Highlights
-
•
Nutrient depletion (ND) can enable survival via the activation of partial (p)-EMT.
-
•
V-ATPase inhibition with ND reduced cell adhesion, exacerbated motility and p-EMT.
-
•
Membrane protrusions via microtubule reorganization led to lysosomal relocalization.
-
•
Lysosomal exocytosis may increase invasive phenotype.
Keywords: Nutrient restriction; Lysosome, cytoskeleton; Partial EMT
Abstract
Introduction
Nutrient restriction in cancer cells can activate a number of stress response pathways for cell survival. We aimed to determine mechanistically how nutrient depletion in colorectal cancer (CRC) cells leads to cellular adaptation.
Materials and methods
Cell survival under nutrient depletion (ND) was evaluated by colony formation and in vivo tumor formation assays. Lysosomes are activated with ND; therefore, we incubated the ND cells with the V-ATPase inhibitor Bafilomycin A1 (ND+Baf). The expression of epithelial and mesenchymal markers with ND+Baf was determined by RNA sequencing and RT-qPCR while motility was determined with an in vivo Chorioallantoic membrane (CAM) assay. Reorganization of cytoskeletal network and lysosomal positioning was determined by immunocytochemistry.
Results
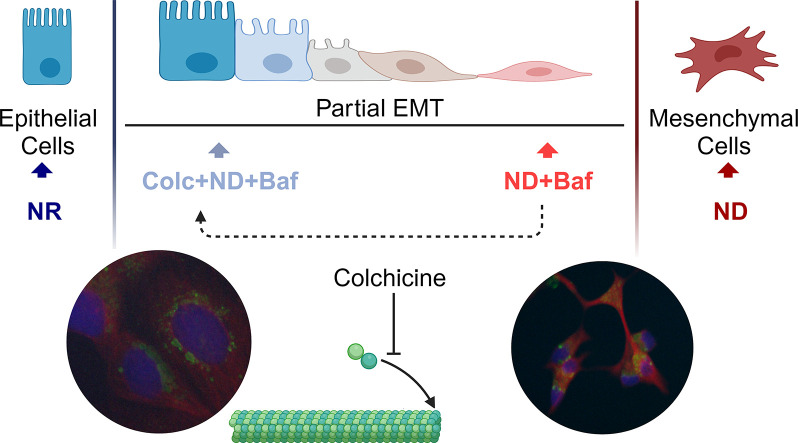
4 different colorectal cancer (CRC) cell lines under ND showed high viability, tumor forming ability and increased expression of one or more epithelial and mesenchymal markers, suggesting the activation of partial (p)-EMT. We observed a further increase in p-EMT markers, numerous membrane protrusions, decreased cell-cell adhesion in 3D, and increased motility in ND+Baf cells. The protrusions in the ND+Baf cells were primarily mediated by microtubules and enabled the relocalization of lysosomes from the perinuclear region to the periphery.
Conclusions
ND activated p-EMT in CRC cells, which was exacerbated by lysosomal alkalinization. The ND+Baf cells also showed numerous protrusions containing lysosomes, which may lead to lysosomal exocytosis and enhanced motility.
Graphical abstract
Introduction
Exogenous nutrient sources can fluctuate greatly during tumor development, which can affect cell cycle progression and proliferation. Cancer cells undergo metabolic adaptations via oncogenic signaling as well as intercellular crosstalk to strengthen their ability to survive and proliferate under low nutrient availability [1]. Such adaptations include autophagy, epithelial to mesenchymal transition (EMT) and chemoresistance, among others [2].
EMT is a highly complex sequence of events that includes loss of contact between adherent epithelial cells, change of morphology and enhanced motility by the acquisition of mesenchymal characteristics [3]. Conventional EMT is characterized by a complete loss of epithelial characteristics such as the expression of the junctional proteins E-cadherin and Occludin and acquisition of mesenchymal properties such as the expression of the cytoskeletal protein Vimentin or transcription factors such as Snail or Slug [4]. Recent studies, however, suggest that rather than the binary process of a complete transition from an epithelial to a mesenchymal phenotype, disseminating cells may acquire a hybrid phenotype whereby there is a gradual conversion of epithelial cells to a mesenchymal form through a number of intermediate stages [5]. Cells that show characteristics of partial EMT (p-EMT) pose a greater risk of metastatic spread compared to cells showing complete EMT and are associated with poor prognosis in cancer [4,6].
The tumor microenvironment (TME), consisting of cells such as fibroblasts, immune cells, endothelial cells and the extracellular matrix (ECM) play a crucial role in the dissemination of cancer cells [7,8]. During EMT, transformed cells can secrete growth factors and cytokines that can recruit stromal cells from the TME, while the stromal cells secrete essential nutrients, ECM remodeling enzymes and growth factors that favor survival and dissemination of cancer cells [9]. Cancer associated fibroblasts (CAF) and tumor associated macrophages (TAM) have been particularly implicated in EMT. CAFs were shown to release growth factors that could activate EMT transcription factors in lung cancer [10] while the inflammatory microenvironment provided by TAMs can partially guide migrating cancer cells [11]. Desmoplastic tumors such as pancreatic ductal adenocarcinomas have extensive ECM that can prevent access to therapeutic agents and thereby contribute to worse prognosis in several different tumor types [12]. The presence of a stiff ECM has been shown to directly enhance the translocation of EMT transcription factors to enhance cellular dissemination [13].
The lysosome is a very dynamic organelle that orchestrates various catabolic processes during nutrient depletion via sensing, signaling, and transcriptional regulation [14]. One of the primary pathways that is activated with low nutrient availability is autophagy, whereby intracellular macromolecules are degraded in the lysosome [15]. A defining characteristic of lysosomes is an internal acidic pH ranging from 4.5 to 5.5 that is maintained by large multimeric channels called vacuolar H+ ATPases (V-ATPase) [16]. A number of hydrolases that have an optimal acidic pH enable the lysosome to hydrolyze a vast repertoire of substrates [17], allowing the recycling of macromolecules and removal of damaged and exhausted organelles. Several studies have shown that lysosomal degradation of key cell-cell junctional proteins [18] or proteins that enable cell-matrix interactions are necessary for the dissemination of cancer cells [19].
The cytoskeleton is a pivotal contributor to the cell's structural framework and is responsible for the mechanical strength needed to establish cell shape and movement. Most solid tumors undergo cytoskeletal rearrangements during EMT, in which cortical actin is re-organized into highly aligned actin stress fibers. This, in turn, plays an important role in changing the elasticity and ability of cancer cells to migrate even through constricted spaces within the TME [20]. Microtubules are also known to be closely involved in cellular dissemination during EMT. For example, detyrosination of α-tubulin in detached mammary epithelial cells undergoing EMT was shown to form micro-tentacles that could help these cells to attach to endothelial cells [21]. Post-translational modifications such as acetylation of a critical lysine residue at the N-terminus of α-tubulin is a marker of stable microtubules [22]. Both acetylation and deacetylation of α-tubulin have been shown to enhance EMT and drug resistance in epithelial cells [23,24].
In the current study we used a nutrient deficiency protocol in which cells were incubated in 10 % of the glucose, glutamine and serum available in complete medium [25]. Nutrient deficiency was associated with the development of quiescence but high survival in several different cell lines. Focusing on Caco-2 cells that showed the highest increase in both epithelial and mesenchymal markers, suggesting the development of p-EMT, we examined the role of the lysosome in nutrient depletion (ND) induced p-EMT. Inhibition of lysosomal acidity with either Bafilomycin A1 (Baf) or NH4Cl led to the ND cells shifting more towards the mesenchymal spectrum, with a remarkable elongation in cell shape that was primarily mediated by the tubulin cytoskeleton. Baf treated ND cells were also significantly more capable of motility in an in vivo chorioallantoic membrane (CAM) assay. Additionally, the lysosomes relocalized to the cellular protrusions where they presumably released their contents, contributing to an invasive phenotype. Overall, our data suggest that ND can lead to increased crosstalk between lysosomal signaling and the microtubule network, enhancing cell motility and survival.
Materials and methods
Cell culture Caco-2, HCT116, T84, RKO and LoVo cells were cultured as described in Supplementary Materials. For nutrient depletion, the cells were incubated for 48 h in glucose and glutamine free DMEM (Caco-2, T84, RKO) or glucose and glutamine free RPMI (LoVo and HCT-116) supplemented with 1 % FBS, 0.1 g/L glucose and 0.2 mM L-glutamine, 1 mM sodium pyruvate, 0.1 mM non-essential amino acids and 1 % penicillin/streptomycin. Caco-2 and T84 cells can undergo confluency dependent differentiation and were therefore cultured at sub-confluency. More details are provided in Supplementary Materials.
Treatments ND or nutrient rich (NR) cells were treated with pre-optimized concentrations of Bafilomycin A1 (Baf) (5 or 100 nM), Cytochalasin B (100 nM) or Colchicine (25 nM) for 6–48 h. More details are provided in Supplementary Materials.
RNA isolation, RT-qPCR and RNA sequencing RNA was isolated from 70 to 80 % confluent cells incubated in ND or NR medium and/or with the drugs described in Supplementary Table 1 using the Macherey Nagel RNA isolation kit. RNA with RIN>8.0 was sent for RNA sequencing (3 replicates per sample). For qRT-PCR, the RNA was treated with DNase I, converted to cDNA, and quantified by qPCR. Further details and primer sequences are provided in Supplementary Materials.
Protein isolation and western blotting Proteins were isolated from 70 to 80 % confluent cells incubated in ND or NR medium using MPER protein isolation kit (Promega) supplemented with protease and phosphatase inhibitors. Western blotting was carried out with 15–20 µg of protein using standard techniques. Further details and the list of antibodies are provided in Supplementary Materials.
CAM assay Fertilized specific-pathogen-free Leghorn eggs were obtained from Tavukçuluk Araştırma Enstitüsü (Ankara, Türkiye). On the 9th day of embryonic development, 1 × 106 viable cell pellets (determined with a Trypan Blue exclusion assay) obtained after incubation in ND or NR medium for 48 h or treated with 100 µM Baf in ND or NR medium (Caco-2 cells only) in 20 µl medium was mixed with 20 µl Matrigel (Corning) and placed on the CAM of the developing embryos. The eggs were placed in an incubator at 37 °C with 60–70 % humidity and incubated for another 5 days, after which the microtumors were harvested for volume measurement or embedding for immunohistochemistry. Liver samples from the developing chicks were isolated after the tumors were removed. Lack of any cross contamination between the tumor and liver samples was ensured. For further details please see Supplementary Materials.
Immunocytochemistry Caco-2 cells incubated in ND or NR medium and/or treated with Baf (100 nM) or colchicine (25 nM) were fixed in 4 % paraformaldehyde and processed for incubation in a primary antibody against LAMP1, or α-Tubulin, followed by incubation with an Alexa Fluor conjugated secondary antibody. Where indicated, the fixed cells were also incubated with Alexa Fluor® 405 Phalloidin to detect F-Actin. Nuclear staining was carried out with DAPI. Further details are provided in Supplementary Materials.
Bioinformatics data analyses
Raw read counts of genes were provided by SZAOMICS Laboratories (Istanbul, Turkiye). tximport package [26] from Bioconductor was used to read the raw data and convert the count data to an expression matrix. DESeq2 package [27] was used for normalization of the data and testing for differential expression (DE). For DE analysis, genes with FDR < 0.01 and logFC < −1 or > 1 were considered significant. Further details are provided in Supplementary Methods.
Statistical analyses All experiments were carried out with at least 2–3 biological replicates with 3–4 technical replicates unless otherwise stated. Individual statistical analyses for the in silico data are provided in the figure legends or in Supplementary Materials. Data analyses and graphing were carried out with Graph Pad (v.9).
Results
Nutrient deficiency selects for a subgroup of cells that are quiescent, but highly viable
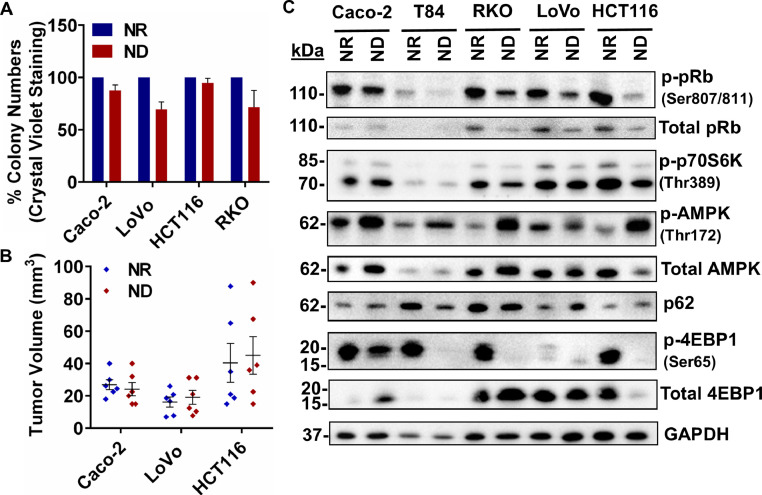
Caco-2, HCT116, LoVo and RKO colon cancer cell lines were incubated in the ND medium for 48 h and the viability of the cells was determined with an in vitro colony formation assay (Fig. 1A, Supplementary Figure 1A) as well as an in vivo CAM assay (Fig. 1B, Supplementary Figure 1B). The colony formation assay indicated that the number of colonies formed in the NR medium was statistically similar to the number of colonies formed in the ND medium, indicating high viability. We have previously reported similar changes in ND T84 [25], HT-29 and DLD1 cells [28]. High cell survival was corroborated by the CAM assay, whereby Caco-2, HCT116 and LoVo cells incubated in the ND medium were capable of forming tumors with comparable size and volume to the cells incubated in the NR medium. Western blot analysis (Fig. 1C) indicated that all cell lines incubated in the ND medium for 48 h showed increased phosphorylation of AMP Kinase (AMPK) at T172 and an inhibition of mTOR, represented by decreased phosphorylation of p70S6K (T389) and 4E-BP1 (S65). Additionally, all cell lines under nutrient depletion showed hypo-phosphorylation of the retinoblastoma protein (pRb) at S807/811, suggesting that the cells were quiescent and arrested at the G1/S phase [29].
Fig 1.
Colorectal cancer cell lines undergoing nutrient deficiency are highly viable and show the activation of nutrient sensing pathways and quiescence. (A) Colony formation assay. (B) Tumor volumes in an in vivo CAM assay (n = 6). (C) Western blot analysis of NR and ND treated CRC cells. Densitometric analyses are shown in Supplementary Figure 1C. Data are represented as mean ± SEM.
Nutrient deficiency enhances partial EMT (p-EMT) in cancer cells
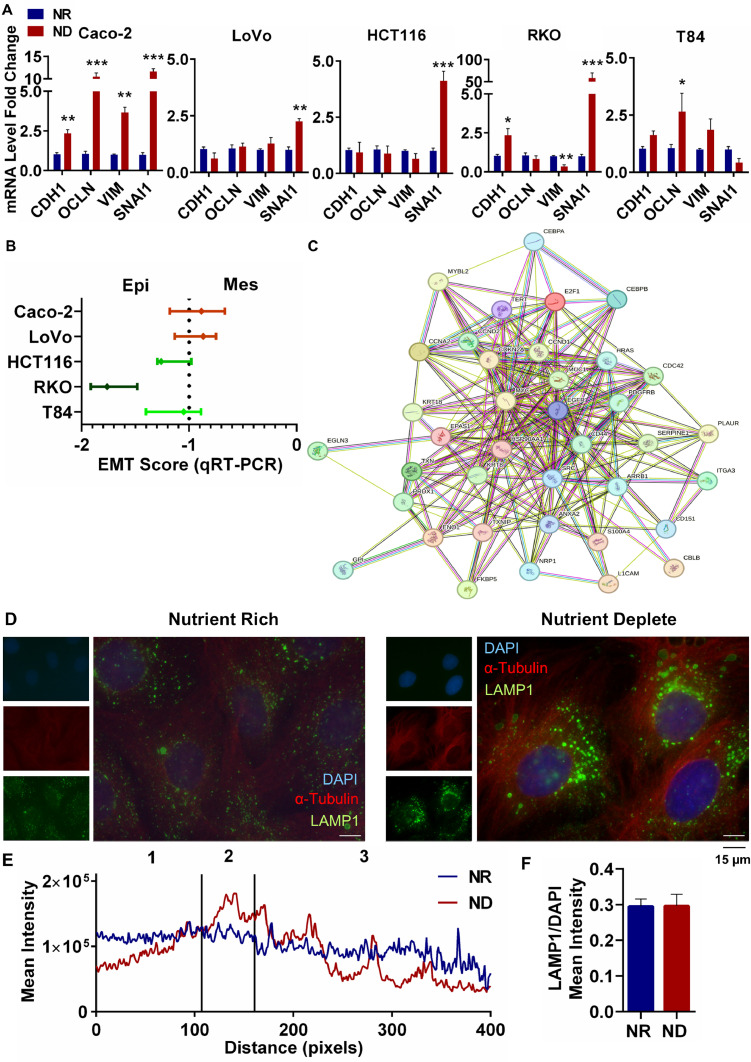
We have recently reported that nutrient restriction in HCT116 colorectal cancer cells was associated with a p-EMT related gene signature [28]. Additionally, we have reported increased drug resistance in several different colorectal cancer cell lines incubated in the ND medium [25]. The highly surviving quiescent cells examined in the current study were capable of tumor formation (Fig. 1A, B), which suggested the activation of oncogenic signaling such as EMT in the ND cells. We carried out a qRT-PCR for the well-established EMT markers CDH1, OCLN, SNAI1 and VIM in 5 different cell lines incubated in the ND or NR medium (Fig. 2A). We observed a significant increase in the expression of one or more epithelial as well as mesenchymal markers in all cell lines under ND. Amongst these cell lines, ND Caco-2 showed a significant increase in expression of all four markers (Fig. 2A); additionally, calculation of an EMT score [30] indicated that ND Caco-2 cells were the most mesenchymal (Fig. 2B).
Fig 2.
Activation of partial EMT in nutrient deficient Caco-2 cells. (A) qRT-PCR of EMT genes in NR and ND treated CRC cells. (B) EMT Score of ND CRC cell lines. (C) STRING analysis of significantly (p<0.01, LFC ±1) DE genes related to EMT. (D) Representative images of IF staining for α-Tubulin and LAMP1 in NR and ND Caco-2 cells. (E) Subcellular localization analysis of LAMP1 signal. Nuclear (1), perinuclear (2), and peripheral (3) regions. (F) Analysis of lysosomal abundance. Data are represented as mean ± SEM (*p < 0.05, **p < 0.01, ***p < 0.001 with respect to NR).
We therefore carried out an RNA sequencing analysis with Caco-2 cells incubated for 48 h in the ND or NR medium. Enrichment analysis of Human Molecular Signature Database (MSigDB) Collection C5 (Ontology gene sets) confirmed significant changes in a number of different gene ontology (GO) terms related to nutrient restriction and quiescence (Supplementary Figure 2A). A STRING analysis of significantly (FDR<0.01, LFC ±1) differentially expressed (DE) genes related to EMT indicated the formation of a highly connected network of EMT proteins (Fig. 2C). Several oncogenic signaling proteins such as EGFR, MYC, SRC, HRAS and PDGFRB formed the core of the interaction network, along with cell cycle related proteins such as CDKN2A, CCND1, CCND2, CCNA2, E2F1, as well as cell-cell adhesion and cytoskeleton related proteins such as ITGA3, KRT18 and L1CAM, among others. This network strongly suggests a robust alteration in several key cellular processes when nutrients are depleted.
One of the organelles that plays a central role in nutrient deficiency is the lysosome [31]. We have reported that T84 cells under ND showed remarkable changes in lysosomal numbers, shape and positioning, increasing in size and moving from the periphery to the perinuclear region [25]. We therefore examined whether ND resulted in any changes in lysosomal numbers or localization in Caco-2 cells. Using immunocytochemistry (Fig. 2D, F) and western blot (Supplementary Figure 2B), we observed that although the expression of the lysosomal surface protein LAMP1 [32] did not change between NR and ND cells, many more lysosomes were located in the perinuclear region in the ND Caco2 cells (Fig. 2D, E). This suggests that nutrient depletion in Caco-2 cells affected the localization of the lysosomes rather than their numbers.
Lysosomal alkalinization exacerbates the p-EMT phenotype
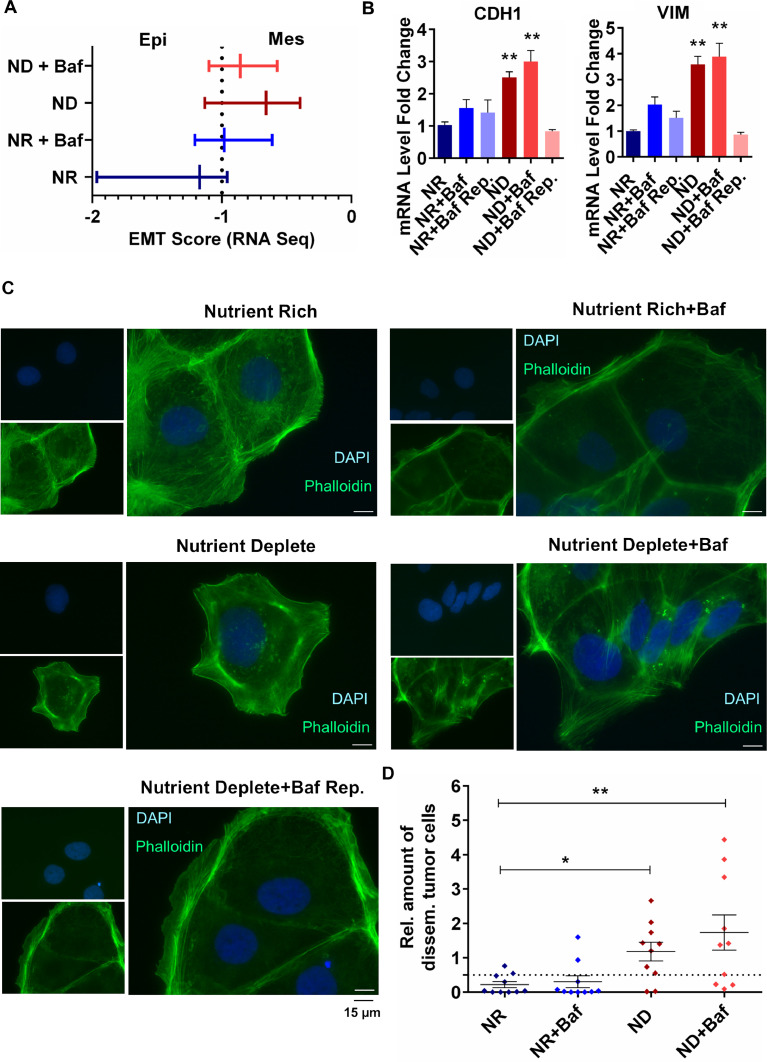
To further explore whether altered lysosomal activity plays a role in the p-EMT phenotype observed in ND Caco-2 cells, we treated the NR and ND Caco-2 cells with 100 nM of the V-ATPase inhibitor Bafilomycin A1 (Baf) that increases the pH of the lysosomes. An EMT score generated from RNA sequencing data from these cells indicated that compared to NR or NR+Baf cells, ND and ND+Baf cells were more mesenchymal (Fig. 3A). This was confirmed by a further increase in CDH1 and VIM expression (Fig. 3B) as well as OCLN and SNAI1 expression (Supplementary Fig. 3A) in ND+Baf cells by qRT-PCR, which could be reverted to control levels when Baf was removed and replaced with complete medium. We also tested whether lysosomal alkalinization led to any alteration in 3D spheroid formation by Caco-2 cells. We observed that NR Caco-2 cells treated with vehicle or Baf were able to form comparable compact spheroids with relatively non-uniform edges (Supplementary Figure 3B). However, with nutrient deficiency, the Caco-2 cells formed loose cell clusters rather than spheroids; this phenotype was further enhanced when the cells were treated with 100 nM Baf (Supplementary Figure 3B), suggesting that lysosomal alkalinization could result in weaker cell-cell adhesion, especially in ND cells. Of note, the loss of cell-cell adhesion in spheroids was irreversible; passaging the cells in complete medium and re-plating in ULA plates could not restore spheroid formation. The spheroids/cell clusters were collected and qRT-PCR was carried out from the RNA isolated from these cells. Similar to the 2D cultured cells, an increase in both epithelial (CDH1 and OCLN) and mesenchymal (VIM and SNAI1) markers was observed when the ND (but not NR) Caco-2 cells were treated with Baf (Supplementary Figure 3C). This was supported by light microscopy images showing an elongation of ND (but not NR) Caco-2 cells treated with either Baf or NH4Cl (Supplementary Figure 3D); moreover, the change in shape could be reversed within 2–6 h when Baf was removed (Supplementary Figure 3E). Further evaluation of these cells with immunocytochemistry also confirmed that the spindle-like shape of the ND+Baf Caco-2 cells was due to the formation of actin stress fibers compared to the ND cells (Fig. 3C). The ND+Baf cells that were located at the edges of clusters showed projections that were rich in actin stress fibers (Supplementary Fig. 3F). Of note, the NR cells incubated with vehicle or Baf did not form actin stress fibers.
Fig 3.
Exacerbation of the partial EMT phenotype with lysosomal alkalinization. (A) EMT Score based on RNA Sequencing of NR and ND Caco-2 cells treated with BafA1 (B) qRT-PCR of CDH1 and VIM in BafA1 treated and replenished cells, t-test. (C) Representative images of immunofluorescence staining for Phalloidin in NR and ND Caco-2 cells treated with BafA1. (D) Relative amount of disseminating tumor cells in the liver of chick embryos assessed by Alu qRT-PCR (n = 10, Mann Whitney U test). The dashed line represents the cut-off for metastasis detection at 0.5. Data are presented as mean ± SEM (*p<0.05, **p<0.01 with respect to NR).
We next wanted to evaluate whether treatment with Baf could lead to enhanced motility of Caco-2 cells. For this, we placed NR, NR+Baf, ND and ND+Baf cells on the CAM of fertilized Leghorn eggs. All of the cell groups led to the formation of tumors, with the tumor volume being highest for the NR+Baf and the lowest for ND+Baf (Supplementary Figure 3 G), which was also supported by Ki67 staining in the tumors (Supplementary Figure 3H) and BrdU proliferation staining in vitro cultured Caco-2 cells (Supplementary Figure 3I). We isolated liver samples from the chicks at the time of collection of tumors, isolated RNA and carried out qRT-PCR for Alu. Since colorectal cancer cells mainly metastasize to the liver, this organ was selected. Alu PCR indicated that most ND and ND+Baf samples exceeded the calculated dissemination threshold of 0.5, suggesting that these cells could disseminate to the chick liver more than the NR and NR+Baf samples (Fig. 3D).
The cytoskeletal network contributes towards p-EMT phenotype in nutrient deficient cells
The formation of actin stress fibers in the ND+Baf Caco-2 cells could be reversed when Baf was removed from the culture medium (Fig. 3C). This pointed towards a rapid post-translation modification of the existing cytoskeletal network. The actin and tubulin cytoskeletal networks are highly dynamic and can both contribute towards cellular motility [33]. To better understand the mechanism behind the remarkable change in shape of ND Caco-2 cells with Baf, we evaluated the RNA sequencing data. We first identified the genes that were exclusively differentially expressed in the ND+Baf Caco-2 cells compared to the NR cells (but not in ND or NR+Baf cells) with LFC of ± 1, adj_p < 0.01. These genes were used for a STRING analysis, and the proteins that had a combined score greater than 0.990 were used for a gene ontology (GO) analysis. We observed significant enrichment in GO terms such as “Microtubule Cytoskeleton Organization” (GO:0,000,226, p = 1.06E-05), for Biological Function, “Microtubule Cytoskeleton” (GO:0,015,630, p = 4.64E-04) for Cellular Location and “Cell Adhesion Molecule Binding” (GO:0,050,839, p = 4.31E-05) for Molecular Function. Additionally, evaluation of the protein products of the significantly differentially expressed genes associated with EMT exclusively in ND+Baf Caco-2 cells showed a rich and highly connected network of proteins (Supplementary Fig. 4A). The proteins in this network were distinct from the proteins that were DE in ND Caco-2 cells, compared to the NR cells (Fig. 2C). The core of the network consisted of key EMT proteins such as TGFB1, TGFB3, TGFBR2 and SMAD4, DNA damage response proteins, immune response proteins, along with critical proteins implicated in development and epigenetic regulation. A number of cell adhesion, cytoskeletal and motor proteins were also identified in this network (Supplementary Figure 4A).
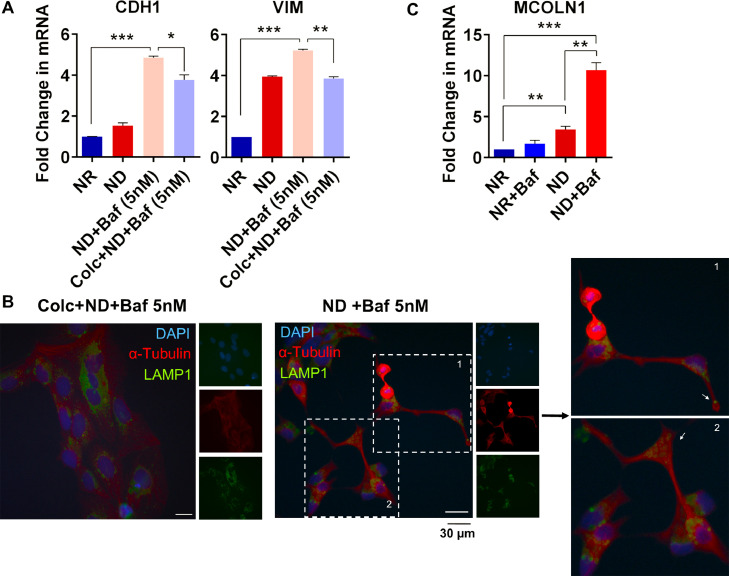
We incubated the ND+Baf or vehicle treated Caco-2 cells with the actin polymerization inhibitor cytochalasin B or the microtubule polymerization inhibitor colchicine. Light microscopic observations indicated that the elongated shape of the ND+Baf cells could be partially reversed with colchicine, but not with cytochalasin B (Supplementary Figure 4B). We carried out qRT-PCR for CDH1 and VIM in ND+Baf Caco-2 cells alone, or with colchicine (Fig. 4A). We observed that treatment with colchicine could modestly but significantly decrease the expression of both CDH1 and VIM. We next carried out an immunocytochemistry assay in the ND+Baf Caco-2 cells. The Baf treated cells again showed an elongated spindle like shape with long extensions that contained microtubules (Fig. 4B). Microtubules and associated motor proteins are responsible for the subcellular localization of lysosomes. Indeed, we observed that LAMP1 positive lysosomes could be located at the long extensions formed in the Baf treated cells (Fig. 4B). A decrease in the formation of protrusions was also observed with colchicine treatment. For this, 50 cells were counted; 44 % of the ND+Baf cells showed protrusions which decreased to 16 % when the cells were pre-treated with colchicine.
Fig 4.
Involvement of the cytoskeletal network in the induction of partial EMT with nutrient deficiency. (A) qRT-PCR of CDH1 and VIM in Colc+BafA1 (5 nM) treated ND Caco-2 cells. Data are presented as mean ± SD. (B) Representative images of IF staining for α-Tubulin and LAMP1 in ND+BafA1 (5 nM) treated Caco-2 cells with or without Colchicine (Colc). Zoomed images are shown in the right panel. White arrow heads indicate the lysosomes in protrusions. (C) qRT-PCR of MCOLN1 in nutrient-restricted and BafA1 (100 nM)-treated Caco-2 cells. Data are presented as mean ± SEM. (*p < 0.05, **p < 0.01, ***p < 0.001, t-test).
To determine whether Ca2+ mediated signaling could be implicated in the fusion of lysosomes with the plasma membrane in ND+Baf cells, we evaluated the RNA sequencing data. For this, we identified the genes that were significantly differentially expressed in the ND+Baf cells compared to the NR cells (LFC±1, adj_p < 0.01) followed by a STRING analysis with a combined score > 0.990 for both up and down regulated genes, separately. A GO analysis revealed significant enrichment of the term “Regulation of Calcium ion dependent exocytosis” (FDR = 0.049) among the upregulated genes. Transient receptor potential cation channel, mucolipin subfamily, member 1 (TRPML1), encoded by MCOLN1, is a cation channel that mediates Ca2+release from lysosomes. We observed that the expression of MCOLN1 increased by around 3 folds in ND cells compared to NR cells (p<0.01, Fig. 4C). Upon treatment with Baf, we observed no significant change in MCOLN1 expression in the NR cells (p>0.05) whereas Baf treatment of the ND cells showed a remarkable and statistically significant (p<0.001) increase in expression.
Discussion
Loss of stimulatory signals such as during nutrient deprivation can cause cells to enter a reversible quiescent state that is characterized by low metabolic activity, exit from the cell cycle in proliferative cells and low rates of transcription and protein synthesis. Such cells activate adaptive mechanisms that help them survive and become more motile to escape challenging metabolic conditions [34]. Energy stress leading to the activation of AMPK was recently shown to enhance cellular motility by decreasing cell-cell adhesion [35]. Supporting this, several different CRC cell lines incubated for 48 h in a nutrient depleted (ND) medium showed increased expression of one or more epithelial and mesenchymal markers suggesting the activation of hybrid/partial EMT in these cells. RNA sequencing analysis of the nutrient rich (NR) and ND Caco-2 cells, which showed the highest EMT score, revealed the activation of autophagy, cell cycle arrest and a decrease in energy generating pathways with ND, as expected, while at the same time differentially regulating a highly connected network of EMT related genes. This network had EGFR and SERPINE1 at its hub, supporting a recent three gene p-EMT signature consisting of EGFR, SERPINE 1 and SOX10 that we identified in nutrient depleted HCT116 cells, which could significantly predict worse disease-free survival (DFS) in multiple CRC patient cohorts [28]. CRC tumors showing the three gene signature were categorized as Consensus Molecular Subtype (CMS) 4, associated with a metastatic and invasive phenotype [36].
The lysosome plays a critical role in response to the stress of nutrient deprivation. We have recently reported that nutrient depletion led to a change in the localization of lysosomes to the perinuclear region; these active lysosomes were capable of trapping chemotherapy drugs, which contributed to drug resistance [25]. We therefore wanted to examine whether lysosomal alkalinization could reverse the effects of nutrient depletion on p-EMT. However, we observed that treatment of Caco-2 cells with either Baf or the weak base NH4Cl led to a remarkable change in the shape of the cells from a cobblestone shape to a spindle like morphology with the formation of actin stress fibers and long protrusions, which could be rapidly reversed when the treatment was removed. Baf treatment of the ND cells also shifted the EMT score to reflect a more mesenchymal phenotype and was supported by the formation of an extensive and well connected EMT related protein-protein interaction network. Modeling studies using a spheroidal model of a tumor have shown that slow fluctuations in nutrient availability could favor the fittest cells for tumor evolution; these cells had low cell-cell adhesion and high motility [37]. We observed that ND+Baf Caco-2 cells grown as spheroids showed low cell-cell adhesion compared to the ND cells. The Baf treated cells were also highly motile and could migrate from the CAM tissue to the liver of chicks in a CAM tumor model.
The cytoskeletal network is known to contribute to the shape of cells [38]. Microtubules were shown to be necessary for the formation of long protrusions in astrocytes, whereas actin stress fibers were mainly responsible for the anchoring of cells via focal adhesions [39]. The same study reported that inhibitors of microtubule dynamics could inhibit the formation of the protrusions, while cytochalasin B (actin polymerization inhibitor) could not [39]. Analysis of RNA sequencing data in the current study indicated that GO terms related to the cytoskeleton were significantly enriched exclusively in ND+Baf Caco-2 cells. We also observed that incubation of the cells with colchicine but not cytochalasin B led to a reversal in the spindle like shape observed in ND+Baf cells, suggesting that the protrusions were mainly formed of microtubules. Of note, we observed a number of LAMP1 positive lysosomes in the long protrusions formed in the ND+Baf cells.
Alkaline lysosomes tend to locate towards cellular periphery; additionally, alkalinization can increase the fusion of multivesicular bodies with the plasma membrane as well as lysosomal exocytosis [40]. The fusion of lysosomes to the plasma membrane is known to be mediated by local alterations in Ca2+ion concentration [41]. Lysosomes are known to sequester Ca2+ at levels that are far higher than the cytosolic levels, but lower than the extracellular levels [42]. V-ATPase inhibition can enhance the release of Ca2+ ion from lysosomes, suggesting that maintenance of a steep Ca2+ gradient may be mediated, at least in part, by the proton gradient [42]. The release of Ca2+ from lysosomes is mediated by cation channels such as TRPML1, which in turn was shown to enhance lysosomal exocytosis [43]. We observed a remarkable increase in the expression of MCOLN1 (gene encoding TRPML1) in the ND+Baf cells (approximately 10-fold) compared to the NR cells.
The interplay between lysosomal function and EMT is likely to be complex and vary in a context- and cell type-dependent manner. Our observation of enhanced expression of p-EMT markers and cellular dissemination upon lysosomal alkalinization with Baf contradicts previous observations showing a role of active lysosomes in the regulation of EMT [14]. Lysosomal degradation of key EMT related proteins has been proposed to be one of the primary regulatory mechanisms in EMT [44]. For example, EMT induction was shown to be accompanied by the degradation and inhibition of recycling of E-cadherin in the lysosome [18]. Release of the lysosomal enzymes such as cathepsins to the extracellular matrix by lysosomal exocytosis was shown to enhance invasion and migration in liver cancer cells [45]. On the other hand, cells with defective autophagy exhibited stabilization of the EMT transcription factor Twist1 upon binding to the adaptor protein SQSTSM1/p62 to enhance EMT [46]. Lysosomal alkalinization with Baf was shown to activate EMT in non-transformed renal podocytes. Mechanistically, increased p62 accumulation with lysosomal dysfunction and a decrease in its phosphorylation via CDK1 was shown to enhance the expression of mesenchymal markers [47]. We did not observe any difference in the accumulation of p62 when ND or NR Caco-2 cells were incubated with Baf (data not shown). We suggest an alternative mechanism whereby lysosomal alkalinization in ND cells led to deregulation of the tubulin cytoskeletal network, followed by the translocation of lysosomes to the periphery, potentially enhancing lysosomal exocytosis. Future experiments will reveal whether the invasive phenotype observed with lysosomal alkalinization of nutrient depleted cells could have resulted from localized alterations in lysosomal Ca2+ homeostasis, causing fusion with the plasma membrane and the release of matrix degrading enzymes.
Conclusions
Research in the past decade has revealed the high metabolic plasticity of cancer cells in obtaining nutrients from extracellular (through micropinocytosis or from non-transformed cells in the TME) or intracellular (via activation of stress response pathways) sources to enable survival. Interest in better understanding how cancer cells survive under nutrient stress is likely to continue in the coming decade. Our research has revealed that nutrient depletion selected a group of cancer cells that were highly adaptive and activated p-EMT. Additionally, nutrient depleted cells revealed an involvement of lysosomes such that the alkalinization of lysosomes in the nutrient depleted cells resulted in the formation of long cellular protrusions via microtubule reorganization where lysosomes were shown to be located. Ca2+ mediated exocytosis of lysosomal constituents may have contributed towards the low cellular adhesion and increased motility.
Ethical approval statement
No ethical approval was necessary for this research.
Funding
The study was funded by TÜBİTAK 1001 project (118Z116) to SB and BAP Project (TEZ-D-108–2022–10899) to HHH and SB. AEGT, HHH, GO, and NSM were supported by BIDEB 2210 and 2211. AN was supported by TÜBİTAK 2209-A (1919B012305767).
Availability of data and materials
RNA sequencing data generated for the current study has been uploaded to GEO (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE245402). The data and R script used in the study will be made available upon request.
CRediT authorship contribution statement
H. Hazal Hüsnügil: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review & editing. Aliye Ezgi Güleç Taşkıran: Conceptualization, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. Ismail Güderer: Data curation, Formal analysis, Investigation. Leman Nur Nehri: Conceptualization, Formal analysis, Investigation, Methodology. Göksu Oral: Investigation, Methodology. Nazlı Şevval Menemenli: Investigation, Methodology. Özün Özcan: Investigation, Methodology. Ariana Noghreh: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. Aytekin Akyol: Formal analysis, Investigation, Methodology. Sreeparna Banerjee: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to acknowledge Dr Regine Schneider-Stock, Dr Chuanpit Hampel, and Dr Kerstin Huebner (FAU Erlangen, Germany), Dr Ayşe Elif Erson Bensan (Orta Dogu Teknik Universitesi, Ankara, Turkiye) and Dr Ozlen Konu (Bilkent Universitesi, Ankara, Turkiye) and Dr Seçil Demirkol-Canlı (Hacettepe Universitesi, Ankara, Turkiye) for advice and useful discussions.
Supplementary materials
Supplementary materials are available for this article.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2023.101860.
Appendix. Supplementary materials
References
- 1.Comito G., Ippolito L., Chiarugi P., Cirri P. Nutritional exchanges within tumor microenvironment: impact for cancer aggressiveness. Front. Oncol. 2020;10:396. doi: 10.3389/fonc.2020.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaziri-Gohar A., et al. Limited nutrient availability in the tumor microenvironment renders pancreatic tumors sensitive to allosteric IDH1 inhibitors. Nat. Cancer. 2022;3(7):852–865. doi: 10.1038/s43018-022-00393-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bocci F., Schneider-Stock R., Banerjee S. Editorial: epithelial to mesenchymal plasticity in colorectal cancer. Front. Cell Dev. Biol. 2022;10 doi: 10.3389/fcell.2022.950980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sinha D., Saha P., Samanta A., Bishayee A. Emerging concepts of hybrid epithelial-to-mesenchymal transition in cancer progression. Biomolecules. 2020;10(11):1–22. doi: 10.3390/biom10111561. 1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saitoh M. Involvement of partial EMT in cancer progression. J. Biochem. 2018;164(4):257–264. doi: 10.1093/jb/mvy047. [DOI] [PubMed] [Google Scholar]
- 6.Pastushenko I., Blanpain C. EMT transition states during tumor progression and metastasis. Trends Cell Biol. 2019;29(3):212–226. doi: 10.1016/j.tcb.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Carloni R., et al. Targeting tumor microenvironment for cholangiocarcinoma: opportunities for precision medicine. Transl. Oncol. 2022;25 doi: 10.1016/j.tranon.2022.101514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rihawi K., et al. Tumor-associated macrophages and inflammatory microenvironment in gastric cancer: novel translational implications. Int. J. Mol. Sci. 2021;22(8):1–15. doi: 10.3390/ijms22083805. 3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Angelo E., et al. Intrinsic and extrinsic modulators of the epithelial to mesenchymal transition: driving the fate of tumor microenvironment. Front. Oncol. 2020;10(1122):1–18. doi: 10.3389/fonc.2020.01122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang W., et al. Crosstalk to stromal fibroblasts induces resistance of lung cancer to epidermal growth factor receptor tyrosine kinase Inhibitors. Clin. Cancer Res. 2009;15(21):6630–6638. doi: 10.1158/1078-0432.CCR-09-1001. [DOI] [PubMed] [Google Scholar]
- 11.Condeelis J., Segall J.E. Intravital imaging of cell movement in tumours. Nat. Rev. Cancer. 2003;3(12):921–930. doi: 10.1038/nrc1231. [DOI] [PubMed] [Google Scholar]
- 12.Henke E., Nandigama R., Ergün S. Extracellular matrix in the tumor microenvironment and its impact on cancer therapy. Front. Mol. Biosci. 2020;6(160):1–24. doi: 10.3389/fmolb.2019.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rice A.J., et al. Matrix stiffness induces epithelial–mesenchymal transition and promotes chemoresistance in pancreatic cancer cells. Oncogenesis. 2017;6(7):e352. doi: 10.1038/oncsis.2017.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim C.Y., Zoncu R. The lysosome as a command-and-control center for cellular metabolism. J. Cell Biol. 2016;214(6):653–664. doi: 10.1083/jcb.201607005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parzych K.R., Klionsky D.J. An overview of autophagy: morphology, mechanism, and regulation. Antioxid. Redox Signal. 2014;20(3):460–473. doi: 10.1089/ars.2013.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banerjee S., Kane P.M. Regulation of V-ATPase activity and organelle pH by phosphatidylinositol phosphate lipids. Front. Cell Dev. Biol. 2020;8(510):1–16. doi: 10.3389/fcell.2020.00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sleat D.E., et al. Extending the mannose 6-phosphate glycoproteome by high resolution/accuracy mass spectrometry analysis of control and acid phosphatase 5-deficient mice*. Mol. Cell. Proteomics. 2013;12(7):1806–1817. doi: 10.1074/mcp.M112.026179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janda E., Nevolo M., Lehmann K., Downward J., Beug H., Grieco M. Raf plus TGFβ-dependent EMT is initiated by endocytosis and lysosomal degradation of E-cadherin. Oncogene. 2006;25(54):7117–7130. doi: 10.1038/sj.onc.1209701. [DOI] [PubMed] [Google Scholar]
- 19.Tang T., et al. The role of lysosomes in cancer development and progression. Cell Biosci. 2020;10(1):1–18. doi: 10.1186/s13578-020-00489-x. 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Basu A., et al. Statistical parametrization of cell cytoskeleton reveals lung cancer cytoskeletal phenotype with partial EMT signature. Commun. Biol. 2022;5(1):1–11. doi: 10.1038/s42003-022-03358-0. 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whipple R.A., et al. Epithelial-to-mesenchymal transition promotes tubulin detyrosination and microtentacles that enhance endothelial engagement. Cancer Res. 2010;70(20):8127–8137. doi: 10.1158/0008-5472.CAN-09-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szyk A., et al. Molecular basis for age-dependent microtubule acetylation by tubulin acetyltransferase. Cell. 2014;157(6):1405–1415. doi: 10.1016/j.cell.2014.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wattanathamsan O., Thararattanobon R., Rodsiri R., Chanvorachote P., Vinayanuwattikun C., Pongrakhananon V. Tubulin acetylation enhances lung cancer resistance to paclitaxel-induced cell death through Mcl-1 stabilization. Cell Death Discov. 2021;7(1):1–13. doi: 10.1038/s41420-021-00453-9. 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu S., et al. Loss of α-tubulin acetylation is associated with TGF-β-induced epithelial-mesenchymal transition. J. Biol. Chem. 2016;291(10):5396–5405. doi: 10.1074/jbc.M115.713123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.A.E. Güleç Taşkıran et al., “Rewiring of endolysosomal signaling with nutrient depletion in cancer cells,” 2023, 10.21203/rs.3.rs-3331567/v1 Preprint.
- 26.Soneson C., Love M.I., Robinson M.D. Differential analyses for RNA-seq: transcript-level estimates improve gene-level inferences. F1000Res. 2016;4(1521):1–19. doi: 10.12688/f1000research.7563.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(550):1–21. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pastorini G., et al. A partial epithelial-mesenchymal transition signature for highly aggressive colorectal cancer cells that survive under nutrient restriction. J . Pathol. 2023 doi: 10.1002/path.6240. [DOI] [PubMed] [Google Scholar]
- 29.Rubin S.M. Deciphering the retinoblastoma protein phosphorylation code. Trends Biochem. Sci. 2013;38(1):12–19. doi: 10.1016/j.tibs.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Demirkol Canlı S., et al. Evaluation of an aldo-keto reductase gene signature with prognostic significance in colon cancer via activation of epithelial to mesenchymal transition and the p70S6K pathway. Carcinogenesis. 2020;41(9):1219–1228. doi: 10.1093/carcin/bgaa072. [DOI] [PubMed] [Google Scholar]
- 31.Tan J.X., Finkel T. Lysosomes in senescence and aging. EMBO Rep. 2023;24(11):57265. doi: 10.15252/embr.202357265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kallunki T., Olsen O.D., Jäättelä M. Cancer-associated lysosomal changes: friends or foes? Oncogene. 2013;32(16):1995–2004. doi: 10.1038/onc.2012.292. [DOI] [PubMed] [Google Scholar]
- 33.Etienne-Manneville S. Actin and microtubules in cell motility: which one is in control? Traffic. 2004;5(7):470–477. doi: 10.1111/j.1600-0854.2004.00196.x. [DOI] [PubMed] [Google Scholar]
- 34.Liu E.Y., Ryan K.M. Autophagy and cancer – issues we need to digest. J. Cell. Sci. 2012;125(10):2349–2358. doi: 10.1242/jcs.093708. [DOI] [PubMed] [Google Scholar]
- 35.Crosas-Molist E., et al. AMPK is a mechano-metabolic sensor linking cell adhesion and mitochondrial dynamics to Myosin-dependent cell migration. Nat. Commun. 2023;14(1):1–22. doi: 10.1038/s41467-023-38292-0. 2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guinney J., et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015;21(11):1350–1356. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenbauer J., Berghoff M., Glazier J.A., Schug A. Multiscale modeling of spheroid tumors: effect of nutrient availability on tumor evolution. J. Phys. Chem. B. 2023;127(16):3607–3615. doi: 10.1021/acs.jpcb.2c08114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li S., et al. Acidic pHe regulates cytoskeletal dynamics through conformational integrin β1 activation and promotes membrane protrusion. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2018;1864(7):2395–2408. doi: 10.1016/j.bbadis.2018.04.019. [DOI] [PubMed] [Google Scholar]
- 39.Etienne-Manneville S., Hall A. Integrin-mediated activation of Cdc42 Controls Cell Polarity in Migrating Astrocytes through PKCζ. Cell. 2001;106(4):489–498. doi: 10.1016/S0092-8674(01)00471-8. [DOI] [PubMed] [Google Scholar]
- 40.Buratta S., et al. Lysosomal exocytosis, exosome release and secretory autophagy: the autophagic- and endo-lysosomal systems go extracellular. Int. J. Mol. Sci. 2020;21(7):1–20. doi: 10.3390/ijms21072576. 2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu H., Ren D. Lysosomal physiology. Annu. Rev. Physiol. 2015;77(1):57–80. doi: 10.1146/annurev-physiol-021014-071649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Christensen K.A., Myers J.T., Swanson J.A. pH-dependent regulation of lysosomal calcium in macrophages. J. Cell. Sci. 2002;115(3):599–607. doi: 10.1242/jcs.115.3.599. [DOI] [PubMed] [Google Scholar]
- 43.Medina D.L., et al. Transcriptional activation of lysosomal exocytosis promotes cellular clearance. Dev. Cell. 2011;21(3):421–430. doi: 10.1016/j.devcel.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gugnoni M., Sancisi V., Manzotti G., Gandolfi G., Ciarrocchi A. Autophagy and epithelial–mesenchymal transition: an intricate interplay in cancer. Cell Death Dis. 2016;7(12):2520. doi: 10.1038/cddis.2016.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lyu L., et al. TBBPA regulates calcium-mediated lysosomal exocytosis and thereby promotes invasion and migration in hepatocellular carcinoma. Ecotoxicol. Environ. Saf. 2020;192(110255):1–9. doi: 10.1016/j.ecoenv.2020.110255. [DOI] [PubMed] [Google Scholar]
- 46.Qiang L., et al. Regulation of cell proliferation and migration by p62 through stabilization of Twist1. Proc. Natl. Acad. Sci. U. S. A. 2014;111(25):9241–9246. doi: 10.1073/pnas.1322913111. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Li G., et al. Enhanced epithelial-to-mesenchymal transition associated with lysosome dysfunction in podocytes: role of p62/sequestosome 1 as a signaling hub. Cell. Physiol. Biochem. Pharmacol. 2015;35(5):1773–1786. doi: 10.1159/000373989. Cellular Physiology and Biochemistry: International Journal of Experimental. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA sequencing data generated for the current study has been uploaded to GEO (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE245402). The data and R script used in the study will be made available upon request.