Abstract
Background
Pathophysiological changes in severely burned patients alter the pharmacokinetics (PK) of anti-infective agents, potentially leading to subtherapeutic concentrations at the target site. Albumin supplementation, to support fluid resuscitation, may affect pharmacokinetic properties by binding drugs. This study aimed to investigate the PK of piperacillin/tazobactam in burn patients admitted to the ICU before and after albumin substitution as total and unbound concentrations in plasma.
Patients and methods
Patients admitted to the ICU and scheduled for 4.5 g piperacillin/tazobactam administration and 200 mL of 20% albumin substitution as part of clinical routine were included. Patients underwent IV microdialysis, and simultaneous arterial plasma sampling, at baseline and multiple timepoints after drug administration. PK analysis of total and unbound drug concentrations under steady-state conditions was performed before and after albumin supplementation.
Results
A total of seven patients with second- to third-degree burns involving 20%–60% of the total body surface were enrolled. Mean (SD) AUC0–8 (h·mg/L) of total piperacillin/tazobactam before and after albumin substitution were 402.1 (242)/53.2 (27) and 521.8 (363)/59.7 (32), respectively. Unbound mean AUC0–8 before and after albumin supplementation were 398.9 (204)/54.5 (25) and 456.4 (439)/64.5 (82), respectively.
Conclusions
Albumin supplementation had little impact on the PK of piperacillin/tazobactam. After albumin supplementation, there was a numerical increase in mean AUC0–8 of total and unbound piperacillin/tazobactam, whereas similar Cmax values were observed. Future studies may investigate the effect of albumin supplementation on drugs with a higher plasma protein binding.
Introduction
The therapeutic management of patients with severe burns is one of the most challenging medical conditions in an ICU, requiring a multidisciplinary and interdisciplinary team of highly skilled specialists within the field of surgery, intensive care, infectiology and nutrition.1,2 Severe medical conditions often lead to life-threatening infections. Due to a loss of skin barrier and an immunocompromised state, burn patients are at constant risk for bloodstream infections, such as sepsis,3 which are associated with a very high mortality.4,5 Hence, beside other intensive care procedures, infection control has been found to be crucial for patient recovery and survival. Inadequate treatment of systemic infections has been shown to result in multiple organ dysfunction syndrome and subsequent death.6–9 Studies have shown that most bacterial infections are caused by Staphylococcus aureus, Pseudomonas aeruginosa and Acinetobacter spp., whereas Candida spp., Aspergillus spp. and Fusarium spp. account for most of the fungal infections.4,5
Due to unique pathophysiological changes as well as therapeutic interventions in critically ill patients (e.g. haemodynamic changes, organ failure, capillary leakage, extracorporeal therapies), particularly in those with major burns, the pharmacokinetics (PK) of many anti-infective agents might be affected. This may lead to changes in drug clearance, volume of distribution (Vd) and protein binding, conditions potentially resulting in a suboptimal drug exposure.10–13 Severe burn injuries are associated with a reduction in plasma albumin levels, which may affect the PK of protein-bound drugs, as only the unbound fraction of an antibiotic exerts a pharmacological effect.14–16 However, the magnitude of impact of change in albumin levels on the dynamically changing concentration-versus-time profile of unbound drugs is still unknown.
Several techniques have been introduced to investigate protein binding and its effect on antibiotic susceptibility in vivo and in vitro.15,17 In the past years, microdialysis (MD) has been used as a minimally invasive and valuable research tool in the field of PK analysis, which allows for continuous measurement of unbound (free) drug concentrations in plasma, as well as in tissues, to assess protein binding in vivo.18–20
The aim of the present study was to detect PK alterations of piperacillin/tazobactam in severely burned patients admitted to the ICU. Further, we investigated protein binding by means of in vivo MD before and after additional albumin supplementation.
Materials and methods
Ethics
This prospective, open-labelled, single-centre study was approved by the responsible Ethics Committee of the Medical University of Vienna before initiation (EC number: 1608/2017) and the Austrian Agency for Health and Food Safety. Further, the study was registered under the EudraCT number 2017-002216-14.
All related study procedures were performed at the Burn ICU (BICU) at the Medical University of Vienna, Austria, in accordance with the International Conference on Harmonisation—Good Clinical Practice (ICH-GCP) guidelines and the Declaration of Helsinki.
The study population included intubated and unconscious intensive care patients. Therefore, all procedures and sampling were performed before informed consent was available. However, consent was obtained from each patient once the subjects were awake and able to understand the full content of the study. The actual sample analysis was carried out after consent was given.
Study medication and equipment
Included patients received piperacillin/tazobactam 4.5 g (Fresenius Kabi Austria GmbH, Graz, Austria) in 100 mL of 0.9% saline solution (Fresenius Kabi Austria GmbH) over 30 min every 8 h) as part of routine care. To ensure steady-state conditions, at least three individual doses were administered prior to the first study day. All study participants underwent regular kidney function testing and showed mean (SD) glomerular filtration rates (GFRs) above 40 mL/min [110.76 (43.12); data not shown]. Hence, no antibiotic dose adjustment was necessary.
Further, the same substances were used for MD calibration and perfusion. Human albumin 20% for supplementation was purchased from CLS Behring GmbH, Vienna, Austria. IV MD catheters and microinfusion pumps (107 microdialysis pump) were supplied by M Dialysis (Stockholm, Sweden).
Study population and procedures
Key inclusion criteria included male and female patients, clinical indication for antibiotic treatment with piperacillin/tazobactam and albumin substitution as part of clinical routine, ≥18 years of age and admission to the BICU due to major burn injury. All participants were sedated and under mechanical ventilation due to respiratory insufficiency.
Exclusion criteria were defined as follows: liver and/or kidney failure requiring organ support, such as renal replacement therapy (RRT) and/or extracorporeal membrane oxygenation (ECMO) and pregnancy (applying to female subjects). For safety reasons, subjects with seropositivity for HIV, HBV (HepB antigen) or HCV (HepC antibody) were not included.
Each included individual was scheduled to undergo two consecutive study days. On Study Day 1, subjects received antibiotic drug treatment with piperacillin/tazobactam prior to plasma sampling. On Study Day 2, albumin substitution of 200 mL of 20% human albumin was infused over 30 min and followed by the next therapeutic antibiotic drug administration. Time between administration of albumin and piperacillin/tazobactam was consistent throughout all patients.
Plasma sampling
Piperacillin 4 g/tazobactam 0.5 g was administered IV via a central venous catheter over 30 min using a volumetric infusion pump. Blood samples were obtained via an arterial catheter. All catheters were implanted by the responsible intensive care clinician as part of clinical routine. For the measurement of the total drug concentrations in plasma, blood samples were collected at baseline (prior to next antibiotic administration), at 0.5 (end of infusion), 1, 2, 4, 6 and 8 h after drug infusion. Plasma sampling on Study Day 2 was performed as on Study Day 1.
MD
MD is a minimally invasive method for the measurement of free drug concentrations in blood and tissues, described in detail elsewhere.18,21–23 MD probes with a membrane length of 30 mm and a molecular weight cut-off of 20 kDa were inserted IV and connected to a microinfusion precision pump. The system was then continuously perfused with a 0.9% saline solution at a flow rate of 2.0 µL/min. Due to the low molecular weight cut-off of the membrane, only the unbound fraction of drugs was able to diffuse through and was ultimately collected in the dialysate.18,24
Samples were calibrated by means of retrodialysis. Two retrodialysis samples were collected after MD sampling for each patient and each study day. The probe was perfused with a solution containing piperacillin/tazobactam (300/37.5 µg/mL) at a flow rate of 2.0 µL/min, to collect two retrodialysis samples over a period of 30 min each.
Assuming that the diffusion of drugs past the membrane is equal in both directions, the fraction of the drug concentration in the dialysate (relative recovery) is calculated using the following equations:
Accordingly, the antibiotic drug concentrations were calculated as follows:
MD sampling
Simultaneous to blood sampling, intravenous MD was used to determine unbound drug concentrations in plasma. After an equilibration period of at least 30 min, MD sampling was initiated at baseline (prior to next antibiotic drug administration) and at 0–0.5, 0.5–1, 1–2, 2–3, 3–4, 4–5, 5–6, 6–7 and 7–8 h after drug administration. After termination of active sampling, all MD probes were calibrated by retrodialysis. Upon completion of all study procedures, the MD probes and venous cannula were removed. All other catheters necessary for further clinical monitoring and/or intensive care treatment were kept in place until removed by the responsible clinicians.
Sample handling
Blood samples were collected in Vacuette® lithium-heparin tubes (Greiner Bio-one, Austria), centrifuged at +4°C and 2600 g for 10 min and aliquoted in polypropylene Nunc Cryotube™ vials (Thermo Scientific, Denmark) within 60 min from collection. MD samples were collected in microvials (M Dialysis) and stored at approximately −20°C. At the end of each study day all samples were transferred to a −80°C freezer until further analysis.
Sample analysis—HPLC-UV method and ultrafiltration
The concentrations of piperacillin and tazobactam were determined by HPLC-UV using a Prominence LC20 modular HPLC system equipped with an SPD-M30A PDA detector (set to 225 nm for piperacillin or 220 nm for tazobactam) and LabSolutions software (Shimadzu, Duisburg, Germany). Piperacillin was determined using a NUCLEOSHELL RP18 2.7 µm 100 × 3 mm column for plasma or a CORTECS T3 2.7 µm 100 × 3 mm column for microdialysate (Waters, Eschborn, Germany) preceded by a guard column (NUCLEOSHELL RP18 2.7 µm 4 × 3 mm, Macherey-Nagel, Düren, Germany). The mobile phase was 0.02 M NaH2PO4/acetonitrile 80:20 (v/v), pH 7.0–7.1. At a flow rate of 0.4 mL/min and a column temperature of 40°C, piperacillin eluted after 3.2 (NUCLEOSHELL) or 4.8 (CORTECS) min. Sample preparation for analysis of piperacillin and tazobactam, as well as determination of tazobactam, was performed as previously described.25 In brief, total drug concentrations were determined after protein precipitation with acetonitrile and removal of acetonitrile by extraction into dichloromethane. Free drug concentrations were determined after ultrafiltration using plasma (300 µL) buffered with 3 M potassium phosphate, pH 7.4 (10 µL), and Vivafree™ 500, 30 kD Hydrosart® centrifugal ultrafiltration device (Vivaproducts Inc., Littleton, MA, USA). Microdialysate was injected directly. The linearity in plasma was shown from 0.1 to 300 mg/L (R > 0.9982) for piperacillin and from 0.125–37.5 mg/L (R > 0.9993) for tazobactam. The respective values in saline as surrogate for microdialysate or ultrafiltrate were R > 0.9996 (concentration 0.03–300 mg/L) for piperacillin and R > 0.9991 (concentration 0.0375–37.5 mg/L) for tazobactam. Based on in-process quality controls (QCs), the intra- and inter-assay imprecision of the determination of total piperacillin/tazobactam in plasma was <2%/<4% (coefficient of variation, CV), the relative error in accuracy was <4%/<1%. The mean (SD) unbound fraction (fu = Cfree/Ctotal × 100%) of piperacillin/tazobactam in these QCs was 86.7% (1.9%)/89.2% (5.3%), corresponding to an inter-assay precision of 2.2%/6.0% (CV). Based on in-process QCs in saline, the intra- and inter-assay precision was <4%/<8% (CV); the accuracy was 100.7%/101.0%.
PK analysis
PK data were analysed with a commercially available computer software program (Phoenix® WinNonlin® Build 8.0, Certara USA, Inc., Princeton, NJ, USA) applying a non-compartmental analysis. Where applicable, data were expressed as mean values.
Maximum plasma concentration (Cmax), time to maximum plasma concentration (Tmax), elimination half-life (t½), Vd, CL and AUC from 0 to 8 h (AUC0–8) were calculated for piperacillin and tazobactam in plasma and intravascular dialysate fluid (recovery corrected).
Pharmacometric data analysis
All quantitative data were analysed using non-linear mixed effects modelling in NONMEM (ICON, Gaithersburg, MD, USA, version 7.5.0) controlled through PsN 5.0.0.26 One- and two-compartment models with linear disposition and elimination were evaluated to describe the plasma PK of piperacillin. To model the unbound concentrations from in vitro ultrafiltration and in vivo MD, a constant unbound fraction of the total plasma concentration was assumed. MD data were modelled using an approach similar to Tunblad et al.27 to account for interval-based sampling.
Inter-individual variability (IIV) of the pharmacokinetic model parameters was assumed to be log-normally distributed. For the residual unexplained variability in plasma, ultrafiltrate and microdialysate samples, an additive, proportional or combined error model was evaluated. Candidate models were evaluated by graphical and numerical criteria [goodness-of-fit plots, visual predictive checks and drop of objective function value (dOFV)]. Parameter uncertainty was evaluated using a log-likelihood profiling-based sampling-importance resampling routine (LLP-SIR), a technique for evaluating parameter uncertainty in small datasets.28
The best fitting model for piperacillin was utilized to perform Monte Carlo simulations to evaluate the suitability of the employed dosing regimen to attain a PK/PD target of 50% and 100% time of the dosing interval that unbound drug concentrations exceed the MIC (fT>MIC)
MICs cannot be determined for tazobactam because it does not exert antibacterial activity on its own. Nevertheless, a threshold drug concentration is needed to effectively neutralize the β-lactamase enzymes. The simulated unbound concentrations were used for calculation of a PK/PD target of 50% or 100% time of the dosing interval that exceeds the threshold concentration (Ct) (fT>Ct).
Results
Study population and study outcome
During a study period from June 2018 to December 2019 a total of seven patients with severe burn injuries admitted to the BICU were enrolled in the study. All participants experienced second- to third-degree burns affecting 20%–60% of the total body surface area (TBSA). Albumin supplementation was indicated in six out of seven patients, due to low albumin values. However, in two patients, a defective MD catheter was identified after the second study day. Hence, due to a lack of reliability, these datasets were removed from further analysis. The final analysis included seven patients on Study Day 1 and four subjects on Study Day 2.
Table 1 and Table S1 (available as Supplementary data at JAC Online) describe the characteristics of the sample. All patients were sedated and mechanically ventilated. No signs of sepsis were detected by the ICU medical staff.
Table 1.
Patient characteristics
| Overall (n = 7) | Study Day 1 (before albumin) (n = 7) | Study Day 2 (after albumin) (n = 6) | |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years), mean (SD) | 52.4 (19.9) | ||
| Female sex, n (%) | 2 (28.6) | ||
| BMI, mean (SD) | 29.6 (3.8) | ||
| Burn characteristics | |||
| Days after burn injury, mean (SD) | 6.5 (4.4) | 8 (3.0) | |
| TBSA (%), n (%) | |||
| 20–30 | 4 (57.1) | ||
| 30–40 | 1 (14.3) | ||
| 40–50 | 1 (14.3) | ||
| 50–60 | 1 (14.3) | ||
| TBSA (%), mean (SD) | 32.4 (11.4) | ||
| Burn degree: II–III, n (%) | 7 (100) | ||
| No. of piperacillin/tazobactam applications, mean (SD) | 15 (10) | 20 (9.9) | |
| Laboratory | |||
| Albumin (g/L), mean (SD) | 24.9 (3.6) | 29.5 (2.3) | |
| Mechanical ventilation | 7 (100) | 6 (100) | |
| Endotracheal tube, n (%) | 4 (57.1) | 3 (50) | |
| Tracheostomy, n (%) | 3 (42.9) | 3 (50) | |
| Patient positioning | |||
| Sand bed, no (%) | 2 (28.6) | 1 (16.7) | |
| Alternating pressure system, n (%) | 5 (71.4) | 5 (83.3) |
Overall, patient characteristics did not reveal essential differences between Study Days 1 and 2. Mean (SD) albumin levels increased from 24.9 (3.6) g/L on Study Day 1 to 29.5 (2.3) g/L after albumin infusions on Study Day 2. Patients received a mean (SD) of 15 (10) single infusions of 4.5 g piperacillin/tazobactam, thus reaching steady-state conditions prior to first study day.
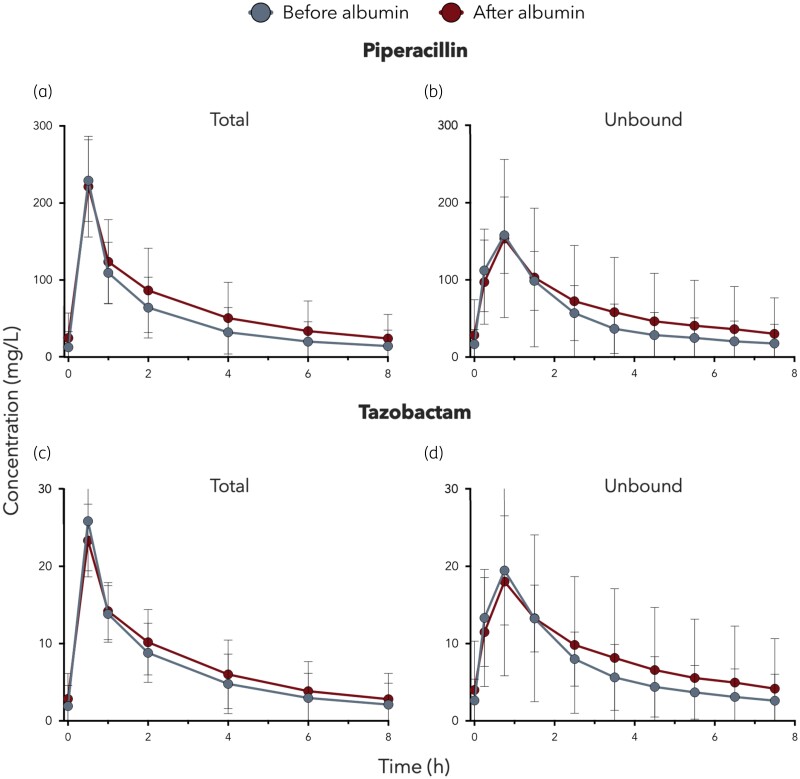
Figure 1 illustrates the mean linear steady-state plasma concentration–time profiles for bound and unbound piperacillin/tazobactam, respectively.
Figure 1.
Mean (SD) concentration–time curves of total (a) and unbound (b) piperacillin and total (c) and unbound (d) tazobactam concentrations in plasma of burn patients after an IV application of 4.5 g of piperacillin/tazobactam at steady-state conditions before and after albumin substitution. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
PK parameters of total and free piperacillin and tazobactam were expressed as mean (SD) and listed in Tables 2 and 3, respectively. Comparable mean (SD) Cmax values were observed before and after albumin substitution for total piperacillin [229.11 (53.04) versus 221.26 (65.44) mg/L)] and tazobactam [25.84 (6.42) versus 23.33 (4.71) mg/L]. Similar results were observed for peak free piperacillin [158.1 (49.22) versus 153.6 (102.48) mg/L] and tazobactam [19.47 (7.06) versus 18.03 (12.21) mg/L].
Table 2.
Key pharmacokinetic parameters of piperacillin in plasma (total concentrations) and microdialysate (free concentrations) after an IV infusion of 4.5 g piperacillin/tazobactam at steady-state conditions before and after albumin substitution
| Piperacillin (4 g) | Plasma before albumin (total concentration) | Plasma after albumin (total concentration) | MD before albumin (free concentration) | MD after albumin (free concentration) |
|---|---|---|---|---|
| C max (mg/L) | 229.11 (53.04) | 221.26 (65.44) | 158.10 (42.22) | 153.6 (102.48) |
| AUClast (hamg/L) | 402.08 (242.35) | 521.80 (363.01) | 398.94 (203.92) | 492.46 (510.815) |
| T max (h) | 0.5 | 0.5 | 0.68 (0.19) | 0.75 |
| t ½ (h) | 2.58 (1.87) | 2.77 (1.85) | 2.01 (1.8)a | 2.95 (2.16) |
| CL (L/h) | 11.75 (6.16) | 10.14 (6.76) | 12.55 (6.58)a | 12.44 (7.72) |
| V d (L) | 31.71 (9.16) | 27.09 (5.73) | 26.37 (10.11)a | 35.22 (14.03) |
Values are expressed as mean (SD).
aResults after exclusion of one outlier (n = 6).
Table 3.
Key pharmacokinetic parameters of tazobactam in plasma (total concentrations) and microdialysate (unbound concentrations) after an IV infusion of 4.5 g piperacillin/tazobactam at steady-state conditions before and after albumin substitution
| Tazobactam (0.5 g) | Plasma before albumin (total concentration) | Plasma after albumin (total concentration) | MDs before albumin (free concentration) | MD after albumin (free concentration) |
|---|---|---|---|---|
| C max (mg/L) | 25.84 (6.42) | 23.33 (4.72) | 19.47 (7.06) | 18.04 (12.21) |
| AUClast (hamg/L) | 53.16 (26.78) | 59.70 (31.63) | 54.48 (25.34) | 64.49 (64.40) |
| T max (h) | 0.5 | 0.5 | 0.75 | 0.75 |
| t ½ (h) | 2.70 (2.00) | 2.93 (1.92) | 2.34 (2.14)a | 3.21 (2.45) |
| CL (L/h) | 10.22 (5.35) | 9.19 (4.67) | 10.38 (4.76)a | 11.09 (6.31) |
| V d (L) | 28.89 (9.43) | 28.20 (3.97) | 25.12 (8.63)a | 34.89 (15.31) |
Values are expressed as mean (SD).
aResults after exclusion of one outlier (n = 6).
Mean (SD) AUC0–8 was numerically higher after albumin substitution for total piperacillin [402.08 (242.35) versus 521.8 (363.01) mg/L) and tazobactam [53.16 (26.78) versus 59.7 (31.63) mg/L]. Similarly, the AUC0–8 of unbound piperacillin [398.92 (203.9) versus 456.35 (438.76) mg/L] and tazobactam [54.48 (25.34) versus 64.49 (82.18) mg/L) increased after albumin supplementation.
One implausible outlier led to a strong distortion of t½, Vd and CL of free piperacillin/tazobactam before albumin substitution. Hence, since these PK parameters are highly susceptible to distortion based on single outliers, we excluded this patient from the analysis for these variables only. The excluded patient did not undergo Study Day 2.
Pharmacometric data analysis
A detailed description of the pharmacometric data analysis can be found in the Supplementary data. A total of 209 samples (plasma: 77, ultrafiltrate: 22, microdialysate: 110) of each drug were available for analysis. Within a patient, parameters for CL, fraction unbound from ultrafiltration and recovery-corrected MD data were modelled separately before and after the supplementation with albumin: The best-fitting models for piperacillin and tazobactam were utilized to predict unbound plasma concentrations through Monte Carlo simulations (n = 500) for the dosing regimen of 4 g piperacillin and 0.5 g tazobactam every 8 h (Figures S1–S4). Pharmacokinetic and protein-binding model parameter estimates for piperacillin and tazobactam can be found in Tables S2 and S3, respectively.
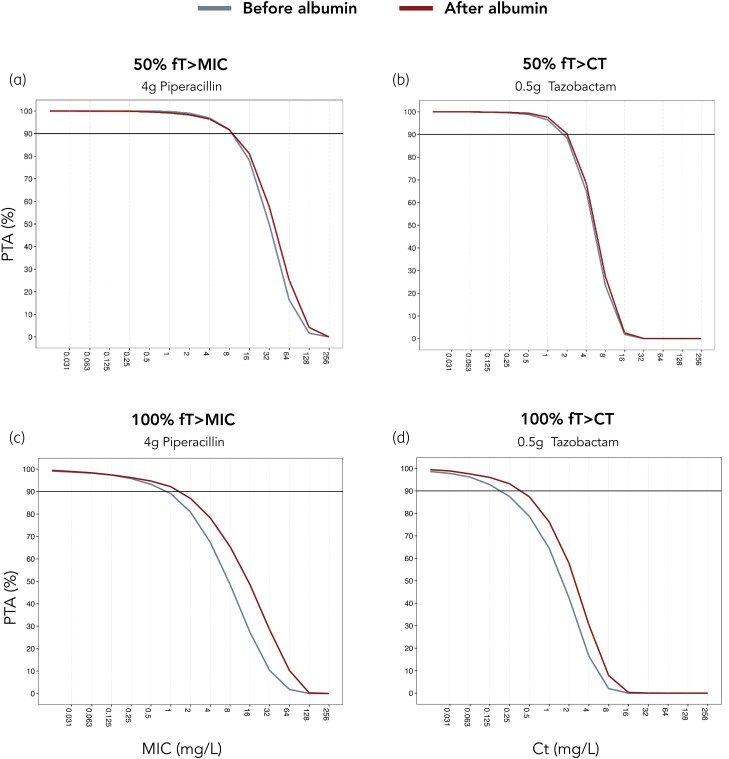
Figure 2 depicts the resulting PTA plots. For piperacillin, at 100% fT>MIC, high PTA (>90%) was achieved at an MIC value of 0.5 mg/L before and 1 mg/L after albumin supplementation. For 50% fT>MIC, high PTA was obtained for MIC values of 8 mg/L.
Figure 2.
PTA plots for 0.5 h infusion of 4.5 g piperacillin/tazobactam q8h under steady-state conditions. (a) PTA of piperacillin for 50% fT>MIC, (b) PTA of tazobactam for 50% fT>Ct, (c) PTA of piperacillin for 100% fT>MIC and (d) PTA of tazobactam for 100% fT>Ct. Comparison between concentrations before albumin supplementation (grey lines) and after albumin supplementation (red lines) with simulated (n = 500) piperacillin/tazobactam concentrations. Horizontal lines denote breakpoints of 90% PTA. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
For tazobactam, at 100% fT>Ct, high PTA (>90%) was attained at a Ct of 0.125 before and 0.25 mg/L after albumin supplementation. For 50% fT>Ct, high PTA was observed at a Ct of 1 mg/L.
Discussion
The present study is the first to investigate PK of anti-infective agents in severely burned patients before and after albumin supplementation, and further to determine the unbound fraction of piperacillin/tazobactam in plasma by means of MD. Our objective was to examine whether an increase in albumin concentration resulting from therapeutic supplementation could lead to a greater bound fraction of the drug and influence antibiotic drug exposure.
Our results show slightly higher mean antibiotic bound and unbound plasma AUC0–8 values after albumin substitution. Comparable plasma Cmax values for both bound and unbound piperacillin/tazobactam were observed. Overall, mentioned data did not show statistical significance (data not illustrated). As a possible explanation we consider the fact that compared with other antibiotic agents, the plasma protein binding (PPB) of piperacillin/tazobactam (approximately 30%) is known to be fairly low.29 Hence, antibiotics showing a higher PPB, such as clindamycin and ceftriaxone, all of which are also selectively used in the BICU, may be affected differently.8 However, our choice of antibiotic was based on prescription frequencies in ICU units, to provide insights into PPB characteristics of more commonly used drugs.
Piperacillin is subject to filtration and substantial tubular secretion in the renal tubules. The unbound fraction is mainly filtered, while the bound fraction can be eliminated through tubular secretion.30 The simultaneous increase in both unbound and bound fractions of piperacillin/tazobactam therefore indicates a simultaneous and proportional rise in filtration and secretion. Despite the consistency in Cmax before and after albumin supplementation, the increase in AUC for both bound and unbound fractions implies a shift in the drug’s clearance. Accordingly, there was a slight decrease in clearance for both bound and unbound piperacillin and total tazobactam, but not for free tazobactam after albumin substitution. Nevertheless, these results should be interpreted with caution, as the increase in AUC was not significant and the variability was high.
Severe burn trauma is associated with unique pathophysiological changes, such as a hypermetabolic response, which initiates >48 h after initial burn trauma, and is proportional to the size of the burn.31 The mean (SD) number of days from burn injury to study initiation was 6.5 (4.4) and the affected %TBSA was >20% in all patients. As a result, all subjects were expected to be in the hypermetabolic phase during the study period. In this state, the metabolism of anti-infective agents may be altered due to factors such as increased cardiac output and hypoalbuminaemia. Accordingly, and in agreement with previous studies investigating PK parameters of piperacillin in severely burned patients, our subjects showed an increased Vd and prolonged t½ when compared with healthy volunteers.29,32
Piperacillin exhibits time-dependent killing, which is achieved when the free fraction of the drug in plasma continuously exceeds the MIC of the target pathogen. Therefore, clinical efficacy is best estimated by the percentage of the dosing interval, in which the free fraction exceeds the MIC of target pathogens (fT>MIC). Assuming a piperacillin dose of 4 g q8h infused over 0.5 h, Monte Carlo simulations predicted a PTA of 50% fT>MIC for MIC values of ≤8 mg/L. No significant difference was observed in PTA before or after albumin supplementation. In contrast, tazobactam does not exert antibacterial properties on its own, but restores the activity of piperacillin. Therefore, MICs cannot be determined for tazobactam alone, but a Ct must be achieved to effectively neutralize β-lactamase enzymes. Monte Carlo simulations predicted a PTA of >90% for achieving 50% fT>Ct for a threshold concentration of 1 mg/L (before and after albumin supplementation).
Common pathogens of infections in burn intensive care include P. aeruginosa and Enterobacterales.3–5 According to EUCAST, the clinical breakpoints for P. aeruginosa and Enterobacterales are 16 and 8 mg/L, respectively. These breakpoints serve as a reference for susceptibility and can guide empirical antibiotic coverage. In our simulation, after albumin supplementation, the PTA of piperacillin achieving 50% fT>MIC amounted to 81% (16 mg/L) and 92% (8 mg/L) for P. aeruginosa and Enterobacterales, respectively.
Although in vivo animal studies have demonstrated that β-lactams require target times of 40%–70% fT>MIC, retrospective analyses suggest that a much larger exposure of 100% fT>4×MIC may be necessary to achieve clinical effectiveness.33 Achieving these targets with a short infusion may prove to be unattainable and could lead to toxic drug exposures. Therefore, when aiming to combat Gram-negative pathogens with elevated MICs, considering extended or continuous drug infusions may emerge as a viable strategy to optimize drug exposure.
We acknowledge the limitation of a relatively small sample size, in which not all subjects underwent complete MD sampling to assess the unbound fraction. This led to relatively high variability in our results. Furthermore, the pharmacometric data analysis did not evaluate covariate effects beyond albumin, as the study size was too small to obtain precise and accurate estimates of covariate effects at sufficiently high power. Nevertheless, this was the first study to successfully establish a complete simultaneous plasma concentration–time profile of total and unbound fractions of piperacillin and tazobactam and to evaluate the effects of albumin supplementation on their PK. Finally, we acknowledge the limitation of having excluded one patient for the calculation of t½, CL and Vd of free piperacillin/tazobactam before albumin substitution. Nevertheless, given the small sample size, we chose to include the data for calculating Cmax, AUC and Tmax of free piperacillin/tazobactam, as well as for generating the corresponding figures and models, since these parameters are less influenced by individual outliers.
Conclusions
Albumin supplementation had little impact on the PK of piperacillin/tazobactam. After albumin supplementation, there was a numerical increase in mean AUC0–8 of total and unbound piperacillin/tazobactam, whereas similar Cmax values were observed. Future studies may investigate the effect of albumin supplementation on drugs with a higher plasma protein binding. Monte Carlo simulations indicate that dosing regimens of 4 g piperacillin/0.5 g tazobactam q8h was sufficient for empirical treatment of common Gram-negative pathogens.
Supplementary Material
Acknowledgements
We thank the entire BICU of the Medical University of Vienna, especially the nursing staff led by Elisabeth Bulant and Diana Raidl, for their active support and collaboration throughout the study process.
Contributor Information
Beatrix Wulkersdorfer, Medical University of Vienna, Department of Clinical Pharmacology, Währinger Gürtel 18-20, 1090 Vienna, Austria; Orthopedic Clinic—SKA Zicksee, Otto-Pohanka-Platz 1, 7161 St.Andrä am Zicksee, Austria.
Felix Bergmann, Medical University of Vienna, Department of Clinical Pharmacology, Währinger Gürtel 18-20, 1090 Vienna, Austria; Medical University of Vienna, Department of Plastic, Reconstructive, and Aesthetic Surgery, Währinger Gürtel 18-20, 1090 Vienna, Austria.
Lisa Amann, University of Hamburg, Department of Clinical Pharmacology, Institute of Pharmacy, Bundesstrasse 45, 20146 Hamburg, Germany.
Alexandra Fochtmann-Frana, Medical University of Vienna, Department of Plastic, Reconstructive, and Aesthetic Surgery, Währinger Gürtel 18-20, 1090 Vienna, Austria.
Valentin Al Jalali, Medical University of Vienna, Department of Clinical Pharmacology, Währinger Gürtel 18-20, 1090 Vienna, Austria.
Elizaveta Kurdina, Medical University of Vienna, Department of Clinical Pharmacology, Währinger Gürtel 18-20, 1090 Vienna, Austria.
Edith Lackner, Medical University of Vienna, Department of Clinical Pharmacology, Währinger Gürtel 18-20, 1090 Vienna, Austria.
Sebastian G Wicha, University of Hamburg, Department of Clinical Pharmacology, Institute of Pharmacy, Bundesstrasse 45, 20146 Hamburg, Germany.
Christoph Dorn, University of Regensburg, Institute of Pharmacy, Universitätsstraße 31, 93053 Regensburg, Germany.
Bruno Schäfer, Medical University of Vienna, Department of Anesthesiology and General Intensive Care, Währinger Gürtel 18-20, 1090 Vienna, Austria.
Gerald Ihra, Medical University of Vienna, Department of Anesthesiology and General Intensive Care, Währinger Gürtel 18-20, 1090 Vienna, Austria.
Thomas Rath, Medical University of Vienna, Department of Plastic, Reconstructive, and Aesthetic Surgery, Währinger Gürtel 18-20, 1090 Vienna, Austria.
Christine Radtke, Medical University of Vienna, Department of Plastic, Reconstructive, and Aesthetic Surgery, Währinger Gürtel 18-20, 1090 Vienna, Austria.
Markus Zeitlinger, Medical University of Vienna, Department of Clinical Pharmacology, Währinger Gürtel 18-20, 1090 Vienna, Austria.
Funding
This project was internally funded by the Department of Clinical Pharmacology at the Medical University of Vienna. All mentioned supplies were provided by the Department of Clinical Pharmacology and in part by the Burn Intensive Care Unit (BICU) at the Medical University of Vienna.
Transparency declarations
None to declare.
Author contributions
All authors have declared full responsibility for the work described in this manuscript and have provided final approval of the version submitted to the journal. Beatrix Wulkersdorfer planned and performed the clinical study, interpreted the data, created tables and figures and wrote the manuscript. Felix Bergmann interpreted the data, created tables and figures and wrote the manuscript. Lisa Amann performed pharmacokinetic modelling, created tables and figures and critically revised the manuscript. Alexandra Fochtmann-Frana performed the clinical study and critically revised the manuscript. Valentin al Jalali performed the clinical study and critically revised the manuscript. Elizaveta Kurdina performed the clinical study and critical revised the manuscript. Edith Lackner planned and performed the clinical study. Sebastian G. Wicha performed pharmacokinetic modelling, created tables and figures and critically revised the manuscript. Christoph Dorn performed the bioanalysis and critically revised the manuscript. Bruno Schäfer planned the clinical study and critically revised the manuscript. Gerald Ihra planned the clinical study and critically revised the manuscript. Thomas Rath planned the clinical study and critically revised the manuscript. Christine Radtke planned the clinical study and critically revised the manuscript. Markus Zeitlinger planned and performed the clinical study, interpreted the data and critically revised the manuscript.
Data and materials availability
The data and materials that support the findings of this study are available from the corresponding author upon reasonable request.
Supplementary data
Pharmacometric data analysis, Figures S1 to S4 and Tables S1 to S3 are available as Supplementary data at JAC Online.
References
- 1. Rex S. Burn injuries. Curr Opin Crit Care 2012; 18: 671–6. 10.1097/MCC.0b013e328359fd6e [DOI] [PubMed] [Google Scholar]
- 2. Lorente JA, Amaya-Villar R. Update in the management of critically ill burned patients. Med Intensiva 2016; 40: 46–8. 10.1016/j.medin.2015.10.009 [DOI] [PubMed] [Google Scholar]
- 3. Brusselaers N, Monstrey S, Snoeij Tet al. Morbidity and mortality of bloodstream infections in patients with severe burn injury. Am J Crit Care 2010; 19: e81–7. 10.4037/ajcc2010341 [DOI] [PubMed] [Google Scholar]
- 4. Branski LK, Al-Mousawi A, Rivero Het al. Emerging infections in burns. Surg Infect 2009; 10: 389–97. 10.1089/sur.2009.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Church D, Elsayed S, Reid Oet al. Burn wound infections. Clin Microbiol Rev 2006; 19: 403–34. 10.1128/CMR.19.2.403-434.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Greenhalgh DG. Management of burns. NEJM 2019; 380: 2349–59. 10.1056/NEJMra1807442 [DOI] [PubMed] [Google Scholar]
- 7. Greenhalgh DG, Saffle JR, Holmes JHet al. American Burn Association Consensus Conference to define sepsis and infection in burns. J Burn Care Res 2007; 28: 776–90. 10.1097/BCR.0b013e3181599bc9 [DOI] [PubMed] [Google Scholar]
- 8. Wang Y, Beekman J, Hew Jet al. Burn injury: challenges and advances in burn wound healing, infection, pain and scarring. Adv Drug Deliv Rev 2018; 123: 3–17. 10.1016/j.addr.2017.09.018 [DOI] [PubMed] [Google Scholar]
- 9. Dries DJ, Marini JJ. Management of critical burn injuries: recent developments. Acute Crit Care 2017; 32: 9–21. 10.4266/kjccm.2016.00969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roberts JA. Using PK/PD to optimize antibiotic dosing for critically ill patients. Curr Pharm Biotechnol 2011; 12: 2070–9. 10.2174/138920111798808329 [DOI] [PubMed] [Google Scholar]
- 11. Roberts JA, Lipman J. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit Care Med 2009; 37: 840–51. 10.1097/CCM.0b013e3181961bff [DOI] [PubMed] [Google Scholar]
- 12. Jaehde U, Sorgel F. Clinical pharmacokinetics in patients with burns. Clin Pharmacokinet 1995; 29: 15–28. 10.2165/00003088-199529010-00003 [DOI] [PubMed] [Google Scholar]
- 13. Jager NG, van Hest RM, Lipman Jet al. Therapeutic drug monitoring of anti-infective agents in critically ill patients. Expert Rev Clin Pharmacol 2016; 9: 961–79. 10.1586/17512433.2016.1172209 [DOI] [PubMed] [Google Scholar]
- 14. Jamal JA, Economou CJ, Lipman Jet al. Improving antibiotic dosing in special situations in the ICU: burns, renal replacement therapy and extracorporeal membrane oxygenation. Curr Opin Crit Care 2012; 18: 460–71. 10.1097/MCC.0b013e32835685ad [DOI] [PubMed] [Google Scholar]
- 15. Beer J, Wagner CC, Zeitlinger M. Protein binding of antimicrobials: methods for quantification and for investigation of its impact on bacterial killing. AAPS J 2009; 11: 1. 10.1208/s12248-008-9072-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Acharya C, Hookers AC, Turkyilmaz GYet al. A diagnostic tool for population models using non-compartmental analysis: the ncappc package for R. Comput Methods Programs Biomed 2016; 127: 83–93. 10.1016/j.cmpb.2016.01.013 [DOI] [PubMed] [Google Scholar]
- 17. Sebille B. Methods of drug protein binding determinations. Fundam Clin Pharmacol 1990; 4Suppl 2: 151s–61s. 10.1111/j.1472-8206.1990.tb00073.x [DOI] [PubMed] [Google Scholar]
- 18. Muller M, Schmid R, Georgopoulos Aet al. Application of microdialysis to clinical pharmacokinetics in humans. Clin Pharmacol Ther 1995; 57: 371–80. 10.1016/0009-9236(95)90205-8 [DOI] [PubMed] [Google Scholar]
- 19. Elmquist WF, Sawchuk RJ. Application of microdialysis in pharmacokinetic studies. Pharm Res 1997; 14: 267–88. 10.1023/A:1012081501464 [DOI] [PubMed] [Google Scholar]
- 20. Brunner M, Langer O. Microdialysis versus other techniques for the clinical assessment of in vivo tissue drug distribution. AAPS J 2006; 8: E263–71. 10.1007/BF02854896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Burian A, Wagner C, Stanek Jet al. Plasma protein binding may reduce antimicrobial activity by preventing intra-bacterial uptake of antibiotics, for example clindamycin. J Antimicrob Chemother 2011; 66: 134–7. 10.1093/jac/dkq400 [DOI] [PubMed] [Google Scholar]
- 22. Zeitlinger MA, Derendorf H, Mouton JWet al. Protein binding: do we ever learn? Antimicrob Agents Chemother 2011; 55: 3067–74. 10.1128/AAC.01433-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Verbeeck RK. Blood microdialysis in pharmacokinetic and drug metabolism studies. Adv Drug Deliv Rev 2000; 45: 217–28. 10.1016/S0169-409X(00)00110-1 [DOI] [PubMed] [Google Scholar]
- 24. Muller M. Science, medicine, and the future: microdialysis. BMJ 2002; 324: 588–91. 10.1136/bmj.324.7337.588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kratzer A, Schiesser S, Matzneller Pet al. Determination of total and free ceftolozane and tazobactam in human plasma and interstitial fluid by HPLC-UV. J Pharm Biomed Anal 2019; 163: 34–8. 10.1016/j.jpba.2018.09.044 [DOI] [PubMed] [Google Scholar]
- 26. Lindbom L, Pihlgren P, Jonsson EN. PsN-Toolkit–a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed 2005; 79: 241–57. 10.1016/j.cmpb.2005.04.005 [DOI] [PubMed] [Google Scholar]
- 27. Tunblad K, Hammarlund-Udenaes M, Jonsson EN. An integrated model for the analysis of pharmacokinetic data from microdialysis experiments. Pharm Res 2004; 21: 1698–707. 10.1023/B:PHAM.0000041468.00587.c6 [DOI] [PubMed] [Google Scholar]
- 28. Broeker A, Wicha SG. Assessing parameter uncertainty in small-n pharmacometric analyses: value of the log-likelihood profiling-based sampling importance resampling (LLP-SIR) technique. J Pharmacokinet. Phar 2020; 47: 219–28. 10.1007/s10928-020-09682-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sorgel F, Kinzig M. Pharmacokinetic characteristics of piperacillin/tazobactam. J. Intensive Care Med 1994; 20Suppl 3: S14–20. 10.1007/BF01745246 [DOI] [PubMed] [Google Scholar]
- 30. Currie GM. Pharmacology, part 2: introduction to pharmacokinetics. J Nucl Med Technol 2018; 46: 221–30. 10.2967/jnmt.117.199638 [DOI] [PubMed] [Google Scholar]
- 31. Jeschke MG, Mlcak RP, Finnerty CCet al. Burn size determines the inflammatory and hypermetabolic response. J Crit Care 2007; 11: R90. 10.1186/cc6102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jeon S, Han S, Lee Jet al. Population pharmacokinetic analysis of piperacillin in burn patients. Antimicrob Agents Chemother 2014; 58: 3744–51. 10.1128/AAC.02089-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Roberts JA, Paul SK, Akova Met al. DALI: defining antibiotic levels in intensive care unit patients: are current β-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis 2014; 58: 1072–83. 10.1093/cid/ciu027 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.