Abstract
In ovo delivery of carvacrol, the primary active compound in oregano essential oil (OEO) has the potential to enhance gut development in broilers. This study aimed to optimize in ovo application of OEO by investigating day and site of injection and delivery of carvacrol to different embryonic tissues. In Experiment 1, 2 d of injection (embryonic day (E) 12 or 17.5) and 3 sites of injection for OEO (air cell, amniotic fluid, or yolk) were evaluated based on hatchability and posthatching performance. Experiment 2 aimed to examine the impact of combining OEO with the nonionic surfactant polysorbate 80 (p80) at ratios to carvacrol of 0:0, 0:1, 0.5:1, and 1:1 on carvacrol concentration in amniotic fluid, blood, and yolk. The concentration of carvacrol was measured at 3, 6, and 9 h after OEO injection either without (0:1) or with (1:1) p80. Injection of OEO on E12 led to a significant lower hatchability compared to E17.5 (P ≤ 0.01; Δ = 9.2%). Injecting OEO into the air cell, amniotic fluid, or yolk at E17.5 did not significantly affect hatchability and posthatching performance. The highest concentrations of carvacrol found in egg tissues were observed when injected together with surfactant at the 1:1 ratio (P ≤ 0.001; 14.45 µM, 16.64 µM, and 124.82 µM, for air cell, amniotic fluid, and yolk, respectively) compared to the 0:0, 0:1 or 0.5:1 ratios. Carvacrol was highest in the amniotic fluid and blood at the first time point (3 h postinjection) and decreased afterward (P ≤ 0.001), whereas the concentration in yolk remained elevated up to 9 h postinjection. In conclusion, the optimization of the in ovo delivery of carvacrol resulted in that early injection (E12) had negative effects on hatchability and should be avoided. The findings also suggest that using a nonionic surfactant was crucial for an effective delivery of carvacrol in ovo and the migration from amniotic fluid to yolk within 3 h. In addition, carvacrol's persistence in yolk may serve as a route for delivery into the gastrointestinal tract via the yolk stalk during the peri-hatching phase, potentially influencing gut development.
Key words: in ovo delivery, diffusion, carvacrol, oregano essential oil, broiler chicken
INTRODUCTION
Enteric diseases can severely affect animal health and welfare, hence requiring antibiotic treatment. The use of antibiotics in intensive animal production systems has been associated with increased antimicrobial resistance which, in turn, has fueled the research on strategies that spare their use (Turnidge, 1999). In broilers, meat-type chickens, essential oil (EO) supplements have been widely studied and reviewed during the posthatching period showing positive effects on gut health among other modes of action (Brenes and Roura, 2010). However, the efficacy and return on investment of in-feed EO supplements are controversial, partially because of a lack of consistent effects on performance, antimicrobial activity and gut health, explaining the limited adoption by producers (Applegate et al., 2010).
In fast-growing modern breeds, broiler chickens spend around one-third of its life during embryonic development. Thus, in ovo interventions have a high potential value which is often ignored (Das et al., 2021). The structures surrounding the embryo are formed during the first week of incubation and are structurally completed by embryonic day (E) 14 (de Oliveira et al., 2008). The most common in ovo intervention adopted by producers is the injection of the herpesvirus Marek's disease vaccine in the amniotic fluid at E17.5. This, in turn, creates an opportunity to inject other compounds at the same time without adding extra costs, provided that they do not interact negatively (Wakenell et al., 2002; Peebles, 2018). For example, in ovo nutrient supplementation (also referred to as in ovo feeding) has been widely studied, using injection procedures around E17.5 in the amniotic fluid, which is ingested by the chicken embryo before hatching (Uni and Ferket, 2004; Jha et al., 2019; Givisiez et al., 2020; Das et al., 2021).
A second in ovo nutritional delivery route widely studied includes the delivery of prebiotics and probiotics through the chorioallantoic membrane by injection in the air cell on E12. At E12, the air cell is highly vascularized and seems to facilitate the transport of prebiotics to the embryo to stimulate gut microbiota development, whereas probiotics stay in the air cell and are ingested when the embryo starts pipping through the air cell membrane prior to hatching (Romanoff and Hayward, 1943; Baggott, 2009; Siwek et al. 2018; Moreira Filho et al., 2019 ). A third option for in ovo interventions relevant to gut development and health relates to the yolk sac contents, which are internalized into the GIT during late incubation (El-Moneim et al., 2020; van der Wagt et al., 2020).
One often used EO during the posthatching phase in broilers is oregano essential oil (OEO) (Basmacioǧlu Malayoǧlu et al., 2010; Hashemipour et al., 2013; Peng et al., 2016). Oregano essential oil has been shown to positively impact GIT development and gut health through enhanced digestion and antimicrobial, antioxidant, and immunodulatory functions (Gholami-Ahangaran et al., 2022; Jin et al., 2022). It can be speculated that in ovo application compared to posthatch intervention could achieve an early and potentially more effective distribution of OEO through developing tissues/GIT, while reducing the economic impact. However, little is known about in ovo application of OEO or carvacrol (the main active compound in OEO) and the tissue distribution of this lipophilic substance once injected. Optimizing the in ovo application of OEO and understanding the flow dynamics into egg compartments and dispersion into embryonic structures is missing.
The current study aimed to optimize the in ovo application of OEO by investigating day and site of injection and enhancing the flow of carvacrol into embryonic tissues. It was hypothesized that injection in the amniotic fluid during late incubation (E17.5) would have no impact on hatchability and that the use of a non-ionic surfactant (i.e., polysorbate 80) would enhance the flow of carvacrol from the amniotic fluid into lipophilic tissues (i.e., yolk) within the first hours after injection.
MATERIAL AND METHODS
Two experiments were conducted under approval certificate number 2019/AE000463 by the Animal Ethics Committee of the University of Queensland (Animal Ethics Unit, St Lucia, QLD, Australia) aligned with the compliance of the Australian code for the use of animals for scientific purposes.
Experiment 1
Experimental Design
This experiment was set up as a 3 × 2 factorial arrangement with 3 sites of injection (air cell, amniotic fluid, or yolk) and 2 stages of embryonic development (E12 and E17.5) (Figure 1). Additionally, 3 control groups were included to establish the baseline for the efficacy of the procedures; a noninjected control group (intact eggs), and two 0.9% saline solution injected controls, one in the air cell at E12 and one in the amniotic fluid at E17.5, which are the most common combinations of day and site of injection (Table 1). Other potential “saline” controls for each day and site combinations were discarded due to limitations in the total number of replicates.
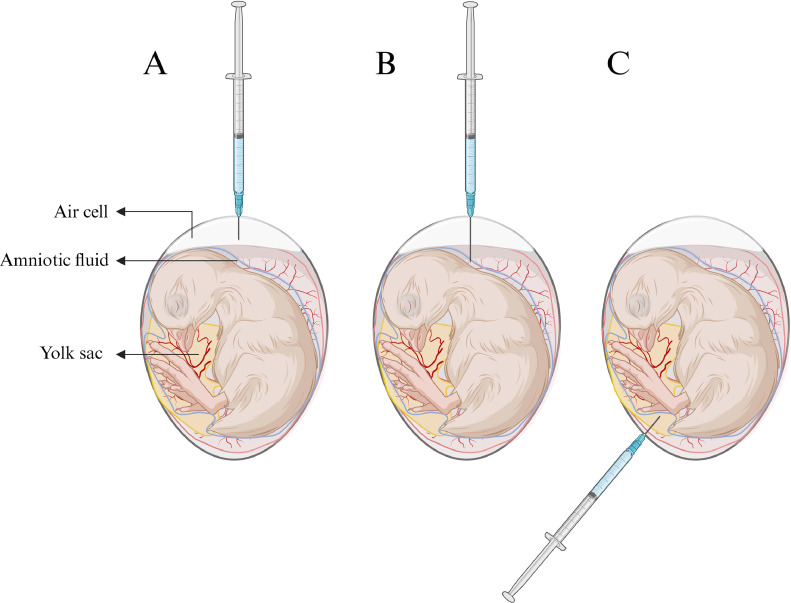
Figure 1.
Illustration of the 3 sites of injection tested in Experiment 1, (A) air cell, (B) amniotic fluid, and (C) yolk sac. Created with BioRender.com.
Table 1.
Design of experiment 1 showing the number of eggs (n) and number of first-grade chicks (n) per noninjected (C1) or injected with 0.9% saline solution (C2 and C3) or oregano essential oil (OEO, T1–T6) in the air cell, amniotic fluid, or yolk during mid-incubation (embryonic day 12 [E12]) or late incubation (embryonic day 17.5 [E17.5]).
| Treatment | Site of injection | Day of injection | Injected solution | Number of eggs (n) | Number of chicks (n) |
|---|---|---|---|---|---|
| C1 | - | - | - | 40 | 34 |
| C2 | Air cell | E12 | Saline | 40 | 33 |
| C3 | Amniotic fluid | E17.5 | Saline | 40 | 32 |
| T1 | Air cell | E12 | OEO | 100 | 74 |
| T2 | Amniotic fluid | E12 | OEO | 100 | 74 |
| T3 | Yolk | E12 | OEO | 100 | 64 |
| T4 | Air cell | E17.5 | OEO | 100 | 74 |
| T5 | Amniotic fluid | E17.5 | OEO | 100 | 69 |
| T6 | Yolk | E17.5 | OEO | 100 | 73 |
Egg Handling, Animals, and Housing
Ross 308 fertile eggs (n = 720) weighing between 60 and 68 g (SD = 4.5 g) from one breeder flock aged 45 wk were obtained from a commercial hatchery (Darwalla group, Allora, QLD, Australia) and transferred to the University of Queensland experimental chicken hatchery (St Lucia, QLD, Australia). The 6 experimental treatments were assigned 100 eggs each (n = 100). The 3 control treatments used to establish the baseline for efficacy of the procedures were assigned 40 eggs each (n = 40) due to a limited incubation capacity. Eggs were blocked within 2 trays for each of the 6 levels of 2 setters (Ova-Easy 580 Advance Series II, Brinsea, FL) making a total of 24 trays. Treatments were separated into 4 groups (25 eggs per group for experimental treatments, and 10 eggs per group for control treatments), and these groups were randomly allocated to one of the trays, with 2 different treatment groups per tray. Eggs were incubated in the setters for 18 d at a set incubator temperature of 37.8°C, a relative humidity of 57%, and a turning interval of 60 min over an angle of 90°. At E18, eggs were candled and infertile eggs removed, all remaining experimental eggs were transferred to 2 hatchers (Greatlander 6BH Six Basket Hatcher, Taabinga, QLD, Australia), consisting of 6 hatching baskets (levels) with each basket divided into 3 compartments, making a total of 36 compartments. Eggs from each control treatment were randomly allocated to 2 compartments and eggs from each experimental treatment to 5 compartments. Eggs were incubated until hatching at a set hatcher temperature of 37.8°C and relative humidity of 70%. From E19 onward, hatchers were checked daily at 8 am and 4 pm for hatched chickens and dry hatched chickens were weighed and transferred to brooders (5-layer Comfortplast Chick Brooder, Cimuka, Turkey), where they had access to ad libitum water and feed. Chickens were housed in 6 brooders with 5 levels per brooder (a total of 30 levels). Hatchability and BW0 were determined at hatch (n = 100 per experimental treatment and n = 40 per control treatment). A maximum of 80 hatched first-grade (chickens with no observed deformities) per experimental treatment were randomly selected from all first-grade chicks and randomly allocated to 4 brooder levels whereas a maximum of 40 first-grade hatched chickens from the control treatments were allocated to 2 brooder levels (exact numbers shown in Table 1). Each level contained a maximum of 20 chickens of the same treatment. On d 7, body weight (BW) and feed intake (FI) were measured as experimental endpoint using the averages per brooder level (n = 4 per experimental treatment and n = 2 per control treatment). Feed conversion ratio (FCR) was calculated, using body weight gain (BWG) and FI between d 0 and d 7. Chickens were euthanized by cervical dislocation.
In Ovo Injection
At E12 and E17.5, saline or OEO solutions were injected following the scheme shown in Table 1. Eggs were sterilized with 70% ethanol and punched, using an egg puncher at sites as determined by each treatment. For injection in the air cell and amniotic fluid, eggs were punched on the broad end of the egg (Figure 1). For injection in the yolk, eggs were punched at the pointy side of the egg, at a 45° diagonal angle. At E12, the following needle sizes were used: for the air cell, a 25G 5/8 (16 mm) precision needle with the addition of a needle guard blocking half of the needle to prevent injection into a lower location whereas for the amniotic fluid and yolk a 23G 1 ¼ (32 mm) precision needle was used. At E17.5, for the air cell and amniotic fluid injection, the same procedure was used as at E12. For the yolk injection at E17.5, a 25G 5/8 (16 mm) precision needle was used. After injection, holes were sealed with commercial nail polish.
Eggs were injected with either 100 µL of 0.9% sterile saline solution (NaCl 0.9% in water, Baxter, Deerfield, IL, CAS: 7647-14-5) or 100 µL OEO solution with a concentration of 0.5% OEO vol/vol (Origanum Oil, doTERRA, Pleasant Grove, UT, CAS: 8007-11-2). Gas Chromatography-Mass Spectrometry (GC-MS) was used to profile OEO in combination with the NIST17 mass spectral library was used to identify individual compounds. The OEO solution was prepared by mixing 100 µL of OEO with 100 µL of the non-ionic surfactant polysorbate 80 (p80) (Tween80, Sigma-Aldrich, St. Louis, MO, CAS: 9005-65-6) for solubilization. Afterwards, 0.9% saline was slowly added to a final volume of 20 mL and shaken properly.
Experiment 2
Experimental Design
Experiment 2 consisted of 2 subexperiments. In subexperiment 2.1, Ross 308 fertile eggs (n = 32) weighing between 60 and 66 g (SD = 3.4 g) were obtained from one breeder flock aged 46 wk (Darwalla group) and transferred to the experimental chicken hatchery (St Lucia, QLD, Australia) to study the impact of 4 OEO solutions with different ratios of p80:OEO (0:0, 0:1, 0.5:1, or 1:1, n = 6 per treatment) on carvacrol transfer to 3 different tissues per egg (amniotic fluid, blood, and yolk). The OEO solutions were injected into the amniotic fluid at E17.5 as explained below. OEO solutions were injected at a concentration of 1.75% vol/vol, based on safety levels reported in rats (Llana-Ruiz-Cabello et al., 2017 ). The 0:0 ratio (control group) was injected with a 0.9% saline solution. To assess the flow of carvacrol through embryonic tissues, samples of the amniotic fluid, blood, and yolk were collected at 9 h after injection.
Subexperiment 2.2 consisted of Ross 308 fertile eggs (n = 56) weighing between 56 and 62 g (SD = 3.5 g), obtained from one breeder flock aged 40 wk (Darwalla group). Based on results from experiment 2.1, subexperiment 2.2 was set up as a 2 × 3 factorial arrangement, consisting of 2 different OEO solutions (p80:OEO ratios 0:1 or 1:1), and 3 time points (different eggs at 3 h, 6 h, and 9 h after injection) with n = 6 per treatment. Carvacrol concentration was measured in 3 different tissues per egg (amniotic fluid, blood, and yolk). Solutions were injected in the amniotic fluid of fertile eggs at E17.5, and OEO was injected at a concentration of 1.75% vol/vol. As a quality control to ensure procedures did not result in carvacrol detection, eggs were injected with 0.9% saline (0:0) and the same samples were collected, but only after 3 h.
Egg Handling
In both subexperiments, the eggs were incubated under the same circumstances as described for Experiment 1. Additionally, spare eggs were incubated to replace infertile eggs or eggs damaged during incubation and injection. Eight eggs were assigned per treatment (n = 8), of which 6 eggs were used for sample collection (n = 6). One setter with 6 levels and 2 trays per level was used. Eggs were only allocated to the middle 2 levels (levels 3 and 4), containing 2 trays per level (4 in total). Eggs were randomly allocated to the right and left sides of the trays, following a randomized block design.
In Ovo Injection
The eggshell was disinfected as described in Experiment 1. A hole was drilled in the eggshell at the blunt side of the egg, using a multi-purpose rotary tool (Ryobi EHT150, Ryobi, Hiroshima, Japan) with an arrow-shaped insert (Dremel High-Speed Cutter 6.4 mm, Dremel, Mount Prospect, IL), keeping the membranes intact. For injection in amniotic fluid, a 23G 1 ¼ (32 mm) precision needle was used. After injection, holes were sealed with beeswax and eggs were returned to the setter.
In Experiment 2.1, eggs were injected with 500 µL of a solution of different ratios of p80:OEO; 0:0, 0:1, 0.5:1, and 1:1, whereas in Experiment 2.2, only the ratios 0:0 (control), 0:1 and 1:1 were used. OEO was used at a concentration of 1.75% vol/vol. Eggs injected with the 0:0 ratio were injected with 500 µL 0.9% saline (NaCl 0.9% in water, Baxter, CAS: 7647-14-5). The 0:1 solution was prepared by mixing 350 µL of OEO (Origanum Oil, doTERRA, CAS: 8007-11-2) with 0.9% saline to a total volume of 20 mL and shaken properly. The 0.5:1 solution was prepared by mixing 175 µL of p80 (Tween80, Sigma-Aldrich, CAS: 9005-65-6) with 350 µL of OEO for solubilization, followed by slowly adding 0.9% saline to a total volume of 20 mL and shaken properly. The 1:1 solution was prepared by mixing 350 µL of p80 with 350 µL of OEO for solubilization, followed by slowly adding 0.9% saline to a total volume of 20 mL and shaking properly. Before loading the syringe, the 0:1 p80:OEO solution was gently shaken to ensure equal OEO distribution in the absence of a surfactant but preventing the formation of air bubbles.
Sample Collection
Samples were collected at 9 h (Exp 2.1) or 3 h, 6 h, and 9 h (Exp. 2.2, different eggs each time point) after injection and treatments were sampled in random order. Screwcap glass storage vials (Agilent Technologies, Santa Clara, CA, Cat. Nr. 5182-0715) were used to prevent volatile losses. The egg was opened on the blunt end, the shell and its membranes were carefully peeled and the embryo with its structures was deposited into a petri dish. Amniotic fluid (0.1–1 mL) was collected using a 23G 1 ¼ precision needle and transferred to a storage vial. Afterward, the remaining amniotic fluid was removed from the embryo using tissue paper. The embryo was euthanized by decapitation, and blood (0.1-1 mL) was collected in a 4 mL K2 EDTA vacutainer (BD, Franklin Lakes, NJ, Cat. Nr. 32007-BD) and properly mixed with EDTA to prevent clotting before transferring the blood to a storage vial. The yolk sac was removed, all yolk contents were gently released initially by gravity into a container and mixed by stirring. After stirring, samples of 1 mL were transferred to a storage vial. All samples were stored at −80°C until analysis.
GC-MS Analysis
Amniotic fluid, blood and yolk samples were thawed on ice for GC-MS analysis. Samples were analyzed in 2 separate GC-MS runs, both following the same procedures. The extraction of carvacrol for GC-MS was performed on ice as much as possible to reduce the vaporization of carvacrol. About 500 µL of dichloromethane and methanol solution (1:1 vol/vol), containing 10 µM patchouli alcohol as an internal control was added to 100 µL of amniotic fluid or blood, or 100 mg of yolk. Samples were vortexed until homogeneous, whereafter 400 µL demineralized water was added and mixed. Samples were sonicated in an ice bath sonicator (Ultrasonic Cleaner, Unisonics, Brookvale, NSW, Australia) for 5 (amniotic fluid and blood) or 10 min (yolk). Afterward, samples were centrifuged (Microcentrifuge 5415R, Eppendorf, Hamburg, Germany) for 10 min at 16,000 × g at 4°C. About 100 µL of the nonpolar phase was transferred to 2 mL screwcap glass vials (Shimadzu Scientific, Kyoto, Japan) with a glass insert (Thermo Fisher Inc., Waltham, MA). One microliter of each sample was injected for GC-MS analysis. Carvacrol detection and quantification were performed on a Shimadzu GC/MS-TQ8050 NX system in Multiple Reaction Monitoring mode using a carvacrol standard (Sigma-Aldrich, CAS: 499-75-2) for calibration (carvacrol retention time was 5.899 min). Carvacrol concentrations were measured in µM.
The extraction efficiency of carvacrol from amniotic fluid, blood, and yolk was determined by adding carvacrol to blank samples of amniotic fluid, blood, and yolk, to achieve concentrations of 2, 11, and 20 µM in all 3 tissues. These initial concentrations were compared to the GC-MS output (average extraction: amniotic fluid; 20.2%, R2 = 0.999, blood; 8.4% R2 = 0.991, yolk; 22.2% R2 = 0.997). The linear regression formulas were used to determine absolute carvacrol concentrations. The lower limits of detection of carvacrol using GC-MS were established at 0.22 µM (amniotic fluid), 0.69 µM (blood), and 0.36 µM (yolk). Lower concentrations were considered 0 for statistical analyses.
Statistical Analyses
All data were analyzed using the statistical software package SAS 9.4 (SAS Institute Inc., Cary, NC). For Experiment 1, the experimental design included the factors day, site, and their interaction.
In Experiment 1, hatchability rates were analyzed with a generalized linear mixed model (Proc Glimmix) procedure, using a binary distribution and a logit link function. A general linear mixed model (Proc Mixed) was used to analyze BW at d 0 and d 7, BWG, FI, and FCR. Model assumptions were approved on both the means and residuals. For hatchability and BW at d 0 eggs or chickens were used as the experimental unit, whereas for BW at d 7, BWG, FI, and FCR, the brooder level was used as the experimental unit.
The basic model used for Experiment 1 was
| (1) |
where Yij = the dependent variable, µ = the overall mean, Dayi = the day of injection (i = E12 or E17.5), Sitej = the site of injection (j = air cell, amniotic fluid, or yolk), Day × Siteij = the interaction between the day of injection and site of injection, and eij = the residual error term. For hatchability and BW at d 0, hatcher, hatcher basket, and hatcher compartment were added to the model as random factors, whereas for BW at d 7, BWG, FI and FCR, brooder and brooder level were added as random factors.
Control treatments (noninjected and saline-injected at E12 in the air cell, and E17.5 in the amniotic fluid) have not been included in the factorial analysis, but all treatments have been compared to the control treatments in a separate analysis without significant outcomes (using Proc Glimmix and Proc Mixed as described before). The basic model used for this was:
| (2) |
where Yij = the dependent variable, µ = the overall mean, Treatmenti = Treatment (i = each of 9 treatments shown in Table 1), and eij = the residual error term.
Data are expressed as LSmeans ± SEM and multiple comparisons between treatments were corrected following the Tukey test. Differences between treatments were considered significant at P ≤ 0.05.
For Experiment 2, a general linear mixed model (Proc Mixed) was used to analyze the concentrations of carvacrol in the amniotic fluid, blood, and yolk. Eggs were used as experimental units. The basic model used for Experiment 2.1 was:
| (3) |
where Y = the dependent variable, µ = the overall mean, Ratioi = the ratio of p80:OEO (i = 0:0, 0:1, 0.5:1 or 1:1), and ei = the residual error term.
The basic model used for Experiment 2.2 was:
| (4) |
where Yij = the dependent variable, µ = the overall mean, Ratioi = the ratio of p80:OEO (i = 0:1 or 1:1), Timej = the time point after injection (j = 3 h, 6 h, or 9 h), Ratio × Timeij = the interaction between the ratio of p80:OEO and time point after injection, and eij = the error term.
Because samples could not all be collected at the same time, there was a variation of 3 h (Experiment 2.1) and 1.5 h (Experiment 2.2) in sampling time. Consequently, the delay in sampling time was added to the model as a covariate. Preliminary analysis showed a lack of significance for the factors GC-MS run (1 or 2), incubator level (3 or 4) incubator tray (1 or 2), and side of the tray (left or right) and consequently, they were omitted from the model.
Data are expressed as LSmeans ± SEM and multiple comparisons between treatments were corrected for using Tukey. Differences between treatments were considered significant at P ≤ 0.05.
RESULTS
The results of the GC-MS analysis of the OEO showed that carvacrol was the main compound (69.76%) at a retention time (RT) of 8.0 min, followed by p-cymene (6.43%, RT 4.6 min), gamma-terpinene (5.36%, RT 5.0 min), linalool (4.64%, RT 5.3 min), beta-bisabolene (2.13%, RT 12.0 min), thymol (1.60%, RT 7.9 min), and caryophyllene (1.56%, RT 10.7 min) and trace amounts of other compounds.
Day and Site of Injection (Experiment 1)
For the control treatments, hatchability rates of 89.7% (noninjected), 89.5% (saline-injected at E12 in the air cell) and 84.6% (saline-injected in the amniotic fluid at E17.5) were found (Table 2).
Table 2.
Experiment 1. Interaction effects of in ovo injection of oregano essential oil at d 12 (E12) or 17.5 (E17.5) of incubation at different sites (air cell, amniotic fluid, yolk) of broiler eggs on hatchability and first-week performance (LSmeans ± SEM). Main effects of day and site of injection are shown in Figure 2.
| Day of injection | Site of injection | Hatchability, %1 | BW d 0, g1 | BW d 7, g1 | BWG d 0–7, g1 | FI d 0–7, g1 | FCR d 0–7, g:g1 |
|---|---|---|---|---|---|---|---|
| Controls2 | |||||||
| Noninjected | - | 89.7 | 46.4 | 160.8 | 114.5 | 136.6 | 1.19 |
| E12 | Air cell | 89.5 | 45.6 | 157.1 | 111.6 | 128.0 | 1.15 |
| E17.5 | Amniotic fluid | 84.6 | 46.3 | 156.5 | 110.0 | 145.7 | 1.32 |
| Treatment | |||||||
| E12 | Air cell | 79.4 | 46.2 | 152.3 | 105.9 | 123.8 | 1.15 |
| E12 | Amniotic fluid | 79.6 | 46.5 | 151.2 | 104.9 | 124.3 | 1.17 |
| E12 | Yolk | 70.1 | 45.9 | 155.7 | 110.0 | 132.9 | 1.22 |
| E17.5 | Air cell | 86.3 | 45.9 | 147.4 | 101.4 | 116.5 | 1.19 |
| E17.5 | Amniotic fluid | 83.9 | 46.2 | 159.1 | 112.8 | 136.4 | 1.17 |
| E17.5 | Yolk | 86.0 | 46.6 | N/A3 | N/A3 | N/A3 | N/A3 |
| SEM | 4.0 | 0.6 | 4.9 | 4.9 | 5.8 | 0.04 | |
| P-value | |||||||
| Day | 0.009 | 0.81 | 0.91 | 0.73 | 0.62 | 0.58 | |
| Site | 0.62 | 0.74 | 0.28 | 0.42 | 0.14 | 0.45 | |
| Day × Site | 0.44 | 0.37 | 0.09 | 0.20 | 0.09 | 0.61 |
Experimental unit for Hatchability and BW d 0: n = 100 eggs per combination of day and site; Experimental units for BW d 7, BWG d 0-7, FI d 0-7, and FCR d 0-7: n = 4 brooder levels per combination of day and site. Average values per bird are shown.
Control treatments (noninjected and saline injected at E12 in the air cell, and E17.5 in the amniotic fluid) are shown on top for reference with their means. These have not been included in the factorial analysis, but all treatments have been compared to the control groups in a separate analysis without significant outcomes.
N/A: Values are missing for BW d 7, BWG d 0-7, FI d 0-7, and FCR d 0-7, because of malfunctioning brooder cages.
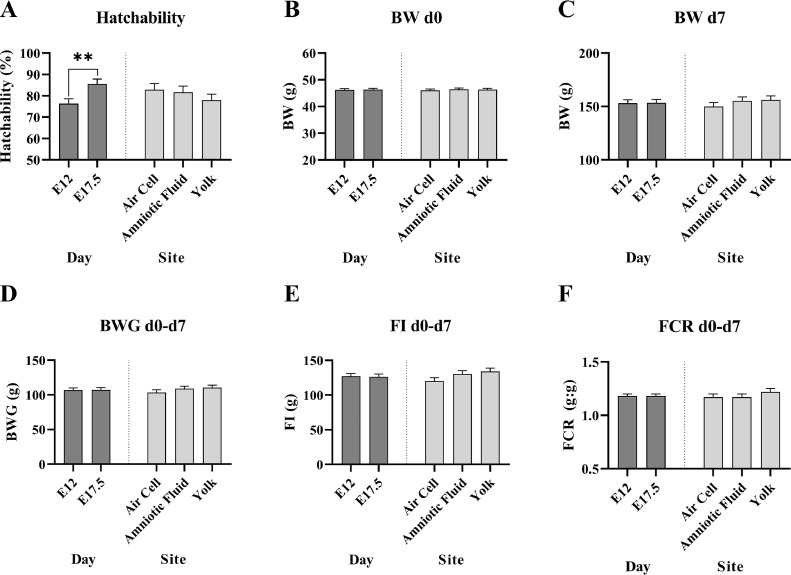
A significant (P ≤ 0.01) effect of the day of injection was found indicating that the E12 injected eggs had lower hatchability (76.3%) compared to the E17.5 (85.5%) injected group (Figure 2A). No other main effect (day or site of injection) or interactions (P > 0.05) were found for any of the variables assessed, hatchability, BW d 0, BW d 7, BWG d 0 to 7, FI d 0 to 7, or FCR d 0 to 7 (Figures 2B–2F, Table 2).
Figure 2.
Experiment 1. Showing the main effects of day of injection of oregano essential oil (d 12 [E12] or 17.5 [E17.5] of incubation) and site of injection (air cell, amniotic fluid, yolk) on hatchability (A) and first-week performance (B–F) of broiler chickens (LSmeans ± SEM). For hatchability and BW d 0: n = 100 eggs per combination of day and site was used; For BW d 7, BWG, FI, and FCR: n = 4 brooder levels per combination of day and site was used. Average values per bird are shown. For main effects, replicates were pooled per day and site.
**P ≤ 0.01.
Effects of Ratio p80:OEO on Carvacrol Concentration per Egg Tissue (Experiment 2.1)
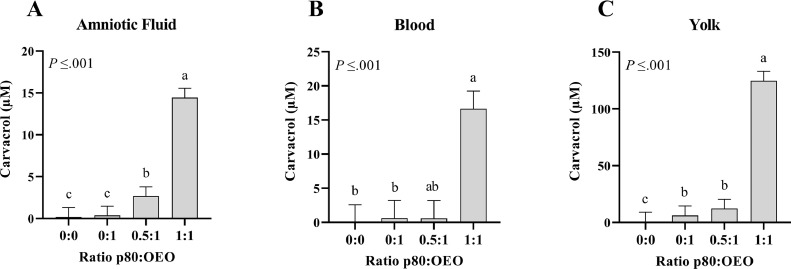
A significant increase in carvacrol concentration was observed in the amniotic fluid after injection of p80:OEO ratios 0.5:1 (P ≤ 0.001; Δ = 2.48 µM) and 1:1 (P ≤ 0.001; Δ = 14.26 µM) compared to the negative control (p80:OEO ratio 0:0). Furthermore, it was observed that injecting the ratio 1:1 resulted in a significant increase in carvacrol concentration compared to the ratio 0.5:1 (P ≤ 0.05; Δ = 11.77 µM) (Figure 3A).
Figure 3.
Experiment 2.1: GC-MS results showing carvacrol concentrations (µM) in amniotic fluid (A), blood (B), and yolk (C) at 9 h after in ovo injection of oregano essential oil (OEO) in combination with polysorbate 80 (p80) at 4 different ratios (ratio p80:OEO 0:0, 0:1, 0.5:1, and 1:1, n = 6 per ratio), injected into the amniotic fluid on incubation d 17.5 (LSmeans ± SEM).
a-cLSmeans within a tissue lacking a common letter differ (P ≤ 0.001).
In blood only after injection of ratio 1:1 (Δ = 16.64 µM) carvacrol concentration was significantly increased compared to the negative control (P ≤ 0.001) (Figure 3B).
In the yolk, a significantly higher carvacrol concentration compared to the negative control was observed irrespective of the level of surfactant, with the ratio 0:1 increasing carvacrol levels by 5.52 µM (P ≤ 0.001), ratio 0.5:1 by 11.45 µM (P ≤ 0.001) and ratio 1:1 by 124.09 µM (P ≤ 0.001). Moreover, the ratio 1:1 resulted in a significantly higher carvacrol concentration compared to ratios 0:1 (P ≤ 0.001; Δ = 118.57 µM) and 0.5:1 (P ≤ 0.001; Δ = 112.64 µM) (Figure 3C).
Distribution of Carvacrol Over Time (Experiment 2.2)
The analysis of the control treatment with saline injection (0:0) showed no presence of carvacrol in the 3 tissues (below the detection threshold of the GC-MS). For the samples injected with OEO solution, the inclusion of p80 at the 1:1 ratio compared to the absence of p80 (0:1 ratio) significantly increased the concentration of carvacrol in amniotic fluid (Δ = 21.20 µM), blood (Δ = 57.89 µM) and yolk (Δ = 149.75 µM) (all P ≤ 0.001; Table 3).
Table 3.
Experiment 2.2. GC-MS results showing carvacrol concentrations (µM) in amniotic fluid, blood, and yolk at 3, 6, or 9 h after in ovo injection of oregano essential oil (OEO) without (0:0) or with (1:1) polysorbate 80 (p80) in amniotic fluid at d 17.5 of incubation (LSmeans ± SEM).
| Ratio p80:OEO | Time after injection (h) | Amniotic fluid (µM)1 | Blood (µM)1 | Yolk (µM)1 |
|---|---|---|---|---|
| 0:0 (Control)2 | 3 | 0 | 0 | 0 |
| 0:1 | 0.98b | 0.51b | 11.32b | |
| 1:1 | 22.19a | 58.40a | 161.10a | |
| SEM | 2.33 | 4.14 | 17.39 | |
| 3 | 24.63a | 49.64a | 78.20 | |
| 6 | 6.52b | 26.47b | 70.34 | |
| 9 | 3.61b | 12.27b | 110.04 | |
| SEM | 2.85 | 5.07 | 21.27 | |
| 0:1 | 3 | 1.61 | 1.18 | 13.29 |
| 0:1 | 6 | 0.53 | 0.27 | 9.26 |
| 0:1 | 9 | 0.81 | 0.08 | 11.41 |
| 1:1 | 3 | 47.66 | 98.09 | 143.12 |
| 1:1 | 6 | 12.50 | 52.68 | 131.43 |
| 1:1 | 9 | 6.40 | 24.43 | 208.66 |
| SEM | 4.04 | 7.19 | 30.13 | |
| P-value | ||||
| Ratio | ≤0.001 | ≤0.001 | ≤0.001 | |
| Time | ≤0.001 | ≤0.001 | 0.37 | |
| Ratio × Time | 0.29 | 0.44 | 0.64 |
For carvacrol concentration in amniotic fluid, blood, and yolk n = 6 eggs per combination of ratio polysorbate 80 and oregano essential oil and time after injection.
The control treatment with saline injection (0:0) showed no presence of carvacrol (below the detection threshold of the GC-MS for all 3 tissues).
LSmeans within a column and treatment lacking a common superscript differ (P ≤ 0.05).
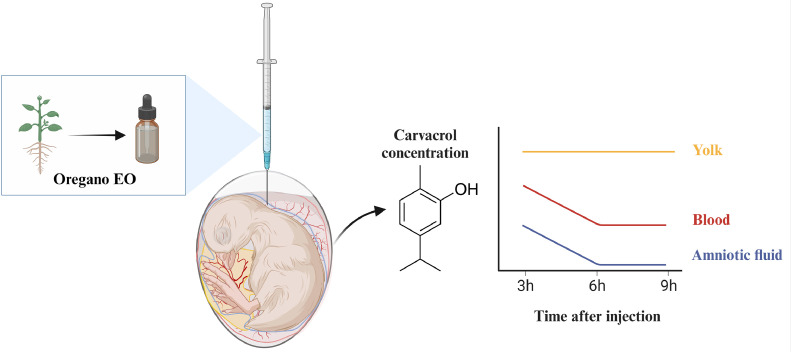
The concentration of carvacrol significantly decreased over time from 3 to 6 h in amniotic fluid (P ≤ 0.01; Δ = 18.11 µM) and blood (P ≤ 0.05; Δ = 23.16 µM) but did not decrease any further between 6 and 9 h after injection (P > 0.05) (Table 3). In contrast, carvacrol concentration was maintained across the 3 time points measured in the yolk (P > 0.05).
DISCUSSION
The current study aimed to optimize in ovo application of OEO investigating day and site of injection as well as the distribution of carvacrol into embryonic tissues (Figure 4). For day and site of injection, the results obtained confirmed that interventions in late embryonic stages (i.e., E17.5) in the amniotic fluid were the most effective treatments. OEO decreased hatchability when injected on E12, but not on E17.5. The embryo is structurally completed around E17.5, but it is still in the middle of development at E12 (de Oliveira et al., 2008). The E12 stage is characterized by rapid development, expressed by a swift increase in embryonic heat production between E10 and E15 (Nangsuay et al., 2017). It can be speculated that OEO injection during early stages of development is more detrimental due to the higher rate of cell division and cell differentiation compared to the later stages of embryonic development. Injecting into the yolk is challenging as it requires turning the eggs upside down and injecting at a 45° angle, and the location of the yolk can differ in each embryo, making it a suboptimal injection site. The air cell may also not facilitate proper transport, as injection of Marek's disease vaccine at E18 into the amniotic fluid resulted in higher protection rates (90%) than injection into the air cell (<50%) (Wakenell et al., 2002). Injection of OEO into the air cell might limit transport of active compounds towards the embryo. Consequently, in ovo injection in the amniotic fluid at E17.5 appeared to have advantages over other sites/days
Figure 4.
Schematic overview highlighting key results of carvacrol dynamics from 3 to 9 h after in ovo injection of oregano essential oil (EO) in the amniotic fluid at d 17.5 of incubation, the concentration of carvacrol decreased from 3 to 6 h in the amniotic fluid and blood but remained consistently elevated in the yolk until at least 9 h after injection. Based on the main effects shown in Table 3. Created with BioRender.com.
This research also addressed the question of the fate of carvacrol once injected into the amniotic fluid. It was anticipated that the hydrophobicity of most of the active compounds in OEO would pose a challenge for an effective distribution inside the egg, particularly in reaching some of the target tissues (i.e., yolk). Nonionic surfactants are used as emulsifiers to improve the dispersion of essential oils in water-based solutions, facilitating diffusion through tissues such as piglet skin (Liu, 2018; Laothaweerungsawat et al. 2020). This study showed that the optimal combination of the nonionic surfactant p80 with OEO was the ratio of 1:1. The results observed with lower ratios did not fully emulsify the OEO based on visual inspection and functional application. The ratio of 1:1 resulted in the highest carvacrol concentration found in the 3 tissues studied (i.e., amniotic fluid, blood, and yolk). The solution injected containing less p80 combined with OEO (0.5:1) increased carvacrol concentration in amniotic fluid and yolk compared to their saline control, whereas injection of OEO without surfactant (0:1) increased carvacrol concentration only in the yolk and to a very limited extent compared to the 1:1 surfactant mix. This indicates that without p80, carvacrol does not distribute well through egg compartments and may not reach embryonic tissues in sufficient amounts. It can be speculated that a p80 to OEO ratio higher than 1:1 could further enhance carvacrol transport, but in this experiment, higher concentrations of p80 were not used due to concerns about potential toxicity (Chassaing et al., 2015). The low carvacrol detection in amniotic fluid and blood samples when administered without or with a lower concentration of surfactant might be related to their water-based environment (Romanoff and Hayward, 1943). Without surfactant, carvacrol is insoluble in water-based environments and may stay in the lipophilic membranes surrounding the amniotic fluid and diffuse only to the lipid-rich yolk but not blood, which, in turn, would explain that carvacrol was identified in yolk when injected without surfactant. Importantly, the current study indicates that carvacrol is less likely to be ingested by the embryo and transported to the circulatory system without p80.
The concentration of carvacrol in amniotic fluid, blood, and yolk was influenced by the time point after injection. The highest concentration of carvacrol in amniotic fluid and blood was observed at the first time point assessed (3 h after injection) and decreased over time. In contrast, carvacrol concentration in yolk did not change throughout the duration of the experiment from 3 to 9 h after injection. These findings suggest that carvacrol diffused to blood and yolk within 3 h after OEO was injected into the amniotic fluid and to a large extent remained in the yolk. If carvacrol was ingested steadily together with the amniotic fluid, the concentration in the amniotic fluid would remain the same. However, the concentration of carvacrol in the amniotic fluid decreased over time, indicating its removal from the fluid through other mechanisms, such as diffusion to other compartments (i.e., yolk). This could be due to the rapid binding of carvacrol to the amniotic membrane or the formation of oil droplets in the amniotic fluid in the absence of a surfactant. With surfactant, carvacrol seemed to persist long enough (3 h) in the amniotic fluid, resulting in partial ingestion and absorption (Zhang et al., 2016). The decline in amniotic fluid after the first 3 h may indicate the diffusion to other compartments. Carvacrol found in the blood may result from absorption after ingestion by the embryo or direct diffusion. The concentration decreased from 3 to 6 h, indicating that carvacrol might be excreted by the kidneys as shown in a postnatal pig model (Michiels et al., 2008).
The high concentration of carvacrol observed in the yolk after injection in the amniotic fluid might be due to 2 possible pathways. One pathway for carvacrol transport to the yolk is diffusion through membranes, facilitated by p80 and the lipophilic nature of carvacrol. However, the molecular weight of carvacrol (150.217 g/mol) is relatively high, which might limit its ability to diffuse through membranes. In a study by Siwek et al. (2018), a water-soluble blue dye with a molecular weight of 504.44 g/mol (E132; indigotin) was injected into the air cell at E12 and transported through the chorioallantoic membrane to the circulatory system within 3 d. Carvacrol has a lower molecular weight and high permeability due to its high lipophilicity, which suggests that it can diffuse through membranes better than blue dye. The formation of micellar complexes by the surfactant and essential oil droplets may also contribute to the improved transfer of carvacrol to the yolk. The use of a surfactant was found to enhance carvacrol transport through membranes, likely by facilitating its dispersion in the amniotic fluid. Micellar complexes have an amphiphilic structure, with hydrophilic heads exposed to the aqueous amniotic fluid and hydrophobic tails surrounding the carvacrol molecules (Perinelli et al., 2020). When micellar complexes encounter phospholipid membranes, such as the amniotic membrane, they fuse with the phospholipid bilayer, thereby facilitating the transport of the emulsified compound to the yolk sac membrane and yolk (Otzen, 2017).
A second potential pathway for carvacrol transport to the yolk is through the yolk stalk. Carvacrol in the amniotic fluid can be ingested by the embryo entering the GIT, which could result in subsequent transport to the yolk via the yolk stalk connection (van der Wagt et al., 2020). However, the yolk stalk connection only opens at approximately E19, which means that it would still be closed within 9 h of injection at E17.5 tested in the current experimental design. Additionally, the yolk stalk appears to facilitate only one-way transfer (from the yolk to the GIT), which suggests that this second route is unlikely (Peebles et al., 1998 ).
Disregarding any practical constraints (i.e., yolk injection compared to other tissues being particularly challenging), the yolk seems to be the best target site for injection of a fat-soluble compound such as carvacrol. However, the current study shows that through injection in the amniotic fluid, carvacrol still ends up primarily in the yolk. Considering that the yolk is absorbed into the embryonic GIT towards the end of incubation and given the sustained presence of carvacrol in the yolk, it can be hypothesized that OEO may gradually reach the GIT through the yolk stalk during the peri-hatching period, thereby having the potential to locally affect gut health.
Based on our findings, it can be concluded that in ovo injection of OEO was more effective at the latest embryonic stage of E17.5. In addition, the use of a nonionic surfactant enhances the transportation of carvacrol to the embryonic structures, following in ovo injection of OEO.
Acknowledgments
DECLARATION OF AI AND AI-ASSISTED TECHNOLOGIES IN THE WRITING PROCESS
During the preparation of this work, the first author used ChatGPT to improve readability and language. After using this tool, the first author reviewed and edited the content as needed and all authors take full responsibility for the content of the publication.
ACKNOWLEDGMENTS
The financial support by AgriFutures Australia's Chicken Meat Program (grant number PRJ-011584) is gratefully acknowledged. The authors would like to acknowledge and thank the steering committee members of AgriFutures, Greg Underwood, Rodney Jenner, and Sheridan Alfirevich, for their valuable contributions to this work and the overall project. The authors thank Kym French and Cora Lau of UQ Biological Resources (Brisbane, Australia) for assisting with animal care and procedures during this experiment, as well as Allan Lisle for statistical advice.
DISCLOSURES
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- Applegate T.J., Klose V., Steiner T., Ganner A., Schatzmayr G. Probiotics and phytogenics for poultry: myth or reality? J. Appl. Poult. Res. 2010;19:194–210. [Google Scholar]
- Baggott G.K. Development of extra-embryonic membranes and fluid compartments. Avian Biol. Res. 2009;2:21–26. [Google Scholar]
- Basmacioǧlu Malayoǧlu H., Baysal Ş., Misirliǒlu Z., Polat M., Yilmaz H., Turan N. Effects of oregano essential oil with or without feed enzymes on growth performance, digestive enzyme, nutrient digestibility, lipid metabolism and immune response of broilers fed on wheat-soybean meal diets. Br. Poult. Sci. 2010;51:67–80. doi: 10.1080/00071660903573702. [DOI] [PubMed] [Google Scholar]
- Brenes A., Roura E. Essential oils in poultry nutrition: main effects and modes of action. Anim. Feed Sci. Technol. 2010;158:1–14. [Google Scholar]
- Chassaing B., Koren O., Goodrich J.K., Poole A.C., Srinivasan S., Ley R.E., Gewirtz A.T. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015;519:92–96. doi: 10.1038/nature14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das R., Mishra P., Jha R. In ovo feeding as a tool for improving performance and gut health of poultry: a review. Front. Vet. Sci. 2021;8 doi: 10.3389/fvets.2021.754246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira J.E., Uni Z., Ferket P.R. Important metabolic pathways in poultry embryos prior to hatch. Worlds Poult. Sci. J. 2008;64:488–499. [Google Scholar]
- El-Moneim A.E.M.E.A., El-Wardany I., Abu-Taleb A.M., Wakwak M.M., Ebeid T.A., Saleh A.A. Assessment of in ovo administration of Bifidobacterium bifidum and Bifidobacterium longum on performance, ileal histomorphometry, blood hematological, and biochemical parameters of broilers. Probiotics Antimicrob. Proteins. 2020;12:439–450. doi: 10.1007/s12602-019-09549-2. [DOI] [PubMed] [Google Scholar]
- Gholami-Ahangaran M., Ahmadi-Dastgerdi A., Azizi S., Basiratpour A., Zokaei M., Derakhshan M. Thymol and carvacrol supplementation in poultry health and performance. Vet. Med. Sci. 2022;8:267–288. doi: 10.1002/vms3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givisiez P.E.N., Moreira Filho A.L.B., Santos M.R.B., Oliveira H.B., Ferket P.R., Oliveira C.J.B., Malheiros R.D. Chicken embryo development: metabolic and morphological basis for in ovo feeding technology. Poult. Sci. 2020;99:6774–6782. doi: 10.1016/j.psj.2020.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemipour H., Kermanshahi H., Golian A., Veldkamp T. Metabolism and nutrition: effect of thymol and carvacrol feed supplementation on performance, antioxidant enzyme activities, fatty acid composition, digestive enzyme activities, and immune response in broiler chickens. Poult. Sci. 2013;92:2059–2069. doi: 10.3382/ps.2012-02685. [DOI] [PubMed] [Google Scholar]
- Jha R., Singh A.K., Yadav S., Berrocoso J.F.D., Mishra B. Early nutrition programming (in ovo and post-hatch Feeding) as a strategy to modulate gut health of poultry. Front. Vet. Sci. 2019;6:1–10. doi: 10.3389/fvets.2019.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X., Huang G., Luo Z., Hu Y., Liu D. Oregano (Origanum vulgare L.) essential oil feed supplement protected broilers chickens against Clostridium perfringens induced necrotic enteritis. Agriculture (Switzerland) 2022;12:18. [Google Scholar]
- Laothaweerungsawat N., Neimkhum W., Anuchapreeda S., Sirithunyalug J., Chaiyana W. Transdermal delivery enhancement of carvacrol from Origanum vulgare L. essential oil by microemulsion. Int. J. Pharm. 2020;579 doi: 10.1016/j.ijpharm.2020.119052. [DOI] [PubMed] [Google Scholar]
- Liu R. 3rd ed. CRC Press; Boca Raton, FL: 2018. Water-Insoluble Drug Formulation. [Google Scholar]
- Llana-Ruiz-Cabello M., Maisanaba S., Puerto M., Pichardo S., Jos A., Moyano R. A subchronic 90-day oral toxicity study of Origanum vulgare essential oil in rats. Food Chem. Toxicol. 2017;101:36–47. doi: 10.1016/j.fct.2017.01.001. [DOI] [PubMed] [Google Scholar]
- Michiels J., Missotten J., Dierick N., Fremaut D., Maene P., De Smet S. In vitro degradation and in vivo passage kinetics of carvacrol, thymol, eugenol and trans-cinnamaldehyde along the gastrointestinal tract of piglets. J. Sci. Food Agric. 2008;88:2371–2381. [Google Scholar]
- Moreira Filho A.L.B., Ferket P.R, Malheiros R.D., Oliveira C.J.B., Aristimunha P.C., Wilsmann D.E., Givisiez P.E.N. Enrichment of the amnion with threonine in chicken embryos affects the small intestine development, ileal gene expression and performance of broilers between 1 and 21 days of age. Poult. Sci. 2019;98:1363–1370. doi: 10.3382/ps/pey461. [DOI] [PubMed] [Google Scholar]
- Nangsuay A., Meijerhof R., van den Anker I., Heetkamp M.J.W., Kemp B., van den Brand H. Effects of breeder age, strain, and eggshell temperature on nutrient metabolism of broiler embryos. Poult. Sci. 2017;96:1891–1900. doi: 10.3382/ps/pew417. [DOI] [PubMed] [Google Scholar]
- Otzen D.E. Biosurfactants and surfactants interacting with membranes and proteins: same but different? Biochim. Biophys. Acta Biomembr. 2017;1859:639–649. doi: 10.1016/j.bbamem.2016.09.024. [DOI] [PubMed] [Google Scholar]
- Peebles E.D. In ovo applications in poultry: a review. Poult. Sci. 2018;97:2322–2338. doi: 10.3382/ps/pey081. [DOI] [PubMed] [Google Scholar]
- Peebles E.D., Sulaiman A., Kellogg T.F., Maslin W.R., Whitmarsh S.K., Gerard P.D. The use of India ink and 51 Cr-labeled microspheres in examining the function of the yolk stalk as a passageway between the yolk sac and intestine in Posthatch Broiler Chicks. Poult. Sci. 1998;77:722–727. doi: 10.1093/ps/77.5.722. [DOI] [PubMed] [Google Scholar]
- Peng Q.Y., Li J.D., Li Z., Duan Z.Y., Wu Y.P. Effects of dietary supplementation with oregano essential oil on growth performance, carcass traits and jejunal morphology in broiler chickens. Anim. Feed Sci. Technol. 2016;214:148–153. [Google Scholar]
- Perinelli D.R., Cespi M., Lorusso N., Palmieri G.F., Bonacucina G., Blasi P. Surfactant self-assembling and critical micelle concentration: one approach fits all? Langmuir. 2020;36:5745–5753. doi: 10.1021/acs.langmuir.0c00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanoff A.L., Hayward F.W. Changes in volume and physical properties of allantoic and amniotic fluids under normal and extreme temperatures. Biol. Bull. 1943;84:141–147. [Google Scholar]
- Siwek M., Slawinska A., Stadnicka K., Bogucka J., Dunislawska A., Bednarczyk M. Prebiotics and synbiotics: in ovo delivery for improved lifespan condition in chicken. BMC Vet. Res. 2018;14:1–17. doi: 10.1186/s12917-018-1738-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnidge, J. 1999. The use of antibiotics in food-producing animals: antibiotic-resistant bacteria in animals and humans. Report of the Joint Expert Technical Advisory Committee on Antibiotic Resistance (JETACAR).
- Uni Z., Ferket R.P. Methods for early nutrition and their potential. Worlds Poult. Sci. J. 2004;60:101–111. [Google Scholar]
- van der Wagt I., de Jong I.C., Mitchell M.A., Molenaar R., van den Brand H. A review on yolk sac utilization in poultry. Poult. Sci. 2020;99:2162–2175. doi: 10.1016/j.psj.2019.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakenell P.S., Bryan T., Schaeffer J., Avakian A., Williams C., Whitfill C. Effect of in ovo vaccine delivery route on herpesvirus of turkeys/SB-1 efficacy and viremia. Avian Dis. 2002;46:274–280. doi: 10.1637/0005-2086(2002)046[0274:EOIOVD]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wang Q.C., Yu H., Zhu J., de Lange K., Yin Y., Wang Q., Gong J. Evaluation of alginate-whey protein microcapsules for intestinal delivery of lipophilic compounds in pigs. J. Sci. Food Agric. 2016;96:2674–2681. doi: 10.1002/jsfa.7385. [DOI] [PubMed] [Google Scholar]