Abstract
Practical relevance Primary hyperaldosteronism is probably the most common adrenocortical disorder in cats. As in humans, it is often unrecognised, which excludes a potentially large number of cats from appropriate treatment.
Patient group Affected cats present at a median age of 13 years (range 5–20 years). A breed or sex predilection has not been documented. The excessive secretion of mineralocorticoids usually leads to hypokalaemia and/or systemic arterial hypertension. Most affected cats present with muscular weakness and/or ocular signs of arterial hypertension.
Diagnostics In any cat presenting with hypokalaemia and/or arterial hypertension, other potential causes should be excluded. The ratio of plasma aldosterone concentration to plasma renin activity (aldosterone:renin ratio) is currently the best screening test for feline primary hyperaldosteronism. Diagnostic imaging is required to differentiate between adrenocortical neoplasia and bilateral hyperplasia, and to detect any distant metastases.
Clinical challenges The differentiation between adrenocortical neoplasia and bilateral hyperplasia is imperative for planning optimal therapy, but the limited sensitivity of diagnostic imaging may occasionally pose a problem. For confirmed unilateral primary hyperaldosteronism, unilateral adrenalectomy is the treatment of choice, and offers an excellent prognosis, but potentially fatal intra- and postoperative haemorrhage is a reported complication and risk factors have yet to be identified.
Evidence base Only a few case reports are available on which to base the optimal diagnostic and therapeutic approach to feline primary hyperaldosteronism. This article reviews the physiology of aldosterone production and the pathophysiology of primary hyperaldosteronism, and summarises the currently available literature on the feline disease. Practical suggestions are given for the diagnostic investigation of cats with suspected primary hyperaldosteronism.
Prevalence and potential barriers to diagnosis
Primary hyperaldosteronism, also referred to as primary aldosteronism or Conn's syndrome, is an adrenocortical disorder characterised by excessive, autonomous secretion of mineralocorticoids, mainly aldosterone, leading to systemic arterial hypertension and/or hypokalaemia. 1 After its first description in man in 1955, 2 Conn suggested that as many as 20% of people with arterial hypertension would have primary hyperaldosteronism. 3 Nevertheless, primary hyperaldosteronism was considered a very rare condition for several decades. 4 With improved screening tests, however, detection has increased and recent studies have shown that the prevalence of primary hyperaldosteronism is quite high indeed: it is found in about 6% of all human patients with arterial hypertension and up to 11% of those selected for therapy-resistant hypertension. 5,6
Primary hyperaldosteronism has also been reported in dogs 7–10 and, much more frequently, in cats. 11–23 It is probably the most common adrenocortical disorder in cats and it may be an important cause of arterial hypertension in this species, as it is in man.
In many cases of arterial hypertension and/or hypokalaemia, chronic renal disease may be wrongly presumed to be the causal disorder.
Although the cat is considered to be the domestic animal in which primary hyperaldosteronism is most prevalent, the disease is not often diagnosed in veterinary practice. It is most likely underdiagnosed, as it is in humans, which excludes a potentially large number of cats from appropriate therapy and possibly a cure for the disease. This may in part be due to the frequent association of arterial hypertension and/or hypokalaemia with chronic renal disease. In many cases of arterial hypertension and/or hypokalaemia, chronic renal disease may be considered the causal disorder, thereby halting further diagnostic efforts — whereas in fact the chronic renal failure may itself be a consequence of primary hyperaldosteronism. 20 Arterial hypertension and hypokalaemia are often treated symptomatically, without a thorough search for the underlying cause. Moreover, arterial blood pressure is not measured routinely, if at all, in many veterinary practices.
Aldosterone actions and metabolism
Aldosterone, or 4-pregnen-11β,21-diol-3,18,20-trione, is a steroid hormone with strong mineralocorticoid activity. It was historically considered to be a purely endocrine agent, produced exclusively in the adrenal cortex. However, recent research has revealed that aldosterone is also produced in tissues other than the adrenal cortex, including the heart, brain and blood vessels (for a review, see Connell et al). 24 In these extra-adrenal tissues aldosterone is thought to act in a paracrine or autocrine mode. Although these new insights contribute to the understanding of a number of long-term complications of primary hyper-aldosteronism, and will be discussed briefly, the main focus of this review is aldosterone as an endocrine agent.
Target organs and tissues
The epithelia of the kidneys, colon and salivary glands are the classic target tissues for circulating aldosterone. It readily passes through the plasma membrane of these epithelial cells and binds to the cytoplasmic mineralocorticoid receptor. Although this receptor has equal affinity for aldosterone and cortisol, and the circulating concentration of cortisol is much higher than that of aldosterone (see box, right), the mineralocorticoid receptor in the classic aldosterone target tissues is preferentially made available to aldosterone by the enzyme 11β-hydroxy-steroid dehydrogenase type 2 (11β-HSD2). This enzyme converts cortisol to cortisone, which has little affinity for the receptor.
The aldosterone—receptor complex is translocated to the nucleus, where it modulates the expression of multiple genes. In epithelial cells of the distal nephron this ultimately results in activation of amiloride-sensitive sodium channels in the apical membrane. The resulting increased sodium influx stimulates the Na+,K+-ATPase in the basolateral membrane. The aldosterone-mediated increase in active sodium reabsorption from the urine generates an electrochemical gradient that facilitates the passive transfer of potassium from the tubular cells into the urine. Thus potassium is not excreted in direct exchange for sodium, but in a manner depending directly on the active reabsorption of sodium (for a review, see Galac et al). 1
In addition to its endocrine effects on classic epithelial target tissues, aldosterone has major effects on other epithelial and non-epithelial tissues. Actions of aldosterone, probably in part non-genomic, on endothelial cells and on cardiac tissue contribute to blood pressure homeostasis (see Connell et al). 24 It appears that aldosterone may increase blood pressure through two main mechanisms: (1) expansion of plasma and extracellular fluid volume; and (2) increased total peripheral resistance. With regard to the non-epithelial actions, long-term mineralocorticoid excess may lead to microangiopathies, with fibrosis and proliferation of endothelial and smooth muscle cells, in tissues such as heart and kidney. 25
Aldosterone biosynthesis.
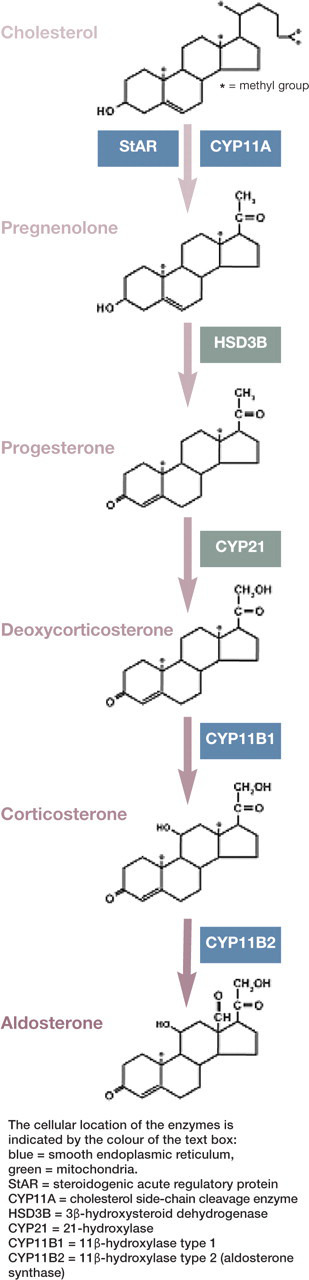
Aldosterone is produced in the zona glomerulosa of the adrenal gland, the outermost zone of the cortex. Unlike cells of the other two zones — the middle zona fasciculata and the inner zona reticularis — glomerulosa cells contain the enzyme aldosterone synthase (CYP11B2), which catalyses the final step in the conversion of cholesterol to aldosterone. Zona glomerulosa cells produce aldosterone ‘on demand', for which purpose they contain cholesterol esters, mainly originating from circulating low-density lipoproteins. On stimulation of aldosterone production, cholesterol esters are rapidly converted to cholesterol and then, in five steps, to aldosterone, which is released into the circulation. As there is no specific binding globulin for aldosterone in the plasma, it is mainly bound with low affinity to albumin. This explains its relatively low circulating concentration in comparison with cortisol.
The lack of a specific binding globulin for aldosterone in the plasma explains its relatively low circulating concentration in comparison with cortisol.
Metabolism
Little is known about the metabolism of aldosterone in cats. The liver is generally considered to be the most important site for inactivation and conjugation of steroid hormones. In cats, cortisol, oestradiol and progesterone are excreted mainly or almost exclusively via the bile into the faeces; 26,27 given the structural similarities, it can be expected that this is also the main excretion route for aldosterone. This assumption is supported by a study of Syme et al, who found that urinary excretion of free aldosterone in cats was 77 times less than in humans and seven times less than in dogs, accompanied by insignificant amounts of aldosterone-18-glucuronide. 28
Pathophysiology
Two pathophysiological mechanisms may lead to hypersecretion of aldosterone. In one, a reduction in the effective arterial blood volume (due, for example, to heart failure or oedema caused by hypoproteinaemia) activates the renin—angiotensin system, which in turn persistently stimulates aldosterone synthesis. This pathophysiological response to hypovolaemia is called secondary hyperaldosteronism or high-renin hyperaldosteronism.
In contrast, aldosterone secretion in primary hyperaldosteronism is associated with suppressed plasma renin activity — and is thus low-renin hyperaldosteronism. Primary hyperaldosteronism is due to autonomous hypersecretion of aldosterone by neoplastic or non-neoplastic zona glomerulosa tissue.
Regulation of aldosterone secretion.
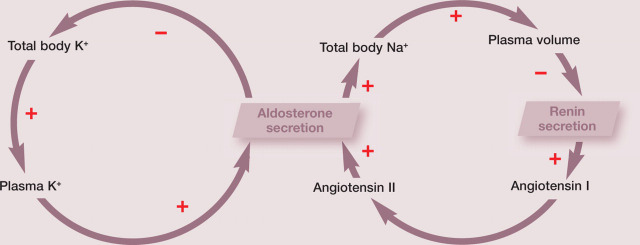
The two primary regulators of aldosterone release are the renin-angiotensin system and potassium. The renin-angiotensin system maintains a constant circulating blood volume by promoting aldosterone-induced sodium retention during periods of hypovolaemia and by decreasing aldosterone-dependent sodium retention during hypervolaemia. Potassium ions directly regulate aldosterone secretion, independent of the renin-angiotensin system. Hyperkalaemia stimulates aldosterone secretion by depolarising (and hypokalaemia inhibits it by repolarising) the membranes of the zona glomerulosa cells. 29 Thus aldosterone secretion is regulated via negative feedback loops for both potassium and the renin-angiotensin system. In addition to these two regulatory mechanisms, aldosterone secretion is influenced by several other factors (adrenocorticotrophic hormone, natriuretic peptides and a variety of neurotransmitters), none of which has direct or indirect negative feedback loops on aldosterone secretion.
The vast majority of the physiological actions of the renin-angiotensin system, such as vasoconstriction and aldosterone production, are mediated by angiotensin II and one of its receptors (AT1R). Angiotensinogen, mainly produced in the liver, is the precursor of several angiotensin peptides. In the circulation, angiotensinogen is cleaved by renin and other enzymes to release angiotensin I. Angiotensin-converting enzyme (ACE) converts the inactive decapeptide angiotensin I to the active octapeptide angiotensin II. ACE-inhibiting compounds are used clinically to disrupt the renin-angiotensin system, as in the treatment of heart failure.
The proteolytic enzyme renin is synthesised in the juxtaglomerular cells of the kidney. Stimulation of renal baroreceptors is the most potent mechanism for its release. These stretch receptors in the afferent arteriole stimulate renin release in response to reduced renal perfusion pressure. Additional regulation is provided by the macula densa, a group of modified cells of the distal tubule near the end of the loop of Henle and intimately associated with the juxtaglomerular cells. Sodium concentration in the tubular lumen is monitored by the cells of the macula densa; low sodium levels trigger communication between the macula densa and the juxtaglomerular cells, resulting in renin release (for a review, see Galac et al). 1
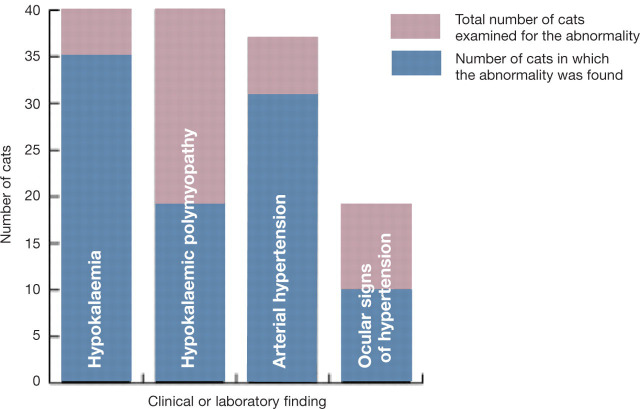
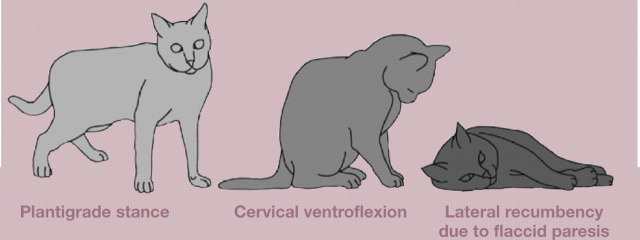
The pathophysiological consequences of excessive aldosterone secretion are related to increased sodium and water retention, and increased renal potassium excretion, which result in systemic arterial hypertension and potassium depletion, respectively. The progressive depletion of potassium and the development of hypokalaemia affect several organ systems, but become particularly manifest in the neuromuscular system by affecting the polarisation of nerve and muscle membranes. Muscle weakness is likely to occur at plasma potassium concentrations of around 2.5 mmol/l, although the severity of muscle weakness is not strictly correlated with the plasma potassium concentration. Affected cats have episodic muscle weakness that results in a plantigrade stance in the hindlimbs, difficulty in jumping and/or a characteristic ventroflexion of the neck; in some cases there is progression to flaccid paresis with hypo-reflexia, muscle hypotonia and difficulty in breathing. In other cats the presenting physical features are dominated by signs of arterial hypertension (ie, loss of vision due to retinal detachment and/or intraocular haemorrhages) (Fig 2). It is important to note that not all cats with primary hyperaldosteronism present with signs of hypokalemia or with signs of arterial hypertension (Fig 3).
FIG 2.
Blindness and muscular weakness in a cat with hypokalaemia associated with primary hyperaldosteronism. The plasma aldosterone concentration was 550 pmol/l, plasma renin activity was 45 fmol/l/sec, and the aldosterone:renin ratio was 12.2. Abdominal ultrasonography revealed an enlarged right adrenal gland
FIG 3.
Four of the clinical and laboratory findings in 40 cats with presumed or confirmed primary hyperaldosteronism
Clinical signs.
Muscle weakness
Muscle weakness is likely to occur at plasma potassium concentrations ~2.5 mmol/l, although the severity of muscle weakness is not strictly correlated with the plasma potassium concentration. Signs of muscle weakness include episodic or acute generalised weakness, a plantigrade stance of the hindlimbs, inability to raise the head (cervical ventroflexion) or to jump, lateral recumbency and collapse.
Ocular complications of arterial hypertension
Ocular complications cause the most striking (and thus usually the presenting) signs of arterial hypertension. They include mydriasis, hyphaema, and loss of vision due to retinal detachment and/or intraocular haemorrhages.
Other, less specific signs
Not all cats with primary hyperaldosteronism present with signs of hypokalaemia or arterial hypertension. Some present with a pendulous abdomen; with polyuria, nocturia and polydipsia; or with rather nonspecific signs such as anorexia, weight loss, depression, restlessness or panting. Physical examination may reveal a palpable mass in the cranial abdomen, pronounced muscle atrophy, cutaneous fragility (especially in the case of combined hyperaldosteronism and hyperprogesteronism), a systolic heart murmur and an irregular cardiac rhythm.
Primary hyperaldosteronism, especially when due to micronodular hyperplasia of the zona glomerulosa, has been associated with cardiovascular and renal complications in both humans 24 and cats. 20 It has been hypothesised that the mild hyperaldosteronism with incomplete renin suppression associated with micronodular hyperplasia of the zona glomerulosa results in the combined deleterious, proinflammatory and profibrotic effects of elevated aldosterone and angiotensin II levels.
Primary hyperaldosteronism in cats — what do we know?
Histopathological diagnosis
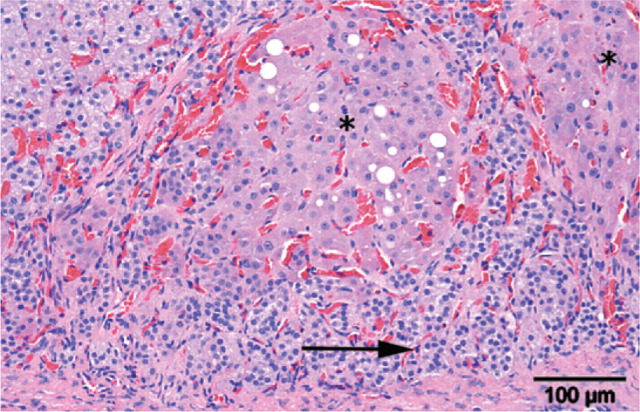
Mineralocorticoid excess in cats mainly occurs in middle and old age. It is caused either by idiopathic bilateral nodular hyperplasia of the zona glomerulosa (Fig 4), in which case plasma renin activity may be incompletely suppressed, or by unilateral or bilateral neoplasia of the zona glomerulosa (Fig 5), usually with complete suppression of renin. 11–13,16–21,23 Histopathological findings indicate that adrenocortical carcinoma (13 reported cases) occurs more often than unilateral (seven cases) and bilateral (two cases) adenoma or bilateral nodular hyperplasia (three cases). These reported figures appear to differ markedly from those in humans, where bilateral hyperplasia of the zona glomerulosa accounts for 60–65% of cases and aldosterone-producing adenomas for 30–35%, unilateral hyperplasia being uncommon and functional adrenocortical carcinomas being rare. 30
FIG 4.
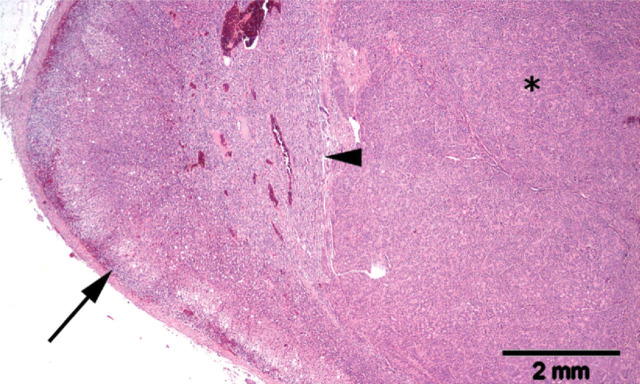
Adrenal of a hypokalaemic cat with multiple cortical hyperplastic nodules (asterisks). Note the pre-existing zona glomerulosa of the adrenal gland (arrow). Haematoxylin and eosin stain
FIG 5.
Histological section of an expansile neoplasm (asterisk) of the adrenal cortex in a cat with primary hyperaldosteronism. Note the compression of the pre-existing adrenal medulla (arrowhead). There is no marked atrophy in the uncompressed areas of the pre-existing adrenal cortex (arrow). Haematoxylin and eosin stain
However, whereas the diagnosis of idiopathic primary hyperaldosteronism can be established clinically in humans, in cats histopathological examination of the adrenal glands is required — and because cats with hyperplasia of the zona glomerulosa are often treated medically, adrenal tissue is not examined histologically, except post mortem. This probably means that idiopathic bilateral nodular hyperplasia of the zona glomerulosa occurs more frequently in cats than suggested by data based on histopathological findings. In line with this supposition, Javadi et al reported 11 cats with primary hyperaldosteronism in which diagnostic imaging failed to demonstrate an adrenal tumour, suggesting that the cause was adrenocortical hyperplasia. 20
Signalment
To date, about 40 cases of presumed or confirmed feline primary hyperaldosteronism have been reported. 11–23 Affected cats were presented at a median age of 13 years (mean 12.4 years; range 5–20 years; n = 34; in the remaining cases the age was not specified). There has been no apparent sex predilection and the breeds have included domestic shorthair (24 cats), domestic longhair (three cats), British shorthair (two cats), and Siamese, Burmese, Burmilla, Tonkinese and Persian (one each).
Physical examination findings
Abnormal physical findings have been mainly related to systemic arterial hypertension and potassium depletion, including elevated arterial blood pressure (31/37 cats in which it was measured); hypertensive ocular signs including intraocular haemorrhage, increased tortuosity of the retinal vessels, retinal oedema and retinal detachment (10/19 cats in which an ophthalmic examination was performed); and hypokalaemic polymyopathy (19 cats) (Fig 3). Other findings included a palpable mass in the cranial abdomen (three cats), pronounced muscle atrophy, cutaneous fragility in two cases of combined hyperaldosteronism and hyperprogesteronism, a systolic heart murmur and an irregular cardiac rhythm. Congestive heart failure was diagnosed in one cat with combined primary hyperaldosteronism and hyperprogesteronism. In two cases no abnormalities were found on initial clinical examination.
Laboratory abnormalities
Abnormalities found on routine laboratory testing included hypokalaemia (35/40 cats; Fig 3), elevations in plasma urea (17/29 cats), creatinine (14/27 cats) and creatinine kinase (15/16 cats), hyperglycaemia (4/4 cats in which blood glucose was specified), hypomagnesaemia (2/2 cats in which blood magnesium was specified), hypochloraemia (3/3 cats in which chloride was specified), hypophosphataemia (2/12 cats) and hyperphosphataemia (1/12 cats). Among all 31 hypertensive cats, 27 were concurrently hypokalaemic and four were normokalaemic, although the plasma potassium concentration in one was at the lower end of the reference interval. Hypernatraemia, combined with hypokalaemia, was found in only one of the 17 cats in which plasma sodium was measured. Plasma progesterone was elevated in two cats and was associated with diabetes mellitus in both.
Plasma aldosterone concentration (PAC) was increased in 33 of 40 reported cases and plasma renin activity was below or within the reference interval in all 22 cats in which it was measured. Although the PAC was within the reference interval in the remaining seven of the 40 cats (in two of which even plasma renin activity was within the reference interval), the aldosterone:renin ratio was elevated in all seven.
Primary hyperaldosteronism should be considered in any cat with elevated arterial blood pressure and/or hypokalaemia, particularly if hypertension and/or hypokalaemia are relatively refractory to treatment…
Investigation of suspected primary hyperaldosteronism
Primary hyperaldosteronism should be considered in any cat found to have an elevated arterial blood pressure and/or hypokalaemia; in particular, if hypertension and/or hypokalaemia are relatively refractory to treatment. Indirect measurement of arterial pressure should be performed using a Doppler or oscillometric technique, and interpreted according to the ACVIM consensus statement on arterial hypertension in dogs and cats. 31 Routine laboratory investigation ideally includes urinalysis and measurement of plasma sodium, potassium, urea, creatinine, glucose, fructosamine, calcium, phosphate and thyroxine.
If, based on the history, physical examination and laboratory results, primary hyper-aldosteronism is considered likely, a screening test for abnormal regulation of aldosterone production should be performed. If regulation is abnormal, the diagnostic investigation should ideally include a test to confirm the diagnosis, diagnostic imaging of the adrenal glands and of predilection sites for metastases, and determination of whether the left or right adrenal is the site of abnormal aldosterone production. Determination of the laterality is necessary for planning treatment, since unilateral primary hyperaldosteronism due to an adrenocortical adenoma or adenocarcinoma can potentially be cured surgically, whereas bilateral primary hyperaldosteronism due to hyperplasia of the zona glomerulosa or a metastasised adrenocortical adenocarcinoma would be controlled medically.
Screening tests
Aldosterone:renin ratio
In both human and feline patients, the ratio of plasma aldosterone concentration (PAC) to plasma renin activity (PRA) (the aldosterone:renin ratio [ARR]) is used to screen for abnormal regulation of aldosterone production. 20,32,33 In cats with unilateral or bilateral zona glomerulosa tumours, the PAC may be very high and PRA is usually completely suppressed. In cats with idiopathic bilateral nodular hyperplasia of the zona glomerulosa, the PAC may be only slightly elevated or within the upper limit of the reference interval. As hypokalaemia is the predominant factor lowering the PAC, the plasma potassium concentration should be considered in evaluating the PAC. In the presence of hypokalaemia, even a mildly elevated aldosterone level can be regarded as inappropriately high. The PRA must also be taken into account. The combination of a high—normal or elevated PAC and a low PRA indicates persistent aldosterone synthesis in the presence of little or no stimulation by the renin—angiotensin system.
… But not all cats with primary hyperaldosteronism present with signs of hypokalaemia, or arterial hypertension.
The diagnostic value of the ARR is mainly determined by the sensitivity of the renin assay. In primary hyperaldosteronism, PRA is low, and therefore a relatively high renin detection limit can have a profound effect on the ARR. 34,35 Furthermore, PRA values should be interpreted in comparison to an appropriate control population. The accuracy of the ARR also depends on preservation of renin activity during sample collection and storage: blood samples should be collected in ice-chilled tubes and centrifuged in a chilled centrifuge, and the plasma should be frozen immediately and kept frozen until assayed.
Although the ARR is currently the gold standard for screening for feline primary hyperaldosteronism, it has some disadvantages. These include the necessity for a large (4 ml) blood sample and instant freezing of the separated plasma. Furthermore, PRA measurements are time consuming and reference values for PRA may differ markedly between laboratories, making comparison difficult. Finally, repeated sampling for the ARR may be required, as a single ARR within the reference interval does not exclude primary hyperaldosteronism in cats. 20
Aldosterone:creatinine ratio
An alternative means of diagnosis is measurement of the urinary aldosterone:creatinine ratio (UACR). Cats excrete smaller quantities of aldosterone and its 18-glucuronidated metabolite in the urine than do humans or dogs, 28 but the UACR can nevertheless be determined. 36 It provides an integrated reflection of aldosterone secretion over time. It has the advantage that the urine sample for measurement of aldosterone does not have to be frozen immediately and can be collected quite easily. Unfortunately, the reference interval for the UACR proved to be quite wide and did not facilitate differentiation between healthy cats and those with primary hyper-aldosteronism. 36 Therefore, for the time being, the ARR remains an indispensable part of the diagnostic protocol for feline primary hyper-aldosteronism.
Confirmatory tests
Several tests have been developed in humans to determine whether an elevated ARR is due to primary hyperaldosteronism. These include a captopril stimulation test, a fludrocortisone suppression test, a saline infusion test and an oral sodium loading test.30,35,37–39 No validated test is available to confirm primary hyperaldosteronism in cats. A test employing a suppressive agent that reduces the UACR in healthy cats but has little or no effect in those with primary hyperaldosteronism would seem to be the best means of showing the presence of a hyperfunction of zona glomerulosa tissue. In a recent study, the oral administration of sodium chloride (0.25 g/kg body weight q12h for 4 consecutive days) did not significantly lower the UACR in 22 healthy cats, but oral administration of fludrocortisone acetate (0.05 mg/kg body weight q12h for 4 consecutive days) reduced the UACR by 44–97% (median 78%) in 15 healthy cats. 36 In a cat with an aldosterone-producing adrenocortical carcinoma, the UACR was within the reference interval and was not lowered by fludrocortisone administration. Hence, further investigation is needed if the oral fludrocortisone suppression test is to be useful in diagnosing idiopathic hyperaldosteronism in cats.
Determining the laterality of hyperaldosteronism.
Adrenal vein sampling was introduced in human medicine in the late 1960s and, despite potentially severe complications, it has become the gold standard for determining the laterality (left or right adrenal) of excessive aldosterone production in humans. 43 Each adrenal vein is cannulated in turn and samples are collected while peripheral venous blood samples are collected simultaneously. 44 The plasma aldosterone and cortisol levels in the adrenal and peripheral venous samples are compared to detect the source of excess aldosterone. Unfortunately, the much smaller vascular dimensions in cats preclude adrenal venous sampling and thus determination of the laterality of primary hyperaldosteronism continues to rely on diagnostic imaging — despite the limitations (see below).
Diagnostic imaging.
Diagnostic imaging techniques such as ultrasonography, magnetic resonance imaging (MRI) and computed tomography (CT) are used to identify adrenal abnormalities and, in the case of neoplasia, to evaluate possible extension into the caudal vena cava and the presence of distant metastases. While the presence of visible tumour in the caudal vena cava indicates that surgical removal may be difficult, failure to detect it by diagnostic imaging is no guarantee of its absence and does not necessarily predict an uncomplicated adrenalectomy. 18,19
This is not the only limitation of conventional diagnostic imaging for determining the optimal treatment strategy for primary hyperaldosteronism. Functional neoplasms of the zona glomerulosa need not be large in order to cause clinically relevant hyperaldosteronism, and may therefore be well below the detection limit of ultrasonography, CT or MRI. Similarly, clinically relevant hyperplasia of the zona glomerulosa may not be revealed by conventional diagnostic imaging techniques. Conversely, nonfunctional adrenocortical neoplasms may grow to considerable proportions and be readily visualised with ultrasound, CT or MRI, but may not cause clinical signs. Therefore, a visible adrenal mass may not be a functional neoplasm of the zona glomerulosa that is causing the clinical signs of primary hyperaldosteronism; and if surgery is planned on the basis of conventional diagnostic imaging alone, the wrong adrenal gland may be removed or the patient may be inappropriately selected for or excluded from adrenalectomy.
Comparing the results of morphological diagnostic imaging techniques (CT and MRI) with adrenal venous sampling in human patients with primary hyperaldosteronism, Kempers et al concluded that CT/MRI results did not accurately identify the source of aldosterone excess in as many as 38% of 950 patients. 40 Although the number of documented cases of primary hyperaldosteronism in cats is small, four instances of inaccurate adrenal diagnostic imaging results have been described. One cat, in which ultrasonography revealed a right adrenal mass and a normal left adrenal gland, was found by postmortem examination 13 days later to have bilateral adrenocortical adenoma. 18 The left adrenal of another cat was removed because ultrasonography and CT indicated asymmetrical thickening, but bilateral nodular hyperplasia of the zona glomerulosa was found. 20 In two other cats ultrasonography and CT revealed no abnormality of the adrenals but nodular hyperplasia was confirmed by histological examination. 20 Distant metastases of adrenocortical adenocarcinomas may be missed on diagnostic images if their size is below the detection limit of the technique. This has been documented in one case, in which thoracic radiography failed to reveal pulmonary metastases 3 mm in diameter. 17
Nuclear medicine imaging of the adrenal glands is a relatively new technique in human endocrinology. Both 11C-metomidate positron emission tomography (PET) scanning and 131I-6β-iodomethyl-19-norcholesterol single photon emission computed tomography (SPECT) have proved useful. 41,42 It is expected that these techniques will eventually become available in veterinary medicine and will prove to be valuable in the diagnosis of feline primary hyperaldosteronism.
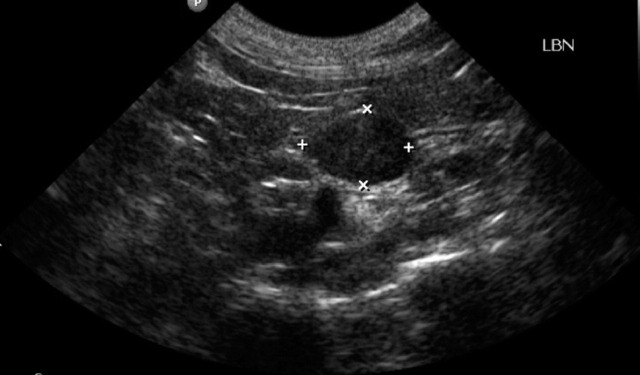
Ultrasonographic image of the left adrenal gland of a cat with primary hyperaldosteronism. The gland appears to be enlarged and hyperechogenic
Treatment and prognosis
Adrenalectomy
Unilateral adrenalectomy is the treatment of choice for confirmed unilateral primary hyperaldosteronism. There have been several reports of successful surgical intervention,12,13,18,23 including the excision of an adrenocortical carcinoma together with its extension into the vena cava. 21
Preoperatively and perioperatively hypokalaemia should be controlled as well as possible by oral and intravenous supplementation. During the first few weeks after surgery a generous dietary intake of sodium can be provided to avoid hyperkalaemia resulting from the chronic suppression of aldosterone secretion in the contralateral adrenal. Temporary administration of fludrocortisone could also be considered, but was not found necessary in the cases that have been reported.
After complete removal of a unilateral non-metastasised mineralocorticoid-producing tumour, the prognosis is excellent, with no medication required in most cases. Most of the cats that survived the immediate postoperative period have continued to be clinically asymptomatic for one to several years. However, perioperative complications were reported in eight of 17 surgical cases. 13,17–19 They included intraoperative or postoperative intra-abdominal haemorrhage (six cases), acute renal failure (one), sepsis (one) and suspected thromboembolism (one). In six cases the outcome was fatal. Furthermore, arterial hypotension and hypoglycaemia occurred in a cat recovering from unilateral adrenalectomy and venous thrombectomy under deliberate hypothermia.
Whereas postoperative sepsis and thromboembolism have also been described in cats following adrenalectomy for other primary conditions, intra- and postoperative haemorrhages have been reported more frequently in association with adrenalectomy for primary hyperaldosteronism. 13,17,18 Risk factors have not yet been identified. Haemorrhage was not specifically related to the type of neoplasia, intravenous tumour extension, or the presence or absence of arterial hypertension as a presenting clinical sign. Therefore, all owners considering surgical management of primary hyperaldosteronism in their cat should be informed of this potential complication.
Medical therapy
If surgery is precluded because of bilateral zona glomerulosa hyperfunction, a non-resectable unilateral adrenocortical neoplasm, distant metastases, financial limitations or comorbid conditions, medical therapy with a mineralocorticoid receptor blocker is instituted, together with potassium supplementation and antihypertensive drugs if needed. The aldosterone receptor blocker most often used in cats is spironolactone. The initial dose is 2 mg/kg body weight orally q12h, increased as needed to control hypokalaemia. A dose in excess of 4 mg/kg may cause anorexia, diarrhoea and vomiting. Persistent arterial hypertension can be treated with the calcium blocker amlodipine, at an initial oral dose of 0.1 mg/kg body weight q24h.
In cats, hyperaldosteronism due to bilateral adrenocortical hyperplasia is usually somewhat milder than that due to neoplasia and normokalaemia may be sustained for long intervals with spironolactone alone or combined with low doses of potassium. 20 However, the prognosis may not be as favourable as that after complete removal of an aldosterone-producing neoplasm, as medical treatment does not abolish the mineralocorticoid excess as definitively as surgery may do.
KEY POINTS.
Primary hyperaldosteronism is probably the most common adrenocortical disorder in cats and is caused by either idiopathic bilateral nodular hyperplasia of the zona glomerulosa or unilateral or bilateral adenoma or adenocarcinoma of the zona glomerulosa.
Presenting physical features are usually — but not always — dominated by signs of hypokalaemia (ie, muscle weakness) and/or signs of arterial hypertension (ie, loss of vision due to retinal detachment and/or intraocular haemorrhages).
The ratio of plasma aldosterone concentration to plasma renin activity (aldosterone:renin ratio) is the best screening test for feline primary hyperaldosteronism. Diagnostic imaging is required to differentiate between adrenocortical neoplasia and bilateral hyperplasia.
Differentiation between unilateral and bilateral hyperaldosteronism and detection of any vascular extension or distant metastases are essential to determine the optimal therapeutic strategy.
For bilateral zona glomerulosa hyperplasia, non-resectable unilateral adrenocortical neoplasia or metastases, medical treatment is possible using a mineralocorticoid receptor blocker together with supplemental potassium and antihypertensive drugs, if needed.
Unilateral adrenalectomy is the treatment of choice for confirmed unilateral primary hyperaldosteronism. Haemorrhage from the caudal vena cava is the most commonly reported, and a potentially lethal, intra- and postoperative complication. However, after complete removal of a unilateral non-metastasised aldosterone-producing neoplasm without complications, the prognosis is excellent without medication in most cases.
Case notes.
Julia, an approximately 8-year-old, neutered female domestic shorthair cat, presented for a head tilt to the right and blepharospasm and hyphaema of the left eye.
Presenting signs The owners noticed a blood clot in the anterior chamber of Julia's left eye, retraction of the globe and squinting.

History Julia had been a stray cat, and was already blind and had a large scar in the left cornea when she entered the animal shelter 7 months earlier. She developed ocular discomfort a month after being adopted by her new owners, prompting referral for an ophthalmic examination.
Physical and ophthalmic examination There was phthisis bulbi of the left eye. Extensive corneal scarring limited examination to the periphery of the anterior chamber, which appeared shallow and contained a small blood clot.
The pupil of the right eye was in full mydriasis and no pupillary reflexes could be elicited. Advanced retinal atrophy was evidenced by generalised hyperreflectivity of the tapetal fundus, a markedly reduced diameter of the retinal blood vessels, and a dark optic disc. Multiple small areas of pronounced narrowing gave the retinal arterioles a beaded appearance. There were two small haemorrhages along the peripheral dorsal vessels.
Thoracic auscultation revealed a gallop rhythm and a systolic murmur with 2/6 intensity at the level of the left atrial valves. Oscillometric measurement of arterial blood pressure revealed an average systolic pressure of 183 mmHg (reference <160 mmHg).
Laboratory findings Analysis revealed plasma urea 16.2 mmol/l (reference interval [RI] 6.1–12.8), plasma creatinine 178 μmol/l (RI 76–164), plasma sodium 155 mmol/l (RI 156–240) and plasma potassium 2.4 mmol/l (RI 3.4–5.2). Values for haematocrit, serum total protein and albumin, and plasma glucose, fructosamine, calcium, phosphate and free T4 were unremarkable, as were the results of urinalysis. Plasma aldosterone was 780 pmol/l (RI 110–540) and plasma renin activity was <40 fmol/l/sec (RI 60–630).
WHAT IS YOUR ASSESSMENT?
1 How should the laboratory findings be interpreted?
-
(a)
The elevated plasma urea and creatinine concentrations indicate chronic primary renal disease, with secondary hyperaldosteronism and hypokalaemia as a consequence.
-
(b)
The combination of an elevated plasma aldosterone concentration, hypokalaemia and suppressed plasma renin activity points towards primary hyperaldosteronism.
-
(c)
Although plasma potassium concentration is markedly decreased, plasma sodium concentration is also slightly decreased and, therefore, either primary or secondary hyperaldosteronism can be ruled out.
2 Which therapy would you preferably prescribe if the adrenal glands were found to have a normal ultrasonographic appearance?
-
(a)
Lifelong administration of an ACE inhibitor supplemented with amlodipine and/or potassium, if needed.
-
(b)
A ‘kidney diet’ supplemented with amlodipine and/or potassium, if needed.
-
(c)
Lifelong administration of spironolactone, supplemented with amlodipine and/or potassium, if needed.
-
(d)
Lifelong administration of amlodipine and potassium.
Answers 1 (b), 2 (c)
Acknowledgments
The authors thank Dr Bruce Belshaw for editing assistance.
References
- 1. Galac S, Reusch CE, Kooistra HS, Rijnberk A. Adrenals. In: Rijnberk A, Kooistra HS, eds. Clinical endocrinology of dogs and cats. 2nd edn. Hannover: Schlütersche, 2010: 93–154. [Google Scholar]
- 2. Conn JW. Primary aldosteronism, a new clinical syndrome. J Lab Clin Med 1955; 45: 3–17. [PubMed] [Google Scholar]
- 3. Conn JW. The evolution of primary aldosteronism: 1954–1967. Harvey Lect 1966–1967; 62: 257–91. [PubMed] [Google Scholar]
- 4. Kaplan NM. Hypokalemia in the hypertensive patient, with observations on the incidence of primary aldosteronism. Ann Intern Med 1967; 66: 1079–90. [DOI] [PubMed] [Google Scholar]
- 5. Fogari R, Preti P, Zoppi A, Rinaldi A, Fogari E, Mugellini A. Prevalence of primary aldosteronism among unselected hypertensive patients: a prospective study based on the use of an aldosterone/renin ratio above 25 as a screening test. Hypertens Res 2007; 30: 111–17. [DOI] [PubMed] [Google Scholar]
- 6. Douma S, Petidis K, Doumas M, et al. Prevalence of primary hyperaldosteronism in resistant hypertension: a retrospective observational study. Lancet 2008; 371: 1921–26. [DOI] [PubMed] [Google Scholar]
- 7. Breitschwerdt EB, Meuten DJ, Greenfield CL, Anson LW, Cook CS, Fulghum RE. Idiopathic hyperaldosteronism in a dog. J Am Vet Med Assoc 1985; 187: 841–45. [PubMed] [Google Scholar]
- 8. Rijnberk A, Kooistra HS, van Vonderen IK, et al. Aldosteronoma in a dog with polyuria as the leading symptom. Domest Anim Endocrinol 2001; 20: 227–40. [DOI] [PubMed] [Google Scholar]
- 9. Johnson KD, Henry CJ, McCaw DL, et al. Primary hyper-aldosteronism in a dog with concurrent lymphoma. J Vet Med A Physiol Pathol Clin Med 2006; 53: 467–70. [DOI] [PubMed] [Google Scholar]
- 10. Machida T, Uchida E, Matsuda K, et al. Aldosterone-, cortico-sterone- and cortisol-secreting adrenocortical carcinoma in a dog: case report. J Vet Med Sci 2008; 70: 317–20. [DOI] [PubMed] [Google Scholar]
- 11. Eger CE, Robinson WF, Huxtable CRR. Primary aldosteronism (Conn's syndrome) in a cat; a case report and review of comparative aspects. J Small Anim Pract 1983; 24: 293–307. [Google Scholar]
- 12. Flood SM, Randolph JF, Gelzer AR, Refsal K. Primary hyper-aldosteronism in two cats. J Am Anim Hosp Assoc 1999; 35: 411–16. [DOI] [PubMed] [Google Scholar]
- 13. MacKay AD, Holt PE, Sparkes AH. Successful surgical treatment of a cat with primary aldosteronism. J Feline Med Surg 1999; 1: 117–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maggio F, DeFrancesco TC, Atkins CE, Pizzirani S, Gilger BC, Davidson MG. Ocular lesions associated with systemic hypertension in cats: 69 cases (1985–1998). J Am Vet Med Assoc 2000; 217: 695–702. [DOI] [PubMed] [Google Scholar]
- 15. Moore LE, Biller DS, Smith TA. Use of abdominal ultrasonography in the diagnosis of primary hyperaldosteronism in a cat. J Am Vet Med Assoc 2000; 217: 213–15, 197. [DOI] [PubMed] [Google Scholar]
- 16. Bruyette DS. Feline endocrinology update. Vet Clin North Am Small Anim Pract 2001; 31: 1063–81, ix. [DOI] [PubMed] [Google Scholar]
- 17. Rijnberk A, Voorhout G, Kooistra HS, et al. Hyperaldosteronism in a cat with metastasized adrenocortical tumour. Vet Q 2001; 23: 38–43. [DOI] [PubMed] [Google Scholar]
- 18. Ash RA, Harvey AM, Tasker S. Primary hyperaldosteronism in the cat: a series of 13 cases. J Feline Med Surg 2005; 7: 173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. DeClue AE, Breshears LA, Pardo ID, Kerl ME, Perlis J, Cohn LA. Hyperaldosteronism and hyperprogesteronism in a cat with an adrenal cortical carcinoma. J Vet Intern Med 2005; 19: 355–58. [DOI] [PubMed] [Google Scholar]
- 20. Javadi S, Djajadiningrat-Laanen SC, Kooistra HS, et al. Primary hyperaldosteronism, a mediator of progressive renal disease in cats. Domest Anim Endocrinol 2005; 28: 85–104. [DOI] [PubMed] [Google Scholar]
- 21. Rose SA, Kyles AE, Labelle P, et al. Adrenalectomy and caval thrombectomy in a cat with primary hyperaldosteronism. J Am Anim Hosp Assoc 2007; 43: 209–14. [DOI] [PubMed] [Google Scholar]
- 22. Briscoe K, Barrs VR, Foster DF, Beatty JA. Hyperaldosteronism and hyperprogesteronism in a cat. J Feline Med Surg 2009; 11: 758–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Renschler JS, Dean GA. What is your diagnosis? Abdominal mass aspirate in a cat with an increased Na:K ratio. Vet Clin Pathol 2009; 38: 69–72. [DOI] [PubMed] [Google Scholar]
- 24. Connell JMC, MacKenzie SM, Freel EM, Fraser R, Davies E. A lifetime of aldosterone excess: long-term consequences of altered regulation of aldosterone production for cardiovascular function. Endocr Rev 2008; 29: 133–54. [DOI] [PubMed] [Google Scholar]
- 25. Joffe HV, Williams GH, Adler GK. Aldosterone and vascular damage. In: Carey RM, ed. Contemporary endocrinology: hypertension and hormone mechanisms. Totowa NJ: Humana Press Inc, 2007: 111–26. [Google Scholar]
- 26. Brown JL, Wasser SK, Wildt DE, Graham LH. Comparative aspects of steroid hormone metabolism and ovarian activity in felids, measured noninvasively in feces. Biol Reprod 1994; 51: 776–86. [DOI] [PubMed] [Google Scholar]
- 27. Graham LH, Brown JL. Cortisol metabolism in the domestic cat and implications for non-invasive monitoring of adrenocortical function in endangered felids. Zoo Biol 1996; 15: 71–82. [Google Scholar]
- 28. Syme HM, Fletcher MGR, Bailey SR, Elliott J. Measurement of aldosterone in feline, canine and human urine. J Small Anim Pract 2007; 48: 202–8. [DOI] [PubMed] [Google Scholar]
- 29. Aguilera G, Catt KJ. Participation of voltage-dependent calcium channels in the regulation of adrenal glomerulosa function by angiotensin II and potassium. Endocrinology 1986; 118: 112–18. [DOI] [PubMed] [Google Scholar]
- 30. Young WF. Primary aldosteronism: renaissance of a syndrome. Clin Endocrinol (Oxf) 2007; 66: 607–18 [DOI] [PubMed] [Google Scholar]
- 31. Brown S, Atkins C, Bagley R, et al. Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med 2007; 21: 542–48. [DOI] [PubMed] [Google Scholar]
- 32. Hiramatsu K, Yamada T, Yukimura Y, et al. A screening test to identify aldosterone-producing adenoma by measuring plasma renin activity. Results in hypertensive patients. Arch Intern Med 1981; 141: 1589–93. [PubMed] [Google Scholar]
- 33. Javadi S, Slingerland LI, van de Beek MG, et al. Plasma renin activity and plasma concentrations of aldosterone, cortisol, adrenocorticotropic hormone, and alpha-melanocyte-stimulating hormone in healthy cats. J Vet Intern Med 2004; 18: 625–31. [DOI] [PubMed] [Google Scholar]
- 34. Montori VM, Young WF., Jr Use of plasma aldosterone concentration-to-plasma renin activity ratio as a screening test for primary aldosteronism. A systematic review of the literature. Endocrinol Metab Clin North Am 2002; 31: 619–32. [DOI] [PubMed] [Google Scholar]
- 35. Young WF., Jr. Primary aldosteronism: management issues. Ann N Y Acad Sci 2002; 970: 61–76. [DOI] [PubMed] [Google Scholar]
- 36. Djajadiningrat-Laanen SC, Galac S, Cammelbeeck SE, van Laar KJ, Boer P, Kooistra HS. Urinary aldosterone to creatinine ratio in cats before and after suppression with salt or fludrocortisone acetate. J Vet Intern Med 2008; 22: 1283–88. [DOI] [PubMed] [Google Scholar]
- 37. Gordon RD, Stowasser M, Tunny TJ, Klemm SA, Rutherford JC. High incidence of primary aldosteronism in 199 patients referred with hypertension. Clin Exp Pharmacol Physiol 1994; 21: 315–18. [DOI] [PubMed] [Google Scholar]
- 38. Stowasser M, Gordon RD, Gunasekera TG, et al. High rate of detection of primary aldosteronism, including surgically treatable forms, after ‘non-selective’ screening of hypertensive patients. J Hypertens 2003; 21: 2149–57. [DOI] [PubMed] [Google Scholar]
- 39. Rossi GP, Seccia TM, Pessina AC. Primary aldosteronism — part I: prevalence, screening, and selection of cases for adrenal vein sampling. J Nephrol 2008; 21: 447–54. [PubMed] [Google Scholar]
- 40. Kempers MJE, Lenders JWM, van Outheusden L, et al. Systematic review: diagnostic procedures to differentiate unilateral from bilateral adrenal abnormality in primary aldosteronism. Ann Intern Med 2009; 151: 329–37. [DOI] [PubMed] [Google Scholar]
- 41. Eriksson B, Örlefors H, Öberg K, Sundin A, Bergström M, Långström B. Developments in PET for the detection of endocrine tumours. Best Pract Res Clin Endocrinol Metab 2005; 19: 311–24. [DOI] [PubMed] [Google Scholar]
- 42. Yen RF, Wu VC, Liu KL, et al. 131I-6β-iodomethyl-19-norcholesterol SPECT/CT for primary aldosteronism patients with inconclusive adrenal venous sampling and CT results. J Nucl Med 2009; 50: 1631–37. [DOI] [PubMed] [Google Scholar]
- 43. Melby JC, Spark RF, Dale SL, Egdahl RH, Kahn PC. Diagnosis and localization of aldosterone-producing adenomas by adrenal-vein catheterization. N Engl J Med 1967; 277: 1050–56. [DOI] [PubMed] [Google Scholar]
- 44. Daunt N. Adrenal vein sampling: how to make it quick, easy, and successful. Radiographics 2005; 25: S143–58. [DOI] [PubMed] [Google Scholar]