Abstract
Background:
Frequent failures observed in some trials comparing the efficacy and safety of osimertinib plus bevacizumab to osimertinib monotherapy in advanced non-small-cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) alterations have brought questions.
Objectives:
To evaluate the efficacy and safety of these two treatment regimens in advanced NSCLC patients harboring EGFR mutations.
Design:
This study is a systematic review and meta-analysis.
Data sources and methods:
PubMed, Embase, Cochrane Library, Web of Science, CNKI, Wanfang, and VIP databases were extensively searched for relevant randomized controlled trials (RCTs) on 14 May 2023. Two researchers independently screened the literature, assessed quality, and extracted data. The primary outcomes were progression-free survival (PFS), overall survival (OS), and objective response rate (ORR). The secondary outcomes were adverse events (AEs) and PFS stratified by patients’ characteristics. STATA 17.0 software (StataCorp LLC, USA) was adopted for meta-analysis.
Results:
A total of four RCTs involving 390 patients were included. Overall, the risk of bias across the studies was moderate to low. Pooled results showed that compared to osimertinib alone, the addition of bevacizumab to osimertinib failed to show prolongation of PFS [hazard ratio (HR) = 1.00, 95% confidence interval (CI): 0.78–1.27], OS (HR = 1.01, 95% CI: 0.73–1.41), or improvement of the ORR (risk ratio = 1.12, 95% CI: 0.90–1.38), while an increased incidence of some AEs was observed, such as nausea, oral mucositis, hypertension, and proteinuria. Notably, combination treatment did significantly prolong the PFS in the subset of smokers (HR = 0.64, 95% CI: 0.44–0.94). A mild trend toward PFS benefit under the combined regimen was also noted in patients with brain metastases and first-line treatment, though not reaching statistical significance.
Conclusion:
Based on the available evidence, the addition of bevacizumab to osimertinib could not provide additional survival benefits with higher but manageable toxicity for EGFR-mutant NSCLC patients. Osimertinib monotherapy remains the prioritized treatment. Further investigation is warranted.
Keywords: bevacizumab, EGFR, non-small-cell lung cancer, osimertinib, TKI
Introduction
Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) have dramatically revolutionized the therapeutic landscape of advanced non-small-cell lung cancer (NSCLC) with driver molecular alterations and have been established as the standard first-line treatment option for this patient population over recent decades. 1 Though the majority of NSCLC patients harboring EGFR mutation initially showed a favorable response to first- and second-generation EGFR-TKIs, they eventually experience disease progression, with acquired EGFR exon 20 T790M being the most common mechanism of resistance. 2 Osimertinib, a third-generation, irreversible EGFR-TKI, has demonstrated activity in both EGFR sensitizing (19del/L858R) and T790M mutations. Across the FLAURA and AURA3 studies, osimertinib provided superior median progression-free survival (PFS) over comparator EGFR-TKIs (gefitinib/erlotinib; FLAURA) or platinum-doublet chemotherapy (AURA3) in patients with advanced EGFR-mutant NSCLC.3,4 However, the survival data in these trials remained unmet expectations of researchers and patients, prompting ongoing efforts to prolong the treatment duration.
Vascular endothelial growth factor (VEGF) is a crucial mediator involved in the angiogenesis of tumor development and growth. Bevacizumab, the first globally approved antitumor angiogenic monoclonal antibody, specifically targets VEGF-A and blocks the signaling pathway of tumor angiogenic cells. To date, bevacizumab has been combined with multiple types of anticancer treatment, including cytotoxic chemotherapy, molecular targeted therapy, and immunotherapy for additional benefit. EGFR-TKIs for lung cancer are no exception. Preclinical data suggested that EGFR mutation was associated with increased expression of VEGF, and dual blockade of these molecular targets elicited a synergistic effect.5–7 Consecutive clinical studies have also been conducted to evaluate the effectiveness of this combination regimen and performed desirable results in recent years.8,9 Currently, erlotinib in combination with bevacizumab has been recommended as a first-line treatment choice for EGFR-mutated NSCLC by the National Comprehensive Cancer Network Practice Guidelines (Version 3.2023).
Despite its potential benefits, concerns have been raised regarding the effectiveness of the combination of osimertinib and bevacizumab due to frequent failures observed in several clinical trials.10–12 Several past meta-analyses have assessed the efficacy of osimertinib plus bevacizumab but they focused only on the smoker population and included other combination regimens.13–15 There is no relevant systematic review yet evaluating the effectiveness and safety of this combination modality. Herein, we performed a systematic review and meta-analysis focusing on the efficacy and safety of osimertinib plus bevacizumab in patients with advanced EGFR-mutated NSCLC.
Methods
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (see Supplemental Material). 16 The protocol for this study is available on the PROSPERO website with the number CRD42023413697.
Search strategy and study selection
We systematically searched seven databases, namely PubMed, Cochrane Library, Embase, Web of Science, and Chinese databases CNKI, Wanfang, and VIP from their inception dates to 14 May 2023, using the subject terms ‘osimertinib’, ‘bevacizumab’, and ‘carcinoma, non-small-cell lung’ along with their synonyms. In addition, the reference lists of included articles in the final selection were reviewed to find any additional trials omitted in the initial search. Detailed search strategies are reported in Supplemental Table A. Eligibility for this study was limited to randomized controlled trials (RCTs) that compared osimertinib plus bevacizumab against osimertinib alone in patients diagnosed with advanced NSCLC harboring EGFR mutations. We excluded conference abstracts, duplicate publications, studies without available results, and studies with poor design.
Data extraction
Two reviewers (GZ and LG) independently performed data extraction using a specifically pre-adapted form. Discrepancies in the research selection and extracting data were resolved through discussion and consensus or by consulting a third author (JC) when necessary. The following data were extracted from each eligible article: author, publication year, country, treatment arms, treatment line, sample size, age distribution, sex distribution, follow-up time, survival outcomes, and adverse events (AEs). The primary outcomes of interest were PFS, overall survival (OS), and objective response rate (ORR). The secondary outcomes were AEs and PFS stratified by patients’ characteristics. If survival parameters were not explicitly available in the article, the Engauge Digitizer v4.1 software (Mark Mitchell, 2002) was applied to extract data from the Kaplan–Meier survival curves based on the methods of Tierney et al. 17
Quality assessment
The methodological quality of the included RCTs was assessed using the Cochrane Collaboration Risk of Bias (ROB) tool that comprises seven aspects: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases. We considered a study to be of ‘high’ quality when more than four items were assessed as low risk, ‘medium’ quality when two to three items were low risk, and ‘low’ quality when less than two low-risk items or more than one high-risk item. 18 ROB graphs were generated using Revman 5.4 software (The Cochrane Collaboration, 2020).
Statistical analysis
All statistical analyses were carried out using the STATA 17.0 software (StataCorp LLC, USA). To estimate the variation of the treatment effect, subgroup analysis was conducted as per the treatment lines. Furthermore, survival data of subgroups across studies, such as brain metastases (BM) and smoking status, were pooled to evaluate the relative effect of combination therapy. Survival data PFS and OS were expressed as hazard ratio (HR) and 95% confidence intervals (CIs), while risk ratio (RR) and 95% CIs were used as effect size for dichotomous data. Q statistics and I 2 statistics were used to assess heterogeneity across studies. A random effect model was used when substantial heterogeneity was observed (I 2 ⩾ 50%); otherwise, a fixed effects model was applied. A leave-one-out sensitivity analysis was performed to test the possibly substantial impact of individual studies on the synthesized results. All statistical tests were two-sided, and a p value of less than 0.05 was regarded as statistically significant.
Results
Study selection and characteristics
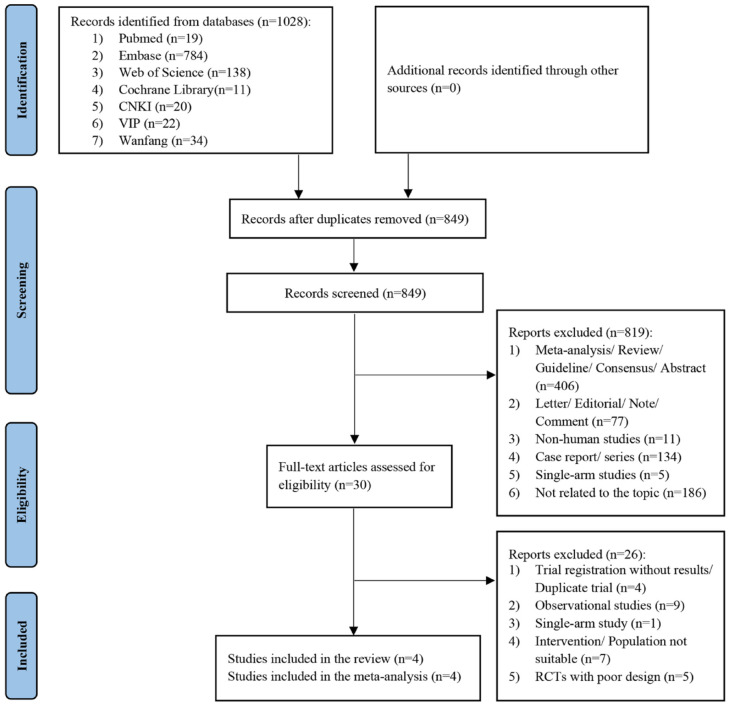
A total of 1028 records were retrieved from seven electronic databases. After removing 179 duplicates, we screened 849 publications for titles and abstracts, of which 819 studies were excluded with reasons, leaving 30 articles to be read in detail. Four RCTs (three from English databases and one from Chinese databases) involving 390 patients were finally included for further analysis.10–12,19 The process of screening and identifying eligible studies is presented in Figure 1.
Figure 1.
Flow diagram of the study selection process for the meta-analysis.
The included studies had publication dates between 2021 and 2022, with sample sizes ranging from 32 to 155. Most recruited patients were from Asia, followed by Europe. Two studies included patients in the first-line setting,12,19 and another two studies10,11 included patients previously treated with first-generation or second-generation EGFR-TKI or cytotoxic chemotherapy. Of the eligible studies, the majority of patients harbored EGFR 19del, L858R, and T790M,10–12 while Feng et al. 19 did not provide a detailed description of concrete types of EGFR mutation. Osimertinib was administered orally at 80 mg once daily in all studies. The experimental arms of most studies used bevacizumab at a regimen of 15 mg/kg intravenously on day 1 of every 3 weeks (q3w),10–12 while one study 19 used intravenous 15 mg/kg as the first dose followed by 7.5 mg/kg q3w. HRs on PFS/OS were obtained directly from three studies, while the PFS of Feng et al. 19 was extracted using the method of Tierney et al. Further characteristics are summarized in Table 1.
Table 1.
Characteristics and bias assessment of each study.
| Study (year) | Country | Arms of treatment | Treatment line | Number of patients (osi + beva/osi) | Age, y (osi + beva/osi) | Gender | Follow-up, m | Outcomes reported | Quality assessment | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Experimental arm | Control arm | osi + beva (M/F) | osi (M/F) | ||||||||
| Akamatsu et al. (2021) | Japan | Osimertinib 80 mg qd + bevacizumab (15 mg/kg d1 + q3w) | Osimertinib 80 mg qd | Pretreated | 81 (40/41) | 68 (43–82)/70 (41–82) | 16/24 | 17/24 | osi + beva 16.0/osi 16.2 a | ①, ②, ③, ④ | Cochrane ROB tool: low risk |
| Soo et al. (2022) | Ireland, The Netherlands, Spain, Korea, Singapore, Switzerland | Osimertinib 80 mg qd + bevacizumab (15 mg/kg d1 + q3w) | Osimertinib 80 mg qd | Pretreated | 155 (78/77) | 68 (34–85)/66 (41/83) | 31/47 | 28/49 | osi + beva 32.6/osi 34.5 a | ①, ②, ③, ④ | Cochrane ROB tool: low risk |
| Kenmotsu et al. (2022) | Japan | Osimertinib 80 mg qd + bevacizumab (15 mg/kg d1 + q3w) | Osimertinib 80 mg qd | Treatment-naïve | 122 (61/61) | 67 (59–74)/66 (60–74) | 24/37 | 23/38 | 30.4 (20.1–32.5) b | ①, ②, ③, ④ | Cochrane ROB tool: low risk |
| Feng et al. (2022) | China | Osimertinib 80 mg qd + bevacizumab (15 mg/kg d1 + 7.5 mg/kg q3w) | Osimertinib 80 mg qd | Treatment-naïve | 32 (16/16) | (>60 y/⩽60 y): (9/7)/(10/6) | 12/4 | 10/6 | 12 c | ①, ③, ④ | Cochrane ROB tool: medium risk |
Outcomes: ①, progression-free survival; ②, overall survival; ③, objective response rate; ④, adverse events.
Median follow-up time.
Median (interquartile range) follow-up time.
This study just reported that the follow-up time was 12 months.
beva, bevacizumab; d1, day 1; F, female; M, male; m, month; osi, osimertinib; qd, once daily; q3w, every 3 weeks; RCT, randomized controlled trial; ROB, risk of bias; y, year.
Quality assessment
In this meta-analysis, the quality of four RCTs was evaluated through the Cochrane ROB tool. Three RCTs were non-masked and considered high risk for performance bias,10–12 while one RCT 19 failed to report adequate blinding methods and was deemed unclear. All RCTs were rated as medium to high quality. The overall quality and details of assessing the ROB are summarized (Table 1; Supplemental Figure A).
Progression-free survival
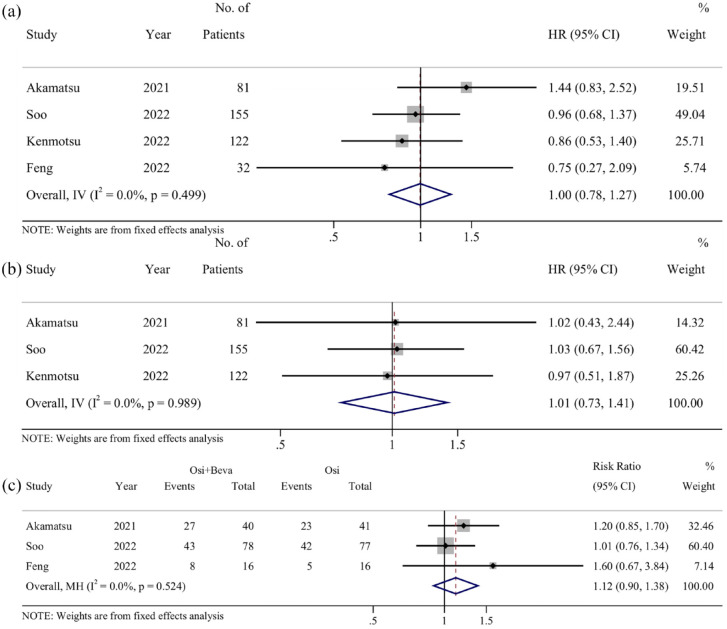
PFS was extracted from all studies10–12,19 with HRs ranging from 0.75 to 1.44. Pooled analysis showed that the HR value was 1.00 (95% CI: 0.78–1.27), (I 2 = 0%, p = 0.499) [Figure 2(a)], indicating that compared to osimertinib monotherapy, osimertinib combined with bevacizumab did not exhibit any advantages in PFS.
Figure 2.
Pooled results of survival outcomes: (a) progression-free survival; (b) overall survival; and (c) objective response rate. HR or risk ratio of less than 1 indicates the results favor osi + beva.
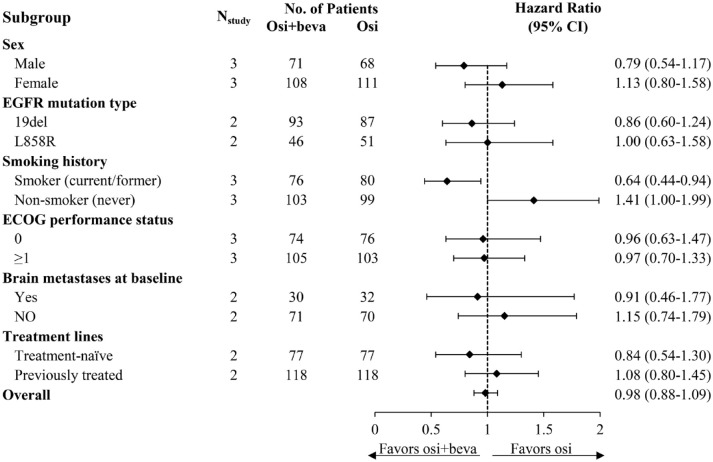
To gain a better understanding of the potential factors influencing PFS, available data from all RCTs were extracted to perform additional subgroup analysis according to patients’ characteristics. The results showed that no statistically significant PFS benefit was apparent in patients without BM, without a smoking history, or in those who were previously treated, as well as in female, patients with EGFR L858R mutation, and in Eastern Cooperative Oncology Group (ECOG) performance status. However, smokers displayed better performance concerning PFS from combination therapy (HR = 0.64, 95% CI: 0.44–0.94, p = 0.292). In addition, a better trend of PFS was found in patients with BM (HR = 0.91, 95% CI: 0.46–1.77, p = 0.744), in the first-line setting (HR = 0.84, 95% CI: 0.54–1.30, p = 0.810), and male (HR = 0.79, 95% CI: 0.54–1.17, p = 0.808) and those harboring EGFR 19del mutation (HR = 0.86, 95% CI: 0.60–1.24, p = 0.273) under combination therapy, albeit not reaching statistical significance. Figure 3 illustrates the details of the subgroup analysis.
Figure 3.
Pooled analysis of subgroups and subgroup analysis as per treatment lines for progression-free survival.
Overall survival
The HRs for OS were reported across three trials,10–12 varying from 0.97 to 1.03. It is worth noting that the OS from one study was immature at the data cutoff. 12 No statistically significant HR was observed between the combination treatment and monotherapy (HR = 1.01, 95% CI: 0.73–1.41) [I 2 = 0%, p = 0.989; Figure 2(b)]. The pooled results revealed that osimertinib combined with bevacizumab did not provide any advantage over osimertinib monotherapy in terms of OS.
Objective response rate
ORR was available in all studies. In the combination arms, the ORR ranged from 50% to 82%, while the osimertinib arms had an ORR varying from 31.3% to 86%. However, one RCT 12 was excluded from the pooled analysis since it reported the ORR based on the per-protocol population rather than the intention-to-treat population. The pooled results showed a RR of 1.12 (95% CI: 0.90–1.38), (I 2 = 0%, p = 0.524), suggesting that no significant difference was observed between the combination therapy and monotherapy with regard to ORR [Figure 2(c)].
Treatment exposure
Three RCTs reported treatment exposure,10–12 with bevacizumab having a median treatment duration of 6.0 months, 10 11.5 cycles, 11 and 33.4 weeks, 12 respectively. In the combination arms, the median duration of exposure to osimertinib across the three studies was 8.5 months, 10 20 cycles, 11 and 94.0 weeks, 12 respectively. The percentage of patients who discontinued bevacizumab due to AEs was 28% in the WJOG8715L study, 10 46% in the BOOSTER study, 11 and 64% in the WJOG9717L study. 12 However, two studies indicated that discontinuation of bevacizumab due to AEs did not affect PFS.10,11 As for post-study further-line treatment, chemotherapy was the common treatment regimen.
Toxicity profiles
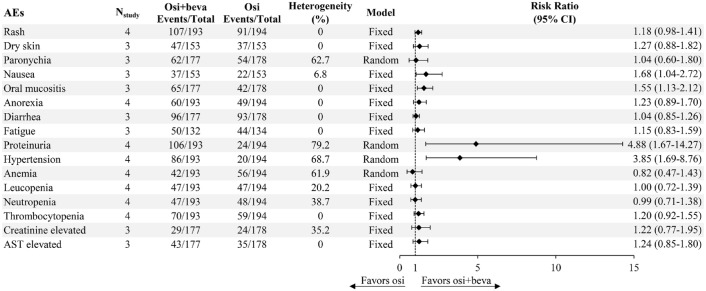
Regarding safety endpoints, we defined rash as including acneiform rash and maculopapular rash in particular. The combination treatment was well-tolerated across the four included RCTs, with no new toxic signals and safety profiles consistent with each agent individually. Common AEs observed in the combination arm were rash (n = 107, 55.4%), proteinuria (n = 106, 54.9%), diarrhea (n = 96, 54.2%), hypertension (n = 86, 44.6%), and oral mucositis (n = 65, 36.7%). Most AEs were generally mild (grades 1–2). Aggregated analyses of all grades of AEs revealed that combination therapy yields a significantly higher incidence of nausea (RR = 1.68, 95% CI: 1.04–2.72, p = 0.342), oral mucositis (RR = 1.55, 95% CI: 1.13–2.12, p = 0.629), proteinuria (RR = 4.88, 95% CI: 1.67–14.27, p = 0.002), and hypertension (RR = 3.85, 95% CI: 1.69–8.76, p = 0.023). However, it is important to note that the CIs of proteinuria and hypertension were wide, suggesting that these findings should be interpreted cautiously. No significant difference was found for dermatologic toxicities (rash, dry skin), fatigue, or hematological toxicities (anemia, leucopenia, neutropenia, and thrombocytopenia). The details of safety are presented in Figure 4.
Figure 4.
Incidence of AEs between osimertinib plus bevacizumab and osimertinib alone.
AEs, adverse events.
Sensitivity analysis
In the sensitivity analysis, the leave-one-out method was used by omitting one included study at a time to determine whether any single omission influenced the overall results. According to Figure 2, Soo et al. contributed the most to the weight in the primary outcomes, suggesting its possible strong impact on the pooled effects. However, sensitivity analysis showed that removing Soo et al. or any other study had little effect on the pooled results, indicating the robustness of the overall results (see Supplemental Figures B–D).
Discussion
This study aimed to compare the efficacy and safety of osimertinib plus bevacizumab against osimertinib alone in patients with advanced NSCLC harboring EGFR alterations. Our findings suggested that compared with osimertinib monotherapy, the addition of bevacizumab to osimertinib contributed less to PFS, OS, and ORR in advanced EGFR-mutant NSCLC. Instead, the combination therapy was associated with a higher but acceptable incidence of certain AEs such as nausea, oral mucositis, proteinuria, and hypertension.
Before delving into the potential reasons behind the negative results, some issues deserve our thinking. The first is the timing of the use of bevacizumab. Across the studies included, bevacizumab was administered concomitantly with the first dose of osimertinib. However, a retrospective study found that patients who received antiangiogenic agents in combination with osimertinib when signs of resistance to osimertinib were observed had longer survival than those given antiangiogenic drugs at the initial dose of osimertinib. 20 The second aspect is the duration of bevacizumab use. In the included RCTs, regardless of whether bevacizumab was used in the first or second line, the median duration of bevacizumab use did not exceed the median PFS of osimertinib in the FLAURA or AURA3 trials, which may contribute to limited benefit from adding bevacizumab. In most studies, the major reason for the short duration of exposure to bevacizumab was toxicity. Although some studies suggested that discontinuation of bevacizumab due to AEs might not impact PFS,10,11 further confirmation is still necessary. In addition, patients who discontinued bevacizumab because of other reasons like disease progression may require further analysis. Third, underlying molecular characteristics should also be taken into consideration. There is scarce evidence indicating that bevacizumab can help osimertinib overcome known resistant mechanisms, such as C797S, exon 20 insertions, and mesenchymal-epithelial transition factor (MET) amplification. 21 All trials included in this meta-analysis were restricted to patients with common EGFR mutations like exon 19del, exon 21 point mutation, and T790M. Thus, further prospective research is needed to estimate the efficacy in these patients. Currently, a study evaluating the combination of osimertinib and bevacizumab in NSCLC patients harboring EGFR 20 insertions is ongoing (NCT04974879).
To assess the variation in PFS, a combined analysis of smoking subgroups was done. The results showed that smokers could derive meaningful prolongation of PFS from the combination treatment (HR = 0.64, 95% CI: 0.44–0.94), while no significant difference in PFS was observed in never-smokers. These findings are in line with conclusions from previous meta-analyses.13–15 The underlying rationale is not yet fully understood. A possible explanation is that TP53 mutations are associated with increased VEGF-A expression, 22 supporting the concept of better efficacy with bevacizumab in tumors with TP53 mutations that are more frequently observed among lung cancer patients with a history of tobacco exposure. 23 It offers hints that this subset of patients may be potential candidates for combination therapy, and future studies should further elucidate the underlying mechanisms.
Favorable penetration of the blood–brain barrier (BBB) for osimertinib had been confirmed by preclinical and clinical evidence,24,25 while bevacizumab has also demonstrated good permeability of BBB with antiproliferative effect on brain metastasis. 26 This provides a rationale for combination therapy that it has the potential to provide encouraging efficacy benefits for patients with BM. Indeed, clinical studies have shown promising activity against central nervous system metastases from combination modality.27,28 Given these thoughts, we conducted a pooled analysis based on BM. The results showed that the combination treatment yielded a mild trend of better PFS in those patients with BM (HR = 0.91, 95% CI: 0.46–1.77), though not reaching statistical significance. This is not the only case for osimertinib plus bevacizumab. A clinical trial investigating the combination of erlotinib and bevacizumab also found pronouncedly better efficacy in the subset of patients with BM, 29 suggesting that the addition of bevacizumab to EGFR-TKI may improve the prognosis of patients with baseline BM. Nonetheless, more robust evidence is needed to support this hypothesis. Several underway studies testing osimertinib and bevacizumab for NSCLC with BM (NCT02971501, NCT05104281), with leptomeningeal metastasis (NCT04148898) may shed further light on this topic.
With respect to treatment lines, Akamatsu et al. 10 found that prior exposure to anti-VEGF inhibitors had a negative impact on the efficacy of second-line osimertinib combined with bevacizumab. Similarly, our study showed a slight trend toward PFS benefit for patients receiving combination therapy as first-line treatment (HR = 0.84, 95% CI: 0.54–1.30). This effect could be likely attributed to changes in the tumor microenvironment caused by exposure to prior treatment and tumor regrowth, leading to resistance to anti-VEGF agents or affecting the efficacy of osimertinib.10,30 Accordingly, we believe that ‘first-line’ here refers more to patients who were not pretreated with antiangiogenic agents. It should be noted that, however, the included RCTs have maybe been quite limited in providing sufficient power to detect a pronounced difference between the two arms for the aforementioned characteristics. A trial testing patients treated in the first-line setting (NCT04181060) is in process.
Interestingly, we also noticed several retrospective cohort studies that compared osimertinib combined with bevacizumab to osimertinib alone in the course of screening literature.31,32 These studies showed that combination treatment leads to significantly better survival outcomes with tolerable side effects. Given the substantial inherent bias of observational studies, we only included RCTs in our analysis, which provide a higher level of evidence.
To the best of our knowledge, no other meta-analysis has been conducted to synthesize the efficacy and safety of osimertinib combined with bevacizumab at present. This meta-analysis provides an overview of the evidence comparing the effectiveness and toxicity of this treatment regimen to the standard first-line monotherapy, that is, osimertinib alone. Based on the current evidence, it may still be premature for clinical practitioners to opt for osimertinib plus bevacizumab for individuals with common EGFR mutation, which will impose a higher economic burden with little survival benefit and increased toxicity.
Some limitations should be acknowledged in our study. First, we only searched a few English and Chinese databases, and the databases we searched may not be fully comprehensive, which could introduce some bias and potential omissions. Next, due to the limited number of eligible RCTs, the statistical power may be not enough to demonstrate the effect of combination modality. Therefore, these results should be interpreted with caution. Third, one of the studies did not report the survival data directly, which may contribute to some inevitable deviation during the data extraction by software. In this respect, data were extracted separately by two authors and checked by a third author to minimize the issue. Last, the population included in this study was mainly Asian and European, which would probably limit the generalizability of these findings to other populations.
Conclusion
In summary, the present evidence suggested that compared to standard osimertinib monotherapy, osimertinib combined with bevacizumab failed to provide additional PFS, OS, and ORR benefits with higher but manageable AEs among advanced NSCLC patients with EGFR mutations. Further studies are required to offer more insights and identify subgroups of patients most likely to benefit from this combination modality.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359241227677 for Comparison of osimertinib plus bevacizumab against osimertinib alone in NSCLC harboring EGFR mutations: a systematic review and meta-analysis by Guojin Zhou, Liuxian Guo, Jing Xu, Kejing Tang and Jie Chen in Therapeutic Advances in Medical Oncology
Acknowledgments
We would like to extend our appreciation to all the authors and patients who were involved in the original studies included in the present systematic review and meta-analysis.
Footnotes
ORCID iDs: Guojin Zhou  https://orcid.org/0000-0002-0802-1661
https://orcid.org/0000-0002-0802-1661
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Guojin Zhou, Department of Pharmacy, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China; School of Pharmaceutical Science, Sun Yat-sen University, Guangzhou, China.
Liuxian Guo, Department of Pharmacy, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China; School of Pharmaceutical Science, Sun Yat-sen University, Guangzhou, China.
Jing Xu, Department of Pharmacy, Dermatology Hospital of Southern Medical University, Guangzhou, China.
Kejing Tang, Department of Pharmacy, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China.
Jie Chen, Department of Pharmacy, The First Affiliated Hospital of Sun Yat-sen University, No. 58, Zhong Shan Er Lu, Guangzhou 510080, China.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Guojin Zhou: Conceptualization; Formal analysis; Investigation; Methodology; Software; Visualization; Writing – original draft.
Liuxian Guo: Formal analysis; Investigation; Methodology; Software; Writing – review & editing.
Jing Xu: Formal analysis; Writing – review & editing.
Kejing Tang: Data curation; Investigation; Writing – review & editing.
Jie Chen: Conceptualization; Funding acquisition; Supervision; Validation; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Basic and Applied Basic Research Foundation of Guangdong Province [grant number 2021A1515220165] and the Wu Jieping Medical Foundation [grant number 320.6750.2022-20-12].
The authors declare that there is no conflict of interest.
Availability of data and materials: The data used and analyzed in the current study are available from the corresponding author upon reasonable request.
References
- 1. Tan AC, Tan DSW. Targeted therapies for lung cancer patients with oncogenic driver molecular alterations. J Clin Oncol 2022; 40: 611–625. [DOI] [PubMed] [Google Scholar]
- 2. Lim SM, Syn NL, Cho BC, et al. Acquired resistance to EGFR targeted therapy in non-small cell lung cancer: mechanisms and therapeutic strategies. Cancer Treat Rev 2018; 65: 1–10. [DOI] [PubMed] [Google Scholar]
- 3. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 2018; 378: 113–125. [DOI] [PubMed] [Google Scholar]
- 4. Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med 2017; 376: 629–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hung MS, Chen IC, Lin PY, et al. Epidermal growth factor receptor mutation enhances expression of vascular endothelial growth factor in lung cancer. Oncol Lett 2016; 12: 4598–4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Naumov GN, Nilsson MB, Cascone T, et al. Combined vascular endothelial growth factor receptor and epidermal growth factor receptor (EGFR) blockade inhibits tumor growth in xenograft models of EGFR inhibitor resistance. Clin Cancer Res 2009; 15: 3484–3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ninomiya T, Takigawa N, Ichihara E, et al. Afatinib prolongs survival compared with gefitinib in an epidermal growth factor receptor-driven lung cancer model. Mol Cancer Ther 2013; 12: 589–597. [DOI] [PubMed] [Google Scholar]
- 8. Hata A, Katakami N, Kaji R, et al. Afatinib plus bevacizumab combination after acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant non-small cell lung cancer: multicenter, single-arm, phase 2 trial (ABC Study). Cancer 2018; 124: 3830–3838. [DOI] [PubMed] [Google Scholar]
- 9. Piccirillo MC, Bonanno L, Garassino MC, et al. Addition of bevacizumab to erlotinib as first-line treatment of patients with EGFR-mutated advanced nonsquamous NSCLC: the BEVERLY multicenter randomized phase 3 trial. J Thorac Oncol 2022; 17: 1086–1097. [DOI] [PubMed] [Google Scholar]
- 10. Akamatsu H, Toi Y, Hayashi H, et al. Efficacy of osimertinib plus bevacizumab vs osimertinib in patients with EGFR T790M-mutated non-small cell lung cancer previously treated with epidermal growth factor receptor-tyrosine kinase inhibitor: West Japan Oncology Group 8715L Phase 2 randomized clinical trial. JAMA Oncol 2021; 7: 386–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Soo RA, Han JY, Dafni U, et al. A randomised phase II study of osimertinib and bevacizumab versus osimertinib alone as second-line targeted treatment in advanced NSCLC with confirmed EGFR and acquired T790M mutations: the European Thoracic Oncology Platform (ETOP 10-16) BOOSTER trial. Ann Oncol 2022; 33: 181–192. [DOI] [PubMed] [Google Scholar]
- 12. Kenmotsu H, Wakuda K, Mori K, et al. Randomized phase 2 study of osimertinib plus bevacizumab versus osimertinib for untreated patients with nonsquamous NSCLC harboring EGFR mutations: WJOG9717L study. J Thorac Oncol 2022; 17: 1098–1108. [DOI] [PubMed] [Google Scholar]
- 13. Dafni U, Soo RA, Peters S, et al. Impact of smoking status on the relative efficacy of the EGFR TKI/angiogenesis inhibitor combination therapy in advanced NSCLC – a systematic review and meta-analysis. ESMO Open 2022; 7: 100507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Qin BD, Jiao XD, Wang Y, et al. Effect of smoking habits on the efficacy of EGFR-TKI plus anti-angiogenic agent in advanced EGFR-mutant NSCLC. Lung Cancer 2022; 170: 91–97. [DOI] [PubMed] [Google Scholar]
- 15. Lee TH, Chen HL, Chang HM, et al. Impact of smoking status in combination treatment with EGFR tyrosine kinase inhibitors and anti-angiogenic agents in advanced non-small cell lung cancer harboring susceptible EGFR mutations: systematic review and meta-analysis. J Clin Med 2022; 11: 3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007; 8: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yi L, Fan J, Qian R, et al. Efficacy and safety of osimertinib in treating EGFR-mutated advanced NSCLC: a meta-analysis. Int J Cancer 2019; 145: 284–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Feng X, Xiao J, Jing M, et al. Observation of clinical efficacy of osimertinib combined with bevacizumab in the first-line treatment of EGFR sensitive mutation-positive advanced non-small cell lung cancer. J Clin Exp Med 2022; 21: 1697–1700. [Google Scholar]
- 20. Feng Y, Huang L, Zhu H, et al. The exploration of three different treatment models of osimertinib plus antiangiogenic agents in non-small cell lung cancer: a real-world study. Thorac Cancer 2022; 13: 2641–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu TC, Lin CC. Antiangiogenesis may not be a universal booster of EGFR tyrosine kinase inhibitors. J Thorac Oncol 2022; 17: 1063–1066. [DOI] [PubMed] [Google Scholar]
- 22. Schwaederle M, Lazar V, Validire P, et al. VEGF-A expression correlates with TP53 mutations in non-small cell lung cancer: implications for antiangiogenesis therapy. Cancer Res 2015; 75: 1187–1190. [DOI] [PubMed] [Google Scholar]
- 23. Gibbons DL, Byers LA, Kurie JM. Smoking, p53 mutation, and lung cancer. Mol Cancer Res 2014; 12: 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ballard P, Yates JW, Yang Z, et al. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res 2016; 22: 5130–5140. [DOI] [PubMed] [Google Scholar]
- 25. Yamaguchi H, Wakuda K, Fukuda M, et al. A phase II study of osimertinib for radiotherapy-naïve central nervous system metastasis from NSCLC: results for the T790M cohort of the OCEAN Study (LOGIK1603/WJOG9116L). J Thorac Oncol 2021; 16: 2121–2132. [DOI] [PubMed] [Google Scholar]
- 26. Masuda C, Sugimoto M, Wakita D, et al. Bevacizumab suppresses the growth of established non-small-cell lung cancer brain metastases in a hematogenous brain metastasis model. Clin Exp Metastasis 2020; 37: 199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yu HA, Schoenfeld AJ, Makhnin A, et al. Effect of osimertinib and bevacizumab on progression-free survival for patients with metastatic EGFR-mutant lung cancers: a phase 1/2 single-group open-label trial. JAMA Oncol 2020; 6: 1048–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang L, You Y, Liu X, et al. Osimertinib combined with bevacizumab as the first-line treatment in non-small cell lung cancer patients with brain metastasis harboring epidermal growth factor receptor mutations. Thorac Cancer 2023; 14: 1355–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee Y, Kim HR, Hong MH, et al. A randomized phase 2 study to compare erlotinib with or without bevacizumab in previously untreated patients with advanced non-small cell lung cancer with EGFR mutation. Cancer 2023; 129: 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cui Q, Hu Y, Cui Q, et al. Osimertinib rechallenge with bevacizumab vs. chemotherapy plus bevacizumab in EGFR-mutant NSCLC patients with osimertinib resistance. Front Pharmacol 2022; 12: 746707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bao J, Wu Z, Zhang C, et al. Efficacy and mechanism of osimertinib combined with bevacizumab in the treatment of postoperative EGFR positive stage II–IIIA lung adenocarcinoma. Am J Transl Res 2022; 14: 633–642. [PMC free article] [PubMed] [Google Scholar]
- 32. Liu S, Pan T, Wang MK, et al. Combination of bevacizumab and osimertinib in patients with EGFR T790M-mutated non-small cell lung cancer. Clin Drug Investig 2022; 42: 459–464. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359241227677 for Comparison of osimertinib plus bevacizumab against osimertinib alone in NSCLC harboring EGFR mutations: a systematic review and meta-analysis by Guojin Zhou, Liuxian Guo, Jing Xu, Kejing Tang and Jie Chen in Therapeutic Advances in Medical Oncology