Abstract
Background
Non-tuberculous mycobacteria (NTM) infections are increasing in incidence and associated mortality. NTM are naturally resistant to a variety of antibiotics, complicating treatment. We conducted a literature assessment on the efficacy of bedaquiline in treating NTM species in vitro and in vivo (animal models and humans); meta-analyses were performed where possible.
Method
Four databases were searched using specific terms. Publications were included according to predefined criteria. Bedaquiline’s impact on NTM in vitro, MICs and epidemiological cut-off (ECOFF) values were evaluated. A meta-analysis of bedaquiline efficacy against NTM infections in animal models was performed. Culture conversion, cure and/or relapse-free cure were used to evaluate the efficacy of bedaquiline in treating NTM infection in humans.
Results
Fifty studies met the inclusion criteria: 33 assessed bedaquiline’s impact on NTM in vitro, 9 in animal models and 8 in humans. Three studies assessed bedaquiline’s efficacy both in vitro and in vivo. Due to data paucity, an ECOFF value of 0.5 mg/mL was estimated for Mycobacterium abscessus only. Meta-analysis of animal studies showed a 1.86× reduction in bacterial load in bedaquiline-treated versus no treatment within 30 days. In humans, bedaquiline-including regimens were effective in treating NTM extrapulmonary infection but not pulmonary infection.
Conclusions
Bedaquiline demonstrated strong antibacterial activity against various NTM species and is a promising drug to treat NTM infections. However, data on the genomic mutations associated with bedaquiline resistance were scarce, preventing statistical analyses for most mutations and NTM species. Further studies are urgently needed to better inform treatment strategies.
Introduction
Non-tuberculous mycobacteria (NTM) is a heterogeneous group of environmental microorganisms that comprise more than 193 species (https://www.bacterio.net/genus/mycobacterium). NTM have the capacity to cause a variety of diseases in humans such as TB-like pulmonary or extrapulmonary disease, cervical lymphadenitis, visceral and disseminated disease in immunocompromised individuals.1,2 NTM infections are a health concern worldwide due to increasing incidence and associated mortality rates.3Mycobacterium avium complex (MAC) and Mycobacterium abscessus are the most common pathogens associated with pulmonary NTM diseases, accounting for >90% of all reported cases.4 Diagnosis of NTM infection is complicated due to the large number of NTM species, the overlap between the symptoms of TB and NTM disease, and the similar microscopic morphology of NTM to Mycobacterium tuberculosis. Treatment of NTM disease is difficult due to the limited number of therapeutic options1,5 as NTM species are naturally resistant to several available antibiotics.6
Following favourable treatment outcomes in several clinical and preclinical trials, the US FDA approved bedaquiline in 2012 for treatment of TB caused by infection with drug-resistant M. tuberculosis.7 In 2012, the WHO listed bedaquiline as one of the three core drugs for treatment of rifampicin-resistant TB.
A limited number of studies have investigated the susceptibility of NTM species to bedaquiline6,8 and have assessed the efficacy of bedaquiline in treating NTM diseases in animal models and humans.9–11 We aimed to systematically review the literature on the in vitro susceptibility of NTM to bedaquiline, including the distribution of MICs, and the efficacy of bedaquiline for treatment of NTM disease in animal and human studies. Moreover, we generated a profile of bedaquiline-resistant mutations identified in various NTM species.
Methods
This systematic review was conducted according to the PRISMA and meta-analysis guidelines12 (PROSPERO CRD42020179792).
Search strategy and selection criteria
We searched four databases: PubMed, Web of Science, Scopus and PubMed European using the search terms: (bedaquiline OR diaryl quinolines OR diarylquinolines OR BDQ OR TMC207 OR R207910 OR Sirturo) AND (nontubercul* mycobacterium OR nontubercul* mycobacteria OR non-tubercul* mycobacterium OR non-tubercul* mycobacteria OR NTM OR atypical mycobacteria OR Mycobacteria other than tuberculosis OR MOTT) AND (abscessus OR avium complex OR chelonae OR fortuitum OR kansasii OR marinum OR scrofulaceum OR smegmatis OR ulcerans OR xenopi OR intracellulare), without language or date restrictions. The last search was conducted in December 2022.
After removing duplicate studies, article titles, abstracts and full texts were independently reviewed by three authors (S.O., M.K. and M.G.W.), using defined inclusion criteria as follows: (i) studies reporting on phenotypic and/or genotypic susceptibility to bedaquiline in NTM species using in vitro or in vivo method(s); and (ii) studies reporting on the efficacy of bedaquiline for treatment of NTM disease, in animal models or human studies. Reviews, conference articles and book chapters were excluded. Studies with both NTM and M. tuberculosis isolates but no stratification by mycobacterial species or microbiological results for individual isolates, as well as articles that discussed the use of bedaquiline for NTM but did not present any primary data, were also excluded. The reference lists of included articles were reviewed for any further relevant publications. When required, conflicts were settled by discussion between the three authors.
Data extraction
Data extraction was performed by one author (S.O.) and reviewed by another author (M.K.) to avoid errors. In case of disagreement between the two authors, a third author was consulted (M.G.W.).
For in vitro studies, the data extracted from each study included year of publication, first author, number of isolates tested (overall and by NTM species), study design, method of drug susceptibility testing (DST) used, bedaquiline MIC concentrations tested, bedaquiline MIC of NTM strains, reported classification as resistant or susceptible, presence of variants in genes that may confer resistance to bedaquiline, and sequencing method. Given the scarcity of data from NTM species, data from both clinical and non-clinical isolates were included.
For human and animal model studies, the data extracted from each study included the year of publication, first author, study design, study location, number of patients/animals included in the study, drug regimen, bedaquiline doses used, bedaquiline treatment duration, route of delivery, treatment outcome, follow-up duration, and participant demographics (age, sex and type of disease). When no raw data were provided, authors were contacted, otherwise data extraction (mean ± standard deviation) from graphs was performed using WebPlotDigitizer (version 4.3).13 However, some data were difficult to extract using software, necessitating manual interpretation. Where data were provided as mean ± standard error of the mean (SEM), standard deviation (SD) was obtained by multiplying the square root of the sample size.
Quality assessment and risk of bias
Two authors (S.O. and M.G.W.) assessed the risk of bias and the quality of individual studies independently using the Quality Assessment of Diagnostic Accuracy Studies tool14 for in vitro studies, the SYstematic Review Centre for Laboratory animal Experimentation (SYRCLE) tool for animal model studies (S.O. and M.G.W.)15 and the Joanna Briggs Institute 2017 Critical Appraisal Checklist for case series and case reports.16 Disagreement between the two authors was resolved by consulting a third author (M.K. for in vitro and human studies, and M.B.N. for animal studies).
Data analysis
Data analysis for in vitro studies and meta-analysis was performed by S.O. and confirmed by Dr Michael McCaul.
In vitro studies
Only isolates with clear MIC99 values and one replicate per isolate were included in the analysis and distribution of WT isolates (excluding non-WT, reference and non-clinical strains) depicted graphically for various NTM species with 50 WT isolates being the minimum number used for a histogram. MIC is defined as the lowest concentration of an antimicrobial agent that inhibits the growth of 99% of an organism. MIC99 was represented as MIC. The most frequent MIC value was used to define the mode. The epidemiological cut-off values (ECOFFs) and/or tentative ECOFF (T-ECOFF) values were estimated visually and/or statistically by using the ECOFFinder program17 and following EUCAST guidelines.18 Isolates reported with MIC values of ‘lower or equal to’ (≤) were considered as equal to the particular MIC reported for simplicity and for the purpose of visualizing the histogram (e.g. ≤0.016 was reported as = 0.016).
Due to the paucity of data related to the genomic mutations associated with phenotypic bedaquiline resistance, a descriptive analysis was performed only.
Animal model studies
To evaluate the treatment outcome ‘decrease in bacterial load’ expressed as log10 cfu, meta-analysis was performed using Cochrane Collaboration Review Manager Software version 5.4.1.19 Due to the variations in mouse strains between animal model studies, the summary effect with 95% CI was calculated using the random-effects model. To account for different treatment durations and variations between studies in bacterial strains/subspecies, we converted the analysis to standardized mean differences (SMDs) and 95% CI was used for meta-analysis of treatment for up to 30 days.20–22 Results were considered statistically significant at P < 0.05. Heterogeneity was measured using I2 (with χ2 and 95% CI) and assessed and interpreted according to the guidelines outlined in the Cochrane Handbook for Systematic Reviews of Interventions.23 Where data were insufficient to perform meta-analysis, a descriptive analysis was considered instead.
Human studies
The efficacy of bedaquiline for treatment of NTM diseases was measured in terms of (time to) culture conversion, cure and/or relapse-free cure. Cure was defined as treatment completed with three or more consecutive negative cultures and symptom improvement with no relapse reported within the study period. Relapse is defined as reverting to culture positivity after initial successful treatment with culture conversion. Treatment failure was defined as the lack of three consecutive negative cultures, lack of symptom improvement, evidence of additional acquired resistance to the drugs, or adverse drug reactions (ADRs).
Results
Search results
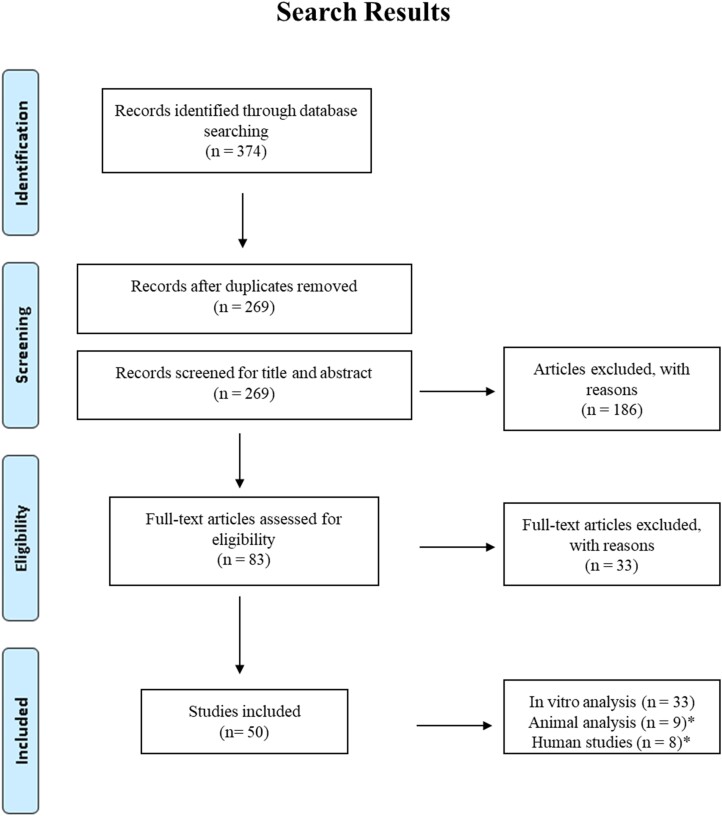
We identified 374 records through the preliminary search. Following deduplication, 269 unique scientific research articles remained for potential inclusion in the review. Screening of the title, abstract and full text identified 50 eligible scientific articles reporting on the effect of bedaquiline on various NTM isolates (Figure 1). Thirty-three studies reported on in vitro experiments6,8,24–54 (Table 1), while only nine animal model studies9,10,55–61 and eight human studies11,62–68 (Table 3) were found, with three studies assessing bedaquiline efficacy both in vitro and in vivo. Thus, these three studies61,62,68 were added to the in vitro analysis to compile a total of 36 studies conducting in vitro analysis. Of the 36 in vitro studies, 13 studies performed genotypic analysis.
Figure 1.
Flow diagram of studies selection. *One animal study and two human studies performed in vitro analysis and were included in our in vitro analysis.
Table 1.
Characteristics of in vitro studies
| Study | Total no. of isolates | Mycobacterium species | Growth | Isolate origin | Phenotypic assay | Genotypic assay | Gene investigated | |
|---|---|---|---|---|---|---|---|---|
| Aguilar-Ayala et al. (2017)6 | 18 | M. abscessus, M. chelonae, M. cosmeticum, M. duvalii, M. flavescens, M. fortuitum, M. franklinii, M. mageritense, M. mucogenicum, M. neoaurum, M. parafortuitum, M. peregrinum, M. phlei, M. smegmatis, M. wolinskyi | Rapid | CCUG strains and UCL strain | MBC MIC | Luria broth, REMA | Sanger sequencing | atpE |
| Alexander et al. (2017)62 | 25 | M. intracellulare | Slow | Clinical | MIC | CAMHB | WGS and targeted | atpE and mmT5 |
| Andries et al. (2005)24 | 26 | M. abscessus, M. avium, M. fortuitum, M. kansasii, M. marinum, M. smegmatis, M. ulcerans | Rapid and slow | Non-clinical | MIC MBC | 7H11, Mueller–Hinton plates | WGS | atpE |
| Asami et al. (2021)25 | 70 | M. abscessus | Rapid | Clinical | MIC | CAMHB | N/A | N/A |
| Brown-Elliott et al. (2017)8 | 104 | MAC and M. avium | Slow | Clinical and reference | MIC | CAMHB | N/A | N/A |
| Cantillon et al. (2022)26 | 2 | M. chimaera, M. abscessus | Rapid and slow | Reference | MIC | REMA | N/A | N/A |
| Chew et al. (2021)27 | 211 | M. abscessus | Rapid | Clinical | MIC | Sensititre plate | N/A | N/A |
| Chew et al. (2021)28 | 32 | M. fortuitum | Rapid | Clinical | MIC | Sensititre plate | N/A | N/A |
| Dupont et al. (2017)29 | 38 | M. abscessus | Rapid | Clinical and non-clinical | MIC | CAMHB | Sanger sequencing | atpE |
| Gumbo et al. (2020)30 | 20 | M. abscessus | Rapid | Clinical and reference | MIC | CAMHB | Sequencing | N/A |
| Gutiérrez et al. (2019)31 | 6 | M. abscessus | Rapid | Non-clinical and reference | MIC | 7H10 | N/A | N/A |
| Huitric et al. (2007)32 | 50 | M. avium, M. intracellulare, M. chelonae, M. fortuitum, M. mageritense, M. phlei, M. vaccae, M. kansasii, M. malmoense, M. gordonae, M. simiae, M. scrofulaceum, M. hiberniae, M. interjectum, M. szulgai, M. terrae, M. conspicuum, M. novocastrense, M. xenopi, M. shimoidei | Rapid and slow | Clinical | MIC | 7H10 | Sanger sequencing | atpE |
| Kim et al. (2017)33 | 2 | M. abscessus | Rapid | Non-clinical and reference | MIC | REMA | N/A | N/A |
| Kim et al. (2019)54 | 307 | M. abscessus, M. avium, M. intracellulare, M. kansasii | Rapid and slow | Clinical | MIC | CAMHB | N/A | N/A |
| Li et al. (2018)34 | 197 | M. abscessus | Rapid | Clinical | MIC | CAMHB | WGS | atpE, MAB_4384 |
| Lin et al. (2022)35 | 111 | M. avium, M. intracellulare, M. marseillense, M. colombiense, M. yongonense, M. vulneris, M. arosiense | Slow | Clinical | MIC | Sensititre plate | N/A | N/A |
| Litvinov et al. (2021)36 | 166 | M. avium, M. intracellulare | Slow | Clinical | MIC | 7H9 | N/A | N/A |
| Lounis et al. (2008)37 | 1 | M. smegmatis | Rapid | Reference | MIC | 7H11, 7H11 + 5% BSA; LJ | N/A | N/A |
| Maeda et al. (2021)38 | 1 | M. smegmatis | Rapid | Non-clinical | MIC | R liquid medium | WGS | atpE, rpoB, prpE |
| Martin et al. (2019)39 | 17 | M. avium, M. chimaera, M. intracellulare, M. kansasii, M. simiae, M. xenopi | Slow | Clinical | MIC | REMA, 7H10 | Sanger sequencing | atpE |
| Pang et al. (2016)40 | 685 | M. abscessus, M. avium, M. fortuitum, M. intracellulare, M. kansasii | Rapid and slow | Clinical | MIC | CAMHB | Sanger sequencing | atpE |
| Ransom et al. (2022)41 | 1 | M. grossiae | Rapid | Clinical | MIC | Not reported | WGS | N/A |
| Richard et al. (2018)43 | 3 | M. abscessus | Rapid | Non-clinical and reference | MIC | CAMHB | N/A | N/A |
| Richard et al. (2018)42 | 7 | M. abscessus | Rapid | Non-clinical and reference | MIC | CAMHB | WGS | MAB_2299c |
| Richter et al. (2018)44 | 1 | M. abscessus | Rapid | Non-clinical and reference | MIC | CAMHB | N/A | N/A |
| Ruth et al. (2019)45 | 28 | M. abscessus, M. avium, M. chimaera, M. fortuitum, M. intracellulare, M. kansasii, M. malmoense, M. simiae, M. xenopi | Rapid and slow | Clinical and reference | MBC MIC | 7H10, CAMHB | N/A | N/A |
| Sarathy et al. (2020)61 | 12 | M. abscessus | Rapid | Clinical and reference | MIC | 7H9, CAMHB | N/A | N/A |
| Schulthess et al. (2022)46 | 62 | M. abscessus | Rapid | Clinical and reference | MIC | CAMHB | N/A | N/A |
| Segala et al. (2012)47 | 37 | M. abscessus, M. fortuitum, M. smegmatis | Rapid | Clinical, non-clinical and reference | MIC | 7H11 | Sequencing | atpE |
| Sorayah et al. (2019)48 | 17 | M. abscessus | Rapid | Clinical | MIC | 7H9 | N/A | N/A |
| Srivastava et al. (2022)49 | 21 | M. kansasii | Slow | Clinical and reference | MIC | 7H9 | N/A | N/A |
| Vesenbeckh et al. (2017)50 | 20 | M. avium, M. intracellulare | Slow | Clinical | MIC | 7H10 | N/A | N/A |
| Vesenbeckh et al. (2017)51 | 20 | M. abscessus | Rapid | Clinical | MIC | 7H10 | N/A | N/A |
| Yu et al. (2019)52 | 260 | M. avium, M. intracellulare, M. kansasii, M. arupense, M. xenopi, M. szulgai, M. fuerth, M. parascrofulaceum, M. terrae, M. malmoense, M. abscessus, M. fortuitum, M. gordonae, M. chelonae, M. holsaticum, M. massiliense, M. asiaticum, M. celatum, M. chimaera, M. gastri, M. microti, M. nonchromogenicum, M. rhodesiae, M. scrofulaceum, M. triviale, M. agri, M. aichiense, M. aurum, M. austroafricanum, M. chitae, M. chubuense, M. cosmeticum, M. diernhoferi, M. fallax, M. farcinogenes, M. flavescens, M. gadium, M. gilvum, M. goodii, M. mucogenicum, M. neoaurum, M. obuense, M. parafortuitum, M. peregrinum, M. phlei, M. porcinum, M. pulveris, M. senegalense, M. septicum, M. simiae, M. sphagni, M. smegmatis, M. thermoresistibile, M. tokaiense, M. vaccae | Rapid and slow | Clinical and reference | MIC | CAMHB | N/A | N/A |
| Zhu et al. (2022)53 | 182 | M. kansasii, M. avium, M. intracellulare, M. abscessus, M. abscessus subsp. massiliense, M. agri, M. aichiense, M. aurum, M. austroafricanum, M. brumae, M. confluentis, M. chelonae, M. chitae, M. chubuense, M. cosmeticum, M. diernhoferi, M. fortuitum, M. fortuitum subsp. fortuitum, M. mucogenicum, M. neworleansense, M. obuense, M. parafortuitum, M. peregrinum, M. pulveris, M. senegalense, M. septicum, M. smegmatis, M. thermoresistibile, M. tokaiense, M. asiaticum, M. arosiense, M. celatum, M. chimaera, M. gastri, M. gadium, M. gordonae, M. interjectum, M. kubicae, M. marinum, M. microti, M. nonchromogenicum, M. parascrofulaceum, M. rhodesiae, M. scrofulaceum, M. szulgai, M. sphagni, M. shimoidei, M. terrae, M. triviale, M. xenopi, M. tuberculosis (H37Rv) | Rapid and slow | Clinical | MIC | CAMHB | N/A | N/A |
| Zweijpfenning et al. (2019)68 | 3 | M. avium | Slow | Clinical | MIC | CAMHB | WGS | atpE; MAV_2512 |
LJ, Löwenstein-Jensen; N/A, not applicable.
Table 3.
Characteristics of human studies
| Study (year) | Study approach | Total no. of patients | No. of patients treated | Sex | Age (years) | Disease | HIV status | Mycobacterium species | Treatment status | In vitro BDQ MIC (mg/L) | Treatment regimen (n) | BDQ dose | Duration of treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alexander et al. (2017)62 | Case series | 7 | 1 | N/R | 54 | NB + C | M. intracellulare | Pre On |
0.004 0.008 | BDQ + EMB + RFB + STR (1) | 200 mg (thrice weekly) | >6 months | |
| 1 | N/R | 60 | N | Pre On |
0.004 0.003 | BDQ + ATM + EMB + RFB + STR (1) | |||||||
| 1 | N/R | 68 | FN + C | Pre On |
0.004 0.008 | BDQ + AMK + EMB + RFB (1) | |||||||
| 1 | N/R | 71 | NB + C | Pre On |
0.004 0.015 | BDQ + AMK + ATM + EMB + STR (1) | |||||||
| 1 | N/R | 58 | N | Pre On |
0.004 2.00 | BDQ + EMB + RFB + STR (1) | |||||||
| 1 | N/R | 66 | NB + C | Pre On |
0.008 0.008 | BDQ + AMK + EMB + RFB (1) | |||||||
| 1 | N/R | 64 | FN + C | Pre | 0.004 | BDQ + CLR + EMB + RFB + STR (1) | |||||||
| Chan et al. (2021)63 | Case study | 1 | 1 | F | 4 | Mycobacterial calcaneal osteomyelitis | No | M. abscessus | BDQ + CLF (1) | 100 mg (thrice weekly) | 8 months | ||
| Erber et al. (2020)64 | Case study | 1 | 1 | F | 20 | Severe soft-tissue infection | No | M. fortuitum complex | Pre | 0.015 | BDQ + LVX (1) | 400 mg (orally once daily for 14 days) | 3.5 month |
| Gil et al. (2021)65 | Case series | 2 | 1 | M | 54 | Disseminated NTM infection, presumed secondary to faecal abdominal cavity contamination | Yes | M. abscessus | Pre | <0.063 | BDQ + CLF + ATM + LZD (1) | 400 mg/day (for 2 weeks, followed by 200 mg thrice monthly) | 21 months |
| 1 | M | 30 | Pyrexia, pancytopenia and lymphadenopathy | Yes | M. avium | BDQ + TDZ + CLF (1) | 400 mg/day (for 2 weeks, followed by 200 mg thrice weekly) | 14 months | |||||
| Meybeck et al. (2021)66 | Case study | 1 | 1 | F | 54 | Cutaneous and subcutaneous nodules, mediastinal lymph nodes with left pulmonary infiltrate. | Yes | M. marinum | BDQ + MFX (1) | 400 mg/day (loading phase for 2 weeks daily followed by a continuation phase of 200 mg thrice weekly) | 12 months | ||
| Pearson et al. (2020)67 | Case series | 4 | 1 | M | 41 | Osteomyelitis and bacteraemia | No | M. abscessus | Pre | 0.12 | BDQ + OMC (1) | 300 mg/day | 24 months BDQ + OMC |
| Philley et al. (2015)11 | Case series | 10 | 1 | M | 36 | N | M. abscessus | AMK + LZD + TGC + BDQ (1) | 400 mg (four tablets of 100 mg once daily with food for the first 2 weeks followed by 200 mg as two tablets of 100 mg) | ≥6 months | |||
| 1 | M | 31 | N | M. abscessus | BDQ + ATM + AMK + TGC + MFX (1) | ≥6 months | |||||||
| 1 | F | 64 | C | M. abscessus | CLR + MFX + ATM + DOX + AMK + TGC + BDQ (1) | ≥6 months | |||||||
| 1 | F | 65 | N | M. abscessus | LZD + LVX + IPM + AMK + BDQ (1) | ≥6 months | |||||||
| 1 | Fa | 58 | N | M. avium complex (M. intracellulare) | RFB + EMB + STR + BDQ (1) | ≥6 months | |||||||
| 1 | Ma | 54 | C | M. avium complex (M. intracellulare) | STR + EMB + RFB + BDQ (1) | ≥6 months | |||||||
| 1 | Ma | 64 | FN + C | M. avium complex (M. intracellulare) | EMB + CLR + RFB + STR + BDQ (1) | ≥6 months | |||||||
| 1 | Fa | 60 | N | M. avium complex (M. intracellulare) | EMB + ATM + AMK + RFB + STR + BDQ (1) | ≥6 months | |||||||
| 1 | M | 65 | C | M. avium complex | EMB + RFB + STR + BDQ (1) | ≥6 months | |||||||
| 1 | F | 73 | C | M. avium complex | EMB + RIF + STR + BDQ (1) | ≥6 months | |||||||
| Zweijpfenning et al. (2019)68 | Case study | 1 | 1 | F | 50 | NB | M. avium | Pre | <0.003 | BDQ + CLF + EMB + CLR (1) | 400 mg/day for 2 weeks then 200 mg thrice weekly | 12 months | |
| On | 0.06 | ||||||||||||
| On | 0.06 |
M, male; F, female; NB, nodular bronchiectasis; C, cavitary; N, nodulary; FN, fibronodular; RIF, rifampicin; AMK, amikacin; BDQ, bedaquiline; CLF, clofazimine; CLR, clarithromycin; IPM, imipenem; RFP, rifapentine; RIF, rifampicin; Q203, telacebec; MFX, moxifloxacin; OMC, omadacycline; TDZ, tedizolid; EMB, ethambutol; STR, streptomycin; RFB, rifabutin; AZM, azithromycin; LZD, linezolid; LVX, levofloxacin; TGC, tigecycline; DOX, doxycycline; pre: pre-treatment; on, on treatment.
aCases reported in two studies.
Description of the included studies
In all 50 included studies, phenotypic MIC data for bedaquiline of 2777 (excluding duplicates) isolates were reported, including 2571 (92.5%) clinical isolates, 59 (2.1%) non-clinical isolates that were generated in vitro, 124 (4.5%) reference strains and 23 (0.8%) for which data on the isolate origin were not available. Out of these 2777 isolates, 1527 (55%) isolates were rapidly growing NTMs (43 species), while 1250 (45%) isolates were slow-growing NTMs (33 species).
M. abscessus species constituted most (84%) of the rapidly growing isolates (1288/1527 isolates, 29 studies), followed by Mycobacterium fortuitum (109 isolates, 7%, 9 studies). Among the slow-growing species, Mycobacterium intracellulare (452/1250 isolates, 36%, 11 studies), M. avium (336 isolates, 27%, 14 studies), Mycobacterium kansasii (246 isolates, 20%, 9 studies) and MAC (110 isolates, 9%, 2 studies) were the most frequently reported species. The remaining species were represented by ≤50 isolates each.
In vitro studies
The characteristics of studies included in in vitro analysis are presented in Table 1. In summary, bedaquiline DST was performed using MIC for 2763 isolates, of which 37 isolates underwent DST using minimum bactericidal concentration (MBC) as well. Bedaquiline MIC was assessed using several methods: CAMHB was the most common method used (17 studies) in which a total of 1995 (72.2%) isolates of different species were characterized, followed by Sensititre plates (345 isolates, 13%, 3 studies), 7H9 (213 isolates, 7.7%, 5 studies), 7H10 (96 isolates, 3.5%, 4 studies) and 7H11 (60 isolates, 2%, 2 studies). Resazurin microtitre assay (REMA), R medium, 7H11 + 5% BSA and Löwenstein-Jensen medium were used in various studies for the remaining isolates, with <50 isolates tested by each method. MBC was assessed using three methods including 7H10, Luria broth agar, and Mueller–Hinton plates, but no method was applied to >50 isolates. One study did not specify the media they used on one isolate (Table S1, available as Supplementary data at JAC Online).
A total of 13 (36%) studies performed genotypic analysis for 1008 isolates (951 clinical and 57 non-clinical) using different sequencing approaches. Sanger sequencing was the most common approach, used for 729 isolates (five studies), followed by WGS (237 isolates, six studies) including targeted sequencing of 25 isolates (one study). In addition, two studies reported on the sequencing of 42 isolates without describing the sequencing approach (Table 1).
Animal model studies
The characteristics of the nine animal studies included in the analysis are shown in Table 2. Six (67%) studies reported on using either bedaquiline alone, followed by combination with other antibiotics, with substantial variation across studies.10,56,58–61 Three studies (33%) reported on the effectiveness of bedaquiline in combination with different antibiotics.9,55,57
Table 2.
Characteristics of animal model studies
| Study (year) | Species | BDQ MIC (mg/L) | Route of infection | Animal strain | Number of mice (N) | BDQ dosage (mg/kg) | Route of drug administration | Treatment regimen (n) | Duration of treatment and follow-up | Outcome measured by |
|---|---|---|---|---|---|---|---|---|---|---|
| Chauffour et al. (2016)55 | M. ulcerans | N/R | Infecting two footpads | Female BALB/c/mice | 60 | 25 | Oral gavage | RFP + BDQ (60) | Up to 4 weeks and follow up till 28 weeks | Lesion index and cfu/footpad |
| Converse et al. (2019)9 | M. ulcerans | 0.125 | Infecting both hind footpads | BALB/c mice | 116 | 25 | Oral gavage | RIF + RFP + CLF + BDQ (29) | Up to 2 weeks and follow-up till 28 weeks | Lesion index and cfu/footpad |
| RFP + BDQ + Q203 (29) | ||||||||||
| RFP + CLF + BDQ + Q203 (29) | ||||||||||
| CLF + BDQ + Q203 (29) | ||||||||||
| Ji et al. (2006)56 | M. ulcerans | N/R | Infecting the left hind footpad subcutaneously | Female BALB/c/mice | 40 | 25 | Oral gavage | BDQ (20) | Up to 8 weeks | cfu/footpad |
| RIF + BDQ (20) | ||||||||||
| Komm et al. (2021)57 | M. ulcerans | N/R | Subcutaneously in both hind footpads | BALB/c mice and Fox Chase SCID Beige mice | 59 | 25 | Oral gavage | BDQ + Q203 (59) | Up to 10 days and follow up till 17 weeks | Lesion index and cfu/footpad |
| Le Moigne et al. (2020)58 | M. abscessus | 0.125 | Intratracheally | C3HeB/FeJ mice | 20 | 30 | Oral gavage | BDQ (10) | Up to 3 weeks | cfu/organ (lung, spleen and liver) |
| BDQ + IPM(10) | ||||||||||
| Lerat et al. (2014)10 | M. abscessus | 0.5 | Intravenously via tail vein | Nude mice | 15 | 25 | Oesophageal gavage | BDQ (15) | Up to 8 weeks | cfu/organ (lung, spleen and kidney) |
| Lounis et al. (2009)59 | M. avium | N/R | Infected intraperitoneally | Female C57BL/6J mice | 80 | 25 | N/R | BDQ (20) | Up to 16 weeks | cfu on the spleen |
| BDQ + CLR (20) | ||||||||||
| BDQ + AMK (20) | ||||||||||
| BDQ + CLR + AMK (20) | ||||||||||
| Obregón-Henao et al. (2015)60 | M. abscessus | 1 ± 0.01 | Intravenously via tail | GKO−/− mice lack IFN and SCID mice | 30 | 30 | Subcutaneous injection | BDQ (15) | Up to 2 weeks | cfu/organ (lung, spleen and liver) |
| BDQ + CLF (15) | ||||||||||
| Sarathy et al. (2020)61 | M. abscessus | N/R | Intranasal | Female NOD.CB17-Prkdcscid/NCrCrl mice | 12 | 20 | Oral gavage | BDQ (12) | Up to 2 weeks | cfu/organ (lung and spleen) |
N, total number of treated mice in BDQ/BDQ-including regimen in the study; n, number of mice in each treatment group; N/R, not reported. RFP, rifapentine; BDQ, bedaquiline; RIF, rifampicin; Q203, telacebec; IPM, imipenem; CLR, clarithromycin.
Four (44%) and five (56%) studies, respectively, reported on the effect of bedaquiline alone or in a combined regimen in treating skin infection caused by Mycobacterium ulcerans species,9,55–57 or disseminated infection caused by either M. abscessus species10,58,60,61 or M. avium species59 (Table 2).
All the animal studies were performed in mice. However, mouse models, the total number of mice, age and sex of mice, the disease induction protocol, the route of drug administration and the treatment duration all varied across the studies (Table 2).
Human studies
Eight descriptive studies were identified that reported on the efficacy of bedaquiline in treating NTM-infected patients. All of the included studies used bedaquiline in combination with other antibiotics (different combinations of amikacin, ethambutol, rifabutin, clarithromycin, streptomycin, linezolid, tigecycline, azithromycin, moxifloxacin or omadacycline).
Of the eight human studies identified, four were case series11,62,65,67 and four were case reports (Table 3).63,64,66,68 Two of the studies were conducted by the same group11,62 and had an overlap of four patients infected with M. intracellulare. However, the analyses done by these studies were completely different. Although Alexander et al.62 reported that 16 cases were enrolled in the case series, the clinical data of only seven patients who experienced relapse were reported, while no clinical data of the remaining nine bedaquiline-treated patients were provided. Therefore, this review reports on results of bedaquiline treatment available for 20 patients (Figure S1).
Of the 20 treated patients, 14 patients had pulmonary disease, and the remaining 6 patients had extrapulmonary diseases, in which 5 had disseminated infection and 1 patient had a severe soft-tissue infection in the lower leg.63 Studies varied in the NTM species investigated, the type of data provided, including bedaquiline DST data, and the measure of patient improvement. Details of the characteristics of the human studies are provided in Table 3.
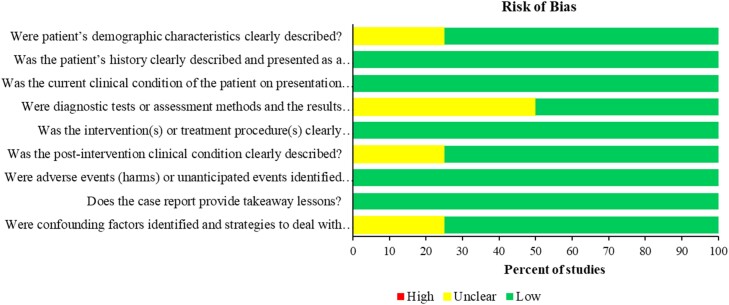
Study quality and risk of bias
In vitro studies
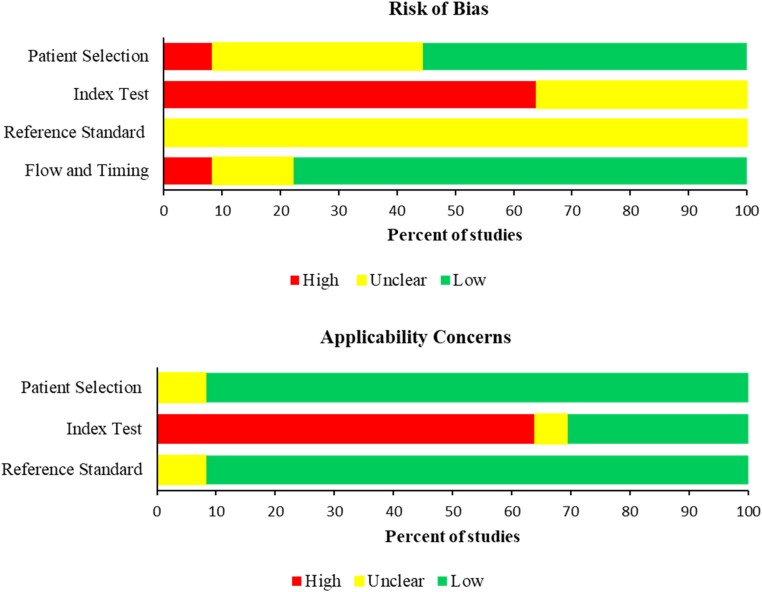
Among studies included in the in vitro analysis, around 64% (23/36) showed a high risk of bias in the index test domain and index test applicability as these studies did not perform the index test. All studies showed unclear risk in the reference test due to either unclear blinding or unclear methodology (e.g. range of the MIC dilutions) used. Most of the studies showed low risk in the reference test applicability with only three studies that showed unclear risk due to unclear reporting of the method used (Figure 2 and Figure S2).
Figure 2.
Risk of bias of the in vitro studies. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Animal model studies
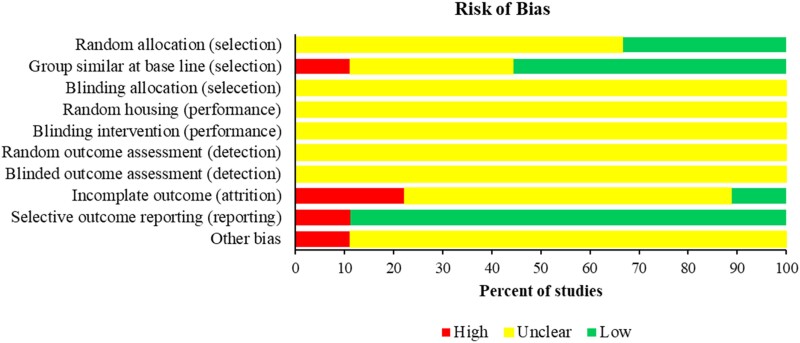
An assessment of selection bias showed that in all studies (100%) it was unclear whether the animals were assigned to groups in a blinded manner (blinding allocation). In six studies (67%), it was unclear whether random allocation was employed. Five (56%) of the studies reported on group similarities at baseline. An assessment of the risk of performance bias demonstrated in all studies (100%) that it was unclear whether the animals were randomly housed, and whether the investigators were blinded during experiments. When evaluated for detection bias, all studies (100%) were unclear on random outcome assessment and whether the outcome assessor was blinded to group allocation. Six studies (67%) had an unclear attrition bias due to poor reporting of the number of experimental animals in methods and results sections, two studies (22%) had a high risk of attrition bias, and one study (11%) had a low risk of bias. The majority of studies (89%; 8/9) had a low risk of selective reporting bias (Figure 3 and Table S2).
Figure 3.
Quality assessment of animal model studies using SYRCLE tool. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Human studies
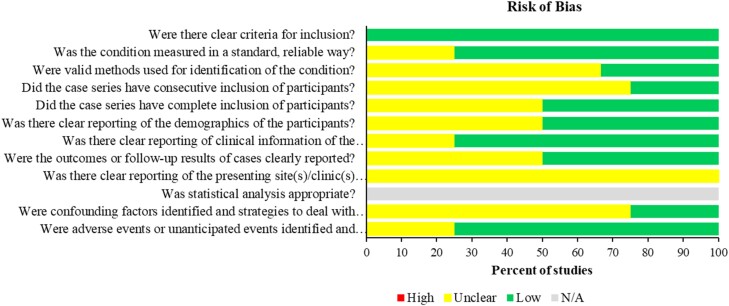
None of the case series studies reported the presenting site(s)/clinic(s) clearly. Three (75%) of the case series were unclear about using consecutive inclusion of participants. Three case-series studies (75%) did not define the confounding factors and strategies to deal with it clearly. Two (50%) of the case-series studies were unclear about the outcome of assessment and follow-up of all the cases and one study was unclear about adverse events (Figure 4 and Table S3). The two case-report studies had low risk of bias in all domains. Both studies had clearly defined the inclusion criteria and identified confounding factors and treatment strategies were discussed. Both studies clearly described patients’ history, clinical conditions, and diagnostic assessment methods. One case report did not describe patient demographics clearly nor provide a clear timeline, and all of the reports were unclear about the confounding factors (Figure 5 and Table S4).
Figure 4.
Risk of bias of case series. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Figure 5.
Risk of bias of case reports. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Data analysis
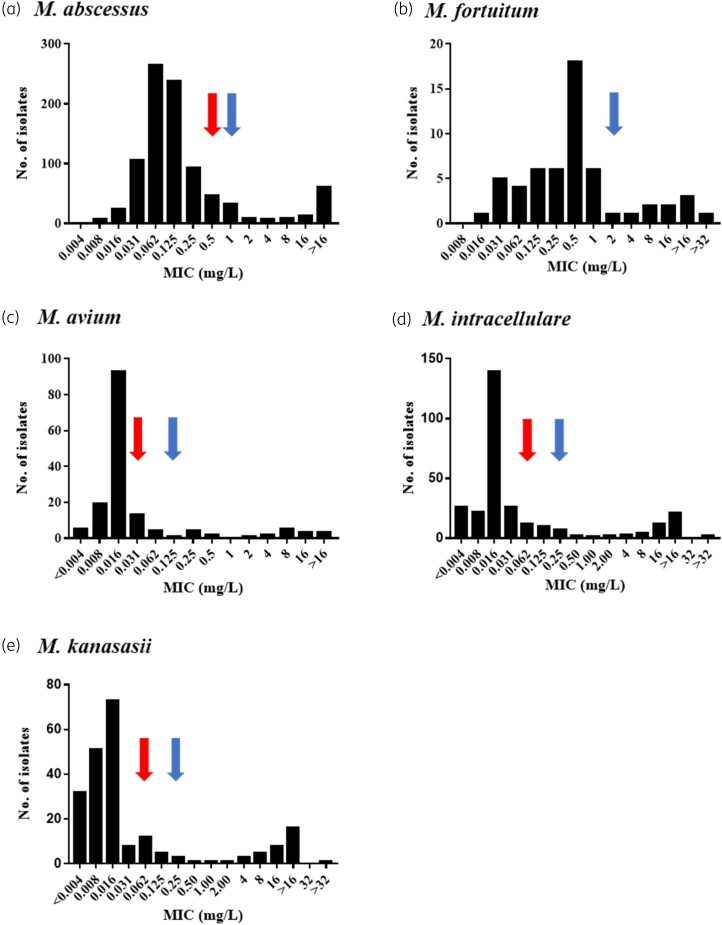
We analysed data from in vitro studies to assess the effect of bedaquiline by using MIC distribution histograms with the aim to propose ECOFF values for various NTM species. However, sufficient data were available for only two rapidly growing species (M. abscessus and M. fortuitum) and three slow-growing species (M. avium, M. intracellulare, M. kanasasii). Data from other species were scarce and thus we were unable to analyse them.
The effect of bedaquiline in treating NTM species in in vitro studies
As MIC distribution can vary across platforms and between species, where data were available, we evaluated bedaquiline MIC distribution of various NTM species accordingly.
Bedaquiline MIC distributions in CAMHB
CAMHB was the most commonly employed medium (19 studies) to define the phenotypic behaviour of 1996 (72%) isolates in the presence of bedaquiline (Table 1). The overall bedaquiline MIC range in CAMHB across NTM species was between 0.008 and >32 mg/L. However, not all MIC histograms formed bimodal distributions, as shown below.
Rapidly growing mycobacteria (RGM)
Of 1996 isolates, 911 WT M. abscessus isolates from eight studies reporting MIC99 values25,29,30,34,40,52–54 were included in the MIC distribution histogram (Figure 6a). The bedaquiline MIC distribution of these isolates ranged from 0.008 to >32 mg/L, with most of the isolates aggregated between 0.031 and 0.25 mg/L, and the mode at 0.062 mg/L with 29% (265/911) isolates. Around 7% (62/911) had an MIC > 16 mg/L. Visual inspection of the M. abscessus MIC distribution suggests that the cut-off value should be 1.0 mg/L (Figure 6a, blue arrow). Thus, 89% (812/911) of isolates were susceptible to bedaquiline, while 11% (99/911) were resistant.
Figure 6.
MIC distribution of the common NTM species in CAMHB. The blue arrow is pointing to the eyeball value and the red arrow is showing the T-ECOFF value. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
MIC data of 56 (56/1681; 3%) M. fortuitum isolates from three studies 40,45,52 were available and included in the MIC distribution histogram (Figure 6b). The MIC distribution ranged between 0.016 and >16.0 mg/L, with the majority (32%) of isolates aggregating at an MIC of 0.5 mg/L. Based on a visual evaluation of the histogram of M. fortuitum isolates, a cut-off value of 2.0 mg/L could be established, with 82% (46/56) susceptible and 18% (10/56) resistant to bedaquiline.
Slow-growing mycobacteria (SGM)
Using CAMHB, the majority of the isolates of the most common slow-growing NTM species aggregated between an MIC of 0.008 and 0.062 mg/L. Of the 155 isolates of M. avium from five studies,40,52–54,68 60% (93/155) had an aggregated MIC (mode) of 0.016 mg/L, with the MIC of one isolate at <0.003 mg/L and another three isolates at ≥16 mg/L. Although the MIC distribution for M. avium was a skew to the right and did not form a clear bimodal distribution, a value of 0.125 mg/L can be proposed as a cut-off point. Applying this value, 86% (134/155) isolates were susceptible and 14% (21/155) were resistant (Figure 6c).
Two hundred and eighty-nine isolates of WT M. intracellulare were evaluated in six studies40,45,53,54,62 using CAMHB. In contrast to M. avium, the MIC histogram of M. intracellulare showed a bimodal distribution. However, the majority of M. intracellulare isolates (48%; 139/289) shared the same mode as M. avium, which was 0.016 mg/L. Visual inspection of the MIC histogram of M. intracellulare suggests a cut-off value of 0.25 mg/L. Applying this value, 81% (235/289) of M. intracellulare were susceptible and 19% (54/289) were resistant to bedaquiline (Figure 6d). Due to the small number of M. intracellulare and M. avium isolates, the MIC distribution was not compiled as one complex.
Data for M. kanasasii revealed that 33% (73/220) of isolates from five studies had a modal MIC of 0.016 mg/L.40,45,52–54 A cut-off value of 0.25 mg/L could also be proposed for M. kanasasii (Figure 6e).
Bedaquiline MIC distribution in Sensititre
Two recent studies reported on the bedaquiline MIC distribution using Sensititre plates. One study reported on bedaquiline MIC distribution of 211 M. abscessus isolates, of which 146 were respiratory isolates,27 using a Sensititre RAPMYCO plate and a customized broth microdilution testing panel (SGPNUHS1 plate) (Sensititre, Thermo Fisher Scientific, Waltham, MA, USA). Bedaquiline MIC ranged from 0.008 to 0.25 mg/L. The majority (96/211; 45%) of isolates had an MIC of 0.06 mg/L. The proposed T-ECOFF value of 99.0% was 0.5 mg/L. Using the same method, the same author reported that the bedaquiline MIC distribution of 32 isolates of M. fortuitum ranged between 0.004 and 0.015 mg/L, with the MIC mode at 0.008 mg/L (Figure S3).28
The other study reported bedaquiline MIC distribution of a total of 111 isolates of five different species of MAC using the Sensititre Myco susceptibility plate for SGM (Thermo Fisher Scientific). Bedaquiline MIC of M. avium and M. intracellulare ranges were 0.03–0.12 and 0.015–0.12 mg/L, respectively. The study showed that majority of MAC isolates were susceptible to bedaquiline and isolates from both species (M. avium and M. intracellulare) aggregated at an MIC of 0.06 mg/L.
Bedaquiline MIC distribution in 7H9
Two studies reported on MIC distribution of SGM species in 7H9.36,49 In one study, the bedaquiline MIC distributions of M. avium and M. intracellulare in 7H9 were mostly in the ranges 0.003–1.0 and 0.003–0.5 mg/L, respectively, with the MIC modes being 0.015 and 0.007 mg/L, respectively (Figure S3). The majority of M. avium (98/124; 79.0%) and M. intracellulare (37/42; 88.1%) isolates had a bedaquiline MIC of <0.03 mg/L. The T-ECOFF values for M. avium and M. intracellulare that were proposed by the authors were 0.12 and 0.06 mg/L, respectively. Using the proposed T-ECOFF value, only two isolates of M. avium and three isolates of M. intracellulare were found to be resistant to bedaquiline in 7H9.36
The other study reported on bedaquiline MIC distribution of only 20 isolates of M. kanasasii, in which most isolates (n = 20) had bedaquiline MIC of 0.03 mg/L and only 2 isolates were resistant to bedaquiline with MIC of 2 mg/L (Figure S3).49
ECOFF value determined by fitting a log-normal distribution (ECOFFinder)
MIC distributions from five studies only25,29,34,53,54 fitted EUCAST criteria and data of 471 isolates were included in ECOFFinder analysis. The estimated values of the ECOFF 95.0%, and 99.0% for M. abscessus were 0.25 and 0.5 mg/L, respectively (red arrow in Figure 6 and Figure S4). These values differ by one 2-fold dilution from the visual estimated values that are accepted. An MIC above the ECOFF 99.9% was categorized as resistance.69,70 Applying an ECOFF of 0.5 mg/L showed that 2% (8/471) of isolates were resistant.
None of the SGM MIC distributions met the EUCAST requirements for acceptable distributions since they were either truncated at the lower end or had too few WT observations (<15) to be certain of their quality. However, when we used ECOFFinder for the available data, T-ECOFF values differed by two 2-fold dilutions from the visual estimated values. Therefore, our findings will remain uncertain and further studies are still required to define the ECOFF value for SGM and to corroborate our findings for M. abscessus species.
Association between genotype and phenotype bedaquiline resistance in NTM species
Genetic variants were identified in seven different genes: 22 mutations in atpE, 11 in MAB_4384, 9 in mmpT5, 6 in MAB_2299c, 1 in MAV_2152, 1 in rpoB and 1 in prpE. In addition, a study identified a new gene cluster called MAB_1135c-MAB_1134c that encodes a new MmpS-MmpL efflux pump system involved in the intrinsic resistance to bedaquiline and clofazamine in M. abscessus. MAB_1135c-1134c expression is also dependent on the MAB_2299c TetR repressor.6,24,29,32,34,38,43,47,52,62,68 Media used for antimicrobial susceptibility tests for these isolates varied across the studies. MIC values for the strains conferring resistance to bedaquiline also varied between 0.004 and >16.0 mg/L, with the highest MIC observed in isolates with mutations in the atpE gene. Mutations in the atpE gene occurred in both clinical and non-clinical isolates (Table 4).
Table 4.
Mutations associated with bedaquiline resistance in NTM species
| Gene | Mutation in amino acid | Mutation in DNA | MIC (mg/L) | No. of isolates | Mycobacterium species | Type of isolate | Study (year) |
|---|---|---|---|---|---|---|---|
| atpE | Ala35Ala | T105C | 0.031 | 3 | M. abscessus | Clinical | Pang et al. (2017)40 |
| 0.62 | 5 | ||||||
| 0.125 | 5 | ||||||
| 0.25 | 2 | ||||||
| 2 | 1 | ||||||
| 4 | 1 | ||||||
| Ala63Met | >2 | 2 | M. flavescens | Clinical | Aguilar-Ayala et al. (2017)6 | ||
| 8 | 1 | M. novocastrense | Clinical | ||||
| 8 | 1 | M. shimoidei | Clinical | Huitric et al. (2007)32 | |||
| 4 | 1 | M. xenopi | Clinical | ||||
| 8 | 1 | ||||||
| 0.5 | 1 | M. smegmatis | Non-clinical | Segala et al. (2012)47 | |||
| Ala63Pro | 0.5 | 1 | M. smegmatis | Non-clinical | Segala et al. (2012)47 | ||
| 16 | 1 | M. abscessus | Non-clinical | ||||
| Ala64Pro | 16 | 1 | M. abscessus | Non-clinical | Dupont et al. (2017)29 | ||
| 0.125 | 1 | ||||||
| Ala65Pro | 0.004 | 1 | M. intracellulare | Clinical on treatment | Alexander et al. (2017)62 | ||
| 2 | 1 | M. intracellulare | Clinical post treatment | ||||
| 2 | 1 | M. intracellulare | Clinical on treatment | ||||
| Aps32Gly | T32C | >0.5 | 1 | M. smegmatis | Non-clinical | Maeda et al. (2021)38 | |
| Asp28Ala | 8 | 7 | M. abscessus | Non-clinical | Segala et al. (2012)47 | ||
| 4 | 9 | M. fortuitum | Non-clinical | ||||
| 16 | 1 | M. smegmatis | Non-clinical | ||||
| Asp28Gly | >16 | 1 | M. smegmatis | Non-clinical | Segala et al. (2012)47 | ||
| Asp28Val | 16 | 1 | M. smegmatis | Non-clinical | Segala et al. (2012)47 | ||
| Asp776Asn & Asp32Ala & Gly31Gly | G776A & T32G & G31A | 0.19 | 1 | M. smegmatis | Non-clinical | Maeda et al. (2021)38 | |
| Asp32Ala & Gly31Gly & Ser465Pro | T32G & G31A & T465C | 0.25 | 1 | M. smegmatis | Non-clinical | ||
| Asp29Val | 16 | 1 | M. abscessus | Non-clinical | Dupont et al. (2017)29 | ||
| 0.125 | 1 | ||||||
| Asp32Val | MIC90 = 3 | 1 | M. smegmatis | Non-clinical | Andries et al. (2014)24 | ||
| MIC90 = 3 | 1 | ||||||
| Glu61Asp | 4 | 1 | M. smegmatis | Non-clinical | Segala et al. (2012)47 | ||
| Glu65Glu | A195G | 0.062 | 2 | M. fortuitum | Clinical | Pang et al. (2017)40 | |
| 0.25 | 1 | ||||||
| 1 | 1 | ||||||
| 16 | 1 | ||||||
| Glu65Asp | T65A | 0.5 | 1 | M. smegmatis | Non-clinical | Maeda et al. (2021)38 | |
| Gly24Gly | T72C | 0.062 | 6 | M. abscessus | Clinical | Pang et al. (2017)40 | |
| 0.12 | 8 | ||||||
| 0.25 | 4 | ||||||
| 0.5 | 6 | ||||||
| >16 | 1 | ||||||
| Gly49Gly | C147T | 0.0156 | 6 | M. intracellulare | Clinical | Pang et al. (2017)40 | |
| 0.031 | 2 | ||||||
| 0.062 | 3 | ||||||
| 0.12 | 2 | ||||||
| 1 | 1 | ||||||
| 4 | 1 | ||||||
| >16 | 1 | ||||||
| Gly62Gly | T186C | 0.031 | 2 | M. kansasii | Clinical | Pang et al. (2017)40 | |
| 0.063 | 3 | ||||||
| 1 | 1 | ||||||
| Ile66Met | 8 | 1 | M. smegmatis | Non-clinical | Segala et al. (2012)47 | ||
| 16 | 8 | ||||||
| Leu59Val | 0.5 | 1 | M. smegmatis | Non-clinical | Segala et al. (2012)47 | ||
| Val62Val | C186A | 0.031 | 1 | M. avium | Clinical | Pang et al. (2017)40 | |
| C186T | 0.062 | 1 | |||||
| C186A | 0.12 | 1 | |||||
| C186A | >16 | 1 | |||||
| Wild type | 0.004–0.03 (0.025) | 22 | M. intracellulare | Clinical | Alexander et al. (2017)62 | ||
| <0.003 | 1 | M. avium | Clinical | Zweijpfenning et al. (2019)68 | |||
| No non-synonymous mutation was found | 0.0078–1 | 197 | M. abscessus | Clinical | Li et al. (2018)34 | ||
| mmpT5 | WT | 0.004 | 5 | M. intracellulare | Clinical | Alexander et al. (2017)62 | |
| Gly66FS | C196_197insC | 0.008 | 1 | M. intracellulare | Clinical | ||
| Val46Gly | T137G | 0.025 | 2 | M. intracellulare | Clinical | ||
| Glu177Lys | G529A | 0.004 | 1 | M. intracellulare | Clinical | ||
| 0.008 | 2 | ||||||
| 0.025 | 1 | ||||||
| Ala23Pro &Glu177Lys | G67C & G529A | 0.008 | 2 | M. intracellulare | Clinical | ||
| Pro104FS | C311_312insC | 0.015 | 2 | M. intracellulare | Clinical | ||
| 0.025 | 2 | ||||||
| Ala162Pro | G484C | 0.004 | 1 | M. intracellulare | Clinical | ||
| 0.008 | 1 | ||||||
| Ilu19Ser & Glu177Lys | T56G & G529A | 0.008 | 1 | M. intracellulare | Clinical | ||
| Val35Gly & Glu177Lys | T104G & G529A | 0.008 | 1 | M. intracellulare | Clinical | ||
| Arg25Pro | G74C | 0.004 | 1 | M. intracellulare | Clinical | ||
| MAB_4384 | Ala152 Glu | 0.062 | 2 | M. abscessus | Clinical | Li et al. (2018)34 | |
| Ala169 Ser | 0.5 | 1 | M. abscessus | Clinical | |||
| Del (MAB_4384 gene) | Del | 0.0078 | 2 | M. abscessus | Clinical | ||
| 0.0156 | 3 | ||||||
| 0.031 | 18 | ||||||
| 0.062 | 39 | ||||||
| 0.125 | 36 | ||||||
| 0.25 | 11 | ||||||
| Gln215 Arg | 0.0078 | 1 | M. abscessus | Clinical | |||
| 0.031 | 1 | ||||||
| 0.062 | 1 | ||||||
| 0.125 | 4 | ||||||
| 0.5 | 1 | ||||||
| Gly125 Asp & Gln215 Arg | 0.062 | 1 | M. abscessus | Clinical | |||
| 0.125 | 2 | ||||||
| His7 Arg &Glu142 Lys | 0.125 | 1 | M. abscessus | Clinical | |||
| 0.25 | 4 | ||||||
| 0.5 | 1 | ||||||
| N1T | 0.062 | 2 | M. abscessus | Clinical | |||
| 0.125 | 1 | ||||||
| WT | 0.007 | 2 | M. abscessus | Clinical | |||
| 0.015 | 1 | ||||||
| 0.031 | 9 | ||||||
| 0.062 | 16 | ||||||
| 0.125 | 19 | ||||||
| 0.25 | 6 | ||||||
| 0.5 | 2 | ||||||
| 1 | 1 | ||||||
| Trp88 Gly | 0.062 | 2 | M. abscessus | Clinical | |||
| 0.125 | 1 | ||||||
| Val31 Ile | 0.031 | 1 | M. abscessus | Clinical | |||
| 0.125 | 2 | ||||||
| 0.25 | 1 | ||||||
| Val31 IleI & Asp120 Asn | 1 | M. abscessus | Clinical | ||||
| Val5 Met & His7 Arg & Glu142 Lys & Ala217 Ser | 1 | M. abscessus | Clinical | ||||
| MAB_2299c | Asp106fs | ins318A | 2 | 1 | M. abscessus | Non-clinical | Richard et al. (2018)43 |
| Glu181stop | C276 | 2 | 1 | ||||
| Gly215Ser | G541T | 2 | 1 | ||||
| Leu151Pro | G643A | 2 | 1 | ||||
| Leu40Trp | T452C | 2 | 1 | ||||
| Pro92fs | T119G | 2 | 1 | ||||
| MAV_2152 | Trp173Arg | A2544950G | 0.06 | 1 | M. avium | Clinical | Zweijpfenning et al. (2019)68 |
| Trp173Arg | A2544950G | 0.125 | 1 | ||||
| rpoB | Asp 776Asn | 0.5 | 1 | M. smegmatis | Non-clinical | Maeda et al. (2021)38 | |
| prpE | Ser465Pro | 0.2 | 1 | M. smegmatis | Non-clinical |
The effect of bedaquiline in treating NTM species in animal models
Four studies evaluated bedaquiline only or bedaquiline-including regimens in treating skin infection (inoculation of footpad) in mice (44%; 4/9).9,55–57 Five studies reported on disseminated infection (56%; 5/9) in lung, liver and spleen.10,58–61 Mortality rate was reported in one study only.10 However, none of the studies that assessed bedaquiline-only efficacy in treating disseminated infections reported on follow-up, relapse, cure or relapse-free cure as treatment outcome.
Treatment outcome of bedaquiline-including regimens
Bedaquiline-including regimens decreased the footpad lesion index in mouse models of skin infection. A mean index of 3 (scale of 0–4) at Day 0 decreased to 1.8 and 1.42 after 4 and 8 weeks of treatment, respectively, in the bedaquiline + rifapentine-treated group and remained stable with no relapse reported.55 Similarly, Converse et al.9 reported a decrease in lesion index to an average of 0.56 ± 0.25 and 0.3 ± 0.2 in all treatment regimens that included bedaquiline after 4 and 8 weeks of treatment, respectively. Komm et al.57 also showed a decrease in the footpad lesions from a median of 2.0 to ≤1.0 within 1 week of bedaquiline + telacebec treatment for 1, 3 and 5 days in two different mouse models (BALB/c and SCID-beige).
A 2 week regimen of rifapentine/clofazimine/bedaquiline (RFP + CLF + BDQ), followed by 12 weeks follow-up resulted in a lesion index of 2.5 in 10% of treated mice. Additionally, a 4 week regimen of RFP + CLF + BDQ plus telacebec (RFP + CLF + BDQ + Q203) showed a lesion index of 1.4 in 10% of treated mice.9
Bedaquiline-including regimens also had the effect of decreasing bacterial burden (mean cfu) from 5.89 ± 0.22 at Day 0 to 1.095 ± 1.0 after 2 weeks of treatment9 and from 6.39 ± 0.30 to 0.19 ± 0.42 after 4 weeks of treatment55 or negative (complete cure).9 Similarly, bacterial load declined from 6.62 ± 0.34 to culture negative within 5 days of initiating a bedaquiline + telacebec treatment regimen.57
There was relapse in 15% (1.5/10 mice) with cfu counts ranging from 3.13 to approximately 5.08 log10 after 2 weeks treatment and 12 weeks follow-up when treated with bedaquiline-including regimens.9 However, no cfu were detected in any other bedaquiline-containing regimen after 4 weeks of treatment and 21 weeks follow-up, suggesting a complete cure of the treated mice using bedaquiline-including regimens.9 Only one (13%) study reported on death as a bedaquiline treatment outcome in 20% (2/10) of mice.10 None of the other studies included data on mortality.
These data suggested that the relapse rate is ∼10%–15% in mice treated with a bedaquiline-containing regimen. However, more data are still required to have a concrete conclusion.
Treatment outcome of bedaquiline alone
The efficacy of bedaquiline alone in treating infection caused by NTM species was evaluated only based on its ability to reduce bacterial burden. While five studies10,58–61 evaluated the efficacy of bedaquiline alone in treating NTM disseminated infection, only one study (13%) looked at its efficacy in treating NTM skin infection.56 For this major outcome, meta-analysis was performed using six studies. To evaluate the pooled effect measure in animals (mice), we performed subgroup meta-analysis stratified by organ and by NTM species. The effect size was measured using SMD as reported in the methods. However, when using mean difference measures, the effect of bedaquiline remains significant with a slight decrease in the effect size.
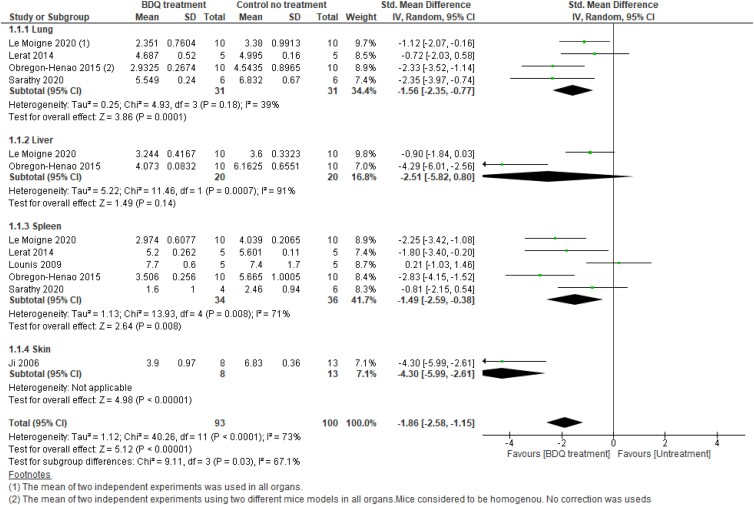
Subgroup analysis by organ
Lung
Subgroup meta-analysis in lungs showed that bedaquiline reduces the bacterial load (log10 cfu) by 1.56 times in the bedaquiline treatment arm compared with no treatment, with low to moderate heterogeneity (SMD 1.56; 95% CI −2.35 to −0.77; P < 0.0001; I2 = 39%; 4 studies; 29 mice) (Figure 7, Analysis 1.1.1). To explore heterogeneity, the Obregón-Henao 2015 study was excluded from the analysis, resulting in decreased heterogeneity of I2 = 20%. However, the effect measure remained significant (SMD −1.26; 95% CI −2.06 to −0.46; P < 0.0001; I2 = 20%).
Figure 7.
Forest plot of bedaquiline (BDQ)-treated animals versus control (no treatment) animals. Outcome 1.1: BDQ effect on bacterial load for each group up to 30 days (subgroup by organs). The effect of BDQ on bacterial load in each group was expressed as log10 cfu ± SD. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Liver
There was no evidence of a difference between bedaquiline treatment and no treatment (SMD −2.51; 95% CI −5.82 to 0.80; P = 0.14; 2 studies; 20 mice) (Figure 7, Analysis 1.1.2). However, there was substantial heterogeneity among the two studies that examined the efficacy of bedaquiline in reducing the bacterial load in the liver (I2 = 91%; P = 0.0007).
Spleen
A forest plot showed a significant difference in reducing the bacterial load in the spleen between bedaquiline treatment and no treatment with high heterogeneity (SMD −1.49; 95% CI −2.59 to −0.38; P = 0.008; I2 = 71%; 5 studies; 34 mice) (Figure 7, Analysis 1.1.3). When the Lounis et al.59 study was excluded from the analysis, the heterogeneity decreased from high to moderate (I2 = 37%; P = 0.19) and the effect measure remained significant (SMD −1.95; 95% CI −2.80 to −1.10; P < 0.00001) (Figure S5). However, when the Lounis et al.59 and Sarathy et al.61 studies were excluded from the analysis, no evidence of heterogeneity was detected and the effect measure remained significant (SMD −2.34; 95% CI −3.11 to −1.58; P < 0.00001; data not shown).
Skin
One study (1/3) compared bedaquiline alone versus a control group and provided data for up to 14, 30 and 60 days56 was included in the meta-analysis. A forest plot showed a significant difference in the reduction in cfu between the treatment and control arms (SMD −4.30; 95% CI −5.99 to −2.61; P < 0.00001; one study, 8 mice, heterogeneity is not applicable) (Figure 7, Analysis 1.1.4).
Overall, the effect of bedaquiline, stratified by organ (lung, liver, spleen and skin) revealed a significant reduction in bacterial load in lung, spleen and skin but not liver within 30 days. Bedaquiline reduced the bacterial load on average by 1.86 times in the bedaquiline treatment arm compared with the no-treatment arm (SMD −1.86; 95% CI −2.58 to −1.15; P < 0.0001; I2 = 73%) (Figure 7). The overall heterogeneity between studies was substantial (I2 = 73%). Similarly, subgroup heterogeneity tests showed a significant difference between organs (P = 0.03; I2 = 67%). This is largely due to the heterogeneity between studies, particularly Ji’s.56 However, when a susceptibility test was performed and the study by Ji was removed, no significant difference in heterogeneity between the three organs (lung, liver and spleen) was detected (Figure S6).
As a part of sensitivity analysis, we conducted the meta-analysis by including data up to 60 days from two studies (Lerat et al.10 and Lounis et al.59). The overall pooled effect measure remains significant with an increase in the effect of bedaquiline, resulting in reduction of the bacterial load on average by 2.29 (SMD −2.29; 95% CI −2.97 to −1.62; P < 0.00001) (Figure S7).
Collectively, this data indicates that bedaquiline can significantly reduce bacterial burden, and it is more effective in the lung and spleen than in the liver. However, depending on the bacterial species and strain, a longer treatment period might be required. Due to the small sample size further studies are still required to confirm this data.
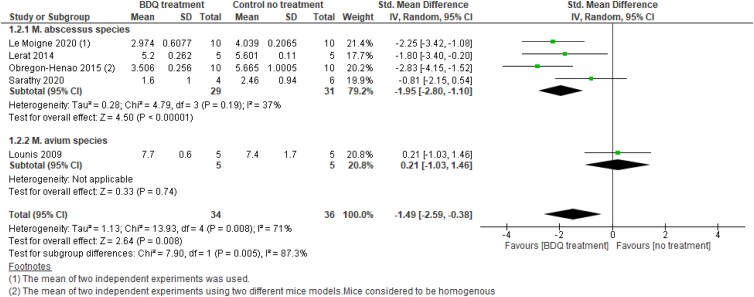
Subgroup analysis by NTM species
We then looked at the effect of bedaquiline stratified by NTM species (M. abscessus, M. avium and M. ulcerans). Since one study59 showed a bedaquiline effect against M. avium species in spleen only, we were only able to perform meta-analysis of bedaquiline treatment efficacy in eradicating bacterial burden in one organ (spleen).
Subgroup analysis by NTM species in spleen
M. abscessus
Subgroup meta-analysis showed that bedaquiline reduces the log10 cfu of M. abscessus significantly by 1.95 times. However, there was moderate heterogeneity between these studies (SMD −1.95; 95% CI −2,80 to −1.10; P < 0.00001; I2 = 37%) (Figure 8, Analysis 1.2.1). Excluding data from Sarathy et al.61 study, decreased heterogeneity (I2 = 0%) within this subgroup (SMD −2.34; 95% CI −3.11 to −1.58; P < 0.00001) (Figure S8, Analysis 1.2.1).
Figure 8.
Forest plot of bedaquiline (BDQ)-treated animals versus control (no treatment) animals. Outcome 1.2: BDQ effect on bacterial load up to 30 days (subgroup by NTM species). The effect of BDQ on bacterial load was expressed as log10 cfu ± SD. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
M. avium
One study59 assessed the effect of bedaquiline against M. avium in the spleen for up to 30 days. There was no difference between bedaquiline treated or untreated arms (SMD −0.21, 95% CI −1.03 to 1.46, P = 0.74) (Figure 8, Analysis 1.2.2).
Overall, within 30 days, stratification by NTM species revealed a significant effect of bedaquiline in reducing the bacterial load of the two NTM species: M. abscessus and M. avium by 1.49 (SMD −1.49; 95% CI −2.59 to −0.38; P = 0.008). However, the overall heterogeneity between studies was high (I2 = 71%; P = 0.008) (Figure 8, Analysis 1.2) with substantial heterogeneity between the subgroups (I² = 87.3%; P = 0.005).
However, when data of 60 days from two studies (Lerat et al.10 and Lounis et al.59) were used in the meta-analysis, the overall bedaquiline efficacy in reducing the bacterial load was increased from 1.49 to 2.54 (SMD −2.54; 95% CI −3.23 to −1.86; P < 0.00001) (Figure S9, Analysis 1.2) with a significant change in the effect on M. avium species treatment (SMD −4.49; 95% CI −7.31 to −1.68; P = 0.002) (Figure S9, Analysis 1.2.2). The overall heterogeneity between subgroups, however, reduced from high to moderate (I2 = 48.8%).
Longer treatment duration with bedaquiline may be required to show a significant effect on NTM species, particularly on M. avium species. However, due to the small sample size, the evidence is uncertain.
The effect of bedaquiline in treating NTM species in humans
Six (30%; 6/20) cases were successfully cured (symptom improvement and culture conversion) with no relapse reported when a bedaquiline-including regimen was used.63–67 All of these cases had NTM extrapulmonary infection caused by either M. abscessus, M. fortuitum, M. avium or Mycobacterium marinum (Table 3), in which one case had cutaneous infection in the leg and four patients had disseminated NTM infection. Three were immunocompromised with HIV coinfection, and one was a child with AML complicated by calcaneal osteomyelitis. Treatment duration varied between 3.5 and 18 months. Although some adverse events such as nausea66 and a mild QTc prolongation in one case63 were reported, bedaquiline was well tolerated.
However, data from the remaining 14 patients with NTM pulmonary diseases fluctuated between symptom improvement and culture conversion11 to a treatment failure.68 Ten of these patients that had potentially life-threatening NTM disease and were failing standard therapy: off-label bedaquiline was added to their current antibiotic regimens as a salvage therapy.11 Various outcomes were observed throughout the study. However, none of these patients had three consecutive negative cultures or were reported to be cured completely, despite the improvement in semi-quantitative sputum culture scores, symptoms and radiographic data, which is not included in our review. Four of the 10 patients, along with 3 other patients who experienced relapse, were reported to have relapsed, despite a positive initial response to the treatment, in the Alexander et al.62 trial. This case series was focused on the genotypic and phenotypic characteristics of the samples from the relapse patients only and did not report on details of the clinical or microbiological data during treatment. Thus, we were unable to conclude treatment outcome from this case series.62 The relapse was associated with a 2- to 8-fold (from 0.004 to 0.03 mg/L) increase in MICs of bedaquiline and in one case the increase in MIC was very high (from 0.004 to 2.0 mg/L). Genetic analysis of the relapse strains from all patients revealed that these strains had non-synonymous mutations in the mmpT5 and atpE genes, in which the atpE variant (Ala65Pro) was reported in only two cases. Moreover, in one patient with MAC lung infection, culture conversion was not achieved upon treatment with a bedaquiline-including regimen. In fact, the patient experienced treatment failure due to the emergence of bedaquiline resistance caused by a mutation in MAV_2152 locus Trp173Arg. The resistant isolate had an increase in bedaquiline MIC (from 0.03 to 0.06 and 0.125 mg/L), within 6 and 12 months of treatment, respectively.68 However, despite the unfavourable treatment outcome, bedaquiline was well tolerated and there were no major side effects reported in all cases with NTM pulmonary diseases. A summary of the treatment outcomes can be found in Table 5.
Table 5.
Summary of findings: human studies treatment outcome
| Study | Disease | Species | Treatment duration (months) with BDQ | Treatment outcome | |||
|---|---|---|---|---|---|---|---|
| Culture conversion | Cure | Relapse | Symptom improvement | ||||
| Alexander et al. (2017)62 | Pulmonary | M. avium complex and M. intracellulare | ≥6 | 0/3 | 0/3 | 3/3 | 0/3 |
| Chan et al. (2021)63 | Extrapulmonary | M. abscessus | 8 | 1/1 | 1/1 | 0/1 | 1/1 |
| Erber et al. (2020)64 | Extrapulmonary | M. fortuitum complex | 3.5 | 1/1 | 1/1 | 0/1 | 1/1 |
| Gil et al. (2021)65 | Extrapulmonary disease | M. abscessus and M. avium | 21,14 | 2/2 | 2/2 | 0/2 | 2/2 |
| Meybeck et al. (2021)66 | A mix between extrapulmonary and pulmonary | M. marinum | 12 | 1/1 | 1/1 | 0/1 | 1/1 |
| Pearson et al. (2020)67 | Extrapulmonary | M. abscessus | > 3.5 | 1/1 | 1/1 | 0/1 | 1/1 |
| Philley et al. (2015)11 | Pulmonary | M. avium complex and M. intracellulare | 6 | 0/10 | 0/10 | 4/10 | 4/10 |
| Zweijpfenning et al. (2019)68 | Pulmonary | M. avium complex | ≥6 | 0/1 | 0/1 | N/A | 0/1 |
N/A, not applicable.
Discussion
Managing the treatment of NTM infections is usually challenging due to the intrinsic resistance of these bacteria to most of the available antibiotics, including some anti-TB drugs.71 Although bedaquiline is an effective therapy for drug-resistant TB, there is little information on its antibacterial efficacy against NTM species in vitro or in vivo. Therefore, we aimed to consolidate all available evidence in the literature regarding bedaquiline activity against various NTM species and evaluate the MIC distribution in vitro and its efficacy in vivo (in animal models and human studies) while scrutinizing the methodological approaches of different studies.
The effect of bedaquiline in treating NTM species in in vitro studies
For most bacterial species, the concept of using MIC distributions among several other tools for determining clinical breakpoints has been extensively adopted by EUCAST.72 Understanding the distribution of MICs throughout the circulating population of mycobacterial isolates is required for DST.73 MIC distributions might fluctuate depending on the antimicrobial susceptibility testing method used. Although bedaquiline MICs of >2000 NTM isolates had been reported using eight different platforms/media, MIC distribution data were insufficient in most of these platforms for most of the NTM species. However, we were able to evaluate and compare the WT MIC distribution of RGM and SGM in CAMHB and determine the ECOFF value for M. abscessus in CAMHB only.
Our study revealed that bedaquiline has a potential in vitro inhibitory activity against the most common clinically relevant species of RGM (M. abscessus and M. fortuitum) as well as SGM (NTM) species (M. avium, M. intracellulare and M. kanasasii), with a higher potency against SGM (NTM) species than RGM. Using CAMHB, the MIC distribution range was wide (≤0.008 to ≥32 mg/mL) for RGM and SGM. While the MICs for the majority of clinically relevant SGM (NTM) species ranged between 0.008–0.031 mg/L, bedaquiline MICs for the majority of RGM isolates (M. abscessus and M. fortuitum) were higher and were in the range 0.031–0.25 mg/L and 0.031–1.0 mg/L, respectively. However, as the number of SGM was insufficient, the finding that SGM seem to be more susceptible to bedaquiline in vitro than RGM species requires further investigation.
Thus far, there is no bedaquiline MIC cut-off value for NTM species. Due to the lack of a standardized method with a defined MIC concentration range and cut-off value specific for bedaquiline susceptibility testing in NTM species, the cut-off or ECOFF value used by various studies was variable. Additionally, studies varied in the control strains used, type of data reported, and MIC range tested. The inconsistent data made it difficult to set proper ECOFFs. These variations, with the rise in the incidence in NTM infection, highlight the necessity for a standardized method with a properly defined range, and quality control measures specific to bedaquiline susceptibility testing for NTM species. Discrepancies in MIC cut-off values for MIC determination in different platforms were reported in M. tuberculosis complex as well.74 Considerable effort has been made by the WHO and EUCAST to establish a standardized method and cut-off values for MIC testing in M. tuberculosis for various antibiotics, including bedaquiline, but little has been done for NTM species.
In this review, we compiled and analysed bedaquiline MIC data from various studies based on MIC methods used, to obtain a more accurate and useful ECOFF value. Despite the challenges in compiling these data, we were able to propose a T-ECOFF value of 0.5 mg/mL for M. abscessus in CAMHB from three acceptable distributions of over 361 isolates from different laboratories across the world. This value was concomitant with the cut-off value proposed by Chew,27 when 211 M. abscessus isolates were evaluated using Sensititre plates.27 However, we should be careful when comparing cut-off values from different methods. The MIC distribution and the visual cut-off value of 0.125 mg/L were similar between SGM species using CAMHB. Due to the insufficient data with truncated/skewed distributions, the ECOFF value of the SGM species could not be confirmed statistically.
In general, the MIC distribution of NTM species was similar to that of the bedaquiline MIC distribution of M. tuberculosis species, especially when using the Middlebrook 7H9 broth microdilution by Ismail et al.75 Critical concentrations of 0.25 and 1.0 mg/L when using 7H11 and MGIT media, respectively, for M. tuberculosis was recommended by the WHO. However, when focusing on M. tuberculosis and SGM MIC distributions and cut-off values, it appears that SGM species are more susceptible to bedaquiline than M. tuberculosis species, implying that bedaquiline is more effective against SGM than M. tuberculosis. Of course, caution must be applied when comparing cut-off values derived using fundamentally different growth media and MIC methods.
Association between genotypic and phenotypic bedaquiline resistance in NTM species
We have generated the most comprehensive dataset of the genotypic mutations associated with bedaquiline phenotype (susceptibility and resistance) in NTM species to date. However, in the absence of a definite cut-off value in different MIC platforms and due to inadequate data, it was difficult to statistically evaluate the association between mutations in various genes and phenotypic resistance to bedaquiline in each NTM species.
Only a single mutation (atpE Ala63Met) is likely to be clinically relevant. This mutation was reported in both RGM (2 Mycobacterium flavescens and 1 Mycobacterium novocastrense) and SGM (2 Mycobacterium xenopi and 1 Mycobacterium shimoidei)32 and these isolates were found to be naturally resistant to bedaquiline with MICs of 4–8 mg/L. Since the Ala63 residue was conserved in many mycobacterial species, this mutation in the atpE gene could be of clinical importance and can be used as a marker of bedaquiline resistance.
Based on our proposed T-ECOFF value (0.5 mg/L) of M. abscessus when utilizing CAMHB, none of the mutations described in clinical isolates could be associated with bedaquiline resistance. However, non-clinical isolates with mutations in the MAB 2299c gene had an MIC of 2.0 mg/mL, suggesting that these mutations could be a relevant resistance marker to consider when treating M. abscessus infections with bedaquiline and clofazimine in clinical settings.43
While the proposed eyeball cut-off value of 0.125 mg/L for SGM (NTM) revealed no mutations associated with bedaquiline resistance, the 0.031 mg/L cut-off value revealed that a mutation (2544950A > G; Trp173Arg) in the MAV 2152 gene of clinical isolates of M. avium could be associated with bedaquiline resistance. This mutation was found in a patient who developed in vivo resistance to bedaquiline within 6 to 12 months of commencing a bedaquiline-containing regimen and had MICs of 0.06 and 0.125 mg/L, which were 20- to 40-fold higher than for the WT isolate.68 Thus, this mutation may have therapeutic significance and could be used as a bedaquiline resistance marker. Although mutations in the mmT5 gene were found in patients who relapsed after ≥3 months of bedaquiline-including therapy, MICs for most variants were within the in vitro bedaquiline susceptible range, except for the mmpT5 T137G (Val46Gly) mutation. This mutation conferred an MIC of 0.03 mg/mL, which was 8-fold higher than for the WT, indicating that this mutation may have clinical implications. However, the in vitro data do not always reflect the in vivo resistance, and further studies are still required to determine the clinical critical concentration for bedaquiline in various NTM species. Unfortunately, because all the abovementioned mutations were only reported once in clinical isolates, this knowledge might not have a significant impact on clinical practice. Thus, further research is still required to identify the relevance of these mutations in bedaquiline resistance in NTM species.
The effect of bedaquiline in treating NTM species in animal models
In the animal model studies included in this review, bedaquiline in vivo activity was evaluated against three NTM species only: M. ulcerans, M. avium and M. abscessus in skin, lung, spleen and liver. Bedaquiline alone showed an effect against M. ulcerans in reducing the lesion index in skin infection after treatment for 8 weeks.56
Overall, our meta-analysis revealed that bedaquiline alone had a significant effect in reducing the log cfu outcome in major organs, except the liver, compared with the control group. The lack of a significant effect in the liver may be explained by the variations between studies, including mouse strain characteristics, bacterial strains with different MICs, and other methodological variations.
Bedaquiline showed a higher effect on M. avium species than on M. abscessus only after 60 days of treatment in the spleen. Despite the limited data, this finding may imply that a short bedaquiline treatment is sufficient to confer a significant effect against M. abscessus infection, whereas a longer bedaquiline treatment duration may be required for M. avium species. This is in line with the difficulty of treating M. avium species in humans but contradicts SGM species’ in vitro susceptibility to bedaquiline. However, in humans, a longer treatment period for at least 1 year was recommended for M. abscessus infection as well.76 Due to insufficient data, we could not perform subgroup analysis on the effect of bedaquiline on NTM species in other organs (lung and liver).
Bedaquiline combined with various antibiotics (rifampicin or rifapentine and/or Q203 or clofazimine) showed high activity against M. ulcerans and significantly reduced the bacterial burden in skin infection9,55–57 with a complete cure without relapse after 2 weeks of treatment.9 The high activity of the BDQ + Q203 + CLF regimen could be due to the fact that these drugs are targeting and disrupting the electron transport chain (ETC) and oxidative phosphorylation in a similar mechanism to what was found in M. tuberculosis.77,78 Similarly, bedaquiline in combination with clarithromycin and/or amikacin reduced the bacterial burden in the spleen against M. avium infection after 3 months of treatment. The effect of this regimen was found to be better than bedaquiline alone.59 Moreover, bedaquiline combined with either imipenem or clofazimine also showed a significant effect in reducing the bacterial burden against M. abscessus species in the major organs.58,60 These drugs have been shown to be extremely effective against both drug-susceptible and drug-resistant M. tuberculosis isolates in animal models and human studies.79–81 Despite the cross-resistance between bedaquiline and clofazimine, and other downsides observed in M. tuberculosis,82 this combination treatment showed a promising effect against M. abscessus species as well.
The time required for bedaquiline to significantly reduce bacterial burden varied between the studies: Obregón-Henao et al.60 showed that bedaquiline reduced bacterial burden after 5 days, Sarathy et al.61 showed a significant effect of bedaquiline within 11 days and Lerat et al.10 showed that bedaquiline had a significant effect only after 2 months of treatment. As mentioned previously, these variations could be due to disparities in the bacterial strain, drug dose, animal model, disease induction protocols, and the analysis approach used in these studies. Although most of the studies evaluating bedaquiline in treating disseminated infection used M. abscessus species, the strain and its in vitro MIC used varied across the studies. Additionally, the bedaquiline dose varied across the studies (Table 3).
Animal models for evaluating antibiotic activity against NTM species infection also varied between studies and included both immunocompetent and immunodeficient mouse strains. Bedaquiline showed a significant effect in decreasing bacterial load in the liver of infected C3HeB/FeJ mice within 25 days in one experiment but not in another experiment of the same strain when using a different infection route.58 Studies that used γ-IFN knockout (GKO) mice demonstrated a substantial effect of bedaquiline treatment in lowering bacterial loads,60 but work in nude mice (athymic mice with depletion of T-cells) failed to show a reduction in bacterial burden and mortality prevention in the first month of treatment and required longer treatment to show an effective reduction in the bacterial load.10 Obregón-Henao et al.60 showed that most mouse models with significant innate or acquired immune deficiencies are nonetheless able to remove a high degree of M. abscessus infection.60 The susceptibility of mouse strains to infection with virulent M. tuberculosis also varied, depending on the genotype, with some strains being more resistant than others, which could affect treatment outcomes.83,84 A C3HeB/FeJ mouse model shows more necrotic granulomas in the lungs after M. tuberculosis infection, which allows bacteria to replicate, and fewer tubercular granulomas in the spleens and livers, resulting in greater bacterial clearance in the liver and spleen.85 These discrepancies in mouse strain susceptibilities emphasize the importance of developing a suitable NTM infection model that provides a high, constant and reproducible bacterial load in organs for therapeutic trials. However, it is important to keep in mind that no animal model can accurately depict the entire clinical condition. Thus, the outcomes of inappropriately designed animal model research may yield indirect results.86
Collectively, bedaquiline alone or bedaquiline-including regimens demonstrated high activity against NTM species in various mouse models. Although the evidence is uncertain due to the limited data, the significant in vivo activity of bedaquiline shown in this review and other studies dictates the need for further research in clinical settings with special attention to the possible cross-resistance when using bedaquiline and clofazamine in combination.
The effect of bedaquiline in treating NTM species in humans
Case-series studies have many limitations, such as restricting researchers from generalizing results, or proving cause-and-effect relationships, with a risk of overinterpretation and publishing bias. Despite these limitations, case reporting has many advantages: the ability to identify novelties and present rare, unusual, uncontrollable observations about symptoms, clinical findings, disease progression, intervention complications, drug side effects, and so on.87 Systematic reviews of observational studies can still be effective to summarize findings and compare case series, as well as identifying methodological concerns.88
Most of the successfully cured cases had NTM-related extrapulmonary diseases with NTM disseminated infection with either HIV coinfection or leukaemia. Despite the severity of the diseases in these cases, bedaquiline proved to be an effective therapeutic option in the treatment of extrapulmonary diseases caused by diverse NTM species. Bedaquiline was used in combination with various antibiotics: levofloxacin, moxifloxacin or tedizolid, and clofazimine, against M. fortuitum complex, M. marinum and M. avium, respectively, or in combination with either omadacycline or clofazimine, against M. abscessus species. In cases of immunocompetent patients, bedaquiline was used in a combination of >2 drugs.
The successful use of bedaquiline-including regimens (Table 6) could be due to the intrinsic activity of bedaquiline. However, we cannot exclude the efficacy of other drugs. In a combination therapy, it is impractical to assign the positive outcomes to a single drug. Bedaquiline in combination with a highly active fluoroquinolone (levofloxacin), which inhibits bacterial DNA replication, to effectively suppress mycobacterial growth could be the essential element of the successful treatment of M. fortuitum complex.64 Omadacycline also showed great efficacy in vitro against M. abscessus species, as well as a wide range of Gram-positive and Gram-negative bacteria,89 through its mechanism of binding to the bacterial ribosome and inhibiting protein synthesis.90 Recently, omadacycline was approved by the FDA for the treatment of skin and skin structure infections, as well as community-acquired pneumonia, after Phase 3 clinical trials revealed good efficacy.91 Moreover, the present cases revealed a high in vivo efficacy of bedaquiline/clofazimine combination against M. abscessus (RGM) and M. avium (SGM), which correlated with the in vitro synergistic effect of these drugs against NTM species as reported by Ruth et al.45 Bedaquiline/clofazimine combination has also proved to be effective against both drug-susceptible and drug-resistant M. tuberculosis isolates.79–81 It has been shown that bedaquiline reduces the time to sputum culture negativity and enhances outcomes in MDR M. tuberculosis patients.65,92 Due to the intrinsic and acquired resistance, NTM species are posing a significant challenge to treat and thus require a prolonged combination therapy, especially for pulmonary diseases.93 The exact time for culture conversion was not reported clearly in human studies, so we could not estimate the time for culture conversion of cured NTM cases. However, the duration required to complete treatment in successful cases varied between 3.5 and 18 months with an average of 9.4 months. Considering the severity of disseminated infection, the treatment regimens with the reported durations in these cases could be a promising therapy for NTM extrapulmonary disease and is worth further study in a larger cohort. Nevertheless, the emergence of bedaquiline resistance should be evaluated carefully.
Table 6.
Summary of findings: effective bedaquiline-including regimens
| In animal model studies | In human studies | ||
|---|---|---|---|
| Regimen | Mycobacterium species | Regimen | Species |
| RIF or RFP and/or Q203 or CLF | M. ulcerans | BDQ + LVX | M. fortuitum |
| CLR and/or AMK | M. avium | BDQ + OMC or CLF | M. abscessus |
| BDQ combined with either IPM or CLF | M. abscessus | BDQ + MFX | M. marinum |
| BDQ + TDZ and CLF | M. avium | ||
RIF, rifampicin; AMK, amikacin; BDQ, bedaquiline; CLF, clofazimine; CLR, clarithromycin; IPM, imipenem; RFP, rifapentine; Q203, telacebec; MFX, moxifloxacin; OMC, omadacycline; TDZ, tedizolid; LVX, levofloxacin.
In the cured cases, bedaquiline MICs for the RGM isolates varied between 0.12 and <0.0625 mg/L for M. abscessus and were 0.015 mg/L for M. fortuitum. Despite the possible variations in MIC platforms, these values are within the susceptible range for bedaquiline proposed in this review and other studies.6,45 Unfortunately, in vitro bedaquiline MIC data for the slow-growing isolates of M. avium and M. marinum were not provided. In the case of M. marinum infections, routine susceptibility testing has not yet been recommended. M. marinum was found to be susceptible to moxifloxacin (MIC90) at 1 to 2 mg/L. Little is known about the relationship between M. marinum in vitro susceptibilities and clinical response.94 In the absence of MIC data for these isolates (M. avium and M. marinum) from clinical cases, it was difficult to identify the correlation between in vitro susceptibilities and clinical response.
In the cases of NTM pulmonary disease, treatment with a bedaquiline-including regimen (Table 3) did not lead to a favourable clinical outcome with a complete cure, despite the initial response in many cases.11,62,68 However, most of these cases had advanced pulmonary disease and had been taking complementary medications for a long time, with little or no response.11,68 Bedaquiline was used as a salvage treatment option in many cases.
Several factors could be contributing to the unfavourable outcome. A major factor seems to be the emergence of resistance to bedaquiline within 3–8 months, which resulted in a 2- to 8-fold increase in in vitro MIC of bedaquiline. WGS showed that the resistant isolates of MAC contained non-synonymous mutations in the mmpT5 and atpE genes, with atpE variations (Ala65Pro) occurring in just two cases,62 and a W173R mutation in the MAV_2512 locus in one case in the study by Zweijpfenning et al.68 These mutations were discussed elsewhere in the review. However, both mmpT5 and MAV_2512 loci encode a transcriptional regulator of the TetR family that regulates the mmpS5-mmpL5 operon in MAC species, which is equivalent to the rv0678 gene in M. tuberculosis. It was shown previously that mutations in the rv0678 gene cause resistance to bedaquiline and cross-resistance to clofazimine in M. tuberculosis.82 A limitation of these studies is that none of them investigated the MIC data or the emergence of resistance in M. abscessus isolates. It should be underlined that for M. tuberculosis, the WHO advises performing phenotypic DST at the very least when treatment is initiated.95
Another possible contributing factor to unfavourable outcomes could be drug–drug interactions and suboptimal drug regimens during coadministration of bedaquiline with other drugs. For example, a combination of rifamycins and bedaquiline, which was used in these studies, is not recommended. Rifamycins are known to activate the CYP450 system pathway, which is responsible for bedaquiline metabolism. Considering that bedaquiline is mostly metabolized by CYP3A, there is a risk of drug interactions when it is combined with CYP3A inducers or inhibitors. According to the FDA, combination of bedaquiline with any strong CYP3A4 inducers should be avoided.96,97 However, according to the included in vitro studies, a bedaquiline and rifabutin combination showed a high bactericidal effect of bedaquiline against M. abscessus and this was boosted when amikacin was added.98 Similarly, bedaquiline in combination with rifampicin showed a significant effect on decreasing bacterial burden of M. abscessus ATCC 19977 in a mouse model.10,55,56 Despite the importance of in vitro and in vivo animal models, they may not always accurately reflect the human system, especially in the case of NTM pulmonary disease.93,99 Moreover, drug–drug interaction between bedaquiline and rifabutin in the presence of other drugs is still unclear and requires further investigation.
WT MAC (which include M. avium isolates) as well as M. abscessus are known to be susceptible to macrolide antibiotics. However, resistance to macrolide therapy, either alone or in combination, could occur within a few months.100 A regimen that includes bedaquiline may be an effective alternative against both M. avium and M. abscessus.
However, treatment outcome of patients with macrolide-resistant MAC pulmonary disease (MR-MAC-PD)101 or M. abscessus infections, with isolates having inducible resistance to macrolides, were found to be poor.102,103 Eight of the 10 cases reported by Philley et al.11 had macrolide-resistant isolates and the fact that bedaquiline was added to already failing regimens, with some cases having macrolide-resistant isolates, may have contributed to the poor outcome of the bedaquiline-including regimens. Interestingly, inducible macrolide resistance after exposure to clarithromycin has been described in M. abscessus subsp. abscessus infection but not in M. abscessus subsp. massiliense infection,102,104 suggesting differential response at subspecies level, which highlights the importance of performing speciation at subspecies level in clinical studies.
NTM-related diseases are known to be difficult to treat, with various therapeutic options available with poor treatment outcome. Due to the small sample size of the included studies, the question of the review regarding the efficacy of bedaquiline-including regimens in the treatment of NTM pulmonary disease could not be answered precisely. However, due to bedaquiline’s bactericidal activity shown in in vitro studies, as well as animal model studies, against various mycobacterial species and its high efficacy in NTM extrapulmonary diseases, bedaquiline may still be a potential treatment option for NTM pulmonary disease. Thus, further studies are still required to evaluate the efficacy of bedaquiline alone or in combination with other drugs in treating NTM pulmonary disease in a randomized clinical setting with a well-optimized regimen and different stages of the disease.
Limitations and strengths
A comprehensive assessment of bedaquiline efficacy from 43 studies in vitro and in vivo (animal models and human) from across the world was performed. We proposed the bedaquiline cut-off value for five of the clinically most important NTM species: two RGM (M. abscessus and M. fortuitum) and three SGM (NTM) species (M. avium, M. intracellulare and M. kanasasii) in CAMHB. We generated a profile of mutations that could be associated with bedaquiline resistance. Furthermore, we performed meta-analysis for the bedaquiline effect on animal models and evaluated the treatment outcome in humans. However, and despite the strengths of our study, several limitations were encountered.
In vitro studies
Firstly, we intended to evaluate bedaquiline efficacy in various clinical NTM species across various MIC methods and identify bedaquiline MIC cut-off values for at least 10 clinically relevant species. However, the high heterogeneity between studies, the lack of a standardized platform for bedaquiline MIC assays in NTM species, the limited MIC99 value data for individual isolates, and insufficient MIC data from various NTM species across various MIC platforms prevented us from achieving our goal.
Secondly, despite the availability of bedaquiline MIC data of over 1600 isolates using CAMHB, a true assessment could only be obtained from a few studies. Moreover, due to data being either skewed or truncated, it was difficult to determine the ECOFF value statistically using the ECOFFinder program used by EUCAST for SGM.
Thirdly, according to the ISO standard, MIC dilutions should be based on 2-fold dilution steps from 1 mg/L, i.e. 1, 0.5, 0.25 and so on and should be reported in two digits.18 Although most of the studies used a 2-fold broth microdilution method as recommended, studies were inconsistent in the MIC concentration range tested, with some using a non-standardized (3-fold) dilution.37 From the data collected, more than 100 isolates had MICs either lower than 0.008 or higher than 16 mg/L, suggesting that the range for bedaquiline MIC tests for NTM species requires further optimization. In addition, there were discrepancies in reporting certain dilutions, such as 0.12 or 0.13 for the concentration 0.125, or 0.0156 rather than 0.016.
Fourthly, most of the in vitro studies’ methodology was not explicitly stated, and some did not report the range of MIC utilized during DST, resulting in an unclear risk of bias in the reference test in the QUADAS tool. This bias affected the review certainty and grading.
Finally, we did not assess the pharmacokinetic/pharmacodynamic (PK/PD) data in relation to bedaquiline. In fact, in absence of a uniform and standardized method for MIC determination that should be used for PK/PD, it would be difficult to obtain sufficient and reliable data for the analysis.
Therefore, we highly recommend that studies should adhere to EUCAST and CLSI guidelines when performing MIC susceptibility testing for NTM species with regard to various methodological aspects, including quality assurance measures, MIC range and dilutions reported. Additionally, studies should report the methodology section clearly to enable establishment of a standardized method specific for bedaquiline MIC with a defined MIC range and QC measures for NTM species, which are still required.
Association between genotypic and phenotypic bedaquiline resistance in NTM species
In all studies, the phenotypic assessment was applied consistently. However, around 54% (13/24) of the studies had a high risk of bias in the index domain and its applicability, due to the lack of conducting the index test (genotypic assessment). Studies that performed phenotypic and genotypic assays had unclear blinding in the genotype assessment, which could affect transparency of the data. Furthermore, not all studies investigated all genes involved in bedaquiline resistance. Variations in MIC platforms and media, the lack of a cut-off value in each MIC platform/media and the small number of isolates being sequenced are all limitations that made it difficult to determine the association between the genotypic variants and bedaquiline phenotypic resistance in various NTM species, as well as their impact on clinical outcome.
Animal model studies
The risk of bias analysis conducted as part of this systematic review indicated that many of the essential methodological details of animal model studies included in our analysis were poorly described. This resulted in an ‘unclear’ risk of bias for most (if not all) studies and ‘high risk’ of incomplete outcomes for some studies. Areas of concern noted in this review include the conflation of observation and experimental units, the representation of single datasets for repeated experiments, and the treatment of non-independent samples as independent. It is critical to highlight the importance of consistent reporting of all details of the experimental design in addition to all treatment outcomes for future animal model studies, as recommended previously in the ARRIVE guidelines.105 Additionally, animal model researchers should strive for standardized preclinical models to permit comparisons. However, considering that studies that do not describe all details may not always be methodologically faulty,86 and because we wanted to provide a comprehensive view of the intervention effect in animal models, we still included the poorly described studies in our meta-analysis.
Due to high costs, preclinical animal model studies are often underpowered, resulting in compromised internal validity and translational value.106 The limited number of the included studies and the high variations between them did not allow us to perform meta-analysis of the overall impact of the intervention. Furthermore, the lack of reporting on treatment follow-ups in most of the studies influenced the strength of this review. All the abovementioned drawbacks impact the validity of the conclusions reached.
The strength of this part of the review arises from being the first exhaustive systematic review, with a meta-analysis, on the effect of bedaquiline on decreasing bacterial burden in animal models. The extensive search and acquisition/extraction of data, the use of animal model data, highlighting the methodological limitations of the animal model studies, the use of random-effects models to account for study heterogeneity and the use of the SMD analysis to account for the variation in bacterial species and duration of treatment in meta-analysis provides context for the interpretation of outcomes of this review.
Human studies
The main limitation of the human studies included in this review is the lack of randomized clinical trials (RCTs) and controlled clinical trials (CCTs), which did not allow us to perform meta-analysis. Thus, the findings of this review cannot be regarded as conclusive evidence regarding the efficacy of bedaquiline treatments and should be interpreted with caution.
Secondly, as the included studies were case series and case reports, the variabilities between studies were very large and there was no uniformity in the methodology, duration of trial, time to culture conversion, duration of follow-up or even the therapeutic regimens used. Thus, because of various variables and discrepancies, comparing the outcomes of the studies was challenging.
Thirdly, due to the incomplete clinical data,62 overlapping data between studies, and lack of microbiological and in vitro susceptibility data in most of the studies, we could not evaluate the exact time to culture conversion or identify the correlation between the in vitro and the in vivo treatment outcome in human studies. MIC values for the isolates provide an initial assessment of antibiotic efficacy; however, in the absence of the in vitro assessment, translation of the MIC to in vivo efficacy remains unclear.
Finally, our review focused on the treatment efficacy only and we did not evaluate the safety of using bedaquiline. Nevertheless, in order to assess the drug safety, we recommend that drug safety and QTc prolongation should be evaluated and reported in more detail, even in case series and case reports.
However, despite the abovementioned limitations, this review is the first study summarizing the evidence of the efficacy of bedaquiline regimens in treating NTM-related diseases caused by the various NTM species in humans. The strengths of this part of the review lie in the extensive search and acquisition of data, and the use of individual patient data stratified by the type of disease and NTM species. The variation among studies may indicate that the conclusions in this review are more broadly applicable.
Conclusions
In this review we compile the available data from more than 40 studies on the effect of bedaquiline on various NTM species in vitro and in vivo (animal models and human studies). We present comprehensive collective data on the MIC distribution and propose the cut-off/ECOFF value of the most clinically relevant NTM species using the CAMHB platform. Overall, bedaquiline demonstrated high antibacterial activity against almost all of the tested clinical isolates of NTM species (RGM and SGM) and was shown to have strong action against NTM species in vitro as well as in vivo. However, bedaquiline’s effectiveness may vary depending on the NTM species, patient disease severity, patient immunity, drug regimen and duration of treatment.
Future research
With the increasing incidence of NTM infection and the emergence of MDR, bedaquiline could be a promising therapeutic drug alone or as part of combination regimens to treat NTM infections, particularly in NTM-related extrapulmonary infection. However, the paucity of data prevented strong conclusions regarding the ECOFF for many NTM species. This highlights the need for future studies to be standardized in terms of various factors including, but not limited to, culturing media and reporting of outcomes, to allow for meta-analysis of data. Furthermore, additional studies are critically needed to evaluate bedaquiline in vitro susceptibility as well as resistance in human studies, to assist in establishing the critical concentration for bedaquiline use against diverse NTM species. WHO guidelines for M. tuberculosis should be strictly followed in NTM infection to prevent bedaquiline-resistant strains from emerging and spreading.
Supplementary Material
Acknowledgements
We would like to express our gratitude to Dr Michael McCaul for his invaluable advice and assistance in evaluating the meta-analysis data, without which this work would not have been possible. We would also like to thank Prof. Annelies van Rie for her assistance in reviewing the study initial protocol. Additionally, we would like to thank: Stellenbosch University, the South African Medical Research Council (SAMRC), the National Research Foundation (NRF) and the European and Developing Countries Clinical Trials Partnership (EDCTP) (TMA2017-1885 and RIA2020I-3305) for providing the opportunity to work on this systematic review.
Contributor Information
Shatha Omar, DST/NRF Centre of Excellence for Biomedical Tuberculosis Research, South African Medical Research Council (SAMRC) Centre for Tuberculosis Research, Division of Molecular Biology and Human Genetics, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa.
Michael G Whitfield, Health Protection Research Unit in Healthcare Associated Infections and Antimicrobial Resistance, National Institute for Health Research, Imperial College London, London, UK.
Margaret B Nolan, DST/NRF Centre of Excellence for Biomedical Tuberculosis Research, South African Medical Research Council (SAMRC) Centre for Tuberculosis Research, Division of Molecular Biology and Human Genetics, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa.
Justice T Ngom, DST/NRF Centre of Excellence for Biomedical Tuberculosis Research, South African Medical Research Council (SAMRC) Centre for Tuberculosis Research, Division of Molecular Biology and Human Genetics, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa.
Nabila Ismail, DST/NRF Centre of Excellence for Biomedical Tuberculosis Research, South African Medical Research Council (SAMRC) Centre for Tuberculosis Research, Division of Molecular Biology and Human Genetics, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa.
Rob M Warren, DST/NRF Centre of Excellence for Biomedical Tuberculosis Research, South African Medical Research Council (SAMRC) Centre for Tuberculosis Research, Division of Molecular Biology and Human Genetics, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa.
Marisa Klopper, DST/NRF Centre of Excellence for Biomedical Tuberculosis Research, South African Medical Research Council (SAMRC) Centre for Tuberculosis Research, Division of Molecular Biology and Human Genetics, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa.
Funding
This study was carried out as part of our routine research at Stellenbosch University. S.O., J.T.N., N.I. and R.W. were supported by both Stellenbosch University and the South African Medical Research Council (SAMRC), and the National Research Foundation (NRF). M.K. was supported by the European and Developing Countries Clinical Trials Partnership (EDCTP) (TMA2017-1885 and RIA2020I-3305). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The content of this manuscript is solely the responsibility of the authors.
Transparency declarations
None to declare.
Data availability
Authors are agreed to share the data of this manuscript. Data will be available upon request. To request the data, contact the corresponding author of the article. Data regarding ECOFF values will be submitted to the EUCAST committee upon publication.
Supplementary data
Figures S1 to S9 and Tables S1 to S4 are available as Supplementary data at JAC Online.
References
- 1. Baldwin SL, Larsen SE, Ordway Det al. The complexities and challenges of preventing and treating nontuberculous mycobacterial diseases. PLoS Negl Trop Dis 2019; 13: e0007083. 10.1371/journal.pntd.0007083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sousa S, Borges V, Joao Iet al. Nontuberculous mycobacteria persistence in a cell model mimicking alveolar macrophages. Microorganisms 2019; 7: 113. 10.3390/microorganisms7050113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brode SK, Daley CL, Marras TK. The epidemiologic relationship between tuberculosis and non-tuberculous mycobacterial disease: a systematic review. Int J Tuberc Lung Dis 2014; 18: 1370–7. 10.5588/ijtld.14.0120 [DOI] [PubMed] [Google Scholar]
- 4. Wu M-L, Aziz DB, Dartois Vet al. NTM drug discovery: status, gaps and the way forward. Drug Discov Today 2018; 23: 1502–19. 10.1016/j.drudis.2018.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yilmaz N, Yilmazel Ucar E, Saglam L. Mycobacterium tuberculosis and nontuberculous mycobacteria coinfection of the lungs. Turk Thorac J 2017; 18: 23–6. 10.5152/TurkThoracJ.2017.16034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aguilar-Ayala DA, Cnockaert M, André Eet al. In vitro activity of bedaquiline against rapidly growing nontuberculous mycobacteria. J Med Microbiol 2017; 66: 1140–3. 10.1099/jmm.0.000537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mahajan R. Bedaquiline: first FDA-approved tuberculosis drug in 40 years. Int J App Basic Med Res 2013; 3: 1–2. 10.4103/2229-516X.112228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brown-Elliott BA, Philley JV, Griffith DEet al. In vitro susceptibility testing of bedaquiline against Mycobacterium avium complex. Antimicrob Agents Chemother 2017; 61: e01798-16. 10.1128/AAC.01798-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Converse PJ, Almeida DV, Tyagi Set al. Shortening Buruli ulcer treatment with combination therapy targeting the respiratory chain and exploiting Mycobacterium ulcerans gene decay. Antimicrob Agents Chemother 2019; 63: e00426-19. 10.1128/AAC.00426-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lerat I, Cambau E, Roth dit Bettoni Ret al. In vivo evaluation of antibiotic activity against Mycobacterium abscessus. J Infect Dis 2014; 209: 905–12. 10.1093/infdis/jit614 [DOI] [PubMed] [Google Scholar]
- 11. Philley JV, Wallace RJ, Benwill JLet al. Preliminary results of bedaquiline as salvage therapy for patients with nontuberculous mycobacterial lung disease. Chest 2015; 148: 499–506. 10.1378/chest.14-2764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Page MJ, McKenzie JE, Bossuyt PMet al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rohatgi A. WebPlotDigitizer. 2020. https://github.com/ankitrohatgi/WebPlotDigitizer.
- 14. Whiting PF. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011; 155: 529–36. 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 15. Hooijmans CR, Rovers MM, de Vries RBet al. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol 2014; 14: 43. 10.1186/1471-2288-14-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Martin J. The Joanna Briggs Institute Critical Appraisal Checklist for Case Series. 2017. https://jbi.global/sites/default/files/2019-05/JBI_Critical_Appraisal-Checklist_for_Case_Series2017_0.pdf.
- 17. Turnidge J, Kahlmeter G, Kronvall G. Statistical characterisation of bacterial wild-type MIC value distributions and the determination of epidemiological cut-off values. Clin Microbiol Infect 2006; 12: 418–25. 10.1111/j.1469-0691.2006.01377.x [DOI] [PubMed] [Google Scholar]
- 18. EUCAST . Standard operating procedure 10.2: MIC distributions and the setting of epidemiological cutoff (ECOFF) values. 2021. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/EUCAST_SOPs/2021/EUCAST_SOP_10.2_MIC_distributions_and_epidemiological_cut-off_value__ECOFF__setting_20211202.pdf.
- 19. RevMan . The Cochrane Collaboration. 2020. https://training.cochrane.org/online-learning/core-software/revman.
- 20. Crossingham I, Turner S, Ramakrishnan Set al. Combination fixed-dose beta agonist and steroid inhaler as required for adults or children with mild asthma. Cochrane Database Syst Rev 2021; issue 5: CD013518. 10.1002/14651858.CD013518.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ferrara R, Imbimbo M, Malouf Ret al. Single or combined immune checkpoint inhibitors compared to first-line platinum-based chemotherapy with or without bevacizumab for people with advanced non-small cell lung cancer. Cochrane Database Syst Rev 2021; issue 4: CD013257. 10.1002/14651858.CD013257.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Janjua S, Carter D, Threapleton CJet al. Telehealth interventions: remote monitoring and consultations for people with chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev 2021; issue 7: CD013196. 10.1002/14651858.CD013196.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Deeks JJ, Higgins JP, Altman DG. Chapter 10: analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane, 2022. [Google Scholar]
- 24. Andries K, Verhasselt P, Guillemont Jet al. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 2005; 307: 223–7. 10.1126/science.1106753 [DOI] [PubMed] [Google Scholar]
- 25. Asami T, Aono A, Chikamatsu Ket al. Efficacy estimation of a combination of triple antimicrobial agents against clinical isolates of Mycobacterium abscessus subsp. Abscessus in vitro. JAC Antimicrob Resist 2021; 3: dlab004. 10.1093/jacamr/dlab004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cantillon D, Goff A, Taylor Set al. Searching for new therapeutic options for the uncommon pathogen Mycobacterium chimaera: an open drug discovery approach. Lancet Microbe 2022; 3: e382–91. 10.1016/S2666-5247(21)00326-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chew KL, Octavia S, Go Jet al. In vitro susceptibility of Mycobacterium abscessus complex and feasibility of standardizing treatment regimens. J Antimicrob Chemother 2021; 76: 973–8. 10.1093/jac/dkaa520 [DOI] [PubMed] [Google Scholar]
- 28. Chew KL, Octavia S, Go Jet al. MIC distributions of routinely tested antimicrobials and of rifabutin, eravacycline, delafloxacin, clofazimine, and bedaquiline for Mycobacterium fortuitum. Antimicrob Agents Chemother 2021; 65: e0059321. 10.1128/AAC.00593-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dupont C, Viljoen A, Thomas Set al. Bedaquiline inhibits the ATP synthase in Mycobacterium abscessus and is effective in infected zebrafish. Antimicrob Agents Chemother 2017; 61: e01225-17. 10.1128/AAC.01225-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gumbo T, Cirrincione K, Srivastava S. Repurposing drugs for treatment of Mycobacterium abscessus: a view to a kill. J Antimicrob Chemother 2020; 75: 1212–7. 10.1093/jac/dkz523 [DOI] [PubMed] [Google Scholar]
- 31. Gutiérrez AV, Richard M, Roquet-Banères Fet al. The TetR family transcription factor MAB_2299c regulates the expression of two distinct MmpS-MmpL efflux pumps involved in cross-resistance to clofazimine and bedaquiline in Mycobacterium abscessus. Antimicrob Agents Chemother 2019; 63: e01000-19. 10.1128/AAC.01000-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huitric E, Verhasselt P, Andries Ket al. In vitro antimycobacterial spectrum of a diarylquinoline ATP synthase inhibitor. Antimicrob Agents Chemother 2007; 51: 4202–4. 10.1128/AAC.00181-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim TS, Choe JH, Kim YJet al. Activity of LCB01-0371, a novel oxazolidinone, against Mycobacterium abscessus. Antimicrob Agents Chemother 2017; 61: e02752-16. 10.1128/AAC.02752-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li B, Ye M, Guo Qet al. Determination of MIC distribution and mechanisms of decreased susceptibility to bedaquiline among clinical isolates of Mycobacterium abscessus. Antimicrob Agents Chemother 2018; 62: e00175-18. 10.1128/AAC.00175-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lin S, Hua W, Wang Set al. In vitro assessment of 17 antimicrobial agents against clinical Mycobacterium avium complex isolates. BMC Microbiol 2022; 22: 175. 10.1186/s12866-022-02582-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Litvinov V, Makarova M, Kudlay Det al. In vitro activity of bedaquiline against Mycobacterium avium complex. J Med Microbiol 2021; 70: 10. 10.1099/jmm.0.001439 [DOI] [PubMed] [Google Scholar]
- 37. Lounis N, Gevers T, Van Den Berg Jet al. Prevention of drug carryover effects in studies assessing antimycobacterial efficacy of TMC207. J Clin Microbiol 2008; 46: 2212–5. 10.1128/JCM.00177-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Maeda T, Kawada M, Sakata Net al. Laboratory evolution of Mycobacterium on agar plates for analysis of resistance acquisition and drug sensitivity profiles. Sci Rep 2021; 11: 15136. 10.1038/s41598-021-94645-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Martin A, Godino IT, Aguilar-Ayala DAet al. In vitro activity of bedaquiline against slow-growing nontuberculous mycobacteria. J Med Microbiol 2019; 68: 1137–9. 10.1099/jmm.0.001025 [DOI] [PubMed] [Google Scholar]
- 40. Pang Y, Zheng H, Tan Yet al. In vitro activity of bedaquiline against nontuberculous mycobacteria in China. Antimicrob Agents Chemother 2017; 61: e02627-16. 10.1128/AAC.02627-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ransom EM, Sawhney SS, Dantas Get al. Draft genome sequence of a Mycobacterium strain isolated from a clinical wound sample. Microbiol Resour Announc 2022; 11: e00170-22. 10.1128/mra.00170-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Richard M, Gutiérrez AV, Viljoen AJet al. Mechanistic and structural insights into the unique TetR-dependent regulation of a drug efflux pump in Mycobacterium abscessus. Front Microbiol 2018; 9: 649. 10.3389/fmicb.2018.00649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Richard M, Gutiérrez AV, Viljoen Aet al. Mutations in the MAB_2299c TetR regulator confer cross-resistance to clofazimine and bedaquiline in Mycobacterium abscessus. Antimicrob Agents Chemother 2018; 63: e01316-18. 10.1128/AAC.01316-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Richter A, Strauch A, Chao Jet al. Screening of preselected libraries targeting Mycobacterium abscessus for drug discovery. Antimicrob Agents Chemother 2018; 62: e00828-18. 10.1128/AAC.00828-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ruth MM, Sangen JJN, Remmers Ket al. A bedaquiline/clofazimine combination regimen might add activity to the treatment of clinically relevant non-tuberculous mycobacteria. J Antimicrob Chemother 2019; 74: 935–43. 10.1093/jac/dky526 [DOI] [PubMed] [Google Scholar]
- 46. Schulthess B, Akdoğan Kittana FN, Hömke Ret al. In vitro bedaquiline and clofazimine susceptibility testing in Mycobacterium abscessus. Antimicrob Agents Chemother 2022; 66: e02346-21. 10.1128/aac.02346-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Segala E, Sougakoff W, Nevejans-Chauffour Aet al. New mutations in the mycobacterial ATP synthase: new insights into the binding of the diarylquinoline TMC207 to the ATP synthase C-ring structure. Antimicrob Agents Chemother 2012; 56: 2326–34. 10.1128/AAC.06154-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sorayah R, Manimekalai MSS, Shin SJet al. Naturally-occurring polymorphisms in QcrB are responsible for resistance to telacebec in Mycobacterium abscessus. ACS Infect Dis 2019; 5: 2055–60. 10.1021/acsinfecdis.9b00322 [DOI] [PubMed] [Google Scholar]
- 49. Srivastava S, Pasipanodya JG, Heysell SKet al. An overview of drugs for the treatment of Mycobacterium kansasii pulmonary disease. J Glob Antimicrob Resist 2022; 28: 71–7. 10.1016/j.jgar.2021.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vesenbeckh S, Schönfeld N, Krieger Det al. Bedaquiline as a potential agent in the treatment of M. intracellulare and M. avium infections. Eur Respir J 2017; 49: 1601969. 10.1183/13993003.01969-2016 [DOI] [PubMed] [Google Scholar]
- 51. Vesenbeckh S, Schönfeld N, Roth Aet al. Bedaquiline as a potential agent in the treatment of Mycobacterium abscessus infections. Eur Respir J 2017; 49: 1700083. 10.1183/13993003.00083-2017 [DOI] [PubMed] [Google Scholar]
- 52. Yu X, Liu P, Liu Get al. The prevalence of non-tuberculous mycobacterial infections in mainland China: systematic review and meta-analysis. J Infect 2016; 73: 558–67. 10.1016/j.jinf.2016.08.020 [DOI] [PubMed] [Google Scholar]
- 53. Zhu R, Shang Y, Chen Set al. In vitro activity of the sudapyridine (WX-081) against non-tuberculous mycobacteria isolated in Beijing, China. Microbiol Spectr 2022; 10: e01372-22. 10.1128/spectrum.01372-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kim DH, Jhun BW, Moon SMet al. In vitro activity of bedaquiline and delamanid against nontuberculous mycobacteria, including macrolide-resistant clinical isolates. Antimicrob Agents Chemother 2019; 63: e00665-19. 10.1128/AAC.00665-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chauffour A, Robert J, Veziris Net al. Sterilizing activity of fully oral intermittent regimens against Mycobacterium ulcerans infection in mice. PLoS Negl Trop Dis 2016; 10: e0005066. 10.1371/journal.pntd.0005066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ji B, Lefrancois S, Robert Jet al. In vitro and in vivo activities of rifampin, streptomycin, amikacin, moxifloxacin, R207910, linezolid, and PA-824 against Mycobacterium ulcerans. Antimicrob Agents Chemother 2006; 50: 1921–6. 10.1128/AAC.00052-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Komm O, Almeida DV, Converse PJet al. Impact of dose, duration, and immune status on efficacy of ultrashort telacebec regimens in mouse models of Buruli ulcer. Antimicrob Agents Chemother 2021; 65: e01418-21. 10.1128/AAC.01418-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Le Moigne V, Raynaud C, Moreau Fet al. Efficacy of bedaquiline, alone or in combination with imipenem, against Mycobacterium abscessus in C3HeB/FeJ mice. Antimicrob Agents Chemother 2020; 64: e00114-20. 10.1128/AAC.00114-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lounis N, Gevers T, Van Den Berg Jet al. ATP synthase inhibition of Mycobacterium avium is not bactericidal. Antimicrob Agents Chemother 2009; 53: 4927–9. 10.1128/AAC.00689-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Obregón-Henao A, Arnett KA, Henao-Tamayo Met al. Susceptibility of Mycobacterium abscessus to antimycobacterial drugs in preclinical models. Antimicrob Agents Chemother 2015; 59: 6904–12. 10.1128/AAC.00459-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sarathy JP, Ganapathy US, Zimmerman MDet al. TBAJ-876, a 3,5-dialkoxypyridine analogue of bedaquiline, is active against Mycobacterium abscessus. Antimicrob Agents Chemother 2020; 64: e02404-19. 10.1128/AAC.02404-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Alexander DC, Vasireddy R, Vasireddy Set al. Emergence of mmpT5 variants during bedaquiline treatment of Mycobacterium intracellulare lung disease. J Clin Microbiol 2017; 55: 574–84. 10.1128/JCM.02087-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chan WY-K, Ho P-L, To KK-Wet al. A child with acute myeloid leukemia complicated by calcaneal osteomyelitis due to Mycobacterium abscessus infection after induction chemotherapy successfully salvaged with bedaquiline and clofazimine. Int J Infect Dis 2021; 103: 9–12. 10.1016/j.ijid.2020.10.102 [DOI] [PubMed] [Google Scholar]
- 64. Erber J, Weidlich S, Tschaikowsky Tet al. Successful bedaquiline-containing antimycobacterial treatment in post-traumatic skin and soft-tissue infection by Mycobacterium fortuitum complex: a case report. BMC Infect Dis 2020; 20: 365. 10.1186/s12879-020-05075-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gil E, Sweeney N, Barrett Vet al. Bedaquiline as treatment for disseminated nontuberculous mycobacteria infection in 2 patients co-infected with HIV. Emerg Infect Dis 2021; 27: 944–8. 10.3201/eid2703.202359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Meybeck A, Tetart M, Baclet Vet al. A disseminated Mycobacterium marinum infection in a renal transplant HIV-infected patient successfully treated with a bedaquiline-containing antimycobacterial treatment: a case report. Int J Infect Dis 2021; 107: 176–8. 10.1016/j.ijid.2021.04.054 [DOI] [PubMed] [Google Scholar]
- 67. Pearson JC, Dionne B, Richterman Aet al. Omadacycline for the treatment of Mycobacterium abscessus disease: a case series. Open Forum Infect Dis 2020; 7: ofaa415. 10.1093/ofid/ofaa415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zweijpfenning SMH, Schildkraut JA, Coolen JPMet al. Failure with acquired resistance of an optimised bedaquiline-based treatment regimen for pulmonary Mycobacterium avium complex disease. Eur Respir J 2019; 54: 1900118. 10.1183/13993003.00118-2019 [DOI] [PubMed] [Google Scholar]
- 69. Ängeby K, Juréen P, Kahlmeter Get al. Challenging a dogma: antimicrobial susceptibility testing breakpoints for Mycobacterium tuberculosis. Bull World Health Org 2012; 90: 693–8. 10.2471/BLT.11.096644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. EUCAST Subcommittee on MIC distributions and epidemiological cut-off values (ECOFFs) . Discussion document, Version 3. 2017. https://eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Consultation/2017/MIC_and_ECOFF/EUCAST_MIC_and_ECOFF_discussion_document_version_3_20170309.pdf.
- 71. Kasperbauer SH, De Groote MA. The treatment of rapidly growing mycobacterial infections. Clin Chest Med 2015; 36: 67–78. 10.1016/j.ccm.2014.10.004 [DOI] [PubMed] [Google Scholar]
- 72. Schon T, Jureen P, Giske CGet al. Evaluation of wild-type MIC distributions as a tool for determination of clinical breakpoints for Mycobacterium tuberculosis. J Antimicrob Chemother 2009; 64: 786–93. 10.1093/jac/dkp262 [DOI] [PubMed] [Google Scholar]
- 73. Burke RM, Coronel J, Moore D. Minimum inhibitory concentration distributions for first- and second-line antimicrobials against Mycobacterium tuberculosis. J Med Microbiol 2017; 66: 1023–6. 10.1099/jmm.0.000534 [DOI] [PubMed] [Google Scholar]
- 74. Schön T, Werngren J, Machado Det al. Antimicrobial susceptibility testing of Mycobacterium tuberculosis complex isolates—the EUCAST broth microdilution reference method for MIC determination. Clin Microbiol Infect 2020; 26: 1488–92. 10.1016/j.cmi.2020.07.036 [DOI] [PubMed] [Google Scholar]
- 75. Ismail NA, Omar SV, Joseph Let al. Defining bedaquiline susceptibility, resistance, cross-resistance and associated genetic determinants: a retrospective cohort study. EBioMedicine 2018; 28: 136–42. 10.1016/j.ebiom.2018.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Griffith DE, Aksamit T, Brown-Elliott BAet al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007; 175: 367–416. 10.1164/rccm.200604-571ST [DOI] [PubMed] [Google Scholar]
- 77. Lamprecht DA, Finin PM, Rahman MAet al. Turning the respiratory flexibility of Mycobacterium tuberculosis against itself. Nat Commun 2016; 7: 12393. 10.1038/ncomms12393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Yano T, Kassovska-Bratinova S, Teh JSet al. Reduction of clofazimine by mycobacterial type 2 NADH:quinone oxidoreductase. J Biol Chem 2011; 286: 10276–87. 10.1074/jbc.M110.200501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cholo MC, Steel HC, Fourie PBet al. Clofazimine: current status and future prospects. J Antimicrob Chemother 2012; 67: 290–8. 10.1093/jac/dkr444 [DOI] [PubMed] [Google Scholar]
- 80. Lenaerts AJ, Hoff D, Aly Set al. Location of persisting mycobacteria in a guinea pig model of tuberculosis revealed by R207910. Antimicrob Agents Chemother 2007; 51: 3338–45. 10.1128/AAC.00276-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Veziris N, Ibrahim M, Lounis Net al. A once-weekly R207910-containing regimen exceeds activity of the standard daily regimen in murine tuberculosis. Am J Respir Crit Care Med 2009; 179: 75–9. 10.1164/rccm.200711-1736OC [DOI] [PubMed] [Google Scholar]
- 82. Andries K, Villellas C, Coeck Net al. Acquired resistance of Mycobacterium tuberculosis to bedaquiline. PLoS One 2014; 9: e102135. 10.1371/journal.pone.0102135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Franzblau SG, DeGroote MA, Cho SHet al. Comprehensive analysis of methods used for the evaluation of compounds against Mycobacterium tuberculosis. Tuberculosis 2012; 92: 453–88. 10.1016/j.tube.2012.07.003 [DOI] [PubMed] [Google Scholar]
- 84. Singh A, Gupta U. Animal models of tuberculosis: lesson learnt. Indian J Med Res 2018; 147: 456. 10.4103/ijmr.IJMR_554_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Henao-Tamayo M, Obregón-Henao A, Creissen Eet al. Differential Mycobacterium bovis BCG vaccine-derived efficacy in C3Heb/FeJ and C3H/HeOuJ mice exposed to a clinical strain of Mycobacterium tuberculosis. Clin Vaccine Immunol 2015; 22: 91–8. 10.1128/CVI.00466-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hooijmans CR, Hlavica M, Schuler FAFet al. Remyelination promoting therapies in multiple sclerosis animal models: a systematic review and meta-analysis. Sci Rep 2019; 9: 822. 10.1038/s41598-018-35734-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Nissen T, Wynn R. The clinical case report: a review of its merits and limitations. BMC Res Notes 2014; 7: 264. 10.1186/1756-0500-7-264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Huisstede BMA, Miedema HS, van Opstal Tet al. Interventions for treating the posterior interosseus nerve syndrome: a systematic review of observational studies. J Peripher Nerv Syst 2006; 11: 101–10. 10.1111/j.1085-9489.2006.00074.x [DOI] [PubMed] [Google Scholar]
- 89. Bax HI, de Vogel CP, Mouton JWet al. Omadacycline as a promising new agent for the treatment of infections with Mycobacterium abscessus. J Antimicrob Chemother 2019; 74: 2930–3. 10.1093/jac/dkz267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Pfaller MA, Huband MD, Shortridge Det al. Surveillance of omadacycline activity tested against clinical isolates from the United States and Europe as part of the 2016 SENTRY Antimicrobial Surveillance Program. Antimicrob Agents Chemother 2018; 62: e02327-17. 10.1128/AAC.02327-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. O’Riordan W, Green S, Overcash JSet al. Omadacycline for acute bacterial skin and skin-structure infections. N Engl J Med 2019; 380: 528–38. 10.1056/NEJMoa1800170 [DOI] [PubMed] [Google Scholar]
- 92. Mbuagbaw L. Review of available evidence on the use of bedaquiline for the treatment of multidrug-resistant tuberculosis: Data analysis report. 2017. https://www.who.int/docs/default-source/documents/tuberculosis/appendix-gdgreport-bedaquiline.pdf?sfvrsn=8852833e_2.
- 93. Daley CL, Iaccarino JM, Lange Cet al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Clin Infect Dis 2020; 71: e1–36. 10.1093/cid/ciaa241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Akram SM, Aboobacker S. Mycobacterium marinum. StatPearls Publishing, 2021. [PubMed] [Google Scholar]
- 95. WHO . Technical Report on critical concentrations for drug susceptibility testing of medicines used in the treatment of drug-resistant tuberculosis. 2018. https://www.who.int/publications-detail-redirect/WHO-CDS-TB-2018.5.
- 96. Svensson EM, Murray S, Karlsson MOet al. Rifampicin and rifapentine significantly reduce concentrations of bedaquiline, a new anti-TB drug. J Antimicrob Chemother 2015; 70: 1106–14. 10.1093/jac/dku504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Winter H, Egizi E, Murray Set al. Evaluation of the pharmacokinetic interaction between repeated doses of rifapentine or rifampin and a single dose of bedaquiline in healthy adult subjects. Antimicrob Agents Chemother 2015; 59: 1219–24. 10.1128/AAC.04171-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lee J, Ammerman N, Agarwal Aet al. Differential in vitro activities of individual drugs and bedaquiline-rifabutin combinations against actively multiplying and nutrient-starved Mycobacterium abscessus. Antimicrob Agents Chemother 2021; 65: e02179-20. 10.1128/AAC.02179-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Jarand J, Levin A, Zhang Let al. Clinical and microbiologic outcomes in patients receiving treatment for Mycobacterium abscessus pulmonary disease. Clin Infect Dis 2011; 52: 565–71. 10.1093/cid/ciq237 [DOI] [PubMed] [Google Scholar]
- 100. CLSI . Susceptibility Testing of Mycobacteria, Nocardiae, and Other Aerobic Actinomycetes—Second Edition: M24. 2011. [PubMed] [Google Scholar]
- 101. Park SC, Kang MJ, Han CHet al. Prevalence, incidence, and mortality of nontuberculous mycobacterial infection in Korea: a nationwide population-based study. BMC Pulm Med 2019; 19: 140. 10.1186/s12890-019-0901-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Choi G-E, Shin SJ, Won C-Jet al. Macrolide treatment for Mycobacterium abscessus and Mycobacterium massiliense infection and inducible resistance. Am J Respir Crit Care Med 2012; 186: 917–25. 10.1164/rccm.201111-2005OC [DOI] [PubMed] [Google Scholar]
- 103. Nash KA, Brown-Elliott BA, Wallace RJ. A novel gene, erm(41), confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from Mycobacterium chelonae. Antimicrob Agents Chemother 2009; 53: 1367–76. 10.1128/AAC.01275-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Richard M, Gutiérrez AV, Kremer L. Dissecting erm(41)-mediated macrolide-inducible resistance in Mycobacterium abscessus. Antimicrob Agents Chemother 2020; 64: e01879-19. 10.1128/AAC.01879-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kilkenny C, Browne WJ, Cuthill ICet al. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 2010; 8: e1000412. 10.1371/journal.pbio.1000412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Koster C, Wever KE, Wagstaff PEet al. A systematic review on transplantation studies of the retinal pigment epithelium in animal models. Int J Mol Sci 2020; 21: 2719. 10.3390/ijms21082719 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Authors are agreed to share the data of this manuscript. Data will be available upon request. To request the data, contact the corresponding author of the article. Data regarding ECOFF values will be submitted to the EUCAST committee upon publication.