Abstract
Toxic epidermal necrolysis (TEN) is a rare severe cutaneous adverse reaction that involves more than 30% of the body surface area. TEN can be accompanied by a series of systemic symptoms and has a high risk of death. Tumor necrosis factor (TNF)-α inhibitors such as adalimumab and etanercept have been shown to be safe and effective for the treatment of TEN in some cases. However, clinical data on the use of TNF-α inhibitors to treat TEN with severe systemic infection are scarce. In the present study, three adult patients who developed TEN with serious active infection were successfully treated with etanercept. One of the three patients had active open pulmonary tuberculosis, and the other two had septicemia and/or fungal sepsis. All patients’ skin lesions significantly improved after several days, and none of the patients developed emerging or re-emerging infectious diseases, adverse reactions, or a similar rash during follow-up. TNF-α inhibitors may be an effective treatment choice for TEN with severe systemic infection. However, further studies with large samples are still required for validation because clinical experience is limited.
Keywords: Toxic epidermal necrolysis, serious active infection, tumor necrosis factor-α inhibitor, etanercept, off-label treatment, case series
Introduction
Toxic epidermal necrolysis (TEN) is a severe cutaneous adverse reaction mainly caused by drugs. It is characterized by detachment of the epidermis and mucous membrane and has an annual incidence of 1 to 5 in 1,000,000 people.1,2 Traditional systemic treatments, including glucocorticoids, human intravenous immunoglobulin (IVIG), cyclosporine, and plasma exchange, have demonstrated efficacy in patients with TEN. Nevertheless, standardized treatment strategies are still lacking and management is very challenging, especially for patients who have comorbid severe active infection. Some research has verified that biologics such as tumor necrosis factor (TNF)-α inhibitors provide safe and effective treatment for TEN, leading to more rapid skin healing and a lower incidence of gastrointestinal side effects compared with systemic corticosteroids.3–6 However, no reports have indicated that TNF-α inhibitors are safe and effective for TEN in patients with severe infection. In this report, we describe three patients who developed TEN with serious active infection, including active open pulmonary tuberculosis, septicemia, and fungal sepsis. All three patients experienced significant remission after anti-TNF-α therapy, and no emerging or re-emerging infectious diseases or adverse reactions were observed during follow-up. These cases demonstrate that short-term therapy with TNF-α inhibitors may have good efficacy in patients with simultaneous TEN and severe systemic infection without aggravating the condition or inducing further infectious diseases.
All three patients provided informed consent to receive off-label treatment before initiation of the TNF-α inhibitor therapy. All patients provided written informed consent for the publication of this report. The requirement for institutional review board approval was waived because of the nature of this study (case report). The reporting of this study conforms to the CARE guidelines. 7
Case presentations
Case 1
A 30-year-old man was prescribed anti-tuberculosis drugs (isoniazid + rifapentine +ethambutol + bicyclol + levofloxacin) by a clinic physician because of secondary pulmonary tuberculosis 2 months before presentation to our hospital. After 1 month of treatment, he developed erythema and itching on his thighs and a high-grade fever. He was therefore prescribed antianaphylaxis and antipyretic therapy (drugs unknown) at a local hospital for drug allergy, but his condition did not improve. The rash gradually worsened until his whole body exhibited generalized dense, point-like, erythematous miliary lesions that had partially fused. This rash was accompanied by edema of the right dorsal foot. Laboratory examinations indicated renal insufficiency, and he therefore stopped the anti-tuberculosis drugs. His symptoms were then partially relieved. Upon restarting anti-tuberculosis drug therapy (ethambutol hydrochloride + pyrazinamide + rifampicin + isoniazid tablets + levofloxacin), he developed an aggravated rash. Two days before presentation to our outpatient department, he developed flushing and epidermal exfoliation. During the clinical course, he had not taken any other drugs and had no history of sun exposure or food or drug allergies.
The patient’s vital signs were as follows: body temperature (T), 39°C; pulse rate (P), 153 beats/minute; respiratory rate (R), 25 breaths/minute; and blood pressure (BP), 124/76 mmHg. Dermatological examination showed widespread erythema all over the body, covering a body surface area (BSA) of >90% according to the Lund–Browder scale, skin peeling lesions covering a BSA of >30%, and mucosal lesions including conjunctival hyperemia and oral and genital mucosal erosions with exudation. Nikolsky’s sign was positive. No other obvious abnormalities were found during the physical examination.
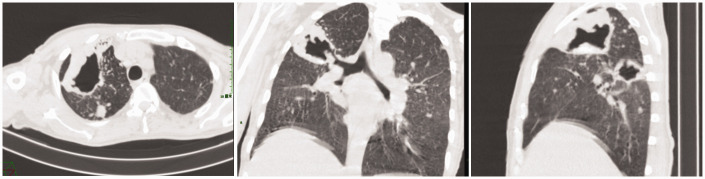
Laboratory examinations on admission revealed hypoalbuminemia (33 g/L; reference range, 35–50 g/L), abnormal routine urinalysis (urine white blood cell (WBC) and qualitative protein levels, 1+; reference value, negative), and increased levels of creatinine (297 µmol/L; reference range, 58–110 µmol/L), uric acid (525 µmol/L; reference range, 208–506 µmol/L), blood urea nitrogen (BUN) (27.2 mmol/L; reference range, 3.2–7.1 mmol/L), serum bicarbonate ( ) (12.5 mmol/L; reference range, 22.0–30.0 mmol/L), C-reactive protein (CRP) (98.9 mg/L; reference range, 0.0–8.0 mg/L), procalcitonin (PCT) (0.47 ng/mL; reference range, 0.0–0.05 ng/mL), and TNF-α (74.30 pg/mL; reference range, 0.0–8.1 pg/mL). Arterial blood gas examination revealed a pH of 7.33 (reference range, 7.35–7.45), PaCO2 of 25 mmHg (reference range, 35–45 mmHg), PaO2 of 81 mmHg (reference range, 80–100 mmHg), and of 13.2 mmol/L (reference range, 22–32 mmol/L) on room air. Blood culture was positive for Staphylococcus sciuri. Chest computed tomography showed active pulmonary tuberculosis (Figure 1).
Figure 1.
Computed tomography scan of Patient 1. Multifocal consolidation and a thick-walled cavity were present in the right lung, and bilateral lymphadenectasis of the pulmonary hilum and mediastinum was observed.
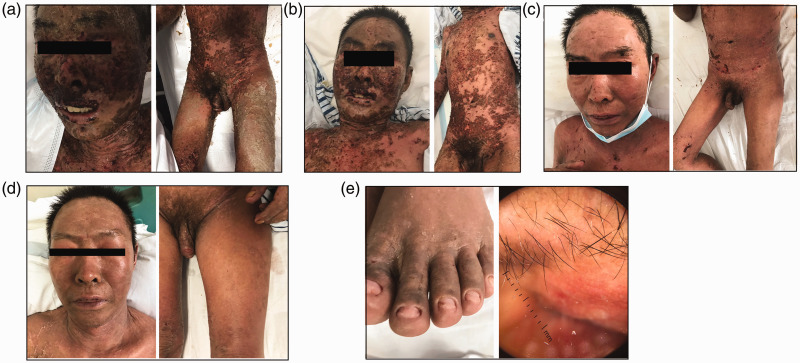
TEN, active pulmonary tuberculosis, and septicemia was diagnosed. The Severity-of-Illness Score for Toxic Epidermal Necrolysis (SCORTEN) showed four risk factors. On the second day of admission, the patient received an injection of 50 mg etanercept after providing informed consent to receive off-label treatment and excluding drug contraindications. At the same time, IVIG was prescribed at a dosage of 10 g for 5 days, and meropenem was given for anti-infection therapy. Appropriate care of the skin and mucosal lesions was ensured, and symptomatic treatment was given. Thereafter, the outcome was favorable. Four days later, the denuded areas gradually resolved and the fever disappeared. Healing of the mucosal lesions as well as the erythematous and skin peeling lesions was basically complete within 10 days. The patient was discharged with complete cutaneous and mucosal regeneration on the 15th day of hospitalization. The changes in the patient’s clinical features after injection of TNF-α inhibitor are shown in Figure 2.
Figure 2.
Changes in clinical features (a) 5 days, (b) 6 days, (c) 8 days, (d) 9 days, and (e) 14 days after injection of TNF-α inhibitor in Patient 1. The mucosal lesions, erythema, and skin peeling lesions gradually healed during the course of treatment, accompanied by loss of toenails and pubic hair.
One week after discharge, the patient’s infectious disease physician prescribed isoniazid monotherapy to further treat his open active pulmonary tuberculosis. However, scattered erythema and itching appeared, and blisters with clear fluid formed on both legs. The isoniazid was discontinued and a topical corticosteroid was instead used to treat his lesions. The lesions then gradually resolved. The patient was thereafter treated with Chinese traditional medicine for tuberculosis at a local clinic for 5 months without relapse of the TEN lesions.
Case 2
A 24-year-old man was treated with intravenous cytarabine and clindamycin because of a cough with sputum production after catching a cold. He then developed painful erythema, blisters on the trunk, and swelling, exudation, and erosion of the oral and ocular mucosa. The patient was treated with dexamethasone, ticarcillin disodium/clavulanate potassium, and acyclovir for 2 days at a local hospital, but his condition did not improve. Methylprednisolone, clindamycin, and amikacin were then prescribed for 3 days. During his clinical course, he had a persistent fever, with the temperature peaking at 40.3°C. Additionally, blood culture was positive for Gram+ bacteria. The diagnosis of TEN with sepsis was considered. He was immediately transferred to our department for further treatment, and 50 mg of etanercept was administered.
Before the treatment, dermatological examination revealed widespread erythema affecting >90% of the BSA and epidermal detachment covering >30% of the BSA, affecting the patient’s face, neck, trunk, limbs, and oral and genital areas with multiple blisters, erosions, and exudation. Nikolsky’s sign was positive. Hemorrhagic crusted erosions of the lips with erosive stomatitis, eyelid edema, and severe bilateral conjunctivitis were also present. His vital signs were as follows: T, 37.7°C; P, 115 beats/minute; R, 20 breaths/minute; and BP, 130/81 mmHg.
Laboratory investigations showed a low WBC count (2.48 × 109/L; reference range, 3.5–9.5 × 109/L) and albumin level (30 g/L). The CRP, PCT, and TNF-α levels were increased at 60.8 mg/L, 8.26 ng/mL, and 66.20 pg/mL, respectively. The BUN, , and serum glucose levels were normal. Arterial blood gas examination revealed a pH of 7.33 (reference range, 7.35–7.45), PaCO2 of 25 mmHg (reference range, 35–45 mmHg), and PaO2 of 81 mmHg (reference range, 80–100 mmHg). Carbapenem-resistant Acinetobacter baumannii was found in oral secretions, and yeast-like spores were found in scrotal secretions. Secretion culture was positive for Staphylococcus aureus.
TEN with severe skin infection was diagnosed. The SCORTEN showed one risk factor. In addition to etanercept, the patient received imipenem/cilastatin and vancomycin to treat the septicemia, and 20 g IVIG was simultaneously administered for 3 days. His condition improved after several days (Figure 3). The erythema and epidermal detachment regressed, and the oral and genital mucosa and ophthalmologic abnormalities gradually resolved. After nearly 2 weeks of treatment, the patient was cured and discharged. No recurrence of the TEN or septicemia was noted during 3 months of follow-up.
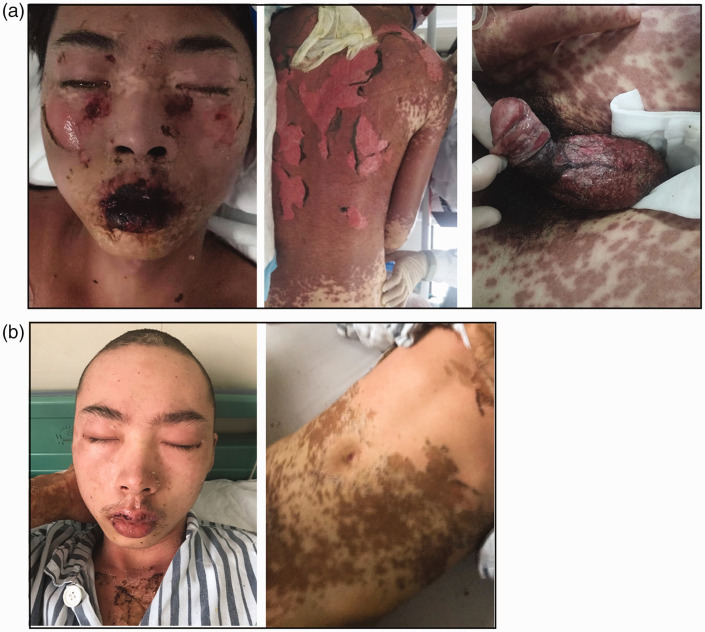
Figure 3.
Photographs of Patient 2 (a) before and (b) 10 days after injection of TNF-α inhibitor. (a) Before TNF-α inhibitor treatment, the patient exhibited widespread erythema and epidermal detachment, multiple blisters and erosions, hemorrhagic crusted erosions of the lips with erosive stomatitis, eyelid edema, and severe bilateral conjunctivitis and (b) Ten days after injection of TNF-α inhibitor, the erythema and epidermal detachment regressed, and the oral and genital mucosal and ophthalmologic abnormalities resolved.
Case 3
A 49-year-old woman was diagnosed with acute myelogenous leukemia and completed the first course of chemotherapy with IA (idarubicin on days 1 and 2 + cytarabine on days 1–7) + venetoclax (days 1–14) 2 months before presentation to our hospital. After the therapy, she developed myelosuppression, agranulocytosis, and fungal sepsis. Caspofungin and fluconazole were prescribed by her hematologist for antifungal and symptomatic treatment. Three days before the second chemotherapy course, the patient developed erythema, papules, and itching all over the body accompanied by epidermal exfoliation. Her hematologist prescribed levocetirizine, but the condition did not improve.
Dermatological examination in our hospital showed multiple foci of erythema with dense blisters and bullae over the whole body (>90% BSA) as well as scattered skin sloughing and denudation (approximately 3% BSA), mainly on the waist, abdomen, and buttocks. Nikolsky’s sign was positive. The oral, ocular, and vulvar mucosa showed no involvement. The patient’s vital signs were as follows: T, 38.2°C; P, 110 beats/minute; R, 20 breaths/minute; and BP, 106/60 mmHg.
Laboratory investigations revealed a WBC count of 10.88 × 109/L and several abnormal serum biochemistry indexes: serum potassium, 6.7 mmol/L (reference range, 3.5–5.1 mmol/L); albumin, 29 g/L; uric acid, 699 μmol/L; creatinine, 408 μmol/L; BUN, 12.1 mmol/L; serum bicarbonate, 10.2 mmol/L; TNF-α, 91.20 pg/mL; PCT, 2.72 ng/mL; and CRP, 109.00 mg/L. Blood culture was positive for Staphylococcus haemolyticus.
After a multidisciplinary consultation, we considered severe drug eruption (with a tendency toward TEN), septicemia, and fungal sepsis. SCORTEN showed four risk factors. The day after hospitalization in the hematology department, further consultation regarding the patient’s lesions led to immediate administration of 50 mg etanercept for treatment of TEN, followed by a 3-day course of 30 g IVIG and a 5-day course of 40 mg methylprednisolone. At the same time, imipenem/cilastatin was given to treat septicemia along with symptomatic and supportive treatment. The patient’s lesions markedly improved in 2 days, and the chemotherapy was continued under her hematologist’s care.
No recurrence of TEN was observed during 1 month of follow-up.
Discussion
TEN is a severe skin and mucosal reaction characterized by extensive exfoliation of the epidermis and mucous membranes. It may be accompanied by multisystem involvement and result in sepsis and death. 8 Several mechanisms have been verified to be associated with the pathogenesis of TEN, including drug-specific T-cell–mediated cytotoxicity, genetic linkage with human leukocyte antigen (HLA)- and non-HLA-genes, T-cell receptor restriction, and cytotoxicity. Additionally, various cytokines have been found to play a key role, including Fas ligand, granular enzyme, TNF-α, and interferon-γ.9,10 All three patients in our report developed generalized flushing, extensive epidermal exfoliation, and severe skin and mucous membrane involvement after oral/intravenous infusion of drugs, which is consistent with the clinical manifestations of TEN. Additionally, each patient’s serum TNF-α level was markedly high, consistent with previous reports. 11
With respect to systemic treatment, although early and adequate corticosteroid therapy has been shown to be useful to control the progression of the disease,12,13 the management of TEN remains difficult, and no consistent standard has been established. TNF-α inhibitors such as adalimumab, infliximab, and etanercept have demonstrated beneficial effects in some cases,14–16 and etanercept may be associated with a lower mortality rate than corticosteroids and other systematic treatments.17,18 Although few reports have described the use of TNF-α inhibitors for TEN with severe infections such as active pulmonary tuberculosis, a prospective comparison study of etanercept versus corticosteroids for the treatment of Stevens–Johnson syndrome/TEN showed that the prolonged duration of skin healing might increase the risk of infection, which was also a common cause of death. Notably, the uses of etanercept reduced the skin healing time, and fewer patients treated with etanercept died of respiratory failure or sepsis than patients treated with corticosteroids. 4 Therefore, considering previous reports of success using TNF-α inhibitors as well as the very high TNF-α levels in our patients, we chose etanercept in all three cases. Etanercept can competitively bind to TNF-α in blood, block its binding to TNF receptors on the cell surface, and reduce the activity of TNF-α, thereby inhibiting the inflammatory response and various biological effects of TNF-α. 19 The patients’ skin lesions significantly improved after using this TNF-α inhibitor, and no emerging or re-emerging infectious diseases, adverse reactions, or similar rashes appeared during follow-up.
TNF-α inhibitors have been shown to be safe and to effectively improve signs and symptoms,5,15,20,21 and they are recommended early in the course of TEN in China. 12 However, clinical experience with TNF-α inhibitors for TEN in the presence of serious active infections is limited because severe infection is a contraindication for TNF-α inhibitor in many cases. In our patients, TEN was complicated by tuberculosis and/or serious infection, and the skin lesions resolved after treatment with the TNF-α inhibitor, indicating good efficacy of this regimen. Moreover, no patients developed obvious adverse reactions, recurrence, or emerging or re-emerging infectious diseases, suggesting that our cases may serve as a reference for treatment of TEN with serious infection. However, our patients were also treated with IVIG, and the third patient was further treated with systemic glucocorticoids, making it difficult to evaluate the efficacy of the TNF-α inhibitor alone. Systemic glucocorticoids and IVIG may be associated with a decreased risk of infection. 22 Therefore, larger numbers of cases and high-quality clinical trials are still required to evaluate the efficacy and adverse reactions of TNF-α inhibitors in the treatment of TEN. Further research is also needed to investigate the risk of infection and the treatment of patients with concurrent TEN and infection using TNF-α inhibitors.
Conclusion
The treatment of TEN with severe infection is challenging. Although TNF-α inhibitors are not currently licensed for patients with TEN and are contraindicated when patients have severe infection, our experience shows that TNF-α inhibitors may be an effective treatment choice for TEN combined with severe infection. However, large-sample clinical trials are still needed for validation.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_03000605231223059 for Tumor necrosis factor-α inhibitor for successful treatment of toxic epidermal necrolysis with severe infection: a case series by Yu-Ting Peng, Jian-Xia Xiong, Bin Wei, Hui Li, Jing Xu, Ai-Jun Chen and Ping Wang in Journal of International Medical Research
Acknowledgements
The authors are grateful to the patients described in this report.
Author contributions: All authors contributed to the study conception and design. Material preparation and data collection and analysis were performed by Yu-Ting Peng, Jian-Xia Xiong, Bin Wei, Hui Li, Ai-Jun Chen, and Jing Xu. The first draft of the manuscript was written by Yu-Ting Peng. Ping Wang reviewed the pertinent raw data on which the results and conclusions of this study are based. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs: Yu-Ting Peng https://orcid.org/0000-0002-7373-8810
Ping Wang https://orcid.org/0000-0001-6181-3556
Authorship
All named authors of this report meet the authorship criteria of the International Committee of Medical Journal Editors (ICMJE), are responsible for the integrity of the entire work, and have approved this version for publication.
Data availability
Data for this study are available and can be made publicly available upon request. To request access to the raw data, please contact: Dr. Ping Wang, wang_ping@hospital.cqmu.edu.cn.
References
- 1.Hasegawa A, Abe R. Recent advances in managing and understanding Stevens-Johnson syndrome and toxic epidermal necrolysis. F1000Res 2020; 9: F1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White KD, Abe R, Ardern-Jones M, et al. SJS/TEN 2017: building multidisciplinary networks to drive science and translation. J Allergy Clin Immunol Pract 2018; 6: 38–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paradisi A, Abeni D, Bergamo F, et al. Etanercept therapy for toxic epidermal necrolysis. J Am Acad Dermatol 2014; 71: 278–283. [DOI] [PubMed] [Google Scholar]
- 4.Wang CW, Yang LY, Chen CB, et al. Randomized, controlled trial of TNF-α antagonist in CTL-mediated severe cutaneous adverse reactions. J Clin Invest 2018; 128: 985–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang S, Tang S, Li S, et al. Biologic TNF-alpha inhibitors in the treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis: a systemic review. J Dermatolog Treat 2020; 31: 66–73. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, Lu CW, Chen CB, et al. Evaluation of combination therapy with etanercept and systemic corticosteroids for Stevens-Johnson syndrome and toxic epidermal necrolysis: a multicenter observational study. J Allergy Clin Immunol Pract 2022; 10: 1295–1304.e6. [DOI] [PubMed] [Google Scholar]
- 7.Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. Headache 2013; 53: 1541–1547. [DOI] [PubMed] [Google Scholar]
- 8.Labib AM, Milroy C. Toxic Epidermal Necrolysis. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC; 2022. [PubMed] [Google Scholar]
- 9.Lerch M, Mainetti C, Terziroli Beretta-Piccoli B, et al. Current perspectives on Stevens-Johnson syndrome and toxic epidermal necrolysis. Clin Rev Allergy Immunol 2018; 54: 147–176. [DOI] [PubMed] [Google Scholar]
- 10.Chen CB, Wang CW, Chung WH. Stevens-Johnson syndrome and toxic epidermal necrolysis in the era of systems medicine. Methods Mol Biol 2022; 2486: 37–54. [DOI] [PubMed] [Google Scholar]
- 11.Wang F, Ye Y, Luo ZY, et al. Diverse expression of TNF-α and CCL27 in serum and blister of Stevens-Johnson syndrome/toxic epidermal necrolysis. Clin Transl Allergy 2018; 8: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adverse Drug Reaction Research Center of Chinese Society of Dermatology. Expert consensus on the diagnosis and treatment of Stevens-Johnson syndrome/toxic epidermal necrolysis. Chin J Dermatol 2021; 54: 376–381. doi: 10.35541/cjd.20201177 [Google Scholar]
- 13.Manvi S, Mahajan VK, Mehta KS, et al. The clinical characteristics, putative drugs, and optimal management of 62 patients with Stevens-Johnson syndrome and/or toxic epidermal necrolysis: a retrospective observational study. Indian Dermatol Online J 2022; 13: 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zárate-Correa LC, Carrillo-Gómez DC, Ramírez-Escobar AF, et al. Toxic epidermal necrolysis successfully treated with infliximab. J Investig Allergol Clin Immunol 2013; 23: 61–63. [PubMed] [Google Scholar]
- 15.Woolridge KF, Boler PL, Lee BD. Tumor necrosis factor alpha inhibitors in the treatment of toxic epidermal necrolysis. Cutis 2018; 101: E15–E21. [PubMed] [Google Scholar]
- 16.Paradisi A, Abeni D, Didona D, et al. A new case series on etanercept treatment for toxic epidermal necrolysis. Eur J Dermatol 2020; 30: 561–568. [DOI] [PubMed] [Google Scholar]
- 17.Jacobsen A, Olabi B, Langley A, et al. Systemic interventions for treatment of Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS/TEN overlap syndrome. Cochrane Database Syst Rev 2022; 3: Cd013130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krajewski A, Maciejewska-Markiewicz D, Jakubczyk K, et al. Impact of multiple medical interventions on mortality, length of hospital stay and reepithelialization time in Toxic Epidermal Necrolysis, Steven-Johnsons Syndrome, and TEN/SJS Overlap – Metanalysis and metaregression of observational studies. Burns 2022; 48: 263–280. [DOI] [PubMed] [Google Scholar]
- 19.Hassett B, Scheinberg M, Castañeda-Hernández G, et al. Variability of intended copies for etanercept (Enbrel®): data on multiple batches of seven products. MAbs 2018; 10: 166–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sachdeva M, Maliyar K, Ponzo MG. A systematic review of efficacy and safety of monotherapy and combination therapy with biologic for Stevens-Johnson syndrome and toxic epidermal necrolysis. J Cutan Med Surg 2021; 25: 598–615. [DOI] [PubMed] [Google Scholar]
- 21.Ao S, Gao X, Zhan J, et al. Inhibition of tumor necrosis factor improves conventional steroid therapy for Stevens-Johnson syndrome/toxic epidermal necrolysis in a cohort of patients. J Am Acad Dermatol 2022; 86: 1236–1245. [DOI] [PubMed] [Google Scholar]
- 22.Kridin K, Brüggen MC, Chua SL, et al. Assessment of treatment approaches and outcomes in Stevens-Johnson syndrome and toxic epidermal necrolysis: insights from a pan-European multicenter study. JAMA Dermatol 2021; 157: 1182–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_03000605231223059 for Tumor necrosis factor-α inhibitor for successful treatment of toxic epidermal necrolysis with severe infection: a case series by Yu-Ting Peng, Jian-Xia Xiong, Bin Wei, Hui Li, Jing Xu, Ai-Jun Chen and Ping Wang in Journal of International Medical Research
Data Availability Statement
Data for this study are available and can be made publicly available upon request. To request access to the raw data, please contact: Dr. Ping Wang, wang_ping@hospital.cqmu.edu.cn.