Abstract
This study compared the effects of a moderate carbohydrate–high fiber (MC–HF) food and a low carbohydrate–low fiber (LC–LF) food on glycemic control in cats with diabetes mellitus. Sixty-three diabetic cats (48 male castrated, 15 female spayed) were randomly assigned to be fed either a canned MC–HF (n=32) food or a canned LC–LF (n=31) food for 16 weeks. Owners were blinded to the type of diet fed. CBC, urinalysis, serum chemistry panel, fructosamine concentration and thyroxine concentration were determined on initial examination, and a complete blood count, serum chemistry panel, urinalysis and serum fructosamine concentration were repeated every 4 weeks for 16 weeks. Insulin doses were adjusted as needed to resolve clinical signs and lower serum fructosamine concentrations. Serum glucose (P=0.0001) and fructosamine (P=0.0001) concentrations significantly decreased from week 0 to week 16 in both dietary groups. By week 16, significantly more of the cats fed the LC–LF food (68%, 22/31), compared to the cats fed the MC–HF food (41%, 13/32), had reverted to a non-insulin-dependent state (P=0.03). Cats in both groups were successfully taken off of insulin regardless of age, sex, type of insulin administered or duration of clinical disease before entering the study. There was no significant difference in the initial or final mean body weights or in the mean change in body weight from week 0 to week 16 between dietary groups. Diabetic cats in this study were significantly more likely to revert to a non-insulin-dependent state when fed the canned LC–LF food versus the MC–HF food.
Diabetes mellitus is a common endocrinopathy in domestic cats. It has been estimated that 1:400 domestic cats in the United States has diabetes mellitus (Panciera et al 1990). The prevalence of type 1 versus type 2 diabetes mellitus in cats remains unknown, but clinical evidence suggests that many cats may develop type 2 diabetes (previously referred to as non-insulin-dependent diabetes mellitus) similar to type 2 diabetes in humans (Johnson et al 1986, Kirk et al 1993, Lutz and Rand 1995, Appleton et al 2001).
Use of high fiber foods for glycemic control in diabetic patients has been suggested for diabetic cats, based on human medical studies showing that fiber may slow carbohydrate absorption in the intestine, alter gastrointestinal transit times and alter insulin sensitivity in peripheral tissues (Anderson and Akanji 1991, Costacou 2003). Nelson et al (2000) reported decreased blood glucose values, pre-prandial and 12 h post-prandial, in diabetic cats fed a canned high fiber food (12% fiber, dry matter) compared to cats fed a food low in fiber (1% fiber, dry matter) and higher in carbohydrates. However, no difference was noted in glycosylated hemoglobin concentrations or insulin requirements between dietary groups.
Both veterinary and human medical researchers have evaluated the effects of various diets on glycemic control in diabetic patients (Brand Miller and Colagiuri 1994, Frank et al 2001, Mazzaferro et al 2003). Low-carbohydrate diets may aid glycemic control in type 2 diabetes by decreasing hyperglycemia and aiding in recovery from glucose toxicity. Decreased blood glucose concentrations, serum fructosamine concentrations, and insulin requirements were documented in diabetic cats fed low-carbohydrate canned foods in two small studies (Frank et al 2001, Mazzaferro et al 2003). Mazzaferro et al (2003) reported improved glycemic control and discontinuation of insulin administration in 60% of diabetic cats given acarbose and a low-carbohydrate canned food. In that study, insulin was discontinued in 66% of the control cats fed a low-carbohydrate food alone, suggesting that perhaps the food itself influenced glycemic control.
As previous studies involved small numbers of cats, larger, prospective clinical studies are needed to evaluate optimal dietary treatment of diabetic cats. The goal of this study was to compare effects of a commercially available moderate carbohydrate–high fiber (MC–HF) food (Hill's Prescription Diet Feline w/d canned, Hill's Pet Nutrition, Inc., Topeka, KS) and low carbohydrate–low fiber (LC–LF) food (Hill's Science Diet Feline Growth canned (Feline Kitten), Hill's Pet Nutrition, Inc., Topeka, KS) with regard to their effect on glycemic control and insulin requirements in cats with naturally occurring diabetes.
Materials and methods
Subjects
Diabetes mellitus was diagnosed in the cats in this study based on appropriate clinical signs, persistent hyperglycemia and persistent glucosuria for at least 2 weeks duration (Feldman and Nelson 2004). Except for hyperglycemia, glucosuria and occasional mild elevations in liver enzymes and cholesterol concentrations, cats were otherwise normal on physical examination, complete blood count, serum chemistry panel and urinalysis. All cats had serum thyroxine concentrations within normal reference range and were negative on FeLV/FIV ELISA assay. Exclusion criteria included prior treatment with glucocorticoids, sex hormones, anabolic steroids or oral hypoglycemic agents 3 months prior to or since the diagnosis of diabetes, concurrent disease (such as, but not limited to, ketoacidosis, hyperthyroidism, hyperadrenocorticism and acromegaly), use of concurrent medications or dietary supplements and refusal to eat the provided study food. Of the 82 cats evaluated for entry into the study, 63 cats were included in the study.
Diet and client protocol
Each cat was randomly assigned to be fed one of two canned study foods for 16 weeks; 32 cats received a canned MC–HF and 31 cats received a canned LC–LF. As each cat was entered into the study, every other one received the MC–HF food or the LC–LF food in an alternating manner. Although the groups were not stratified upon entry, the distribution of cats based on age, sex, weight, duration of disease, insulin treatment and entry glucose and fructosamine concentrations was balanced in both food groups. On a caloric basis, the MC–HF food was higher in carbohydrates and fiber, marginally higher in protein, and lower in fat compared with the LC–LF food (Table 1). Because commercial foods were used, each food contained different quantities of nutrients and trace elements. The main carbohydrate component in the MC–HF food was ground corn whereas the LC–LF food contained soybean meal and corn gluten meal.
Table 1.
Nutrient analysis of the moderate carbohydrate–high fiber study food (MC–HF) and the low carbohydrate–low fiber study food (LC–LF) shown on an as-fed, caloric basis, as provided by the manufacturer
| As-fed, caloric basis (g/100 kcal) | Percentage of metabolizable energy (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| CHO | Fiber | Protein | Fat | CHO | Fiber | Protein | Fat | |
| Moderate carbohydrate–high fiber (MC–HF) | 7.6 | 3.1 | 11.5 | 4.8 | 26 | 0 | 40 | 41 |
| Low carbohydrate–low fiber (LC–LF) | 3.5 | 0.1 | 10.6 | 6.0 | 12 | 0 | 37 | 51 |
CHO=carbohydrates.
Clients remained blinded to the type of food assigned to their cat for the 4-month duration of the study. Clinical investigators were aware of the type of food each cat received so that they could ensure that each cat received an equivalent daily caloric intake (60–65 kcal/kg/day) (Hand et al 2000) regardless of the type of food they received. Clients were instructed to feed the calculated quantity of canned food divided into two meals per day at approximately 12-h intervals. Clients administered a prescribed dose of insulin to their cat, subcutaneously, along the lateral trunk, rotating injections sites between administrations, while the cat ate or just after the cat had finished each meal. Owners were instructed not to feed any other types of cat food, table scraps, treats or supplements during the 4-month study period. If the cat lived in a multi-cat household, enough study food was provided for all cats in the house to prevent exposure to any other food. All animals in the house were meal-fed and monitored by the clients to prevent the diabetic cats from stealing food from others. Each owner was required to sign an informed consent form as well as a contract to abide by these dietary guidelines. Daily food and insulin amounts were recorded by the clients as well as subjective assessments of water intake, urine output and energy level.
Clinical and laboratory evaluations
A physical examination, complete blood count, serum chemistry panel, serum thyroxine and fructosamine concentrations, urinalysis and urine culture were done upon entry into the study. Each cat was re-examined every 4 weeks for a total of 16 weeks. At each examination, a complete blood count, serum chemistry panel, urinalysis and serum fructosamine concentration were evaluated.
Body condition was evaluated in 17 cats (8 MC–HF, 9 LC–LF) on week 0 and week 16, via dual X-ray adsorption scan (DEXA: Hologic QD 1000/W with software version 5.71P, Bedford, MA) when the DEXA scanner became available later in the study. After initial screening, these 17 cats underwent DEXA scanning to establish baseline body composition on week 0. A scan was then repeated at the end of the study on week 16. Each cat had an intravenous catheter placed and was anesthetized with intravenous propofol (PropoFlo; Abbott Laboratories, N. Chicago, IL) (4–7 mg/kg IV bolus, then continuous infusion to effect) for each DEXA scan. Supplemental oxygen was administered and routine anesthetic monitoring was performed. Cats were positioned in sternal recumbency with the hindlimbs extended caudally, and DEXA measurements were performed using a whole body scanner operated in single-beam mode, as previously described (Mazzaferro et al 2003). Calibration of the unit was verified by scanning a calibration phantom before each individual scan was performed. Commercially available pediatric software was used to analyze the scans. Body mass (lean and fat) was obtained, and the percentage of body fat calculated. Scans took approximately 10 min; the scan was repeated if any gross movement was detected.
For the purpose of this study, glycemic control was primarily evaluated based on clinical signs (decreased water consumption, stable body weight, and increased physical activity) and change in serum fructosamine concentrations. A random serum blood glucose concentration was determined from the blood sample obtained at each examination to identify hypoglycemia that might suggest poor regulation, Somogyi response and inappropriate insulin administration or defective insulin. Insulin dosages were never increased based on the presence of hyperglycemia found in a single blood sample. However, if hypoglycemia was present, the insulin dosage was decreased by 1 unit per injection or discontinued if the cat was receiving only 1 unit per injection. Similarly, urine was analyzed for presence of ketones or absence of glucose. Insulin dosages were never increased based on these results, but if glucosuria was absent on analysis, the insulin dosage was decreased by 1 unit per injection. No urine was collected in those cats presenting without a palpable bladder. Urine glucose was measured via urine dipstick as 0, 1+, 2+, 3+ or 4+.
The cats in this study received a variety of types of insulin from various manufacturers (NPH, Lente, Ultralente, Protamine Zinc Insulin (PZI)). Cats were either continued on their prior insulin product or switched to a single PZI (Blue Ridge Pharmaceuticals Raleigh, NC). PZI was recommended because bovine insulin more closely approximates feline insulin compared to human recombinant insulin. In addition, an effort was made to try to use a common insulin product when possible so that insulin dosages could be compared statistically. Thus, the majority of the cats received a specific PZI for the duration of the study. This enabled us to compare mean PZI insulin doses from week 0 to week 16 between the dietary groups. Insulin doses were adjusted based on client reports on clinical signs, serum glucose and fructosamine concentrations, and urine dipstick results as described above. Doses were decreased by 1 unit per injection or discontinued in cats receiving only 1 unit per injection when the owners reported that the clinical signs had resolved completely (normal water intake, normal appetite, stable body weight, increased energy and range of activity) or when owners reported glucose-responsive ataxia, disorientation or recumbency. Doses were decreased similarly when serum fructosamine concentration was <400 μmol/l, serum glucose concentration was <60 mg/dl on a glucometer or <80 mg/dl by enzyme oxidation, or when glucosuria was absent. Insulin dose was increased when owners reported continued clinical signs, when weight loss was recorded or when serum fructosamine concentration was >475 μmol/l without resolution of clinical signs and without observed signs of hypoglycemia suggesting possible Somogyi response. If fructosamine was >475 μmol/l but the owner was unsure of changes in clinical signs, the insulin dose was first decreased 1 unit to assess clinical response and then increased if clinical signs worsened. The same criteria for insulin dose adjustments were used for each cat regardless of the food provided.
In this study, responders were defined as cats able to maintain a stable body weight, normal water intake, normal appetite and a normal activity level with a serum fructosamine concentration <400 μmol/l without exogenous insulin administration. All cats requiring insulin from week 1 through week 16 were designated non-responders. Cats were considered ‘well-regulated’ if they were able to maintain stable body weight, normal water intake, normal appetite and a normal energy level with a serum fructosamine concentration <400 μmol/l with or without continued insulin administration. Clients were asked to assess appetite and food intake and to estimate the number and size of urine clumps in the litter box. If possible, clients were asked to measure the amount of water ingested on a daily basis. If this could not be done accurately due to the presence of other animals in the house, clients were asked to compare the amount of water that disappeared from the water bowl on a daily basis and note any changes. Clients were asked to keep a daily log of this information.
Statistical analysis
Serum glucose and fructosamine concentrations, PZI insulin doses and DEXA measurements are reported as mean±standard deviation. Data were analyzed with commercial statistical analysis software (Statview for Windows v.5.0.1, SAS Institute Inc, Cary, NC 27513). The Kolmogorov–Smirnov test was performed to assess normality of distribution for serum glucose and fructosamine concentrations at weeks 0, 4, 8, 12 and 16. A repeated measures ANOVA was performed to determine differences in serum glucose and fructosamine concentrations at each weekly interval. Fisher's least significant difference test was used as a post hoc analysis for individual means comparisons. An unpaired t-test was used to compare body weight and percentage of body fat between dietary groups on week 0 and week 16. An unpaired t-test was used to compare change in percentage of body fat between dietary groups from week 0 to week 16. Median urine glucose values are reported. Because urine was not readily available for all cats, statistics were not performed on these results.
Cats were divided into short (≤45 days), moderate (46 days–12 months), and long-term (>12 months) disease groups to analyze the influence that duration of disease might have on the likelihood that a cat might revert to a non-insulin-dependent state. Contingency table analysis was used to determine whether duration of diagnosed illness, insulin dose at week 0, insulin type or type of diet affected discontinuation of insulin administration; P<0.05 was considered significant. A logistic model coefficients (Wald test P value) analysis was used to calculate the odds ratio for effect of diet on number of responders versus non-responders and number of well-regulated cats versus not well-regulated cats.
All applicable statistics were analyzed for the LC–LF group compared with the MC–HF group, the LC–LF responder cats compared to the MC–HF responder cats and the LC–LF non-responder cats (those on insulin week 0 through week 16) compared to the MC–HF non-responder cats.
Results
Sixty-three cats (48 male castrated, 15 female spayed) completed the dietary trial (32 MC–HF, 31 LC–LF). Nineteen cats (9 MC–HF, 10 LC–LF) were excluded due to concurrent disease or refusal to eat the assigned study food. Only 2 of these 19 cats had participated in the study beyond the first 4 weeks at the time they were dropped from the study. One cat was excluded from the study after he died approximately 12 weeks into the study due to acute pancreatitis (diagnosed on post-mortem histopathology) and ketosis. This cat had been in the MC–HF dietary group and had been diagnosed with diabetes approximately 6 months prior to entering the study. One cat developed acute renal failure and constipation approximately 3 weeks into the study and was dropped from the study after the first 4-week recheck. That cat had been in the LC–LF group and was subsequently switched to a high fiber diet in an attempt to better regulate intestinal motility. The cat continued to require insulin while receiving the MC–HF food.
Nineteen cats (10 MC–HF, 9 LC–LF) had been diagnosed with diabetes more than 12 months prior to entry (14–72 months). Twenty-four cats had been diagnosed (13 MC–HF, 11 LC–LF) between 46 days and 12 months prior to entry and, 19 cats (eight MC–HF, 11 LC–LF) had been diagnosed within 45 days prior to entry. Duration of illness was not recorded for one of the cats in the MC–HF group.
Cats were fed an extreme variety of diets prior to their entry into the study and 87% of the cats had been provided with at least a portion of dry food. Therefore, data could not be analyzed based on initial diet composition. There was no statistical difference between cats allocated to the MC–HF and LC–LF food groups with regard to age, sex, mean body weight, duration of illness or treatment, insulin type, mean PZI insulin dose at week 0, initial mean fructosamine concentration, or initial mean serum glucose concentration (Figs 1 and 2; Table 2). Median initial urine glucose concentration on urine dipstick (MC–HF: 3+, LC–LF: 3+, range 2–4+) was similar in cats in both groups. The body composition of 17 cats (8 MC–HF, 9 LC–LF) was evaluated by DEXA scan at the time of entry into the study (week 0). Of these cats, there was no difference in the initial mean percentage of body fat between dietary groups (MC–HF (n=8): 32%±10.8; LC–LF (n=9): 30.5%±11.7).
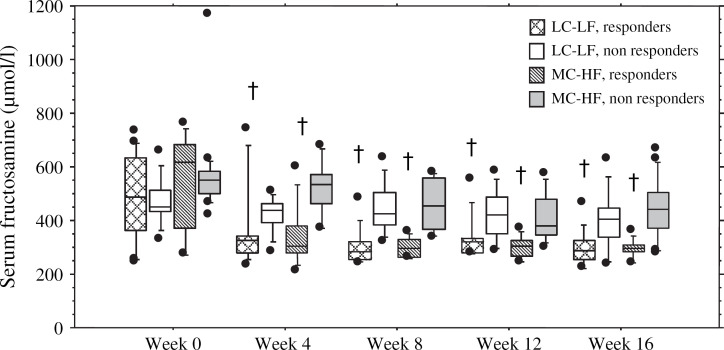
Fig 1.
Box plots of mean serum fructosamine concentrations at weeks 0, 4, 8, 12 and 16 in cats fed a low carbohydrate–low fiber food (LC–LF) and in cats fed a moderate carbohydrate–high fiber food (MC–HF). The responder groups consist of cats eventually taken off of insulin. The non-responder groups consist of cats that continued to require insulin throughout the study. Each box represents the 25th to 75th percentile range of the data. The whiskers represent the 5th to 95th percentile range of data. The horizontal bar within the box indicates the median concentration. Outlying data points are shown as closed circles. Significance (P<0.05) in comparison to week 0 is denoted with a cross above the box plot.
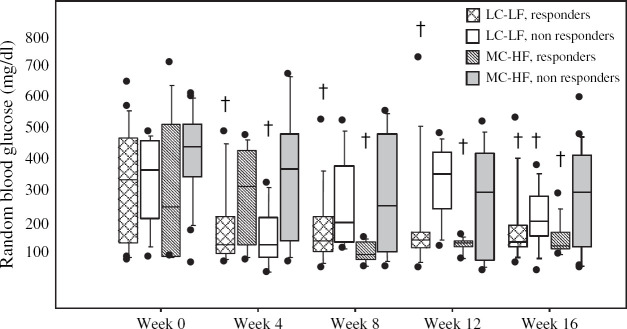
Fig 2.
Box plots of mean serum glucose concentrations at weeks 0, 4, 8, 12 and 16 in cats fed a low carbohydrate–low fiber food (LC–LF) and in cats fed a moderate carbohydrate–high fiber food (MC–HF). The responder groups consist of cats eventually taken off of insulin. The non-responder groups consist of cats that continued to require insulin throughout the study. Each box represents the 25th to 75th percentile range of the data. The whiskers represent the 5th to 95th percentile range of data. The horizontal bar within the box indicates the median concentration. Outlying data points are shown as closed circles. Significance (P<0.05) in comparison to week 0 is denoted with a cross above the box plot.
Table 2.
Initial measurements (week 0) and final measurements (week 16) for each dietary group are summarized as mean±standard deviation
| LC–LF food (n=31) | MC–HF food (n=32) | P value | |
|---|---|---|---|
| Sex | 10F:21M | 9F:23M | |
| Mean age (years) | 10.05±3.04 | 11.54±2.81 | 0.2 |
| Time since diagnosis | 1 Unknown | 0.75 | |
| ≤45 days | 9 | 10 | |
| 46 days–12 months | 11 | 13 | |
| >12 months | 11 | 8 | |
| Weight, week 0 (kg) | 6.37±1.42 | 5.98±1.86 | 0.34 |
| Weight, week 16 (kg) | 6.50±1.31 | 6.33±1.75 | 0.20 |
| Insulin used in study | |||
| PZI | 25 | 28 | |
| Ultralente | 0 | 1 | |
| Lente | 4 | 2 | |
| NPH | 2 | 1 | |
| Mean PZI q12h (units/kg/dose), week 0 | 0.34±0.13 | 0.46±0.17 | 0.22 |
| Mean PZI q12h (units/kg/dose), week 16 | 0.52±0.43 | 0.6±0.31 | 0.08 |
| Mean glucose, week 0 (mg/dl) | 316±30.5 | 365±32.5 | 0.98 |
| Mean glucose, week 16 (mg/dl) | 160.8±19.6 | 208.8±25.8 | 0.64 |
| Mean fructosamine, week 0 (μmol/l) | 497±24.6 | 565±29.2 | 0.45 |
| Mean fructosamine, week 16 (μmol/l) | 332.7±16.7 | 373±20.2 | 0.53 |
All cats in either dietary group were included in these calculations except for the final PZI dose category; only those cats still receiving insulin on week 16 were included for calculation of final, mean PZI dose. P<0.05 is considered significant.
Eighty-three percent of the cats (52 cats: 25 MC–HF, 27 LC–LF) had been treated with some type of injectable insulin (NPH, Lente, Ultralente or PZI) before beginning the study; the other 17% had not been treated for their clinical signs. Average pre-study insulin dosage varied with the type of insulin used: 0.7 units/kg/dose Lente, 0.8 units/kg/dose NPH, 0.5 units/kg/dose PZI and 0.4 units/kg/dose Ultralente. Fifty-four cats (85%) received PZI (27 MC–HF, 27 LC–LF) and 9 cats (14%) received human recombinant NPH (2), Lente (6), or Ultralente (1) insulin for the duration of the study. Of those cats already receiving insulin, 67% were switched to PZI upon entry and 33% continued to receive the same insulin on which they were referred. The number of cats that reverted to a non-insulin-dependent state was not statistically different between those cats that entered the study on insulin therapy and those cats that had not been treated with insulin prior to the start of the study (P=0.719).
LC–LF responders versus MC–HF responders
At the end of the study (week 16), there was no statistical difference between dietary groups with regard to mean body weight, final PZI insulin dose for those cats still receiving insulin, final serum fructosamine concentration and final serum glucose concentration (Figs 1 and 2; Table 2). Of the 17 cats that were evaluated by DEXA scan at weeks 0 and 16, there was no difference in final mean percentage of body fat between the dietary groups at week 16 (MC–HF: 32.6%±11.4, LC–LF: 30.2%±9.1). There was no statistical difference between dietary groups in the change in mean percent body fat from week 0 to week 16 (LC–LF: −1.9±6.2, MC–HF: 2.1±9.8). There was no difference in the change in percentage of body fat from week 0 to week 16 between those cats that reverted to a non-insulin-dependent state versus those that did not.
Nine of the 11 cats that reverted to a non-insulin-dependent state were cats that entered the study with >30% body fat and, seven out of those nine cats decreased their body fat during the study (4 MC–HF, 3 LC–LF). However, in this small subset of cats, approximately the same percentage of cats on either diet responded by the end of this study; 5/8 (62%) MC–HF DEXA cats reverted to a non-insulin-dependent state versus 6/9 (67%) LC–LF DEXA cats.
Serum glucose and fructosamine concentrations decreased significantly from week 0 to week 16 in responder cats in both dietary groups (Figs 1 and 2). A significant decrease was seen in both serum fructosamine (P=0.004) and serum glucose (P=0.020) concentrations in the responder cats of both the LC–LF and the MC–HF groups by week 4 (Figs 1 and 2). Serum fructosamine concentrations continued to decrease progressively throughout the study in the responder cats in both dietary groups though there was no significant difference between weeks 4, 8, 12 and 16. Both the serum glucose concentrations (P=0.033) and the serum fructosamine concentrations (P=0.001) from week 0 to week 16 decreased more markedly in the responder cats than in the non-responder cats.
Cats in both dietary groups reverted to a non-insulin-dependent state throughout the study. Of the cats that no longer needed insulin, 29% (3/13 MC–HF, 7/21 LC–LF) were discontinued by week 4, 18% (3/13 MC–HF, 3/21 LC–LF) responded by week 8, 21% (4/13 MC–HF, 3/21 LC–LF) responded by week 12 and the final 32% (3/13 MC–HF, 8/21 LC–LF) responded by week 16. There was no difference in the number of responders versus non-responders based on duration of illness (P=0.6, Table 3). Within the subset of cats that had been diabetic for >12 months (14–72 months) prior to their entry into the study, all of the cats that reverted to a non-insulin-dependent state had been diabetic for a period of 14–30 months. None of the cats that had been diabetic for a period of 36–72 months reverted to a non-insulin-dependent state in this study. There were significantly more responders in the LC–LF group (68%, 21/31) than in the MC–HF group (41%, 13/32) (P=0.031). Cats fed the LC–LF food had 1.7 times greater chance of achieving a non-insulin-dependent state than cats fed the MC–HF food (odds ratio 3.1; 95% CI=1.1–8.6; Wald test P value=0.030)
Table 3.
The 63 cats in the study are listed as responders and non-responders based on duration of illness
| Duration of illness | Responders | Non-responders |
| ≤45 Days | 11 (5 MC–HF, 6 LC–LF) | 8 (5 MC–HF, 3 LC–LF) |
| 46 Days–12 months | 11 (3 MC–HF, 8 LC–LF) | 13 (10 MC–HF, 3 LC–LF) |
| >12 Months (14–72 months) | 9 (3 MC–HF, 6 LC–LF) | 10 (5 MC–HF, 5 LC–LF) |
The parenthetical numbers indicate the subset of cats fed either a moderate carbohydrate–high fiber diet (MC–HF) or a low carbohydrate–low fiber diet (LC–LF).
Well-regulated LC–LF cats versus well-regulated MC–HF cats
Only 10% (6/63: 2/32 MC–HF, 4/31 LC–LF) of the cats were well-regulated at the time of entry into the study based on clinical signs and the presence of a serum fructosamine concentration <400 μmol/l. By the end of the study, 81% (25/31; 4 on insulin, 21 off insulin) of cats fed the LC–LF food were well-regulated compared to 56% (18/32; 5 on insulin, 13 off insulin) of cats fed the MC–HF food (P=0.048). Cats fed the LC–LF food had 1.5 times greater chance of achieving adequate glycemic regulation whether or not they still required insulin than cats fed the HC food (odds ratio 2.9; 95% CI=1.3–6.4; Wald test P value=0.012)
LC–LF non-responders versus MC–HF non-responders
By the end of the 4-month study period, 59% (19/32) of MC–HF cats and 32% (10/31) of the LC–LF cats continued to require insulin to maintain glycemic control. Of the cats still requiring insulin at week 16, 26% (5/19) on the MC–HF diet and 40% (4/10) on the LC–LF diet were considered well-regulated based on clinical signs and a serum fructosamine concentration <400 μmol/l. Of the cats still requiring insulin, 89% (17/19) of the MC–HF cats and all of the LC–LF cats (10/10) had improved diabetic regulation based on clinical signs alone (decreased water consumption, stable body weight, increased energy level and range of activity) when compared to their initial clinical signs on week 0. At week 16, there were no statistical differences in mean serum fructosamine concentration (MC–HF: 434.1±109.5, LC–LF: 400.9±107.7), mean serum glucose concentration (MC–HF: 253.9±165.6, LC–LF: 190.9±108.5) and mean PZI dose (MC–HF: 0.60±0.31 units/kg/dose, LC–LF: 0.52±0.43 units/kg/dose) between the non-responder cats in different dietary groups (19 MC–HF, 10 LC–LF). There was no statistical difference in the initial mean PZI dose and the final PZI dose of those cats still requiring insulin at week 16 (19/32 MC–HF, 10/31 LC–LF; P=0.82). Nor was there a significant change in serum glucose or serum fructosamine concentrations between weeks 0, 4 and 8 (Figs 1 and 2). Although there was no significant difference in serum glucose concentrations from week 0 to week 16 in the MC–HF non-responder group, the LC–LF non-responder group did show a significant decline in serum glucose concentration by week 16 when compared with week 0 (Fig 2). There was no significant difference in the change in body fat from week 0 to week 16 in the non-responders of each dietary group. Of the 17 cats that were evaluated with DEXA scan, 4 out of the 6 cats that continued to require insulin entered the study with <30% body fat and increased their body fat during the study (2 MC–HF, 2 LC–LF; Table 3). Although there was a trend for insulin doses to progressively increase in all of the non-responder cats over the course of this study and a trend for their serum fructosamine concentrations to decline, the primary change noted between week 0 and week 16 was a subjective improvement in clinical signs as reported by the owners.
Discussion
The results of this study would suggest that 41–68% of feline diabetes mellitus may indeed be type 2 diabetes, previously called non-insulin-dependent diabetes, which can be modulated by change in diet, exercise and weight loss. Cats fed canned MC–HF and canned LC–LF foods were able to revert to a non-insulin-dependent state in this study, suggesting that either type of food may contribute to glycemic regulation and increased remission. In this study, insulin was successfully discontinued in 68% of the cats fed the LC–LF food and 41% of the cats fed the MC–HF food, suggesting that the LC–LF food may be a better alternative for the management of cats with diabetes mellitus.
Type 2 diabetes mellitus is a multifactorial endocrinopathy affecting both humans and cats (Kirk et al 1993, Costacou and Mayer-Davis 2003). Decreased insulin secretion, decreased insulin sensitivity in peripheral tissues, decreased glucose transporter translocation, defective post-receptor insulin signaling and increased amyloid production and deposition within pancreatic beta cells have all been implicated in the pathogenesis of type 2 diabetes in humans and models for human type 2 diabetes (Ciaraldi et al 1991, DeFronzo et al 1992, Papa et al 1997). The prevalence of cats with type 2 diabetes is not known. However, studies suggest that many cats with naturally occurring diabetes demonstrate a pathogenesis similar to that seen in type 2 diabetes mellitus in humans (O'Brien et al 1985, Nelson et al 1990, Lutz and Rand 1993, Biourge et al 1997, Appleton et al 2001, Costacou and Mayer-Davis 2003, Mazzaferro et al 2003).
Diet and exercise play an essential role in management of type 2 diabetes mellitus in humans (Marshall et al 1991, 1993, Feskens et al 1994, Costacou and Mayer-Davis 2003). Both low fat, low carbohydrate, high protein diets and diets high in fiber can improve glycemic regulation in humans with glucose intolerance and type 2 diabetes (Parker et al 2002, Costacou and Mayer-Davis 2003). Studies with diabetic cats have reported improved glycemic parameters or decreased insulin resistance with diets high in insoluble fiber (Nelson et al 2000) and diets high in protein or low in carbohydrates (Frank et al 2001, Mazzaferro et al 2003).
In this study, the MC–HF diet was higher in fiber and carbohydrates, marginally higher in protein and lower in fat than the LC–LF diet, allowing us to explore the potential benefit of either a LC–LF diet or a MC–HF diet. Cats were randomly allocated to dietary groups without stratification for dependent variables. There were no differences in age, sex, body weight, percent body fat, duration of illness, insulin type, initial PZI insulin dose, initial serum glucose, initial serum fructosamine, or initial urine glucose concentration between groups at the beginning of the study.
Although clients were blinded to the type of diet assigned to their cat, the clinical investigators were not. As the caloric density varied between study foods, the investigators needed to know the food being fed so as to determine the appropriate quantity of food given to each cat. Each cat received approximately the same daily caloric intake based on individual body weight (60–65 kcal/kg/day) (Hand et al 2000). Investigator knowledge of assigned foods could have allowed possible bias, but this is unlikely to have occurred. Protocols were predetermined for all cats, regardless of the diet they were receiving. The goal for every cat was control of clinical signs with the lowest possible insulin dosage. Furthermore, the criteria used for alterations of insulin dosages were identical for all cats.
For the purpose of this study, glycemic control was primarily evaluated based on clinical signs (decreased water consumption, stable body weight, increased physical activity) and change in serum fructosamine concentrations. Blood glucose curves were not obtained due to potential error in interpretation secondary to stress and daily fluctuations (Carlstead et al 1993, Link and Rand 1998, Plier and Grindem 1998, Rand et al 2002, Fleeman and Rand 2003). Serum fructosamine concentration is not affected by stress hyperglycemia or daily fluctuations and has been shown to be a reliable indicator of short-term (1–3 weeks) glycemic control when interpreted in light of clinical signs (Kaneko et al 1992, Reusch and Hoyer 1995, Crenshaw et al 1996, Thoresen and Bredal 1996, Plier and Grindem 1998). As serum fructosamine concentration has also been shown to be an equal predictor of glycemic control when compared to a 12-h mean blood glucose concentration, the latter was not obtained in this study (Elliot et al 1999). Thus, serum fructosamine concentration was used as a cost-effective, time-efficient determination of glycemic control. Serum fructosamine concentration was always interpreted in light of clinical signs and results of the complete blood count, serum chemistry panel and urine glucose concentration.
Most of the cats in this study were overweight. Increased body weight and body fat contribute to insulin resistance in both humans and cats, and changes in either may affect the degree of insulin resistance present and, therefore, the amount of insulin required (Ciaraldi 1991, Hand et al 2000). As a result, the study was designed to maintain body weight over the 16-week study period as best as possible. Any change in insulin requirements would then be directly attributable to dietary influences rather than weight gain or weight loss. None of the cats entered into this study were markedly underweight, despite reported weight loss. Thus, none of the cats were maintained at a suboptimal body weight over the course of the study. At the end of the study period, it was recommended that those cats which were overweight be started on a weight loss regimen to aid in alleviation of glucose intolerance and insulin resistance.
Body composition can change without an apparent change in body weight. In the study by Mazzaferro et al (2003), diabetic cats fed a low-carbohydrate diet decreased percent body fat as measured on DEXA scan, without decreasing body weight. Sixty-six percent of the control cats in that study, fed a low-carbohydrate diet, reverted to a non-insulin-dependent state suggesting that a low-carbohydrate food might influence insulin resistance and insulin requirements by decreasing body fat and increasing lean mass, thereby maintaining body weight. We found no statistical difference in the initial (week 0) or final (week 16) percent body fat, nor a difference in the change in percentage of body fat between dietary groups in the subset of cats that were evaluated by DEXA scan in this study. In the small subset of cats that were evaluated by DEXA scan, 64% of the cats that reverted to a non-insulin-dependent state entered the study with >30% body fat and lost body fat over the course of the study. These data would suggest that cats high in body fat, which are able to decrease body fat, are more likely to revert to a non-insulin-dependent state. However, in the small subset of cats evaluated with DEXA scan, cats with >30% body fat, fed either of the study foods provided, were equally as likely to decrease body fat and revert to a non-insulin-dependent state. It is possible that this represents a type 2 error as the study population evaluated accurately for body composition was small. In the study performed by Mazzaferro et al (2003), the cats that reverted to a non-insulin-dependent state while fed a low-carbohydrate food were initially >30% body fat and had decreased body fat at the time their insulin administration was discontinued. These data would suggest that a low-carbohydrate food might affect insulin requirements in obese cats by altering body composition and decreasing body fat. Further studies are required to evaluate the effect that high fiber or low-carbohydrate foods have on change in body composition, in diabetic cats, over time.
Aside from the alteration of body composition, dietary carbohydrates can influence insulin secretion and glycemic parameters. Both the glycemic index of dietary carbohydrates as well as the amount of dietary carbohydrates can affect the post-prandial rise in glucose and subsequent insulin secretion and insulin requirements. In a previous study, healthy cats fed a diet for 4 weeks with approximately 50% of energy from carbohydrate demonstrated a significantly higher mean glucose concentration and glucose area under the curve compared to cats fed diets with 25% of energy from carbohydrate (Farrow et al 2002). These results suggest that decreasing the quantity of carbohydrates might aid in glycemic control in cats with type 2 diabetes. The foods used in this study contained different carbohydrate sources as well as different quantities of carbohydrates. This difference in glycemic load likely affected the change seen in glycemic parameters as well as the likelihood for remission in the cats in this study. In order to elucidate the role that carbohydrate quantity versus carbohydrate quality might play in the glycemic control of cats with diabetes further investigation is required.
Most of the cats in this study, regardless of food type, exhibited improved glycemic control within the 16-week study period (Figs 1 and 2). This was anticipated as the goal was to improve diabetic regulation in all cats, and insulin doses were adjusted accordingly. The results also suggest that although feeding a low-carbohydrate diet is more likely to lead to remission of clinical diabetes and good diabetic control, either diet used in this study can be effective in the management of diabetes mellitus in cats. However, for the cats that did not revert to a non-insulin-dependent state, the type of diet provided did not affect final serum glucose concentrations, serum fructosamine concentrations or insulin dosages in cats. If the study period were longer than 4 months, it is possible that diet associated modifications in glycemic parameters or insulin requirements may have become more apparent in non-responders. More of the cats that were still not well-regulated at the end of this study may have achieved good glycemic control within a longer time frame. Perhaps, then, there would have been a difference in insulin requirements between dietary groups once the ideal insulin dosages were reached. It is also possible that the non-responder population may have been too small to statistically reflect differences in insulin requirements between dietary groups.
Although cats in both dietary groups experienced improved clinical signs and achieved lower serum fructosamine concentrations, significantly more cats in the LC–LF group demonstrated clinical resolution of their diabetes mellitus. Overall, more of the LC–LF cats were well-regulated at the end of the study than MC–HF cats. While it is impossible to determine which dietary constituent(s) most influenced the positive effect of these diets, carbohydrates may be the most important factor as the diets differed most in the amount of carbohydrate present on an as-fed, caloric basis. In support of this theory, the majority (87%) of the cats in this study, prior to their entry into the study, had been fed at least some dry food in their diet. The vast majority of dry foods are higher in carbohydrate content compared to an equivalent canned product. Thus, many of these cats may have ingested fewer carbohydrates when switched to a strictly canned food diet upon entry into the study. This decrease in carbohydrate content may have contributed to improvements seen in cats in both dietary groups. Further studies using controlled, experimental foods are required to better understand the role each nutrient plays in the management of diabetes mellitus in cats.
Cats can have transient diabetes triggered by stress, drugs or concurrent disease that will go into remission with treatment of glucose toxicity and resolution of concurrent stress. Resolution of transient illness or stress-induced feline diabetes generally occurs within the first 4–6 weeks after initial diagnosis is made (Feldman and Nelson 2004). Therefore, cats diagnosed within this approximate 45-day window were grouped together to determine if they were more likely to go into remission. Mortality rate of diabetic cats is reportedly higher within the first 6–12 months of diagnosis. One study reported a median survival time of 17 months and a mean survival time of 24 months for cats diagnosed with diabetes mellitus (Goossens et al 1998) whereas others report good quality of life for more than 5 years (Feldman and Nelson 2004). In order to retain enough cats with long-term disease duration for analysis, the cut-off for the moderate duration disease group was placed at 12 months. Cats diagnosed with diabetes mellitus more than 12 months (14–72 months) prior to entry into the study were thus placed in the long-term duration group.
Although it is possible that the diabetes in the cats included in this study went into remission, regardless of the diet provided, many of the responders in this study did not revert to a non-insulin-dependent state until weeks 12 through 16. Transient undiagnosed illness or stress-induced diabetes would be more unlikely in those cats. In addition, the majority of the cats in this study had moderate or long-term disease. These cats had been diagnosed with diabetes, and treated for their diabetes, for months to years before entering this study. Cats that had been diagnosed with diabetes up to 30 months prior reverted to a non-insulin-dependent state in this study. Because there were significantly more responders amongst the cats eating the LC–LF diet, it suggests that the diet truly was a contributing factor in glycemic control and clinical resolution. It is important to note that none of the cats that had been diabetic for more than 30 months went into remission. These cats may have lost any residual pancreatic beta cell function over the long-term duration of their disease. It remains unknown as to whether these cats were type 1 or type 2 diabetics.
It is possible that more stringent monitoring, frequent client communication, client compliance and appropriate insulin dose adjustment succeeded in maintaining lower blood glucose concentrations in these cats and reversing glucose toxicity. This likely contributed to the rate of diabetic remission seen in this study. However, each cat was monitored in a similar manner, and dosage adjustments were made for each cat based on the criteria outlined in the Materials and methods. Thus, these factors cannot explain the difference in the number of remissions recorded within each of the dietary groups.
In conclusion, the results of this study suggest that dietary therapy may play a discernable role in the control of clinical signs and the need for exogenous insulin in cats with diabetes mellitus. Cats fed the canned LC–LF diet were significantly more likely to revert to a non-insulin-dependent state than cats fed the canned MC–HF diet (68% versus 41% of cats). In addition, cats were significantly more likely to be well-regulated on the canned LC–LF diet than cats fed the canned MC–HF diet (81% versus 56% of cats). Both canned high fiber and low-carbohydrate foods are acceptable alternatives in the management of diabetes mellitus in cats. However, cats may be more likely to be well-regulated or revert to a non-insulin-dependent state when fed a canned low-carbohydrate food.
References
- Anderson J.W., Akanji A.O. Dietary fiber – an overview, Diabetes Care 14, 1991, 1126–1131. [DOI] [PubMed] [Google Scholar]
- Appleton D.J., Rand J.S., Sunvold G.D. Insulin sensitivity decreases with obesity, and lean cats with low insulin sensitivity are at greatest risk of glucose intolerance with weight gain, Journal of Feline Medicine and Surgery 3, 2001, 211–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biourge V., Nelson R.W., Feldman E.C., Willits N.H., Morris J.G., Rogers Q.R. Effect of weight gain and subsequent weight loss on glucose intolerance and insulin response in healthy cats, Journal of Veterinary Internal Medicine 11, 1997, 86–91. [DOI] [PubMed] [Google Scholar]
- Miller J.C. Brand, Colagiuri S. The carnivore connection: dietary carbohydrate in the evolution of NIDDM, Diabetologia 37, 1994, 1280–1286. [DOI] [PubMed] [Google Scholar]
- Carlstead K., Brown J.L., Strawn W. Behavioral and physiological correlates of stress in laboratory cats, Applied Animal Behavioral Science 38, 1993, 143–158. [Google Scholar]
- Ciaraldi T.P., Molina J.M., Olefsky J.M. Insulin action kinetics in adipocytes from obese and non-insulin-dependent diabetes mellitus subjects: identification of multiple cellular defects in glucose transport, Journal of Clinical Endocrinology and Metabolism 72, 1991, 876–882. [DOI] [PubMed] [Google Scholar]
- Costacou T., Mayer-Davis E.J. Nutrition and prevention of type 2 diabetes, Annual Review of Nutrition 23, 2003, 147–170. [DOI] [PubMed] [Google Scholar]
- Crenshaw K.L., Peterson M.E., Heeb L.A., Moroff S.D., Nichols R. Serum fructosamine concentration as an index of glycemia in cats with diabetes mellitus and stress hyperglycemia, Journal of Veterinary Internal Medicine 10, 1996, 360–364. [DOI] [PubMed] [Google Scholar]
- DeFronzo R.A., Bonadonna R.C., Ferrannini E. Pathogenesis of NIDDM. A balanced overview, Diabetes Care 15, 1992, 318–368. [DOI] [PubMed] [Google Scholar]
- Elliot D.A., Nelson R.W., Reusch C.E., Feldman E.C., Neal L.A. Comparison of serum fructosamine and blood glycosolated hemoglobin concentrations for assessment of glycemic control in cats with diabetes mellitus, Journal of the American Veterinary Medical Association 214, 1999, 1794–1798. [PubMed] [Google Scholar]
- Farrow H.A., Rand J.S., Sunvold G.D. (2002) The effect of high protein, high fat or high carbohydrate diets on postprandial glucose and insulin concentrations in normal cats. Abstract No 133, Proceedings 20th ACVIM, 794.
- Feldman E.C., Nelson R.W. Canine and Feline Endocrinology and Reproduction, 2004, Saunders: St Louis, MS. [Google Scholar]
- Feskens E.J.M., Loeber J.G., Kromhut D. Diet and physical activity as determinants of hyperinsulinemia, American Journal of Epidemiology 140, 1994, 350–360. [DOI] [PubMed] [Google Scholar]
- Fleeman L.M., Rand J.S. Evaluation of day-to-day variability of serial blood glucose concentration curves in diabetic dogs, Journal of the American Veterinary Medical Association 22, 2003, 317–321. [DOI] [PubMed] [Google Scholar]
- Frank G., Anderson W., Pazak H., Hodgkins E., Ballam J., Laflamme D. Use of a high-protein food in the management of feline diabetes mellitus, Veterinary Therapeutics 2, 2001, 238–246. [PubMed] [Google Scholar]
- Goossens M.M.C., et al. Response to insulin treatment and survival in 104 cats with diabetes mellitus (1985–1995), Journal of Veterinary Internal Medicine 12, 1998. [DOI] [PubMed] [Google Scholar]
- Hand M.S., Thatcher C.D., Remillard R.L., Roudebush P. Small Animal Clinical Nutrition, 4th edn, 2000, Mark Morris Institute: Topeka, KS. [Google Scholar]
- Johnson K.H., Hayden D.W., O'Brien T.D., Westermark P. Spontaneous diabetes-mellitus-islet amyloid complex in adult cats, American Journal of Pathology 125, 1986, 416–419. [PMC free article] [PubMed] [Google Scholar]
- Kaneko J.J., Kawamoto M., Heusner A.A., Feldman E.C., Koizumi I. Evaluation of serum fructosamine concentration as an index of blood glucose control in cats with diabetes mellitus, American Journal of Veterinary Research 53, 1992, 1797–1801. [PubMed] [Google Scholar]
- Kirk C.A., Feldman E.C., Nelson R.W. Diagnosis of naturally acquired type-I and type-II diabetes mellitus in cats, American Journal of Veterinary Research 54, 1993, 463–467. [PubMed] [Google Scholar]
- Link K.R.J., Rand J.S. Glucose tolerance status of 57 cats from southeastern Queensland, Journal of the American Veterinary Medical Association 213, 1998, 492–496. [PubMed] [Google Scholar]
- Lutz T.A., Rand J.S. A review of new developments in type 2 diabetes in humans and in cats, British Veterinary Journal 149, 1993, 527–536. [DOI] [PubMed] [Google Scholar]
- Lutz T.A., Rand J.S. Pathogenesis of feline diabetes mellitus, Veterinary Clinics of North America Small Animal Practice 25, 1995, 527–552. [DOI] [PubMed] [Google Scholar]
- Marshall J.A., Hamman R.F., Baxter J. High-fat, low-carbohydrate diet and the etiology of non-insulin-dependent diabetes mellitus: the San Luis Valley Diabetes Study, American Journal of Epidemiology 134, 1991, 590–603. [DOI] [PubMed] [Google Scholar]
- Marshall J.A., Weiss N.S., Hamman R.F. Role of dietary fiber in the etiology of non-insulin-dependent diabetes mellitus, Annals of Epidemiology 3, 1993, 18–26. [DOI] [PubMed] [Google Scholar]
- Mazzaferro E.M., Greco D.S., Turner A.S., Fettman M.J. Treatment of feline diabetes mellitus using an α-glucosidase inhibitor and a low-carbohydrate diet, Journal of Feline Medicine and Surgery 5, 2003, 183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R.W., Himsel C.A., Feldman E.C., Bottoms G.D. Glucose tolerance and insulin response in normal weight and obese cats, American Journal of Veterinary Research 51, 1990, 1357–1362. [PubMed] [Google Scholar]
- Nelson R.W., Scott-Moncrieff J.C., Feldman E.C., DeVries-Concannon S.E., Kass P.H., Davenport D.J., Kiernan C.T., Neal L.A. Effect of dietary insoluble fiber on control of glycemia in cats with naturally acquired diabetes mellitus, Journal of the American Veterinary Medical Association 216, 2000, 1082–1088. [DOI] [PubMed] [Google Scholar]
- O'Brien T.D., Hayden D.W., Johnson K.H., Stevens J.B. High-dose intravenous glucose tolerance test and serum insulin and glucagon levels in diabetic and non-diabetic cats: relationships to insular amyloidosis, Veterinary Pathology 22, 1985, 250–261. [DOI] [PubMed] [Google Scholar]
- Panciera D.L., Thomas C.B., Eicker S.W., Atkins C.E. Epizootiologic patterns of diabetes mellitus in cats, 333 cases (1980–1986), Journal of the American Veterinary Medical Association 197, 1990, 1504–1508. [PubMed] [Google Scholar]
- Papa P.C., Seraphim P.M., Machado U.F. Loss of weight restores GLUT 4 content in insulin-sensitive tissues of monosodium glutamate-treated obese mice, International Journal of Obesity 21, 1997, 1065–1070. [DOI] [PubMed] [Google Scholar]
- Parker B., Noakes M., Luscombe N., Clifton P. Effect of a high-protein, high-monosaturated fat weight loss diet on glycemic control and lipid levels in type 2 diabetes, Diabetes Care 25, 2002, 425–430. [DOI] [PubMed] [Google Scholar]
- Plier M.L., Grindem C.B. Serum fructosamine concentration in non-diabetic and diabetic cats, Veterinary Clinical Pathology 27, 1998, 34–39. [DOI] [PubMed] [Google Scholar]
- Rand J.S., Kinnaird E., Baglioni A., Blackshaw J., Priest J. Acute stress hyperglycemia in cats associated with struggling and increased concentrations of lactate and norepinephrine, Journal of Veterinary Internal Medicine 16, 2002, 123–132. [DOI] [PubMed] [Google Scholar]
- Reusch C., Hoyer O.M. The importance of fructosamine analysis in the control of diabetes mellitus. Investigations in normal and diabetic cats as well as with so-called stress induced hyperglycemia, Kleintierpraxis 40, 1995, 95–100. [Google Scholar]
- Thoresen S.I., Bredal W.P. Clinical usefulness of fructosamine measurements in diagnosing and monitoring feline diabetes mellitus, Journal of Small Animal Practice 37, 1996, 64–68. [DOI] [PubMed] [Google Scholar]