Abstract
Rapid start of antiretroviral therapy (ART) has been associated with improvement in several HIV-related outcomes in clinical trials as well as demonstration projects, but how regional and contextual differences may affect the effectiveness of this intervention necessitates further study. In this study of a large, urban, Southern US clinic-based retrospective cohort, we identified 544 patients with a new diagnosis of HIV during 2016 to 2019 and compared HIV care continuum outcomes for the first 12 months of care before and after rapid start implementation. Kaplan-Meier time-to-event curves were used to summarize time to virologic suppression, and stepwise Cox, linear, and logistic regression models were used to create multivariate models to evaluate the association between rapid start and time to virologic suppression, medication adherence, and retention in care and sustained virologic suppression, respectively. We found that rapid start was significantly associated with improved medication adherence scores (+15.37 points, 95% confidence interval [CI] 9.36-21.39, P < .01) and retention in care (adjusted odds ratio = 1.51, 95% CI 1.05-2.19, P = .03). Time to virologic suppression (median 2.46 months before, 2.56 months after rapid start) and sustained virologic suppression were not associated with rapid start in our setting. Though rapid start was associated with improved medication adherence and retention in care, more support may be needed to achieve the same outcomes seen in other studies and sustained over the entire HIV care continuum, especially in settings with significant patient and systemic barriers to care such as unstable housing, lack of Medicaid expansion, and frequent coverage interruptions.
Keywords: rapid start, rapid initiation of ART, HIV care continuum
Introduction
Despite the wide availability of HIV care and antiretroviral therapy (ART) in the United States, gaps in the HIV care continuum persist. 1 People with HIV who are not linked to care, do not receive ART or do not achieve viral suppression experience higher morbidity, mortality, 2 and have a higher risk of transmitting HIV to others. 3 In order to end the HIV epidemic, increased implementation and evaluation of evidence-based interventions in real-world settings are critical to improving the HIV care continuum across the United States. 4
In the last decade there has been increasing evidence that immediate initiation of antiretroviral therapy (rapid start) after HIV diagnosis compared to delaying treatment significantly benefits individuals 5 and substantially decreases the transmission of HIV in communities. 3 Randomized controlled trials comparing ART initiation on the day of HIV diagnosis or first clinic presentation to usual care were completed in Haiti, 6 Lesotho, 7 and South Africa 8 demonstrating improved retention in care 6 and virologic suppression.6–8 Based on these data the World Health Organization now defines rapid initiation as within 7 days from the date of HIV diagnosis and has recommended offering ART universally and as early as the same day of diagnosis. 9 Similarly, in the United States, rapid start is becoming more widely adopted after several observational studies have demonstrated the effectiveness of this approach, including in San Francisco, CA,10–12 New Orleans, LA,13,14 and Atlanta, GA. 15 These sites demonstrated shorter time to virologic suppression,11,13,15 high rates of retention in care, 14 and prolonged virologic suppression.10,14 Rapid start is now also recommended by the International Antiviral Society—USA 16 and US Department of Health and Human Services. 17
As momentum builds toward ending the HIV epidemic in the United States, it is important to not only implement rapid start widely, but to assess and refine this intervention to address disparities in HIV-related health outcomes. The United States is a diverse place, with unique policy, access, population, and geographical differences that may influence the deployment and success of rapid start programs. Within the United States, the South is disproportionately affected by HIV, accounting for 52% (n = 16,700) of new diagnoses in 2021, and the state of Texas had the second largest number of new HIV infections. 18 Even within the US South, each state has unique issues that affect the epidemiology of HIV and HIV care. For example, the presence or absence of Medicaid expansion, differences in the administration of the Ryan White HIV/AIDS Program (RWHAP), and the demographic make-up of patient populations (racial/ethnicity, gender, age, primary language) may require unique program adaptations to achieve universal and sustained improvements in clinical outcomes. Moreover, there is structural racism that affects these policies and programs, which continues to drive HIV inequities in the US South. More data, including detailed information about patient demographics and social determinants of health, are needed from diverse settings to improve our understanding and optimization of rapid start.
In this study, we describe and assess a rapid start program in a large, Southern US, urban safety net clinic in Texas, a state without Medicaid expansion. We aimed to: (a) compare HIV care continuum outcomes for all patients with newly diagnosed HIV presenting for care during the first year of the rapid start program to historical controls; and (b) assess for independent associations between various factors (demographic, socio-economic, and behavioral) and HIV clinic outcomes in the setting of rapid start.
Methods
Study Population/Context
Parkland HIV Services (PHS) is the largest HIV program in Dallas, TX and comprises 4 HIV clinics that care for ∼6300 individual patients per year as part of a county safety-net hospital system. About 60% of newly diagnosed patients are referred from within Parkland Health (ED Routine HIV Testing program, Parkland Hospital, ambulatory clinics), and the remainder are from external referrals (clinics, hospitals, jail, health department) from Dallas and surrounding counties. PHS receives funding from the RWHAP parts A-D, and >50% of our patients are funded primarily through RWHAP, while the remainder have Medicare or Medicaid, and a small minority have commercial insurance. The PHS rapid start program was implemented in October 2018. This study presents a retrospective analysis of a clinic-based cohort for HIV care continuum outcomes and medication adherence among newly diagnosed (within 12 months of HIV diagnosis) people with HIV (PWH) who initiated care between October 1, 2016 and September 31, 2019 at PHS.
Rapid Start Program Protocol
After referral to PHS, newly diagnosed patients complete the following: (1) a clinic intake by case management (typically same day as referral, at most within 7 days) for confirmation of HIV diagnosis, post-test counseling and assessment of physical and psychosocial needs; (2) a financial counselor visit to assess medical coverage and apply for appropriate programs, though not a criterion for the initial clinic visit since newly diagnosed patients in Texas are able to obtain conditional RWHAP coverage for 30 days; (3) a medical provider visit the same day whenever possible, for which 2 to 4 appointment slots are reserved per day; (4) same day ART prescription unless there is a contraindication; (5) a meeting with a medication access specialist, who ensures immediate and ongoing coverage of medications through a combination of insurance, pharmaceutical assistance programs, and the AIDS Drug Assistance Program (ADAP); (6) bloodwork; and (7) pick-up ART at the pharmacy. Initial ART selection is left up to the evaluating clinician, but most patients were started on bictegravir/emtricitabine/tenofovir alafenamide. Patients typically return to clinic 4 to 6 weeks later for lab work and see a provider shortly thereafter.
Data Collection
Data were collected from the electronic medical record (EMR), Epic (Verona, WI). All endpoints as well as demographics and baseline characteristics were abstracted from routinely collected data in the EMR.
Baseline Characteristics
Age was determined on the date of the first clinic visit. Gender was defined as male, female, or transgender. Race and ethnicity were combined, and patients were stratified into non-Hispanic Black, non-Hispanic White, and Hispanic/other. Primary language was defined as English or non-English. HIV risk factor was categorized into either men who have sex with men (MSM), heterosexual, or injection drug use (IDU) or other. IDU was considered the primary risk factor for those who reported multiple risk factors. Marital status was considered as single/separated for those who reported being single, widowed, legally separated, divorced, other, or unknown. Highest education level was classified as less than high school, completed high school (including GED), or greater than high school. Insurance was classified as charity (including RWHAP)/self-pay (referring to those whose coverage has lapsed), Medicare/Medicaid, and commercial insurance. History of mental illness and substance use, as well as monthly family income were determined by a case manager as part of the intake process. Those who were unstably housed included those who reported that they were homeless, lived in shelters, substance use disorder treatment facilities, or transitional housing. Initial CD4 count was used to identify those with baseline <200 cells/µL. As a marker of concern about HIV disclosure and stigma, we included an indicator variable for those who requested confidentiality with phone calls.
Outcomes
Linkage to care is defined as completing a visit with an HIV provider within 12 months of case management intake. Initiated on ART is defined as having any prescription for ART sent to a pharmacy within 12 months of case management intake. Time to virologic suppression was defined as the number of days between intake visit with case management and the first VL <200 copies/mL. Retained in care (“annual retention in care”) is defined by the US Health Resources and Services Administration (HRSA) and refers to the percentage of patients with a diagnosis of HIV who had at least 2 HIV medical care encounters (medical visit with a prescribing provider or HIV viral load test (VL)) at least 90 days apart within a 12-month measurement year. At least 1 of the 2 HIV medical care encounters needs to be a medical visit with a provider with prescribing privileges. 19 Continuous retention in care was also calculated using the HRSA HIV medical visit frequency performance measure which requires at least one medical visit in each 6-month period with a minimum of 60 days between medical visits. 19 Medication adherence is defined according to the Parkland Score for Adherence to Medication (P-SAM), a score from 0 to 100 that represents the proportion of days the patient has had a medication available over a 12-month period. It is based on pharmacy pick-up data and was calculated at 12 months after case management intake by dividing the actual number of days the medication has been dispensed by the potential number of days dispensed since the medication was prescribed. P-SAM scores are connected to the majority of local commercial pharmacies as well as our Parkland pharmacies. 20 Maintained ART pickup at 12 months is defined as having a recorded pharmacy pickup between 6 and 12 months after case management intake, according to the P-SAM score. Sustained virologic suppression is defined as a VL <200 copies/mL between 6 and 12 months after case management intake date. Those without a VL measurement in this period are considered to not be suppressed.
All endpoints are measured from the date of case management intake since this represents the first time the patient interfaces with the clinic.
Data Analysis
Patients were stratified based on whether they enrolled in our clinic before rapid start (October 1, 2016 to September 31, 2018) or after implementation of the rapid start protocol (October 1, 2018 to September 31, 2019). Outcomes data were collected for the first 12 months after each patient's case management intake. Baseline characteristics are reported as proportions based on available data for each variable. Since nearly 100% achieved linkage to care and were initiated on ART within 12 months of our study, pre-rapid start and rapid start groups were compared with Chi-squared tests only for these 2 outcomes, without multivariate modeling. Stepwise Cox, linear, and logistic regression models were used to create multivariate models to evaluate the association of rapid start on time to virologic suppression, medication adherence (P-SAM) score, and retention in care, continuous retention in care, and sustained virologic suppression, respectively. Stepwise models were created using the baseline variables that had a univariate association with the outcome in which P ≤ .20, and where one's pre-rapid or post-rapid start status was forced into the models in order to evaluate the strength of the association between our rapid start program and the outcomes while adjusting for significant covariates. Kaplan-Meier time-to-event curves were used to summarize the distribution of times to the first event of virologic suppression. All analyses were performed in SAS, version 9.4.
Ethical Approval and Informed Consent
The Institutional Review Board at UT Southwestern waived the need for ethics approval and the need to obtain consent for the collection, analysis, and publication of the retrospectively obtained and anonymized data for this noninterventional study.
Results
Demographics and Baseline Characteristics
There were 298 patients who initiated care in PHS between October 1, 2016 and September 30, 2018 (pre-rapid start), and 246 who initiated care between October 1, 2018 and September 30, 2019 (postimplementation of rapid start). Most of the demographic characteristics were similar between the 2 groups: overall, they had a mean age of 35.2 years, 75.6% were male, 46% were Black, 39.5% identified as Hispanic, and 22.2% of patients were non-English speakers, most of whom spoke Spanish as their primary language. The most common risk factor for HIV was being an MSM (55.8%), followed by heterosexual (39.1%), and IDU (5.1%). Over half (58.2%) reported their highest education level as high school or less and mean monthly family income was $930.10. The proportion presenting with CD4 < 200 at baseline was stable around 28% before and after rapid start.
Significant demographic differences between pre-rapid start and rapid start included a higher proportion of single/separated PWH (90.2% vs 84.6%), charity/self-pay (80.9% vs 71.7%), mental illness (23.8% vs 16.2%), and unstable housing (11.6% vs 5.1%) in the rapid start group compared to the pre-rapid start. A significantly lower proportion of PWH also requested confidentiality with phone calls under the rapid start program compared to pre-rapid start (77.6% vs 90.9%) (Table 1).
Table 1.
Baseline Characteristics for Individuals Newly Diagnosed With HIV Before and After Implementation of Rapid Start.
| Characteristic | Total n = 544 |
Pre-rapid start n = 298 |
Rapid start n = 246 |
P-valuea |
|---|---|---|---|---|
| Mean age (SD) | 35.2 (11.1) | 35.5 (11.2) | 34.8 (11.1) | .42 |
| Gender | .78 | |||
| Male | 411 (75.6%) | 225 (75.5%) | 186 (75.6%) | |
| Female | 114 (21.0%) | 64 (21.5%) | 50 (20.3%) | |
| Transgender | 19 (3.5%) | 9 (3.0%) | 10 (4.1%) | |
| Race/ethnicity | .14 | |||
| Non-Hispanic Black | 250 (46.0%) | 126 (42.3%) | 124 (50.4%) | |
| Hispanic/other | 215 (39.5%) | 128 (43.0%) | 87 (35.4%) | |
| Non-Hispanic White | 79 (14.5%) | 44 (14.8%) | 35 (14.2%) | |
| Primary language | .57 | |||
| English | 423 (77.8%) | 229 (76.9%) | 194 (78.9%) | |
| Non-English | 121 (22.2%) | 69 (23.2%) | 52 (21.1%) | |
| HIV risk factor | .12 | |||
| MSM | 298 (55.8%) | 163 (55.4%) | 135 (56.3%) | |
| Heterosexual | 209 (39.1%) | 121 (41.2%) | 88 (36.7%) | |
| IDU/Other | 27 (5.1%) | 10 (3.4%) | 17 (7.1%) | |
| Marital status: single/separated | 474 (87.1%) | 252 (84.6%) | 222 (90.2%) | .05 |
| Highest education level | .92 | |||
| Less than high school | 110 (21.0%) | 59 (20.3%) | 51 (21.8%) | |
| Completed high school | 195 (37.2%) | 109 (37.6%) | 86 (36.8%) | |
| Greater than high school | 219 (41.8%) | 122 (42.1%) | 97 (41.5%) | |
| Insurance | .05 | |||
| Charity/self-pay | 398 (76.0%) | 203 (71.7%) | 195 (80.9%) | |
| Medicare/medicaid | 77 (14.7%) | 49 (17.3%) | 28 (11.6%) | |
| Commercial | 49 (9.4%) | 31 (11.0%) | 18 (7.5%) | |
| History of mental Illness | 104 (19.6%) | 47 (16.2%) | 57 (23.8%) | .03 |
| History of substance use | 158 (29.9%) | 86 (29.7%) | 72 (30.3%) | .88 |
| Homeless or unstably housed | 43 (8.0%) | 15 (5.1%) | 28 (11.6%) | <.01 |
| Employed | 227 (41.7%) | 127 (42.6%) | 100 (40.7%) | .64 |
| Mean monthly family income (SD) | $930.10 (3387.24) | $1012.36 (2756.21) | $830.46 (4025.11) | .55 |
| Baseline CD4 < 200 cells/mm3 | 153 (28.3%) | 85 (28.6%) | 68 (28.0%) | .87 |
| Requested confidentiality with phone calls | 452 (84.8%) | 261 (90.9%) | 191 (77.6%) | <.01 |
Percentages are based on the denominator of persons with available data for each characteristic.
Student's t-tests and chi-square tests were performed for continuous and categorical data, respectively.
Abbreviations: IDU, injection drug use; MSM, men who have sex with men; SD, standard deviation.
HIV Care Continuum
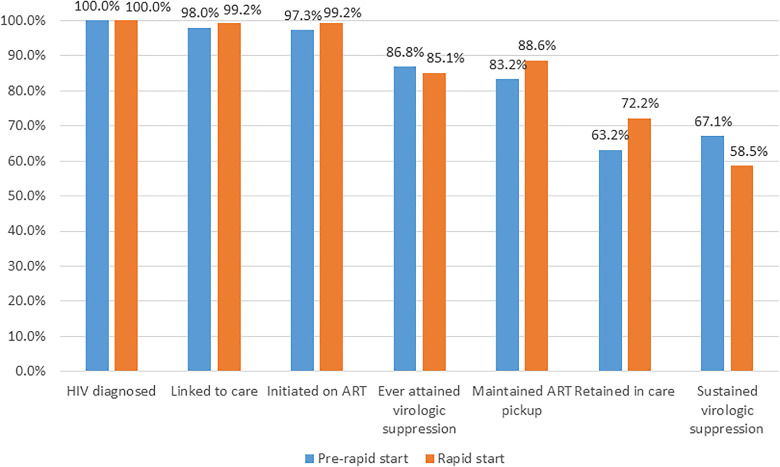
The overall HIV care continuum is depicted in Figure 1, stratified by pre-rapid start and rapid start periods.
Figure 1.
HIV care continuum for individuals newly diagnosed with HIV before and after rapid start, 12 month outcomes. HIV diagnosed includes all individuals with a confirmed new diagnosis of HIV within 12 months who initiated care by completing a case management (CM) intake, n = 298 pre-rapid start, and n = 246 rapid start. Linked to care = completing a visit with an HIV provider. Initiated on antiretroviral therapy (ART)= having any prescription for ART sent to a pharmacy. Ever attained virologic suppression = ever attained an HIV viral load <200 copies/mL. Maintained ART pickup = having any recorded ART pharmacy pickups between 6 and 12 months after CM intake. Retained in care = completing at least 2 HIV medical care encounters (medical visit with a prescribing provider or HIV viral load test) at least 90 days apart, at least one of which is a medical visit. Sustained virologic suppression = measured HIV viral load <200 copies/mL between 6 and 12 months after CM intake. All outcomes take place within 12 months of CM intake unless otherwise specified.
Linkage to Care
In the pre-rapid start and rapid start cohorts, 98.0% and 99.2% (P = .30), respectively, were linked to care within 12 months (Figure 1). Under rapid start, a significantly larger proportion of patients were linked to care within 7 days of diagnosis (18.4% vs 1.0%, P < .01) and 30 days of diagnosis (50.4% vs 20.5%, P < .01). The median time from diagnosis to linkage to care was 30 days under rapid start, compared to 71 days before rapid start. When measuring linkage to care from the date of case management intake, 84.8% were linked to care within 7 days under rapid start compared to 18.8% pre-rapid start (P < .01) (Table 2).
Table 2.
Selected Linkage and ART Initiation Outcomes Before and After Initiation of Rapid Start.
| Outcome | Total n = 544 |
Pre-rapid start n = 298 |
Rapid start n = 246 |
P-valuea |
|---|---|---|---|---|
| Linked to care within 7 days of diagnosis | 48 (8.9%) | 3 (1.0%) | 45 (18.4%) | <.01 |
| Linked to care within 30 days of diagnosis | 183 (34.1%) | 60 (20.5%) | 123 (50.4%) | <.01 |
| Linked to care within 7 days of CM intake | 262 (48.8%) | 55 (18.8%) | 207 (84.8%) | <.01 |
| Median (IQR) days from diagnosis to linkage to care | 53 (23, 173) | 71 (37, 222) | 30 (11, 94) | <.01 |
| Median (IQR) days from CM intake to ART initiation | 8 (0,16) | 14 (8, 23) | 0 (0, 5) | <.01 |
Wilcoxon rank-sum tests and chi-square tests were used for continuous and categorical data, respectively.
Abbreviations: ART, antiretroviral therapy; CM = case management; IQR, interquartile range.
Initiated on ART
Within the first 12 months, 97.3% (pre-rapid start) and 99.2% (rapid start) had been initiated on ART (P = .12) (Figure 1). The median time from case management intake to ART initiation decreased from 14 days to 0 days under rapid start (P < .01) (Table 2).
Time to Virologic Suppression
There was no significant difference in the time to virologic suppression (P = .51), with a median of 2.46 months before and 2.56 months after rapid start was begun (Figure 2); the probability of ever attaining virologic suppression at 12 months was 86.8% and 85.1%, respectively (Figure 1). In the Cox regression model, rapid start was not significantly associated with the time to virologic suppression, but history of substance abuse (adjusted hazard ratio [aHR] 0.73; 95% confidence interval [CI] 0.59,0.90; P < .01) and unstable housing (aHR 0.51; 95% CI 0.35,0.77; P < .01) were significantly associated with less likelihood of achieving virologic suppression (Table 3A).
Figure 2.
Time from clinic enrollment/case management intake to virologic suppression, before and after implementation of rapid start.
Table 3.
Multivariate Analyses for Various HIV Care Outcomes.
| (A) Time to virologic suppression, stepwise Cox proportional hazards model, n = 521 | |||
|---|---|---|---|
| Characteristic | aHR | 95% CI | P-value |
| Rapid start | 0.96 | (0.79, 1.16) | .67 |
| History of substance use | 0.73 | (0.59, 0.90) | <.01 |
| Unstably housed | 0.51 | (0.35, 0.77) | <.01 |
| (B) Medication adherence score, stepwise linear regression, n = 459 | |||
| Characteristic | Estimate ± std err | 95% CI | P-value |
| Rapid start | 15.37 ± 3.06 | (9.36, 21.39) | <.01 |
| Race/Ethnicity | |||
| Hispanic/Other | ref | ||
| Non-Hispanic Black | −14.10 ± 3.38 | (−20.74, −7.46) | <.01 |
| Non-Hispanic White | −14.71 ± 4.74 | (−24.06, −5.45) | <.01 |
| Unstably housed | −14.89 ± 5.85 | (−26.39, −3.39) | .01 |
| Gender | |||
| Female | ref | ||
| Male | −5.80 ± 3.90 | (−13.46, 1.85) | .14 |
| Transgender | −22.83 ± 8.64 | (−39.80, −5.85) | <.01 |
| (C) Retention in care at 12 months, stepwise logistic regression, n = 537 | |||
| Characteristic | OR | 95% CI | P-value |
| Rapid start | 1.51 | (1.05, 2.19) | .03 |
| (D) Continuous retention in care at 12 months, stepwise logistic regression, n = 536 | |||
| Characteristic | aOR | 95% CI | P-value |
| Rapid start | 0.78 | (0.53, 1.16) | .22 |
| Race/ethnicity | |||
| Hispanic/other | ref | ||
| Non-Hispanic Black | 0.48 | (0.31, 0.77) | <.01 |
| Non-Hispanic White | 0.34 | (0.19, 0.61) | <.01 |
| Unstably housed | 0.41 | (0.21, 0.79) | <.01 |
| Gender | |||
| Female | ref | ||
| Male | 0.50 | (0.29, 0.84) | .01 |
| Transgender | 0.57 | (0.18, 1.84) | .35 |
| (E) Sustained virologic suppression at 12 months, stepwise logistic regression, n = 524 | |||
| Characteristic | aOR | 95% CI | P-value |
| Rapid start | 0.75 | (0.52, 1.09) | .13 |
| Race/ethnicity | |||
| Hispanic/other | ref | ||
| Non-Hispanic Black | 0.48 | (0.32, 0.73) | <.01 |
| Non-Hispanic White | 0.29 | (0.17, 0.51) | <.01 |
| Coverage | |||
| Charity/self-pay | ref | ||
| Commercial | 2.52 | (1.23, 5.16) | .01 |
| Medicaid/medicare | 1.70 | (0.98, 2.94) | .06 |
Abbreviations: aHR, adjusted hazard ratio; aOR, adjusted odds ratio; CI, confidence interval.
Medication Adherence
There was not a significant difference in the proportion who maintained ART pickups at 6 to 12 months (88.6% rapid start vs 83.2% pre-rapid start, P = .07) (Figure 1). However, in the multivariate model, rapid start was associated with a 15.37 point (95% CI 9.36,21.39; P < .01) increase in medication adherence score. Non-Hispanic Black patients and non-Hispanic White patients had significantly lower medication adherence scores (–14.10; 95% CI −20.74,–7.46; P < .01 and −14.71; 95% CI −24.06,−5.45; P < .01, respectively), as did unstably housed (–14.89; 95% CI −26.39,–3.39; P = .01) and transgender individuals (–22.83; 95% CI −39.80,–5.85; P < .01) compared to their counterparts (Table 3B).
Retained in Care
Overall, 63.2% were retained in care before rapid start and 72.2% during rapid start (P = .03) (Figure 1). Rapid start was associated with significantly higher odds of being retained in care at 12 months (odds ratio [OR] 1.51; 95% CI 1.05,2.19; P = .03) (Table 3C). No other variables met criteria for inclusion in the stepwise model.
Continuous Retention in Care
Under this alternate metric, 67.1% achieved continuous retention in care under rapid start compared to 75.8% pre-rapid start (P = .02) (data not shown). In the multivariate model, rapid start was not significantly associated with higher odds of continuous retention in care at 12 months (P = .22). However, being non-Hispanic Black or non-Hispanic White was associated with significantly lower odds of continuous retention in care compared to those identifying as Hispanic/other (adjusted OR [aOR] 0.48; 95% CI 0.31,0.77; P < .01 and 0.34; 95% CI 0.19,0.61; P < .01, respectively). Being homeless or unstably housed was also associated with lower odds of continuous retention in care (aOR 0.41; 95% CI 0.21,0.79; P < .01). Finally, males had lower odds of continuous retention in care compared to females (aOR 0.50; 95% CI 0.29,0.84; P = .01) (Table 3D).
Sustained Virologic Suppression
Rapid start was characterized by lower rates of sustained virologic suppression compared to the pre-rapid start era (58.5% vs 67.1%, P = .04) (Figure 1). In multivariate analysis, rapid start was no longer significantly associated with sustained virologic suppression (aOR 0.75; 95% CI 0.52,1.09; P = .13). Both non-Hispanic Black and non-Hispanic White patients had lower odds of sustained virologic suppression (aOR 0.48; 95% CI 0.32,0.73; P < .01 and 0.29; 95% CI 0.17,0.51; P < .01, respectively), while those with either commercial (aOR 2.52; 95% CI 1.23,5.16; P = .01) or Medicaid/Medicare (aOR 1.70; 95% CI 0.98,2.94; P = .06) had higher odds of sustained virologic suppression at 12 months than other groups (Table 3E).
Discussion
In our large, clinic-based cohort in the US South, we present a comprehensive analysis of the HIV care continuum before and after rapid start implementation and found that rapid start was associated with improved medication adherence and retention in care. Time to virologic suppression, sustained virologic suppression, and continuous retention in care were not associated with rapid start in our setting. To our knowledge, our findings represent the largest US clinic-based cohort of rapid start and provide additional real-world data on whether rapid start is effective, and outcomes are durable. Additionally, ours is the only rapid start study to include medication adherence as an outcome, which in other studies has been a key proxy for virologic suppression, 21 especially if individuals are not consistently attending clinic and lab visits.
Due to the lack of a standard definition 22 and limited existing data on the complete HIV care continuum in rapid start, 14 we evaluated 2 different measures of retention in care. In our study, rapid start was associated with improved retention in care when measured by the current HRSA definition (one clinic visit and one viral load at least 90 days apart) but did not impact continuous retention in care (one medical visit in each 6-month period at least 60 days apart). These findings may be explained by the observation that patients in our rapid start era were less likely to come to clinic appointments, but still completed viral loads and thus satisfied the definition of retention in care.
Contrary to prior observational studies on rapid start in the United States,11,13,15,23 we did not find a significant change in time to virologic suppression or sustained virologic suppression. A possible explanation is that our rapid start protocol does not dictate the exact timing of the first follow-up viral load, instead leaving it to the discretion of the provider. The mean time between the first ART prescription and the subsequent VL measurement was 1.97 months pre-rapid start and 2.19 months after rapid start implementation, likely missing an earlier timepoint of initial suppression among the rapid start group which may occur as early as 28 days 24 after starting an integrase strand transfer inhibitor. Furthermore, it is possible that the rate of sustained virologic suppression may underestimate actual virologic suppression (with missing viral load = failure). Since the rapid start cohort had improved medication adherence, it may be that they prioritized medication pickups over lab testing. This suggests that more support is needed beyond the initial rapid start visit to help patients continue to remain engaged in their clinical care.
Our outcomes may also be partially explained by the unique context of our clinic and the demographics of our patient population. Unlike most other published US rapid start demonstration projects, our clinic is in a state without Medicaid expansion, with the majority of newly diagnosed patients relying on the RWHAP for outpatient coverage and on ADAP for medication coverage (which both require recertification every 6 months). Furthermore, ADAP in Texas takes over 8 weeks to process the initial application, 25 requiring enrollment in pharmaceutical assistance programs until ADAP is approved. Challenges in obtaining, switching between, and maintaining these coverage programs may explain why our outcomes may be less favorable relative to other published US cohorts. Additionally, during the rapid start era, the study cohort had a higher prevalence of comorbid psychosocial issues including mental illness, unstable housing, and lack of medical insurance. The number of newly diagnosed individuals initiating care in our clinic was much higher in the rapid start era compared to before: 298 in 24 months pre-rapid start versus 246 in just 12 months after rapid start. These numbers are much larger than in other studies,10,11,13,15 straining limited resources, and also suggest that the rapid start protocol is capturing a larger and more vulnerable group of patients. Many in this group may never have made it to the first medical appointment in the pre-rapid start paradigm, supported by a 2014 study in our clinic which found that 26% missed this critical initial visit. 26
Our study does have some limitations. The study is based on a single site, which may limit its generalizability, though PHS is likely comparable to other urban clinics at the epicenter of the HIV epidemic. The single site data source also means that we were unable to account for people who moved or transferred care elsewhere. Second, as with any study using a historical comparator group, we are unable to account for unmeasured historical confounders that may differ between the 2 groups. However, our authors include 2 clinicians who were in the clinic during the entire study period, and there are no historical confounders that they are aware of. Next, our expanded definition of rapid start, which aimed to have patients seen within 7 days of case management intake (instead of date of diagnosis) may incorporate more patients than other studies, since accepting referrals from all across Dallas County may result in delays in care engagement. In order for clinics such as ours to fully realize true rapid start, a jurisdiction-wide coordinated effort may be needed, such as that seen in San Francisco.11,12 Finally, the COVID-19 pandemic, during which routine lab and clinic visits were deferred, may have impacted the latter rapid-ART era outcomes, since our follow-up period for our rapid start group continued through September 2020.
While rapid start protocols share a common focus on starting PWH on ART within days of diagnosis, it is important to recognize the heterogeneity in the processes of the published US demonstration projects to date, including observed administration of first dose of ART in clinic,11,13 providing a 5-day ART starter pack,10,11 variable follow-up practices including follow-up calls from clinical staff withing 1 to 2 weeks,10,11 and patient navigation. 13 As a result, it is unclear which components of these interventions are most effective, and which processes best support ongoing retention and virologic suppression. Future evaluations should identify best practices for successful and sustained rapid start programs, acknowledging that there is likely a “care bundle effect.” 27
Conclusions
Our study has shown that rapid start is associated with key outcomes of improved medication adherence and retention in care in a large, safety-net clinic in a high-incidence jurisdiction prioritized by the federal Ending the HIV Epidemic initiative. Rapid start connected a greater proportion, and a potentially harder-to-reach group, of newly diagnosed PWH to ART. However, though comprehensive, immediate attention to HIV appears to be effective in achieving positive clinical outcomes for many PWH, more support is needed to sustain the entire HIV care continuum, particularly for vulnerable groups such as those with unstable housing or interruptions in insurance coverage. Future studies are needed to determine the optimal strategies to provide longitudinal support after rapid start and ensure universal improvement in long-term outcomes.
Footnotes
Authorship Contribution Statement: JYC was involved in conceptualization, methodology, writing—original draft preparation, and funding acquisition. CA contributed to methodology, formal analysis, writing—review & editing. AG was involved in formal analysis, software, data curation, writing—review & editing. AEN was involved in conceptualization, methodology, writing—review & editing.
JYC and AEN are recipients of an Investigator Sponsored Research grant from Gilead Sciences which funded this project.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by an Investigator Sponsored Research grant from Gilead Sciences (grant number IN-US-380-5711).
ORCID iD: Jeremy Y. Chow https://orcid.org/0000-0002-3027-3806
References
- 1.Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas, 2019. HIV Surveillance Supplemental Report. 2021;26(2). http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Accessed Accessed 6/27/2023. [Google Scholar]
- 2.Lee JS, Cole SR, Richardson DB, et al. Incomplete viral suppression and mortality in HIV patients after antiretroviral therapy initiation. AIDS. 2017;31(14):1989–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen MS, Chen YQ, McCauley M, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med. 2016;375(9):830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fauci AS, Redfield RR, Sigounas G, Weahkee MD, Giroir BP. Ending the HIV epidemic: a plan for the United States. JAMA. 2019;321(9):844–845. [DOI] [PubMed] [Google Scholar]
- 5.Group ISS, Lundgren JD, Babiker AG, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373(9):795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koenig SP, Dorvil N, Devieux JG, et al. Same-day HIV testing with initiation of antiretroviral therapy versus standard care for persons living with HIV: a randomized unblinded trial. PLoS Med. 2017;14(7):e1002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Labhardt ND, Ringera I, Lejone TI, et al. Effect of offering same-day ART vs usual health facility referral during home-based HIV testing on linkage to care and viral suppression among adults with HIV in Lesotho: the CASCADE randomized clinical trial. JAMA. 2018;319(11):1103–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosen S, Maskew M, Fox MP, et al. Initiating antiretroviral therapy for HIV at a patient’s first clinic visit: The RapIT randomized controlled trial. PLoS Med. 2016;13(5):e1002015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guidelines for Managing Advanced HIV Disease and Rapid Initiation of Antiretroviral Therapy . 2017. WHO Guidelines Approved by the Guidelines Review Committee .
- 10.Coffey S, Bacchetti P, Sachdev D, et al. RAPID antiretroviral therapy: high virologic suppression rates with immediate antiretroviral therapy initiation in a vulnerable urban clinic population. AIDS. 2019;33(5):825–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pilcher CD, Ospina-Norvell C, Dasgupta A, et al. The effect of same-day observed initiation of antiretroviral therapy on HIV viral load and treatment outcomes in a US public health setting. J Acquir Immune Defic Syndr. 2017;74(1):44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bacon O, Chin J, Cohen SE, et al. Decreased time from human immunodeficiency virus diagnosis to care, antiretroviral therapy initiation, and virologic suppression during the citywide RAPID initiative in San Francisco. Clin Infect Dis. 2021;73(1):e122–e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halperin J, Butler I, Conner K, et al. Linkage and antiretroviral therapy within 72 hours at a federally qualified health center in New Orleans. AIDS Patient Care STDS. 2018;32(2):39–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halperin J, Conner K, Butler I, et al. A care continuum of immediate ART for newly diagnosed patients and patients presenting later to care at a federally qualified health center in New Orleans. Open Forum Infect Dis. 2019;6(4):ofz161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colasanti J, Sumitani J, Mehta CC, et al. Implementation of a rapid entry program decreases time to viral suppression among vulnerable persons living with HIV in the southern United States. Open Forum Infect Dis. 2018;5(6):ofy104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gandhi RT, Bedimo R, Hoy JF, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2022 recommendations of the international antiviral society-USA panel. JAMA. 2023;329(1):63–84. [DOI] [PubMed] [Google Scholar]
- 17.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Department of Health and Human Services; 2023. Accessed August 16, 2023. https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv
- 18.Centers for Disease Control and Prevention. Estimated HIV incidence and prevalence in the United States, 2017–2021. HIV Surveillance Suppll Rep. 2023;28(3). May 2023. Accessed December 12, 2023. http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html [Google Scholar]
- 19.Health Resources and Services Administration. HIV/AIDS Bureau Performance Measures. 2019. Accessed November 22, 2022. https://hab.hrsa.gov/sites/default/files/hab/clinical-quality-management/coremeasures.pdf
- 20.Alvarez K, Townsend C, McNulty JN, et al. Design and implementation of an electronic tool to measure medication adherence at the point of care. Clin Diabetes. 2020;38(4):382–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saberi P, Caswell N, Amodio-Groton M, Alpert P. Pharmacy-refill measure of adherence to efavirenz can predict maintenance of HIV viral suppression. AIDS Care. 2008;20(6):741–745. [DOI] [PubMed] [Google Scholar]
- 22.Rehman N, Wu M, Garcia C, et al. Measures of retention in HIV care: a study within a review. AIDS Patient Care STDS. 2023;37(4):192–198. [DOI] [PubMed] [Google Scholar]
- 23.O'Shea JG, Gallini JW, Cui X, Moanna A, Marconi VC. Rapid antiretroviral therapy program: development and evaluation at a veterans affairs medical center in the southern United States. AIDS Patient Care STDS. 2022;36(6):219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walmsley SL, Antela A, Clumeck N, et al. Dolutegravir plus Abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med. 2013;369(19):1807–1818. [DOI] [PubMed] [Google Scholar]
- 25.THMP- Moving Forward Together - FAQ. Accessed May 28, 2023. https://www.dshs.texas.gov/hiv-std-program/texas-dshs-hiv-std-program-texas-hiv-medication-program/texas-hiv-medication-program-10
- 26.Nijhawan AE, Liang Y, Vysyaraju K, et al. Missed initial medical visits: predictors, timing, and implications for retention in HIV care. AIDS Patient Care STDS. 2017;31(5):213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lavallee JF, Gray TA, Dumville J, Russell W, Cullum N. The effects of care bundles on patient outcomes: a systematic review and meta-analysis. Implement Sci. 2017;12(1):142. [DOI] [PMC free article] [PubMed] [Google Scholar]