Abstract
Objective: To investigate the dosimetric effects of using individualized silicone rubber (SR) bolus on the target area and organs at risk (OARs) during postmastectomy radiotherapy (PMRT), as well as evaluate skin acute radiation dermatitis (ARD). Methods: A retrospective study was performed on 30 patients with breast cancer. Each patient was prepared with an individualized SR bolus of 3 mm thickness. Fan-beam computed tomography (FBCT) was performed at the first and second fractions, and then once a week for a total of 5 times. Dosimetric metrics such as homogeneity index (HI), conformity index (CI), skin dose (SD), and OARs including the heart, lungs, and spinal cord were compared between the original plan and the FBCTs. The acute side effects were recorded. Results: In targets' dosimetric metrics, there were no significant differences in Dmean and V105% between planning computed tomography (CT) and actual treatments (P > .05), while the differences in D95%, V95%, HI, and CI were statistically significant (P < .05). In OARs, there were no significant differences between the Dmean, V5, and V20 of the affected lung, V5 of the heart and Dmax of the spinal cord (P > .05) except the V30 of affected lung, which was slightly lower than the planning CT (P < .05). In SD, both Dmax and Dmean in actual treatments were increased than plan A, and the difference was statistically significant (P < .05), while the skin-V20 and skin-V30 has no difference. Among the 30 patients, only one patient had no skin ARD, and 5 patients developed ARD of grade 2, while the remaining 24 patients were grade 1. Conclusion: The OR bolus showed good anastomoses and high interfraction reproducibility with the chest wall, and did not cause deformation during irradiation. It ensured accurate dose delivery of the target and OARs during the treatment, which may increase SD by over 101%. In this study, no cases of grade 3 skin ARD were observed. However, the potential of using OR bolus to reduce grade 1 and 2 skin ARD warrants further investigation with a larger sample size.
Keywords: postmastectomy radiotherapy, dosimetry, bolus, fan beam computed tomography, planning target volume, organ at risk, acute radiation dermatitis
Introduction
Postmastectomy radiotherapy (PMRT) has been found to significantly reduce the local recurrence rate (LRR) and prolong the overall survival (OR).1,2 Bolus, a tissue-equivalent material, has been widely used to improve the chest wall dose during radiation treatment. By overcoming the influence of the dose-building effect, bolus can achieve suitable target dose distribution, which is essential for cancer treatment. Many cancer centers have adopted bolus as a routine technique during PMRT. This approach has shown promising results in reducing the LRR and improving patient outcomes. With continued research and development, bolus is expected to become an increasingly important tool in the fight against cancer.3,4
Traditional tissue-equivalent materials, such as pig skin, silicone, vaseline, and others, have been used in radiotherapy but often fail to accurately fit with the postoperative scar surface of patients, leading to poor reproducibility and even exacerbating skin side effects.5,6 Similarly, a three-dimensional (3D) printed bolus commonly used in breast cancer radiotherapy may reduce the air gap and dose deviation but requires a second computed tomography (CT) scan and Prolong the waiting time for treatment.7–9 Early studies by Babic et al 10 have shown that dental impressions materials with excellent plasticity can be utilized in radiotherapy, but proper storage methods must be employed, such as keeping them in a water-filled container. Therefore, it is crucial to develop a bolus with high individualized conformal for clinical practice.
In previous studies, the dosimetric effects of bolus application on the target area, lung, heart, and other organs at risk (OAR) were generally evaluated based on treatment plans and modes.9,11,12 However, there was limited discussion on the real dosimetry analysis during the course of treatment. To address this problem, diagnostic fan-beam computed tomography (FBCT) images are obtained using the CT-Linac (United Imaging Healthcare Co., Ltd, uCT-linac 506c) for dose calculation. FBCT follows the same scanning parameters as planned CT, which can be used not only for positioning calibration, but also directly for planning design. This approach allows for a more accurate assessment of the radiation delivery to the OARs and helps to optimize treatment plans.
Silicone rubber (SR) is a dental impression material that possesses superior shaping and fitting properties. Our center has independently developed an individualized SR bolus in radiation therapy. This study retrospectively analyzed the dosimetric effects of using SR bolus on target areas and OARs, as well as assessed patients' acute radiation dermatitis (ARD).
Materials and Methods
Patient Data
A total of 30 patients who were treated at our institution between July 25, 2022, and March 25, 2023, were selected consecutively for the study. The patients range in age from 32 to 73 years old, with a median age of 51 years. All patients signed an informed consent form before treatment, and they agreed to have their radiotherapy imaging data used for analysis and scientific publication, which was approved by the Ethics Committee on Biomedical Research. We have de-identified all patient details. The reporting of this retrospective study conforms to STROBE guidelines. 13
Make Individualized Silicone Rubber Bolus
All patients were immobilized with styrofoam. The manufacturing process of the SR bolus is as follows: (1) the clinician delineates the parts of the patient's chest that need to be covered with the SR bolus, and customizes the length and width; (2) the matrix and catalyst from Meijiayin elastomeric initial impression (HUGE, Shanghai, China) are mixed in a 1: 1 ratio, and placed in the customized mold (Figure 1a) with a thickness of 3 mm for flattening; (3) taking out the mixture and placing it on the patient's chest, shaping it according to the delineation range, and applying pressure with a wet towel and elastic bandage to make the mixture fit the chest wall skin; and (4) after 2 min, the SR bolus with a thickness of 3 mm was cooled and molded, and then the positioning marks were drawn on the skin and the bolus. The OR bolus is shown in Figure 1b and c.
Figure 1.
(a) The mold for individualized silicone rubber bolus, 1 is a removable stainless steel plate that can be adjusted to the width of the bolus, 2 is a stainless steel tank with the length, width, and height of 25 × 25 × 0.3 cm, and 3 can roll the mixed materials into a bolus with a thickness of 3 mm; (b) the front of bolus; and (c) the back of bolus.
CT Simulation and Treatment Planning
The plan CT of 30 patients used Philips 16-slice CT with a 5 mm slice thickness and 140 kV tube voltage, from the upper edge of the second cervical vertebra to the lower edge of the second lumbar vertebra. Each patient underwent 2 CT scans, one with an individualized SR bolus (CT1) and the other without it (CT2). The images were then imported into the treatment planning system (TPS, uRT-TPOIS R001), where intensity-modulated radiation therapy (IMRT) was used for treatment.
The prescribed dose was 50 Gy/25 fractions, which was divided into Plan A and Plan B. Plan A required the SR bolus during treatment, and Plan B did not. All patients were irradiated with 7 conventional fields. Plan A with the prescribed dose of 36 Gy/18 fractions was generated by TPS based on CT1, which followed our clinical standard for target coverage and surface dose. The target areas included the chest wall, internal mammary nodes, axillary lymph nodes, and supraclavicular lymph nodes. The superficial 3 mm of the chest wall target volume was delineated as the skin region of interest (ROI). The skin was shrunk by 0 mm to generate a 3 mm ring and then intersected with the chest wall target area. Plan B delineated the target on CT2, which had the same dose volume limits for the target and OARs as Plan A, with a prescribed dose of 14 Gy/7 fractions.
All patients were treated on the CT-integrated linear accelerator uRT-linac 506c, which features a diagnostic-quality helical CT system compactly fixed behind the gantry of a C-arm linear accelerator. The patient is sent through the scanner by moving the couch longitudinally. The integration of CT and linear accelerator enables a seamless workflow from simulation to treatment on one device. Compared to kV-CBCT, which is commonly used for image-guided radiation therapy (IGRT), the helical kV-FBCT provides both slice-by-slice comparison of the patient's anatomy with planning CT and accurate CT numbers for online adaptive planning.
Data Acquisition
The FBCT was performed for IGRT at the first and second fractions of Plan A, and then once a week for a total of 5 times in the whole treatment. A total of 150 FBCT images of OR bolus coverage were obtained in 30 patients. The contours including planning tumor volume (PTV) and clinical target volume (CTV) were registered from plan A, and the OARs were delineated by auto-segmentation. The new dose distribution was recalculated by copying the original plan onto the FBCT1 to FBCT5. All FBCTs were only used in this study to analyze the dosimetry of PTV and OARs during treatment.
Evaluation index
Target: D50%, D98%, D2%, D95%, Dmean, V95%, V105%, VPTV, VPres, and VPres-ptv in PTV were obtained. The PTV constraints protocol is as follows: V95% ≥ 95%, V105% ≤ 25%, and D95% > 95%. The homogeneity index (HI) of PTV was calculated as HI = (D2% − D98%)/D50%. The conformity index (CI) of the PTV was evaluated as CI = (VPres-ptv)2/(VPTV × VPres), where VPres-ptv is the target volume of the prescribed isodose value, VPTV is the target volume, and VPres is the volume of the prescribed isodose value.
OARs: The OARs constraints protocol is as follows: spinal cord Dmax < 40 Gy; ipsilateral lung V20 < 30%, V30 < 20%, V5 < 50%, and Dmean < 15 Gy; heart of left breast cancer V5 < 45%; and heart of right breast cancer V5 < 30%.
Skin dose (SD) and ARD: For each individual patient, we analyzed the skin dosimetry within a depth of 3 mm, where mean dose, maximum dose, skin-V20, and skin-V30 were recorded. In addition, we evaluated the skin's ARD according to the RTOG acute radiation morbidity scoring criteria. The scale classifies the clinical symptoms of dermatitis into 5 grades, from no skin change (grade 0) to severe ulcerative tissue necrosis (grade 4). 14
Statistical Analysis
SPSS 26.0 (IBM, USA) was used for statistical analysis. The target, OARs, and skin parameters of the FBCT1-5 were compared with Plan A. Data that conformed to a normal distribution (mean ± standard deviation) were analyzed using One-way repeated measures analysis of variance, while data that did not (median [interquartile spacing]) were analyzed using the Friedman Test. Statistical significance was defined as P < .05.
Results
Dosimetric Evaluation of Target Area and OARs
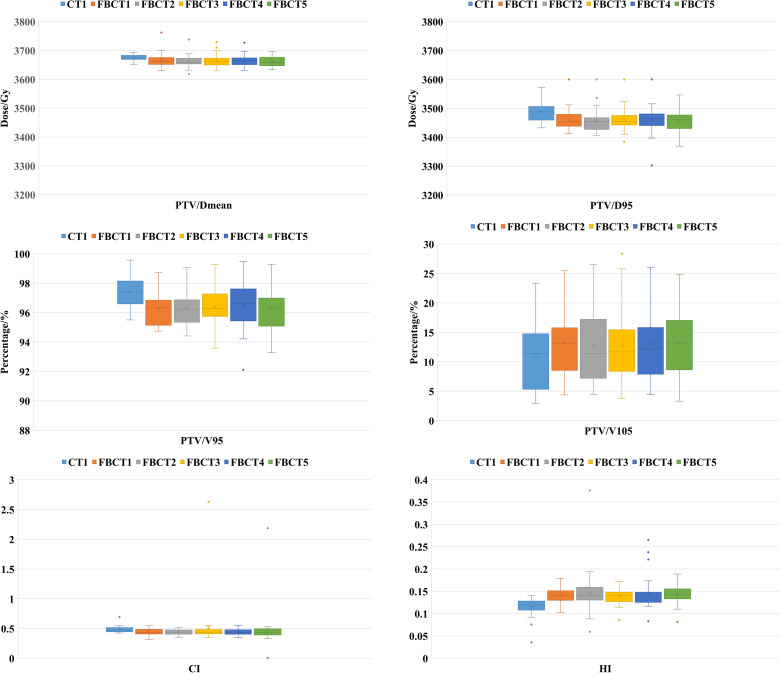
The target dosimetry characteristics of the patient's 5 FBCTs and plan A are shown in Table 1 and Figure 2. There was no statistical significance in the results of Dmean and V105%. Although D95% and V95% of PTV in the actual treatment were slightly lower than those in plan A, the difference was statistically significant (P < .05), but the target coverage still met clinical requirements. HI and CI of plan A are better than FBCTs.
Table 1.
The PTV Evaluation Parameter.
| PTV/parameter | Dmean (Gy) | D95% (Gy) | V95% (%) | V105% (%) | HI (0-1) | CI (0-1) |
|---|---|---|---|---|---|---|
| CT1 | 36.74 (14.31) | 34.88 (46.99) | 97.38 ± 0.96 | 10.43 (9.23) | 0.12 (0.02) | 0.47 (0.07) |
| FBCT1 | 36.63 (23.73) | 34.54 (44.43)* | 96.25 ± 1.09* | 13.12 (7.65) | 0.14 (0.02)* | 0.44 (0.07)* |
| FBCT2 | 36.60 (19.61)* | 34.54 (40.60)* | 96.33 ± 1.14* | 11.72 (10.61) | 0.14 (0.03)* | 0.43 (0.07)* |
| FBCT3 | 36.61 (23.07)* | 34.53 (32.79)* | 96.41 ± 1.30* | 11.97 (8.13) | 0.14 (0.02)* | 0.43 (0.07)* |
| FBCT4 | 36.64 (23.49) | 34.65 (40.42)* | 96.48 ± 1.48* | 12.28 (8.29) | 0.13 (0.02)* | 0.43 (0.07)* |
| FBCT5 | 36.58 (29.33)* | 34.60 (46.69)* | 96.28 ± 1.34* | 13.24 (8.97) | 0.14 (0.02)* | 0.43 (0.10)* |
| P | .007 | <.001 | <.001 | .07 | <.001 | <.001 |
Note: Abbreviations: PTV, planning tumor volume; FBCT, fan-beam computed tomography; CT, computed tomography.
* represents a statistically significant difference compared to CT1 (P < .05).
Figure 2.
The planning tumor volume (PTV) evaluation parameter.
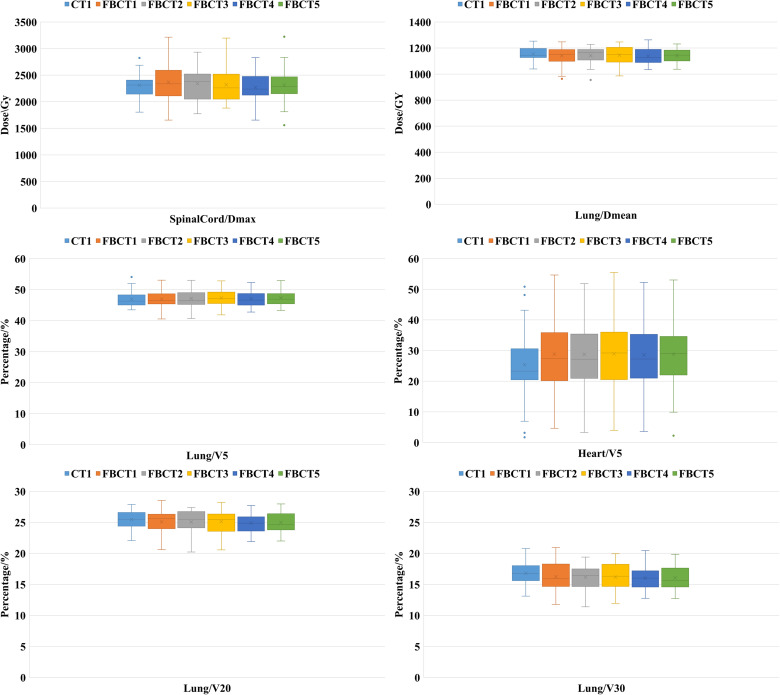
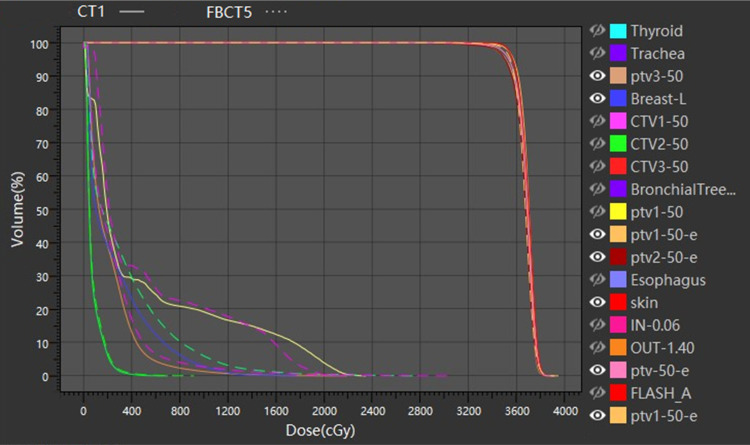
The OAR dosimetry characteristics of the patient's 5 FBCTs and plan A are shown in Table 2 and Figure 3. Compared with plan A and FBCTs, there were no significant differences between the Dmean, V5, and V20 of the ipsilateral lung, V5 of the heart, and Dmax of the spinal cord (P > .05) except the V30 of the affected lung, which was slightly lower than the plan A (P < .05). As shown in Figure 4, target areas and OARs' dose and volume histogram (DVH) of plan A and FBCT5 were close to each dose indicator curve, with highly consistent and little difference.
Table 2.
The OARs Evaluation Parameter.
| OARs/parameter | Lung (ipsilateral) | Heart V5 (%) | Spinal cord Dmax (Gy) | |||
|---|---|---|---|---|---|---|
| Dmean (Gy) | V5 (%) | V20 (%) | V30 (%) | |||
| CT1 | 11.45 (0.70) | 46.30 (3.24) | 25.46 ± 1.38 | 16.80 ± 1.94 | 25.39 ± 11.99 | 23.11 ± 2.14 |
| FBCT1 | 11.51 (0.89) | 46.47 (3.28) | 25.07 ± 1.92 | 16.23 ± 2.28 | 28.84 ± 12.05 | 23.68 ± 3.28 |
| FBCT2 | 11.65 (0.80) | 46.45(3.77) | 25.07 ± 1.75 | 16.16 ± 1.90 | 28.77 ± 12.07 | 23.42 ± 3.27 |
| FBCT3 | 11.50 (1.14) | 47.11 (3.67) | 25.14 ± 1.96 | 16.19 ± 2.35 | 28.93 ± 11.98 | 23.20 ± 3.20 |
| FBCT4 | 11.29 (1.01) | 46.62 (3.70) | 24.89 ± 1.55 | 16.02 ± 1.96 | 28.58 ± 11.70 | 22.65 ± 2.48 |
| FBCT5 | 11.45 (0.83) | 46.95 (3.25) | 24.98 ± 1.59 | 16.07 ± 2.02* | 28.82 ± 11.76 | 23.12 ± 3.23 |
| P | .394 | .523 | .386 | .018 | .342 | .330 |
Note: Abbreviations: OARs, organs at risk; FBCT, fan-beam computed tomography; CT, computed tomography.
* represents a statistically significant difference compared to CT1 (P < .05).
Figure 3.
The organs at risk (OARs) evaluation parameter.
Figure 4.
DVH diagram of CT1 and FBCT5.
Abbreviations: DVH, dose–volume histogram; FBCT, fan-beam computed tomography; CT, computed tomography.
Skin Acute Radiation Dermatitis and Dosimetric Assessment
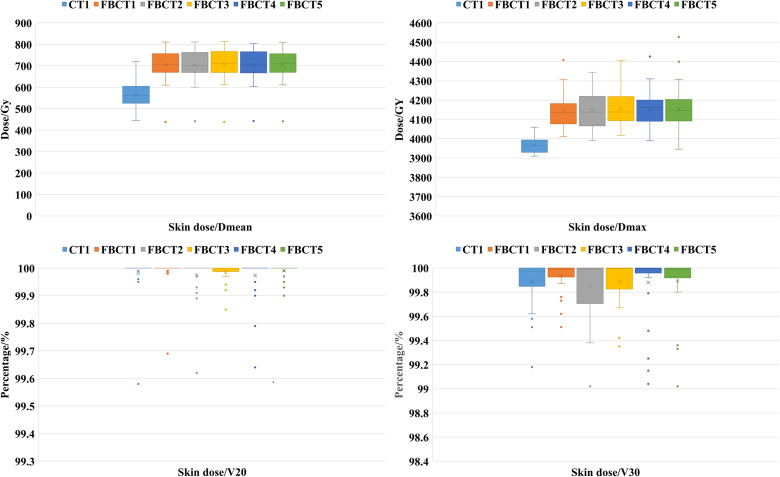
The skin dosimetry characteristics of the patient's 5 FBCTs and plan A are shown in Table 3 and Figure 5. Compared with plan A, both Dmax and Dmean were increased in actual treatments, and the difference was statistically significant (P < .05), while the skin-V20 and skin-V30 had no difference.
Table 3.
The Skin Dose Evaluation Parameter.
| Skin dose parameter | Dmean (Gy) | Dmax (Gy) | V20 (%) | V30 (%) |
|---|---|---|---|---|
| CT1 | 37.04 (0.31) | 39.47 (0.67) | 99.98 ± 0.08 | 99.89 ± 0.19 |
| FBCT1 | 37.22 (0.29)* | 40.43 (0.84)* | 99.99 ± 0.06 | 99.93 ± 0.12 |
| FBCT2 | 37.21 (0.25) | 40.46 (1.13)* | 99.98 ± 0.07 | 99.85 ± 0.25 |
| FBCT3 | 37.22 (0.35)* | 40.85 (0.95)*a | 99.99 ± 0.03 | 99.89 ± 0.20 |
| FBCT4 | 37.29 (0.32)*b | 40.61 (0.86)* | 99.97 ± 0.08 | 99.88 ± 0.27 |
| FBCT5 | 37.24 (0.29)* | 40.62 (0.98)* | 99.99 ± 0.02 | 99.89 ± 0.23 |
| P | .000 | .000 | .507 | .470 |
Note: Abbreviations: FBCT, fan-beam computed tomography; CT, computed tomography.
* represents a statistically significant difference compared to CT1 (P < .05); a represents a statistically significant difference compared to FBCT1 (P < .05); b represents a statistically significant difference compared to FBCT2 (P < .05).
Figure 5.
The skin dose evaluation parameter.
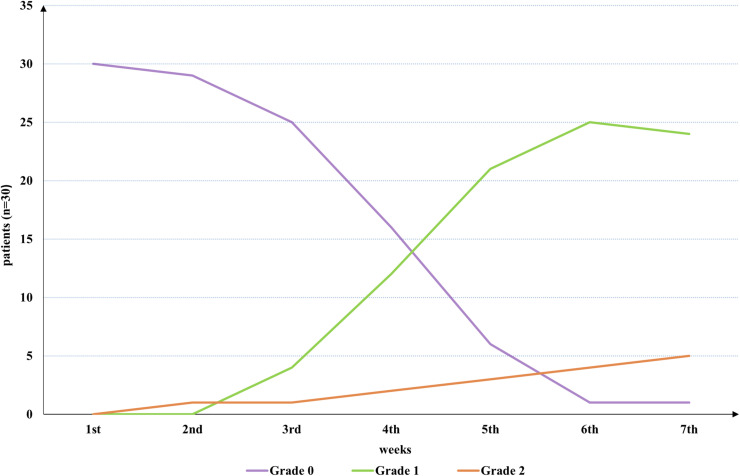
Thirty patients were evaluated for skin ARD during treatment and the results are shown in Figure 6. Twelve patients (40%) had grade 1 skin ARD in the fourth week, and 9 (30%) had grade 1 skin ARD in the fifth week. Grade 2 skin ARD occurred in one patient in the second, fourth, fifth, and sixth weeks. One patient developed a grade 1 skin ARD in the first week after treatment ended (sixth week), and one patient progressed from grade 1 to grade 2 skin ARD in the first week after treatment ended (seventh week). Only one patient had no skin ARD (grade 0), and no patients with grade 3 or 4 ARD were recorded in this study. Except for 2 patients with slight pigmentation, all patients recovered fully within 1 to 2 months of ending treatment. The average follow-up time was 6 months after the end of treatment.
Figure 6.
Assessment of skin: the curves show the probability of 3 grades (0, 1, and 2) of skin acute radiation dermatitis (ARD) for different weeks. Peaks in grades 1 and 2 skin ARD occurred in the sixth week (83.3%) and seventh week (20.8%), respectively.
Discussion
The purpose of the bolus is to increase the standard deviation while ensuring that the chest wall is adequately irradiated, thereby reducing the LRR.15,16 However, a thick bolus can affect the SD and exacerbate the symptoms of ARD. Studies have shown that when bolus isn't used, the average SD can be reduced by 20% to 30%. 17 Yang et al 18 proposed that a thickness of 3 mm had the highest CI when studying the use strategy of bolus in PMRT, which could provide a sufficient dose to the chest wall target. Kaidar-Person et al 4 believed that placing a bolus with a thickness of 3 to 5 mm in line with the body contour was conducive to reducing air gaps and ensuring the SD. Based on these findings, the SR bolus with a thickness of 3 mm used in this study can meet the actual clinical requirements.
This study was conducted using CT-Linac, which is compatible with Philips 16-slice CT. The interfraction FBCT images obtained can be used to accurately evaluate the dose distribution and the influence of individualized SR bolus on PTV and OARs during actual treatment. During the treatment, the Dmean and D95 of 5 FBCTs reached > 99.6% and 99.2%, respectively, of plan A, and all D95 values were > 95% (34.20 Gy) of the prescribed dose. The V105% showed no difference, which further ensured the accurate delivery of the target dose. HI, as a tool for quantifying dose uniformity within the target volume, exhibited excellent uniformity within the target area (within 0.14) in both planned and actual treatments. CI increased with improved target conformity, and the median value fluctuated in the range of 0.43 to 0.47. In OARs, the V30 (16.07% vs 16.83%) of the ipsilateral lung could be reduced, effectively protecting normal tissue.
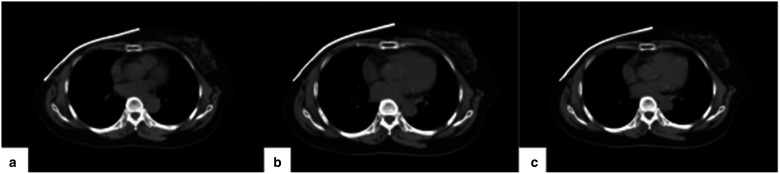
Several studies have suggested that air gaps can affect the dose distribution in the target area, resulting in lower-than-expected doses and decreased uniformity.19,20 However, in this study, the use of SR bolus resulted in a high degree of uniformity in the target area while also meeting clinical requirements for target dosimetry, which may be due to the materials used for the bolus. SR is made using an elastic mold system, which has high flexibility, strong plasticity, resistance to warping and deformation, and low shrinkage. It closely fits the chest wall, reducing unstable air gaps and ensuring their relative positions remain stable. By capturing images of the bolus at the same level in plan A, third, and fifth FBCT of the same patient (Figure 7), it was found that there were minimal changes. Previously, most scholars' studies21,22 on bolus focused on physical measurement or dose analysis during treatment planning without investigating dosimetry differences caused by interfraction reproducibility.
Figure 7.
The patient scanned the same level of the FBCT image at different fractions: a is a bolus of CT1; b is a bolus of FBCT3; and c is a bolus of FBCT5.
Abbreviations: FBCT, fan-beam computed tomography; CT, computed tomography.
The study also aimed to evaluate the actual skin SD of SR bolus in patients during treatment. The results showed that the Dmean of the skin reached 101%, which was equal to the planned value, and the Dmax exceeded the prescribed dose by 2.4% to 3.5%. This indicates that SR bolus can significantly increase the skin surface dose. In a previous study by Gong et al, 9 the skin surface dose of thermoplastic elastomer bolus could reach > 90% of the prescribed dose, while in another study by Fiedler et al 23 with a 3 mm polymer-gel type bolus, the skin dose was 94.8%. Wong et al 17 defined the same skin ROI as this study and reported that the Dmean could reach 95% of the prescribed dose, and the Dmax exceeded the prescribed dose by 5% to 6%. Overall, our results were better than those of the studies mentioned above.
In addition, the advantages of silicone rubber personalized bolus are also reflected in the low incidence of dermatitis in patients. Only 16.6% of patients developed grade 2 ARD, with Dmean exceeding plan A by −0.2% to 1.5%, and Dmax exceeding plan A by −0.5% to 7.5%. The SR bolus was cut to an appropriate size, reducing the side effects of normal skin outside the target area, and no skin infections occurred. One patient had local effusion in the chest wall during treatment, resulting in poor fitting of bolus. After communicating with the radiologist, the effusion was extracted and the bolus was well-fitted again. In the literature on the use of conventional bolus during radiotherapy, Abel et al 24 identified that among 50 patients using a 5 mm bolus, 32 patients developed grade 2 ARD, accounting for 60.4%, and patients with grade 3 accounted for 5%; Das et al 5 found that the proportion of patients with grade 2 and grade 3 treated with a 2 mm bolus was 71.4% and 12.2%, respectively. Dahn et al 3 summarized 27 studies and calculated that the adverse reaction of grade 3 caused by bolus was 9.6%. The proportion of skin ARD reported in the above studies was higher than this result, which may be due to the fact that conventional boluses placed on the chest wall may exceed the target area. Kaidar-Person et al 4 concluded in the international consensus recommendations that customized bolus can be considered in a limited area to reduce unnecessary toxicity and attain a better dose delivery. In addition, the chest wall becomes thinner after surgery and more sensitive as radiotherapy progresses. When multiple people repeatedly used the same bolus, there might be cross-infection, which wasn't conducive to the recovery of chest wall skin. However, whether the occurrence of skin ARD is related to material and thickness requires further verification.
3D printed bolus, which usually requires a secondary CT simulation, is costly in terms of time and more expensive than conventional bolus.7,25,26 There are also significant differences in softness, hardness, friction coefficient, and other aspects compared to soft tissues of the human body.27,28 Therefore, it's extremely important to develop an individualized bolus with high reproducibility. SR bolus belongs to medical oral impression material, which can be directly in contacted with the patient's skin surface. We have several recommendations for using SR bolus in the workflow: (1) when there is an air gap between the patient's skin and the chest wall due to adverse reactions such as edema, disposable medical tape can be used to stick diagonally on the bolus to increase its adhesion to the skin; and (2) the complete treatment process requires retaining the skin markers of the bolus and placing it in the exact area. The limitation of this study lies in the absence of power calculation for sample size estimation and the small number of patients. Additionally, we didn't measure the volume of the air gap or establish a control group. In subsequent studies, we will conduct a multicenter study with an increased sample size to compare and analyze the bolus made of different materials.
Conclusion
SR bolus production is a convenient and fast workflow, with high adhesion to the chest wall and the ability to be reused. During radiotherapy, FBCTs obtained a reasonable dose distribution in the target volume and OARs compared to the planned CT, maintaining high interfraction stability and an increased skin dose of > 101%. In comparison to previous literature, this study found no grade 3 skin ARD in patients treated with SR bolus. This finding suggests that the use of SR bolus may be worth promoting in clinical practice. However, further discussion is needed to determine whether it can effectively reduce the incidence of skin ARD (grades 1 and 2).
Abbreviations
- SR
silicone rubber
- OAR
organs at risk
- PMRT
postmastectomy radiotherapy
- ARD
acute radiation dermatitis
- FBCT
fan-beam computed tomography
- HI
homogeneity index
- CI
conformity index
- SD
skin dose
- CT
computed tomography
- LRR
local recurrence rate
- OR
overall survival
- 3D
three-dimensional
- PTV
planning tumor volume
- CTV
clinical target volume
- DVH
dose-volume histogram
Footnotes
Authors' Contributions: Xue-mei Chen and Chen-di Xu carried out the data collection, and statistical analysis and drafted the manuscript. Li-ping Zeng and Xiao-tong Huang participated in the data collection and statistical analysis. Ao-qiang Chen, Lu Liu, and Liu-wen Lin reviewed the image registration. Le-cheng Jia and Hua Li exported the data. Xiao-bo Jiang revised and finally approved the manuscript. All authors contributed to the article and approved the submitted version.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The Ethics Committee on Biomedical Research, Sun Yat-sen University Cancer Center, approved this study (Number: B2022-516).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the crosswise project for Shanghai United Imaging Healthcare Co., Ltd (ZLDL-UIH-2022007).
ORCID iDs: Xue-mei Chen https://orcid.org/0000-0003-1909-8530
References
- 1.Remick J, Amin NP. Postmastectomy Breast Cancer Radiation Therapy. In: StatPearls. StatPearls Publishing; 2023:1-12. [PubMed] [Google Scholar]
- 2.Kim D, Kim JH, Kim IA, et al. Impact of postmastectomy radiation therapy on breast cancer patients according to pathologic nodal status after modern neoadjuvant chemotherapy. Cancer Res Treat. 2023;55(2):592-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dahn HM, Boersma LJ, de Ruysscher D, et al. The use of bolus in postmastectomy radiation therapy for breast cancer: a systematic review. Crit Rev Oncol Hematol. 2021;163(7):3-8. [DOI] [PubMed] [Google Scholar]
- 4.Kaidar-Person O, Dahn HM, Nichol AM, et al. A Delphi study and international consensus recommendations: the use of bolus in the setting of postmastectomy radiation therapy for early breast cancer. Radiother Oncol. 2021;164(11):115-121. [DOI] [PubMed] [Google Scholar]
- 5.Das LC, Golden DW, Perevalova E, et al. A feasibility study of 2-mm bolus for postmastectomy radiation therapy. Pract Radiat Oncol. 2017;7(3):161-166. [DOI] [PubMed] [Google Scholar]
- 6.Al-Rahbi ZS, Cutajar DL, Metcalfe P, et al. Dosimetric effects of brass mesh bolus on skin dose and dose at depth for postmastectomy chest wall irradiation. Phys Med. 2018;54(10):84-93. [DOI] [PubMed] [Google Scholar]
- 7.Robar JL, Moran K, Allan J, et al. Intrapatient study comparing 3D printed bolus versus standard vinyl gel sheet bolus for postmastectomy chest wall radiation therapy. Pract Radiat Oncol. 2018;8(4):221-229. [DOI] [PubMed] [Google Scholar]
- 8.Craft DF, Kry SF, Balter P, et al. Material matters: analysis of density uncertainty in 3D printing and its consequences for radiation oncology. Med Phys. 2018;45(4):1614-1621. [DOI] [PubMed] [Google Scholar]
- 9.Gong P, Dai G, Wu X, et al. Application of thermoplastic elastomer (TPE) bolus in postmastectomy radiotherapy. Breast. 2022;66(6):317-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Babic S, Kerr AT, Westerland M, et al. Examination of Jeltrate Plus as a tissue equivalent bolus material. J Appl Clin Med Phys. 2002;3(3):170-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wake JR, Chen FQ, Ashworth S, et al. Verification using in vivo optically stimulated luminescent dosimetry of the predicted skin surface dose in patients receiving postmastectomy radiotherapy. Med Dosim. 2021;46(2):e1-e6. [DOI] [PubMed] [Google Scholar]
- 12.Fagerstrom JM. Dosimetric characterization of a rigid, surface-contour-specific thermoplastic bolus material. Med Dosim. 2019;44(4):401-404. [DOI] [PubMed] [Google Scholar]
- 13.Von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573-577. [DOI] [PubMed] [Google Scholar]
- 14.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the radiation therapy oncology group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31(5):1341-1346. [DOI] [PubMed] [Google Scholar]
- 15.Recht A, Comen EA, Fine RE, et al. Postmastectomy radiotherapy: an American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of surgical oncology focused guideline update. J Clin Oncol. 2016;34(36):4431-4442. [DOI] [PubMed] [Google Scholar]
- 16.Yap ML, Tieu M, Sappiatzer J, et al. Outcomes in patients treated with post-mastectomy chest wall radiotherapy without the routine use of bolus. Clin Oncol (R Coll Radiol). 2018;30(7):427-432. [DOI] [PubMed] [Google Scholar]
- 17.Wong G, Lam E, Bosnic S, et al. Quantitative effect of bolus on skin dose in postmastectomy radiation therapy. J Med Imaging Radiat Sci. 2020;51(3):462-469. [DOI] [PubMed] [Google Scholar]
- 18.Yang C, Yang ZZ, Hu WG, et al. Effects of different bolus strategies on doses in postmastectomy radiotherapy. Chin J Radiol Med Prot. 2023;43(1):30-35. [Google Scholar]
- 19.Dilson L, Challapalli S, Sourjya B, et al. Estimation of surface dose in the presence of unwanted air gaps under the bolus in postmastectomy radiation therapy: a phantom dosimetric study. Asian Pac J Cancer Prev. 2022;23(9):2973-2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lobo D, Banerjee S, Srinivas C, et al. Influence of air gap under bolus in the dosimetry of a clinical 6 MV photon beam. J Med Phys. 2020;45(3):175-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hou YJ YUJP, Wang YQ, et al. Fabrication and pre-clinical application of patient-specific 3D silicone rubber bolus for chest wall. Chin J Radiat Oncol. 2018;27(9):835-838. [Google Scholar]
- 22.Tian XM, Shen ZW, Dong WL, et al. Dosimetric differences in radiotherapy using wet gauze versus bolus for giant cell tumor of tendon sheath. Chin J Med Phys. 2020;37(6):680-684. [Google Scholar]
- 23.Fiedler DA, Hoffman S, Roeske JC, et al. Dosimetric assessment of brass mesh bolus and transparent polymer-gel type bolus for commonly used breast treatment delivery techniques. Med Dosim. 2021;46(3):e10-e14. [DOI] [PubMed] [Google Scholar]
- 24.Abel S, Renz P, Trombetta M, et al. Local failure and acute radiodermatological toxicity in patients undergoing radiation therapy with and without postmastectomy chest wall bolus: Is bolus ever necessary? Pract Radiat Oncol. 2017;7(3):167-172. [DOI] [PubMed] [Google Scholar]
- 25.Baltz GC, Chi PM, Wong PF, et al. Development and validation of a 3D-printed bolus cap for total scalp irradiation. J Appl Clin Med Phys. 2019;20(3):89-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kong Y, Yan T, Sun Y, et al. A dosimetric study on the use of 3D-printed customized boluses in photon therapy: a hydrogel and silica gel study. J Appl Clin Med Phys. 2019 Jan;20(1):348-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawamoto T, Shikama N, Kurokawa C, et al. Dosimetric assessment of bolus for postmastectomy radiotherapy. Med Dosim. 2021;46(1):e1-e4. [DOI] [PubMed] [Google Scholar]
- 28.Lu Y, Shi QY, Wang Y, et al. Research progress on bolus materials used for radiotherapy. Chin J Radiat Oncol. 2022;31(5):488-492. [Google Scholar]