showed no main effects on the G to S path regardless of whether it was given first or second, but there were big differences on the S to G path that depended on order. The S to G path showed clear effects of phenytoin at both doses, but the magnitude of the effect was larger when the S to G path was first. This showed that the S to G path better differentiated the effects of the drug when run in the absence of prior experience in the G to S path. Hence, the data showed that the G to S path was not adding value, in fact it reduced group differences on the S to G path due to transfer of training effects and was, therefore, counterproductive. Accordingly, we only use the S to G path.
-
F.
Escape assistance: The third set of offspring from these litters was used to test the effects of assisted versus unassisted escape when rats reached the trial time limit without finding the goal. Both phenytoin groups were significantly impaired under both conditions, but the rats tested with unassisted escape made more errors than those with assisted escape. Since the point of most experiments is to test for treatment effects, the data showed that unassisted escape better differentiated the effects of the drug than assisted escape. In addition, unassisted escape saved time and eliminated human interactions with the rats during testing. This removed the risk of experimenter effects as discussed earlier (Vorhees et al., 1991).
-
G.
Interim Summary: At this point, we had improved the maze significantly over Biel’s design and procedure. Our refined CWM9 method used only the S to G path with unassisted escape when a rat reached the time limit. Rats received 2 trials/day for 5–6 days with a 5 min rest between trial-1 and trial-2 if it reached the time limit or back-to-back trials if it found the goal in <5 min on the first trial of the day. With this procedure, Sprague Dawley control rats make about 30 errors on trial-1 of day-1 and get down to about 1 error by trial-12 on day-6.
Fig. 3.
Biel water maze (BWM).
Fig. 4.
CWM9. A, the maze with 9T-shaped cul-de-sacs. S = Start, G = Goal. B (right), shows stem errors when started at G and swimming to S; B (left) shows T errors when started at S and swimming to G. Note there are 9 unique stem error types in the version on the right and 18 unique T arm error types in the version on the left.
What the CWM measures
As we began testing different compounds in the CWM9 and MWM we saw divergence where an effect was larger in one maze than the other. This led us to investigate the basis of these differences which led to a question about what kind of L&M the CWM9 reflected. Much was known about the MWM as a test of spatial learning and it was backed by many studies where it was shown to depend on activation of place cells in the hippocampus and grid cells in the entorhinal cortex that create cognitive maps of a subject’s surroundings (Morris et al., 1982, Morris et al., 1986a, Morris et al., 1986c, Morris et al., 1986b, Morris, 1989, Eichenbaum et al., 1990, Morris et al., 1990, Davis et al., 1992, Jeffery and Morris, 1993, Stewart and Morris, 1993, Morris et al., 2006, Ekstrom et al., 2014, Moser et al., 2014, Brown et al., 2016, Eichenbaum, 2017, Hardcastle et al., 2017, Moser et al., 2017). However, we knew little about what brain region or type of navigation the CWM was measuring. It was known that spatial L&M relies on distal cues to active place cells in the hippocampus, therefore, one of us (MTW) decided to test whether eliminating distal cues would reveal whether rats used distal cues in the CWM9. First, we tried using red lights. This slowed the rate of learning, but the effect was minor. Next, we tried hoods to cover the rat’s eyes. This failed because as soon as thehoods got wet, rats pried them off. Next, we turned the room lights off and evaluated performance wearing night vision goggles. Rats took longer to learn than under red light, but infrared goggles hum and it appeared to provide rats an auditory reference point to find their way through the maze. Next, we eliminated the night vision googles and mounted an infrared sensitive camera above the maze and used infrared LEDs to illuminate the maze and fed the image to a monitor in an adjacent room where the experimenter evaluated performance (Atanasova et al., 2022). This also eliminated the need to have a person in the room during testing. Rats now made more errors but still solved the maze efficiently. Next, we stood in the room until fully dark-adapted to look for sources of light. It became evident that light was leaking around the door, so we had the door sealed on all sides. Now the rats made many more errors and showed no signs of orienting to the door or any other feature in the room.
What brain region did rats use to find their way through the maze in complete darkness? There were studies implicating head direction cells in the thalamus with connections to the striatum and the hippocampus, therefore, one of us (MTW) suggested using 6-hydroxydopamine injected into the dorsal striatum, dorsolateral striatum, dorsomedial striatum, ventral striatum (nucleus accumbens), and PFC to determine which regions were important for this form of learning (Braun et al., 2012, Braun et al., 2015, Braun et al., 2016a). Without describing these experiments in detail, the data showed that striatal and nucleus accumbens dopamine damage caused large impairments in CWM9 learning in the dark. There were also MWM deficits in these rats, but proportionately the MWM deficits were less severe than those in the CWM. 6-Hydroxydopamine injections into the PFC had no effects. It takes rats significantly longer to learn in total darkness, 18–21 days, versus 5–6 days under visible light (CWM9 or CWM10). Since adopting the infrared testing procedure, we have seen more examples of treatments that affect the CWM differently than the MWM. Later, it was shown that there are egocentric sensitive cells in the striatum, analogous to place cells in the hippocampus (Hinman et al., 2019) and that egocentric learning also depends on head direction cells (Bjerknes et al., 2018, Gofman et al., 2019). Collectively, the data show that the CWM under infrared lighting assesses egocentric or route-based L&M as distinct from the spatial or allocentric L&M assessed in the MWM.
-
A.
Age Effects: We typically test rats in the CWM between P50 and P180. At these ages, rats learn the maze well with no age-dependent differences. We know rats learn the MWM even at P360, and rats of this age can probably learn the CWM as well, but we have not tested this. At the other end of the spectrum, young rats have difficulty learning the CWM. We tested rats at P30, and they learned it only if we reduced the complexity from 9 to 7 T-shaped cul-de-sacs (CWM7). At this level of complexity, P30 rats could learn the maze under infrared lighting (Jablonski et al., 2017). Since the BWM is simpler than the CWM7, P30 rats should be able to learn the BWM under infrared lighting as well if one wanted to use it that way. There are no published data for the CWM or BWM for rats younger than P30. Extrapolating from available data suggests that P25 rats should be able to learn the CWM7 under visible light and maybe under infrared light, however, it would be advisable to conduct a preliminary experiment to prove this before adopting it for use. Also, for young rats, a rest period between trials is needed since at these ages, rats, like mice, lose body heat rapidly.
-
B.
Retesting: There is no evidence that using the same test at two ages offers advantages over testing two different types of learning at different ages. One cannot rule out cases where a chemical might result in an effect at P25 with recovery by the time they reach P60. Or the opposite, one could imagine a worse effect at P60 than at P25 if the compound had cumulative effects, however, this should be interpreted cautiously if using the same maze at both ages because once rats learn a maze they remember it well making the retest less sensitive to any treatment effect. For this reason, it is preferable to use different tests if one is going to test L&M at different ages.
-
C.
Trials: In the CWM with 2 trials/day under visible light, adult rats learn in 5–6 days, similarly 2 trials/day under infrared light, it takes at least 18 days and 21 days is better.
-
D.
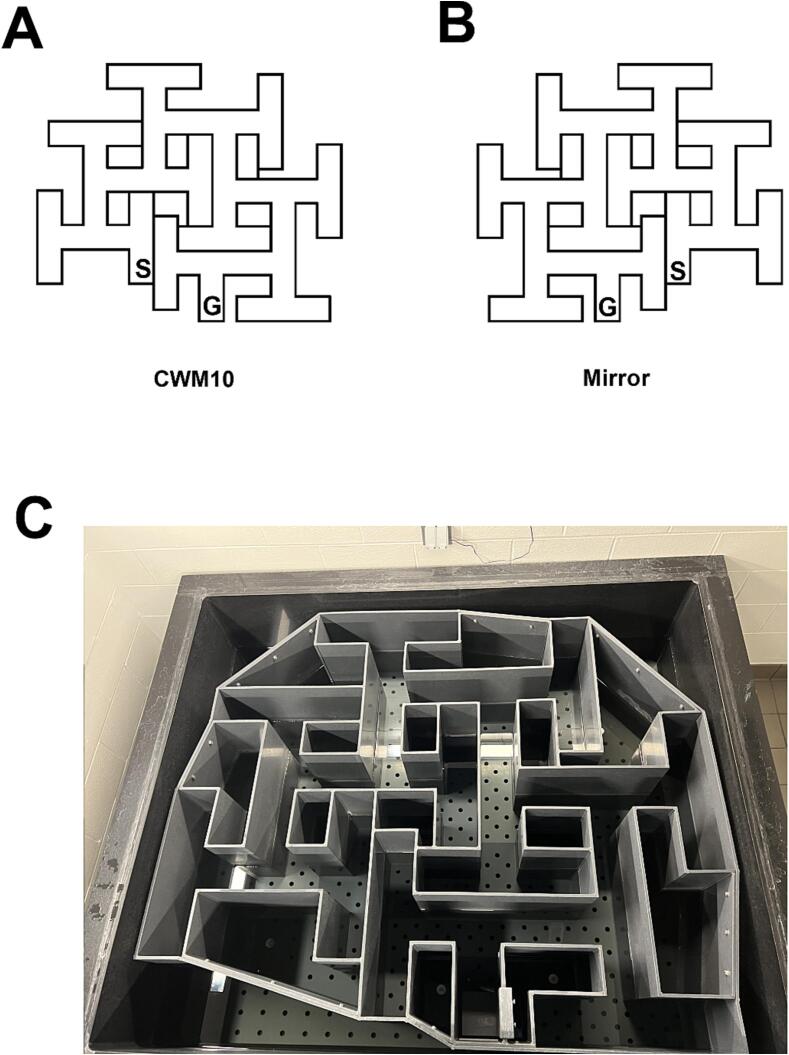
CWM10: After using the CWM9 for a number of years it needed to be replaced. We used the opportunity to make design refinements. If you examine the CWM9 design, you will notice that it can accommodate an additional T-shaped cul-de-sac at the start. Therefore, we had the new maze fabricated with 10 multiple Ts rather than 9 (Fig. 5A,C). As one would predict, rats make more errors in the 10 T version, have longer latencies, and take more days to become proficient than in the CWM9. In fact, the CWM10 is sufficiently more difficult that rats hit the time limit on multiple days (4–6) before finding the goal for the first time. This is why 21 days of testing is preferable to 18 days. Observationally, the CWM10 is approaching the limit of what rats can learn, if it were made any more complex more rats would fail so often that they might stop searching and create non-learner problems. Because the maze is asymmetric, we also constructed a mirror image version (Fig. 5B) in order to test a kind of reversal learning.
-
E.
Scoring: Originally, an error was defined as a rat swimming to the end of a T-shaped cul-de-sac, turning right or left and entering one of the arms. We observed that as rats learn, at first they enter all the way into the arms before turning around, but as they learn they enter the stem, stop, and turn around and start backtracking. Therefore, we now also define stem entries as errors. Rats learn in stages through a process of gradual elimination of errors. They do not exhibit sudden improvements but gradually improve as they recognize certain turns as incorrect. They do this gradually until they make mostly stem and few arm errors, until they eventually make few errors of either type.
-
F.
Failures to Escape: One issue in complex mazes occurs when a rat stops searching and treads water. These rats make few errors, perhaps 10, whereas rats that actively search can easily make 80 errors. If data such as these are analyzed with some making many errors and with some making few errors and never finding the way out it skews the data and adds error variance. Latency does not have this issue since a rat that stops searching has a latency of 5 min, the same as a rat that actively searches but fails to find the goal. Hence, the time limit truncates latency data. Errors should be scaled similarly for rats that reach the time limit and stop searching during the trial. We do this by assigning such rats an error score that reflects the upper boundary of number of errors made by the rat that makes the most errors. This increases the correlation between errors and latency, but we still find effects that are significant on one measure and not the other.
-
G.
Recommendations: The CWM9 and CWM10 are more sensitive than the BWM. The G to S path is counterproductive and reduces the sensitivity of the test and is not recommended. The S to G path is more sensitive and sufficient in and of itself. Two trials/day with a 5 min time limit works well and prevents fatigue on trials where rats reach the time limit. If rats reach the goal in < 5 min they should receive trial-2 immediately. If rats reach the time limit, they should be removed from the maze without help and rested for at least 5 min before trial-2. If tested in the light, the maze is a mixed egocentric/allocentric test. To optimize it as a test of egocentric L&M, it should be run in darkness under infrared LED lighting. Under infrared lighting, rats should be tested for 18–21 days recording errors and latency and for analyses errors should have a correction. Rats as young as P30 can be tested using the first 7 Ts (CWM7). If time constraints make testing under infrared light prohibitive we recommend the CWM under lighted conditions which requires only 5 days for either the CWM9 or CWM10. A summary of the CWM10 procedures is provided in Table 2.
-
H.
If two test ages are needed, we recommend the MWM starting at P30 in 4 phases and the CWM for adults, preferably under infrared lighting. However, CWM7 can be used in rats as young as P30. Start rats in the CWM9 or CWM10 at P50 or later and only after pre-maze trials in a straight swimming channel.
-
I.
BWM vs. CWM: It is clear from the data that the CWM10 and CWM9 are more sensitive than the BWM. There are no data comparing the BWM to the CWM7 or the BWM to the CWM under infrared lighting but from existing data it may be inferred that the BWM is less sensitive than the CWM7 which is less sensitive than the CWM9 or 10 under visible or infrared light. Since these mazes are more sensitive under infrared than visible light, the easiest way to increase sensitivity is to test under infrared light. Would the BWM under infrared light have adequate sensitivity? Perhaps, but there are no data on this. For adult rats, the CWM9 or CWM10 under infrared light are the best choices.
Fig. 5.
CWM10. A, Schematic of the CWM10 where S is the start and G is the goal. B, Schematic of the mirror image CWM10. C, photograph of the CWM10. The perforated floor is to allow water to flow to a canonical subfloor to a drain connected to a room floor drain for cleaning.
Table 2.
Cincinnati water maze (CWM).
| Factor | Specification |
|---|---|
| Tank size-adults | Rectangular 94 × 72 in. |
| Maze dimensions | See Fig. 6 |
| Tank depth | 76 cm to subfloor; 49 cm to perforated floor at edge |
| Construction | 2.54 cm black polypropylene |
| Lighting | Infrared (940 nm wavelength) |
| Water depth | 25 cm |
| Platform size | 15 cm × 15 cm submerged |
| Platform color | Black polypropylene |
| Water | Tap water (nothing added) |
| Water temperature | 19–21 °C, allow at least 12 h to equilibrate to room temp |
| Platform depth | 1.5 cm below water surface |
| Time limit | 5 min per trial |
| Intertrial interval | Back-to-back If goal found on trial-1 of each day, 5 min if rat reaches time limit; or in rotation in cohorts |
| Trial failures | Remove rat and place in cage with paper towels (unassisted escape) |
| Training | 4 trials in 244 cm straight channel day before maze trials |
| Trials/day | 2 |
| Number of days | 18–21 (or 5 if run in visible light) |
| Rat strain | SD, LE, Lister, F344, Wistar, Dark Agouti and more |
| Measures | Latency and errors (errors corrected for rats reaching the time limit) |
| Data analysis | Mixed general linear ANOVA-RM, Group × Sex × Day; if developmental include litter as a random factor |
If a regulatory agency is flexible, start testing at P30 with straight channel training trials the day before maze trials would be better than testing at ages younger than this. If different ages are tested, use a CWM10 maze for the adults and block off channels to make it a CWM7 maze for young rats.
-
J.
Infrared Lighting: Most infrared LEDs come in one of two wavelengths, 850 and 940 nm. We recommend using 940 nm infrared lights because they are invisible to humans, and presumably to rodents, whereas 850 nm infrared lights give off a faint red glow. We have not tested 850 nm infrared lights to determine if rats can detect such light but since 940 nm infrared lights are readily available these are the safer choice.
CWM Details
To illustrate how the CWM is constructed, we provide details in Fig. 6.
Fig. 6.
CWM10. Top, layout of the CWM showing the dimensions of the maze in inches for the benefit of American fabricators and the dimensions of the outer tank and ancillary specification. Front, the profile view shows the height and internal structure and dimensions including the perforated floor and sloping subfloor to the drain and the overflow outlet to prevent flooding if someone fails to shut off the water when refilling the maze after cleaning. Not shown are the PVC drain pipes from the drain at the bottom of the subfloor and overglow that extend under the maze to a shutoff valve and then to a floor drain.
Value of Two Tests: The value of two tests of L&M does not arise from testing at different ages, but rather from assessing two L&M systems. In this context, testing egocentric and allocentric L&M would achieve this. Two tests add value since they tap different brain regions and provide a broader assessment of higher brain function. If two ages are required by the regulatory agency, then it can be the MWM in younger rats and CWM in older rats, or vice versa. When the CWM is used it is done around P30 and the CWM is the most sensitive under infrared light.
Conclusions
Regulatory guidelines that encourage two tests of L&M are taking a significant step forward in safety assessment for higher brain function, functions more relevant to human health than more rudimentary tests included in most current neurobehavioral test batteries. We recommend two tests of L&M that are supported by evidence and have proven records of detecting neurotoxic effects without being overly burdensome in terms of time or resources and have clear homology to the equivalent functional domains in humans. These characteristics facilitate interpretation of any effect observed and are a significant advantage over many other tests. While both tests we recommend are water escape tests and are aversively motivated, they are less aversive than foot-shock tests because swimming is an innate behavior in rodents, it requires no intervention unlike food deprivation, and rats learn these tests efficiently. Of course, no test is perfect, but we suggest that comparatively, water mazes offer distinct advantages compared with appetitive, shock, and spontaneous tests. Appetitive tests require food deprivation with daily adjustment, electric shock is highly aversive and can cause non-performing animals, and tests that rely on spontaneous behaviors have narrow dynamic ranges that are subject to influences unrelated to the independent variable. Moreover, MWM reversal testing captures cognitive flexibility, an assessment otherwise requiring time-consuming operant methods.
Drawbacks to water mazes are few. The CWM error scoring does require adjustment for non-searching rats, but the adjustment is straightforward. Water mazes require regular cleaning and temperature regulation but are otherwise free from other implementation issues. The mazes we recommend are just that, recommendations. We are not suggesting there are no other tests of L&M that are sensitive to neurotoxic effects, we are simply presenting the case for two L&M tests that offer a number of advantages for regulatory safety studies and would be improvements over the current practice of having only one test of L&M.
In closing we quote compelling new experimental evidence of the central importance of hippocampally mediated higher cognitive function: “We developed a brain-machine interface to test whether rats can do so [navigate] by controlling their hippocampal activity in a flexible, goal-directed, and model-based manner. We found that rats can efficiently navigate or direct objects to arbitrary goal locations within a virtual reality arena solely by activating and sustaining appropriate hippocampal representations of remote places” (Lai et al., 2023). There is no clearer evidence than this that rats have cognitive abilities that approximate those of people, making them suitable predictors of these functions in humans.
Author contributions
This paper was conceived and drafted by the first author with critical input and editing by the second author. The creation of this paper was made possible by the research on which it is founded that was and is supported by research grants from the U.S. National Institutes of Health and currently NIH R01 ES032270 and P30 ES006096.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Charles Vorhees reports financial support was provided by National Institute of Environmental Health Sciences. Michael Williams reports financial support was provided by National Institute of Environmental Health Sciences. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Charles V. Vorhees, Email: Charles.vorhees@cchmc.org.
Michael T. Williams, Email: Michael.williams@cchmc.org.
Data availability
Data will be made available on request.
References
- Able J.A., Gudelsky G.A., Vorhees C.V., Williams M.T. 3,4-Methylenedioxymethamphetamine in adult rats produces deficits in path integration and spatial reference memory. Biol. Psychiatry. 2006;59:1219–1226. doi: 10.1016/j.biopsych.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amos-Kroohs R.M., Davenport L.L., Atanasova N., Abdulla Z.I., Skelton M.R., Vorhees C.V., Williams M.T. Developmental manganese neurotoxicity in rats: Cognitive deficits in allocentric and egocentric learning and memory. Neurotoxicol. Teratol. 2017;59:16–26. doi: 10.1016/j.ntt.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annett L.E., McGregor A., Robbins T.W. The effects of ibotenic acid lesions of the nucleuc accumbens on spatial learning and extinction in the rat. Behav. Brain Res. 1989;31:231–242. doi: 10.1016/0166-4328(89)90005-3. [DOI] [PubMed] [Google Scholar]
- Atanasova N.A., Williams M.T., Vorhees C.V. In: The Tools of Neuroscience Experiment: Philosophical and Scientific Perspectives. Bickle J., Craver C.F., Barwich A.-.-A., editors. Routledge, Taylor and Francies Group; New York: 2022. Science in practice in neuroscience: Cincinnati water maze in the making; pp. 56–82. [Google Scholar]
- Bach M.E., Hawkins R.D., Osman M., Kandel E.R., Mayford M. Impairment of spatial but not contextual memory in CaMKII mutant mice with selective loss of hippocampal LTP in the range of the µ frequency. Cell. 1995;81:905–915. doi: 10.1016/0092-8674(95)90010-1. [DOI] [PubMed] [Google Scholar]
- Bannerman D.M., Niewoehner B., Lyon L., Romberg C., Schmitt W.B., Taylor A., Sanderson D.J., Cottam J., Sprengel R., Seeburg P.H., Kohr G., Rawlins J.N. NMDA receptor subunit NR2A is required for rapidly acquired spatial working memory but not incremental spatial reference memory. J. Neurosci. 2008;28:3623–3630. doi: 10.1523/JNEUROSCI.3639-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker G.R., Bird F., Alexander V., Warburton E.C. Recognition memory for objects, place, and temporal order: a disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. J. Neurosci. 2007;27:2948–2957. doi: 10.1523/JNEUROSCI.5289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes C.A. Memory deficits associated with senescence: A neurophysiological and behavioral study in the rat. J. Comp. Physiol. Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Baumann O., Mattingley J.B. Medial parietal cortex encodes perceived heading direction in humans. J. Neurosci. 2010;30:12897–12901. doi: 10.1523/JNEUROSCI.3077-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekinschtein P., Cammarota M., Igaz L.M., Bevilaqua L.R., Izquierdo I., Medina J.H. Persistence of long-term memory storage requires a late protein synthesis- and BDNF- dependent phase in the hippocampus. Neuron. 2007;53:261–277. doi: 10.1016/j.neuron.2006.11.025. [DOI] [PubMed] [Google Scholar]
- Bevins R.A., Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study 'recognition memory'. Nat. Protoc. 2006;1:1306–1311. doi: 10.1038/nprot.2006.205. [DOI] [PubMed] [Google Scholar]
- Biel W.C. Early age differences in maze performance in the albino rat. J. Genet. Psychol. 1940;56:439–453. [Google Scholar]
- Bjerknes T.L., Dagslott N.C., Moser E.I., Moser M.B. Path integration in place cells of developing rats. PNAS. 2018;115:E1637–E1646. doi: 10.1073/pnas.1719054115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blokland A., Geraerts E., Been M. A detailed analysis of rats' spatial memory in a probe trial of a Morris task. Behav. Brain Res. 2004;154:71–75. doi: 10.1016/j.bbr.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Brandeis R., Brandys Y., Yehuda S. The use of the Morris water maze in the study of memory and learning. Int. J. Neurosci. 1989;48:29–69. doi: 10.3109/00207458909002151. [DOI] [PubMed] [Google Scholar]
- Braun A.A., Graham D.L., Schaefer T.L., Vorhees C.V., Williams M.T. Dorsal striatal dopamine depletion impairs both allocentric and egocentric navigation in rats. Neurobiol. Learn. Mem. 2012;97:402–408. doi: 10.1016/j.nlm.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A.A., Amos-Kroohs R.M., Gutierrez A., Lundgren K.H., Seroogy K.B., Skelton M.R., Vorhees C.V., Williams M.T. Dopamine depletion in either the dorsomedial or dorsolateral striatum impairs egocentric Cincinnati water maze performance while sparing allocentric Morris water maze learning. Neurobiol. Learn. Mem. 2015;118:55–63. doi: 10.1016/j.nlm.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A.A., Amos-Kroohs R.M., Gutierrez A., Lundgren K.H., Seroogy K.B., Vorhees C.V., Williams M.T. 6-Hydroxydopamine-induced dopamine reductions in the nucleus accumbens, but not the medial prefrontal cortex, impair cincinnati water maze egocentric and morris water maze allocentric navigation in male sprague-dawley rats. Neurotox. Res. 2016;30:199–212. doi: 10.1007/s12640-016-9616-6. [DOI] [PubMed] [Google Scholar]
- Braun A.A., Amos-Kroohs R.M., Gutierrez A., Lundren K.H., Seroogy K.B., Vorhees C.V., Williams M.T. 6-Hydroxydopamine reductions in the nucleus accumbens, but not the medial prefrontal cortex, impair Cincinnati water maze egocentric and Morris water maze allocentric navigation in male Sprague-Dawley rats. Neurotox. Res. 2016 doi: 10.1007/s12640-016-9616-6. [DOI] [PubMed] [Google Scholar]
- Brown T.I., Whiteman A.S., Aselcioglu I., Stern C.E. Structural differences in hippocampal and prefrontal gray matter volume support flexible context-dependent navigation ability. J. Neurosci. 2014;34:2314–2320. doi: 10.1523/JNEUROSCI.2202-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T.I., Carr V.A., LaRocque K.F., Favila S.E., Gordon A.M., Bowles B., Bailenson J.N., Wagner A.D. Prospective representation of navigational goals in the human hippocampus. Science. 2016;352:1323–1326. doi: 10.1126/science.aaf0784. [DOI] [PubMed] [Google Scholar]
- Buresova O., Bures J. Radial maze as a tool for assessing the effect of drugs on the working memory of rats. Psychopharmacology (Berl) 1982;77:268–271. doi: 10.1007/BF00464578. [DOI] [PubMed] [Google Scholar]
- Burgess N., Maguire E.A., O'Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- Burwell R.D., Saddoris M.P., Bucci D.J., Wiig K.A. Corticohippocampal contributions to spatial and contextual learning. J. Neurosci. 2004;24:3826–3836. doi: 10.1523/JNEUROSCI.0410-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher R.E. Learning impairment associated with maternal phenylketonuria. Nature. 1970;226:555–556. doi: 10.1038/226555a0. [DOI] [PubMed] [Google Scholar]
- Butcher R.E., Vorhees C.V., Berry H.K. A learning impairment associated with induced phenylketonuria. Life Sci. 1970;9:1261–1268. doi: 10.1016/0024-3205(70)90266-3. [DOI] [PubMed] [Google Scholar]
- Buzsaki G., Moser E.I. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat. Neurosci. 2013;16:130–138. doi: 10.1038/nn.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carman H.M., Mactutus C.F. Proximal versus distal cue utilization in spatial navigation: The role of visual acuity? Neurobiol. Learn. Mem. 2001;78:332–346. doi: 10.1006/nlme.2002.4062. [DOI] [PubMed] [Google Scholar]
- Carman H.M., Mactutus C.F. Proximal versus distal cue utilization in spatial navigation: the role of visual acuity? Neurobiol. Learn. Mem. 2002;78:332–346. doi: 10.1006/nlme.2002.4062. [DOI] [PubMed] [Google Scholar]
- Clark R.E., Zola S.M., Squire L.R. Impaired recognition memory in rats after damage to the hippocampus. J. Neurosci. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commins S., Cunningham L., Harvey D., Walsh D. Massed but not spaced training impairs spatial memory. Behav. Brain Res. 2003;139:215–223. doi: 10.1016/s0166-4328(02)00270-x. [DOI] [PubMed] [Google Scholar]
- Conrad C.D. A critical review of chronic stress effects on spatial learning and memory. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;34:742–755. doi: 10.1016/j.pnpbp.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Conrad C.D., Roy E.J. Dentate gyrus destruction and spatial learning impairment after corticosteroid removal in young and middle-aged rats. Hippocampus. 1995;5:1–15. doi: 10.1002/hipo.450050103. [DOI] [PubMed] [Google Scholar]
- Cornwell B.R., Johnson L.L., Holroyd T., Carver F.W., Grillon C. Human hippocampal and parahippocampal theta during goal-directed spatial navigation predicts performance on a virtual Morris water maze. J. Neurosci. 2008;28:5983–5990. doi: 10.1523/JNEUROSCI.5001-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravens R.W. Effects of maternal undernutrition on offspring behavior: Incentive value of a food reward and ability to escape from water. Dev. Psychobiol. 1974;7:61–69. doi: 10.1002/dev.420070110. [DOI] [PubMed] [Google Scholar]
- Crawley J.N. Edition Edition. John Wiley & Sons; Hoboken, NJ: 2007. What's Wrong with My Mouse: Behavioral Phenotyping of Transgenic and Knockout Mice, Second. [Google Scholar]
- Crawley J.N. Mouse behavioral assays relevant to the symptoms of autism. Brain Pathol. 2007;17:448–459. doi: 10.1111/j.1750-3639.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S., Butcher S.P., Morris R.G.M. The NMDA receptor antagonist D-2-amino-5-phophonopentanoate (D-AP5) impairs spatial learning and LTP in vivo at intracerebral concentrations comparable to those that block LTP in vitro. J. Neurosci. 1992;12:21–34. doi: 10.1523/JNEUROSCI.12-01-00021.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon R.M., Bannerman D.M., Kirby B.P., Croucher A., Rawlins J.N. Effects of cytotoxic hippocampal lesions in mice on a cognitive test battery. Behav. Brain Res. 2002;133:57–68. doi: 10.1016/s0166-4328(01)00451-x. [DOI] [PubMed] [Google Scholar]
- Devan B.D., Blank G.S., Petri H.L. Place navigation in the Morris water task: Effects of reduced platform interval lighting and pseudorandom plaform positioning. Psychobiology. 1992;20:120–126. [Google Scholar]
- Dhawan S.S., Tait D.S., Brown V.J. More rapid reversal learning following overtraining in the rat is evidence that behavioural and cognitive flexibility are dissociable. Behav. Brain Res. 2019;363:45–52. doi: 10.1016/j.bbr.2019.01.055. [DOI] [PubMed] [Google Scholar]
- Dupret D., Revest J.M., Koehl M., Ichas F., Costet P., Abrous D.N., Piazza P.V. Spatial relational memory requires hippocampal adult neurogenesis. PLoS One. 2008;3:e1959. doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- During M.J., Cao L., Zuzga D.S., Francis J.S., Fitzsimons H.L., Jiao X., Bland R.J., Klugmann M., Banks W.A., Drucker D.J., Haile C.N. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat. Med. 2003;9:1173–1179. doi: 10.1038/nm919. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Memory: organization and control. Annu. Rev. Psychol. 2017;68:19–45. doi: 10.1146/annurev-psych-010416-044131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H., Stewart C., Morris R.G. Hippocampal representation in place learning. J. Neurosci. 1990;10:3531–3542. doi: 10.1523/JNEUROSCI.10-11-03531.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom A.D., Arnold A.E., Iaria G. A critical review of the allocentric spatial representation and its neural underpinnings: toward a network-based perspective. Front. Hum. Neurosci. 2014;8:803. doi: 10.3389/fnhum.2014.00803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmann A. Impaired long-term memory and NR2A-type NMDA receptor-dependent synaptic plasticity in mice lacking c-Fos in the CNW. J. Neurosci. 2003;23:9116–9122. doi: 10.1523/JNEUROSCI.23-27-09116.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouquet C., Babayan B.M., Watilliaux A., Bontempi B., Tobin C., Rondi-Reig L. Complementary roles of the hippocampus and the dorsomedial striatum during spatial and sequence-based navigation behavior. PLoS One. 2013;8:e67232. doi: 10.1371/journal.pone.0067232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs R.B., Johnson D.A. Sex-specific effects of gonadectomy and hormone treatment on acquisition of a 12-arm radial maze task by Sprague Dawley rats. Endocrinology. 2008;149:3176–3183. doi: 10.1210/en.2007-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gofman X., Tocker G., Weiss S., Boccara C.N., Lu L., Moser M.B., Moser E.I., Morris G., Derdikman D. Dissociation between Postrhinal Cortex and Downstream Parahippocampal Regions in the Representation of Egocentric Boundaries. Curr. Biol. 2019;29(2751–2757):e2754. doi: 10.1016/j.cub.2019.07.007. [DOI] [PubMed] [Google Scholar]
- Grant S.G.N., O'Dell T.J., Karl K.A., Stein P.L., Soriano P., Kandel E.R. Impaired long-term potentiation, spatial learning, and hippocampal development in fyn mutant mice. Science. 1992;258:1903–1910. doi: 10.1126/science.1361685. [DOI] [PubMed] [Google Scholar]
- Guderian S., Dzieciol A.M., Gadian D.G., Jentschke S., Doeller C.F., Burgess N., Mishkin M., Vargha-Khadem F. Hippocampal volume reduction in humans predicts impaired allocentric spatial memory in virtual-reality navigation. J. Neurosci. 2015;35:14123–14131. doi: 10.1523/JNEUROSCI.0801-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez A., Williams M.T., Vorhees C.V. A single high dose of methamphetamine reduces monoamines and impairs egocentric and allocentric learning and memory in adult male rats. Neurotox. Res. 2018;33:671–680. doi: 10.1007/s12640-018-9871-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski J.F., Setlow B., Wagner E.K., McGaugh J.L. Experience-dependent gene expression in the rat hippocampus after spatial learning: A comparison of the immediate-early genes Arc, c-fos, and zif268. J. Neurosci. 2001;21:5089–5098. doi: 10.1523/JNEUROSCI.21-14-05089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C.J., O'Tuathaigh C.M., van Trigt L., Quinn J.J., Fanselow M.S., Mongeau R., Koch C., Anderson D.J. Trace but not delay fear conditioning requires attention and the anterior cingulate cortex. PNAS. 2003;100:13087–13092. doi: 10.1073/pnas.2132313100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardcastle K., Ganguli S., Giocomo L.M. Cell types for our sense of location: where we are and where we are going. Nat. Neurosci. 2017;20:1474–1482. doi: 10.1038/nn.4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison F.E., Hosseini A.H., McDonald M.P. Endogenous anxiety and stress responses in water maze and Barnes maze spatial memory tasks. Behav. Brain Res. 2009;198:247–251. doi: 10.1016/j.bbr.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb D.O., Williams K. A method of rating animal intelligence. J. Gen. Psychol. 1946;34:59–65. doi: 10.1080/00221309.1946.10544520. [DOI] [PubMed] [Google Scholar]
- Herring N.R., Schaefer T.L., Gudelsky G.A., Vorhees C.V., Williams M.T. Effect of (+)-methamphetamine on path integration learning, novel object recognition, and neurotoxicity in rats. Psychopharmacology (Berl) 2008;199:637–650. doi: 10.1007/s00213-008-1183-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyser C.J., McNaughton C.H., Vishnevetsky D., Fienberg A.A. Methylphenidate restores novel object recognition in DARPP-32 knockout mice. Behav. Brain Res. 2013;253:266–273. doi: 10.1016/j.bbr.2013.07.031. [DOI] [PubMed] [Google Scholar]
- Hinman J.R., Chapman G.W., Hasselmo M.E. Neuronal representation of environmental boundaries in egocentric coordinates. Nat. Commun. 2019;10:2772. doi: 10.1038/s41467-019-10722-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges H. Maze procedures: The radial-arm and water maze compared. Cog Brain Res. 1996;3:167–181. doi: 10.1016/0926-6410(96)00004-3. [DOI] [PubMed] [Google Scholar]
- Hoh T., Beiko J., Boon F., Weiss S., Cain D.P. Complex behavioral strategy and reversal learning in the water maze without NMDA receptor-dependent long-term potentiation. J. Neurosci. 1999;19(RC2) doi: 10.1523/JNEUROSCI.19-10-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holscher C. Stress impairs performance in spatial watermaze learning tasks. Behav. Brain Res. 1999;100:225–235. doi: 10.1016/s0166-4328(98)00134-x. [DOI] [PubMed] [Google Scholar]
- Iivonen H., Nurminen L., Harri M., Tanila H., Puolivali J. Hypothermia in mice tested in Morris water maze. Behav. Brain Res. 2003;141:207–213. doi: 10.1016/s0166-4328(02)00369-8. [DOI] [PubMed] [Google Scholar]
- Ingram D.K. Complex maze learning in rodents as a model of age-related memory impairment. Neurobiol. Aging. 1988;9:475–485. doi: 10.1016/s0197-4580(88)80101-5. [DOI] [PubMed] [Google Scholar]
- Inman-Wood S.L., Williams M.T., Morford L.L., Vorhees C.V. Effects of prenatal cocaine on Morris and Barnes maze tests of spatial learning and memory in the offspring of C57BL/6J mice. Neurotoxicol. Teratol. 2000;22:547–557. doi: 10.1016/s0892-0362(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Jablonski S.A., Schiffino F.L., Stanton M.E. Role of age, post-training consolidation, and conjunctive associations in the ontogeny of the context preexposure facilitation effect. Dev. Psychobiol. 2012;54:714–722. doi: 10.1002/dev.20621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski S.A., Williams M.T., Vorhees C.V. Learning and memory effects of neonatal methamphetamine exposure in rats: Role of reactive oxygen species and age at assessment. Synapse. 2017;71:1–16. doi: 10.1002/syn.21992. [DOI] [PubMed] [Google Scholar]
- Jablonski S.A., Williams M.T., Vorhees C.V. Learning and memory effects of neonatal methamphetamine exposure in sprague-dawley rats: test of the role of dopamine receptors D1 in mediating the long-term effects. Dev. Neurosci. 2019;41:44–55. doi: 10.1159/000498884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery K.J., Morris R.G.M. Cumulative long-term potentiation in the rat dentate gyrus correlates with, but does not modify, performance in the water maze. Hippocampus. 1993;3:133–140. doi: 10.1002/hipo.450030205. [DOI] [PubMed] [Google Scholar]
- Jo Y.S., Park E.H., Kim I.H., Park S.K., Kim H., Kim H.T., Choi J.S. The medial prefrontal cortex is involved in spatial memory retrieval under partial-cue conditions. J. Neurosci. 2007;27:13567–13578. doi: 10.1523/JNEUROSCI.3589-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney J.W., Gould T.J. Modulation of hippocampus-dependent learning and synaptic plasticity by nicotine. Mol. Neurobiol. 2008;38:101–121. doi: 10.1007/s12035-008-8037-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelstrup K.B., Solstad T., Brun V.H., Hafting T., Leutgeb S., Witter M.P., Moser E.I., Moser M.B. Finite scale of spatial representation in the hippocampus. Science. 2008;321:140–143. doi: 10.1126/science.1157086. [DOI] [PubMed] [Google Scholar]
- Knowlton B.J., Wenk G.L., Olton D.S., Coyle J.T. Basal forebrain lesions produce a dissociation of trial-dependent and trial-independent memory performance. Brain Res. 1985;345:315–321. doi: 10.1016/0006-8993(85)91008-x. [DOI] [PubMed] [Google Scholar]
- Kolarik B.S., Shahlaie K., Hassan A., Borders A.A., Kaufman K.C., Gurkoff G., Yonelinas A.P., Ekstrom A.D. Impairments in precision, rather than spatial strategy, characterize performance on the virtual Morris Water Maze: A case study. Neuropsychologia. 2016;80:90–101. doi: 10.1016/j.neuropsychologia.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong M.S., Kim N., Jo K.I., Kim S.P., Choi J.S. Differential encoding of trace and delay fear memory in the entorhinal cortex. Exp Neurobiol. 2023;32:20–30. doi: 10.5607/en22042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer P.J., Brown R.W., Baldwin S.A., Scheff S.W. Validation of a single-day Morris Water Maze procedure used to assess cognitive deficits associated with brain damage. Brain Res. Bull. 1996;39:17–22. doi: 10.1016/0361-9230(95)02028-4. [DOI] [PubMed] [Google Scholar]
- Kubik S., Fenton A.A. Behavioral evidence that segregation and representation are dissociable hippocampal functions. J. Neurosci. 2005;25:9205–9212. doi: 10.1523/JNEUROSCI.1707-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C., Tanaka S., Harris T.D., Lee A.K. Volitional activation of remote place representations with a hippocampal brain-machine interface. Science. 2023;382:566–573. doi: 10.1126/science.adh5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberty Y., Gower A.J. Simplifying environmental cues in a Morris-type water maze improves place learning in old NMRI mice. Behav. Neural Biol. 1991;56:89–100. doi: 10.1016/0163-1047(91)90315-h. [DOI] [PubMed] [Google Scholar]
- Lashley K.S. In: Brain Mechanisms and Intelligence: A Quantitative Study of Injuries to the Brain. Lashley K.S., editor. University of Chicago Press; Chicago: 1929. The influence of cerebral lesions upon the capacity to learn; pp. 26–75. [Google Scholar]
- Levin E.D. Psychopharmacological effects in the radial-arm maze. Neurosci. Biobehav. Rev. 1988;12:169–175. doi: 10.1016/s0149-7634(88)80008-3. [DOI] [PubMed] [Google Scholar]
- Levin E.D., Addy N., Nakajima A., Christopher N.C., Seidler F.J., Slotkin T.A. Persistent behavioral consequences of neonatal chlorpyrifos exposure in rats. Brain Res. Dev. Brain Res. 2001;130:83–89. doi: 10.1016/s0165-3806(01)00215-2. [DOI] [PubMed] [Google Scholar]
- Levin E.D., Addy N., Baruah A., Elias A., Christopher N.C., Seidler F.J., Slotkin T.A. Prenatal chlorpyrifos exposure in rats causes persistent behavioral alterations. Neurotoxicol. Teratol. 2002;24:733–741. doi: 10.1016/s0892-0362(02)00272-6. [DOI] [PubMed] [Google Scholar]
- Mactutus C.F., Booze R.M. Accuracy of spatial navigation: The role of platform and tank size. Soc. Neurosci. Abst. 1994;20 [Google Scholar]
- Mandel R.J., Gage F.H., Thal L.J. Enhanced detection of nucleus basalis magnocellularis lesion-induced spatial learning deficit in rats by modification of training regimen. Behav. Brain Res. 1989;31:221–229. doi: 10.1016/0166-4328(89)90004-1. [DOI] [PubMed] [Google Scholar]
- McDonald R.J., White N.M. Parallel information processing in the water maze: Evidence for independent memory systems involving dorsal striatum and hippocampus. Behav. Neural Biol. 1994;61:260–270. doi: 10.1016/s0163-1047(05)80009-3. [DOI] [PubMed] [Google Scholar]
- McNaughton N., Morris R.G.M. Buspirone produces a dose-related impairment of spatial navigation. Pharmacol. Biochem. Behav. 1992;43:167–171. doi: 10.1016/0091-3057(92)90653-w. [DOI] [PubMed] [Google Scholar]
- Miyakawa T., Yamada M., Duttaroy A., Wess J. Hyperactivity and intact hippocampus-dependent learning in mice lacking the M1 muscarinic acetylcholine receptor. J. Neurosci. 2001;21:5239–5250. doi: 10.1523/JNEUROSCI.21-14-05239.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen J., Pedersen T.K., Holm S., Bang L.E. Prefrontal cortical mediation of rats' place learning in a modified water maze. Brain Res. Bull. 1995;38:425–434. doi: 10.1016/0361-9230(95)02009-g. [DOI] [PubMed] [Google Scholar]
- Morford L.L., Inman-Wood S.L., Gudelsky G.A., Williams M.T., Vorhees C.V. Impaired spatial and sequential learning in rats treated neonatally with d-fenfluramine. Eur. J. Neurosci. 2002;16:491–500. doi: 10.1046/j.1460-9568.2002.02100.x. [DOI] [PubMed] [Google Scholar]
- Morris R.G.M. Spatial localization does not require the presence of local cues. Learn. Motiv. 1981;12:239–260. [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Morris R.G.M. Synaptic plasticity and learning: Selective impairment of learning in rats and blockade of long-term potentiation in vivo by the N-methyl-D-aspartate receptor antagonist AP5. J. Neurosci. 1989;9:3040–3057. doi: 10.1523/JNEUROSCI.09-09-03040.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R.G., Garrud P., Rawlins J.N., O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Morris R.G., Hagan J.J., Rawlins J.N. Allocentric spatial learning by hippocampectomised rats: a further test of the “spatial mapping” and “working memory” theories of hippocampal function. Q. J. Exp. Psychol. 1986;38B:365–395. [PubMed] [Google Scholar]
- Morris R.G.M., Hagan J.J., Rawlins J.N.P. Allocentric spatial learning by hippocampectomized rats: A further test of the “spatial mapping” and “working memory” theories of hippocampal function. Quart J Exp Psychol. 1986;38B:365–395. [PubMed] [Google Scholar]
- Morris R.G.M., Anderson E., Lynch G.S., Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate antagonist, AP5. Nature. 1986;329:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Morris R.G., Schenk F., Tweedie F., Jarrard L.E. Ibotenate lesions of hippocampus and/or subiculum: dissociating components of allocentric spatial learning. Eur. J. Neurosci. 1990;2:1016–1028. doi: 10.1111/j.1460-9568.1990.tb00014.x. [DOI] [PubMed] [Google Scholar]
- Morris R.G., Inglis J., Ainge J.A., Olverman H.J., Tulloch J., Dudai Y., Kelly P.A. Memory reconsolidation: sensitivity of spatial memory to inhibition of protein synthesis in dorsal hippocampus during encoding and retrieval. Neuron. 2006;50:479–489. doi: 10.1016/j.neuron.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Moser E.I., Roudi Y., Witter M.P., Kentros C., Bonhoeffer T., Moser M.B. Grid cells and cortical representation. Nat. Rev. Neurosci. 2014;15:466–481. doi: 10.1038/nrn3766. [DOI] [PubMed] [Google Scholar]
- Moser E.I., Moser M.B., McNaughton B.L. Spatial representation in the hippocampal formation: a history. Nat. Neurosci. 2017;20:1448–1464. doi: 10.1038/nn.4653. [DOI] [PubMed] [Google Scholar]
- Nagelhus A., Andersson S.O., Cogno S.G., Moser E.I., Moser M.B. Object-centered population coding in CA1 of the hippocampus. Neuron. 2023;111(2091–2104):e2014. doi: 10.1016/j.neuron.2023.04.008. [DOI] [PubMed] [Google Scholar]
- Nasello A.G., Ramirez O.A. Open field and Lashley III maze behaviour of the offspring of amphetamine-treated rats. Psychopharmacology (Berl) 1978;58:171–173. doi: 10.1007/BF00426902. [DOI] [PubMed] [Google Scholar]
- Obenhaus, H.A., Zong W., Jacobsen, R.I., Rose, T., Donato, F., Chen, L., Cheng, H., Bonhoeffer, T., Moser, M.B., Moser, E.I. 2022. Functional network topography of the medial entorhinal cortex. Proc. Natl. Acad. Sci. U. S. A. 119. [DOI] [PMC free article] [PubMed]
- Olton D.S. The radial arm maze as a tool in behavioral pharmacology. Physiol. Behav. 1987;40:793–797. doi: 10.1016/0031-9384(87)90286-1. [DOI] [PubMed] [Google Scholar]
- Otnaess M.K., Brun V.H., Moser M.-B., Moser E.I. Pretraining prevents spatial learning impairment after saturation of hippocampal long-term potentiation. J. Neurosci. 1999;19(RC49):1–5. doi: 10.1523/JNEUROSCI.19-24-j0007.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard M.G., McGaugh J.L. Double dissociation of fornix and caudate nucleus lesions on acquisition of two water maze tasks: Further evidence for multiple memory systems. Behav. Neurosci. 1992;106:439–446. doi: 10.1037//0735-7044.106.3.439. [DOI] [PubMed] [Google Scholar]
- Pitzer E.M., Sugimoto C., Gudelsky G.A., Huff Adams C.L., Williams M.T., Vorhees C.V. Deltamethrin exposure daily from postnatal day 3–20 in sprague-dawley rats causes long-term cognitive and behavioral deficits. Toxicol. Sci. 2019;169:511–523. doi: 10.1093/toxsci/kfz067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polidora V.J., Boggs D.E., Waisman H.A. A behavioral deficit associated with phenylketonuria in rats. Proc. Soc. Exp. Biol. Med. 1963;113:817–820. doi: 10.3181/00379727-113-28500. [DOI] [PubMed] [Google Scholar]
- Polidora V.J., Cunningham R.F., Waisman H.A. Dosage parameters of a behavioral deficit associated with phenylketonuria in rats. J. Comp. Physiol. Psychol. 1966;61:436–441. doi: 10.1037/h0023257. [DOI] [PubMed] [Google Scholar]
- Polidora V.J., Cunningham R.F., Waisman H.A. Phenylketonuria in rats: Reversibility of behavioral deficit. Science. 1966;151:219–221. doi: 10.1126/science.151.3707.219. [DOI] [PubMed] [Google Scholar]
- Possin K.L., Sanchez P.E., Anderson-Bergman C., Fernandez R., Kerchner G.A., Johnson E.T., Davis A., Lo I., Bott N.T., Kiely T., Fenesy M.C., Miller B.L., Kramer J.H., Finkbeiner S. Cross-species translation of the Morris maze for Alzheimer's disease. J. Clin. Invest. 2016;126:779–783. doi: 10.1172/JCI78464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsaran A.I., Wang Y., Golbabaei A., Aleshin S., de Snoo M.L., Yeung B.A., Rashid A.J., Awasthi A., Lau J., Tran L.M., Ko S.Y., Abegg A., Duan L.C., McKenzie C., Gallucci J., Ahmed M., Kaushik R., Dityatev A., Josselyn S.A., Frankland P.W. A shift in the mechanisms controlling hippocampal engram formation during brain maturation. Science. 2023;380:543–551. doi: 10.1126/science.ade6530. [DOI] [PubMed] [Google Scholar]
- Rein B., Ma K., Yan Z. A standardized social preference protocol for measuring social deficits in mouse models of autism. Nat. Protoc. 2020;15:3464–3477. doi: 10.1038/s41596-020-0382-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy J.W., Paylor R. Development of interocular equivalence of place learning in the rat requires convergence sites established prior to training. Behav. Neurosci. 1987;101:732–734. doi: 10.1037//0735-7044.101.5.732. [DOI] [PubMed] [Google Scholar]
- Rudy J.W., Paylor R. Reducing the temporal demands of the Morris place-learning task fails to ameliorate the place-learning impairment of preweanling rats. Psychobiology. 1988;16:152–156. [Google Scholar]
- Rudy J.W., Stadler-Morris S., Albert P. Ontogeny of spatial navigation behaviors in the rat: Dissociation of “proximal” and “distal”-cue-based behaviors. Behav. Neurosci. 1987;101:62–73. doi: 10.1037//0735-7044.101.1.62. [DOI] [PubMed] [Google Scholar]
- Safaei F., Farimaneh J., Rajabi Mohammad Abad A., Iranmanesh E., Arabpour F., Doostishoar F., Taherizadeh Z. The effect of silver nanoparticles on learning and memory in rodents: “a systematic review”. J. Occup. Med. Toxicol. 2023;18:15. doi: 10.1186/s12995-023-00381-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandi C., Loscertales M., Guaza C. Experience-dependent facilitating effect of corticosterone on spatial memory formation in the water maze. Eur. J. Neurosci. 1997;9:637–642. doi: 10.1111/j.1460-9568.1997.tb01412.x. [DOI] [PubMed] [Google Scholar]
- Schaefer T.L., Braun A.A., Amos-Kroohs R.M., Williams M.T., Ostertag E., Vorhees C.V. A new model of Pde4d deficiency: genetic knock-down of PDE4D enzyme in rats produces an antidepressant phenotype without spatial cognitive effects. Genes Brain Behav. 2012;11:614–622. doi: 10.1111/j.1601-183X.2012.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk F. Development of place navigation in rats from weaning to puberty. Behav. Neural Biol. 1985;43:69–85. doi: 10.1016/s0163-1047(85)91510-9. [DOI] [PubMed] [Google Scholar]
- Schindler C.W. Acquisition of a noe-poke response in rats as an operatn. Bull. Psychon. Soc. 1993;31:291–294. [Google Scholar]
- Silverman J.L., Nithianantharajah J., Der-Avakian A., Young J.W., Sukoff Rizzo S.J. Lost in translation: At the crossroads of face validity and translational utility of behavioral assays in animal models for the development of therapeutics. Neurosci. Biobehav. Rev. 2020;116:452–453. doi: 10.1016/j.neubiorev.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sircar R. Postnatal phencylidine-induced deficit in adult water maze performance is associated with N-methyl-D-aspartate receptor regulation. Int. J. Dev. Neurosci. 2003;21:159–167. doi: 10.1016/s0736-5748(03)00026-1. [DOI] [PubMed] [Google Scholar]
- Skelton M.R., Williams M.T., Schaefer T.L., Vorhees C.V. Neonatal (+)-methamphetamine increases brain derived neurotrophic factor, but not nerve growth factor, during treatment and results in long-term spatial learning deficits. Psychoneuroendocrinology. 2007;32:734–745. doi: 10.1016/j.psyneuen.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton M.R., Schaefer T.L., Herring N.R., Grace C.E., Vorhees C.V., Williams M.T. Comparison of the developmental effects of 5-methoxy-N, N-diisopropyltryptamine (Foxy) to (+/-)-3,4-methylenedioxymethamphetamine (ecstasy) in rats. Psychopharmacology (Berl) 2009;204:287–297. doi: 10.1007/s00213-009-1459-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skosnik P.D., Edwards C.R., O'Donnell B.F., Steffen A., Steinmetz J.E., Hetrick W.P. Cannabis use disrupts eyeblink conditioning: evidence for cannabinoid modulation of cerebellar-dependent learning. Neuropsychopharmacology. 2008;33:1432–1440. doi: 10.1038/sj.npp.1301506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprowles J.L., Hufgard J.R., Gutierrez A., Bailey R.A., Jablonski S.A., Williams M.T., Vorhees C.V. Perinatal exposure to the selective serotonin reuptake inhibitor citalopram alters spatial learning and memory, anxiety, depression, and startle in Sprague-Dawley rats. Int. J. Dev. Neurosci. 2016;54:39–52. doi: 10.1016/j.ijdevneu.2016.08.007. [DOI] [PubMed] [Google Scholar]
- Sprowles J.L.N., Hufgard J.R., Gutierrez A., Bailey R.A., Jablonski S.A., Williams M.T., Vorhees C.V. Differential effects of perinatal exposure to antidepressants on learning and memory, acoustic startle, anxiety, and open-field activity in Sprague-Dawley rats. Int. J. Dev. Neurosci. 2017;61:92–111. doi: 10.1016/j.ijdevneu.2017.06.004. [DOI] [PubMed] [Google Scholar]
- Stewart C.A., Morris R.G.M. In: Behavioural Neuroscience, Volume i, A Practical Approach. Sahgal A., editor. IRL Press at Oxford University Press; Oxford: 1993. The watermaze; pp. 107–122. [Google Scholar]
- Strange B.A., Witter M.P., Lein E.S., Moser E.I. Functional organization of the hippocampal longitudinal axis. Nat. Rev. Neurosci. 2014;15:655–669. doi: 10.1038/nrn3785. [DOI] [PubMed] [Google Scholar]
- Suthana N.A., Ekstrom A.D., Moshirvaziri S., Knowlton B., Bookheimer S.Y. Human hippocampal CA1 involvement during allocentric encoding of spatial information. J. Neurosci. 2009;29:10512–10519. doi: 10.1523/JNEUROSCI.0621-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland R.J., Kolb B., Whishaw I.Q. Spatial mapping: definitive disruption by hippocampal or medial frontal cortical damage in the rat. Neurosci. Lett. 1982;31:271–276. doi: 10.1016/0304-3940(82)90032-5. [DOI] [PubMed] [Google Scholar]
- Tees R.C., Buhrmann K., Hanley J. The effect of early experience on water maze spatial learning and memory in rats. Dev. Psychobiol. 1990;23:427–439. doi: 10.1002/dev.420230505. [DOI] [PubMed] [Google Scholar]
- Teixeira C.M., Pomedli S.R., Maei H.R., Kee N., Frankland P.W. Involvement of the anterior cingulate cortex in the expression of remote spatial memory. J. Neurosci. 2006;26:7555–7564. doi: 10.1523/JNEUROSCI.1068-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonkiss J., Shultz P., Galler J.R. Long-Evans and Sprague-Dawley rats differ in their spatial navigation performance during ontogeny and at maturity. Dev. Psychobiol. 1992;25:567–579. doi: 10.1002/dev.420250804. [DOI] [PubMed] [Google Scholar]
- Topic B., Willuhn I., Palomero-Gallagher N., Zilles K., Huston J.P., Hasenohrl R.U. Impaired maze performance in aged rats is accompanied by increased density of NMDA, 5-HT1A, and alpha-adrenoceptor binding in hippocampus. Hippocampus. 2007;17:68–77. doi: 10.1002/hipo.20246. [DOI] [PubMed] [Google Scholar]
- Tsuji R., Crofton K.M. Developmental neurotoxicity guideline study: issues with methodology, evaluation and regulation. Congenit Anom (Kyoto) 2012;52:122–128. doi: 10.1111/j.1741-4520.2012.00374.x. [DOI] [PubMed] [Google Scholar]
- Ulsaker-Janke I., Waaga T., Waaga T., Moser E.I., Moser M.B. Grid cells in rats deprived of geometric experience during development. Proc. Natl. Acad. Sci. U. S. A. 2023;120 doi: 10.1073/pnas.2310820120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upchurch M., Wehner J.M. DBA/2Ibg mice are incapable of cholinergically-based learning in the Morris water task. Pharmacol. Biochem. Behav. 1988;29:325–329. doi: 10.1016/0091-3057(88)90164-5. [DOI] [PubMed] [Google Scholar]
- Upchurch M., Wehner J.M. Differences between inbred strains of mice in Morris water maze performance. Behav. Genet. 1988;18:55–68. doi: 10.1007/BF01067075. [DOI] [PubMed] [Google Scholar]
- Upchurch M., Wehner J.M. Effects of N-methyl-D-aspartate antagonism on spatial learning in mice. Psychopharmacology (Berl) 1990;100:214. doi: 10.1007/BF02244408. [DOI] [PubMed] [Google Scholar]
- Van Dam D., Lenders G., De Deyn P.P. Effect of Morris water maze diameter on visual-spatial learning in different mouse strains. Neurobiol. Learn. Mem. 2006;85:164–172. doi: 10.1016/j.nlm.2005.09.006. [DOI] [PubMed] [Google Scholar]
- van Rijzingen I.M.S., Gispen W.H., Spruijt B.M. Olfactory bulbectomy temporarily impairs Morrs maze performance: An ACTH (4–9) analog accelerates return of function. Physiol. Behav. 1995;58:147–152. doi: 10.1016/0031-9384(95)00032-e. [DOI] [PubMed] [Google Scholar]
- Vorhees C.V. Influence of early testing on postweaning performance in untreated F344 rats, with comparisons to Sprague-Dawley rats, using a standardized battery of tests for behavioral teratogenesis. Neurobehav. Toxicol. Teratol. 1983;5:587–591. [PubMed] [Google Scholar]
- Vorhees C.V. Maze learning in rats: A comparison of performance in two water mazes in progeny prenatally exposed to different doses of phenytoin. Neurotoxicol. Teratol. 1987;9:235–241. doi: 10.1016/0892-0362(87)90008-0. [DOI] [PubMed] [Google Scholar]
- Vorhees C.V., Weisenburger W.P., Acuff-Smith K.D., Minck D.R. An analysis of factors influencing complex water maze learning in rats: Effects of task complexity, path order and escape assistance on performance following prenatal exposure to phenytoin. Neurotoxicol. Teratol. 1991;13:213–222. doi: 10.1016/0892-0362(91)90013-m. [DOI] [PubMed] [Google Scholar]
- Vorhees C.V., Morford L.L., Inman S.L., Reed T.M., Schilling M.A., Cappon G.D., Moran M.S., Nebert D.W. Genetic differences in spatial learning between Dark Agouti and Sprague-Dawley strains: possible correlation with the CYP2D2 polymorphism in rats treated neonatally with methamphetamine. Pharmacogenetics. 1999;9:171–181. [PubMed] [Google Scholar]
- Vorhees C.V., Williams M.T. Value of water mazes for assessing spatial and egocentric learning and memory in rodent basic research and regulatory studies. Neurotoxicol. Teratol. 2014;45:75–90. doi: 10.1016/j.ntt.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Vorhees C.V., Reed T.M., Skelton M.R., Williams M.T. Exposure to 3,4-methylenedioxymethamphetamine (MDMA) on postnatal days 11–20 induces reference but not working memory deficits in the Morris water maze in rats: implications of prior learning. Int. J. Dev. Neurosci. 2004;22:247–259. doi: 10.1016/j.ijdevneu.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Vorhees C.V., Skelton M.R., Williams M.T. Age-dependent effects of neonatal methamphetamine exposure on spatial learning. Behav. Pharmacol. 2007;18:549–562. doi: 10.1097/FBP.0b013e3282ee2abe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees C.V., Herring N.R., Schaefer T.L., Grace C.E., Skelton M.R., Johnson H.L., Williams M.T. Effects of neonatal (+)-methamphetamine on path integration and spatial learning in rats: effects of dose and rearing conditions. Int. J. Dev. Neurosci. 2008;26:599–610. doi: 10.1016/j.ijdevneu.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees C.V., Williams M.T. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees C.V., Schaefer T.L., Skelton M.R., Grace C.E., Herring N.R., Williams M.T. (+/-)3,4-Methylenedioxymethamphetamine (MDMA) dose-dependently impairs spatial learning in the morris water maze after exposure of rats to different five-day intervals from birth to postnatal day twenty. Dev. Neurosci. 2009;31:107–120. doi: 10.1159/000207499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees C.V., Williams M.T. Assessing spatial learning and memory in rodents. ILAR J. 2014;55:310–332. doi: 10.1093/ilar/ilu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees C.V., Jablonski S., Gutierrez A., Tee T., Suttling K., Williams M. Neonatal (+)-methamphetamine exposure impairs egocentric, allocentric, and working memory in rats. Neurotoxicol. Teratol. 2015;49 [Google Scholar]
- Wade S.E., Maier S.F. Effects of individual housing and stressor exposure upon the acquisition of watermaze escape. Learn. Motiv. 1986;17:287–310. [Google Scholar]
- Wahlsten D., Cooper S.F., Crabbe J.C. Different rankings of inbred mouse strains on the Morris maze and a refined 4-arm water escape task. Behav. Brain Res. 2005;165:36–51. doi: 10.1016/j.bbr.2005.06.047. [DOI] [PubMed] [Google Scholar]
- Wesierska M., Macias-Gonzalez R., Bures J. Differential effect of ketamine on the reference and working memory versions of the Morris water maze task. Behav. Neurosci. 1990;104:74–83. doi: 10.1037//0735-7044.104.1.74. [DOI] [PubMed] [Google Scholar]
- Whishaw I.Q. Spatial mapping takes time. Hippocampus. 1998;8:122–130. doi: 10.1002/(SICI)1098-1063(1998)8:2<122::AID-HIPO4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Whishaw I.Q. Place learning in hippocampal rats and the path integration hypothesis. Neurosci Biobehav Res. 1998;22:209–220. doi: 10.1016/s0149-7634(97)00002-x. [DOI] [PubMed] [Google Scholar]
- Whishaw I.Q., Hines D.J., Wallace D.G. Dead reckoning (path integration) requires the hippocampal formation: evidence from spontaneous exploration and spatial learning tasks in light (allothetic) and dark (idiothetic) tests. Behav. Brain Res. 2001;127:49–69. doi: 10.1016/s0166-4328(01)00359-x. [DOI] [PubMed] [Google Scholar]
- Whishaw I.Q., Tomie J.-A. Perseveration on place reversals in spatial swimming pool tasks: Further evidence for place learning in hippocampal rats. Hippocampus. 1997;7:361–370. doi: 10.1002/(SICI)1098-1063(1997)7:4<361::AID-HIPO2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Whishaw I.Q., McKenna J.E., Maaswinkel H. Hippocampal lesions and path integration. Curr. Opin. Neurobiol. 1997;7:228–234. doi: 10.1016/s0959-4388(97)80011-6. [DOI] [PubMed] [Google Scholar]
- Williams M.T., Vorhees C.V., Boon F., Saber A.J., Cain D.P. Methamphetamine exposure from postnatal days 11 to 20 causes impairments in both behavioral strategies and spatial learning in adult rats. Brain Res. 2002;958:312–321. doi: 10.1016/s0006-8993(02)03620-x. [DOI] [PubMed] [Google Scholar]
- Williams M.T., Moran M.S., Vorhees C.V. Refining the critical period for methamphetamine-induced spatial deficits in the Morris water maze. Psychopharmacology (Berl) 2003;168:329–338. doi: 10.1007/s00213-003-1433-y. [DOI] [PubMed] [Google Scholar]
- Williams M.T., Blankenmeyer T.L., Schaefer T.L., Brown C.A., Gudelsky G.A., Vorhees C.V. Long-term effects of neonatal methamphetamine exposure in rats on spatial learning in the Barnes maze and on cliff avoidance, corticosterone release, and neurotoxicity in adulthood. Dev. Brain Res. 2003;147:163–175. doi: 10.1016/j.devbrainres.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Williams M.T., Morford L.L., Wood S.L., Wallace T.L., Fukumura M., Broening H.W., Vorhees C.V. Developmental d-methamphetamine treatment selectively induces spatial navigation impairments in reference memory in the Morris water maze while sparing working memory. Synapse. 2003;48:138–148. doi: 10.1002/syn.10159. [DOI] [PubMed] [Google Scholar]
- Williams M.T., Braun A.A., Amos-Kroohs R., McAllister J.P.I., Lindquist D.M., Mangao F.T., Vorhees C.V., Yuan W. Kaolin-induced ventriculomegaly at weaning produces long-term learnng, memory, and motor deficits in rats. Int. J. Dev. Neurosci. 2014;35:7–15. doi: 10.1016/j.ijdevneu.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M.T., Sugimoto C., Regan S.L., Pitzer E.M., Fritz A.L., Mascia A.E., Sertorio M., Vatner R.E., Perentesis J.P., Vorhees C.V. Whole brain proton irradiation in adult Sprague Dawley rats produces dose dependent and non-dependent cognitive, behavioral, and dopaminergic effects. Sci. Rep. 2020;10:21584. doi: 10.1038/s41598-020-78128-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M.T., Sugimoto C., Regan S.L., Pitzer E.M., Fritz A.L., Sertorio M., Mascia A.E., Vatner R.E., Perentesis J.P., Vorhees C.V. Cognitive and behavioral effects of whole brain conventional or high dose rate (FLASH) proton irradiation in a neonatal Sprague Dawley rat model. PLoS One. 2022;17:e0274007. doi: 10.1371/journal.pone.0274007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfer D.P., Stagljar-Bozicevic M., Errington M.L., Lipp H.-P. Spatial memory and learning in transgenic mice: Fact or artifact? News Physiol. Sci. 1998;13:118–123. doi: 10.1152/physiologyonline.1998.13.3.118. [DOI] [PubMed] [Google Scholar]
- Zhang M., Moon C., Chan G.C., Yang L., Zheng F., Conti A.C., Muglia L., Muglia L.J., Storm D.R., Wang H. Ca-stimulated type 8 adenylyl cyclase is required for rapid acquisition of novel spatial information and for working/episodic-like memory. J. Neurosci. 2008;28:4736–4744. doi: 10.1523/JNEUROSCI.1177-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S.J., Zhu M.E., Shu D., Du X.P., Song X.H., Wang X.T., Zheng R.Y., Cai X.H., Chen J.F., He J.C. Preferential enhancement of working memory in mice lacking adenosine A(2A) receptors. Brain Res. 2009;1303:74–83. doi: 10.1016/j.brainres.2009.09.082. [DOI] [PubMed] [Google Scholar]
- Zlomuzica A., Ruocco L.A., Sadile A.G., Huston J.P., Dere E. Histamine H1 receptor knockout mice exhibit impaired spatial memory in the eight-arm radial maze. Br. J. Pharmacol. 2009;157:86–91. doi: 10.1111/j.1476-5381.2009.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.