Abstract
Purpose:
To determine differences between women and men in the repair of rhegmatogenous retinal detachments (RRD) in the United States.
Design:
Retrospective cohort study.
Methods:
Setting:
A large insurance claims database.
Participants:
Subjects with an incident RRD between 2007–2015.
Procedures:
Demographic data, comorbid ocular conditions associated with RRD, systemic comorbidities, and surgical intervention (pneumatic retinopexy (PR), pars plana vitrectomy (PPV), laser barricade, or scleral buckle (SB)) were collected.
Main outcome measures:
Odds of receipt of surgical intervention for incident RRD, time to repair, type of intervention, and the rate of reoperation by sex.
Results:
The study period included 133 million eligible records with 61,071 cases of incident RRD meeting inclusion criteria among which 43% (n = 26,289) were women. Women had 34% reduced odds of receipt of surgical repair of an RRD (OR 0.66, 95% CI 0.59 – 0.73, p<0.001) after adjusting for confounders. This effect persisted in all sensitivity models. Among patients that received repair, women were more often delayed (0.17 days, p = 0.04). Women were more likely to undergo primary laser barricade (RRR 1.68, p < 0.001), primary SB (RRR 1.15, p < 0.001), and PR (RRR 1.07, p < 0.04) than men. The odds of reoperation were lower in women (OR 0.91, 95% CI 0.85 – 0.96, p=0.002) after adjustment.
Conclusions:
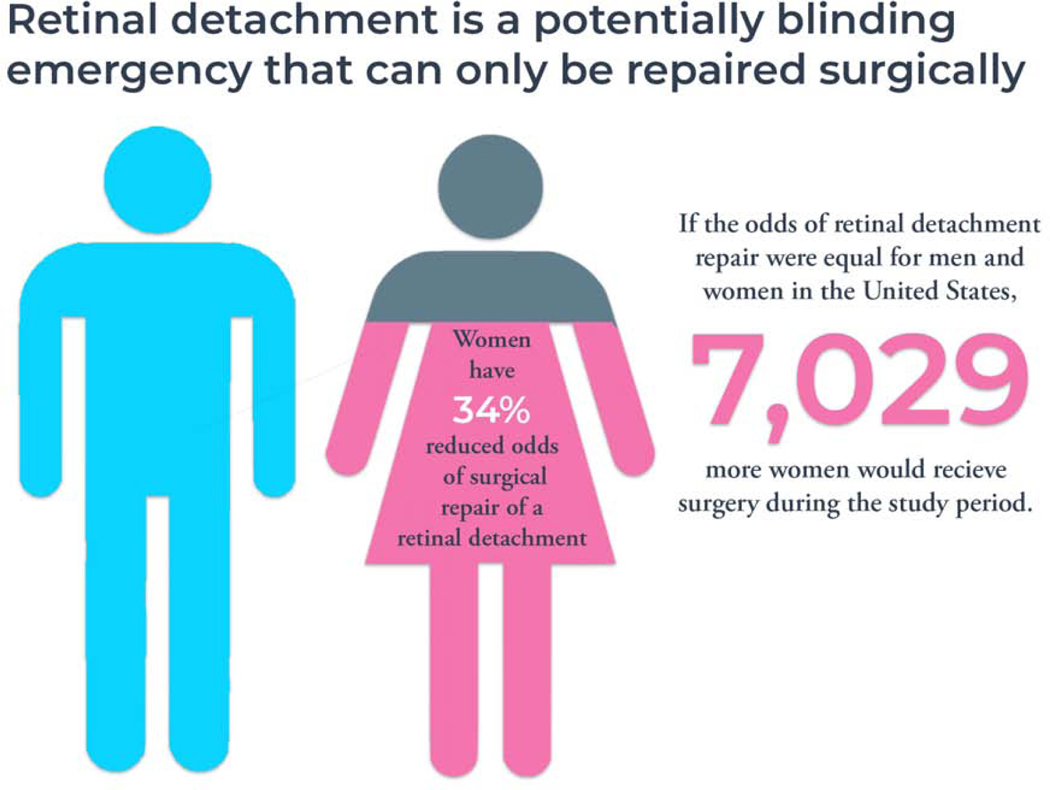
Insured women are less likely than insured men to receive surgical intervention for an RRD. If the odds of repair were equal between women and men in the U.S. then 781 more women would receive surgery each year, or 7,029 more during the study period. Women are more likely to have the repair performed with scleral buckle and laser barricade. The reason for these sex differences in RRD repair remains unknown and requires further investigation.
Rhegmatogenous retinal detachment (RRD)is a potentially blindingophthalmic emergency requiring intervention with an incidence of more than 1 in 10,000 people.1-7RRD is the most common type of retinal detachment and it occurs in the presence of a retinal break (rhegmain Greek) that allows for the passage of vitreous fluid into the subretinal space. Timely repair of RRDs is critical to optimize vision outcomes and minimize long-term visual disability.8,9 Population-based studies have identified several risk factors for RRD development including, but not limited to: age, ethnicity, myopia, lattice degeneration, retinal breaks, trauma, pseudophakia, family history of retinal detachment, and genetic syndromes.3,5,6,10–12
Research on RRD has primarily focused on the biological risk factors and surgical techniques affecting the delivery and outcomes of repair, however, relatively few studies have reported the real-world practices in the United States. Studies in other fields of medicine have found disparities between female and male patients for urgent surgical interventions that persist after controlling for confounding variables, most notably in cardiology related to repair of abdominal aortic aneurysms13,14 and valve surgery.15,16 These studies report a decreased rate of surgical intervention for women, even in the inpatient setting15,16, as well as delayed diagnosis17, increased peri-operative complications18, and increased morbidity and mortality.13,19,20
In ophthalmology, studies on RRD repair have suggested in secondary analyses that there may be differences in the rates of trauma associated presentation and treatment of RRD by sex, however, no study has specifically examined the relationship of sex to RRD repair.21,22The impetus for this study was to determine whether sex differences impacted the repair of RRDs in the United States. The study question was assessed using a large outpatient insurance claims database that represents the majority of commercially insured patients in the United States. We hypothesize that the rate of surgical repair of primary RRD is lower for women, that women are more often delayed and repaired with different surgical procedures.
METHODS
The Stanford University School of Medicine Institutional Review Board (IRB)/Ethics Committee ruled this secondary analysis of de-identified administrative data to be exempt from IRB approval (IRB#53203). A waiver of informed consent for this retrospective analysis was granted. All research adhered to the tenets of the Declaration of Helsinki. This study represents a retrospective cohort study using administrative insurance claims from a large commercial administrative claims database.
Commercial Claims and Encounters Database
The Truven Health MarketScan Commercial Claims and Encounters database (Truven Health Analytics, San Jose, CA)contains administrative data from over 150 million United States beneficiaries across more than 350 insurance carriers from a wide variety of private payers including fee-for-service, managed care, and consumer-directed payer plans. The dataset represents roughly 50% of the U.S. population covered by employee-sponsored insurance and includes beneficiaries from all 50 states. The database contains longitudinal claims data for enrollees until the insurance is discontinued due to loss of insurance, change in insurer, or transition to Medicare. Beneficiary information includes demographic characteristics, diagnosis procedural, and billing codes. Administrative insurance claims data from the Truven Health MarketScan database from January 1, 2007 to December 31, 2015 were used for this study.
Sample Selection and Predictor Variables
Records from beneficiaries with an incident RRD diagnosis and at least one year of longitudinal data before the diagnosis and at least 30 days following the index diagnosis of RRD during the study period were included. Baseline demographic characteristics including sex, year, and comorbid ocular conditions associated with retinal detachment(myopia, pseudophakia, lattice degeneration, and vitreous hemorrhage) were collected as predictor variables. The diagnoses were identified using International Classification of Diseases 9th and 10th editions (ICD-9 and ICD-10) diagnosis codes available for each year. Ocular comorbidities were any diagnosis codes for the aforementioned conditions within the one year prior to incident RRD diagnosis or 30-days following diagnosis.
Surgical intervention was identified by current procedural terminology (CPT) codes for pneumatic retinopexy (PR), pars plana vitrectomy (PPV), laser barricade, or scleral buckle (SB). CPT coding does not permit the differentiation of PPV with and without a SB, thus SB here represents the procedure alone and PPV represents vitrectomy with or without a SB. If multiple surgeries were coded on the same day, they were classified by the most invasive procedure. Laser barricade was considered the least invasive followed by PR, SB, then PPV as most invasive. Laterality data was also collected; however, this was only used in sensitivity analysis because laterality data was available for 12.9% (n=7,882) of all records with a diagnosis of RRD and only 4.5% (n=2,775) of confirmed RRD diagnosis records used for the primary outcome reporting model.
The year of diagnosis was also included as a predictor variable because prior studies have reported increasing trends in PPV utilization over time.1Patients with prior documented RRD during the one-year mandatory look-back period were excluded to reduce the rate of miscoding related to a historic retinal detachment and isolate incident RRD. CPT codes for complex retinal detachments were excluded to isolate RRD. These include records with diagnoses of tractional, exudative, and serous retinal detachments, proliferative diabetic retinopathy, retinopathy of prematurity, sickle cell, retinoschisis, chorioretinitis, endophthalmitis, ruptured globe, retinal vein occlusion, and choroidal hemorrhage or rupture.
Primary and Secondary Outcomes
The primary outcome was defined as the odds of receipt of surgical intervention for women compared to men after controlling for baseline characteristics, ocular comorbidities, year of surgery, surgical procedure, and Elixhauser comorbidities. The Elixhauser comorbidity index represents a validated method for assessing systemic health originally developed for use in the inpatient setting but has since been used in ophthalmic studies as a proxy for general health.23
Secondary outcomes for beneficiaries who received repair of incident RRD included the time to surgical repair (days) after diagnosis of RRD, the type of surgical intervention, and the rate of reoperation after controlling for the confounders described above. Reoperation was defined as a secondary RRD intervention among beneficiaries who met incident RRD criteria above and had a minimum of 30 days follow-up after the initial surgery.
Statistical Analysis
The primary outcome was a binary variable classified as yes or no for the receipt of surgical intervention after incident RRD diagnosis. Logistic regression modeling on the full cohort to assess the receipt of surgical intervention for women and men was performed controlling for age, ocular comorbidities, total Elixhauser comorbidities index, and year of surgery. For beneficiaries who met inclusion criteria and who underwent repair of an incident RRD the time from diagnosis of first RRD to the time of surgical intervention was collected and compared between women and men after controlling for the aforementioned potential confounders using linear regression modeling for this continuous outcome. Time was recorded in days as was available in the database. Time analysis was performed using all available data on beneficiaries and secondarily restricting to within 90 days of index diagnosis. The type of surgery was treated as a categorical variable modeled with multinomial logistic regression for all patients who received repair. Reoperation was a binary variable and analyzed with logistic regression modeling.
Increasingly stringent sensitivity models were then performed for all of the aforementioned outcomes. Model 1 is the full incident RRD cohort adjusted for confounders. Model 2 is the pre specified outcome reporting model that requires a second confirmatory diagnosis of RRD by an ophthalmic provider prior to surgical intervention to reduce the chance of the miscoding of an RRD. A prior study of RRDs using this database reported significant improvement in surgical repair rate to over 90% with confirmatory diagnosis criteria and thus this model was pre specified as the primary outcome reporting model.22Model 3 is a sensitivity analysis including only patients with laterality coding that was congruent between the diagnosis and surgical intervention side to avoid including patients who had an RRD in the other eye. Finally, Model 4 required both a confirmatory RRD diagnosis and laterality. We sequentially evaluated the models as 1) the full cohort, 2) requiring confirmatory RRD diagnosis, 3) laterality, and 4) both laterality and confirmatory RRD.
Statistical analysis was performed using Stata (StataCorp, College Station, Texas) version 14.2 and a two-tailed alpha with a P< 0.05 was considered statistically significant.
RESULTS
Sample Characteristics
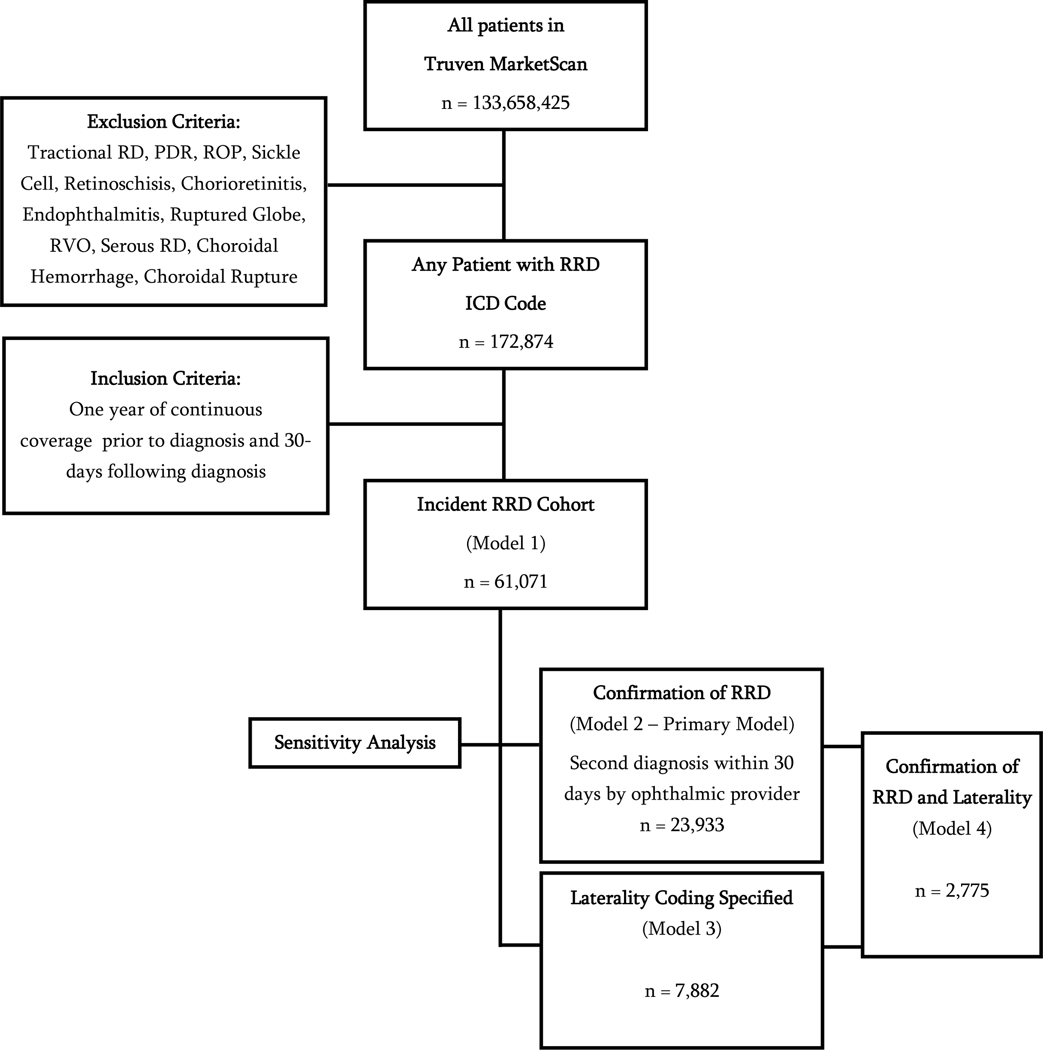
From a population of 133,658,425 beneficiaries, there were 61,071 retinal detachment records that met study criteria and were included in the full incident RRD cohort (Model 1) analysis (Figure 1). Among records that met inclusion criteria for the study, 43% (n = 26,289) were women. Sixty-three percent (n=38,537) of beneficiaries in the full incident RRD cohort received any surgery. The confirmatory model requiring a second diagnosis of RRD contained 23,938 records (62.1% of the full cohort). Among these 93.1% (n = 22,275) received surgery. Laterality was recorded in only 7,882 (12.9% of the full cohort) of beneficiaries eligible for the study.
Figure 1. Study Inclusion Flow Diagram.
RRD = rhegmatogenous retinal detachment; RD = retinal detachment; PDR = proliferative retinal detachment; ROP = retinopathy of prematurity; RVO = retinal vein occlusion; ICD = international classification of diseases
The baseline and clinical characteristics of the cohort are shown in Table 1. There were fewer women than men in the full cohort (43% vs 57%). The mean age at diagnosis was slightly, but significantly, younger for women by 0.3 years (p=0.02). There was a statistically significant difference in the distribution of ocular comorbidities between women and men with women being more likely to have a diagnosis of myopia (4.7% vs 3.4%, p<0.01) and lattice degeneration (17.5% vs 15.3%, p<0.01) and less likely to have vitreous hemorrhage (11.3% vs 12.7%, p<0.01) than men. For most of the records the diagnosing provider was unknown (70.6%), but when it was known the most common diagnosing provider was a retinal specialist diagnosing 21.2% of the cases. In the full cohort (Model 1) the percentage with surgical repair for incident RRD was 63.1%, similar to prior claims database studies on RRD repair.1,22Among patients diagnosed with an RRD, women did not undergo repair as frequently as men (41.8% vs 33.2%, p<0.01) in the full unadjusted sample. When a second confirmatory RRD diagnosis was required (Model 2) the rate of repair increased to 93.1%.In the confirmatory model women represent 39% of RRDs (n = 9.339) and men 61% (n = 14,599) and the absolute repair rate was 91.3% for women and 94.2% for men. In the unadjusted comparison, there was a statistically significant difference in the type of surgical repair performed between women and men (p =<0.01).
Table 1.
Baseline Demographic and Clinical Characteristics Among Patients with Primary Rhegmatogenous Retinal Detachment (RRD) By Sex in the Truven MarketScan Database from 2007–2015
| Characteristic | All Patients n = 61,071 | Female n = 26,289 (43.0%) | Male n = 34,782 (57.0%) | p-value |
|---|---|---|---|---|
|
| ||||
| Age (years) [mean (SD)] | 57.2 (14.5) | 57.1 (15.0) | 57.4 (14.1) | 0.02 |
|
Ocular Comorbidities [n, (%)] Myopia Pseudophakia Lattice Degeneration Vitreous Hemorrhage |
2,419 (4.0) 1,175 (1.9) 9,916 (16.2) 7,385 (12.1) |
1,237 (4.7) 484 (1.8) 4,594 (17.5) 2,958 (11.3) |
1,182 (3.4) 691 (2.0) 5,322 (15.3) 4,427 (12.7) |
<0.01 0.20 <0.01 <0.01 |
|
Diagnosing Provider [n, (%)] Unknown Optometrist Ophthalmologist Retina Specialist |
43,104 (70.6) 2,953 (4.8) 2,061 (3.4) 12,953 (21.2) |
18,508 (70.4) 1,349 (5.1) 887 (3.4) 5,545 (21.1) |
24,596 (70.7) 1,604 (4.6) 1,174 (3.4) 7,408 (21.3) |
0.03 |
|
Type of RRD Repair [n, (%)] None Pneumatic Retinopexy Scleral Buckle Laser Barricade Pars Plana Vitrectomy |
22,534 (36.9) 5,240 (8.6) 3,870 (6.3) 7,044 (11.5) 22,383 (36.7) |
10,999 (41.8) 1,966 (7.5) 1,590 (6.1) 3,485 (13.3) 8,249 (31.4) |
11,535 (33.2) 3,274 (9.4) 2,280 (6.6) 3,559 (10.2) 14,134 (40.6) |
<0.01 |
| Received any Surgery [n, (%)] | 38,537 (63.1%) | |||
| Second Confirmatory RRD Diagnosis in Record [n, (%)] | 23,938 (39.2%) | |||
| Received Surgery with Confirmatory RRD [n, (%)] | 22,275 (93.1%) | |||
Standard Deviation (SD), Rhegmatogenous retinal detachment (RRD)
Women Are Less Likely to Receive Surgical Intervention for a Retinal Detachment
Adjusted regression modeling for the primary outcome of interest, odds of receipt of retinal detachment repair, in the full and sensitivity models, is shown in Table 2and graphically represented in Figure 2. In all models women had a statistically significant reduced odds ratio (OR) of undergoing RRD repair with 32% reduced odds in the full cohort (Model 1, OR 0.68, p<0.001), 34% reduced odds in the confirmatory RRD model (Model 2, OR 0.66, p<0.001), 28% reduced odds in the laterality sensitivity model (Model 3, OR 0.72, p<0.00001), and 39% reduced odds in the most stringent sensitivity model requiring both confirmatory RRD and laterality coding (Model 4, OR 0.61, p>0.0001).
Table 2.
Multinomial Logistic Regression Model for Likelihood of Receipt of Any Rhegmatogenous Retinal Detachment (RRD) Repair within 30 Days of Diagnosis in the Truven MarketScan Database 2007–2015
| Full Incident Cohort (n = 61,071) | Cohort with Second Confirmation of Incident RRD (n = 23,933) | Cohort with RRD with Laterality (n = 7,882) | Cohort with Second Confirmation of RRD and Laterality (n = 2,775) | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Variables | *Adjusted OR (95% CI) | p-value | *Adjusted OR (95% CI) | p-value | *Adjusted OR (95% CI) | p-value | *Adjusted OR (95% CI) | p-value |
|
| ||||||||
| Sex | ||||||||
| Male | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Female | ||||||||
| 0.68 (0.66–0.71) | < 0.001 | 0.66 (0.59–0.73) | < 0.001 | 0.72 (0.66–0.80) | < 0.001 | 0.61 (0.47–0.79) | < 0.001 | |
| Ocular Comorbidities | ||||||||
| Myopia | 0.86 (0.79–0.94) | 0.001 | 0.87 (0.68–1.1) | 0.246 | 0.61 (0.5–0.76) | < 0.001 | 0.84 (0.49–1.44) | 0.518 |
| Pseudophakia | ||||||||
| 0.66 (0.58–0.75) | < 0.001 | 0.73 (0.53–1) | 0.051 | 1.25 (1.08–1.44) | 0.002 | 0.95 (0.65–1.37) | 0.767 | |
| Lattice Degeneration | ||||||||
| 1.79 (1.7–1.88) | < 0.001 | 1.55 (1.35–1.79) | < 0.001 | 1.67 (1.49–1.87) | < 0.001 | 1.71 (1.24–2.36) | 0.001 | |
| Vitreous Hemorrhage | ||||||||
| 2.1 (1.98–2.23) | < 0.001 | 1.1 (0.94–1.28) | 0.258 | 2.02 (1.77–2.31) | < 0.001 | 0.6 (0.44–0.82) | 0.001 | |
| Age at Diagnosis | ||||||||
| < 30 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| 31–40 | ||||||||
| 0.95 (0.86–1.05) | 0.99 (0.76–1.27) | 0.77 (0.57–1.03) | 0.92 (0.47–1.83) | |||||
| 41–50 | 0.326 | 0.923 | 0.078 | 0.822 | ||||
| 1.58 (1.45–1.72) | 1.64 (1.31–2.05) | 1.37 (1.08–1.75) | 2.27 (1.21–4.24) | |||||
| 51–60 | ||||||||
| < 0.001 | < 0.001 | 0.011 | 0.010 | |||||
| 1.99 (1.84–2.15) | 2.29 (1.86–2.81) | 1.55 (1.24–1.94) | 2.01 (1.18–3.42) | |||||
| > 60 | ||||||||
| < 0.001 | < 0.001 | < 0.001 | 0.011 | |||||
| 1.44 (1.33–1.55) | 2.24 (1.8–2.79) | 1.01 (0.81–1.27) | 1.83 (1.05–3.21) | |||||
| < 0.001 | < 0.001 | 0.908 | 0.034 | |||||
| Year of Diagnosis | 0.92 (0.91–0.93) | < 0.001 | 0.96 (0.94–0.98) | 0.001 | 1.24 (1.11–1.39) | < 0.001 | 1.3 (0.96–1.76) | 0.085 |
Analysis adjusted for: sex, ocular comorbidities, age at diagnosis, and year of diagnosis. Confidence interval (CI), Odds ratio (OR), Rhegmatogenous retinal detachment (RRD).
Figure 2. Women Have Reduced Odds of Repair of Retinal Detachment.
In this study reviewing 133 million administrative claims records over a 9-year study period women had 34% lower odds of receiving surgical repair for a rhegmatogenous retinal detachment compared to men after controlling for age, year of diagnosis, ocular comorbidities, and systemic comorbidities. The number of women that would be repair annually if the odds were equivalent was calculated using prior epidemiologic data from the United States estimating the incidence of RRD as 17.9 per 100,000 person-years and the U.S. Census Bureau report from the last included study year (2015) estimating 350 million insured working adults in the United States where this dataset would be applicable. Using these numbers, 781 more women would be repaired annually if the odds of repair were equivalent between the sexes. Over the nine-year study period 7,029 more insured women would receive repair if the odds were equal.
Other variables also demonstrated a statistically significant association with receipt of repair. Older age(OR 2.24, 95% CI 1.8 – 2.79, p <0.001 for 60+ years) by decade was associated with higher odds of repair that persisted in most sensitivity modeling. Lattice degeneration (OR 1.55, 95% CI 1.35 – 1.79, p < 0.001 in Model 2) increased the odds of receipt of repair in all models while vitreous hemorrhage (OR 2.1, 95% CI 1.98 – 2.23, p < 0.001) increased the odds of receipt of repair in all but Model 2 where it had no statistically significant effect. Year of diagnosis and Elixhauser comorbidity index were both statistically significant in all models (p < 0.001)
Timing of Rhegmatogenous Retinal Detachment Repair
When evaluating all records that met criteria, women had a 2.95 day delay in the receipt of RRD repair compared to men (95% CI for beta: 0.61 – 5.29, p = 0.01) in the adjusted model (Table 3). Adjusted analysis and restricting the time to repair to within30, 60, and 90 days after index diagnosis found a 0.06, 0.22, and 0.14 day delay for women, respectively, in the receipt of repair after controlling for age, year of diagnosis, ocular comorbidities, and Elixhauser comorbidities. This delay was again present in the sensitivity model requiring confirmatory RRD diagnosis. Time to repair was reduced in the three older decades of age across all models. Ocular comorbidities had variable effects on time to repair.
Table 3.
Linear Regression Model for Time to Repair of Rhegmatogenous Retinal Detachment Among Patients Who Received Surgery in the Truven MarketScan Database 2007–2015
| Full Cohort (n = 38,537) | Patients with Repair within 30 Days of RRD Diagnosis (n = 37,111) | Patients with Repair within 60 Days of RRD Diagnosis (n = 37,535) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variable | *Adjusted Beta Coefficient (95% CI) | p-value | *Adjusted Beta Coefficient (95% CI) | p-value | *Adjusted Beta Coefficient (95% CI) | p-value |
|
| ||||||
| Sex | 2.95 (0.61–5.29) | 0.014 | 0.06 (−0.02–0.14) | 0.114 | 0.22 (0.10–0.34) | < 0.001 |
| Female | ||||||
|
| ||||||
| Patients with Repair within 90 Days of RRD Diagnosis (n = 37,963) | Patients with Repair within 90 Days and with Second Confirmation RRD (n = 22,156) | Patients with Repair within 90 Days and with Laterality (n = 3,372) | ||||
|
| ||||||
| Variables | *Adjusted Beta Coefficient (95% CI) | p-value | *Adjusted Beta Coefficient (95% CI) | p-value | *Adjusted Beta Coefficient (95% CI) | p-value |
|
| ||||||
| Sex | 0.14 (−0.01–0.30) | 0.065 | 0.17 (0.01–0.32) | 0.035 | 0.25 (−0.34–0.85) | 0.401 |
| Female | ||||||
| Ocular Comorbidities | ||||||
| Myopia | 0.12 (−0.28–0.52) | 0.557 | −0.19 (−0.59–0.20) | 0.337 | −0.17 (−1.54–1.2) | 0.809 |
| Pseudophakia | ||||||
| 1.44 (0.80–2.10) | < 0.001 | 1.20 (0.63–1.78) | <0.001 | 0.63 (−0.22–1.48) | 0.149 | |
| Lattice Degeneration | ||||||
| −0.54 (−0.74 to −0.35) | < 0.001 | −0.36 (−0.55 to −0.16) | < 0.001 | −0.65 (−1.3–0) | 0.050 | |
| Vitreous Hemorrhage | ||||||
| −0.05 (−0.26 to −0.16) | 0.657 | −0.30 (−0.52 to −0.08) | 0.009 | 0.18 (−0.54–0.9) | 0.625 | |
| Age at Diagnosis | ||||||
| < 30 | 1.00 | 1.00 | 1.00 | |||
| 31–40 | ||||||
| 0.01 (−0.49–0.51) | −0.53 (−1.03 to −0.04) | 0.86 (−1.05–2.77) | ||||
| 41–50 | 0.965 | 0.034 | 0.379 | |||
| −1.36 (−1.76 to −0.96) | −2.03 (−2.43 to −1.63) | −1.19 (−2.7–0.33) | ||||
| 51–60 | ||||||
| < 0.001 | < 0.001 | 0.126 | ||||
| −2.32 (−2.69 to −1.95) | −2.76 (−3.13 to −2.39) | −2.37 (−3.76 to −0.98) | ||||
| > 60 | ||||||
| < 0.001 | < 0.001 | 0.001 | ||||
| −1.82 (−2.20 to −1.44) | −2.29 (−2.67 to −1.90) | −2.41 (−3.86 to −0.96) | ||||
| < 0.001 | < 0.001 | 0.001 | ||||
| Year of Diagnosis | 0.04 (0.00–0.07) | 0.03 | 0.01 (−0.03–0.04) | 0.755 | 0.74 (0.03–1.46) | 0.042 |
| Elixhauser | 0.15 (0.12–0.19) | <0.001 | 0.12 (0.08–0.15) | <0.001 | ||
| Comorbidities | ||||||
Adjusted analysis adjusts for: sex, ocular comorbidities, age at diagnosis, and year of diagnosis. Confidence interval (CI), Odds ratio (OR), Rhegmatogenous Retinal Detachment (RRD)
Sex Differences in the Type of Surgical Intervention for Retinal Detachments
The selection of surgical intervention for RRD differed between women and men (Table 4). Women were more likely to undergo primary laser barricade (RRR 1.68, p < 0.001), primary SB (RRR 1.15, p < 0.001), and PR (RRR 1.07, p < 0.04) than men compared to PPV after controlling for age, ocular comorbidities, year of diagnosis, and Elixhauser comorbidities. Increased age by decade reduced the relative risk ratio (RRR) of receiving surgical intervention with primary laser barricade or SBbut had an increased RRR of receipt of PR (RRR 2.28 for those >60 years of age, p < 0.001). The ocular comorbidities that may impact surgeon decision making in regards to surgical selection were consistent with the findings in the model: the diagnosis of myopia (RRR 1.3, p=0.003) and lattice degeneration (RRR 1.3, p < 0.001) increased the likelihood of SB while pseudophakia (RRR 0.15, <0.001) and vitreous hemorrhage (RRR 0.33, <0.001) reduced the likelihood of SB. Pseudophakia, lattice degeneration, and vitreous hemorrhage all reduced the RRR of primary repair with PR (all p-values < 0.001). Lattice degeneration (RRR 1.25, p < 0.001) and vitreous hemorrhage (RRR 1.37, p < 0.001) increased the likelihood of repair with primary laser barricade while pseudophakia reduced the risk (RRR 0.42, p < 0.001).
Table 4.
Multinomial Logistic Regression Model for Type of Retinal Detachment Repair Among All Patients with Repair within 30 Days of Diagnosis in the Truven MarketScan Database 2007–2015 (n = 38,537)
| Primary Scleral Buckle (n = 3,870) | Primary Pneumatic Retinopexy (n = 5,240) | Primary Laser Barricade (n = 7,044) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variables | *Adjusted RRR (95% CI) | p-value | *Adjusted RRR (95% CI) | p-value | *Adjusted RRR (95% CI) | p-value |
|
| ||||||
| Sex | ||||||
| Male | 1.00 | 1.00 | 1.00 | |||
| Female | ||||||
| 1.15 (1.07–1.23) | < 0.001 | 1.07 (1.00–1.14) | 0.036 | 1.68 (1.59–1.77) | < 0.001 | |
| Ocular Comorbidities | ||||||
| Myopia | 1.30 (1.10–1.53) | 0.003 | 1.10 (0.93–1.31) | 0.257 | 1.06 (0.92–1.23) | 0.398 |
| Pseudophakia | ||||||
| 0.15 (0.06–0.36) | < 0.001 | 0.58 (0.42–0.79) | 0.001 | 0.42 (0.31–0.58) | < 0.001 | |
| Lattice Degeneration | ||||||
| 1.30 (1.19–1.41) | < 0.001 | 0.75 (0.69–0.82) | < 0.001 | 1.25 (1.16–1.34) | < 0.001 | |
| Vitreous | ||||||
| 0.33 (0.29–0.39) | < 0.001 | 0.56 (0.51–0.62) | < 0.001 | 1.37 (1.27–1.47) | < 0.001 | |
| Hemorrhage | ||||||
| Age at Diagnosis | ||||||
| < 30 | 1.00 | 1.00 | 1.00 | |||
| 31–40 | ||||||
| 0.57 (0.48–0.68) | 1.67 (1.22–2.30) | 0.78 (0.66–0.92) | ||||
| 41–50 | < 0.001 | 0.002 | 0.003 | |||
| 0.27 (0.23–0.31) | 2.23 (1.71–2.91) | 0.39 (0.34–0.45) | ||||
| 51–60 | ||||||
| < 0.001 | < 0.001 | < 0.001 | ||||
| 0.19 (0.16–0.21) | 2.59 (2.00–3.35) | 0.43 (0.37–0.48) | ||||
| > 60 | ||||||
| < 0.001 | < 0.001 | < 0.001 | ||||
| 0.13 (0.11–0.15) | 2.28 (1.76–2.96) | 0.35 (0.31–0.4) | ||||
| < 0.001 | < 0.001 | < 0.001 | ||||
| Year of Diagnosis | 0.90 (0.88–0.91) | < 0.001 | 0.96 (0.95–0.98) | < 0.001 | 0.99 (0.97–1.00) | 0.025 |
| Elixhauser | 0.97 (0.95–0.99) | 0.001 | 0.96 (0.94–0.97) | <0.001 | 0.98 (0.97–1.00) | 0.015 |
| Comorbidities | ||||||
Pars plana vitrectomy (PPV) is the reference group. Adjusted analysis adjusts for: sex, ocular comorbidities, age at diagnosis, year of diagnosis, and Elixhauser comorbidities. Confidence interval (CI), Relative risk ratio (RRR).
Reoperation After Surgical Intervention for Incident RRD
The odds of reoperation within 30 days of index surgical intervention for primary RRD is shown in Table 5. For the overall cohort, the odds of reoperation are lower in women (OR 0.91, 95% CI 0.85 – 0.96, p=0.002) after adjustment. Women were less likely than men to undergo reoperation after primary PR (OR 0.7, 95% CI 0.62 – 0.79, p < 0.001). There was no statistically significant difference between women and men in regard to reoperation for beneficiaries undergoing primary SB (OR 0.97, 95% CI 0.8 – 1.17, p = 0.74) or PPV (OR 0.99, 95% CI 0.92 – 1.08, p = 0.86).
Table 5.
Multinomial Logistic Regression Model for Odd of Reoperation within 30 Days Following Retinal Detachment Repair in the Truven MarketScan Database 2007–2015
| Overall Reoperation Rate for All Surgeries (n = 30,322) | Primary Scleral Buckle (n = 3,765) | Primary Pneumatic Retinopexy (n = 5,185) | Primary Pneumatic Retinopexy (n = 5,185) | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Variables | *Adjusted OR (95% CI) | p-value | *Adjusted OR (95% CI) | p-value | *Adjusted OR (95% CI) | p-value | *Adjusted OR (95% CI) | p-value | |
|
| |||||||||
| Sex | |||||||||
| Male | 1.00 | 1.00 | 1.00 | 1.00 | |||||
| Female | |||||||||
| 0.91 (0.85–0.96) | 0.002 | 0.97 (0.8–1.17) | 0.741 | 0.7 (0.62–0.79) | < 0.001 | 0.99 (0.92–1.08) | 0.857 | ||
| Ocular Comorbidities | |||||||||
| Myopia | 1.12 (0.96–1.31) | 0.159 | 1.57 (1.1–2.26) | 0.014 | 1.18 (0.85–1.62) | 0.321 | 0.98 (0.79–1.22) | 0.886 | |
| Pseudophakia | |||||||||
| 1.11 (0.87–1.43) | 0.401 | 1 (0–0) | < 0.001 | 1.88 (1.03–3.42) | 0.038 | 1.11 (0.83–1.48) | 0.493 | ||
| Lattice Degeneration | |||||||||
| 0.97 (0.9–1.05) | 0.495 | 1.03 (0.83–1.28) | 0.788 | 1.13 (0.95–1.34) | 0.153 | 1.01 (0.91–1.12) | 0.909 | ||
| Vitreous Hemorrhage | |||||||||
| 1.28 (1.18–1.4) | < 0.001 | 2.26 (1.62–3.16) | < 0.001 | 1.81 (1.5–2.18) | < 0.001 | 1.27 (1.15–1.4) | < 0.001 | ||
| Age at Diagnosis | |||||||||
| < 30 | 1.00 | 1.00 | 1.00 | 1.00 | |||||
| 31–40 | |||||||||
| 1.05 (0.85–1.28) | 1.06 (0.73–1.55) | 1.17 (0.63–2.17) | 0.82 (0.62–1.07) | ||||||
| 41–50 | 0.664 | 0.747 | 0.618 | 0.143 | |||||
| 0.91 (0.77–1.07) | 1.02 (0.73–1.41) | 1.07 (0.64–1.81) | 0.55 (0.44–0.69) | ||||||
| 51–60 | |||||||||
| 0.265 | 0.913 | 0.793 | < 0.001 | ||||||
| 0.79 (0.68–0.92) | 0.98 (0.73–1.32) | 0.71 (0.43–1.18) | 0.5 (0.4–0.61) | ||||||
| > 60 | |||||||||
| 0.003 | 0.900 | 0.183 | < 0.001 | ||||||
| 0.82 (0.7–0.96) | 0.98 (0.7–1.38) | 0.69 (0.41–1.15) | 0.55 (0.44–0.67) | ||||||
| 0.014 | 0.918 | 0.157 | < 0.001 | ||||||
| Year of Diagnosis | 0.98 (0.97–0.99) | 0.003 | 0.94 (0.9–0.98) | 0.003 | 1 (0.97–1.03) | 0.972 | 0.99 (0.97–1) | 0.126 | |
| Elixhauser | 1.01 (1–1.03) | 0.078 | 1.05 (1–1.1) | 0.073 | 1.03 (1–1.06) | 0.023 | 1.01 (0.99–1.03) | 0.208 | |
| Comorbidities | |||||||||
Adjusted analysis adjusts for: sex, ocular comorbidities, age at diagnosis, year of diagnosis, and Elixhauser comorbidities. Confidence interval (CI), Odds ratio (OR).
Myopia(OR 1.57, 95% CI 1.1 – 2.26, p = 0.01) and vitreous hemorrhage (OR 2.26, 95% CI 1.62 – 3.16, p < 0.001)were associated with increased odds of reoperation for patients undergoing primary SB. Pseudophakia (OR 1.88, 95% CI 1.03 – 3.42, p = 0.04) and vitreous hemorrhage (OR 1.81, 95% CI 1.5 – 2.18, p = <0.001) were associated with increased odds of reoperation among patients undergoing primary PR. Vitreous hemorrhage (OR 1.27, 95% CI 1.15 – 1.4, p < 0.001) increased the odds of reoperation after primary PPV. Increased age (OR 0.55, 95% CI 0.44 – 0.67, p < 0.001 for 60+ years) was associated with a decreased odds of reoperation among patients who were treated with primary PPV.
DISCUSSION
We report the results of a large outpatient claims database analysis representing 133million U.S. commercially insured patients from all 50 states and find that women are less likely to receive surgical intervention for incident RRD. After adjusting for age, ocular comorbidities, year of diagnosis, and systemic comorbidities, women have 34% reduced odds of receipt of surgery for this vision-threatening emergent condition. The dataset is a nationally representative sample containing approximately one-third of all insured adults in the United States. If the odds of repair between women and men were equal, based on our results, we estimate781 more women would have surgery each year, or 7,029 more during the study period (using previously reported U.S. RRD incidence rates of 17.9 per 100,000 persons and the U.S. Census Bureau report from 2015 estimating 350 million insured working adults in the United States where this dataset would be applicable).24
Secondary outcomes of this study investigate differences between women and men who do undergo surgical repair of the RRD including the time to surgical intervention, type of repair, and reoperation rate. The time from diagnosis to repair is slightly longer for women, but this effect is less than one day in all models. Women are more likely to undergo repair with scleral buckle and laser barricade than men and women are less likely to undergo reoperation.
Disparities in the delivery of healthcare and outcomes between women and men are increasingly reported and of significant concern to our profession. These include delays in the diagnosis of life-threatening conditions such as myocardial infarction25,26, a reduced rate of emergent procedures26, an increased complication rate18,27, and increased morbidity and mortality13,25,27–29. Differences between women and men in the setting of life-threatening conditions is the most striking, but they have also been reported for other healthcare delivery and outcomes including prescription practices by physicians30and rates of elective surgical interventions31.
In ophthalmology, there is limited data available on the role of sex in retinal detachment management because it is usually controlled for in regression modeling. When one controls for a variable in a model, this suggests that the variable is a confounder of the relationship between the exposure and outcome. A nationwide registry review of primary RRD in Korea reported reduced odds of surgical intervention for women after controlling for available confounders.21A study on RRD repair trends over time that controlled for sex found reduced odds of repair for women in the final adjusted model.1 Another study from the United States population, that also controlled for sex in their modeling, focused on the relative impact of patient, physician, and geographic factors on variation in RRD management and secondarily reported a reduced odds of repair for women.22These prior studies focused on different aspects of RRD management and secondarily identified reduced odds of repair for women. This study represents the first to focus on the difference between women and men in the treatment of an emergent potentially blinding condition. We report a reduced rate of receipt of surgical intervention for primary RRD for women in the United States that persists in all sensitivity modeling and upon review of prior published U.S. epidemiologic RRD studies has been present all along.1,22
Why is there a difference in the receipt of retinal detachment repair between women and men in the United States? Any investigation of differences in healthcare utilization and outcomes between women and men warrants a review of the difference between sex and gender. The World Health Organization (WHO) defines sex as the biological and physiologic characteristics of males and females driven by their chromosomal composition while the term gender refers to a complex interplay of societally constructed roles and norms of men and women that affect relational, hierarchical, historical, contextual and institutional elements of behavior.32 Although sex is often inextricably connected to gender, it is not defined by it and both sex and gender can impact healthcare outcomes. In this study, the variable available for analysis was sex as self-reported by the patient during insurance claim registration.
Men and women may present with different types of RRDs. Several epidemiologic studies report a higher incident RRD rate among men for reasons that remain poorly understood.2–4,10,33Proposed hypotheses for this sex difference include an increased risk of trauma, a more traumatic posterior vitreous detachment with abnormal vitreoretinal adhesions, and increased axial length among men.4,34In this study 57% of incident RRDs occurred in men. Despite these possible biological differences in the incidence of RRD, there is a paucity of data on the difference between men and women’s type of retinal detachment that may influence timing and surgical selection. One study found no significant gender difference between macula on and off detachment status between women and men, which would likely be the most crucial element in the timing of retinal detachment repair.35In the present study, there was a statistically significant difference in time of repair with a delay for women, however, the effect was less than one day for most models and is thus likely not clinically significant. When all the records were taken together for any available follow-up time the delay increased to 2.95 days suggesting that women are more likely to undergo late repair of RRD or that there are other unmeasured confounders contributing to this late delay. When the time frame was restricted to the 30 or 90-day period the time delay for surgical intervention remained statistically significant, but was much less clinically significant representing less than a one day difference in repair.
Interestingly, in this study women were more likely to be repaired with laser barricade or scleral buckle compared to vitrectomy than men and their rate of reoperation was slightly lower. This suggests that women and men may present with different retinal detachments or ocular comorbidities that lead surgeons to choose one procedure over another with similar and even improved success. Indeed, women in this study had higher rates of myopia and lattice degeneration and these are both conditions that are more likely to be repaired with a scleral buckle or laser barricade for a low lying peripheral retinal detachment as can occur with myopia. Because of the nature of this data we are unable to know the location or visual outcomes of RRD that may explain the difference in surgical repair selection. The lower rate of reoperation observed may be related to biological factors or it could be due to better adherence with post-operative recommendations such as improved adherence with positioning, which has been reported.36It could also be that women are not returning for or being offered secondary procedures and that there is also a reduced rate of receipt of reoperation. These potential contributing factors cannot be assessed using claims data alone and require further study.
Although there may be biological differences in incident RRDs between women and men that explain the secondary outcomes of this study, the most notable finding cannot be explained by biological differences: women are not getting to the operating room at the same rate as men. This difference in receipt of repair is potentially related to gender. Women are more often caregivers for children, parents, spouses at every stage of their lives and this informal responsibility carries with it a significant physical and emotional burden.37 The stress of caregiving has been associated with an increased rate of depression, anxiety, burnout, and delays or avoidance of seeking medical care.37 Surveys of female cancer caregivers report delays in seeking interventional treatment for their own medical needs because of fear of complication that would limit their ability to care for their dependent family member.38 In RRD repair, where vision may be decreased for weeks due to a gas or oil tamponade, women may delay or not undergo surgery to preserve vision and fulfill their caretaker responsibilities. The caretaker role and fear of complications may also contribute to the differential surgical selection seen in this study as scleral buckle and laser barricade are less likely to have the same temporary visual reduction as a vitrectomy with tamponade.
In addition to their role as caretakers, women may be less likely to voice concerns or challenge authority, here the physician, related to the procedure or timing of surgery and this may result in “no-shows” on the date of surgery. Women also tend to outlive their husbands39 and are more likely to be widowed so they may not have the support to travel to the numerous appointments associated with surgery.40 These barriers could be addressed with interventions by the physician and organization if they were better understood. Female patients may benefit from a shared-decision making model that offers flexibility of scheduling, transportation, and prescription delivery services. Finally, provider bias cannot be excluded as a possibility for the difference in receipt of repair.
Strengths and Limitations
The strengths of this study derive from the size and representative coverage area of the dataset. The Truven MarketScan dataset represents all 50 states, all geographic regions, multiple insurance providers, and has longitudinal enrollee data that can be followed for the duration of coverage to track a beneficiary over time. 133 million records were queried and the study findings remained robust in all sensitivity models designed to reduce the contribution from miscoding. Confounding would only be expected to affect the outcomes of this study if the confounders were different between women and men and resulted in differential misclassification. Importantly, all patients in the dataset were insured reducing the concern that financial barriers limited a beneficiary’s ability to receive surgical repair of an RRD promptly.
The limitations of this study are those inherent to any administrative claims study. The analysis and resultsrely on the accuracy of data inputvia ICD and CPT coding where there is a possibility of some coding and billing errors. Sensitivity models were used to address this issue by requiring confirmation of RRD diagnosis, laterality, and both of the above. In the full cohort model, RRD diagnosis underwent repair in 63.1% of subjects which is seemingly low, but consistent with prior epidemiologic evaluations. When a secondary RRD diagnosis was required this increased to 93.1% suggesting this was the most accurate model and was used for cumulative reporting in this study. Laterality was missing in the vast majority of records and was used for sensitivity analysis purposes. With claims data we cannot determine why surgery was or was not performed from this data nor can we identify characteristics of the detachment that may contribute to surgical selection. We cannot determine if there is a difference in visual acuity in any of the groups because this information is not available in the database. Although the claims database is one of the largest in the United States, the beneficiaries represent only insured patients and cannot be applied to Medicare beneficiaries, or children. Further, uninsured patients are not represented and the results of this analysis may be different in this population where the financial burden of surgical repair may play a more important role in decision making for the patient. As women are more likely to be living below the poverty line and uninsured, the odds of receipt of repair may be even lower in this population. Finally, this dataset does not contain a variable for race or ethnicity and this is likely an important confounder when evaluating healthcare outcomes.
Conclusions and Implications
In summary, among 133 million records of insured adult beneficiaries from a broadly representative U.S. commercial claims database women were less likely to receive surgical intervention for RRD and intervention was more often delayed compared with their male counterparts. Among patients with RRD who received surgical repair, women were more likely to undergo repair with scleral buckle and laser barricade and were less likely to undergo reoperation. The reason for this difference between women and men remains poorly understood, but may be secondary to a complex and multifaceted interplay of access to care, societal gender roles, and possibly biological differences in types of RRDs. This study is the first to focus on differences in receipt of repair for a vision-threatening ophthalmic emergency. A better understanding of differences in retinal detachments between women and men and the barriers to the operating room requires further investigation.
Highlights.
Retinal detachment is a potentially blinding ophthalmic emergency that requires prompt intervention to prevent vision loss
Several fields in medicine report differences between women and men for surgical interventions and emergency treatment
This study queried one of the largest U.S. administrative claims databases and found that women have 34% reduced odds of receiving retinal detachment repair in the US and they are repaired with different methods than men
If the odds of women and men receiving repair for retinal detachment were equal then 781 more women would receive surgery each year
Acknowledgment:
The authors would like to thank Tanya Fijalkowski for her design of Figure 2 “Women Have Reduced Odds of Repair of Retinal Detachment” Infographic.
Funding/Support:
This work was supported by the Heed Ophthalmic Foundation and Michels Fellowship Foundation awarded to Natalia F. Callaway, MD, MS, Stanford Medical Scholars awarded to Daniel Vail, Vinit B. Mahajan, MD, Ph.D., is supported by NIH grants [R01EY026682, R01EY024665, R01EY025225, and R01EY024698] and the department by an unrestricted grant from Research to Prevent Blindness and NEI P30-EY026877.
Biography

Darius M. Moshfeghi, MD, is a Professor of Ophthalmology and Chief of Retina at the Byers Eye Institute, Stanford University School of Medicine. He founded the Stanford Vitreoretinal Surgery Fellowship and the Stanford University Network for Diagnosis of Retinopathy of Prematurity (SUNDROP). He is entrepreneurial and serves on the Board of Directors of 1800 Contacts and the American Academy of Ophthalmology Telemedicine Working Group. His works with ethical, innovative people to solve important ophthalmic problems.
Footnotes
Financial Disclosures: Natalia F. Callaway: no financial disclosures. Daniel Vail : no financial disclosure. Ahmad Al-Moujahed: no financial disclosure. Cassie Ludwig: no financial disclosure. Marco H. Ji: no financial disclosure. Vinit B. Mahajan: no financial disclosure. Suzann Pershing: Acumen Consultant. Darius M. Moshfeghi: 1–800 Contacts, Aldeyra Therapeutics, Apellis, CMEOutfitters.com, dStentz, Inc. Genetech, Grand Legened Technology, Linc, Pr3vent, Promisight, Pykus, Retina Technologies LLC, Visunex.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Reeves MG, Pershing S, Afshar AR. Choice of Primary Rhegmatogenous Retinal Detachment Repair Method in US Commercially Insur2ed and Medicare Advantage Patients, 2003–20216. Am J Ophthalmol. 2018;196:82–90. doi: 10.1016/j.ajo.2018.08.024 [DOI] [PubMed] [Google Scholar]
- 2.Van de Put MAJ, Hooymans JMM, Los LI, Dutch Rhegmatogenous Retinal Detachment Study G. The incidence of rhegmatogenous retinal detachment in The Netherlands. Ophthalmology. 2013;120(3):616–622. doi: 10.1016/j.ophtha.2012.09.001 [DOI] [PubMed] [Google Scholar]
- 3.Li X, Beijing Rhegmatogenous Retinal Detachment Study G. Incidence and epidemiological characteristics of rhegmatogenous retinal detachment in Beijing, China. Ophthalmology. 2003;110(12):2413–2417. doi: 10.1016/S0161-6420(03)00867-4 [DOI] [PubMed] [Google Scholar]
- 4.Bechrakis NE, Dimmer A. [Rhegmatogenous retinal detachment : Epidemiology and risk factors]. Ophthalmologe. 2018;115(2):163–178. doi: 10.1007/s00347-017-0647-z [DOI] [PubMed] [Google Scholar]
- 5.Mitry D, Charteris DG, Fleck BW, Campbell H, Singh J. The epidemiology of rhegmatogenous retinal detachment: geographical variation and clinical associations. Br J Ophthalmol. 2010;94(6):678–684. doi: 10.1136/bjo.2009.157727 [DOI] [PubMed] [Google Scholar]
- 6.Chen SN, Lian Ie B, Wei YJ. Epidemiology and clinical characteristics of rhegmatogenous retinal detachment in Taiwan. Br J Ophthalmol. 2016;100(9):1216–1220. doi: 10.1136/bjophthalmol-2015-307481 [DOI] [PubMed] [Google Scholar]
- 7.Park SJ, Choi NK, Park KH, Woo SJ. Five year nationwide incidence of rhegmatogenous retinal detachment requiring surgery in Korea. PLoS One. 2013;8(11):e80174. doi: 10.1371/journal.pone.0080174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim JD, Pham HH, Lai MM, Josephson JW, Minarcik JR, Von Fricken M. Effect of symptom duration on outcomes following vitrectomy repair of primary macula-off retinal detachments. Retina. 2013;33(9):1931–1937. doi: 10.1097/IAE.0b013e3182877a27 [DOI] [PubMed] [Google Scholar]
- 9.Mowatt L, Shun-Shin GA, Arora S, Price N. Macula off retinal detachments. How long can they wait before it is too late? Eur J Ophthalmol. 2005;15(1):109–117. doi: 10.1177/112067210501500117 [DOI] [PubMed] [Google Scholar]
- 10.Mitry D, Singh J, Yorston D, et al. The predisposing pathology and clinical characteristics in the Scottish retinal detachment study. Ophthalmology. 2011;118(7):1429–1434. [DOI] [PubMed] [Google Scholar]
- 11.Clark A, Morlet N, Ng JQ, Preen DB, Semmens JB. Whole population trends in complications of cataract surgery over 22 years in Western Australia. Ophthalmology. 2011;118(6):1055–1061. doi: 10.1016/j.ophtha.2010.11.001 [DOI] [PubMed] [Google Scholar]
- 12.Chandra A, Banerjee P, Davis D, Charteris D. Ethnic variation in rhegmatogenous retinal detachments. Eye (Lond). 2015;29(6):803–807. doi: 10.1038/eye.2015.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deery SE, Soden PA, Zettervall SL, et al. Sex differences in mortality and morbidity following repair of intact abdominal aortic aneurysms. J Vasc Surg. 2017;65(4):1006–1013. doi: 10.1016/j.jvs.2016.08.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Rango P, Simonte G, Manzone A, et al. Mortality Risk for Ruptured Abdominal Aortic Aneurysm in Women. Ann Vasc Surg. 2017;39:143–151. doi: 10.1016/j.avsg.2016.06.023 [DOI] [PubMed] [Google Scholar]
- 15.Wong SC, Yeo I, Bergman G, et al. The influence of gender on in-hospital clinical outcome following isolated mitral or aortic heart valve surgery. Cardiovasc Revasc Med. 2018. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-de-Andres A, Mendez-Bailon M, Perez-Farinos N, et al. Gender differences in incidence and in-hospital outcomes of surgical aortic valve replacement in Spain, 2001–15. Eur J Public Health. 2019. [DOI] [PubMed] [Google Scholar]
- 17.Bushnell C, Howard VJ, Lisabeth L, et al. Sex differences in the evaluation and treatment of acute ischaemic stroke. Lancet Neurol. 2018;17(7):641–650. doi: 10.1016/S1474-4422(18)30201-1 [DOI] [PubMed] [Google Scholar]
- 18.Dey S, Parthasarathi R, Sabnis SC, et al. Laparoscopic management of recurrent ventral hernia: an experience of 222 patients. Hernia. 2019. [DOI] [PubMed] [Google Scholar]
- 19.Lo RC, Bensley RP, Hamdan AD, et al. Gender differences in abdominal aortic aneurysm presentation, repair, and mortality in the Vascular Study Group of New England. J Vasc Surg. 2013;57(5):1261–1268, 1268 e1261–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Udell JA, Fonarow GC, Maddox TM, et al. Sustained sex-based treatment differences in acute coronary syndrome care: Insights from the American Heart Association Get With The Guidelines Coronary Artery Disease Registry. Clin Cardiol. 2018;41(6):758–768. doi: 10.1002/clc.22938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park SJ, Cho SC, Choi NK, Park KH, Woo SJ. AGE, SEX, AND TIME-SPECIFIC TRENDS IN SURGICAL APPROACHES FOR RHEGMATOGENOUS RETINAL DETACHMENT: A Nationwide, Population-Based Study Using the National Claim Registry. Retina. 2017;37(12):2326–2333. doi: 10.1097/IAE.0000000000001485 [DOI] [PubMed] [Google Scholar]
- 22.Vail D, Pershing S, Reeves MG, Afshar AR. The Relative Impact of Patient, Physician, and Geographic Factors on Variation in Primary Rhegmatogenous Retinal Detachment Management. Ophthalmology. 2019. [DOI] [PubMed] [Google Scholar]
- 23.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004 [DOI] [PubMed] [Google Scholar]
- 24.Rowe JA, Erie JC, Baratz KH, et al. Retinal detachment in Olmsted County, Minnesota, 1976 through 1995. Ophthalmology. 1999;106(1):154–159. doi: 10.1016/S0161-6420(99)90018-0 [DOI] [PubMed] [Google Scholar]
- 25.Lanz J, Wyss D, Raber L, et al. Mechanical complications in patients with ST-segment elevation myocardial infarction: A single centre experience. PLoS One. 2019;14(2):e0209502. doi: 10.1371/journal.pone.0209502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kosmidou I, Redfors B, Selker HP, et al. Infarct size, left ventricular function, and prognosis in women compared to men after primary percutaneous coronary intervention in ST-segment elevation myocardial infarction: results from an individual patient-level pooled analysis of 10 randomized trials. Eur Heart J. 2017;38(21):1656–1663. doi: 10.1093/eurheartj/ehx159 [DOI] [PubMed] [Google Scholar]
- 27.Wong SC, Yeo I, Bergman G, et al. The Influence of Gender on In-Hospital Clinical Outcome Following Isolated Mitral or Aortic Heart Valve Surgery. Cardiovasc Revasc Med. 2019;20(6):468–474. doi: 10.1016/j.carrev.2018.08.004 [DOI] [PubMed] [Google Scholar]
- 28.Nevidomskyte D, Shalhub S, Singh N, Farokhi E, Meissner MH. Influence of Gender on Abdominal Aortic Aneurysm Repair in the Community. Ann Vasc Surg. 2017;39:128–136. doi: 10.1016/j.avsg.2016.06.012 [DOI] [PubMed] [Google Scholar]
- 29.Boitano LT, Iannuzzi JC, Tanious A, et al. Predicting Postoperative Destination Through Preoperative Evaluation in Elective Open Aortic Aneurysm Repair. J Surg Res. 2019;235:543–550. doi: 10.1016/j.jss.2018.10.039 [DOI] [PubMed] [Google Scholar]
- 30.Khurshid S, Weng LC, Hulme OL, Ellinor PT, Lubitz SA. Factors Associated with Anticoagulation Delay Following New-Onset Atrial Fibrillation. Am J Cardiol. 2017;120(8):1316–1321. doi: 10.1016/j.amjcard.2017.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roh YH, Yun YH, Kim DJ, Nam M, Gong HS, Baek GH. Prognostic factors for the outcome of arthroscopic capsular repair of peripheral triangular fibrocartilage complex tears. Arch Orthop Trauma Surg. 2018;138(12):1741–1746. doi: 10.1007/s00402-018-2995-9 [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization Terms: Sex, Gender, Equity, and Human Rights. 2014; https://www.who.int/gender-equity-rights/understanding/gender-definition/en/. Accessed April 19, 2019.
- 33.Mitry D, Tuft S, McLeod D, Charteris DG. Laterality and gender imbalances in retinal detachment. Graefes Arch Clin Exp Ophthalmol. 2011;249(7):1109–1110. doi: 10.1007/s00417-010-1529-0 [DOI] [PubMed] [Google Scholar]
- 34.Mukhtar MA, Mehboob MA, Islam QU, Ishaq M. Comparison of Clinical Characteristics of Rhegmatogenous Retinal Detachment in Pseudophakic and Phakic Eyes. J Coll Physicians Surg Pak. 2017;27(5):288–291. [PubMed] [Google Scholar]
- 35.Potic J, Bergin C, Giacuzzo C, Daruich A, Konstantinidis L, Wolfensberger TJ. Primary rhegmatogenous retinal detachment: risk factors for macular involvement. Graefes Arch Clin Exp Ophthalmol. 2018;256(3):489–494. doi: 10.1007/s00417-017-3880-x [DOI] [PubMed] [Google Scholar]
- 36.Suzuki K, Shimada Y, Seno Y, Mizuguchi T, Tanikawa A, Horiguchi M. Adherence to the face-down positioning after vitrectomy and gas tamponade: a time series analysis. BMC Res Notes. 2018;11(1):142. doi: 10.1186/s13104-018-3257-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Macera CA, Eaker ED, Jannarone RJ, Davis DR, Stoskopf CH. A measure of perceived burden among caregivers. Eval Health Prof. 1993;16(2):205–211. doi: 10.1177/016327879301600205 [DOI] [PubMed] [Google Scholar]
- 38.Lund-Nielsen B, Midtgaard J, Rorth M, Gottrup F, Adamsen L. An avalanche of ignoring--a qualitative study of health care avoidance in women with malignant breast cancer wounds. Cancer Nurs. 2011;34(4):277–285. doi: 10.1097/NCC.0b013e3182025020 [DOI] [PubMed] [Google Scholar]
- 39.Life Expectancy at birth, at age 65, and at age 75, by sex, race, and Hispanic origin: United States, selected years 1900–2016: Centers for Disease Control and Prevention;2017. [Google Scholar]
- 40.Bodnar-Deren DCaS. Gender, Aging, and Widowhood. International Hadnbook of Population Aging: Spring Science; 2009. [Google Scholar]